Abstract
Bitter taste perception is a conserved chemical sense against the ingestion of poisonous substances in mammals. A multigene family of G-protein-coupled receptors, T2R (so-called TAS2R or TRB) receptors and a G-protein α subunit (Gα), gustducin, are believed to be key molecules for its perception, but little is known about the molecular basis for its interaction. Here, we use a heterologous expression system to determine a specific domain of gustducin necessary for T2R coupling. Two chimeric Gα16 proteins harboring 37 and 44 gustducin-specific sequences at their C termini (G16/gust37 and G16/gust44) responded to different T2R receptors with known ligands, but G16/gust 23, G16/gust11, and G16/gust5 did not. The former two chimeras contained a predictedβ6 sheet, anα5 helix, and an extreme C terminus of gustducin, and all the domains were indispensable to the expression of T2R activity. We also expressed G16 protein chimeras with the corresponding domain from other Gαi proteins, cone-transducin (Gαt2), Gαi2, and Gαz (G16/t2, G16/i2, and G16/z). As a result, G16/t2 and G16/i2 produced specific responses of T2Rs, but G16/z did not. Because Gαt2 and Gαi2 are expressed in the taste receptor cells, these G-protein αi subunits may also be involved in bitter taste perception via T2R receptors. The present Gα16-based chimeras could be useful tools to analyze the functions of many orphan G-protein-coupled taste receptors.
Keywords: bitter taste, T2R receptor, G-protein α subunit, gustducin, Gα chimera, calcium imaging
Introduction
Taste perception is initially mediated by multiple signaling pathways in the taste receptor cells (TRCs) within taste buds in the oral epithelium. Bitter taste, as well as sweet taste, is believed to be detected by G-protein-coupled receptors (GPCRs), and the signaling pathways of TRCs have been the subject of intense speculation (for review, see Gilbertson et al., 2000; Lindemann, 2001; Margolskee, 2002; Montmayeur and Matsunami, 2002). α-Gustducin is a transducin-like Gαi protein selectively expressed in 20 -30% of TRCs (McLaughlin et al., 1992). In vitro biochemical assays and in vivo physiological studies using knock-out mice have demonstrated that gustducin plays a key role in TRC responses to numerous bitter compounds (Wong et al., 1996). However, gustducin knock-out mice still retained substantial sensitivity to bitter compounds in physiological and behavioral assays (Wong et al., 1996; He et al., 2002). In contrast, the second family of taste receptors identified, T2R (so-called TAS2R or TRB), is a large GPCR multigene family of ∼30 members in humans and rodents (Adler et al., 2000; Matsunami et al., 2000). The genes map to regions of human and mouse chromosomes implicated genetically in sensitivity to various bitter compounds and are coexpressed with gustducin, suggesting that T2R receptors could function as gustducin-linked bitter taste receptors. Currently, there are two T2R receptors that display ligand responses with an affinity range compatible with behavioral sensitivity: mouse T2R5 (mT2R5) for cycloheximide (Chandrashekar et al., 2000) and human T2R16 (hT2R16) for salicin (Bufe et al., 2002). However, the other T2R receptors remain orphan receptors with no known ligands.
To measure T2R activity, previous studies used a heterologous expression system using human embryonic kidney 293/Gα15 (HEK293/Gα15) cells (Chandrashekar et al., 2000; Bufe et al., 2002). These cells stably express the α subunit of the mouse G-protein α subunit (Gα) protein Gα15, which is thought to indiscriminately couple to many GPCRs (Offermanns and Simon, 1995). In this strategy, transfection of Gα15 into the cell system potentially allows measurements of elevated levels of intracellular calcium [Ca2+]i, giving a simple readout for agonist activation, although there is evidence that bitter taste transduction is mediated by Gαi-coupled receptors. However, Gα15 cannot be considered as a true universal adapter for GPCRs, because ∼18% of the total number of Gαi-coupled GPCRs examined to date cannot activate its human ortholog Gα16 (Mody et al., 2000; Kostenis, 2001). Moreover, T2R receptors are believed to couple with gustducin in the native TRCs. In the present study, we constructed a variety of chimeric Gα proteins to determine the specific domain of gustducin necessary for T2R coupling and demonstrated that a specific domain of gustducin is indispensable to ligand responses compatible with behavioral sensitivity.
Materials and Methods
Materials. Animals were obtained from Shizuoka Laboratory Animal Center (Shizuoka, Japan). The human leukemic cell line, HL60, was obtained from the Japan Collection Research Bioresources Cell Bank (Japan). Reagents for reverse transcription PCR (RT-PCR) were obtained from Invitrogen (Carlsbad, CA) and Applied Biosystems (Branchburg, NJ). Cycloheximide was purchased from Biomol Research Laboratories (Plymouth Meeting, PA). Salicin, serum, culture media, and anti-FLAG M2 and anti-opsin (Clone RET-P1) monoclonal antibodies were from Sigma (St. Louis, MO) unless otherwise noted.
