Abstract
Previous studies addressing the role of the transcription factor cAMP response element-binding protein (CREB) in mammalian long-term synaptic plasticity and memory by gene targeting were compromised by incomplete deletion of the CREB isoforms. Therefore, we generated conditional knock-out strains with a marked reduction or complete deletion of all CREB isoforms in the hippocampus. In these strains, no deficits could be detected in lasting forms of hippocampal long-term potentiation (LTP) and long-term depression (LTD). When tested for hippocampus-dependent learning, mutants showed normal context-dependent fear conditioning. Water maze learning was impaired during the early stages, but many mutants showed satisfactory scores in probe trials thought to measure hippocampus-dependent spatial memory. However, conditioned taste aversion learning, a putatively hippocampus-independent memory test, was markedly impaired. Our data indicate that in the adult mouse brain, loss of CREB neither prevents learning nor substantially affects performance in some hippocampus-dependent tasks. Furthermore, it spares LTP and LTD in paradigms that are sensitive enough to detect deficits in other mutants. This implies either a species-specific or regionally restricted role of CREB in the brain and/or a compensatory upregulation of the cAMP response element modulator (CREM) and other as yet unidentified transcription factors.
Keywords: CREB, synaptic plasticity, LTP, LTD, learning, memory, water maze, fear conditioning, hippocampus, conditioned taste aversion
Introduction
It has become widely accepted that the formation of long-term memory (LTM) and the expression of long-term synaptic plasticity (LTSP) require an activation of transcription and the de novo synthesis of certain proteins (Agranoff et al., 1966; Krug et al., 1984; Frey et al., 1988; Kandel and Pittenger, 1999; Schafe et al., 1999). The most prominent types of synaptic plasticity are long-term potentiation (LTP) and long-term depression (LTD), a robust decline in transmission usually observed after long trains of low-frequency stimulation at 1–5 Hz (Malenka, 1994). Of particular relevance in this context are mechanisms that control the consolidation to lasting forms of synaptic plasticity and memory. A prominent role in these processes has been attributed to the transcription factor cAMP response element (CRE)-binding protein 1 (CREB1), which binds to a regulatory DNA sequence known as CRE (Silva et al., 1998; Mayr and Montminy, 2001). CRE sequences are present in the regulatory regions of many cAMP-responsive genes as well as genes stimulated through other pathways.
Data showing that associative conditioning in Aplysia and LTM of Drosophila are impaired by suppressing CREB-dependent gene transcription (Yin et al., 1994; Bartsch et al., 1995) were apparently confirmed in mice soon afterward (Bourtchuladze et al., 1994). Thus, mice with a targeted Creb hypomorphic mutation were reported to show profound deficits in LTP and LTM. In addition, evidence that CREB also has a critical role in LTD has been presented (Deisseroth et al., 1996; Ahn et al., 1999). Together, these findings endorsed the hypothesis that CREB represents a “memory modulator,” which acts as a “molecular switch” (Yin et al., 1995) in the consolidation of LTSP and the formation of LTM.
However, the findings in hypomorphic Creb mice (Bourtchuladze et al., 1994) could only be partially replicated (Gass et al., 1998). In particular, LTP was not impaired, and water maze learning revealed a genetically dose-dependent effect of hypomorphic alleles on thigmotaxis (wall hugging) but not on probe trial scores. In these mice, the two major physiological isoforms of CREB, α and Δ, were disrupted, whereas a third isoform, CREBβ, as well as several activator and repressor forms of cAMP response element modulator (CREM) were upregulated (Hummler et al., 1994; Blendy et al., 1996). Thus, some characteristics of the phenotype observed in these mice might be attributed to a compensatory upregulation of related transcription factors. Likewise, results might have been biased by the effects of the genetic background (Lipp and Wolfer, 1998; Wolfer and Lipp, 2000), which was not controlled in the first study by Bourtchuladze et al. (1994).
To clarify the role of CREB in hippocampal synaptic plasticity and hippocampus-dependent LTM, we reanalyzed hypomorphic CREB mutants but added two mutant mouse strains with conditional deletion of all CREB isoforms, either throughout the brain or restricted to the CA1 region of the hippocampus and other forebrain areas, resulting in a 70–80% reduction of CREB-containing neurons. Here, we report that neither a marked reduction of hippocampal CREB nor its complete loss in the mouse brain significantly altered hippocampal LTP and LTD. Conditional deletion or reduction of CREB only modestly impaired early stages of water maze learning but did not interfere with contextual fear conditioning. In contrast, it markedly perturbed conditioned taste aversion (CTA), a putatively hippocampus-independent learning task.
Materials and Methods
Generation of mice strains. The generation of the CrebαΔ and Creb- alleles used to raise CrebαΔ and Crebcomp mice is described here and has also been described previously (Hummler et al., 1994; Blendy et al., 1996; Gass et al., 1998; Rudolph et al., 1998). Conditional Creb mutant mice were generated by flanking Creb exon 10 with loxP sites (Mantamadiotis et al., 2002). Mice homozygous for the CrebloxP allele were crossed with transgenic mice possessing a transgene for Cre recombinase, under the control of either the nestin promoter and enhancer (Tronche et al., 1999) or the calcium–calmodulin-dependent protein kinase IIα (CamKIIα) promoter (Otto et al., 2001). Conditionally mutant mice were always homozygous CrebloxP/loxP and carried one Cre transgenic allele, whereas control mice were CrebloxP/loxP. Genotyping was performed by PCR on tail DNA samples as described previously (Mantamadiotis et al., 1998).
Animals. We always coinvestigated groups of control and mutant mice, respectively. The genetic background of the mice was a mixture of 129SvEv and C57BL/6. All experiments were done with 2- to 6-month-old mice, and a similar number of males and females were used. Mice were kept on a 12 hr light/dark cycle. All experiments were performed during the light phase of the cycle, with the exception of the water maze studies, which were conducted during the dark cycle.
Immunohistochemistry. For analysis of CREB expression, mice were perfused with cold 4% paraformaldehyde (PFA), and brains were dissected and postfixed for 16 hr in PFA at 4°C before embedding in 2% agarose in PBS. Agarose-embedded brains were sectioned using a Vibratome cutter at a thickness of 50 μm. CREB antibodies (recognizing an epitope in the N-terminal half; residues 136–150) were used at a dilution of 1:3000 (Bleckmann et al., 2002). Sections were immunostained using the ABC-Vectastain kit (Vector Laboratories, Burlingame, CA) according to the instructions of the manufacturer.
Electrophysiological long-term recordings. Electrophysiological recordings were performed as described previously (Gass et al., 1998). For LTP recordings, slices were incubated in an interface chamber at 32°C. After a 4–5 hr preincubation period, two recording electrodes were positioned in the CA1 dendritic and cell body layer, respectively. For stimulation, two monopolar stainless-steel electrodes were placed in different sublayers of the stratum radiatum. The stimulation strength was adjusted to elicit a population spike of 25% of the maximum. Once stable responses were obtained for 60 min, LTP was induced by three stimulus trains of 100 pulses at 100 Hz, with a 10 min intertrain interval (duration, 0.2 msec per polarity). Four 0.2 Hz biphasic constant-current pulses (0.1 msec per polarity) were used for testing 1, 3, 5, 11, 15, 21, 25, and 30 min after tetanus, and thereafter once every 15 min for ≥6 hr.
