Abstract
The nitric oxide (NO)-cGMP pathway has been implicated as playing a crucial role in the induction of cerebellar long-term depression (LTD). The amplitude and duration of the cGMP signal is controlled by cyclic nucleotide phosphodiesterases (PDEs). Here we identify PDE5 and PDE1B as the two major cGMP-hydrolyzing PDEs specifically and differentially expressed in the Purkinje neurons of mouse cerebellum. PDE5 was found in all Purkinje neurons, whereas PDE1B was detected only in a subset of these cells, suggesting that individual Purkinje cells may differentially regulate cGMP, depending on the PDE isozymes expressed.
Although expression of guanylate cyclase and/or cGMP-dependent protein kinase (PKG) in Purkinje cells have been reported, neither cGMP accumulation nor PKG activation in these cells in vivo has been demonstrated. To determine if changes in PKG activation and PDE5 regulation occur in vivo we have examined the phosphorylation of PDE5 in mouse cerebellar Purkinje cells by immunocytochemistry and Western blot analyses using a phosphospecific PDE5 antibody. Injection of sodium nitroprusside or selective PKG activators into the lateral ventricle of mouse brain induced PDE5 phosphorylation in vivo, but was completely missing in Purkinje cell-specific PKG I knock-out mice. In cerebellar slices, treatment with sildenafil or IBMX led to different levels of phospho-PDE5 accumulation and activation of PDE5. These results suggest that phosphorylation of PDE5 in Purkinje neurons after cGMP-PKG activation performs a critical role in the termination of the cGMP signal during LTD progression; moreover, PDE5 phosphorylation may be used as an in vivo indicator for PKG activation.
Keywords: cerebellum, Purkinje cells, PDEs, cyclic nucleotide phosphodiesterase, PDE5, cGMP-specific phosphodiesterase, PDE1B, calmodulin-stimulated cyclic nucleotide phosphodiesterase, phosphorylation, PKG, cyclic GMP-dependent protein kinase, long-term depression
Introduction
Long-term depression (LTD), a decrease of synaptic transmission at the synapses between parallel fibers and Purkinje cells in the cerebellum, has been suggested as a mechanism for regulation of motor learning (Ito, 2000). The induction of LTD involves accumulation of intracellular cytosolic Ca2+ through activation of voltage-gated calcium channels and glutamate receptors. Elevated Ca2+ triggers a metabolic cascade of not yet fully understood cellular responses, including nitric oxide (NO) production. Although Purkinje neurons do not produce NO, they express all downstream components of the cGMP-dependent protein kinase (PKG) pathway, which can be activated by NO: nitric oxide-stimulated soluble guanylate cyclase and PKG (Lohmann et al., 1981; Ariano et al., 1982; Giuili et al., 1994) and cGMP-specific PDE (PDE5) (Kotera et al., 1997; Juilfs et al., 1999).
There has been growing evidence that the NO—cGMP-PKG pathway is involved in modification of synaptic efficacy in the cerebellum during LTD. Electrophysiological studies in cerebellar slices have shown that NO donors and cGMP analogs could induce an LTD-like effect in the synaptic connection between parallel fibers and Purkinje neuron dendrites (Crepel and Jaillard, 1990; Ito and Karachot, 1992; Daniel et al., 1993). LTD was also induced when NO donors or cGMP were directly dialyzed into Purkinje cells (Daniel et al., 1993; Hartell, 1994; Lev-Ram et al., 1997). Recently, a genetically encoded, fluorescent cGMP indicator was used to demonstrate transient increases in cGMP levels induced by NO or electrical stimulation of parallel fibers in Purkinje neurons. In the presence of a nonspecific PDE inhibitor, IBMX, these increases in cGMP levels were sustained, providing direct evidence that one or more PDEs regulate intracellular cGMP levels in Purkinje cells (Honda et al., 2001). Another recent study has indicated that PDE5 may be primarily responsible for cGMP hydrolysis in Purkinje cells. In this study, the decay of a fluorescent cGMP analog infused into Purkinje cells after application of the partially selective PDE5 inhibitors (zaprinast or dipyridamole) was found to be diminished, although uncertainties remain about the ability of other PDE isoforms to hydrolyze this fluorescent cGMP analog (Hartell et al., 2001). This study also showed that application of an NO donor accelerated the hydrolysis of the fluorescent cGMP analog, suggesting the possibility that cGMP elevation triggered by NO may induce an activation of PDE5, perhaps because of its phosphorylation by PKG. However, the phosphorylation of PDE5 by PKG has not been demonstrated in cerebellar Purkinje neurons. Recently, the phosphorylation status of PDE5 in intact smooth muscle cells was determined by using a phosphospecific PDE5 antibody, and it was suggested that analysis of phospho-PDE5 accumulation in intact cells and tissues may serve as an indicator of PKG activation (Rybalkin et al., 2002).
In this study, we have identified two cGMP hydrolyzing PDEs, PDE1B and PDE5, specifically expressed in Purkinje neurons in the mouse cerebellum, with PDE1B expression restricted to a subset of Purkinje neurons. By using a phosphospecific PDE5 antibody, we determined the phosphorylation status of PDE5 after PKG activation in cerebellar Purkinje neurons in vivo and in situ.
Materials and Methods
Materials. Sodium nitroprusside (SNP), 8-Br-cGMP, 8-bromo-cAMP (8-Br-cAMP), forskolin, and 3-isobutyl-1-methylxanthine (IBMX) were obtained from Sigma (St. Louis, MO). 8-para-chlorophenylthio cyclic GMP(8-pCPT-cGMP),7-deacetyl-7-[O-(N-methylpiperazino)-γ-butyryl]forskolin (MPB-forskolin), 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), and N-[2-((p-bromocinnamyl)amino)ethyl]-5-isoquinolinesulfonamide (H-89) were obtained from Calbiochem (San Diego, CA). Sildenafil was a gift from Pfizer Central Research (Sandwich, Kent, UK). All other reagents were obtained from Sigma. Antibodies used in this study, including a rabbit polyclonal phosphospecific PDE5 antibody, a rabbit polyclonal PDE5 antibody (total PDE5 antibody), and a mouse monoclonal PDE5 antibody, were produced, purified, and characterized as described previously (Rybalkin et al., 2002). Briefly, the antibodies were raised against a synthetic phosphopeptide (corresponding to the N-terminal part of bovine PDE5A1, aa 85-98, with a phosphorylated serine 92), a synthetic peptide (corresponding to bovine PDE5A1, aa 836-852), and purified recombinant PDE5 fragment (corresponding to bovine PDE5A1, aa 125-539), respectively. Isoform-specific antibodies for PDE1B and PDE1C were purified and characterized as described previously (Rybalkin et al., 1997).
Animals. C57BL/6J male mice (8-12 weeks) were used for most experiments. The mice were maintained on a 12 hr light/dark schedule with lights on at 6:00 A.M. and ad libitum access to food and water. Purkinje cell-specific PKG I knock-out mice were generated by using the Cre/loxP recombination system and were used at >20 weeks of age (R. Feil, W. Wolfsgruber, and F. Hofmann, unpublished observations); control mice carried modified PKG alleles and the Cre transgene in a combination that did not produce a PKG I knock-out.
