Abstract
The rat medial frontal cortex (MFC) has been implicated in allowing animals to work harder to receive larger rewards. However, it is unknown what role the individual MFC regions [anterior cingulate cortex (ACC) and prelimbic-infralimbic cortex (PL-IL)] play in such decision making. To investigate this, we trained rats on a T-maze cost-benefit task with two possible courses of action, shown previously to be affected by complete MFC lesions. One response involved climbing a 30 cm barrier to obtain a large quantity of reward (high cost-high reward), whereas the other had a lower energetic demand but also a smaller reward gain (low cost-low reward). Before surgery, all animals preferred to select the high cost-high reward option. However, after excitotoxic ACC lesions, there was a complete reversal of behavior, with the ACC group selecting the low cost-low reward response on nearly every trial. In contrast, both control animals and rats with PL-IL lesions continued to choose to climb the barrier for the larger reward. When the same rats were tested on a delayed match-to-sample paradigm however, it was the PL-IL group that was significantly impaired at learning the response rule, with the performance of ACC rats being comparable with controls. This double dissociation indicates that the ACC is the important region within the MFC when evaluating how much effort to expend for a specific reward.
Keywords: cingulate cortex, decision making, cost-benefit, effort, prelimbic cortex, reward
Introduction
The medial frontal cortex (MFC) forms part of an extended frontostriatal circuit with direct influence over both the mesolimbic dopamine and motor systems; as such, it is in a prime position to influence behavioral choice. In a previous study, we demonstrated that rat MFC, including the anterior cingulate cortex (ACC) and prelimbic and infralimbic areas (PL-IL), is important for allowing animals to put in more work to receive greater rewards (Walton et al., 2002). Specifically, whereas animals typically chose to put in work for an increased quantity of food, after lesions to the MFC, there was a complete reversal in behavior, the lesioned rats always selecting the response involving less work and smaller reward. This was not caused by insensitivity to costs and benefits, however, because reducing the energetic demands or increasing the reward associated with the high-effort response caused the animals with MFC lesions to return to the high cost-high reward option.
However, it is not clear what role the individual areas of the MFC play in overcoming effort constraints to obtain greater reward. All of the MFC projects to the nucleus accumbens (NAc) (Berendse et al., 1992), which is also known to be involved in evaluating the costs and benefits of actions (Salamone et al., 1997; Cardinal et al., 2001). Moreover, although lesions to various parts of the MFC have been shown to cause impairments in behavioral flexibility and attention (Muir et al., 1996; Bussey et al., 1997; Brown and Bowman, 2002), it has proved difficult thus far to show functional dissociations on separate tasks between these areas. In particular, there has been little consistent evidence of what role the ACC might play.
There is some indication from primate studies suggesting that the ACC might be a good candidate for influencing effort-based decision making. Cells have been reported in this region that respond while working toward or receiving rewards (Akkal et al., 2002; Shidara and Richmond, 2002). Moreover, ACC lesions cause impairments in the ability to link particular movements with reinforcers (Hadland et al., 2003).
The aim of the present study, therefore, was to investigate the effects of either ACC (including both Cg1 and Cg2 fields of ACC) (Zilles, 1985) or PL-IL lesions on the ability of rats to choose how much effort to exert to obtain a particular size of reward. Initially, we tested rats on the T-maze cost-benefit paradigm used previously (Salamone et al., 1994; Walton et al., 2002), in which animals could elect either to a obtain small reward in one arm or to climb a barrier to receive high reward in the other. Subsequently, the same animals were run on a delayed match-to-sample (DMTS) task, which has been shown to be sensitive to MFC lesions (Dias and Aggleton, 2000).
Materials and Methods
Animals. Thirty male Lister hooded rats, ∼2 months of age at the start of testing, were used for both experiments. All animals were housed in groups of three under a 12 hr light/dark cycle (lights on at 7:00 A.M.). At surgery, rats weighed 300-380 gm. The experiments described were conducted in accordance with the UK Animals Scientific Procedures Act (1986), under project license number PPL 30/1505.
