Abstract
Long-term memory for habituation to tap in Caenorhabditis elegans depends on glr-1, a homolog of mammalian non-NMDA glutamate receptors; mutations in glr-1 blocked long-term memory formation. Green fluorescent protein (GFP) constructs were used to visualize glr-1 expression in the interneurons of the mechanosensory circuit and synaptobrevin in the tap sensory neurons of trained and untrained worms. Trained animals had less GLR-1::GFP expression than untrained animals; there was no difference in the vesicle marker synaptobrevin. Heat shock during training blocked both the behavioral expression of long-term memory and the change in GLR-1::GFP expression. Thus, long-term memory in C. elegans is dependent on glr-1 and likely involves changes in the expression or localization of glutamate receptors.
Keywords: C. elegans, habituation, long-term memory, glutamate receptors, synaptobrevin, LTD
Introduction
Glutamate receptor trafficking has been hypothesized as a mechanism of plasticity in the mammalian brain (for review, see Lüscher and Frerking, 2001; Malinow and Malenka, 2002). Stimuli that produce long-term potentiation produce an increase in AMPA-type glutamate receptors inserted into neuronal membranes, whereas stimulation that produces long-term depression (LTD) produces a decrease in the AMPA receptors. Thus far, receptor trafficking as a result of learning has not been shown in any preparation. In this paper, we report that glutamate receptors are critical for long-term memory for habituation in Caenorhabditis elegans and that glutamate receptor expression is altered as a function of training.
C. elegans shows protein synthesis-dependent retention of habituation of the tap withdrawal response 24 hr after distributed training (Beck and Rankin 1995, 1997; Rose et al., 2002). The primary elements of the neural circuit for tap are five sensory neurons and four pairs of interneurons (as well as a large number of motor neurons) (Chalfie et al., 1985; Wicks and Rankin, 1995). The tap response is mediated by electrical connections and modulated by chemical connections (Chalfie et al., 1985; Wicks and Rankin, 1995). The gene EAT-4 encodes a vesicular glutamate transporter and is expressed in the tap circuit sensory neurons (ALMs, AVMs, and PLMs) (Avery 1993; Bellocchio et al., 1998; Lee et al., 1999). Mutation of EAT-4 alters the expression of long-term memory (LTM) for habituation (Rose et al., 2002). When a tap stimulus was used for training, eat-4(ky5) worms did not exhibit LTM for habituation; however, if trained with a stronger stimulus, a train of taps, LTM was observed. From this, we hypothesize that, with a strong enough stimulus, sufficient glutamate is released from the mechanosensory neurons to produce LTM for habituation.
The necessity of presynaptic glutamate release suggests that postsynaptic glutamate receptors play an important role in LTM for habituation. GLR-1 (glutamate receptor 1) encodes a receptor subunit of a non-NMDA excitatory ionotropic glutamate receptor subtype; it has 40% homology with mammalian AMPA receptors. The two glr-1 alleles identified in C. elegans are expressed on the tap circuit interneurons (AVAs, AVBs, AVDs, and PVCs). glr-1(n2461) is a deletion mutation, and glr-1(ky176) is a loss-of-function mutation produced by a premature stop codon (Hart et al., 1995; Maricq et al., 1995).
To investigate the role of glr-1 receptors in LTM for habituation training in C. elegans, wild-type (N2), glr-1(n2461), glr-1(ky176), and N2 worms treated with an AMPA receptor antagonist were given distributed habituation training and were tested for LTM. In addition, the effects of LTM training on the genes expressed in the tap sensory neurons and command interneurons were examined using two green fluorescent protein (GFP) constructs (Nonet, 1999; Rongo and Kaplan, 1999).
Parts of this work have previously been published in abstract form (Rose et al., 2001).
