Abstract
Interneurons in the olfactory bulb (OB) are generated not only in the developing embryo but also throughout the postnatal life of mammals from neuronal precursor cells migrating from the anterior subventricular zone (SVZa) of the mammalian forebrain. We discovered that the OB secretes a diffusible activity that attracts these neuronal precursor cells. The attractive activity is present in specific layers in the OB, including the glomerular layer but not the granule cell layer. The attractive activity and the neuronal responsiveness persist from embryonic through neonatal to adult stages. Removal of the rostral OB significantly reduces SVZa migration toward the OB, an effect that can be rescued by a transplant of the OB but not by that of the neocortex. The activity in the OB is not mimicked by the known attractants. These results provide an explanation for the continuous migration of SVZa neurons toward the OB, demonstrate an important role of the OB in neuronal migration, and reveal the existence of a new chemoattractant.
Keywords: neuronal migration, olfactory bulb, attraction, precursor cells, olfaction, neural development
Introduction
The olfactory bulb (OB) plays a central role in relaying olfactory information from the olfactory epithelium to the olfactory cortex (Farbman, 1991; Greer, 1991; Axel, 1995; Buck, 1996; Dulac, 1997; Mori et al., 1999; Mombaerts, 2001; Marin et al., 2002; Wong et al., 2002). A fascinating feature of the OB known for a long time is that the OB is continuously supplied with newly generated interneurons even during the adult life.
The major interneurons in the OB, the periglomerular and granule cells, are derived from neuronal precursor cells that migrate from the lateral ganglionic eminences (LGE) in the embryo (Wichterle et al., 2001). Postnatally, they are derived from neuronal precursor cells that migrate in the rostral migratory pathway (RMS) from the anterior subventricular zone (SVZa) in the lateral ventricles of the forebrain (Hinds, 1968; Altman, 1969; Bayer, 1983; Corotto et al., 1993; Luskin, 1993; Lois et al., 1994, 1996). The continuous generation of SVZa neurons and their migration to the OB in adult mammals has been found not only in rodents (Altman and Das, 1966; Hinds, 1968; Altman, 1969; Bayer, 1983; Lois et al., 1994, 1996) but also in primates including New World monkeys (McDermott and Lantos, 1990), Old World monkeys (Lewis, 1968; Kaplan, 1983; Gould et al., 1999; Kornack and Rakic, 2001; Pencea et al., 2001), and humans (Pincus et al., 1998; Kukekov et al., 1999; Weickert et al., 2000). In primates, SVZa neurons have to migrate several centimeters to reach the OB (Kornack and Rakic, 2001; Pencea et al., 2001). Persistent neuronal migration has also been demonstrated in other regions of the brain (Eriksson et al., 1998); therefore, the OB provides a useful model to understand a process of general importance in the brain (Altman and Das, 1966; Hinds, 1968; Altman, 1969; Bayer, 1983; Kishi, 1987; Luskin, 1993; Lois and Alvarez-Buylla, 1994; Menezes et al., 1995; Alvarez-Buylla, 1997; Goldman and Luskin, 1998; Wichterle et al., 2001).
Despite the importance of neuronal migration to the OB, its mechanisms are still not well understood. At the cellular level, the septum in the forebrain and the ventricular zone contains repulsive activities for the SVZa neurons (Hu and Rutishauser, 1996; Zhu et al., 1999). At the molecular level, the polysialylated neural cell-adhesion molecule is important for SVZa migration (Tomasiewicz et al., 1993; Bonfanti and Theodosis, 1994; Cremer et al., 1994; Ono et al., 1994; Rousselot et al., 1995; Hu et al., 1996; Wichterle et al., 1997; Chazal et al., 2000). Recent evidence suggests that Slit, a secreted repellent for axons (Brose et al., 1999; Kidd et al., 1999; Li et al., 1999; Wong et al., 2002), is expressed in the septum and ventricular zone (Wu et al., 1999; Zhu et al., 1999) and can repel both the postnatal SVZa neurons (Hu, 1999; Wu et al., 1999) and embryonic LGE neurons (Zhu et al., 1999). However, there is no evidence that Slit can guide neuronal migration in the entire RMS (Wu et al., 1999; Chen et al., 2001). In vitro, Slit can only act on neurons within the distance of 1 mm (Wu et al., 1999), and it is possible that Slit is involved in driving newly generated neuronal precursor cells from the subventricular zone (Zhu et al., 1999) but not in guiding their migration later in the pathway. It is therefore unknown how neuronal precursor cells are targeted to the OB. Here, we report that the OB contains a diffusible attractant for the SVZa cells, which is in contrast to previous conclusions that the OB has neither attractive nor repulsive activities (Hu and Rutishauser, 1996; Kirschenbaum et al., 1999). Removal of the rostral OB significantly reduces the migration of SVZa cells in the RMS toward the OB. This chemotropic activity in the OB persists from embryonic to adult brains. Our results demonstrate a critical role for the OB-derived attractive activity in directing the SVZa neurons to the OB.
Materials and Methods
Animals. Timed pregnant and adult Sprague Dawley rats were obtained from Charles River Laboratories (Wilmington, MA). For postnatal staging, the date of birth was regarded as postnatal day (P) 0.
Dissection of explants and coculture experiments. Brains from prenatal and postnatal rats were dissected and embedded in 4% low melting-point agarose prepared in PBS. Coronal and sagittal sections of 200-300 μm were obtained by a vibratome. Tissues from the SVZa, RMS, and subventricular zone of the LGE were isolated, as described previously (Wu et al., 1999; Zhu et al., 1999), and trimmed into blocks of 100-200 μm. The trimmed tissues and explants were embedded in the mixture gel (3:2:1 of collagen:matrigel:medium) and cultured with DMEM (Invitrogen, San Diego, CA) containing 10% fetal calf serum (FCS) and 100 U/ml of penicillin and 100 μg/ml of streptomycin at 37°C in an incubator with 5% CO2 for 12-18 hr. When coculturing the different layers of the OB with the SVZa, OB explants were wrapped in a membrane filter (0.45 μm; Millipore, Bedford, MA) to prevent cells from migrating from the granular cell layer (GCL) of the OB, which interfered with the observation and counting of cells migrating from the SVZa or the RMS. Explants from different layers of the OB were made, and some were used for the coculture experiments whereas others were fixed in 4% paraformaldehyde (PFA) at 4°C overnight. The 20 μm sections were made by cryostat sectioning of frozen samples. Cresyl violet was used to stain these sections.
For coculturing cell aggregates with neural explants, we used human embryonic kidney (HEK) cells stably expressing netrin-1 and stromal derived factor (SDF-1) and HEK cells transiently transfected with Semaphorin 3A, Semaphorin 3B, and β-netrin cDNA. Aggregates of cells were made by the hanging-drop method (Fan and Tessier-Lavigne, 1994).
Slice assay. Sagittal sections of postnatal rat brains of 200 μm were cut with a vibratome. Those containing the OB, RMS, SVZa, septum, and entire neocortex were collected and transferred onto a piece of Millipore filter (HABG 01300; 0.45 μm pore size; 13 mm diameter). A small piece of 1,1-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) crystal (Molecular Probes, Eugene, OR) was inserted into the middle part of the RMS. The slices were cultured with DMEM containing 10% FCS, 100 U/ml of penicillin, and 100 μg/ml of streptomycin at 37°C in an incubator with 5% CO2 for 15-20 hr. The tip of the OB was removed by using a tungsten needle. In the rescue experiments, we transplanted either a piece of the neocortex or a tip of the OB from another slice to a slice from which the tip of the OB was removed.
