Abstract
The establishment of memory requires coordinated signaling between presynaptic and postsynaptic terminals in the CNS. The integrins make up a large family of cell adhesion receptors that are known to mediate bidirectional signaling between cells or between cells and their external environment. We show here that many different integrins, including α3 and α5, are expressed broadly in the adult mouse brain and are associated with synapses. Mice with genetically reduced expression of α3 integrin fail to maintain long-term potentiation (LTP) generated in hippocampal CA1 neurons. Mice with reduced expression of the α3 and α5 integrins exhibit a defect in paired-pulse facilitation. Mice with reduced expression of α3, α5, and α8 are defective in hippocampal LTP and spatial memory in the water maze but have normal fear conditioning. These results demonstrate that several different integrins are involved in physiological plasticity and provide the first evidence of their requirement for behavioral plasticity in vertebrates.
Keywords: integrins, synaptic plasticity, PPF, LTP, learning and memory, behaviors
Introduction
Learning occurs via experience-dependent alterations in the strength of synaptic connections, which, in turn, occur from changes in the biochemistry and molecular biology underlying synaptic transmission. The presynaptic terminal contains a complex array of molecules including cytoskeletal proteins that tether neurotransmitter vesicles, ion channels, and sensors that function to release neurotransmitter in response to calcium influx (Garner et al., 2000; Sudhof, 2000; Martin, 2002). The postsynaptic side of the synapse contains molecular machinery of equal complexity that detects neurotransmitter release, provides for the trafficking of neurotransmitter receptors, and produces ion influx across the postsynaptic membrane at fast synapses and the activation of second messenger cascades at slow synapses (Kennedy, 2000; Greengard, 2001; Sheng, 2001). Basal synaptic communication and the changes attributable to experience require that the presynaptic and postsynaptic faces of the synapse communicate via adhesive and signaling events.
One family of cell adhesion receptors with attractive features for mediating changes in synaptic communication is the integrins. Integrins are heterodimers of noncovalently linked α- and β-subunits. Each subunit has a single transmembrane domain connecting the extracellular ligand-binding domain to cytoplasmic tails that are linked to the actin cytoskeleton (Humphries, 2000; van der Flier and Sonnenberg, 2001). Currently 19 different α-subunits and 8 β-subunits are known in vertebrates, and >20 different αβ heterodimers have been described.
Recent studies have begun to reveal that integrins help to form the synapse and that they function in synaptic change and learning. Two vertebrate integrins, β8 and α8, have been localized to dendritic spines of pyramidal neurons where they are associated with the postsynaptic density (PSD) (Einheber et al., 1996; Nishimura et al., 1998). The α5 integrin has been shown to distribute preferentially to apical dendrites of pyramidal cells of the hippocampus and neocortex (Bi et al., 2001), and four different integrins of Drosophila have been localized to the presynaptic and/or postsynaptic side of the larval neuromuscular junction (Prokop, 1999). Evidence for a potential role in synaptic plasticity has been gathered by attenuating the stability of hippocampal long-term potentiation (LTP), using broad-spectrum peptide inhibitors of the integrins or other pharmacological reagents (Bahr et al., 1997; Staubli et al., 1998; Chun et al., 2001; Kramar et al., 2002). A direct link of integrin function to memory formation was demonstrated in Drosophila, in which the disruption of Volado, a gene encoding for two forms of α-integrin, impairs olfactory learning (Grotewiel et al., 1998). Furthermore, disruption of the integrin-associated protein, IAP, produces memory deficits in mice (Huang et al., 1998; Chang et al., 1999, 2001). Despite the growing evidence linking integrin function to synaptic change and learning, the identification in vertebrates of the relevant integrin proteins from the numerous family members has not been made. In addition, clear links between these integrins and their hypothetical roles in synaptic change and behavior are lacking.
The present study was designed to determine the importance of selected integrins to mouse learning and synaptic plasticity.
We find that heterozygous mutants of the integrin gene α3, but not of α5 or α8, reduce the magnitude of NMDA receptor-dependent hippocampal LTP. A deficiency in spatial memory, however, is produced only when the expression of the three integrin genes, α3, α5, and α8, is reduced simultaneously. The results provide the first evidence that the integrin class of cell adhesion receptors mediate behavioral plasticity.
Materials and Methods
Mice and genotyping
All integrin knock-outs used in this study were kept as out-crossed stocks to C57Bl/6 (Jackson Laboratories, Bar Harbor, ME) or separately to 129SvEv (Bradley strain). The experiments reported here used animals out-crossed for a minimum of six generations. All experiments were performed on animals out-crossed to C57Bl/6 with the exception of that shown in Figure 3B. Mice were housed in conventional animal cages and maintained on a 12 hr light/dark cycle. Integrins mutants were genotyped by genomic blotting and PCR assays of tail DNA with the use of oligonucleotide primers representing the neomycin gene and genomic regions flanking the insertions. All animals were handled and treated during the experiments in ways approved by the Baylor College of Medicine Institutional Animal Care and Use Committee and according to national regulations and policies.
Figure 3.
