Abstract
Egg-laying behavior in Caenorhabditis elegans is activated by signaling through the G-protein Gρq and inhibited by signaling through a second G-protein, Gρo. Activation of egg laying depends on the serotonergic hermaphrodite-specific neurons (HSNs), but the neurotransmitter(s) and cell(s) that signal to inhibit egg laying are not known. Mutants for G-protein signaling genes have well characterized defects in egg laying. Here we present an analysis of mutants for other genes reported to lack inhibition of egg laying. Of the nine strongest, six have morphological defects in the ventral-type C (VC) neurons, which synapse onto both the HSNs and the egg-laying muscles and are thus the third cell type comprising the egg-laying system. Laser-ablating VC neurons could also disrupt the inhibition of egg laying. The remaining three mutants (unc-4, cha-1, and unc-17) are defective for synthesis or packaging of acetylcholine in the VCs. The egg-laying defects of unc-4, cha-1, and unc-17 were rescued by VC-specific expression of the corresponding cDNAs. In addition, increasing synaptic acetylcholine by reducing acetylcholinesterase activity, with either mutations or the inhibitor aldicarb, decreased egg laying. Finally, we found that a knock-out for the HSN-expressed receptor G-protein-coupled acetylcholine receptor 2 (GAR-2) shows a partial defect in the inhibition of egg laying and fails to respond to aldicarb. Our results show that acetylcholine released from the VC neurons inhibits egg-laying behavior. This inhibition may be caused, in part, by acetylcholine signaling onto the HSN presynaptic terminals, via GAR-2, to inhibit neurotransmitter release.
Keywords: acetylcholine, egg-laying behavior, VC neuron, Caenorhabditis elegans, unc-4, cha-1
Introduction
Egg-laying behavior in Caenorhabiditis elegans is controlled by neurotransmission through heterotrimeric G-proteins and has been used as a model for analyzing the mechanism of this type of neural signaling. Mutations in the highly conserved C. elegans Gρo ortholog GOA-1 result in hyperactive egg laying (Mendel et al., 1995; Sègalat et al., 1995). Egg laying is regulated by environmental stimuli, and Gρo signaling appears to be the mechanism normally used to reduce its frequency (Dong et al., 2000). Genetic studies suggest that GOA—1 signaling inhibits presynaptic neurotransmitter release (Lackner et al., 1999; Nurrish et al., 1999). Extensive genetic analysis demonstrates that Gρo signaling in C. elegans is antagonized by signaling through Gρq, a second highly conserved neural G-protein (Wilkie, 2000). Gρq signaling is thus a mechanism to increase the frequency of egg laying. Our understanding of these signaling pathways is based heavily on the behavioral effects of mutations in Gρo and Gρq signaling components; however, this understanding is severely limited because the neurotransmitters and cells that signal to increase and decrease egg laying have not been fully defined. Thus fundamental issues, such as whether the Gρo and Gρq signaling pathways operate in the same cells to directly antagonize each other, have not been resolved. Delineating the cells and signals that use these G-protein signaling pathways to regulate egg laying could provide insights into neural G-protein signaling that will generalize to other behaviors in C. elegans and to the corresponding signaling in human neurons.
Egg laying serves as an excellent model behavior because it has been extensively characterized, can be effectively quantitated, and has a simple anatomical basis. Eggs are laid when the two hermaphrodite-specific neurons (HSNs) stimulate the contraction of 16 egg-laying muscle cells to push eggs through the uterus and out the vulva (Desai et al., 1988). Only one other type of neuron synapses onto the egg-laying muscles: the six ventral-type C (VC) neurons, which also synapse onto the HSNs (White et al., 1986). The function of the VC neurons is not clear.
Mutations affecting egg laying can result in one of two opposite phenotypes: egg-laying defective (Egl) or hyperactive egg laying. Many Egl mutants have Gρq signaling defects (Brundage et al., 1996; Koelle and Horvitz, 1996; Miller et al., 1999). Others cannot stimulate egg laying because their HSNs are absent or anatomically abnormal (Desai et al., 1988). There are hyperactive egg-laying mutants that cannot properly inhibit egg laying because of Gρo signaling defects (Mendel et al., 1995; Sègalat et al.,1995;
Hajdu-Cronin et al., 1999; Nurrish et al., 1999); however, hyperactive egg-laying mutants have not been systematically examined for anatomical defects that might reveal the identity of the cell(s) or signal(s) that inhibits egg laying. Our analysis suggests that the VC neurons, by releasing the neurotransmitter acetylcholine, inhibit egg-laying behavior.
Materials and Methods
Nematode strains. The wild-type strain was Bristol N2. Mutations used were as follows: ace-1(p1000); ace-2(g72); cha-1(p1152); dgk-1(sy428); eat-16(ad702); gar-2(ok520); goa-1(n1134); lin-15(765ts); unc-2(e55); unc-4(wd1); unc-5(e53); unc-8(e49); unc-10(e102); unc-17(e245); unc-20(e112); unc-29(e403); unc-32(e189); unc-34(e566); unc-35(e259); unc-37(e262); unc-38(e293); unc-42(e270); unc-58(e665); unc-73(e936); unc-74(e883); unc-75 (e950); unc-76(e911); unc-115(e2225). Worms were cultured at 20°C under standard conditions, and double-mutant strains were generated using standard genetic techniques (Brenner, 1974).
Egg-laying assays. The average number of unlaid eggs and the percentage of early-stage eggs laid were quantified as described (Koelle and Horvitz, 1996). The staged adults used in all assays were obtained by collecting late fourth larval stage (L4) animals and culturing at 20°C for 36 hr. In the unlaid egg assay, 30 staged adults were individually dissolved in 5% sodium hypochlorite, and their eggs, which survive because of their protective eggshells, were counted. In the early-stage egg assay, 25 staged adults were placed on a thin lawn of OP50 bacteria on a nematode growth medium (NGM) agar plate (Brenner, 1974) and allowed to lay eggs for 30 min. This was repeated with new sets of staged animals until a total of at least 100 eggs were laid. This population assay allowed us to obtain samples of sufficient numbers of eggs so that differences between strains could be accurately measured. Each egg was examined under a Leica M420 dissecting microscope and categorized as having fewer than or equal to eight cells or more than eight cells. Eggs with eight cells or fewer were classified as “early stage.”
VC-specific promoter transgenes. We generated a vector to drive VC-specific expression: pMD64 contains a 2.1 kb PacI-ClaI fragment of the lin-11 promoter described by Cameron et al. (2002) inserted into pPD49.26 (gift from A. Fire, Carnegie Institute of Washington). When green fluorescent protein (GFP) coding sequences were inserted between the EcoRV and NcoI sites of pMD64, the resulting construct gave robust, relatively non-mosaic expression in the six VC neurons, as well as additional expression in cells of the posterior intestine and in the secondary cells of the vulva. Animals carrying the construct showed wild-type egg-laying behavior. pMD64 was also used in the unc-4, cha-1, and unc-17 rescue experiments.
unc-4, unc-17, and cha-1 cDNAs were isolated by RT-PCR using mixed-stage poly-A-selected mRNA. These cDNAs were inserted between the EcoRV and NcoI sites of pMD64.
To visualize the VC neurons, a VC promoter derived from that in pMD64 was used to drive GFP expression. pDM4 contained a 500 bp VC enhancer fragment from lin-11 amplified by the primers 5′-GACCGCATGCGTGGTGTAATCTGATCTG and 5′-GAGAAGGCCTTGCTCTATTCAATCATCC cloned upstream of the basal pes-10 promoter and the GFP coding sequences in the vector pPD97.78 vector (gift of A. Fire). pDM4 drove GFP expression in only the six VC neurons and a few cells of the posterior intestine. pDM4 was injected into lin-15(n765ts) animals at 80 ng/μl along with the lin-15 rescuing plasmid pL15EK at 50 ng/μl; the resulting extrachromosomal transgene was chromosomally integrated using γ irradiation and selecting a strain in which the transgene was stably inherited. This strain was outcrossed four times, and the resulting integrated transgene vsIs13 was used to visualize the VC neurons in Unc mutant backgrounds and for laser ablation experiments. unc-4, unc-17, and cha-1 rescue experiments. VC::cDNA constructs were coinjected (at 80 ng/μl) with the lin-15 rescuing plasmid pL15EK (at 50 ng/μl) into unc-4, unc-17, or cha-1 mutant backgrounds carrying the lin-15(n765ts) mutation. Five transgenic lines for each injection were isolated, and staged non-Muv adults were used in the early-stage egg assay. Results of each transgene experiment are shown as an average and SD for the five lines.
