Abstract
Hippocampal place cells show spatially localized activity that can be modulated by both geometric information (e.g., the distances and directions of features in the environment) and nongeometric information (e.g., colors, odors, and possibly behaviors). Nongeometric information may allow the discrimination of different spatial contexts. Understanding how nongeometric (or contextual) information affects hippocampal activity is important in light of proposals that the hippocampus may play a role in constructing a representation of spatial context. We investigated the contextual modulation of place cell activity by recording hippocampal place cells while rats foraged in compound contexts comprising black or white color paired with lemon or vanilla odor. Some cells responded to the color or odor changes alone, but most responded to varying combinations of both. Thus, we demonstrate, for the first time, that there is a heterogeneous input by contextual inputs into the hippocampus. We propose a model of contextual remapping of place cells in which the geometric inputs are selectively activated by subsets of contextual stimuli. Because it appears that different place cells are affected by different subsets of contextual stimuli, the representation of the entire context would require activity at the population level, supporting a role for the hippocampus in constructing a representation of spatial context.
Keywords: hippocampus, single-unit, place cells, context, remapping, spatial representation
Introduction
Place cells in the rat hippocampus show spatially localized firing (O'Keefe and Dostrovsky, 1971), and ensembles of these cells are thought to form spatial representations that can be used in navigation (O'Keefe and Nadel, 1978; Maren and Holt, 2000). After changes to the environment, either the firing rate of the cells or the region of the environment in which they fire (the place field) may alter: both are examples of the phenomenon known as “remapping” (Muller and Kubie, 1987; Muller et al., 2001) and imply the activation of a new place representation.
Although it is well established that place cell activity is governed by constellations of sensory cues (O'Keefe and Speakman, 1987; Shapiro et al., 1997), exactly how these cues act is still unclear. One recent view is that sensory stimuli are functionally differentiated into geometric and nongeometric inputs. Geometric inputs can be manipulated by changing the shape of the environment, causing subtle changes in the shape or location of some of the place fields (O'Keefe and Burgess, 1996; but see Lever et al., 2002), suggesting that the cues govern where a cell should fire. In contrast, changes to nongeometric aspects of the environment, such as alterations to its color (Bostock et al., 1991; Kentros et al., 1998; Jeffery et al., 2003) or the internal state of the rat (Markus et al., 1995; Wood et al., 2000), often cause sudden large changes to many or all of the place fields simultaneously. This kind of remapping seems qualitatively different from geometric remapping. We refer to these nongeometric cues as “contextual” and have suggested previously that they act by selecting which geometric cues will drive a place cell in a given environment (Jeffery and Anderson, 2003).
If contextual inputs are functionally discriminable from other kinds of input, it becomes of interest to know how they are constructed, and this question forms the subject of the current study. There are two broad possibilities (Fig. 1). The first is that place cell activity is modulated by a single, “monolithic” context signal that has been assembled previously, which effectively tells the cells, as a collective, which environment they are in. Alternatively, information about the contextual stimuli might be passed to the place cells in fragments, so that different cells receive different subsets of the available contextual information. Which of these two alternatives is correct speaks to the issue of whether a representation of spatial context is constructed by the hippocampus itself (Nadel and Willner, 1980; Nadel et al., 1985; Myers and Gluck, 1994; Rudy and O'Reilly, 1999; O'Reilly and Rudy, 2001), by some other structure, or even not at all. To distinguish between these possibilities, we created four unique environments characterized by two elements: one of two colors (black or white, referred to as colors for simplicity) paired with one of two odors. If all place cells receive a unitary context signal, then each environment should elicit a completely new representation and all of the cells should remap together. If different cells receive different elements of the compound context, then only a subset of cells should remap. Such partial remapping to a nonspatial sensory change, which has not been reported previously, would support the hypothesis that a unique representation of context occurs at or after the level of the place cells.
Figure 1.
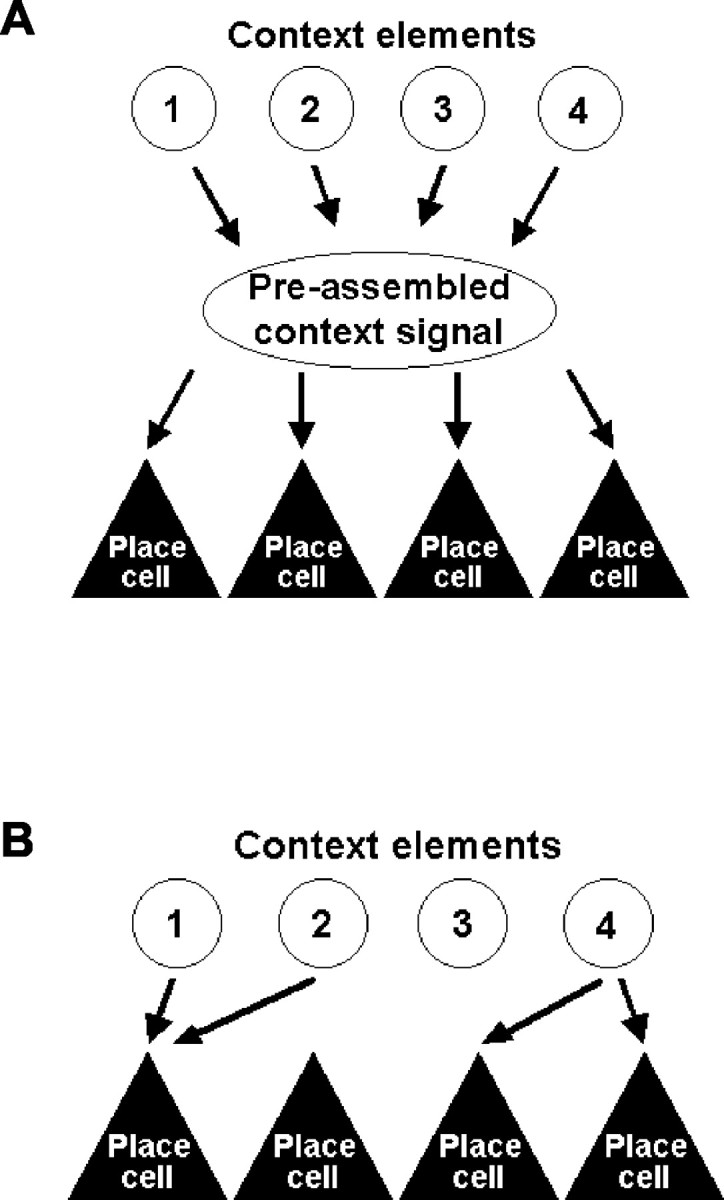
Pictorial representations of how contextual stimuli may modulate place cell activity. A, In the first possibility, all place cells are modulated by the same presynthesized context signal. B, In the second possibility, some place cells are modulated by different subsets of the contextual stimuli. Note that these pictures illustrate only how the context signal might be constructed, not how place cells remap after changes to these stimuli (a model of the latter is proposed in Fig. 4).
