Abstract
The study aimed to evaluate the fluorescent molecular-imaging probe 2-(1-{6-[(2-fluoroethyl)(methyl)amino]-2-naphthyl}ethylidene)malononitrile (FDDNP) for its ability to selectively and reproducibly label prion plaques in fixed, paraffin-embedded cerebellar sections from patients of confirmed Gerstmann-Sträussler-Scheinker disease, sporadic Creutzfeldt-Jacob disease (CJD) with kuru plaques, and variant CJD (vCJD). FDDNP is a highly hydrophobic, viscosity-sensitive, solvent-sensitive, fluorescent substance, whose radiofluorinated analog [18F]FDDNP has recently been successfully used to label senile plaques and neurofibrillary tangles in the living brain of Alzheimer's disease patients with positron emission tomography. Our results show that FDDNP reliably identifies all prion plaques, including small cluster-plaques in vCJD. This finding may open new in vivo diagnostic possibilities for vCJD.
Keywords: fluorescent labeling, human, neuroimaging, TSE, variant Creutzfeldt-Jacob disease, amyloid
Introduction
Transmissible spongiform encephalopathies (TSE), also known as prion diseases, are a group of invariably fatal neurodegenerative disorders that affect both humans and animals (Prusiner, 1998). Despite their rarity (∼1 case per million per year), human TSE have a dramatic impact because of their clinical profile and complete absence of effective treatment. Concerns of possible transmission of prion diseases through surgical and dental procedures, tissue transplantation, and blood transfusion have been fueled by a recent emergence of a new variant Creutzfeldt-Jacob disease (vCJD) (Will et al., 1996) and evidence of its association with the epidemic of bovine spongiform encephalopathy.
Definitive diagnosis of a prion disease requires a postmortem pathomorphological inspection of the brain, which is usually combined with immunological detection and characterization of the pathological prion protein PrPSc (DeArmond et al., 2002). There are currently a few antemortem investigations that can consolidate a clinical suspicion of a prion disease (e.g., EEG, magnetic resonance brain imaging, immunoassays for a range of nonspecific markers). None of them, however, has an ideal sensitivity and specificity profile. In particular, these investigative techniques do not appear to be decisive in securing the diagnosis of vCJD. Therefore, invasive procedures (i.e., CNS or lymphoreticular tissue biopsy) have been used to confirm the presence of PrPSc in suspected vCJD (Ironside et al., 2002).
Recently, Barrio et al. (1999) developed 2-(1-{6-[(2-[18F]fluoroethyl) (methyl)amino]-2-naphthyl}ethylidene)malononitrile ([18F]FDDNP; [18F], t1/2 = 110 min) for positron emission tomography (PET) scan detection of amyloid deposits in Alzheimer's disease (Agdeppa et al., 2001). More recently, a modified version of thioflavin-T labeled with 11C (t1/2 = 20 min) was also introduced with similar intent (Bacskai et al., 2002). [18F]FDDNP crosses the blood-brain barrier and reveals the localization and load of senile plaques and neurofibrillary tangles in the living brain of Alzheimer's disease patients (Agdeppa et al., 2001; Shoghi-Jadid et al., 2002). A PET molecular imaging probe that could similarly reveal prion plaques would be particularly relevant for the diagnosis of vCJD, in which massive prion plaques are a hallmark of the disease and have a characteristic distribution within the CNS (Will et al., 1996; Zeidler et al., 1997; DeArmond et al., 2002; Ironside et al., 2002).
FDDNP is a hydrophobic substance that binds to its ligands through hydrophobic interactions (Agdeppa et al., 2001). It can be excited in the visible part of the spectrum between 440 and 490 nm. Its fluorescent emission is environmentally sensitive, being poorly fluorescent in water and of considerably stronger fluorescence when in viscous environments, or when bound (Jacobson et al., 1996; Petric et al., 1998; Agdeppa et al., 2001; Shoghi-Jadid et al., 2002). Aβ(1-40) fibril-bound FDDNP peak emission is at ∼500 nm (Agdeppa et al., 2001).
The aim of this study was to use the nonradioactive FDDNP and determine its in vitro potential for plaque labeling in human prion diseases with amyloid plaques, with particular emphasis on vCJD.
