Abstract
Presence of the glutamate receptor 2 (GluR2) subunit prevents calcium influx through AMPA-receptor complexes; deletion of this subunit results in enhanced hippocampal long-term potentiation. We investigated whether mice lacking the GluR2 subunit [gria2 knock-out (KO) mice] displayed impairments in learning stimulus-reward associations, and the subsequent ability of reward-paired cues to control motivated behavior. Both gria2 KO and wild-type (WT) mice learned to associate a light/tone stimulus with food delivery, as evidenced by approach toward the food magazine after the presentation of the cues (pavlovian conditioning). Subsequently, the cues also served to reinforce an operant response in both KO and WT mice (conditioned reinforcement), although response rates were greater in gria2 KOs. Responding for conditioned reinforcement was enhanced after 0.5 mg/kg amphetamine administration in WT mice, but not in KO mice. The ability of the cues to elicit approach behavior (conditioned approach) and to enhance responding for the reward (pavlovian-to-instrumental transfer; PIT) were also impaired in gria2 KO mice. This pattern of behavior resembles that seen after lesions of the central nucleus of the amygdala (CeA), an area rich in GluR2-containing AMPA receptors. Immunostaining revealed reduced GluR1 expression within both the basolateral amygdala and the CeA, suggesting that the behavioral deficits observed were unlikely to be caused by compensatory changes in GluR1. These results suggest that GluR2-containing AMPA receptors, possibly within the CeA, are critical for the formation of stimulus-reward associations necessary for PIT and conditioned approach, but are not involved in the plastic processes underlying the attribution of motivational value to the conditioned stimulus (CS).
Keywords: learning, pavlovian association, conditioned reinforcement, pavlovian to instrumental transfer, pavlovian approach, discriminated approach, AMPA receptor, GluR-A, GluR-B, gria1, amygdala, amphetamine
Introduction
Conditioned associations between environmental stimuli and rewarding events are important in controlling and maintaining appropriate behavioral responses; they may also contribute to aberrant behaviors, including drug addiction. Cues associated with drug taking initiate and control behavior in both abstaining addicts and animal models of drug abuse, increasing drug craving (Childress et al., 1999) and drug seeking (Shaham et al., 2003). Consequently, treatment strategies for relapse prevention include the removal of an addict from situations in which exposure to drug-paired cues is likely (O'Brien et al., 1998). Therefore, understanding the mechanisms that underlie the formation and expression of such associations has both theoretical and practical importance.
Models of associative learning implicate glutamatergic neurotransmission through ionotropic NMDA and AMPA receptors as the molecular basis of learning, long-term potentiation (LTP) (for review, see Nicoll, 2003). In particular, the expression of LTP is associated with enhanced glutamatergic transmission through AMPA receptors, of which GluR1 (encoded by the gria1 gene) and GluR2 (encoded by the gria2 gene) subunits are major components. Because drug addiction has been viewed as a form of aberrant learning (Everitt et al., 2001), the glutamate system is a prime candidate for studies of processes underlying addiction. Previously, we have reported that mice lacking the GluR1 subunit of the AMPA receptor [gria1 knock-out (KO) mice] display specific deficits in stimulus-reward learning (Mead and Stephens, 2003). Although they are capable of forming an association between a discrete cue and the presentation of a food reward, as evidenced by their ability to use the cue as a discriminative stimulus (pavlovian conditioning), show approach to the cue (conditioned approach; Tomie et al., 1999), and are responsive to the ability of the cue to enhance ongoing operant behavior [pavlovian-to-instrumental transfer (PIT); Dickinson, 1994], when presented with the opportunity to obtain the cue through an instrumental response (conditioned reinforcement; Mackintosh, 1974), gria1 KOs failed to respond (Mead and Stephens, 2003). This deficit, an inability to attribute motivational value to the cue, was also reflected in a deficit in responding under a second-order schedule of reinforcement (Mead and Stephens, 2003), a task also dependent on the conditioned reinforcing properties of the cue (Whitelaw et al., 1996). This pattern of deficits mirrors that seen after the occurrence of lesions of the basolateral region of the amygdala (BLA) (Everitt et al., 2000), an area also rich in GluR1 expression (McDonald, 1996), leading us to suggest that the deficits observed in gria1 KO mice may be caused by impaired neurotransmission in or via the BLA (Mead and Stephens, 2003).
However, deletion of GluR1 subunits in the gria1 KO mouse affects the distribution of other AMPA-receptor subunits in both the hippocampus (Zamanillo et al., 1999) and the BLA (Mead and Stephens, 2003) [although not the neighboring central nucleus of the amygdala (CeA)]. Thus, it was unclear whether the deficits observed in the gria1 KO may be caused by associated changes in GluR2. Therefore, the present set of experiments investigated the effects of GluR2 deletion on stimulus-reward learning, using the gria2 KO mouse (Jia et al., 1996). This mutant shows facilitated AMPA-dependent LTP in hippocampal slices, and enhanced calcium permeability of AMPA receptors, consistent with the known role of GluR2 in preventing calcium influx through AMPA-receptor-gated channels (Jia et al., 1996). Here, we investigate the effects of GluR2 deletion on pavlovian conditioning, conditioned approach, PIT, and conditioned reinforcement, in addition to examining possible changes in GluR1 expression within the amygdala subregions.
Materials and Methods
Animals. Wild-type (WT) and gria2 KO littermates were bred at the University of Sussex from heterozygous parents obtained from The Jackson Laboratory (Bar Harbor, ME; strain name, STOCK Gria2tm1Rod; stock number, 002913). The genotypes of the offspring were identified using PCR analysis. Mice were housed two or three to a cage and were allowed at least 2 weeks of habituation to the home cages before the beginning of experimental sessions. Holding rooms were maintained at a constant temperature (21 ± 2°C) and humidity (50 ± 10%), and lights were on for 12 hr starting at 7:00 A.M.). Except when specified, mice were allowed access to standard lab chow and water ad libitum. Between five and eight WT and gria2 KO mice were used in each phase of the experiment. All testing took place during the light phase, between 8:00 A.M. and 6:00 P.M. The experiment used a within-subject design, whereby all mice were used for each stage of the study in the order described. All experiments were approved by the institutional ethics committee and were performed under United Kingdom legislation on animal experimentation (Animal Scientific Procedures Act, 1986).
