Abstract
Ionotropic glutamate receptors of the kainate and AMPA subtypes share a number of structural features, both topographical and in terms of stoichiometry. In addition, AMPA and kainate receptors share similar pharmacological and biophysical properties in that they are activated by common agonists and display rapid activation and desensitization characteristics. However, we show here that in contrast to AMPA receptor-mediated responses (native or recombinant GluR3 receptor), the response of native and recombinant (GluR6) kainate receptors to glutamate was drastically reduced in the absence of extracellular Na+ (i.e., when replaced by Cs+). Removal of Na+ increases the rate of desensitization, indicating that external Na+ modulates channel gating. Whereas the size of the substituting cation is important in mimicking the action of Na+ (Li+>K+>Cs+), modulation was voltage independent. These results indicate the existence of different gating mechanisms for AMPA and kainate receptors. By using chimeric AMPA-kainate receptors derived from GluR3 and GluR6, we have identified a key residue in the S2 segment of GluR6 (M770) that is largely responsible for the sensitivity of the receptor to external Na+. Thus, these results show the existence of a specific kainate receptor gating mechanism that requires external Na+ to be operative.
Keywords: kainate, glutamate, GluR6, KA2, GluR3, AMPA, channel gating, structure-function, allosteric modulation, receptors, chimeras
Introduction
Ionotropic glutamate receptors are ligand-gated ion channels that mediate most of the transfer of excitatory information in the brain. Among these glutamate receptors, the kainate and AMPA type share many pharmacological and structural properties (for review, see Dingledine et al., 1999; Madden, 2002). These receptor types can be activated by the same family of compounds: kainate activates both kainate and AMPA receptors, whereas different subtypes of kainate receptors are also sensitive to AMPA and its derivatives (for review, see Lerma, 2003). Similarly, it is well known that certain compounds antagonize both types of receptor. The structure of these receptor channels is also similar, and not only is there significant homology at the amino acid level, but also the residues within the segments lining the receptor channel impose similar biophysical properties. Moreover, the behavior of both receptor types is modified by mechanisms of RNA editing (Mass et al., 1997; Seeburg, 2002). These similarities are manifested in the analogous properties of ion permeability and channel blockade. The gating of AMPA and kainate receptor channels is also similar in that both activate and desensitize rapidly on glutamate binding with similar kinetics (Lerma, 1997; Bowie and Lange, 2002). Therefore, it might be expected that these receptors would rely on a common gating mechanism in which similar conformational changes of the agonist-binding domain must operate channel opening and closing. Little is known about the three-dimensional structure of the binding site formed by kainate receptors. However, it has been found that residues in the S2 domain influence their sensitivity to agonists (Swanson et al., 1997). Indeed, from the analysis of the numerous GluR6 receptor mutants, it appears that residues S689 and A721 in the S2 segment play a significant role in defining the gating properties of kainate receptors (Swanson et al., 1997, 1998).
During a series of experiments aimed at elucidating the channel permeability of native kainate receptors, we observed that kainate receptor-mediated current is undetectable in the absence of extracellular Na+. In contrast, AMPA receptor-mediated responses were unaffected. This result indicated that different gating mechanisms were imposed by the presence or absence of Na+. Indeed, Bowie (2002) has recently reported that external anions and cations influence the gating properties of recombinant kainate receptors. Therefore, we set out to reproduce this phenomenon in recombinant receptors to elucidate the molecular determinants and mechanisms involved. We found that kainate receptor channel gating depends on the presence of external Na+ and that it is associated with a key residue in the S2 segment (M770) of GluR6 subunits, which is largely responsible for this sensitivity. Thus, we provide further evidence for the existence of a kainate receptor-specific gating mechanism.
Materials and Methods
Generation of chimera constructs and of single-point mutants. The chimeras R6TM1R3, R6TM3R3, and R6(R3S2) have been described previously (Stern-Bach et al., 1994; Ayalon and Stern-Bach, 2001). The other chimeras used in this study were all constructed by a similar approach. The PCR templates GluR3(Q, flip) and GluR6(VCQ) were originally obtained from S. F. Heinemann (Salk Institute, La Jolla, CA). The PCR products were first subcloned into the pGEMHE vector (a gift from E. Liman, University of Southern California, Los Angeles, CA) for expression in Xenopus oocytes and subsequently subcloned into pCDNA3 (Invitrogen, San Diego, CA) for expression in HEK293 cells. The entire region amplified by PCR was verified by double-strand DNA sequencing. GluR6 single-point mutations were made on a GluR6(VCQ) template in pRK5 (obtained from Dr. P. H. Seeburg, Max-Planck Institute, Heidelberg, Germany) using the QuickChange method (Stratagene, La Jolla, CA). Subsequently, a small fragment containing the mutation was moved to a clean background and sequenced in full. Numbering of amino acids starts from the first methionine of the ORF.
Transient cDNA transfection and injection of Xenopus oocytes. PRK5 plasmids encoding GluR6 (VCR or VCQ versions), GluR5-2a, and KA2 were a gift from Dr. Seeburg, and Dr. T. Hughes (Yale University, New Haven, CT) kindly supplied the plasmid encoding the GFP. CDNAs encoding the wild-type, chimeric, and single-point mutant subunits were cotransfected with a GFP expression vector into HEK293 cells by electroporation (Gene Pulser; Bio-Rad, Hercules, CA). Cells were seeded in Petri dishes in DMEM supplemented with 10% FCS and antibiotics and maintained in a humidified incubator at 37°C and 5% CO2. Highly fluorescent cells (Marshall et al., 1995) were identified and selected for recording.
The isolation and maintenance of oocytes has been described previously (Kushner et al., 1988). Each oocyte was injected with 1-10 ng of cRNA, which was synthesized using an in vitro transcription kit (Stratagene; or Ambion, Austin, TX). Plasmids were linearized at the 3′ untranslated end of the cDNA insert with NheI (pGEMHE) or SalI (pRK5) before transcription with T7 or SP6 RNA polymerase, respectively.
Electrophysiological recordings and perfusion procedures. Electrophysiological experiments in 293 cells were carried out 24-48 hr after electroporation. Membrane currents were recorded at a given membrane potential under the whole-cell configuration of the patch-clamp technique using a List EPC-7 amplifier. Series resistance was compensated by 50-60%. Patch electrodes were fabricated from borosilicate glass with a resistance of 2-5 MΩ. Currents were filtered (two-pole Butterworth filter, -12 dB/octave) and transferred into a personal computer at a sampling rate of 1-5 kHz for analysis using pCLAMP software (Axon Instruments, Foster City, CA). The cells were rapidly perfused using a linear array of eight glass tubes placed 200-300 μm from the cell body. Ringer's solution with and without agonist flowed from adjacent barrels, and solution changes were achieved by displacing the whole perfusion array laterally using a motorized device controlled by a personal computer (Lerma et al., 1998). Electrophysiological recordings in Xenopus oocytes were performed 2-5 d after injection under a two-electrode voltage clamp with an Axoclamp 2A amplifier or GeneClamp500 (Axon Instruments).
