Abstract
In mammals, the ventilatory response to decreased oxygen tension in the arterial blood is initiated by excitation of specialized O2-sensitive chemoreceptor cells in the carotid body that release neurotransmitters to activate endings of the sinus nerve afferent fibers. We investigated the role of ATP acting via ionotropic P2X receptors in the carotid body function and ventilatory response to hypoxia in mice. Mice deficient in P2X2 receptor subunit showed a markedly attenuated ventilatory response to hypoxia, whereas the response to hypoxia in P2X3-deficient mice was comparable with that seen in wild-type controls. P2X2 and P2X3 receptor subunit deficiency did not affect the ventilatory responses to hypercapnia. P2X2 subunit deficiency resulted in a dramatic reduction in the responses of the carotid sinus nerve to hypoxia in the in vitro carotid body-sinus nerve preparation. ATP and its stable analog α,β-methyleneATP both evoked rapid excitation of sinus nerve afferents, and the P2 receptor antagonist PPADS (pyridoxal-5′-phosphate-6-azophenyl-2′,4′-disulphonic acid) (100 μm) blocked hypoxia-induced increase in sinus nerve discharge. Immunoreactivities for P2X2 and P2X3 subunits were both detected on afferent terminals surrounding clusters of glomus cells in the wild-type animals but were absent in mice deficient in P2X2 and P2X3 receptor subunits. These observations provide the first definitive evidence that, in the carotid body, ATP is a key transmitter released by chemoreceptor cells to activate endings of the sinus nerve afferent fibers. We conclude that P2X receptors containing the P2X2 subunit play a pivotal role in carotid body function and in mediating ventilatory responses to hypoxia.
Keywords: ATP, carotid body, chemosensitivity, hypercapnia, hypoxia, knock-out, P2X, purines, respiration, ventilation
Introduction
Oxygen sensing is necessary for the adaptation of animals and humans to variable environmental and physiological conditions. Physiological responses to the reduction of oxygen in the arterial blood or the airways involve activation of highly specialized oxygen-sensing neurosecretory cells located in the carotid and the aortic bodies and in the pulmonary neuroepithelial bodies (NEBs) (Gonzalez et al., 1994; Forster et al., 2000; Prabhakar, 2000; Peers and Kemp, 2001). In the adult, however, glomus cells of the carotid body are the major peripheral chemosensitive elements (Gonzalez et al., 1994; Prabhakar, 2000; Lahiri et al., 2001). These cells detect changes in arterial PO2 and transmit this information to the afferent nerve fibers of the carotid sinus nerve, which relays to the brainstem respiratory centers to produce adaptive changes in ventilation. To date, the identity of the transmitter(s) mediating the activation of chemoreceptor afferents is uncertain (Lahiri et al., 2001). Carotid bodies synthesize and release a range of transmitters, including dopamine, acetylcholine (ACh), serotonin, nitric oxide, and neuropeptides (Fitzgerald, 2000; Vicario et al., 2000; Kim et al., 2001). Until recently, it has, however, proved impossible to block the afferent nerve responses to hypoxia using receptor antagonists of many putative transmitters.
There has been increasing interest in the role of purinergic signaling in peripheral sensory transduction (Burnstock, 2000). ATP activates sensory neurons via P2X receptors, a family of ligand-gated ion channels (North and Surprenant, 2000; North, 2002). Two P2X subunits, P2X2 and P2X3, forming either homomeric P2X2 and P2X3 or heteromeric P2X2/3 receptors, mediate the rapid excitation of sensory neurons by ATP (Lewis et al., 1995; Thomas et al., 1998; Dunn et al., 2001). ATP stimulates chemoreceptor afferents, and, recently, experiments in a coculture preparation consisting of dispersed rat carotid body type 1 (glomus) cells and dissociated petrosal neurons showed that ATP might be involved in chemotransmission in the carotid body (Zhang et al., 2000; Prasad et al., 2001). Zhang et al. (2000) showed that a hypoxic stimulus was sensed at the level of glomus cells and that the transmission of this information to nearby petrosal neurons was blocked by a coapplication of the P2 receptor antagonist suramin with a nicotinic ACh receptor blocker. This suggests that the corelease of ATP and ACh may play a role in chemotransmission in the carotid body. However, suramin has a wide spectrum of pharmacological actions. In addition, McQueen et al. (1998) showed that some cardiorespiratory responses evoked by P2X agonists are not blocked by suramin. Earlier, Spergel and Lahiri (1993) concluded that the carotid body has surface ATP receptors that may mediate the chemosensory response to nicotine but not to hypoxia.
To obtain the conclusive evidence for the role of ATP-mediated purinergic signaling in the carotid body, we investigated the ventilatory responses and the carotid body afferent nerve sensitivity to hypoxia in mice with selective deletion of genes encoding P2X2 (P2X2-/-), P2X3 (P2X3-/-), or both subunits (P2X2/P2X3Dbl-/-). We show that mice deficient in P2X2 receptor subunit have an attenuated ventilatory response to hypoxia and an impaired responsiveness of the carotid sinus nerve to decrease in PO2, suggesting that ATP plays a pivotal role in the function of peripheral chemoreceptors.
Materials and Methods
Animals. P2X3-/- mice have been described previously in detail (Cockayne et al., 2000). P2X2-/- mice were generated by introducing a deletion encompassing exons 2-11 into the mouse P2X2 gene (Cockayne et al., 2002). P2X2/P2X3Dbl-/- mice were generated by conventional breeding of P2X2-/- and P2X3-/- mice to eventually generate mice carrying a deletion in both the P2X2 and P2X3 genes. All mutant mice used in this study were on the 129Ola × C57BL/6 genetic background and were derived from homozygous breeding pairs, in which each mutant line was compared with its own filial generation-matched wild-type controls. A description of the generation and general characterization of the P2X2-/- and P2X2/P2X3Dbl-/- mice has been presented previously (Cockayne et al., 2002).