Pavlovian conditioning. Mice were food-restricted to maintain their body weight at ∼85% of free feeding body weight. During 2 hr sessions, mice were placed into mouse operant chambers (Med Associates, E. Fairfield, VT) with the levers retracted, and 20 mg food pellets (Noyes Precision pellets, Formula P; Research Diets, Inc., New Brunswick, NJ) were delivered at random intervals [mean, 2 min; variable interval (VI), 120 sec schedule], preceded and immediately followed by the cues consisting of a 10 sec illumination of two flashing lights (1 Hz) and the onset of a tone (2.9 kHz; 5 dB above background). The two stimulus lights were located above and to either side of the food magazine. The tone generator was located centrally on the opposite wall of the chamber. The cues were presented for 10 sec and the food pellet was delivered 5 sec after the cue onset. Initial training consisted of 10 sessions, although an additional three training sessions were given after the tests for conditioned reinforcement (sessions 11-13) and one additional training session was performed after the tests for conditioned approach and cue breakdown (session 14). Infrared detectors across the entrance to the food magazine allowed the latency between cue onset and reward retrieval to be measured, along with the total number of food magazine entries. These measures assessed the ability of mice to use the cues as a signal for reward availability.
Conditioned approach. To assess the ability of the cues to elicit conditioned approach, infrared detectors were placed across the entrances to the two cue lights. The cue lights were also moved to the opposite wall of the chamber at a lower height. This was done to enable nose poking into the apertures to occur more easily, and to place the apertures as far as possible from the food magazine. The tone generator was located behind one of the cue lights [conditioned stimulus (CS)]. The second cue light [control (CTRL)] was never illuminated during the test, and was used to assess baseline levels of nose-poking behavior. During a 30 min session, the cues were presented for 60 sec every 2 min, and approach toward the cues (into the CS aperture), and nonspecific approach behavior (into the CTRL aperture) were recorded.
CS components. To assess the ability of the mice to use either the visual or auditory components of the CS alone, an additional pavlovian conditioning session was performed. This session was identical to those described above, except that three CS conditions were included. Either the compound CS consisting of the tone and stimulus lights together (as used in earlier pavlovian conditioning sessions), or single-modality CSs consisting of either the tone or the CS lights alone, were presented 5 sec before food delivery. Reward retrieval latency and CS% (the percentage of total food-magazine entries occurring during the CS presentation) were recorded for the three stimulus conditions.
Conditioned reinforcement. To assess the ability of the cues to act as a conditioned reward, two levers were extended into the operant chambers. Responding on one lever [conditioned reinforcement (CR) lever] resulted in a brief 1 sec presentation of the CS, whereas responding on the alternative lever [no conditioned reinforcement (NCR) lever] had no programmed consequences. During two sessions, responses on each lever were monitored during a 3 hr session after the injection of either saline (10 ml/kg, i.p) or amphetamine (0.5 mg/kg, i.p.). All mice were tested under each condition, and the order of treatment was counterbalanced.
Instrumental responding. As a control for responding for CR, the ability of mice to perform an operant response for the primary reinforcer was also examined. Mice were initially trained to respond for 20 mg food pellets (Noyes Precision pellets, Formula P; Research Diets) on a fixed-ratio (FR1) schedule during a 120 min session. The schedule was then progressively increased to an FR10 schedule. After acquisition, the schedule was changed to a VI 30 sec schedule, and increased daily to a VI 120 sec schedule. After 3 d on a VI 120 sec schedule, mice were tested on a series of different VI schedules (VI 30, 60, 120, 240, 360, and 480 sec) in a random order.
PIT. To assess PIT, mice from the instrumental responding stage were retrained on a VI 240 sec schedule for one session of 120 min. Both KO and WT mice responded at a stable rate on this schedule, although the overall rate of lever pressing was higher in KO mice. This VI schedule was chosen for PIT testing because it was at this schedule that WT mice responded at the lowest rates. The following day, mice were again allowed to respond on a VI 240 sec schedule for food pellets for a total of 60 min. In addition, an extended 60 sec CS was presented on a fixed-interval 5 min schedule (cue location and conditions exactly as for pavlovian conditioning stage). The rate of responding was then compared during the presence of the cues (CS period) and the absence of the cues (VI period).
Immunohistochemistry. For immunohistochemical analysis of GluR1, adult mice were anesthetized with tribromoethanol (Avertin, 20 mg/kg) and transcardially perfused with 4% paraformaldehyde. Brains were removed and stored in 4% paraformaldehyde for 24 hr, before placement in 0.1 m phosphate buffer containing 30% sucrose for 48 hr. Brains were then frozen in isopentane at -45°C, and stored at -80°C until sectioning. Coronal sections (30 μm) were taken using a cryostat, and sections were washed in PBS. Endogenous peroxidase was quenched by immersion in 0.3% hydrogen peroxide, and sections were washed in PBS before blocking in 1.5% normal goat serum (Vector Laboratories, Peterborough, UK). After additional washing in PBS, sections were incubated in 0.25 μg/ml anti-GluR1 (06-306; Upstate UK, Botolph Claydon, UK) overnight. Sections were then washed in PBS, and incubated in a 1:600 dilution of biotinylated secondary antibody (BA-1000; Vector Laboratories) for 60 min, before being washed again. Sections were subsequently incubated in ABC complex (Vectastain ABC elite kit: PK6100; Vector Laboratories), and washed in PBS; staining was visualized using the nickel-DAB glucose (D-5637 and G-2133; Sigma Aldrich, Gillingham, UK) method. Sections were slide-mounted, dehydrated, and coverslipped before analysis. For the analysis of sections, images were captured using a Sony (Tokyo, Japan) DSC-S75 digital camera mounted on a Zeiss (Oberkochen, Germany) Axioskop 2 microscope. Negative controls for optical density analysis standardization were run using exactly the same protocol except that the primary antibody was omitted.
Statistical analysis
Pavlovian conditioning. Two measures were analyzed to assess pavlovian conditioning behavior. First, the latency between cue onset and reward retrieval (latency) was compared between genotypes; second, the percentage of total food-magazine entries occurring during the CS presentation (CS%) was compared. Two-way ANOVA was performed, with training sessions (within subjects) and genotype (between subjects) as factors. Post hoc analysis was performed using independent-sample t tests.
Conditioned approach. For analysis of conditioned approach, nose-poke rates into the CS aperture were assessed during the CS (CS+) and compared with rates when the CS was not presented (CS-). Similarly, nose-poke rates into the CTRL aperture were compared. Data for this measure were square root transformed to gain homogeneity of variance and to allow parametric analysis. Three-way ANOVA was performed, with CS state (either CS+ or CS-), aperture (within subjects) and genotype (between subjects) as factors.
CS components. For the analysis of CS component data, latency and CS% were measured. Two-way ANOVA was performed, with CS condition (within subjects) and genotype (between subjects) as factors. When appropriate, post hoc analysis was performed using the Student-Newman-Keuls test.