For recording LTD, slices were kept in a submerged-type chamber. A glass electrode filled with artificial CSF (1–4 MΩ) was positioned in the apical dendritic layer to record field EPSPs (fEPSPs). For stimulation, a lacquer-coated stainless-steel stimulating electrode was placed into the CA1 stratum radiatum ∼200 μm apart. The stimulation strength was adjusted to evoke an fEPSP slope of 35% of the maximum. A robust electrical LTD was induced by triple application of a low-frequency stimulation (LFS) of 2000 stimuli at 2 Hz every 10 min (counted from the end of the preceding LFS train). Immediately after every LFS train, four single recordings (spaced by 10 sec) were taken at 1, 4, 7, and 10 min (only three time points in CrebNesCre mice). Thereafter, the recording interval was 5 min. In all experiments, the recording of slices from mutant mice was interleaved by experiments with wild-type controls.
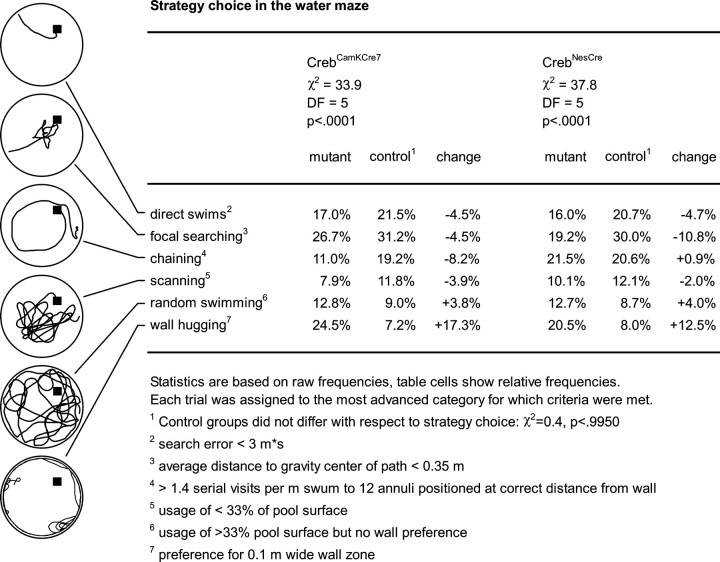
Water maze studies. Water maze studies were conducted as described previously (Gass et al., 1998). In brief, mice were trained to find the platform in a 150-cm-diameter pool by running two sessions per day for 14 consecutive days. The maximal duration of the session was confined to 60 sec. The first daily session was followed by an intersession interval of 60 sec that was spent on the platform. Probe trials (free swimming without the platform in the pool) were conducted on days 10 and 15. The probe trial on day 10 was followed by two regular training trials after an interval of ∼1 hr. The swim paths of the mice were recorded using a Noldus EthoVision video tracking system (Noldus Information Technology, Wageningen, The Netherlands). x–y coordinates were imported for off-line analysis to the custom-developed public domain program Wintrack (Wolfer et al., 2001) (available at www.dpwolfer.ch/wintrack). The following measures were calculated to assess acquisition: escape latency, path length, search error (sum of distances to goal taken every second minus the value obtained for an ideal direct swim) (Gallagher et al., 1993), and percentage of time in a 10-cm-wide wall zone. Spatial selectivity during probe trials was quantified using the following parameters: percentage of time in quadrant, percentage of time in a circular target zone comprising one-eighth of the pool surface, annulus crossings (number of crossings over the target minus the average of crossings over control sites, divided by distance swum), proximity (average distance to target) (Gallagher et al., 1993), and polar error (average angle between lines pointing from the maze center to the subject and target, respectively). Categorization of trials according to predominant strategy was done by an automatic algorithm implemented in Wintrack, using the criteria detailed in Figure 5.
Figure 5.
Strategy choice by CrebCamKCre7 and CrebNesCre mice in the water maze. The path recordings on the left exemplify the six exclusive categories of swim patterns that have been defined to classify the gradual improvement of spatial precision and efficiency during the learning process (from bottom to top). Note that these categories describe the successive stages of water maze learning occurring “on average,” across a group of mice. Thus, not every category will be seen during an individual learning trial. DF, Degrees of freedom.
Context-dependent fear conditioning. The conditioning system (TSE Systems, Bad Homburg, Germany) consisted of a soundproof box (58 × 30 × 27 cm 3) containing a light at the ceiling, a fan, a speaker, and a Plexiglas chamber (35 × 20 × 20 cm), which was placed on a shock grid made of stainless-steel rods. Experiments were performed according to the protocol described previously (Bourtchuladze et al., 1994; Gass et al., 1998). For contextual conditioning, mice were placed into the Plexiglas chamber for 2 min before the onset of the conditioned stimulus (CS; tone, 2.8 Hz; 85 dB) that lasted 30 sec. At the end of the tone, animals received the unconditioned stimulus (US; foot shock, 0.75 mA for 2 sec). Animals were left in the conditioning chamber for another 30 sec and then placed back into their home cages. Context conditioning was assessed in the same box 24 hr later by measuring freezing over a period of 5 min.
Conditioned taste aversion. After they were adapted to a restricted drinking schedule (two times for 20 min per day), animals were exposed to a saccharin solution (CS; 0.5%) during the first drinking session, followed 1 hr later by a malaise-inducing injection of LiCl (US; 0.14 m; 2% BW). Control mice were injected with vehicle solution [2% body weight (BW)]. Beginning 48 hr after conditioning, mice could freely choose to drink either saccharin solution or tap water during three daily choice tests (ct1–ct3). The amount of saccharin intake expressed as the percentage of total fluid consumed [(saccharin/saccharin + water) × 100] was taken as an aversion index.
Results
Generation of mice
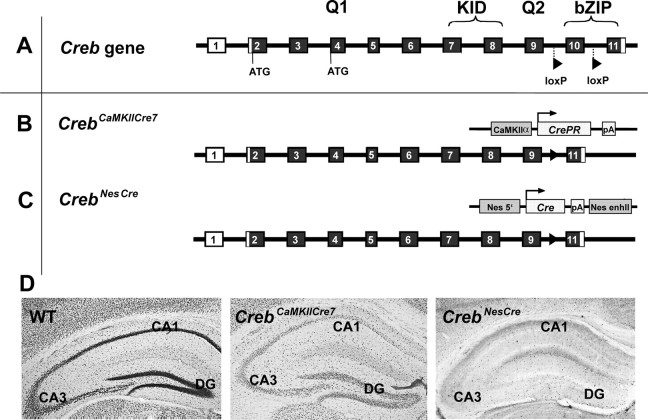
Crebcomp mice were generated by crossing CrebaΔ mice (Hummler et al., 1994) with Creb+/- mice (Rudolph et al., 1998), resulting in a mouse with only one Creb hypomorphic allele from which only CREBβ is expressed (Gass et al., 1998). CrebloxP/loxP mice were generated by flanking Creb exon 10 with loxP sites (Mantamadiotis et al., 2002). Cre-recombinase activity results in the specific excision of exon 10 and in the loss of all CREB isoforms in Cre-expressing cells (see Fig. 1 for gene-targeting strategies). Because we were particularly interested in the role of CREB in hippocampus-dependent LTM and LTSP, we crossed CrebloxP/loxP mice with mutants expressing Cre-recombinase postnatally under the control of the CamKIIα promoter, thereby restricting the time of Creb recombination to the first weeks after birth. CrebCamKCre7 mice showed CREB loss in ∼70–80% of CA1 neurons (Fig. 1B,D). To generate mutants that are completely devoid of CREB in the brain, CrebloxP/loxP mice were crossed into mice harboring a nestin-driven Cre-recombinase transgene (Fig. 1C). Because the nestin promoter induces Cre expression early, before separation of neuronal and glial lineages, CrebNesCre mice exhibited a loss of CREB in all brain regions during early neuronal development (Fig. 1D) but were upregulated in CREM, as were all other CREB-deficient strains generated thus far. At the level of general behavior and morphology, all mutant strains appeared normal except CrebNesCre mice, which had a dwarf phenotype attributable to a hypothalamic neuroendocrine dysfunction (T. Mantamadiotis, unpublished observations).
