Abstract
Netrin-1 is a bifunctional guidance cue that directs migrating neurons and axons based on specific receptors expressed on the cell surface. Attraction occurs through the receptor Deleted in Colorectal Cancer (DCC) and repulsion occurs through a receptor complex of DCC and UNC5H, the vertebrate homolog to Caenorhabditis elegans UNC-5, but how the specific surface expression of these receptors is achieved remains unknown. Here, we demonstrate that surface expression of UNC5H1 is regulated in neurons by protein interacting with C kinase-1 (PICK1) and protein kinase C (PKC), and show that one mechanism by which cells control their response to netrin-1 is by changing the surface availability of receptors. We identified PICK1 as a binding partner for UNC5H1 using the yeast two-hybrid system and found that the extreme three C-terminal amino acids of UNC5H1 interact with the PSD-95/Dlg/ZO-1 (PDZ) domain of PICK1. Coexpression of UNC5H1 and PICK1 in heterologous cells results in the recruitment of PICK1 to UNC5H1 clusters. Endogenous UNC5H1 and PICK1 coimmunoprecipitate from extracts of cultured hippocampal neurons and P4 cortices, and immunohistochemistry shows that UNC5H1, PICK1, and PKC are all present in growth cones. PKC activation induces the formation of UNC5H1/PICK1/PKC complexes and leads to the specific removal of UNC5H1, but not DCC, from the surface of neurons and growth cones via a PICK1/PKC-dependent mechanism. Lastly, we demonstrate that activating PKC, which decreases surface expression of UNC5H1, inhibits netrin-1-dependent collapse of hippocampal growth cones. Together, our results suggest that by regulating the surface expression of UNC5Hs, an axon can modulate its repellent response to netrin-1.
Keywords: UNC5H, netrin, PICK1, PKC, axon guidance, receptor trafficking
Introduction
In the developing nervous system, cells and axons respond to a variety of attractive and repellent guidance cues en route to their final destination. Often, they follow a complicated trajectory using multiple cues. As a result, some cells switch responsiveness to a particular cue or respond to new cues in the environment (Bloch-Gallego et al., 1999; Alcantara et al., 2000; Finger et al., 2002; Shewan et al., 2002). Studies show that concentration changes in global signaling molecules, like cAMP and calcium, affect the response of a cell to guidance molecules (for review, see Song and Poo, 1999; Petersen and Cancela, 2000). However, little is known about receptor-mediated mechanisms controlling this process at the cell surface.
The secreted molecule netrin-1 is a guidance cue capable of bidirectional signaling. Netrin-1 promotes attraction of neurons and axons through the receptor Deleted in Colorectal Cancer (DCC) (Keino-Masu et al., 1996) and repulsion through a receptor complex of DCC and UNC5Hs, the vertebrate homolog to Caenorhabditis elegans UNC-5 (Hong et al., 1999). The vertebrate UNC5Hs (UNC5H1, 2, 3, and 4) (Ackerman et al., 1997; Leonardo et al., 1997; Engelkamp, 2002), the C. elegans UNC-5 (Hedgecock et al., 1990; Leung-Hagesteijn et al., 1992), and Drosophila UNC5 (Keleman and Dickson, 2001) are members of the Ig superfamily of transmembrane receptors that directly bind netrin-1. In addition to their role in netrin-1-dependent axon guidance, the UNC5Hs also mediate netrin-1-independent apoptosis (Llambi et al., 2001; Tanikawa et al., 2003; Williams et al., 2003). Currently, little is known about the downstream molecules that bind UNC5Hs and affect their signal transduction (Tong et al., 2001; Williams et al., 2003).
Protein interacting with C kinase-1 (PICK1) was initially identified by its interaction with the catalytic subunit of protein kinase Cα (PKCα) (Staudinger et al., 1995). It contains a coiled-coil domain that mediates homotypic dimerization and a PDZ domain that mediates its interaction with the C termini of proteins (Staudinger et al., 1995, 1997). PICK1 is best characterized for its role in clustering and trafficking the AMPA receptor during synaptic plasticity (Xia et al., 1999; Chung et al., 2000; Daw et al., 2000; Perez et al., 2001; Hirbec et al., 2003). It is thought that PICK1 facilitates AMPA-receptor phosphorylation by bringing it in close proximity to PKC (Xia et al., 1999; Chung et al., 2000). Phosphorylation of the AMPA receptor by PKC rapidly changes its subcellular localization, which affects its ability to function at synapses (Chung et al., 2000; Daw et al., 2000). These and other studies on PICK1 suggest that it functions as a targeting and transport protein, directing the activated form of PKC to receptors.
Here, we show that the netrin-1 receptor UNC5H1 associates with PICK1 and PKC. Furthermore, PKC stimulation promotes the removal of UNC5H1 from the cell surface of hippocampal neurons and growth cones, and decreases the ability of netrin-1 to induce the collapse of growth cones. The discovery that surface levels of UNC5H1 are regulated via PICK1 and PKC suggests that one mechanism by which cells and axons alter their response to guidance cues is by modulating receptor availability at the cell surface.
Materials and Methods
Constructs and reagents. The original full-length, rat Unc5h1, Unc5h2, and Unc5h3 constructs in pSecTagB (Invitrogen, San Diego, CA) (Leonardo et al., 1997; Hong et al., 1999; Williams et al., 2003) were modified by PCR cloning to move the Myc tag from the C terminus to the N terminus of the UNC5Hs. Full-length PICK1 was isolated in the yeast two-hybrid screen and cloned into pcDNA3.1 with a C-terminal Flag tag. MycUnc5h1Δ3C and Pick1-FlK27A,D28A mutants were generated using Stratagene (La Jolla, CA) QuikChange Site Directed Mutagenesis kit according to the manufacturer's protocol. MycUnc5h1 and Pick1-Fl DNA, including a consensus Kozak and start site, were cloned into the viral vector pSinRep5 (Invitrogen) using the XbaI site. All PCR products and mutations were verified by sequencing.
Yeast two-hybrid analysis and glutathione S-transferase pull-down assay. The E15.5 mouse brain library was made according to manufacturer's instructions using a Stratagene cDNA synthesis kit and ligated into the EcoRI/XhoI sites in pACT2 (Clontech, Cambridge, UK) that contains a Gal4-activating domain. The UNC5H1 intracellular bait (H1ICD) in pBTM116 was fused in-frame with a LexA DNA-binding domain (Hong et al., 1999). DNA was transformed into the L40 yeast strain stably transfected with a LexA-driven HIS3 gene and LexA-driven LacZ gene (Vojtek et al., 1993). Transformants were selected for the ability to grow on minus histadine plates supplemented with 30 mm 3-amino1,2,4-triazole and subsequently screened for the ability to produce LacZ. Glutathione S-transferase (GST) pull-down assays were performed as described previously (Williams et al., 2003).
Cell culture and transfections. COS cells were maintained in DMEM supplemented with 10% FBS and transfected using FuGENE (Roche, Hertfordshire, UK). Sindbis virus expressing MycUnc5h1 or Pick1-Fl was packaged in baby hamster kidney (BHK) cells grown in MEM as described by the manufacturer's protocol (Invitrogen) and virus was diluted 1:40 to infect hippocampal neurons.
Hippocampal cultures and infection. Hippocampal neurons were harvested from embryonic day 18 rat tissue as described previously (Osten et al., 1998), plated on coverslips at 9.5 × 103 cells/cm2 for immunohistochemistry or 2.5 × 105 cells/cm2 for immunoprecipitation and biotinylation experiments, and maintained in Neurobasal A medium with B27 supplement (Invitrogen, Gaithersburg, MD). Pseudovirion-containing medium was prepared from electroporated BHK cells as directed by the Sindbis Expression System (Invitrogen). Cells were incubated with infectious medium for 1 hr, returned to Neurobasal medium without virus, and immunostained 30 hr later.
Immunoprecipitation and Western blotting. COS cells or hippocampal neurons were immunoprecipitated as described previously (Hong et al., 1999). Briefly, cells were incubated in lysis buffer (50 mm Tris, 150 mm NaCl, 5 mm EDTA, 1% Triton X-100, and 10% glycerol, pH 7.5) containing aprotinin, leupeptin, and PMSF at 1 μg/ml each. The samples were rocked at 4°C for 40 min and then pelleted in a microfuge at 14,000 × g (full speed) for 20 min. The supernatant was then incubated with antibody prebound to protein A/G (Santa Cruz Biotechnology, Santa Cruz, CA) for 3 hr at 4°C. Western blots were visualized using ECL detection (Amersham Biosciences, Arlington Heights, IL). For the endogenous coimmunoprecipitations, ∼1.5 × 107 hippocampal neurons grown 7 d in vitro (DIV) or two postnatal day 4 (P4) mouse cortices were used per immunoprecipitation. Cortices were homogenized in lysis buffer using progressively smaller-bore Pasteur pipettes; then immunoprecipitations were performed as for cultured cells. The following antibodies were diluted to 1 μg/ml and used for Western blots: anti-Myc 9E10, anti-PICK1 N18 (Santa Cruz Biotechnology), anti-UNC5H1 3E5, anti-Flag M2 (Sigma, St. Louis, MO), and anti-DCC Ab-1 (Oncogene Sciences, Uniondale, NY).