Conditioned reinforcement. For the analysis of CR, the total number of lever presses was used as the dependent variable. Three-way ANOVA was performed, with lever and drug treatment (within subjects) and genotype (between subjects) as factors. Post hoc analysis was performed using repeated measures or independent-sample t tests when appropriate. In addition, ANOVA was also performed with testing order as a factor. This analysis was performed to rule out the possibility that the order of drug treatment influenced responding for conditioned reinforcement, because previous work has shown that even a single amphetamine administration can enhance responding on subsequent tests for CR (Mead et al., 2003).
Instrumental responding. For the analysis of instrumental responding, the number of food pellets received and total lever presses during the session were taken as the dependent variables. Two-way ANOVA was performed, with VI schedule (within subjects) and genotype (between subjects) as factors. Post hoc analysis was performed using independent-sample t tests.
PIT. For the analysis of PIT, response rates during the CS were divided by response rates during the VI period to produce a ratio (for which a ratio of 1 indicates identical response rates during the CS and in the absence of the CS). Two-way ANOVA was performed, with lever (within subjects) and genotype (between subjects) as factors. Post hoc comparisons were made using repeated-measures t tests. Magazine approach during the PIT session was also analyzed by comparing nose-poke rates into the magazine during the CS with rates during the intervening VI period. Two-way ANOVA was performed, with CS state (within subjects) and genotype (between subjects) as factors. Posthoc comparisons were made using repeated-measures t tests.
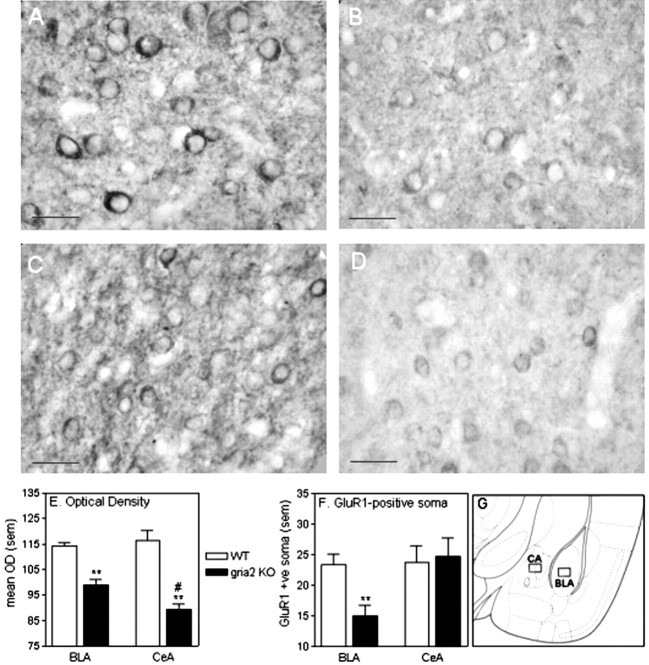
Immunohistochemistry. The analysis of GluR1 immunoreactivity was performed by counting the number of GluR1-positive soma and analyzing optical density within a 130 × 170 μm region of the BLA and CeA. The regions selected are indicated in Figure 5G, and represent regions from within the basolateral amygdaloid nucleus and the central amygdaloid nucleus (Franklin and Paxinos, 1997). For the quantitative analysis of GluR1-positive soma, two independent observers scored each section, and were unaware of the condition. For the analysis of optical density of GluR1 staining, mean optical density values were obtained using Scion-Image (Scion Corp., Frederick, MD), rating each pixel with a value of 0 to 255. Mean optical densities for each section were then corrected for nonspecific binding by subtracting optical density values from negative controls. Two-way ANOVA was then performed with region (within subjects) and genotype (between subjects) as factors. Post hoc comparisons were performed using independent-sample t tests when appropriate.
Figure 5.
Immunohistochemical analysis of GluR1 in WT and gria2 KO mice. A, B, GluR1 immunoreactivity within the BLA of WT (left) and gria2 KO (right) mice. C, D, GluR1 immunoreactivity within the CeA of WT (left) and gria2 KO (right) mice. Scale bars, 20 μm. E, Optical density analysis of GluR1 immunoreactivity within a 130 × 70 μm region of the BLA and CeA. **p < 0.01 compared with WT; #p < 0.05 compared with BLA. F, Quantitative analysis of GluR1-positive soma within a 130 × 70 μm region of the BLA and CeA. **p < 0.01 compared with WT. G, Amygdaloid regions in which quantitative analysis of GluR1-positive soma was conducted. This image was modified from Franklin and Paxinos (1997); it represents a coronal section at the bregma - 1.22 mm.
Results
Pavlovian conditioning
Figure 1A indicates that when trained to associate a tone/light cue with the delivery of a food reward, by presenting the cues immediately before the randomly timed delivery of a reward, both WT and KO mice learned the association. This is indicated by an increase in the percentage of total nose-pokes into the food magazine during the CS presentation (CS%) (main effect of session, F(13,182) = 25.14, p < 0.01). Differences were observed between genotypes during the later sessions only (session by genotype interaction, F(13,182) = 3.12, p < 0.01), and post hoc analysis revealed that the CS% was significantly lower in KO mice for sessions 11-14 (p < 0.05). However, the analysis of total nose-pokes into the food magazine during the CS and intervening random interval (RI) periods revealed that the differences observed in sessions 11-14 were caused by a small difference in the number of irrelevant nose-pokes during the RI period, rather than a reduction in nose-pokes during the CS period (Fig. 1C). Analysis of reward retrieval latency after the CS onset (Fig. 1B) also indicated acquisition of the CS-unconditioned stimulus (US) relationship across sessions (main effect of session, F(13,182) = 7.37, p < 0.01), although no between-genotype differences were observed for this measure.
Figure 1.