Surgical procedures. Rats received excitotoxic bilateral ACC lesions (n = 10), PL-IL lesions (n = 10), or sham surgery (n = 10) after training on the cost-benefit task. Assignment of lesion groups was counterbalanced according to preoperative performance and the right-left orientation of the rewards. Lesions were produced by infusing quinolinic acid (0.09 m) through a 10 μl syringe with a specially adapted 34 gauge needle mounted onto the stereotaxic frame. All animals were anesthetized with avertin (0.29 gm/kg, i.p.) and placed in a stereotaxic frame with the head level between bregma and lambda. An incision was made along the mid-line, and a craniotomy was performed before injections were made at the following coordinates relative to bregma or dura for dorsoventral (DV) coordinates (volume of 0.2 μl unless specified): for ACC lesion, antero-posterior (AP), +2.3, mediolateral (ML), ±0.5; DV, -1.5; AP, +1.6; ML, ±0.5; DV, -2.0; AP, +0.9; ML, ±0.5; DV, -2.0; AP, +0.2; ML, ±0.5; DV, -2.0; for PL-IL lesion, AP, +3.3; ML, ±0.5; DV, -3.5; AP, +2.6; ML, ±0.5; DV, -3.5 (0.25 μl). Infusions were made manually at a rate of 0.1 μl every 30 sec, with a 30 sec interval between each 0.1 μl infusion. The needle was then left in place for another 3 min to ensure that diffusion occurred away from the injection site. Sham animals received only a craniotomy. After completion of surgery, all animals were sutured and a topical antibiotic powder (P.E.P. 2% powder; Intervet Laboratories, Cambridge, UK) was sprinkled over the wound.
Histology. At the conclusion of behavioral testing, rats were anesthetized with sodium pentobarbitone (200 mg/kg) and perfused transcardially with physiological saline and 10% formal saline. The brains were removed and placed into a formal saline solution before being stored in a sucrose-formalin solution for 24 hr, frozen, and then sectioned coronally (50 μm). All sections were mounted and stained with cresyl violet. The lesions are described in terms of the nomenclature and classification of cortical areas adopted by Paxinos and Watson (1998).
Experiment 1: cost-benefit T-maze
Apparatus. Rats were tested on a high-sided wooden T-maze, consisting of one start arm and two goal arms, each being 60 cm long, 10 cm wide, and 30 cm high. Food rewards (45 mg food-reinforcement pellets, Formula A/I; P. J. Noyes, Lancaster, NH) were placed in raised metal wells 2 cm from the far end of each goal arm. Barriers were constructed from wire mesh in the shape of a three-dimensional triangle. These were placed at the midpoint of each goal arm as required, meaning that animals had to scale the vertical side and descend the slope corresponding to the hypotenuse to obtain rewards. On “forced” trials, a 30-cm-high, 10-cm-wide wooden block was placed to prevent access to one goal arm.
Training and testing procedures. For detailed methods on habituation and training schedule, see the study by Walton et al. (2002). In brief, after habituation, four food pellets were placed in one goal arm [high reward (HR)] and two pellets in the other [low reward (LR)]. For one-half of the animals, the HR arm was to the left. Once all animals had been trained to choose the HR arm on ≥ 80% trials, a 15 cm barrier was introduced into the HR arm. When all animals returned to selecting the HR arm on ≥ 80% of trials, the barrier size was increased by 5 cm, and then by 5 cm every 2 d up to a maximum of 30 cm. For the first two trials occurring each day, and on all subsequent testing, the rats were forced in opposite directions. They were then given 10 choice trials, with an intertrial interval of ∼5 min.
During prelesion and the first postlesion testing block (blocks A and B, each consisting of3dof10 choice trials), the 30 cm barrier was placed in the HR arm while the LR arm was vacant. For the second postlesion testing block (block C), identical 30 cm barriers were present in both arms to measure whether any deficit was caused by a spatial or motor impairment or by an inability to remember reward quantity.