Experimental solutions. The normal external solution for HEK293 cells was (in mm): 160 NaCl, 2.5 KCl, 1.8 CaCl2, 2 MgCl2, 10 glucose, and 10 HEPES, pH 7.4. Pipettes were filled with (in mm): 140 cesium methanesulfonate, 5 CsCl, 0.5 CaCl2, 5 MgCl2, 10 EGTA, and 10 HEPES, pH 7.4. Osmolarity was adjusted by adding sucrose, when necessary, to 330 and 314 mOsm for extracellular and intracellular solutions, respectively. Xenopus oocytes were continuously perfused with (in mm): 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, and 10 HEPES, pH 7.6; and voltage-recording and current-injecting electrodes were filled with 1 m KCl and had a resistance of ∼1MΩ. In experiments in which external Na+ was removed, this ion was completely substituted by Cs+, K+, or Li+. Because the current responses mediated by fast desensitizing receptors may escape detection in oocyte experiments, all expressed constructs were assayed in the presence of concanavalin A (Sigma, St. Louis, MO) or cyclothiazide (Lilly Research laboratory, Indianapolis, IN) to block desensitization of kainate and AMPA receptors, respectively. Kainate was obtained from either Sigma or Tocris Cookson (Bristol, UK). Monosodium glutamate was from Sigma, and other salts were obtained from either Sigma or Merck (Darmstadt, Germany).
Generation of three-dimensional models of GluR6 S1-S2. The three-dimensional homology models were generated using the Swiss-Model server (http://www.expasy.org/swissmod) (Peitsch, 1996) based on the template structure of GluR2 S1-S2 with glutamate-bound and apo states [Protein Data Bank (PDB) identification numbers 1FTJ and 1FTO, respectively] (Armstrong and Gouaux, 2000). The 251 aa sequence of GluR6 S1-S2 used for modeling (counting from the first M of the ORF) includes S427-K544 (1-113) for S1 and S670-N802 (119-251) for S2, linked by 5 aa, GTPIA (114-118), as in the GluR2 S1-S2 construct (Armstrong et al., 1998). The secondary structure of the models was also confirmed by comparison to the other relevant crystal structures. For a model without providing a specific template structure, the Swiss-Model server chose as templates the many available crystal structures of the GluR2 S1-S2 glutamate-binding core (PDB identification numbers 1FTJ, 1FTK, 1FTL, 1FTM, 1FTO, 1FW0, 1GR2, 1LB8, 1LB9, 1LBB, and 1LBC; 50.9-53.4% identity) as well as the bacterial glutamine binding protein (QBP; PDB identification numbers 1GGG and 1WDN; 27.6% identity). To model the dimer structure shown in Figure 7, the monomer was superimposed onto the second monomer of the glutamate-binding dimer (PDB identification number 1FTJ) using the program TOP (http://gamma.mbb.ki.se/~guoguang/export/doctop.html), which yielded a root mean square deviation of 0.4. Pictures of all three-dimensional models were made in BOBSCRIPT 2.4.
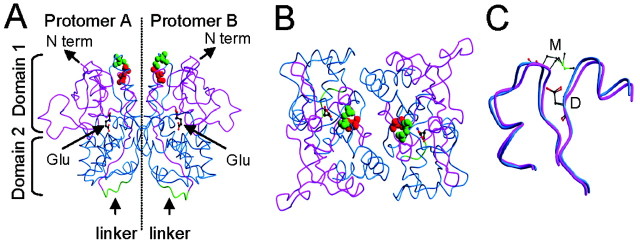
Figure 7.
The three-dimensional model of GluR6 S1-S2. A, Side view of the ribbon model of GluR6 S1-S2 dimer (see Materials and Methods) in complex with glutamate. In each monomer, S1 is magenta, S2 is blue, and the artificial S1-S2 linker is green. The glutamate molecule (Glu; balls-and-sticks model) is shown for each monomer at positions corresponding to those of the GluR2-bound structure (PDB identification number 1FTJ). The relative positions of domain 1 (composed of S1 and the C-terminal region of S2) and domain 2 (formed by the N-terminal region of S2) are marked as well as the position of the N terminus (N term) and the S1-S2 artificial linker. Residues D528 (red) and M770 (green) are depicted as space-filling models. These residues are shown to be located distant from the predicted dimer interface. B, Top view of the dimer in A, looking down the twofold axis, showing the dimer interface more clearly. C, Superposition of the two models of GluR6 S1-S2 corresponding to the template structures of glutamate-bound and unbound states of GluR2. Only the backbone trace of residues Y521-P532 and V766-T779 are shown. The unbound state is depicted as blue ribbon, whereas the bound state is magenta. Residues D528 and M770 of both states are drawn as ball-and-stick models, with carbon atoms (gray), oxygen atoms (red), nitrogen atoms (blue), and sulfur atoms (green). In both binding conformations, D538 and M770 are situated in a similar relative position.
Results
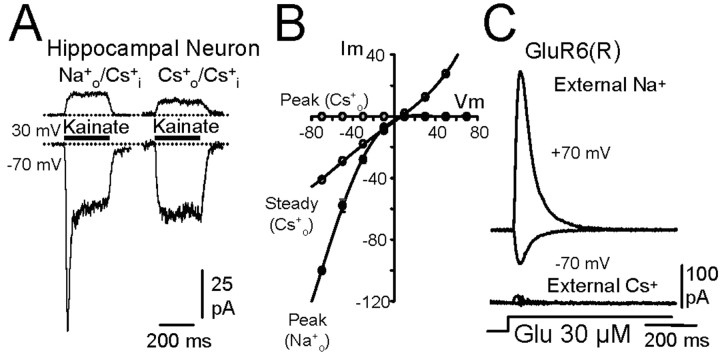
In young hippocampal neurons, rapid application of kainate activates both transient and sustained currents. It has been shown that the transient response corresponds to the activation of kainate receptors, whereas the sustained currents are generated through the direct stimulation of AMPA receptors (Lerma et al., 1993; Paternain et al., 1995). The I-V relationships presented by both types of response differed in that the peak transient current strongly rectified, whereas the steady response did not (Fig. 1B). This behavior is consistent with the fact that young hippocampal cells mostly express the unedited (Q) kainate receptors (Ruano et al., 1995). Despite the fact that kainate and AMPA receptor channels are equally permeable to Cs+ (Burnashev et al., 1996), the peak response was abolished when Cs+ substituted for extracellular Na+ (Fig. 1A,B). This result suggests that extracellular Na+ makes differential gating between native kainate and AMPA receptors.
Figure 1.
Effects of removing external Na+ on kainate and AMPA receptor-mediated responses. A, In a young culture of hippocampal neurons, application of kainate (300 μm) at -70 mV evoked two responses (the bar above the records indicates the time during which the cell was exposed to agonist). The transient current was caused by activation of kainate receptors, whereas the steady current was caused by the activation of AMPA receptors (Lerma et al., 1993). Note that the transient response was not active at depolarized potentials and disappeared when external Na+ was substituted completely with Cs+. B, Current-voltage relationships for peak and steady kainate-induced responses in the presence of extracellular Na+ or Cs+ in the neuron shown in A. C, Both inward and outward currents evoked by glutamate (Glu) on GluR6(R) kainate receptors expressed in HEK293 cells were practically abolished when extracellular Na+ was substituted by Cs+ (symmetrical Cs+).