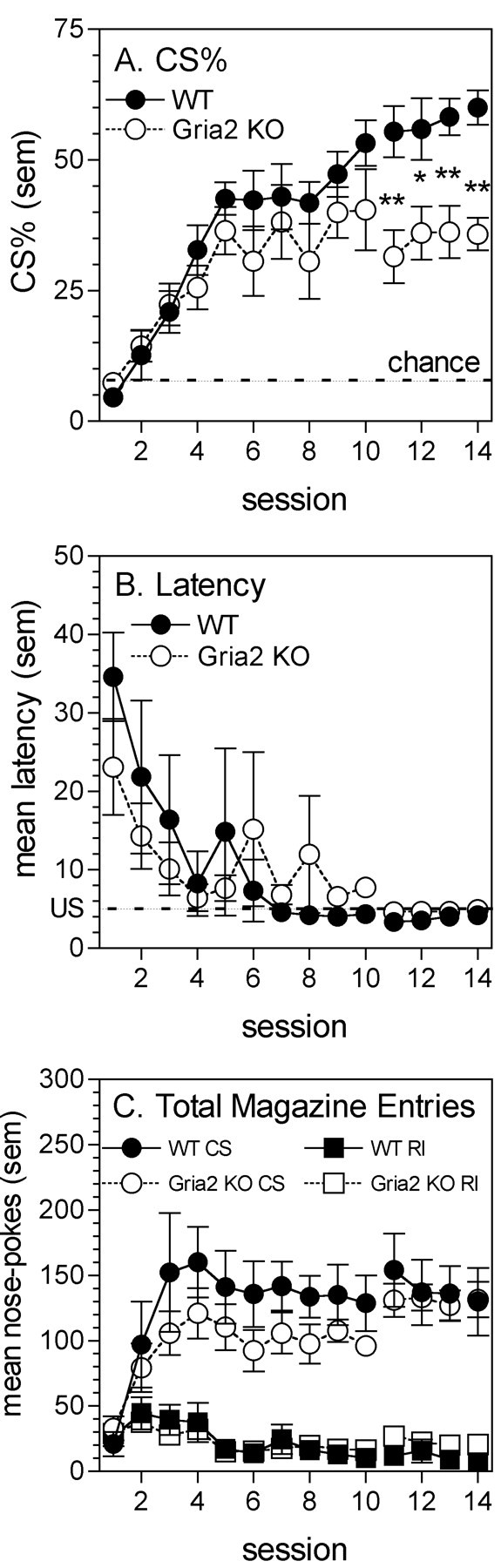
Pavlovian conditioning in WT and gria2 KO mice. A, Percentage of total nose-pokes into the food magazine occurring during the CS presentation. The chance level (i.e., equal rates of nose-poking during the CS and between CS presentations) is indicated by the dashed line. *p < 0.05; **p < 0.01 for effect of genotype during session. B, Reinforcer retrieval latency (seconds) after the cue onset. Reward (20 mg food pellet) presentation occurred at 5 sec, as indicated by the dashed line. C, Rate of nose-pokes (per hour) into the food magazine during the cue presentation (CS) and the intervening RI period.
Conditioned approach
Once the cue light had been relocated in the wall opposite to the food magazine, WT mice displayed conditioned approach toward the CS, specifically during the CS presentation (Fig. 2A). This effect was also specific to the CS location, because approaches into a control aperture were unaffected by the CS state (CS state by aperture interaction; F(1,7) = 9.40, p < 0.05). In contrast, KO mice displayed no selective approach toward the CS aperture, and any approaches made were not related to the CS state (main effect of aperture, F(1,7) = 0.39, main effect of CS state, F(1,7) = 0.51; CS state by aperture interaction, F(1,7) = 1.13; all not significant).
Figure 2.
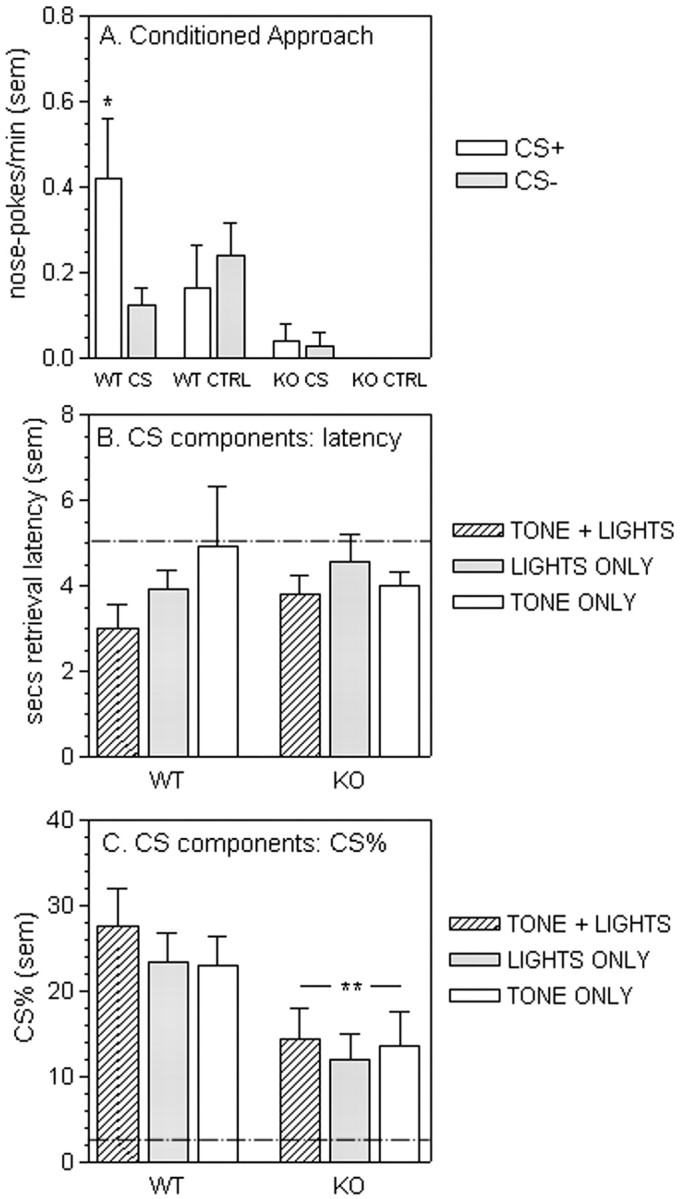
Pavlovian approach and discriminated approach to individual cue elements in WT and gria2 KO mice. A, Conditioned approach toward the cues. Data show mean nose-poke rates (per minute) into an aperture containing the cue light (CS) or toward a control aperture (CTRL) during the cue presentations (CS+) and the intervening periods (CS-). The cues were presented every 2 min for 60 sec. *p < 0.05; significant CS state by aperture interaction for WT mice. B, Effect of individual cue elements on discriminated approach behavior. Reinforcer retrieval latency (seconds) after the compound CS (TONE + LIGHTS), visual only CS (LIGHTS ONLY), or auditory only CS (TONE ONLY) onset. Reward (20 mg food pellet) presentation occurred at 5 sec, as indicated by the dashed line. C, Effect of individual cue elements on discriminated approach behavior. Percentage of total nose-pokes into the food magazine occurring during the compound CS (TONE + LIGHTS), visual only CS (LIGHTS ONLY), or auditory only CS (TONE ONLY) presentation. The chance level (i.e., equal rates of nose-poking during the CS and between CS presentations) is indicated by the dashed line. **p < 0.01 for significant main effect of genotype.