Experiment 2: DMTS
Apparatus and testing. Rats were run on an elevated, low-walled T-maze (2 cm high, with the maze 40 cm above floor level) of otherwise identical size to that used in experiment 1. Testing began ∼4 months after the end of experiment 1 and took place in a different room containing novel distal cues. Rats were habituated to the maze in the same manner as in experiment 1. During testing, each trial consisted of a sample run in which the animal was forced by a wooden block to select a particular goal arm for a single food pellet reward, followed by a choice run in which both goal arms were open. A correct trial, rewarded with two food pellets, required making the same response in the choice as in the sample run (i.e., matching-to-position). The direction of the sample response was generated pseudorandomly for each animal, with equal numbers of left and right turns across 10 trials and no more than three successive turns in the same direction. There was a delay of ∼10 sec between the sample and choice phases. A trial was considered to be concluded once the animal had visited one of the two food wells. Testing was divided into sessions of 10 trials and continued until 24 sessions had been completed.
Results
Histology
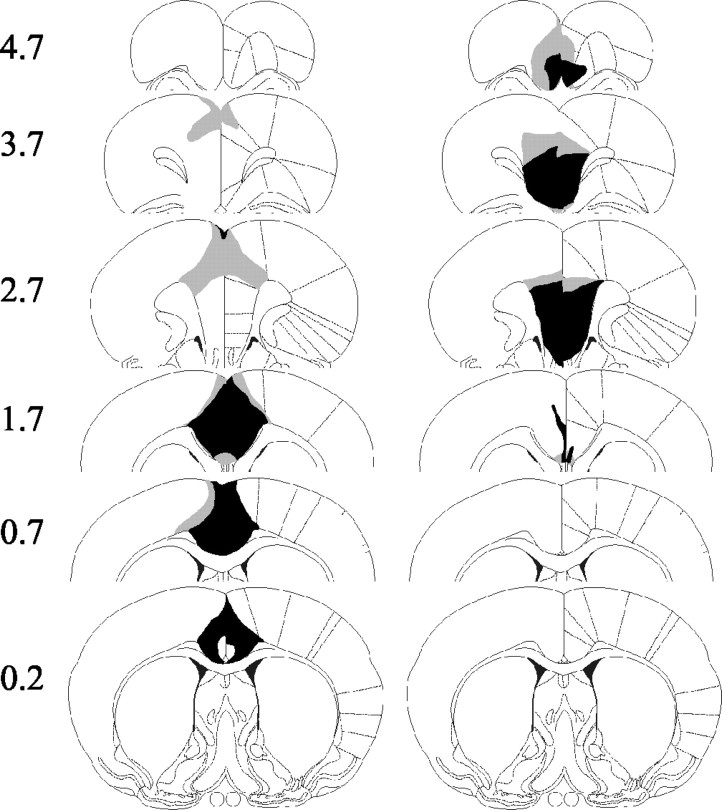
Both sets of lesions were highly restricted, and there was little overlap between the groups. ACC lesions were all consistent and reproducible, with little overall difference in size between animals (Figs. 1, left column, 2, middle row). Generally, any variation consisted of a rostrocaudal shift in the lesion, with the small lesion depicted in Figure 1 representing the furthest posterior starting point of cell damage. The lesion produced extensive cell loss in the entire ACC, extending back to ∼0.8-0.2 mm anterior to bregma. This is ∼0.5-1 mm more anterior than the extent of ACC damage in our previous study (Walton et al., 2002). In all animals, there was some sparing of the rostral ACC in the most anterior sections (3.7 mm anterior to bregma). At supracallosal levels, there was also partial damage to secondary motor cortex.
Figure 1.
Representations of the maximal (gray shading) and minimal (black shading) examples of both the ACC (left) and PL-IL (right) lesions.
Figure 2.