Fluorescence microscopy. Worms were staged as late-L4 larvae and then cultured at 20°C for 20-24 hr. They were fixed in a 6 μl drop of 4% paraformaldehyde on a glass slide for ∼5 min, until movement ceased, and then rinsed with 100 μl of M9 buffer (Brenner, 1974). Worms were left in ∼5 μl buffer, covered with a coverslip, and examined with a Zeiss Axioskop. Images were processed using Openlab software. Defects in VC morphology were quantitated for each strain by analyzing three separate sets of 15 animals, each set at a different magnification (10×, 40×, and 100× objectives), for a total of 45 worms per strain analyzed.
Laser ablation. Ablations were performed as described previously (Bargmann and Avery, 1995). Briefly, L4 larvae carrying the vsIs13 transgene (expressing GFP in the VCs) were placed in 3 μl of M9 buffer on a 2% agarose pad containing 1 mm sodium azide to induce reversible paralysis. GFP-positive cells were identified using a Zeiss Axioskop equipped with a Micropoint Laser System (Photonic Instruments, Inc.), and their nucleoli were repeatedly targeted with the laser until they appeared ruptured. Mock-ablated animals were placed on the same pad and exposed to fluorescence excitation light for the same period of time, but not shot with the laser. The animals were recovered, cultured on NGM agar plates, and examined 24-30 hr later with a Zeiss M2BIO fluorescence dissecting microscope to ensure absence of GFP-positive cells. Thirty hours after the ablation procedure, the early-stage egg assay was performed, and data for at least 15 eggs were collected for each individual animal.
Aldicarb and levamisole assays. To measure the acute effects of drugs on egg-laying behavior, we measured the rate at which eggs were laid after worms were placed on plates containing various drug concentrations. This assay revealed acute changes in behavior in animals of the same genotype, regardless of how many unlaid eggs animals of that genotype contained before drug treatment. We did not measure the stage of the eggs laid in response to drugs because that predominantly reflected the steady-state accumulation of eggs in the genotype being analyzed rather than the effects of drug. Plates containing aldicarb were prepared as described by Miller et al. (1996). Briefly, a 105 mm stock solution of aldicarb in ethanol was prepared, and the drug was added to varying final concentrations to NGM agar media (Brenner, 1974) after autoclaving. Plates were poured and dried overnight, and OP50 bacteria was spread on the plates and given 2 d at room temperature to grow to a thin lawn. The plates were then stored at 4°C and used within 1 week. Levamisole plates were prepared similarly, but the 100 mm levamisole stock solution was prepared in water and used immediately. Furthermore, levamisole plates were used within 4 d of preparation. Before use, a drop of 4 m fructose was spread around the edge of each plate, which created an osmotic barrier and kept worms from crawling up the sides of the plate. Egg-laying rates were determined by placing 10 adult animals on each plate for 1 hr and counting the number of eggs laid. The animals used were staged as L4 larvae and assayed 36 hr later. For each condition tested, the assay was repeated 12 times, thus using a total of 120 animals. The data presented are the average and SE of the 12 trials.
Results
Certain neural G-protein signaling mutants exhibit a hyperactive egg-laying phenotype
We have performed a systematic analysis of hyperactive egg-laying mutants. We begin our report of this analysis with a description of the hyperactive egg-laying phenotype and two methods for its quantitation. C. elegans hermaphrodites produce internally self-fertilized eggs. In wild-type animals, the fertilized eggs spend ∼2.5 hr developing within the uterus before being released by periodic episodes of egg-laying behavior (Waggoner et al., 1998). A wild-type worm has a steady state of ∼12 eggs retained within its body (Fig. 1A), and its eggs have reached about the 100-cell stage of development by the time they are laid (Fig. 1C). Hyperactive egg-laying mutants engage in egg-laying behavior more frequently than do the wild type. As a result, they accumulate very few eggs within the uterus (Fig. 1B) and lay eggs that are at an early stage of development (Fig. 1D). We can quantify both aspects of this phenotype to assess its strength (Fig. 1A, B,E), although measuring the percentage of early-stage eggs laid provides the most sensitive gauge of the hyperactive egg-laying phenotype.
Figure 1.
The hyperactive egg-laying phenotype, as illustrated in mutants with defects in Gρo signaling. A, Wild-type adult hermaphrodite. B, Loss-of-function mutant for goa-1, the C. elegans ortholog of the G-protein Gρo. Arrows indicate unlaid eggs; asterisks indicate the vulva (through which eggs are laid). Average numbers of unlaid eggs are above the animals. Wild-type animals retain fertilized eggs for ∼2.5 hr before laying them, whereas hyperactive egg-laying mutants, such as goa-1, engage in egg-laying behavior so frequently that few eggs are retained. C, Freshly laid multicellular egg from a wild-type animal. D, Freshly laid two-cell egg from a goa-1 mutant. Eggs laid by the wild type have developed for ∼2.5 hr and typically contain 50-100 cells, whereas eggs laid by hyperactive egg-laying mutants are much younger and thus often contain fewer than eight cells. E, Percentage of early-stage eggs (8 cells or fewer) laid by wild-type or mutant strains. The three G-protein signaling mutants shown fail to inhibit egg laying and thus exhibit the hyperactive egg-laying phenotype.
Null mutants for three G-protein signaling genes, goa-1, eat-16, and dgk-1, lay almost entirely early-stage eggs (Fig. 1E). Genetic studies indicate that neurotransmitter(s) signals through the neural Gρo protein GOA-1 to inhibit egg laying and that the regulator of G-protein signaling protein EAT-16 and the diacylglycerol kinase DGK-1 contribute to Gρo signaling (Mendel et al., 1995; Sègalat et al., 1995; Hajdu-Cronin et al., 1999; Nurrish et al., 1999). We have looked for other mutants with similarly strong hyperactive egg-laying phenotypes that might help define the cells and molecules that regulate the egg-laying system.
Strong hyperactive egg laying is observed in mutants that are defective either for neural development or for acetylcholine signaling
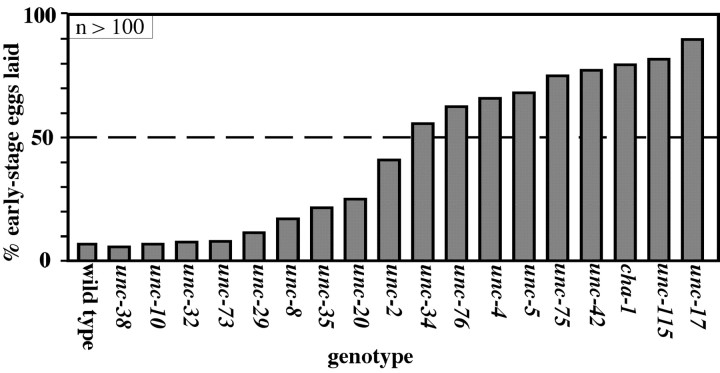
There have been reports of a number of mutants, besides Gρo signaling mutants, that are unable to properly inhibit egg laying (Riddle et al., 1997). These mutants have been described as “egg-laying constitutive” (Egl-C) on the basis of qualitative observations that they (1) laid early-staged eggs or (2) continued to lay eggs in the absence of bacteria or in liquid medium, conditions under which the wild type suppresses egg laying. We characterized a panel of 18 such mutants to see whether they exhibited the hyperactive egg-laying phenotype illustrated in Figure 1. Specifically, we looked for mutants that showed hyperactive egg laying comparable with that of the G-protein signaling mutants that laid >90% early-stage eggs (Fig. 1E). Some mutants showed no detectable defects by the early-stage egg assay, whereas others showed defects of varying strengths (Fig. 2). We have chosen to pursue the nine mutants from the panel that meet the arbitrary criterion of laying >50% early-stage eggs.
Figure 2.
Quantitation of the hyperactive egg-laying phenotype in a panel of uncoordinated mutants reported to have defects in inhibition of egg laying. The mutants showed defects in the early-stage egg assay ranging from extremely mild to severe. We chose to further analyze only those mutants that laid >50% early-stage eggs (dotted line).