Materials and Methods
Subjects
Subjects were five male Lister hooded rats (weighing 250-350 gm), which were housed singly in Plexiglas cages. The lights in the animal room were at half strength from 7 to 8 A.M. (simulated dawn), full strength from 8 A.M. to 7 P.M., half strength again from 7 to 8 P.M. (simulated dusk), then off from 8 P.M. to 7 A.M. Each rat was given sufficient food to maintain 90% of its free-feeding weight and allowed ad libitum access to water. All procedures in this study were licensed by the UK Home Office, subject to the restrictions and provisions contained in the Animals (Scientific Procedures) Act of 1986.
Electrode implantation
Rats were implanted with four tetrodes in the neocortex overlying the dorsal hippocampus (-3.8 mm, anteroposterior relative to bregma; 2.2 mm, mediolateral; the deepest tetrode positioned 1.5 mm below brain surface). The tetrodes were held in a microdrive that allowed them to be advanced together through the brain in steps as small as 25 μm. The assembly was held in place by means of jewelry screws fixed to the skull, and dental acrylic. Rats were allowed at least 1 week to recover before recording began. During this time, they were fed honeyed rice grains in their cages in addition to their regular food to accustom them to the behavioral reward. At the end of the experiment, the tetrode tracks were reconstructed after histological processing; the tracks in all of the rats were clearly visible in hippocampal area CA1.
Apparatus
All testing was conducted in a quiet room in a secure laboratory. Throughout the experiment, the rats could see all available cues in the testing room, such as the stand holding the recording equipment, a colored door, the unchanging position of the experimenter, etc.
The recording environment for each trial was configured from one of two Plexiglas square boxes (width, 60 cm; height, 50 cm), which was wiped with lemon or vanilla food-flavoring. Each Plexiglas box was dedicated to the lemon or vanilla odor throughout the experiment. To change their apparent color, the Plexiglas boxes were placed inside slightly larger black or white painted wooden boxes, allowing the color to show through the Plexiglas without affecting the odor of the Plexiglas boxes.
Early in the experiment, a slightly different version of the recording arena was used: the Plexiglas boxes were created from separate wall and floor pieces. The box walls were constructed by clamping the corners of the wall pieces together with L-shaped clamps, one to each corner; the walls were then set on the floor piece. Black or white vinyl was placed behind the walls and the floor. This box permitted intertrial rearrangement of the surfaces so that the wall pieces were pseudorandomly mixed, and the floor piece rotated, allowing us to check that local odor cues on the walls and floor were not controlling place cell firing. The solid Plexiglas boxes were introduced to facilitate testing after it was clear that local odor cues did not control place cell firing (such cues were nevertheless eliminated as much as possible by wiping the walls and floor with the food flavoring before each trial). This box was rotated pseudorandomly (by 0, 90, 180, or 270°) between trials to further ensure that any place cell remapping was not attributable to localized odor cues. There were no apparent differences in place cell firing or remapping behavior between the two versions of the recording arena.
Before each trial, any extraneous matter (e.g., sawdust, fecal material) was removed, and the inner surfaces of the walls and floor of the recording box were liberally wiped with food-flavoring (lemon or vanilla).
The remainder of the room, including apparatus not being used in a given trial, was always arranged in the same way during recording sessions to prevent the possibility of remapping to distal cues.
Place cell recording
Each rat was placed on a holding platform (a cage bottom) sitting on a pedestal and located ∼1 m from the experimental box. The rat was connected to the recording equipment (Axona, Herts, UK) via lightweight hearing-aid wires and a socket that connected to the microdrive plug. The potentials recorded on each of the 16 electrodes of the four tetrodes were passed through AC-coupled unity gain operational amplifiers mounted on the rat's head and led to the multichannel recording system, in which the signal was amplified (20,000-40,000 times) and bandpass filtered (500 Hz to 7 kHz). Each of the four wires of one tetrode could be recorded differentially with respect to one of the wires of any other tetrode. One of the recording channels was dedicated to EEG recording.
The microdrive was advanced by 25-200 μm daily until complex spike cells appeared on one or more of the tetrodes. When such activity appeared and stabilized, the rat was carried from the holding platform and placed into the center of the recording box. The experimenter, who stood in the same position near the box throughout the experiment, started a recording session by pressing a key on the computer keyboard and then threw honeyed rice grains into the box to encourage the rat to forage (they learned to forage all over the box during the first trial). During the recording, the position of the rat was monitored by a monochrome video camera mounted directly above the apparatus. The video image was passed to a tracking system (Axona) that detected an infrared light mounted on the rat's headstage and stored this location at a sampling rate of 50 Hz. Each spike event was stamped with the time elapsed since the start of the recording and the simultaneous location of the rat.
Each recording session comprised two trials in each of the four conditions (i.e., two trials each of black lemon, black vanilla, white lemon, and white vanilla). Each trial lasted 4 min, with an intertrial interval of ∼2 min (the time it took to reconfigure the recording environment). In most recording sessions, the trial order was varied in a pseudorandom manner; some sessions, however, were conducted in an ordered manner in which both trials in a pair of the same-condition trials were consecutive. This was done to demonstrate that taking the rat out of the environment and putting it back in did not in itself trigger place cell remapping. Recording twice in each condition (and with intervening trials of other conditions in most sessions) enabled us to verify the stability of within-condition place fields.
If there were no place fields in the recording box in the first trial, the session was stopped, the electrodes were moved down by 25-50 μm, and the rat was returned to its home cage.
Data analysis
Data analysis was initially done using Tint analysis software (Axona). The path of the rat was smoothed using a boxcar algorithm with a boxcar width of 400 msec. The collected waveforms were displayed as clusters by plotting the peak-to-peak amplitude of each spike on one electrode against that on each of the other three. The clusters were separated by hand on the first trial and then by an automatic clustering algorithm using parameters derived from the initial manual cut. These parameters were the centroid of the cluster (in multidimensional space) and a cluster boundary formed by an ellipse, the long axis of which passed through the origin and the length and width of which were 3 SDs from the centroid. Both putative interneurons and complex spiking cells were isolated and discriminated on the basis of spike shape, firing rate, and firing location (see below). All eight trials in a session were cut before additional analysis took place.
After cutting was finished, eight firing rate maps for each unit were visualized using Tint. Firing rate maps were constructed by dividing the box floor into square pixels of side ∼2.5 cm, and the firing rate for a given cell in each pixel was determined by dividing the number of spikes in that pixel by the amount of time the rat spent there. The firing rate maps were smoothed using an algorithm that replaced the value in each pixel with the average of the value in that plus the adjacent eight pixels. These pixels were then entered into the remapping analysis (see below). Place fields were visualized as contour maps with five levels, each level representing a 20% portion of the peak firing rate for that map.
