Abstract
Accumulation of the amyloid-β (Aβ) peptide depends on both its generation and clearance. To better define clearance pathways, we have evaluated the role of the tissue plasminogen activator (tPA)-plasmin system in Aβ degradation in vivo. In two different mouse models of Alzheimer's disease, chronically elevated Aβ peptide in the brain correlates with the upregulation of plasminogen activator inhibitor-1 (PAI-1) and inhibition of the tPA-plasmin system. In addition, Aβ injected into the hippocampus of mice lacking either tPA or plasminogen persists, inducing PAI-1 expression and causing activation of microglial cells and neuronal damage. Conversely, Aβ injected into wild-type mice is rapidly cleared and does not cause neuronal degeneration. Thus, the tPA-plasmin proteolytic cascade aids in the clearance of Aβ, and reduced activity of this system may contribute to the progression of Alzheimer's disease.
Keywords: Alzheimer's disease, neurodegeneration, protease, amyloid-β degradation, plasmin, PAI-1
Introduction
Alzheimer's disease (AD), the most common form of human amyloidosis, is the leading cause of cognitive decline and dementia in aged individuals. AD patients exhibit characteristic pathologies including extracellular senile plaques composed mainly of the amyloid-β (Aβ) peptide, intracellular neurofibrillary tangles of hyperphosphorylated tau protein, and localized deposition of amyloid in the blood vessels of the brain. The Aβ peptide comes from the proteolytic processing of a larger molecule, the Aβ precursor protein (AβPP). Excessive production or reduced degradation of the Aβ peptide can lead to its accumulation and the formation of senile plaques in Alzheimer's disease. Various proteases have been implicated in Aβ degradation, including neprilysin, insulin-degrading enzyme (IDE), and plasmin (Selkoe, 2001).
Plasminogen activators (PAs) are serine proteases, the main function of which is to activate plasminogen into plasmin. There are two types of mammalian PAs: tissue-type (tPA) and urokinase-type (uPA) (Collen, 2001). tPA is expressed in various regions of the mouse brain, including the hippocampus, amygdala, cerebellum, and hypothalamus (Sappino et al., 1993), where it participates in both normal and pathological events (Baranes et al., 1998; Madani et al., 1999; Tsirka et al., 1995). In particular, tPA is highly expressed in the hippocampus, a region important for learning and memory and often involved in AD pathology.
Numerous reports have suggested that the tPA-plasmin system may play a role in AD. For example, in vitro studies have linked the tPA-plasmin system with Aβ turnover (Kingston et al., 1995; Tucker at al, 2000a,b). Enzymatic studies have shown that fibrillar Aβ stimulates tPA activity in vitro (Kingston et al., 1995), and Aβ can stimulate tPA mRNA expression in cell culture, resulting in the production of plasmin and subsequent Aβ degradation (Tucker et al., 2000a,b). Because the in vivo significance of the tPA-plasmin system in AD is not known, we investigated this in mouse models of AD and in mice deficient for either tPA or plasminogen. The results indicate that the tPA-plasmin system contributes to Aβ degradation and pathology.
Materials and Methods
AD transgenic mouse models. Transgenic mice were obtained from Taconic Farms (Tg2576) (Hsiao et al., 1996) and Drs. M. A. Chishti and D. Westaway (University of Toronto, Toronto, Ontario) (TgCRND8) (Chishti et al., 2001). The Tg2576 mice express AβPP695 with the Swedish mutation driven by the hamster prion promoter (PrP). The TgCRND8 mice express AβPP695 with both the Swedish and V717F mutations also driven by the hamster PrP. Brains from 3-month-old TgCRND8 and 12-month-old Tg2576 mice (ages when overt amyloid deposition is present) and from their respective age-matched nontransgenic litter-mates were obtained from animals that were anesthetized with 2.5% avertin (15 μl/g mouse body weight) in sterile saline and transcardially perfused with ice-cold sterile PBS. Brains were quickly embedded in Optimum Cutting Temperature medium (Sakura Finetek, Torrance, CA), frozen with dry ice, and cut into 20 μm coronal sections for either immunohistochemistry or in situ zymography. In situ zymography to assess tPA activity was performed according to Sappino et al. (1993). Zymographic activity of tPA was viewed using dark-field microscopy. Lytic zones in specific brain regions (hippocampus, amygdala) were measured using NIH Image software.