Figure 1.
Schematic representation of the targeting strategy used for the generation of mouse strains with a progressive reduction of CREB in the brain. A, Genomic structure of the Creb gene. Coding exons are indicated by filled boxes; noncoding exons are indicated by open boxes. 5′- and 3′-flanking sequences and introns are given as lines. Q1, KID, Q2, bZIP, Domains of the CREB protein; Q, glutamine-rich transactivation domain; b, basic region; ZIP, leucine zipper dimerization domain; ATG, start condon. B, CrebCamKCre7, LoxP-flanked Creb exon 10 was excised by a constitutively active Cre-recombinase fused with the C-terminal-truncated ligand binding domain of the progesterone receptor fusion (Kellendonk et al., 1996) expressed under the control of a CamKIIα promoter. Filled triangles indicate the loxP site remaining after excision of exon 10. pA, Polyadenylation signal. C, CrebNesCre, Exon 10 was deleted under the control of the brain-specific promoter nestin (Nes). enhII, Enhancer II. D, CREB immunostaining in the different Creb mutant and wild-type lines at 8–12 weeks of age. WT, Wild type; DG, dentate gyrus.
Reduction of the Creb gene dose does not prevent a robust LTP in the hippocampal CA1 region
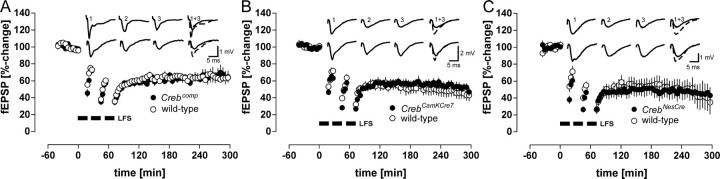
Conflicting data have been reported about the functional impact of deleting two of the three CREB isoforms on hippocampal LTP (Bourtchuladze et al., 1994; Gass et al., 1998). Therefore, we used the hippocampal slice preparation to test whether a major reduction of all CREB isoforms had any functional impact on a robust LTP in the hippocampal CA1 region, which was induced by repeated tetanization. Slices from CrebCamKCre7 mice lacking CREB in ∼70–80% of CA1 neurons (Fig. 1D) displayed a robust maintenance of LTP of ≥6 hr duration (mutant, 156.2 ± 19.8%, n = 4; wild type, 139.0 ± 10.4%, n = 4) (Fig. 2B). Wild-type slices showed a nonsignificant trend to a higher initial magnitude of potentiation. If there is any functional deficit resulting from the reduction of CREB, it should be most easily detectable in mice that do not have any residual CREB in the CA1 area. As evidenced by Figure 2C, robust LTP can even be maintained in the absence of CREB in CrebNesCre mice (6 hr mutants, 140.3 ± 16.6%, n = 6; wild type, 133.9 ± 18.3%, n = 7). In comparison, we analyzed CrebαΔ mice with the same genetic background as examined in a previous study (Bourtchuladze et al., 1994) but different from a later investigation (Gass et al., 1998). Also, in these mutants, we could not observe any differences from wild-type controls (6 hr mutants, 169.1 ± 23.6%, n = 9; wild type, 165.2 ± 13.4%, n = 5) (Fig. 2D). Because of this, our data corroborate previous findings using CrebαΔ mice with a B6/FVB F1 background (Gass et al., 1998) and a recent study in which inhibition of CRE-dependent gene transcription in the dorsal hippocampus by a dominant-negative CREB allele (K-CREB) had no effect on electrically induced LTP (Pittenger et al., 2002).
Figure 2.
LTP in the CA1 region is unchanged in mutant strains with a progressive deletion of all CREB isoforms in the hippocampus. A, Scheme of electrode placement in the hippocampal CA1 region. SC, Schaffer collaterals; MF, mossy fibers; PP, perforant path; DG, dentate gyrus; stimul., stimulation; fimbr, fimbria; fiss. hipp, fissura hippocampi. B, LTP in CrebCamKCre7 mice lacking CREB in ∼80% of CA1 neurons. Both groups expressed a stable LTP of ≥6 hr duration (mutant, 156.2 ± 19.8%, n = 4; wild type, 139 ± 10.4%, n = 4). C, Normal LTP was even obtained in CrebNesCre mice, which do not have any residual CREB in the CA1 area (mutants, n = 6; wild type, n = 7). D, CrebαΔ mice (n = 9) with a genetic background of an undefined mixture of C57BL/6 and 129SvEv displayed robust LTP (wild types, n = 5). Insets show representative recordings of a mutant (top row) and a wild-type mouse (bottom row) taken during baseline recording (1), 10 min after the third 100 Hz train (2), and at the end of the recording time (3). HFS, High-frequency stimulation.
Deletion of Creb has no effect on robust LTD
CREB phosphorylation is triggered not only by LTP-inducing stimuli but also by long trains of low-frequency stimulation that result in the induction of LTD in the hippocampus (Deisseroth et al., 1996). An involvement of CREB in LTD has also been inferred from studies in cultured Purkinje neurons (Ahn et al., 1999). First, we induced LTD in slices from Crebcomp mice that showed a robust depression indistinguishable from wild-type controls (Fig. 3A). However, slices from mutant mice appeared to be more susceptible to low-frequency stimulation, showing a significantly larger depression immediately after the first LFS train compared with wild-type controls (Crebcomp, 45.4 ± 4.4%, n = 7; wild type, 55.4 ± 3.3%, n = 11; p < 0.05; Mann–Whitney U test). Between the second and third LFS train, neither mutant nor wild-type slices showed any additional significant depression (Crebcomp: second train, 35.1 ± 3.6%; third train, 34.9 ± 3.8%; wild-type: second train, 37.9 ± 3.5%; third train, 35.8 ± 2.9%; Wilcoxon test), indicating a saturation of depression. The same feature was observed in CrebCamKCre7 mice (Fig. 3B), whereas controls decayed in a stepwise manner. This resulted in a larger depression of mutants after the second LFS train (CREBloxPCamKIICre7, 28.2 ± 1.6%, n = 12; wild type, 39.6 ± 3.3%, n = 9; p < 0.01; Mann–Whitney U test). Even complete loss of CREB in CrebNesCre mice did not interfere with LTD (Fig. 3C). As found with other CREB mutant strains, LTD of mutants was saturated after the second LFS train (second train, 26.4 ± 3.2%; third train, 27.2 ± 5.8%; n = 7), whereas wild-type mice exhibited a step-wise depression up to the third train (53.7 ± 6.6, 40.2 ± 3.9, and 26.4 ± 2.6%, respectively; n = 7), resulting in a significantly larger depression in mutants after the second train (p < 0.05; Mann–Whitney U test). The higher susceptibility of CREB mutants to LFS was also confirmed by a nonlinear regression of characteristic data points of the induction phase (last baseline value; each of the three 1 min values after cessation of LFS). Curve fitting of a one-phase exponential decay to these data yielded a mean decay time constant (τ) of 16.6 min in wild-type controls but 11.3 min in CREB mutant mice (i.e., only 68.1% of the value calculated for wild types).
Figure 3.