Materials and Methods
Sections of paraformaldehyde-fixed, paraffin-embedded human cerebellar samples from patients with confirmed sporadic CJD (sCJD) with kuru plaques, Gerstmann-Sträussler-Scheinker (GSS) syndrome (with the P102L mutation of the PRNP gene; generously provided by Dr. Herbert Budka, Institute of Neurology, University of Vienna, and Austrian Reference Centre for Human Prion Diseases, Vienna, Austria; Hainfellner at al., 1995), and vCJD (generously provided by Dr. James Ironside, Neuropathology Laboratory, CJD Surveillance Unit, University of Edinburgh, Edinburgh, UK) were used in the study. In addition, tissue sections of equally processed brain of a deceased individual (normal control) known not to have had a prion disease or any other neurological disorder was used as a negative control. The CJD and vCJD (but not GSS or normal) cerebellar samples were immersed in 96% formic acid for 1 hr after fixation in paraformaldehyde.
The 5-μm-thick sections were either used immediately after deparaffination or after antigen retrieval procedure involving 30 min autoclaving at 121°C in distilled water and subsequent 5 min incubation in 96% formic acid, which is the standard recommended pretreatment for PrP Sc immunodetection in tissue samples (Hegyi et al., 1997).
Monoclonal antibody (MAb) 3F4 was used (Dako, Glostrup, Denmark) for immunofluorescent (IF) and immunohistochemical (IHC) detection of PrP Sc. Both immunodetection procedures were indirect, using biotinylated secondary antibody and streptavidin-fluorophore conjugate (IF) or avidin-biotin-peroxidase with 3′,3-diaminobenzidine (IHC). Briefly, the sections were rinsed in buffer before a 4% normal horse serum was applied in buffer, pH 7.2, for 20 min to block the nonspecific binding of the secondary antibody. Incubation with the primary anti-PrP MAb 3F4 (3-10 μg/ml) was performed overnight at 4°C. Incubation in the biotinylated horse anti-mouse secondary antibody (1:1000; Vector Laboratories, Burlingame, CA) was followed by incubation in the avidin-biotin-peroxidase complex (ABC Standard Elite Kit; Vector Laboratories) and chromogen (3′,3-diaminobenzidine; Sigma, Deisenhofen, Germany) solution, or streptavidin-Alexa 488 (Molecular Probes, Eugene, OR). In IHC, hematoxilin counterstaining was used to visualize the cell nuclei.
FDDNP was synthesized as described previously (Agdeppa et al., 2001). Labeling was performed on deparaffinated sections stained with hematoxilin, rinsed in water, and immersed for 5 min in 1% Sudan black solution in 70% ethanol to reduce the autofluorescence of lipofuscin (Schnell et al., 1999) and other tissue components. Sections were rinsed with water, and excess Sudan black was removed by dipping the slides in 70% ethanol. The sections were then incubated with a freshly prepared 10 μm FDDNP in aqueous 1% ethanol (v/v) in the dark at room temperature (Agdeppa et al., 2001). The stained tissue was rinsed with water and coverslipped with glycerol before viewing under the fluorescent microscope.
Labeling of the same plaques with FDDNP and 3F4 immunofluorescence was performed in sequence, and the FDDNP was applied first to a freshly deparaffinated section. The stained section was photographed, rinsed in ethanol to remove FDDNP, and pretreated for immunodetection of PrP Sc aggregates, as described above. Before applying 3F4 to these sections, we verified that no remaining FDDNP fluorescence was detected in the plaques. In the final series, sections were first stained with FDDNP, photographed, rinsed in ethanol, and stained with periodic acid-Schiff (PAS). PAS was chosen over the more amyloid-specific Congo red staining because of the better labeling profile of smaller plaques with the former dye. After collection of photographs of the PAS-stained plaques, we exposed the same sections to the antigen retrieval protocol and processed them for IHC detection of PrP Sc with 3F4.
Photomicrographs were taken on a Nikon Eclipse E600 light and fluorescent microscope equipped with appropriate filter (EX 465-695, DM 505, BA 515-555), with a Nikon DXM 1200 digital camera, and connected to a personal computer image analysis station. MCID Image Analysis System software (Image Analysis System, St. Catharines, Ontario, Canada) was used to identify individual plaques, to verify their labeling with different techniques, and to determine the signal surface area in FDDNP-, PAS-, and 3F4-IHC-labeled sections. Briefly, a microscopic image was digitized, and a signal threshold was determined for each of the labeling methods applied to the same loci (n = 4 fields at 200× magnification). The signal surface area was then measured, and FDDNP and PAS results were expressed as percentage (average ± SD) of the 3F4-IHC measurement result. Final images were created by pseudocoloring the original green fluorescent signal yellow in accordance with the requirements for digital art preparation.