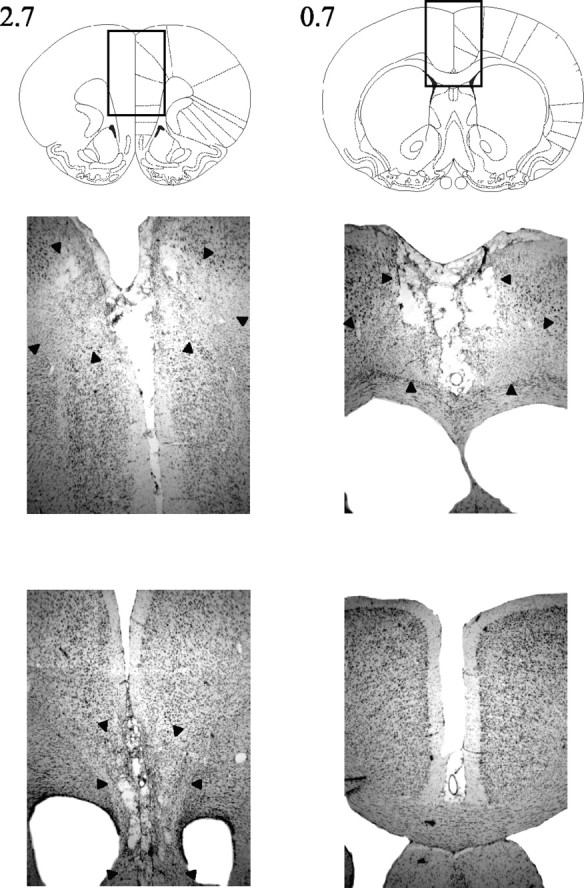
Photomicrographs of coronal sections showing typical cell loss for a representative ACC-lesioned (middle) and PL-IL-lesioned (bottom) animal. The boxed regions in the top panels (brain outlines) are shown at high magnification in the middle and bottom panels. The extent of the lesion is indicated by the black arrowheads. Note that both ACC and PL-IL lesions are complete and separate at sections 2.7 mm anterior to bregma.
Two of the PL-IL lesions showed extensive sparing of tissue in either one or both hemispheres and were thus excluded from analysis. However, the eight remaining lesions were as intended, centered on the PL-IL, and again showed little variation between animals (Figs. 1, right column, 2, bottom row). The majority of lesions had some sparing of the PL in the most anterior sections. Otherwise, the lesion completely removed the PL-IL in all animals, except one for which there was slight sparing of the dorsal-most portion of the PL throughout. In anterior sections, cell loss included parts of the medial orbital cortex, extending in the largest case into the ventral orbital cortex. Damage also extended ventrally to include the dorsopeduncular cortex.
Experiment 1: cost-benefit T-maze
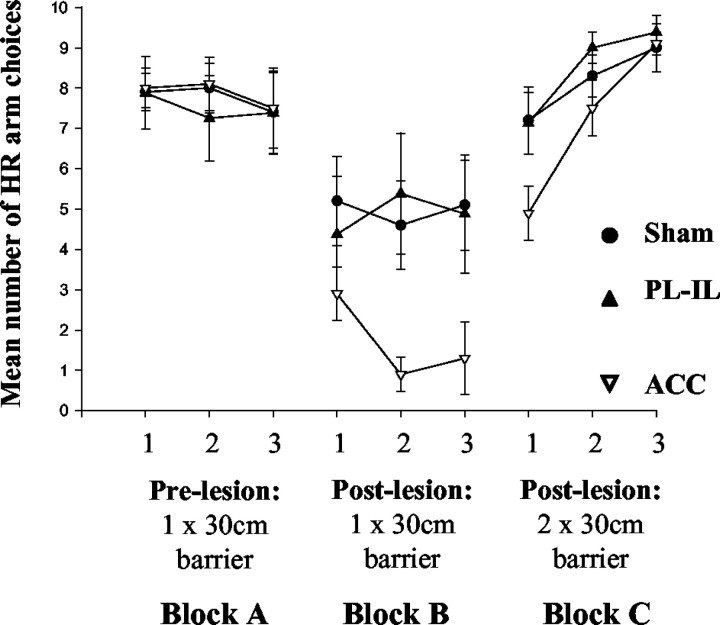
The rats were divided into three groups on the basis of their prelesion performance. As can be seen in Figure 3 (block A), all three groups showed a strong preference to climb the barrier to obtain the high reward. After surgery, there was a marked change in behavior of the group with ACC lesions, with all animals selecting the low-reward option on most trials. In contrast, although there was a tendency for more LR choices than during the prelesion testing, the majority of rats in both the sham and PL-IL groups continued to prefer the HR arm (Fig. 3, block B). This was confirmed by an ANOVA that showed a significant interaction between testing block (blocks A and B) and lesion group (F(2,25) = 5.81; p < 0.01). To explore this more closely, an additional ANOVA was run on postlesion data (block B). This confirmed the significant difference between the groups (F(2,25) = 3.42; p < 0.05), and post hoc Fisher's least significant difference (LSD) tests showed that this was caused by ACC animals making significantly more LR arm choices than both the sham and PL-IL groups (p < 0.05). However, there were no differences in choices between the sham and PL-IL animals.