LTD in the CA1 region of the hippocampus is not altered by a progressive reduction of Creb gene dosage. A, Crebcomp mice with only one allele coding for the β-isoform of CREB display a robust depression. The depression obtained after the first LFS train was larger in Crebcomp mice, as in wild-type controls (Crebcomp, 45.4 ± 4.4%, n = 7; wild type, 55.4 ± 3.3%, n = 11; p < 0.05; Mann–Whitney U test). B, LTD in CrebCamKCre7 mice. Note that recordings of wild-type mice (n = 9) displayed a stepwise decline with every additional LFS train, whereas the depression of mutants (n = 12) was already saturated after the second LFS train. C, Normal LTD in CrebNesCre mice (n = 7). The depression was already saturated after the second LFS train and significantly larger, as in wild-type littermates (n = 7) (p < 0.05; Mann–Whitney U test). Insets show representative LTD recordings arranged as in Figure 2.
CREB deficiency increases wall hugging in the water maze
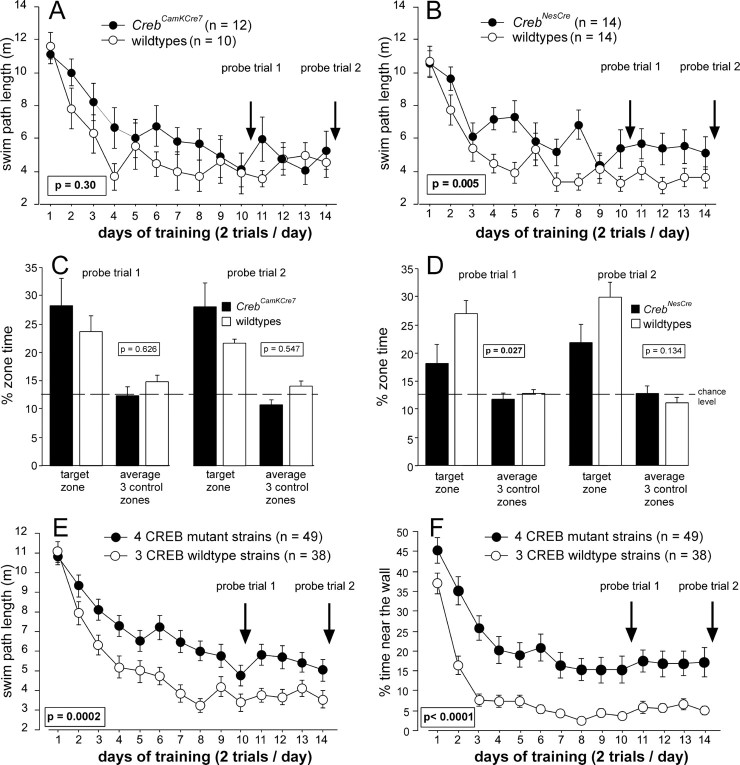
Because lasting forms of hippocampal LTP and LTD were found to be intact in CREB-deficient mice, we investigated whether this will go along with normal hippocampus-dependent LTM. To address this question, we used the Morris water maze test, in which the mouse must learn to find a submerged platform using distal visual cues. Acquisition of spatial memory is tested in a probe trial during which the platform is removed. We examined the water maze learning of CrebCamKCre7 and CrebNesCre mice using a protocol similar to the schedule of Kogan et al. (1996), as described in more detail by Gass et al. (1998). CrebCamKCre7 mice lacking CREB in ∼70–80% of forebrain neurons showed on average longer swim paths than littermate controls, although this difference did not reach statistical significance (Fig. 4A). Complete loss of CREB in CrebNesCre mice was associated with a small but statistically significant increase in swim path length (Fig. 4B).
Figure 4.
CREB deficiency impairs water maze learning, which is predominantly attributable to a marked increase in wall hugging (thigmotaxis). A, Water maze learning in CrebCamKCre7 was indistinguishable from wild-type littermates. B, CrebNesCre mice displayed a significantly increased swim path length. C, Marked reduction of CREB in the forebrain did not impair spatial memory. D, Apparent deficits of spatial memory in CrebNesCre mice during probe trial 1. E, Pooling of four Creb mutant strains (CrebCamKCre7, CrebNesCre, CrebαΔ, and Crebcomp) and three wild-type strains, respectively, contrasts the performance deficits of CREB-deficient mutants with their littermate controls. F, In mutant strains, the percentage of thigmotaxis is markedly increased. Two-way ANOVA with repeated measures with days 1–14 and genotype (mutant and wild type) as factors was used for the analysis of the values depicted in Figure 4. The p values represent the genotype effect.
We also analyzed strategy choice during training by categorizing each individual trial according to the predominant swim pattern. Six exclusive categories were defined to capture the gradually improving spatial precision and efficiency during the learning process (Fig. 5): wall hugging, random swimming, scanning, chaining, focal searching, and direct swims. χ 2 statistics revealed a highly significant change in strategy choice in both CrebCamKCre7 and CrebNesCre mice, whereas the respective control groups were indistinguishable in this respect (Fig. 5). In both mutants, the most striking change was a threefold increase in wall-hugging trials at the expense of more modest reductions in direct swims and focal searching. Trials in the latter category were more strongly reduced in CrebNesCre than in CrebCamKCre7 mice. Finally, spatial retention was assessed in two probe trials. To maximize possible CREB-dependent differences, we used the percentage of time in a circular target zone comprising one-eighth of the pool surface. This is a more stringent measure of spatial selectivity than the commonly used percentage of time in quadrant. When tested in an initial probe trial after 10 d of training, CrebCamKCre7 mice attained scores equaling or even exceeding those of wild-type controls (Fig. 4C). In contrast, CrebNesCre mice showed significantly lower average scores than wild-type litter-mates (Fig. 4D) but still tended to spend slightly more time in the trained zone than in the control zone (p < 0.109; t test). After an additional 4 d of training, probe trial performance of CrebNesCre mice had improved. Mutants now spent significantly more time in the trained zone than in the control zone (p < 0.0253; t test), and their inferiority with respect to the control group was no longer statistically significant. To determine how many of the CREB-deficient mice would qualify for an excellent probe trial score, we computed the percentage of time spent in the trained zone (chance level, 12.5%) by a population of 209 control animals pooled from different studies. We found that 50% of the subjects in this reference population spent >27% of their time in the trained zone. Using this value as criterion for excellent probe trial performance, we found that among the CrebNesCre mice in the present study, 3 of 13 (23%) mutants and 6 of 14 (43%) controls met this criterion. Among the CrebCamKCre7 mice, scores meeting this criterion were observed for 5 of 11 (45%) mutants and 3 of 10 (30%) wild types. This shows that both mutant lines included CREB-deficient mice with excellent spatial retention, although this number was clearly smaller in the CrebNesCre line. Finally, we analyzed to what extent strong initial wall hugging was predicting low probe trial scores. Among the CrebNesCre mice, we identified four mutants in which persistent wall hugging was associated with probe trial scores at chance level, whereas another three mutants performed poorly despite normal acquisition. Likewise, within the CrebCamKCre7 mice, we identified three animals showing persistent wall hugging and low probe trial scores, whereas two other low-scoring animals were characterized by other nonspatial search strategies (see above).
