Abstract
The genetic analysis of larval neuromuscular junctions (NMJs) of Drosophila has provided detailed insights into molecular mechanisms that control the morphological and physiological development of these glutamatergic synapses. However, because of the chronic defects caused by mutations, a time-resolved analysis of these mechanisms and their functional relationships has been difficult so far. In this study we provide a first temporal map of some of the molecular and cellular key processes, which are triggered in wild-type animals by natural larval locomotor activity and then mediate experience-dependent strengthening of larval NMJs. Larval locomotor activity was increased either by chronically rearing a larval culture at 29°C instead of 18 or 25°C or by acutely transferring larvae from a culture vial onto agar plates. Within 2 hr of enhanced locomotor activity, NMJs showed a significant potentiation of signal transmission that was rapidly reversed by an induced paralysis of the temperature-sensitive mutant parats1. Enhanced locomotor activity was also associated with a significant increase in the number of large subsynaptic translation aggregates. After 4 hr, postsynaptic DGluR-IIA glutamate receptor subunits started to transiently accumulate in ring-shaped areas around synapses, and they condensed later on, after chronic locomotor stimulation at 29°C, into typical postsynaptic patches. These NMJs showed a reduced perisynaptic expression of the cell adhesion molecule Fasciclin II, an increased number of junctional boutons, and significantly more active zones. Such temporal mapping of experience-dependent adaptations at developing wild-type and mutant NMJs will provide detailed insights into the dynamic control of glutamatergic signal transmission.
Keywords: larval locomotion, experience-dependent strengthening, time-resolved analysis, synaptic protein synthesis, glutamate receptor, bouton-outgrowth, neuromuscular junction, Drosophila
Introduction
Over the last decade the developing neuromuscular junction (NMJ) of Drosophila larvae has gained much attention as a simple synaptic model system for the genetic analysis of activity-dependent processes at glutamatergic synapses (Budnik et al., 1990; Zhong et al., 1992; Jareki and Keshishian, 1995; Davis et al., 1996, 1998; Schuster et al., 1996a, b; Stewart et al., 1996; Petersen et al., 1997; Saitoe et al., 1997, 2001; Sigrist et al., 2000, 2002; Thomas et al., 2000; Wan et al., 2000; Aberle et al., 2002; Pennetta et al., 2002; Sanyal et al., 2002; for review, see Broadie and Richmond, 2002). Such mutation studies have provided a detailed picture of some of the key mechanisms that control the morphological and physiological development of glutamatergic NMJs in vivo. However, because of the mostly chronic nature of genetic manipulations, the temporal sequence of these mechanisms and their functional relationship have been difficult to resolve in model genotypes of activity-dependent plasticity in Drosophila.
In contrast, a time-resolved characterization of the physiological alterations that are involved in different forms of short-term and long-term plasticity has been well established at neuromuscular junctions of crayfish and other crustaceans (Atwood et al., 1975; Wojtowicz and Atwood, 1985, 1986, 1988; Wojtowicz et al., 1988, 1994; Delaney et al., 1989; Zucker, 1999; Beaumont et al., 2001, 2002). In these preparations, alterations in the functional state of the glutamatergic synapses were typically induced and observed in vitro by applying various stimulation protocols to the cut motor nerve and recording from the targeted muscle fiber. These studies have provided a wealth of physiological and biochemical information about the signaling pathways involved in the induction and maintenance of various forms of synaptic plasticity. However, compared with Drosophila, the genetic and molecular analyses of these processes appear restricted in these model systems.
In the present study, we therefore established an experimental framework to uncover the temporal sequence of events involved in activity-dependent alterations at developing NMJs of Drosophila larvae. Our results show that the locomotor activity of wild-type and mutant larvae can be controlled experimentally in an acute and chronic manner. Increased locomotor activity of wild-type larvae results in a characteristic sequence of physiological and molecular key events that are involved in the long-term strengthening of junctional signal transmission in vivo. Thus, the experimental control of larval locomotor activity now allows a time-resolved dissection of the molecular and genetic mechanisms underlying experience-dependent processes at glutamatergic synapses.
Materials and Methods
Genetics
We used the w- strain CS10 (Yin et al., 1995) as wild type, which has been out-crossed for at least 10 generations to Canton S. Further used genotypes include the following: knock-out mutant of the postsynaptic glutamate receptor subunit DGluR-IIA (Schuster et al., 1991), dglurIIA-ko: dglurIIAg9/df(2L)clh4 (Petersen et al., 1997); loss-of-function allele of the poly(A)-binding protein gene (pabpP970) used as heterozygote over CS10 (pabpP970/+) (Sigrist et al. (2000); temperature-sensitive mutant of the voltage-gated sodium channel Paralytic (parats-1) (Ganetzky, 1984).
Larval culture and developmental matching
We have standardized our larval culture to generate consistent and reproducible conditions for larval development with as few as possible individual variances. This was achieved by using a reproducible number of developing larvae (24 hr egg collections from 40 fertilized females at 25°C) and by controlling their rearing conditions in standard 26-mm-diameter fly stock vials [temperature (18, 25, 29°C), humidity (65%), food quality (10 ml of fresh cornmeal food: 6.4% corn meal, 6.4% malt extract, 1.44% dry yeast, 0.8% soy bean meal, 1.76% molasses, 0.64% agar, 0.5% propionic acid)]. Larvae from such 24 hr egg collections rapidly convert the upper few millimeters of the solid cornmeal food into soft slurry, in which the feeding and developmental conditions appear to be very similar for each individual animal.
Because the rearing temperature considerably affects the speed of larval development, we could not use the elapsed time after egg deposition to match the developmental status of larvae. However, the rearing temperature did not alter the animal size shortly before pupation [average length ± SD of the mean (SDM) of wandering stage third instar larvae reared at 18°C: 4.1 ± 0.4 mm, n = 25; 25°C: 4.2 ± 0.5 mm, n = 25; 29°C: 4.1 ± 0.2 mm, n = 23]. We therefore used the larval body size as a staging criterion to developmentally match mid third instar male larvae (feeding stage) before processing.
Analysis of larval locomotion
Size-matched mid third instar larvae (feeding stage) were video taped directly either from the wall of culture vials (average larval length ± SDM: 3.6 ± 0.6 mm, n = 40) or from the surface of agar plates (average larval length: 3.7 ± 0.2 mm, n = 13), onto which they have been transferred after a short rinse in water. The temperature of agar plates was controlled with a custom-made water bath, in which the bottom of the agar plate dipped into circulating water of 18, 25, or 29°C. Video sessions started 20-60 min after the transfer of larvae onto agar plates, and they typically lasted 10-15 min; in a few experiments, they lasted up to 90 min. These recordings were used for off-line analysis of (1) larval locomotor parameters during stretches of fast-forward locomotion and (2) the total crawling distance over time.
Locomotor parameters. From each recorded animal we first selected two stretches of fast-forward locomotion, which is characterized by a straight and uninterrupted crawling path. We measured the larval length (ll), the moved distance (d1 and d2), the time spent (t1 and t2), and the number of performed contractions (c1 and c2). From these measurements we calculated the stride length [(d1/c1 + d2/c2)/2ll], i.e., the moved distance per contraction wave, the stride frequency [(c1/t1 + c2/t2)/2], and the speed [(d1/t1 + d2/t2)/2ll].
Crawling distance. This is a measurement of the length of the entire crawling path covered within 45 min (see Fig. 2a,b) or 10 min (see Fig. 2c-e) after an equilibration period of 20-60 min.
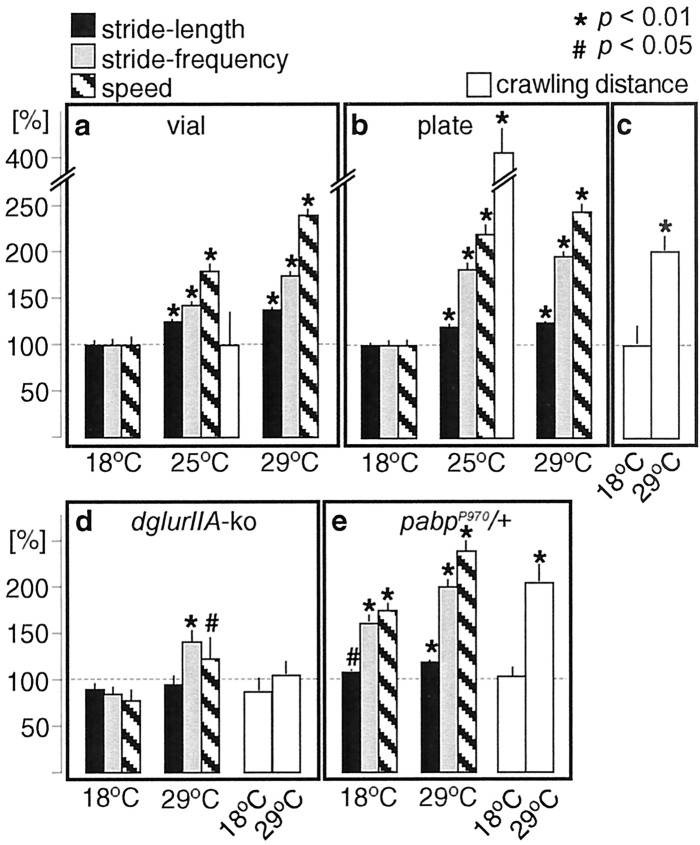
Figure 2.
