Abstract
Transplantation of olfactory ensheathing cells into spinal cord lesions promotes regeneration of cut axons into terminal fields and functional recovery. This repair involves the formation of a peripheral nerve-like bridge in which perineurial-like fibroblasts are organized into a longitudinal stack of parallel tubular channels, some of which contain regenerating axons enwrapped by Schwann-like olfactory ensheathing cells. The present study examines whether cut retinal ganglion cell axons will also respond to these cells, and if so, whether they form the same type of arrangement. In adult rats, the optic nerve was completely severed behind the optic disc, and a matrix containing cultured olfactory ensheathing cells was inserted between the proximal and distal stumps. After 6 months, the transplanted cells had migrated for up to 10 mm into the distal stump. Anterograde labeling with cholera toxin B showed that cut retinal ganglion cell axons had regenerated through the transplants, entered the distal stump, and elongated for 10 mm together with the transplanted cells. Electron microscopy showed that a peripheral nerve-like tissue had been formed, similar to that seen in the spinal cord transplants. However, in contrast to the spinal cord, the axons did not reach the terminal fields, but terminated in large vesicle-filled expansions beyond which the distal optic nerve stump was reduced to a densely interwoven mass of astrocytic processes.
Keywords: regeneration, CNS, adult, vision, eye, OECs, transplantation
Introduction
Lesions of the retinal ganglion cell (RGC) axons have been extensively used as models for various repair strategies in the adult mammalian CNS (for review, see Chierzi and Fawcett, 2001). Retrobulbar section of adult rodent RGC axons results in abortive sprouting (Zeng et al., 1995) and progressive cell death of the parent population (Villegas-Perez et al., 1993; Berkelaar et al., 1994). Anastomosis of Schwann cell-containing peripheral nerves on to the optic nerve stump has been shown to rescue axotomized RGCs (Villegas-Pérez et al., 1988) and to mediate regrowth of their severed axons from behind the optic disc to innervate pretectum and superior colliculus (Vidal-Sanz et al., 1987; Avilés-Trigueros et al., 2000), where they form functional synapses (Keirstead et al., 1989; Sauvé et al., 1995). Numerous studies have shown the stimulatory effects of various exogenous and endogenous factors on the survival of axotomized RGCs (for review, see Yip and So, 2000) and regeneration of their axons (Cui et al., 1999; Leon et al., 2000; Fischer et al., 2001).
Olfactory ensheathing cells (OECs) cultured from the adult rat olfactory bulb and transplanted into the spinal cord induce regrowth of severed long spinal axons and recovery of function (Li et al., 1997, 2003; Ramón-Cueto et al., 1998, 2000). The axonal repair is associated with formation of a peripheral nerve-like structure in which Schwann-like OECs enwrap the regenerating axons and are themselves enclosed within a perineurial-like sheath of fibroblasts (Li et al., 1998). In the present study we examined whether transplanted OECs would have a growth-promoting effect on severed adult RGC axons and whether they would establish similar peripheral nerve-like arrangements to those seen in the repaired corticospinal tract.
Materials and Methods
Cell culture. Tissue was dissected from the outer nerve and glomerular layers of locally inbred (syngeneic) adult female Albino Swiss (AS) rat olfactory bulbs, trypsinized (1% trypsin for 30 min at 37°C), and plated on to 35 mm dishes coated with poly-l-lysine and cultured for 14 -17 d in DMEM-F-12 Nutrient Mix and 10% fetal calf serum (Invitrogen, Gaithersburg, MD) to a density of ∼1.5 × 106 in a 35 mm culture dish, then washed in the same medium without serum (Li et al., 1998). This is based on the method of Ramón-Cueto and Nieto-Sampedro (1992), but without the purification step. The initial plating density was critical for the cells to produce a sufficiently firm matrix to allow transfer into the lesion site. The optimal cell mixture was obtained between 14 and 17 d culture in DMEM-F-12 medium including 10% fetal calf serum (Invitrogen 3133-028), at which time each dish yielded ∼1.5 million cells, of which ∼50% are p75-positive OECs and 50% are fibronectin-positive olfactory nerve fibroblasts (ONFs) (Li et al., 1998), embedded in a matrix of their own production, and covering the dish to a thickness of ∼20 μm. This cell-containing matrix was scraped off the dish with a polythene scraper (3010; Costar, Cambridge, MA), gathered into a mass of ∼5 mm and cut into four or five pieces. Using number 5 watchmaker's forceps (Dumont), the pieces were lifted out of the dish and transferred directly into the lesion area. Compared with injections of cells in suspension, this “endogenous matrix transfer method” (Li et al., 2003) provided a semisolid mass that facilitated efficient harvesting of the cultured cells from the dish, enabled the transplant to be formed into a physical bridge between the cut nerve ends, and retained the cells in place during the crucial early postoperative period.