Construction of Gα proteins and chimeras. A variety of Gα subunits were obtained from a human cell line and rat tissues by RT-PCR. Human Gα16 was obtained from HL60 cells. Rat α-gustducin and Gαi2 cDNAs were from rat lingual tissues containing circumvallate papillae. Similarly, rat Gαt2 and Gαz cDNAs were obtained from the retina and brain, respectively. All of the chimeras were constructed by PCRs using human Gα16 and rat-appropriated Gα cDNAs as templates. We first constructed a series of Gα16/gustducin (G16/gust) chimeras by incorporating different lengths of gustducin amino acid sequences at the C terminus of Gα16: G16/gust44, G16/gust37, G16/gust23, G16/gust11, and G16/gust5. In addition, we also constructed Gα16-based chimeras by replacing 44 amino acid sequences at the C terminus of Gα16 with those of Gαt2, Gαz, or Gαi2 (G16/t2, G16/z, and G16/i2). All full-length α-subunit cDNAs were subcloned into a pcDNA3.1(+) mammalian expression vector (Invitrogen). The G16/gust chimeras were also tagged by a FLAG epitope and cloned into the vector for expression assay and Western blot analysis.
Construction of T2R receptors. Mouse T2R5 and human T2R16 were amplified from mouse and human genomic DNAs, respectively. We subcloned its open reading frame into pME18S-FL3 containing the first 39 amino acids of bovine rhodopsin in the frame. The sequences allow immunohistochemical detection and facilitate expression of the recombinant chemosensory receptors on the cell surface.
Transfection of HEK293T cells. HEK293T cells were cultured with DMEM and supplemented with 10% FCS (v/v) at 37°C in humidified air with 5% CO2. For transfection, cells were seeded onto 100 mm dishes or uncoated glass coverslips in 35 mm chambers. After 24 hr at 37°C, cells were washed in DMEM medium and transfected with Gα and T2R using LipofectAmine 2000 reagent (Invitrogen). The transfection efficiencies were estimated by cotransfection of a GFP reporter plasmid or by immunohistochemistry and were typically >70%.
Western blot analysis. For Western blot analysis, a series of FLAG-tagged G16/gust chimeras was used. Cells were grown on 100 mm dishes to 70-80% confluence. Transfection was performed on 35 mm dishes with proper adjustments for the volumes and amounts of the reagents used. After 36 hr in normal growth conditions, cells were spun down briefly, resuspended in a lysis buffer (20 mm Tri-HCl, pH 7.4, 0.1% SDS, 1% Triton X-100, 1% sodium decoxycholate) containing one protease inhibitor cocktail tablet (complete mini; Roche Products, Mannheim, Germany), lysed by one cycle of freeze-thawing followed by 10 passages through a 27-gauge needle at 4°C, and centrifuged at 15,000 rpm for 30 min. Collected supernatants were used, and the protein concentrations were determined using a Bio-Rad (Hercules, CA) protein assay kit. Next, 50 μg of each protein sample was resolved on a 10% SDS-polyacrylamide gel and transferred to Immobilon-P transfer membrane (Millipore, Bedford, MA) via electroblotting. FLAG-tagged Gα chimeras were detected by an anti-FLAG antibody followed by an alkaline phosphatase-labeled anti-mouse IgG secondary antibody and then visualized by a phosphatase reaction using nitro blue tetrazolium chloride and 5-bromo-4-chlor-indolyl-phosphate (Roche Products).