Temperature dependence of larval locomotor activity. Larvae were reared at the indicated temperatures and video taped as mid third instar larvae (feeding stage) either from the wall of culture vials (a) or from the surface of isothermal agar plates (b-e). Shown are the following locomotor parameters during stretches of fast-forward locomotion (see Materials and Methods) of size-matched animals: moved distance per contraction wave (stride-length), frequency of contraction waves (stride-frequency), and the average speed of locomotion. To estimate the overall locomotor activity of larvae, we monitored the crawling distances (see Materials and Methods) of size-matched animals over 45 min (a, b) or 10 min (c-e). a, b, At a given temperature, wild-type animals showed similar locomotor parameters during stretches of fast-forward locomotion on both culture vials and agar plates. However, because of the strong differences of larval behavior within the food slurry of culture vials and on agar plates, the overall larval crawling distance was significantly larger on 25°C agar plates than in 25°C food vials. At 29°C the measured parameters of fast-forward locomotion were significantly higher than at 18°C, resulting in a significantly larger larval crawling distance per 10 min interval on 29°C agar plates versus 18°C agar plates (c). d, In dglurIIA-ko mutants the stride length remained unaltered at 18 and 29°C, whereas stride frequency and speed of locomotion showed significant temperature-dependent changes. However, these temperature-dependent differences did not significantly alter the crawling distance. e, pabpP970/+ mutants showed enhanced locomotor parameters during fast-forward locomotion at 18°C; however, the crawling distance over 10 min remained similar to wild type. Rearing at 29°C further increased the locomotor parameters and resulted in similarly larger crawling distances as in wild-type larvae. The number of animals was as follows: locomotor parameters: a, 21, 40, 42; b, 11, 13, 25; d, 5, 6; e, 11, 18; crawling distance: a, 4; b, 20, c,: 9, 8; d, 9, 19; e, 16, 11. Data represent means ± SEM.
NMJ size
Size-matched mid third instar male larvae (feeding stage) were filleted and processed for immunofluorescence with antibodies recognizing the cell adhesion molecule Fasciclin II (Fas II) [monoclonal antibody (mAb) 1D4] or the anti-HRP-epitope (Sigma, St. Louis, MO) as described previously (Schuster et al., 1996). The number of type I boutons was counted from muscles 6/7 of abdominal segment 2 or 3, the dimensions (length × width of the exposed muscle surface) of which were comparable as measured with an eyepiece micrometer (average inner muscle 6/7 surface area ± SDM: 43.9 ± 5.9 eyepiece units2, n = 200; 1 U2 ≈ 1400 μm2). To obtain a value that expresses the size of a given NMJ per muscle surface area (see Fig. 1, “# of boutons/muscle surface area”), we divided the counted number of boutons by the measured muscle surface area of that muscle.
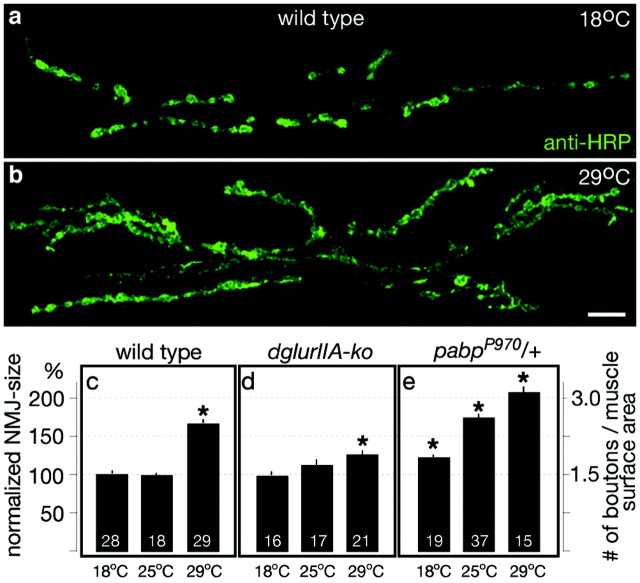
Figure 1.
Increased outgrowth of NMJs at 29°C rearing temperature. a,b, Confocal images of anti-HRP-labeled NMJs (muscle 6/7, abdominal segment 2) of wild-type larvae reared at 18°C (a) or 29°C (b). Scale bar, 20 μm. c-e, Quantification of NMJ size on muscle 6/7 of abdominal segment 2 (see Materials and Methods) in wild-type larvae (c), dglur-IIA-ko animals (dglurIIAAD9/df(2L)clh4) (d), and pabpP970/+ larvae (e) that have been reared at the indicated temperatures. Larvae reared at 29°C developed significantly larger NMJs than animals raised at 25 or 18°C (*p < 0.001). This effect was particularly prominent in pabpP970/+ larvae but significantly suppressed in dglur-IIA-ko animals (p << 0.001). Note that pabpP970/+ larvae showed a strong increase in bouton outgrowth at 25°C, whereas NMJs of wild-type and dglur-IIA-ko animals developed simple NMJs at this rearing temperature. Data represent means ± SEM.
Electrophysiology
Experiments were performed on size-matched mid third instar male larvae at 22°C. All recordings of evoked excitatory junctional currents (eEJCs) and miniature EJCs (mEJCs) were performed in two-electrode voltage clamp mode as described previously (Reiff et al., 2002). In short, muscle cells of dissected larvae were impaled with two 15-30 MΩ microelectrodes filled with 2 m KCl. Cells with a resting potential more negative than -60 mV and an input resistance Rin of ∼5 MΩ were selected for further analysis in hemolymph-like solution (HL3) solution (Stewart et al., 1994) containing 1 mm Ca2+. In two-electrode current clamp mode, the Rin was calculated by measuring the steady-state voltage drop (ΔU) caused by injection of ΔI = -2 nA (Rin = ΔU/ΔI). For stimulation the cut end of the intersegmental nerve was placed into a suction electrode, and suprathreshold current pulses were applied at 0.1 Hz. We recorded 30-50 eEJCs (voltage clamp at -60 mV) and 90 sec of mEJCs (voltage clamp at -70 mV) per cell. Intracellular recordings of spontaneous and evoked excitatory membrane potentials (mEJPs and eEJPs) were performed in HL3 containing 1.5 mm Ca2+ using bridge-mode recordings. Data analysis was performed off-line (pClamp6, Axon Instruments; Jaejin Software). In addition to the amplitudes of evoked and spontaneous vesicle release events, we determined the frequency of spontaneous events from all recordings. The average frequency of mEJCs and mEJPs recorded in these experiments was highly variable between individual preparations ranging from ∼1.5 to 8 Hz. This large variability was observed in all animal populations and experimental conditions and therefore was not a useful parameter to characterize the functional properties of the analyzed NMJs in our work.
Antibodies
We used the following antibodies: Fasciclin II: mAb 1D4 (gift of Corey S. Goodman, University of California Berkeley, Berkeley, CA); DGluR-IIA: DM2 (gift from Yoshiaki Kidokoro, Gunma University, Gunma, Japan); eukaryotic initiation factor 4e (eIF4e) (gift of Paul Lasko, University of Montreal, Montreal, Canada); anti-HRP (Sigma).
Quantification of immunofluorescence signals
Size-matched mid third instar male larvae (feeding stage) of 18 and 29°C reared wild-type Drosophila were filleted and processed in the same vial for immunofluorescence with antibodies recognizing DGluR-IIA (DM2) and Fas II (mAb 1D4) as described previously (Schuster et al., 1996). Three isolated type Ib boutons (muscle 6/7, abdominal segment 2) were randomly selected in the anti-Fas II channel from a recorded confocal image stack, and the average fluorescence signal of this selection was determined in both channels. From these mean pixel values we subtracted the mean background pixel value obtained similarly from a nearby muscle region of the same shape. This quantification of the mean pixel values was performed at six nonoverlapping areas of a given NMJ and subsequently averaged. We analyzed six animals per experimental condition.
Electron microscopy
Ultrastructural examinations were performed as described previously (Sigrist et al., 2002). In short, serial ultrathin sections of boutons (muscle 6/7, segment A2) were photographed at 21.000-fold enlargement, scanned, and reconstructed. We determined the surface area by measuring the bouton perimeters in every section and integrating them over the depth of the reconstruction (87 nm thickness per section). The sizes of individual synapses (see Fig. 6, dense areas between arrowheads) were determined similarly. We also scored the presence or absence of presynaptic dense bodies (see Fig. 6a, T-bars, arrow). From these raw data we derived the values given in Figure 6b and Table 1.
Figure 6.
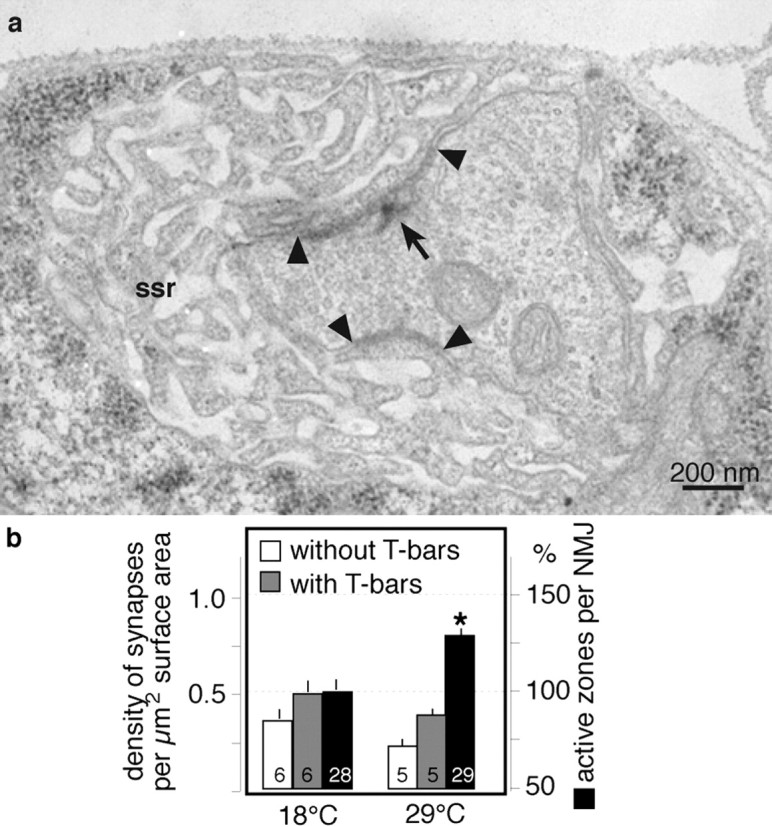
Ultrastructural effects of elevated locomotor activity. a, Representative transmission electron microscopy image of a type Ib bouton of a wild-type larva (muscle 6/7, abdominal segment 2). Marked are synapses (dense areas between arrowheads), the subsynaptic reticulum (ssr), and a presynaptic dense body (T-bar, arrow). Large sequential series of such images were used to reconstruct junctional branches and analyze ultrastructural changes in larvae reared at 18 or 29°C (Table 1; see Materials and Methods). b, Rearing at 18 and 29°C resulted in a similar density of synapses with T-bars (i.e., active zones; gray bars) and without T-bars (white bars). Because 29°C larvae develop more synapse-harboring boutons, this lead to a significant increase in the total number of active zones per NMJ compared with 18°C reared larvae (black bars; p < 0.001). Data are taken from Table 1 and given as means ± SEM.