Figure 3.
Mean ± SE number of choices per day (maximum of 10) that the ACC, PL-IL, and sham groups selected the HR arm when performing the cost-benefit T-maze task. The left panel represents prelesion performance (block A), and the middle panel represents postlesion performance with a single 30 cm barrier in the HR arm (block B). Data in the right panel correspond to postlesion testing with an identical 30 cm barrier in each goal arm (block C).
The introduction of a second identical 30 cm barrier in the LR arm caused all three groups to return to selecting the HR arm on nearly every trial (Fig. 3, block C). An ANOVA comparing the postlesion one-barrier condition and two-barrier condition (blocks B and C) across all groups showed a significant testing block by day by group interaction (F(2,36) = 4.58; p < 0.05) caused by this change in behavior and the fact that the animals with ACC lesions shifted further than either of the other groups. By days 2 and 3 of the two-barrier block, there were no differences between the three groups (both F(2,27) < 2; NS).
Experiment 2: DMTS
Acquisition of the match-to-sample rule was designated by the achievement of 85% correct across two sessions of testing (equivalent to a score of ≥ 17 of 20). For the purposes of analysis, any animals that did not reach this criterion were counted as taking the maximum 24 sessions. Because this caused clear violations of normality assumptions, comparisons between the groups using the criterion measure were made using nonparametric statistics.
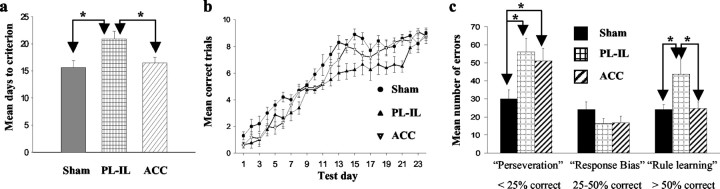
The average number of sessions to reach criterion can be seen in Figure 4a. It is clear that the rats in both the sham and ACC groups learned the task in a comparable amount of time, whereas the PL-IL group took markedly longer. A significant difference between the groups is borne out by a Kruskal-Wallis test (H = 8.20; p < 0.05), and subsequent Mann-Whitney tests demonstrated that PL-IL animals took significantly more sessions to achieve criterion than either the ACC or sham groups (both p < 0.05; two-tailed).
Figure 4.
a, Mean ± SE number of sessions required by ACC, PL-IL, and sham groups to achieve a criterion of ≥17 of 20 correct trials across two testing sessions on the DMTS task. Asterisks denote a significant difference between the groups at the p < 0.05 level. b, Pattern of acquisition of the matching rule in all three groups across all 24 testing sessions as measured by the mean number of correct trials in each session. c, Number of errors committed on DMTS divided into three phases: a perseverative phase (score of ≤5 of 20 across 2 test sessions), a response bias phase (scores of between 6 and 10 of 20), and finally, a rule-learning phase (≥11 of 20 to criterion). Asterisks denote a significant difference between the groups at the p < 0.05 level.