All nine strong hyperactive egg-laying mutants show uncoordinated locomotion (eight are named “unc” genes as a result of this; cha-1 is named for the choline acetyltransferase it encodes rather than its Unc mutant phenotype). Uncoordinated locomotion in C. elegans can result from defects in neural development, in neural signaling, or in muscles. Seven of the nine strong mutants are known to have defects in neural structure, including defects in axonal guidance, fasiculation, synaptic choice, and axon sprouting. Specifically, unc-115, unc-34, and unc-5 function in netrin signaling to regulate axon guidance (Colavita and Culotti, 1998; Lundquist et al., 1998; Gitai et al., 2003). unc-4 (Winnier et al., 1999) and unc-42 (Wightman et al., 1997; Baran et al., 1999) encode homeodomain transcription factors that regulate the fate and synaptic connections of many neurons. unc-76 encodes a protein kinase C-binding protein that is necessary for the proper extension and bundling of most or all neurons (Bloom and Horvitz, 1997; Kuroda et al., 1999). unc-75 encodes a nuclear RNA binding protein that affects synaptic transmission and axonal sprouting (O. Hobert, personal communication).
The remaining two mutants (cha-1 and unc-17) are not known to cause developmental defects in the nervous system but rather are defective for acetylcholine signaling. cha-1 encodes the enzyme that synthesizes acetylcholine (Alfonso et al., 1994), and unc-17 encodes the only acetylcholine vesicular transporter in C. elegans (Alfonso et al., 1993). Null mutations of cha-1 and unc-17 are lethal (Rand and Russell, 1984), so partial loss-of-function mutations were used for our analysis (Fig. 2). Comparing two cha-1 alleles showed that the stronger the reduction of CHA-1 function, the stronger the hyperactivity of egg-laying behavior (data not shown).
Six hyperactive egg-laying mutants are defective for VC neuron structure
The fact that seven of the hyperactive mutants have defects in neuronal structure suggests that there might be one class of neuron that, when physically disrupted in these mutants, causes hyperactivity of egg-laying behavior. There are only two types of neurons, HSN and VC, that innervate the egg-laying muscles (White et al., 1986). Defects in the HSNs are known to cause the egg-laying defective phenotype, the opposite of the hyperactive egg-laying phenotype (Desai et al., 1988). The VC neurons synapse onto the same egg-laying muscles as the HSNs but have a poorly understood role in the regulation of egg laying. Therefore, we investigated the morphology of the VC neurons in the seven hyperactive Unc mutants.
To visualize the VC neurons, we constructed a transgene that expresses the GFP specifically in the six VC neurons. We modified the lin-11 promoter in a previously characterized lin-11::gfp transgene (Cameron et al., 2002) to eliminate vulval cell expression that obscured visualization of VC cell bodies and processes near the vulva. Our modified “VC::GFP” transgene was chromosomally integrated and showed expression only in the six VC neurons and in some posterior cells of the intestine (Fig. 3, top panels). The VC::GFP reporter was crossed into the genetic background of each of the seven hyperactive Unc mutants known to cause defects in neuronal structure.
Figure 3.
Representative morphological defects seen in the VC neurons of certain hyperactive egg-laying mutants. Animals shown express a GFP reporter transgene in the VCs and are seen at increasing objective magnifications in A-C to illustrate different types of defects. A, Wild-type control (top panel) and an unc-5 mutant (bottom panel). Arrows indicate VC cell bodies; asterisks indicate the vulva. Although six VC neurons are present in the wild type, only four can be visualized in the unc-5 mutant shown, two of which are dim. B, Wild-type control (top panel) and an unc-42 mutant (bottom panel). Brackets indicate VC axonal processes between two VC cell bodies. The unc-42 mutant shown has completely lost the processes between the VC5 and VC6 cell bodies. C, Ventral views of the vulval region of a wild-type control (top panel) and an unc-115 mutant (bottom panel). The wild type shows processes that completely circle the vulva and that display varicosities at the sites of synaptic connections. The unc-115 mutant shown has breaks in these processes, which trail off laterally. The defects shown are representative of those seen at varying penetrance in six different hyperactive egg-laying mutants.
Using fluorescence microscopy, we saw frequent defects in the gross morphology of the VC neurons in six of the seven strains (Fig. 3). The defects fell into three major categories. First, although there were always the expected six brightly labeled cell bodies in the wild type (Fig. 3A, top panel), we noticed that in many Unc mutant animals fewer than six cell bodies could be seen. For example, the unc-5 mutant shown (Fig. 3A, bottom panel) had only four labeled VC cell bodies. Second, we found that the mutants frequently showed defects in the VC axonal processes. In the wild type, each VC extends processes to the vulva that form synapses onto other VC processes, the HSN processes, and the vulval muscles (White et al., 1986). The VC processes were visualized as a continuous line of fluorescence between the VC cell bodies (Fig. 3B, top panel). Gaps in this fluorescence, as illustrated in the unc-42 mutant shown (Fig. 3B, bottom panel), suggested that the hyperactive egg-laying mutants had defective VC processes and that the VCs were thus unable to signal properly. Finally, observing the vulval region under higher magnification revealed frequent aberrant VC synaptic connections in the mutants. The wild-type vulva is typically fully encircled in VC processes that make synapses onto the HSN neurons and the vm2 class of egg-laying muscles (Fig. 3C, top panel). In the hyperactive mutants the observed defects were varied, but frequently included gaps in the fluorescent processes approaching and encircling the vulva, as illustrated in the unc-115 mutant shown (Fig. 3C, bottom panel).
VC defects in the hyperactive mutants were highly penetrant. Most mutant animals had some morphological defects of the VC neurons. The mutant strains exhibiting the strongest hyperactive egg-laying behavior showed the most frequent and severe VC defects (Table 1). The exception was unc-4, the one mutant of the seven examined that did not show highly penetrant VC defects.
Table 1.
Morphological defects in VC neurons observed using VC::GFP transgenes
|
Genetic backgrounda |
Type of protein mutated |
Number of VC cell bodies per wormb |
Number of VC process gaps per wormb |
% Worms with normal VC processes at vulvab |
|---|---|---|---|---|
| Wild type | 6.0 ± 0 | 0.0 | 93 | |
| unc-34 | Transcription factor | 5.2 ± 0.3 | 0.1 ± 0.1 | 67 |
| unc-76 | PKC binding protein | 5.4 ± 0.2 | 0.3 ± 0.2 | 67 |
| unc-4 | Transcription factor | 6.0 ± 0.1 | 0.4 ± 0.2 | 93 |
| unc-5 | Netrin receptor | 5.7 ± 0.2 | 0.9 ± 0.2 | 47 |
| unc-75 | RNA-binding protein | 5.3 ± 0.2 | 1.1 ± 0.3 | 40 |
| unc-115 | Actin binding protein | 4.7 ± 0.3 | 1.1 ± 0.3 | 13 |
|
unc-42
|
Transcription factor
|
5.7 ± 0.2
|
0.7 ± 0.2
|
20
|
PKC, Protein kinase C.
Strains are listed in increasing order of egg-laying hyperactivity.
n ≥ 15 for analysis at each magnification. When indicated, errors represent SEM.
We also examined the structure of the VC neurons in the cha-1 mutant and as expected saw no gross morphologic defects (data not shown). This suggests that acetylcholine is not necessary for proper morphology of the VCs.
Our results suggested the possibility that physical disruption of the VC neurons, and thus disruption of their function, may be the cause of the hyperactive egg laying observed in the mutants. We hypothesized that the VC neurons provide a signal that inhibits egg laying, opposing the stimulation of egg laying provided by the HSN neurons. The existence of an inhibitory signal for egg laying had already been suggested by the fact that Gρo mutants are hyperactive for egg laying, and our hypothesis builds on this idea.
Ablation of VC neurons can cause hyperactive egg laying
We sought to determine whether defects specifically in the VC neurons cause hyperactive egg laying and thus could be the common basis of the hyperactive behavior in the six Unc mutants with defects in VC structure. These mutants are each known to have multiple classes of neurons with structural defects, and it is not clear whether there are neurons besides the VCs affected by all six. A laser microbeam can be used to ablate specific neurons in living animals without affecting other cells (Bargmann and Avery, 1995). Any defects seen in VC-ablated animals could thus be attributed directly to loss of the VCs.
