Abstract
Microglia are the immune cells of the CNS. In the normal adult mammalian brain, the majority of these cells is quiescent and exhibits a ramified morphology. Microglia are perhaps best known for their swift transformation to an activated ameboid morphology in response to pathological insults. Here we have observed the responsiveness of these cells to events surrounding the normal activation of neurosecretory neurons in the hypothalamic supraoptic nucleus (SON), a well studied model of structural plasticity in the CNS. Neurons in the SON were activated by substituting 2% saline for drinking water. Brain sections were collected from four experimental groups [controls (C), 2 d-dehydrated (2D), 7 d-dehydrated (D7), and 7 d-dehydrated/21 d-rehydrated animals (R21)] and stained with Isolectin-B4-HRP to visualize microglial cells. Based on morphological criteria, we quantified ramified, hypertrophied, and ameboid microglia using unbiased stereological techniques. Statistical analyses showed significant increases in the number of hypertrophied microglia in the D2 and D7 groups. Moreover, there was a significant increase in the number of ameboid microglia in the D7 group. No changes were seen across conditions in the number of ramified cells, nor did we observe any significant phenotypic changes in a control area of the cingulate gyrus. Hence, increased morphological diversity of microglia was found specifically in the SON and was reversible with the cessation of stimulation. These results indicate that phenotypic plasticity of microglia may be a feature of the normal structural remodeling that accompanies neuronal activation in addition to the activation that accompanies brain pathology.
Keywords: plasticity, microglia, SON, hypothalamus, magnocellular neurons, stereology
Introduction
The mammalian CNS is the site of extensive structural plasticity, not only during development but also throughout the lifespan of the organism (Bennett et al., 1964; Greenough, 1988; Greenough et al., 1992). The hypothalamic supraoptic nucleus (SON), is one especially well studied model of activity-dependent plasticity (Tweedle and Hatton, 1976) (for review, see Hatton, 1997; Salm et al., 1998; Theodosis and Poulain, 1999). The SON contains magnocellular neuroendocrine cells (MNCs) that synthesize the peptides oxytocin (OX) and vasopressin (VP). When the SON becomes active, these peptides are transported to axonal endings in the posterior pituitary, where they are released into the circulation. Stimulation of MNCs during dehydration, lactation, parturition, initiation of maternal behaviors, and restraint stress have variously been reported to result in extensive structural changes of SON astrocytes in relation to adjacent neurons (Tweedle and Hatton, 1976; Theodosis et al., 1981; Salm et al., 1988; Miyata et al., 1994). Simply stated, at these times astrocytic coverage of neuronal somata, dendrites, and axons decreases. This results in an increase in neuronal membrane apposition enabling activated MNCs to form dendritic bundles and receive novel synaptic contacts. Gap junctions also appear between dendrites, presumably enhancing intercellular communication and excitability of the MNCs (Andrew et al., 1981). In the neurohypophysis, axon terminals undergo similar uncovering caused by the retraction of pituitary astrocyte processes, and this is thought to facilitate the delivery of neuropeptides to the fenestrated capillaries (Tweedle and Hatton, 1980). Remarkably, all these changes are reversible with the cessation of stimulation. Together with the morphological changes, the glial populations in the SON and posterior pituitary show a basal proliferation that increases with increased physiological demands (Murray, 1968; Paterson and LeBlond 1977; Murugaiyan and Salm, 1995).
Despite the large number of studies of astrocytic structural plasticity in the SON, the possible contribution of SON microglia to structural remodeling in this nucleus has received little attention. Generally, microglia are known to serve an immune function and are involved in pathological states (Matsumoto et al., 1992; Maeda and Sobel, 1996). Ramified microglia, with an oval cell body and long branched processes, account for the majority of microglia in the adult CNS. When activated, microglia transform to an ameboid phenotype and are functionally similar to macrophages (Davis et al., 1994; Streit, 2000). In the SON, Mander and Morris (1995) reported three distinct populations of microglia in the hypothalamo-neurohypophysial system (HNS) using an array of four different antibodies. In the SON they identified ramified microglia with long radially branched processes. Elongated non-ramified microglia were also found throughout the HNS in association with blood vessels; these were described as perivascular. Here we extend their seminal observations by investigating a possible role for microglia in the plasticity that occurs in the SON after activation of the MNCs. We provide evidence that microglia, contrary to being quiescent bystanders under normal conditions, undergo activity-dependent changes in the activated SON.
Some of these data have been presented in preliminary form (Ayoub and Salm, 2000).
Materials and Methods
Histochemical staining. Twenty-four adult male Sprague Dawley rats (300-350 gm) of matching age were equally divided into four groups: controls (C), 2 d-dehydrated (D2), 7 d-dehydrated (D7), and 7 d-dehydrated/21 d-rehydrated (R21; n = 6 each group). We induced the activation of MNCs by substitution of 2% saline for drinking water (Jones and Pickering, 1969). The R21 group experienced conditions similar to the D7 experimental group for the first 7 d and then had access to tap water for a 21 day period. Rats were anesthetized with 150 mg/kg sodium pentobarbital, then transcardially perfused with 0.1 m PBS followed by 4% paraformaldehyde. We sectioned the brains at 50 μm with a vibrating microtome (VT1000S; Leica, Nussloch, Germany) and then processed the sections for histochemical staining with Isolectin-B4-HRP from Griffonia simplicifolia (L5391; Sigma, St. Louis, MO), which recognizes α-d-galactose-containing glycoconjugates on the surface of microglial cells in brain tissue (Streit and Kreutzberg, 1987; Streit, 1990). After rinsing in PBS containing 10% methanol and 0.5% H2O2 for 10 min to quench endogenous peroxidase activity and a 5 min rinse in PBS and 0.1% Triton X-100, sections were incubated in a solution of Isolectin-B4-HRP (20 μg/ml in 0.1 m PBS) for 2 hr at room temperature. Three washes of 5 min each in PBS preceded the final reaction in 3,3′-diaminobenzidine (Streit and Kreutzberg, 1987). For negative controls, we treated the sections with 0.1 m melibiose (6-O-α-d-galactopyranosyl-d-glucose; Sigma, M5500) for 30 min before the incubation in lectin (Wu et al., 1997). As visualized by this method, we were able to classify microglial cells based on morphology as ramified, hypertrophied, or ameboid, with good agreement between the two investigators. Ramified microglia were defined as having a normal appearing soma with thin, delicate, and radially projecting processes. Hypertrophied microglia were defined as a having an enlarged, darkened soma and shorter, thicker, and less branched, processes. Ameboid microglia were defined as having densely stained, enlarged cell bodies, a few short processes if any, and often several filopodia (Davis et al., 1994) (Fig. 1). Intermediate morphologies were rare, but were excluded from the analysis when encountered.