Results
FDDNP identifies typical prion plaques in human prion diseases
Standard IHC revealed diverse prion aggregates in the cerebellar tissue sections from all the prion disease cases used in this study: a kuru plaque in sCJD (Fig. 1a), multicentric plaque in GSS (Fig. 1c), and typical spiked-ball plaque in vCJD (Fig. 1e). In adjacent sections, different prion plaques were consistently and intensely labeled with FDDNP in the same pattern characteristic for each of the three prion diseases (Fig. 1b, d, and f, respectively). A simultaneously processed section from a normal cerebellum (Fig. 1g-i) displayed no detectable fluorescent labeling with FDDNP (Fig. 1j).
Figure 1.
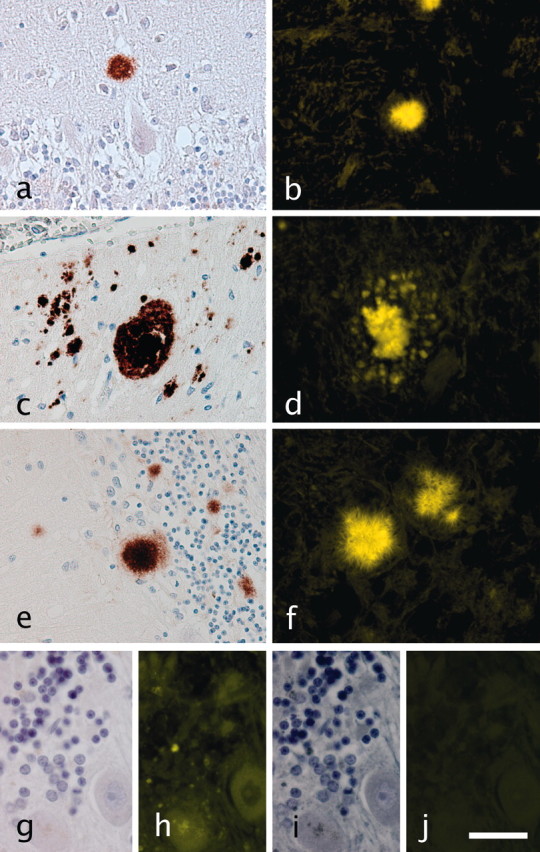
a, b, Comparison of standard immunohistochemistry and FDDNP plaque labeling. IHC with 3F4 MAb reveals typical prion aggregates in the cerebellar tissue sections from all the prion disease cases used in this study: kuru plaques in sCJD (a), multicentric plaques in GSS (b), and typical spiked-ball plaques in vCJD (c). Different prion plaques show intense labeling with FDDNP in the same general pattern characteristic for each of the three prion diseases (d-f, respectively). g-j, Normal control cerebellar section stained only with hematoxilin (g) displays considerable autofluorescence (h) that was quenched by Sudan black staining (i, j). In addition, absence of fluorescent signal after FDDNP labeling of the same section is shown in j. Green fluorescent signal was pseudocolored yellow. Scale bars: a-f, 50 μm; g-j, 15 μm.