Immunostaining and antibodies. Hippocampal neurons were treated with 100 ng/ml TPA (phorbol 12-myristate 13-acetate; Sigma) for 1 hr. For live labeling of surface proteins, cells were incubated at 37°C with primary antibody plus 0.1% sodium azide to prevent internalization for 30 min. Cells were washed with PBS, fixed, and permeabilized with methanol at -20°C for 15 min. Background staining was prevented by blocking in PBS + 3% heat-inactivated goat serum (Invitrogen) for 30 min. To label intracellular proteins, cells were next incubated with the appropriate primary antibody for 1 hr at room temperature or overnight at 4°C. Cells were incubated with secondary antibody at room temperature for 45 min, washed thoroughly with PBS, and coverslipped using Fluoromount G (Southern Biotechnology, Alabaster, AL). Antibodies for immunostaining were used as follows: MycH1 live label; chicken anti-Myc 1 μg/ml (Aves Labs, Tigard, OR), MycH1 fixed label; rabbit anti-Myc (A-14) 1 μg/ml (Santa Cruz Biotechnology), 0.4 μg/ml anti-Flag M2 (Sigma), 1.7 μg/ml anti-PICK1 (Affinity BioReagents, Golden, CO), 0.6 μg/ml anti-PKCα (BD Biosciences, San Jose, CA), and 5 μg/ml 2026 anti-UNC5H1.
Biotinylation. Twenty-four hours after infection, hippocampal cells were treated with 100 ng/ml TPA and incubated at 37°C for 60 min. Cells were washed twice with ice-cold HEPES-buffered Ringers solution (HBR: 10 mm HEPES, 150 mm NaCl, 7.2 mm KCl, and 2.25 mm CaCl2, pH 7.5). The cells were then incubated in 1 mg/ml Sulfo-biotin (Pierce, Rockford, IL) in HBR at 4°C for 20 min. To quench the excess biotin, cells were washed twice for 20 min each in HBR + 100 mm glycine. Finally, cells were washed with cold Tris-buffered saline, lysed with immunoprecipitation buffer, and immunoprecipitated using Immobilized NeutrAvidin agarose beads (Pierce). Controls were performed to confirm that intracellular proteins were not biotin-labeled.
Collapse assay. Two hours after plating, hippocampal neurons were infected with a Sindbis virus containing both Unc5h1 and green fluorescent protein (GFP) on a single plasmid (Invitrogen). Twenty-four hours later, the cells were treated with TPA (100 ng/ml) or a control and incubated at 37°C for 1 hr. Next, purified netrin-1 (300 ng/ml) was added to some cultures for 45 min. Cells were then fixed in PBS containing 4% PFA and 4% sucrose for 15 min. Single cells with clearly defined axons expressing GFP were examined for growth cone collapse using 63× phase-contrast microscopy on a Zeiss (Oberkochen, Germany) Axiovert 200. For quantitation, a growth cone was considered collapsed if it had no lamellipodia and two or fewer filopodia. Each experimental condition was repeated three or more times and 50 random growth cones were examined per experiment.
Statistics and image analysis. For the quantitative immunofluorescence assay, all images were acquired with a Zeiss LSM 5 Pascal confocal microscope at 63× magnification. The laser intensity, exposure, and gain settings were the same for each image. Segments of individual axons and dendrites were excised from the images, and color channels were split and converted to grayscale using Adobe Photoshop (San Jose, CA). Images were thresholded (150 on a scale from 0 to 255) to exclude background staining and then analyzed with Scion (Frederick, MD) Image 1.63. Fluorescent surface area from each antibody (intracellular or extracellular) was calculated from a constant total cell area. Mean cell segment values (n ≥2 per cell) were calculated. This was repeated on multiple cells from independent rat hippocampal preparations (n ≥15 cell;, n ≥3 rats). Biotinylation experiments were quantified using NIH ImageJ software(NIH, Bethesda, MD. Multiple Western blot exposures from several experiments (n = 5) were scanned, and band intensities were analyzed. Statistics were performed using Excel (Microsoft, Seattle, WA) software.
Results
UNC5H1 binds PICK1 through a PDZ interaction
To identify molecules involved in UNC5H1 signal transduction, we performed a yeast two-hybrid screen on an E15.5 murine brain library using the intracellular domain of UNC5H1 (H1ICD) as bait. One molecule identified was PICK1, a PDZ protein known to regulate AMPA-receptor trafficking (Xia et al., 1999; Boudin et al., 2000; Chung et al., 2000; Daw et al., 2000; Perez et al., 2001; Hanley et al., 2002; Hirbec et al., 2003). We initially mapped the interaction between UNC5H1 and PICK1 using the yeast two-hybrid system. Deleting the C-terminal death domain of UNC5H1 (H1ΔDD) abolished the interaction with PICK1 (Fig. 1A). Previous studies demonstrated that PICK1 typically interacts with receptors through PDZ-binding domains at their extreme C terminals (Torres et al., 1998, 2001; Xia et al., 1999; Boudin et al., 2000; Dev et al., 2000; Lin et al., 2001; Duggan et al., 2002; Hirbec et al., 2003). To test whether this is true for UNC5H1, we truncated the last three amino acids (H1Δ3C) and found that this was sufficient to prevent the interaction with PICK1 (Fig. 1A). A construct expressing only the death domain, including the last three amino acids, still interacts, although more weakly, with PICK1 (Fig. 1A). These results indicate that the UNC5H1-PICK1 interaction requires the last three amino acids of the UNC5H1 cytoplasmic tail.
Figure 1.
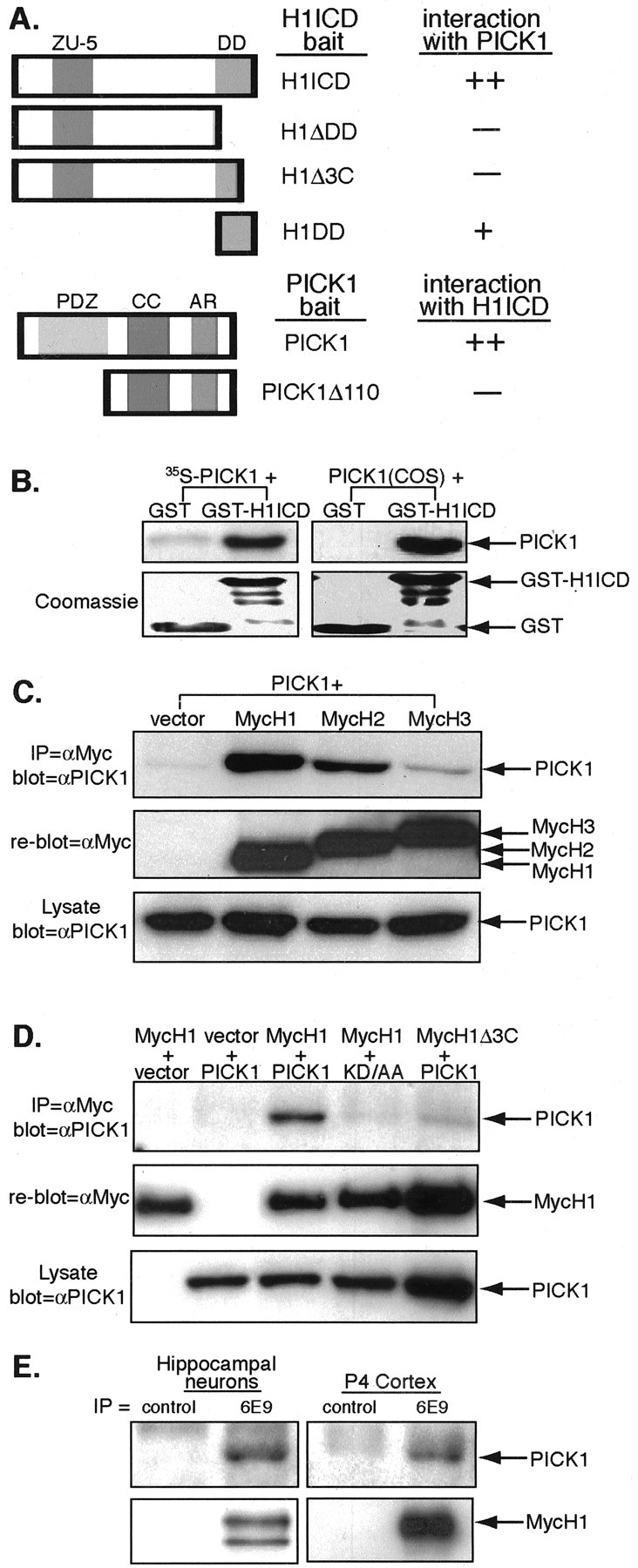
UNC5H1 directly interacts with PICK1 in vitro and in vivo. A, The schematic diagram shows UNC5H1 intracellular domain (H1ICD) and PICK1 deletion constructs. ++, Excellent ability to grow on histidine selection; +, some growth; -, no growth. B, GST or GST-H1ICD were incubated with in vitro-translated 35S-PICK1 or lysate from COS cells transiently transfected with Pick1. Glutathione agarose beads were used to pull down the GST molecules, and the samples were separated by SDS-PAGE. Autoradiography was used to detect coprecipitating 35S-PICK1 (left top) and a Western blot using anti-PICK1 antibodies was used to detect PICK1 from COS lysates (right top). The Coomassie-stained gels show that equivalent amounts of GST proteins were used (bottom). Some degradation products of GST-H1ICD are visible, but the full-length protein predominates. C, D, COS cells were cotransfected with the indicated MycUnc5h and Pick1 constructs and immunoprecipitated with anti-Myc antibodies. The top panels show coprecipitated PICK1 using anti-PICK1 antibodies. The membranes were reprobed with anti-Myc antibodies to indicate UNC5H expression (middle). The bottom panels show equal amounts of total cell lysate probed with anti-PICK1 antibodies as a control for transfection efficiency. C, MycH1 interacts strongly with PICK1, MycH2 interacts less well, and MycH3 shows little interaction. D, MycH1 but not MycH1Δ3C interacts with PICK1. PICK1 but not PICK1kd/aa interacts with MycH1. E, UNC5H1 and PICK1 interact in vivo. Extracts of rat hippocampal neurons or P4 mouse cortex were immunoprecipitated with 6E9, an antibody specific to UNC5H1. The top panels show coprecipitated PICK1 after Western blotting with an anti-PICK1 antibody. The bottom panels show the same gel blotted with anti-UNC5H1. An UNC5H1 doublet, present here in hippocampal cultures, is frequently seen for the UNC5Hs and likely represents receptor glycosylation.
