Abstract
The thalamocortical network is characterized by rhythmic burst activity during natural sleep and tonic single-spike activity during wakefulness. The change between these two activity modes is partially governed by transmitters acting on leak K+ currents in the thalamus, although the nature of the constituting ion channels is not yet known. In the present study, the contribution of members of the two-pore domain K+ channel family to the leak current was investigated using whole-cell patch-clamp techniques and molecular biological techniques. RT-PCR and in situ hybridization revealed the expression of TWIK-related acid-sensitive K+ channel 1 (TASK 1) and TASK3 channels in the rat dLGN. Voltage-clamp recordings of thalamocortical relay neurons in slice preparations demonstrated the existence of a current component sensitive to the TASK channel blocker bupivacaine, which reversed at the presumed K+ equilibrium potential, showed outward rectification, and contributed ∼40% to the standing outward current at depolarized values of the membrane potential (-28 mV). The pharmacological profile was indicative of TASK channels, in that the current was sensitive to changes in extracellular pH, reduced by muscarine and increased by halothane, and these effects were occluded by a near-maximal action of bupivacaine. Pharmacological manipulation of this current under current-clamp conditions resulted in a shift between burst and tonic firing modes. It is concluded that TASK1 and TASK3 channels contribute to the muscarine- and halothane-sensitive conductance in thalamocortical relay neurons, thereby contributing to the change in the activity mode of thalamocortical networks observed during the sleep-wake cycle and on application of inhalational anesthetics.
Keywords: whole-cell patch clamp, RT-PCR, in situ hybridization, thalamic activity mode, anesthetics, muscarinic receptor, sleep-wake cycle
Introduction
The thalamocortical neuronal network is characterized by two fundamentally different states of activity (for review, see Sherman and Guillery, 1996; Steriade, 1997): whereas low-frequency (<15 Hz) oscillatory activity occurs during natural sleep, deep anesthesia, and epileptic seizures, high-frequency oscillatory activity in the gamma range and tonic activity prevail during wakefulness. On the cellular level, slow oscillatory activity is associated with rhythmic bursts of action potentials in thalamocortical relay neurons, whereas tonic activity is associated with sequences of single-action potentials. The latter is thought to underlie the faithful transfer of sensory information from the periphery to the cortex (Sherman and Guillery, 2001). The switch between the two states of thalamic activity is accompanied by a depolarizing shift of the membrane potential of thalamocortical relay neurons and governed by the release of neurotransmitters from thalamic terminals of the ascending brainstem system during wakeup (for review, see McCormick, 1992b): ACh, noradrenalin, and 5-HT are released during increased activity of neurons whose cell bodies are located in brainstem cholinergic nuclei, the locus coeruleus, and the raphe nuclei, respectively. One crucial step leading to depolarization of thalamocortical relay neurons is the decrease of a leak K+ conductance (IKL), for instance, on activation of muscarinic (mACh) and α-adrenergic receptors (McCormick and Prince, 1987, 1988). In addition, inhalational anesthetics hyperpolarize the membrane potential and decrease the excitability of thalamocortical neurons through a conductance shunt by rising a IKL (Ries and Puil, 1999a,b). Until now, the molecular nature of the channels underlying the IKL in thalamocortical neurons is unknown.
IKL are crucial in stabilizing the resting potential in most neurons. With the discovery of a novel family of two-pore domain (2P) K+ channels, molecular correlates of K+ background conductances have been identified in various types of cells (for review, see Goldstein et al., 2001). Their name relates to the membrane topology of the channel subunits: two-pore-forming P domains, flanked by four transmembrane regions, are arranged in tandem. Functionally, most 2P K+ channels give rise to time- and voltage-independent K+ background currents. Channel activity is regulated in a complex way by various stimuli (e.g., temperature, pH, phospholipids, anesthetics). Some family members are further inhibited by G-protein-mediated signaling cascades. On the basis of their sensitivity to extracellular pH and sequence homology, pH-dependent 2P K+ channels are classified as acidosis inhibited (TWIK-related acid-sensitive K+ channel 1 (TASK1), TASK3, TASK5) and alkalosis activated (TASK2, TASK4/TWIK-related acid-sensitive K+ channel 1 (TALK1), TALK2) (Brown, 2000; Karschin et al., 2001; Lesage, 2003). In three neuronal cell types of the mammalian CNS, the transmitter-sensitive resting current has been narrowed down to the species of TASK1 channels (Millar et al., 2000; Talley et al., 2000) and, in addition, TASK3 channels (Han et al., 2002; Washburn et al., 2002).
The present study combines molecular biological and electrophysiological techniques to investigate the possible contribution of TASK channels to K+ background current in thalamocortical relay neurons in the dLGN, the major thalamic station of the primary visual pathway.
Materials and Methods
Preparation. Rats (postnatal days 12-20) were anesthetized with halothane and decapitated. A block of tissue containing the thalamus was removed and placed in ice-cold saline containing (mm): sucrose, 200; PIPES, 20; KCl, 2.5; NaH2PO4, 1.25; MgSO4, 10; CaCl2, 0.5; dextrose, 10; pH 7.35, with NaOH. Thalamic slices were prepared as coronal sections on a vibratome. Before recording, slices were kept submerged in standard ACSF containing (mm): NaCl, 125; KCl, 2.5; NaH2PO4, 1.25; NaHCO3, 24; MgSO4, 2; CaCl2, 2; dextrose, 10; pH adjusted to 7.35 by bubbling with a mixture of 95% O2 and 5% CO2.
Whole-cell patch clamp. Recordings were performed on thalamocortical relay neurons of the dLGN at room temperature. Slices were recorded in a solution containing: NaCl, 120; KCl, 2.5; NaH2PO4, 1.25; HEPES, 30; MgSO4, 2; CaCl2, 2; dextrose, 10; pH 7.2 or 6.4 was adjusted with HCl. Individual cells were visually identified by infrared differential interference contrast video-microscopy (Dodt and Zieglgänsberger, 1990). Membrane currents were measured with pipettes pulled from borosilicate glass (GC150T-10; Clark Electromedical Instruments, Pangbourne, UK), connected to an EPC-10 amplifier (HEKA Elektronik, Lamprecht, Germany), and filled with (in mm): K-gluconate, 95; K3-citrate, 20; NaCl, 10; HEPES, 10; MgCl2, 1; Ca Cl2, 0.5; BAPTA, 1; Mg-ATP, 3; and Na-GTP, 0.5. The internal solution was set to a pH of 7.25 with KOH and an osmolarity of 295 mOsm/kg.
Some experiments were performed in nominal Na+-free external and internal solutions of the following composition (mm): (1) external solution: N-methyl-D-glucamine (NMDG)-Cl, 100; KCl, 2.5; KH2PO4, 1.25; HEPES, 30; MgSO4, 3.5; Ca Cl2, 0.5; dextrose, 10; TEA-Cl, 20; 4-AP, 6; pH 7.2 or 6.4 was adjusted with HCl; (2) internal solution: K-gluconate, 95; K3-citrate, 20; NMDGCl, 10; HEPES, 10; MgCl2, 1; Ca Cl2, 0.5; BAPTA, 1; Mg-ATP, 3. The internal solution was set to a pH of 7.25 with KOH and an osmolarity of 295 mOsm/kg.
For current-clamp experiments, a pipette solution containing 5 mm EGTA and 0.5 mm CaCl2 was used. Typical electrode resistance was 2-3 MΩ, with an access resistance in the range of 5-15 MΩ. Series resistance compensation of >40% was routinely used. Voltage-clamp experiments were governed by Pulse software (HEKA Elektronik) operating on an IBM-compatible personal computer. A liquid junction potential of 8 ± 1 mV (n = 6) was measured and taken into account according to Neher (1992).
For recordings of high-voltage-activated Ca2+ (Meuth et al., 2002) and fast transient Na+ currents (Budde and White, 1998) in acutely isolated neurons, isolation procedures and recording conditions were used as described previously.
All results were presented as mean ± SEM. Substance effects were tested for statistical significance using the nonparametric Mann-Whitney test (Graph Pad Prism software; Graph Pad, San Diego, CA). Where applicable (because of a sufficient number of observations, a Gaussian distribution could be demonstrated for the amplitude of the standing outward current, the effect of extracellular acidification, and the effect of bupivacaine), the parametric t test was used (Origin software). Differences were considered statistically significant if p < 0.05.
Drugs. Bupivacaine and muscarine were obtained from Sigma (Deisenhofen, Germany), prepared as stock solutions in distilled water, and added to the perfusion medium. Halothane was administered by perfusion. One percent of fluothane (Zeneca, Plankstadt, Germany) was added to the extracellular solution at room temperature, followed by 1-min sonication. For comparison of halothane concentrations used in situ with those used for anesthesia in vivo, the study by Ries and Puil (1999a) offers some considerations.
In situ hybridization. Long-Evans rats (18-21 d old) were sacrificed as described previously, and complete brains were frozen in -50°C isopentane. Cryostat coronal sections of 14 μm thickness were cut at the level of the dLGN, thaw-mounted onto silane-coated slide glasses, and air dried.