Mouse surgery and Aβ peptide injection. tPA-/- and plg-/- mice were obtained from Drs. D. Collen and P. Carmeliet (Catholic University of Leuven, Leuven, Belgium). Both tPA-/- and plg-/- mice were backcrossed to the C57BL/6 background for nine generations. Animals were anesthetized with 2.5% avertin (15 μl/g body weight) and atropine (2 μl/g body weight). For injection into the hippocampus, the coordinates relative to bregma (anterior-posterior -2.5 mm, lateral 1.7 mm, and 1.8 mm depth) were taken from Franklin and Paxinos (1997) and measured using a Kopf Company stereotaxic instrument. Aβ40 peptide (American Peptide Company, Sunnyvale, CA) freshly solubilized to a final concentration of 2 μg/μl in PBS or PBS alone was injected into the CA1 region of the hippocampus of 10- to 12-week-old female C57BL/6, tPA-/-, and plg-/- mice. The Aβ40 peptide was injected into the hippocampus at a rate of 200 nl/min using a 33 gauge Hamilton syringe. The mice were allowed to recover for 1, 2, or 3 d before brain isolation.
Immunohistochemistry and histology. For immunostaining, sections were fixed in 4% paraformaldehyde/PBS and blocked with PBS containing 10% goat serum, 1% BSA, and 0.5% Triton X-100. Sections were incubated with rabbit polyclonal anti-Aβ antibody (1:1000) (Biosource, Camarillo, CA), polyclonal anti-plasminogen activator inhibitor 1 (PAI-1) antibody (provided by Dr. D. Loskutoff, Scripps Institute, San Diego, CA), rat anti-mouse F4/80 antibody (Serotec, Raleigh, NC), or P2-1, a monoclonal antibody directed against the N-terminal end of AβPP (gift from Dr. William E. Van Nostrand, University of Stony Brook, Stony Brook, NY), and placed in a humidified chamber at 4°C overnight. The next day, sections were rinsed with PBS/Triton X-100 and incubated with the appropriate secondary antibody (1:500) followed by either the FITC-conjugated goat anti-rabbit IgG (Vector Laboratories, Burlingame, CA) or the ABC kit according to the manufacturer's protocol (Vector Laboratories). Immunoreactivity was visualized using either fluorescence microscopy or Nova-Red according to the manufacturer's protocol (Vector Laboratories). The monoclonal antibody P2-1 was used with the Mouse on Mouse Staining kit according to manufacturer's protocol (Vector Laboratories). When using fluorescence microscopy, all images were viewed and photographed using identical exposures and magnifications. The images were imported into Photoshop and converted to grayscale. The area and intensity of Aβ deposition and F4/80 immunoreactivity were quantified using NIH Image, and the mean and SEM were calculated for each animal group (at least two sections per animal; n = 5-7 per experimental group).
Fluoro-Jade B staining was performed according to the protocol by Schmued and Hopkins (2000) and viewed under fluorescence microscopy. Briefly, sections were immersed sequentially in 1% NaOH in 80% alcohol for 2 min, 70% alcohol for 2 min, water for 2 min, 0.06% potassium permanganate for 10 min, water for 2 min, and 0.0004% Fluoro-Jade B in 1% acetic acid for 20 min, and rinsed in water three times. The sections were then dried, immersed in xylene, and mounted with p-xylene bis-(N-pyridinium bromide). The sections were viewed under blue-green excitation light with a fluorescent microscope. Images were obtained using identical magnification and exposures and quantified as above.
Statistical analysis. The data were analyzed by ANOVA and Newman-Keuls post hoc comparison. A value for p < 0.05 was considered significant.
Results
Decreased tPA activity in transgenic AD mouse models
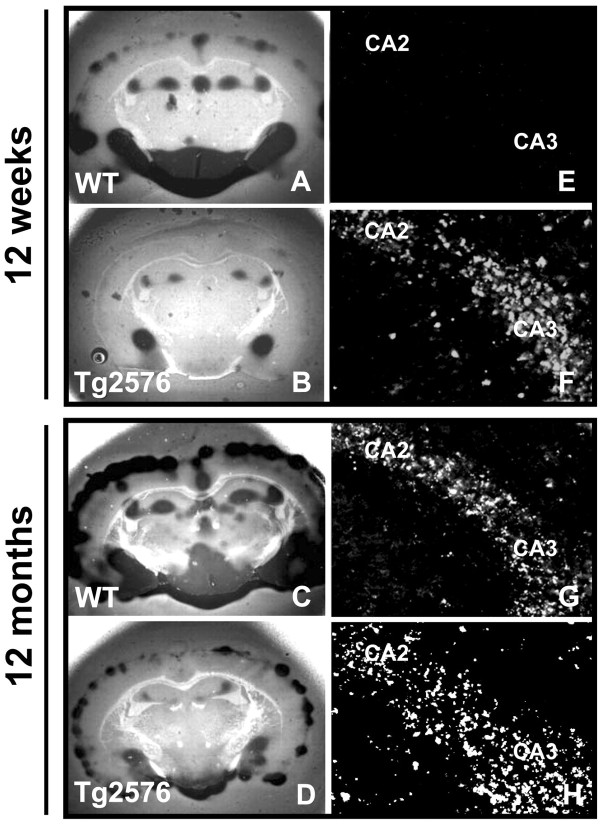
Enzymatic studies have shown that fibrillar Aβ stimulates tPA activity (Kingston et al., 1995), and in cell culture, Aβ can stimulate tPA mRNA expression resulting in the production of plasmin and subsequent Aβ degradation (Tucker et al., 2000a,b). To investigate the in vivo significance of the tPA-plasmin system in clearing Aβ, we examined the level of tPA activity in transgenic AD mouse models. One model was the Tg2576 mouse, which exhibits amyloid plaques and cognitive deficit at 12 months of age (Hsiao et al., 1996). Tg2576 and age-matched control mice were used for in situ zymography, which measures tPA activity in a brain section through plasmin cleavage of casein in the overlay gel (Sappino et al., 1993). tPA activity in the amygdala, cortex, hypothalamus, and hippocampus was decreased in both 12-week-old (Fig. 1B) and 12-month-old Tg2576 mice (Fig. 1D) as compared with age-matched controls (Fig. 1A,C) (quantified in Fig. 2, open bars).