The failure of H1Δ3C to interact with PICK1 suggested that the interaction is mediated through the PDZ domain of PICK1. To test this, we deleted the PDZ domain of PICK1 (PICK1Δ110) and observed no interaction with H1ICD (Fig. 1A). Although, the C-terminal amino acids of UNC5H1, alanine, glutamic acid, and cysteine (AEC), are not a typical consensus PDZ-binding domain, several studies show that PICK1 interacts with multiple PDZ-binding sequences, including atypical motifs (Lin et al., 2001; Hirbec et al., 2002).
To determine whether the interaction between UNC5H1 and PICK1 results from direct protein binding, we performed a GST pull-down assay. Purified GST-H1ICD strongly interacted with in vitro-translated, 35S-labeled PICK1, whereas GST alone showed little nonspecific binding (Fig. 1B). This result demonstrates that a direct interaction occurs between UNC5H1 and PICK1. We then tested the interaction between UNC5H1 and PICK1 by precipitating Pick1-transfected COS cells with GST-H1ICD or GST alone and Western blotting with an anti-PICK1 antibody. Again, PICK1 was precipitated by GST-H1ICD and not by GST alone (Fig. 1B).
Next, we tested whether other members of the UNC5H family interact with PICK1 by coimmunoprecipitation. COS cells were transiently transfected with full-length Myc-tagged Unc5h1, Unc5h2, or Unc5h3 and full-length Pick1. PICK1 was immunoprecipitated with anti-Myc antibodies when MycUNC5H1 (MycH1), but not empty vector, was cotransfected (Fig. 1C). In addition, we found that MycH2 interacted with PICK1, although slightly less well than MycH1, and that MycH3 showed very little interaction with PICK1 (Fig. 1C). These results were not surprising, based on sequence comparisons of the last three amino acids of the UNC5Hs. The C terminus of UNC5H2, GDC, is similar to the AEC of UNC5H1 in that they both contain an acidic residue followed by a cysteine residue, whereas the amino acids at the C terminus of UNC5H3, GQY, do not share these characteristics.
We then tested whether truncating the last three amino acids of UNC5H1 would prevent the interaction with PICK1 in cells by coimmunoprecipitation. Very little PICK1 coimmunoprecipitated with MycH1Δ3C compared with the amount coprecipitated by full-length MycH1 (Fig. 1D). A PICK1 double point mutant K27A,D28A (PICK1kd/aa) was also tested for its ability to interact with MycH1 in cells. These mutations in PICK1kd/aa reside in the carboxylate-binding loop of the PDZ domain and interfere with PDZ interactions, including the interaction between PICK1 and PKCα (Staudinger et al., 1997; Xia et al., 1999). Accordingly, we found that PICK1kd/aa did not coimmunoprecipitate with MycH1 (Fig. 1D). These observations indicate that the interaction between UNC5H1 and PICK1 is likely to occur between a PDZ-binding domain at the C terminus of UNC5H1 and the PDZ domain of PICK1.
Lastly, we examined whether UNC5H1 binds to PICK1 in vivo. Endogenous UNC5H1 from both rat hippocampal neurons and murine P4 cortices was immunoprecipitated with 6E9, an antibody specific to UNC5H1 (Williams et al., 2003). In both cases, PICK1 coimmunoprecipitated with anti-UNC5H1 but not with a control antibody (Fig. 1E), indicating that UNC5H1 and PICK1 associate in vivo.
UNC5H1 and PICK1 colocalize in heterologous cells
Expression of UNC5H1 in heterologous cells resulted in a punctate pattern of UNC5H1 localization (Fig. 2A) (Huang et al., 2002). In contrast, PICK1 expression was normally diffuse throughout the cytoplasm (Fig. 2B) but became punctate when coexpressed with cognate receptors (Torres et al., 1998; Xia et al., 1999; Boudin et al., 2000). Therefore, we asked whether coexpression of UNC5H1 and PICK1 affects the localization of either protein. Cotransfection and immunostaining of MycH1, together with Flag-tagged PICK1 (PICK1-Fl), showed PICK1-Fl in a punctate pattern overlapping with UNC5H1 expression (Fig. 2C-E). Coexpressing either the MycH1Δ3C mutant with wild-type PICK1-Fl (Fig. 2F-H) or wild-type MycH1 with PICK1-Flkd/aa (Fig. 2I-K) did not result in a similar recruitment of PICK1 to clusters, but instead PICK1 remained diffuse in the cytoplasm. These data, demonstrating colocalization of UNC5H1 and PICK1, further support our finding that UNC5H1 and PICK1 interact when expressed together in cells.
Figure 2.
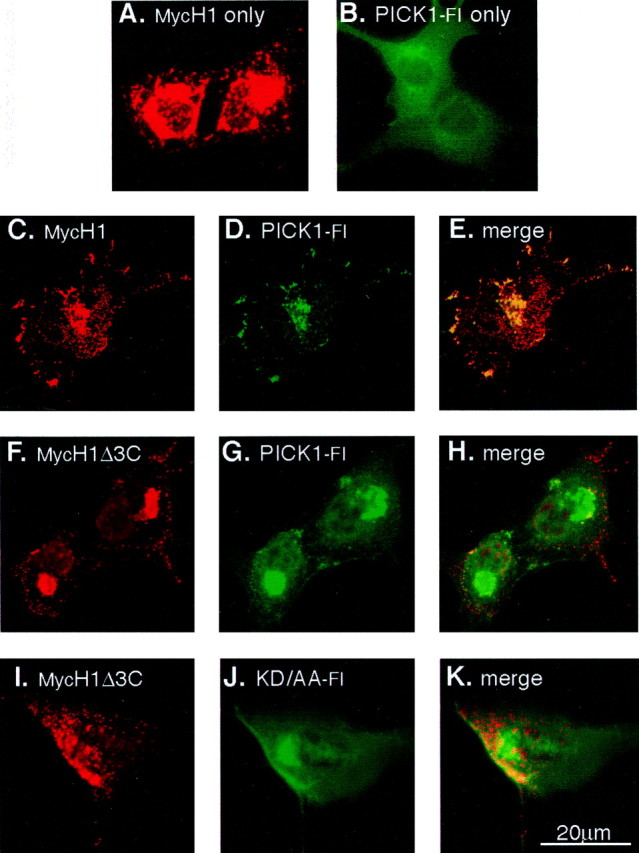
PICK1 is recruited to UNC5H1 clusters in COS cells. A-K, COS cells were transfected with the indicated MycUnc5h1 and Pick1-Fl constructs. To identify MycH1 expressed on the cell surface, cells were live-labeled with anti-Myc antibodies (A, C, F, I, red). Next, the cells were fixed, permeabilized, and immunostained with anti-Flag antibodies to label PICK1-Fl expression (B, D, G, J, green); colocalization is shown in yellow (E, H, K). A, MycH1 is expressed at the cell membrane in a punctate pattern in the absence of exogenous PICK1. B, PICK1-Fl is expressed in a diffuse cytoplasmic pattern in the absence of exogenous UNC5H1. C-E, MycH1 and PICK1-Fl colocalize in clusters along the cell membrane. F-H, When MycH1Δ3C and Pick1-Fl were cotransfected, PICK1-Fl remains diffuse and is not recruited to MycH1 clusters. I-K, Similarly, when MycH1 and PICK1-Flkd/aa were cotransfected, PICK1-Flkd/aa remains diffuse and is not recruited to MycH1 clusters.
UNC5H1, PICK1, and PKC colocalize in the growth cone
Next, we determined whether endogenous levels of UNC5H1 and PICK1 are present together in the growth cone. After 2 DIV, rat hippocampal neurons plated at low density begin to send out axonal projections that have, at their tip, a leading growth cone (Fig. 3A,E). To examine endogenous UNC5H1 expression, we generated a rabbit polyclonal antibody against the intracellular domain of UNC5H1 (2026); 2026 specifically recognized UNC5H1, but not UNC5H2 or UNC5H3, by immunohistochemistry in transfected COS cells (data not shown).