Table 1.
Summary of ultrastructural analysis
|
|
Rearing condition |
||
|---|---|---|---|
| Characteristic
|
18°C
|
29°C
|
|
| Number of branches | 6 | 5 | |
| Total length of branches (μm) | 40.1 | 48.7 | |
| Total junction surface (μm2) | 420.9 | 506.9 | |
| Number of dense areas | 222 | 191 | |
| Number of dense areas with one T-bar (% of all) | 116 (52.3%) | 95 (49.7%) | |
| Number of dense areas with more than one T-bar (% of all) | 19 (8.6%) | 30 (15.7%) | |
| Number of dense areas without T-bar (% of all) | 87 (39.2) | 66 (34.6%) | |
| Mean size of dense areas (μm2) | 0.33 ± 0.04 | 0.35 ± 0.02 | |
| Number of dense areas per surface area (μm2) | 0.56 ± 0.03 | 0.42 ± 0.06 | |
| Number of dense areas with T-bars per surface area (μm2) | 0.32 ± 0.04 | 0.27 ± 0.02 | |
| Number of dense areas with more than one T-bar per surface area (μm2) | 0.05 ± 0.01 | 0.06 ± 0.01 | |
| Number of dense areas without T-bar per surface area (μm2) | 0.23 ± 0.03 | 0.16 ± 0.03 | |
| Size of entire NMJ (arbitrary units) (number of boutons per muscle surface area) | 1.50 ± 0.06 | 2.49 ± 0.07* | |
| Total number of active zones per entire NMJ
|
100% ± 4%
|
129% ± 4%*
|
|
Summary of scored and derived data obtained from serial reconstructions of junctional branches (Sigrist et al., 2002). NMJs of wild-type larvae, which have been raised at either 18 or 29°C, distribute their active zones (dense areas with presynaptic T-bars) at similar densities (dense areas with T-bars per surface area). Note the unproportionally high number of complex synapses (number of synapses with more than 1 T-bar) in 29°C reared larvae. Larvae reared at 29°C develop larger NMJs and therefore harbor significantly more active zones than NMJs of 18°C reared animals (*p<0.01). The total number of active zones per NMJ was extrapolated by multiplying the measured NMJ sizes of each rearing condition (Fig. 1c) with the respective mean density of active zones. All data were obtained from individual branches and then combined to mean values (±SEM) per rearing condition.
Results
Rearing temperature can alter the morphological outgrowth of developing Drosophila NMJs
The morphological analysis of larval NMJs of Drosophila has revealed that the number of synaptic boutons varies even within an isogenic population of animals of comparable age and size (unpublished observations). This variability in NMJ size can be reduced by controlling the culture conditions of the animals to be examined (Schuster et al., 1996a,1996b; Sigrist et al., 2000; 2002; Reiff et al., 2002; Sanyal et al., 2002). These observations have suggested that the morphological and presumably the physiological development of NMJs is sensitive to yet uncharacterized environmental factors. We therefore used for all experiments in this and previous studies a standardized larval culture (see Materials and Methods) to generate consistent and reproducible conditions for larval development with as few as possible individual variances. On the basis of this larval rearing we set out to define conditions that could be used experimentally to reproducibly affect the morphological and functional development of NMJs in wild-type larvae.
We first tested whether the rearing temperature affects the development of NMJs. Although the NMJ morphologies of mid third instar wild-type larvae reared at 18 or 25°C were relatively simple (Fig. 1a), size-matched animals reared at 29°C showed consistently larger and apparently more complex NMJs on similarly sized larval muscles (Fig. 1b). In fact, quantification of muscle 6/7 NMJ size by counting the number of synaptic boutons and measuring exposed muscle surface in the filet preparations area of the muscle 6/7 pair revealed a significant increase in the normalized NMJ size in 29°C reared animals compared with larvae raised at 18 or 25°C (Fig. 1c). These temperature treatments of developing Drosophila larvae affected neither the size of larval body wall muscles nor the viability and fertility of emerging adult flies, suggesting that 29°C rearing of Drosophila larvae triggers cellular signals that can accumulate in an over-proportional outgrowth of junctional boutons on larval muscles of similar sizes.
Temperature-induced bouton outgrowth requires intact synaptic signal transmission
The temperature-induced bouton outgrowth at developing NMJs of wild-type larvae was similar in shape and dimension to that described previously for model genotypes of activity-dependent junctional plasticity, such as the hyperactive potassium channel mutant eag1, Sh102 (Budnik et al., 1990; Schuster et al., 1996b) or animals with genetically elevated subsynaptic protein synthesis (pabpP970/+) (Sigrist et al., 2000). We have shown recently that the additional bouton outgrowth seen in the above mutants requires an increased synaptic expression of the postsynaptic glutamate receptor subunit DGluR-IIA (Reiff et al., 2002; Sigrist et al., 2002), suggesting that DGluR-IIA-mediated signal transmission at NMJs is involved in the regulation of the morphological development of larval NMJs. We therefore examined the size of NMJs in dglurIIA-ko mutants, which have a compromised junctional signal transmission showing significantly reduced quantal sizes (Petersen et al., 1997; DiAntonio et al., 1999) and an enhanced depression of evoked signal transmission during high-frequency stimulation (Reiff et al., 2002).
Rearing of dglurIIA-ko mutants at 18 or 25°C revealed no difference in the morphological development of NMJs compared with wild-type controls (Fig. 1c,d). Rearing of dglurIIA-ko mutants at 29°C resulted in a small but significant (p < 0.01) increase in the number of presynaptic boutons relative to 18 or 25°C reared mutants (Fig. 1d, right bar). However, compared with similarly raised wild-type animals (Fig. 1c, right bar), the stimulation of bouton outgrowth at 29°C was significantly suppressed in this mutant (p << 0.001). These results show that the temperature-induced outgrowth of wild-type NMJs depends primarily on an intact synaptic expression of the DGluR-IIA subunit.
On the basis of the specific defects in synaptic signal transmission described for this postsynaptic glutamate receptor mutant (Petersen et al., 1997; DiAntonio et al., 1999; Reiff et al., 2002), it appears likely that the temperature-induced bouton outgrowth is not caused simply by potential pleiotropic effects arising, e.g., from a generally enhanced cellular metabolism at elevated temperatures. Instead, our data suggest that intact synaptic signal transmission at NMJs is directly or indirectly involved in the regulation of temperature-induced bouton outgrowth. This interpretation is consistent with our behavioral data of the dglurIIA-ko mutant (see Fig. 3), which show that larval locomotor behavior is considerably compromised in this mutant.
Figure 3.

Experience-dependent strengthening of Drosophila NMJs. Two-electrode voltage-clamp recording of eEJCs and mEJCs from muscle 6 of abdominal segment 2. a, Shown are representative traces of mEJC recordings (top panels) and average traces of 10 consecutively recorded eEJCs of animals raised at either 18 or 29°C. b, All locomotor-stimulated animals showed a significantly larger junctional quantal content and thus enhanced junctional signal transmission compared with controls. This effect was already maximal after 2 hr of enhanced locomotor activity. Larval locomotor activity was acutely enhanced by transferring 18°C reared mid third instar larvae (feeding stage) onto agar plates (29°C) for 2 and 4 hr; chronic locomotor enhancement was achieved by continuously rearing larvae at 29°C (Fig. 2). From these animals we measured the muscle 6 input resistances Rin as an estimate of the relative muscle sizes (bottom panel, gray bars) and the amplitudes of spontaneous mEJCs and stimulation evoked eEJCs (top panel, gray and black bars). The derived junctional quantal content (mean eEJC amplitude/mean mEJC amplitude) (bottom panel, black bars) gives an estimate of the number of presynaptic vesicles released per action potential and thus represents a measure of the efficacy of evoked junctional signal transmission. Note that the slight but significant reduction of mEJCs in 4 hr stimulated animals is likely caused by the somewhat smaller muscle cells (larger Rin) of this animal population. Because the eEJC amplitudes are reduced proportionally in these cells, the quantal content is restored. The number of analyzed cells per experimental condition is given in b, below the top panel. c, Intracellular recordings of mEJPs and eEJPs in wild-type larvae and the temperature-sensitive paralytic mutant parats1 revealed that 2 hr of enhanced larval locomotor activity at permissive temperature (22°C agar plates) results in a consistent and significant strengthening of junctional signal transmission in both genotypes (wild type, n = 7; parats1, n = 6) compared with their 18°C reared siblings (wild type, n = 11; parats1, n = 9). These larvae were then treated with a temperature-shift protocol (20 min at 34°C followed by maintained 29°C on agar plates) that results in immediate paralysis of parats1 mutants, whereas wild-type larvae continue vigorous locomotion. Within 2 hr of paralysis the eEJP amplitudes of parats1 mutants dropped to the control value (n = 5), whereas signal transmission at wild-type NMJs was enhanced further (n = 6). The mEJP amplitudes of all three experimental conditions were similar (wild type 18°C:1.17 ± 0.07 mV; 2 hr 22°C plate:1.06 ± 0.08 mV; +2 hr paralysis protocol: 1.06 ± 0.05 mV; parats1 18°C: 1.02 ± 0.12 mV; 22°C plate: 0.91 ± 0.06 mV; +2 hr paralysis protocol: 1.14 ± 0.11 mV). d, The quantal content (expressed as percentage of control genotypes) is plotted as a function of the normalized NMJ size. Wild-type larvae that have been chronically reared at either 18 or 29°C develop NMJs with a typical relationship between NMJ size and transmission strength (black and white pentagons). A similar relationship has been described previously in several model genotypes of junctional plasticity, such as the transgenic overexpression of the glutamate receptor subunit DGluR-IIA (black and white circles) and the pabpP970/+ mutant (black square and white triangle) (Sigrist et al., 2002). However, wild-type animals that experienced acute locomotor stimulation showed enhanced junctional signal transmission in the absence of additional growth (white squares). Data represent means ± SEM. t test results: *p < 0.001; #p < 0.005.