Materials and Methods
Animals. Worms were maintained on nematode growth medium (NGM) agar seeded with Escherichia coli (OP50) (Brenner, 1974). N2 C. elegans Bristol (N2) and glr-1(n2461) were obtained from the Caenorhabditis Genetics Center (University of Minnesota, Minneapolis, MN), glr-1(ky176) was from V. Maricq (University of Utah, Salt Lake City, UT), GLR-1::GFP was from J. Kaplan (Harvard University, Boston, MA), and pMEC-7::SNB-1::GFP was from M. Nonet (Washington University, St. Louis, MO).
Behavioral procedures. Habituation stimuli (taps-trains) were delivered using a Grass Instruments (Quincy, MA) S88 stimulator driving a mechanical tapper exerting 1-2 N of force to the side of the Petri plate. For studies of short-term habituation, 20 single tap or train (train is six taps in 600 msec) stimuli were delivered to single worms on blank NGM agar plates at a 60 sec interstimulus interval (ISI). For studies of LTM for habituation groups of 10-15 3-d-old worms were placed on E. coli seeded Petri plates 12-18 hr before training. On the training day, experimental worms were given distributed habituation training (four blocks of 20 taps or trains at a 60 sec ISI separated by 1 hr rest periods); control worms were given a single tap or train. One hour after training, all worms were transferred to individual E. coli streaked plates. During testing (24-28 hr after training), each worm received 10 taps or trains (whichever was the same as that worm's training) at 60 sec ISI. The responses to the 10 test taps were scored and used in the data analysis. In studies in which heat shock was used, plates were sealed with Parafilm and submerged in a water bath at 32°C for 40 min during the rest period between training blocks. Sham heat shock involved submerging plates in a water bath at 21°C in the same procedure.
For studies on the role of 6,7-dinitroquinoxaline-2,3(1H,4H)-dione (DNQX) on LTM, the drug was dissolved in 35 mm NaOH to prepare 10 mm DNQX. Pilot experiments showed no differences in the reversal behaviors of worms treated with different dilutions of DNQX; therefore, a high drug concentration was used to ensure drug penetration. Two hours before training, 500 μl of either 10 mm DNQX or 35 mm NaOH (vehicle) was placed onto the surface of agar plates bearing E. coli. For the drug groups, the 500 μl of 10 mm DNQX applied to 10 ml of agar made the effective external concentration in the worm's environment 476 μm. One hour before training, four groups of 10-15 4-d-old wild-type worms were transferred to drug-treated plates. Groups of worms were given either taps in the distributed training protocol at a 60 sec ISI or the single-tap control and then 1 hr later were transferred to individual drug-free plates for testing with 10 taps (at a 60 sec ISI) the next day. Drug exposure was <6 hr, and all testing was conducted ≥20 hr after removal of drug.
All behavioral data were scored using stop-frame video analysis to trace the distance traveled backward to each tap or train onto acetate sheets. Tracings were scanned into the computer and measured using NIH Image software. Occasionally, worms swam rapidly forward in response to tap; these accelerations forward are qualitatively different from reversals and were scored as missing data points (<10% of the data).
Imaging procedures. For imaging, GFP transgenic worm strains were mounted onto welled slides using 12 μl of 2,3-butanedione monoxime for paralysis mixed with medium Sephadex beads to prevent worms from being crushed. A Nikon (Tokyo, Japan) Optiphot-2 microscope with an MRC 600 confocal system (Bio-Rad, Hercules, CA) equipped with a krypton-argon laser was used for imaging. GFP was excited using a 488 nm wavelength laser setting with emitted light collected passing through a ∼510-550 nm bandpass filter. Images were captured in a 768 × 512 pixel field of view, with optical sections collected at 0.5 μm intervals using a 60 × oil lens.
Twenty-four hours before imaging, GLR-1::GFP and pmec-7::SNB-1::GFP worms were given taps in the distributed training protocol or the single-tap control. One hour after training, worms were transferred to individual plates for imaging 24 hr later.