Figure 3.
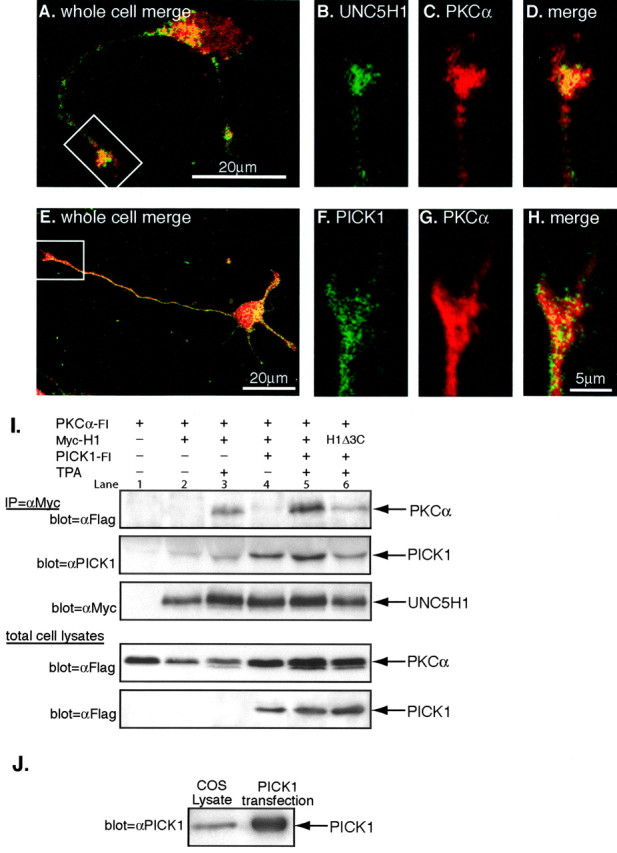
UNC5H1, PICK1, and PKCα are expressed in the growth cone and form a complex in cells. A-H, 2 DIV rat hippocampal neurons were immunostained with the indicated antibodies. A-D, Endogenous UNC5H1 (B, green) and PKCα (C, red) colocalize in 2 DIV hippocampal neurons. The merged image is shown (D, yellow). E-H, Endogenous PICK1 (F, green) also colocalizes with PKCα (G, red) in the growth cone as seen in the merged image (H, yellow). Growth cones from the whole cell pictured in A and E, indicated by the white box, are magnified in B-D and F-H. I, COS cells were cotransfected with the indicated MycUnc5h1, PKCα-Flag, Pick1-Flag constructs and immunoprecipitated with anti-Myc antibodies to precipitate UNC5H1. The top panel shows immunoprecipitates blotted with an anti-Flag antibody to identify coprecipitating PKCα-Fl (Note that PKCα-Fl and PICK1-Fl can be distinguished because of the difference in their molecular weights; PKCα-Fl runs at 80 and PICK1-Fl at 50 kDa.) The second panel shows the same immunoprecipitates blotted with an anti-PICK1 antibody to identify coprecipitating endogenous PICK1 and PICK1-Fl. The immunoprecipitates were reprobed with an anti-Myc antibody to confirm equivalent UNC5H1 expression in all lanes. Also shown are total cell lysates probed with anti-Flag to confirm that PKCα-Fl and PICK1-Fl expression is equivalent in the indicated lanes. J, COS cells endogenously express PICK1. Total protein lysates from untransfected and Pick1-Fl-transfected COS cells were Western blotted using anti-PICK1 antibodies.
We used 2026 to immunostain 2 DIV hippocampal neurons and observed UNC5H1 expression in growth cones (Fig. 3A,B). We also coimmunostained the neurons for PKCα because PICK1 functions as an adaptor molecule for PKCα, bringing it in close proximity with transmembrane receptors (Staudinger et al., 1995, 1997). Our results showed that PKCα was present at sites of UNC5H1 expression in the growth cone (Fig. 3A-D). Next, we immunostained 2 DIV hippocampal neurons using antibodies to PICK1 and PKCα and found that PICK1 also colocalized with PKCα (Fig. 3E-H). Thus, UNC5H1, PICK1, and PKCα are expressed endogenously in growth cones.
UNC5H1, PICK1, and PKCα form a complex after PKC activation
Previous work suggests that PICK1 dimers function as a scaffolding molecule, bringing PKCα in close proximity to receptors (Staudinger et al., 1997; Xia et al., 1999; Chung et al., 2000). Given that UNC5H1, PICK1, and PKCα were simultaneously expressed in the growth cone (Fig. 3A-H), we used coimmunoprecipitation to test whether these molecules associate with each other to form a higher-order complex. COS cells were transfected with various combinations of MycUnc5h1, PKCα-Flag, and Pick1-Flag cDNAs. Cells were immunoprecipitated using an anti-Myc antibody and Western blotted to identify coprecipitating PKCα-Fl and PICK1-Fl. We found that PKCα-Fl coimmunoprecipitated with MycH1 only if PKC was first activated by treating the cells with the phorbol ester TPA (Fig. 3I, top, lanes 2, 3). Because COS cells endogenously express PICK1 (Fig. 3J), we also probed these immunoprecipitations with an anti-PICK1 antibody to determine whether endogenous PICK1 also precipitated in this experiment. We observed that endogenous PICK1 coprecipitated with MycH1 and PKCα-Fl (Fig. 3I, middle), suggesting that PICK1 acts as an adapter molecule to support a complex between UNC5H1 and PKCα. To test this hypothesis, we kept MycH1 and PKCα-Fl levels constant while increasing PICK1-Fl expression by transfection. Coimmunoprecipitation under this condition showed an increased amount of PKCα-Fl in a complex with MycH1 in TPA-treated cells (Fig. 3I, top, lanes 4, 5). Furthermore, we showed that MycH1Δ3C, which had a reduced ability to interact with PICK1-Fl, also had a reduced ability to precipitate PKCα-Fl (Fig. 3I, top, lane 6). Together, these results suggest that the interaction between UNC5H1 and PKCα is not direct but instead, requires PICK1, which acts as an adapter molecule to bring PKCα to UNC5H1.
UNC5H1 is internalized after PKC activation by a PICK1-dependent mechanism
PICK1, in conjunction with PKC, regulates the surface expression of several transmembrane receptors to control their activity (Daw et al., 2000; Xia et al., 2000; Kim et al., 2001; Torres et al., 2001; Hirbec et al., 2003). Because UNC5H1 colocalized with both PICK1 and PKC in hippocampal growth cones, we tested whether the interaction between UNC5H1 and PICK1 regulates the level of UNC5H1 on the cell surface in a PKC-dependent manner. At 14 DIV, hippocampal neurons were infected with viruses expressing MycH1 and Pick1-Fl. To stimulate PKC, TPA was added to some cultures. Because the Myc tag on MycH1 is extracellular, we examined the surface expression of MycH1 by live-labeling the cells without permeabilization using a chicken anti-Myc antibody. To examine the intracellular MycH1, the same cells were then fixed, permeabilized, and incubated with a rabbit anti-Myc antibody. Confocal microscopy showed that when MycH1 and PICK1-Fl were coexpressed without TPA, MycH1 showed high levels of both intracellular and surface expression (Fig. 4A,B). However, in the presence of TPA the surface expression of MycH1 was significantly reduced, whereas the intracellular expression of MycH1 remained high (Fig. 4, compare B,D). Furthermore, we found that internalization of UNC5H1 by TPA was dependent on the interaction of UNC5H1 with PICK1. When MycH1Δ3C and PICK1-Fl, which have a weak affinity for binding, were coexpressed, the surface expression of MycH1 did not change in the presence of TPA (Fig. 4E,F). Similarly, when MycH1 and PICK1-Flkd/aa, which also have a weak affinity for binding, were coexpressed, the surface expression of UNC5H1 did not change in the presence of TPA (Fig. 4G,H). To quantify these results, the total area of MycH1 surface fluorescence and MycH1 intracellular fluorescence was determined from neurons that also expressed PICK1-Fl. These data were graphed as a ratio of MycH1 surface expression over MycH1 intracellular expression and referred to as the S/I ratio (Fig. 4I). The S/I ratio of neurons expressing wild-type MycH1 and PICK1-Fl decreased significantly from 1.8 ± 0.29 to 0.7 ± 0.05 (p < 0.001) after adding TPA (Fig. 4I). This corresponds to a 60% decrease in MycH1 surface expression. In contrast, when either MycH1Δ3C (S/I ratio, 1.8 ± 0.21) or PICK1-Flkd/aa (S/I ratio, 1.6 ± 0.08) mutants were used in the assay, no significant decrease in surface expression of MycH1Δ3C or MycH1, respectively, was observed (Fig. 4I). These results indicate that UNC5H1 is removed from the cell surface after PKC stimulation and this removal requires an interaction between UNC5H1 and PICK1.
Figure 4.