CS components
To determine whether the failure of KO mice to approach the CS was caused by a sensory impairment, we examined the ability of the individual cue components (light alone or tone alone) to elicit discriminated approach. The analysis of retrieval latency after the CS onset (Fig. 2B) revealed that the individual CS elements were equally effective as discriminative stimuli as the compound CS used previously (no significant effect of CS type, F(2,28) = 1.86, not significant; or CS by genotype interaction, F(2,28) = 1.24, not significant). The analysis of CS% revealed differences between genotypes and CS types, but no interaction (main effect of CS, F(2,28) = 5.67, p < 0.01; main effect of genotype, F(1,14) = 4.95, p < 0.05). The reduced CS% observed in the KO mice for all CS types was consistent with that seen in the final four sessions of the discriminated approach stage. Post hoc analysis also revealed that both WT and KO mice displayed a reduced CS% when CS elements were presented alone (lights alone or tone alone) than when the compound CS was presented (tone plus lights). Although these results suggest that the compound cue is more effective as a discriminative stimulus than the individual cue components alone, they indicate that both WT and KO mice are capable of using either the visual or auditory elements of the CS as discriminative stimuli, and that the deficit observed in the KOs during the conditioned approach task was unlikely to be caused by general sensory deficits in these mice.
Conditioned reinforcement
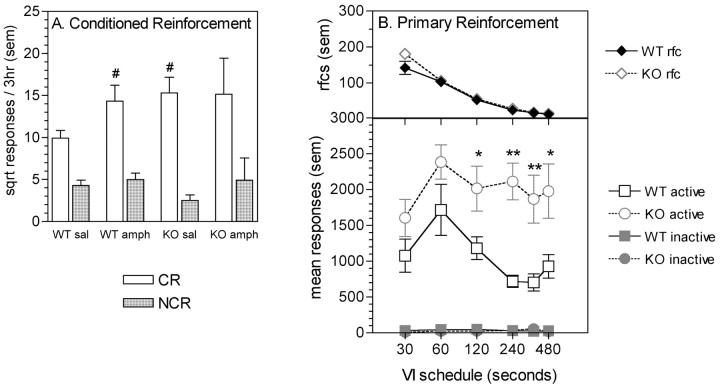
The analysis of lever pressing for the presentation of the CS indicated that the CS attained conditioned reinforcing properties in both WT and KO mice (main effect of lever, F(1,12) = 43.19, p < 0.01) (Fig. 3A). ANOVA also indicated a three-way interaction between genotype, drug treatment, and lever (F(1,12) = 4.86, p < 0.05). Additional investigation of this interaction revealed that WT mice responded at higher levels on the CR lever after amphetamine administration than after saline administration, without concurrent changes in responding on the NCR lever (drug treatment by lever interaction; F(1,7) = 9.98, p < 0.05). However, in KO mice, there was no effect of drug treatment on response levels on either lever (main effect of drug treatment, F(1,5) = 0.22; drug treatment by lever interaction, F(1,5) = 0.69, both not significant). Independent-sample t tests revealed that after saline administration, the response rate on the CR lever was significantly higher in KO mice than in WT mice (t12 = 2.80, p < 0.05) whereas response rates on the NCR lever did not differ (t12 = 1.90, not significant). However, after amphetamine administration, response rates on either the CR or NCR lever did not differ between genotypes (t12 = 0.19, t12 = 0.02, respectively, not significant). The order in which drug treatment was administered had no effect on response rates (main effect of order, F(1,12) = 1.51; order by genotype interaction, F(1,10) = 0.67, both not significant).
Figure 3.
Responding for conditioned and primary reinforcement in WT and gria2 KO mice. A, Mean square-root responses on a lever resulting in the cues presentation (CR) and on a control lever with no consequences (NCR) during a 3 hr session. Mice received either saline (sal) or 0.5 mg/kg amphetamine (amph) before the test session. #p < 0.05 compared with responding on the CR lever in the WT sal condition. B, Responding for primary reinforcement (20 mg food pellet) on VI schedules. Data show mean responses and total reinforcers earned during a 2 hr session. *p < 0.05; **p < 0.01, compared with WT active responses on the indicated schedule.
Instrumental responding
Response rates for primary reinforcement are shown in Figure 3B. Analysis of the number of reinforcers obtained (top) revealed that as the VI schedule increased, the number of reinforcers earned decreased (main effect of schedule, F(5,70) = 219.08, p < 0.01). However, there were no differences between genotypes on this measure. In contrast, analysis of active lever responses (bottom) indicated not only an effect of increasing the VI schedule, but also effects of genotype and a genotype-by-schedule interaction (main effect of genotype, F(1,14) = 8.67, p < 0.05; genotype-by-schedule interaction, F(5,70) = 2.79, p < 0.05). Post hoc analysis revealed that KO mice responded at significantly higher rates on the active lever than WT mice at VI schedules at or above 120 sec. There were no differences in inactive lever responses. Therefore, although KO mice obtained the same number of reinforcers as WT mice, they were emitting a greater number of responses to obtain these reinforcers.
PIT
Analysis of PIT was performed by comparing rates of responding during the CS with rates of responding during the inter-CS intervals (VI periods). These data are expressed as a ratio for clarity (Fig. 4A). ANOVA revealed a significant two-way interaction (lever by genotype interaction, F(1,13) = 9.34, p < 0.01), indicating that in WT mice, the CS increased response rates on the active lever compared with the inactive (control) lever (t7 = 9.35, p < 0.01). However, in KO mice, the rate of responding on the active lever did not differ from the rate of responding on the inactive lever (t6 = 0.49, not significant). Analysis of nose-poking rates into the food-magazine during the PIT test also revealed between-genotype differences (CS state by genotype interaction, F(1,13) = 20.11, p < 0.01) (Fig. 4C). Post hoc tests indicated that WT mice displayed significantly higher rates of magazine approach during the CS than during the VI period (t7 = 4.92, p < 0.01). However, KO mice displayed equal rates of magazine approach during the CS and VI periods.
Figure 4.