Surgical procedures. In 122 adult female AS rats (220 -240 gm body weight) under intraperitoneal tribromoethanol anesthesia (20 mg/100 gm body weight), the optic nerve was exposed, and the optic nerve sheath was incised intraorbitally at ∼2 mm from the optic disc, the nerve was lifted from the sheath to allow complete transection with fine scissors. Completeness of transection was confirmed visually by complete separation of the proximal and distal stumps. The control group consisted of 49 lesioned rats without transplants. In 73 rats, one or two pellets of OECs in matrix were inserted into this cavity. The opening in the optic nerve sheath was closed with 10/0 Ethicon W2970 sutures (Johnson & Johnson, Edinburgh, UK), and this had the effect of closing the gap by drawing the cut proximal and distal ends of the nerve in proximity with each other.
Animal groups. Of the 73 rats with lesions and transplants, 39 were used for immunohistochemistry at survival times of 2 weeks (n = 10), 3 weeks (n = 12), 1 (n = 4),3(n = 4),8(n = 5), and 9 months (n = 4), 26 for cholera toxin B (CTB) labeling of RGC axons at survival times of 5 (n = 8), 6 (n = 10), and 7 months (n = 8), 4 for electron microscopy at a survival time of 7 months, and 4 for green fluorescent protein (GFP) labeling of the transplanted cells at a survival time of 10 d. Of the 49 rats with lesion alone, 14 were used for immunohistochemistry at survival times of 4 (n = 5), 6 (n = 4), and 7 months (n = 5), and 35 for CTB labeling of RGC axons at survival times of 2 weeks (n = 4), 1 (n = 5), 2 (n = 5), 3 (n = 5), 4 (n = 6), 6 (n = 6), and 8 months (n = 4).
Immunohistochemistry. Under deep terminal pentobarbitone anesthesia, rats were perfused with 0.1 m PBS, and 16 μm longitudinal cryostat sections were immunostained for glial fibrillary acidic protein (GFAP), neurofilament, p75 low-affinity neurotrophin receptor, fibronectin, and PO, a marker of peripheral myelin (for details, see Li et al., 1998).
CTB application. Under anesthesia, a glass micropipette with a 30 -50 μm tip was inserted tangentially through the sclera behind the lens, and two 4 -5 μl aliquots of a 1% aqueous solution of CTB (List Biologic, Campbell, CA) were injected into the vitreous. Detection of CTB was achieved using the protocol of Angelucci et al. (1996) with minor modifications. Briefly, 5 d after CTB injection, the rats were perfused with 4% paraformaldehyde. Sixteen micrometer cryostat sections were incubated in 0.1 m glycine for 30 min and blocked in a PBS solution containing 2.5% bovine serum albumin (BSA) (Boehringer Mannheim, Mannheim, Germany) and 0.5% Triton X-100, followed by a solution containing goat anti-CTB antibody (List Biologic) diluted 1:4000 in a PBS containing 2% normal rabbit serum (NRS), 2.5% BSA, and 2% Triton X-100 overnight at room temperature, washed in PBS, and incubated in biotinylated rabbit anti-goat IgG antibody (Vector Laboratories, Burlingame, CA) diluted 1:200 in 2% NRS, 2.5% BSA, and 2% Triton X-100 in PBS for 2 hr at room temperature, followed by 1:200 streptavidin-green (Alexa Fluor 438; Molecular Probes, Eugene, OR) in PBS for 2 hr at room temperature, washed in PBS, and counterstained with propidium iodide for confocal microscopy.