Additional analysis was conducted using programs custom-written in Matlab (The MathWorks, Natick, MA). A unit was defined as having a place field if its peak rate (taken from the pixel with the highest rate) after smoothing was ≥1 Hz and the number of spikes in its cluster was ≥20. If the unit did not meet these criteria in a given trial, it was considered to have not fired in that trial and the map was shown empty.
A unit was removed from additional analysis at this stage for any of the following reasons: (1) if its peak rate in each trial was <1 Hz, (2) if its spike count in each trial was <20, or (3) if its “field” in each trial occupied a proportion of space >0.75 of the total floor area of the recording box. Reason 3 eliminated interneurons from additional analysis. In other words, to be considered for additional analysis, a place field had to take up <0.75 of the total floor area in both trials of a given box condition. If (rarely, as it happened) in one condition, both fields occupied a proportion of space >0.75, but in another condition, both fields occupied a proportion of space ≤0.75, the unit was considered to have lost its spatial tuning in the former condition but not the latter.
The next stage of analysis involved determining whether the two firing rate maps generated by a given cell in two different trials were the same or different. This was done using pairwise Pearson's correlations between the pixels in the two maps. The maps were aligned by ensuring that the top left corner of each map occupied the same point in space. Only pixels in which the cell had fired in at least one of the trials were correlated, to prevent artificially high correlations from being generated by large numbers of zero-zero correlations.
Correlations were first calculated between trials of the same condition: these are henceforth called “same-condition r values.” If any of the four same-condition r values were ≥0.4 (a user-defined value determined after assessing many maps by eye), then the unit was considered to have a sufficiently stable field, in at least one condition, for remapping assessment. Units with no same-condition r values of ≥0.4 were removed from additional analysis.
Remapping analysis
Having survived the above selection processes, units were then tested for remapping between trials of different conditions. This was done in a stepwise manner as follows: (1) test for loss of a field; (2) test for shift of field location; and (3) tests for change in peak firing rate (other than switching off and on), field size, and field coherence (Sharp, 1997).
Test for loss of a field. If the firing of a cell in a particular condition fell consistently below a peak rate of 1 Hz, or to <20 spikes per trial, it was considered to have remapped by switching off.
Test for shift of field location. All pairwise correlations between maps in different conditions were calculated as described above; these are termed “different-condition r values.” The next stage was to determine whether a cell with high same-condition r values (indicating stability) also had high different-condition r values, indicating a preservation of that field in the changed condition, or whether it had low different-condition r values, indicating a remapping. One-sample t tests were conducted, using the mean of the same-condition r values as the test value and the different-condition r values as the test variable for each possible pairing of maps to detect significant differences (p < 0.05) between the correlations. If a significant difference was found between two conditions, the place fields were considered to have remapped between those conditions.
Tests for change in peak firing rate, field size, and field coherence. A parameter analysis of remapping was conducted using one-way ANOVAs (with post hoc Tukey tests if the ANOVA showed a significant main effect) with condition as the factor and peak firing rate, field size, and field coherence as dependent variables; this stage detected significant differences in the dependent variable list that may not have been detected by the correlational analysis.
The results from the above remapping analyses permitted the construction of a remapping (or nonremapping) profile for each unit. There was a high correspondence between remapping as detected by the algorithm and the experimenter's visual judgment of whether a unit had remapped (see Fig. 2, legend, for sample correlation values).
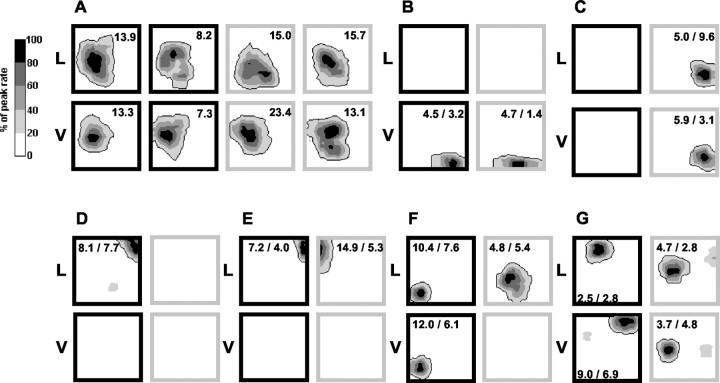
Figure 2.
The remapping patterns of seven place cells (A-G) across the contextual configurations (all complete data sets). A, A unit that had the same place field in all of the contexts (same condition r values: black lemon, 0.89; white lemon, 0.84; black vanilla, 0.91; white vanilla, 0.85; median different condition r values: black lemon and white lemon, 0.72; black lemon and black vanilla, 0.86; black lemon and white vanilla, 0.90; white lemon and black vanilla, 0.57; white lemon and white vanilla, 0.76; black vanilla and white vanilla 0.86). For this unit, all eight trials are displayed. For the units in B-G, only four trials are displayed, one for each context; these units had the same place fields in both trials of each context; the extra trials are omitted to save space. The letter to the left of each figure panel is a description of the context odor (L, lemon; V, vanilla) and refers to each map in that row. The context color is indicated by the dark and light boxes bordering each map (a dark box for the black contexts, a light box for the white contexts). The number inside each box in A indicates the peak firing rate for the corresponding map (in hertz); the two numbers inside each box in B-G indicate the peak firing rates for both trials in the corresponding context, with the rate for the displayed trial given first. Place fields are displayed as contour maps with five levels, each level representing a 20% portion of the peak firing rate for that map (color bar). B, A unit that fired only in the vanilla contexts (black and white), expressing the same place field in both vanilla contexts. C, A unit that fired only in the white contexts (lemon and vanilla). It had the same place field in both white contexts. D, A unit that fired only in the black lemon context. E, A unit that fired only in the lemon contexts (black and white) and remapped between them (compare with the unit in B). F, A unit that had the same place field in the black contexts (lemon and vanilla) but that showed a different place field in the white lemon context and did not fire in the white vanilla context (same condition r values: black lemon, 0.94; white lemon, 0.77; black vanilla, 0.80; median different condition r values: black lemon and white lemon, -0.05; black lemon and black vanilla, 0.74; white lemon and black vanilla, -0.08). G, A unit that fired in all contexts, with the same place field in the white contexts but two new fields in the black lemon and black vanilla contexts.