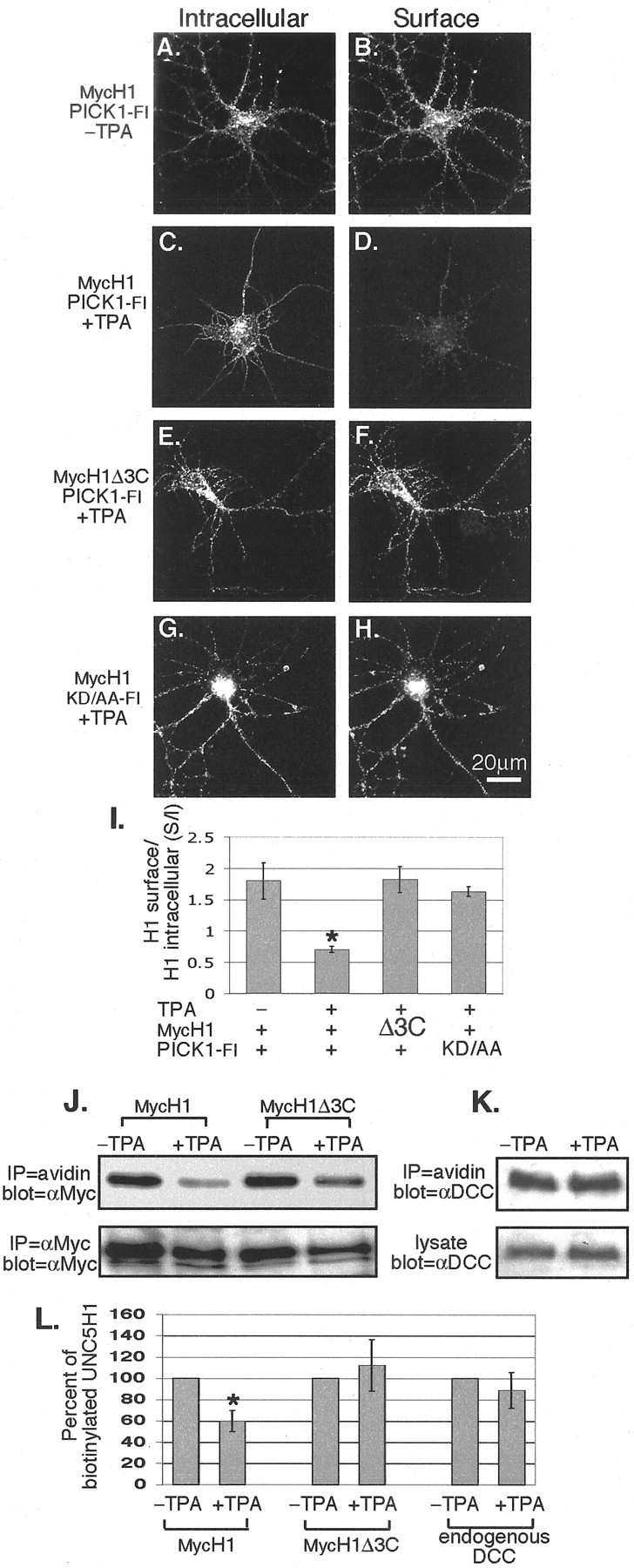
UNC5H1 is internalized after PKC activation in mature neurons. A-H, 14 DIV hippocampal neurons were infected with the indicated MycH1 and Pick1-Fl Sindbis viruses. Cells were live-labeled to immunostain surface MycH1 (B, D, F, H), then fixed and permeabilized to immunostain internal MycH1 (A, C, E, G). The addition of 100 ng/ml TPA visibly reduced the surface expression of MycH1 in cells coinfected with MycH1 and Pick1-Fl (compare B, D). When MycH1Δ3C (E, F) or PICK1-Flkd/aa (G, H) were expressed in the assay, then MycH1Δ3C or MycH1, respectively, was not internalized. I, The results from A-H were quantified by determining the total fluorescence intensity of both the surface-labeled MycH1 and intracellular-labeled MycH1 over the same defined area. Data are graphed as a ratio of surface fluorescence over intracellular fluorescence (S/I). The addition of TPA when MycH1 and PICK1-Fl were expressed resulted in a significant decrease in cell surface expression of MycH1. *p < 0.001; t test. Expression of either MycH1Δ3C with PICK1-Fl or MycH1 with PICK1-Flkd/aa shows no significant change in the S/I ratio after adding TPA. Error bars indicate SEM, and in all cases n > 10. J, K, Surface biotinylation experiments show that MycH1 is removed from the cell surface after the addition of TPA, whereas DCC remains on the surface. Hippocampal neurons were infected with MycH1 or MycH1Δ3C, surface proteins were biotinylated, and cells were precipitated with streptavidin beads. J, Western blotting for MycH1 shows a reduced level of biotinylated MycH1 in the presence of TPA compared with control cultures incubated in the absence of TPA. Western blotting for H1Δ3C shows little change in the levels of biotinylated MycH1Δ3C in the presence or absence of TPA. A portion of the total cell lysate was immunoprecipitated and Western blotted using anti-Myc antibodies to determine infection efficiency. K, Western blotting with an anti-DCC antibody shows that the surface expression of DCC does not change in the presence or absence of TPA. As a loading control, equal amounts of cell lysates were also Western blotted with anti-DCC. L, The results from the biotinylation experiments were quantified. The amount of biotinylated MycH1 or MycH1Δ3C in cultures without TPA was quantified and normalized as 100%. After the addition of TPA, significantly less MycH1 is biotinylated compared with controls (p < 0.001; t test), whereas MycH1Δ3C shows no significant difference between minus and plus TPA treated cultures. DCC also shows no significant difference between minus and plus TPA treated cultures. (n ≥ 3). Error bars indicate SD.
Next, we performed a biotinylation assay to confirm that the surface expression of MycH1 decreases after PKC activation. Hippocampal neurons infected with either MycH1 or MycH1Δ3C were incubated with or without TPA at 37°C for 1 hr to promote internalization, and then placed on ice to stop cellular trafficking. Next, surface proteins were labeled with biotin. After quenching the biotin reaction, cells were lysed and biotinylated proteins were immunoprecipitated using streptavidin-agarose beads. The biotinylated proteins were separated by SDS-PAGE and Western blotted with anti-Myc antibodies. As a control, one fifth of the lysate was saved and immunoprecipitated using anti-Myc antibodies to account for the efficiency of infection. Figure 4J shows that TPA visibly reduced the amount of biotinylated MycH1, suggesting that MycH1 was internalized after PKC activation. To quantify this assay, the amount of biotin-labeled UNC5H1 on untreated cultures was considered 100% of the surface UNC5H1. After PKC stimulation, the surface expression of UNC5H1 was reduced by 40% (p < 0.001) (Fig. 4L). The surface expression of MycH1Δ3C appeared slightly less after PKC stimulation; however, after normalizing to the total MycH1Δ3C expressed in each sample, quantification revealed that surface expression of MycH1Δ3C did not significantly change after adding TPA (Fig. 4 J,L). We also examined whether the coreceptor of UNC5H1, DCC, was internalized by a similar mechanism in hippocampal cultures. Under the same conditions used for UNC5H1 biotinylation experiments, we found that the surface expression of DCC did not significantly change in the presence or absence of TPA (Fig. 4K,L). These results support the quantitative immunofluorescence analysis and, together, strongly suggest that the removal of UNC5H1, but not DCC, from the cell surface depends on the interaction of UNC5H1 with PICK1 and the activation of PKC.
UNC5H1 undergoes PICK1/PKC-dependent trafficking in growth cones
Because UNC5H1 intracellular trafficking is regulated in mature hippocampal neurons, we tested if a similar mechanism functions in growth cones. Using quantitative immunofluorescence, we determined the surface versus internal MycH1 expression levels in growth cones of 2 DIV hippocampal neurons. Similar to our results on mature neurons grown 14 DIV, when cells were infected with MycH1 and PICK1-Fl, high levels of clustered MycH1 staining were visible both inside and on the surface of growth cones (Fig. 5A-C). The addition of TPA significantly reduced the amount of MycH1 on the surface (Fig. 5D-F). Analysis of the S/I ratio of UNC5H1 expression showed that there was 70% less MycH1 on the surface of growth cones in TPA-treated cultures (1.8 ± 0.24 vs 0.6 ± 0.07, p < 0.001) (Fig. 5M). Furthermore, infection of either MycH1Δ3C or PICK1-Flkd/aa did not result in any significant decrease in surface expression of MycH1Δ3C or MycH1, respectively (Fig. 5G-M). These results indicate that UNC5H1 is removed from the surface of growth cones after PKC activation by a mechanism involving PICK1.
Figure 5.