Figure 1.
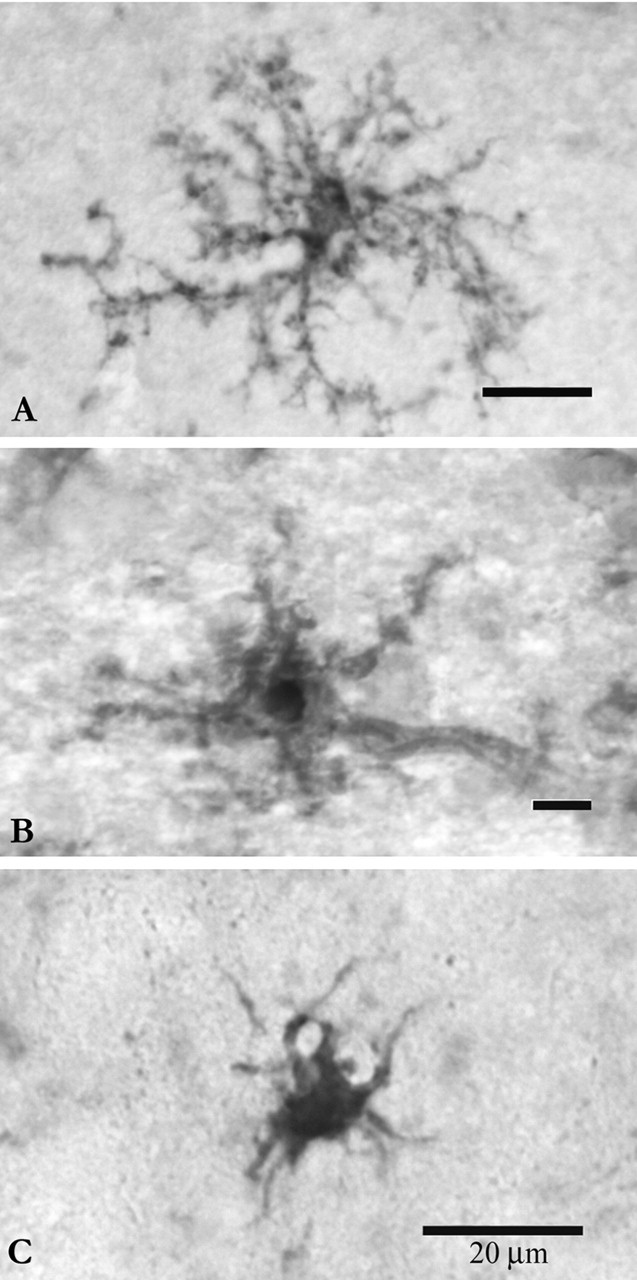
Microglial cells were classified as ramified (A), hypertrophied (B), or ameboid (C). Ramified microglia were defined as having a normal-appearing soma with thin, delicate, and radially projecting processes. Hypertrophied microglia were defined as a having an enlarged, darkened soma and shorter, thicker, and less branched processes. Ameboid microglia were defined as having densely stained, enlarged cell bodies, a few short processes, if any, and often several filopodia. Intermediate morphologies were rare, but excluded from the analysis when encountered. Scale bars, 20 μm.
Stereological analysis. To determine relative SON volumes and quantify microglia, we used an AX70 Olympus (Melville, NY) light microscope, interfaced with a personal computer (Micron PC, ID) and the StereoInvestigator software package (MicroBrightField, Colchester, VT). As determined by StereoInvestigator, we chose four sections through the rostrocaudal extent of the SON by systemic random sampling. This number of sections proved sufficient to provide reliable estimates of relative SON volumes based on an average section thickness of 20-23 μm after histochemical processing. Area measures of the SON included the SON proper containing the magnocellular neurons, plus the underlying dendritic zone and ventral glial limitans. These areas were later used by StereoInvestigator to compute volumes. The number of cells with each of the above morphologies in the SON, and a cortical control area of the cingulate gyrus were then quantified. Before counting, all slides were coded by a colleague to prevent experimenter bias. We used the “Optical Fractionator” method (Bjugn and Gundersen, 1993; Peterson, 1999) to achieve unbiased estimates of the number of cells. Cells were counted on the monitor at a final magnification of 400×. The number and density of cells (number of cells per cubic micrometer) was then determined for each region. We designated an area of the cingulate cortex close to the pial surface as the control, because similar to the SON it is adjacent to the subarachnoid space. For each section, a similarly sized area from the cingulate cortex was quantified in the same manner.
Data analysis. GraphPad (San Diego, CA) Prism software was used to analyze parametric data by an ANOVA followed by post hoc Tukey's t test comparisons between groups when appropriate. Bartlett's statistic was used to identify nonparametric data, which was analyzed with a Kruskal-Wallis test followed by Dunn's multiple comparisons.
Results
Volume of the SON
A significant change in the relative volume of the SON (SON proper, plus dendritic zone and underlying ventral glial limitans) was found (F(3,24) = 19.24; p < 0.001) across hydration states. Further comparisons showed that this was caused by a 36% increase in the volume of the D7 group relative to controls (q = 10.04; p < 0.001). SON volumes were as follows: C: 7.8 × 1007 μm3; D2: 8.8 × 1007 μm3; D7: 1.2 × 1008 μm3; and R21: 8.7 × 1007 μm3.