Digoxigenin-labeled antisense and sense riboprobes were generated by in vitro transcription from vectors containing cDNA of rat TASK1 channels (corresponding to bp 574-1242) and TASK3 channels (bp 502-1431). In situ hybridization was performed as described previously (Stork et al., 2000) with minor modifications. In brief, sections were washed in 3× PBS for 5 min after fixation in 4% paraformaldehyde, acetylated, and prehybridized for 2 hr at 55°C. The prehybridization solution consisted of 50% formamide, 5× SSC, 1× Denhardt's solution, 0.5 mg/ml yeast tRNA, and 1.0 mg/ml total yeast RNA. For hybridization, sections were exposed to 50% formamide, 1× Denhardt's solution, 0.1 mg/ml yeast tRNA, 0.1 mg/ml total yeast RNA, 10% Dextran sulfate, 0.125% SDS, 10 mm Tris-HCl, pH 7.5, 1 mm EDTA, and 300 mm NaCl. Digoxigenin-labeled RNA probes were added (with a final concentration of 20 ng/ml prehybridization buffer), and sections were incubated at 55°C for 16-18 hr. For all steps, RNase-free solutions and sterile 6-well plates were used. After hybridization, sections were subjected to washes of increasing stringency including 2× SSC at room temperature (once for 15 min), 50% formamide/2× SSC at 70°C (twice for 60 min), 2× 50% formamide/0.2× SSC at 70°C (twice for 60 min), and 0.1× SSC at 70°C (once for 60 min). Labeled cells were detected with an anti-digoxigenin antibody tagged with alkaline phosphatase (Roche Molecular Biochemicals, Mannheim, Germany). Staining was performed using 4-nitro blue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl phosphate as substrates (Roche Molecular Biochemicals).
Specificity of the hybridization reaction was verified by substituting labeled sense probes for the antisense probes. No labeling was observed under these conditions. The density of positively labeled cells was determined using the NeuroLucida system.
RT-PCR assays. Poly(A) mRNA was prepared from freshly dissected tissue by extraction with Trizol reagent according to the manufacturer's instructions (Oligotex; Qiagen GmbH, Hilden, Germany). First-strand cDNA was primed with oligo(dT) from 0.5-1 μg of mRNA and synthesized using the SuperScript II enzyme (Gibco BRL Life Technologies, Karlsruhe, Germany) at 42°C for 50 min. The cDNAs from different tissues were amplified with 0.75 U of HotStarTag polymerase (Qiagen GmbH), 1.5 mm MgCl2, and 0.2 mm of each dNTP in 30 reaction mixture using 50 pmol of forward and reverse specific oligonucleotide primers. For amplification of TASK templates, the initial cDNA concentration (1 ng) was 10 times higher as for the other cDNA species. Amplification protocols included 35 cycles of 95°C for 1 min, Tann for 1 min, and 72°C for 1 min after predenaturation at 95°C for 15 min. The annealing temperature Tann was calculated for every primer pair from G/C and A/T content of the primers used in each reaction. Normalization of cDNAs was done using primers specific for the housekeeping genes β-actin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The following primers were used: β-actin (nt 253-1080; accession no. NM_031144), forward ATTTGGCACCACACTTTCTACAAT, reverse CTGCTTGCTGATCCACATCTGC; GAPDH (nt 561-1012; accession no. AB017801), forward ACCACAGTCCATGCCATCAC, reverse TCCACCACCCTGTTGCTGTA; Kir 3.4 (nt 358-888; accession no. X83584), forward CACGTGGGTGACCAAGAGTG, reverse CTGCTCCAGTTGAGCACGAG; Kir 3.3 (nt 522-1058; accession no. L77929), forward CACCTGGAGGACACCGCGTG, reverse GTCGTCCCTCTCGAGGGCGC; Kir 3.2 (nt 121-390; accession no. X83583), forward GACCTGCCAAGACACATCAGCC, reverse CGAGGGGTCCTCTATGTGGTCCA; Kir 3.1 (nt 124-436; accession no. U01141), forward TCGTCCAGCGGTTCGGGCTTGCAG, reverse GAGTGTAGTTGCCGACATGGGC; Kir 2.3 (nt 623-952; accession no. X87635), forward CAGGCCCACGTGCCCAGGCGGA, reverse TACATGCATGATACACGGTTTG; mAChR2 (nt 1133-1501; accession no. J03025), forward CAAGACCCAGTATCTCCAAGTCTG, reverse CGACGACCCAACTAGTTCTACAGT; mAChR3 (nt 854-1414; accession no. M16407), forward ACAGAAGCGGAGGCAGAAAACTTT, reverse CTTGAAGGACAGAGGTAGAGTAGC; TASK1 (nt 220-735; accession no. AB048823), forward CACCGTCATCACCACAATCG, reverse TGCTCTGCATCACGCTTCTC; TASK2 (nt 330-959; accession no. AF259395), forward TGGGCGCCTCTTCTGTGTCTTCTA, reverse TCCCCTCCCCCACTTGTTTTCATT; TASK3 (nt 188-602; accession no. AF192366), forward ATGAGATGCGCGAGGAGGAGAAAC, reverse ACGAGGCCCATGCAAGAAAAGAAG; TASK5 (nt 137-700; accession no. AF294353), forward GAGCCTGGGCGAGCGTCTGAAC, reverse CGGGCCCGGAGTCTGTCTGG.
Results
Expression of TASK channels, inward rectifier K+ channels, and mACh receptor subtypes
Using a RT-PCR assay of rat dLGN tissue, PCR fragments from TASK1 and TASK3 were detected in significant amounts in the dLGN, whereas only faint bands were seen for TASK2 (Fig. 1A). For TASK5, no PCR product was detected in the dLGN, whereas cerebellar cDNA used as positive control (Karschin et al., 2001) revealed a clear expression signal (Fig. 1B). The expression of TASK4 in dLGN could not be tested, because of the lack of a complete rat mRNA sequence.
Figure 1.
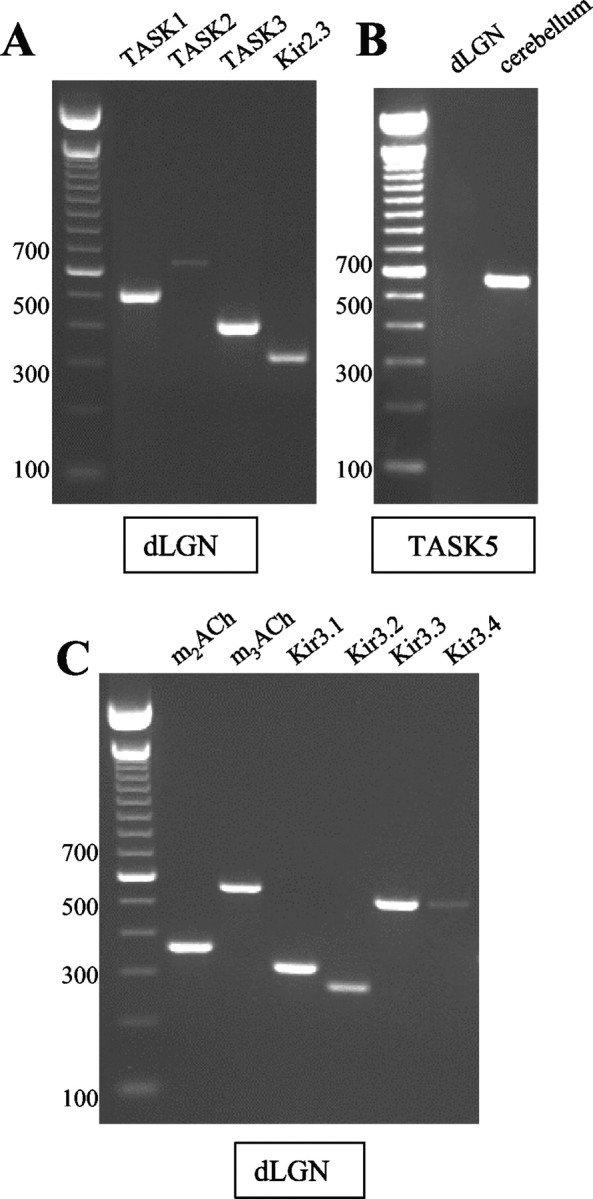
RT-PCR analysis of different rat brain regions. A, Expression of TASK1-3 and Kir2.3 channels in dLGN. B, Expression of TASK5 channels in dLGN and cerebellum. C, Expression of Kir3.1-3.4 channels and ACh receptors (m2-m3AchR) in dLGN. The sizes of the DNA marker bands are indicated in the left margin. When H2O and brain mRNA not subjected to reverse transcription were used as negative controls instead of cDNA, no PCR bands could be detected because of the lack of adequate substrates for the DNA polymerase (data not shown).
An analysis of inward rectifier K+ (Kir) channel expression in dLGN revealed the presence of Kir2.3 (Fig. 1A) and all four members of the Kir3 family (Kir3.1-3.4; Fig. 1C), although the signal for Kir3.4 was very faint. In addition, PCR products for m2ACh and m3ACh receptors were detected (Fig. 1C). These data demonstrate the presence of Kir channels sensitive to changes in extracellular pH (Kir2.3) and modulated via mACh receptors (Kir3 family).