For experiments involving free hand microinjection to the lateral ventricle, mice were anesthetized with ketamine (Phoenix Pharmaceutical Inc., St. Joseph, MO) and xylazine (Phoenix Pharmaceutical Inc.), and injected with various compounds dissolved in sterile artificial CSF (aCSF) (5 μl/mouse) using a 27-gauge, 1/2-inch needle attached to a 25 μl Hamilton syringe (Hamilton Company, Reno, NV). The needle was fitted with polyethylene tubing (8 mm length) leaving 4 mm of the needle tip exposed. The point of injection was 0.5 mm posterior to the bregma that can be identified by touching a skull with a needle tip, and 1.5 mm lateral from the midsagittal sinus. The compounds were delivered at a steady rate, taking 30 sec to complete, and the needle remained inserted for 15 sec after the delivery to allow the solution to be absorbed. Animals in the control group were injected with 5 μl of sterile aCSF. For the injection of glutamate, the animals were anesthetized with methoxyflurane (Schering-Plough Animal Health, Union, NJ) instead of ketamine and xylazine. These anesthetics did not affect the basal level of phosphorylated PDE5.
Immunocytochemistry. Animals destined for immunocytochemistry were anesthetized with phenobarbital and perfused with 0.2% heparin-PBS, followed by 4% paraformaldehyde in 0.1 m phosphate buffer, pH 7.4. After perfusion, brains were removed from the skull, and the cerebellum was excised. The tissues were postfixed for 5 hr in the same 4% paraformaldehyde fixative then cryoprotected by incubating with consecutive concentrations of sucrose (10, 20, and 30%) in PBS buffer. All tissues were then frozen in OCT compound and stored at -70°C until cryostat sectioning at -20°C. Each cerebellum was sectioned at 30 μm. Free-floating sections were preincubated in PBS containing 5% goat serum, 1 mg/ml BSA, and 0.05% Triton X-100 for 1 hr and incubated with either phosphospecific PDE5 antibody or total PDE5 antibody in PBS containing 1% goat serum, 1 mg/ml BSA, and 0.05% Triton X-100 overnight at 4°C under continuous gentle agitation on a rotary shaker. The sections were washed in PBS containing 0.05% Tween (3 × 20 min) then incubated with Alexa 488-conjugated goat anti-rabbit IgG (1:500; Molecular Probes, Eugene, OR) for 2 hr at room temperature. After additional washes in PBS, the sections were counterstained with propidium iodide. For double immunostaining for PDE5 and PDE1B, monoclonal PDE5 antibody was used. The antibody was visualized with secondary FITC-conjugated donkey anti-mouse IgG (1:500; Jackson ImmunoResearch, West Grove, PA). PDE1B antibody was visualized with secondary Alexa 594-conjugated goat anti-rabbit IgG (1:500). The sections were observed with a confocal microscope (Bio-Rad, Hercules, CA).
Preparation and stimulation of cerebellar slices. Mice were killed, and the cerebellum was rapidly excised and cooled in Krebs-Ringer's solution bicarbonate (KRB) buffer containing (in mm): 120 NaCl, 3.5 KCl, 1.3 MgSO4, 2.5 CaCl2, 1.25 NaH2PO4, 25.6 NaHCO3, and 10 glucose aerated with 95% O2 and 5% CO2, pH 7.4, at 0-4°C. The cerebellum was glued to a mounting block, and sagittal slices (400 μm) were cut with a Vibro-slice (Campden Instruments) in cold oxygenated KRB buffer and further cut in half. The slices were placed in KRB buffer at a temperature of 37°C for 5 min and were transferred to wells (12-well cell culture cluster; Costar) containing 1.5 ml of KRB buffer, where incubations with different reagents were performed in an O2-enriched atmosphere at 37°C. Each treatment contained two or three slices. To examine the effects of H-89 and ODQ, slices were preincubated with either H-89 or ODQ for 15 min and further incubated with 8-Br-cGMP, sildenafil, or IBMX in the presence of the inhibitors. Incubations were terminated by transferring the slices to 60 μl of the homogenization buffer, and they were briefly sonicated. After centrifugation at 1000 × g for 10 min at 4°C, the protein concentration in supernatant was estimated. The supernatant was used for SDS-PAGE or immunoprecipitation as described below. When the slices were analyzed by immunocytochemistry, they were immediately transferred to 4% paraformaldehyde fixative after stimulation, incubated for 30 min at 4°C, and further incubated in 4% paraformaldehyde-10% sucrose solution for 90 min. The slices were then cryoprotected by incubating with consecutive concentrations of sucrose (20 and 30%) in PBS buffer. All tissues were then frozen in OCT compound and sectioned at 20 μm. Immunostaining with the phosphospecific PDE5 antibody was performed as described above.
Western blot analysis of phosphorylated PDE5. After the brain was injected with different agents and treated for varying lengths of time, the cerebellum was rapidly removed and homogenized by Polytron with 1 ml of homogenization buffer containing 100 mm phosphate buffer, pH 7.4, 50 mm NaCl, 1 mm EDTA, 0.1% Triton X-100, 50 mm sodium fluoride, 100 μm sodium orthovanadate, 10 nm caliculin A, and a protease inhibitor cocktail (Boehringer Mannheim, Indianapolis, IN). The homogenate was centrifuged at 1000 × g for 10 min at 4°C. Protein concentration was measured by the Bradford method. The samples were separated on SDS-PAGE, transferred to a nitrocellulose membrane, and analyzed by Western blot analysis using either purified phosphospecific PDE5 antibody or purified total PDE5 antibody and enhanced chemiluminescent detection system (Pierce, Rockford, IL).
Immunoprecipitation of PDE5. The supernatants of cerebellar slice homogenates (containing 6 mg protein/ml) were obtained as described above. The supernatants (300 μl) were incubated overnight at 4°C with 30 μl of monoclonal PDE5 antibody. After incubation with 20 μl of protein G agarose beads (Oncogene, Cambridge, MA) for 1 hr at 4°C, the antigen-antibody complex was sedimented and washed four times. Final immunocomplexes were suspended in 50 μl of the homogenization buffer and used directly in PDE activity assays. Samples were then boiled for 1 min and chilled before addition of 10 μl of snake venom (2.5 mg/ml) containing 5′-nucleotidase activity. Samples were incubated for 10 min at 30°C, and an equal volume of 20 mm Tris-HCl, pH 6.8, was added. Fractions were then chromatographed on DEAE-Sephadex A-25 columns, and the effluent was counted in aqueous scintillant.
PDE assays and protein determinations. Phosphodiesterase assays were performed at 30°C using either 1 μm cAMP or 1 μm cGMP as substrates in the presence of either 1 mm EGTA or 0.8 mm CaCl2 and 4 μg/ml calmodulin (Rybalkin et al., 1997). Protein concentrations were determined by the Bradford method.
High-performance anion-exchange chromatography. The cerebellum was rapidly removed and homogenized in a Polytron with 2 ml of homogenization buffer containing 50 mm Tris-HCl, pH 7.5, 2.0 mm EDTA, 0.1 mm Na3VO4, 1 mm DTT, 10 μg/ml aprotinin, 5 μg/ml pepstatin, 20 μg/ml leupeptin, and 1 mm benzamidine. The homogenate was centrifuged at 10,000 × g for 50 min at 4°C. Anion-exchange chromatography was performed as described (Rybalkin et al., 1997). The proteins in the supernatant were separated on a Mono Q anion-exchange column HR 5/5. Fractions of 0.25 ml were collected and assayed for PDE activity as described above. Aliquots of each fraction were used for Western blot analysis for either PDE5, PDE1B, or PDE1C.
Data analysis. Optical densities of PDE5-immunoreactive bands from Western blot analysis were measured by LabWorks software (UVP, Inc, Upland, CA). Relative values after densitometric analysis were calculated as a percentage of control (unstimulated group) and represent the mean ± SEM values for each treatment group. Significant differences were determined using the Wilcoxon-Mann-Whitney U test. A p value of <0.05 was considered statistically significant.