To further study the dependence of gating on external Na+, we determined the effect of removing extracellular Na+ on recombinant kainate receptors. Because young cultured hippocampal neurons mostly express GluR6 (Ruano et al., 1995), this subunit was expressed in HEK293 cells. When Cs+ replaced Na+ in the perfusion fluid, both the inward and the larger outward currents of GluR6(R) were almost abolished in these cells (Fig. 1C). The effect of Na+ removal was independent of the channel permeability characteristics, because the response was reduced to a similar extent in receptors composed of either edited (R) or unedited (Q) subunits. On average, with approximately symmetrical Cs+, 7.9±.9% of the peak response was observed in cells expressing edited subunits (n = 9), whereas 8.2 ± 1.3% (n = 19) was found in unedited receptors. Similar results were observed when Na+ was removed from the extracellular fluid in the presence of intracellular Na+ (data not shown), indicating that it is the presence of this ion at the extracellular face of the receptor that is critical for channel opening and permeation. The lack of response was not a result of an inhibitory effect of extracellular Cs+ because essentially identical results were obtained when we substituted Na+ by N-methyl-d-glucamine (data not shown).
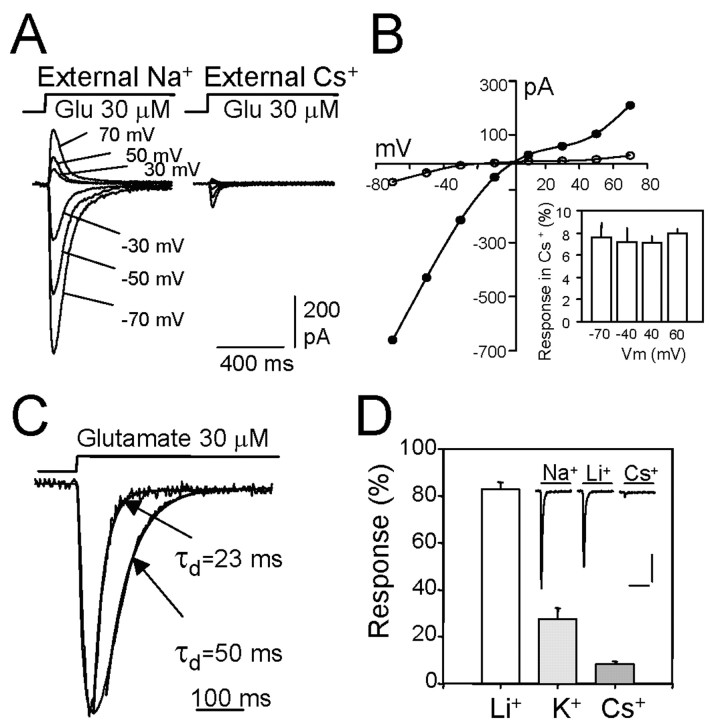
The possibility that this effect is a consequence of charge screening or ion channel blockage is unlikely because modulation was insensitive to the membrane potential. The same degree of current loss was observed at negative and positive membrane voltages [e.g., 7.3 ± 0.6% (n = 4) of the current remained at -40 mV, compared with 7.2 ± 0.6% (n = 6) at +40 mV; Fig. 2B]. Interestingly, the rate of desensitization was clearly accelerated when Cs+ replaced Na+ in the extracellular solution (Fig. 2C). At normal extracellular [Na+], the desensitization process was well fitted with one or two exponentials, the faster one being predominant. With 1 mm glutamate, the average time constant of the faster exponential decay was 17.7 ± 1.6 msec (n = 13), whereas at lower agonist concentrations (30 μm) it was 49 ± 2.3 msec (n = 14). When external Na+ was substituted with Cs+, the decay rate doubled because the time constants were <50% of those observed originally (7.7 ± 0.4 msec and 21.6 ± 1.1 msec at 1 mm and 30 μm glutamate, respectively). This result further indicates that the gating machinery may be modified in the absence of Na+.
Figure 2.
The characteristics of Na+ sensitivity of activation of GluR6 (Q) receptors in HEK293 cells. A, Typical responses obtained in normal extracellular Na+ (left) and when Cs+ substituted for Na+ (right). Responses were obtained at different membrane potentials and are superimposed. B, Removal of external Na+ did not affect the reversal potential of GluR6 receptors but reduced the response in a voltage-independent manner. The inset corresponds to a summary of experiments showing the response remaining in extracellular Cs+ expressed as a percentage of the current evoked in the presence of Na+. Data represent the mean ± SEM from four to eight cells. C, The desensitization rate of GluR6 receptors is increased in the absence of Na+. Responses to glutamate with and without extracellular Na+ are shown superimposed after normalization. The indicated time constants (τs) are from the single exponential fit (superimposed) to the desensitization process. D, The effect of substituting external Na+ with equimolar concentrations of alkali metals of different size. Data represent mean ± SEM from four to eight experiments. Calibration: 200 pA, 400 msec.
We then estimated the sensitivity of kainate receptor-mediated current to the removal of Na+ by measuring the responses at different [Na+], substituted with equimolar concentrations of Cs+. Under these circumstances and given that the channels are equally permeable to Na+ and Cs+, there was no apparent change in the reversal potential. Data pooled from 3-10 cells were fitted to the logistic equation considering basal activity as a parameter. The channel half activity was detected at a Na+ concentration of 86.8 mm (r = 0.98; SE of estimation, <5%), and the curve was quite steep, with a slope coefficient of 2.9 (SE of estimation, <10%; data not shown).
A plausible explanation for these results is that Na+ acts as an allosteric modulator of channel gating, probably by occupying a zone of the receptor molecule involved in the translation of binding to gating. To examine this hypothesis, we substituted Na+ with monovalent cations of different sizes. We found that 86 ± 1.3% (n = 20) of the current remained when Na+ was substituted with Li+, 24 ± 2% (n = 10) when it was replaced by K+, and this finally decreased to ∼8% when Na+ was substituted by the larger cation, Cs+ (Fig. 2D). These results indicate that the size of the cation is important in mimicking the role played by Na+.