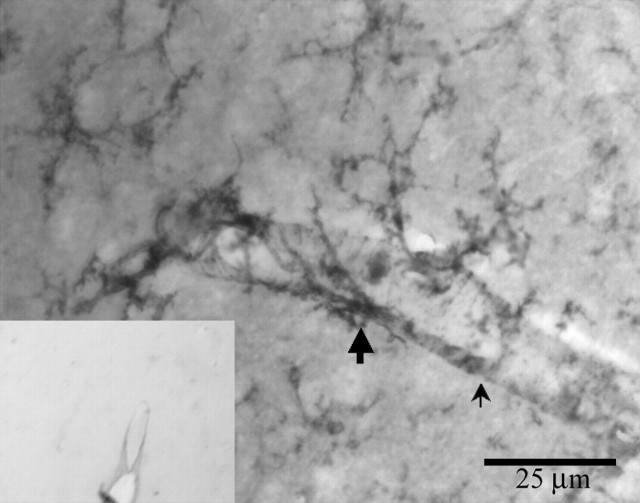
Isolectin B4 stains microglial cells in the adult CNS and also has a lower affinity to vascular endothelial cells (Streit and Kreutzberg, 1987). Although staining of blood vessels was minimal in all areas of the brain, it was more pronounced in the SON but not to the point of obscuring stained microglia (Figs. 2, 3, 4). Cells were easily discriminated from underlying vasculature by observing them at 400× magnification and focusing through the sections. Sections treated with melibiose (negative controls) before the incubation with isolectin, to saturate the α-d-galactosidase residues, did not show staining (Fig. 3, inset).
Figure 2.
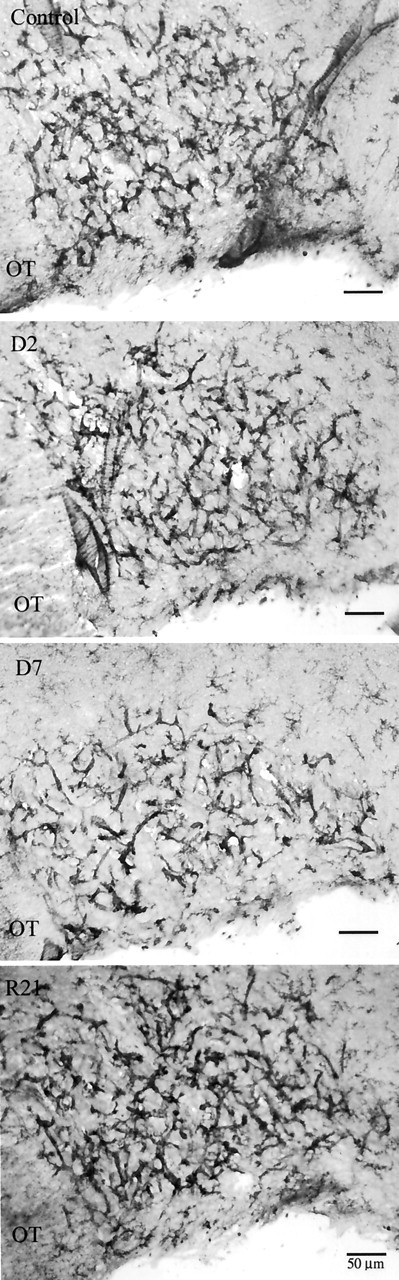
Representative sections from the SON from control, D2, D7, and R21 experimental groups (top to bottom). At low magnification it can be appreciated that the majority of microglia are in the vicinity of SON capillaries. Microglia were easily distinguished from capillary endothelia at higher magnification (Fig. 4). OT, Optic tract.
Figure 3.
Vascular endothelium in the SON stains slightly with isolectin-B4 (thin arrow). Clearly identifiable ramified microglia (thick arrow) are also visible. Inset, A negative control section pretreated with melibiose does not show stained microglia.
Figure 4.
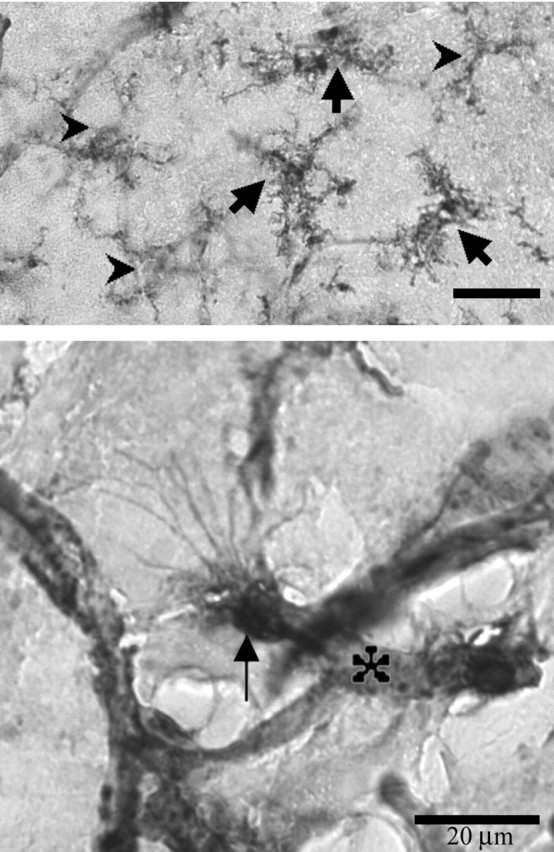
Example of microglia in the SON taken at high magnification and showing all three phenotypes: carets, ramified; thick arrows, hypertrophied; thin arrow, ameboid; asterisk, blood vessel (images edited with Adobe Photoshop; Adobe Systems Inc., San Jose, CA). Notice extensive filopodia protruding from the ameboid cell in the bottom panel.
Ramified microglia
Ramified microglia were evenly distributed throughout the SON and the brain parenchyma (Fig. 5). The numbers and densities of these cells did not change among experimental groups in the SON (F(3,21) = 0.76; p > 0.05, numbers) (F(3,21) = 0.391; p > 0.05, densities).
Figure 5.