Results
Characterization of cGMP-hydrolyzing PDEs in Purkinje cells
The cerebellum is a highly organized tissue consisting of four different types of neurons: Purkinje cells, granule cells, Golgi cells, and stellate/basket cells. The complex anatomical structure of cerebellum presents difficulties in identification and analysis of PDE isoforms in particular types of neurons. In this study we applied two methods (HPLC chromatography and immunocytochemistry) to investigate which PDEs besides PDE5 are expressed in cerebellar neurons.
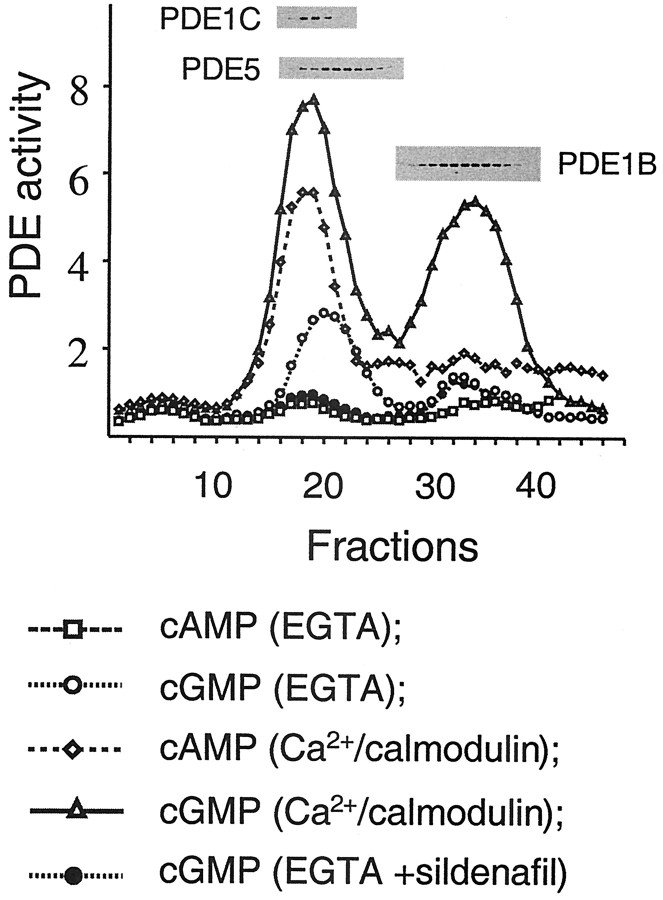
First, total mouse cerebellar cytosolic PDE activity, which represents 70% of total activity, was separated by anion exchange chromatography on a Mono Q column, and PDE activity was measured at 1 μm cGMP or 1 μm cAMP in the presence or absence of Ca2+-calmodulin (Fig. 1). The first peak eluted from the column was identified as PDE1C. This Ca2+-calmodulin-stimulated PDE could hydrolyze cGMP as well as cAMP equally well (Rybalkin et al., 1997). PDE5 was eluted slightly later than PDE1C and was the major calcium-independent cGMP-hydrolyzing activity. When cGMP-hydrolyzing activity was assayed in the presence of 40 nm sildenafil, it resulted in almost complete blockage of PDE5 activity. Two small peaks of cGMP-hydrolyzing activity were not inhibited by sildenafil. The remaining cGMP-hydrolyzing activity in the fractions 24-30 was found to be basal, unstimulated PDE1C. Another peak in the fractions 36-46 also contained cGMP hydrolyzing activity, was greatly activated by Ca2+-calmodulin, and identified as PDE1B by Western blot analysis.
Figure 1.
Anion-exchange chromatography of PDE activity from mouse cerebellum. PDE activity was assayed using either 1 μm cGMP or 1 μm cAMP in the presence of either 1 mm EGTA (EGTA) or 1 mm CaCl2 and 4 μg/ml calmodulin (Ca2+-calmodulin). Immunoblots with detectable bands of PDE5, PDE1B, and PDE1C are shown above their corresponding chromatographic fractions. PDE activity is expressed as picomoles of cGMP (or cAMP) per minute per 50 μl.
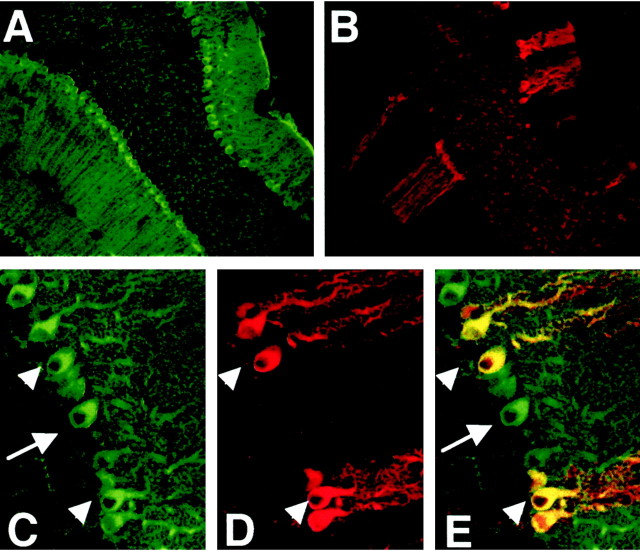
To study which of these PDEs were specifically expressed in Purkinje cells, cerebellar sections were immunostained with PDE5, PDE1B, and PDE1C antibodies. PDE5 was localized only in Purkinje neurons and not in other cerebellar cells (Fig. 2A). PDE1B immunoreactivity was also seen only in Purkinje neurons. However, unlike PDE5, PDE1B was not expressed in the entire Purkinje cell layer, but was seen in specific subsets of Purkinje cells (Fig. 2B). Because PDE1B and PDE5 were expressed specifically in Purkinje cells, we could compare their hydrolytic activities by analyzing fractions of crude cerebellum extract separated by Mono Q chromatography. The fully Ca2+-calmodulin-stimulated cGMP hydrolytic activity of PDE1B was comparable with the cGMP hydrolytic activity of PDE5, as measured at 1 μm cGMP.
Figure 2.
Localization of PDE5 and PDE1B in Purkinje cells in mouse cerebellum. Mouse cerebellar sections were double-stained for PDE5 (green) with mouse monoclonal antibody and PDE1B (red) with rabbit polyclonal antibody. PDE5 is expressed in the entire Purkinje cell layer (A), whereas PDE1B is expressed in subsets of Purkinje neurons (B). C and D, enlarged images of A and B, respectively. E, Merged image of C and D. Arrows indicate the cells containing only PDE5. Arrowheads indicate the cells containing both PDE5 and PDE1B.
Immunostaining with PDE1C antibody did not reveal any specific pattern, showing diffuse expression of PDE1C (data not shown), indicating that PDE1C is most likely expressed in both the granule cells and Purkinje cells. Previously high levels of PDE1C mRNAs were found in both the neurons of the granule cell layer and in Purkinje cells (Yan et al., 1996).
These results suggest that in Purkinje neurons PDE5 and PDE1B represent most of the cytosolic cGMP hydrolyzing PDE activity as analyzed at 1 μm cGMP, and their differential expression may provide a means for an individual Purkinje neuron to exercise a precise control over cGMP degradation.