In situ hybridization techniques using digoxigenin-labeled antisense RNA probes revealed a dense distribution of cells expressing TASK1 (Fig. 2A) and TASK3 (Fig. 2B) in the dLGN. Positively labeled cells were found in a density of 542 ± 32 cells/mm2 and 729 ± 24 cells/mm2 (n = 6) for TASK1 and TASK3, respectively. Nissl-stained sections were used to assess the overall density of neurons. The average cell density in Nissl staining was 808 ± 31 cells/mm2 (n = 6), indicating that 67 and 90% of the cells express TASK1 and TASK3, respectively, thereby providing evidence for at least partial overlapping expression. Control experiments using a sense cRNA construct showed no signals (data not shown). Assuming similar efficiencies of the two antisense RNA probes, TASK3 expression was at a higher level compared with TASK1, corresponding to the difference in intensity of RTPCR bands obtained for TASK1 and TASK3 channels (Fig. 1A).
Figure 2.
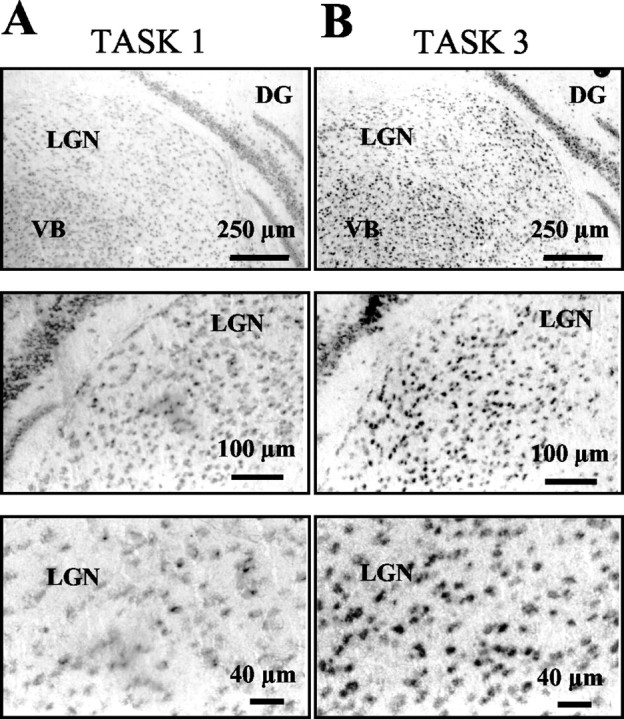
Expression of TASK channel transcripts in dLGN. In situ hybridization was performed on sections of the rat brain using digoxigenin-labeled RNA probes complementary to TASK1 (A) and TASK3 (B). Coronal sections of the thalamus show moderate expression levels of TASK1 and high levels of TASK3 expression at different magnification. DG, Dentate gyrus; VB, ventrobasal thalamic complex.
Pharmacological profile of the leak conductance in thalamocortical relay neurons
At holding potentials between -58 and -68 mV, thalamocortical relay neurons displayed a standing outward current with an amplitude of 71 ± 4 pA (n = 10) (cf. Budde et al., 1997). In a first experimental step, a pharmacological profile of this current was obtained by application of ions (H+, Ba2+) known to block TASK channels. Lowering the extracellular pH from 7.2 to 6.4 and further application of Ba2+(150 μm) resulted in a two-stage reduction of the outward current (data not shown). In addition, when bupivacaine (20 μm) was added to the control recording solution, the standing outward current was greatly reduced, revealing current amplitudes of 29 ± 3 pA (n = 7; data not shown).
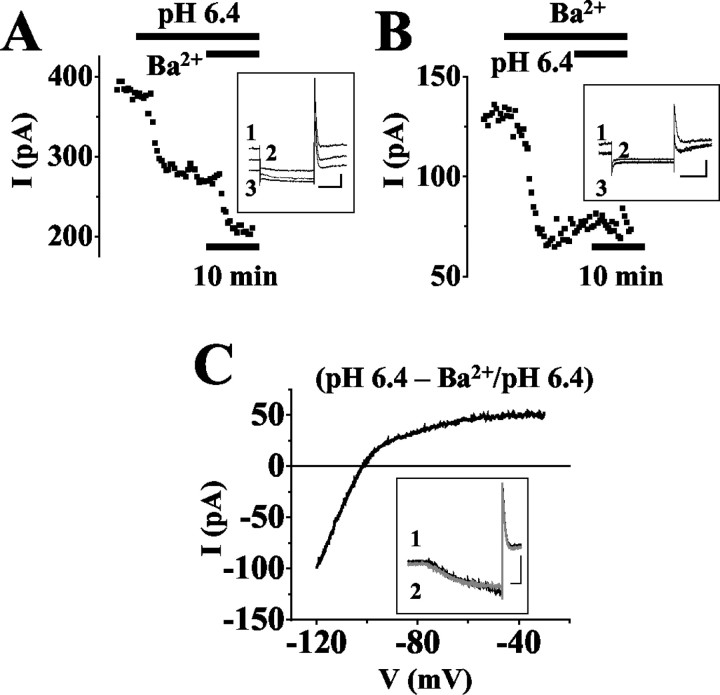
To increase outward current amplitudes, recordings were obtained at more depolarized values of the membrane potential. At -28 mV, the standing outward current averaged 348 ± 11 pA (n = 89). Stepping the membrane potential from -28 to -68 mV for a duration of 500 msec every 20 sec resulted in a step-wise decrease in membrane outward current (Fig. 3A, inset), indicating the presence of channels with fast gating properties. Changing the external pH from 7.2 to 6.4 induced a decrease of the current at -28 mV by 41 ± 3% (n = 4; Fig. 3A). The addition of Ba2+ (150 μm; Fig. 3A) lead to an additional decrease by 47 ± 5% of the remaining current (n = 4), respectively. These drug effects were statistically significant with respect to the previous recording condition (p < 0.004). The opposite experimental approach with applying Ba2+ (150 μm; Fig. 3B) before lowering the external pH resulted in a significant (p < 0.0001) decrease in the standing outward current amplitude by 49 ± 4% (n = 4). Thereafter, external acidification had no significant (p = 0.49) effect on the current amplitude. The current-voltage (I-V) relationship of the Ba2+-sensitive current during extracellular acidification was obtained by ramping the membrane potential in 800 msec from -30 mV to -120 mV (Fig. 3C). The data were obtained by subtraction of the currents obtained in the presence of Ba2+ at pH 6.4 (Fig. 3C, inset, gray trace, 2) from those recorded at pH 6.4 (Fig. 3C, inset, black trace, 1). The I-V relationship was characterized by an inward rectification and a reversal potential of -102 ± 2 mV (n = 3; Fig. 3C), i.e., close to the expected K+ equilibrium potential (EK = -104 mV).
Figure 3.
Effects of Ba2+ and extracellular acidification on the standing outward current in thalamocortical relay neurons. A, B, Amplitude of the net outward current plotted against time in one relay neuron recorded under voltage-clamp conditions at -28 mV (black data points). Periods of extracellular acidification, pH 6.4, and application of Ba2+ (150 μm) are indicated by horizontal lines. Insets, Original traces of membrane responses to a voltage step from a holding potential of -28 mV to -68 mV for 500 msec under control conditions (1) and application of drugs (A: 2 = pH 6.4, 3 = pH 6.4, Ba2+; B: 2 = Ba2+, 3 = Ba2+, pH 6.4). In B, current traces recorded in Ba2+ and Ba2+ at pH 6.4 are indistinguishable. C, I-V relationship of the Ba2+-sensitive current obtained by graphical subtraction of ramp currents during Ba2+ action at pH 6.4 from currents recorded at pH 6.4 (see term near trace). Inset, Currents evoked by ramping the membrane from -30 mV to -120 mV over 800 msec at pH 6.4 (black trace) and application of Ba2+ at pH 6.4 (gray trace). Scale bars in the insets: A, B, 200 msec/100 pA; C, 100 msec/500 pA.
These results demonstrate the presence of a pH-sensitive component that can be blocked by Ba2+, which is indicative of TASK channels, and are in agreement with the contribution of inward rectifier channels to the standing outward current in thalamocortical neurons.
Outward current indicative of TASK channels
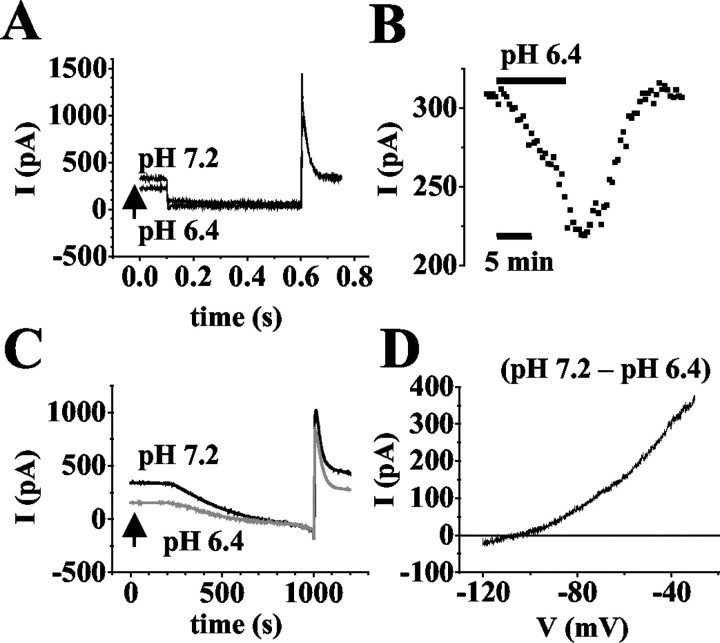
In a next experimental step, the possible contribution of TASK channels to the standing outward current was tested by analyzing the pH-sensitive component in more detail. Throughout the experiments, ZD 7288 was present to eliminate possible contamination by pH-sensitive Ih channels (Munsch and Pape, 1999). Changing the external pH from 7.2 to 6.4 significantly (p < 0.0001) reduced the outward current at -28 mV by 40 ± 2% (n = 35; Fig. 4A). This effect was fully reversible (Fig. 4B) and in some cells was accompanied by a positive overshoot of the current beyond control value (data not shown). Current-voltage relationships were obtained by ramping the membrane potential in 800 msec from -30 mV to -120 mV (Fig. 4C). The rate of hyperpolarization (0.11 mV/ms) was sufficiently slow to allow the outward current to reach steady state at each potential (cf. Watkins and Mathie, 1996; Millar et al., 2000). The I-V relationship obtained by subtracting currents evoked in solutions at pH 6.4 (Fig. 4C, gray trace) from those at pH 7.2 (Fig. 4C, black trace) revealed a reversal potential close to EK (Vrev = -103 ± 2 mV; n = 3) and outward rectification (Fig. 4D).