Adult (4-6 months) P2X2-/-, P2X3-/-, and P2X2/P2X3Dbl-/- mice and their wild-type littermates were used for whole-body plethysmograghy and in vitro sinus nerve recordings. All experiments were performed in accordance with the United Kingdom Animals (Scientific Procedures) Act of 1986.
Whole-body plethysmography. Respiratory rate (fR, breaths per minute) and tidal volume (VT, milliliters per kilogram of body weight) in conscious mice were measured by whole-body plethysmography according to the method described previously (Onodera et al., 1997; Kline et al., 2002). The mouse was placed in a Plexiglas recording chamber that was flushed continuously with a mixture of 79% nitrogen and 21% oxygen (unless otherwise required by the protocol) at a rate of 500 ml/min. Respiratory rate (fR) and tidal volume (VT) were determined by the pressure changes in the chamber. Concentrations of O2 and CO2 in the chamber were monitored online using a fast-response O2-CO2 monitor (Morgan Medical, Rainham, UK). The animals were allowed at least 30 min to acclimatize to the chamber environment at normoxia-normocapnia (21% O2, 79% N2, and <0.3% CO2) before measurements of baseline ventilation were taken. After measurements of baseline ventilation were made in conditions of normoxia, the O2 concentration in the inspired air was reduced in steps of 2.5%, down to a level of 7.5%. Similarly, in separate experiments, hypercapnia was induced by titrating CO2 into the respiratory mixture in steps of 1.5%, up to a level of 6%. Each step was maintained until ventilation had stabilized. The pressure signal was amplified, filtered, recorded, and analyzed offline using Spike 2 software (Cambridge Electronic Design, Cambridge, UK). Hypoxia- or hypercapnia-induced changes in the fR, VT and minute ventilation (VE; fR × VT; milliliters per minute per kilogram) relative to the baseline were averaged and expressed as means ± SE. Comparisons among experimental groups were made using one-way ANOVA, followed by the post hoc Scheffe's test. p values <0.05 were considered significant.
In vitro sinus nerve recording. Animals were killed by exposure to increasing concentrations of inspired CO2 and were decapitated at the cervical level. The head was placed in a dissecting chamber with circulating ice-cold Krebs' solution saturated with 95% O2 plus 5% CO2. The carotid bifurcation region containing the carotid body and the attached sinus nerve was carefully dissected under a microscope and was placed into a recording chamber (3 ml). The tissue was superfused with oxygenated Krebs' solution. Perfusion rate was 15 ml/min, and the chamber temperature was kept at 34°C. PO2 in the perfusate was monitored online (World Precision Instruments, Sarasota, FL). The sinus nerve was desheathed, and recordings were made using a suction electrode. Nerve activity was amplified (20,000×) and filtered (200-3000 Hz), and recordings were stored by computer using a Spike 2 data capture and analysis program. Single-unit analysis was performed using the spike-sorting function of the Spike 2 program. ATP (1-3 mm) and α,β-methyleneATP (α,β-meATP) (0.1-1 mm) were applied by injection as a bolus (0.3 ml) into the chamber. P2 receptor antagonist pyridoxal-5′-phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS) was applied by switching the superfusate to O2-saturated Krebs' solution containing the compound (30-100 μm). The carotid body was challenged with hypoxic solutions (Krebs' solution saturated with 95% N2 plus 5% CO2) for a period of 3 min at the intervals of 15 min. Comparisons among experimental groups were made using Student's t test. p values <0.05 were considered significant.
Immunohistochemistry. The carotid bifurcation region was fixed in 4% paraformaldehyde for 2 hr, and 10 μm serial sections were cut using a Leica (Nussloch, Germany) cryostat. Sections were incubated for 30 min at room temperature in a preblock solution (10% normal horse serum and 0.1% Triton X-100 in PBS), followed by three 5 min washes in PBS. Sections were then incubated for 12 hr at room temperature with the primary antibodies, rabbit-anti-rat P2X2 IgG (1:800; Roche Bioscience, Palo Alto, CA), or rabbit-anti-rat P2X3 IgG (1:500; Roche Bioscience). After three 10 min washes in PBS, the sections were incubated for 1 hr in donkey anti-rabbit IgG conjugated to Cy3 (1:500 dilution; Jackson ImmunoResearch, West Grove, PA). Sections were then washed three times for 10 min each in PBS, air dried onto glass slides, mounted under a coverslip using VectaMount (Vector Laboratories, Burlingame, CA), and viewed under a Zeiss (Jena, Germany) Axioplan fluorescent microscope using the appropriate filter sets or under a Zeiss inverted laser-scanning confocal microscope (model 510 CLSM).
Results
P2X2-/- mice, like the P2X3-/- mice generated previously (Cockayne et al., 2000), are outwardly healthy and show no signs of gross pathology. In contrast, P2X2/P2X3Dbl-/- mice show a high degree of mortality, with >90% of these mice dying between birth and weaning. Postmortem examination has shown pulmonary infection and bronchial pneumonia in these mice. Hence, the P2X2/P2X3Dbl-/- mice used in this study represent the ∼10% of the double-mutant mice that survived through weaning. These mice showed no gross pathological abnormalities.