Immunocytochemistry. Tissues were fixed with 4% PFA overnight at 4°C. After washing in TBST (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 0.1% Triton X-100) three times, the explants were treated by the blocking buffer (5% normal goat serum in TBST) for 1 hr at room temperature. Incubation with the primary antibody [mouse monoclonal class III β-tubulin (TuJ1) at 1:200, or rabbit polyclonal anti-GABA antibody at 1:1000, in TBST containing 5% BSA] was performed overnight at 4°C. After three washes in TBST, goat anti-mouse conjugated to Cy3 and goat anti-rabbit conjugated to Cy2 in TBST was added for 1 hr at room temperature and washed before being mounted with glycerol.
Quantitative analysis. Explants were fixed with 4% PFA and stained with Hoeschst 33258 (Sigma, St. Louis, MO). Images of Hoeschst-stained cells were obtained from a Spot digital camera and saved in the computer. The number of cells in the proximal and distal quadrant was counted, and the proximal/distal ratios were calculated as described previously (Zhu et al., 1999). Images of DiI-labeled cell migrating in slices were taken and stored. Anterior/posterior ratios were calculated from the number of cells in the part anterior to the DiI insertion site divided by those in the part posterior to the DiI insertion site. Data were analyzed using Student's t test.
Results
The presence of a chemoattractive activity in the OB
In our efforts to study mechanisms guiding neuronal migration to the OB, we tested whether the OB could regulate SVZa migration by using explant coculture assays (Hu and Rutishauser, 1996; Wu et al., 1999). When explants of the SVZa from P4 rats were cultured for 24 hr in the mixture gel, cells migrating from the explants were distributed symmetrically around the circumference of each explant (Fig. 1B). In contrast, after SVZa explants were cocultured with the tip of P4 OB, the distribution of migrating cells was asymmetric with a higher number of cells in the quadrant proximal to the OB than that in distal quadrant (with asymmetric migration observed in 539 of 627 explants) (Fig. 1D). When SVZa explants were cocultured with an explant of the neocortex, the distribution of migrating cells was symmetric (129 of 137 explants) (Fig. 1C). To quantify the distribution of migrating cells, the area surrounding each explant was divided into four quadrants; the number of cells in the proximal quadrant was compared with that in the distal quadrant. The proximal/distal ratio of cells migrating from SVZa-OB coculture is significantly higher than that of SVZa-neocortical explant cocultures (Fig. 1H). The neuronal and GABAergic nature of the cells migrating from the SVZa explants was confirmed by immunocytochemistry with the TuJ1 antibody and the anti-GABA antibody (Fig. 1E-G). These results indicate that the OB contained a chemoattractive activity for SVZa neurons.
Figure 1.

Presence of a chemoattractive activity in the OB. A, A diagram of the sagittal section of neonatal rat forebrain. X, SVZa; Y, region of neocortex from which cortical explants were isolated and used in C; Z, tip of the OB from which explants were isolated and used in D-G. B, An SVZa explant was cultured for 24 hr. Cells migrated symmetrically from the explant. C, Coculture of an SVZa explant with a neocortical explant (CTX). Migrating SVZa cells were symmetrically distributed around the circumference of the SVZa explant (n = 129 of 137). D, Effect of the OB on the migration of cells from SVZa explants. Asymmetric distribution of migrating cells with a higher number of cells in the quadrant proximal to the OB than that in distal quadrant (n = 539 of 627) is shown. Scale bar, 100 μm. E, The same explant as in D, showing staining with the TuJ1 antibody. F, The same explant as in D, showing staining with anti-GABA antibodies. G, Superimposition of E and F. H, Effects of cortical and OB explants on the distribution of SVZa cell migration. Proximal/distal ratios were calculated from the numbers of cells in the proximal quadrants divided by those in the distal quadrants. Cell numbers were counted from 33 cocultures with the OB explants and 16 cortical (CTX) explants. CTX, Cortex.
To determine whether the OB is only attractive to cells at the SVZa or also to cells migrating along the RMS, the tip of P4 OB was also cocultured with explants from either the anterior or the posterior parts of the RMS (Fig. 2A). The OB attracted cells migrating both in the anterior and posterior parts of the RMS (with 312 asymmetric explants of 372 anterior RMS explants, and 236 asymmetric explants of 291 posterior RMS explants) (Fig. 2). These results indicate the OB is attractive to SVZa cells both at their origin and migrating within the RMS.
Figure 2.
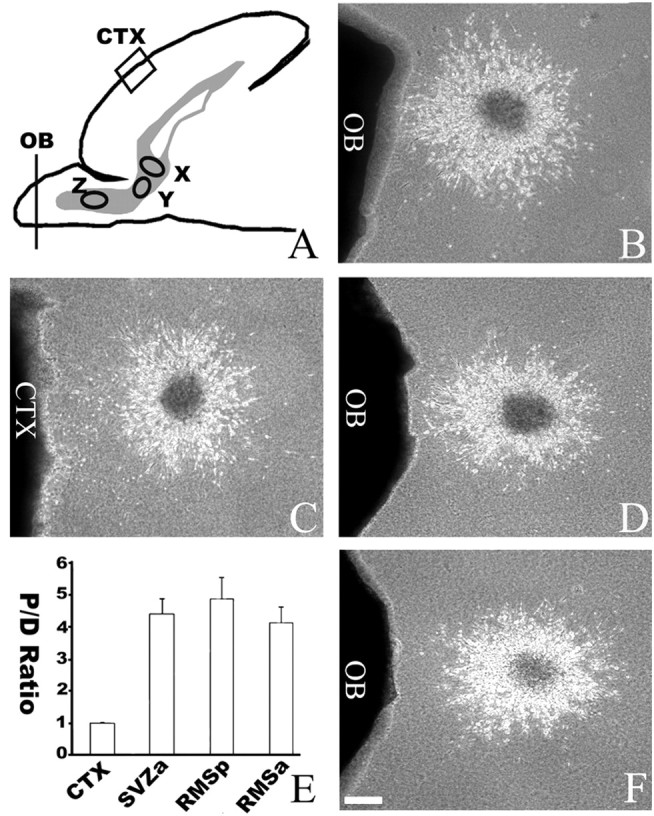
Attractive effects of the OB on cells migrating along the RMS. A, A diagram of the sagittal section of neonatal rat forebrain showing different parts of the RMS from which explants were isolated and cocultured. X, SVZa; Y, posterior part of RMS; Z, anterior part of the RMS. B, Asymmetric distribution of cells migrating from an SVZa explant cocultured with the OB. C, Symmetric distribution of cells migrating from an SVZa explant cocultured with a cortical explant. D, Asymmetric distribution of cells migrating from an explant from the posterior part of the RMS cocultured with the OB (n = 236 of 291). E, Effects of cortical and OB explants on the distribution of cells migrating out of explants of the SVZa and RMS. The cell numbers were counted from 33 SVZa explants, 32 posterior RMS explants, and 36 anterior RMS explants. CTX, Cortex; RMSp, posterior RMS; RMSa, anterior RMS; P/D, proximal/distal. F, Asymmetric distribution of cells migrating from an anterior RMS explant cocultured with the OB (n = 312 of 372). Scale bar, 100 μm.