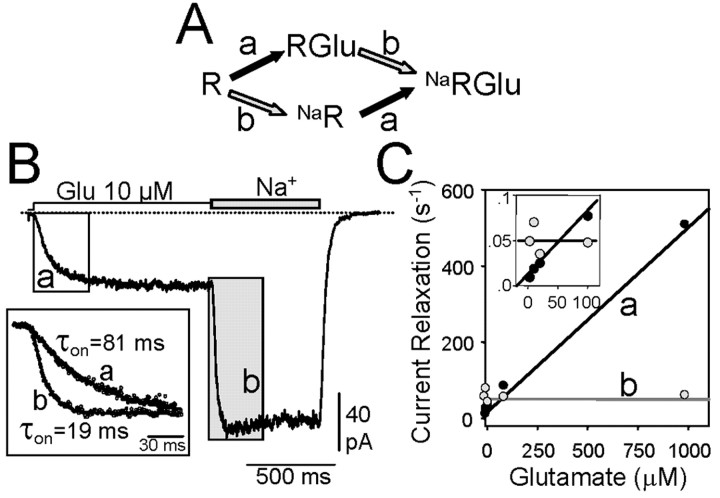
The binding of glutamate to amino acid transporters is dependent on the presence of Na+ (Zhang et al., 1998), and, thus, we wanted to determine whether Na+ affects glutamate binding to the kainate receptor. We took advantage of the fact that by treating GluR6-expressing cells with concanavalin A (ConA), not only was desensitization abolished, but enough current remained in the absence of Na+, thus allowing to study current relaxation. After treating the cells with ConA (0.3 μg/ml, 5 min), 26.2 ± 2.1% of the current remained in the absence of Na+ (n = 14). We established a simple scheme to test the possible effects of Na+ on glutamate binding (Fig. 3A). If glutamate could not bind to the kainate receptor in the absence of Na+, then the increase in current on rapidly introducing Na+ would result from the ensuing binding of glutamate (process a) rather than the binding of Na+ to receptors with or without bound glutamate (process b). Although it is difficult to distinguish between Na+ binding and channel opening in terms of kinetics, it is possible to detect glutamate binding by making it the rate-limiting step. The on-rate kinetics (measured in the presence of Cs+, Fig. 3B, trace a) were directly related to the glutamate concentration (Fig. 3C). However, the kinetics of current relaxation on switching to a Na+-containing solution (Fig. 3B, trace b) was independent of the concentration of glutamate in the perfusion medium (Fig. 3C). In principle, these results exclude the possibility that the loss of current observed in the absence of Na+ is caused by an impairment of glutamate binding. The degree of current deficit was not correlated to the concentration of glutamate used. In the absence of Na+, the fraction of remaining current was 7.4 ± 0.9% (n = 17) when assayed with 30 μm glutamate and 8.2 ± 1.3% at 1 mm glutamate. This indicates that a significant change in glutamate affinity does not take place by lowering [Na+] (cf. Bowie, 2002).
Figure 3.
The absence of extracellular Na+ does not prevent glutamate binding to kainate receptors. A, B, In the absence of external Na+ (Cs+), low concentrations of glutamate were applied to make the agonist binding rate limiting in cells expressing GluR6(Q) that were treated with ConA. Then, a solution containing Na+ instead of Cs+ was rapidly applied. Current relaxation rates were measured by fitting single exponentials (B, inset). Note that the first current relaxation represents binding of glutamate to the receptor (A, step a), whereas current development on Na+ introduction represents step b in the scheme and must correspond to the process triggered by Na+, either receptor modulation or Na-dependent binding of glutamate. C, The rate of current relaxation induced by glutamate was concentration dependent, as expected for a first-order binding process, whereas the rate of current increase on Na+ introduction was constant over a wide range of glutamate concentrations. The inset shows the results at low agonistconcentrations.
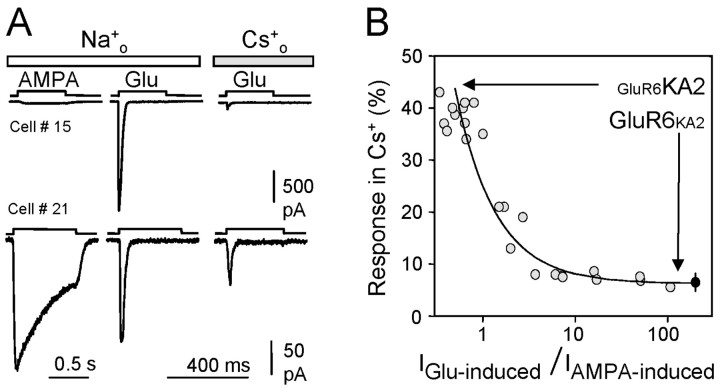
Removing Na+ produced a similar effect on channel gating of GluR5-KA2 heteromers, in which only 29 ± 3% (n = 4) of the response remained in extracellular Cs+. This value is significantly higher than that obtained for homomeric GluR6 receptors. However, in our hands, GluR5 subunits do not form functional homomeric channels, and, thus, we could not evaluate the action of Na+ removal on GluR5 receptors per se. To determine the influence of heteromerization on the effect of Na+ on channel gating, we tested the effect of Na+ removal on heteromeric GluR6-KA2 receptors, expressing the two subunits at different ratios. As shown previously, KA2 subunits do not form functional receptors. However, inserting KA2 into AMPA-insensitive GluR6 receptors enables GluR6-KA2 heteromeric channels to be gated by AMPA. Therefore, a good parameter to quantify the amount of functional GluR6-KA2 receptors present in the cell membrane would be the ratio of current amplitude induced by glutamate (which activates both homomeric and heteromeric receptors) over the current induced by AMPA (which activates only heteromeric receptors). The more heteromeric receptors that are present, the lower this ratio would be. Extreme examples of this are shown in Figure 4A, where the cDNAs for GluR6 and KA2 were cotransfected at ratios of 10:1 (top recordings) and 1:10 (bottom traces). However, because expression of these two plasmids and, therefore, formation of heteromeric receptors varies from cell to cell, we plotted all cases against the current amplitude ratio, irrespective of the cDNA ratio used. As can be seen, heteromeric receptors are less sensitive to Na+ removal, and sensitivity increases exponentially as the ratio increased (i.e., the number of heteromeric receptors falls; Fig. 3B), approaching the values registered for a pure population of homomeric GluR6 channels in Cs+. Altogether, these results indicate that the incorporation of KA2 into GluR6 receptors, and probably as well in GluR5 receptors (which we could not directly address), partially relieves the dependency on extracellular Na+ for channel gating.
Figure 4.
Effect of Na+ removal on heteromeric GluR6/KA2 receptors. A, Typical responses induced by glutamate and AMPA in the presence and absence of extracellular Na+ in HEK293 cells expressing different proportions of GluR6 and KA2 subunits. The density of heteromeric receptors was assessed by the amplitude of AMPA-induced currents with respect to the current activated by glutamate. B, A plot comparing the response to glutamate that remained in the absence of external Na+ versus the density of heteromeric GluR6-KA2 receptors. The heteromeric receptor density was quantified as the ratio of glutamate to AMPA-induced current amplitudes in each cell. The curve is the result of fitting an exponential function to the data points. The black dots represent the values of current remaining in Cs+ in cells expressing homomeric GluR6 receptors.