Detection of PDE5 phosphorylation in cerebellar Purkinje cells in vivo
To examine whether phosphorylation of PDE5 by PKG occurs in cerebellar Purkinje cells in vivo, we injected a PKG activator, 8-Br-cGMP (125 nmol/mouse), into the lateral ventricle of mouse brain. Fifteen minutes after injection, the cerebellum was removed, and cerebellar sections were prepared and immunostained with a phosphospecific PDE5 antibody. This immunohistochemical study revealed that 8-Br-cGMP induced significant phosphorylation of PDE5 in the cell bodies and dendrites of Purkinje neurons (Fig. 3C). No specific staining of phosphorylated PDE5 was observed when aCSF alone was injected (Fig. 3D). An antibody that recognizes all forms of PDE5 (total PDE5) showed the same amount of PDE5 protein in Purkinje cell bodies and dendrites in both 8-Br-cGMP and aCSF injections (Fig. 3A,B). Injections of another PKG activator, 8-pCPT-cGMP (125 nmol), showed a similar pattern of phosphorylated PDE5 immunoreactivity to that of 8-Br-cGMP in Purkinje neurons (Fig. 3E). However, very little immunoreactivity of phosphorylated PDE5 in Purkinje cells was detected in the cerebellum treated with 8-Br-cAMP (125 nmol, data not shown) or MPB-forskolin (100 nmol) (Fig. 3F).
Figure 3.
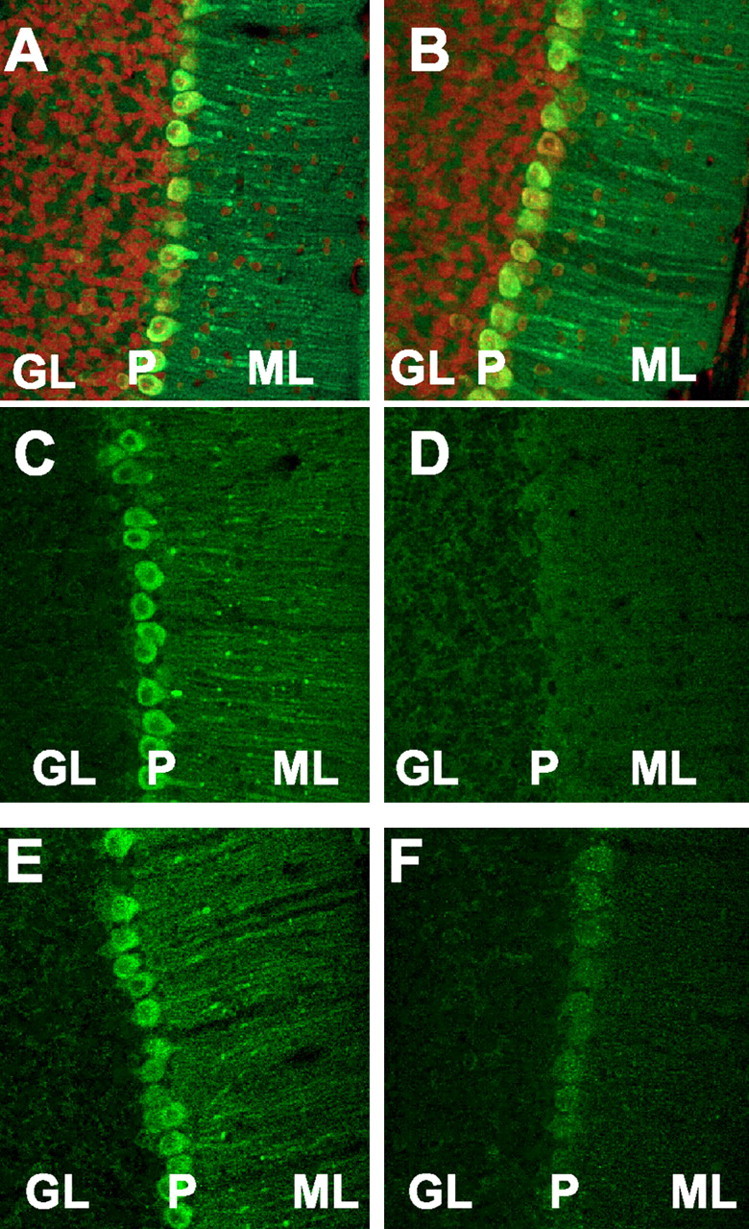
Activation of PKG in mouse Purkinje neurons in vivo leads to the phosphorylation of PDE5. Fifteen minutes after microinjections into the lateral ventricle with 125 nmol 8-Br-cGMP (A, C), aCSF (B, D), 125 nmol 8-pCPT-cGMP (E) and 100 nmol MPB-forskolin (F) cerebellar sections were prepared and immunostained with phospho-PDE5 antibody (green) (C-F) or total PDE5 antibody (green) (A, B) and counterstained with propidium iodide (red) (A, B). Tissue sections from the mice treated with 8-Br-cGMP (A, C) and aCSF (B, D) were processed simultaneously. Immunostaining for the phospho-PDE5 was found only in mice treated 8-Br-cGMP (C) and 8-pCPT-cGMP (E), but not with aCSF (D) or MPB-forskolin (F). GL, Granule cell layer; ML, molecular layer; P, Purkinje cell layer.
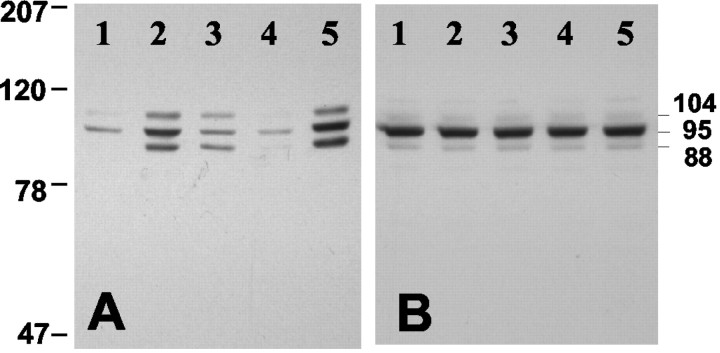
PDE5 phosphorylation was also analyzed by Western blot analysis after lateral ventricle injection of different NO-cGMP stimulators. Injections with either 8-Br-cGMP (125 nmol) or 8-pCPT-cGMP (125 nmol), but not 8-Br-cAMP (250 nmol), resulted in an increase of immunoreactive bands of phosphorylated PDE5. Injections of SNP (500 nmol) also induced an increase in phosphorylated PDE5 (Fig. 4A). The phosphospecific PDE5 antibody detected a prominent immunoreactive band at ∼95 kDa, which was consistent with a single band labeled with the total PDE5 antibody (Fig. 4B). There also were two less prominently labeled bands. These three bands may represent different PDE5 isoforms. However, the lower band also may be a result of partial proteolytic degradation. A similar PDE5 phosphorylation pattern has been reported during phosphorylation of mouse uterine rings (Rybalkin et al., 2002).
Figure 4.
Detection of phospho-PDE5 in mouse cerebellum after injections with cAMP and cGMP-elevating agents in vivo. Fifteen minutes after microinjection of either aCSF (1), 125 nmol 8-Br-cGMP (2), 125 nmol 8-pCPT-cGMP (3), 250 nmol 8-Br-cAMP (4), or 500 nmol SNP (5), the cerebellum was dissected, and samples were prepared in SDS sample buffer for Western blot analysis. Proteins were immunoblotted with either the phosphospecific PDE5 antibody (A) or total PDE5 antibody (B). The numbers on the left side of the panels indicate the molecular weight (in kilodaltons) of standard markers.