We similarly analyzed larvae that were heterozygous mutant for the poly(A)-binding-protein locus (pabpP970/+) (Sigrist et al., 2000), an RNA-binding protein that has been shown to function in the initiation process of mRNA translation (Gallie, 1998). We have reported recently that the pabpP970/+ mutant generates significantly more subsynaptic eIF4e aggregates (Sigrist et al., 2002), presumably because of a sensitization of the subsynaptically localized protein synthesis machinery (Sigrist et al., 2000). In addition, this mutant develops significantly larger NMJs with an increased strength of junctional signal transmission (Sigrist et al., 2000, 2002; Reiff et al., 2002). These phenotypes were mostly suppressed in a dose-dependent manner by removal of one or both dglurIIA gene copies (Sigrist et al., 2002). The significantly larger NMJs of pabpP970/+ mutants at 25°C compared with wild type (Fig. 1, compare c, e) appear to be caused by an increased efficiency of this mutant in triggering subsynaptic protein synthesis at this temperature. Because these phenotypes of pabpP970/+ mutants require intact DGluR-IIA-mediated synaptic signal transmission (Sigrist et al., 2002), it seems that the regulation of the temperature-dependent changes of NMJ development involves temperature-dependent changes in junctional signal transmission itself. According to such a model, the temperature-dependent changes of NMJ activity at 25°C would efficiently induce bouton outgrowth in pabpP970/+ mutants, whereas they remain subthreshold in wild-type animals.
Experimental control of larval locomotor activity
Our above results suggest that the observed morphological phenotypes require intact synaptic signal transmission at NMJs. NMJs couple neuronal excitation to muscle contractions, which in the case of the single-fibered body wall muscles of Drosophila larvae drive body movements and ultimately larval crawling. It is therefore likely that the neuronal activity, which is transmitted by NMJs, is reflected in the crawling behavior of larvae. Crawling of Drosophila larvae is a simple locomotor behavior that has been analyzed extensively to detect alterations in the locomotor pattern in wild-type and mutant animals (Sokolowski, 1980; Pereira et al., 1995; Wang et al., 1997, 2002). However, these studies have generally been performed at room temperature. We therefore quantified the influence of temperature on the larval locomotor pattern both on the wall of larval culture vials (Fig. 2a) and on agar plates (Fig. 2b-e). Our analysis included an examination of locomotor parameters during uninterrupted stretches of fast-forward locomotion, such as the moved distance per contraction wave (stride-length) (Fig. 3, black bars), the frequency of contraction waves (stride-frequency) (Fig. 3, gray bars), and the speed of locomotion (Fig. 3, hatched bars). We also quantified the length of the crawling path over time (crawling distance) (Fig. 3, white bars) as an estimate of the overall larval locomotor activity.
At a given rearing temperature, wild-type animals showed similar stride lengths, frequencies of contraction waves, and crawling speeds during stretches of fast-forward locomotion either on walls of culture vials or on agar plates (Fig. 2a,b). These findings suggest that fast-forward locomotion is a rather stereotypic locomotor behavior that appears to be used by larvae on relatively clean surfaces. It is important to note, however, that feeding-stage third instar larvae, which are kept in the soft food slurry of culture vials, stay feeding within the food most of the time and only occasionally leave the food for short stretches of fast-forward locomotion on the vial wall. In contrast, larvae transferred onto isothermal agar plates show an almost continuous locomotor performance with only short resting phases, presumably for reorientation. These obvious behavioral differences of feeding stage third instar larvae in food vials and on agar plates are well reflected in the crawling distances measured over 45 min (Fig. 2a,b, white bars) (see Materials and Methods), which are significantly longer on agar plates (160.8 ± 10.6 cm; n = 20) than in food slurry (39.8 ± 13.8 cm; n = 4). We therefore conclude that larvae that have been transferred onto isothermal agar plates have a significantly enhanced locomotor activity compared with their siblings in culture vials.
We found further that increased larval rearing temperatures are associated with significant increases of all analyzed locomotor parameters in vials and on plates (Fig. 2a,b). These changes are well reflected in the crawling distances measured over 10 min on agar plates (Fig. 2c, white bars), with a significantly enhanced locomotor activity of larvae on 29°C agar plates compared with those at 18°C. These results demonstrate that the locomotor activity of size-matched third instar larvae is subject to temperature-dependent and environmental changes, which can be controlled experimentally.
Elevated locomotor activity induces NMJ outgrowth
A similar behavioral analysis of dglurIIA-ko mutants revealed an unaltered stride length at 29 and 18°C but significant changes in the frequency of contraction waves and the speed of locomotion (Fig. 2d, gray and hatched bars). However, these significant changes did not translate into a similarly large increase of the measured crawling distance over 10 min as seen in wild-type animals at 29°C (Fig. 2c,d, white bars). This observation may be explained by the obviously altered locomotor pattern of dglurIIA-ko mutants, which shows only short stretches of uninterrupted locomotion and longer and more frequent resting phases. dglurIIA-ko mutants still developed slightly larger NMJs at 29°C than at 18°C (Fig. 1d); however, this additional bouton outgrowth at 29°C was significantly suppressed compared with that observed in wild-type animals (Fig. 1c). These data suggest that the specific defects in synaptic signal transmission at NMJs are responsible for both the failure of dglurIIA-ko mutants to significantly enhance the overall locomotor activity at 29°C and the strong suppression of induced bouton outgrowth. They also suggest that the temperature-dependent changes of some of the locomotor parameters are sufficient to induce NMJ growth at a low rate. We therefore conclude that it is not the increased rearing temperature itself that causes the observed large-scale overgrowth of NMJs in wild-type larvae; rather, it appears that elevated temperature results in enhanced overall locomotor activity, which then seems to trigger the outgrowth of additional boutons.
To test this idea, we morphologically analyzed NMJs of mid third instar wild-type larvae that were raised at 25°C and experienced increased locomotor activity on 25°C agar plates for 12-18 hr (see Materials and Methods) or that were kept for the same time in 25°C culture vials. As mentioned above, the crawling distance covered by larvae on agar plates is approximately fourfold at 25°C compared with that of their siblings within the food slurry. Larvae that experienced such enhanced locomotor activity for 12-18 hr developed slightly but significantly more boutons per muscle surface area than animals with lower locomotor activity over the entire period (normalized NMJ size in vials: 2.25 ± 0.09; 100 ± 4%, n = 23; on plates: 2.49 ± 0.06; 111 ± 3%, n = 50; p = 0.04). These on average slightly larger NMJs in animals with acutely increased locomotor activity show that bouton outgrowth can indeed be induced in the absence of temperature shifts. However, compared with chronic treatments of larvae, e.g., by continuous 29°C rearing or mutant backgrounds (e.g., pabpP970/+ or eag, Sh, or transgenic DGluR-IIA overexpression), which typically result in >50% larger and more complex NMJs (Fig. 1c,e) (Sigrist et al., 2002), the observed acute bouton outgrowth appears to be rather mild (11%). This small morphological change after up to 18 hr of locomotor stimulation offers insight into the temporal dynamics of morphological regulation at developing NMJs, suggesting that the induction or execution of additional bouton outgrowth is a rather slow process.
We finally analyzed the locomotor behavior of the abovementioned mutant of the pabp gene, pabpP970/+, which enhances growth stimuli and results in larger NMJs than wild-type animals (Fig. 1c) at all tested rearing temperatures (Fig. 1e) presumably because of its sensitized subsynaptic protein synthesis machinery (Sigrist et al., 2000). According to such a model, one might expect that this mutant overreacts to signals originating from locomotor activity. Consistent with this idea we found that 29°C reared pabpP970/+ mutants exhibited similar locomotor parameters and crawling distances (Fig. 2b,c) than wild-type animals at 29°C (Fig. 2b) but significantly larger NMJs (Fig. 1e). A similar situation was observed at 18°C: pabpP970/+ larvae showed strengthened locomotor parameters during stretches of fast-forward locomotion and unaltered crawling distances over 10 min (Fig. 2e) compared with wild-type animals, but again significantly larger NMJs (Fig. 1e). These results support the idea that pabpP970/+ animals convert signals from increased locomotor activity more efficiently into morphological changes. In summary, our findings suggest that enhanced locomotor activity is a potent but slow regulator of NMJ morphology in wild-type Drosophila larvae.
Experience-dependent strengthening of junctional signal transmission is rapid and reversible
Several recent reports have suggested that the morphological development of larval NMJs is tightly correlated with the strength of junctional signal transmission (Budnik et al., 1990; Schuster et al., 1996b; Cheung et al., 1999; Sigrist et al., 2000, 2002; Reiff et al., 2002; Sanyal et al., 2002). We therefore recorded eEJCs and mEJCs from wild-type animals reared at either 18 or 29°C or that had been transferred onto 29°C agar plates for 2 or 4 hr (Fig. 3). The average amplitudes of mEJCs (Fig. 3a, top traces) and thus quantal sizes were indistinguishable among most analyzed animals (Fig. 3b, top panel, white bars). This finding shows that the physiological properties of individual synapses, such as the amount of glutamate released per vesicle and the postsynaptic sensitivity to released glutamate, were affected neither by elevated rearing temperature nor by enhanced locomotor activity at the indicated time points. However, all animals with enhanced locomotor activity showed substantially bigger evoked responses (Fig. 3b, top panel, black bars) and thus significantly increased quantal contents compared with 18°C reared control animals (Fig. 3b, bottom panel). Interestingly, this experience-dependent strengthening of evoked signal transmission was already maximal after an acute temperature shift of 2 hrs, well before the first morphological changes could be detected at NMJs (Fig. 3d). These results suggest that enhanced larval locomotor activity leads to a fast and substantial strengthening of junctional signal transmission of wild-type NMJs.