Figure 4.
Analysis of the pH-sensitive current component. A, Original traces of membrane responses to a voltage step from a holding potential of -28 mV to -68 mV for 500 msec at pH 7.2 and pH 6.4 (as indicated near traces). B, Plot of the current amplitude at -28 mV against time (period of extracellular acidification indicated by horizontal bar). C, Currents evoked by ramping the membrane potential from -30 mV to -120 mV over 800 msec at pH 7.2 (black trace) and pH 6.4 (gray trace). D, I-V relationship of the pH-sensitive current obtained by graphical subtraction of ramp currents recorded at pH 6.4 from pH 7.2 (see term near trace).
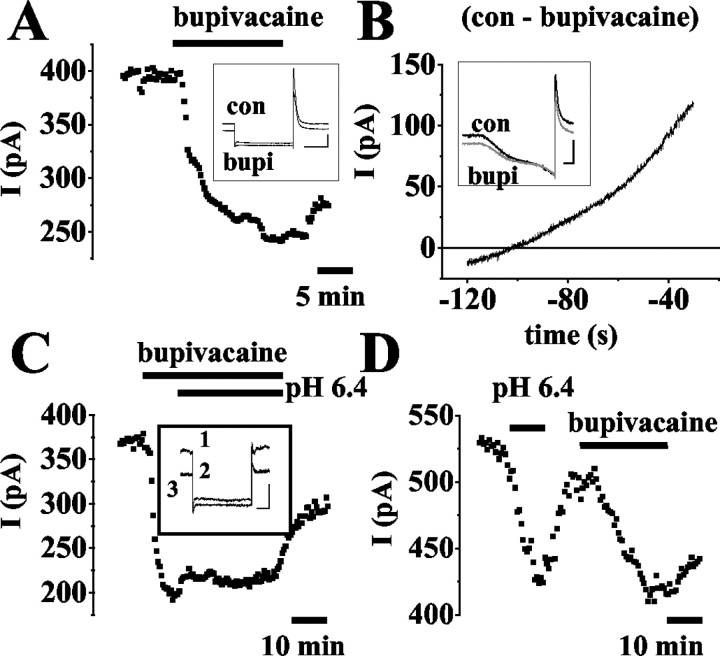
In the following, the local anesthetic bupivacaine, which is known to block TASK channels, was used (Leonoudakis et al., 1998; Kindler et al., 1999; Brown, 2000; Goldstein et al., 2001; Meadows and Randall, 2001). During application of bupivacaine at different concentrations, the standing outward current at -28 mV was significantly (p < 0.03) reduced by 12 ± 4% (5 μm, n = 3; data not shown), 38 ± 3% (20 μm, n = 29; Fig. 5A), and 28 ± 4% (50 μm, n = 5; data not shown). These effects were only partially reversible (Fig. 5A) and not significantly (p = 0.06) different between 20 and 50 μm. For comparison, the effect of bupivacaine (20 μm) was tested on the fast transient outward K+ current (cf. Budde et al., 1992) in the slice preparation, as well as fast Na+ currents (cf. Budde and White, 1998) and high-voltage-activated Ca2+ currents (cf. Meuth et al., 2002) after acute isolation. The reduction of the transient K+ currents (9 ± 2%; n = 6), Na+ currents (11 ± 4%; n = 3), and Ca2+ currents (6 ± 2%; n = 5) were significantly (p < 0.005) smaller as compared with the standing outward current (data not shown). These data indicate a maximal blocking effect of bupivacaine on the standing outward current, together with minor effects on other currents, at a concentration of 20 μm, and, thus, bupivacaine at this concentration was used in the following. It is notable that, in three of 18 cells, application of bupivacaine resulted in a ∼10% increase in the outward current at -28 mV. These cells were not included for analysis.
Figure 5.
Current components sensitive to application of bupivacaine (20 μm) and changes in pH during action of bupivacaine. A, C, D, Plot of current amplitude at -28 mV against time (drug application indicated by horizontal bar). Insets, Original traces of membrane responses to a voltage step from a holding potential of -28 mV to -68 mV for 500 msec under control conditions (con, 1) and lowering pH and/or application of drugs (as indicated near traces; C: 2 = bupivacaine, 3 = bupivacaine, pH 6.4). In C, current traces recorded in bupivacaine (bupi) at pH 7.2 and pH 6.4 are indistinguishable. B, I-V relationship of the bupivacaine-sensitive current obtained by graphical subtraction of currents during drug action from control currents (see term near trace). Inset, Currents evoked by ramping the membrane from -30 mV to -120 mV over 800 msec under control conditions (black trace) and in the presence of bupivacaine (gray trace). Scale bars in the insets: A, 200 msec/200 pA; B, 100 msec/300 pA; C, 100 msec/150 pA.
Current-voltage relationships of bupivacaine-sensitive currents (Fig. 5B) were obtained by ramp protocols and graphical subtraction of the currents obtained during bupivacaine (Fig. 5B, inset, gray trace) from those under control conditions (Fig. 5B, inset, black trace). The I-V relationship revealed a reversal potential of -103 ± 1mV (n = 9; Fig. 5B), i.e., close to the expected K+ equilibrium potential (EK = -104 mV), outward rectification, and was, thus, very similar to the pH-sensitive ramp currents. Next, the effect of external acidification was tested during bupivacaine action (Fig. 5C). After administration of bupivacaine, the outward current was significantly reduced by 45 ± 2% as before (n = 7; Fig. 5C). After a steady-state effect of bupivacaine had been reached, lowering the pH from 7.2 to 6.4 did not result in an additional reduction, but in a small, statistically not significant (p = 0.37) increase in outward current amplitude by 4 ± 1% (n = 7; Fig. 5C). This effect most likely reflected the pH dependency of bupivacaine block of TASK channels (Kindler et al., 1999), as indicated by the similar outward rectification and reversal potential of the bupivacaine-sensitive ramp currents obtained under control conditions and the pH-sensitive ramp currents in the presence of bupivacaine (data not shown).
Next, the effects of extracellular acidification and bupivacaine were compared in individual cells. As before, changing the pH from 7.2 to 6.4 resulted in a significant (p < 0.008) reduction of the standing outward current at -28 mV by 27 ± 3% (n = 5; Fig. 5D). After recovery of the pH effect, the addition of bupivacaine (20 μm) again significantly (p < 0.01) reduced the outward current by 28 ± 6% (n = 5; Fig. 5D). The magnitude of current reduction induced by acidification and bupivacaine were not significantly (p < 0.95) different. Taken together, these data indicate that acidification and bupivacaine block the same current component. Furthermore, TASK channels contribute to the leak K+ outward current in thalamocortical neurons and constitute the substrates of its pH sensitivity.