Partial data sets
The remapping analysis detailed above highlighted some units that had one or more unstable conditions. Conditions were considered unstable for the following reasons: (1) despite robust firing in both maps in the condition, the same-condition r value was <0.4; (2) the peak rate in one map of the condition was ≥1 Hz but was <1 Hz in the other; (3) the spike count in one map of the condition was ≥20 spikes but was <20 spikes in the other; and (4) the field in one map of the condition occupied a proportion of space ≤0.75 but occupied a proportion of space >0.75 in the other.
Units with two or three stable conditions can still provide information on how the contextual stimuli affect place cell behavior (for example, a unit with stable firing in black lemon and black vanilla only, which remapped between the two conditions, could be said to remap robustly to the odor stimuli in the black environment). For this reason, units with at least two stable conditions (which could be one condition with firing and one condition in which the unit stopped firing) were analyzed as partial data sets.
Results
Cells were recorded from five rats as they foraged for cooked rice in the box configured in each of its contexts; all cells were recorded from hippocampal area CA1 (confirmed after histological processing). Rats began to forage for the rice from the first trial (they had been accustomed previously to the rice), ensuring good positional sampling of the box. A recording session comprised two trials in each context, making a total of eight trials. Rat 1 experienced 21 sessions, rat 2 experienced 9 sessions, rat 3 experienced 19 sessions, rat 4 experienced 8 sessions, and rat 5 experienced 4 sessions. Recordings started from the first sessions each rat experienced under the experimental protocol described above. Two rats (rats 1 and 3), however, had some experience in the same experimental set-up before the current experiment began, but this experience involved odor changes only (one session here comprised four trials in a black lemon box and four trials in a black vanilla box, making a total of eight trials). Rat 1 experienced 14 of these sessions, and rat 3 experienced 7 sessions.
A total of 286 U, which satisfied the acceptance criteria described in Materials and Methods, were recorded from rats 1-5 (147, 51, 36, 45, and 7 U, respectively). Every unit was recorded in eight trials (two trials of each condition), which were usually varied pseudorandomly. The number of subsequently accepted units that was recorded in a single session ranged from 1 to 12 (mean units per session ± SEM, 5.15 ± 0.36).
Complete data sets
A total of 132 U, which satisfied the acceptance criteria described in Materials and Methods and had complete data sets (i.e., maintained reliable and stable firing over all eight trials), were recorded from rats 1-5 (68, 28, 17, 16, and 3 U, respectively).
Each unit was analyzed to determine whether it remapped between one box type and the next (see Materials and Methods). After applying the remapping algorithm to each unit, it was found that 11 (8%) did not remap in any condition (i.e., they showed the same firing pattern in every trial). Of the 121 (92%) units that did remap, nine (7%) remapped when the color alone was changed, four (3%) remapped when the odor alone was changed, and 108 cells (82%) remapped to color and odor changes in more complex ways. These data are displayed separately for each rat in Table 1, and examples of each kind of remapping are shown in Figure 2. Remapping was seen from the first session of recording.
Table 1.
Numbers of place cells remapping in response to changes in each of the various stimulus modalities (complete data sets only)
|
|
Rat 1 |
Rat 2 |
Rat 3 |
Rat 4 |
Rat 5 |
All rats |
|---|---|---|---|---|---|---|
| Odor only | 1 | 3 | 4 | |||
| Color only | 3 | 5 | 1 | 9 | ||
| Complex | 64 | 16 | 16 | 9 | 3 | 108 |
| No remapping | 0 | 7 | 0 | 4 | 0 | 11 |
| Total
|
68
|
28
|
17
|
16
|
3
|
132
|
“Complex” refers to cells that showed an influence of both odor and color.
Figure 2A shows a unit that did not remap in any context, Figure 2B shows a unit that remapped purely to odor changes, and Figure 2C shows a unit that remapped purely to color changes. Units that responded to complex odor and color changes showed a variety of interesting remapping patterns (Fig. 2D-G). The unit in Figure 2D fired only in the black lemon context, showing that it required a combination of black and lemon to fire. The unit in Figure 2E fired only in the lemon contexts but had different fields between the black and white lemon contexts. The units in Figure 2F,G are apparently more complex: the unit in F had the same field in both black contexts but remapped in the white lemon context and switched off in the white vanilla context; the unit in G fired in all contexts, with the same place field in the white contexts but two new fields in the black lemon and black vanilla contexts (i.e., three different place fields across the four contexts).
Of all of the units, remapping or not, 12 (9%) had place fields in one condition only, 24 (18%) had place fields in two conditions only, 18 (14%) had place fields in three conditions only, and 78 (59%) had place fields in all four conditions.
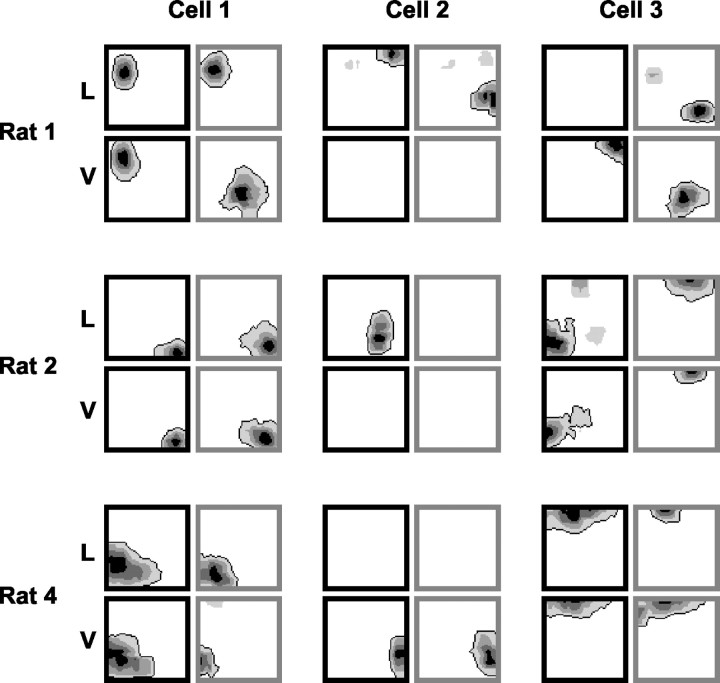
Figure 3 displays three sets of simultaneously recorded units (3 U in each set), with each set from a different rat. This figure illustrates two important findings: first, that simultaneously recorded units had different remapping profiles from one another, and second, that this heterogeneous remapping was consistently observed in different rats. Also of interest are the remapping patterns that we failed to observe. We never saw a cell that discriminated between contexts that did not also show spatially localized firing. Cells that did respond to contextual changes were always place cells; for example, no unit with field sizes of >0.75 in every trial (34 U, some of which are putative interneurons) switched on or off between contexts. Some of the remapping cells responded to a contextual change by switching off the field in one location and switching on another field in a different location. Despite the ability of such cells to express either field, they never expressed both together. Finally, we did not reliably see cells that had identical fields in two nonoverlapping contexts (e.g., white lemon and black vanilla) without also having the same fields in one or both of the other conditions. These absences, together with the data presented above, provide important constraints on the possible explanations for contextual remapping, which we discuss below.