Calcium imaging. We used a cell-based reporter system to examine T2R-Gα interaction (Chandrashekar et al., 2000; Bufe et al., 2002). In this system, receptor activation leads to increases in intracellular calcium [Ca 2+]i, which can be monitored at the single-cell level using the fura-2 AM calcium indicator dye. HEK293T cells were transiently transfected with a rhodopsin-tagged T2R receptor with a Gα16- or Gα16-based Gα chimera using LipofectAmine 2000 reagent. After 24 -30 hr, transfected cells were loaded with 5 μm fura-2 AM for 30 min at room temperature. The loading solution was washed out, and cells were incubated in 500 μl of assay buffer (10 mm HEPES, 130 mm NaCl, 10 mm glucose, 5 mm KCl, 2 mm CaCl2, and 1.2 mm MgCl2, pH 7.4) and stimulated with tastants using a bath perfusion system at a flow rate of 5 ml/min. We recorded [Ca 2+]i changes using an Olympus IX-70 (Olympus Optical, Tokyo, Japan) equipped with the ARGUS-HiSCA system (Hamamatsu, Shizouka, Japan). Aquisition and analysis of the fluorescence images were done using ARGUS-HiSCA version 1.65 software. Generally, [Ca 2+]i response was measured by sequentially illuminating cells at 340 and 380 nm and monitoring the fluorescence emission at 510 nm using a cooled CCD camera. At the beginning of each experiment, 10 μm isoproterenol was applied for 10 sec. A 180 sec interval was then left between each tastant application to ensure that cells were not desensitized as a result of the previous application of tastants. In all cases, we measured the entire camera field. As a control, we used isoproterenol (10 μm) to stimulate endogenous β-adrenergic receptors, proving that the Gα16-dependent signal transduction cascade was functional. Approximately 70-80% of all cells in the camera field responded to isoproterenol, whereas ∼15-20% of all cells in the field showed dose-dependent responses to agonists in the transient transfection experiments. The proportion of responders was about half of that found by immunohistochemistry, which was similar to that in a previous study using HEK/Gα15 cells (Bufe et al., 2002).
Results
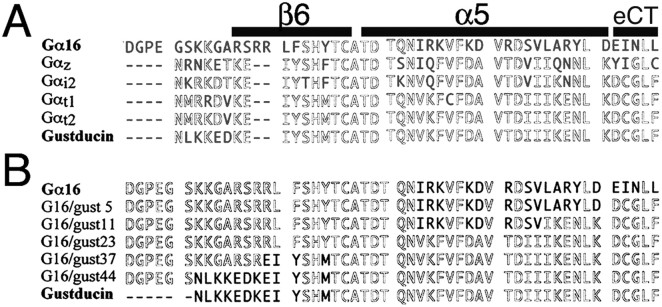
Figure 1A shows the alignment of the C-terminal amino acid sequences of the Gα used. Murine Gα15 has been successfully used to determine the function of two T2R receptors when stably expressed in HEK293 cells. Because its human ortholog, Gα16, is also known to interact with a wide variety of GPCRs, we first examined whether Gα16 could couple to T2R receptors in our transient expression system. Although we expressed Gα16 with mT2R5, a cycloheximide receptor in the mouse, by transient transfection in HEK293T cells, the T2R receptor did not respond to the cycloheximide (Fig. 2). Similarly, hT2R16, which has been reported to react with a plant bitter tastant salicin, failed to respond to it (Fig. 2). In both cases, 10 μm isoproterenol increased [Ca2+]i by activating an endogenous β-adrenergic receptor present in HEK293T cells. Untreated cells and cells without Gα16 did not respond to isoproterenol, indicating that human Gα16 could mediate intracellular calcium mobilization but could not couple to T2R receptors in the present assay system. We thus used Gα16 as the basis of Gα chimeras for a functional assay on the basis of calcium imaging.
Figure 1.
A, B, Alignments of C-terminal amino acid sequences of Gα16, Gαz, Gαi2, Gαt1, Gαt2, and α-gustducin (A) and a variety of G16/gust chimeras (B). Conserved sequences are in gray, and the sequences that differ from the majority are in black. Putative secondary structures on the basis of the Gαt1 crystal structure are indicated by shaded bars above the Gα16 sequence in A. eCT, Extreme C terminus.
Figure 2.
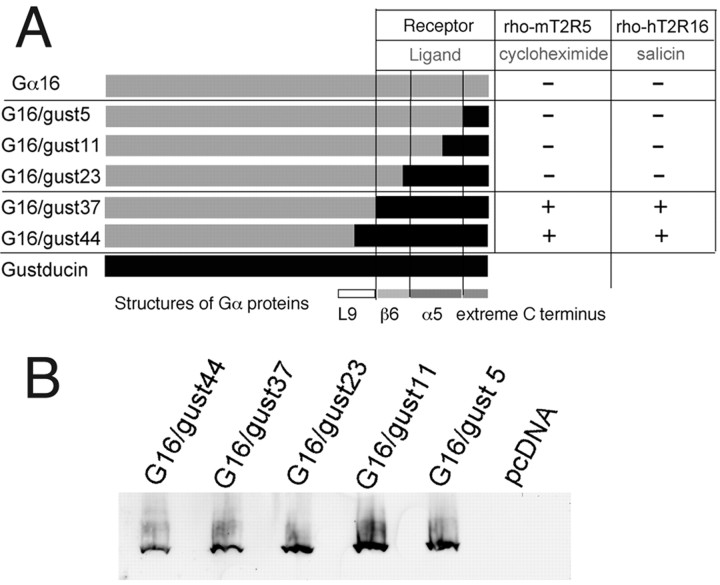
A, Schematic illustrations of chimeric G16/gust proteins with different lengths of C terminal amino acids found in gustducin and their abilities to couple to T2Rs. +, Specifically responded to the ligand in dose-dependent manner; -, did not exhibit any responses. B, Immunoblot analysis of FLAG-tagged chimeric G16/gust subunits expressed in HEK293T cells, stained with the anti-FLAG M2 monoclonal antibody. The FLAG epitope tag did not influence the functional activity of G16/gust chimeras.