We used the VC::GFP reporter described above (Fig. 3A) to visualize and thus conveniently ablate the VC neurons in the L4 larval stage, when the VC neurons begin to extend their axonal processes. Initially we ablated all six VC neurons because all six make synapses onto the egg-laying muscles, but these experiments repeatedly resulted in small adults that produced few eggs and therefore could not be analyzed for egg-laying behavior. Thus we chose to ablate just VC4 and VC5, the two neurons that directly flank the vulva and make the most extensive synapses onto the egg-laying muscles (White et al., 1986). Of 20 VC4/5 ablated animals, six were strongly hyperactive for egg laying, as defined by laying >50% early-stage eggs. Mock-ablated animals never exhibited strong hyperactive egg-laying behavior. The variable hyperactivity in VC4/5 ablated animals could result if the remaining four VC neurons were variably successful in making functional connections with the egg-laying muscles in the absence of VC4/5. Our results showed that loss of the VC neurons can result in the hyperactive egg-laying phenotype, but our inability to analyze animals ablated for all six VCs limits the interpretability of this experiment. It is unclear why the ablation of all six VC neurons had such dramatic impact on the adult morphology; either the VC neurons play a role in proper adult development or there was significant collateral damage caused by the ablations. Below we present a more definitive analysis of the role of the VC neurons in egg laying using transgenes expressed specifically in all six VCs to manipulate their ability to signal.
The VC neurons release acetylcholine to inhibit egg laying
Above we described mutations in six Unc genes that result in defects in the structure of VC neurons. We now turn to the remaining three of the nine strongly hyperactive egg-laying mutants from our panel: unc-4, cha-1, and unc-17. All three of these genes are required for acetylcholine signaling. The CHA-1 choline acetyltransferase synthesizes acetylcholine, and the UNC-17 vesicular acetylcholine transporter loads acetylcholine into synaptic vesicles (Alfonso et al., 1993, 1994). It was reported recently that mutants for the UNC-4 transcription factor have substantially reduced expression of CHA-1 and UNC-17 (Lickteig et al., 2001). UNC-4 functions in a complex with the Groucho-like transcription factor UNC-37 to regulate transcription (Winnier et al., 1999), and unc-37 mutants, like unc-4 mutants, show reduced expression of CHA-1 and UNC-17 (Lickteig et al., 2001). We assayed egg laying in an unc-37 partial loss-of-function mutant and observed a mild hyperactive egg-laying phenotype of 32% early-stage eggs laid. The UNC-4 complex thus appears to affect egg laying by regulating cha-1 and unc-17 gene expression. Our results show that acetylcholine acts to inhibit egg laying.
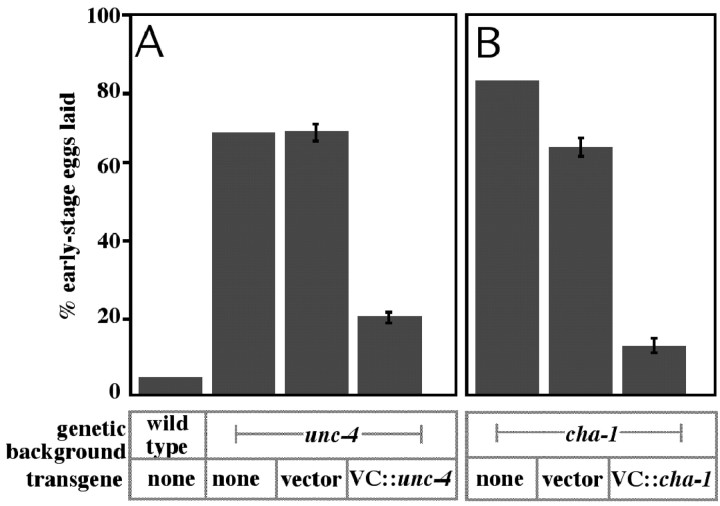
Because the VC neurons appear to inhibit egg laying and are cholinergic, we tested whether the VCs release acetylcholine to inhibit egg laying. The VCs are the only cells of the egg-laying system that express the UNC-4 complex, CHA-1, and UNC-17 (Lickteig et al., 2001); however, because unc-4, cha-1, and unc-17 are each expressed in other neurons, it was necessary to determine whether mutations in these genes cause hyperactive egg laying specifically attributable to their effects on the VC neurons. For this purpose, we expressed the unc-4, cha-1,or unc-17 cDNAs in the VC neurons and determined whether this rescued the hyperactive egg-laying defects of the corresponding mutants. To direct VC expression, we used a modified lin-11 promoter similar to that used to express GFP in Figure 3A (see Materials and Methods). Expression of the unc-4 cDNA using this promoter rescued the hyperactive egg-laying defect of unc-4 mutants, returning the percentage of early-stage eggs laid to near-wild-type levels (Fig. 4A). Furthermore, expressing the cha-1 cDNA in the VC neurons of cha-1 mutants also rescued their hyperactive egg-laying phenotype (Fig. 4B). Similar experiments with unc-17 gave analogous results (data not shown). Restoring the inhibition of egg laying by restoring the ability of the VC neurons to signal with acetylcholine provides our most compelling evidence that it is the VC neurons that inhibit egg laying.
Figure 4.
Effects of VC-expressed unc-4 or cha-1 cDNAs in unc-4 or cha-1 mutants, respectively. A vector containing a VC-expressed promoter derived from the lin-11 gene was used to generate constructs for VC expression of the unc-4 and cha-1 cDNAs (VC::unc-4 and VC::cha-1). A, Percentage early-stage eggs laid by unc-4 mutants and by transgenic strains carrying the vector or VC::unc-4 construct. B, Percentage early-stage eggs laid by cha-1 mutants and by transgenic strains carrying the vector or VC::cha-1 construct. The wild type is included in A for comparison. Five independent lines were analyzed for each transgene, and averages and SDs for the five lines are shown. VC expression of the unc-4 or cha-1 cDNAs rescued the hyperactive egg-laying defects of the corresponding mutants.
In summary, all nine strong hyperactive Unc mutants had either morphological or functional defects in their VC neurons. Our results argue that the release of acetylcholine from the VC neurons normally inhibits egg-laying behavior.
Increasing synaptic acetylcholine inhibits egg laying
If acetylcholine released from the VCs normally inhibits egg laying, then exogenously applied acetylcholine agonists might also inhibit egg laying. In opposition to this expectation, the reported action of the nicotinic acetylcholine agonists levamisole and nicotine is to stimulate egg laying (Trent et al., 1983; Weinshenker et al., 1995; Kim et al., 2001). We analyzed egg laying in wild-type worms placed on agar plates containing levamisole in the presence of bacterial food. Under these conditions both inhibition and stimulation of egg-laying rates can be observed. As we increased S the levels of levamisole in the agar, we saw an increased rate of egg laying (Fig. 5A), confirming the previous reports.
Figure 5.
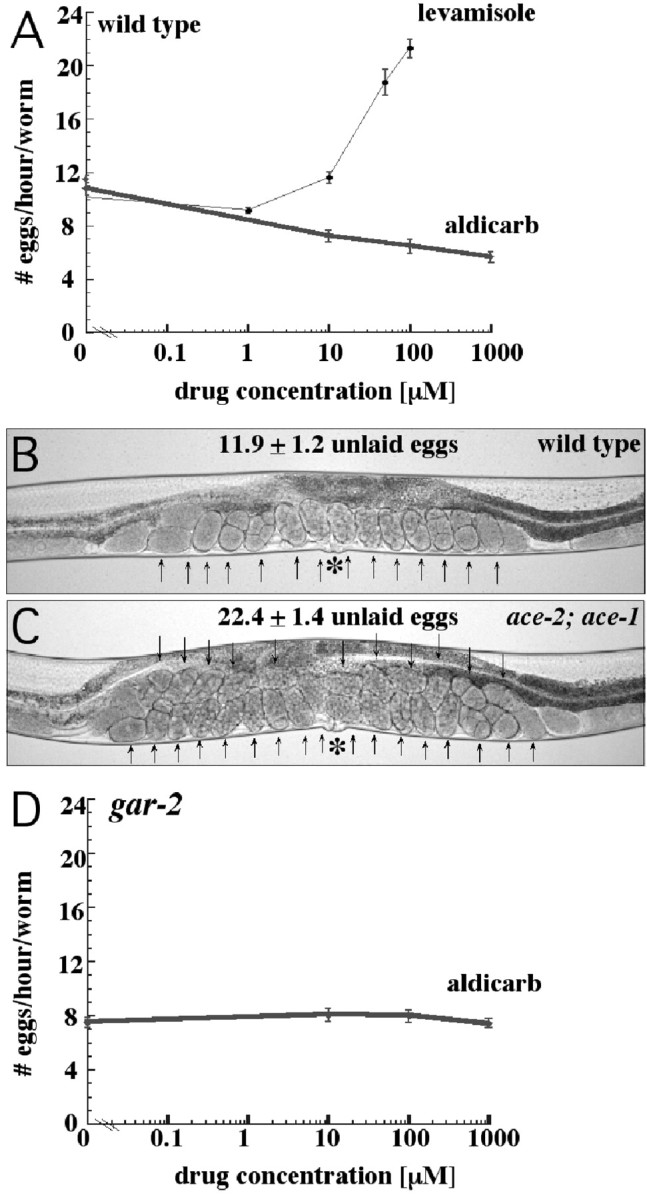
Effects of increasing synaptic acetylcholine on egg-laying behavior. A, Rates of egg-laying of wild-type worms on plates containing varying concentrations of levamisole and aldicarb. For each condition tested, the number of eggs laid in 1 hr by 10 adult animals was determined, and this was repeated 12 times. The data presented are the average and SE of the 12 trials. Levamisole stimulates nicotinic acetylcholine receptors, whereas aldicarb, by inhibiting acetylcholinesterase activity, amplifies all endogenous acetylcholine signaling. Levamisole stimulates egg-laying rates, whereas aldicarb slows the rate of egg laying. B, Wild-type adult hermaphrodite. C, ace-2; ace-1 double mutant, lacking function of two acetylcholinesterase genes and therefore lacking most acetylcholinesterase activity. Arrows indicate unlaid eggs; asterisks indicate the vulva. Average numbers of unlaid eggs are indicated above the animals. The ace-2; ace-1 mutant has higher endogenous levels of acetylcholine because acetylcholine is not efficiently removed from synapses. The mutant shows increased accumulation of unlaid eggs, indicating increased inhibition of egg laying. D, Rate of egg laying of gar-2 mutants on plates containing varying concentrations of aldicarb. In contrast to the wild type, the gar-2 mutant does not respond to aldicarb, suggesting that acetylcholine inhibits egg laying by signaling, at least in part, through the muscarinic GAR-2 receptor.