The GLR-1::GFP strain expressed GFP along the ventral cord, and images were collected along the posterior portion from the tail to the vulva. Images of GLR-1::GFP expression were composed of 10-15 optical sections. A researcher blind to the treatment groups measured images. Collected projection images were viewed and measured using NIH Image 1.61. GFP expression was measured as the length of GFP clusters because ventral cord width was relatively uniform across animals.
GFP expressed in the pmec-7::SNB-1::GFP worms was captured in a single image composed of ∼12-18 optical sections. Because the GFP in these worms was often faint, the microscope was set to maximal sensitivity for all worms. A researcher blind to the treatment groups measured images. In NIH Image, a threshold adjustment produced high-contrast images in black and white to allow for viewing of faint GFP. Area measurements for each region of GFP expression were calculated by outlining the GFP-expressing region and using the area measure function. Final figures were generated using Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA).
Results
Behavioral analyses of glr-1 in long-term memory
Both of the glr-1 alleles were originally hypothesized to lead to null mutations (Hart et al., 1995; Maricq et al., 1995); however, our data showing differences in response magnitude to different stimuli suggests that the truncated protein produced by ky176 is not totally lacking in function. ky176 showed almost no reversal to tap, whereas n2461 showed reversal responses to tap similar to wild-type worms (response to tap in pixels: n = 20 glr-1(n2461) worms, 220.03 ± 49.18; n = 10 glr-1(ky176) worms, 5.95 ± 4.04). Both strains reversed in response to a stronger stimulus, a train of taps (response to train: n = 19 glr-1(n2461) worms, 744.64 ± 50.59; n = 16 glr-1(ky176) worms, 542.88 ± 65.00). Therefore, for assessing both short- and long-term habituation, glr-1(n2461) was tested with both trains and taps, whereas glr-1(ky176) was only tested with trains.
Despite differences in initial response magnitude, both glr-1 strains showed significant short-term habituation to 20 stimuli at a 60 sec ISI (Fig. 1), suggesting that the gene does not play a direct role in short-term habituation. To examine the role of glr-1 in LTM for habituation, we used a between-groups distributed training protocol (training, four blocks of 20 stimuli at a 60 sec ISI each separated by 1 hr rest periods; testing, 10 stimuli at a 60 sec ISI 24 hr after training) to test wild-type N2, n2461, and ky176 worms. On day 2, when we compared the test responses of trained wild-type worms to trained glr-1 mutant worms, we found that N2 worms showed LTM for both trains and taps, and neither glr-1 allele showed LTM for either taps or trains (Fig. 2a). An ANOVA comparing the trained and control groups was significant (F(5,108) = 3.17; p = 0.01). PLSD post hoc comparisons showed that wild-type worms showed LTM in the form of significantly lower responses than control when trained with both taps (p = 0.01) and trains (p = 0.01); none of the glr-1 trained groups differed significantly from control (p > 0.05 in all cases). In addition, the responses of glr-1(n2461) trained worms were significantly larger than the responses of trained wild-type worms with both taps (p = 0.009) and trains (p = 0.03), and the responses of glr-1(ky176) trained worms were significantly larger than the responses of trained wild-type worms with trains (p = 0.04).
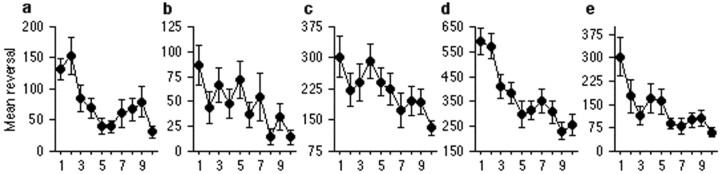
Figure 1.