Impaired ventilatory responses to hypoxia in P2X2-/- and P2X2/P2X3Dbl-/- mice
Adult mice lacking P2X2, P2X3, or both P2X subunits were exposed to graded levels of hypoxia (15, 10, and 7.5% O2 in the inspired air), and fR and VT were monitored. The resting ventilation during normoxia was identical in all groups of animals. With decreasing levels of O2, wild-type mice showed increased rate and depth of breathing and hence an increased minute volume (Fig. 1A). The response to mild hypoxia (15% O2 in the inspired air) was not significantly different (p > 0.05) in the P2X2-/-, P2X3-/-, and P2X2/P2X3Dbl-/- mice compared with their wild-type counterparts. However, when O2 concentration in the inspired air was lowered further, P2X2-/- and P2X2/P2X3Dbl-/- mice showed a markedly diminished ventilatory response compared with P2X2+/+ and P2X2/P2X3Dbl+/+ mice, respectively (Fig. 1A). Thus, in the atmosphere of 10% O2, minute ventilation (VE) in P2X2+/+ mice increased by 1008 ± 154 ml · min-1 · kg-1 and in P2X2/P2X3Dbl+/+ mice by 979 ± 161 ml · min-1 · kg-1, whereas in P2X2-/- and P2X2/P2X3Dbl-/- mice, VE increased by only 261 ± 202 ml · min-1 · kg-1 (p = 0.017) and 129 ± 154 ml · min-1 · kg-1 (p = 0.003), respectively. When the O2 concentration was lowered to 7.5%, there was no additional increase in ventilation in the wild-type animals, although VE remained higher than during normoxia. In P2X2-/- and P2X2/P2X3Dbl-/- mice profound respiratory depression was observed. The VE decreased below prehypoxic levels by 606 ± 209 ml · min-1 · kg-1 (p = 0.002) and 555 ± 137 ml · min-1 · kg-1 (p = 0.003) in P2X2-/- and P2X2/P2X3Dbl-/- mice, respectively. The ventilatory response of P2X3-/- mice to hypoxia was not significantly different to that of P2X3+/+ mice (Fig. 1A).
Figure 1.
The P2X2 receptor subunit is essential for the normal ventilatory response to hypoxia in mice. A, Respiratory responses to varying levels of hypoxia in conscious P2X2-deficient mice (P2X2-/-), in P2X3-deficient mice (P2X3-/-), in mice deficient in both P2X2 and P2X3 receptor subunits (P2X2/P2X3Dbl-/-), and in the respective wild-type control mice (P2X2+/+, P2X3+/+, and P2X2/P2X3Dbl+/+). B, Respiratory responses to varying levels of hypercapnia in P2X2/P2X3Dbl-/- and P2X2/P2X3Dbl+/+ mice. Data represents the mean ± SE. Numbers in parentheses indicate sample sizes. fR, Respiratory rate; VT, tidal volume; VE, minute ventilation (fR × VT). *p < 0.05 indicates significant difference (ANOVA; post hoc Scheffe's test).
Hypoxia-induced decreases in body temperature in P2X2-/- and P2X2/P2X3Dbl-/- mice were not significantly different to those observed in the respective wild-type animals. Thus, in the atmosphere of 7.5% O2, body temperature in P2X2+/+ mice decreased by 0.8 ± 0.1°C, in P2X2-/- mice by 0.9 ± 0.2°C (p > 0.05), in P2X2/P2X3Dbl+/+ mice by 1.1 ± 0.3°C, and in P2X2/P2X3Dbl-/- mice by 1.1 ± 0.2°C (p > 0.05).
Hypercapnia induced a profound increase in ventilation in all groups of animals (Fig. 1B). However, there were no significant differences in any measures of ventilation (fR and VT) among P2X2/P2X3Dbl-/- and their respective wild-type mice when the concentration of CO2 in the inspired air increased to 3% and then to 6% (Fig. 1B).
Impaired carotid body function in P2X2-/- and P2X2/P2X3Dbl-/- mice
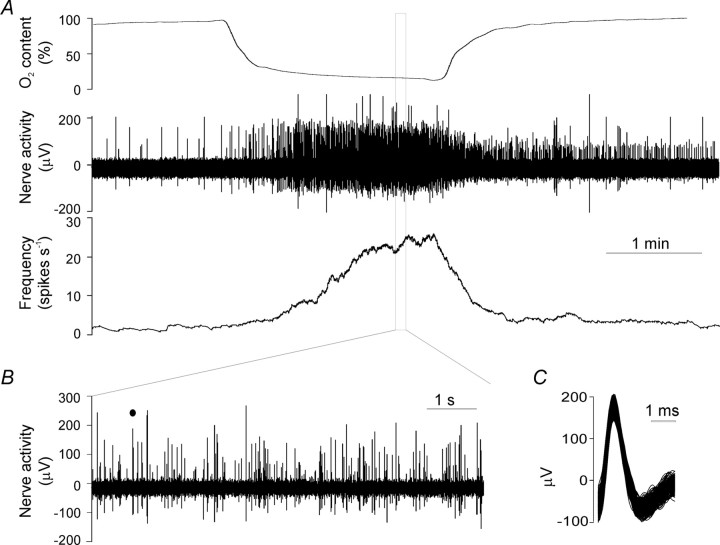
We recorded electrophysiologically the activity of the carotid sinus nerve in the carotid body-sinus nerve preparation taken from wild-type and P2X2-/-, P2X3-/-, or P2X2/P2X3Dbl-/- mice. Figure 2 is a representative recording of the afferent nerve response to hypoxia in a preparation taken from a wild-type animal. In agreement with previous reports, carotid body afferents exhibited ongoing activity under normal conditions. This discharge sharply increased when PO2 fell below 50% and rose progressively with the additional fall in PO2. The level of ongoing activity and the amplitude of the responses to intermittent hypoxia (at intervals of ∼15 min) usually stabilized after two to three hypoxic challenges and remained stable for 1-3 hr (see Fig. 6A).
Figure 2.
A representative recording of the afferent nerve responses to hypoxia in the isolated carotid body-sinus nerve preparation taken from the wild-type mouse. A, Typical traces of changes in PO2, raw nerve activity, and the firing frequency. B, Expanded view of the nerve activity during hypoxia shows that the afferent recording consists of discharges of several single units that can be discriminated using the Spike2 program. C, Superimposed action potentials of a single unit marked (filled circle) in B.