We hypothesized that acetylcholine might have opposing stimulatory and inhibitory roles in egg laying by acting through different types of receptors. Acetylcholine signals through two types of receptors, nicotinic and muscarinic. Nicotinic receptors are ligand-gated channels that open in response to acetylcholine binding (Clementi et al., 2000), and levamisole specifically activates nicotinic receptors (Lewis et al., 1980). Muscarinic receptors are G-protein-coupled receptors that initiate a G-protein-mediated signal transduction pathway after acetylcholine binding (Felder, 1995). Because egg laying is under the regulation of two antagonistic G-protein signaling pathways, muscarinic acetylcholine receptors could function in these pathways. Therefore, we investigated the role that the muscarinic receptors play in regulating egg-laying behavior.
C. elegans has three known muscarinic receptor homologs, but two of the three do not respond to the agonists or antagonists that affect vertebrate muscarinic receptors, and the third does so only weakly (Hwang et al., 1999; Lee et al., 1999, 2000). In the absence of available drugs to specifically stimulate C. elegans muscarinic receptors, we turned to the acetylcholinesterase inhibitor aldicarb, which potentiates signaling through both muscarinic and nicotinic receptors (Brenner 1974). Acetylcholinesterase acts to clear acetylcholine released in synaptic clefts, thus ensuring that signaling is rapidly terminated. Aldicarb, by inhibiting acetylcholinesterase activity, causes acetylcholine to accumulate in synapses and allows us to investigate the effects of increasing all acetylcholine signaling.
We exposed animals to plates containing increasing doses of aldicarb and assayed egg laying in the same manner as in the levamisole-response experiments. We observed that increasing aldicarb caused decreasing rates of egg laying (Fig. 5A). The aldicarb-induced inhibition of egg laying demonstrates that the overall effect of acetylcholine signaling is to inhibit egg laying. It is possible that this inhibition is caused by muscarinic signaling that overrides the demonstrated nicotinic stimulation of egg laying.
We saw similar results by using mutations rather than aldicarb to decrease acetylcholinesterase activity. There are four acetylcholinesterase genes in C. elegans, and loss of three is lethal (Johnson et al., 1988; Combes et al., 2000); however, animals mutant for the genes that encode the two major acetylcholinesterases, ace-1 and ace-2, are mildly egg-laying defective (Fig. 5B,C). This suggests that a buildup of acetylcholine at synapses can inhibit egg laying.
GAR-2 is one of the three C. elegans muscarinic acetylcholine receptors and is expressed on the HSNs (Lee et al., 2000). GAR-2 is the only identified muscarinic acetylcholine receptor known to be expressed in any cell of the egg-laying system (Lee et al., 2000); therefore, GAR-2 could be a receptor mediating the inhibitory effects of acetylcholine on egg laying. To test this idea, we obtained a mutant carrying a deletion in the gar-2 gene that removes the last third of the gene. The resulting mutant protein lacks most of the large intracellular loop thought to interact with G-proteins and is missing the last two of the predicted seven transmembrane domains. Therefore, this gar-2 deletion is likely to be a strong reduction-of-function or null mutation.
We found that the gar-2 mutants were mildly hyperactive for egg laying. In the early-stage egg assay, these mutants laid 30% of their eggs at an early developmental stage, showing that gar-2 mutants are impaired in their ability to inhibit egg laying. This hyperactive phenotype was mild compared with that seen in mutants lacking acetylcholine signaling (e.g., cha-1), however, suggesting that not all of the inhibitory effects of acetylcholine on egg laying are the result of GAR-2 signaling.
We also placed the gar-2 mutants on aldicarb plates, hypothesizing that if acetylcholine inhibits egg laying by signaling through GAR-2, then the gar-2 mutants should fail to inhibit egg laying in response to aldicarb. This in fact is what we observed; despite increasing concentrations of aldicarb, the rate of egg laying in gar-2 mutants was unchanged (Fig. 5D). This is not because of an inability to respond to exogenous drugs, because the animals were still stimulated to lay eggs by levamisole (data not shown). The failure of gar-2 mutants to inhibit egg laying in response to aldicarb and the hyperactive egg-laying phenotype of gar-2 mutants both suggest that GAR-2 mediates some of the inhibitory effects of acetylcholine on egg laying.
Acetylcholine released by the VCs may inhibit egg laying by inhibiting HSN function
The fact that GAR-2 is expressed in the HSN suggests that acetylcholine may act on the HSN to inhibit egg laying. Because the HSN stimulates egg laying, acetylcholine would have to act by inhibiting HSN function (Fig. 6A). This model predicts that loss of acetylcholine would have no effect on egg laying in animals lacking HSNs. In egl-1 mutants the HSNs undergo aberrant cell death (Desai et al., 1988). As a result, these worms are unable to stimulate egg laying and retain a large number of eggs (Fig. 6C). We tested whether the unc-4 and cha-1 mutations, which lead to a failure of acetylcholine signaling from the VCs, have effects on egg laying in the egl-1 mutant background.
Figure 6.