As numerous studies on Gαi subunits have attested to the importance of the C-terminal tail of the α subunit as one of the major receptor contact regions, we constructed a series of Gα16/gustducin chimeras by incorporating different lengths of gustducin sequences at the C terminus of Gα16 (Fig. 1B). First, because the α5 helix is a known contact region for receptors (Lichtarge et al., 1996), we replaced the entire α5 helix of Gα16 with that of gustducin. The resultant chimera was named G16/gust23; for G16/gust23 and subsequent chimeras, the number after the latter gust indicates the number of gustducin residues present in the C terminus of the construct. Second, another region determining coupling selectivity is the extreme C terminus (also called C-terminal turn or β-turn) (Conklin et al., 1993; Blahos et al., 2001). G16/gust5 contains the minimum sequences of gustducin that correspond to the region decisive for coupling of Gα proteins with specific receptors. G16/gust11 was designed from a Gα COOH-terminal minigene vector to explore the coupling mechanisms of receptors (Gilchrist et al., 2002). Last, the region between α4 and α5 helices that includes the L9 loop and β6 sheet is also involved in coupling selectivity, probably by directly interacting with the receptors of the rhodopsin-like family (family 1 GPCRs) (Noel et al., 1993). G16/gust44 contains all the structures, an L9 loop, a β6 sheet, an α5 helix, and an extreme C terminus of gustducin, whereas G16/gust37 includes the latter three structures. We also made FLAG epitope-tagged G16/gust chimeras, but the results obtained using these epitope-tagged chimeras were identical to those using the Gα chimeras without epitope tags.
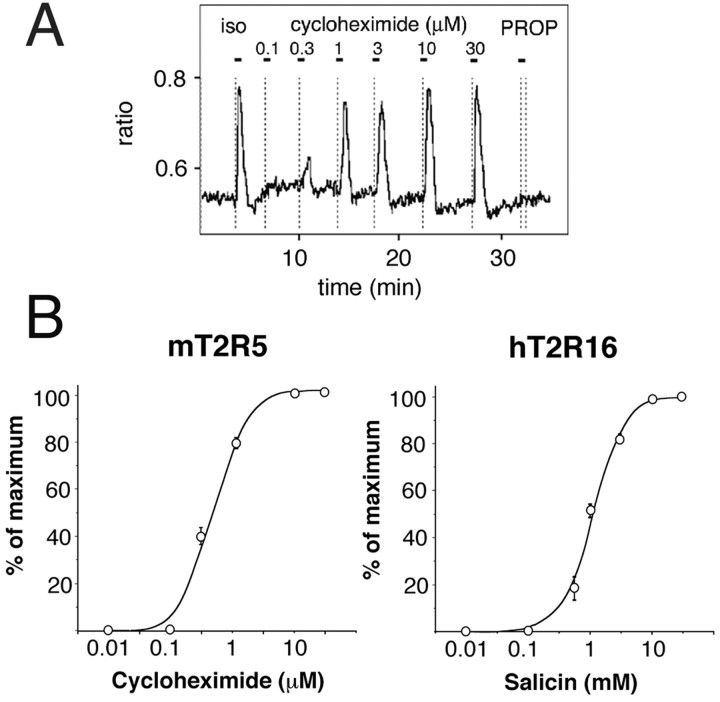
We used a well established transient expression system to examine the ability of the G16/gust chimeras to interact with T2R receptors (see Materials and Methods). We first examined the response against cycloheximide in HEK293T cells coexpressing the rhodopsin-mT2R5 (rho-mT2R5) with G16/gust44, G16/gust37, G16/gust23, G16/gust11, or G16/gust5. When transfected with T2R and either G16/gust44 or G16/gust37, cells specifically responded to cycloheximide (Figs. 2 and 3). The response was receptor- and Gα chimera-dependent, because cells lacking either of these components did not cause a [Ca2+]i increase, even at a 1000-fold higher cycloheximide concentration. In this assay, the EC50 value of mT2R5 was 0.5 μm, and the threshold was ∼0.2 μm (Fig. 3). These responses resembled those obtained from behavioral experiments with rodents (sensitivity threshold, ∼ 0.25 μm), indicating that G16/gust44 and G16/gust37 can functionally couple to mT2R5. In contrast, the other G-protein chimeras (G16/gust23, G16/gust11, and G16/gust5) failed to mediate the cycloheximide response under identical experimental conditions (Fig. 2). These G16/gust chimeras did not mediate any responses, even at 1000-fold higher ligand concentration. Hence, we checked the expression of the G16 chimeras by Western blot analysis using an anti-FLAG M2 monoclonal antibody for the immunodetection. As shown Figure 2, all chimeras were detected by the antibody in proteins prepared from HEK293T transfected with the FLAG-tagged chimeras. There were no differences in protein expression between them (Fig. 2).