Halothane-sensitive currents
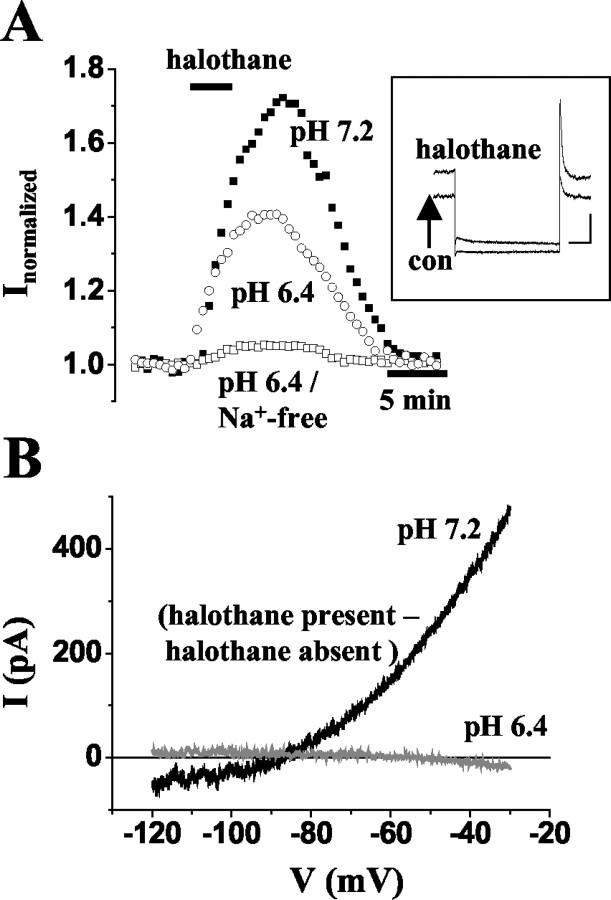
Another feature of TASK channels is their sensitivity to inhalational anesthetics (Patel et al., 1999). Indeed, the addition of 1% halothane to the external recording solution for 3 min resulted in a significant (p < 0.0001) and reversible increase (64 ± 3%; n = 6) in outward current amplitude at -28 mV (Fig. 6A, filled squares). Halothane-sensitive ramp currents were obtained by subtracting currents recorded in the absence of halothane from those recorded in the presence of the anesthetic. The I-V relationship revealed a rather linear current-voltage relationship with some outward rectification at voltages positive to about -45 mV (data not shown). The reversal potential of -68 ± 4mV (n = 3) was positive to the expected reversal potential for K+ currents (EK = -104 mV), indicating the contribution of a mixture of ions (data not shown). In external solutions with the pH set to 6.4, the halothane-induced increase of the current was 42 ± 2% (n = 4; Fig. 6A, open circles) and, thus, significantly (p < 0.006) smaller compared with that at pH 7.2, and the reversal potential was shifted to -49 ± 2 mV (data not shown; n = 3). This finding is in agreement with the assumption that a K+ current is no longer part of the halothane-sensitive component. To further determine the ionic nature of the halothane-sensitive current, hyperpolarizing voltage ramps were applied in an external solution containing TEA (20 mm), 4-AP (6 mm), TTX (1 μm), and ZD 7288 (100 μm). In addition, Na+ was removed from the external and internal recording solutions, and extracellular Ca2+ was reduced to 0.5 mm. Under these conditions, the halothane-sensitive current revealed outward rectification and a reversal potential of -92 ± 1 mV (Fig. 6B, black trace; n = 3), which was close to the expected reversal potential for K+ currents (EK = -94 mV). With the external pH set to 6.4, the halothane effect was nearly completely blocked (Fig. 6A, open squares; B, gray trace; n = 3). These data indicate that the halothane-sensitive current is carried by current through TASK channels and as yet uncharacterized Na+ and/or Ca2+ channels.
Figure 6.
Current components sensitive to application of halothane. A, Plot of current amplitude at -28 mV against time during halothane application (indicated by horizontal bar) at pH 7.2 (filled squares), at pH 6.4 (open circles), and at pH 6.4 during isolation of K+ currents (open squares; see below). Inset, Examples of current responses evoked by stepping the membrane from -28 mV to -68 mV for 500 msec under control conditions and during action of halothane (as indicated near trace). The scale bars represent 100 msec and 200 pA. B, I-V relationship of halothane-sensitive currents evoked by ramping the membrane from -30 mV to -120 mV over 800 msec at pH 7.2 (black trace) and pH 6.4 (gray trace). Currents were obtained by subtracting ramp currents in the absence of halothane from those in its presence (see term near traces). Recordings were performed during the presence of TEA (20 mm), 4-AP (6 mm), TTX (1 μm), and ZD 7288 (100 μm), removal of Na+ from the external and internal recording solutions, and reduction of extracellular Ca2+ to 0.5 mM. To achieve a reversible effect, halothane (1%) was applied for 3 min.
mACh receptor-mediated responses
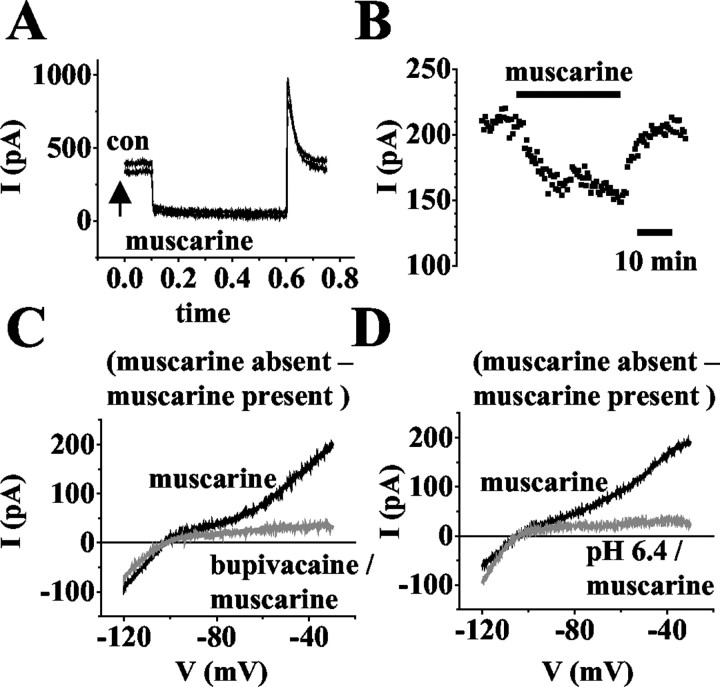
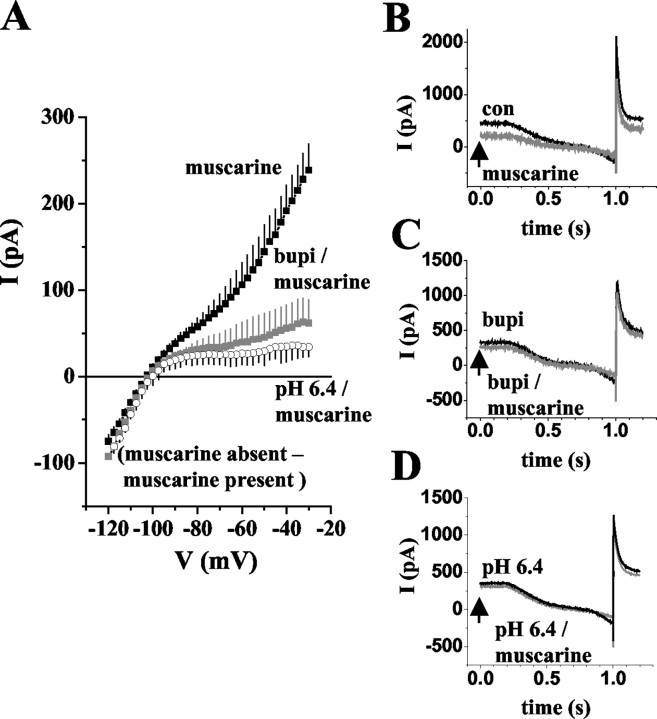
The block of a K+ leak conductance is of important functional significance for the change of thalamic activity modes induced by activation of mACh receptors (McCormick, 1992a). To link this leak conductance to TASK channels, the effects of bupivacaine and extracellular acidification on responses to ACh and muscarine were tested in thalamocortical relay neurons. Application of ACh (50 μm) or muscarine (50 μm) significantly (p < 0.0001) reduced the outward current at -28 mV by 35 ± 3% (n = 3; data not shown) and 34 ± 4% (n = 14; Fig. 7A,B), respectively. It should be noted here that in two of 16 cells that were tested, application of muscarine resulted in an ∼10% increase in the current amplitude. These cells were not included for analysis. Muscarine-sensitive ramp currents were obtained by subtracting currents recorded in the presence of muscarine (Fig. 8B, gray trace) from those under control conditions (Fig. 8B, black trace) and yielded an I-V relationship exhibiting both inward and outward rectification, and a reversal potential at the expected K+ equilibrium potential (Erev = -104 ± 3 mV; n = 4; Fig. 8A, filled squares). Next, bupivacaine was used to block TASK channels. In the presence of bupivacaine, application of muscarine resulted in a small but significant (p < 0.002) decrease in the outward current at -28 mV by 4 ± 9% (n = 12; data not shown). The I-V relationship of the muscarine-sensitive current was constructed by subtracting the ramp currents in the presence of the drug (Fig. 8C, gray trace) from those in the absence of the drug (Fig. 8C, black trace) and revealed an inwardly rectifying K+ current (Erev = -104 ± 2 mV; n = 5; Fig. 8 A, gray squares). In addition, extracellular acidification was used to block TASK channels. As for bupivacaine, the construction of the muscarine-sensitive I-V relationship from ramp protocols (Fig. 8 D) revealed an inwardly rectifying current that reversed (Erev = -103 ± 2 mV; n = 3; Fig. 8 A, open circles) close to the expected EK of -104 mV.
Figure 7.
Current components sensitive to application of muscarine (50 μm) during absence/presence of bupivacaine (20 μm) and at pH 7.2/pH 6.4. A, Original traces of membrane responses to a voltage step from a holding potential of -28 mV to -68 mV for 500 msec under control conditions (con) and application of muscarine (as indicated near traces). B, Plot of current amplitude at -28 mV against time (drug application indicated by horizontal bar). C, D, I-V relationship of the drug-sensitive current obtained by graphical subtraction of currents during drug action from control currents. Currents were evoked by ramping the membrane from -30 mV to -120 mV over 800 msec. Note that muscarine blocks an inwardly and outwardly rectifying current under control conditions (black traces) and an inwardly rectifying current in bupivacaine (C; gray trace) and at pH 6.4 (D; gray traces) in individual cells.
Figure 8.