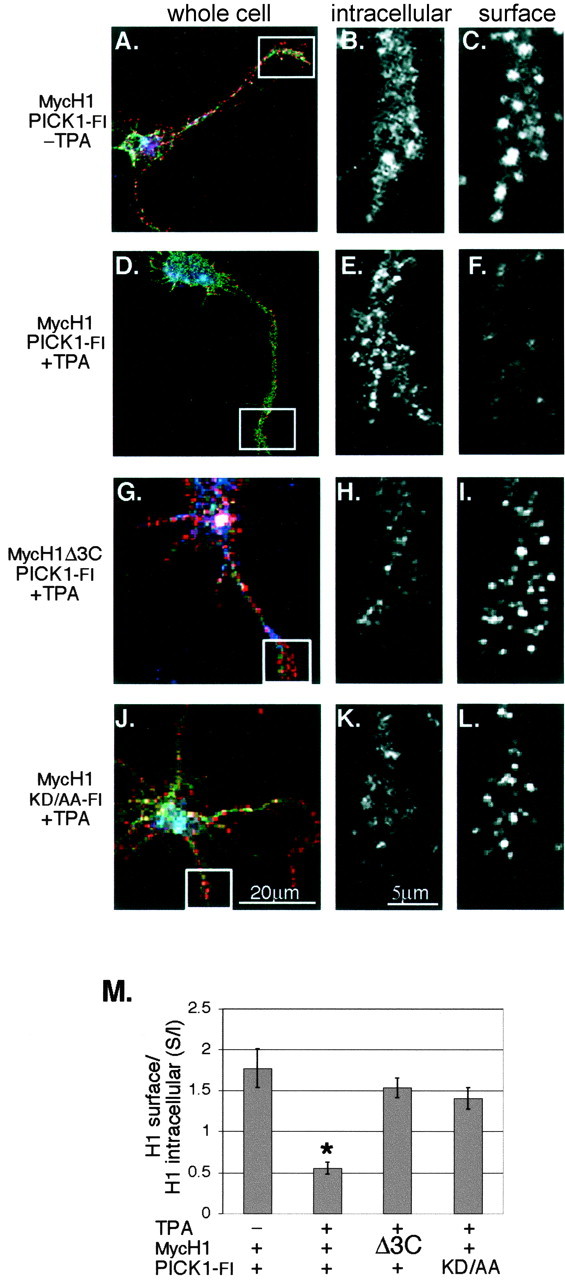
UNC5H1 is internalized after PKC activation in growth cones. A-L, 2 DIV hippocampal neurons were infected with the indicated MycH1 and Pick1-Fl Sindbis viruses. Cells were live-labeled to immunostain the surface MycH1 (C,F, I, L) and then fixed and permeabilized to immunostain the internal MycH1 (B, E, H, K). The first panel in each row (A, D, G, J) shows an entire cell triple labeled for surface MycH1 (red), intracellular MycH1 (green), and PICK1-Fl (blue). The following smaller panels show magnified images of the growth cone from the corresponding whole cell. The addition of 100 ng/ml of TPA visibly reduces the surface expression of MycH1 at the growth cone in cells coexpressing MycH1 and PICK1-Fl (compare C, F). When cells expressing MycH1Δ3C (G-I) or PICK1-Flkd/aa (J-L) were analyzed, MycH1Δ3C or MycH1, respectively, were not internalized. M, The results from A-H were quantified by determining the total fluorescence intensity of both the surface-labeled MycH1 and intracellular-labeled MycH1 over the growth cone. Data are graphed as a ratio of surface fluorescence over intracellular fluorescence (S/I). The addition of TPA results in a significant decrease in cell-surface MycH1 when MycH1 and PICK1-Fl are coexpressed. *p < 0.001; t test. Expression of either MycH1Δ3C or Pick1-Flkd/aa shows no significant change in the S/I ratio after adding TPA. Error bars indicate SEM, and in all cases n > 10.
PKC activation inhibits netrin-1-dependent growth cone collapse
To test whether PICK1/PKC-dependent removal of UNC5H1 from the surface of growth cones affects the response of an axon to netrin-1, we performed collapse assays on hippocampal growth cones. Hippocampal neurons were infected with Sindbis virus expressing MycH1 2 hr after plating; 24 hr later, the cells extended axons with normal growth cones at the tip (Fig. 6A). When netrin-1 was added to the media, 74 ± 6.0% of the growth cones collapse, as evidenced by a nearly complete loss of lamellipodia and filopodia (Fig. 6B,E). In contrast, when the cells were pretreated with TPA, to stimulate the PKC-dependent removal of UNC5H1 from the surface of growth cones, significantly fewer growth cones underwent netrin-1-mediated collapse (35 ± 2.1%; p < 0.005) compared with cells treated with netrin-1 only (Fig. 6C,E). As a control, we tested whether TPA alone had any effect on growth cone collapse and found that it did not (Fig. 6D,E). Thus, our data suggest that controlling the surface expression of UNC5H1 through a mechanism involving PICK1 and PKC is one way cells regulate the response of a migrating axon to netrin-1.
Figure 6.
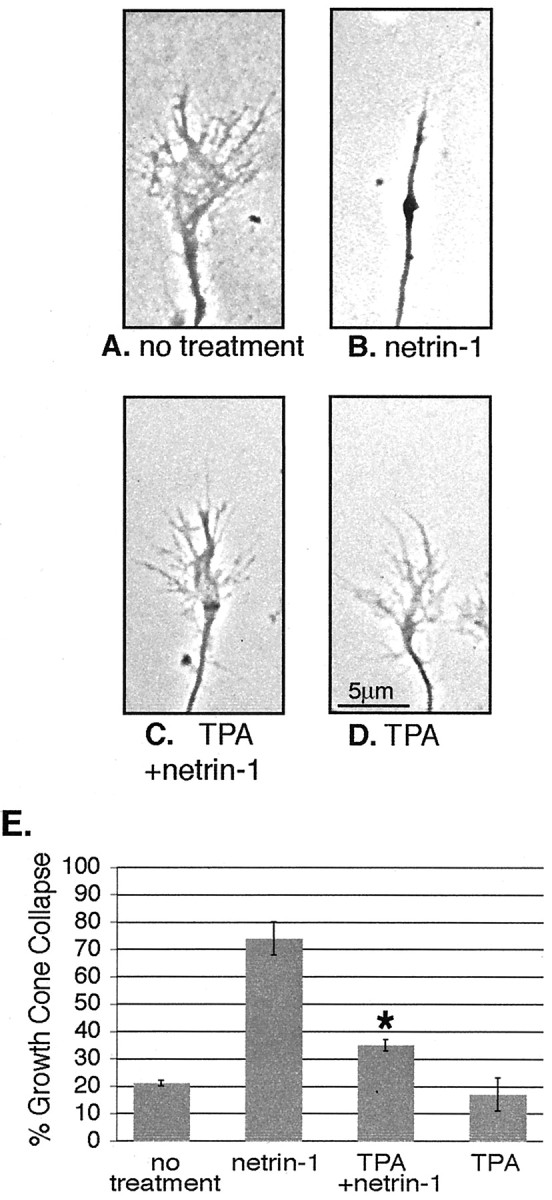
Decreasing surface expression of UNC5H1 after PKC activation prevents netrin-1-dependent growth cone collapse. A-D, Phase contrast images of 1 DIV hippocampal growth cones were treated as indicated. E, The results from A-D were quantified and the percent of collapsed growth cones is shown. n = 50 cells from three separate experiments. *p < 0.005; t test compared with treatment with netrin-1 alone.
Discussion
Very few details are known about netrin-1 signaling as it is mediated specifically through its repellent receptors, the UNC5Hs. By performing a yeast two-hybrid screen, we sought to identify proteins that directly affect UNC5H1 signaling. In this study, we report a PDZ-mediated interaction between the netrin-1 receptor UNC5H1 and PICK1. The interaction between UNC5H1 and PICK1 facilitates the removal of UNC5H1 from the cell surface of neurons and, more specifically, from growth cones through a PKC-dependent mechanism. Because PICK1 forms homodimers (Staudinger et al., 1995, 1997) it may function as a bridge between UNC5H1 and PKC by binding both molecules simultaneously. In support of this idea, we show that the ability of UNC5H1 to associate with PKC depends on the expression level of PICK1, and mutations in PICK1 that interfere with UNC5H1 and PKC binding prevent TPA-induced removal of UNC5H1 from the cell surface. The intracellular domain of UNC5H1 contains several conserved PKC phosphorylation sites and can be phosphorylated by PKC in vitro (our unpublished results). Thus, phosphorylation of UNC5H1 by PKC may be the mechanism by which PICK1 promotes the internalization of UNC5H1. This is consistent with a role for PICK1 priming UNC5Hs for endocytosis, perhaps by releasing it from an interaction with cell-surface scaffolding proteins such as GRIP (glutamate receptor-interacting protein), similar to one of the proposed models for PICK1 regulation of AMPA-receptor trafficking (Chung et al., 2000).
Regulated endocytosis generates mechanical forces to facilitate cell migration
Recently, it was discovered that activating PKC at the growth cone induces a repulsive turning response (Xiang et al., 2002). It was found that this repulsion is dependent on local concentrations of intracellular messengers at growth cones because increasing the concentration of cGMP converts PKC-dependent repulsion to attraction (Xiang et al., 2002). Similarly, many other studies have shown that the intracellular environment, for example the level of Ca2+ or cAMP, is critical during axon guidance and influences the turning response for many guidance cues (for review, see Song and Poo, 1999; Petersen and Cancela, 2000). Despite these numerous studies on the effects of altering the concentration of global, intracellular second messengers, the actual receptor-mediated mechanism through which the repellent response is executed remains unknown.