Inspection of acquisition indicated that there were several phases involved in learning the DMTS task (Fig. 4b). Following Dias and Aggleton (2000), we divided the data for each animal into an initial “perseveration” phase, in which animals tended to respond according to their innate nonmatching preference (≤25%, or ≤5 of 20 correct across two test sessions), a “response bias” phase, in which animals adopted a strategy of always turning in the same direction during the choice run (between 25 and 50% correct), and finally, a “rule-learning” phase, during which the rats acquired the matching rule (≥ 50% correct). The number of errors made during each phase can be seen in Figure 4c. An ANOVA showed a significant group by test phase interaction (F(4,50) = 3.34; p < 0.05). Subsequent analyses demonstrated that there were significant group differences during perseveration (F(2,27) = 4.56; p < 0.05) and rule learning (F(2,27) = 4.46; p < 0.05). Fisher's LSD tests indicated that during the perseveration phase, this was caused by both lesion groups making more errors than sham animals (p < 0.05). However, only the PL-IL group was impaired at learning the rule (p < 0.05, compared with sham and ACC groups); animals with ACC lesions performed similarly to controls.
Discussion
The results from experiment 1 demonstrate that the ACC is the essential region of the rat MFC that allows animals to exert effort to obtain a larger reward. Excitotoxic lesions of the ACC caused rats to switch from climbing a barrier to obtain a larger reward to selecting the low effort-low reward option on nearly every trial, replicating our findings with large MFC lesions (Walton et al., 2002). In contrast, rats with lesions to adjacent regions of the MFC (namely, the PL-IL) performed identically to the control animals. These results cannot be attributed to a simple spatial or motor deficit, or to an inability to remember reward quantity, because all ACC animals returned to choosing the high-reward option when the energetic demands were equated by putting an identical barrier in both goal arms. Moreover, the behavioral change in the ACC group relative to the PL-IL group was not caused by a larger lesion size, because when the same rats were tested on a spatial matching-to-sample task, the ACC group learned the rule in a similar amount of time to the controls; in contrast, the PL-IL animals were impaired at this task.
The bias toward low-effort responses in rats with ACC lesions but not those with PL-IL lesions on the cost-benefit task is interesting for several reasons. First, a similar set of studies by Salamone et al. (1994) and Cousins et al. (1996) showed that dopamine depletions of the NAc also reduced the preference of animals to work for higher reward. However, not only the ACC but also the PL-IL projects to the NAc (Berendse et al., 1992; Brog et al., 1993), and both have direct influence on the origin of the mesolimbic dopamine system (the ventral tegmental area) through reciprocal connections (Uylings and van Eden, 1990). One possibility is that both top-down and bottom-up interactions between the ACC and subcortical centers with influence over the production of monoaminergic neurotransmitters are crucial for allowing an animal to overcome effort constraints to achieve a larger reward.
Furthermore, it seems that the ACC is not needed in all situations requiring an assessment of costs and benefits. A recent study by Cardinal et al. (2001) examining impulsive choice in rats found that only lesions to the NAc core, but not to either the ACC or the PL-IL, induced a shift toward choosing the immediate low-reward option when faced with a choice between this and a delayed but larger reward. This raises the intriguing possibility that the ACC might be important only when assessing how much effort to expend for a specific reward and not when evaluating delay-based costs (or more generally, only when ascribing value to courses of action).
Such a description of ACC function is bolstered by findings that there are cells in this region of primate cortex that appear to be concerned with selecting responses on the basis of their reinforcing outcome (Shima and Tanji, 1998; Procyk et al., 2000), and one of the few lesion studies of the primate ACC found a selective impairment in using rewards to guide action (Hadland et al., 2003). Similarly, several human neuroimaging experiments have reported ACC activity when choosing between and monitoring the consequences of actions with different potential sizes of reward (Bush et al., 2002; Gehring and Willoughby, 2002). Finally, and of particular relevance to the present study, Shidara and Richmond (2002) found that nearly one-third of neurons in the rostral ACC progressively increased their firing as animals advanced through a fixed schedule of trials for reward. However, these responses disappeared if the length of the schedule was randomized, suggesting that they were concerned with the amount of work required to obtain an expected outcome.