Persistent chemoattractive activity in the OB and neuronal responsiveness from embryonic to adult stages
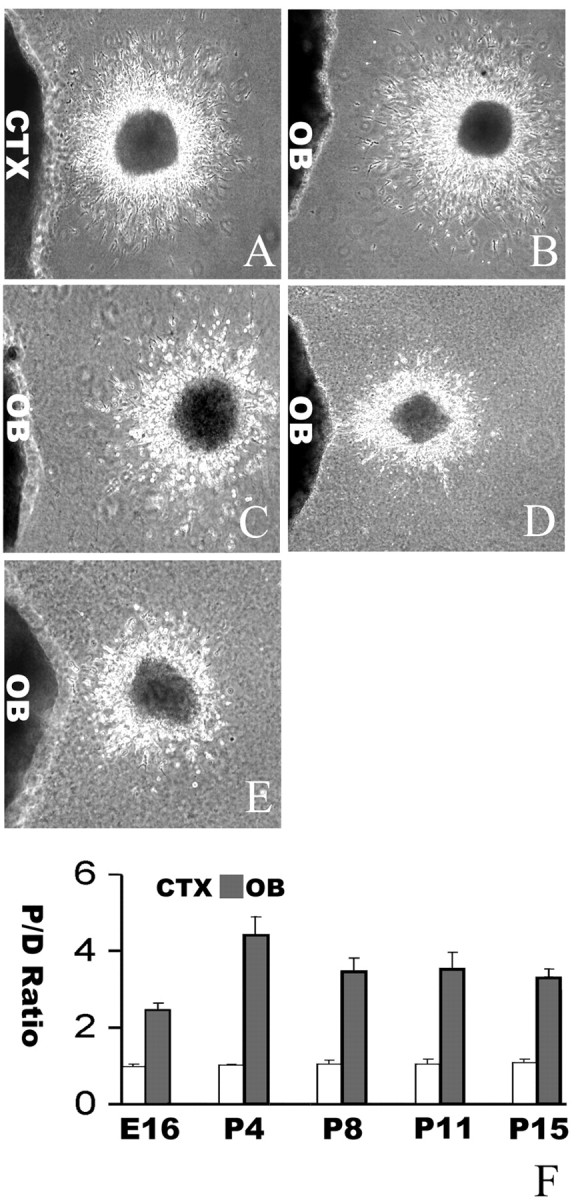
We examined the stages when the chemoattractive activity in the OB and neuronal responsiveness to the OB are present. We isolated explants from the OB at different stages [embryonic day (E) 16, E20, P4, P8, P11, and adult] and cocultured them separately with SVZa explants from P4 rat brains. SVZa cells were migrated toward the OB explants from all stages (89 explants of 98 SVZa explants were asymmetric when cocultured with the E16 OB; 103 explants of 108 SVZa explants were asymmetric when cocultured with the E20 OB; 539 explants of 627 SVZa explants were asymmetric when cocultured with the P4 OB; 137 explants of 146 SVZa explants were asymmetric when cocultured with the P8 OB; 112 explants of 132 SVZa explants were asymmetric when cocultured with the P11 OB; and 64 explants of 80 SVZa explants were asymmetric when cocultured with the adult OB) (Fig. 3).
Figure 3.

Persistent chemoattractive activity in the OB from embryonic to adult stages. A, Effect of an E16 rat cortical explant on cells migrating from a P4 SVZa explant. B, Effect of an E16 OB explant on cells migrating from a P4 SVZa explant. C, Effect of an E20 OB explant on cells migrating from a P4 SVZa explant. D, Effect of a P4 OB explant on cells migrating from a P4 SVZa explant. E, Effect of a P8 OB explant on cells migrating from a P4 SVZa explant. F, Effect of a P11 OB explant on cells migrating from a P4 SVZa explant. G, Effect of an adult OB explant on cells migrating from a P4 SVZa explant. Scale bar, 100 μm. H, The proximal/distal (P/D) ratios of cell numbers were counted from 21 cortical and 31 E16 OB explants of E16 rats, 25 cortical and 25 OB explants of E20 rats, 16 cortical and 33 OB explants of P4 rats, 26 cortical and 22 OB explants of P8 rats, 22 cortical and 19 OB explants of P11 rats, and 26 cortical and 25 OB explants of adult rats. The difference between cortical and OB explants was statistically very significant (p < 0.0001). CTX, Cortex.
When the P4 SVZa explants were cocultured with explants for the neocortex from different stages, the SVZa cells migrated symmetrically surround the explants (42 explants of 46 SVZa explants were symmetric when cocultured with the E16 neocortex; 42 explants of 43 SVZa explants were symmetric when cocultured with the E20 neocortex; 129 explants of 137 SVZa explants were symmetric when cocultured with the P4 neocortex; 86 explants of 95 SVZa explants were symmetric when cocultured with the P8 neocortex; 58 explants of 64 SVZa explants were symmetric when cocultured with the P11 neocortex; and 55 explants of 59 SVZa explants were symmetric when cocultured with the adult neocortex) (Fig. 3). Quantitative analysis of the proximal/distal ratios indicated very significant differences between the OB and the neocortical explants at all six stages (p < 0.001) (Fig. 3). The result suggests that the chemoattractive activity in the OB was persisted and has an effect on neuronal migration during brain development.
We also examined the responsiveness of neuronal precursor cells migrating to the OB by culturing neuronal explants of different stages with explants of P4 OB. During embryogenesis, interneurons in the OB migrated in the RMS from the LGE (Wichterle et al., 2001). When E16 LGE explants were cocultured with explants of either P4 OB or neocortex, they were attracted by the OB (Fig. 4B) (141 asymmetric explants of 206 explants) but not by the neocortex (Fig. 4A) (113 symmetric explants of 128 explants). When the number of cells was counted in proximal and distal quadrants, the proximal/distal ratio for cells migrating in LGE cocultures with the OB explants is significantly different from that in cocultures of LGE with neocortical explants (p < 0.001). Similarly, P4 OB was attractive to SVZa of P8, P11, and P15 rats (Fig. 4) (number of asymmetric explants of the total explants: 157 of 164 for P8, 62 of 68 for P11, and 132 of 146 for P15). Explants of the P4 neocortex did not attract or repel cells from any stages of SVZa (number of symmetric explants of the total explants: 78 of 80 for P8, 78 of 86 for P11, and 87 of 90 for P15). These results indicate that the neurons remain responsive to the OB throughout embryonic and postnatal stages.
Figure 4.
Persistent neuronal responsiveness to the OB chemoattractant(s). A, Effect of a P4 cortical explant on cells migrating from an E16 LGE explant. B, Effect of a P4 OB explant on cells migrating from an E16 LGE explant. C, Effect of a P4 OB explant on cells migrating from a P8 SVZa explant. D, Effect of a P4 OB explant on cells migrating from a P11 SVZa explant. E, Effect of a P4 OB on cells migrating from a P15 SVZa explant. Scale bar, 100 μm. F, The proximal/distal (P/D) ratios of cell numbers were counted from 25 P4 cortical and 21 P4 OB cocultures with E16 LGE explants, 16 cortical and 33 OB cocultures with P4 SVZa explants, 32 cortical and 30 OB cocultures with P8 SVZa explants, 24 cortical and 21 OB cocultures with P11 SVZa explants, and 25 cortical and 24 OB cocultures with P15 SVZa explants. The difference between cortical and OB cocultures was statistically very significant (p < 0.0001).
Presence of the chemoattractive activity in the glomerular layer and other layers in the OB
The OB is a distinctly laminated structure composed of five layers, including the glomerular layer (GL), external plexiform layer, mitral cell layer (MCL), internal plexiform layer, and granular cell layer (Fig. 5). To determine the layers containing the chemoattractive activity, sagittal sections of P4 rat brains were made. After repeated efforts, it was possible to separate the layers in three parts: GL (Fig. 5F,G), GCL (Fig. 5J), and MCL in combination with part of the external and internal plexiform layers (Fig. 5H,I). It was not possible to separate MCL from the plexiform layers, and the results shown below for simplicity for MCL are those for the mitral layer and plexiform layers.
Figure 5.