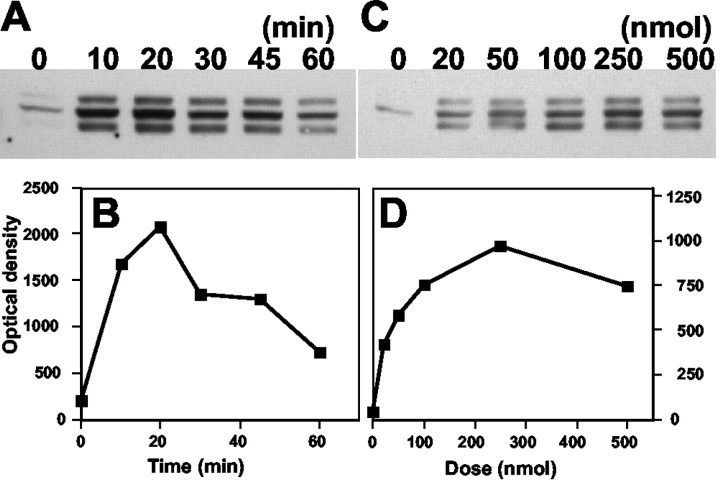
As shown in Figure 5A, injection of SNP (250 nmol) into the lateral ventricle produced a time-dependent change in the phosphorylation of PDE5. The intensity of phosphorylated PDE5 was maximal at 20 min after the injection of SNP (Fig. 5B). The PDE5 phosphorylation level was decreased to basal levels by 60 min after SNP injection. These results indicate that in the absence of a constant supply of cGMP (because of slow diffusion and spontaneous decay of injected SNP) phospho-PDE5 could undergo dephosphorylation (Fig. 5B). These data also point to the importance of a phosphatase in the regulation of PDE5 phosphorylation induced by transient increases in intracellular cGMP concentration. Phosphorylation of PDE5 was increased in a concentration-dependent manner between 20 and 250 nmol of SNP (Fig. 5C,D). These results suggest that the phosphorylation level of PDE5 depends on the degree of cGMP-PKG activation.
Figure 5.
Time and concentration dependence of SNP-induced phospho-PDE5 accumulation in cerebellar Purkinje cells. A, SNP (250 nmol) was injected into the lateral ventricle, and after various time periods (10-60 min) the cerebellum was removed, and samples were prepared and analyzed by Western blot. C, Mice were injected with different doses of SNP (10-500 nmol), and after 20 min the cerebellum was dissected, and samples were prepared in SDS sample buffer. A, C, Proteins were immunoblotted with the phosphospecific PDE5 antibody. B, D, Phospho-PDE5 bands were quantified by densitometry and calculated as a percentage of control.
Modulation of PDE5 phosphorylation in cerebellar Purkinje cells in situ
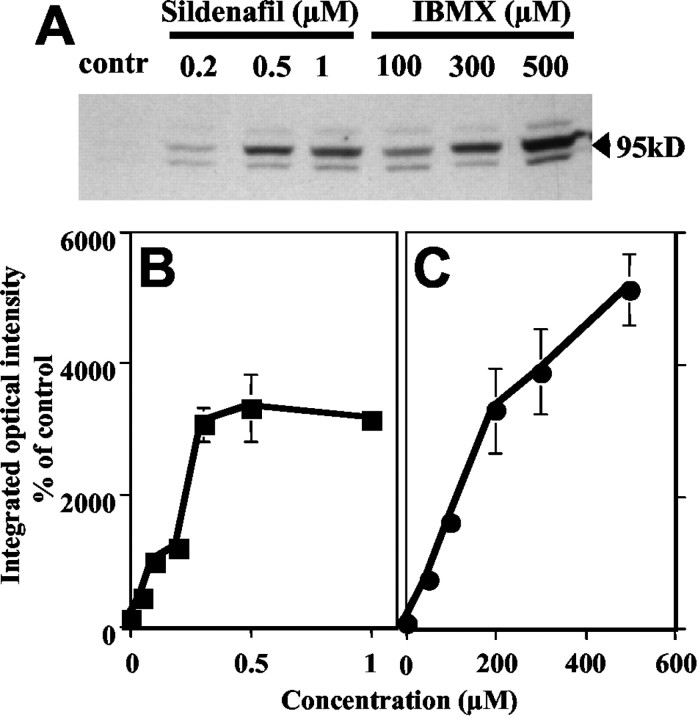
The effect of PDE inhibitors on the phosphorylation level of PDE5 was examined in cerebellar slices. When the slices were incubated with either a specific PDE5 inhibitor, sildenafil (Fig. 6A,B), or a nonselective PDE inhibitor, IBMX (Fig. 6A,C), both inhibitors induced a concentration-dependent increase in PDE5 phosphorylation. The result that a PDE inhibitor alone can induce phosphorylation of PDE5 suggests that the basal levels of both PDE and guanylate cyclase activities are relatively high in Purkinje neurons. Western blotting of these same samples using the total PDE5 antibody demonstrated the presence of approximately equivalent amounts of PDE5 in each slice preparation (data not shown). The level of increased PDE5 phosphorylation reached a plateau at 300 nm sildenafil, whereas it showed a linear change between 50 and 500 μm IBMX. The fact that higher concentrations of IBMX induced more phosphorylation than that induced by the highest concentration of sildenafil suggests that other PDEs, such as PDE1B, may participate in the regulation of cGMP concentration in Purkinje cells. Another explanation might be that an increase in cAMP caused by inhibition of cAMP-PDEs by IBMX may be partially associated with the IBMX-induced increase in PDE5 phosphorylation. However, this possibility is not likely because the phosphorylation of PDE5 induced by IBMX at 300 μm was not increased by coincubation with 8-Br-cAMP (1 mm) or forskolin (100 μm). 8-Br-cAMP (1 mm) was also coincubated with sildenafil (300 nm); however, no additional phosphorylation was seen on the sildenafil-induced PDE5 phosphorylation (data not shown).
Figure 6.
Phosphorylation of PDE5 after treatments of mouse cerebellar slices with PDE inhibitors in situ. A shows a representative Western blot for phospho-PDE5 in slices treated with different concentrations of sildenafil (0.05-1 μm) or IBMX (50-500 μm) for 15 min. Quantification of phospho-PDE5 after treatments with sildenafil (B) and IBMX (C) was done by densitometry and calculated as a percentage of control (unstimulated slices; contr). Data represent mean ± SEM of five separate experiments.
Immunostaining for phosphorylated PDE5 was also detected in Purkinje cell bodies and dendrites when slices were incubated with either sildenafil (500 nm) (Fig. 7C) or IBMX (500 μm) (Fig. 7D) for 15 min. Pretreatment with ODQ, a guanylate cyclase inhibitor, completely abolished the staining for phosphorylated PDE5 in Purkinje neurons in these slices (Fig. 7E,F, respectively).
Figure 7.
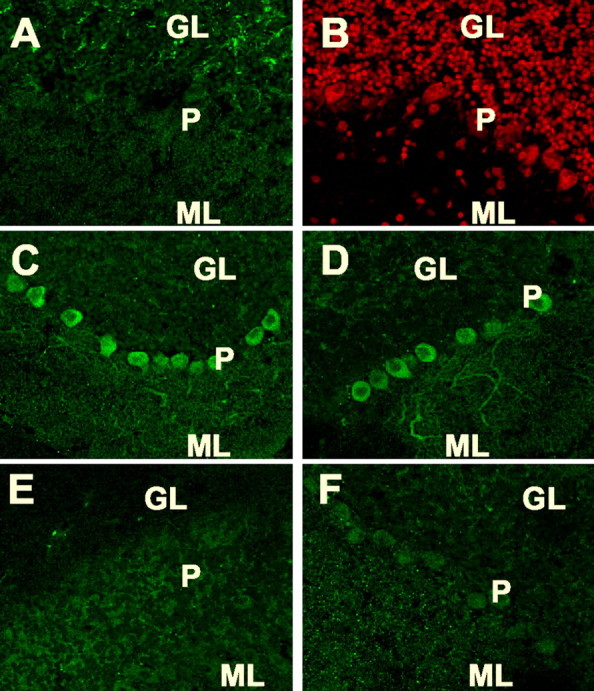
Phospho PDE5 immunostaining in mouse cerebellar slices after treatments with PDE inhibitors in situ. Slices were preincubated with 10 μm ODQ (E, F) or without ODQ (C, D) for 15 min, followed by addition of 500 nm sildenafil (C, E) or 500 μm IBMX (D, F) for 15 min. The slices were fixed and immunostained with the phospho-PDE5 antibody (green) and counterstained with propidium iodide (red) (B), as described in Materials and Methods. Phospho-PDE5 was detected in Purkinje cell bodies and dendrites in slices treated with sildenafil (C) and IBMX (D). ODQ inhibited the phosphorylation of PDE5 induced by sildenafil (E) and IBMX (F). No detectable immunostaining for phosphorylated PDE5 was seen in an unstimulated slice (A). Counterstaining with propidium iodide shows three layer structure of the cerebellar cortex in the same area as A (B). GL, Granule cell layer; ML, molecular layer; P, Purkinje cell layer.