Mutations in glr-1 do not impair short-term habituation at a 60 sec ISI. Mean response magnitude in pixels for wild-type (a) and n2461 (b) worms given 20 taps at a 60 sec ISI (ky176 does not respond to taps). Data are presented averaged into 10 bins of two responses each. Both groups show a similar gradual decrease in response magnitude with repeated stimulation. Mean response magnitude in pixels for wild-type (c), n2461 (d), and ky176 (e) worms given 20 trains of taps (1 train is equivalent to 6 taps within 600 msec) at a 60 sec ISI. Data are presented averaged into 10 bins of two responses each, as above. All groups show a similar gradual decrease in response magnitude with repeated stimulation. Paired comparisons between bin 1 (average response to the first two stimuli) and bin 10 (average response across the last two stimuli) were significant in all cases with adjustment for multiple comparisons (N2 taps, t(19) = 4.73, p < 0.01; n2461 taps, t(19) = 3.21, p < 0.05; N2 trains, t(17) = 3.82, p < 0.05; n2461 trains, t(18) = 5.11, p < 0.01; ky176 trains, t(15) = 4.33, p < 0.01).
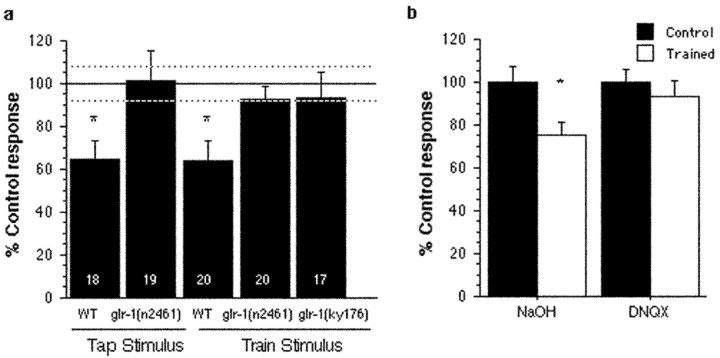
Figure 2.
The role of glr-1 glutamate receptors in memory. a, Mutations in glr-1 (results from 2 alleles, n2461 and ky176) eliminate long-term memory for habituation. Mean percentage of control response magnitude for LTM for habituation training of the tap withdrawal response in wild-type (WT) and glr-1(n2461) and glr-1(ky176). Average reversal magnitude across the test trial for trained groups (black bars) expressed as percentage of control response (top black line across graph; dotted lines represent average ± SEM for control groups). White numbers in bars represent the number of worms in each trained group. Both wild-type (WT) groups showed significant LTM; none of the glr-1 groups showed significant LTM to either taps or trains. b, Exposure to the non-NMDA glutamate receptor antagonist DNQX during training blocks LTM for habituation of the tap withdrawal response in wild-type worms, and exposure to the vehicle control NaOH during training does not affect formation of LTM.
To confirm the importance of glr-1 receptors in LTM, N2 worms were treated with the competitive non-NMDA glutamate receptor antagonist DNQX (Sigma, St. Louis, MO). DNQX blocks neurotransmission at the Aplysia sensory neuron to motor neurons synapse (Dale and Kandel, 1993; Chitwood et al., 2001). DNQX does not block short-term homosynaptic depression (the cellular analog of habituation) (Armitage and Siegelbaum, 1998) but does block lasting memory for habituation in Aplysia (Ezzeddine and Glanzman, 2002). In C. elegans, neither DNQX nor the vehicle control NaOH blocked significant short-term habituation to 20 taps at a 60 sec ISI (t test for mean of responses 1 and 2 vs mean of responses 19 and 20: NaOH, t(11) = 3.2, p = 0.008; DNQX, t(11) = 3.3, p = 0.007). N2 worms were given distributed training on agar plates treated with 35 mm NaOH (vehicle; n = 22) (Fig. 2b) or 10 mm DNQX (n = 27) dissolved in NaOH. Matched control groups (n = 21 for NaOH; n = 24 for DNQX) underwent the same drug treatments but received only one tap during the training phase. An ANOVA comparing trained DNQX and trained NaOH worms against untrained control worms was significant (F(2,69) = 4.1; p = 0.02). PLSD post hoc tests showed significant differences between NaOH trained and control worms (p = 0.007) and between NaOH trained and DNQX trained worms (p = 0.04); there was no significant difference between DNQX trained worms and control worms (p = 0.45). Thus, exposure to vehicle during training had no effect on expression of LTM, and exposure to vehicle plus DNQX during training blocked the development of LTM for habituation.