Electron microscopy. The rats were perfused with a mixture of 1% paraformaldehyde and 1% glutaraldehyde, and the optic nerves were dissected out and embedded in resin (for details, see Li and Raisman, 1995).
GFP labeling of transplanted cells. One day before transplantation the cells were transfected with an adenoviral vector harboring enhanced GFP (Clontech, Palo Alto, CA). This labels 25-50% of the cells and provides a strong fluorescent label for up to 12-14 d after transplantation (Ruitenberg et al., 2002). After 10 d survival, these rats were perfused with 4% paraformaldehyde, and 20 μm cryostat sections were examined in the fluorescent microscope.
Results
Lesions alone
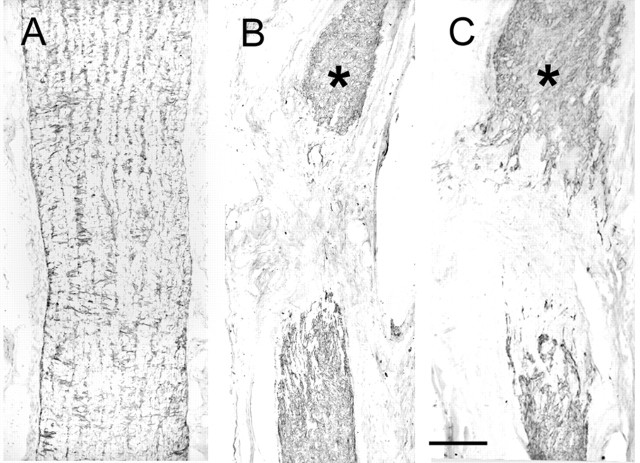
In the intact animal, the two injections of CTB into the vitreous body label a substantial contingent of retinal ganglion cell axons dispersed evenly throughout the optic nerve. One month after retrobulbar section of the optic nerve, numerous CTB-labeled axons are still present in the segment of the nerve attached to the retina. For the last 200 μm before the cut, the axons emit numerous collaterals at right angles to the main axis and then terminate as a neuromatous tangle at the cut surface. Despite the fact that the gap between the cut proximal and distal stumps becomes progressively physically bridged by a 2-3 mm segment of vascularized connective tissue, no axons are ever seen to advance into this bridge, and none reach the distal stump. At increasing survival times, there is a major reduction in the numbers of axons present in the proximal nerve, with only a very small number remaining at 8 months (Fig. 1). Compared with the intact nerve, there is a markedly increased density of GFAP staining in both proximal and distal stumps. This obscures the normal linear arrangement of astrocytic rows and transverse processes (Fig. 2A,B), and is associated with the formation of a dense meshwork of interweaving processes (see Fig. 7C)
Figure 1.

CTB labeling of RGC axons (green) showing progressive decrease from 1, 3, 6-8 months (m) after lesion without transplant. d, Distal stump; p, proximal stump. Counterstained with propidium iodide (red). Longitudinal confocal images. Scale bar, 250 μm.
Figure 2.
GFAP staining of the intact optic nerve (A), lesioned optic nerve without transplant (B), and with transplant (C). Survival times, 7 months in B, 8 months in C. Asterisks, the cut end of the proximal stump. Longitudinal sections. Scale bar, 250 μm.
Figure 7.

A, Huge axon varicosity (a) densely packed with mitochondria and synaptic and dense core vesicles, and making direct contact with massive collagen bundles (c). B, An axon terminal varicosity (a) retaining a partial contact with the process of a Schwann-like OEC (arrow). C, Densely packed astrocytic processes (s) in the optic nerve stump distal to the furthest extension of the transplanted OECs and associated RGC axon growth. Longitudinal sections. Survival time, 7 months. Scale bar, 1 μm.