Figure 1.
tPA activity is decreased and PAI-1 expression increased in Tg2576 mice. Tg2576 and age-matched wild-type (WT) mice brain sections (n = 5-7) were subjected to in situ zymography to measure tPA activity. Dark, lytic zones are smaller in the hippocampus, amygdala, and hypothalamus of 12-week-old (B) and 12-month-old (D) Tg2576 mice as compared with wild-type mice (A, C). Twelve-week-old Tg2576 mice show increased PAI-1 immunoreactivity (F), whereas age-matched littermate controls do not (E). At 12 months, both Tg2576 (H) and littermate nontransgenic “wild-type” (G) show similar levels of PAI-1 expression in the hippocampus. CA2 and CA3 indicate respective hippocampal subregions.
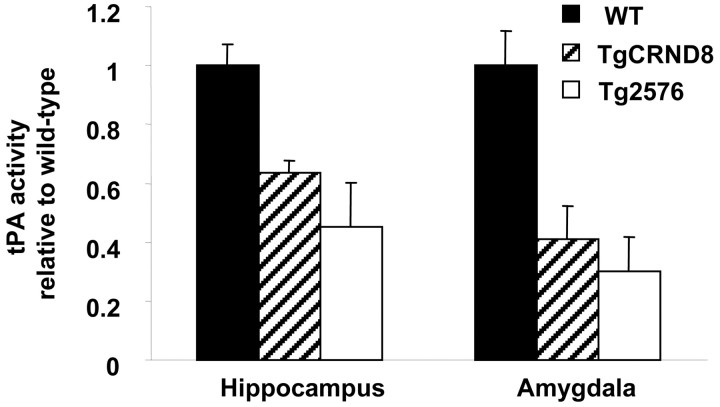
Figure 2.
Quantification of tPA activity in the hippocampus and amygdala of TgCRND8 mice (hatched bars), Tg2576 mice (open bars), and their age-matched littermate nontransgenic controls (WT, solid bars). The two sets of controls were equivalent and averaged for this figure. See Results for detailed statistical analysis.
This decrease in tPA activity was also observed in the hippocampus and amygdala in another AD mouse model, the TgCRND8 mice (Fig. 2, hatched bars); this mouse exhibits AD-like pathology and cognitive deficiency at 3 months (Chishti et al., 2001). ANOVA revealed a strong effect of the genotype on tPA activity in both the amygdala and hippocampus (F(2,9) = 10.00, p < 0.05, and F(2,9) = 9.98, p < 0.005, respectively). Newman-Keuls post hoc comparison showed a difference between wild-type and both Tg2576 (p < 0.05 for amygdala and p < 0.01 for hippocampus) and TgCRND8 mice (p < 0.05 for amygdala and p < 0.01 for hippocampus). These results are consistent with the report that plasmin levels are decreased in the brains of AD patients (Ledesma et al., 2000).
Increased PAI-1 levels in AD transgenic mice
In Tg2576 mice, tPA mRNA expression is reportedly elevated (Tucker et al., 2000b), and the observed decrease in tPA activity could be caused by overexpression of an inhibitor. Therefore, we investigated the expression of the serpin PAI-1, a main inhibitor of tPA. PAI-1 is an acute-phase protein often upregulated during inflammation, a response normally triggered in AD brains (Podor et al., 1992). Immunohistochemistry for PAI-1 was performed to identify specific brain regions that expressed this protein. PAI-1 expression was elevated in 12-week-old Tg2576 mice (Fig. 1F) but not in age-matched wild-type mice (Fig. 1E). PAI-1 was elevated in regions where tPA activity was depressed, such as the CA regions of the hippocampus. This result indicates that increased Aβ levels correlate with the upregulation of PAI-1. At 12 months, wild-type and Tg2576 mice showed increased PAI-1 levels (Fig. 1G,H); however, wild-type mice compensated for elevated PAI-1 expression with an increase in tPA activity, because there was no change in plasminogen activation (Fig. 1A,C). AD transgenic mice exhibit the elevation of PAI-1 and concomitant depression of tPA activity before evident Aβ plaque formation, but in an environment of increased Aβ load.