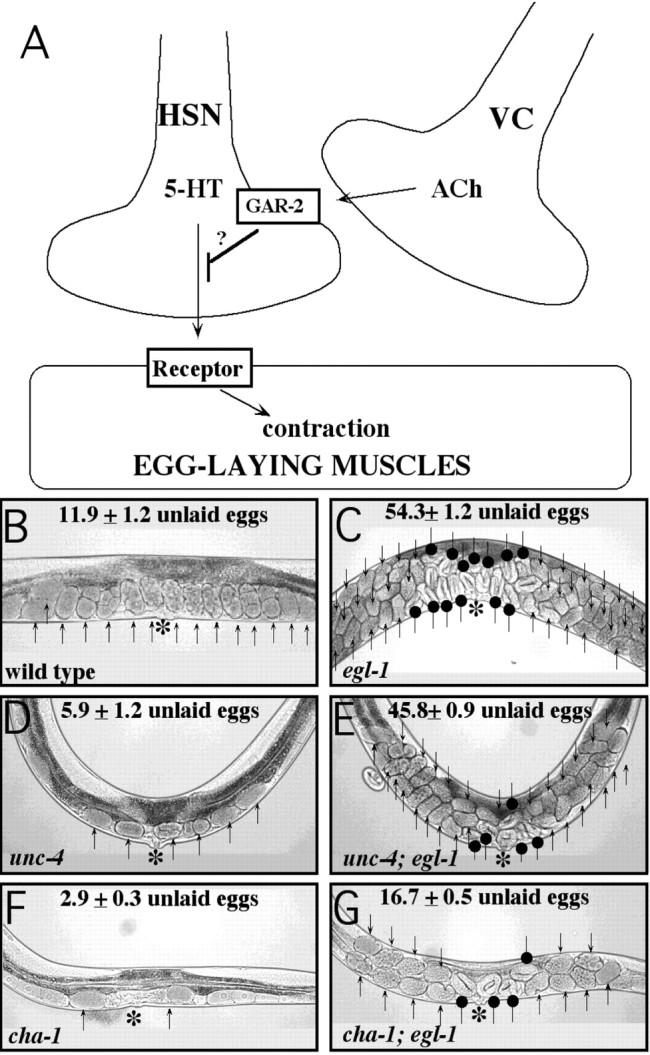
Effects of loss of acetylcholine in animals with and without HSNs. A, One model for the inhibition of egg laying by VC-derived acetylcholine. In this model, acetylcholine acts on the HSN through the GAR-2 receptor to inhibit release of neurotransmitters, including serotonin (5-HT). B, Wild-type adult hermaphrodite. C, Gain-of-function egl-1 mutant. The egl-1 mutant lacks the HSN neurons; it cannot stimulate egg-laying and retains a large number of eggs. D, Null unc-4 mutant. The unc-4 mutant is defective for synthesis and packaging acetylcholine in its VC neurons. E, unc-4; egl-1 double mutant. The hyperactive unc-4 single mutant retained very few eggs, whereas the unc-4; egl-1 double mutant was extremely defective in egg laying. F, Reduction-of-function cha-1 mutant, containing <1% normal acetylcholine levels. G, cha-1; egl-1 double mutant. The hyperactive cha-1 single mutant retained very few eggs, whereas the cha-1; egl-1 double mutant retains five times as many eggs, including eggs that are of a late developmental stage. Arrows indicate unlaid eggs, asterisks indicate the vulva, and round-tipped arrows indicate late-stage eggs. Average numbers of unlaid eggs are indicated for each strain. These results show that animals fail to lay eggs in the absence of HSNs, regardless of the presence or absence of acetylcholine.
Our results showed egl-1 is epistatic to unc-4 or cha-1; that is, the double-mutant animals showed the egg-laying defective phenotype of egl-1 single mutants, not the hyperactive egg-laying phenotype of unc-4 or cha-1 single mutants. The unc-4; egl-1 double mutants were similar to egl-1 mutants (Fig. 6C,E). They accumulated a large number of unlaid eggs, demonstrating an inability to lay eggs. Analysis of cha-1 is more complex because cha-1 mutations cause relatively few eggs to be produced (brood size is 135 ± 13 compared with ∼300 for the wild type) and thus do not generate enough eggs to accumulate them in large numbers; however, the cha-1; egl-1 double mutants did accumulate five to six times as many unlaid eggs as did cha-1 single mutants (Fig. 6F,G). Furthermore, the cha-1; egl-1 double mutant retained late-stage eggs (Fig. 6G, round-tipped arrows). These eggs were at least 7.5 hr old, as judged by the “twofold” morphology of the larvae developing within them (Wood, 1988). The accumulation of late-stage eggs also occurred in egl-1 single mutants (Fig. 6C) as well as unc-4; egl-1 double mutants (Fig. 6E) and was an indicator of the severe egg-laying defects in these strains. In contrast, unc-4 and cha-1 single mutants laid early-stage eggs within 2 hr of fertilization, and the few unlaid eggs that they retained were never of a late stage (Fig. 6D,F).
These experiments distinguish between alternative models for the relationship between the HSN and VC neurons in regulating egg-laying behavior. The results are consistent with the model shown in Figure 6A in which acetylcholine inhibits egg laying by acting on the HSNs to inhibit neurotransmitter release. These results cannot rule out a second possibility, in which the HSN and VC neurons both signal in parallel onto the egg-laying muscles, with the HSNs causing contraction and the VCs causing relaxation. They do, however, rule out a third model in which the VCs release acetylcholine onto the egg-laying muscles to relax them, and the role of the HSNs is to signal onto the VCs to inhibit acetylcholine release.
Discussion
Several lines of evidence presented in this paper lead to the conclusion that acetylcholine released by the VC neurons inhibits egg-laying behavior. First, six Unc mutants hyperactive for egg-laying behavior exhibited defects in the structure of the VC neurons. Second, ablation of VC4 and VC5 led to strongly hyperactive egg laying in some animals. Third, mutants that cannot properly synthesize (cha-1) or package acetylcholine into synaptic vesicles (unc-17) were strongly hyperactive for egg laying. In addition, mutants lacking the homeodomain transcription factor unc-4, which promotes the expression of both unc-17 and cha-1 in the VC neurons, were strongly hyperactive for egg laying. Fourth, expression of cDNAs for cha-1, unc-17, or unc-4 in the VC neurons rescued the egg-laying defects in the corresponding mutants. Fifth, we observed a decrease in egg laying when synaptic acetylcholine was increased, either by using the acetylcholinesterase inhibitor aldicarb or by using mutant animals deficient for acetylcholinesterases.
Inhibition is the predominant effect of acetylcholine on egg laying
Although the above results demonstrate that acetylcholine inhibits egg laying, one observation suggests that acetylcholine might also stimulate egg laying: the nicotinic acetylcholine agonist levamisole stimulates egg laying. Kim et al. (2001) showed that this effect requires the ion channel that serves as the levamisole receptor; however, levamisole receptor mutants are not appreciably defective in egg laying (Waggoner et al., 2000a; Kim et al., 2001), demonstrating that stimulation of this receptor is sufficient but not necessary for egg laying. In fact, nicotinic stimulation of egg laying could have little physiological significance. The fact that reducing all acetylcholine signaling (with unc-17 or cha-1 mutations) leads to hyperactive egg laying, and increasing all acetylcholine signaling (with aldicarb or acetylcholinesterase mutations) decreases egg laying, demonstrates that the overriding effect of acetylcholine signaling is to inhibit egg laying. Therefore, any stimulation of egg laying through nicotinic receptors is at most a secondary effect.
This work is the first to suggest that acetylcholine or the VC neurons inhibit egg laying. Earlier literature focused on the possibility that the normal physiological role of acetylcholine was to stimulate egg laying, as suggested by the effects of levamisole (Weinshenker et al., 1995; Waggoner et al., 1998; Kim et al., 2001). In our experiments, we detected the extreme hyperactive egg-laying phenotype of animals lacking acetylcholine or VC neuron function using the early-stage egg assay. Previously, other egg laying assays were used that could not easily detect this phenotype. For example, egg laying was measured when animals were placed in liquid medium, a condition that strongly inhibits egg laying. Thus only stimulation, not inhibition, of egg laying could be detected (Trent et al., 1983; Weinshenker et al., 1995). A second assay measured the temporal pattern of egg release by animals on agar plates, their normal laboratory culture conditions (Waggoner et al., 1998). Although an important advance, use of this assay presupposes that the animals being measured contain eggs to lay. Because mutants extremely hyperactive for egg laying retain very few eggs, the pattern of egg release in these animals is probably limited by the rate of egg production rather than by egg-laying behavior. For example, the goa-1(n1134) mutant shows an extreme hyperactive egg-laying phenotype by the early-stage egg assay (Fig. 1), but shows only modest and complex effects on the pattern of egg release (Waggoner et al., 2000b). In addition, the strong hyperactivity that we observed in a subset of VC-ablated animals was not evident when similar animals were analyzed for their pattern of egg release (Waggoner et al., 1998; Kim et al., 2001). Through the use of the early-stage egg assay we have been able to recognize clearly for the first time the hyperactive egg-laying phenotype of animals lacking acetylcholine or VC function.
VC-derived acetylcholine may inhibit egg laying by inhibiting neurotransmitter release from the HSN presynaptic terminals
Having shown that the VC neurons release acetylcholine to inhibit egg laying, we can formulate a model that places the genetically defined Gρo and Gρq signaling pathways into the physical context of the cells and signals that regulate the egg-laying system. Extensive genetic analysis has shown Gρo signaling inhibits egg laying, whereas Gρq signaling stimulates egg laying (Wilkie, 2000). A major weakness underlying this work is that the cells and neurotransmitters carrying out Gρo and Gρq signaling in the egg-laying system have not been identified.