Training alters GLR-1::GFP expression
Together, the experiments with the glr-1 worms and the wild-type worms exposed to DNQX show that the glr-1 receptor is critical for LTM of habituation in the tap withdrawal circuit. This suggested that habituation training might alter glr-1 expression. To investigate this possibility, we tested worms carrying chimeric receptors made up of GLR-1 tagged with green fluorescent protein (GLR-1::GFP) to visualize changes in glr-1 expression (Rongo and Kaplan, 1999). In GLR-1::GFP worms, fluorescence is localized to clusters along the ventral cord neurites and in the nerve ring (corresponding to anatomical synaptic markers reported in EM studies) (White et al., 1986). Confocal imaging was used to visualize the GLR-1::GFP. We compared the amount of GLR-1::GFP expression in the ventral cord in trained worms and control worms. Worms that received distributed training 24 hr earlier showed no difference in the number of GLR-1::GFP clusters in the ventral cord; however, the overall length of GFP clusters per worm in the trained worms was significantly shorter than in the control worms (Fig. 3a). These data led us to hypothesize that long-term habituation training did not produce a change in the number of synapses but did reduce the number of glr-1 receptors expressed per synapse on the interneurons.
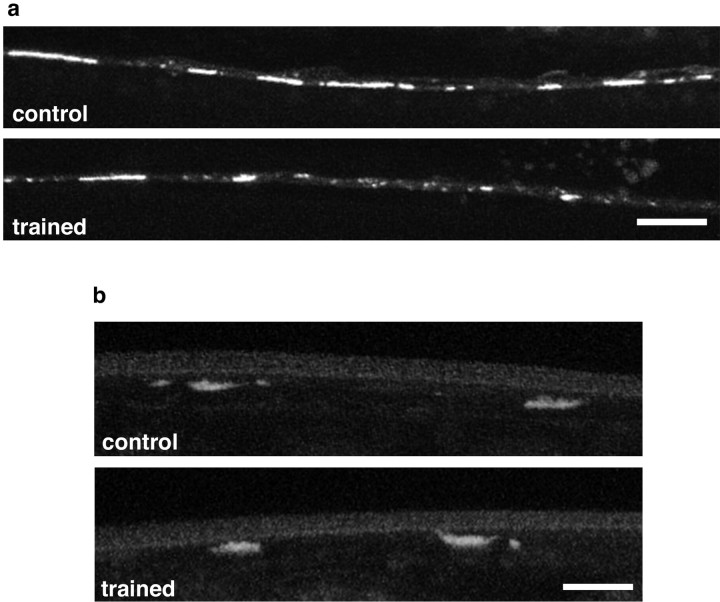
Figure 3.
Confocal images of GFP expression in trained and control worms. a, Confocal images of postsynaptic expression of glr-1 receptors on processes of the interneurons in the posterior ventral nerve cord visualized with GLR-1::GFPshowing a single-tap control worm (control) and a worm that received distributed habituation training 24 hr earlier (trained; same procedure as Fig. 2). The training produced behavioral LTM in another group of these transgenic worms (t(44) = 2.5; p = 0.02; data not shown). There were significantly smaller GFP clusters in the trained worms (x = 280.95 ± 32.37; n = 21) than in the single-stimulus control worms (x = 463.70 ± 43.74; n = 16; t(35) = 3.44; p = 0.002), although the number of clusters between groups did not differ (trained, 62.2 ± 7.14; control, 69.3 ± 10.3; t(35) = 0.59; p = 0.56). Scale bar, 20 μm. b, Confocal images of the vesicles in tap sensory neuron terminals visualized with a synaptobrevin GFP marker showing a single-tap control worm (control) and a worm that received distributed habituation training 24 hr earlier (trained). The training produced behavioral LTM in another group of these transgenic worms (t(44) = 2.45; p = 0.02; data not shown). There was no difference in measured GFP expression between the trained worms (x = 41.00 ± 3.47; n = 12) and the single-stimulus control worms (x = 42.5 ± 5.4; n = 12; t(22) = 0.41; p = 0.69). Scale bar, 20 μm.