Subunit domains involved in Na+ modulation of channel gating
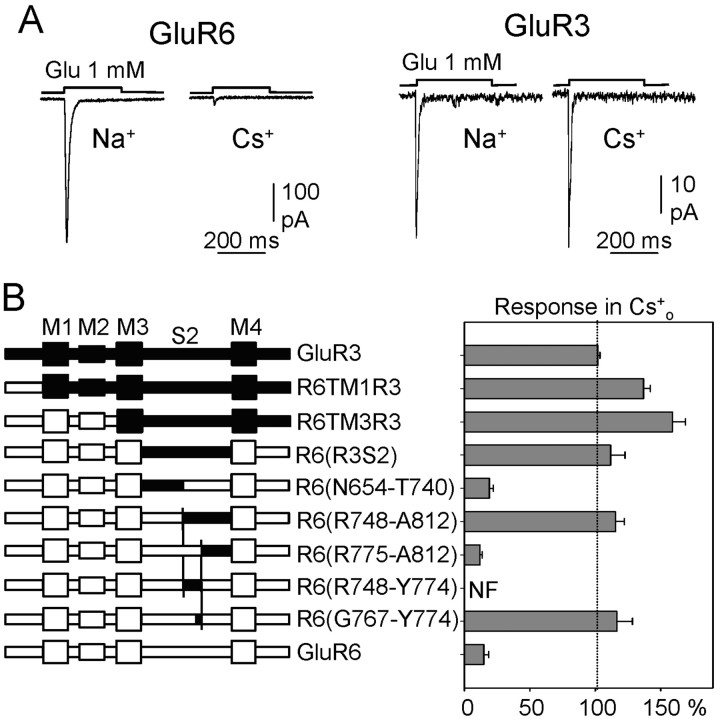
To determine which domain is involved in the interaction with Na+, we took advantage of the observation that AMPA receptors (e.g., GluR3 subunits) were insensitive to changes in extracellular [Na+] (Figs. 1A, 5A). Thus, we tested the effects of removing Na+ on chimeras made of GluR3 and GluR6 (Stern-Bach et al., 1994; Ayalon and Stern-Bach, 2001). HEK293 cells were transfected with chimeras in which the GluR3 region extending from M1 (chimera R6TM1R3), or M3 (chimera R6TM3R3), or that is confined to S2 [chimera R6(R3S2)] was incorporated into a GluR6 backbone, and, in response to glutamate, these chimeras behaved as GluR3 (Fig. 5B). Based on these results, two additional chimeras were generated in which the GluR3 substitution was restricted to either the N-terminal or the C-terminal half of GluR6-S2 [chimeras R6(N654-T740) and R6(R748-A812)], respectively. These chimeras were assayed in Xenopus oocytes because they were poorly expressed in HEK293 cells, and, to avoid rapid desensitization, the oocytes were treated with ConA. Although the N-terminal substitution (N654-T740) generated receptors sensitive to Na+, the chimera with the C-terminal substitution (R748-A812) was insensitive to the removal of this cation. We then analyzed the segment identified more closely, producing the chimera R6(R775-A812) that included a 53-aa-long segment of GluR3 confined to the flip-flop region. Interestingly, this chimera was sensitive to the Na+ removal, presenting a GluR6 phenotype. Because chimera R6(R748-A812) was like GluR3 and chimera R6(R775-A812) was like GluR6, we concluded that a motif important for Na+ interaction must be within the 29 aa segment that differentiates these two constructs. Unfortunately, the chimera containing this segment from GluR3 [R6(R748-Y774)] was not functional. Therefore, we generated additional mutants, splitting this segment in several parts (data not shown). Their analysis identified an 8 aa segment of GluR3, the insertion of which into the corresponding position in GluR6 produced insensitivity to the removal of Na+. To validate this result in the expression system used for most of our analyses, we expressed this chimera in HEK293 cells without treating them with ConA and found that, as in oocytes, the glutamate response was preserved in the absence of Na+ (84.4 ± 23%; n = 9; Fig. 6). Indeed, there was a remarkable agreement between quantitative data obtained in oocytes and HEK cells.
Figure 5.
Identification of molecular determinants responsible for sensitivity to external Na+ of kainate receptors. A, The responses mediated by kainate (GluR6), but not by AMPA (GluR3) receptors are modulated by external Na+ in HEK cells. B, left, Schematic representation of GluR3 (top), GluR6 (bottom), and the chimeras. In each chimera, GluR3 regions are black and GluR6 regions are white. In the chimeras with small GluR3 substitutions, the exchanged region in GluR6 is indicated by the first and last amino acids exchanged. In chimera R6(R3S2), the exchanged region in GluR6 extends from N654 to A812. Right, Glutamate-induced responses in the presence of external Cs+ of receptors expressed in Xenopus oocytes, as a percentage of the current evoked in the presence of external Na+. Data are the mean ± SEM of 10-15 experiments. NF, Nonfunctional.
Figure 6.
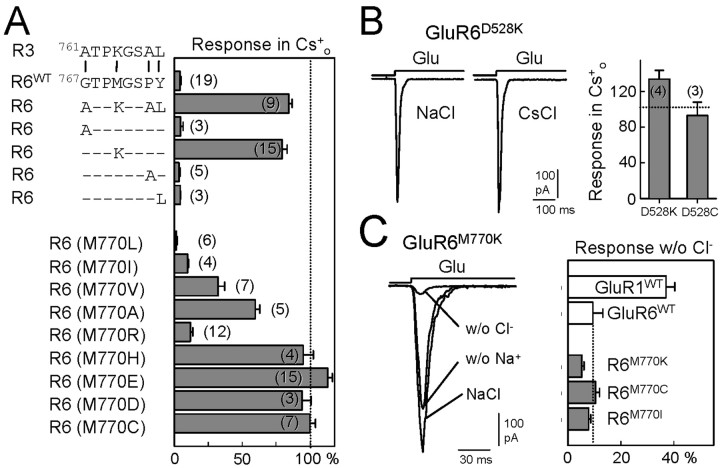
Identification of a single amino acid important for the sensitivity to Na+ removal in GluR6 receptors. A, The GluR3 critical segment identified from chimera R6(G767-Y774) differs from GluR6 in the four residues indicated. In GluR6, these were substituted by their counterparts from GluR3 (top). The histogram on the right represents the magnitude of the responses in the absence of Na+ as a fraction of the current obtained under normal ionic conditions (recorded in HEK293 cells). Note that only the substitution of residue M770 was able to abolish the sensitivity to Na+ removal. The bottom of the figure shows a summary of experiments in which M770 was replaced by different amino acids. Data are the mean ± SEM of the number of experiments indicated in parentheses. B, Substitution of D528 also relieves the requirement for external cations for channel functionality. The histogram shows the percent of the response when Na+ was substituted by Cs+. C, Effects of Cl- removal on GluR6 receptors. As it happens with Na+ removal, GluR6wt responses were largely depressed in the absence of Cl- (when substituted by methylsuphonate). The requirement for anions (Cl-) persisted in those GluR6 mutants that lose their sensitivity to Na+ removal. Bars are the mean ± SEM of four to six measurements. Traces on the left correspond to responses in normal solution and in the complete absence of Na+ (w/o Na+) or Cl- (w/o Cl-s) in the indicated mutant.