Transplantation of OECs
Cells
GFP labeling of the transplanted OECs confirms that the endogenous matrix transfer method (Li et al., 2003) allows efficient retention of a dense mass of cells in the lesion cavity. By 10 d after operation the cells have taken up their typically elongated form, ∼100 -150 μm in length, and aligned along the axis of the nerve. As in the rats with nontransplanted lesions, the 2-3 mm gap between the cut proximal and distal surfaces of the nerve becomes progressively bridged by vascularized connective tissue. The fluorescent signal from the adenovirally transduced GFP shows that this bridge is densely infiltrated by the elongated transplanted cells (Fig. 3) (in which case we estimate there are ∼15,000 transplanted cells). The fluorescent signal from the adenovirally transduced GFP label fades progressively after 2 weeks, so that subsequent detection of the transplanted cells relies on p75 and fibronectin immunohistochemistry and electron microscopy.
Figure 3.

A, Low-power photomicrograph to show the location of the transplant (T; immunostained for fibronectin) within the repaired optic nerve sheath (s). B, GFP labeling (green) to show the aligned mass of transplanted OECs crossing the lesion from proximal (p) to distal (d) stumps. A smaller population of OECs track out into the area of the sutures (arrows). Counterstained with propidium iodide (red). Survival times, 2 weeks (A), 10 d (B). Longitudinal confocal images. Scale bars, 250 μm.
Axons
At 2 weeks neurofilament immunohistochemistry shows many small bundles of axons already penetrating throughout the transplant area. At 5-8 months after operation, CTB labeling shows bundles of optic nerve axons leaving the proximal optic nerve stump, crossing the entire 2-3 mm length of the transplant, and entering the distal stump, where the fibers are distributed throughout the cross-sectional area of the optic nerve, but with some preference for the periphery (Fig. 4). The regenerating fibers are straight, unbranched, and of fine diameter (see EM below). As they proceed distally, the axons aggregate into larger fascicles within which individual axons are too closely packed to be separately resolved by light microscopy. From the point at which they were cut, the regenerating axons extend overall for ∼10 mm into the distal stump, but never reach the optic chiasma or tract, or the terminal fields. Estimates of the numbers of regenerating fibers taken at a distance of 2 mm below the transplant, in the distal part of the nerve, in each of the five animals at 6 months survival were 224, 252, 350, 266 (by CTB labeling), and 230 (by electron microscopy.)
Figure 4.

CTB labeling (green) of regenerating RGC axons induced by OEC transplants (T). Counterstained with propidium iodide (red). Longitudinal confocal images. Survival time, 6 months in A; 7 months in B. Scale bar, 500 μm.
The GFAP labeling pattern shows an intensification broadly similar to that after lesion alone, although with some suggestion of a more “open” texture at the proximal interface (Fig. 2B,C, asterisks, compare the cut end of the proximal stump).
Electron microscopy
Semithin and ultrathin sections show that the proximal and distal stumps are joined by a slender bridge of ∼100 -150 μm diameter and 300 - 400 μm length, consisting of strands of compact, highly vascularized tissue enclosed within the repaired outer optic nerve sheath (Fig. 5A,B).
Figure 5.
Transplanted OECs (arrow) forming a slender, highly vascularized tissue bridge (arrows) across the lesion. A, Longitudinal section; B, cross section. s, Repaired optic nerve sheath. Resin sections (1- to 2-μm-thick) stained with methylene blue and Azur II. Survival time, 7 months. Scale bars: A, 200 μm; B, 300 μm.
The transplants contain two types of cells: OECs and ONFs. As in the olfactory nerves in situ, and in culture, the p75-positive (“Schwann-like”) OECs are always accompanied by approximately equal numbers of fibronectin-positive (“fibroblast-like”) ONFs (Li et al., 2003). As in the transplants into the corticospinal tract (Li et al., 1998), the OECs are Schwann-like cells that enwrap the axons (although never myelinating in the case of the present optic nerve transplants). They do not make direct contact with any other cell type and are surrounded on their abaxonal surfaces by basal lamina. The ONFs are fibroblast-like cells that lie in close proximity to each other and coalesce to form an outer sheath around channels containing the OECs are their associated axons. The ONFs do not make direct contact with either axons or OECs, from which they are separated by a collagen containing extracellular space.