One possible receptor-mediated mechanism for repellent migration involves PKC activation inducing growth cone collapse or in some instances, repulsion, by stimulating receptor-mediated endocytosis. Endocytosis, in turn, generates mechanical forces that pull membrane off the cell surface to cause retraction. For example, the repellent cue ephrin stimulates endocytosis through Rac1 to generate cellular forces that result in growth cone collapse (Jurney et al., 2002). This may represent one mechanism whereby growth cones use regulated endocytosis to produce mechanical forces that generate directional cytoskeletal changes during axon guidance (Condic and Letourneau, 1997; Jurney et al., 2002). As for UNC5Hs, one hypothesis is that with PKC activation, UNC5Hs are internalized, generating a force that promotes growth cone collapse away from a source of netrin-1. Although it is unknown whether UNC5H is recycled back to the cell surface, it is possible that successive rounds of endocytosis may continue to generate forces required to propel the cell away from netrin-1. We predict that this repulsion would occur in an intracellular environment low, but not absent, in cAMP, cGMP, and Ca2+ because many studies have shown that this intracellular environment is conducive to repellent migration (Lohof et al., 1992; Ming et al., 1997; Song et al., 1997; Hopker et al., 1999; Hong et al., 2000).
A second way that regulated removal of surface receptors may generate motile forces is by increasing or decreasing adhesion between the cell and the extracellular environment. This was shown for integrin, whose expression on the neuronal cell surface is dynamically regulated by the concentration of extracellular ligand, providing a mechanism whereby neurons maintain proper adhesive interactions over a range of ligand concentrations (Condic and Letourneau, 1997). Because chemotactic ligands are often present in gradients, maintaining appropriate surface expression of receptors is likely critical during axon guidance. UNC5Hs and DCC each contain multiple immunoglobulin domains, a common motif found in adhesive molecules; therefore, their removal from the cell surface could facilitate repulsion through a loss of adhesive contacts with the extracellular matrix. In support of this, the loss of neogenin, a netrin-1 receptor homologous to DCC, results in a decrease of cell adhesion in the mammary gland (Srinivasan et al., 2003). Most likely, the endocytic removal of UNC5H1 from the cell surface promotes repulsion through a combination of membrane collapse and loss of extracellular matrix contact.
Regulating endocytosis of the UNC5Hs to direct a switch between repulsion and attraction
In addition to playing a mechanical role by generating motile forces required for migration, the regulation of receptors on the cell surface may also play an instructive role during axon guidance by dictating directionality. Our data show that UNC5H1 is specifically internalized by a mechanism involving PICK1 and PKC, leaving DCC on the cell surface. In this circumstance, if the intracellular environment (i.e., levels of cAMP and Ca2+) is appropriate for attraction, then the specific internalization of UNC5Hs, but not DCC, could lead to a switch from repulsion to attraction in a netrin-1 gradient. This switch may be relevant because netrins are bidirectional guidance cues that both attract and repel neurons and their growth cones (Hong et al., 1999, 2000; Hopker et al., 1999; Shewan et al., 2002). Consequently, the way cells respond to netrin-1 at intermediate choice points may depend on the availability of different receptors at the cell surface. For example, the availability of the receptor Robo on the surface of axons crossing the midline regulates their responsiveness to the repellent cue Slit (Keleman et al., 2002; Myat et al., 2002). Thus, intracellular trafficking of receptors is one means by which navigating growth cones quickly adjust their sensitivity to extracellular guidance cues at intermediate choice points.
Sequestering UNC5H1 could prevent unnecessary apoptosis
The discovery that maintaining UNC5H1 on the cell surface does not occur simply by default but, instead, is a regulated cellular process has implications toward understanding how a cell can modulate the various functions of netrin-1 and its receptors. Not only do UNC5Hs function during netrin-1-dependent cell and axon migration but they also induce netrin-1-independent apoptosis (Llambi et al., 2001; Tanikawa et al., 2003; Williams et al., 2003). Subcellular trafficking of UNC5H1 may be important for separating these two distinct functions. Constitutive expression of UNC5H1 on the surface of cells leads to programmed cell death (Llambi et al., 2001; Tanikawa et al., 2003; Williams et al., 2003). However, if UNC5H1 were shuttled to endosomal compartments, then UNC5Hs may not be able to send an apoptotic signal. In support of this hypothesis, we find that expression of the intracellular domain of UNC5H1 as a cytoplasmic protein results in significantly less apoptosis than UNC5H1 that is expressed at the cell membrane (our unpublished results). Thus, maintaining UNC5H1 in intracellular stores would allow the receptor to be readily available for mobilization during axon guidance, but sequestered to prevent unintentional apoptosis.
In sum, we find that the internalization of UNC5H1 is regulated by a mechanism involving PICK1 and PKC and there are at least two models for how this could control axon guidance. One is that internalization of UNC5H after PKC activation mediates repulsion by generating mechanical forces away from a source of netrin-1. The second is that specific internalization of UNC5H after PKC activation mediates attraction when DCC remains on the cell surface in a netrin-1 gradient. These models are not mutually exclusive and the outcome likely reflects the intracellular signaling environment. Because UNC5H1 functions in diverse cellular processes including cell and axon repulsion and apoptosis, regulated subcellular trafficking of this receptor may provide one mechanism to control the final outcome of UNC5H signaling.
Footnotes
This work was supported by National Institutes of Health Grants NS39572-01 (L.H.) and MH12813-02 (M.E.W.) and March of Dimes Birth Defects Foundation Grant 5-FY99-765. We thank David Feldheim for donating Sindbis virus reagents, Alex Toker for providing the PKCα-Flag pCMV5 construct, and Yishi Jin for use of her confocal microscope.
Correspondence should be addressed to Dr. Lindsay Hinck, Sinsheimer Laboratories, University of California, Santa Cruz, CA 95064. E-mail: hinck@biology.ucsc.edu.
Copyright © 2003 Society for Neuroscience 0270-6474/03/2311279-10$15.00/0
M.E.W., S.C.-Y.W., and W.L.M. contributed equally to this work.
References
- Ackerman SL, Kozak LP, Przyborski SA, Rund LA, Boyer BB, Knowles BB ( 1997) The mouse rostral cerebellar malformation gene encodes an UNC-5-like protein. Nature 386: 838-842. [DOI] [PubMed] [Google Scholar]
- Alcantara S, Ruiz M, De Castro F, Soriano E, Sotelo C ( 2000) Netrin 1 acts as an attractive or as a repulsive cue for distinct migrating neurons during the development of the cerebellar system. Development 127: 1359-1372. [DOI] [PubMed] [Google Scholar]
- Bloch-Gallego E, Ezan F, Tessier-Lavigne M, Sotelo C ( 1999) Floor plate and netrin-1 are involved in the migration and survival of inferior olivary neurons. J Neurosci 19: 4407-4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudin H, Doan A, Xia J, Shigemoto R, Huganir RL, Worley P, Craig AM ( 2000) Presynaptic clustering of mGluR7a requires the PICK1 PDZ domain binding site. Neuron 28: 485-497. [DOI] [PubMed] [Google Scholar]
- Chung HJ, Xia J, Scannevin RH, Zhang X, Huganir RL ( 2000) Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J Neurosci 20: 7258-7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condic ML, Letourneau PC ( 1997) Ligand-induced changes in integrin expression regulate neuronal adhesion and neurite outgrowth. Nature 389: 852-856. [DOI] [PubMed] [Google Scholar]
- Daw MI, Chittajallu R, Bortolotto ZA, Dev KK, Duprat F, Henley JM, Collingridge GL, Isaac JT ( 2000) PDZ proteins interacting with C-terminal GluR2/3 are involved in a PKC-dependent regulation of AMPA receptors at hippocampal synapses. Neuron 28: 873-886. [DOI] [PubMed] [Google Scholar]
- Dev KK, Nakajima Y, Kitano J, Braithwaite SP, Henley JM, Nakanishi S ( 2000) PICK1 interacts with and regulates PKC phosphorylation of mGLUR7. J Neurosci 20: 7252-7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan A, Garcia-Anoveros J, Corey DP ( 2002) The PDZ domain protein PICK1 and the sodium channel BNaC1 interact and localize at mechanosensory terminals of dorsal root ganglion neurons and dendrites of central neurons. J Biol Chem 277: 5203-5208. [DOI] [PubMed] [Google Scholar]