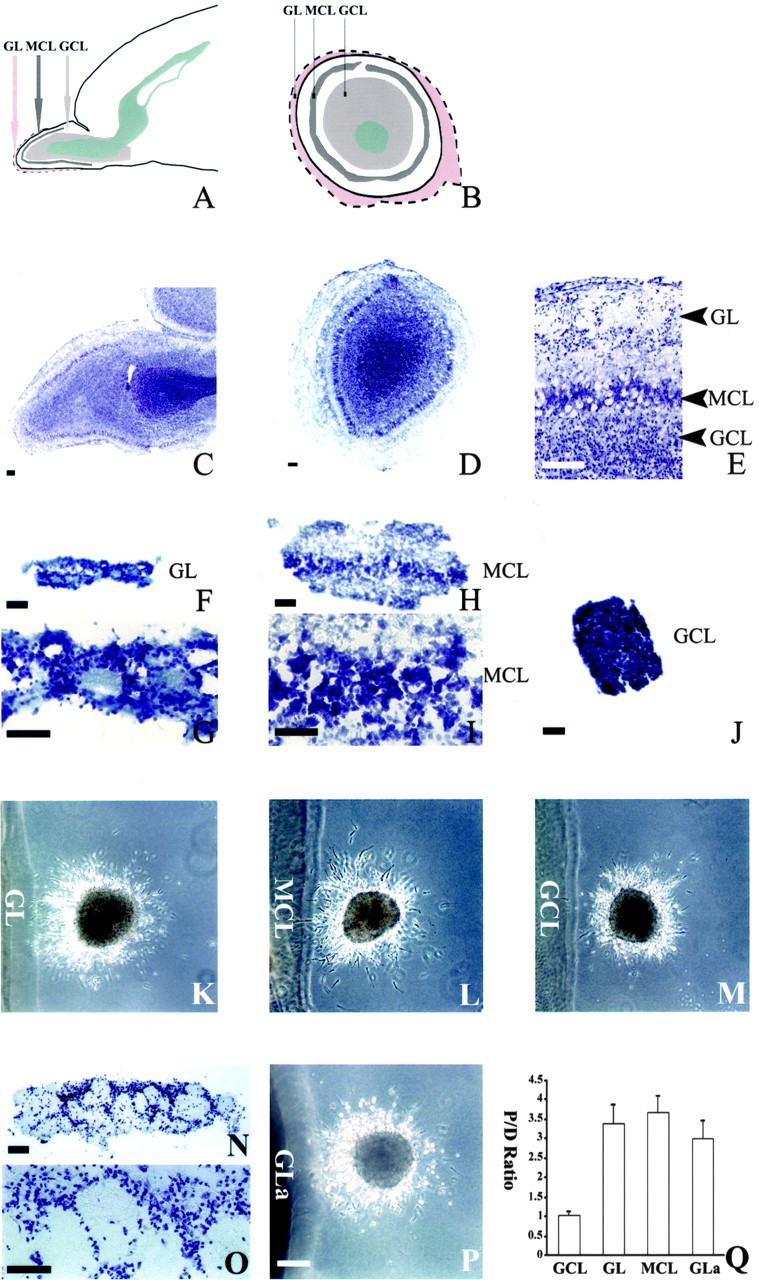
Dissection of OB layers with the chemoattractive activity. A, A diagram of the sagittal section of neonatal forebrain showing the anatomic layers of the OB. B, A diagram of the coronal section of the neonatal OB (outlined by dashed line). The layer between the GL and MCL is the external plexiform layer, whereas the layer between the MCL and GCL is the internal plexiform layer. C, A sagittal slice of the P4 OB stained with cresyl violet showing the different layers in the OB. D, A coronal slice of P4 OB stained with cresyl violet. E, A higher magnification view of C showing the GL, external plexiform layer, MCL, internal plexiform layer, and GCL. F, A GL explant dissected from a P4 OB was stained with cresyl violet. G, A higher magnification view of F showing that it contains GL. H, An explant dissected from a P4 OB was stained with cresyl violet, showing that it contains the external plexiform layer, MCL, and internal plexiform layer. I, A higher magnification view of H. J, A GCL explant from a P4 OB was stained with cresyl violet, showing that it contains only GCL but not the other layers in the OB. K, Effect of the GL explants on cells migrating from a P4 SVZa explant (n = 66 of 74). L, Effect of the MCL explants (including the MCL and parts of the external and internal plexiform layers) on cells migrating from a P4 SVZa explant (n = 58 of 61). M, Effect of GCL explants on cells migrating from a P4 SVZa explant (n = 69 of 77). N, A GL explant dissected from the adult OB was stained with cresyl violet, showing that it contains only the GL. O, A higher magnification view of N. P, Effect of an adult GL explant on cells migrating from a P4 SVZa explant (n = 87 of 93). GLa, Adult GL. Q, Effects of different layers from the OB on the distribution of cells migrating out of the SVZa explants. The cell numbers were counted from 24 P4 GCL explants, 26 P4 GL explants, 22 P4 MCL explants, and 25 adult GL (GLa) explants. Scale bars, 100 μm.
When the GCL from P4 brains was cocultured with RMS explants of the same stage, the distribution of migrating neurons was symmetric (69 symmetric explants of 77 total explants) (Fig. 5M,Q). In contrast, both the GL (Fig. 5K,Q) and the MCL explants were attractive (number of asymmetric explants of the total explants: 66 of 74 for the GL, and 58 of 61 for the MCL) (Fig. 5L,Q). The proximal/distal ratio of GCL cocultures is significantly different from those with the GL and the MCL (p < 0.001). We also dissected the GL from the OB of adult rats. The adult GL can be very cleanly dissected without any other layers (Fig. 5N,O). It contained the attractive activity, with 87 asymmetric explants of 93 explants (Fig. 5P,Q). These results indicate that the GL is attractive throughout the stages, whereas the GCL is not. There is also an attractive activity in the mitral layer or the external or internal plexiform layers, although it is not possible to locate the source because of physical limit in dissection.
Essential role of the OB in directing neuronal migration in the RMS
Although the in vitro coculture assays provided strong evidence that the OB is attractive to the neurons migrating in the RMS from the SVZa toward the OB, they could not establish the role of the OB in vivo. To investigate a possible role of the OB in directing neuronal migration in the RMS, we used the slice assay in which a sagittal section of the P4 rat brain containing the entire RMS from the SVZa to the OB was cultured (Fig. 6A) (Wu et al., 1999; Zhu et al., 1999). A crystal of the lipophilic dye DiI was inserted into the RMS to label neuronal precursors migrating in the RMS from the SVZa to the OB (Fig. 6B) (Wu et al., 1999; Zhu et al., 1999). DiI crystals placed at the juncture of the anterior and posterior parts of the RMS revealed cells migrating both anteriorly and posteriorly (Fig. 6B). This allows us to measure the ratio of cells migrating anteriorly over those migrating posteriorly (the anterior/posterior ratio). There are normally more cells migrating anteriorly toward the OB with an anterior/posterior ratio of ∼2 (Fig. 6B,D). When the rostral end of the OB was removed (Fig. 6A), the distribution of migrating cells was changed with an anterior/posterior ratio of ∼0.6 (Fig. 6C,D).
Figure 6.