Electron micrographs taken across these tissue bridges show that they consist of a mass of channels varying between 1 and 10 μm diameter, which are formed by overlapping hollow cylindrical sheets of electron-dense fibroblast cytoplasm and which are filled with collagen fibrils (Fig. 6A,B). The channels and the collagen fibrils are aligned along the longitudinal axis of the nerve (Fig. 6C). The fibroblastic sheets are ∼0.5-1.0 μm in width; at higher power they can be seen to be closely adherent at numerous electron-dense junctions (Fig. 6D). At least six of the channels shown in Figure 6A contain nerve bundles consisting of masses of unmyelinated axons, ranging in diameter from 0.1 to 1.5 μm, also aligned longitudinally, and partially or completely en-wrapped in thin cytoplasmic sheets arising from the elongated OECs (Fig. 6B). The remaining channels contain only collagen. Facing the collagen-containing space, the outward-facing surfaces of the cytoplasmic sheets of the OECs, and also less completely, the inner-facing surfaces of the cell processes making up the fibroblastic sheath are clothed by a basal lamina. No myelination was seen in any of the samples, either in semithin or ultrathin sections, or with P0 immunostaining.
Figure 6.
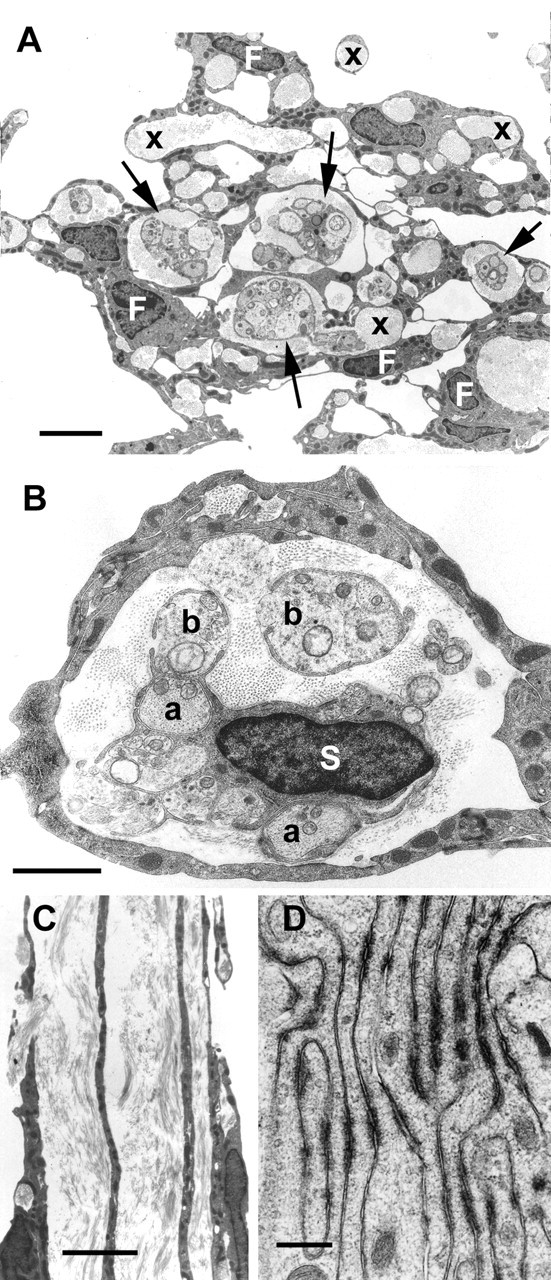
Electron micrographic samples from the bridge of transplanted OECs. A is a cross section, showing multiple tissue channels formed by the processes of the fibroblast-like cells (F). Some channels (arrows) contain regenerating RGC axons enwrapped in thin processes of Schwann-like OECs (S in B) that lie in a collagen-filled space, other channels (x) contain only collagen. B, Cross section of a single fibroblastic channel containing the nucleated perikaryon of a Schwann-like OEC (S) that gives rise to fine cytoplasmic sheets completely (a) or partially (b) enclosing regenerating RGC axons and other, probably astrocytic processes. C is a longitudinal section showing the linear fibroblastic processes forming the walls of adjacent collagen-containing tubular channels. D, High-power micrograph showing the numerous symmetrical electron-dense junctions between tightly apposed fibroblastic processes. Survival, 7 months. Scale bars: A, 4 μm; B, 2 μm; C, 6 μm; D, 0.5 μm.