- Engelkamp D ( 2002) Cloning of three mouse Unc5 genes and their expression patterns at mid-gestation. Mech Dev 118: 191-197. [DOI] [PubMed] [Google Scholar]
- Finger JH, Bronson RT, Harris B, Johnson K, Przyborski SA, Ackerman SL ( 2002) The netrin 1 receptors unc5h3 and dcc are necessary at multiple choice points for the guidance of corticospinal tract axons. J Neurosci 22: 10346-10356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley JG, Khatri L, Hanson PI, Ziff EB ( 2002) NSF ATPase and alpha/beta-SNAPs disassemble the AMPA receptor-PICK1 complex. Neuron 34: 53-67. [DOI] [PubMed] [Google Scholar]
- Hedgecock EM, Culotti JG, Hall DH ( 1990) The unc-5, unc-6 and unc-40 genes guide the circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans Neuron 4: 61-85. [DOI] [PubMed] [Google Scholar]
- Hirbec H, Perestenko O, Nishimune A, Meyer G, Nakanishi S, Henley JM, Dev KK ( 2002) The PDZ proteins PICK1, GRIP, and syntenin bind multiple glutamate receptor subtypes. Analysis of PDZ binding motifs. J Biol Chem 277: 15221-15224. [DOI] [PubMed] [Google Scholar]
- Hirbec H, Francis JC, Lauri SE, Braithwaite SP, Coussen F, Mulle C, Dev KK, Couthino V, Meyer G, Isaac JT, Collingridge GL, Henley JM ( 2003) Rapid and differential regulation of AMPA and kainate receptors at hippocampal mossy fibre synapses by PICK1 and GRIP. Neuron 37: 625-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K, Hinck L, Nishiyama M, Poo MM, Tessier-Lavigne M, Stein E ( 1999) A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell 97: 927-941. [DOI] [PubMed] [Google Scholar]
- Hong K, Nishiyama M, Henley J, Tessier-Lavigne M, Poo M ( 2000) Calcium signalling in the guidance of nerve growth by netrin-1. Nature 403: 93-98. [DOI] [PubMed] [Google Scholar]
- Hopker VH, Shewan D, Tessier-Lavigne M, Poo M, Holt C ( 1999) Growth-cone attraction to netrin-1 is converted to repulsion by laminin-1. Nature 401: 69-73. [DOI] [PubMed] [Google Scholar]
- Huang X, Cheng HJ, Tessier-Lavigne M, Jin Y ( 2002) MAX-1, a novel PH/MyTH4/FERM domain cytoplasmic protein implicated in netrin-mediated axon repulsion. Neuron 34: 563-576. [DOI] [PubMed] [Google Scholar]
- Jurney WM, Gallo G, Letourneau PC, McLoon SC ( 2002) Rac1-mediated endocytosis during ephrin-A2- and semaphorin 3A-induced growth cone collapse. J Neurosci 22: 6019-6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keino-Masu K, Masu M, Hinck L, Leonardo ED, Chan SS, Culotti JG, Tessier-Lavigne M ( 1996) Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell 87: 175-185. [DOI] [PubMed] [Google Scholar]
- Keleman K, Dickson BJ ( 2001) Short and long-range repulsion by the Drosophila Unc5 netrin receptor. Neuron 32: 605-617. [DOI] [PubMed] [Google Scholar]
- Keleman K, Rajagopalan S, Cleppien D, Teis D, Paiha K, Huber LA, Technau GM, Dickson BJ ( 2002) Comm sorts robo to control axon guidance at the Drosophila midline. Cell 110: 415-427. [DOI] [PubMed] [Google Scholar]
- Kim CH, Chung HJ, Lee HK, Huganir RL ( 2001) Interaction of the AMPA receptor subunit GluR2/3 with PDZ domains regulates hippocampal long-term depression. Proc Natl Acad Sci USA 98: 11725-11730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardo ED, Hinck L, Masu M, Keino-Masu K, Ackerman SL, Tessier-Lavigne M ( 1997) Vertebrate homologues of C. elegans UNC-5 are candidate netrin receptors. Nature 386: 833-838. [DOI] [PubMed] [Google Scholar]
- Leung-Hagesteijn C, Spence AM, Stern BD, Zhou Y, Su MW, Hedgecock EM, Culotti JG ( 1992) UNC-5, a transmembrane protein with immunoglobulin and thrombospondin type 1 domains, guides cell and pioneer axon migrations in C. elegans Cell 71: 289-299. [DOI] [PubMed] [Google Scholar]
- Lin WJ, Chang YF, Wang WL, Huang CY ( 2001) Mitogen-stimulated TIS21 protein interacts with a protein-kinase-Cα-binding protein rPICK1. Biochem J 354: 635-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llambi F, Causeret F, Bloch-Gallego E, Mehlen P ( 2001) Netrin-1 acts as a survival factor via its receptors UNC5H and DCC. EMBO J 20: 2715-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohof AM, Quillan M, Dan Y, Poo MM ( 1992) Asymmetric modulation of cytosolic cAMP activity induces growth cone turning. J Neurosci 12: 1253-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Song HJ, Berninger B, Holt CE, Tessier-Lavigne M, Poo MM ( 1997) cAMP-dependent growth cone guidance by netrin-1. Neuron 19: 1225-1235. [DOI] [PubMed] [Google Scholar]
- Myat A, Henry P, McCabe V, Flintoft L, Rotin D, Tear G ( 2002) Drosophila Nedd4, a ubiquitin ligase, is recruited by commissureless to control cell surface levels of the roundabout receptor. Neuron 35: 447-459. [DOI] [PubMed] [Google Scholar]
- Osten P, Srivastava S, Inman GJ, Vilim FS, Khatri L, Lee LM, States BA, Einheber S, Milner TA, Hanson PI, Ziff EB ( 1998) The AMPA receptor GluR2 C terminus can mediate a reversible, ATP-dependent interaction with NSF and alpha- and beta-SNAPs. Neuron 21: 99-110. [DOI] [PubMed] [Google Scholar]
- Perez JL, Khatri L, Chang C, Srivastava S, Osten P, Ziff EB ( 2001) PICK1 targets activated protein kinase Cα to AMPA receptor clusters in spines of hippocampal neurons and reduces surface levels of the AMPA-type glutamate receptor subunit 2. J Neurosci 21: 5417-5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen OH, Cancela JM ( 2000) Attraction or repulsion by local Ca(2+) signals. Curr Biol 10: R311-R314. [DOI] [PubMed] [Google Scholar]
- Shewan D, Dwivedy A, Anderson R, Holt CE ( 2002) Age-related changes underlie switch in netrin-1 responsiveness as growth cones advance along visual pathway. Nat Neurosci 5: 955-962. [DOI] [PubMed] [Google Scholar]
- Song HJ, Poo MM ( 1999) Signal transduction underlying growth cone guidance by diffusible factors. Curr Opin Neurobiol 9: 355-363. [DOI] [PubMed] [Google Scholar]
- Song HJ, Ming GL, Poo MM ( 1997) cAMP-induced switching in turning direction of nerve growth cones. Nature 388: 275-279. [DOI] [PubMed] [Google Scholar]
- Srinivasan K, Strickland P, Valdes A, Shin GC, Hinck L ( 2003) Netrin-1/neogenin interaction stabilizes multipotent progenitor cap cells during mammary gland morphogenesis. Dev Cell 4: 371-382. [DOI] [PubMed] [Google Scholar]
- Staudinger J, Zhou J, Burgess R, Elledge SJ, Olson EN ( 1995) PICK1: a perinuclear binding protein and substrate for protein kinase C isolated by the yeast two-hybrid system. J Cell Biol 128: 263-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger J, Lu J, Olson EN ( 1997) Specific interaction of the PDZ domain protein PICK1 with the COOH terminus of protein kinase C-alpha. J Biol Chem 272: 32019-32024. [DOI] [PubMed] [Google Scholar]
- Tanikawa C, Matsuda K, Fukuda S, Nakamura Y, Arakawa H ( 2003) p53RDL1 regulates p53-dependent apoptosis. Nat Cell Biol 5: 216-223. [DOI] [PubMed] [Google Scholar]
- Tong J, Killeen MT, Steven R, Binns KL, Culotti JG, Pawson T ( 2001) Netrin stimulates tyrosine phosphorylation of the UNC-5 family of netrin receptors and induces Shp2 binding to the RCM cytodomain. J Biol Chem 276: 40917-40925. [DOI] [PubMed] [Google Scholar]
- Torres GE, Yao WD, Mohn AR, Quan H, Kim KM, Levey AI, Staudinger J, Caron MG ( 2001) Functional interaction between monoamine plasma membrane transporters and the synaptic PDZ domain-containing protein PICK1. Neuron 30: 121-134. [DOI] [PubMed] [Google Scholar]
- Torres R, Firestein BL, Dong H, Staudinger J, Olson EN, Huganir RL, Bredt DS, Gale NW, Yancopoulos GD ( 1998) PDZ proteins bind, cluster, and synaptically colocalize with Eph receptors and their ephrin ligands. Neuron 21: 1453-1463. [DOI] [PubMed] [Google Scholar]
- Vojtek AB, Hollenberg SM, Cooper JA ( 1993) Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell 74: 205-214. [DOI] [PubMed] [Google Scholar]
- Williams ME, Strickland P, Watanabe K, Hinck L ( 2003) UNC5H1 induces apoptosis via its juxtamembrane region through an interaction with NRAGE. J Biol Chem 278: 17483-17490. [DOI] [PubMed] [Google Scholar]
- Xia J, Zhang X, Staudinger J, Huganir RL ( 1999) Clustering of AMPA receptors by the synaptic PDZ domain-containing protein PICK1. Neuron 22: 179-187. [DOI] [PubMed] [Google Scholar]
- Xia J, Chung HJ, Wihler C, Huganir RL, Linden DJ ( 2000) Cerebellar long-term depression requires PKC-regulated interactions between GluR2/3 and PDZ domain-containing proteins. Neuron 28: 499-510. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Li Y, Zhang Z, Cui K, Wang S, Yuan XB, Wu CP, Poo MM, Duan S ( 2002) Nerve growth cone guidance mediated by G protein-coupled receptors. Nat Neurosci 5: 843-848. [DOI] [PubMed] [Google Scholar]