We propose a model in which the VC neurons release acetylcholine onto the presynaptic terminals of the HSN neurons, signaling through muscarinic acetylcholine receptors, including GAR-2, to inhibit HSN function (Fig. 6A). These receptors would activate Gρo signaling in the HSNs to inhibit neurotransmitter release, the same effect that Gρo has been shown to have on other C. elegans motor neurons. The HSNs release serotonin and other neurotransmitters onto the egg-laying muscles to stimulate their contraction and thus egg laying. Therefore inhibition of HSN function by the VCs, as proposed in our model, would inhibit egg laying. Acetylcholine acts on presynaptic terminals in vertebrate sympathetic neurons to inhibit neurotransmitter release (Boehm and Kubista, 2002), and our model suggests that it plays a similar role in the C. elegans egg-laying system.
The expression patterns and known functions of GAR-2 and Gρo are consistent with our model. Both GAR-2 and the Gρo protein GOA-1 are expressed in HSNs (Mendel et al., 1995; Sègalat et al., 1995; Lee et al., 2000), and gar-2 and goa-1 mutations both cause hyperactive egg laying. GOA-1 is expressed in all neurons, and previous studies have shown that it acts in presynaptic terminals of ventral cord motor neurons to inhibit neurotransmitter release (Nurrish et al., 1999). Genetic analysis suggests that GOA-1 signaling results in decreased diacylglycerol levels (Miller et al., 1999; Nurrish et al., 1999), which in turn decrease recruitment of the diacylglycerol-binding protein UNC-13 to sites of synaptic vesicle release (Nurrish et al., 1999). UNC-13 is essential for priming synaptic vesicles for exocytic release (Brose et al., 2000), and therefore by inhibiting UNC-13 recruitment, Gρo signaling inhibits neurotransmitter release. In our model, we simply suggest that Gρo functions in HSNs in the same way it that functions in ventral cord motor neurons.
The gar-2 mutant showed a hyperactive egg-laying phenotype and failed to inhibit egg laying in response to the acetylcholinesterase inhibitor aldicarb. This is consistent with our model, which suggests that GAR-2 mediates inhibitory effects of acetylcholine on HSN function. We note, however, that GAR-2 cannot fully account for these effects: the hyperactivity observed in the gar-2 mutant was not as strong as that seen in mutants (cha-1, unc-17) defective for acetylcholine signaling. One possibility is that other muscarinic acetylcholine receptors function in parallel to GAR-2 in the HSNs to mediate acetylcholine signaling. C. elegans has at least two other muscarinic receptors (Hwang et al., 1999; Lee et al., 1999, 2000), and their expression patterns and functions have not been fully investigated.
The anatomy of the egg-laying system is consistent with our model, because the VC neurons synapse onto the HSN processes (White et al., 1986). The VCs also make synapses onto the egg-laying muscles. These occur near the sites of HSN synapses onto the same muscles, and it is possible that acetylcholine released by the VCs at neuromuscular junctions acts, through diffusion, on the nearby HSN presynaptic terminals. This type of “heterosynaptic” signaling is a widespread signaling mechanism in other species (Miller, 1998) but has not been studied in C. elegans.
Our model provides a mechanism for inhibition of egg laying by VC-derived acetylcholine but does not attempt to explain other signaling that also occurs in the egg-laying system. Because the VCs synapse directly onto the egg-laying muscles, acetylcholine could act directly on the muscles to relax them. Such an effect would be unprecedented; acetylcholine typically acts on muscle through nicotinic receptors to cause contraction. As noted above, such nicotinic signaling could occur but be subordinate to the inhibitory effects of acetylcholine on egg laying. HSN signaling also remains to be fully explained. The HSNs release serotonin onto the egg-laying muscles to stimulate their contraction. Less understood are the functional consequences of the fact that the HSNs contain other neurotransmitters, including acetylcholine, and that they also synapse onto the VCs (Duerr et al., 2001). Finally, the cells and signals responsible for the Gρq signaling that stimulates egg laying remain unidentified. One possibility is that unidentified signal(s) acts on the HSN presynaptic terminals to activate Gρq. The C. elegans Gρq protein EGL-30 is expressed in all neurons, including the HSNs, and by inducing production of diacylglycerol can directly oppose the inhibitory effect of Gρo signaling on neurotransmitter release (Lackner et al., 1999).
We now have a model that defines roles for some of the neurotransmitters and cells that regulate egg-laying behavior. Testing and refining this model will allow the detailed understanding of one specific behavior that may serve as a model for understanding presynaptic inhibition and for understanding the mechanisms used by the opposing Gρo and Gρq signaling pathways to control neural activity.
Footnotes
This work was supported by National Institutes of Health (NIH) Grant NS36918 and a Leukemia and Lymphoma Society Scholar Award (M.R.K.). We thank Diana Mandelker for help with VC-specific promoters, Michael Stern for helpful discussions and advice on laser ablations, Valerie Reinke for C. elegans mRNA, David Miller for the unc-4 (wd1) mutant, the Oklahoma and Vancouver groups of the C. elegans Gene Knockout Consortium for the gar-2(ok520) mutant, and the Caenorhabditis Genetics Center, which is supported by the NIH National Center for Research Resources, for additional strains.
Correspondence should be addressed to Dr. Michael Koelle, Yale University School of Medicine, Department of Molecular Biophysics and Biochemistry, 333 Cedar Street, SHM CE30, New Haven, CT 06520-8024. E-mail:michael.koelle@yale.edu.
M.-Q. Dong's present address: Department of Cellular and Molecular Medicine, University of California, San Diego, La Jolla, CA 92093.
Copyright © 2003 Society for Neuroscience 0270-6474/03/238060-10$15.00/0
References
- Alfonso A, Grundahl K, Duerr JS, Han HP, Rand JB ( 1993) The Caenorhabditis elegans unc-17 gene: a putative vesicular acetylcholine transporter. Science 261: 617-619. [DOI] [PubMed] [Google Scholar]
- Alfonso A, Grundahl K, McManus JR, Rand JB ( 1994) Cloning and characterization of the choline acetyltransferase structural gene (cha-1) from C. elegans. J Neurosci 14: 2290-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran R, Aronoff R, Garriga G ( 1999) The C. elegans homeodomain gene unc-42 regulates chemosensory and glutamate receptor expression. Development 126: 2241-2251. [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Avery L ( 1995) Laser killing of cell in Caenorhabditis elegans. In: Methods in cell biology, Vol 48, Caenorhabditis elegans: modern biological analysis of an organism (Epstein HF, Shakes DC, eds), pp 225-250. New York: Academic. [DOI] [PMC free article] [PubMed]
- Bloom L, Horvitz HR ( 1997) The Caenorhabditis elegans gene unc-76 and its human homologs define a new gene family involved in axonal outgrowth and fasciculation. Proc Natl Acad Sci USA 94: 3414-3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm S, Kubista H ( 2002) Fine tuning of sympathetic transmitter release via ionotropic and metabotropic presynaptic receptors. Pharmacol Rev 54: 43-99. [DOI] [PubMed] [Google Scholar]
- Brenner S ( 1974) The genetics of Caenorhabditis elegans. Genetics 77: 71-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose N, Rosenmund C, Rettig J ( 2000) Regulation of transmitter release by Unc-13 and its homologues. Curr Opin Neurobiol 10: 303-311. [DOI] [PubMed] [Google Scholar]
- Brundage L, Avery L, Katz A, Kim UJ, Mendel JE, Sternberg PW, Simon MI ( 1996) Mutations in a C. elegans Gqalpha gene disrupt movement, egg laying, and viability. Neuron 16: 999-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron S, Clark SG, McDermott JB, Aamodt E, Horvitz HR ( 2002) PAG-3, a Zn-finger transcription factor, determines neuroblast fate in C. elegans. Development 129: 1763-1774. [DOI] [PubMed] [Google Scholar]