Aβ40 peptide can increase PAI-1 expression
To test whether Aβ40 could induce expression of PAI-1 and thereby reduce tPA activity in the brain, we injected 2 μg of Aβ40 (in 1 μl PBS) into the CA1 region of the hippocampus (Shin et al., 1997) of 10- to 12-week-old tPA-/-, plg-/-, and C57BL/6 female mice. For 3 d, brains of injected mice were isolated daily, and coronal sections were prepared. PAI-1 was only weakly induced in the wild-type mice (Fig. 3A), but it was strongly upregulated in the Aβ40-injected areas of tPA-/- and plg-/- mice (Fig. 3B,C).
Figure 3.
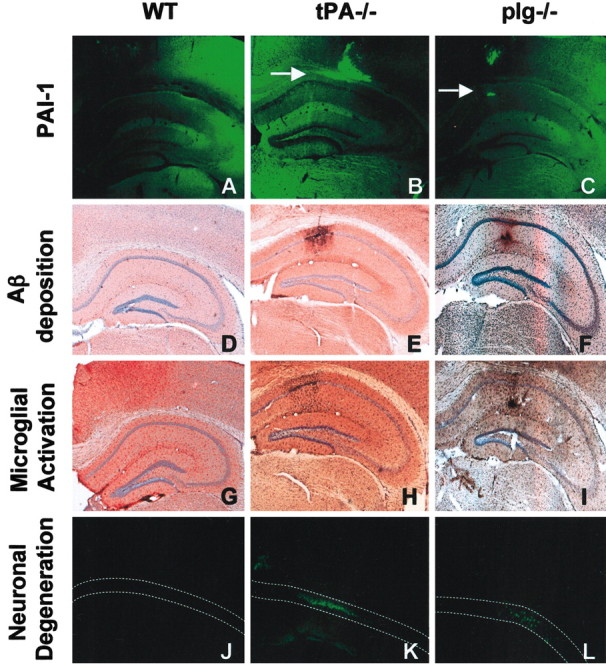
Mice deficient for tPA or plasminogen show PAI-1 induction and persistent Aβ deposition after Aβ injection. tPA-/-, plg-/-, and C57BL/6 (WT) mice (n = 5-7) were injected with Aβ40 into the CA1 region of the hippocampus. PAI-1 expression (bright green fluorescence, highlighted with arrows) was upregulated in the presence of Aβ in the tPA-/- (B) and plg-/- (C) mice but not in wild-type mice (A). Immunohistochemical analysis shows the presence of Aβ in tPA-/- (E) and plg-/- (F) but not in wild-type (D) mice 3 d after injection. The Aβ40 deposited in tPA-/- and plg-/- mice causes microglial activation as assessed by F4/80 staining (H and I, respectively; compare with G) and neuronal degeneration as assessed by Fluoro-Jade B staining (K and L, respectively; compare with J).
Rapid clearance of the Aβ40 peptide from the mouse hippocampus requires tPA and plasminogen
One reason for the lack of PAI-1 induction in wild-type mice could be rapid degradation of Aβ by the tPA-plasminogen system. Given that an increase in Aβ level was correlated with a decrease in tPA activity, we tested whether the tPA-plasminogen system could degrade Aβ40 in the parenchyma. After intrahippocampal injection in wild-type mice, Aβ immunoreactivity gradually disappeared for the first 2 d (data not shown) and was completely cleared by day 3 (Fig. 3D). In contrast, Aβ immunoreactivity was evident in both tPA-/- and plg-/- mice (Fig. 3E,F) 3 d after injection, indicating that the tPA-plasmin system participates in Aβ degradation. Aβ immunoreactivity was ∼10-fold higher in both tPA-/- and plg-/- as compared with wild-type mice 3 d after injection (Fig. 4A). ANOVA showed an effect of the genotype on Aβ clearance (F(2,16) = 10.58; p < 0.01). Newman-Keuls post hoc comparison showed a significant difference between wild-type and tPA-/- (p < 0.01) and wild-type and plg-/- (p < 0.01) mice. By day 7 after Aβ injection, the peptide was cleared from the brains (data not shown), indicating that other proteases contribute to Aβ degradation. Thus, there was a positive correlation between the duration of Aβ40 deposition, the induction of PAI-1, and the inhibition of tPA, suggesting a role for the tPA-plasmin system in Aβ clearance.