To exclude any potential temperature effects on the development of the junctional transmission strength, we included an analysis of the temperature-sensitive paralytic mutant parats1 (Ganetzky, 1984), which appears to develop normally at the permissive temperature of ≤22°C. At restrictive temperatures, however, such as 29°C or higher, the voltage-gated sodium channel encoded by the gene para fails to operate and eliminates action potential propagation and larval locomotion. We found that 2 hr of surface locomotion on agar plates at permissive 22°C resulted in a consistent strengthening of junctional signal transmission in both genotypes, as indicated by their significantly larger eEJPs compared with animals that remained in the food slurry at 18°C (Fig. 3c). After 3 hr of locomotor stimulation on 22°C agar plates, we transferred the larvae onto agar plates at 34°C for 20 min and then maintained them at 29°C. This protocol resulted in an immediate and persisting paralysis of parats1 mutants, whereas wild-type animals continued vigorous surface locomotion. Within 2 hr of paralysis the eEJP amplitudes of parats1 mutants dropped significantly to a value close to the one before the experiment, whereas signal transmission at wild-type NMJs remained enhanced. These results demonstrate that experience-dependent strengthening of junctional signal transmission does not rely on a temperature shift to 29°C. They instead suggest that enhanced locomotor activity on agar plates can trigger a rapid and reversible strengthening of junctional signal transmission. Moreover, within the up to 6 hr time window of these experiments, we did not observe obvious morphological changes at NMJs, demonstrating that the rapid physiological changes occur in the absence of large-scale morphological growth (Fig. 3d). This finding is consistent with our morphological results, which suggested that the acute induction or execution of additional bouton outgrowth is a rather slow process.
Taken together, our results show that enhanced larval locomotor activity can trigger a rapid and reversible strengthening of junctional signal transmission even in the absence of large-scale morphological alterations. Bouton outgrowth appears to start with a considerable delay relative to the acutely induced physiological changes, so that chronic locomotor stimulation results in NMJs with a typical relationship between transmission strength and NMJ size (Fig. 3d). It therefore appears that the tight correlation between NMJ size and transmission strength that has been described for several genetic backgrounds (Budnik et al., 1990; Schuster et al., 1996b; Cheung et al., 1999; Sigrist et al., 2000, 2002; Reiff et al., 2002; Sanyal et al., 2002) reflects a long-term adaptation of NMJs that is preceded by rapid and reversible physiological changes.
Experience-dependent regulation of subsynaptic protein synthesis
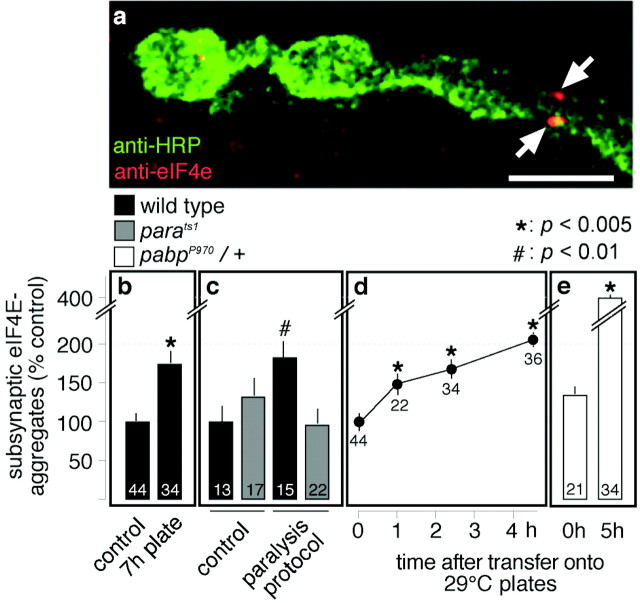
Because of the presumed major importance of local subsynaptic protein synthesis in the spatial organization of synaptic and morphological changes of neuronal connectivity (Martin et al., 2000; Sigrist et al., 2000; Jiang and Schuman, 2002), we determined whether the number of subsynaptically localized aggregates of eIF4e (Fig. 4a) (Sigrist et al., 2000) is altered in wild-type larvae as a result of enhanced locomotor activity. These experiments were performed with larvae reared in standardized culture at 18°C to third instar stage and then transferred onto agar plates either at 18°C (Fig. 4b)or at 29°C (Fig. 4c-e). We found that surface locomotion on agar plates induced a rapid increase in the number of large subsynaptic eIF4e aggregates that was already significant after 1 hr of enhanced locomotor activity at 29°C (Fig. 4b,d).
Figure 4.
Experience-dependent regulation of subsynaptic protein synthesis. a, Large eIF4e aggregates (Sigrist et al., 2000) appear locally within and close to the subsynaptic reticulum of junctional boutons (arrows). Scale bar, 5 μm. b-e, Quantification of large subsynaptic eIF4e aggregates (Sigrist et al., 2002). b, Larvae that showed vigorous locomotor activity on 18°C agar plates for 7 hr developed significantly more eIF4e-positive boutons than controls in standardized culture vials at 18°C. c, After a temperature-shift protocol, which can be used to paralyze the temperature-sensitive mutants parats1 for ∼3 hr (20 min at 34°C, then 29°C), wild-type animals showed vigorous locomotor activity and a significant increase in the number of eIF4e-positive boutons compared with 18°C reared animals (black bars). In contrast, the number of large subsynaptic eIF4e aggregates remained unaltered in paralyzed parats1 mutants compared with those at permissive 18°C (gray bars). d, Time course of subsynaptic eIF4e accumulation after the experimental induction of high locomotor activity by transferring larvae from standardized 18°C cultures vials onto agar plates (29°C). We observed a significant increase in the number of eIF4e-labeled boutons within 1 hr of high locomotor activity. e,pabpP970/+ mutants show a slight but not significant increase in the number of subsynaptic eIF4e aggregates in 18°C reared animals; rearing at 29°C results in a very large and highly significant increase of eIF4e aggregates. Data are plotted as means ± SEM; the numbers of analyzed segments are within bars or below symbols.
To control for potential temperature effects on general protein synthesis that are not triggered by locomotion, we included the temperature-sensitive paralytic mutant parats-1 (Ganetzky, 1984), which could be reversibly paralyzed at increased temperatures. After 1-2 hr of 29°C plate locomotion, wild-type NMJs showed a significant increase in the number of subsynaptic eIF4e aggregates (Fig. 4c, black bars), whereas the paralyzed parats1 mutants showed an apparent slight decline of postsynaptic translation (Fig. 4c, gray bars). These results demonstrate that neither the larval exposure to the agar surface nor the temperature treatment itself is responsible for the observed increase in subsynaptic translation levels. Instead, they strongly suggest that the larval locomotor activity can efficiently stimulate local subsynaptic protein synthesis.
We performed a similar analysis with pabpP970/+ mutants, which showed a very large increase in the number of subsynaptic eIF4e aggregates after 5 hr of sustained surface locomotion on 29°C agar plates (Fig. 4e). This finding is consistent with the idea that the subsynaptic translation machinery in pabpP970/+ mutants is genetically sensitized (Sigrist et al., 2000) and therefore leads to an “over-interpretation” of inductive signals caused by increased locomotor activity. Consequently, this results in an “over-proportional” stimulation of junctional growth in pabpP970/+ larvae (Fig. 1e). Taken together, these data show that subsynaptic protein synthesis can be regulated efficiently and dynamically in an experience-dependent manner by controlling the locomotor activity of Drosophila larvae.
Experience-dependent upregulation of postsynaptic DGluRIIA expression precedes the downregulation of perisynaptic Fasciclin II
We recently showed that the strengthening of junctional signal transmission that can be observed in animals with genetically enhanced subsynaptic protein synthesis (Sigrist et al., 2000) depends on an increased synaptic accumulation of the glutamate receptor subunit DGluR-IIA (Sigrist et al., 2002). It further results in a reduced junctional expression of the cell adhesion molecule Fas II, which is required for morphological growth of NMJs (Schuster et al., 1996a,1996b; Davis and Goodman, 1998; Sanyal et al., 2002). Similar to these genetically induced results, we found that the chronic rearing of wild-type larvae at 29°C is associated with a strongly increased synaptic DGluR-IIA immunoreactivity and a consistently reduced perisynaptic Fas II expression compared with NMJs of 18°C reared animals (Fig. 5c,d). Consistent with previous results (Sigrist et al., 2000, 2002), we found that the enhanced accumulation of DGluR-IIA subunits did not result in larger quantal sizes, a finding that supports the idea that the quantal size and its variation are determined primarily by the size of presynaptic vesicles and thus by the amount of released transmitter glutamate. Our observations provide further evidence that the temperature-induced strengthening of signal transmission and the enhanced bouton outgrowth in wild-type larvae rely on similar molecular and cellular key mechanisms as in previously described mutants (Sigrist et al., 2000, 2002; Reiff et al., 2002).
Figure 5.