FDDNP plaque identification is confirmed by prion-specific immunolabeling
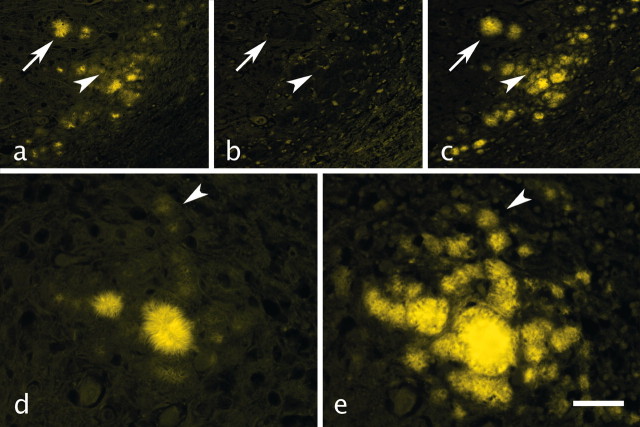
Sequential application of prion immunolabeling and FDDNP to the same tissue sections permitted a direct control of prion plaque detection and labeling with FDDNP. Absence of antigen retrieval techniques precludes successful immunodetection of most prion aggregates with anti-PrP antibodies on formaldehyde-fixed, paraffin-embedded tissue sections. Thus, harsh antigen retrieval has to be used to allow optimal immunodetection of prion aggregates (Hegyi et al., 1997). Such tissue pretreatment was determined not to be compatible with FDDNP plaque labeling (data not shown). Therefore, FDDNP was applied first onto freshly deparaffinated sections and found to selectively and reproducibly label prion plaques of various sizes, including smaller cluster-plaques in vCJD cerebellar sections (Fig. 2a). FDDNP binding is reversible, and no plaque fluorescence could be detected after several ethanol rinses just before 3F4 MAb application to the same section (Fig. 2b). IF detection of prion aggregates with 3F4 at the same location previously labeled with FDDNP revealed a qualitatively very similar picture: intensely labeled large plaques and numerous smaller, less intensely labeled cluster-plaques (Fig. 2c). All of the IF-labeled plaques (Fig. 2c,e) can be identified in the FDDNP-labeled section (Fig. 2a,d). Quantitatively, however, there was a difference in prion deposit visualization with FDDNP (Fig. 2d), and 3F4 immunofluorescence (Fig. 2e) in favor of the immunodetection method, which depends on very effective signal amplification.
Figure 2.
Comparison of FDDNP prion plaque labeling with IF detection of prions in the same section of vCJD cerebellum. FDDNP strongly labeled large plaques (a, arrow). However, smaller cluster-plaques (a, arrowhead) could also be discerned against the tissue background. The same plaques displayed no residual fluorescence after ethanol rinsing to remove FDDNP (b, arrow and arrowhead). After antigen retrieval, 3F4 MAb binding visualized by indirect immunofluorescence with Alexa 488 revealed prion aggregates in the same area (c), showing the same labeling pattern as FDDNP (c, arrow and arrowhead) but a stronger, amplified fluorescence of the smaller aggregates. A higher-magnification view of another cluster of vCJD cerebellar plaques labeled with FDDNP (d) and subsequently with 3F4-IF (e) allows better insight into details of plaque labeling with the two methods (for comparison, the same small plaque in d and e is indicated by an arrowhead). All fluorescent images were pseudocolored. Scale bars: a-c, 200 μm; d, e, 50 μm.
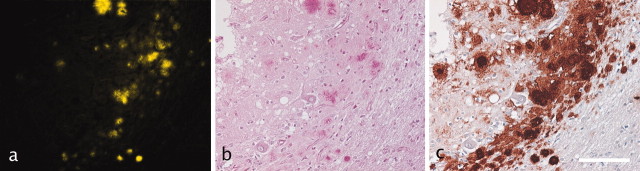
Small cluster-plaques in vCJD cerebellum are readily detected by FDDNP but not PAS
Sequential labeling of the same sections with FDDNP (Fig. 3a), PAS (Fig. 3b), and eventually with specific anti-PrP MAb 3F4-IHC (Fig. 3c) permitted a rough comparison of the extent of the same prion plaque labeling with the three methods. Although the standard IHC with intense signal amplification and chromogen precipitation proved superior to both nonspecific methods, FDDNP showed an apparent advantage over PAS in visualizing the small cluster-plaques in the cerebellum.
Figure 3.
Comparison of consecutive labeling of the same locus of a single tissue section with FDDNP (a, yellow pseudocolored plaques), PAS (b, magenta-labeled plaques), and IHC to PrPSc (c, brown precipitate). FDDNP labeled both larger amyloid and smaller cluster-plaques, whereas PAS readily identifies mostly larger amyloid plaques. Effective antigen retrieval and signal amplification of prion immunolabeling with anti-PrP MAb allows visualization of all prion deposits over a wide area in the same location. Scale bar, 200 μm.
The differences between plaque labeling techniques visible in Figures 2 and 3 were roughly quantified by expressing FDDNP- and PAS-labeled surface area as percentage of the surface area of the same tissue section labeled with 3F4-IHC: FDDNP signal surface area was 16 ± 4%, whereas PAS signal surface area covered 6 ± 2% of the surface area labeled with 3F4-IHC.