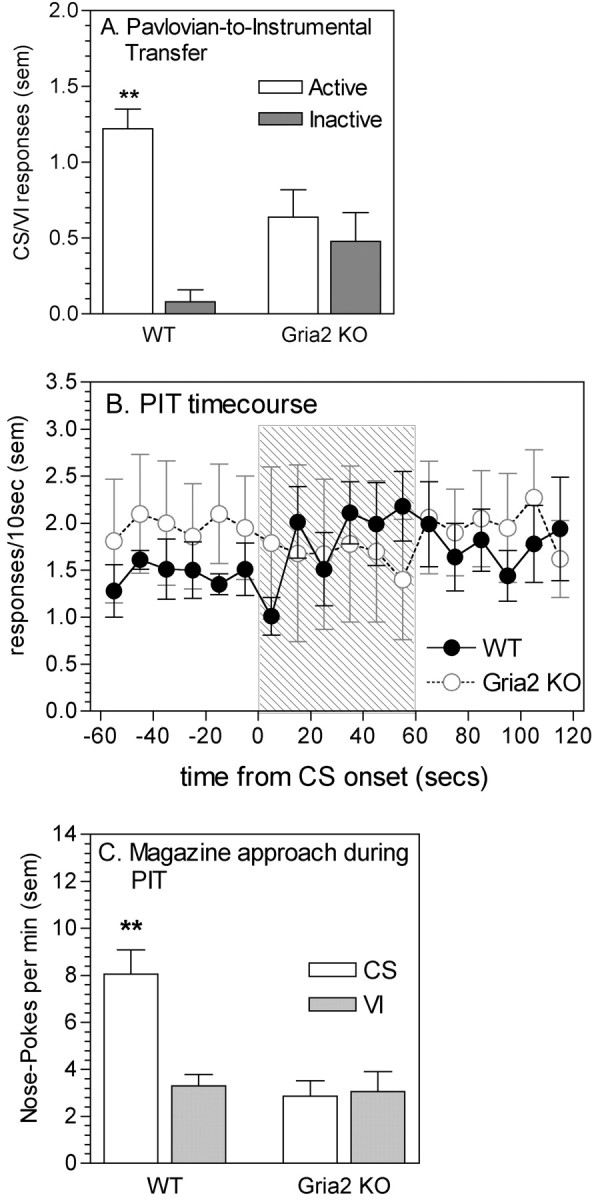
PIT in WT and gria2 KO mice. A, Mean response rates during the CS presentation as a ratio of response rates during intervening VI periods, during a 1 hr session. **p < 0.01 compared with responding on the inactive lever. B, Time course of responding during test for PIT. Data show mean active lever response rates 60 sec before, during, and after the CS presentation. Shaded area indicates the time of the CS presentation. C, Mean approaches toward the food magazine (expressed as nose-pokes into the magazine) during the CS presentation and the intervening variable interval periods. **p < 0.01 compared with approach rates during the VI period.
Immunohistochemistry
Analysis of the mean optical density of GluR1 immunoreactivity revealed that levels of GluR1 were reduced in both the BLA and CeA of gria2 KO mice (main effect of genotype, F(1,8) = 41.29, p < 0.01) (Fig. 5E). However, the magnitude of this decrease was greater in the CeA (genotype by region interaction, F(1,8) = 14.11, p < 0.01). Quantitative analysis of GluR1-positive soma revealed that there was a significant reduction in the number of GluR1-containing neurons in the BLA of gria2 KO mice, but not in the CeA (genotype by region interaction, F(1,8) = 5.36, p < 0.05) (Fig. 5F).
Discussion
In the present experiments, we demonstrate that targeted deletion of the gria2 gene encoding the GluR2 subunit of the AMPA receptor leads to specific deficits in stimulus-reward learning. Namely, deletion of gria2 results in impairments in conditioned approach and PIT, without affecting discriminated approach performance during pavlovian conditioning. In addition, gria2 KOs display enhanced responding for conditioned reinforcement, but insensitivity to the rate-enhancing effects of amphetamine in this task. Finally, GluR1 expression in amygdala subregions is disrupted after the deletion of gria2.
Gria2 KO mice display normal acquisition of a cue-reward association, as shown by their ability to use the cues as a signal for reward availability (pavlovian conditioning). However, it is not clear which aspects of the cue-reward association are necessary for appropriate responding of this type. After extensive training, magazine approach behavior in gria2 KO mice became less accurate than that seen in WT mice, with regard to the number of magazine approaches during the CS as a percentage of total approaches (CS%). Although this may reflect a performance deficit in the KO mice, it appears more likely that it is a reflection of the motor impairments seen in these mice. First, no deficits were observed on retrieval latency after CS onset, indicating that KO mice were equally capable of using the CS as a signal to retrieve the reward. Second, gria2 KO mice display marked motor coordination deficits, resulting in increased passivity and failure to perform the rotarod task (Jia et al., 1996; Gerlai et al., 1998). Analysis of total magazine entries indicated that the difference in CS% was attributable to a small increase in the number of magazine approaches during the inter-CS periods, rather than decreased approaches during the CS. Although WT mice typically move around the operant chambers during sessions, KO mice were observed to spend most of their time close to the food magazine, thereby increasing the probability of incorrect magazine entries.
In addition, gria2 deletion does not impair the ability of the mouse to attribute motivational value to the reward-paired cues, as indicated by intact responding for conditioned reinforcement. In fact, gria2 KOs display enhanced responding for the cues, suggesting that the CS has gained increased motivational value. One explanation for this finding would be that the processes involved in the attribution of motivational value to appetitive cues have been facilitated after the deletion of gria2. This is consistent with data showing that AMPA receptors lacking the GluR2 subunit display enhanced calcium permeability (Hollmann et al., 1991; Mishina et al., 1991), and removal of this subunit results in facilitated LTP (Gerlai et al., 1998), perhaps implying enhanced learning ability. If this were the case, it would also be expected that gria2 KO mice would display enhanced rates of learning on similar tasks, because deletion of GluR2 occurs throughout all brain regions. However, rates of acquisition on pavlovian conditioning did not differ after the deletion of gria2, suggesting that these mice do not simply display enhanced learning of all stimulus-reward associations. It is also possible that the increased responding for conditioned reinforcement may be a result of increased motivational value being attributed to the US, because gria2 KO mice also responded at higher rates for the primary reinforcer (food) when responding was maintained on a VI ratio. Response rates during VI schedules of reinforcement are suggested to be indicative of, and influenced by, the frequency and magnitude of reinforcement, whereby increased rates of responding are associated with increased frequency or magnitude of reinforcement (Herrnstein, 1970; Heyman et al., 1987). However, this explanation for the enhanced responding for conditioned reinforcement relies on the assumption that the motivational value of the US is quantitatively related to the motivational value of an associated CS. Although we are not aware of any direct tests of this assumption, indirect support is provided by the observation that response rates during the first predrug period of a second-order schedule for cocaine are directly related to the cocaine dose associated with the CS (Arroyo et al., 1998).