Figure 3.
A, [Ca 2+]i responses in HEK293T cells expressing G16/gust37 and rho-mT2R5 when treated with multiple pulses of 10 μm isoproterenol (iso), cycloheximide, and 3 mm PROP (6-n-propylthiouracil). Isoproterenol was used to ascertain that the G16-dependent signaling cascade was functional. Horizontal bars above the traces indicate the time and duration of tastant pulses. Cycloheximide triggered robust receptor activation, but PROP did not. Similar results were obtained when G16/gust44 was used in place of G16/gust37. B, Dose-dependent curves of the effects of the ligands on the [Ca 2+]i in cells expressing G16/gust37 and the T2R receptor indicated.
The ability of G16/gust44 and G16/gust37 to interact productively with the mT2R5 receptor prompted us to further investigate their capacity to functionally associate with another T2R receptor. HEK293T cells were cotransfected with either G16/gust44 or G16/gust37 and rho-hT2R16. In transfected cells, stimulation of the ligand for hT2R16 (salicin) significantly increased [Ca2+]i (Fig. 2). Such [Ca2+]i increases were receptor- and Gα chimera-dependent and were in a dose-dependent manner. The EC50 and threshold were ∼2 and 0.2 mm, respectively (Fig. 3). These closely resembled those obtained in experiments with human subjects reported previously (Bufe et al., 2002). Thus, G16/gust44 and G16/gust37 successfully interacted with hT2R16 in addition to mT2R5. However, the other chimeras (G16/gust23, G16/gust11, and G16/gust5) did not mediate any ligand responses via hT2R16. Thus, the C-terminal 37 amino acid residues containing the predicted β6 sheet, α5 helix, and extreme C terminus of gustducin may be necessary for productive functional expression of T2R taste receptors.
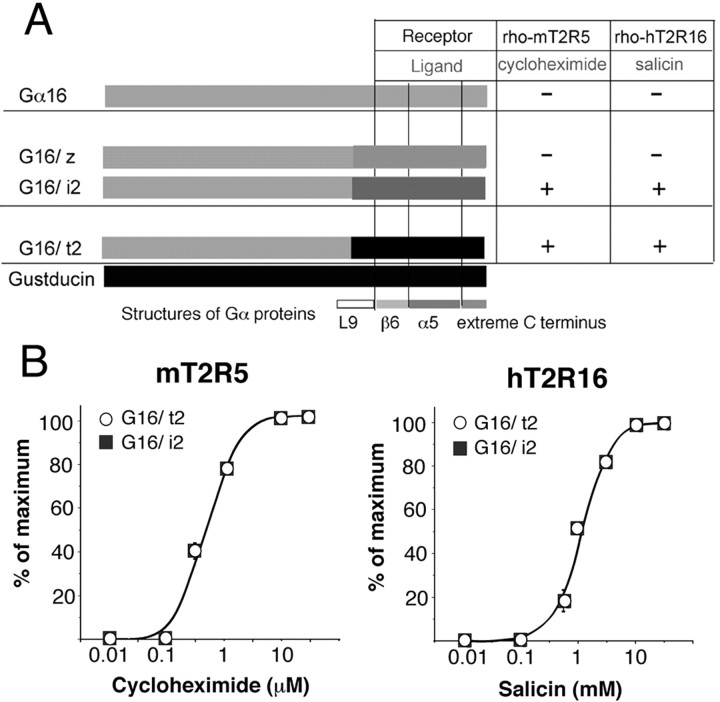
It has been suggested that in native mammalian TRCs, bitter taste may also be mediated by Gα proteins other than gustducin. We tested whether the T2Rs studied could associate with the C-terminal domain of other Gα proteins corresponding to the β6 sheet, α5 helix, and extreme C terminus of gustducin. We constructed Gα16 chimeras with 44 amino acid C termini found in a variety of Gαi proteins, including Gαt2, Gαi2, and Gαz (Fig. 1A) (G16/t2, G16/i2 and G16/z), and assayed them under identical experimental conditions to the experiments of G16/gust chimeras. As a result, G16/t2 and G16/i2 exhibited effective couplings with both mT2R5 and hT2R16 whereas G16/z did not (Fig. 4A). The dose-dependent curves were similar to those obtained from G16/gust chimeras that coupled to these receptors (Fig. 4B). There are no significant differences in the potency and efficacy between G16/t2 and G16/i2.