Discussion
This study aimed to evaluate the fluorescent molecular probe FDDNP for its ability to selectively and reproducibly label prion plaques in fixed, paraffin-embedded cerebellar sections from patients of confirmed GSS, sCJD with kuru plaques, and vCJD. Our results show that FDDNP reliably identifies prion plaques in all tissue samples. Its ability to selectively detect prion plaques of various sizes in the tested tissue samples was confirmed by IF identification of the same plaques with a standard anti-PrP antibody, 3F4. In vCJD samples, FDDNP allowed detection of smaller plaques better than PAS staining of the same prion aggregates, which was confirmed by subsequent IHC detection of the full extent of PrPSc deposits at the same locus of individual tissue sections.
Amyloid plaques in prion diseases
The “protein only” hypothesis states that the infectious agent causing TSE is a conformational isomer of PrPC, a host protein, the function of which is still poorly understood (Prusiner, 1998). The pathological isomer, PrPSc, has high β-sheet content, is largely protease-resistant, and forms aggregates, which accumulate in the brain (Prusiner, 1998). Different types of prion aggregates and their anatomical distribution are characteristic for different prion diseases (DeArmond et al., 2002). Although prion deposits can be of various sizes and structures, prions frequently form amyloid plaques, which are a hallmark of some prion diseases such as GSS, sCJD with kuru plaques, and vCJD (DeArmond et al., 2002). The latter disease is a prime candidate condition for noninvasive in vivo procedures, which would aid diagnosis.
Suspected vCJD: a diagnostic challenge
Currently, the clinical course is key to establishing a working diagnosis of vCJD. Emotional and behavioral changes occur early in vCJD and frequently involve depression or psychosis. The lack of marked organic features often leads to a psychiatric diagnosis (Zeidler et al., 1997). The suspicion of vCJD is raised if ataxia and forgetfulness develop (Zeidler et al., 1997). Diagnosis of vCJD may be confirmed by detecting PrPSc in biopsy specimens, but the nonuniform tissue distribution of typical “florid” plaques in the CNS and low concentration of PrPSc in lymphoreticular tissue has occasionally resulted in negative biopsy results (Ironside et al., 2002). Thus, definitive diagnosis requires a postmortem detection of severe spongiform changes in the basal ganglia, thalamus, and cerebellum, as well as the presence of prion plaques.
The most remarkable feature of vCJD is the obligatory massive amount of prion amyloid plaques, many with features of kuru plaques, and the huge number of primitive plaque-like deposits that are PAS nonreactive (DeArmond et al., 2002). All regions of the cerebral cortex contain prion plaques, but the highest concentration occurs in the cerebellum (DeArmond et al., 2002; Ironside et al., 2002). Therefore, a PET scan probe, which would reliably and sensitively label prion plaques in brains of living patients, would be of great potential value.
Potential for molecular-imaging of amyloid plaques with [18F]FDDNP-PET in vivo
A molecular probe such as FDDNP that binds to amyloid plaques with high affinity (Agdeppa et al., 2001; Shoghi-Jadid et al., 2002) is likely to meet the requirement of sufficient sensitivity. FDDNP is a hydrophobic molecule that forms induced dipoles between the donor group (e.g., dialkyl amino) and acceptor moiety (e.g., malononitrile) in various environmental conditions. We hypothesize that these dipoles would form hydrogen bond interactions with groups on the hydrophobic surfaces of β-amyloid aggregates (or tau aggregates in neurofibrillary tangles), giving rise to the unique binding properties of this new probe. FDDNP is not the only molecular probe that has been developed for the purpose of in vivo neuroimaging of amyloid plaques (Bacskai et al., 2002; Kung et al., 2002). However, [18F]FDDNP it is the only substance that had been successfully used to localize and assess the burden of senile plaques and neurofibrillary tangles in living brains of Alzheimer's patients.
The lack of specificity of FDDNP for prion plaques over amyloid plaques of Alzheimer's disease, or other amyloid deposits in the brain, might be viewed as an apparent disadvantage. However, an indication for a plaque-detecting PET scan in a candidate vCJD patient would be based on a thorough clinical evaluation and patient history, thus considerably reducing the likelihood of confusing vCJD with other diseases with amyloid deposition in the brain, such as Alzheimer's disease. Furthermore, a preponderance of plaque load in the cerebellum would distinguish the PET scan result of a vCJD patient from virtually all differential diagnoses.
FDDNP labels all 3F4-identified prion plaques in vitro
In our study, FDDNP was compared with one of the routine histological methods for amyloid staining, PAS (DeArmond at al., 2002). When applied to a section of vCJD cerebellar tissue, FDDNP was shown to label not only large amyloid plaques but also small cluster-plaques that were not reliably visualized with subsequent application of PAS onto the same section. It has been shown previously that certain plaques, in particular the smaller cluster-plaques representing a significant proportion of plaque load in vCJD, do not display typical tinctorial properties of amyloid (e.g., they are not eosinophylic or PAS reactive) (DeArmond at al., 2002).