At progressively more distal levels, the axons become more closely packed together, and the relative number of OEC processes decreases. This is the ultrastructural equivalent of the dense bundling seen in the CTB confocal pictures (Fig. 4d). The advance of the axons is coextensive with the migration of the OECs and ONFs. At the furthest distances (∼10 mm) from the level of the original cut where the migration of the transplanted OECs into the distal stump ceases, the nerve fibers escape from the ensheathing processes of the OECs and expand into huge terminal varicosities densely filled with clear and dense core vesicles (Fig. 7A,B). There is no indication of synaptic contacts with any postsynaptic element. The terminals are directly apposed to massive interweaving collagen bundles that mark the transition to the distal part of the denervated optic nerve stump, which, at 7 months, consists of densely packed astrocytic processes (Fig. 7C). Compared with the axon-containing proximal stump, where the astrocytes still have a degree of longitudinal orientation, the much more numerous, finer astrocytic processes of the distal, empty stump (beyond the furthest axon extension) form a random meshwork from which oligodendrocytes and myelin have disappeared.
Discussion
These observations show that transplanted adult OECs survive within a complete transection of the adult optic nerve and, in addition, migrate into the adjacent proximal and distal stumps of the optic nerve. They are able to stimulate and guide regeneration of cut retinal ganglion cell axons for up to 10 mm into the distal stump. The peripheral nerve-like organization of the transplanted tissue in the optic nerve is strikingly similar to that which we observed after transplantation of OECs into the lesioned corticospinal tract (Li et al., 1998). In both the corticospinal tract and the optic nerve, the fibroblast-like ONFs play a key role. Aggregations of cell bodies give rise to thin cytoplasmic sheets, shaped into hollow cylinders and tightly bound by numerous membrane adhesions. The effect is to produce a parallel stack of tubular, collagen-containing channels aligned with the long axis of the host tract. These ONF channels act as conduits for the Schwann-like OECs and their enclosed axons.
A number of studies have shown the structural and above all functional repair of spinal cord injuries by transplants of OECs (Li et al., 1997, 2003; Ramón-Cueto et al., 1998, 2000; Lu et al., 2002). In the spinal cord, the regenerating axons are able to leave the transplant and re-enter the host tracts. In the optic nerve, however, the regenerating RGC axons terminate in large expansions and do not continue further distally in the optic nerve stump. In the electron micrographs of the optic nerve transplants, the axons are never seen unaccompanied by OEC and ONF processes. They are never found on their own extending into the astrocytic territory of the distal stump. Thus, in the optic nerve, the advance of the axons is coextensive with the migration of the OECs and ONFs and their processes.
Because the mammalian optic nerve consists almost entirely of retinofugal axons, axotomy causes effectively total loss of axons from the distal stump, and this results in the death of the oligodendrocytes (for references, see Li et al., 1999), which are removed (together with the myelin debris) by tissue phagocytes. The distal stump is reduced to a densely interwoven mass of fine astrocytic processes (Fig. 7C) that does not retain any of the original longitudinally aligned elements that could have provided a pathway to enable the axons to bridge the long distances to the terminal fields.
In contrast, regenerating corticospinal axons not only cross the bridge of transplanted OECs, but also re-enter the distal part of the corticospinal tract, where they become myelinated by host oligodendrocytes from adjacent fiber tracts. As a working hypothesis, we are inclined to attribute this success to this possibility of access to an aligned glial tract structure (Suzuki and Raisman, 1992). In the case of the regenerating retinal ganglion cell axons, there are no other fibers present in the distal optic nerve stump, so the aligned tract structure is completely lost, oligodendrocytes are absent, and the astrocytes and their processes lose all longitudinal alignment.