Figure 4.
A, Schematic illustrations of chimeric G16 proteins with 44 C terminal amino acids of different Gα proteins and their ability to couple to T2Rs. +, Specifically responded to the ligand in a dose-dependent manner; -, did not exhibit any responses. B, Dose-dependent curves of the effects of ligands on the [Ca 2+]i in cells expressing G16/t2 or G16/i2 and the T2R receptor indicated.
Discussion
To determine essential domains for coupling to GPCR, many loss-of-function-type mutation studies on G-proteins have been done. However, mutations of structurally important G-protein domains can also inhibit functional coupling with GPCRs, although the mutation domains themselves are not binding sites for GPCRs. In the present study, we constructed a variety of chimeric Gα proteins to better understand the domains involved in the interactions between T2R and gustducin. In addition, these chimeric Gα proteins enabled us to analyze gustducin-linked GPCRs on common robust assays that are amenable to high throughput-screening analysis. We monitored [Ca2+]i increases caused by activation of signaling cascade T2R-heterotrimeric G-protein (Gαβγ)-phospholipase C-inositol 3,4,5 triphosphate receptor. From the G-protein α, β, and γ subunits, probably both Gα and βγ dimers contact the receptors. The Gα subunit is likely to play a decisive role in discriminating between different receptor subtypes (Savarese and Fraser, 1992; Bourne, 1997; Wess, 1997). Here, we demonstrated that G16/gust44 and G16/gust37 successfully coupled to T2R receptors for their signal transduction. These responses were comparable with those obtained from in vivo experiments, and there is evidence that mT2R5 can activate intact gustducin in vitro (Chandrashekar et al., 2000), indicating that our system is capable of reproducing signaling transduction of T2R receptors in native TRCs. In contrast, G16/gust23 that contained the α5 helix of gustducin appeared not to associate, although numerous studies have attested to the importance of the α5 helix in receptor coupling. Similarly, G16/gust11 and G16/gust5 did not cause T2R activity. These results indicated that the α5 helix and extreme C terminus of gustducin were insufficient for detection of T2R activities, and the β6 sheet, in addition to the α5 and C-terminal β-sheet, is indispensable for signal transduction of T2Rs.
We next tested whether T2Rs could couple to domains including the β6 sheet, α5, and extreme C terminus from other G-protein α subunits. As a result, we revealed that some G16 chimeras constructed from other Gα proteins, G16/t2 and G16/i2, functionally coupled with the T2R receptors examined. Rod-α-transducin (Gαt1) and cone-α-transducin (Gαt2) are present in vertebrate taste cells. The former has been reported to transduce bitter taste by coupling taste receptor(s) to taste cell phosphodiesterase (Ruiz-Avila et al., 1995). In addition, gustducin and rod-transducin are biochemically indistinguishable in their in vitro interactions with retinal phosphodiesterase, rhodopsin (retinal GPCR), and G-portion βγ subunits (Hoon et al., 1995). Because the amino acid sequences of the β6 sheet and α5 helix in Gαt1 and Gαt2 are almost identical to those of gustducin (Fig. 1A), the C-terminal region (β6, α5, and extreme C terminus) conserved could be one of the most important domains for α-gustducin, Gαt1, and Gαt2 to interact with GPCRs.
In the present study, functional expression of T2R receptors was also observed in HEK293T cells coexpressing G16/i2 and T2R receptors, suggesting that T2Rs cannot only couple to gustducin and transducin but also to the G-protein αi2 subunit. One or more G-protein α subunits may play a role in bitter taste transduction, because α-gustducin knock-out mice retain residual responsiveness to bitter compound. In addition, transgenic expression of a dominant-negative form of α-gustducin from the gustducin promoter further decreased the residual responses of α-gustducin knock-out mice apparently by inhibiting T2R/TRB interactions with other TRC-expressed G-protein α subunits (Margolskee, 2002). It has been reported that Gαi2 subunit is expressed in subsets of TRCs. The frequency of Gαi2 expression appears to be higher than that of gustducin, and some Gαi2-positive cells also express α-gustducin (Kusakabe et al., 2000). Gαi2 could thus function as “backup” for gustducin in T2R-gustducin-expressing TRCs. In contrast, several TRCs that are immunoreactive for Gαi2 but not for gustducin responded to cycloheximide in an in vivo recording using mouse lingual slices (Caicedo et al., 2002). This suggests that Gαi2 may be involved in gustducin-independent bitter taste transduction via other G-protein-coupled receptors as well as T2Rs. On the basis of in situ hybridization with a mix of 10 different T2R probes, it was concluded that T2R genes are selectively expressed in gustducin-expressing taste receptor cells (Adler et al., 2000). However, a recent genomic study has shown that mouse T2R (Tas2r) family is composed of at least 36 full-length genes (Shi et al., 2003). Additional studies are required to determine whether all of the T2R genes are exclusively expressed in gustducin-expressing cells.