Figure 3.
Heterogeneous remapping of simultaneously recorded place cells. The remapping patterns of three sets of simultaneously recorded cells are shown (three cells in each set), with each set from a different rat (rats 1, 2, and 4). The remapping profile of each cell is illustrated with a four-map grouping, as used in Figure 2 (the same figure conventions are used here). Each row contains the simultaneously recorded cell set from one rat (the rat number is given to the left of the row). They are shown to illustrate that even simultaneously recorded cells demonstrated heterogeneous remapping profiles, and that these profiles were consistently observed in different rats. L, Lemon; V, vanilla.
Partial data sets
A total of 154 U, which satisfied the acceptance criteria described in Materials and Methods and had partial data sets (i.e., either two or three stable conditions), were recorded from rats 1-5 (79, 23, 19, 29, and 4 U, respectively).
The stable conditions were analyzed to determine whether each unit remapped between one box type and the next (see Materials and Methods). The units with two stable conditions (47 U) may show stability in conditions of the same color (e.g., black lemon and black vanilla), of the same odor (e.g., black lemon and white lemon), or of nonoverlapping colors and odors (e.g., black lemon and white vanilla). Hence, we can determine, at least for some of these units, whether they are remapping solely to color changes, solely to odor changes, or whether they do not remap at all. Twenty-one units were stable in conditions of the same color (and hence different odor), with 12 (26%) remapping between the conditions and nine (19%) not remapping; 15 U were stable in conditions of the same odor (and hence, different color), with 12 (26%) remapping between the conditions and three (6%) not remapping. It is not possible to determine whether the remaining 11 U remap to color changes, odor changes, or both, because they were stable in conditions with no overlapping color or odor, with eight (17%) remapping between these conditions and three (6%) not remapping.
Similarly, we can assess for units with three stable conditions (107 U) what determines their remapping patterns. These units, as a consequence of having three stable conditions, will fire in overlapping pairs of conditions of the same color (e.g., black lemon and black vanilla) and of the same odor (black lemon and white lemon). Therefore, we can determine whether units remap solely to color changes, solely to odor changes, or to color and odor changes in more complex ways, or whether they do not remap at all. Eight units (7%) did not remap between any of these conditions and therefore were not influenced by odor or color changes; 21 U (20%) remapped solely to color changes (e.g., showed the same field in black lemon and black vanilla but a new field in white lemon); 10 U (9%) remapped solely to odor changes (e.g., showed the same field in black lemon and white lemon but a new field in white vanilla); and 68 U (64%) remapped in all three conditions and therefore respond to color and odor changes in more complex ways.
It was clear also, for partial data sets as for complete data sets, that simultaneously recorded units nearly always had different remapping profiles from one another and that this pattern was observed in different rats.
Observed remapping profiles
Ignoring specific contexts and focusing only on the number of conditions in which a cell could potentially fire and what remapping patterns it could show between these conditions, 11 remapping profiles are possible: (1) a cell that fires in one condition only (which we will call profile A); (2) a cell that fires in two conditions only, where the fields could be the same or different (profiles B1 and B2, respectively); (3) a cell that fires in three conditions only, where the fields could be all the same or all different, or two fields the same and one field different (profiles C1, C2, and C3, respectively); and (4) a cell that fires in all four conditions, where the fields could be all the same or all different, or three fields the same and one field different, or two fields the same and two new fields, or two fields the same and two fields different from the first fields but the same as each other (profiles D1, D2, D3, D4, and D5, respectively). The frequencies with which we observed each remapping profile for the cells with complete data sets are displayed in Table 2. Twenty-eight of the 132 U (one that fired in three conditions and 27 that fired in four conditions) could not be classified in this way because of a logical anomaly with the remapping profile [the anomaly is illustrated as follows: if field a = field b (meaning “the place field in condition a is the same as the place field in condition b”) and field a = field c, how do we classify the cell if field b ≠ field c?]. A χ2 test on the classified units (104 in total) showed that the observed frequencies of these remapping profiles are significantly different from the expected frequencies (χ2 = 19.1; df = 10; p < 0.05): some remapping profiles are more or less common than expected.
Table 2.
Frequencies and proportions of the observed remapping profiles (complete data sets only)
|
Profile |
A1 |
B1 |
B2 |
C1 |
C2 |
C3 |
D1 |
D2 |
D3 |
D4 |
D5 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of units | 12 | 9 | 15 | 4 | 8 | 5 | 11 | 7 | 10 | 18 | 5 |
| %
|
11.5
|
8
|
14
|
4
|
8
|
5
|
10.5
|
7
|
10
|
17
|
5
|
If one does take into account the different contexts (i.e., black lemon, black vanilla, white lemon, and white vanilla) as well as the number of conditions in which a cell could potentially fire, we calculate that there are 51 possible remapping profiles that any unit might show. There are not enough cells to decide which remapping profile was most often seen here. As stated above, we did not find any simultaneously recorded units that behaved in the same way as one another across the contexts. This is attributable presumably to the relatively small numbers of simultaneously recorded units compared with the large number of possible remapping profiles.
A model of heterogeneous contextual remapping
We turn now to the question of how the inputs to place cells might be organized to account for the heterogeneous remapping patterns we observed in this experiment. First, might the remapping be induced, not by a separate, unitary contextual signal but by geometric signals that are tied to the sensory characteristics of the walls by which a cell is driven? For example, perhaps the cell in Figure 2D remaps not because the context as a whole (the “blackness” or “lemon-ness” of the box) changed but because the cell is programmed to respond when the rat is (for example) 10 cm south of a north, lemon-scented black wall and 10 cm west of an east, lemon-scented black wall [compare the color modulation of “boundary vector cells” (Burgess and Hartley, 2002)]. We consider this unlikely, because we have demonstrated previously (Jeffery and Anderson, 2003) that changes to the aspects of a testing arena that do not localize fields (i.e., the floor) can induce place cell remapping in the absence of changes to aspects that do localize these fields (i.e., the walls). Thus, we believe the change of the box in color, odor, or both in the current experiment exerted its effects via a nonlocal, diffuse contextual signal rather than via a local geometric signal. This interpretation is also supported by other published observations of contextual remapping (Markus et al., 1995; Wood et al., 2000), although these studies found remapping to changes in behavior rather than to the perceptual changes we studied here.