The results of strategy choice analysis raised the question of whether the lack of significant mutation effects in CrebNesCre mice could reflect insufficient statistical power rather than a true biological dissociation, especially with respect to swim path length. Therefore, we extended our analysis by enlarging the set of measures for training and probe trial performance as well as including data of CrebαΔ and Crebcomp mice that had been collected previously under exactly the same experimental conditions (Gass et al., 1998). Combining the data sets was valid, because control groups were indistinguishable in all measures of both training and probe trial performance (Table 1, column 1). We then compared the four mutant groups against each other (CrebαΔ, Crebcomp, CrebCamKCre7, and CrebNesCre). They were homogeneous with respect to all training parameters, including wall hugging, but not with respect to probe trial performance (Table 1, column 2). However, homogeneity of mutant groups with respect to probe trial performance could be restored by removing CrebCamKCre7 mice from the comparison (Table 1, column 3). To confirm a possible dissociation between CrebCamKCre7 mice and the other lines in which the mutation was not driven by a CamKIIα promoter, we used a twoway ANOVA design (deficient vs not deficient; CamKII-driven vs other mutation types). All measures of acquisition showed a highly significant effect of CREB deficiency, which was independent of mutation type and associated with strongly increased wall hugging (Table 1, columns 4,5; Fig. 4E,F). In contrast, probe trial analysis showed no overall effect of CREB deficiency but significant ANOVA interactions, statistically confirming a dissociation between CrebCamKCre7 mice, which were normal in the probe trials, and other mutants that were, on average, impaired.
Table 1.
Comparison of training and probe trial performance of CREB mutant mice in the water maze
|
|
All controls n = 38 one-way ANOVA Linea |
All mutants n = 49 one-way ANOVA Lineb |
Mutants excl CamKCre7 n = 37 one-way ANOVA Line |
All animals n = 87 two-way ANOVA Genotypec |
Genotype × selectivityd |
All controls n = 38 mean ± SE |
αΔ n = 10 mean ± SE |
comp n = 13 mean ± SE |
NesCre n = 14 mean ± SE |
CamKCre7 n = 12 mean ± SE |
|---|---|---|---|---|---|---|---|---|---|---|
| Training | ||||||||||
| Escape latencye (sec) | ns | ns | ns | p<0.0007 | ns | 22.77 ± 1.07 | 27.17 ± 4.28 | 32.08 ± 2.85 | 31.06 ± 2.66 | 33.87 ± 3.91 |
| Path length (m) | ns | ns | ns | p<0.0031 | ns | 5.00 ± 0.22 | 6.52 ± 0.93 | 7.60 ± 0.57 | 6.44 ± 0.51 | 6.38 ± 0.83 |
| Search error (m × sec) | ns | ns | ns | p<0.0008 | ns | 12.09 ± 0.70 | 17.04 ± 3.31 | 20.90 ± 2.37 | 17.99 ± 2.13 | 19.48 ± 3.16 |
| Wall hugging (%) | ns | ns | ns | p<0.0008 | ns | 8.52 ± 0.71 | 20.90 ± 6.46 | 28.14 ± 5.76 | 17.63 ± 3.71 | 18.19 ± 4.24 |
| Probe trial 1 | ||||||||||
| Percentage of time in trained quadrant | ns | p<0.0656 | ns | ns | p<0.0234 | 35.78 ± 2.03 | 28.5 ± 2.11 | 26.39 ± 2.00 | 31.11 ± 3.70 | 40.87 ± 6.49 |
| Percent time in trained zone | ns | p<0.0226 | ns | ns | p<0.0121 | 24.58 ± 1.53 | 14.40 ± 2.24 | 14.49 ± 1.81 | 18.06 ± 3.53 | 28.12 ± 5.02 |
| Crossing preference (x/m) | ns | p<0.0149 | ns | ns | p<0.0016 | 0.19 ± 0.06 | -0.01 ± 0.07 | 0.05 ± 0.05 | 0.16 ± 0.09 | 0.44 ± 0.16 |
| Proximity (m) | ns | p<0.0346 | ns | ns | p<0.0364 | 0.49 ± 0.01 | 0.59 ± 0.02 | 0.59 ± 0.03 | 0.57 ± 0.04 | 0.46 ± 0.04 |
| Polar error (°) | ns | p<0.0368 | ns | ns | p<0.0613 | 77.10 ± 2.71 | 84.03 ± 2.18 | 88.87 ± 2.95 | 84.06 ± 5.11 | 69.06 ± 7.36 |
| Probe trial 2 | ||||||||||
| Percent time in trained quadrant | ns | p<0.0628 | ns | ns | p<0.0077 | 38.88 ± 2.49 | 32.83 ± 4.09 | 25.97 ± 2.25 | 34.78 ± 3.21 | 40.99 ± 5.56 |
| Percent time in trained zone | ns | p<0.0502 | ns | ns | p<0.0101 | 26.26 ± 1.97 | 19.13 ± 4.02 | 14.26 ± 1.94 | 21.88 ± 3.26 | 27.90 ± 4.33 |
| Crossing preference (x/m) | ns | ns | ns | ns | ns | 0.22 ± 0.07 | 0.24 ± 0.11 | 0.10 ± 0.06 | 0.14 ± 0.11 | 0.41 ± 0.16 |
| Proximity (m) | ns | ns | ns | ns | p<0.0432 | 0.48 ± 0.02 | 0.56 ± 0.05 | 0.58 ± 0.03 | 0.51 ± 0.03 | 0.47 ± 0.04 |
| Polar error (°)
|
ns
|
p<0.0408
|
ns
|
ns
|
p<0.0197
|
73.19 ± 3.13
|
83.33 ± 5.66
|
88.43 ± 3.29
|
77.52 ± 3.78
|
69.6 ± 6.04
|
ns, Not significant
αΔ + comp versus CamKCre7 versus NesCre
αΔ versus comp versus CamKCre7 versus NesCre
CREB deficient versus wild type
Forebrain selective (CamKCre7) versus whole NS (αΔ, comp, NesCre)
Escape latency and other training measures averaged over all acquisition trials
CREB deletion does not affect context-dependent fear conditioning
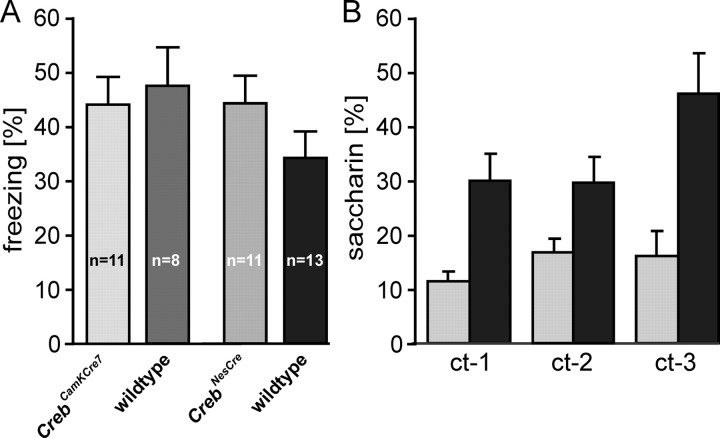
Next, we examined context-dependent fear conditioning, a hippocampus-dependent type of associative learning. In this task, the animals developed an immobility response (freezing) after exposure to electric foot shock. When placed again in the experimental chamber after 24 hr, they showed the freezing response again, indicating long-term recognition memory of the test chamber. Variations in the freezing scores are thought to reflect the strength of the memory trace. The conditioning protocol was identical to the procedures used in previous studies (Bourtchuladze et al., 1994; Gass et al., 1998). As shown in Figure 6A, neither forebrain-specific reduction of CREB in CrebCamKCre7 mice nor its complete deletion in CrebNesCre mutants resulted in significant alterations in freezing scores when tested 24 hr after training.
Figure 6.