The segment that we had identified differed in four residues between GluR3 and GluR6; the GluR6 residues G767, M770, P773, and Y774 correspond to A761, K764, A767, and L768 in GluR3 (Fig. 6). By mutating these residues individually in GluR6 to the corresponding GluR3 equivalent, we found that the conversion of M770 to K was necessary and sufficient to abolish the dependence of GluR6 receptors on external Na+ (79.4 ± 3.7% of the current remained; n = 15; Fig. 6). Interestingly, only by replacing this M770 residue was Na+ sensitivity abolished; the triple mutant G767A/P773A/Y774L was still sensitive to Na+ (data not shown). Because the corresponding residue in GluR3 is a charged amino acid, we explored the role of the charge at this site. Surprisingly, not all positively charged residues were equivalent in eliminating the sensitivity to Na+, because only 12 ± 1.8% (n = 15) of the current remained in the absence of Na+ when R substituted for M770. However, when M770 was mutated to histidine (H), which at physiological pH has a positive charge, the response to glutamate remained intact in the absence of extracellular Na+ (95 ± 7.5%; n = 4). Similar observations were made when this key residue was replaced by negatively charged amino acids, glutamate (E) or aspartate (D), as well as when the substitution was to the uncharged but polar cysteine (99.9 ± 4.1%; n = 7; Fig. 6). These results indicate that the Na+ dependency of kainate receptors channel gating is because of the presence of a nonpolar residue at position 770. This was further confirmed by substituting M770 with other nonpolar residues. Replacement of M770 by isoleucine (I) or leucine (L) generated receptors that behaved similarly to the wild type [9.8 ± 0.7% (n = 4) and 1.5 ± 0.7% (n = 6), respectively]. Interestingly, substitutions with nonpolar amino acids of smaller side chains tended to relieve the Na+ dependency of channel gating because M770V and M770A mutants developed significant responses in the absence of Na+ [32 ± 4.8% (n = 7) and 59.7 ± 3.2% (n = 5) of the control current, respectively]. These results indicate that the bulkiness of the side chain is decisive for the modulation of gating by extracellular Na+ ions. Very similar results were obtained when the mutants were tested in Xenopus oocytes (data not shown).
Using the recently solved crystal structure of the S1-S2 binding domain of GluR2 AMPA receptor as a template, we modeled the structure of GluR6 S1-S2 in both the open (unbound) and closed (glutamate bound) conformations (Armstrong and Gouaux, 2000). We noted that in both cases GluR6 M770 situates in space close to residue D528 located in S1 (Fig. 7). This amino acid is conserved in AMPA, NMDA, and kainate receptor subunits, and we decided to explore the possibility that it also plays a role in Na+-dependent gating. Unfortunately, D528 appears to be critical for receptor functionality because mutations D528E and D528A produced nonfunctional channels. We observed a normal level of current responses from GluR6 carrying the D528K and D528C mutations, and the response remained in the absence of external Na+ in both cases [134 ± 10% (n = 4) and 93 ± 15% (n = 3), respectively; Fig. 6B]. Therefore, although limited, these results indicate that D528 may also contribute to the domain that it is critical for translating agonist binding into channel gating.
It could be hypothesized that, in kainate receptors, the presence of M at position 770 (instead of K that occurs in AMPA receptors) would require another means of neutralizing the negative charge of D528 (or others) to favor channel opening, a mechanism probably requiring external cations of a given size. To test this hypothesis, we substituted K764 in GluR3 for M and studied whether these mutant AMPA receptors were conferred with sensitivity to Na+ removal. Unfortunately, M764 appears to be critical for AMPA receptor function, because the substitution of this residue yielded nonfunctional receptors where assayed in HEK cells. However, in oocytes treated with cyclothiazide, GluR3(K764M) did yield small responses (∼10% of wild-type GluR3), and this was still insensitive to Na+ removal (data not shown). Similar results were obtained when all four different amino acids in this region were substituted in GluR3, or when we combined the K764M mutation with the exchange of the entire N-terminal domain of GluR3 for that of GluR6. Whereas these experiments did not allow a direct conclusion, it could be that this small current represents the current that remains in GluR6WT in the absence of Na+.
It has been shown that not only external cations but also anions could regulate the gating behavior of kainate but not AMPA receptors (Bowie, 2002). Indeed, replacement of external Cl- with other monovalent anions modulated GluR6 response amplitude and decay kinetics in a voltage-independent manner with no apparent shift in the reversal potential (Bowie, 2002). To test whether sensitivity to both ions was affected by the same mutations, we studied glutamate-induced responses in the absence of extracellular Cl-, by substituting it with methanesulphonate. Under this situation, whereas AMPA receptor-mediated responses were partially inhibited (37 ± 3.4% of the response remained in GluR1 receptors; n = 5), GluR6 responses were largely depressed (9.4 ± 3.4% remained, n = 4). However, the effect of Cl- removal persisted in those GluR6 mutants that lose their sensitivity to Na+ removal (e.g., M770K, M770C; Fig. 6C). Therefore, these results illustrate that the site for cations and anions must be independent, suggesting the existence of multiple regulatory sites for ions in kainate receptors.
Discussion
Here, we show that in contrast to AMPA receptors, agonist binding to either native or recombinant kainate receptors in the absence of external Na+ does not provoke the passage of current. This is a remarkable fact, because both types of ionotropic glutamate receptors share most of their structural characteristics (e.g., Madden, 2002). The absence of extracellular Na+ does not appear to affect the pore permeation of kainate receptors, nor does it screen out surface charge. Moreover, the effect of Na+ removal was voltage independent, and the reversal potential did not shift in symmetrical Cs+ conditions when compared with the normal Na+out/Cs+in situation. Nor was the loss of this kainate receptor-mediated response on Na+ removal dependent on agonist binding, because we found that glutamate bound normally to kainate receptors in the absence of Na+. External Na+ ions not only influenced the overall response of kainate receptors but also their desensitization kinetics. Assuming that desensitization kinetics and channel closure are coupled (Partin et al., 1996; Bowie, 2002; Bowie and Lange, 2002), the reduced response in low Na+ probably arises from a radical change in the open probability. Therefore, we conclude that external Na+ affects the gating mechanism of these channels, a property that is not conserved in AMPA receptors.
As indicated recently (Bowie, 2002), extracellular cations and anions can modulate kainate receptor channel gating, as occurs in other voltage-gated channels (Hille 1992). In the latter, however, this phenomenon was previously thought to be largely caused by screening the surface charge in the vicinity of voltage sensor rather than to a real allosteric effect. It is also known that in terms of permeation, ions may stabilize the open state, an effect described for K+ channels as “foot in the door” (Yeh and Armstrong, 1978). It is unlikely that this is the case for kainate receptors for several reasons. First, Cs+ and Na+ are both equally permeable in kainate receptors. Second, a single residue far from the conduction pathway (see below) confers sensitivity of kainate receptors to extracellular cations. Finally, the effect of cations and anions on kainate receptors channel gating could be abolished independently, supporting that there are two different and independent sites for each ionic class. Rather, the situation is more closely related to the incapacity of potassium channels to close in the absence of Ca2+ in squid neurons (Armstrong and Lopez-Barneo, 1987). Therefore, Na+ (and Cl-) seems to be an essential cofactor in the gating of kainate receptor channels.