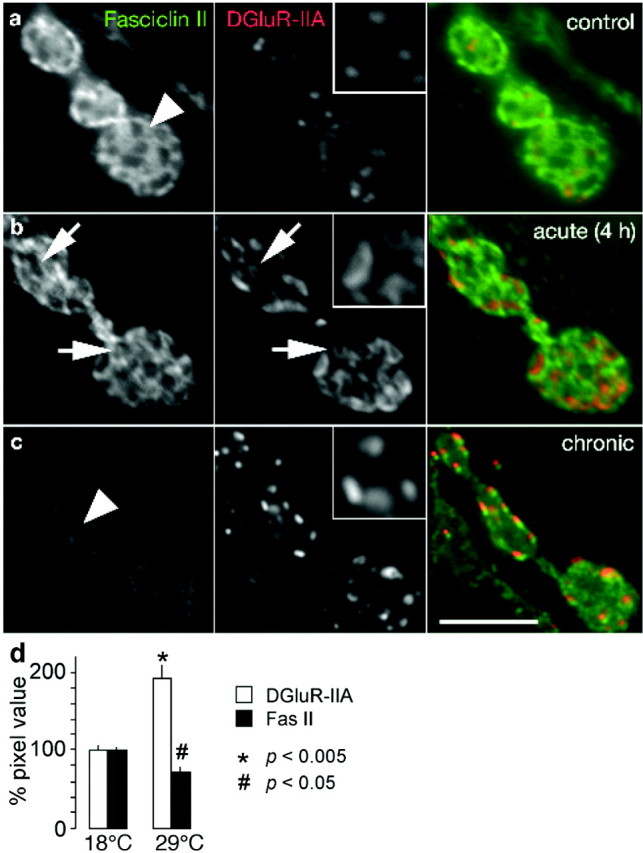
Experience-dependent upregulation of postsynaptic DGluR-IIA expression precedes the downregulation of Fasciclin II (Fas II). Shown are confocal images of boutons double labeled with antibodies recognizing the cell adhesion molecule Fasciclin II (left panels and green channel) and the postsynaptic glutamate receptor subunit DGluR-IIA (middle panels with enlarged insets and red channel). a, Control animals, which have been reared at constant 18°C in standardized larval cultures, show strong perisynaptic Fas II expression (arrowhead) and few DGluR-IIA positive synapses. b, After 4 hr of vigorous locomotor activity on 29°C agar plates, the perisynaptic Fas II expression was essentially unaltered (compare green channels), whereas DGluR-IIA showed an increased, ring-shaped immunoreactivity (arrows) at preexisting synapses (holes in Fas II expression, arrows). c, Larval rearing at 29°C, which is associated with chronically enhanced locomotor activity, leads to an enhanced overall DGluR-IIA immunoreactivity at an increased number of postsynaptic patches. The perisynaptic Fas II immunoreactivity is significantly downregulated (arrowhead, compare green channels). Scale bar, 5 μm. d, Quantification of immunofluorescence signals at NMJs (see Materials and Methods) revealed a significantly enhanced immunoreactivity of postsynaptic DGluR-IIA and a reduced immunoreactivity of Fas II in animals reared at 29°C compared with their 18°C reared sibling. Note that the enhanced DGluR-IIA immunoreactivity is caused by an increased number of DGluR-IIA patches and obviously stronger fluorescence signals per DGluR-IIA patch.
A time-resolved analysis of these events revealed that the DGluR-IIA immunoreactivity at NMJs increases visibly already after 4 hr of surface locomotion on 29°C agar plates (Fig. 5b, red channel). At this time point of acute locomotor stimulation, the DGluR-IIA immunoreactivity often showed a ring-shaped appearance at the outer edge of presumably preexisting synapses (Fig. 5b, inset). This has been rarely or never observed in control larvae (Fig. 5a), chronically stimulated animals (Fig. 5c), or mutants (Sigrist et al., 2002). This observation suggests that the rapid ring-like accumulation of DGluR-IIA, which likely relies on an enhanced subsynaptic translation of postsynaptically stored mRNAs encoding DGluR-IIA subunits (Sigrist et al., 2000), may reflect a transient stage of incorporating DGluR-IIA-containing receptor complexes into individual synaptic sites. However, at this point of the analysis it remains to be investigated whether the ring-like DGluR-IIA immunofluorescence originates from receptor complexes that are already inserted in the perisynaptic plasma membrane to facilitate a lateral exchange with complexes of postsynaptic patches (Young and Poo, 1983; Akaaboune et al., 1999; Meier et al., 2001; Borgdorff and Choquet, 2002). Alternatively, the ring-shaped DGluR-IIA immunofluorescence could also reflect intracellular accumulations of receptor subunits shortly before entering the perisynaptic plasma membrane.
Interestingly, 4 hr after acute locomotor stimulation the perisynaptic Fas II immunoreactivity remained unaltered (Fig. 5b, green channel). A visible downregulation of perisynaptic Fas II was detectable only after chronic locomotor stimulation at 29°C (Fig. 5c,d, green channel). This suggests that Fas II-mediated morphological changes are induced with a significant delay after alterations to postsynaptic glutamate receptors. Taken together, our results demonstrate that the experimental control of larval locomotor activity allows insights into the dynamics of molecular and physiological changes that are involved in the execution of experience-dependent plasticity at larval NMJs.
Experience-dependent strengthening of NMJs involves an increase in the number of active zones per NMJ
On the basis of extensive ultrastructural analyses in several mutations that enhance junctional signal transmission and morphological growth, it has been suggested recently that the number and density of active zones within NMJs are tightly regulated throughout development (Meinertzhagen et al., 1998; Reiff et al., 2002; Sigrist et al., 2002). From these studies it emerged that genetically and thus chronically strengthened signal transmission relies on an upregulation of the total number of active zones per NMJ and their even distribution in newly grown boutons (Reiff et al., 2002; Sigrist et al., 2002). We therefore assessed whether the enhanced signal transmission at NMJs of 29°C reared wild-type larvae (Fig. 3) involves a similar morphological consolidation by comparing the ultrastructure of serially reconstructed boutons of wild-type larvae raised at 18 or 29°C (Fig. 6a) (see Materials and Methods). Our analysis revealed that the density of active zones, i.e., synapses with a T-shaped presynaptic dense body that are thought to represent synapses with a high probability of vesicle release (Wojtowicz et al., 1994; Cooper et al., 1995, 1996; Atwood and Wojtowicz, 1999), is slightly but not significantly reduced in boutons of 29°C reared animals (Fig. 6, gray bars, Table 1). However, the percentage of complex synapses, active zones with more than one presynaptic T-bar, which may represent synapses with a very high vesicle release probability (Wojtowicz et al., 1994), has roughly doubled from 8.6% at 18°C rearing to 15.7% in 29°C animals (Table 1). Given that the number of boutons is strongly increased in 29°C reared animals, we extrapolated a significant elevation of the total number of active zones per NMJ (Fig. 6, filled bars). This finding is essentially consistent with our previous results (Reiff et al., 2002; Sigrist et al., 2002), and it provides further evidence for the idea that larval NMJs tend to rapidly consolidate strengthened signal transmission by the addition of simple and complex synaptic release sites to the synaptic system of NMJs.
Discussion
So far, activity-dependent changes at Drosophila NMJs have been analyzed almost exclusively in mutants. Such mutant animals were affected in their neural excitability or in one of the potential downstream mechanisms that sense, signal, or execute alterations at NMJs. These studies have provided detailed insights into the functional relevance of individual genes for NMJ plasticity. However, because of the mostly chronic defects of mutations, the temporal sequence of the identified mechanisms remained elusive. This study therefore aimed at providing an experimental framework to uncover the temporal sequence of events involved in activity-dependent alterations at developing larval NMJs. We show that locomotor activity of larvae can be controlled experimentally and that enhanced locomotor activity triggers a cascade of, in part, reversible events that result in the long-term strengthening of larval NMJs.
Temperature, locomotor activity, and experience-dependent adaptations at larval NMJs
One of the prerequisites for a time-resolved analysis of experience-dependent adaptations at Drosophila NMJs was the tight control of larval locomotor activity. Acute enhancement of larval locomotor activity was achieved by transferring larvae from food vials onto agar plates (Fig. 2c,d), a procedure that has been used extensively before as a locomotor reference in the genetic analysis of larval foraging behavior (Shaver et al., 2000; Sokolowski, 2001). In addition, larval locomotor activity was persistently modified at different temperatures (18, 25, and 29°C) (Fig. 2a-c) that were well within the natural temperature range of Drosophila development (12-32°C) (Ludwig and Cable, 1933). Both paradigms enabled us to control larval locomotor activity and therefore allowed a time-resolved analysis of experience-dependent adaptations at developing NMJs of Drosophila.
Three independent lines of evidence suggest that the morphological and physiological changes at NMJs described here are triggered by enhanced larval locomotor activity and not caused by temperature treatment or plate transfer itself. First, the considerable bouton outgrowth seen in wild-type larvae reared at 29°C was significantly suppressed in 29°C reared dglurIIA-ko mutants (Fig. 1), which show defective postsynaptic signal transmission (Petersen et al., 1997; DiAntonio et al., 1999), rapid depression of spike train-evoked junctional signal transmission (Reiff et al., 2002), reduced locomotor activity (Fig. 2c), and reduced subsynaptic protein synthesis (Sigrist et al., 2002). Second, exposing wild-type and parats1 larvae to permissive 22°C agar plates for 2-3 hr resulted in a significant and similar strengthening of junctional signal transmission in both genotypes that was rapidly reversed by induced paralysis in parats1 animals (Fig. 3c). Third, wild-type larvae reared at 25°C and exposed to 25°C agar plates for up to 18 hr showed significantly enhanced locomotor activity (Fig. 2a,b), and they developed more boutons than comparable animals that remained in the food slurry. These experiments show that whenever synaptic signal transmission and larval locomotor activity was compromised, such as in dglurIIA or paralyzed parats1 mutants, the junctional phenotypes were strongly suppressed. We therefore conclude that the acute and chronic exposure of Drosophila larvae to elevated temperatures or agar plates leads to an enhanced larval locomotor activity (Fig. 2), which results initially in reversible physiological changes (Fig. 3) and later on in molecular (Figs. 4, 5) and cellular adaptations (Fig. 1) that ensure persistently enhanced junctional signal transmission and efficient muscle contraction.
Experience-dependent strengthening of Drosophila NMJs
One of the first obvious consequences of enhanced locomotor activity was the fast enhancement of evoked junctional signal transmission, which was already maximal after 2-4 hr of locomotor stimulation on agar plates and was rapidly reversed by paralysis (Fig. 3d). The observation that the quantal sizes remained unaltered at the indicated time points of locomotor stimulation, whereas evoked junctional responses increased significantly, strongly suggests that the phases of experience-dependent strengthening of Drosophila NMJs are based primarily on an enhanced release of presynaptic vesicles per NMJ (Fig. 3b).