Mean muscarine-sensitive (50 μm) ramp currents under different recording conditions. A, Mean I-V relationship of the muscarine-sensitive current under control conditions (filled squares), in the presence of bupivacaine (open squares), and at pH 6.4 (open circles). I-V relationships were obtained by graphical subtraction of currents during muscarine action from control currents and determining current amplitudes in 2.5 mV intervals. B-D, Currents evoked by ramping the membrane from -30 mV to -120 mV over 800 msec in the presence (gray traces) and absence (black traces) of muscarine (20 μm) as indicated near the current traces.
To further strengthen these results, the effect of muscarine was tested in individual cells under different recording conditions. In six thalamocortical neurons, the muscarine-sensitive I-V relationship revealed inward and outward rectification with a reversal at -104 ± 1 mV (n = 6; Fig. 7C,D, black traces) as before. After recovery from the first muscarine application, muscarine was tested a second time in the presence of bupivacaine and at pH 6.4, respectively. For both recording conditions, the muscarine-sensitive I-V relationship was inwardly rectifying and reversed close to EK (muscarine in bupivacaine: Erev = -105 ± 1 mV, n = 3; muscarine at pH 6.4: Erev = -103 ± 1 mV, n = 3). These data indicate that activation of mACh receptors results in an inhibition of TASK as well as inwardly rectifying Kir type channels.
Regulation of firing patterns
The possible functional significance of modulation of TASK channels for firing patterns of thalamocortical relay neurons was analyzed under current-clamp conditions. Recordings were obtained at slightly hyperpolarized values of the membrane potential from rest (-73 mV ± 1 mV, n = 12 vs -71 ± 1 mV, n = 24) using DC injection. Under these conditions, depolarizing current steps elicited typical burst responses with two to five action potentials riding on top of a low threshold Ca2+ spike (Fig. 9A,B,D). A frequency analysis including the first two action potentials of a burst response revealed an intra-burst frequency of 148 ± 7 Hz (n = 12). The addition of bupivacaine (20 μm) resulted in a depolarization of the membrane to -42 ± 3 mV, accompanied by a change in firing mode from burst to tonic generation of action potentials (Fig. 9A; n = 3). The frequency of tonic action potential firing as derived from the first two spikes was 38 ± 9 Hz. Similar results were obtained on changes of the extracellular pH from 7.2 to 6.4 in the presence of ZD 7288 (100 μm; membrane potential, -52 ± 3 mV; firing frequency, 32 ± 10 Hz; n = 6; Fig. 9B) and application of muscarine (50 μm; membrane potential, -49 ± 2 mV; firing frequency, 37 ± 4 Hz; n = 3; Fig. 9D). All effects were reversible. The effect of halothane was tested at slightly depolarized values of the membrane potential (61 ± 2 mV; n = 4) using DC injection. Depolarizing current pulses consistently elicited tonic action potential firing (Fig. 9C). The application of halothane (1%) induced a hyperpolarizing shift of the membrane potential to -68 ± 1 mV (n = 4). This hyperpolarization was accompanied by a change in firing pattern from tonic to low-threshold Ca2+ spikes (Fig. 9C). The Ca2+ spike typically triggered a single-action potential rather than a burst of action potential, most likely because of a decrease in input resistance and inhibition of T-type Ca2+ currents associated with the action of halothane (Takenoshita and Steinbach, 1991; Ries and Puil, 1999a). Indeed, during prolonged action of halothane, a purely passive response of the cells to the same depolarizing current injection was observed (data not shown).
Figure 9.
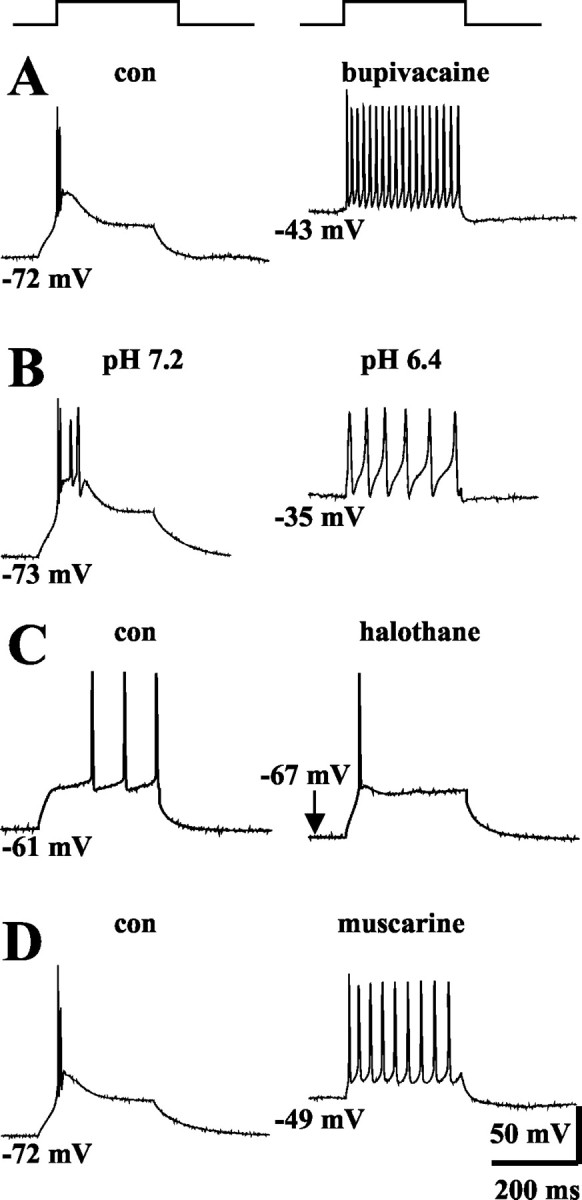
Effects of bupivacaine (A), lowering of extracellular pH from 7.2 to 6.4 (B), halothane (C), and muscarine (D) on the firing mode of thalamocortical relay neurons. Cells were recorded under whole-cell current-clamp conditions and were challenged using a 100-200 pA depolarizing current pulse (300 msec duration). From hyperpolarized potentials (indicated near traces), depolarizing pulses induced robust burst firing (A, B, D, left panel). Application of bupivacaine (20 μm; A), extracellular acidification in the presence of ZD7288 (B), and application of muscarine (50 μm; D) result in a depolarizing shift of the membrane potential and generation of tonic trains of action potentials in response to the same depolarizing current pulse. Application of halothane (1%; C) at -61 mV results in a hyperpolarization and replacement of tonic firing by a low-threshold action potential triggering one spike.
Discussion
Contribution of TASK channels to the leak conductance in thalamocortical neurons
A significant proportion of the leak current in thalamocortical neurons at membrane potentials close to rest (approximately -65 mV) or at more positive values (approximately -30 mV) seems to be carried by TASK channels, as indicated by the sensitivity to bupivacaine at 20 μm, which has been used to block TASK channels in various types of cells (Leonoudakis et al., 1998; Brown, 2000; Buckler et al., 2000; Lesage and Lazdunski, 2000; Goldstein et al., 2001; Meadows and Randall, 2001). This conclusion is supported by the overall pharmacological profile of this current component, i.e., sensitivity to extracellular acidification, increase and decrease on application of halothane and stimulation of mACh receptors (Patel et al., 1999; Millar et al., 2000), respectively. Furthermore, the isolated bupivacaine-sensitive current reversed at the presumed K+ equilibrium potential and displayed outward rectification, resembling that of TASK currents in whole-cell recordings (Rajan et al., 2000). One point of consideration relates to Kir-type channels, which contribute to leak K+ conductances in various types of cells (Hille, 2001). In this respect, Kir2.3 channels and members of the Kir3 family are of special interest, because these channels are blocked by lowering the extracellular pH (Coetzee et al., 1999; Brown, 2000), are activated by ACh receptor stimulation (Ruppersberg, 2000), and are expressed in the dLGN (present study). In thalamocortical relay neurons, most of the pH-sensitive K+ current seems to be carried through TASK channels, as indicated by the occlusion of the effects of extracellular acidification (in ZD 7288) by a near-maximal effect of bupivacaine. In support of this is the finding that the isolated pH-sensitive current displayed outward rectification similar to that of the bupivacaine-sensitive current in dLGN neurons and that of TASK currents in other types of cells (Buckler et al., 2000). Ba2+ blocks Kir-type as well as TASK channels, and the Ba2+-sensitive current component of thalamocortical relay neurons recorded in the present study is carried by both TASK and Kir channels. This conclusion is supported by the finding that a Ba2+-sensitive K+ conductance is existing in thalamocortical neurons (McCormick and Prince, 1987; Williams et al., 1997) and by the observation made during the present study that there is a Ba2+-sensitive inwardly rectifying current in acidic extracellular solutions, but no pH-sensitive component in Ba2+-containing solutions.