Cerebellar slices were also examined by Western blot analysis after preincubation with 8-Br-cGMP, ODQ, and PKA activators (Fig. 8A). Treatment with 8-Br-cAMP (1 mm) or forskolin (100 μm) did not induce PDE5 phosphorylation in slices, consistent with the in vivo results. There was no synergistic effect of cGMP and cAMP when the slice was incubated with both 8-Br-cGMP and 8-Br-cAMP. ODQ (10 μm) completely blocked the increases in PDE5 phosphorylation induced by sildenafil and also significantly decreased PDE5 phosphorylation induced by IBMX (Fig. 8B,C). The catalytic activity of PDE5 was also analyzed in cerebellar slices treated with either 1 mm 8-Br-cGMP or 500 nm sildenafil for 15 min (Fig. 8D). Immunoprecipitated total PDE5 activity was higher after treatments with 8-Br-cGMP and sildenafil by 46 and 26%, respectively.
Figure 8.
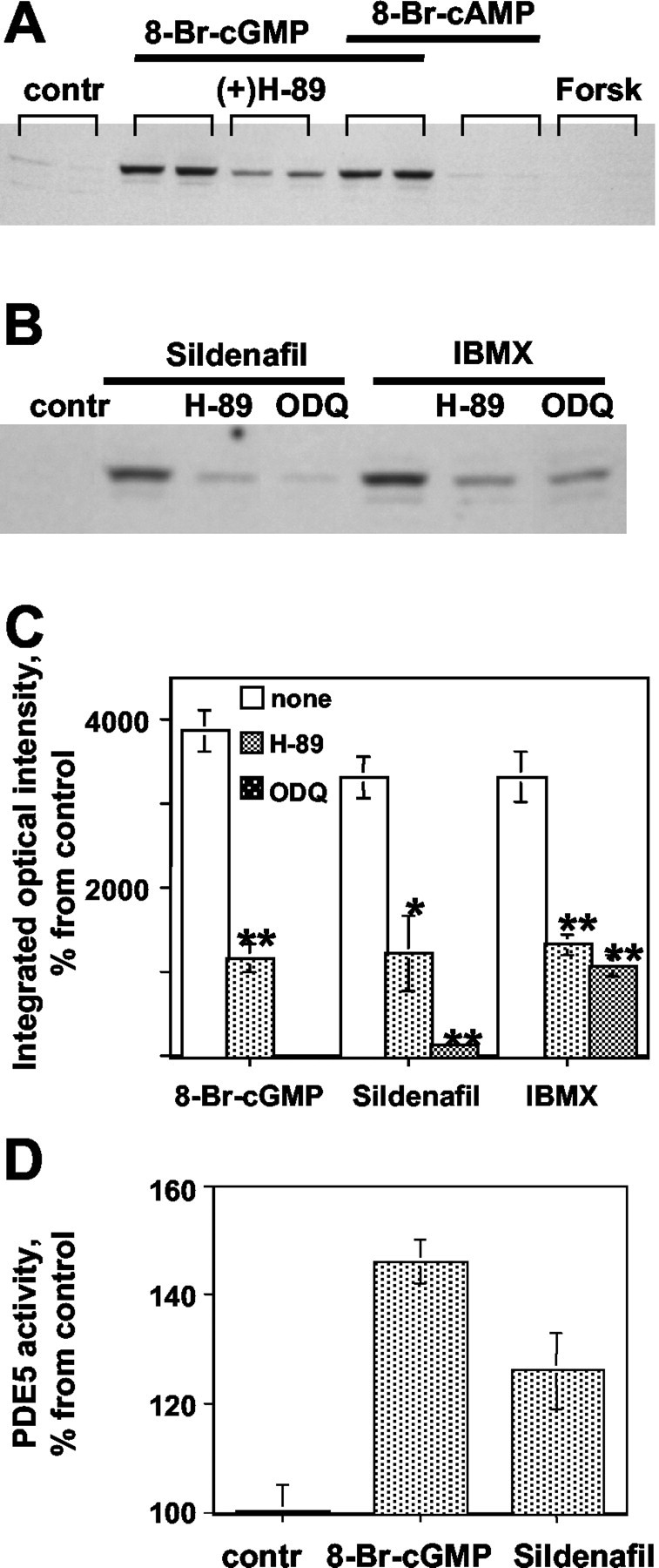
Effects of ODQ and H-89 on PDE5 phosphorylation in cerebellar slices treated with cyclic nucleotide analogs. A and B show representative Western blots for phospho-PDE5 in slices incubated with either 1 mm 8-Br-cGMP, 1 mm 8-Br cAMP, both of them, forskolin (Forsk, 100 μm), 0.5 μm sildenafil, or 200 μm IBMX for 15 min. Slices were preincubated with 50 μm H-89 or 10 μm ODQ for 15 min, followed by addition of 8-Br-cGMP, sildenafil, or IBMX for 15 min. C, Phospho-PDE5 bands from A and B were quantified by densitometry and calculated as a percentage of control (unstimulated slices; contr). D, PDE5 activity is increased in slices treated with 8-Br-cGMP or sildenafil in situ. Cerebellar slices were incubated with either 1 mm 8-Br-cGMP or 500 nm sildenafil for 15 min, and PDE5 was immunoprecipitated using total monoclonal PDE5 antibody, as described in Materials and Methods. PDE5 activity was calculated as a percentage of control (untreated slices). Data represent mean ± SEM of three separate experiments. *p < 0.05, **p < 0.01 relative to controls.
To further examine cGMP-induced PDE5 phosphorylation, H-89, a nonspecific cyclic nucleotide protein kinase inhibitor, was applied. This inhibitor could inhibit PDE5 phosphorylation in slices, treated with PDE inhibitors (Fig. 8A,B). A 15 min preincubation of slices with H-89 (50 μm) decreased phosphorylation of PDE5 induced by 8-Br-cGMP, sildenafil, and IBMX, by 70, 63, and 60%, respectively (Fig. 8B,C). Neither ODQ nor H-89 alone affected PDE5 phosphorylation levels.