Many Gαi-coupled GPCRs share the ability to inhibit adenylyl cyclase via the pertussis toxin-insensitive Gαz (Chan et al., 1995; Lai et al., 1995). The incorporation of a Gαz-specific sequence into a Gα16 backbone (G16/z) successfully improved the recognition of a variety of Gαi-coupled receptors (Mody et al., 2000). However, the G16/z chimera was incapable of responding to the T2Rs studied in the present experiments, indicating that T2R receptors have specific sequences for interacting with gustducin and Gαi2. Within the β6 sheet and α5 helix (37 amino acids), there are five amino acids that are conserved in gustducin and Gαi2 but not in Gαz: V333, K346, D350, C351, and F354 in gustducin (at position -23, -10, -5, -4, and -1, respectively; the residues -1 being the last one) (Fig. 1A). In particular, the latter three amino acids are contained in the extreme C terminus of the Gα protein. Indeed, a gustducin mutant containing a glycine-to-proline substitution at position -3 can bind to taste receptor Gβγ subunits and the effector, but it cannot be activated by receptors (Ruiz-Avila et al., 2001). Therefore, the extreme C terminus may also play an important role in transduction via the gustducin, Gαt1, Gαt2, and Gαi2 of T2R taste receptors.
In conclusion, we found that 37 C terminal amino acids (β6, α5, and extreme C terminus) of gustducin and Gαi2 are indispensable for the detection of T2R activity. Because T2Rs have the greatest conservation in their cytoplasmic loops and adjacent transmembrane segments, which are the predicted sites for G-protein interaction, the present chimeric G16/gust proteins could be powerful tools to analyze orphan gustducin-linked taste receptors.
Footnotes
This work was supported by research grants from the Japan Society for the Promotion of Science.
Correspondence should be addressed to Takashi Ueda, Department of Molecular Morphology, Graduate School of Medical Sciences, Nagoya City University, 1 Kawasumi, Mizuho-cho, Mizuho-ku, Nagoya 467-8601, Japan. E-mail: tueda@med.nagoya-cu.ac.jp.
Copyright © 2003 Society for Neuroscience 0270-6474/03/237376-05$15.00/0
References
- Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJP, Zucker CS ( 2000) A novel family of mammalian taste receptors. Cell 100: 693-702. [DOI] [PubMed] [Google Scholar]
- Blahos J, Fischer T, Brabet I, Stauffer D, Rovelli G, Bockaert J, Pin J-P ( 2001) A novel site on the Gα-protein that recognizes heptahelical receptors. J Biol Chem 276: 3262-3269. [DOI] [PubMed] [Google Scholar]
- Bourne HR ( 1997) How receptors talk to trimeric G-proteins. Curr Opin Cell Biol 9: 134-142. [DOI] [PubMed] [Google Scholar]
- Bufe B, Hofmann T, Krautwurst D, Raguse J-D, Meyerhof W ( 2002) The human TAS2R16 receptor mediates bitter taste in response to β-glucopyranosides. Nat Genet 32: 397-401. [DOI] [PubMed] [Google Scholar]
- Caicedo A, Pereira E, Margolskee RF, Roper SD ( 2002) Gα-proteins involved in bitter taste detection. Poster presented at the 32nd Annual Meeting of the Society for Neuroscience, Orlando, FL, November.