Identifying domains important for ion sensitivity of channel gating
In a similar manner to kainate receptors and external Na+, RCK4 potassium channels require external K+ for current to permeate through the channel (Pardo et al., 1992). The region that mediates this K+ sensitivity coincides with the segment responsible for TEA sensitivity and N-type inactivation, suggesting that there is a conserved cation-binding site in the outer mouth of these channels (Heginbothan and McKinnon, 1992; Pardo et al., 1992). Thus, permeation rather than gating is altered in these channels when external K+ is removed. Significantly, the critical M770 residue for ion-sensitive kainate receptor-mediated current is far from the conduction pore and agonist-binding site (Fig. 7), as well as from the predicted dimer interface, shown to play a role in AMPA receptor desensitization (Sun et al., 2002). All kainate receptor subunits hold a nonpolar amino acid at this position (I, M, V, and L in GluR5, GluR6/7, KA1, and KA2, respectively). Interestingly, this M770 residue is a positively charged lysine (K) in all AMPA (and NMDA) receptor subunits that are not sensitive to the modulation by external ions (Bowie, 2002; the present study). Given the available structural information for the GluR2 binding domain (Armstrong and Gouaux, 2000), it could be determined that this positive charge situates in the space close to a conserved aspartate (D514 in GluR3; D528 in GluR6) located in S1 (Fig. 7). It is possible that this negative charge must be counteracted for the correct structural rearrangements to activate channel gating. Indeed, it has recently been proposed that intramolecular coupling of channel gating in the α-subunit of GABAA receptors occurs through electrostatic interactions of similar class (Kash et al., 2003). The absence of this K in kainate receptors would require that another means of neutralizing the negative charge of D528 must be used to favor channel opening, a mechanism probably requiring external cations of appropriate size. Were this hypothesis correct, substitution of K764 by a nonpolar amino acid should confer AMPA receptors with sensitivity to Na+ removal. Unfortunately, this hypothesis could not be tested because this residue appears to be critical for AMPA receptor function. Alternatively, kainate receptors may be more reminiscent of ACh receptors, in which hydrogen bonding replaces electrostatic interactions (Unwin et al., 2002). Thus, external Na+ may be required to facilitate these interactions and to stabilize channel opening. Favoring this interpretation, replacement of D528 in GluR6 receptors by K or C did abolish Na+ dependence, and it seems that introducing a polar residue at GluR6(770) is generally sufficient to make the kainate receptor mostly independent of external Na+. Hence, the residue we have identified appears to belong to a segment that links agonist binding to gating, which is stabilized by unknown interactions in the presence of Na+. Unfortunately, the chimera approach to identify domains important for a given phenomenon ignores conserved residues that might also play a role in receptor behavior. Worth mentioning is that in the crystallized GluR2 binding domain there seems to be a number of subsites complementary to the agonist-binding pocket (Madden, 2002). It might be that, as for water molecules that can be found in these unused subsites in AMPA receptors (Armstrong and Gouaux, 2000), kainate receptors need to complex Na+ or other ions at one or several of these subsites to transmit the effect of agonist binding to the channel gate. Thus, the mechanism by which external Na+ affects channel gating awaits verification from crystallographic studies of kainate receptors. Meanwhile, our data provide a structural foundation for the different gating mechanisms in AMPA and kainate receptors. Therefore, although AMPA and kainate receptors are constructed from a similar modular design, agonist-binding results in conformational changes for channel opening and desensitization that are specific to kainate receptors.
Is Na+ dependence of channel gating physiologically relevant?
One question that arises when considering the dependence of kainate receptor channel gating on external Na+ is whether physiological or pathological situations exist where the variation in external Na+ accounts for alterations in kainate receptor function. In other words, are there situations in which the gating of kainate receptors would be compromised because of their dependence on external Na+? It is well known that the extracellular ion concentration may change under particular situations, such as intense neuronal activity and pathological spreading depression, a process that involves a rapid redistribution of ions between intracellular and extracellular compartments (Somjen, 2001). During intense activity, cations are not exchanged one for one, but rather the Na+ decrease is greater than the increase in external K+. Indeed, there is a net gain of solutes, because cells become swollen as a consequence of a water drain. Variations in extracellular ions have been measured both in vitro and in vivo, and the Na+ concentration drops by ∼100 mm in 2-3 sec (Herreras and Somjen, 1993). This means that during spreading depression, external Na+ reaches a concentration as low as 40-60 mm. According to our results, at these concentrations the response of kainate receptors should be significantly depressed. It is more difficult to calculate to what extent the external concentration of Na+ declines during normal repetitive neuronal activity. Using ion-sensitive electrodes, extracellular Na+ drops of up to 20 mm have been measured (Dietzel et al., 1982; Dietzel and Heinemann, 1986). However, Na+ depletion in the vicinity of kainate receptors (i.e., within the synaptic cleft) must be much more profound. Recent data have pointed out that activity-dependent depletion of extracellular Ca2+ has important consequences for synaptic signaling (Rusakov, 2001; Rusakov and Fine, 2003). Because the activity-evoked Ca2+ decrease is mainly caused by activation of nonselective NMDA receptor channels (Rusakov and Fine, 2003), significant reductions in external Na+ could also be assumed. Thus, although it must be studied, kainate receptors could be less sensitive to glutamate released by later action potentials during repetitive firing. Therefore, Na+ sensitivity of channel gating may constitute a novel mechanism to modulate kainate receptors during burst or repetitive neuronal activity.
Footnotes
Correspondence should be addressed to Dr. J. Lerma, Instituto Cajal, Consejo Superior de Investigaciones Científicas, Av. Doctor Arce 37, 28002 Madrid, Spain. E-mail: Lerma@cajal.csic.es.
This work was supported by grants to J.L. from the Dirección General de Ensen̄anza Superior e Investigación Cientifica (PM99-0106) and the European Union (QLG3-CT2001-00929) and to Y.S.-B. from The Israel Science Foundation (496/00-2), the Israeli Ministry of Science, and the Spanish Ministry of Foreign Affairs in the form of an Israeli-Spanish Scientific Cooperation grant. A.V.P. is a postdoctoral fellow of the Community of Madrid. We are grateful to Dr. P. H. Seeburg for the GluR6, GluR3, and KA2 pRK plasmids; Dr. G. Swanson for providing GluR6M770I mutant; M. Kosloff and H. Dvir for help in modeling; and Dr. M. Morales, who collaborated in the preliminary stages of the work.