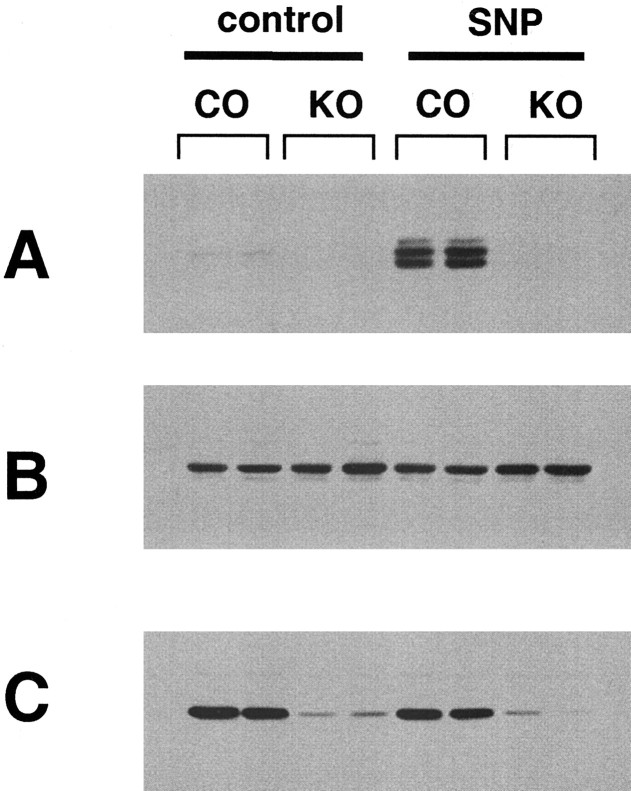
To prove that PKG is the protein kinase responsible for PDE5 phosphorylation, PKG I knock-out mice specifically targeted to Purkinje cells were used (R. Feil, W. Wolfsgruber, and F. Hofmann, unpublished observations). Immunohistochemical analysis indicated that PKG I was deleted in >95% of Purkinje cells of these knock-out mice. The small amount of PKG detectable in total cerebellum extract (Fig. 9C) from the PKG I knock-out mice is likely caused by the residual expression of PKG in some Purkinje cells. However, this level of PKG expression was not sufficient to sustain the low basal level of PDE5 phosphorylation detectable without any treatments (Fig. 9A). Moreover, injection of 250 nmol SNP (Fig. 9A) into the lateral ventricle of mice with PKG I-deficient Purkinje cells did not produce any phosphorylation of PDE5 in these cells. Similar data were also obtained when 8-Br-cGMP (125 nmol) was used for injections (data not shown).
Figure 9.
No PDE5 phosphorylation in PKG I-deficient Purkinje cells. Fifteen minutes after microinjection of 250 nmol SNP into the lateral ventricle of brain of Purkinje cell-specific PKG I knock-out mice (KO) and control mice (CO) the cerebellum was dissected, and samples were prepared in SDS sample buffer for Western blot analysis. Proteins were immunoblotted with the phosphospecific PDE5 antibody (A), total PDE5 antibody (B), and PKG antibody (C).
Taken together these results show that activation of the cGMP-PKG pathway plays a major role in the PDE5 phosphorylation event in Purkinje neurons.
Discussion
In this study, we have demonstrated that in slices in situ and in vivo the cGMP-PKG pathway plays a major role in the phosphorylation of PDE5. The most unambiguous results suggesting the role of PKG in PDE5 phosphorylation were obtained using Purkinje cell-specific PKG I knock-out mice. No phosphorylation of PDE5 was detected when either SNP or 8-Br-cGMP was injected into the lateral ventricle of PKG I knock-out mice. Furthermore, mice with this tissue specific knock-out showed a significant reduction in LTD and impaired adaptation of the vestibulo-ocular reflex (Feil, Wolfsgruber, and Hofmann, unpublished observations).
Our immunocytochemical studies showed differential expression of PDE1B and PDE5 in mouse cerebellar Purkinje neurons (Fig. 2). PDE5 immunoreactivity was seen in almost all Purkinje cells, whereas PDE1B immunoreactivity existed in particular subsets of Purkinje neurons. There are a number of reports about heterogeneous gene expression in Purkinje cells, including insulin-like growth factor I (Aguado et al., 1994), low-affinity nerve growth factor receptor (Dusart et al., 1994), and subunits of the NMDA receptor channel (Nakagawa et al., 1996). However, the physiological implications of Purkinje cell heterogeneity are still not known. Functionally, all these neurons provide output for the cerebellar cortex, although it is likely that the individual response of a Purkinje neuron might be affected by their surrounding environment. Phosphorylated PDE5 with its maximum accumulation at the peak of cGMP-PKG activation is likely to provide a negative feedback regulation of cGMP concentration in all Purkinje neurons and thereby attenuate the duration of the LTD response. We can speculate that in the cells expressing both PDE5 and PDE1B the amplitude and duration of the cGMP signal would be even smaller, because of PDE1B activation by Ca2+ influx at the onset of LTD. These findings stress the importance of PDE isozyme identification for electrophysiological studies that are conducted on single cells. Clearly, different PDE compositions in individual cells may substantially affect the Purkinje neuron response to NO-cGMP stimulation during development of LTD. For example, it is likely that zaprinast at 5 μm used in some studies in cerebellar Purkinje neurons as specific PDE5 inhibitor (Hartell, 1996; Hartell et al., 2001) could also inhibit PDE1B in cells expressing both PDE5 and PDE1B. In fact zaprinast has been used as a PDE1 inhibitor in studies with other types of neurons (Calabresi et al., 1999).
There have been several attempts to measure cGMP in Purkinje cells in the cerebellum. Immunocytochemistry for cGMP has never shown a specific localization of cGMP in Purkinje neurons, even after a stimulation with a NO donor (de Vente et al., 1989; de Vente and Steinbusch, 1992). Recently, a new approach to visualize cGMP in living Purkinje neurons has been successfully implemented by using a genetically encoded fluorescent indicator for cGMP. When a NO donor or the stimulation of parallel fibers was applied to cerebellar slices, transient increases in cGMP were observed in Purkinje cells (Honda et al., 2001). However, the range of changes in fluorescence intensity of the indicator were small, and further efforts are needed to develop a more sensitive indicator.
Recently, it was shown that direct cGMP binding to the GAF A domain of PDE5 converts PDE5 into an activated state even in the absence of phosphorylation (Rybalkin et al., 2003). Because PKG can phosphorylate PDE5 only in this state, it is likely that the phosphorylation status of PDE5 would correlate with changes in intracellular concentrations of cGMP and subsequently PKG activation. Thus, the phosphorylation of PDE5 observed in this report by using phospho-PDE5 specific antibodies can be used as an in vivo indicator for PKG activation in Purkinje cells.
It has been reported that PKG can phosphorylate a number of proteins in Purkinje neurons, including AMPA receptors (Nakazawa et al., 1995), IP3 receptors (Haug et al., 1999), and G-substrate (Endo et al., 1999). Although the roles of AMPA and IP3 receptors in LTD are well established, the functional significance of their phosphorylation by PKG has not yet determined. G-substrate, a 24 kDa protein, which is specifically expressed in Purkinje cells (Detre et al., 1984), represents a likely physiological target of PKG. The phosphorylated G-substrate was found to be an effective protein phosphatase inhibitor (Ki = 1.51 μm for PP1, and Ki = 0.27 μm for PP2A), whereas the dephospho G-substrate did not have any significant effects on either PP1 or PP2A. By inhibiting endogenous phosphatase activities, phospho G-substrate might control different signal transduction pathways, including PKC, protein tyrosine kinases, or MAPKs (Ito, 2001) and affect clathrin-mediated internalization of AMPA receptors, which appears to be required for cerebellar LTD (Wang and Linden, 2000).
Taken together, our data suggest that the phosphorylation and activation of PDE5 by PKG may be an important molecular mechanism for regulation of neuronal excitability and synaptic plasticity by the cGMP-PKG pathway in Purkinje neurons. Differential expression of PDE1B and PDE5 in these cells may further modify the LTD response in different subsets of Purkinje neurons to Ca2+-NO-cGMP stimulation.
Footnotes
This work was supported by National Institutes of Health Grant DK 21723.
Correspondence should be addressed to Joseph. A. Beavo, Department of Pharmacology, Box 357280, University of Washington, Seattle, WA 98195. E-mail: beavo@u.washington.edu.