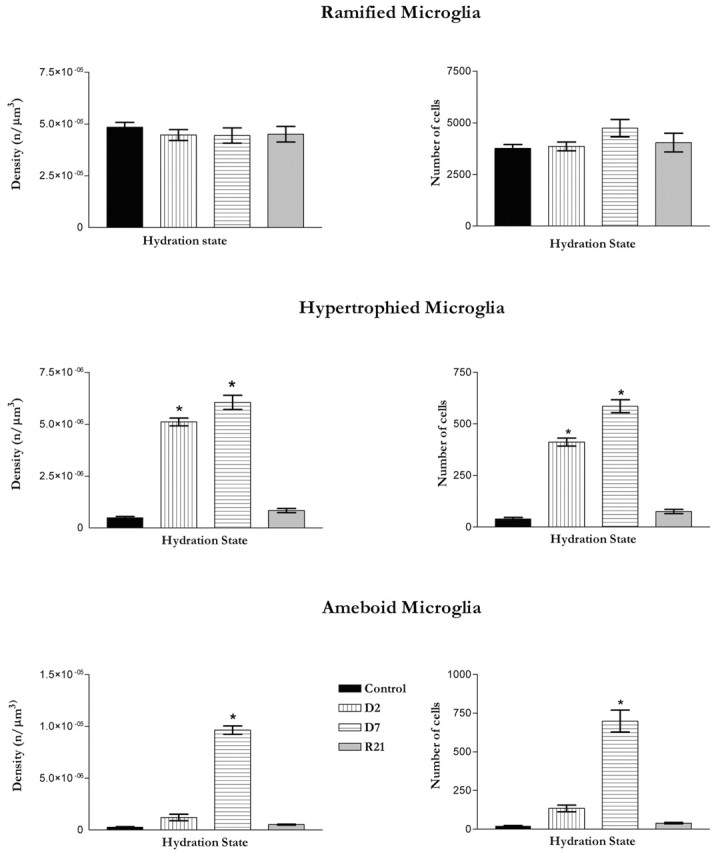
Graphs depicting densities and numbers of microglial phenotypes in the SON across hydration states. n = 6 or 7 in each group. The number of ramified microglia did not show significant change with stimulation. In addition, the density of ramified microglia did not change across hydration states. The number of hypertrophied microglia significantly increased (p < 0.001) in the D2 and D7 groups compared with the control and R21 groups. The density of hypertrophied microglia showed a similar increase. The number of ameboid microglia increased significantly (p < 0.001) in the D7 group compared with the control, D2, and R21 groups. The density of ameboid microglia was also significantly higher in the D7 group, but not in any of the other experimental groups. Error bars indicate SEMs for ramified microglia and minimum-maximum values for hypertrophied and ameboid microglia.
Hypertrophied microglia
Significant changes were observed in the numbers and densities of hypertrophied microglia (H3 = 21.0; p = 0.0001, numbers) (H3 = 20.9; p < 0.0001, densities) (Fig. 5). These changes were apparent, relative to controls by D2 (p < 0.05 both number and density). Further significant increases were seen with prolonged dehydration to D7 (p values <0.001). For both numbers and densities of hypertrophied microglia, no differences were found between controls and the R21 group, indicating that changes in this phenotype were totally reversed with rehydration.
Ameboid microglia
Significant changes were also observed in the numbers and densities of ameboid microglia in the activated SON (H3 = 18.3 and 20.8, respectively; p values <0.0001) (Fig. 5). The density, but not the number, of hypertrophied microglia was significantly increased by D2 (p < 0.05) relative to controls. However, the changes from D2 to D7 were robust (p values <0.001, number and density). After prolonged access to normal drinking water after 7 d of saline, there were no differences in numbers or densities of ameboid microglia in the R21 group versus controls. Hence, changes in ameboid microglia were also reversible with the cessation of stimulation.
Cingulate cortex
No changes were found in the microglial population in the control area of the cingulate cortex (Fig. 6). Microglia of the cingulate cortex almost exclusively exhibited ramified morphology, and this did not change with hydration state (F(3,21) = 0.39, ramified; 2.19, hypertrophied; 0.66, ameboid: p > 0.05 all cases). Thus, the effect of hydration was localized to the SON and did not affect the control area of the cingulate cortex.
Figure 6.

A, Graphs depicting densities and numbers of microglial phenotypes in the control area of the cingulate cortex across hydration states. An area of the ipsilateral cingulate cortex, of dimensions equal to the SON of that section, was quantified as a per-section-control. As shown in this graph, no significant changes in the density of any phenotype was detected in the area of the cingulate cortex. Error bars indicate SEMs. B, A representative section from a D2 animal showing the control area of the cingulate cortex. The majority of microglia identified in this area had a ramified phenotype in each experimental group.
Total microglia in the SON
To better understand the changes in microglial populations in the SON we also compared total numbers of isolectin-stained microglia in the SON across conditions (Fig. 7a). We found that there was a significant increase in the numbers of microglia (F(3,20) = 7.40; p < 0.002) and that this was not apparent until D7 (q = 6.15; p < 0.01). At this time there was a 37.0% increase in the total number of cells. Again, this change was reversed relative to controls in the R21 group (q = 0.98; p > 0.05).
Figure 7.