Mechanisms that can result in a fast increase in the number of released vesicles include an enhanced presynaptic Ca2+ influx (Mallart, 1993), alterations in the Ca2+ sensitivity of the presynaptic release process (Atwood and Karunanithi, 2002), activation of presynaptic metabotropic glutamate receptors (Zhang et al., 1999), or signaling mediated by the presynaptic G-protein-coupled receptor Methuselah (Song et al., 2002). These mechanisms are typically involved in transient short-term enhancements of synaptic signal transmission. It appears likely that these or similar mechanisms are active during early phases of the experience-dependent junctional strengthening described here; however, their exact involvement as well as their temporal regulation remain to be investigated.
The number of released presynaptic vesicles can also increase at NMJs with a larger number of active release sites (Reiff et al., 2002; Sigrist et al., 2002). Our ultrastructural and morphological analysis of NMJs revealed that animals that experienced persistently enhanced locomotor activity (rearing at 29°C) developed larger NMJs (Fig. 1) with an increased total number of T-bar-harboring active zones and an unaltered density of active zones per bouton (Fig. 6b, Table 1). Because active zones represent synapses with a high probability of vesicle release (Wojtowicz et al., 1994), this mechanism could account for the observed strengthening of junctional signal transmission at larger NMJs. In fact, such a typical relationship between the number of active zones and the number of junctional boutons is readily apparent in the consistently observed correlation between junctional transmission strength and NMJ size (Fig. 3d). These data suggest that the fast-developing NMJs of Drosophila larvae consolidate induced functional changes by recruiting active zones and controlling their density by growing additional boutons. Recent experiments have shown that such NMJs not only transmit single stimuli more efficiently than control NMJs, they also show an enhanced faithfulness in the transmission of high-frequency stimuli (Reiff et al., 2002).
It is intriguing to note that the scored electrophysiological parameters were indistinguishable among most locomotor-stimulated animals (Fig. 3). This included larvae, which experienced 2-6 hr of locomotor stimulation. NMJs of these larvae showed no detectable bouton outgrowth compared with their controls (Fig. 3d, white squares), suggesting that this early phase of junctional strengthening does not rely on large-scale morphological alterations. We have shown recently that dglurIIA-ko mutants are unable to greatly enlarge their NMJs by bouton addition (Reiff et al., 2002; Sigrist et al., 2002). This mutant mediates enhanced presynaptic vesicle release (Petersen et al., 1997) by increasing the number of active zones; however, these additional active zones are squeezed into a smaller number of preexisting boutons compared with wild type (Reiff et al., 2002). It is therefore tempting to speculate that within 2 hr of locomotor stimulation NMJs start to increase the number of active zones by de novo synaptogenesis and by rapidly recruiting presynaptic T-bars (dense bodies) onto a large reservoir of preexisting and T-bar-free synapses. In fact, such a fast recruitment of presynaptic dense bodies to synapses has been proposed for synapses in the fly visual system (Brandstatter and Meinertzhagen, 1995). It is therefore possible that experience-dependent strengthening of junctional signal transmission is mediated primarily by the functional recruitment of additional active zones, which are later distributed in newly grown boutons at their typical density. These processes would leave the efficacy of junctional signal transmission unchanged even during the morphological expansion of NMJs. Unfortunately, because of the current lack of probes that could specifically recognize T-bars or active zones in vivo or in light-microscopic preparations, we have been unable to address these potentially highly dynamic processes at larval NMJs of Drosophila.
A temporal map of experience-dependent alterations at Drosophila NMJs
Our results show that enhanced locomotor activity results within 2 hr in a rapid and reversible enhancement of evoked junctional signal transmission and in a similarly fast stimulation of local subsynaptic protein synthesis (Fig. 4). Although it remains to be investigated whether localized subsynaptic protein synthesis could play an instructive role during these early physiological events, we have found recently that the mRNA encoding the glutamate receptor subunit DGluR-IIA (Schuster et al., 1991) is stored within the subsynaptic compartment of NMJs (Sigrist et al., 2000). It therefore represents a likely substrate of localized subsynaptic protein synthesis. We found that DGluR-IIA-specific immunoreactivity increased visibly after 4 hr of locomotor stimulation (Fig. 5), first in the form of ring-shaped accumulations at the edge of preexisting synapses (Fig. 5b) and after chronic stimulation within typical postsynaptic patches (Fig. 5c). Given that several ionotropic neurotransmitter receptors perform lateral diffusion movements into and out of postsynaptic complexes (Young and Poo, 1983; Akaaboune et al., 1999; Meier et al., 2001; Borgdorff and Choquet, 2002), it appears likely that the ring-shaped DGluR-IIA accumulations described here similarly reflect a transient step in the maturation of postsynapses. Thus, experience-induced subsynaptic protein synthesis seems to instruct the DGluR-IIA-mediated functional maturation of postsynapses, which together with added presynaptic dense bodies mediates the observed increase in the total number of active zones. Finally, NMJs grow more boutons to reestablish the typical active zone density to consolidate the earlier induced physiological alterations. On the basis of this first temporal map of processes contributing to experience-dependent plasticity at Drosophila NMJs, future experiments will incorporate the behavioral assays introduced here to uncover the dynamic control of glutamatergic signal transmission.
Footnotes
This work was funded by the Max-Planck-Society. We thank C. S. Goodman (University of California Berkeley, Berkeley, CA) and J. Kidokoro (Gunma University, Gunma, Japan) for kindly providing reagents, E. Illgen and M. Langegger for excellent technical assistance, and S. Marella for critical comments on this manuscript.
Correspondence should be addressed to C. M. Schuster, Friedrich-Miescher-Laboratorium der Max-Planck-Gesellschaft, Spemannstrasse 39, 72076 Tübingen, Germany. E-mail: christoph.schuster@tuebingen.mpg.de.
S. J. Sigrist's current address: European Neuroscience Institute Göttingen, Max-Planck-Institute for Biophysical Chemistry, Waldweg 33, 37073 Göttingen, Germany.
D. F. Reiff's current address: Max-Planck-Institute of Neurobiology, Neuronale Informationsverarbeitung, 82152 Martinsried, Germany.
Copyright © 2003 Society for Neuroscience 0270-6474/03/236546-11$15.00/0
S.J.S. and D.F.R. contributed equally to this work.
References
- Aberle H, Haghighi AP, Fetter RD, McCabe BD, Magalhaes TR, Goodman CS ( 2002) Wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila Neuron 33: 545-558. [DOI] [PubMed] [Google Scholar]
- Akaaboune M, Culican SM, Turney SG, Lichtman JW ( 1999) Rapid and reversible effects of activity on acetylcholine receptor density at the neuromuscular junction in vivo. Science 286: 503-507. [DOI] [PubMed] [Google Scholar]
- Atwood HL, Karunanithi S ( 2002) Diversification of synaptic strength: presynaptic elements. Nat Rev Neurosci 3: 497-516. [DOI] [PubMed] [Google Scholar]
- Atwood HL, Wojtowicz JM ( 1999) Silent synapses in neural plasticity: current evidence. Learning Memory 6: 542-571. [DOI] [PubMed] [Google Scholar]
- Atwood HL, Swenarchuk LE, Gruenwald CR ( 1975) Long-term synaptic facilitation during sodium accumulation in nerve terminals. Brain Res 100: 198-202. [DOI] [PubMed] [Google Scholar]
- Beaumont V, Zhong N, Fletcher R, Froemke RC, Zucker RS ( 2001) Phosphorylation and local presynaptic protein synthesis in calcium- and calcineurin-dependent induction of crayfish long-term facilitation. Neuron 32: 489-501. [DOI] [PubMed] [Google Scholar]
- Beaumont V, Zhong N, Froemke RC, Ball RW, Zucker RS ( 2002) Temporal synaptic tagging by I-h activation and actin: involvement in long-term facilitation and cAMP-induced synaptic enhancement. Neuron 33: 601-613. [DOI] [PubMed] [Google Scholar]
- Borgdorff AJ, Choquet D ( 2002) Regulation of AMPA receptor lateral movements. Nature 417: 649-653. [DOI] [PubMed] [Google Scholar]
- Brandstatter JH, Meinertzhagen IA ( 1995) The rapid assembly of synaptic sites in photoreceptor terminals of the fly's optic lobe recovering from cold shock. Proc Natl Acad Sci USA 92: 2677-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadie KS, Richmond JE ( 2002) Establishing and sculpting the synapse in Drosophila and C. elegans Curr Opin Neurobiol 12: 491-498. [DOI] [PubMed] [Google Scholar]
- Budnik V, Zhong Y, Wu CF ( 1990) Morphological plasticity of motor axon terminals in Drosophila mutants with altered excitability. J Neurosci 10: 3754-3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung US, Shayan AJ, Boulianne GL, Atwood HL ( 1999) Drosophila larval neuromuscular junctions responses to reduction of cAMP in the nervous system. J Neurobiol 40: 1-13. [DOI] [PubMed] [Google Scholar]
- Cooper RL, Marin L, Atwood HL ( 1995) Synaptic differentiation of a single motor neuron: conjoint definition of transmitter release, presynaptic calcium signals, and ultrastructure. J Neurosci 15: 4209-4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper RL, Winslow JL, Govind CK, Atwood HL ( 1996) Synaptic structural complexity as a factor enhancing probability of calcium-mediated transmitter release. J Neurophysiol 75: 2451-2466. [DOI] [PubMed] [Google Scholar]