It is known that bupivacaine blocks Na+ (Brau et al., 1998), high-voltage-activated Ca2+ (Liu et al., 2001), and voltage-activated potassium channels (Komai and McDowell, 2001) in different neuronal preparations. Therefore, the effect of bupivacaine on these ion channels was analyzed in thalamocortical relay neurons. It was found that at a concentration of 20 μm the blocking effect of bupivacaine on the standing outward current (∼40%) was about four times higher as compared with Na+, Ca2+, and fast transient K+ currents (∼10%). Therefore, it is concluded that depending on the individual recording conditions and the concentration used, bupivacaine can act as a marker of currents through TASK channels. In the present study, this view is supported by two major arguments. First, the isolated bupivacaine-sensitive current is reversed at the presumed K+ equilibrium potential, indicating that a contamination could only arise from other K+ currents. One important voltage-dependent K+ current that is active below threshold and can, thus, influence thalamic activity modes is a fast transient outward current (Huguenard et al., 1991; Budde et al., 1992). However, the reduction of this current is significantly lower compared with the standing outward current (see above). Second, extracellular acidification and bupivacaine block current components exhibiting very similar properties with respect to reversal, waveform, and amplitude, indicating an effect on the same current component.
The leak conductance in thalamocortical neurons at membrane potentials ranging from rest to approximately -30 mV seems to have strong contributions of TASK and Kir channels. A TTX-sensitive component, most likely representing the persistent Na+ current activated at membrane potentials slightly positive to rest (Parri and Crunelli, 1998), a ZD 7288-sensitive component, representing current through Ih channels (BoSmith et al., 1993; Maccaferri and McBain, 1996), and a TEA/4-AP-sensitive component representing current through delayed rectifier (Huguenard and Prince, 1991; Budde et al., 1992)/slow A-type K+ channels (McCormick, 1991) also make detectable contributions (data not shown). TASK channels contribute ∼40% to the leak conductance as deduced from the bupivacaine- and pH-sensitive components. The contribution of several voltage-dependent ion channels (persistent Na+ channels, TEA/4-AP-sensitive K+ channels) seem to increase with more positive values of the membrane potential.
Involvement of different TASK channel subtypes
TASK1 and TASK3, together with the possibly nonfunctional TASK5 subunits, form a subfamily of 2P K+ channels that are structurally related and inhibited by acidosis. In addition, a subgroup including TASK2, TASK4 (TALK1), and TALK2 subunits are recognized based on their sequence homology and more alkaline range of activation (TASK1-5) (Goldstein et al., 2001; Karschin et al., 2001; Lesage, 2003). Whereas TASK1 and TASK3 are significantly expressed in dLGN, no TASK5 expression could be detected in this visual thalamic nucleus. These results are in agreement with the recently determined tissue distribution of 2P domain K+ channels in brain, because TASK3 is the major channel species in principal thalamic relay nuclei, and TASK5 is predominantly associated with the central auditory pathway (Karschin et al., 2001; Talley et al., 2001). The uniformly low expression of TASK2 in brain is in accordance with the barely detectable PCR signal of TASK2 described here. Because the expression of TASK4 was not assessed in the present study and bupivacaine is known to block these acid-sensitive channels, a contribution of TASK4 (and other members of the TALK channel subgroup) cannot be excluded. However, as judged from the pH dependency of TASK4 channels in oocytes (Decher et al., 2001), it is questionable whether they are open under the conditions of the present study. Taken together, it seems that TASK1 and TASK3 channels constitute most of the pH-sensitive K+ conductance, although the contribution of additional acid-sensing K+ channels (Han et al., 2002) cannot be excluded based on available data.
Contribution of TASK channels to halothane and mACh receptor-mediated effects
Activation of mACh receptors in thalamocortical neurons inhibited an outwardly and inwardly rectifying K+ current (McCormick, 1993). The finding that bupivacaine and extracellular acidification were able to largely block the muscarine effect on the outwardly rectifying, but spared the inwardly rectifying, component of ramp currents indicates that the outward rectification is largely carried by current through TASK channels. Furthermore, application of halothane to thalamocortical relay neurons resulted in a large increase in outward current amplitude. The reversal of the halothane-sensitive ramp current was positive to the expected K+ reversal potential, indicating that additional ions contribute. Likely candidates are Na+ and/or Ca2+ channels, as indicated by the finding that the halothane-sensitive ramp current showed clear outward rectification and reversed at the expected K+ equilibrium potential after current through Na+ and Ca2+ channels had been minimized. Moreover, the current under these conditions was blocked by extracellular acidification, indicating mediation by TASK channels. An enhancement of a K+ conductance (Ries and Puil, 1999b) and the depression of postsynaptic potentials (Sugiyama et al., 1992) by halothane in thalamocortical relay neurons has been described previously. Similar to the present findings, the halothane-sensitive current in the study by Ries and Puil (1999b) also deviated from a pure K+ conductance, in that additional ions with positive equilibrium potential contributed.
Possible functional implication of TASK channel modulation
The shift from periods of synchronized electroencephalogram (EEG) activity, such as during slow wave sleep, to the desynchronized EEG pattern of wakefulness is associated with tonic depolarization of thalamocortical relay neurons (for review, see Steriade et al., 1997; Sherman and Guillery, 2001). This state-dependent change in activity mode is associated with an abolition of rhythmic burst activity in thalamocortical relay cells, enables the faithful transmission of synaptic signals through the thalamus, and is largely controlled by ascending inputs from the upper brainstem core, of which cholinergic fibers from the tegmental nuclei comprise an important part. The release of ACh has been shown to act on both nicotinic and mACh receptors, which, in the dLGN results in a strong depolarization and shift from burst to tonic firing patterns in identified X- and Y-cells (Eysel et al., 1986; McCormick and Prince, 1987; McCormick, 1992a). Activation of mACh receptors is associated with a slow depolarization due to a decrease in a leak K+ conductance (for review, see McCormick, 1992b), and the results of the present study demonstrate that TASK1/TASK 3 channels are major constituents of this response.
In contrast, activation of the same type of channels by halothane will strongly disfacilitate conditions of faithful signal transfer in the thalamocortical system, in that the hyperpolarization in relay neurons shifts the membrane out of the range of tonic action potential firing. Furthermore, the shunting effect of the increased K+ conductance on postsynaptic potentials, Na+/K+ action potentials, and low-threshold Ca2+ spikes (Sugiyama et al., 1992; Ries and Puil, 1999a), will block the transfer of all sensory and motor activity coded as tonic or burst firing (Fanselow et al., 2001; Weyand et al., 2001), resulting in analgesia, loss of awareness, and the suppression of motor activity. The 2P domain K+ channels in thalamocortical relay neurons, therefore, seem to make an important contribution to the clinical effects of inhalational anesthetics.
Footnotes
This work was supported by Deutsche Forschungsgemeinschaft (DFG) Grant BU 1019/5-1, Leibniz Program (H.-C. P.). S.G.M. was a member of the DFG-Graduiertenkolleg “Biological basis of central nervous system diseases.” Part of this work was done in partial fulfillment of a doctoral thesis by S.G.M. We thank R. Ziegler and A. Jahn for excellent technical assistance. We also thank Prof. A. Karschin for a kind gift of in situ hybridization probes for TASK1 and TASK3.
Correspondence should be addressed to Dr. Thomas Budde, Faculty of Medicine, Institute of Physiology, Otto-von-Guericke Universität, Leipziger Str.44, D-39120 Magdeburg, Germany. E-mail: thomas.budde@medizin.unimagdeburg.de.
Copyright © 2003 Society for Neuroscience 0270-6474/03/236460-•$15.00/0
S.G.M. and T.B. contributed equally to this work.
References
- BoSmith RE, Briggs I, Sturgess NC ( 1993) Inhibitory actions of ZENECA ZD7288 on whole-cell hyperpolarization activated inward current (If) in guinea-pig dissociated sinoatrial node cells. Br J Pharmacol 110: 343-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brau ME, Vogel W, Hempelmann G ( 1998) Fundamental properties of local anesthetics: half-maximal blocking concentrations for tonic block of Na+ and K+ channels in peripheral nerve. Anesth Analg 87: 885-889. [DOI] [PubMed] [Google Scholar]
- Brown DA ( 2000) Neurobiology: the acid test for resting potassium channels. Curr Biol 10: R456-R459. [DOI] [PubMed] [Google Scholar]
- Buckler KJ, Williams BA, Honore E ( 2000) An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J Physiol 525: 135-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde T, White JA ( 1998) The voltage-dependent conductances of rat neocortical layer I neurons. Eur J Neurosci 10: 2309-2321. [DOI] [PubMed] [Google Scholar]
- Budde T, Mager R, Pape H-C ( 1992) Different types of potassium outward current in relay neurons acutely isolated from the rat lateral geniculate nucleus. Eur J Neurosci 4: 708-722. [DOI] [PubMed] [Google Scholar]
- Budde T, Biella G, Munsch T, Pape H-C ( 1997) Lack of regulation by intracellular Ca2+ of the hyperpolarization-activated cation current in rat thalamic neurons. J. Physiol (Lond) 503.1: 79-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de Miera E, Rudy B ( 1999) Molecular diversity of K+ channels. Ann N Y Acad Sci 868: 233-285. [DOI] [PubMed] [Google Scholar]
- Decher N, Maier M, Dittrich W, Gassenhuber J, Bruggemann A, Busch AE, Steinmeyer K ( 2001) Characterization of TASK-4, a novel member of the pH-sensitive, two-pore domain potassium channel family. FEBS Lett 492: 84-89. [DOI] [PubMed] [Google Scholar]
- Dodt HU, Zieglgänsberger W ( 1990) Visualizing unstained neurons in living brain slices by infrared DIC-videomicroscopy. Brain Res 537: 333-336. [DOI] [PubMed] [Google Scholar]
- Eysel UT, Pape H-C, Van Schayck R ( 1986) Exitatory and differential disinhibitory actions of acetylcholine in the lateral geniculate nucleus of the cat. J Physiol (Lond) 370: 233-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow EE, Sameshima K, Baccala LA, Nicolelis MA ( 2001) Thalamic bursting in rats during different awake behavioral states. Proc Natl Acad Sci USA 98: 15330-15335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein SA, Bockenhauer D, O'Kelly I, Zilberberg N ( 2001) Potassium leak channels and the KCNK family of two-P-domain subunits. Nat Rev Neurosci 2: 175-184. [DOI] [PubMed] [Google Scholar]
- Han J, Truell J, Gnatenco C, Kim D ( 2002) Characterization of four types of background potassium channels in rat cerebellar granule neurons. J Physiol (Lond) 542: 431-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B ( 2001) Ion channels of excitable membranes. Sunderland: Sinauer.