Copyright © 2003 Society for Neuroscience 0270-6474/03/238641-08$15.00/0
References
- Armstrong CM, Lopez-Barneo J ( 1987) External calcium ions are required for potassium channel gating in squid neurons. Science 236: 712-714. [DOI] [PubMed] [Google Scholar]
- Armstrong N, Gouaux E ( 2000) Mechanisms for activation and antagonism of an AMPA-sensitive glutamate receptor: crystal structures of the GluR2 ligand binding core. Neuron 28: 165-181. [DOI] [PubMed] [Google Scholar]
- Armstrong N, Sun Y, Chen GQ, Gouaux E ( 1998) Structure of a glutamate-receptor ligand-binding core in complex with kainate. Nature 395: 913-917. [DOI] [PubMed] [Google Scholar]
- Ayalon G, Stern-Bach Y ( 2001) Functional assembly of AMPA and kainate receptors is mediated by several discrete protein-protein interactions. Neuron 31: 103-113. [DOI] [PubMed] [Google Scholar]
- Bowie D ( 2002) External anions and cations distinguish between AMPA and kainate receptor gating mechanisms. J Physiol (Lond) 539: 725-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie D, Lange GD ( 2002) Functional stoichiometry of glutamate receptor desensitization. J Neurosci 22: 3392-3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnashev N, Villarroel A, Sakmann B ( 1996) Dimensions and ion selectivity of recombinant AMPA and kainate receptor channels and their dependence on Q/R site residues. J Physiol (Lond) 496: 165-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzel I, Heinemann U ( 1986) Dynamic variations of the brain cell microenvironment in relation to neuronal hyperactivity. Ann N Y Acad Sci 481: 72-86. [DOI] [PubMed] [Google Scholar]
- Dietzel I, Heinemann U, Hofmeier G, Lux HD ( 1982) Stimulus-induced changes in extracellular Na+ and Cl- concentration in relation to changes in the size of the extracellular space. Exp Brain Res 46: 73-84. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF ( 1999) The glutamate receptor ion channels. Pharmacol Rev 51: 7-61. [PubMed] [Google Scholar]
- Heginbotham L, MacKinnon R ( 1992) The aromatic binding site for tetraethylammonium ion on potassium channels. Neuron 8: 483-491. [DOI] [PubMed] [Google Scholar]
- Herreras O, Somjen GG ( 1993) Propagation of spreading depression among dendrites and somata of the same cell population. Brain Res 610: 276-282. [DOI] [PubMed] [Google Scholar]
- Hille B ( 1992) Ionic channels of excitable membranes. Sunderland, MA: Sinauer.
- Kash TL, Jenkins A, Kelley JC, Trudell JR, Harrison NL ( 2003) Coupling of agonist binding to channel gating in the GABA(A) receptor. Nature 421: 272-275. [DOI] [PubMed] [Google Scholar]
- Kushner L, Lerma J, Zukin RS, Bennett MV ( 1988) Coexpression of N-methyl-d-aspartate and phencyclidine receptors in Xenopus oocytes injected with rat brain mRNA. Proc Natl Acad Sci USA 85: 3250-3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J ( 1997) Kainate reveals its targets. Neuron 19: 1155-1158. [DOI] [PubMed] [Google Scholar]
- Lerma J ( 2003) Roles and rules of kainate receptors in synaptic transmission. Nat Rev Neurosci 4: 481-495. [DOI] [PubMed] [Google Scholar]
- Lerma J, Paternain AV, Naranjo JR, Mellstrom B ( 1993) Functional kainate-selective glutamate receptors in cultured hippocampal neurons. Proc Natl Acad Sci USA 90: 11688-11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J, Paternain AV, Salvador N, Somohano F, Morales M, Casado M ( 1998) Excitatory amino acid-activated channels. In: Ion channel pharmacology (Soria B, Ceña V, eds), pp 399-421. Oxford: Oxford UP.
- Maas S, Melcher T, Seeburg PH ( 1997) Mammalian RNA-dependent deaminases and edited mRNAs. Curr Opin Cell Biol 9: 343-349. [DOI] [PubMed] [Google Scholar]
- Madden DR ( 2002) The structure and function of glutamate receptor ion channels. Nat Rev Neurosci 3: 91-101. [DOI] [PubMed] [Google Scholar]
- Marshall J, Molloy R, Moss GW, Howe JR, Hughes TE ( 1995) The jellyfish green fluorescent protein: a new tool for studying ion channel expression and function. Neuron 14: 211-215. [DOI] [PubMed] [Google Scholar]
- Pardo LA, Heinemann SH, Terlau H, Ludewig U, Lorra C, Pongs O, Stuhmer W ( 1992) Extracellular K+ specifically modulates a rat brain K+ channel. Proc Natl Acad Sci USA 89: 2466-2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partin KM, Fleck MW, Mayer ML ( 1996) AMPA receptor flip/flop mutants affecting deactivation, desensitization, and modulation by cyclothiazide, aniracetam, and thiocyanate. J Neurosci 16: 6634-6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paternain AV, Morales M, Lerma J ( 1995) Selective antagonism of AMPA receptors unmasks kainate receptor-mediated responses in hippocampal neurons. Neuron 14: 185-189. [DOI] [PubMed] [Google Scholar]
- Peitsch MC ( 1996) ProMod and Swiss-Model: internet-based tools for automated comparative protein modeling. Biochem Soc Trans 24: 274-279. [DOI] [PubMed] [Google Scholar]
- Ruano D, Lambolez B, Rossier J, Paternain AV, Lerma J ( 1995) Kainate receptor subunits expressed in single cultured hippocampal neurons: molecular and functional variants by RNA editing. Neuron 14: 1009-1017. [DOI] [PubMed] [Google Scholar]
- Rusakov DA ( 2001) The role of perisynaptic glial sheaths in glutamate spillover and extracellular Ca(2+) depletion. Biophys J 81: 1947-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusakov DA, Fine A ( 2003) Extracellular Ca2+ depletion contributes to fast activity-dependent modulation of synaptic transmission in the brain. Neuron 37: 287-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg PH ( 2002) A-to-I editing: new and old sites, functions and speculations. Neuron 35: 17-20. [DOI] [PubMed] [Google Scholar]
- Somjen GG ( 2001) Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol Rev 81: 1065-1096. [DOI] [PubMed] [Google Scholar]
- Stern-Bach Y, Bettler B, Hartley M, Sheppard PO, O'Hara PJ, Heinemann SF ( 1994) Agonist selectivity of glutamate receptors is specified by two domains structurally related to bacterial amino acid-binding proteins. Neuron 13: 1345-1357. [DOI] [PubMed] [Google Scholar]
- Sun Y, Olson R, Horning M, Armstrong N, Mayer M, Gouaux E ( 2002) Mechanism of glutamate receptor desensitization. Nature 417: 245-253. [DOI] [PubMed] [Google Scholar]
- Swanson GT, Gereau 4th RW, Green T, Heinemann SF ( 1997) Identification of amino acid residues that control functional behavior in GluR5 and GluR6 kainate receptors. Neuron 19: 913-926. [DOI] [PubMed] [Google Scholar]
- Swanson GT, Green T, Heinemann SF ( 1998) Kainate receptors exhibit differential sensitivities to (S)-5- iodowillardiine. Mol Pharmacol 53: 942-949. [PubMed] [Google Scholar]
- Unwin N, Miyazawa A, Li J, Fujiyoshi Y ( 2002) Activation of the nicotinic acetylcholine receptor involves a switch in conformation of the α subunits. J Mol Biol 319: 1165-1176. [DOI] [PubMed] [Google Scholar]
- Yeh JZ, Armstrong CM ( 1978) Immobilisation of gating charge by a substance that simulates inactivation. Nature 273: 387-389. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Bendahan A, Zarbiv R, Kavanaugh MP, Kanner BI ( 1998) Molecular determinant of ion selectivity of a (Na+ + K+)-coupled rat brain glutamate transporter. Proc Natl Acad Sci USA 95: 751-755. [DOI] [PMC free article] [PubMed] [Google Scholar]