Figure 4.
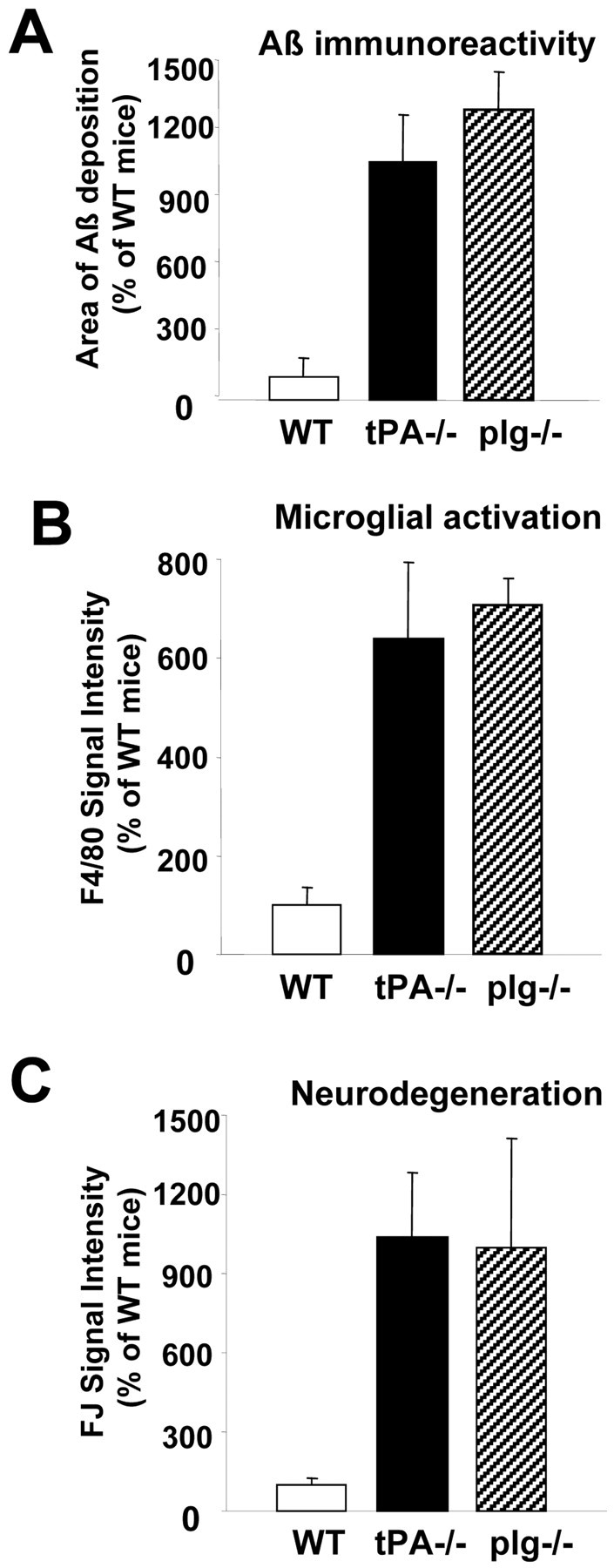
Quantification of Aβ immunoreactivity (A), microglial activation (B), and neurodegeneration (C) in wild-type (open bars), tPA-/- (solid bars), and plg-/- mice (hatched bars). See Results for detailed statistical analysis.
Microglial activation and neurodegeneration in tPA-/- and plg-/- mice after Aβ40 injection
The deposition of Aβ in the parenchyma triggers an inflammatory response in the adjacent parenchyma and eventually causes neuronal death (Frautschy et al., 1998). In both tPA-/- and plg-/- mice, activated microglial cells were detected near the sites of Aβ injection after 3 d (Fig. 3H,I), whereas control animals did not show activated microglia (Fig. 3G). The intensity of microglial staining was at least sixfold greater in both tPA-/- and plg-/- mice as compared with wild-type mice (Fig. 4B). ANOVA also showed genotype effects in the activation of microglial cells (F(2,10) = 7.97; p < 0.01). Newman-Keuls post hoc comparison also showed differences between wild-type and tPA-/- (p < 0.05) and wild-type and plg-/- (p < 0.05) mice.