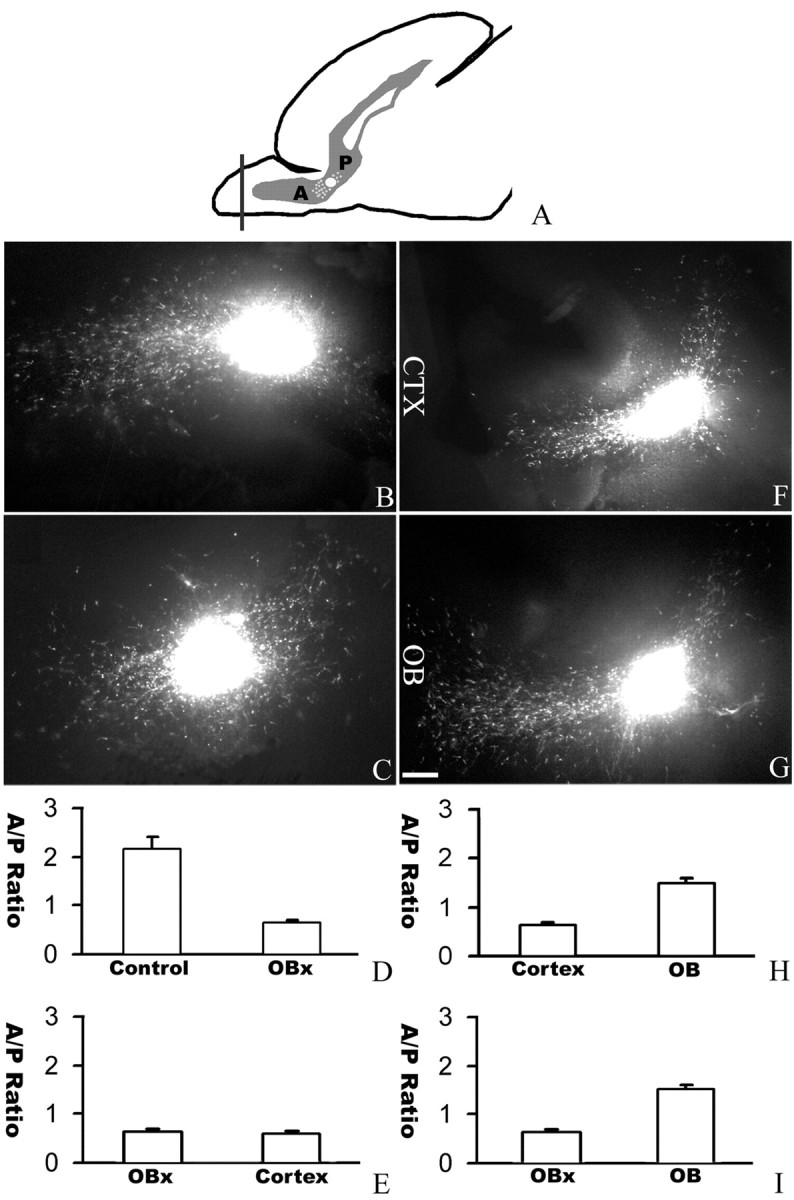
An essential role of the OB in directing neuronal migration in the RMS. A, A diagram of the sagittal section of a neonatal forebrain showing the position of DiI insertion at the juncture of the anterior (A) and posterior (P) parts of the RMS. B, In a normal slice, more cells migrating anteriorly toward the OB. C, When the rostral end of the OB was removed, the distribution of migrating cells was changed with reduced anterior migration and increased posterior migration. D, Effect of OB removal in directing neuronal migration in the RMS. The cell numbers were counted from 20 intact sagittal slices and 32 sagittal slices without the rostral end of the OB. E, Cortical transplants could not functionally replace the rostral end of the OB in directing anterior migration. The cell numbers were counted from 32 slices with the rostral end of their OB removed and nine cortical transplants placed at the rostral end of the OB. F, When a cortical transplant was used to replace the rostral end of the OB, the distribution of migrating cells could not be rescued. G, When an OB transplant was placed at the rostral end of the OB after the original rostral end of the OB was removed, the distribution of migrating cells was changed with more cells migrating anteriorly toward the OB. H, I, The cell numbers were counted from 32 sagittal slices without the rostral end of the OB, nine cortical transplants, and seven OB transplants placed at the rostral end of the OB. CTX, Cortex; A/P, anterior/posterior. Scale bar, 100 μm.
To rule out that the observed effect was attributable to mechanic damage, we placed an explant of the OB or the neocortex to the anterior end of the slices from which the rostral OB was removed. The transplanted OB was able to rescue the defect of OB removal and attract more cells to migrate anteriorly (Fig. 6F,G). The anterior/posterior ratio after the placement of an OB transplant was 1.5, which was significantly different from the anterior/posterior ratio of 0.6 after the placement of a neocortical transplant (Fig. 6E,H,I). Together, results from the OB removal and rescue experiments demonstrated an essential role for the OB in directing neurons in the RMS migrating toward the OB in their natural pathway.
Inability of known chemoattractants to mimic the attractive activity in the OB
We tested whether any of the known neuronal chemoattractants can mimic the activity in the OB. There are five known families of molecules that can guide axon projection and neuronal migration. Netrins are the best known neuronal chemoattractant but can function as chemorepellents for some axons (Colamarino and Tessier-Lavigne, 1995). Semaphorins usually function as chemorepellents but can also be attractants (Kolodkin, 1996; Raper, 2000). Ephrins and Slit are axonal repellents (Flanagan and Vanderhaeghen, 1998; Klein, 2001; Wong et al., 2002). Chemokines, originally identified as attractants for leukocytes, are also attractants for axons and neurons (Klein et al., 2001; Lu et al., 2001; Bagri et al., 2002; Lu et al., 2002; Xiang et al., 2002; Zhu et al., 2002).
Because Slit is known to repel SVZa neurons (Wu et al., 1999) and the ephrins are membrane-attached repellents, they are not candidates for the OB attractant(s). We tested whether representative members of the other three families could be attractants for the SVZa neurons by coculturing SVZa explants with aggregates of HEK cells transfected with cDNA expressing netrin-1, sema3, and the chemokine SDF-1. Although netrin-1 could attract axons from the dorsal spinal cord (Fig. 7A), it repelled SVZa neurons (Fig. 7B). β-netrin, another member of the netrin family (Koch et al., 2000; Yin et al., 2000), did not affect SVZa migration (data not shown). SDF-1 attracted cerebellar precursor neurons from the upper rhombic lip (Fig. 7C), but neither attracted nor repelled SVZa neurons (Fig. 7D). Sema 3A repelled axons from the dorsal root ganglion (Fig. 7E) but did not affect SVZa migration (Fig. 7F). Sema 3B did not affect SVZa migration (data not shown). These results suggest that the OB attractant is unlikely to be a known neuronal attractant.
Figure 7.

Inability of known chemoattractants to mimic the attractive activity in the OB. A, Chemoattraction of E12 commissural axons by netrin-1 expressed from a stable HEK cell line. dSP, Dorsal spinal cord. B, Chemorepulsion of SVZa cells by netrin-1 from the same HEK cell line as those used in A. C, Chemoattraction of E15 upper rhombic lip (URL) by SDF-1 expressed from a stable HEK cell line. D, Absence of guidance activity on SVZa neurons by SDF-1 from the same cell line as that in C. E, Chemorepulsion of E14 dorsal root ganglion (DRG) axon by Sema 3A transiently expressed from an HEK cell line. F, Absence of guidance activity on SVZa neurons by Sema 3A transiently expressed from an HEK cell line. Scale bar, 100 μm.
Discussion
Our results provide the first evidence that the OB secretes a chemotropic activity, and that this activity is important for guiding neurons migrating rostrally from the SVZa toward the OB. The persistence of the attractive activity in the OB from embryonic to adult stages suggests a mechanism that underlies the continuous migration of neurons into the OB. Because newly generated neurons and their migration are important for neural function and plasticity in the olfactory (Gheusi et al., 2000; Cecchi et al., 2001) and other systems (Shors et al., 2001), the finding of the OB activity not only helps our understanding of OB development but also reveals a new process that can potentially be involved in regulating olfactory signaling and plasticity.
Essential role of an attractive activity in the OB
Despite its central role in olfactory information processing, mechanisms that control OB development and plasticity are not well understood. The migration of SVZa cells through the RMS to the OB is important for OB development. A continuous supply of new neurons may allow maximization of odor discrimination, adaptation to changing environmental conditions, and renewing of memories (Gheusi et al., 2000; Cecchi et al., 2001). Our results indicate that cell migration toward the OB is controlled by attractant(s) in the OB.