Putting the above data together, we propose the following explanation for the observed remapping patterns across the different contexts. We assume two sets of inputs driving the activity of place cells in a given environment. The first set, the inputs that determine where place cells fire in an environment, are inferred from work showing that place cells apparently use the distance from two or more walls in a walled environment to determine the location and shape of place fields (O'Keefe and Burgess, 1996; Hartley et al., 2000; Burgess and Hartley, 2002). Because of the above-mentioned dissociation of geometric and contextual influences, we suggest that these geometric determinants of place cell firing [referred to here as “boundary cells;” the firing of boundary cells may be determined by inputs from several of the boundary vector cells of Hartley et al. (2000) which, in sets, govern the location of individual place fields] are selectively activated by the second set of inputs, the contextual inputs. This set comprises the stimuli in the environment that act as distinguishers of context, such as the color and odor stimuli manipulated here. Note that it is possible that environmental geometry can also act as a distinguisher of context, depending on how or where in the brain it is processed; this idea is elaborated in the Discussion. In the present experiment, however, given that the environmental geometry did not change, the only possible distinguishers of context are the color and odor stimuli. In our model, when the rat is placed into a new environment, elemental contextual stimuli are rapidly connected to the boundary cells of coactive place cells by a Hebbian process (the model does not currently specify where this happens other than upstream of the place cells). In this way, each boundary cell comes to be affected by its own private subset of the available contextual elements. This model is reminiscent of that proposed by Sharp (1999), in which structures outside of the hippocampus, such as the entorhinal cortex, supply it with context-free spatial information, to which the hippocampus adds a contextual modulation.
Why the necessity for the binding of contextual cues to the boundary cell rather than to the place cell itself? The reason derives from the observation that in individual cells with different place fields in different contexts, the fields frequently behaved independently of one another (Fig. 2F). This implies that it is one of the boundary cells rather than the entire place cell that receives the discriminative contextual stimuli. For cells showing different place fields in different contexts, we suggest that different boundary cells are selectively activated by different contextual elements. In such a way, remapping patterns such as that shown in Figure 2F can be explained by groups of contextual elements selecting one boundary cell rather than another.
The idea that place cells are activated by contextually selected boundary cells can also account for the two ways in which a place cell can remap, which is either by shifting the location of its field or by switching its field on or off without changing its location. Our model proposes that in the former case, these place cells receive contextually selected inputs from more than one boundary cell so that when the context changes, one set switches off and another switches on, resulting in a shift of the firing location of the cell. In the latter case, place cells receive contextually selected inputs from only one boundary cell and thus switch on and off between contexts. We believe that some mechanism must exist for competition between the boundary cells impinging on a given place cell, because we did not reliably see instances of fields, driven by different but overlapping subsets of contextual inputs, that were simultaneously expressed by the cell in the overlapping context. The usual explanation for this observation is that the activity of a given place cell is part of an attractor state (Samsonovich and McNaughton, 1997; Tsodyks, 1999; Doboli et al., 2000; Kali and Dayan, 2000), in which the collective activity of a simultaneously active population of cells is mutually reinforcing and also inhibits other, alternative forms of activity. However, such attractor models less easily explain patterns such as the one we observed, in which arbitrary combinations of place fields are activated by a given selection of stimuli.
In summary, our results show a heterogeneous modulation of the place cell population by subsets of contextual elements, suggesting that geometric and contextual information about the environment is combined in or upstream of the hippocampus to form a holistic representation of the environment.
Discussion
In this study, we characterized the contextual remapping expressed by place cells while rats foraged in four different compound contexts that were each created from two elements (an odor and a color). We found that simultaneously recorded cells showed a variety of remapping patterns, indicating that they did not all receive the same information about the context changes. Some units remapped only to changes in color and some only to changes in odor, and a large majority remapped to varying combinations of color and odor changes. No set of simultaneously recorded cells contained units that showed the same remapping patterns across the four contexts, implying a heterogeneous modulation of place cell firing by the contextual stimuli.
This is the first report of partial remapping after a sensory but nonspatial change to the environment, and it is important for two reasons. First, it adds to the growing body of evidence suggesting that place cell populations do not always remap in an “all-or-nothing” manner but often show discordant behavior after environmental changes (Shapiro et al., 1997; Tanila et al., 1997; Skaggs and McNaughton, 1998; Lever et al., 2002; Knierim, 2003). Such findings challenge the widely held assumption that place cell representations are held in a coherent form by attractor dynamics (Samsonovich and McNaughton, 1997; Tsodyks, 1999; Kali and Dayan, 2000) and support the alternative notion that place cells are relatively individualistic and can remap independently of their neighbors. In fact, these two views may not be mutually exclusive, as we discuss below.
Second, and more important, our findings show that the nongeometric, contextual stimuli that characterize a particular environment do not influence place cells en bloc, but rather, different subsets of stimuli drive different place cells. This observation is significant in light of recent suggestions that geometric and nongeometric stimuli exert different effects on place cells (O'Keefe and Burgess, 1996; Hartley et al., 2000; Jeffery and Anderson, 2003). Partial remapping in the studies cited previously occurred in response to changes to spatial stimuli. Our finding that place cells also respond heterogeneously to nongeometric (context) changes rather than showing complete remapping provides significant constraints on the kinds of models that can explain contextual remapping.
The suggestion that sensory cues exert different effects on place cells, depending on whether they convey geometric or contextual information, derives from several experimental observations. Place cells sometimes respond differently to changes in geometric versus nongeometric cues, showing subtle shifts in field location and shape in response to the former (O'Keefe and Burgess, 1996) and sudden jumps in location or rate in response to the latter. Furthermore, there is an asymmetry with respect to spatial and nonspatial cues in that although many hippocampal cells show place-specific firing, there have been no reliable reports of cells firing solely to some nonspatial aspect of the environment (i.e., no “context cells”), suggesting that spatial cues have a preeminent role in the driving of hippocampal cells. And finally, the notion that some sensory cues can provide abstract, geometric information provides a parsimonious account for why place fields are so resistant to large sensory changes (like sudden darkness) and coalesce so quickly in novel environments when a rat first faces one way (seeing one view) and then the other (seeing a different view).
If sensory cues are indeed divided into geometric and nongeometric classes, it becomes of interest to know how these interact. Hartley et al. (2000) suggested that the geometric inputs from the boundaries of the environment are provided by the firing of boundary cells (O'Keefe and Burgess, 1996; Hartley et al., 2000; Burgess and Hartley, 2002), which tell a place cell how far the rat is from the walls. We elaborated this model to account for contextual remapping by proposing that these boundary inputs are selectively activated by the contextual stimuli (Jeffery and Anderson, 2003). The present study explored how these contextual stimuli might arrive at their place cell targets. We initially assumed, because complete remapping is so commonly observed after context changes, that contextual cues would act as a single signal that switches geometric inputs on and off en bloc. We were surprised, therefore, to discover that multimodal context changes, instead of eliciting complete remapping, induced a fragmentary remapping in which different cells responded to different cue changes. This suggests that the cells are not in receipt of a single, preprocessed context signal but receive the elements of the context independently. We proposed a model of how these changes might occur (Fig. 4). To explain the complex remapping patterns observed here, some boundary cells would need to be activated by more than one contextual input. Our data suggest that the contextual signal must be bound to the boundary cells upstream of the place cell to explain our observations that the two different fields of a cell could remap independently, as long as they never co-occurred in the same environment.