Loss of CREB does not affect hippocampus-dependent learning but severely impairs associative learning that depends on the activation of extrahippocampal brain regions. A, Reduction of CREB does not result in significant changes in context-dependent fear conditioning, a task that is contingent on the functional integrity of the hippocampus. The two mutant strains show freezing scores similar to those obtained for their respective littermate groups, indicating unimpaired LTM. B, Creb mutants display a significant attenuation of conditioned taste aversion, a hippocampus-independent associative learning paradigm. CrebNesCre mice (filled bars) avoided saccharin less than wild types (p < 0.0001) during three choice tests separated by 24 hr (ct-1–ct-3). Animals of both genotypes developed the same preference for the saccharin solution when its first consumption during conditioning was followed by vehicle injection not inducing malaise (saccharin preference is indicated by the gray bar). Mean ± SEM are shown.
Reduction of CREB attenuated the development of a conditioned taste aversion
To determine any clear effects on memory processes, we tested CrebNesCre mice in a CTA paradigm, a task that is rarely affected by hippocampal lesions, at least in rats. CTA represents a form of classical conditioning with a malaise-inducing substance (e.g., lithium chloride) as the US and a taste stimulus (e.g., saccharin solution) as the CS. Association of the CS with the US during a single conditioning trial leads to an avoidance of the CS in future sessions. Although not yet shown for mice, in many species, CTA probably involves at least two complex extrahippocampal neuronal networks mediating malaise and gustatory processes. In the forebrain, it depends on activation of the insular cortex, the amygdala, and parts of the posteromedial thalamus (for review, see Welzl et al., 2001). Recent findings suggest a functional interaction of CTA with LTP-like mechanisms (Escobar et al., 1998; Escobar and Bermudez-Rattoni, 2000). As illustrated in Figure 6B, CrebNesCre mice had a significantly attenuated CTA (i.e., they avoided the saccharin solution to a lesser degree compared with wild-type controls) (ANOVA; F(1,21) = 10.72; p = 0.0036). This difference is not attributable to a general alteration of fluid consumption or gustation, because control and CrebNesCre mice drank comparable amounts of the CS during conditioning, and both preferred the saccharin solution over tap water during the choice tests when not being injected with the malaise-inducing US during conditioning (F(1,7) = 3.398 × 10-4; p = 0.986; not significant).
Discussion
Our data, obtained with mutant mice harboring conditional deletions of all CREB isoforms, corroborate previous findings that a reduction of the CREB gene dose has no effect on hippocampal LTSP and only subtle effects on the water maze task. There, it primarily increases the propensity to adopt inappropriate search strategies rather than impairing indices of spatial memory (Gass et al., 1998). In contrast, a role for memory processing of CREB was revealed for conditioned taste aversion.
CREB deficiency and synaptic plasticity
Our study failed to demonstrate impairments in lasting forms of hippocampal LTP and LTD in CrebCamKCre7 mice and mutants devoid of all CREB isoforms in the brain (CrebNesCre). This is in contrast to the report by Bourtchuladze et al. (1994) but is in accordance with studies of hippocampal LTP by Gass et al. (1998) and Pittenger et al. (2002). The latter study suggested a different role for CREB, depending on the mode of LTP induction. Thus, mice carrying K-CREB expressed normal LTP if high-frequency stimulation was used for induction but were compromised in chemical potentiation generated by the application of forskolin (Pittenger et al., 2002). Congruent with our data, normal LTP and depotentiation were described in the basolateral amygdala of transgenic mice expressing CREBS133A, a dominant-negative form of CREB (Rammes et al., 2000).
In the present experiments, we found no evidence that a progressive reduction of CREB perturbs LTD. The induction protocol that was used generated a robust LTD maintained for ≥5 hr, the longest duration ever reported in vitro. The only difference that was consistently obtained in mutant mice was a higher susceptibility of Creb mutant strains to LFS, indicating a slight shift in the induction mechanism rather than a dramatic change.
Behavioral consequences of CREB loss or deficiency
Water maze data
Our water maze experiments revealed a clear dissociation between effects of CREB deficiency on training performance and spatial retention during probe tests. Both CrebCamKCre7 and CrebNesCre mice showed strongly increased wall hugging and impaired escape performance during training. This impairment of acquisition was indistinguishable from the one observed previously in CrebαΔ and Crebcomp mice (Gass et al., 1998) and is in line with other studies of water maze learning in CREB-deficient mice (Bourtchuladze et al., 1994). In contrast, spatial retention during probe trials was partially impaired only in CrebNesCre mice but barely or not at all in CrebCamKCre7 mice. This indicates that the mutation affects training performance and spatial retention independently, corresponding to a principal component analysis of water maze data from >3000 mice carrying different mutations (Wolfer and Lipp, 2000). In contrast, it is evident that CrebCamKCre7 and CrebNesCre mice differ in several ways. In CrebCamKCre7 mice, the mutation spares early development and is restricted to the forebrain but also leaves CREB expression intact in ∼20% of forebrain neurons. Although the available data do not allow us to distinguish whether developmental–temporal differences or efficiency of recombination is responsible for the absence of a spatial retention deficit, our results suggest that the residual expression of CREB in CrebCamKCre7 mice suffices to support normal spatial retention in a sensitive water maze protocol. In agreement with this, CrebαΔ mice on a B6/129 genetic background, which still harbor the β-isoform of CREB, were reported recently to show nearly normal performance in the water maze (Graves et al., 2002). Furthermore, even if CREB is completely absent in the brain, as in CrebNesCre mice, the observed retention deficit can be partially overcome by extended training. In two other studies, the inhibition of CRE-dependent gene transcription was confined to the dorsal hippocampus, either by applying CREB antisense oligodeoxynucleotides (ODNs) into the dorsal hippocampus (Guzowski and McGaugh, 1997) or by using transgenic mice expressing K-CREB only in the dorsal part (Pittenger et al., 2002). Both approaches resulted in deteriorated probe trial performance in the water maze, but only the published surface occupancy plots of the second study (Pittenger et al., 2002) allow an evaluation of the spatial strategy. K-CREB transgenic mice clearly show a circular search strategy during probe trials (Fig. 5, chaining) after almost identical escape latencies during training. Such search strategies can feign severe memory deficits during the probe trial, as shown previously in two vole species (Clethrionomys glareolus and Microtus oeconomus) with intact spatial memory (Pleskacheva et al., 2000).
In any case, the relatively high number of CrebCamKCre7 mice showing excellent probe trial scores suggests that this behavioral score is fairly insensitive to the presence or absence of CREB, at least in this mutant line. Together, all water maze studies of CREB-deficient mice so far indicate that the most predictable effect is an increase in less-directed search strategies, whereas specific effects on LTM still need to be demonstrated.
Fear conditioning
In the present study, even the complete loss of CREB did not produce an overt deficiency in context-dependent fear conditioning. This is in line with the lack of statistically significant deficits in mice with the αΔ Creb mutation on a B6/FVB F1 background (Gass et al., 1998) and in transgenic mice expressing a dominant-negative form of CREB in the forebrain (Rammes et al., 2000). In contrast, Crebcomp mice, CrebαΔ mice on a B6/129 F1 hybrid background, and mutants with an inducible CREB repressor were reported to be impaired in contextual fear conditioning (Gass et al., 1998; Graves et al., 2002; Kida et al., 2002). A similar discrepancy exists between studies in which CRE-dependent gene transcription was specifically blocked in the dorsal hippocampus, either by infusion of CRE decoy ODN, resulting in an impairment of contextual conditioning (Athos et al., 2002), or by a dominant-negative Creb allele having no effect (Pittenger et al., 2002).