Consistent with these findings, the hippocampi of tPA-/- and plg-/- mice exhibited neuronal degeneration at the sites around the Aβ deposition as assessed by Fluoro-Jade B (Fig. 3K,L) and Cresyl violet staining (data not shown), whereas wild-type mice did not show neuronal death (Fig. 3J). Again, neurodegeneration was almost nonexistent in wild-type mice, whereas both tPA-/- and plg-/- mice exhibited ∼10-fold greater neuronal damage (Fig. 4C). Statistical analysis showed a difference in genotype response to neuronal damage (F(2,19) = 3.943; p < 0.05), and Newman-Keuls post hoc analysis indicated differences in wild-type versus either tPA-/- (p < 0.05) or plg-/- (p < 0.05) neurodegeneration. Microglial activation and Fluoro-Jade B staining was negligible in the needle tracks of animals injected with PBS (data not shown). Therefore, the tPA-plasmin system was required to efficiently clear Aβ injected into the hippocampus and prevent neuronal degeneration.
Discussion
Proteases take part in both the generation and catabolism of the Aβ peptide and therefore are logical targets for drugs attenuating Alzheimer's disease. The Aβ peptide is produced by the sequential proteolytic processing of AβPP by the β-site APP cleaving enzyme (Vassar et al., 1999) and presenilins (Wolfe et al., 1999). Presenilin-null neurons are deficient in Aβ production (Zhang et al., 2000), and presenilin inhibitors are effective in preventing Aβ formation in cell culture (Beher et al., 2001); however, the clinical use of these inhibitors might lead to side effects such as decreased Notch processing (Geling et al., 2002; Roncarati et al., 2002).
Proteases that degrade Aβ are being considered as possible targets for inhibiting AD pathogenesis; neprilysin, IDE, and uPA have been implicated in Aβ clearance (Selkoe, 2001). Both uPA and tPA activate the zymogen plasminogen to the active protease plasmin, which cleaves Aβ in vitro; however, a role for plasminogen activation in Alzheimer's disease has not been studied in vivo.
In an environment of increased Aβ load in mouse models of AD, we found a dramatic reduction in tPA activity in the hippocampus and amygdala, areas affected in AD patients. This reduction of tPA activity (and diminished plasminogen to plasmin conversion) was caused by upregulation of PAI-1, which was elevated in AD mouse models and induced at the sites of Aβ injection. Consistent with these results, PAI-1 levels in the cerebrospinal fluid are elevated in certain neurological pathologies, including dementia caused by AD (Sutton et al., 1994). Therefore, the inhibition of the tPA-plasmin system by PAI-1 could facilitate Aβ deposition by slowing down the clearance of the peptide (Fig. 4). This mechanism could start a vicious cycle of accumulating Aβ, leading to increased PAI-1 expression, further tPA inhibition, and therefore even more Aβ deposition. Although the increase in PA activity could be attributable to uPA in wild-type mice as compared with AβPP transgenic mice, the addition of amiloride, an inhibitor of uPA but not tPA (Vassalli and Belin, 1987), into the overlay gel for wild-type mice did not decrease PA activity (data not shown), indicating that the activity was attributable to tPA. Another possibility is that the decrease in tPA activity in AβPP transgenic mice was attributable to increased AβPP and not Aβ. The two longer isoforms of AβPP can be alternatively processed into protease nexin II, a serpin that could inhibit tPA (Van Nostrand et al., 1989, 1991). Both of the transgenic lines used here, however, express the shorter 695 amino acid isoform of AβPP. Additionally, immunostaining for both AβPP and tPA did not colocalize in the AD transgenic mice (data not shown).
There is precedence for both a harmful or protective role of the tPA-plasmin system in neurological insults, depending on the primary substrate specific for a given pathology. For example, when the blood-brain barrier (or the blood-nerve barrier in the peripheral nervous system) breaks down and fibrin is deposited, the tPA-plasmin system accelerates clearance of fibrin and can be protective (Akassoglou et al., 2002). Similarly, in stroke models induced by obstructing brain arteries with fibrin-rich clots, the tPA-plasmin system helps recanalize the vessels and protects the brain from damage (Tabrizi et al., 1999).
tPA, however, can play a deleterious role as well (Pawlak and Strickland, 2002). Excitotoxic insult leads to the induction of tPA mRNA and protein expression (Qian et al., 1993; Tsirka et al., 1996), which results in the cleavage of laminin by plasmin and neuronal damage (Chen and Strickland, 1997). In a mouse model for stroke without the formation of clots or during a permanent occlusion, the tPA-plasmin system can be deleterious if it leaks into the brain parenchyma (Wang et al., 1998; Nagai et al., 1999).
Alzheimer's disease represents a situation of pathological deposition of a substrate for the tPA-plasmin system in the brain. The data here indicate that the tPA-plasmin system contributes to the clearance of Aβ injected into the mouse brain and the prevention of neurotoxicity of the peptide that may be important in slowing the progression of Alzheimer's disease.