- Clementi F, Fornasari D, Gotti C ( 2000) Neuronal nicotinic receptors, important new players in brain function. Eur J Pharmacol 393: 3-10. [DOI] [PubMed] [Google Scholar]
- Colavita A, Culotti JG ( 1998) Suppressors of ectopic UNC-5 growth cone steering identify eight genes involved in axon guidance in Caenorhabditis elegans. Dev Biol 194: 72-85. [DOI] [PubMed] [Google Scholar]
- Combes D, Fedon Y, Grauso M, Toutant J-P, Arpagaus M ( 2000) Four genes encode acetylcholinesterases in the nematodes Caenorhabditis elegans and Caenorhabditis briggsae. cDNA sequences, genomic structures, mutations and in vivo expression. J Mol Biol 300: 727-742. [DOI] [PubMed] [Google Scholar]
- Desai C, Garriga G, McIntire S, Horvitz HR ( 1988) A genetic pathway for the development of the Caenorhabditis elegans HSN motor neurons. Nature 336: 638-646. [DOI] [PubMed] [Google Scholar]
- Duerr JS, Gaskin J, Rand JB ( 2001) Identified neurons in C. elegans coex-press vesicular transporters for acetylcholine and monoamines. Am J Physiol Cell Physiol 280: C1616-C1622. [DOI] [PubMed] [Google Scholar]
- Dong MQ, Chase D, Patikoglou GA, Koelle MR ( 2000) Multiple RGS proteins alter neural G protein signaling to allow C. elegans to rapidly change behavior when fed. Genes Dev 14: 2003-2014. [PMC free article] [PubMed] [Google Scholar]
- Felder CC ( 1995) Muscarinic acetylcholine receptors: signal transduction through multiple effectors. FASEB J 9: 619-625. [PubMed] [Google Scholar]
- Gitai Z, Yu TW, Lundquist EA, Tessier-Levine M, Bargmann CI ( 2003) The Netrin receptor UNC-40/DCC stimulates axon attraction and outgrowth through Enabled and, in parallel, Rac and UNC-115/AbLIM. Neuron 37: 53-65. [DOI] [PubMed] [Google Scholar]
- Hajdu-Cronin YM, Chen WJ, Patikoglou G, Koelle MR, Sternberg PW ( 1999) Antagonism between Goρ and Gqρ in Caenorhabditis elegans: the RGS protein EAT-16 is necessary for Goρ signaling and regulates Gqρ activity. Genes Dev 13: 1780-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JM, Chang DJ, Kim US, Lee Y-S, Park Y-S, Kaang B-K, Cho NJ ( 1999) Cloning and functional characterization of a Caenorhabditis elegans muscarinic acetylcholine receptor. Receptors Channels 6: 415-424. [PubMed] [Google Scholar]
- Johnson CD, Rand JB, Herman RK, Stern BD, Russell RL ( 1988) The acetylcholinesterase genes of C. elegans: identification of a third gene (ace-3) and mosaic mapping of a synthetic lethal phenotype. Neuron 1: 165-173. [DOI] [PubMed] [Google Scholar]
- Kim J, Poole DS, Waggoner LE, Kempf A, Ramirez DS, Treschow PA, Schafer WR ( 2001) Genes affecting the activity of nicotinic receptors involved in Caenorhabditis elegans egg-laying behavior. Genetics 157: 1599-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelle MR, Horvitz HR ( 1996) EGL-10 regulates G protein signaling in the C. elegans nervous system and shares a conserved domain with many mammalian proteins. Cell 84: 115-125. [DOI] [PubMed] [Google Scholar]
- Kuroda S, Nakagawa N, Tokunaga C, Tatematsu K, Tanizawa K ( 1999) Mammalian homologue of the Caenorhabditis elegans UNC-76 protein involved in axonal outgrowth is a protein kinase Cζ-interacting protein. J Cell Biol 144: 403-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner MR, Nurrish SJ, Kaplan JM ( 1999) Facilitation of synaptic transmission by EGL-30 Gqρ and EGL-8 PLCβ: DAG binding to UNC-13 is required to stimulate acetylcholine release. Neuron 24: 335-346. [DOI] [PubMed] [Google Scholar]
- Lee Y-S, Park Y-S, Chang DJ, Hwang JM, Min CK, Kaang B-K, Cho NJ ( 1999) Cloning and expression of a G protein-linked acetylcholine receptor from Caenorhabditis elegans. J Neurochem 72: 58-65. [DOI] [PubMed] [Google Scholar]
- Lee Y-S, Park Y-S, Nam S, Suh S, Lee J, Kaang B-K, Cho NJ ( 2000) Characterization of GAR-2, a novel G protein-linked acetylcholine receptor from Caenorhabditis elegans. J Neurochem 75: 1800-1809. [DOI] [PubMed] [Google Scholar]
- Lewis JA, Wu C-H, Levine JH, Berg H ( 1980) Levamisole-resistant mutants of the nematode Caenorhabditis elegans appear to lack pharmacological acetylcholine receptors. Neuroscience 5: 967-989. [DOI] [PubMed] [Google Scholar]
- Lickteig KM, Duerr JS, Frisby DL, Hall DH, Rand JB, Miller III DM Regulation of neurotransmitter vesicles by the homeodomain protein UNC-4 and its transcriptional corepressor UNC-37/groucho in Caenorhabditis elegans cholinergic motor neurons. J Neurosci 21: 2001-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist EA, Herman RK, Shaw JE, Bargmann CI ( 1998) UNC-115, a conserved protein with predicted LIM and actin-binding domains, mediates axon guidance in C. elegans. Neuron 21: 385-392. [DOI] [PubMed] [Google Scholar]
- Mendel JE, Korswagen HC, Liu KS, Hajdu-Cronin YM, Simon MI, Plasterk RHA, Sternberg PW ( 1995) Participation of the protein Go in multiple aspects of behavior in C. elegans. Science 267: 1652-1655. [DOI] [PubMed] [Google Scholar]
- Miller KG, Alfonso A, Nguyen M, Crowell JA, Johnson CD, Rand JB ( 1996) A genetic selection for Caenorhabditis elegans synaptic transmission mutants. Proc Natl Acad Sci USA 93: 12593-12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KG, Emerson MD, Rand JB ( 1999) Goalpha and diacylglycerol kinase negatively regulate the Gqalpha pathway in C. elegans. Neuron 24: 323-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RJ ( 1998) Presynaptic receptors. Annu Rev Pharmacol Toxicol 38: 201-227. [DOI] [PubMed] [Google Scholar]
- Nurrish S, Sègalat L, Kaplan JM ( 1999) Serotonin inhibition of synaptic transmission: Gρo decreases the abundance of UNC-13 at release sites. Neuron 24: 231-242. [DOI] [PubMed] [Google Scholar]
- Rand JB, Russell RL ( 1984) Choline acetyltransferase-deficient mutants of the nematode Caenorhabditis elegans. Genetics 106: 227-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle DL, Blumenthal T, Meyer BJ, Priess JR ( 1997) C. elegans II. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory. [PubMed]
- Sègalat L, Elkes DA, Kaplan JM ( 1995) Modulation of serotonin-controlled behaviors by Go in Caenorhabditis elegans. Science 267: 1648-1651. [DOI] [PubMed] [Google Scholar]
- Trent C, Tsung N, Horvitz HR ( 1983) Egg-laying defective mutants of the nematode Caenorhabditis elegans. Genetics 104: 619-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner LE, Zhou GT, Schafer RW, Schafer WR ( 1998) Control of alternative behavioral states by serotonin in Caenorhabditis elegans. Neuron 21: 203-214. [DOI] [PubMed] [Google Scholar]
- Waggoner LE, Dickinson KA, Poole DS, Tabuse Y, Miwa J, Schafer WR ( 2000a) Long-term nicotine adaptation in Caenorhabditis elegans involves PKC-dependent changes in nicotinic receptor abundance. J Neurosci 20: 8802-8811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner LE, Hardaker LA, Golik S, Schafer WR ( 2000b) Effect of a neuropeptide gene on behavioral states in Caenorhabditis elegans egg-laying. Genetics 154: 1181-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker D, Garriga G, Thomas JH ( 1995) Genetic and pharmacological analysis of neurotransmitters controlling egg laying in C. elegans J Neurosci 15: 6975-6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S ( 1986) The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci 314: 1-340. [DOI] [PubMed] [Google Scholar]
- Wightman B, Baran R, Garriga G ( 1997) Genes that guide growth cones along the C. elegans ventral nerve cord. Development 124: 2571-2580. [DOI] [PubMed] [Google Scholar]
- Wilkie TM ( 2000) G-protein signaling: Satisfying the basic necessities of life. Curr Biol 10: R853-R856. [DOI] [PubMed] [Google Scholar]
- Winnier AR, Meir JY-J, Ross J, Tavernarakis N, Driscoll M, Ishihara I, Miller III DM ( 1999) UNC-4/UNC-37-dependent repression of motor neuron-specific genes controls synaptic choice in Caenorhabditis elegans. Genes Dev 13: 2774-2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood WB ( 1988) Embryology. In: The nematode Caenorhabditis elegans (Wood WB, ed), pp 215-241.Cold Spring Harbor, NY: Cold Spring Harbor Laboratory.