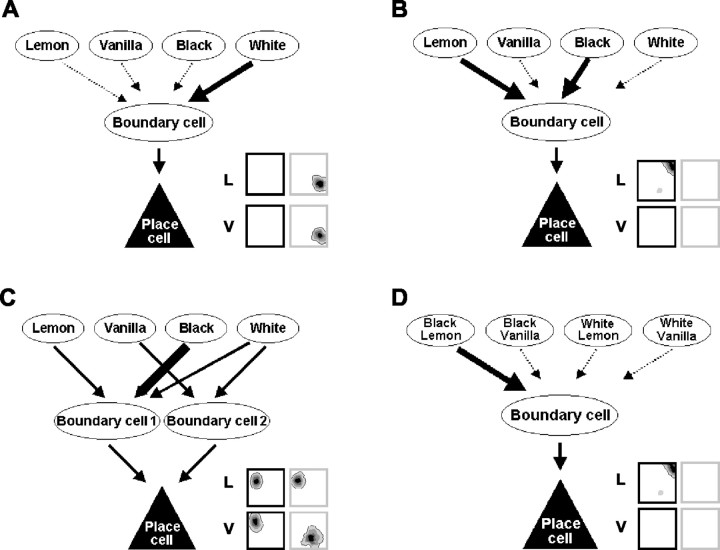
Figure 4.
A model of the contextual remapping of place cells. In our model, the spatial inputs from the boundaries of the environment to a place cell, which are represented in the firing of what we call the boundary cell, are selectively activated by the contextual stimuli. In each panel, an example of a place cell from the present study is shown, with a figurative description of how the model can explain the remapping pattern that the unit shows. Heavy lines indicate strong connections, medium-weight lines indicate medium-strength connections, and dotted lines indicate connections that are not required to explain the remapping pattern of the unit. A, A unit that fires in both white contexts (lemon and vanilla) only. Its remapping pattern can be explained by its single boundary cell being activated by an elemental representation of white. B, To explain the complex remapping patterns observed here, some boundary cells would need to be activated by more than one contextual input so that the coactivity of both black and lemon contextual elements for this unit would be required to activate its boundary cell. C, Units that remap by showing different fields in different contexts (as opposed to units that remap by switching off in a particular context) require more than one boundary cell, activated by different contextual elements, to explain their remapping patterns. D, An alternative explanation of the remapping pattern for the cell shown in B. Subsets of contextual elements may be precombined before reaching the boundary cell level. Because we did not reliably see cells with identical fields in two nonoverlapping contexts only, we favor the explanation in B. L, Lemon; V, vanilla.
We note that our hypothetical boundary cells are empirically similar to cells recorded in the medial entorhinal cortex (MEC) (Quirk et al., 1992). MEC cells show spatially localized firing similar to place cells, and their firing fields “stretch” to fit a recording arena of a different shape. Quirk et al. (1992) concluded that “the positional firing of MEC cells is more `sensory bound' than hippocampal cells, and that the ability to discriminate different environments, although present in the hippocampus, is not yet present in its input from MEC.” Such properties might be expected of boundary cells. We predict that structures upstream of the hippocampus might also yield nonspatially specific context-sensitive cells.
Our model raises the important question of what types of environmental cues are contextual and what types are geometric. If it has the right properties, the same cue might be processed as both a contextual and a geometric cue, because there is nothing in principle to prevent it from being processed in parallel along two neural routes. For example, Lever et al. (2002) recently reported partial remapping in place cell populations subjected to repeated switching between square and circular environments of similar size. Arguably, the switching between square and circle involved both a geometric change (in the shape of the environment) and a contextual change [for example, one environment having vertical features (corners) that made it appear different]. We suggest, based on our model, that only some place cells in the experiment by Lever et al. (2002) received the changing contextual information, explaining why only some cells contextually remapped early in experience of the two environments. The place fields of cells that did not contextually remap would be expected to deform in response to the geometric changes, as indeed they frequently did (C. Lever, personal communication).
The factors governing whether contextual remapping is partial or complete are unknown at present, but we postulate here that complete remapping to context might be more likely if the rat has had extensive experience with one of the contexts, thus strengthening connections between all of the available contextual elements and all of the place cells. In support of this idea, rats with much experience in a particular environment showed complete remapping to environmental changes (Bostock et al., 1991; Jeffery et al., 2003), whereas in the present study, in which rats had no more experience in one environment than another, partial remapping occurred instead. We propose that early in the experience of an environment, the direct connections from the entorhinal cortex to CA1 determine predominantly how place cells in CA1 fire, whereas later on, the connections from CA3 to CA1 become more influential. Hippocampal area CA3 is where attractor dynamics are presumed to be neurologically realized. In this sense, attractor architecture may increasingly (with repeated experience of the environment in question) determine place cell remapping behavior.
An important consequence of contextual remapping is that the same space can be represented in different ways by the hippocampus, implying that the output sent by the hippocampus to the rest of the brain is not a map of space alone but rather a map of spatial context (Sharp, 1999). Our experimental findings lead us to propose an operational definition of contextual stimuli: those stimuli that switch on or off the geometric inputs to place cells. It follows that a given context could be thought of as that collection of stimuli that evokes a unique firing pattern across the place cell population. The well established finding that the hippocampus is necessary for many kinds of contextual learning (Kim and Fanselow, 1992; Phillips and LeDoux, 1992; Maren and Holt, 2000; Hall et al., 2001; Barrientos et al., 2002) suggests that the hippocampus has the major role in representing spatial context. In this light, our findings are consistent with the proposal that a unitary representation of spatial context is constructed at the population level (Nadel and Willner, 1980) using geometric inputs that are selectively activated by particular contextual elements.
Footnotes
This work was supported by Wellcome Trust and Biotechnology and Biological Sciences Research Council grants to K.J. We thank Kazuo Inoue and Viji Maharalingam for help with the unit recording and Roger Bunce and Jim Donnett for technical assistance. We also thank Neil Burgess and Tom Hartley for their comments on this manuscript and Subhojit Chakraborty, Robin Hayman, Colin Lever, and John O'Keefe for helpful discussion.
Correspondence should be addressed to Kathryn J. Jeffery, Department of Psychology, University College London, 26 Bedford Way, London, WC1H OAP, UK. E-mail: k.jeffery@ucl.ac.uk.