There are several factors that may account for such discrepancies. First, the divergent phenotypes of several CrebαΔ strains in contextual fear conditioning clearly point to genetic background as one major factor. The phenotypic disparity indicates modifier genes in a particular genetic background having a decisive influence not only on viability (Graves et al., 2002; J. A. Blendy and G. Schütz, unpublished observations) but also on behavior. Thus, the inherent selection for viability in mutant mouse lines may increasingly mask modest behavioral phenotypes observed in the first mutant generation. Second, it is known that even the same promoter will produce differential quantitative and spatial expression patterns of a mutation–transgene across various mouse lines generated (Kida et al., 2002; Pittenger et al., 2002; Mantamadiotis and Schütz, unpublished observations). This, in turn, may produce differential deficits in brain regions that are involved in contextual fear conditioning (e.g., the hippocampus, amygdala, and periaqueductal gray) (Impey et al., 1998; Fendt and Fanselow, 1999; Graves et al., 2002). Third, most previous studies with CREB-deficient mice were done using constitutive mutants in which developmental yet behaviorally relevant side effects unrelated to CREB could not be excluded. Last, the method of using a tamoxifen-inducible CREB repressor (Kida et al., 2002) affects not only CREB but also CRE-mediated gene transcription in general and may additionally involve extrahippocampal CTA-like mechanisms induced by the high concentration of tamoxifen (Wogan, 1997).
At the present stage of knowledge, it appears to be impossible to identify a single specific factor explaining the reported phenotypical differences of CREB mutants in contextual fear conditioning (see below for additional aspects).
Conditioned taste aversion
Loss of CREB resulted in a marked deterioration of CTA, which is in line with studies reporting an increase in phospho CREB immunoreactivity in the lateral amygdala during CTA (Swank, 2000) and an attenuation of this learning task after application of CREB-directed antisense oligonucleotides (Lamprecht et al., 1997).
General discussion
The view that hippocampal LTSP is not affected in CREB-deficient mice has received support by recent studies and thus shall not be discussed further. However, as supported by our data and published data, a deficiency in CREB alone appears to entail nonreplicable or fairly moderate effects on often-used behavioral indices of hippocampus-dependent types of LTM. The reasons might be the following: (1) multiple pathways and mechanisms and (2) an upregulation of CREM and/or as yet undiscovered transcription factors. For multiple pathways and mechanisms, there are different kinds of memory that are likely to be contingent on different mechanisms and stored with different time constants of consolidation in different brain regions. In view of our CTA results, it appears reasonable to assume that several parallel molecular pathways are responsible for changes in long-term memory storage. Only some of these pathways might be directly dependent on an activation of the known members of the CREB family (Lonze and Ginty, 2002). An upregulation of CREM and/or as yet undiscovered transcription factors could compensate for the progressive CREB deficiency. Recent studies revealed that in vertebrates, CREB family members have a close functional relationship (Blendy et al., 1996; Bleckmann et al., 2002; Mantamadiotis et al., 2002). Thus, a reduction in CREB by gene targeting will induce an upregulation of CREM, which may compensate many functional deficits, at least partially. In theory, this issue might be addressed by studying double mutants for CREM and CREB. Such studies are underway (D. Balschun, J. U. Frey, P. Gass, H.-P. Lipp, H. Welzl, and D. P. Wolfer, unpublished data) but are complicated by the fact that these mutant lines display a progressive neuronal degeneration because of increased apoptosis, especially in the hippocampal CA1 region (Mantamadiotis et al., 2002). Hence, any interpretation of CREB–CREM-related memory functions should take into account the now well documented role of CREB–CREM in neuronal survival and degeneration (Bonni et al., 1999).
Conclusion
This study and our previous studies have not verified the anticipated functions of CREB in hippocampal LTSP and LTM, although we used standard procedures that are widely accepted for the examination of mutant mice. However, we do not rule out the possibility that an exhaustive examination of other paradigms may reveal more subtle, hitherto undiscovered effects of CREB on hippocampal functions. The failure to demonstrate substantial effects of CREB deficiency on classical hippocampal tasks, combined with the finding of rather robust effects on (putatively extrahippocampally mediated) CTA, suggests either a minor function of CREB in the hippocampus or a hippocampus-specific compensatory upregulation of other transcription factors such as CREM.
Footnotes
This work was supported by Deutsche Forschungsgemeinschaft Grants SFB 426 (D.B. and J.U.F.) and 427/4-1 (P.G.), by the Swiss National Science Foundation, by European Community Grant BIO4CT980297–BBW98.0125, and by the National Center of Competence in Research Neural Plasticity and Repair. We gratefully acknowledge the excellent technical assistance of Inger Drescher, Sabine Hartmann, Diana Koch, and Rosemarie Lang. We thank J. Leutgeb and W. Schmid for critical suggestions.
Correspondence should be addressed to Dr. Detlef Balschun, Leibniz Institute for Neurobiology, Brenneckestrasse 6, 39118 Magdeburg, Germany. E-mail: balschun@ifn-magdeburg.de.
Copyright © 2003 Society for Neuroscience 0270-6474/03/236304-11$15.00/0
D.B. and D.P.W. contributed equally to this work.
References
- Agranoff BW, Davis RE, Brink JJ ( 1966) Chemical studies on memory fixation in goldfish. Brain Res 1: 303–309. [DOI] [PubMed] [Google Scholar]
- Ahn S, Ginty DD, Linden DJ ( 1999) A late phase of cerebellar long-term depression requires activation of CaMKIV and CREB. Neuron 23: 559–568. [DOI] [PubMed] [Google Scholar]
- Athos J, Impey S, Pineda VV, Chen X, Storm DR ( 2002) Hippocampal CRE-mediated gene expression is required for contextual memory formation. Nat Neurosci 5: 1119–1120. [DOI] [PubMed] [Google Scholar]
- Bartsch D, Ghirardi M, Skehel PA, Karl KA, Herder SP, Chen M, Bailey CH, Kandel ER ( 1995) Aplysia CREB2 represses long-term facilitation: relief of repression converts transient facilitation into long-term functional and structural change. Cell 83: 979–992. [DOI] [PubMed] [Google Scholar]
- Bleckmann SC, Blendy JA, Rudolph D, Monaghan AP, Schmid W, Schutz G ( 2002) Activating transcription factor 1 and CREB are important for cell survival during early mouse development. Mol Cell Biol 22: 1919–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blendy JA, Kaestner KH, Schmid W, Gass P, Schutz G ( 1996) Targeting of the CREB gene leads to up-regulation of a novel CREB mRNA isoform. EMBO J 15: 1098–1106. [PMC free article] [PubMed] [Google Scholar]
- Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME ( 1999) Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 286: 1358–1362. [DOI] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ ( 1994) Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell 79: 59–68. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Bito H, Tsien RW ( 1996) Signaling from synapse to nucleus: postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron 16: 89–101. [DOI] [PubMed] [Google Scholar]