Figure 6.
Effects of the P2 receptor antagonist PPADS on the responses of chemoreceptor afferents to hypoxia. A, PPADS (30 μm) reduces afferent discharge in response to hypoxia reversibly. B, Accumulative dose of PPADS (100 μm) virtually abolishes the afferent response to hypoxia. C, PPADS reduces the peak discharge frequency of single afferent units (n = 12) during hypoxia. **p < 0.01 (paired Student's t test).
In preparations taken from wild-type mice, sinus nerve discharge increased from a baseline of 6.19 ± 1.52 spikes/sec to a peak of 130.82 ± 10.79 spikes/sec (n = 22) during hypoxia (Fig. 3A). The hypoxia-induced increase in sinus nerve activity was similar in preparations from P2X2+/+ (n = 7), P2X3+/+ (n = 7), and P2X2/P2X3Dbl+/+ (n = 8) mice. However, the afferent discharge in preparations from P2X2-/- mice only reached a peak of 58.13 ± 9.40 spikes/sec (n = 7; p < 0.001 compared with P2X2+/+), and the response was even smaller in preparations from P2X2/P2X3Dbl-/- mice (peak discharge, 32.80 ± 6.97 spikes/sec; n = 9; p < 0.001 compared with P2X2/3+/+ or P2X2-/-). The increase in sinus nerve activity evoked by hypoxia was similar in preparations from P2X3-/- (peak discharge, 112.04 ± 22.87 spikes/sec; n = 7; p > 0.05 compared with P2X3+/+) and P2X3+/+ mice.
Figure 3.
Impaired carotid body function in P2X2-deficient mice. The plot of average firing rate (in spikes per second) of the whole nerve activity during hypoxic challenges. Data from P2X2+/+ (n = 7), P2X3+/+ (n = 7), and P2X2/P2X3Dbl+/+ (n = 8) mice are combined. *p < 0.05 and **p < 0.01 indicate significant difference compared with the wild-type animals (Student's t test).
In many experiments, recordings of the afferent activity consisted of several units with distinguishable spike amplitude and shape (Fig. 2B,C). This allowed analysis and comparison of single-unit activity between wild-type and knock-out animals. Thus, the average peak firing rate of single units during hypoxia was 7.96 ± 0.88, 8.22 ± 0.74, and 7.76 ± 0.99 spikes/sec in the carotid body-sinus nerve preparations from P2X2+/+ (n = 30), P2X3+/+ (n = 38), and P2X2/P2X3Dbl+/+ (n = 36) mice, respectively (p > 0.05). The peak activity induced by hypoxia was significantly lower in preparations taken from P2X2-/- mice (1.64 ± 0.11 spikes/sec; n = 55; p < 0.001 compared with P2X2+/+) and in preparations taken from P2X2/P2X3Dbl-/- mice (1.19 ± 0.10 spikes/sec; n = 47; p < 0.001 compared with P2X2/P2X3Dbl+/+ and p < 0.05 compared with P2X2-/-). The single-unit response to hypoxia in preparation from P2X3-/- mice (7.73 ± 0.69 spikes/sec; n = 48) was not significantly different from that in P2X3+/+ mice (p > 0.05).
Pharmacological evidence that during hypoxia ATP activates chemoreceptor afferents of the sinus nerve in the carotid body
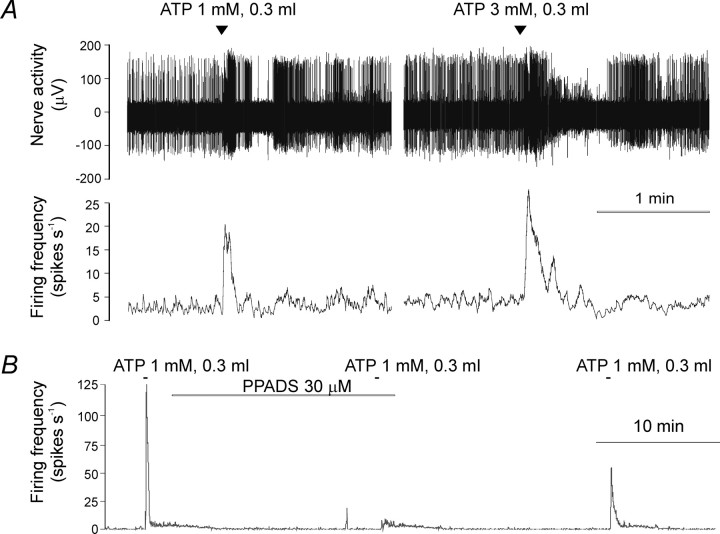
To investigate the effects of ATP and its stable analog α,β-meATP on the sinus nerve activity, we applied the agonists (0.3 ml) into the bath medium. In preparations taken from the wild-type mice, both ATP and α,β-meATP evoked an immediate burst of discharge (Figs. 4, 5). The initial burst of discharge was followed by a short period of inhibition in 6 of 15 preparations and a sustained phase of increased firing (lasting 70-260 sec) in the remaining nine preparations. The response to ATP or α,β-meATP could be blocked by the P2 receptor antagonist PPADS (Fig. 4B). In preparations taken from P2X2-/- mice (Fig. 5), ATP and α,β-meATP also evoked increases in afferent activity that lasted only 5-20 sec. In preparations taken from P2X3-/- mice (Fig. 5), ATP, but not α,β-meATP, evoked a rapid increase in sinus nerve discharge. In preparations from P2X2/P2X3Dbl-/- mice (Fig. 5), ATP, as well as α,β-meATP, induced only a moderate, delayed (65-120 sec after administration) and sustained increase in sinus nerve afferent activity. This delayed response to ATP or α,β-meATP appeared to be more prominent in the P2X2/P2X3Dbl-/- than in the wild-type preparations. None of the P2X2/P2X3Dbl-/- preparations showed a rapid response to ATP or α,β-meATP.