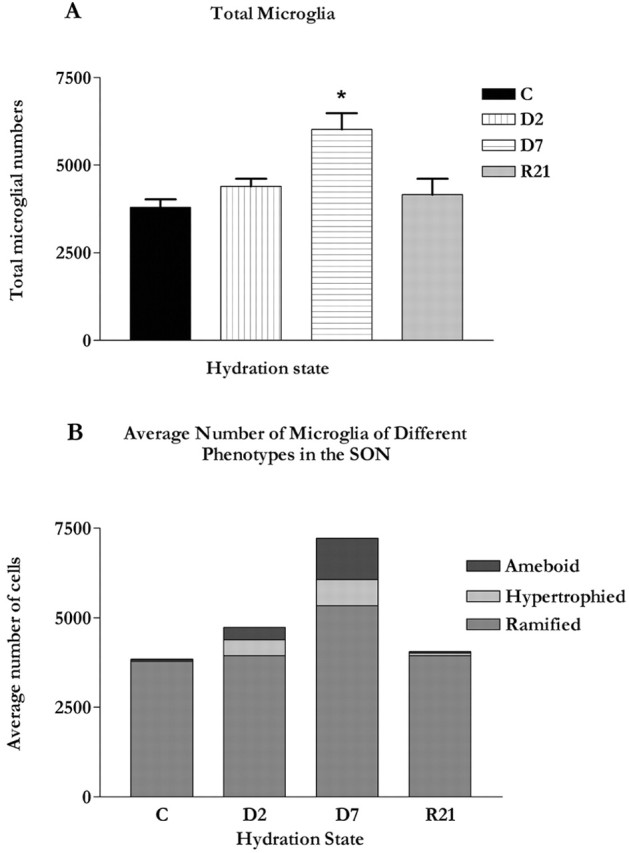
A, Graph showing the total numbers of microglia in the SON across hydration state. Significantly more microglia were observed in the SON at D7 (p < 0.01) relative to control and R21. Error bars indicate SEMs. B, Composite graph showing the relative numbers of each microglial phenotype in the SON across hydration states (graph generated using Microsoft Excel; Microsoft Corp., Seattle, WA).
When considered as a percentage of total cells (Fig. 7b), numbers of hypertrophied microglia increased from 1% in controls to 9.3 and 9.7% in D2 and D7 groups, respectively. Numbers of ameboid cells increased from a negligible 0.5% in controls to 11.6% of the total in the D7 group.
Discussion
Here we have shown that consumption of 2% saline instead of drinking water leads to increased morphological diversity of microglia in the SON. This manifests as a significant increase in the hypertrophied phenotype in the D2 and D7 groups and the ameboid phenotype in the D7 group. The changes we observed lasted as long as the dehydration stimulus and reversed with rehydration. Numbers of ramified microglia did not change across hydration state. However, with 7 d of dehydration, there was a significant increase in total microglia. This was accompanied by an overall significant enlargement of the SON at D7. Both of these effects were reversed with rehydration. The reversible increase in relative SON volume was not unexpected because it is known that MNCs hypertrophy when activated (Hatton and Walters, 1973). Interestingly, the values for total SON volume obtained in this study with systematic random sampling of four sections per animal agree well with those of a similar study in our laboratory in which we sampled approximately four times as many sections (Hawrylak and Salm, 1999), underscoring the efficiency of the new stereologic methods to estimate volumes (Gundersen et al., 1988). We did not detect phenotypic changes in microglia residing in the control area: the cingulate cortex.
Whether morphological transformation of microglia in the SON is accompanied by the biochemical transformation that microglia undergo in response to brain injury and disease was not addressed in this study. We used isolectin B4 to visualize these cells, because it enabled us to study many more microglia, including ramified microglia that ordinarily do not stain well with antibodies to biochemical markers of activation such as cytokines, CD4, ED1, and MHCII antigens (Flaris et al., 1993). Likewise, OX-42 antibodies did not label all SON microglia. Therefore, we have adhered to morphological descriptors in lieu of terms such as “resting,” “reactive”, and “activated.” Nevertheless, to our knowledge phenotypic and biochemical-functional transformation generally go hand in hand.
Of interest is the source of increased numbers of hypertrophied and ameboid microglia in the activated SON. Sequential transformation of ramified microglia into hypertrophied and then ameboid phenotypes is likely. This type of transformation occurs in response to disease or peripheral nerve injury (Streit and Graeber, 1993; Gehrmann et al., 1995). That significant increases in the numbers of hypertrophied microglia at D2 preceded significant increases in ameboid cells at D7 agrees with phenotypic transformation. However, the finding that numbers of ramified microglia did not decrease, and in fact remained stable, contradicts their being transformed. We propose that some ramified cells re-entered the cell cycle and supplied daughter cells to the hypertrophied and ameboid populations. On rare occasions we did see dividing hypertrophied microglia (data not shown). Lawson et al. (1992) have shown a turnover of microglia in normal mouse brain and increased DNA synthesis after 2.5% saline administration in neurohypophysial microglia (Lawson et al., 1993). We do not know if SON microglia proliferate, however, both Murray (1968) and Paterson and LeBlond, (1977) demonstrated proliferation of non-neuronal cells in the SON induced by saline substitution for drinking water, but attributed the increase in [3H]-thymidine labeling to division of astrocytes and endothelial cells.
Another source of increased SON microglia, as well as the selective increases in hypertrophied and ramified forms, may be an infiltration into the nucleus of circulating monocytes. Based on F4/80 labeling, Lawson et al. (1992) suggested cells can be recruited from the circulating monocyte pool, enter the brain through an intact blood-brain barrier, and differentiate into ramified microglia. Two days of dehydration is also a time when we have seen a reduction in the extent of the basal lamina (Salm and Bobak, 1999). These observations suggest that some of our ameboid microglia could belong to a population of “perivascular” circulating monocytes that exist near blood vessels outside of the basal lamina (Graeber and Streit, 1990). To determine if this is the case, we performed six separate attempts to stain control and activated SON tissues with antibodies to the perivascular cell marker ED2 (Graeber and Streit, 1990) according to the methods of Grossmann et al. (2002) (data not shown). To date, we have not detected ED2-positive microglia in the SON. Although negative data are difficult to interpret, our observations are consistent with those of Mander and Morris (1995), who found only negligible numbers of ED2-positive cells near the SON in the subjacent ventral glial limitans.
Alternatively, those microglia seen astride SON vasculature may belong to the “juxtavascular” population of parenchymal microglia recently studied by Grossmann et al. (2002). Using time-lapse observations of tissue slices, this group documented a highly mobile population of OX42+, ED2-microglia that was recruited to the surface of blood vessels. There these cells actively retracted their processes to assume an ameboid appearance. Migrating microglia coming from elsewhere in the CNS parenchyma could deposit α-d-galactose-containing glycoconjugate residues on the capillaries, accounting for the enhanced SON capillary labeling with isolectin B4.