To look for the presynaptic changes after training, we measured GFP expression of the gene for C. elegans synaptobrevin (snb-1), a protein associated with synaptic vesicles, in the tap sensory neurons. SNB-1::GFP was expressed under the control of the mec-7 promoter that restricts GFP expression to the six mechanosensory neurons of the tap circuit (Nonet, 1999). In these worms, we focused on an accumulation of fluorescence in areas that corresponded with a specific set of tail-touch cell synaptic connections seen in EM studies (White et al., 1986). The fluorescence we measured appeared as one or two patches in the ventral cord just posterior to the vulva (Nonet, 1999). We measured the total area of the patches in the ventral cords of worms that received distributed training and control worms and found that there was no difference in the amount of SNB-1::GFP expression in the two groups (Fig. 3b). Thus, LTM for habituation did not affect the expression of synaptobrevin in these sensory neuron terminals.
Because the behavioral expression of LTM for habituation can be blocked by exposure to heat shock in the 1 hr rest intervals between training blocks (Beck and Rankin, 1977; Rose et al., 2002), we tested whether the heat shock would alter the effect of training on the expression of GLR-1::GFP. We found that, 24 hr after training, the mean length of GLR-1::GFP clusters in worms that received heat shock (32°C for 40 min after each training block) during distributed habituation training on day 1 did not differ from worms that received only a single tap on day 1 (trained with heat shock, n = 18, x = 496.95 ± 50.2; control with heat shock, n = 16, x = 468.75 ± 54.26; t(31) = 0.380; p = 0.71). In contrast, worms that received distributed training with sham heat shock (21°C for 40 min after each training block) showed decreased mean length of GLR-1::GFP clusters 24 hr after training when compared with their single-tap controls (trained with sham shock, n = 18, x = 374.8 ± 26.38; control with sham shock, n = 18, x = 485.26 ± 33.47; t(34) = 2.6; p = 0.01). Thus, heat shock blocked the alteration in GLR-1::GFP expression usually seen after distributed habituation training, without affecting any other aspect of GLR-1::GFP expression (compare GLR-1::GFP levels in control heat shock and sham shock groups).
Discussion
In behavioral studies, we showed that mutations in the excitatory glutamate receptor gene glr-1 did not affect short-term memory for habituation in C. elegans but blocked LTM. A glutamate receptor antagonist also blocked LTM for habituation. Therefore, the receptor encoded by the glr-1 gene is hypothesized to play a critical role in LTM for habituation in the tap withdrawal response in C. elegans. Interestingly, in the rat, bilateral infusion of an AMPA-receptor antagonist immediately after training impaired LTM for habituation to a novel environment (Vianna et al., 2000).
When we used confocal imaging to examine expression of GLR-1::GFP 24 hr after LTM training, we found no decrease in the number of GFP clusters observed but a significant decrease in the overall amount of GFP expressed. This suggested that, although there was no change in the number of synapses, there was a change in the density of receptors at the synapses. The observation that there was no change in the amount of synaptobrevin in the terminals of the sensory neurons suggests that training and the decrease of postsynaptic glutamate receptors did not alter the number of vesicles in the presynaptic terminal. This was an unexpected result because, in Aplysia, long-term memory for habituation is correlated with a presynaptic decrease in the number of synapses, size of synapses, and number of vesicles in active zones; no studies have looked postsynaptically (Bailey and Chen, 1983). One possible explanation for the differences is that, in our experiments, we used only a short training regimen that produces behavioral LTM; the Aplysia received extensive training, much more than would be needed to produce behavioral changes. We recently collected pilot data that suggests that, if worms are overtrained, there are decreases in the number of vesicles in the sensory neuron terminals.