Apart from the absence of aligned channels, the failure of axon growth may be caused by the absence of growth-promoting stimuli or the presence of inhibitors. It seems unlikely that oligodendrocytes or oligodendrocytic myelin can be the cause of the failure of the RGC axons to leave the transplants because neither oligodendrocytes nor myelin are present in the denervated optic nerve stump. Moreover, they are present in the corticospinal tract, where re-entry and regeneration does occur. On the other hand, the astrocytic meshwork of the distal optic nerve stump would be rich in inhibitory molecules such as chondroitin sulfate proteoglycans (Davies et al., 1997; Bradbury et al., 2002; Grimpe and Silver, 2002). Possibly the migratory limits of the transplanted OECs are established by the progression of events associated with the degeneration and reactive gliosis, so that by the time the cells have reached 10 mm, the gliotic reaction has reached a level that precludes further migration.
Other studies have indicated that regeneration can be enhanced by stimulatory events within the eye as well as by manipulating the environment through which the axons run (Cui et al., 1999; Leon et al., 2000; Yip and So, 2000; Fischer et al., 2001). Here we show a special permissive role for OECs to limit parent cell loss and support axon growth both across the gap and into the distal stump. It is clear, however, that to achieve full repair, other components are needed.
Footnotes
This work was supported by the British Neurological Research Trust, the International Spinal Research Trust, and the Wellcome Trust. Dr. Maria Cristina Cenni and members of Professor Maffei's team in Pisa generously gave us instruction in cholera toxin B labeling. Dr. Pauline Field provided important collaboration. Laiwen Fu is acknowledged for histology and Grant Roalfe for cell culture.
Correspondence should be addressed to Dr. Geoffrey Raisman, Division of Neurobiology, National Institute for Medical Research, The Ridgeway, Mill Hill, London NW7 1AA, UK. E-mail: graisma@nimr.mrc.ac.uk.
Copyright © 2003 Society for Neuroscience 0270-6474/03/237783-06$15.00/0
References
- Angelucci A, Clasca F, Sur M ( 1996) Anterograde axonal tracing with the subunit B of cholera toxin: a highly sensitive immunohistochemical protocol for revealing fine axonal morphology in adult and neonatal brains. J Neurosci Methods 65: 101-112. [DOI] [PubMed] [Google Scholar]
- Avilés-Trigueros M, Sauvé Y, Lund RD, Vidal-Sanz M ( 2000) Selective innervation of retinorecipient brainstem nuclei by retinal ganglion cell axons regenerating through peripheral nerve grafts in adult rats. J Neurosci 20: 361-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkelaar M, Clarke DB, Wang YC, Bray GM, Aguayo AJ ( 1994) Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J Neurosci 14: 4368-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LDF, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB ( 2002) Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416: 636-640. [DOI] [PubMed] [Google Scholar]
- Chierzi S, Fawcett JW ( 2001) Regeneration in the mammalian optic nerve. Restor Neurol Neurosci 19: 109-118. [PubMed] [Google Scholar]
- Cui Q, Lu Q, So KF, Yip HK ( 1999) CNTF, not other trophic factors, promotes axonal regeneration of axotomized retinal ganglion cells in adult hamsters. Invest Ophthalmol Vis Sci 40: 760-766. [PubMed] [Google Scholar]
- Davies SJA, Fitch MT, Memberg SP, Hall AK, Raisman G, Silver J ( 1997) Regeneration of adult axons in white matter tracts of the central nervous system. Nature 390: 680-683. [DOI] [PubMed] [Google Scholar]
- Fischer D, Heiduschka P, Thanos S ( 2001) Lens-injury-stimulated axonal regeneration throughout the optic pathway of adult rats. Exp Neurol 172: 257-272. [DOI] [PubMed] [Google Scholar]
- Grimpe B, Silver J ( 2002) The extracellular matrix in axon regeneration. Prog Brain Res 137: 333-349. [DOI] [PubMed] [Google Scholar]
- Keirstead SA, Rasminsky M, Fukuda Y, Carter DA, Aguayo AJ, Vidal-Sanz M ( 1989) Electrophysiologic responses in hamster superior colliculus evoked by regenerating retinal axons. Science 246: 255-256. [DOI] [PubMed] [Google Scholar]