From where does the signal leading to morphological transformation (or infiltration) of hypertrophied and ameboid microglia arise? We hypothesized that it might emanate from the CSF in the nearby subarachnoid space, but the absence of microglial changes in the cingulate cortex argues against this possibility. This is also adjacent to the subarachnoid space and has a much thinner glial limitans than does the SON (A. K. Salm, unpublished observations). Therefore the CSF is an unlikely source of any transformation signal, which more than likely originates in the SON instead.
Assuming some SON microglia undergo morphological transformation, it seems plausible that they do so in response to neurotransmitters released in the activated SON. These include GABA, glutamate, noradrenaline, and acetylcholine (for review, see Hatton and Li, 1998; Shibuya et al., 2000; Li et al., 2001). Microglia express the AMPA glutamate receptor (Noda et al., 2000), β-adrenergic receptors (Fujita et al., 1998), GABA receptors (Banati et al., 1997), and muscarinic cholinergic receptors (Hirayama and Kuriyama, 2001). Stimulation of any or all of these might well result in morphological changes of microglia.
An intriguing possibility is that SON microglia respond to activity-dependent release of interleukin-1β from dendrites of MNCs. Watt and Hobbs (2000) recently showed both oxytocinergic and vasopressinergic MNCs are immunoreactive for this cytokine, which is stored in granules distinct from neurosecretory granules. Furthermore, stores of IL-1β in neurosecretory terminals were depleted by 2% saline administration. IL-1β could be a transformative factor if it is also released into the nucleus via dendrites, similar to OX and VP (Landgraf et al., 1995). Consistent with this, Basu et al. (2002) showed that the type 1 interleukin receptor is essential for activation of microglia in response to brain injury.
What might be the functional significance of increased microglial diversity in the SON? Clearly the physiologically activated SON is undergoing significant changes, including structural remodeling, increased synaptic activity, and proliferation of endothelia and astrocytes (Murray, 1968; Paterson and LeBlond, 1977; Hatton, 1997). Microglia could remove cellular debris generated by both remodeling and the death of proliferating daughter cells. In support of this, in our electron microscopic studies of the SON (Bobak and Salm, 1996; Salm and Bobak, 1999) we have seen cells with the appearance of ameboid microglia (data not shown). These cells had vacuoles and filopodia and appeared to be phagocytosing nearby dying astrocytes (Bobak et al., 1997). In this study we observed increased numbers of hypertrophied microglia at 2 d 3of dehydration. It may be significant that at D2 there is also a dissolution of the basal lamina that precedes, and may be permissive for, the ensuing reorientation of glial processes, formation of novel synaptic contacts, and dendritic bundling (for review, see Salm, 2000). SON microglia may be participating in the degradation of basal lamina proteins, especially laminin (Stolzing et al., 2002).
SON microglia may also play a pivotal role in regulating peptides and other substances released in the activated nucleus. Ameboid microglia, increased at D7, provide a source of cytokines such as interleukin 1 (Watt and Hobbs, 2000; Chauvet et al., 2001). This cytokine excites MNCs (Li et al., 1992), upregulates MNC c-fos expression (Chang et al., 1993), and stimulates dendritic release of OX and VP (Landgraf et al., 1995). They may also take up locally released OX and VP, as is the case for activated posterior pituitary microglia (Pow et al., 1989).
Microglial cells are best known for their swift immunoeffector response to pathological states, including autoimmune and other diseases of the CNS and traumatic injuries (Booth and Thomas, 1991; Matsumoto et al., 1992; Streit and Graeber, 1993; McRae et al., 1997; Wu et al., 1997; Bruce-Keller, 1999). Although the neurons of the SON respond to increases in plasma osmolality (Richard and Bourque, 1995), we do not consider the physiologically activated SON as “pathological.” There is neither necrosis nor apoptosis of cellular constituents caused by this level and source of stimulation, and although microglia can respond to even small changes in CNS homeostasis (Streit, 2000), we did not observe these changes in brain areas uninvolved with hydration homeostasis. Our observations of a reversible increase in the morphological diversity of microglia in the SON suggest a novel function for these phenotypes in the normally functioning brain and adds to the remarkable list of structural changes that occur in the SON with stimulation.
Footnotes
This work was supported by National Science Foundation Grant 9514574.
Correspondence should be addressed to Dr. A. K. Salm, Department of Neurobiology and Anatomy, P. O. Box 9128, West Virginia University School of Medicine, Morgantown, WV 26506-9128. E-mail: asalm@hsc.wvu.edu.