The observation that heat shock blocks the behavioral expression of LTM after distributed but not massed training has been used to support the hypothesis that the LTM observed at 24 hr after training is protein synthesis dependent. Heat shock is thought to stop ongoing protein synthesis and block new protein synthesis from occurring for a critical period of time after each training block (Beck and Rankin, 1975; Rose et al., 2002). The finding that heat shock also blocks the change in GLR-1::GFP expression suggests that this too is protein synthesis dependent.
This is the first study to simultaneously examine both presynaptic and postsynaptic anatomical changes in the nervous system of living animals induced using the same stimulus protocol that produces behavioral expression of long-term memory. The data from these experiments suggest the hypothesis that long-term memory for habituation in C. elegans is mediated not by a change in the number of synapses but by a downregulation of non-NMDA-type glutamate receptors on the postsynaptic interneurons that weakens the strength of the sensory to interneuron synapses. This is consistent with data on AMPA receptor trafficking that is being reported in mammalian LTD preparations in which electrical stimulation or drug treatments that produce a weakening of synaptic strength also produce a downregulation of surface-expressed AMPA receptors (for review, see Bear and Linden, 2001; Lüscher and Frerking, 2001; Malinow and Malenka, 2002). Heynen et al. (2000) have suggested that memory is encoded by experience-dependent assignment of glutamate receptors to synapses. C. elegans offers an exciting in vivo system to study glutamate receptor trafficking as it relates to behavioral changes and experience.
Footnotes
This work was funded by a Natural Sciences and Engineering Research Council of Canada (NSERC) operating grant (C.H.R.), an NSERC postgraduate scholarship B (J.K.R.), and Michael Smith Foundation for Health Research and Canadian Institutes of Health Research fellowships (J.K.R.). We thank Amelia Wan and Stephan Steidl for scoring data and Lindsay Arscott and Mark McConkey for data on response to tap and trains.
Correspondence should be addressed to Catharine H. Rankin, Department of Psychology and Brain Research Centre, University of British Columbia, 2136 West Mall, Kennedy Building, Vancouver, British Columbia, Canada, V6T 1Z4. E-mail: crankin@psych.ubc.ca.
Copyright © 2003 Society for Neuroscience 0270-6474/03/239595-05$15.00/0
References
- Armitage BA, Siegelbaum SA ( 1998) Presynaptic induction and expression of homosynaptic depression at Aplysia sensorimotor neuron synapses. J Neurosci 18: 8770-8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery L ( 1993) The genetics of feeding in Caenorhabditis elegans. Genetics 133: 897-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CH, Chen M ( 1983) Morphological basis of long-term habituation and sensitization in Aplysia. Science 220: 91-93. [DOI] [PubMed] [Google Scholar]
- Bear MF ( 2001) The mechanisms and meaning of long-term synaptic depression in the mammalian brain. In: Synapses (Cowan WM, Sudhof TC, Stevens CF, eds), pp 455-517. Baltimore: Johns Hopkins UP.