Despite displaying normal pavlovian conditioning and responding for CR, gria2 KO mice were clearly impaired on the other aspects of cue-maintained behaviors studied here. Whereas WT mice displayed selective approach toward the stimulus light when it was presented (conditioned approach), gria2 KOs showed no such approach. This effect could not be attributed to a visual deficit in the gria2 KOs because these mice were capable of using the light CS as a discriminative stimulus for predicting food delivery as effectively as WT mice. In addition, the ability of the cues to enhance responding on an ongoing operant task (PIT) was abolished after the deletion of GluR2. Although the magnitude of the PIT effect was not as large as we have reported previously in the WT mice in our gria1 KO experiment (possibly because the background strain of the gria2 KO mouse was CD1m whereas that for the gria1 KO was C57Bl), there was still a clear increase in response rates after the CS onset (Fig. 4B). One likely explanation for the weaker effect is that WT mice also displayed enhanced magazine approach during the CS, the appropriate response from earlier pavlovian conditioning sessions. This magazine approach resulted in an initial decrease in responding (response competition), followed by an increase (PIT). However, no increase in response rates during the CS was observed in gria2 KO mice, and because no increase in magazine approach was observed, this lack of PIT cannot be attributed to response competition.
Deficits observed in the gria1 KO mouse were attributed to impaired BLA function (Mead and Stephens, 2003) because of the similarities in behavioral impairments seen after gria1 deletion and BLA lesions, a striking similarity is seen between deletion of gria2 and lesions of the CeA (Table 1). CeA lesions impair conditioned approach and PIT, while leaving intact pavlovian conditioning and responding for conditioned reinforcement (Everitt et al., 2000). Furthermore, CeA lesions abolish the amphetamine-induced potentiation of responding for conditioned reinforcement (Robledo et al., 1996), as does the deletion of gria2. Therefore, our data indicate a double dissociation in the roles of GluR1 and GluR2 in the behavioral responses to appetitive cues, similar to that reported previously with lesions of the BLA and CeA.
Table 1.
Comparison of the behavioral consequences of gria1 or gria2 deletion with lesions of the basolateral or central regions of the amygdala on stimulus-reward learning tasks and control measures
|
Behavior |
gria1 KOa |
BLA lesionsb |
gria2 KO |
CeA lesionsb |
|---|---|---|---|---|
| Pavlovian conditioning | Normal | Normal | Normal | Normal |
| Conditioned approach | Normal | Normal | Impaired | Impaired |
| Pavlovian-to-instrumental transfer | Normal | Normal | Impaired | Impaired |
| CR | Impaired | Impaired | Enhanced | Normal |
| Primary reinforcement (instrumental) | Enhanced | Normalc | Enhanced | Normalc |
| Amphetamine-potentiation of CR | Not tested | Normald | Impaired | Impairede |
| Second-order schedules of reinforcement
|
Impaired
|
Impairedf
|
Not tested
|
Normalg
|
The effects of gria1 deletion resemble BLA lesions, whereas gria2 deletion resembles lesion of the CeA. Pavlovian conditioning is defined as the ability to use a cue as a signal for reward availability. Responding for primary reinforcement as assessed using a VI schedule of reinforcement for food reward.
In the case of the gria1 KOs, the deficits could not be conclusively attributed to GluR1 deletion, because we also observed increased GluR2 expression in the BLA of these mice. Previous reports noted no overall changes in levels of GluR1, GluR3, or GluR4 in the brains of gria2 KO mice (Jia et al., 1996), but no studies on possible compensation within specific brain regions have been performed previously. To examine whether compensatory changes in AMPA-receptor subunits occurred after gria2 deletion, we examined GluR1 expression within the amygdala of gria2 KO mice. Although changes in the overall levels of GluR1 were observed in the CeA of gria2 KO mice, no alterations in the number of neurons expressing GluR1 were observed. However, in the BLA, both a reduction in the number of GluR1-positive neurons, and the overall density of GluR1 was found. These observations raise the possibility that the “CeA like” behavioral deficits seen in the gria2 KO mice may have been caused by alterations in GluR1 as well as the deletion of GluR2. However, the fact that a similar downregulation of GluR1 was observed in the BLA suggests that this explanation is unlikely, because BLA-dependent tasks were unaffected. However, it should be noted that the overall decrease in levels of GluR1 in the CeA was significantly greater than that seen in the BLA; therefore, we cannot rule out the possibility that the behavioral deficits observed were caused by altered GluR1 expression.
Although our findings with the gria2 KO mice parallel behavioral deficits seen in rats with CeA lesions, it cannot be concluded from the present data that the deficits we see are attributable to impaired transmission in the CeA. The CeA receives inputs from a large number of brain regions, including cortical and thalamic sensory areas, and other amygdala nuclei. It is likely that neurotransmission in these pathways is also affected by the gria2 deletion. Moreover, there are substantial connections from the CeA to the dopaminergic neurons of the ventral tegmental area (VTA) (Fudge and Haber, 2000) and it has been suggested that it is this projection that plays an important role in conditioned approach (Everitt et al., 2000). Because up to 84% of VTA dopamine neurons carry GluR2 subunits (Chen et al., 2001), it is possible that our results reflect disruption of this pathway rather than disruption of transmission within the CeA itself.
In summary, gria2 KO mice display deficits in both conditioned approach and PIT. Although responding for CR is enhanced in gria2 KO mice, the rate-enhancing effects of amphetamine on responding for CR are absent. The results reported here have interesting implications for understanding the mechanisms underlying relapse in drug abuse. By dissociating the effects of reward cues on behavior at the level of receptor subunits, in addition to the previously demonstrated anatomical dissociation, the ability to prevent cues from influencing certain aspects of behavior without interfering with all stimulus-controlled behaviors becomes more realistic. The ability of reward-paired cues to elicit enhanced pursuit of the associated reward (PIT) has been cited by some as a critical element of the relapse process (Wyvell and Berridge, 2001), and as such, GluR2-containing AMPA receptors may be a suitable target for antagonism when developing treatments for relapse prevention.
Footnotes
This work was supported by funding from the United Kingdom Biotechnology and Biological Sciences Research Council. We thank G. Brown, Dr. A. Chiter, Dr. S. Rulten, and Dr. L. Mayne for genotyping and Dr. P. Acostas for immunohistochemistry assistance.
Correspondence should be addressed to Dr. D. N. Stephens, Laboratory of Experimental Psychology, University of Sussex, Brighton BN1 9QG, United Kingdom. E-mail: dns@biols.susx.ac.uk.