Copyright © 2003 Society for Neuroscience 0270-6474/03/237759-08$15.00/0
References
- Andrew RD, MacVicar BA, Dudek FE, Hatton GI ( 1981) Dye transfer through gap junctions between neuroendocrine cells of rat hypothalamus. Science 211: 1187-1189. [DOI] [PubMed] [Google Scholar]
- Ayoub A, Salm AK ( 2000) Morphological plasticity of microglia in the activated supraoptic nucleus. Soc Neurosci Abstr 26: 1943. 2000. [Google Scholar]
- Banati RB, Myers R, Kreutzberg GW ( 1997) PK (“peripheral benzodiazepine”)-binding sites in the CNS indicate early and discrete brain lesions: microautoradiographic detection of [3H]PK11195 binding to activated microglia. J Neurocytol 26: 77-82. [DOI] [PubMed] [Google Scholar]
- Basu A, Krady JK, O'Malley M, Styren SD, DeKosky ST, Levison SW ( 2002) The type 1 interleukin-1 receptor is essential for the efficient activation of microglia and the induction of multiple proinflammatory mediators in response to brain injury. J Neurosci 22: 6071-6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett EL, Diamond MC, Krech D, Rosenzweig MR ( 1964) Chemical and anatomical elasticity of brain. Science 146: 610-619. [DOI] [PubMed] [Google Scholar]
- Bjugn R, Gundersen HJ ( 1993) Estimate of the total number of neurons and glial and endothelial cells in the rat spinal cord by means of the optical disector. J Comp Neurol 328: 406-414. [DOI] [PubMed] [Google Scholar]
- Bobak JB, Salm AK ( 1996) Plasticity of astrocytes of the ventral glial limitans subjacent to the supraoptic nucleus. J Comp Neurol 376: 188-197. [DOI] [PubMed] [Google Scholar]
- Bobak JB, Sedlmeyer T, Hawrylak N, Salm AK ( 1997) Evidence of astrocyte death in the glial limitans of the supraoptic nucleus (SON) of normal and dehydrated adult rats. Soc Neurosci Abstr 23: 2377. [Google Scholar]
- Booth PL, Thomas WE ( 1991) Evidence for motility and pinocytosis in ramified microglia in tissue culture. Brain Res 548: 163-171. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ ( 1999) Microglial-neuronal interactions in synaptic damage and recovery. J Neurosci Res 58: 191-201. [DOI] [PubMed] [Google Scholar]
- Chang SL, Ren T, Zadina JE ( 1993) Interleukin-1 activation of FOS protooncogene protein in the rat hypothalamus. Brain Res 617: 123-130. [DOI] [PubMed] [Google Scholar]
- Chauvet N, Palin K, Verrier D, Poole S, Dantzer R, Lestage J ( 2001) Rat microglial cells secrete predominantly the precursor of interleukin-1 beta in response to lipopolysaccharide. Eur J Neurosci 14: 609-617. [DOI] [PubMed] [Google Scholar]
- Davis EJ, Foster TD, Thomas WE ( 1994) Cellular forms and functions of brain microglia. Brain Res Bull 34: 73-78. [DOI] [PubMed] [Google Scholar]
- Flaris NA, Densmore TL, Molleston MC, Hickey WF ( 1993) Characterization of microglia and macrophages in the central nervous system of rats: definition of the differential expression of molecules using standard and novel monoclonal antibodies in normal CNS and in four models of parenchymal reaction. Glia 7: 34-40. [DOI] [PubMed] [Google Scholar]
- Fujita H, Tanaka J, Maeda N, Sakanaka M ( 1998) Adrenergic agonists suppress the proliferation of microglia through beta 2-adrenergic receptor. Neurosci Lett 242: 37-40. [DOI] [PubMed] [Google Scholar]
- Gehrmann J, Matsumoto Y, Kreutzberg GW ( 1995) Microglia: intrinsic immuneffector cell of the brain. Brain Res Brain Res Rev 20: 269-287. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Streit WJ ( 1990) Perivascular microglia defined. Trends Neurosci 13: 366. [DOI] [PubMed] [Google Scholar]
- Greenough WT ( 1988) The turned-on brain: developmental and adult responses to the demands of information storage. In: From message to mind (Easter SS, Barald KF, Carlson BM, eds), pp 288-302. Sunderland, MA: Sinauer.
- Greenough W, Alcantara A, Hawrylak N, Anderson B, Karr T, Weiler IJ ( 1992) Determinants of brain readiness for action: experience shapes more than neuronal form. Brain Dysfunction 5: 129-149. [Google Scholar]
- Grossmann R, Stence N, Carr J, Fuller L, Waite M, Dailey ME ( 2002) Juxtavascular microglia migrate along brain microvessels following activation during early postnatal development. Glia 37: 229-240. [PubMed] [Google Scholar]
- Gundersen HJG, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A, West MJ ( 1988) The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS 96: 857-881. [DOI] [PubMed] [Google Scholar]
- Hatton GI ( 1997) Function-related plasticity in hypothalamus. Annu Rev Neurosci 20: 375-397. [DOI] [PubMed] [Google Scholar]
- Hatton GI, Li Z ( 1998) Mechanisms of neuroendocrine cell excitability. Adv Exp Med Biol 449: 79-95. [DOI] [PubMed] [Google Scholar]
- Hatton GI, Walters JK ( 1973) Induced multiple nucleoli, nucleolar margination, and cell size changes in supraoptic neurons during dehydration and rehydration in the rat. Brain Res 59: 137-154. [DOI] [PubMed] [Google Scholar]
- Hawrylak N, Salm AK ( 1999) Stereological analysis of the activated supraoptic nucleus (SON) and subjacent ventral glial limitans. Soc Neurosci Abstr 25: 1902. [Google Scholar]
- Hirayama M, Kuriyama M ( 2001) MK-801 is cytotoxic to microglia in vitro and its cytotoxicity is attenuated by glutamate, other excitotoxic agents and atropine. Possible presence of glutamate receptor and muscarinic receptor on microglia. Brain Res 897: 204-206. [DOI] [PubMed] [Google Scholar]
- Jones CW, Pickering BT ( 1969) Comparison of the effects of water deprivation and sodium chloride inhibition on the hormone content of the neurohypophysis of the rat. J Physiol (Lond) 203: 449-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf R, Neumann I, Holsboer F, Pittman QJ ( 1995) Interleukin-1 beta stimulates both central and peripheral release of vasopressin and oxytocin in the rat. Eur J Neurosci 7: 592-598. [DOI] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Gordon S ( 1992) Turnover of resident microglia in the normal adult mouse brain. Neuroscience 48: 405-415. [DOI] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Gordon S ( 1993) Microglial responses to physiological change: osmotic stress elevates DNA synthesis of neurohypophyseal microglia. Neuroscience 56: 929-938. [DOI] [PubMed] [Google Scholar]