Our previous studies suggested that the secreted protein Slit can repel the SVZa neurons and its expression in the ventricular zone in the SVZa, and other areas may drive SVZa neurons from their origin and keep them in the normal migratory pathways (Wu et al., 1999; Zhu et al., 1999; Wong et al., 2001). Our present study of the OB complements previous studies in revealing an attractant in the OB. It appears that there is both a force to push and a force to pull the SVZa cells toward the OB. The localization of the activity in the glomerular and mitral cell layers also suggests a mechanism underlying the targeting of the SVZa cells into specific layers in the OB.
A previous study with cocultures of the OB and the SVZa led to the conclusion that the OB could not attract or repel SVZa cells (Hu and Rutishauser, 1996). Although the exact explanations for the different results are not clear, the size and position of explants used in the cocultures may contribute to the differences. SVZa explants (100-200 μm in diameter) used in our cocultures are small, whereas the OB explants are 3-4 times bigger than the SVZa explants. The position of the OB explant is important because the glomerular layer should be accessible to the SVZa explants.
In our experiments, removal of the OB in the brain slices significantly reduced the migration of neurons toward the OB. In a previous study, the OB was removed from rats, and SVZa neuronal precursors were found to continue to proliferate in the SVZa and migrate in the RMS (Kirschenbaum et al., 1999). It was concluded that the OB was not required for SVZa migration toward the OB. Interpretation of results from the previous experiments could have been complicated by the relatively long-term side effects of OB removal, which caused volume changes in the RMS, and by the absence of quantitative analysis (Kirschenbaum et al., 1999). In our assays, each experiment was internally controlled, because the anterior/posterior ratios were analyzed in each slice. Qualitatively, there were still cells migrating rostrally after OB removal. However, quantitative analyses revealed that there was a significant reduction of rostral neuronal migration. Another difference between our studies and the previous studies is that most of the previous experiments were performed with BrdU labeling, and the results about migration could therefore have been influenced by cell proliferation. The only experiment that did not use BrdU labeling is that shown in Figure 6 of Kirschenbaum et al. (1999), in which lacZ-expressing SVZa cells from one animal were transplanted into the SVZa of a host animal. In this case, the number of cells migrating anteriorly toward the olfactory bulb appeared to be reduced when the olfactory bulb was removed [Fig. 6 in Kirschenbaum et al. (1999), compare C, D, E], which is what we found here. Therefore, the in vivo data in Kirschenbaum et al. (1999) could be viewed as agreeing with our conclusion that the olfactory bulb attracted the SVZa cells.
Localization of the source of the OB attractant(s) and its implications for SVZa migration
The OB activity functions as a diffusible long-distance attractant. This conclusion is supported by several results. First, OB explants can attract cells from the SVZa explants, even when these explants are not in contact with each other (Fig. 1). Second, the OB can attract cells from explants from the origin of SVZa as well as from other locations in the RMS (Fig. 2). Third, when the rostral OB is removed, rostral migration cells in the middle of the RMS was reduced (Fig. 6), indicating the OB functions at a distance in the native RMS.
Dissection of the layers in the OB indicate that the glomerular layer clearly has the activity, whereas the granule layer does not. The glomerular layer activity can explain why SVZa cells migrate to this layer to form periglomerular cells. Because SVZa cells also migrate to the granule layer to form granule cells, the absence of any attractive activity in the granule layer is also quite interesting. Because the granule layer is situated between the glomerular layer and the RMS, one possibility is that some SVZa cells end up in the granule layer when they are trapped in the granule layer by a stop signal on their way to the glomerular layer.
Unlike our conclusion about the glomerular and granule layers, we cannot conclude the precise location of attractive activities in the mitral cell layer or the external or internal plexiform layers. This is because of the limited precision of manual dissections: the glomerular and granule cell layers can be cleanly dissected, whereas the mitral cell layer and the external and internal plexiform layers cannot. Molecular and genetic approaches may be essential to further localize the sources of guidance activities in these layers.
Molecular novelty of the OB attractant(s)
The molecular nature of the OB attractant(s) appears to be novel, because previously known attractants do not behave as attractants for the SVZa neurons. Time-lapse recordings have shown directed migration in the RMS and that the inhibition of netrin signaling through its receptor deleted-in-colorectal-cancer by a function-blocking antibody reduced the directionality of some cells in the RMS (Murase and Horwitz, 2002). Because netrins are usually attractants (Kennedy et al., 1994; Serafini et al., 1996; Yee et al., 1999; Alcantara et al., 2000), those authors suggested that netrin was an attractant for the SVZa neurons (Murase and Horwitz, 2002). However, netrin is unlikely to be the OB attractant, both because netrin-1 is not present in the OB continuously (Murase and Horwitz, 2002) and because netrin-1 is now shown to be a repellent for SVZa neurons (Fig. 7). Others have shown that netrin-2 does not attract SVZa neurons (Hu and Rutishauser, 1996; Mason et al., 2001), whereas we found that β-netrin is expressed in the OB, but it neither attracts nor repels the SVZa neurons.
The chemokine SDF-1 has recently been found to be a chemoattractant for cerebellar granule cells and their precursor cells (Klein et al., 2001; Lu et al., 2001; Zhu et al., 2002). However, results obtained here indicate that SDF-1 did not attract or repel the SVZa cells.
An astrocyte-derived migration-inducing activity (MIA), which induces the migration of SVZa cells, was identified recently (Mason et al., 2001). However, MIA does not seem to be the OB attractant, because MIA by itself does not attract SVZa cells, and it is present in the RMS. It is possible that the OB activity can also be a motility-promoting factor, which functions in a concentration-dependent manner to cause the effect observed in Figures 1, 2, 3, 4. However, data in Figure 6 indicate that this activity is clearly an attractant, because the ratio of cells migrating rostrally toward the OB over those migrating caudally is reduced. It remains to be formally tested whether the OB activity can enhance the motility of SVZa cells in addition to attracting them.
Because directed migration of neurons is essential to regeneration after injury (Magavi et al., 2000) and neurodegeneration (Nakatomi et al., 2002), studies of new guidance activities will be useful in designing therapeutic strategies. At this time, there are more known repellents than attractants for axons and neurons. Because neither of the known attractants, netrin and SDF-1, can mimic the activity, our results indicate that there is a novel attractant present in the OB. It will be interesting to molecularly identify the OB attractant(s).
Our slice assays with DiI labeling indicate that, in addition to cells migrating rostrally toward the OB, there are also cells in the RMS that can migrate caudally. This adds a new dimension to neuronal migration in the RMS. It will be interesting to investigate the origin of the rostrally migrating cells and their destination and eventual function. It also suggests that other guidance cues may control caudal migration.
Footnotes
This work was supported by the National Institutes of Health and the Klingenstein Foundation.
Correspondence should be addressed to Yi Rao, Department of Anatomy and Neurobiology, Washington University School of Medicine, P.O. Box 8108, 660 South Euclid Avenue, St. Louis, MO 63110. E-mail: raoyi@thalamus.wustl.edu.