- Davis GW, Goodman CS ( 1998) Synapse-specific control of synaptic efficacy at the terminals of a single neuron. Nature 392: 82-86. [DOI] [PubMed] [Google Scholar]
- Davis GW, Schuster CM, Goodman CS ( 1996) Genetic dissection of structural and functional components of synaptic plasticity: III. CREB is necessary for presynaptic functional plasticity. Neuron 17: 669-679. [DOI] [PubMed] [Google Scholar]
- Davis GW, DiAntonio A, Petersen SA, Goodman CS ( 1998) Postsynaptic PKA controls quantal size and reveals a retrograde signal that regulates presynaptic transmitter release in Drosophila Neuron 20: 305-315. [DOI] [PubMed] [Google Scholar]
- Delaney KR, Zucker RS, Tank DW ( 1989) Calcium in motor nerve terminals associated with post-tetanic potentiation. J Neurosci 9: 3558-3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiAntonio A, Petersen SA, Heckmann M, Goodman CS ( 1999) Glutamate receptor expression regulates quantal size and quantal content at the Drosophila neuromuscular junction. J Neurosci 19: 3023-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie DR ( 1998) A tale of two termini: a functional interaction between the termini of a mRNA is a prerequisite for efficient translation initiation. Gene 216: 1-11. [DOI] [PubMed] [Google Scholar]
- Ganetzky B ( 1984) Genetic studies of membrane excitability in Drosophila: lethal interaction between two temperature-sensitive paralytic mutations. Genetics 108: 897-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jareki J, Keshishian H ( 1995) Role of neural activity during synaptogenesis in Drosophila J Neurosci 15: 8177-8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang CG, Schuman EM ( 2002) Regulation and function of local protein synthesis in neuronal dendrites. Trends Biochem Sci 27: 506-513. [DOI] [PubMed] [Google Scholar]
- Ludwig D, Cable RM ( 1933) The effect of alternating temperatures on the pupal development of Drosophila melanogaster. Physiol Zool 6: 493-508. [Google Scholar]
- Mallart A ( 1993) Calcium-dependent modulation of the facilitation of transmitter release at neuromuscular junctions of Drosophila J Physiol (Lond) 87: 83-88. [DOI] [PubMed] [Google Scholar]
- Martin KC, Barad M, Kandel ER ( 2000) Local protein synthesis and its role in synapse-specific plasticity. Curr Op Neurobiol 10: 587-592. [DOI] [PubMed] [Google Scholar]
- Meier J, Vannier C, Serge A, Triller A, Choquet D ( 2001) Fast and reversible trapping of surface glycine receptors by gephyrin. Nat Neurosci 4: 253-260. [DOI] [PubMed] [Google Scholar]
- Meinertzhagen IA, Govind CK, Stewart BA, Carter JM, Atwood HL ( 1998) Regulated spacing of synapses and presynaptic active zones at larval neuromuscular junctions in different genotypes of the flies Drosophila and Sarcophaga J Comp Neurol 393: 482-492. [DOI] [PubMed] [Google Scholar]
- Pennetta G, Hiesinger PR, Fabian-Fine R, Meinertzhagen IA, Bellen HJ ( 2002) Drosophila VAP-33A directs bouton formation at neuromuscular junctions in a dosage-dependent manner. Neuron 35: 291-306. [DOI] [PubMed] [Google Scholar]
- Pereira HS, MacDonald DE, Hilliker AJ, Sokolowski MB ( 1995) Chaser (Csr), a new gene affecting larval foraging behavior in Drosophila melanogaster. Genetics 141: 263-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SA, Fetter RD, Noordermeer JN, Goodman CS, DiAntonio A ( 1997) Genetic analysis of glutamate receptors in Drosophila reveals a retrograde signal regulating presynaptic transmitter release. Neuron 19: 1237-1248. [DOI] [PubMed] [Google Scholar]
- Reiff DF, Thiel PR, Schuster CM ( 2002) Differential regulation of active zone density during long-term strengthening of Drosophila neuromuscular junctions. J Neurosci 22: 9399-9409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoe M, Tanaka S, Takata K, Kidokoro Y ( 1997) Neural activity affects distribution of glutamate receptors during neuromuscular junction formation in Drosophila embryos. Dev Biol 184: 48-60. [DOI] [PubMed] [Google Scholar]
- Saitoe M, Schwarz TL, Umbach JA, Gundersen CB, Kidokoro Y ( 2001) Absence of junctional glutamate receptor clusters in Drosophila mutants lacking spontaneous transmitter release. Science. 293: 514-517. [DOI] [PubMed] [Google Scholar]
- Sanyal S, Sandstrom DJ, Hoeffer CA, Ramaswami M ( 2002) AP1 functions upstream of CREB to control synaptic plasticity in Drosophila Nature 416: 870-874. [DOI] [PubMed] [Google Scholar]
- Schuster CM, Ultsch A, Schloss P, Cox JA, Schmitt B, Betz H ( 1991) Molecular cloning of an invertebrate glutamate receptor subunit expressed in Drosophila muscle. Science 254: 112-114. [DOI] [PubMed] [Google Scholar]
- Schuster CM, Davis GW, Fetter RD, Goodman CS ( 1996a) Genetic dissection of structural and functional components of synaptic plasticity. I. Fasciclin II controls synaptic stabilization and growth. Neuron 17: 641-654. [DOI] [PubMed] [Google Scholar]
- Schuster CM, Davis GW, Fetter RD, Goodman CS ( 1996b) Genetic dissection of structural and functional components of synaptic plasticity. II. Fasciclin II controls presynaptic structural plasticity. Neuron 17: 655-667. [DOI] [PubMed] [Google Scholar]
- Shaver SA, Riedl CAL, Parkes TL, Sokolowski MB, Hilliker AJ ( 2000) Isolation of larval behavioral mutants in Drosophila melanogaster J Neurogenet 14: 193-205. [DOI] [PubMed] [Google Scholar]
- Sigrist JS, Thiel PR, Reiff DF, Lachance PED, Lasko P, Schuster CM ( 2000) Postsynaptic translation affects the efficacy and morphology of neuromuscular junctions. Nature 405: 1062-1065. [DOI] [PubMed] [Google Scholar]
- Sigrist JS, Thiel PR, Reiff DF, Schuster CM ( 2002) The postsynaptic glutamate receptor subunit DGluR-IIA mediates long-term plasticity in Drosophila J Neurosci 22: 7362-7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowski MB ( 1980) Foraging strategies of Drosophila melanogaster: a chromosomal analysis. Behav Genet 10: 291-302. [DOI] [PubMed] [Google Scholar]
- Sokolowski MB ( 2001) Drosophila: Genetics meets behaviour. Nat Rev Genet 2: 879-890. [DOI] [PubMed] [Google Scholar]
- Song W, Ranjan R, Dawson-Scully K, Bronk P, Marin L, Seroude L, Lin YJ, Nie ZP, Atwood HL, Benzer S, Zinsmaier KE ( 2002) Presynaptic regulation of neurotransmission in Drosophila by the G protein-coupled receptor Methuselah. Neuron 36: 105-119. [DOI] [PubMed] [Google Scholar]
- Stewart BA, Atwood HL, Renger JJ, Wang J, Wu CF ( 1994) Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J Comp Physiol [A] 175: 179-191. [DOI] [PubMed] [Google Scholar]
- Stewart BA, Schuster CM, Goodman CS, Atwood HL ( 1996) Homeostasis of synaptic transmission in Drosophila with genetically altered nerve terminal morphology. J Neurosci 16: 3877-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas U, Ebitsch S, Gorczyca M, Koh YH, Hough CD, Woods D, Gundelfinger ED, Budnik V ( 2000) Synaptic targeting and localization of Discs-large is a stepwise process controlled by different domains of the protein. Curr Biol 10: 1108-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan HI, DiAntonio A, Fetter RD, Bergstorm K, Strauss R, Goodman CS ( 2000) Highwire regulates synaptic growth in Drosophila Neuron 26: 313-329. [DOI] [PubMed] [Google Scholar]
- Wang JW, Sylwester AW, Reed D, Wu DA, Soll DR, Wu CF ( 1997) Morphometric description of the wandering behavior in Drosophila larvae: aberrant locomotion in Na+ and K+ channel mutants revealed by computer-assisted motion analysis. J Neurogenet 11: 231-254. [DOI] [PubMed] [Google Scholar]
- Wang JW, Soll DR, Wu CF ( 2002) Morphometric description of the wandering behavior in Drosophila larvae: a phenotypic analysis of K+ channel mutants. J Neurogenet 16: 45-63. [DOI] [PubMed] [Google Scholar]
- Wojtowicz JM, Atwood HL ( 1985) Correlation of presynaptic and postsynaptic events during establishment of long-term facilitation at crayfish neuromuscular junction. J Neurophysiol 54: 220-230. [DOI] [PubMed] [Google Scholar]
- Wojtowicz JM, Atwood HL ( 1986) Long-term facilitation alters transmitter releasing properties at the crayfish neuromuscular junction. J Neurophysiol 55: 484-498. [DOI] [PubMed] [Google Scholar]
- Wojtowicz JM, Atwood HL ( 1988) Presynaptic long-term facilitation at the crayfish neuromuscular junction: voltage-dependent and ion-dependent phases. J Neurosci 8: 4667-4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtowicz JM, Parnas I, Parnas H, Atwood HL ( 1988) Long-term facilitation of synaptic transmission demonstrated with macro-patch recording at the crayfish neuromuscular junction. Neurosci Lett 90: 152-158. [DOI] [PubMed] [Google Scholar]
- Wojtowicz JM, Marin L, Atwood HL ( 1994) Activity-induced changes in synaptic release sites at the crayfish neuromuscular junction. J Neurosci 14: 3688-3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin JC, Del Vecchio M, Zhou H, Tully T ( 1995) CREB as a memory modulator: induced expression of a dCREB2 activator isoform enhances longterm memory in Drosophila Cell 81: 107-115. [DOI] [PubMed] [Google Scholar]
- Young SH, Poo MM ( 1983) Rapid lateral diffusion of extrajunctional acetylcholine receptors in the developing muscle membrane of Xenopus tadpole. J Neurosci 3: 225-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DW, Kuromi H, Kidokoro Y ( 1999) Activation of metabotropic glutamate receptors enhances synaptic transmission at the Drosophila neuromuscular junction. Neuropharmacology 38: 645-657. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Budnik V, Wu CF ( 1992) Synaptic plasticity in Drosophila memory and hyperexcitable mutants: role of cAMP cascade. J Neurosci 12: 644-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS ( 1999) Calcium- and activity-dependent synaptic plasticity. Curr Opin Neurobiol 9: 305-313. [DOI] [PubMed] [Google Scholar]