- Beck CDO, Rankin CH ( 1995) Heat-shock disrupts long-term memory consolidation in Caenorhabditis elegans Learn Mem 2: 161-177. [DOI] [PubMed] [Google Scholar]
- Beck CDO, Rankin CH ( 1997) Long-term habituation is produced by distributed training at long ISIs and not by massed training or short ISIs in Caenorhabditis elegans Anim Learn Behav 25: 446-457. [Google Scholar]
- Bellocchio EE, Hu H, Pohorille A, Chan J, Pickel VM, Edwards RH ( 1998) The localization of the brain-specific inorganic phosphate transporter suggests a specific presynaptic role in glutamatergic transmission. J Neurosci 18: 8648-8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S ( 1974) The genetics of Caenorhabditis elegans. Genetics 77: 71-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M, Sulston JE, White JG, Southgate E, Thomson JN, Brenner S ( 1985) The neural circuit for touch sensitivity in Caenorhabditis elegans. J Neurosci 5: 956-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood RA, Li Q, Glanzman DL ( 2001) Serotonin facilitates AMPA-type responses in isolated siphon motor neurons of Aplysia in culture. J Physiol (Lond) 534: 501-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N, Kandel E ( 1993) L-glutamate may be the fast excitatory transmitter of Aplysia sensory neurons. Proc Natl Acad Sci USA 90: 7163-7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzeddine YM, Glanzman DL ( 2002) Role of protein synthesis and protein kinase activity in long-term habituation of the gill-withdrawal reflex in Aplysia Soc Neurosci Abstr 28: 376.6. [Google Scholar]
- Hart AC, Sims S, Kaplan JM ( 1995) Synaptic code for sensory modalities revealed by C. elegans GLR-1 glutamate receptor. Nature 378: 82-85. [DOI] [PubMed] [Google Scholar]
- Heynen AJ, Quinlan EM, Bae DC, Bear MF ( 2000) Bidirectional, activity-dependent regulation of glutamate receptors in the adult hippocampus in vivo. Neuron 28: 527-536. [DOI] [PubMed] [Google Scholar]
- Lee RY, Sawin ER, Chalfie M, Horvitz HR, Avery L ( 1999) EAT-4, a homolog of a mammalian sodium-dependent inorganic phosphate co-transporter, is necessary for glutamatergic neurotransmission in Caenorhabditis elegans. J Neurosci 19: 159-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C, Frerking M ( 2001) Restless AMPA receptors: implications for synaptic transmission and plasticity. Trends Neurosci 24: 665-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R, Malenka RC ( 2002) AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci 25: 103-126. [DOI] [PubMed] [Google Scholar]
- Maricq AV, Peckol E, Driscoll M, Bargmann CI ( 1995) Mechanosensory signalling in C. elegans mediated by the GLR-1 glutamate receptor. Nature 378: 78-81. [DOI] [PubMed] [Google Scholar]
- Nonet ML ( 1999) Visualization of synaptic specializations in live C. elegans with synaptic vesicle protein-GFP fusions. J Neurosci Methods 89: 33-40. [DOI] [PubMed] [Google Scholar]
- Rongo C, Kaplan JM ( 1999) CaMKII regulates the density of central glutamatergic synapses in vivo. Nature 402: 195-199. [DOI] [PubMed] [Google Scholar]
- Rose JK, Chen S, Kaun KR, Rankin CH ( 2001) Glutamate and AMPA-receptor function are necessary for long-term memory in C. elegans Soc Neurosci Abstr 27: 87.9. [Google Scholar]
- Rose JK, Kaun KR, Rankin CH ( 2002) A new group-training procedure for habituation demonstrates that presynaptic glutamate release contributes to long-term memory in C. elegans Learn Mem 9: 130-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianna MR, Alonso M, Viola H, Quevedo J, de Paris F, Furman M, de Stein ML, Medina JH, Izquierdo I ( 2000) Role of hippocampal signaling pathways in long-term memory formation of a nonassociative learning task in the rat. Learn Mem 7: 333-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JE, Southgate E, Thomson JN, Brenner S ( 1986) The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci 314: 1-340. [DOI] [PubMed] [Google Scholar]
- Wicks SR, Rankin CH ( 1995) Integration of mechanosensory stimuli in Caenorhabditis elegans. J Neurosci 15: 2434-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]