- Escobar ML, Bermudez-Rattoni F ( 2000) Long-term potentiation in the insular cortex enhances conditioned taste aversion retention. Brain Res 852: 208–212. [DOI] [PubMed] [Google Scholar]
- Escobar ML, Alcocer I, Chao V ( 1998) The NMDA receptor antagonist CPP impairs conditioned taste aversion and insular cortex long-term potentiation in vivo Brain Res 812: 246–251. [DOI] [PubMed] [Google Scholar]
- Fendt M, Fanselow MS ( 1999) The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev 23: 743–760. [DOI] [PubMed] [Google Scholar]
- Frey U, Krug M, Reymann KG, Matthies H ( 1988) Anisomycin, an inhibitor of protein synthesis, blocks late phases of LTP phenomena in the hippocampal CA1 region in vitro Brain Res 452: 57–65. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M ( 1993) Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci 107: 618–626. [DOI] [PubMed] [Google Scholar]
- Gass P, Wolfer DP, Balschun D, Rudolph D, Frey U, Lipp HP, Schutz G ( 1998) Deficits in memory tasks of mice with CREB mutations depend on gene dosage. Learn Mem 5: 274–288. [PMC free article] [PubMed] [Google Scholar]
- Graves L, Dalvi A, Lucki I, Blendy JA, Abel T ( 2002) Behavioral analysis of CREB αΔ mutation on a B6/129 F1 hybrid background. Hippocampus 12: 18–26. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, McGaugh JL ( 1997) Antisense oligodeoxynucleotide-mediated disruption of hippocampal cAMP response element binding protein levels impairs consolidation of memory for water maze training. Proc Natl Acad Sci USA 94: 2693–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummler E, Cole TJ, Blendy JA, Ganss R, Aguzzi A, Schmid W, Beermann F, Schutz G ( 1994) Targeted mutation of the CREB gene: compensation within the CREB/ATF family of transcription factors. Proc Natl Acad Sci USA 91: 5647–5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impey S, Smith DM, Obrietan K, Donahue R, Wade C, Storm DR ( 1998) Stimulation of cAMP response element (CRE)-mediated transcription during contextual learning. Nat Neurosci 1: 595–601. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Pittenger C ( 1999) The past, the future and the biology of memory storage. Philos Trans R Soc Lond B Biol Sci 354: 2027–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellendonk C, Tronche F, Monaghan AP, Angrand PO, Stewart F, Schutz G ( 1996) Regulation of Cre recombinase activity by the synthetic steroid RU 486. Nucleic Acids Res 24: 1404–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida S, Josselyn SA, de Ortiz SP, Kogan JH, Chevere I, Masushige S, Silva AJ ( 2002) CREB is required for the stability of new and reactivated fear memories. Nat Neurosci 5: 348–355. [DOI] [PubMed] [Google Scholar]
- Kogan JH, Frankland PW, Blendy JA, Coblentz J, Marowitz Z, Schutz G, Silva AJ ( 1996) Spaced training induces normal long-term memory in CREB mutant mice. Curr Biol 7: 1–11. [DOI] [PubMed] [Google Scholar]
- Krug M, Lossner B, Ott T ( 1984) Anisomycin blocks the late phase of long-term potentiation in the dentate gyrus of freely moving rats. Brain Res Bull 13: 39–42. [DOI] [PubMed] [Google Scholar]
- Lamprecht R, Hazvi S, Dudai Y ( 1997) cAMP response element-binding protein in the amygdala is required for long- but not short-term conditioned taste aversion memory. J Neurosci 17: 8443–8450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp HP, Wolfer DP ( 1998) Genetically modified mice and cognition. Curr Opin Neurobiol 8: 272–280. [DOI] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD ( 2002) Function and regulation of CREB family transcription factors in the nervous system. Neuron 35: 605–623. [DOI] [PubMed] [Google Scholar]
- Malenka RC ( 1994) Synaptic plasticity in the hippocampus: LTP and LTD. Cell 78: 535–538. [DOI] [PubMed] [Google Scholar]
- Mantamadiotis T, Taraviras S, Tronche F, Schutz G ( 1998) PCR-based strategy for genotyping mice and ES cells harboring loxP sites. Biotechniques 25: 968–970, 972. [DOI] [PubMed] [Google Scholar]
- Mantamadiotis T, Lemberger T, Bleckmann SC, Kern H, Kretz O, Martin VA, Tronche F, Kellendonk C, Gau D, Kapfhammer J, Otto C, Schmid W, Schutz G ( 2002) Disruption of CREB function in brain leads to neurodegeneration. Nat Genet 31: 47–54. [DOI] [PubMed] [Google Scholar]
- Mayr B, Montminy M ( 2001) Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol 2: 599–609. [DOI] [PubMed] [Google Scholar]
- Otto C, Kovalchuk Y, Wolfer DP, Gass P, Martin M, Zuschratter W, Grone HJ, Kellendonk C, Tronche F, Maldonado R, Lipp HP, Konnerth A, Schutz G ( 2001) Impairment of mossy fiber long-term potentiation and associative learning in pituitary adenylate cyclase activating polypeptide type I receptor-deficient mice. J Neurosci 21: 5520–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger C, Huang YY, Paletzki RF, Bourtchouladze R, Scanlin H, Vronskaya S, Kandel ER ( 2002) Reversible inhibition of CREB/ATF transcription factors in region CA1 of the dorsal hippocampus disrupts hippocampus-dependent spatial memory. Neuron 34: 447–462. [DOI] [PubMed] [Google Scholar]
- Pleskacheva MG, Wolfer DP, Kupriyanova IF, Nikolenko DL, Scheffrahn H, Dell'Omo G, Lipp HP ( 2000) Hippocampal mossy fibers and swimming navigation learning in two vole species occupying different habitats. Hippocampus 10: 17–30. [DOI] [PubMed] [Google Scholar]
- Rammes G, Steckler T, Kresse A, Schutz G, Zieglgansberger W, Lutz B ( 2000) Synaptic plasticity in the basolateral amygdala in transgenic mice expressing dominant-negative cAMP response element-binding protein (CREB) in forebrain. Eur J Neurosci 12: 2534–2546. [DOI] [PubMed] [Google Scholar]
- Rudolph D, Tafuri A, Gass P, Hammerling GJ, Arnold B, Schutz G ( 1998) Impaired fetal T cell development and perinatal lethality in mice lacking the cAMP response element binding protein. Proc Natl Acad Sci USA 95: 4481–4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Nadel NV, Sullivan GM, Harris A, LeDoux JE ( 1999) Memory consolidation for contextual and auditory fear conditioning is dependent on protein synthesis, PKA, and MAP kinase. Learn Mem 6: 97–110. [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S ( 1998) CREB and memory. Annu Rev Neurosci 21: 127–148. [DOI] [PubMed] [Google Scholar]
- Swank MW ( 2000) Phosphorylation of MAP kinase and CREB in mouse cortex and amygdala during taste aversion learning. NeuroReport 11: 1625–1630. [DOI] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G ( 1999) Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet 23: 99–103. [DOI] [PubMed] [Google Scholar]
- Welzl H, D'Adamo P, Lipp HP ( 2001) Conditioned taste aversion as a learning and memory paradigm. Behav Brain Res 125: 205–213. [DOI] [PubMed] [Google Scholar]
- Wogan GN ( 1997) Review of the toxicology of tamoxifen. Semin Oncol 24: S87–S97. [PubMed] [Google Scholar]
- Wolfer DP, Lipp HP ( 2000) Dissecting the behaviour of transgenic mice: is it the mutation, the genetic background, or the environment? Exp Physiol 85: 627–634. [PubMed] [Google Scholar]
- Wolfer DP, Madani R, Valenti P, Lipp H ( 2001) Extended analysis of path data from mutant mice using the public domain software Wintrack. Physiol Behav 73: 745–753. [DOI] [PubMed] [Google Scholar]
- Yin JC, Wallach JS, Del Vecchio M, Wilder EL, Zhou H, Quinn WG, Tully T ( 1994) Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila Cell 79: 49–58. [DOI] [PubMed] [Google Scholar]
- Yin JC, Del Vecchio M, Zhou H, Tully T ( 1995) CREB as a memory modulator: induced expression of a dCREB2 activator isoform enhances long-term memory in Drosophila Cell 81: 107–115. [DOI] [PubMed] [Google Scholar]