Such a conclusion is based on the assumption of homology between the rodent and primate MFC. Although there is contention over whether the PL-IL should be compared with the ventromedial or dorsolateral prefrontal cortex in primates (Preuss, 1995; Brown and Bowman, 2002), there is good anatomical correspondence between ACC regions in the two species, with both projecting to analogous regions of the mediodorsal nucleus of the thalamus, and both sharing similar subcortical connections (Uylings and van Eden, 1990; Bachevalier et al., 1997). Moreover, in addition to the functional similarities in terms of reward processing described above, both the rat and primate ACC, unlike any other frontal regions, contain neurons that respond to noxious stimulation and play a role in processing pain-related unpleasantness (Devinsky et al., 1995; Johansen et al., 2001).
Although lesions to the PL-IL had no discernable effect on rats' ability to make effort-based decisions, they did cause a notable impairment in learning the DMTS task, a finding that concurs with the previous study of the effects of MFC lesions using this paradigm (Dias and Aggleton, 2000). As reported by Dias and Aggleton (2000), there appeared to be two different phases of impairment. Initially, both lesion groups persisted in responding using their innate nonmatching preference compared with control animals. However, it was only the PL-IL group, and not ACC animals, who made significantly more errors in switching from a subsequent side-bias strategy to learning the matching rule. This pattern of results for ACC animals was similar to that observed by Dias and Aggleton (2000), although their ACC lesion only encompassed the pregenual ACC dorsal and rostral to the corpus callosum, whereas cell loss in the present study also included supracallosal ACC regions. It is unlikely that either impairment in the PL-IL group reflects spatial working memory problems, because all animals were initially able to perform with a non-matching bias. Moreover, the rapid change in behavior observed in all groups on the cost-benefit task when energetic requirements were equated (experiment 1, block C) argues against a simple deficit in response reversal. Rather, our findings are consistent with those of several other groups indicating that the PL-IL, rather than other areas of the MFC, is involved in using recently acquired information to guide actions and switch strategies (Delatour and Gisquet-Verrier, 1999; Ragozzino et al., 1999; Birrell and Brown, 2000; Dias and Aggleton, 2000).
The combination of results from experiments 1 and 2 provides the first direct demonstration of a double dissociation between two regions of the MFC using the same rats being tested on different tasks. The selective nature of the deficit on both tasks reinforces the notion that despite their similar anatomy and large number of interconnections, ACC and PL-IL are functionally independent (Passetti et al., 2002). Furthermore, the discovery of a particular role for the ACC in evaluating the costs and benefits of working for a larger reward opens up several avenues for research into how this region participates in choosing between multiple courses of action.
Footnotes
This work was supported by the Medical Research Council and a Wellcome Prize Studentship to M.E.W. D.B. was funded from a Wellcome Project Grant awarded to J. N. P. Rawlins. We thank Greg Daubney for his assistance with histology.
Correspondence should be addressed to Mark Walton, Department of Experimental Psychology, South Parks Road, Oxford, OX1 3UD, UK. E-mail: mark.walton@psy.ox.ac.uk.