- Leon S, Yin Y, Nguyen J, Irwin N, Benowitz LI ( 2000) Lens injury stimulates axon regeneration in the mature rat optic nerve. J Neurosci 20: 4615-4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Raisman G ( 1995) Sprouts from cut corticospinal axons persist in the presence of astrocytic scarring in long-term lesions of the adult rat spinal cord. Exp Neurol 134: 102-111. [DOI] [PubMed] [Google Scholar]
- Li Y, Field PM, Raisman G ( 1997) Repair of adult rat corticospinal tract by transplants of olfactory ensheathing cells. Sci 277: 2000-2002. [DOI] [PubMed] [Google Scholar]
- Li Y, Field PM, Raisman G ( 1998) Regeneration of adult rat corticospinal axons induced by transplanted olfactory ensheathing cells. J Neurosci 18: 10514-10524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Field PM, Raisman G ( 1999) Death of oligodendrocytes and microglial phagocytosis of myelin precede immigration of Schwann cells into the spinal cord. J Neurocytol 28: 417-427. [DOI] [PubMed] [Google Scholar]
- Li Y, Decherchi P, Raisman G ( 2003) Transplantation of olfactory ensheathing cells into spinal cord lesions restores breathing and climbing. J Neurosci 23: 727-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Féron F, Mackay-Sim A, Waite PME ( 2002) Olfactory ensheathing cells promote locomotor recovery after delayed transplantation into transected spinal cord. Brain 125: 14-21. [DOI] [PubMed] [Google Scholar]
- Ramón-Cueto A, Nieto-Sampedro M ( 1992) Glial cells from adult rat olfactory bulb: immunocytochemical properties of pure cultures of ensheathing cells. Neurosci 47: 213-220. [DOI] [PubMed] [Google Scholar]
- Ramón-Cueto A, Plant GW, Avila J, Bunge MB ( 1998) Long-distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants. J Neurosci 18: 3803-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramón-Cueto A, Cordero MI, Santos-Benito FF, Avila J ( 2000) Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing glia. Neuron 25: 425-435. [DOI] [PubMed] [Google Scholar]
- Ruitenberg MJ, Plant GW, Christensen CL, Blits B, Niclou SP, Harvey AR, Boer GJ, Verhaagen J ( 2002) Viral vector-mediated gene expression in olfactory ensheathing glia implants in the lesioned rat spinal cord. Gene Ther 9: 135-146. [DOI] [PubMed] [Google Scholar]
- Sauvé Y, Sawai H, Rasminsky M ( 1995) Functional synaptic connections made by regenerated retinal ganglion cell axons in the superior colliculus of adult hamsters. J Neurosci 15: 665-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Raisman G ( 1992) The glial framework of central white matter tracts: Segmented rows of contiguous interfascicular oligodendrocytes and solitary astrocytes give rise to a continuous meshwork of transverse and longitudinal processes in the adult rat fimbria. Glia 6: 222-235. [DOI] [PubMed] [Google Scholar]
- Vidal-Sanz M, Bray GM, Villegas-Pérez MP, Thanos S, Aguayo AJ ( 1987) Axonal regeneration and synapse formation in the superior colliculus by retinal ganglion cells in the adult rat. J Neurosci 7: 2894-2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas-Perez MP, Vidal-Sanz M, Rasminsky M, Bray GM, Aguayo AJ ( 1993) Rapid and protracted phases of retinal ganglion cell loss follow axotomy in the optic nerve of adult rats. J Neurobiol 24: 23-36. [DOI] [PubMed] [Google Scholar]
- Villegas-Pérez MP, Vidal-Sanz M, Bray GM, Aguayo AJ ( 1988) Influences of peripheral nerve grafts on the survival and regrowth of axotomized retinal ganglion cells in adult rats. J Neurosci 8: 265-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip HK, So KF ( 2000) Axonal regeneration of retinal ganglion cells: effect of trophic factors. Prog Retin Eye Res 19: 559-575. [DOI] [PubMed] [Google Scholar]
- Zeng BY, Anderson PN, Campbell G, Lieberman AR ( 1995) Regenerative and other responses to injury in the retinal stump of the optic nerve in adult albino rats: transection of the intracranial optic nerve. J Anat 186: 495-508. [PMC free article] [PubMed] [Google Scholar]