Copyright © 2003 Society for Neuroscience 0270-6474/03/236651-09$15.00/0
References
- Alcantara S, Ruiz M, Castro FD, Soriano E, Sotelo C ( 2000) Netrin 1 acts as an attractive or a repulsive cue for distant migrating neurons during the development of the cerebellar system. Development 127: 1359-1372. [DOI] [PubMed] [Google Scholar]
- Altman J ( 1969) Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol 137: 433-457. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD ( 1966) Autoradiographic and histological studies of postnatal neurogenesis. I. A longitudinal investigation of the kinetics, migration and transformation of cells incorporating tritiated thymidine in neonate rats, with special reference to postnatal neurogenesis in some brain regions. J Comp Neurol 126: 337-389. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A ( 1997) Mechanism of migration of olfactory bulb interneurons. Cell Dev Biol 8: 207-213. [DOI] [PubMed] [Google Scholar]
- Axel R ( 1995) The molecular logic of smell. Sci Am 4: 154-159. [DOI] [PubMed] [Google Scholar]
- Bagri A, Gurney T, He X, Zou Y-R, Littman DR, Tessier-Lavigne M, Pleasure SJ ( 2002) The chemokine SDF1 regulates migration of dentate granule cells. Development 129: 4249-4260. [DOI] [PubMed] [Google Scholar]
- Bayer SA ( 1983) 3H-thymidine-radiographic studies of neurogenesis in the rat olfactory bulb. Exp Brain Res 50: 329-340. [DOI] [PubMed] [Google Scholar]
- Bonfanti L, Theodosis DT ( 1994) Expression of polysialylated neural cell adhesion molecule by proliferating cells in the subependymal layer of the adult rat, in its rostral extension and in the olfactory bulb. Neuroscience 62: 291-305. [DOI] [PubMed] [Google Scholar]
- Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T ( 1999) Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell 96: 795-806. [DOI] [PubMed] [Google Scholar]
- Buck LB ( 1996) Information coding in the vertebrate olfactory system. Annu Rev Neurosci 19: 517-544. [DOI] [PubMed] [Google Scholar]
- Cecchi GA, Petreanu LT, Alvarez-Buylla A, Magnasco MO ( 2001) Unsupervised learning and adaptation in a model of adult neurogenesis. J Comput Neurosci 11: 175-182. [DOI] [PubMed] [Google Scholar]
- Chazal G, Durbec P, Jankovski A, Rougon G, Grmer H ( 2000) Consequences of neural cell adhesion molecule deficiency on cell migration in the rostral migratory stream of the mice. J Neurosci 20: 1446-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JH, Wen L, Dupuis S, Wu JY, Rao Y ( 2001) The N-terminal leucine-rich regions in Slit are sufficient to repel olfactory bulb axons and subventricular zone neurons. J Neurosci 21: 1548-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colamarino SA, Tessier-Lavigne M ( 1995) The role of the floor plate in axon guidance. Annu Rev Neurosci 18: 497-529. [DOI] [PubMed] [Google Scholar]
- Corotto FS, Henegar JA, Maruniak JA ( 1993) Neurogenesis persists in the subependymal layer of the adult mouse brain. Neurosci Lett 149: 111-114. [DOI] [PubMed] [Google Scholar]
- Cremer H, Lange R, Christoph A, Plomann M, Vopper G, Roes J, Brown R, Baldwin S, Kraemer P, Scheff S, Barthels D, Rajewsky K, Wille W ( 1994) Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature 367: 455-459. [DOI] [PubMed] [Google Scholar]
- Dulac C ( 1997) How does the brain smell? Neuron 19: 477-480. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn A, Nordborg C, Peterson DA, Gage FH ( 1998) Neurogenesis in the adult human hippocampus. Nat Med 4: 1313-1317. [DOI] [PubMed] [Google Scholar]
- Fan C-M, Tessier-Lavigne M ( 1994) Patterning of mammalian somites by surface ectoderm and notochord: evidence for sclerotome induction by a Hedgehog homolog. Cell 79: 1175-1186. [DOI] [PubMed] [Google Scholar]
- Farbman AI ( 1991) Developmental neurobiology of the olfactory system. In: Smell and taste in health and disease (Getchell TV, ed), pp. 19-32. New York: Raven.
- Flanagan JG, Vanderhaeghen P ( 1998) The ephrins and Eph receptors in neural development. Annu Rev Neurosci 21: 309-345. [DOI] [PubMed] [Google Scholar]
- Gheusi G, Cremer H, McLean H, Chazal G, Vincent JD, Lledo PM ( 2000) Importance of newly generated neurons in the adult olfactory bulb for odor discrimination. Proc Natl Acad Sci USA 97: 1823-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SA, Luskin MB ( 1998) Strategies utilized by migrating neurons of the postnatal vertebrate forebrain. Trends Neurosci 21: 107-114. [DOI] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Graziano MSA, Gross CG ( 1999) Neurogenesis in the neocortex of adult primates. Science 286: 548-552. [DOI] [PubMed] [Google Scholar]
- Greer CA ( 1991) Structural organization of the olfactory system. In: Smell and taste in health and disease (Getchell TV, ed), pp. 65-79. New York: Raven.
- Hinds JW ( 1968) Autoradiographic study of histogenesis in the mouse olfactory bulb. I. Time of origin of neurons and neuroglia. J Comp Neurol 134: 287-304. [DOI] [PubMed] [Google Scholar]
- Hu H ( 1999) Chemorepulsion of neuronal migration by Slit2 in the developing mammalian forebrain. Neuron 23: 703-711. [DOI] [PubMed] [Google Scholar]
- Hu H, Tomasiewicz H, Magnuson T, Rutishauser U ( 1996) The role of polysialic acid in migration of olfactory bulb interneuron precursors in the subventricular zone. Neuron 16: 735-743. [DOI] [PubMed] [Google Scholar]
- Hu HY, Rutishauser U ( 1996) A septum-derived chemorepulsive factor for migrating olfactory interneuron precursors. Neuron 16: 933-940. [DOI] [PubMed] [Google Scholar]
- Kaplan MS ( 1983) Proliferation of subependymal cells in the adult primate CNS: differential uptake of DNA labeled precursors. J Hirnforsch 24: 23-33. [PubMed] [Google Scholar]
- Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M ( 1994) Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell 87: 175-185. [DOI] [PubMed] [Google Scholar]
- Kidd T, Bland KS, Goodman CS ( 1999) Slit is the midline repellent for the Robo receptor in Drosophila Cell 96: 785-794. [DOI] [PubMed] [Google Scholar]
- Kirschenbaum B, Doetsch F, Lois C, Alvarez-Buylla A ( 1999) Adult subventricular zone neuronal precursors continue to proliferate and migrate in the absence of the olfactory bulb. J Neurosci 19: 2171-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi K ( 1987) Golgi studies on the development of granule cells of the rat olfactory bulb with references to migration in the subependymal layer. J Comp Neurol 258: 112-124. [DOI] [PubMed] [Google Scholar]
- Klein R ( 2001) Excitatory Eph receptors and adhesive ephrin ligands. Curr Opin Cell Biol 13: 196-203. [DOI] [PubMed] [Google Scholar]
- Klein RS, Rubin JB, Gibson HD, DeHaan EN, Alvarez-Hernandez X, Segal RA, Luster AD ( 2001) SDF-1α induces chemotaxis and enhances Sonic hedgehog-induced proliferation of cerebellar granule cells. Development 128: 1971-1981. [DOI] [PubMed] [Google Scholar]
- Koch M, Murrell JR, Hunter DD, Olson PF, Jin W, Keene DR, Brunken WJ, Burgeson RE ( 2000) A novel member of the netrin family, beta-netrin, shares homology with the beta chain of laminin: identification, expression, and functional characterization. J Cell Biol 151: 221-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodkin AL ( 1996) Growth cones and the cues that repel them. Trends Neurosci 19: 507-513. [DOI] [PubMed] [Google Scholar]