Copyright © 2003 Society for Neuroscience 0270-6474/03/236452-08$15.00/0
References
- Aguado F, Sanchez-Franco F, Rodrigo J, Cacicedo L, Martinez-Murillo R ( 1994) Insulin-like growth factor I-immunoreactive peptide in adult human cerebellar Purkinje cells: co-localization with low-affinity nerve growth factor receptor. Neuroscience 59: 641-650. [DOI] [PubMed] [Google Scholar]
- Ariano MA, Lewicki JA, Brandwein HJ, Murad F ( 1982) Immunohistochemical localization of guanylate cyclase within neurons of rat brain. Proc Natl Acad Sci USA 79: 1316-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Gubellini P, Centonze D, Sancesario G, Morello M, Giorgi M, Pisani A, Bernardi G ( 1999) A critical role of the nitric oxide/cGMP pathway in corticostriatal long-term depression. J Neurosci 19: 2489-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepel F, Jaillard D ( 1990) Protein kinases, nitric oxide and long-term depression of synapses in the cerebellum. NeuroReport 1: 133-136. [DOI] [PubMed] [Google Scholar]
- Daniel H, Hemart N, Jaillard D, Crepel F ( 1993) Long-term depression requires nitric oxide and guanosine 3′:5′ cyclic monophosphate production in rat cerebellar Purkinje cells. Eur J Neurosci 5: 1079-1082. [DOI] [PubMed] [Google Scholar]
- de Vente J, Steinbusch HW ( 1992) On the stimulation of soluble and particulate guanylate cyclase in the rat brain and the involvement of nitric oxide as studied by cGMP immunocytochemistry. Acta Histochem 92: 13-38. [DOI] [PubMed] [Google Scholar]
- de Vente J, Bol JG, Steinbusch HW ( 1989) Localization of cGMP in the cerebellum of the adult rat: an immunohistochemical study. Brain Res 504: 332-337. [DOI] [PubMed] [Google Scholar]
- Detre JA, Nairn AC, Aswad DW, Greengard P ( 1984) Localization in mammalian brain of G-substrate, a specific substrate for guanosine 3′,5′-cyclic monophosphate-dependent protein kinase. J Neurosci 4: 2843-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusart I, Morel MP, Sotelo C ( 1994) Parasagittal compartmentation of adult rat Purkinje cells expressing the low-affinity nerve growth factor receptor: changes of pattern expression after a traumatic lesion. Neuroscience 63: 351-356. [DOI] [PubMed] [Google Scholar]
- Endo S, Suzuki M, Sumi M, Nairn AC, Morita R, Yamakawa K, Greengard P, Ito M ( 1999) Molecular identification of human G-substrate, a possible downstream component of the cGMP-dependent protein kinase cascade in cerebellar Purkinje cells. Proc Natl Acad Sci USA 96: 2467-2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuili G, Luzi A, Poyard M, Guellaen G ( 1994) Expression of mouse brain soluble guanylyl cyclase and NO synthase during ontogeny. Brain Res Dev Brain Res 81: 269-283. [DOI] [PubMed] [Google Scholar]
- Hartell NA ( 1994) cGMP acts within cerebellar Purkinje cells to produce long term depression via mechanisms involving PKC and PKG. NeuroReport 5: 833-836. [DOI] [PubMed] [Google Scholar]
- Hartell NA ( 1996) Inhibition of cGMP breakdown promotes the induction of cerebellar long-term depression. J Neurosci 16: 2881-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartell NA, Furuya S, Jacoby S, Okada D ( 2001) Intercellular action of nitric oxide increases cGMP in cerebellar Purkinje cells. NeuroReport 12: 25-28. [DOI] [PubMed] [Google Scholar]
- Haug LS, Jensen V, Hvalby O, Walaas SI, Ostvold AC ( 1999) Phosphorylation of the inositol 1, 4, 5-trisphosphate receptor by cyclic nucleotide-dependent kinases in vitro and in rat cerebellar slices in situ. J Biol Chem 274: 7467-7473. [DOI] [PubMed] [Google Scholar]
- Honda A, Adams SR, Sawyer CL, Lev-Ram V, Tsien RY, Dostmann WR ( 2001) Spatiotemporal dynamics of guanosine 3′, 5′-cyclic monophosphate revealed by a genetically encoded, fluorescent indicator. Proc Natl Acad Sci USA 98: 2437-2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M ( 2000) Mechanisms of motor learning in the cerebellum. Brain Res 886: 237-245. [DOI] [PubMed] [Google Scholar]
- Ito M ( 2001) Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiol Rev 81: 1143-1195. [DOI] [PubMed] [Google Scholar]
- Ito M, Karachot L ( 1992) Protein kinases and phosphatase inhibitors mediating long-term desensitization of glutamate receptors in cerebellar Purkinje cells. Neurosci Res 14: 27-38. [DOI] [PubMed] [Google Scholar]
- Juilfs DM, Soderling S, Burns F, Beavo JA ( 1999) Cyclic GMP as substrate and regulator of cyclic nucleotide phosphodiesterases (PDEs). Rev Physiol Biochem Pharmacol 135: 67-104. [DOI] [PubMed] [Google Scholar]
- Kotera J, Yanaka N, Fujishige K, Imai Y, Akatsuka H, Ishizuka T, Kawashima K, Omori K ( 1997) Expression of rat cGMP-binding cGMP-specific phosphodiesterase mRNA in Purkinje cell layers during postnatal neuronal development. Eur J Biochem 249: 434-442. [DOI] [PubMed] [Google Scholar]
- Lev-Ram V, Jiang T, Wood J, Lawrence DS, Tsien RY ( 1997) Synergies and coincidence requirements between NO, cGMP, and Ca2+ in the induction of cerebellar long-term depression. Neuron 18: 1025-1038. [DOI] [PubMed] [Google Scholar]
- Lohmann SM, Walter U, Miller PE, Greengard P, De Camilli P ( 1981) Immunohistochemical localization of cyclic GMP-dependent protein kinase in mammalian brain. Proc Natl Acad Sci USA 78: 653-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Watanabe M, Inoue Y ( 1996) Altered gene expression of the N-methyl-d-aspartate receptor channel subunits in Purkinje cells of the staggerer mutant mouse. Eur J Neurosci 8: 2644-2651. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Mikawa S, Hashikawa T, Ito M ( 1995) Transient and persistent phosphorylation of AMPA-type glutamate receptor subunits in cerebellar Purkinje cells. Neuron 15: 697-709. [DOI] [PubMed] [Google Scholar]
- Rybalkin SD, Bornfeldt KE, Sonnenburg WK, Rybalkina IG, Kwak KS, Hanson K, Krebs EG, Beavo JA ( 1997) Calmodulin-stimulated cyclic nucleotide phosphodiesterase (PDE1C) is induced in human arterial smooth muscle cells of the synthetic, proliferative phenotype. J Clin Invest 100: 2611-2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybalkin SD, Rybalkina IG, Feil R, Hofmann F, Beavo JA ( 2002) Regulation of cGMP-specific phosphodiesterase (PDE5) phosphorylation in smooth muscle cells. J Biol Chem 277: 3310-3317. [DOI] [PubMed] [Google Scholar]
- Rybalkin SD, Rybalkina IG, Shimizu-Albergine M, Tang XB, Beavo JA ( 2003) PDE5 is converted to an activated state upon cGMP binding to the GAF A domain. EMBO J 22: 469-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YT, Linden DJ ( 2000) Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron 25: 635-647. [DOI] [PubMed] [Google Scholar]
- Yan C, Zhao AZ, Bentley JK, Beavo JA ( 1996) The calmodulin-dependent phosphodiesterase gene PDE1C encodes several functionally different splice variants in a tissue-specific manner. J Biol Chem 271: 25699-25706. [DOI] [PubMed] [Google Scholar]