Copyright © 2003 Society for Neuroscience 0270-6474/03/238827-09$15.00/0
References
- Barrientos RM, O'Reilly RC, Rudy JW ( 2002) Memory for context is impaired by injecting anisomycin into dorsal hippocampus following context exploration. Behav Brain Res 134: 299-306. [DOI] [PubMed] [Google Scholar]
- Bostock E, Muller RU, Kubie JL ( 1991) Experience-dependent modifications of hippocampal place cell firing. Hippocampus 1: 193-205. [DOI] [PubMed] [Google Scholar]
- Burgess N, Hartley T ( 2002) Orientational and geometric determinants of place and head-direction. In: Advances in neural information processing systems (Dietterich TG, Becker S, Ghahramani Z, eds), pp 165-172. Cambridge, MA: MIT.
- Doboli S, Minai AA, Best PJ ( 2000) Latent attractors: a model for context-dependent place representations in the hippocampus. Neural Comput 12: 1009-1043. [DOI] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ ( 2001) Cellular imaging of zif268 expression in the hippocampus and amygdala during contextual and cued fear memory retrieval: selective activation of hippocampal CA1 neurons during the recall of contextual memories. J Neurosci 21: 2186-2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley T, Burgess N, Lever C, Cacucci F, O'Keefe J ( 2000) Modeling place fields in terms of the cortical inputs to the hippocampus. Hippocampus 10: 369-379. [DOI] [PubMed] [Google Scholar]
- Jeffery KJ, Anderson MI ( 2003) Dissociation of the spatial and contextual influences on place cells. Hippocampus, in press. [DOI] [PubMed]
- Jeffery KJ, Gilbert A, Burton S, Strudwick A ( 2003) Preserved performance in a hippocampal-dependent spatial task despite complete place cell remapping. Hippocampus 13: 133-147. [DOI] [PubMed] [Google Scholar]
- Kali S, Dayan P ( 2000) The involvement of recurrent connections in area CA3 in establishing the properties of place fields: a model. J Neurosci 20: 7463-7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentros C, Hargreaves E, Hawkins RD, Kandel ER, Shapiro M, Muller RV ( 1998) Abolition of long-term stability of new hippocampal place cell maps by NMDA receptor blockade. Science 280: 2121-2126. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS ( 1992) Modality-specific retrograde amnesia of fear. Science 256: 675-677. [DOI] [PubMed] [Google Scholar]
- Knierim JJ ( 2003) Dynamic interactions between local surface cues, distal landmarks, and intrinsic circuitry in hippocampal place cells. J Neurosci 22: 6254-6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever C, Wills T, Cacucci F, Burgess N, O'Keefe J ( 2002) Long-term plasticity in hippocampal place-cell representation of environmental geometry. Nature 416: 90-94. [DOI] [PubMed] [Google Scholar]
- Maren S, Holt W ( 2000) The hippocampus and contextual memory retrieval in Pavlovian conditioning. Behav Brain Res 110: 97-108. [DOI] [PubMed] [Google Scholar]
- Markus EJ, Qin YL, Leonard B, Skaggs WE, McNaughton BL, Barnes CA ( 1995) Interactions between location and task affect the spatial and directional firing of hippocampal neurons. J Neurosci 15: 7079-7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RU, Kubie JL ( 1987) The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci 7: 1951-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RU, Poucet B, Rivard B ( 2001) Sensory determinants of hippocampal place cell firing fields. In: The neural basis of navigation: evidence from single-cell recording (Sharp PE, ed), pp 1-22. Norwell, MA: Kluwer Academic.
- Myers CE, Gluck MA ( 1994) Context, conditioning, and hippocampal rerepresentation in animal learning. Behav Neurosci 108: 835-847. [DOI] [PubMed] [Google Scholar]
- Nadel L, Willner J ( 1980) Context and conditioning: a place for space. Physiol Psychol 8: 218-228. [Google Scholar]
- Nadel L, Willner J, Kurz EM ( 1985) Cognitive maps and environmental context. In: Context and learning (Balsam PD, Tomie A, eds), pp 385-406. Hillsdale, NJ: Lawrence Erlbaum.
- O'Keefe J, Burgess N ( 1996) Geometric determinants of the place fields of hippocampal neurons. Nature 381: 425-428. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Dostrovsky J ( 1971) The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely moving rat. Brain Res 34: 171-175. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L ( 1978) The hippocampus as a cognitive map. Oxford: Oxford UP.
- O'Keefe J, Speakman A ( 1987) Single unit activity in the rat hippocampus during a spatial memory task. Exp Brain Res 68: 1-27. [DOI] [PubMed] [Google Scholar]
- O'Reilly RC, Rudy JW ( 2001) Conjunctive representations in learning and memory: principles of cortical and hippocampal function. Psychol Rev 108: 311-345. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE ( 1992) Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci 106: 274-285. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Muller RU, Kubie JL, Ranck JB ( 1992) The positional firing properties of medial entorhinal neurons: description and comparison with hippocampal place cells. J Neurosci 12: 1945-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, O'Reilly RC ( 1999) Contextual fear conditioning, conjunctive representations, pattern completion, and the hippocampus. Behav Neurosci 113: 867-880. [DOI] [PubMed] [Google Scholar]
- Samsonovich A, McNaughton BL ( 1997) Path integration and cognitive mapping in a continuous attractor neural network model. J Neurosci 17: 5900-5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro ML, Tanila H, Eichenbaum H ( 1997) Cues that hippocampal place cells encode: dynamic and hierarchical representation of local and distal stimuli. Hippocampus 7: 624-642. [DOI] [PubMed] [Google Scholar]
- Sharp PE ( 1997) Subicular cells generate similar spatial firing patterns in two geometrically and visually distinctive environments: comparison with hippocampal place cells. Behav Brain Res 85: 71-92. [DOI] [PubMed] [Google Scholar]
- Sharp PE ( 1999) Complimentary roles for hippocampal versus subicular/entorhinal place cells in coding place, context, and events. Hippocampus 9: 432-443. [DOI] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL ( 1998) Spatial firing properties of hippocampal CA1 populations in an environment containing two visually identical regions. J Neurosci 18: 8455-8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanila H, Shapiro ML, Eichenbaum H ( 1997) Discordance of spatial representation in ensembles of hippocampal place cells. Hippocampus 7: 613-623. [DOI] [PubMed] [Google Scholar]
- Tsodyks M ( 1999) Attractor neural network models of spatial maps in hippocampus. Hippocampus 9: 481-489. [DOI] [PubMed] [Google Scholar]
- Wood ER, Dudchenko PA, Robitsek RJ, Eichenbaum H ( 2000) Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron 27: 623-633. [DOI] [PubMed] [Google Scholar]