Figure 4.
Activation of chemoreceptor afferent fibers by ATP. A, Effects of ATP (1 and 3 mm, 0.3 ml) on the sinus nerve activity. Note that a short period of inhibition after the initial burst of discharge was seen in the afferent units with larger spikes. B, The P2 receptor antagonist PPADS (30 μm) reversibly blocks the effects of ATP.
Figure 5.
Effects of ATP and its stable analog α,β-meATP on the carotid sinus nerve activity in wild-type mice and in P2X2- and/or P2X3-deficient mice. Note that ATP and α,β-meATP evoke different patterns of changes in the afferent activity in the wild-type (A) and P2X2 and/or P2X3 (B-D)-deficient preparations.
We also investigated the effects of the P2 receptor antagonist PPADS on the responses of chemoreceptor afferents to hypoxia in carotid body-sinus nerve preparations taken from the wild-type animals. We found that PPADS attenuates afferent responses to hypoxia. At 30 μm, PPADS reversibly reduced sinus nerve background discharge (from 5.43 ± 0.74 to 1.30 ± 0.06 spikes/sec; n = 5; p < 0.001) and also attenuated the response to hypoxia (from 144 ± 27.9 to 76.8 ± 13.5 spikes/sec; n = 5; p < 0.001) (Fig. 6). At a concentration of 100 μm, PPADS induced a profound and irreversible reduction in the background activity and virtually blocked the hypoxia-induced increase in sinus nerve afferent discharge (Fig. 6). Likewise, the carotid sinus nerve single-unit response to hypoxia was dose dependently blocked by PPADS (Fig. 6C).
Immunohistochemical identification of the P2X2 and P2X3 receptor subunits in the carotid body
The hematoxylin and eosin (HE) staining of the carotid bifurcation region observed under the light microscope did not show significant differences in the morphology of the carotid bodies between the wild-type and knock-out mice (Fig. 7). Consistent with previous studies in rats (Prasad et al., 2001), extensive staining for both P2X2 and P2X3 receptor subunits was found in the carotid bodies of wild-type mice (Fig. 7). The patterns of staining for P2X2 and P2X3 receptor subunits were similar in that both appear to be on the afferent terminals surrounding clusters of glomus cells rather than on the surface of the glomus cells themselves. In the P2X2-/- animals, P2X2 immunoreactivity was not detected in the carotid bodies, whereas P2X3 staining remained. Conversely, only P2X2 immunoreactivity was detected in the carotid bodies of P2X3-/- mice. In P2X2/P2X3Dbl-/- animals, neither P2X2 nor P2X3 immunoreactivities were detected.
Figure 7.
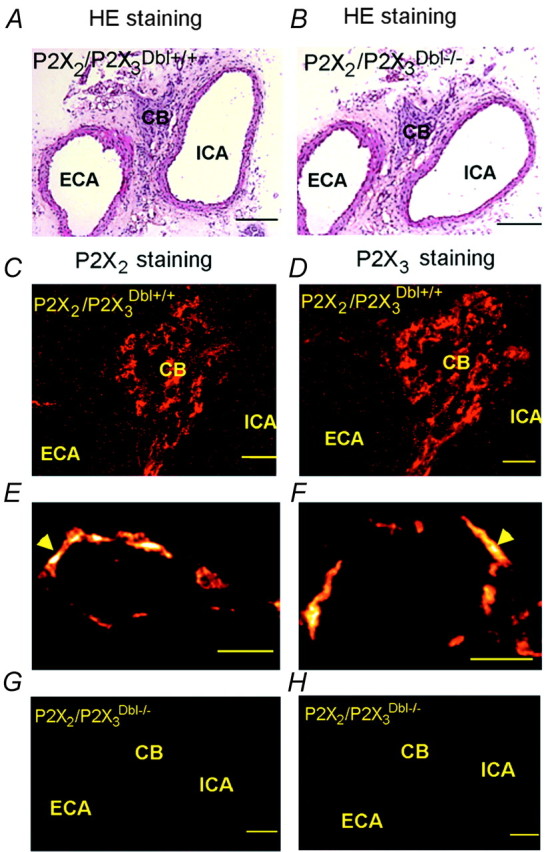
Immunolocalization of P2X2 and P2X3 receptor subunits in the mouse carotid body. A, B, HE staining of the carotid bifurcation region of a P2X2/P2X3Dbl+/+ and a P2X2/P2X3Dbl-/- mouse. Scale bars, 100 μm. C, D, Immunofluorescent staining for P2X2 and P2X3 subunit in coronal sections of the carotid bifurcation region taken from a P2X2/P2X3Dbl+/+ mouse. Scale bars, 20 μm. E, F, Immunofluorescence for P2X2 and P2X3 subunit observed under a confocal microscope. Note the ring-shaped staining pattern. Scale bar, 10 μm. G, H, Absence of P2X2 and P2X3 immunoreactivity in the carotid body of a P2X2/P2X3Dbl-/- mouse. Scale bars, 100 μm. CB, Carotid body; ECA, external carotid artery; ICA, internal carotid artery.