Copyright © 2003 Society for Neuroscience 0270-6474/03/236475-05$15.00/0
References
- Akkal D, Bioulac B, Audin J, Burbaud P ( 2002) Comparison of neuronal activity in the rostral supplementary and cingulate motor areas during a task with cognitive and motor demands. Eur J Neurosci 15: 887-904. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Meunier M, Lu MX, Ungerleider LG ( 1997) Thalamic and temporal cortex input to medial prefrontal cortex in rhesus monkeys. Exp Brain Res 115: 430-444. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Galis-de Graaf Y, Groenewegen HJ ( 1992) Topographical organization and relationship with ventral striatal compartments of pre-frontal corticostriatal projections in the rat. J Comp Neurol 316: 314-347. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ ( 2000) Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci 20: 4320-4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS ( 1993) The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol 338: 255-278. [DOI] [PubMed] [Google Scholar]
- Brown VJ, Bowman EM ( 2002) Rodent models of prefrontal cortical function. Trends Neurosci 25: 340-343. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR ( 2002) Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci USA 99: 523-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Muir JL, Everitt BJ, Robbins TW ( 1997) Triple dissociation of anterior cingulate, posterior cingulate, and medial frontal cortices on visual discrimination tasks using a touchscreen testing procedure for the rat. Behav Neurosci 111: 920-936. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ ( 2001) Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science 292: 2499-2501. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Atherton A, Turner L, Salamone JD ( 1996) Nucleus accumbens dopamine depletions alter relative response allocation in a T-maze cost/benefit task. Behav Brain Res 74: 189-197. [DOI] [PubMed] [Google Scholar]
- Delatour B, Gisquet-Verrier P ( 1999) Lesions of the prelimbic-infralimbic cortices in rats do not disrupt response selection processes but induce delay-dependent deficits: evidence for a role in working memory? Behav Neurosci 113: 941-955. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA ( 1995) Contributions of anterior cingulate cortex to behaviour. Brain 118: 279-306. [DOI] [PubMed] [Google Scholar]
- Dias R, Aggleton JP ( 2000) Effects of selective excitotoxic prefrontal lesions on acquisition of nonmatching- and matching-to-place in the T-maze in the rat: differential involvement of the prelimbic-infralimbic and anterior cingulate cortices in providing behavioural flexibility. Eur J Neurosci 12: 4457-4466. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR ( 2002) The medial frontal cortex and the rapid processing of monetary gains and losses. Science 295: 2279-2282. [DOI] [PubMed] [Google Scholar]
- Hadland KA, Rushworth MF, Gaffan D, Passingham RE ( 2003) The anterior cingulate and reward-guided selection of actions. J Neurophysiol 89: 1161-1164. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Fields HL, Manning BH ( 2001) The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc Natl Acad Sci USA 98: 8077-8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir JL, Everitt BJ, Robbins TW ( 1996) The cerebral cortex of the rat and visual attentional function: dissociable effects of mediofrontal, cingulate, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cereb Cortex 6: 470-481. [DOI] [PubMed] [Google Scholar]
- Passetti F, Chudasama Y, Robbins TW ( 2002) The frontal cortex of the rat and visual attentional performance: dissociable functions of distinct medial prefrontal subregions. Cereb Cortex 12: 1254-1268. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C ( 1998) The rat brain in stereotaxic coordinates, Ed 4. San Diego: Academic.
- Preuss TM ( 1995) Do rats have prefrontal cortex? The Rose-Woolsey-Akert program reconsidered. J Cogn Neurosci 7: 1-24. [DOI] [PubMed] [Google Scholar]
- Procyk E, Tanaka YL, Joseph JP ( 2000) Anterior cingulate activity during routine and non-routine sequential behaviors in macaques. Nat Neurosci 3: 502-508. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP ( 1999) Involvement of the prelimbicinfralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci 19: 4585-4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Bucher S ( 1994) Anhedonia or anergia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behav Brain Res 65: 221-229. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Snyder BJ ( 1997) Behavioral functions of nucleus accumbens dopamine: empirical and conceptual problems with the anhedonia hypothesis. Neurosci Biobehav Rev 21: 341-359. [DOI] [PubMed] [Google Scholar]
- Shidara M, Richmond BJ ( 2002) Anterior cingulate: single neuronal signals related to degree of reward expectancy. Science 296: 1709-1711. [DOI] [PubMed] [Google Scholar]
- Shima K, Tanji J ( 1998) Role for cingulate motor area cells in voluntary movement selection based on reward. Science 282: 1335-1338. [DOI] [PubMed] [Google Scholar]
- Uylings HB, van Eden CG ( 1990) Qualitative and quantitative comparison of the prefrontal cortex in rat and in primates, including humans. Prog Brain Res 85: 31-62. [DOI] [PubMed] [Google Scholar]
- Walton ME, Bannerman DM, Rushworth MF ( 2002) The role of rat medial frontal cortex in effort-based decision making. J Neurosci 22: 10996-11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K ( 1985) The cortex of the rat. Berlin: Springer.