- Kornack DR, Rakic P ( 2001) The generation, migration, and differentiation of olfactory neurons in the adult primate brain. Proc Natl Acad Sci USA 98: 4752-4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukekov VG, Laywell ED, Suslov O, Davies K, Scheffler B, Thomas LB, O'Brien TF, Kusakabe M, Steindler DA ( 1999) Multipotent stem/progenitor cells with similar properties arise from two neurogenic regions of adult human brain. Exp Neurol 156: 333-344. [DOI] [PubMed] [Google Scholar]
- Lewis PD ( 1968) Mitotic activity in the primate subependymal layer and the genesis of gliomas. Nature 217: 974-975. [DOI] [PubMed] [Google Scholar]
- Li H, Chen J, Wei W, Fagaly T, Zhou L, Yuan W, Dupuis S, Jiang Z, Nash W, Gick C, Ornitz DM, Wu JY, Rao Y ( 1999) Vertebrate slit, a secreted ligand for the transmembrane protein roundabout, is a repellent for olfactory bulb axons. Cell 96: 807-818. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A ( 1994) Long-distance neuronal migration in the adult mammalian brain. Science 264: 1145-1148. [DOI] [PubMed] [Google Scholar]
- Lois C, Garcia-Verdugo JM, Alvarez-Buylla A ( 1996) Chain migration of neuronal precursors. Science 271: 978-981. [DOI] [PubMed] [Google Scholar]
- Lu M, Grove EA, Miller RJ ( 2002) Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. Proc Natl Acad Sci USA 99: 7090-7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Sun EE, Klein RS, Flanagan JG ( 2001) Ephrin-B reverse signaling is mediated by a novel PDZ-RGS protein and selectively inhibits G protein-coupled chemoattraction. Cell 105: 69-79. [DOI] [PubMed] [Google Scholar]
- Luskin MB ( 1993) Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron 11: 173-189. [DOI] [PubMed] [Google Scholar]
- Magavi SS, Leavitt BR, Macklis JD ( 2000) Induction of neurogenesis in the neocortex of adult mice. Nature 405: 951-955. [DOI] [PubMed] [Google Scholar]
- Marin EC, Jefferis GS, Komiyama T, Zhu H, Luo L ( 2002) Representation of the glomerular olfactory map in the Drosophila brain. Cell 109: 243-255. [DOI] [PubMed] [Google Scholar]
- Mason HA, Ito S, Corfas G ( 2001) Extracellular signals that regulate the tangential migration of olfactory bulb neuronal precursors: inducers, inhibitors, and repellents. J Neurosci 21: 7654-7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott KWG, Lantos PL ( 1990) Cell-proliferation in the subependymal layer of the postnatal Marmoset, Callithrix-Jacchus. Dev Brain Res 57: 269-277. [DOI] [PubMed] [Google Scholar]
- Menezes JR, Smith CM, Nelson KC, Luskin MB ( 1995) The division of neuronal progenitor cells during migration in the neonatal mammalian forebrain. Mol Cell Neurosci 6: 496-508. [DOI] [PubMed] [Google Scholar]
- Mombaerts P ( 2001) How smell develops. Nat Neurosci 4(Suppl): 1192-8. [DOI] [PubMed] [Google Scholar]
- Mori K, Nagao H, Yoshihara Y ( 1999) The olfactory bulb: coding and processing of odor molecule information. Science 286: 711-715. [DOI] [PubMed] [Google Scholar]
- Murase S, Horwitz AF ( 2002) Deleted in colorectal carcinoma and differentially expressed integrins mediate the directional migration of neural precursors in the rostral migratory stream. J Neurosci 22: 3568-3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M ( 2002) Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell 110: 429-441. [DOI] [PubMed] [Google Scholar]
- Ono K, Tomasiewicz H, Magnuson T, Rutishauser U ( 1994) N-CAM mutation inhibits tangential neuroal migration and is phenocopied by enzymatic removal of polysialic acid. Neuron 13: 595-609. [DOI] [PubMed] [Google Scholar]
- Pencea V, Bingaman KD, Freedman LJ, Luskin MB ( 2001) Neurogenesis in the subventricular zone and rostral migratory stream of the neonatal and adult primate forebrain. Exp Neurol 172: 1-16. [DOI] [PubMed] [Google Scholar]
- Pincus DW, Keyoung HM, Harrison-Restelli C, Goodman RR, Fraser RAR, Edgar M, Sakakibara S, Okano H, Nedergaard M, Goldman SA ( 1998) Fibroblast growth factor-2/brain-derived neurotrophic factor-associated maturation of new neurons generated from adult human subependymal cells. Ann Neurol 43: 576-585. [DOI] [PubMed] [Google Scholar]
- Raper JA ( 2000) Semaphorins and their receptors in vertebrates and invertebrates. Curr Opin Neurobiol 10: 88-94. [DOI] [PubMed] [Google Scholar]
- Rousselot R, Lois C, Alvarez-Buylla A ( 1995) Embryonic (PSA) N-CAM reveals chains of migrating neuroblasts between the lateral ventricle and the olfactory bulb of adult mice. J Comp Neurol 351: 51-61. [DOI] [PubMed] [Google Scholar]
- Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M ( 1996) Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell 87: 1001-1014. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E ( 2001) Neurogenesis in the adult is involved in the formation of trace memories. Nature 410: 372-376. [DOI] [PubMed] [Google Scholar]
- Tomasiewicz H, Ono K, Yee D, Thompson C, Goridis C, Rutishauser U, Magnuson T ( 1993) Genetic deletion of a neural cell adhesion molecule variant (N-CAM-180) produces distinct defects in the central nervous system. Neuron 11: 1163-1174. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Webster MJ, Colin SM, Herman MM, Hyde TM, Weinberger DR, Kleinman JE ( 2000) Localization of epidermal growth factor receptors and putative neuroblasts in human subependymal zone. J Comp Neurol 423: 359-372. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Garcia-Verduge JM, Alvarez-Buylla A ( 1997) Direct evidence for homotypic, glia-independent neuronal migration. Neuron 18: 779-791. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A ( 2001) In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development 128: 3759-3771. [DOI] [PubMed] [Google Scholar]
- Wong AM, Wang JW, Axel R ( 2002) Spatial representation of the glomerular map in the Drosophila protocerebrum. Cell 109: 229-241. [DOI] [PubMed] [Google Scholar]
- Wong K, Ren XR, Huang YZ, Xie Y, Liu G, Saito H, Tang H, Wen L, Brady-Kalnay SM, Mei L, Wu JY, Xiong WC, Rao Y ( 2001) Signal transduction in neuronal migration: roles of GTPase activating proteins and the small GTPase Cdc42 in the Slit-Robo pathway. Cell 107: 209-221. [DOI] [PubMed] [Google Scholar]
- Wu W, Wong K, Chen JH, Jiang ZH, Dupuis S, Wu J, Rao Y ( 1999) Directional guidance of neuronal migration in the olfactory system by the protein Slit. Nature 400: 331-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Li Y, Zhang Z, Cui K, Wang S, Yuan XB, Wu CP, Poo MM, Duan S ( 2002) Nerve growth cone guidance mediated by G protein coupled receptors. Nat Neurosci 5: 843-848. [DOI] [PubMed] [Google Scholar]
- Yee KT, Simon HH, Tessier-Lavigne M, O'Leary DM ( 1999) Extension of long leading processes and neuronal migration in the mammalian brain directed by the chemoattractant netrin-1. Neuron 24: 607-622. [DOI] [PubMed] [Google Scholar]
- Yin Y, Sanes JR, Miner JH ( 2000) Identification and expression of mouse netrin-4. Mech Dev 96: 115-119. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Li HS, Zhou L, Wu JY, Rao Y ( 1999) Cellular and molecular guidance of GABAergic neuronal migration from an extra-cortical origin to the neocortex. Neuron 23: 473-485. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Yu T, Zhang X-C, Nagasawa T, Wu JY, Rao Y ( 2002) Role of the chemokine SDF-1 as the meningeal attractant for embryonic cerebellar neurons. Nat Neurosci 5: 719-720. [DOI] [PMC free article] [PubMed] [Google Scholar]