Copyright © 2003 Society for Neuroscience 0270-6474/03/239500-08$15.00/0
References
- Arroyo M, Markou A, Robbins TW, Everitt BJ ( 1998) Acquisition, maintenance and reinstatement of intravenous cocaine self-administration under a second-order schedule of reinforcement in rats: effects of conditioned cues and continuous access to cocaine. Psychopharmacology (Berl) 140: 331-344. [DOI] [PubMed] [Google Scholar]
- Burns LH, Robbins TW, Everitt BJ ( 1993) Differential effects of excitotoxic lesions of the basolateral amygdala, ventral subiculum and medial prefrontal cortex on responding with conditioned reinforcement and locomotor activity potentiated by intra-accumbens infusions of d-amphetamine. Behav Brain Res 55: 167-183. [DOI] [PubMed] [Google Scholar]
- Chen LW, Wei LC, Lang B, Ju G, Chan YS ( 2001) Differential expression of AMPA receptor subunits in dopamine neurons of the rat brain: a double immunocytochemical study. Neuroscience 106: 149-160. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP ( 1999) Limbic activation during cue-induced cocaine craving. Am J Psychiatry 156: 11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A ( 1994) Instrumental conditioning. In: Animal learning and cognition (Mackintosh N, ed), pp 45-78. London: Academic Press.
- Everitt BJ, Cador M, Robbins TW ( 1989) Interactions between the amygdala and ventral striatum in stimulus-reward associations: studies using a second-order schedule of reinforcement. Neuroscience 30: 63-75. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Cardinal R, Hall J, Parkinson J, Robbins TW ( 2000) Differential involvement of amygdala subsystems in appetitive conditioning and drug addiction. In: The amygdala, ed 2 (Aggleton J, ed), pp 353-390. New York: Oxford UP.
- Everitt BJ, Dickinson A, Robbins TW ( 2001) The neuropsychological basis of addictive behaviour. Brain Res Brain Res Rev 36: 129-138. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW ( 2003) Appetitive behavior: impact of amygdala-dependent mechanisms of emotional learning. In: The amygdala in brain function: basic and clinical approaches (Cahill L, ed), pp 233-250. New York: New York Academy of Sciences. [PubMed]
- Franklin K, Paxinos G ( 1997) The mouse brain in stereotaxic coordinates. San Diego: Academic Press.
- Fudge JL, Haber SN ( 2000) The central nucleus of the amygdala projection to dopamine subpopulations in primates. Neuroscience 97: 479-494. [DOI] [PubMed] [Google Scholar]
- Gerlai R, Henderson JT, Roder JC, Jia Z ( 1998) Multiple behavioral anomalies in GluR2 mutant mice exhibiting enhanced LTP. Behav Brain Res 95: 37-45. [DOI] [PubMed] [Google Scholar]
- Herrnstein R ( 1970) On the law of effect. J Exp Anal Behav 13: 243-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman GM, Monaghan MM, Clody DE ( 1987) Low doses of cis-flupentixol attenuate motor performance. Psychopharmacology (Berl) 93: 477-482. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M ( 2003) Double dissociation of the effects of lesions of basolateral and central amygdala on conditioned stimulus-potentiated feeding and pavlovian-instrumental transfer. Eur J Neurosci 17: 1680-1694. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Hartley M, Heinemann S ( 1991) Ca2+ permeability of KA-AMPA-gated glutamate receptor channels depends on subunit composition. Science 252: 851-853. [DOI] [PubMed] [Google Scholar]
- Jia Z, Agopyan N, Miu P, Xiong Z, Henderson J, Gerlai R, Taverna FA, Velumian A, MacDonald J, Carlen P, Abramow-Newerly W, Roder J ( 1996) Enhanced LTP in mice deficient in the AMPA receptor GluR2. Neuron 17: 945-956. [DOI] [PubMed] [Google Scholar]
- Mackintosh N ( 1974) The psychology of animal learning. London: Academic Press.
- McDonald AJ ( 1996) Localization of AMPA glutamate receptor subunits in subpopulations of non-pyramidal neurons in the rat basolateral amygdala. Neurosci Lett 208: 175-178. [DOI] [PubMed] [Google Scholar]
- Mead AN, Stephens DN ( 2003) Selective disruption of stimulus-reward learning in glutamate receptor gria1 knock-out mice. J Neurosci 23: 1041-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead AN, Crombag HS, Rocha BA ( 2003) Sensitization of psychomotor stimulation and conditioned reward in mice: differential modulation by contextual learning. Neuropsychopharmacology, in press. [DOI] [PubMed]
- Mishina M, Sakimura K, Mori H, Kushiya E, Harabayashi M, Uchino S, Nagahari K ( 1991) A single amino acid residue determines the Ca2+ permeability of AMPA-selective glutamate receptor channels. Biochem Biophys Res Commun 180: 813-821. [DOI] [PubMed] [Google Scholar]
- Nicoll RA ( 2003) Expression mechanisms underlying long-term potentiation: a postsynaptic view. Philos Trans R Soc Lond B Biol Sci 358: 721-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, Ehrman R, Robbins SJ ( 1998) Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol 12: 15-22. [DOI] [PubMed] [Google Scholar]
- Robledo P, Robbins TW, Everitt BJ ( 1996) Effects of excitotoxic lesions of the central amygdaloid nucleus on the potentiation of reward-related stimuli by intra-accumbens amphetamine. Behav Neurosci 110: 981-990. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J ( 2003) The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 168: 3-20. [DOI] [PubMed] [Google Scholar]
- Tomie A, Brooks W, Zito B ( 1999) Sign-tracking: the search for reward. In: Contemporary learning theory (Klein S, Mowers R, eds), pp 191-226. Hillsdale, NJ: Lawrence Erlbaum.
- Whitelaw RB, Markou A, Robbins TW, Everitt BJ ( 1996) Excitotoxic lesions of the basolateral amygdala impair the acquisition of cocaine-seeking behaviour under a second-order schedule of reinforcement. Psychopharmacology (Berl) 127: 213-224. [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC ( 2001) Incentive sensitization by previous amphetamine exposure: increased cue-triggered “wanting” for sucrose reward. J Neurosci 21: 7831-7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanillo D, Sprengel R, Hvalby O, Jensen V, Burnashev N, Rozov A, Kaiser KM, Koster HJ, Borchardt T, Worley P, Lubke J, Frotscher M, Kelly PH, Sommer B, Andersen P, Seeburg PH, Sakmann B ( 1999) Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science 284: 1805-1811. [DOI] [PubMed] [Google Scholar]