Footnotes
This work was funded by a grant from the Institute for the Study of Aging (S.S.), grants from the National Institutes of Health (S.S.), and a National Institute on Aging National Research Service Award postdoctoral fellowship (J.P.M.). We thank Peter Mercado and Yuliya Keptsi for technical assistance and Dr. Zu-Lin Chen and the Strickland laboratory members for critical reading of this manuscript. We also thank Dr. M. Azhar Chishti and Dr. David Westaway for the TgCRND8 mice, Dr. David Loskutoff for the anti-PAI-1 antibody, and Dr. William E. Van Nostrand for the anti-AβPP antibody, P2-1.
Correspondence should be addressed to Sidney Strickland, Laboratory of Neurobiology and Genetics, The Rockefeller University, 1230 York Avenue, New York, NY 10021. E-mail: strickland@rockefeller.edu.
Copyright © 2003 Society for Neuroscience 0270-6474/03/238867-05$15.00/0
References
- Akassoglou K, Yu WM, Akpinar P, Strickland S ( 2002) Fibrin inhibits peripheral nerve remyelination by regulating Schwann cell differentiation. Neuron 33: 861-875. [DOI] [PubMed] [Google Scholar]
- Baranes D, Lederfein D, Huang YY, Chen M, Bailey CH, Kandel ER ( 1998) Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron 21: 813-825. [DOI] [PubMed] [Google Scholar]
- Beher D, Wrigley JD, Nadin A, Evin G, Masters CL, Harrison T, Castro JL, Shearman MS ( 2001) Pharmacological knock-down of the presenilin 1 heterodimer by a novel gamma-secretase inhibitor: implications for presenilin biology. J Biol Chem 276: 45394-45402. [DOI] [PubMed] [Google Scholar]
- Chen ZL, Strickland S ( 1997) Neuronal death in the hippocampus is promoted by plasmin-catalyzed degradation of laminin. Cell 91: 917-925. [DOI] [PubMed] [Google Scholar]
- Chishti MA, Yang DS, Janus C, Phinney AL, Horne P, Pearson J, Strome R, Zuker N, Loukides J, French J, Turner S, Lozza G, Grilli M, Kunicki S, Morissette C, Paquette J, Gervais F, Bergeron C, Fraser PE, Carlson GA, George-Hyslop PS, Westaway D ( 2001) Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem 276: 21562-21570. [DOI] [PubMed] [Google Scholar]
- Collen D ( 2001) Ham-Wasserman lecture: role of the plasminogen system in fibrin-homeostasis and tissue remodeling. Hematology 2001: 1-9. [DOI] [PubMed] [Google Scholar]
- Franklin KJ, Paxinos G ( 1997) The mouse brain in stereotaxic coordinates. San Diego: Academic.
- Frautschy SA, Horn DL, Sigel JJ, Harris-White ME, Mendoza JJ, Yang F, Saido TC, Cole GM ( 1998) Protease inhibitor coinfusion with amyloid beta-protein results in enhanced deposition and toxicity in rat brain. J Neurosci 18: 8311-8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geling A, Steiner H, Willem M, Bally-Cuif L, Haass C ( 2002) A gamma-secretase inhibitor blocks Notch signaling in vivo and causes a severe neurogenic phenotype in zebrafish. EMBO Rep 3: 688-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G ( 1996) Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science 274: 99-102. [DOI] [PubMed] [Google Scholar]
- Kingston IB, Castro MJ, Anderson S ( 1995) In vitro stimulation of tissue-type plasminogen activator by Alzheimer amyloid beta-peptide analogues. Nat Med 1: 138-142. [DOI] [PubMed] [Google Scholar]
- Ledesma MD, Da Silva JS, Crassaerts K, Delacourte A, De Strooper B, Dotti CG ( 2000) Brain plasmin enhances APP alpha-cleavage and Abeta degradation and is reduced in Alzheimer's disease brains. EMBO Rep 1: 530-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madani R, Hulo S, Toni N, Madani H, Steimer T, Muller D, Vassalli JD ( 1999) Enhanced hippocampal long-term potentiation and learning by increased neuronal expression of tissue-type plasminogen activator in transgenic mice. EMBO J 18: 3007-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai N, De Mol M, Lijnen HR, Carmeliet P, Collen D ( 1999) Role of plasminogen system components in focal cerebral ischemic infarction: a gene targeting and gene transfer study in mice. Circulation 99: 2440-2444. [DOI] [PubMed] [Google Scholar]
- Pawlak R, Strickland S ( 2002) Tissue plasminogen activator and seizures: a clot-buster's secret life. J Clin Invest 109: 1529-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podor TJ, Joshua P, Butcher M, Seiffert D, Loskutoff D, Gauldie J ( 1992) Accumulation of type 1 plasminogen activator inhibitor and vitronectin at sites of cellular necrosis and inflammation. Ann NY Acad Sci 667: 173-177. [DOI] [PubMed] [Google Scholar]