Therefore, in addition to comparing FDDNP with PAS, we also tested the ability of FDDNP to identify plaques against optimized immunodetection of prion deposits by a standard anti-PrP MAb, 3F4. The intent was to obtain a rough quantitative assessment of the proportion of all prion deposits that can be detected by FDDNP. Our results clearly show that FDDNP labeled all plaques that were subsequently identified by amplified immunofluorescence of the anti-PrP antibody binding. The observed differences in the intensity of labeling by FDDNP and 3F4 immunofluorescence should be interpreted in the context of the differences in the signal amplification between the two methods. Use of PrP-specific antibodies on adequately prepared tissue and appropriate signal amplification techniques allow visualization of most, even the finest (e.g., synaptic) prion deposits. In contrast, FDDNP should be expected to accumulate predominantly in those plaques with amyloid ultrastructure. Visualization of small PAS-nondetectable plaques with FDDNP (subsequently confirmed with 3F4 binding) reveals a favorable prion plaque labeling profile of FDDNP.
Prion detection with 3F4 immunofluorescence and FDDNP were both somewhat limited by the autofluorescent background of aldehyde-fixed tissue, which required quenching (Schnell et al., 1999). However, a less than ideal signal-to-noise ratio attributable to tissue autofluorescence is an artifact of the in vitro conditions under which FDDNP was tested in this study. Radiolabeled FDDNP used as a PET probe would not be likely to encounter these problems. The latter statement could be tested in vitro by determining the specificity and sensitivity of prion amyloid aggregate detection by a radiolabeled FDDNP. In addition, transgenic mice (Scott et al., 1999) or primates (Lasmezas et al., 2001) inoculated with vCJD should be used to test the [18F]FDDNP PET scan detection of prion plaques in vivo. Finally, the present evidence of strong in vitro prion plaque labeling with FDDNP coupled with the documented successful and safe use of [18F]FDDNP to detect plaque load in living Alzheimer's disease patients (Shoghi-Jadid et al., 2002) could stimulate preparations for the first [18F]FDDNP PET scans of confirmed and suspected vCJD patients.
Footnotes
This work was supported by the Republic of Slovenia Ministry of Health; Ministry of Education, Science and Sport Grants 0381-518, L3-3435 (M.B. and M.P.), P0-503-0103, and Slo-US 2001/34 (A.P.); and the European Union-funded project “Human Transmissible Spongiform Encephalopathies: The Neuropathology Network (PRIONET)” (M.P.). We acknowledge the assistance of Dr. Herbert Budka (Institute of Neurology, University of Vienna, and Austrian Reference Centre for Human Prion Diseases, Vienna, Austria) and Dr. James Ironside (Neuropathology Laboratory, CJD Surveillance Unit, University of Edinburgh, Edinburgh, UK), who kindly provided the samples of GSS and vCJD cerebellar tissue.
Correspondence should be addressed to Dr. Mara Bresjanac, Institute of Pathophysiology, Zaloska 4, SI-1000 Ljubljana, Slovenia. E-mail: maja.bresjanac@mf.uni-lj.si.