- Li DP, Pan YZ, Pan HL ( 2001) Acetylcholine attenuates synaptic GABA release to supraoptic neurons through presynaptic nicotinic receptors. Brain Res 920: 151-158. [DOI] [PubMed] [Google Scholar]
- Li Z, Inenage K, Kawano S, Kannan H, Yamashita H ( 1992) Interleukin-1 beta directly excites hypothalamic supraoptic neurons in rats in vitro. NeuroReport 3: 91-93. [DOI] [PubMed] [Google Scholar]
- Maeda A, Sobel RA ( 1996) Matrix metalloproteinases in the normal human central nervous system, microglial nodules, and multiple sclerosis lesions. J Neuropathol Exp Neurol 55: 300-309. [DOI] [PubMed] [Google Scholar]
- Mander TH, Morris JF ( 1995) Immunophenotypic evidence for distinct populations of microglia in the rat hypothalamo-neurohypophysial system. Cell Tissue Res 280: 665-673. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Ohmori K, Fujiwara M ( 1992) Immune regulation by brain cells in the central nervous system: microglia but not astrocytes present myelin basic protein to encephalitogenic T cells under in vivo-mimicking conditions. Immunology 76: 209-216. [PMC free article] [PubMed] [Google Scholar]
- McRae A, Dahlstrom A, Ling EA ( 1997) Microglial in neurodegenerative disorders: emphasis on Alzheimer's disease. Gerontology 43: 95-108. [DOI] [PubMed] [Google Scholar]
- Miyata S, Itoh T, Matsushima O, Nakashima T, Kiyohara T ( 1994) Not only osmotic stress but also repeated restraint stress causes structural plasticity in the supraoptic nucleus of the rat hypothalamus. Brain Res Bull 33: 669-675. [DOI] [PubMed] [Google Scholar]
- Murray M ( 1968) Effects of dehydration on the rate of proliferation of hypothalamic neuroglia cells. Exp Neurol 20: 460-468. [DOI] [PubMed] [Google Scholar]
- Murugaiyan P, Salm AK ( 1995) Dehydration-induced proliferation of identified pituicytes in fully adult rats. Glia 15: 65-76. [DOI] [PubMed] [Google Scholar]
- Noda M, Nakanishi H, Nabekura J, Akaike N ( 2000) AMPA-kainate subtypes of glutamate receptor in rat cerebral microglia. J Neurosci 20: 251-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson JA, Leblond CP ( 1977) Increased proliferation of neuroglia and endothelial cells in the supraoptic nucleus and hypophysial neural lobe of young rats drinking hypertonic sodium chloride solution. J Comp Neurol 175: 373-390. [DOI] [PubMed] [Google Scholar]
- Peterson DA ( 1999) Quantitative histology using confocal microscopy: implementation of unbiased stereology procedures. Methods 18: 493-507. [DOI] [PubMed] [Google Scholar]
- Pow DV, Perry VH, Morris JF, Gordon S ( 1989) Microglia in the neurohypophysis associate with and endocytose terminal portions of neurosecretory neurons. Neuroscience 33: 567-578. [DOI] [PubMed] [Google Scholar]
- Richard D, Bourque CW ( 1995) Synaptic control of rat supraoptic neurones during osmotic stimulation of the organum vasculosum lamina terminalis in vitro. J Physiol (Lond) 489: 567-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salm AK ( 2000) Mechanisms of glial retraction in the hypothalamoneurohypophysial system of the rat. Exp Physiol 85S: 197S-202S. [DOI] [PubMed] [Google Scholar]
- Salm AK, Bobak JB ( 1999) Dehydration-associated changes in the ventral glial limitans subjacent to the supraoptic nucleus include a reduction in the extent of the basal lamina but not astrocytic process shrinkage. Exp Neurol 160: 425-432. [DOI] [PubMed] [Google Scholar]
- Salm AK, Modney BK, Hatton GI ( 1988) Alterations in supraoptic nucleus ultrastructure of maternally behaving virgin rats. Brain Res Bull 21: 685-691. [DOI] [PubMed] [Google Scholar]
- Salm AK, Hawrylak N, Bobak J, Hatton GI, Aoki C ( 1998) Recent evidence from around the brain for structural plasticity of astrocytes in the adult CNS. In: Glial cells: their role in behaviour (Laming P, Sykova E, Reichen-bach A, Hatton GI, Bauer H, eds), pp 291-314. Cambridge: Cambridge UP.
- Shibuya I, Kabashima N, Ibrahim N, Setiadji SV, Ueta Y, Yamashita H ( 2000) Pre- and postsynaptic modulation of the electrical activity of rat supraoptic neurones. Exp Physiol 85S: 145S-151S. [DOI] [PubMed] [Google Scholar]
- Stolzing A, Wengner A, Grune T ( 2002) Degradation of oxidized extracellular proteins by microglia. Arch Biochem Biophys 400: 171-179. [DOI] [PubMed] [Google Scholar]
- Streit WJ ( 1990) An improved staining method for rat microglial cells using the lectin from Griffonia simplicifolia (GSA I-B4). J Histochem Cytochem 38: 1683-1686. [DOI] [PubMed] [Google Scholar]
- Streit WJ ( 2000) Microglial response to brain injury: a brief synopsis. Toxicol Pathol 28: 28-30. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Graeber MB ( 1993) Heterogeneity of microglial and perivascular cell populations: insights gained from the facial nucleus paradigm. Glia 7: 68-74. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Kreutzberg GW ( 1987) Lectin binding by resting and reactive microglia. J Neurocytol 16: 249-260. [DOI] [PubMed] [Google Scholar]
- Theodosis DT, Poulain DA ( 1999) Contribution of astrocytes to activity-dependent structural plasticity in the adult brain. Adv Exp Med Sci 468: 175-182. [DOI] [PubMed] [Google Scholar]
- Theodosis DT, Poulain DA, Vincent J-D ( 1981) Possible morphological bases for synchronisation of neuronal firing in the rat supraoptic nucleus during lactation. Neuroscience 6: 919-929. [DOI] [PubMed] [Google Scholar]
- Tweedle CD, Hatton GI ( 1976) Ultrastructural comparisons of neurons of supraoptic and circularis nuclei in normal and dehydrated rats. Brain Res Bull 1: 103-121. [DOI] [PubMed] [Google Scholar]
- Tweedle CD, Hatton GI ( 1980) Evidence for dynamic interactions between pituicytes and neurosecretory axons in the rat. Neuroscience 5: 661-667. [DOI] [PubMed] [Google Scholar]
- Watt JA, Hobbs NK ( 2000) Interleukin-1 β immunoreactivity in identified neurons of the rat magnocellular neurosecretory system: evidence for activity-dependent release. J Neurosci Res 60: 478-489. [DOI] [PubMed] [Google Scholar]
- Wu CH, Wang HJ, Wen CY, Lien KC, Ling EA ( 1997) Response of amoeboid and ramified microglial cells to lipopolysaccharide injections in postnatal rats- a lectin and ultrastructural study. Neurosci Res 27: 133-141. [DOI] [PubMed] [Google Scholar]