Discussion
It has been shown previously that ATP can stimulate carotid body chemoreceptor afferents (Spergel and Lahiri, 1993; McQueen et al., 1998; Alcayaga et al., 2000) and that the P2 receptor antagonist suramin, when coapplied with nicotinic ACh receptor antagonist, can block hypoxia-induced increase in chemoreceptor afferent neuron discharge (Zhang et al., 2000). Immunoreactivities for P2X2 and P2X3 receptor subunits have also been localized to the rat carotid body afferents (Prasad et al., 2001). However, this study provides the first definitive evidence that ATP, acting via P2X receptors that contain the P2X2 subunit, plays a pivotal role in the normal carotid body function. We demonstrate that, in mice, P2X2 deficiency results in an attenuation of the ventilatory response to hypoxia and in a dramatic reduction in the responses of the carotid sinus nerve to a decrease in PO2 in the in vitro carotid body-sinus nerve preparation. We show that ATP and its stable analog α,β-meATP evoke rapid excitation of the sinus nerve afferents and that the P2 receptor antagonist PPADS can virtually abolish hypoxia-induced increase in sinus nerve discharge.
Of the seven cloned P2X receptor subtypes, P2X2 and P2X3 subunits are believed to be most associated with sensory function (Burnstock, 2000; Dunn et al., 2001). P2X3 receptor subunit is almost exclusively expressed in small-diameter sensory fibers, and P2X2 subunit can coassemble with P2X3 subunits to form heteromeric P2X2/3 receptors (Lewis et al., 1995; Thomas et al., 1998). Subtype-selective P2X receptor antagonists are not yet readily available, making it difficult to determine the receptor subtypes that mediate the biological action of extracellular ATP. Comparison of the effects of ATP and α,β-meATP on sinus nerve discharge in mice with selective deletion of P2X2 and/or P2X3 subunits allows us to conclude that homomeric P2X2, P2X3, and heteromeric P2X2/3 receptors may all be involved in mediating the excitation of sinus nerve evoked by hypoxia or ATP application. Indeed, in preparations taken from wild-type mice, both ATP and α,β-meATP evoke an immediate burst of discharge, precluding homomeric P2X2 receptors being solely responsible for that response. In the preparations from P2X2-/- mice, ATP and α,β-meATP evoke very short increases in duration of afferent activity. This is consistent with a homomeric P2X3-mediated rapidly desensitizing current as observed in sensory neurons. In preparations taken from P2X3-/- mice, ATP, but not α,β-meATP, evokes an increase in sinus nerve discharge, indicating that homomeric P2X2 receptors probably mediate this response. Furthermore, although hypoxia-induced sinus nerve activity in P2X3-/- mice is similar to that in the wild-type mice, the afferent response evoked by hypoxia is much smaller in carotid body-sinus nerve preparations taken from P2X2/P2X3Dbl-/- mice compared with that in P2X2-/- mice, indicating that the P2X3 receptor subunit is also involved in chemosensory transmission. Finally, consistent with previous studies in rats (Prasad et al., 2001), we found that both P2X2 and P2X3 subunit immunoreactivities are present on afferent terminals of the sinus nerve surrounding clusters of glomus cells. Thus, these data indicate that sinus nerve terminals contain functional homomeric P2X2 and P2X3 receptors, as well as heteromeric P2X2/3 receptors. This conclusion is supported by the evidence that variable proportions of homomeric P2X2, P2X3, and heteromeric P2X2/3 receptors mediate ATP-evoked responses in sensory neurons (Thomas et al., 1998; Dunn et al., 2001).
ATP is unlikely to be involved in the primary oxygen-sensing mechanism (i.e., the excitation of chemoreceptor cells in conditions of low PO2) because, in the carotid body, P2X2 and P2X3 immunoreactivities were detected on nerve terminals rather than on the glomus cells. This conclusion is further supported by a recent report that ATP had no effects on dissociated type 1 cells and evokes only a transient rise in intracellular Ca2+ in type 2 cells through activation of P2Y2 receptors (Xu et al., 2003). Incidentally, we observed a delayed increase in afferent nerve activity after the rapid burst of discharge induced by ATP or α,β-meATP. This delayed response remains and appears to be enhanced in the absence of P2X2 and P2X3 subunits (Fig. 5). Whether P2Y2 receptors (or other P2Y receptors) on type 2 cells mediate this delayed afferent response by modulating the excitability of type 1 cells has to be determined.
Obviously, ATP cannot be viewed as the only transmitter involved in chemotransmission in the carotid body, given the overwhelming evidence that the carotid body can synthesize and release a range of transmitters, including ACh, dopamine, 5-HT, nitric oxide, and neuropeptides (Fitzgerald, 2000; Vicario et al., 2000; Kim et al., 2001; Lahiri et al., 2001). We found that hypoxia induces some small increase in carotid sinus nerve discharge even in P2X2/P2X3Dbl-/- mice (Fig. 3), indicating that other transmitters may mediate this residual response. Zhang et al. (2000) have demonstrated using cocultures of the rat carotid body chemoreceptors and petrosal neurons that both ATP and ACh may act as cotransmitters during chemotransduction in the carotid body. However, recent evidence obtained in the rabbit carotid body indicates that hypoxia may in fact inhibit ACh release by the glomus cells (Kim et al., 2003). Thus, given the available data, it seems justified at present to consider ATP as the key transmitter acting postsynaptically on sensory nerve endings. Other transmitters may act as presynaptic and/or postsynaptic modulators. In this sense, whereas P2X2 and P2X3 subunits are expressed exclusively on nerve endings, receptors for ACh, dopamine, and 5-HT were found on both presynaptic and postsynaptic membranes (Lahiri et al., 2001).