Copyright © 2003 Society for Neuroscience 0270-6474/03/238029-05$15.00/0
References
- Agdeppa ED, Kepe V, Liu J, Flores-Torres S, Satyamurthy N, Petric A, Cole GM, Small GW, Huang SC, Barrio JR ( 2001) Binding characteristics of radiofluorinated 6-dialkylamino-2-naphthylethylidene derivatives as positron emission tomography imaging probes for β-amyloid plaques in Alzheimer's disease. J Neurosci 21: RC189(1-5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacskai JB, Klunk EW, Mathis AC, Hyman TB ( 2002) Imaging amyloid-β deposits in vivo. J Cereb Blood Flow Metab 22: 1035-1041. [DOI] [PubMed] [Google Scholar]
- Barrio JR, Huang SC, Cole GM, Satyamurthy N, Petric A, Phelps ME, Small GW ( 1999) PET Imaging of tangles and plaques in Alzheimer's disease. J Nucl Med 40: 70P-71P.9935060 [Google Scholar]
- DeArmond SJ, Kretzschmar HA, Prusiner SB ( 2002) Prion diseases. In: Greenfield's neuropathology, Ed 7, Vol 2 (Graham DI, Lantos PE, eds), pp 273-323. London: Arnold. [Google Scholar]
- Hainfellner JA, Brantner-Inthaler S, Cervenáková L, Brown P, Kitamoto T, Tateishi J, Diringer H, Liberski PP, Regele H, Feucht M, Mayr N, Wessely P, Summer K, Seitelberger F, Budka H ( 1995) The original Gerstmann-Sträussler-Scheinker family of Austria: divergent clinicopathological phenotypes but constant PrP genotype. Brain Pathol 5: 201-211. [DOI] [PubMed] [Google Scholar]
- Hegyi I, Hainfellner JA, Flicker H, Ironside JW, Hauw JJ, Tateishi J, Haltia M, Bugiani O, Aguzzi A, Budka H ( 1997) Prion protein immunocytochemistry: reliable staining protocol, immunomorphology, and diagnostic pitfalls. Clin Neuropathol 16: 262-263. [Google Scholar]
- Ironside JW, McCardle L, Horsburgh A, Lim Z, Head MW ( 2002) Pathological diagnosis of variant Creutzfeldt-Jakob disease. APMIS 110: 79-87. [DOI] [PubMed] [Google Scholar]
- Jacobson A, Petric A, Hogenkamp D, Sinur A, Barrio JR ( 1996) 1,1-Dicyano-2-(6-dimethylaminonaphthalen-2-yl)propene (DDNP): a solvent polarity-and viscosity-sensitive fluorophore for fluorescence microscopy. J Am Chem Soc 118: 5572-5579. [Google Scholar]
- Kung MP, Hou C, Zhuang ZP, Zhang B, Skovronsky D, Trojanowski JQ, Lee VM, Kung HF ( 2002) IMPY: an improved thioflavin-T derivative for in vivo labeling of β-amyloid plaques. Brain Res 956: 202-210. [DOI] [PubMed] [Google Scholar]
- Lasmezas CI, Fournier JG, Nouvel V, Boe H, Marce D, Lamoury F, Kopp N, Hauw JJ, Ironside J, Bruce M, Dormont D, Deslys JP ( 2001) Adaptation of the bovine spongiform encephalopathy agent to primates and comparison with Creutzfeldt-Jakob disease: implications for human health. Proc Natl Acad Sci USA 98: 4142-4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petric A, Jacobson AF, Barrio JR ( 1998) Functionalization of a viscosity-sensitive fluorophore for probing of biological systems. Bioorg Med Chem Lett 8: 1455-1460. [DOI] [PubMed] [Google Scholar]
- Prusiner SB ( 1998) Prions. Proc Natl Acad Sci USA 95: 13363-13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell SA, Staines WA, Wessendorf MW ( 1999) Reduction of lipofuscin-like autofluorescence in fluorescently labeled tissue. J Histochem Cytochem 47: 719-730. [DOI] [PubMed] [Google Scholar]
- Scott MR, Will R, Ironside J, Nguyen HO, Tremblay P, DeArmond SJ, Prusiner SB ( 1999) Compelling transgenetic evidence for transmission of bovine spongiform encephalopathy prions to humans. Proc Natl Acad Sci USA 96: 15137-15142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoghi-Jadid K, Small GW, Agdeppa ED, Kepe V, Ercoli LM, Siddarth P, Read S, Satyamurthy N, Petric A, Huang SC, Barrio JR ( 2002) Localization of neurofibrillary tangles and β-amyloid plaques in the brains of living patients with Alzheimer disease. Am J Geriatr Psychiatry 10: 24-35. [PubMed] [Google Scholar]
- Will RG, Ironside JW, Zeidler M, Cousens SN, Estibeiro K, Alperovitch A, Poser S, Pocchiari M, Hofman A, Smith PG ( 1996) A new variant of Creutzfeldt-Jakob disease in the UK. Lancet 347: 921-925. [DOI] [PubMed] [Google Scholar]
- Zeidler M, Stewart GE, Barraclough CR, Bateman DE, Bates D, Burn DJ, Colchester AC, Durward W, Fletcher NA, Hawkins SA, Mackenzie JM, Will RG ( 1997) New variant Creutzfeldt-Jakob disease: neurological features and diagnostic tests. Lancet 350: 903-907. [DOI] [PubMed] [Google Scholar]