- Chan JSC, Chiu TT, Wong YH ( 1995) Activation of type II adenylyl cyclase by the cloned μ-opioid receptor: coupling to multiple G-proteins. J Neurochem 65: 2682-2689. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zucker CS, Ryba NJP ( 2000) T2Rs function as bitter taste receptors. Cell 100: 703-711. [DOI] [PubMed] [Google Scholar]
- Conklin BR, Farfel Z, Lustig KD, Julius D, Bourne HR ( 1993) Substitution of three amino acids switches receptor specificity of Gqα to that of Giα. Nature 363: 274-276. [DOI] [PubMed] [Google Scholar]
- Gilbertson TA, Damark S, Margolskee RF ( 2000) The molecular physiology of taste transduction. Curr Opin Neurobiol 10: 519-527. [DOI] [PubMed] [Google Scholar]
- Gilchrist A, Li A, Hamm HE ( 2002) Gα COOH-terminal minigene vectors dissect heterotrimeric G-protein signaling. Sci STKE 2002: PL1. [DOI] [PubMed] [Google Scholar]
- He W, Danilova V, Zou S, Hellekant G, Max M, Morgolskee RF, Damak S ( 2002) Partial rescue of taste responses of α-gustducin null mice by transgenic expression of α-transducin. Chem Senses 27: 719-727. [DOI] [PubMed] [Google Scholar]
- Hoon MA, Northup JK, Margolskee RF, Ryba NJP ( 1995) Functional expresssion of the taste specific G-protein, α-gustducin. Biochem J 309: 629-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostenis E ( 2001) Is Gα16 the optimal tool for fishing ligands of orphan G-protein-coupled receptors? Trends Pharmacol Sci 22: 560-564. [DOI] [PubMed] [Google Scholar]
- Kusakabe Y, Yasuoka A, Asano-Miyoshi M, Iwabuchi K, Matsumoto I, Arai S, Emori Y, Abe K ( 2000) Comprehensive study on G-protein α-subunits in taste bud cells, with special reference to the occurrence of Gαi2 as a major Gα species. Chem Senses 25: 525-531. [DOI] [PubMed] [Google Scholar]
- Lai HWL, Minami M, Satoh M, Wong YH ( 1995) Gz coupling to the rat κ-opioid receptors. FEBS Lett 360: 97-99. [DOI] [PubMed] [Google Scholar]
- Lichtarge O, Bourne HR, Cohen FE ( 1996) Evolutionarily conserved Galphabetagamma binding surfaces support a model of the G-protein-receptor complex. Proc Natl Acad Sci USA 93: 7507-7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann B ( 2001) Receptors and transduction in taste. Nature 413: 219-225. [DOI] [PubMed] [Google Scholar]
- Margolskee RF ( 2002) Molecular mechanisms of bitter and sweet taste transduction. J Biol Chem 277: 1-4. [DOI] [PubMed] [Google Scholar]
- Matsunami H, Montmayeur J-P, Buck LB ( 2000) A family of candidate taste receptors in human and mouse. Nature 404: 601-603. [DOI] [PubMed] [Google Scholar]
- McLaughlin SK, McKinnon PJ, Margolskee RF ( 1992) Gustducin is a taste-cell-specific G-protein closely related to transducins. Nature 357: 563-569. [DOI] [PubMed] [Google Scholar]
- Mody SM, Ho MKC, Joshi SA, Wong YH ( 2000) Incorporation of Gαz-specific sequence at the carboxyl terminus increases the promiscuity of Ga16 toward Gi-coupled receptors. Mol Pharmacol 57: 13-23. [PubMed] [Google Scholar]
- Montmayeur J-P, Matsunami H ( 2002) Receptors for bitter and sweet taste. Curr Opin Neurobiol 12: 366-371. [DOI] [PubMed] [Google Scholar]
- Noel JP, Hamm HE, Sigler PB ( 1993) The 2.2 A crystal structure of transducin-alpha complexed with GTP gamma S. Nature 366: 654-663. [DOI] [PubMed] [Google Scholar]
- Offermanns S, Simon MI ( 1995) Gα15 and Gα16 couple a wide variety of receptors to phospholipase C. J Biol Chem 270: 15175-15180. [DOI] [PubMed] [Google Scholar]
- Ruiz-Avila L, McLaughlin SK, Wildman D, McKinnon PJ, Robichon A, Spickofsky N, Margolskee RF ( 1995) Coupling of bitter receptor to phosphodiesterase through transducin in taste receptor cells. Nature 376: 80-85. [DOI] [PubMed] [Google Scholar]
- Ruiz-Avila L, Wong GT, Damak S, Margolskee RF ( 2001) Dominant loss of responsiveness to sweet and bitter compounds caused by a single mutation in α-gustducin. Proc Natl Acad Sci USA 98: 8868-8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarese TM, Fraser CM ( 1992) In vitro mutagenesis and the search for structure-function relationships among G-protein-coupled receptors. Biochem J 283: 1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi P, Zhang J, Yang H, Zhang Y ( 2003) Adaptive diversification of bitter taste receptor genes in mammalian evolution. Mol Biol Evol 20: 805-814. [DOI] [PubMed] [Google Scholar]
- Wess J ( 1997) G-protein-coupled receptors: molecular mechanisms involved in receptor activation and selectivity of G-protein recognition. FASEB J 11: 346-354. [PubMed] [Google Scholar]
- Wong GT, Gannon KS, Margolskee RF ( 1996) Transduction of bitter and sweet taste by gustducin. Nature 381: 796-800. [DOI] [PubMed] [Google Scholar]