Detection of arterial PO2 levels by chemoreceptors and the transmission of the information to sensory nerve endings are the early steps in a series of events underlying the coordinated ventilatory response to hypoxia. Accordingly, we found that mice deficient in P2X2 subunit showed a significantly attenuated ventilatory response to hypoxia as well as an impaired responsiveness of the sinus nerve to decrease in PO2. However, the unexpected finding of the present study was that, in mice, the ventilatory response to increasing levels of CO2 in the inspired air was unaffected by P2X2 (and P2X3) receptor subunit deficiency. This seems to contradict the findings of Prasad et al. (2001), who showed that the afferent nerve response to hypercapnia could be blocked by P2 receptor antagonist suramin, and our results (Thomas et al., 1999) showing that suramin also attenuates the ventilatory response to hypercapnia when applied into the rostral ventrolateral medulla, the primary central chemosensitive site. It is generally believed that the ventilatory response to hypercapnia is primarily preserved in experimental animals after denervation of the carotid and aortic bodies. Approximately 80% of the CO2-evoked response is mediated by the action of CO2 at the brainstem chemosensitive sites (Heeringa et al., 1979). Hence, one explanation for the normal ventilatory response to hypercapnia as opposed to the impaired response to hypoxia in P2X2-deficient mice might be that central chemoreceptors are able to compensate fully for the loss of peripheral CO2 sensitivity in these mice. On the other hand, taking into account the evidence that ATP, through interactions with certain P2X receptors, is a mediator of central CO2 chemosensitivity as well (Spyer and Thomas, 2000), we also conclude that P2X receptors other than P2X2 or P2X3 receptors might be involved in mediating hypercapnia-induced changes in ventilatory activity.
There is evidence that ATP acting via P2X receptors modulates respiratory (phrenic and hypoglossal) motoneuron activity (Funk et al., 1997; Miles et al., 2002). Thus, it is possible that the reduced ventilatory responses to hypoxia in P2X2-deficient mice could be partly attributable to the absence of this modulation. However, this possibility can be ruled out because no difference in hypercapnia-induced increase in ventilation was found among P2X2/P2X3Dbl-/- and the respective wild-type mice, indicating that P2X2 deficiency does not impair the ability of mutant animals to mount an adequate ventilatory response to stimuli other than hypoxia.
In addition to the carotid bodies, the aortic bodies also function as peripheral chemoreceptors detecting acute changes in arterial PO2 levels, although the presence and extent of the aortic bodies is highly variable (for review, see Daly, 1997). Furthermore, the NEBs detect airway O2 levels (Cutz and Jackson, 1999; Peers and Kemp, 2001) and have been implicated in ventilatory control in neonates, when the carotid body is not yet fully developed (Carroll et al., 1993; Bolle et al., 2000). It is conceivable that chemoreceptive cells in the NEBs might also transmit information about levels of inspired O2 through the release of ATP to stimulate sensory endings. This is supported by the study of Brouns et al. (2000), which showed intensive P2X3 subunit immunoreactivity on vagal afferent terminals innervating the NEBs. Furthermore, these authors also showed that NEB cells accumulate quinacrine, an indicator of ATP-secreting cells. It is thus possible that impaired NEB and carotid body chemosensory function might be responsible for the high mortality among preweaning mice deficient in both P2X2 and P2X3 subunits, whereby deficits in the control of breathing in postnatal mice may lead to improper clearing of the lungs and render these mice susceptible to infection and subsequent pneumonia.
The major conclusion of this study, however, remains that ATP acting via P2X receptors that contain the P2X2 subunit, with or without P2X3 subunit, contributes in a significant manner to the transmission of the sensitivity of the carotid body to changes in arterial PO2. The role of P2X receptors in peripheral and central mechanisms of CO2 chemosensitivity remains to be determined.
Footnotes
This work was supported by the Biotechnology and Biological Sciences Research Council (A.V.G., K.M.S.) and The Welcome Trust (W.R., Z.X.). We thank Prof. M. Duchen for his expert help with confocal microscopy.
Correspondence should be addressed to Prof. G. Burnstock, Autonomic Neuroscience Institute, Royal Free and University College Medical School, Rowland Hill Street, London NW3 2PF, UK. E-mail: ucgageb@ucl.ac.uk.
Copyright © 2003 Society for Neuroscience 0270-6474/03/2311315-•$15.00/0
W.R. and A.V.G. contributed equally to this work.
References
- Alcayaga J, Cerpa V, Retamal M, Arroyo J, Iturriaga R, Zapata P ( 2000) Adenosine triphosphate-induced peripheral nerve discharges generated from the cat petrosal ganglion in vitro. Neurosci Lett 282: 185-188. [DOI] [PubMed] [Google Scholar]
- Bolle T, Lauweryns JM, Lommel AV ( 2000) Postnatal maturation of neuroepithelial bodies and carotid body innervation: a quantitative investigation in the rabbit. J Neurocytol 29: 241-248. [DOI] [PubMed] [Google Scholar]
- Brouns I, Adriaensen D, Burnstock G, Timmermans JP ( 2000) Intraepithelial vagal sensory nerve terminals in rat pulmonary neuroepithelial bodies express P2X3 receptors. Am J Respir Cell Mol Biol 23: 52-61. [DOI] [PubMed] [Google Scholar]
- Burnstock G ( 2000) P2X receptors in sensory neurones. Br J Anaesth 84: 476-488. [DOI] [PubMed] [Google Scholar]
- Carroll JL, Bamford OS, Fitzgerald RS ( 1993) Postnatal maturation of carotid chemoreceptor responses to O2 and CO2 in the cat. J Appl Physiol 75: 2383-2391. [DOI] [PubMed] [Google Scholar]
- Cockayne D, Dunn PM, Burnstock G, Ford A ( 2002) Generation and electrophysiological characterization of P2X2 and P2X2/P2X3 knockout (KO) mice. Soc Neurosci Abstr 28: 52.12. [Google Scholar]
- Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford AP ( 2000) Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 407: 1011-1015. [DOI] [PubMed] [Google Scholar]
- Cutz E, Jackson A ( 1999) Neuroepithelial bodies as airway oxygen sensors. Respir Physiol 115: 201-214. [DOI] [PubMed] [Google Scholar]
- Daly M ( 1997) Peripheral arterial chemoreception and respiratory-cardiovascular integration. Monograph for the Physiological Society. Oxford: Oxford UP.