- Qian Z, Gilbert ME, Colicos MA, Kandel ER, Kuhl D ( 1993) Tissue-plasminogen activator is induced as an immediate-early gene during seizure, kindling and long-term potentiation. Nature 361: 453-457. [DOI] [PubMed] [Google Scholar]
- Roncarati R, Sestan N, Scheinfeld MH, Berechid BE, Lopez PA, Meucci O, McGlade JC, Rakic P, D'Adamio L ( 2002) The gamma-secretase-generated intracellular domain of beta-amyloid precursor protein binds Numb and inhibits Notch signaling. Proc Natl Acad Sci USA 99: 7102-7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sappino AP, Madani R, Huarte J, Belin D, Kiss JZ, Wohlwend A, Vassalli JD ( 1993) Extracellular proteolysis in the adult murine brain. J Clin Invest 92: 679-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ ( 2000) Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res 874: 123-130. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ ( 2001) Clearing the brain's amyloid cobwebs. Neuron 32: 177-180. [DOI] [PubMed] [Google Scholar]
- Shin RW, Ogino K, Kondo A, Saido TC, Trojanowski JQ, Kitamoto T, Tateishi J ( 1997) Amyloid β-protein (Aβ) 1-40 but not Aβ1-42 contributes to the experimental formation of Alzheimer disease amyloid fibrils in rat brain. J Neurosci 17: 8187-8193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton R, Keohane ME, VanderBerg SR, Gonias SL ( 1994) Plasminogen activator inhibitor-1 in the cerebrospinal fluid as an index of neurological disease. Blood Coagul Fibrinolysis 5: 167-171. [DOI] [PubMed] [Google Scholar]
- Tabrizi P, Wang L, Seeds N, McComb JG, Yamada S, Griffin JH, Carmeliet P, Weiss MH, Zlokovic BV ( 1999) Tissue plasminogen activator (tPA) deficiency exacerbates cerebrovascular fibrin deposition and brain injury in a murine stroke model: studies in tPA-deficient mice and wild-type mice on a matched genetic background. Arterioscler Thromb Vasc Biol 19: 2801-2806. [DOI] [PubMed] [Google Scholar]
- Tsirka SE, Gualandris A, Amaral DG, Strickland S ( 1995) Excitotoxin-induced neuronal degeneration and seizure are mediated by tissue plasminogen activator. Nature 377: 340-344. [DOI] [PubMed] [Google Scholar]
- Tsirka SE, Rogove AD, Strickland S ( 1996) Neuronal cell death and tPA. Nature 384: 123-124. [DOI] [PubMed] [Google Scholar]
- Tucker HM, Kihiko-Ehmann M, Wright S, Rydel RE, Estus S ( 2000a) Tissue plasminogen activator requires plasminogen to modulate amyloid-beta neurotoxicity and deposition. J Neurochem 75: 2172-2177. [DOI] [PubMed] [Google Scholar]
- Tucker HM, Kihiko M, Caldwell JN, Wright S, Kawarabayashi T, Price D, Walker D, Scheff S, McGillis JP, Rydel RE, Estus S ( 2000b) The plasmin system is induced by and degrades amyloid-β aggregates. J Neurosci 20: 3937-3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nostrand WE, Wagner SL, Suzuki M, Choi BH, Farrow JS, Geddes JW, Cotman CW, Cunningham DD ( 1989) Protease nexin-II, a potent anti-chymotrypsin, shows identity to amyloid beta-protein precursor. Nature 341: 546-549. [DOI] [PubMed] [Google Scholar]
- Van Nostrand WE, Schmaier AH, Farrow JS, Cunningham DD ( 1991) Platelet protease nexin-2/amyloid beta-protein precursor. Possible pathologic and physiologic functions. Ann NY Acad Sci 640: 140-144. [DOI] [PubMed] [Google Scholar]
- Vassalli JD, Belin D ( 1987) Amiloride selectively inhibits the urokinase-type plasminogen activator. FEBS Lett 214: 187-191. [DOI] [PubMed] [Google Scholar]
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, et al. ( 1999) Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286: 735-741. [DOI] [PubMed] [Google Scholar]
- Wang YF, Tsirka SE, Strickland S, Stieg PE, Soriano SG, Lipton SA ( 1998) Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat Med 4: 228-231. [DOI] [PubMed] [Google Scholar]
- Wolfe MS, De Los Angeles J, Miller DD, Xia W, Selkoe DJ ( 1999) Are presenilins intramembrane-cleaving proteases? Implications for the molecular mechanism of Alzheimer's disease. Biochemistry 38: 11223-11230. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Nadeau P, Song W, Donoviel D, Yuan M, Bernstein A, Yankner BA ( 2000) Presenilins are required for gamma-secretase cleavage of beta-APP and transmembrane cleavage of Notch-1. Nat Cell Biol 2: 463-465. [DOI] [PubMed] [Google Scholar]