- Huguenard JR, Prince DA ( 1991) Slow inactivation of a TEA-sensitive K current in acutely isolated rat thalamic relay neurons. J Neurophysiol 66: 1316-1328. [DOI] [PubMed] [Google Scholar]
- Huguenard JR, Coulter DA, Prince DA ( 1991) A fast transient potassium current in thalamic relay neurons: kinetics of activation and inactivation. J Neurophysiol 66: 1304-1315. [DOI] [PubMed] [Google Scholar]
- Karschin C, Wischmeyer E, Preisig-Muller R, Rajan S, Derst C, Grzeschik KH, Daut J, Karschin A ( 2001) Expression pattern in brain of TASK-1, TASK-3, and a tandem pore domain K(+) channel subunit, TASK-5, associated with the central auditory nervous system. Mol Cell Neurosci 18: 632-648. [DOI] [PubMed] [Google Scholar]
- Kindler CH, Yost CS, Gray AT ( 1999) Local anesthetic inhibition of baseline potassium channels with two pore domains in tandem. Anesthesiology 90: 1092-1102. [DOI] [PubMed] [Google Scholar]
- Komai H, McDowell TS ( 2001) Local anesthetic inhibition of voltage-activated potassium currents in rat dorsal root ganglion neurons. Anesthesiology 94: 1089-1095. [DOI] [PubMed] [Google Scholar]
- Leonoudakis D, Gray AT, Winegar BD, Kindler CH, Harada M, Taylor DM, Chavez RA, Forsayeth JR, Yost CS ( 1998) An open rectifier potassium channel with two pore domains in tandem cloned from rat cerebellum. J Neurosci 18: 868-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage F ( 2003) Pharmacology of neuronal background potassium channels. Neuropharmacology 44: 1-7. [DOI] [PubMed] [Google Scholar]
- Lesage F, Lazdunski M ( 2000) Molecular and functional properties of two-pore-domain potassium channels. Am J Physiol Renal Physiol 279: F793-F801. [DOI] [PubMed] [Google Scholar]
- Liu BG, Zhuang XL, Li ST, Xu GH, Brull SJ, Zhang JM ( 2001) Effects of bupivacaine and ropivacaine on high-voltage-activated calcium currents of the dorsal horn neurons in newborn rats. Anesthesiology 95: 139-143. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ ( 1996) The hyperpolarization-activated current (Ih) and its contribution to pacemaker activity in rat CA1 hippocampal stratum oriens-alveus interneurones. J. Physiol (Lond) 497 1: 119-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA ( 1991) Functional properties of a slowly inactivating potassium current in guinea pig dorsal lateral geniculate relay neurons. J Neurophysiol 66: 1176-1189. [DOI] [PubMed] [Google Scholar]
- McCormick DA ( 1992a) Cellular mechanisms underlying cholinergic and noradrenergic modulation of neuronal firing mode in the cat and guinea pig dorsal lateral geniculate nucleus. J Neurosci 12: 278-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA ( 1992b) Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog Neurobiol 39: 337-388. [DOI] [PubMed] [Google Scholar]
- McCormick DA ( 1993) Actions of acetylcholine in the cerebral cortex and thalamus and implications for function. Prog Brain Res 98: 303-308. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Prince DA ( 1987) Actions of acetylcholine in the guineapig and cat medial and lateral geniculate nuclei, in vitro J Physiol (Lond) 392: 147-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Prince DA ( 1988) Noradrenergic modulation of firing pattern in guinea pig and cat thalamic neurons, in vitro J Neurophysiol 59: 978-996. [DOI] [PubMed] [Google Scholar]
- Meadows HJ, Randall AD ( 2001) Functional characterisation of human TASK-3, an acid-sensitive two-pore domain potassium channel. Neuropharmacology 40: 551-559. [DOI] [PubMed] [Google Scholar]
- Meuth S, Pape H-C, Budde T ( 2002) Modulation of Ca2+ currents in rat thalamocortical relay neurons by activity and phosphorylation. Eur J Neurosci 15: 1603-1614. [DOI] [PubMed] [Google Scholar]
- Millar JA, Barratt L, Southan AP, Page KM, Fyffe RE, Robertson B, Mathie A ( 2000) A functional role for the two-pore domain potassium channel TASK-1 in cerebellar granule neurons. Proc Natl Acad Sci USA 97: 3614-3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munsch T, Pape H-C ( 1999) Modulation of the hyperpolarization-activated cation current of rat thalamic relay neurones by intracellular pH. J Physiol (Lond) 519: 493-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E ( 1992) Correction for liquid junction potentials in patch-clamp experiments. Methods Enzymol 207: 123-131. [DOI] [PubMed] [Google Scholar]
- Parri HR, Crunelli V ( 1998) Sodium current in rat and cat thalamocortical neurons: role of a noninactivating component in tonic and burst firing. J Neurosci 18: 854-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AJ, Honore E, Lesage F, Fink M, Romey G, Lazdunski M ( 1999) Inhalational anesthetics activate two-pore-domain background K+ channels. Nat Neurosci 2: 422-426. [DOI] [PubMed] [Google Scholar]
- Rajan S, Wischmeyer E, Xin Liu G, Preisig-Muller R, Daut J, Karschin A, Derst C ( 2000) TASK-3, a novel tandem pore domain acid-sensitive K+ channel. An extracellular histiding as pH sensor. J Biol Chem 275: 16650-16657. [DOI] [PubMed] [Google Scholar]
- Ries CR, Puil E ( 1999a) Mechanism of anesthesia revealed by shunting actions of isoflurane on thalamocortical neurons. J Neurophysiol 81: 1795-1801. [DOI] [PubMed] [Google Scholar]
- Ries CR, Puil E ( 1999b) Ionic mechanism of isoflurane's actions on thalamocortical neurons. J Neurophysiol 81: 1802-1809. [DOI] [PubMed] [Google Scholar]
- Ruppersberg JP ( 2000) Intracellular regulation of inward rectifier K+ channels. Pflügers Arch 1-21. [DOI] [PubMed]
- Sherman SM, Guillery RW ( 1996) Functional organization of thalamocortical relays. J Neurophysiol 76: 1367-1395. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW ( 2001) Exploring the thalamus. San Diego: Academic.
- Steriade M ( 1997) Synchronized activities of coupled oscillators in the cerebral cortex and thalamus at different levels of vigilance. Cereb Cortex 7: 583-604. [DOI] [PubMed] [Google Scholar]
- Steriade M, Jones EG, McCormick DA ( 1997) Thalamus. Amsterdam: Elsevier.
- Stork O, Welzl H, Wolfer D, Schuster T, Mantei N, Stork S, Hoyer D, Lipp H, Obata K, Schachner M ( 2000) Recovery of emotional behaviour in neural cell adhesion molecule (NCAM) null mutant mice through transgenic expression of NCAM180. Eur J Neurosci 12: 3291-3306. [DOI] [PubMed] [Google Scholar]
- Sugiyama K, Muteki T, Shimoji K ( 1992) Halothane-induced hyperpolarization and depression of postsynaptic potentials of guinea pig thalamic neurons in vitro Brain Res 576: 97-103. [DOI] [PubMed] [Google Scholar]
- Takenoshita M, Steinbach JH ( 1991) Halothane blocks low-voltage-activated calcium current in rat sensory neurons. J Neurosci 11: 1404-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley EM, Lei Q, Sirois JE, Bayliss DA ( 2000) TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron 25: 399-410. [DOI] [PubMed] [Google Scholar]
- Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA ( 2001) CNS distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci 21: 7491-7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn CP, Sirois JE, Talley EM, Guyenet PG, Bayliss DA ( 2002) Serotonergic raphe neurons express TASK channel transcripts and a TASK-like pH- and halothane-sensitive K+ conductance. J Neurosci 22: 1256-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins CS, Mathie A ( 1996) A non-inactivating K+ current sensitive to muscarinic receptor activation in rat cultured cerebellar granule neurons. J Physiol (Lond) 491: 401-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand TG, Boudreaux M, Guido W ( 2001) Burst and tonic response modes in thalamic neurons during sleep and wakefulness. J Neurophysiol 85: 1107-1118. [DOI] [PubMed] [Google Scholar]
- Williams SR, Turner JP, Hughes SW, Crunelli V ( 1997) On the nature of anomalous rectification in thalamocortical neurones of the cat ventro-basal thalamus in vitro. J Physiol (Lond) 505: 727-747. [DOI] [PMC free article] [PubMed] [Google Scholar]