- Dunn PM, Zhong Y, Burnstock G ( 2001) P2X receptors in peripheral neurons. Prog Neurobiol 65: 107-134. [DOI] [PubMed] [Google Scholar]
- Fitzgerald RS ( 2000) Oxygen and carotid body chemotransduction: the cholinergic hypothesis—a brief history and new evaluation. Respir Physiol 120: 89-104. [DOI] [PubMed] [Google Scholar]
- Forster HV, Pan LG, Lowry TF, Serra A, Wenninger J, Martino P ( 2000) Important role of carotid chemoreceptor afferents in control of breathing of adult and neonatal mammals. Respir Physiol 119: 199-208. [DOI] [PubMed] [Google Scholar]
- Funk GD, Kanjhan R, Walsh C, Lipski J, Comer AM, Parkis MA, Housley GD ( 1997) P2 receptor excitation of rodent hypoglossal motoneuron activity in vitro and in vivo: a molecular physiological analysis. J Neurosci 17: 6325-6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A, Rigual R ( 1994) Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev 74: 829-898. [DOI] [PubMed] [Google Scholar]
- Heeringa J, Berkenbosch A, de Goede J, Olievier CN ( 1979) Relative contribution of central and peripheral chemoreceptors to the ventilatory response to CO2 during hyperoxia. Respir Physiol 37: 365-379. [DOI] [PubMed] [Google Scholar]
- Kim DK, Summers BA, Prabhakar NR, Kumar GK ( 2001) Neurotransmitter release from the rabbit carotid body: differential effects of hypoxia on substance P and acetylcholine release. Adv Exp Med Biol 499: 39-43. [DOI] [PubMed] [Google Scholar]
- Kim DK, Prabhakar NR, Kumar GK ( 2003) Acetylcholine release from the carotid body by hypoxia: evidence for the involvement of autoinhibitory receptors. J Appl Physiol, in press. [DOI] [PubMed]
- Kline DD, Overholt JL, Prabhakar NR ( 2002) Mutant mice deficient in NOS-1 exhibit attenuated long-term facilitation and short-term potentiation in breathing. J Physiol (Lond) 539: 309-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri S, Rozanov C, Roy A, Storey B, Buerk DG ( 2001) Regulation of oxygen sensing in peripheral arterial chemoreceptors. Int J Biochem Cell Biol 33: 755-774. [DOI] [PubMed] [Google Scholar]
- Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A ( 1995) Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature 377: 432-435. [DOI] [PubMed] [Google Scholar]
- McQueen DS, Bond SM, Moores C, Chessell I, Humphrey PP, Dowd E ( 1998) Activation of P2X receptors for adenosine triphosphate evokes cardiorespiratory reflexes in anaesthetized rats. J Physiol (Lond) 507: 843-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles GB, Parkis MA, Lipski J, Funk GD ( 2002) Modulation of phrenic motoneuron excitability by ATP: consequences for respiratory-related output in vitro. J Appl Physiol 92: 1899-1910. [DOI] [PubMed] [Google Scholar]
- North RA ( 2002) Molecular physiology of P2X receptors. Physiol Rev 82: 1013-1067. [DOI] [PubMed] [Google Scholar]
- North RA, Surprenant A ( 2000) Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol 40: 563-580. [DOI] [PubMed] [Google Scholar]
- Onodera M, Kuwaki T, Kumada M, Masuda Y ( 1997) Determination of ventilatory volume in mice by whole body plethysmography. Jpn J Physiol 47: 317-326. [DOI] [PubMed] [Google Scholar]
- Peers C, Kemp PJ ( 2001) Acute oxygen sensing: diverse but convergent mechanisms in airway and arterial chemoreceptors. Respir Res 2: 145-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar NR ( 2000) Oxygen sensing by the carotid body chemoreceptors. J Appl Physiol 88: 2287-2295. [DOI] [PubMed] [Google Scholar]
- Prasad M, Fearon IM, Zhang M, Laing M, Vollmer C, Nurse CA ( 2001) Expression of P2X2 and P2X3 receptor subunits in rat carotid body afferent neurones: role in chemosensory signalling. J Physiol (Lond) 537: 667-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spergel D, Lahiri S ( 1993) Differential modulation by extracellular ATP of carotid chemosensory responses. J Appl Physiol 74: 3052-3056. [DOI] [PubMed] [Google Scholar]
- Spyer KM, Thomas T ( 2000) Sensing arterial CO2 levels: a role for medullary P2X receptors. J Auton Nerv Syst 81: 228-235. [DOI] [PubMed] [Google Scholar]
- Thomas S, Virginio C, North RA, Surprenant A ( 1998) The antagonist trinitrophenyl-ATP reveals co-existence of distinct P2X receptor channels in rat nodose neurones. J Physiol (Lond) 509: 411-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T, Ralevic V, Gadd CA, Spyer KM ( 1999) Central CO2 chemoreception: a mechanism involving P2 purinoceptors localized in the ventrolateral medulla of the anaesthetized rat. J Physiol (Lond) 517: 899-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicario I, Rigual R, Obeso A, Gonzalez C ( 2000) Characterization of the synthesis and release of catecholamine in the rat carotid body in vitro. Am J Physiol Cell Physiol 278: C490-C499. [DOI] [PubMed] [Google Scholar]
- Xu J, Tse FW, Tse A ( 2003) ATP triggers intracellular Ca2+ release in type II cells of the rat carotid body. J Physiol (Lond) 549: 739-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Zhong H, Vollmer C, Nurse CA ( 2000) Co-release of ATP and ACh mediates hypoxic signalling at rat carotid body chemoreceptors. J Physiol (Lond) 525: 143-158. [DOI] [PMC free article] [PubMed] [Google Scholar]