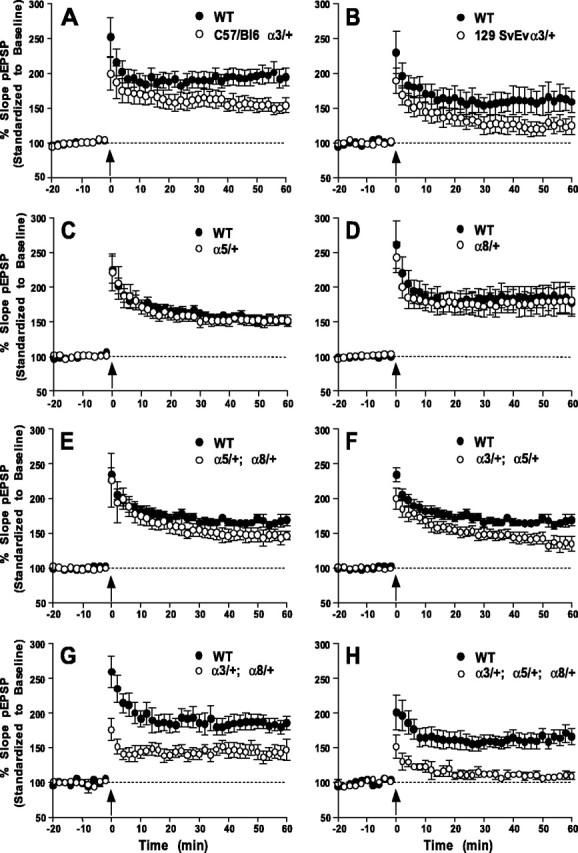
Effects of integrin deficiency on hippocampal LTP in area CA1. LTP was induced by using 100 Hz stimulation (arrow). Data points were normalized to the average response during an initial 20 min pretetanus stimulation period. A, B, Similar deficits in LTP were observed at 60 min post-tetanus in mice heterozygous for integrin α3 knock-out in either C57/Bl6 (n = 7; WT, n = 14) or 129/SvEv (n = 7; WT, n = 7) backgrounds. C-E, Normal LTP was recorded in α5/+ (n = 9; WT, n = 9), α8/+ (n = 11; WT, n = 8), and α5/+;α8/+ (n = 9; WT, n = 9) heterozygotes. F-H, LTP immediately after HFS was normal in α3/+;α5/+ (n = 9; WT, n = 14) heterozygotes but was reduced in α3/+;α8/+ (n = 8; WT, n = 8) and α3/+;α5/+;α8/+ heterozygotes (n = 5; WT, n = 9). LTP measured at 60 min after HFS was deficient in all three genotypes. No LTP was present in the triple heterozygote. Significant differences in pEPSP were found between the mutant and WT animals at t = 60 min in A, B and F-H.
Reverse transcriptase-PCR
Total RNA was extracted from 20-30 mg of isolated brain tissue from 3-month-old C57Bl/6 animals by using RNeasy Mini Kit (Qiagen, Chats-worth, CA) according to the protocol provided by the manufacturer. Total RNA (4 μg) was reverse transcribed in a 20 μl reaction volume containing 500 μg/ml oligo-dT and (in mm) 50 Tris-HCl, pH 8.3, 75 KCl, 3 MgCl2 plus 0.01 m DTT, 500 μm dNTP mix, and 200 U of Superscript II Reverse Transcriptase (RT; Invitrogen, San Diego, CA) and was incubated at 42°C for 50 min. For PCR amplification, 2 μl of first-strand cDNA was added to 25 μl of reaction volume containing (in mm) 10 Tris-HCl, pH 8.3, 50 KCl, 1.5 MgCl2, 2 dNTP mix plus 0.1 μm forward and reverse primers and 1 U of Taq DNA polymerase. The temperature cycling conditions were initial melting at 95°C for 5 min, followed by 30 cycles of 94°C for 30 sec, and annealing at 57, 60, or 63°C for 1 min, at 72°C for 1 min, and a final extension at 72°C for 4 min. PCR products were visualized by 1% agarose gel electrophoresis. The PCR primers that were used [forward, reverse (in 5′ to 3′ direction)] included the following: α1 (CCTGTACTGTACCCAATTGGATGG, GTGCTCTTATGAAAGTCGGTTTCC); α2 (TCTGCGTGTGGACATCAGTTTGGA, GATAACCCCTGTCGGTACTTCTGC); α3 (ACTACTCCTTACCTCTGCGCATGC, CACTTGCCATTGGTAACTTCATAG-); α4 (GCGAAGAAAACGAGCCAGAAACAT, GGGAGCAGGGGACAGGGGAGAGGA); α5 (CTGCAGCTGCATTTCCGAGTCTGG, GAAGCCGAGCTTGTAGAGGACGTA); α6 (GAGGAATATTCCAAACTGAACTAC, GGAATGCTGTCATCGTACCTAGAG); α7 (CCAGGACCTGGCCATCCGTG, CTATCCTTGCGCAGAATGAC); αV (GTGCCAGCCCATTGAGTTTGATTC, TTCACCACCATAGGGAGCAGCAAT); α9 (GACTGGAGAGGAGGAGAGGGAACT, CGATTAGGGAAAGAGATGCTGACA); αE(TGGAGGAGGAAGACGAGGAG,TTTGGGGAGTTGATGACAGT);β1 (GTGACCCATTGCAAGGAGAAGGAC, GTCATGAATTATCATTAAAAGTTT); β3 (CTGGTGTTTACCACTGATGCCAAG, TGTTGAGGCAGGTGGCATTGAAGG); β4 (CGTTTGTGTTCCAGGTCTTTGAGC, CAGAGAGGAGACGGGGAAATACTT); β5 (ACTTGGAGAACATCCGGAGC, TTGAAGCTGTCGACTCTGTC); β6 (GCTTGGCTCCCGGCTGGC, AGTTAATGGCAAAATGTGCT); β7 (TGGATGGCTACTACGGGGCTCTGT, CCTTCTCTTGGGGTCTCACTCTCA).
Synaptosomes and protein analysis
Cortical and hippocampal tissues were removed rapidly from freshly dissected brain and homogenized in 10 volumes of ice-cold homogenization buffer (320 mm sucrose, 5 mm HEPES, pH 7.4) with 9-12 strokes of a pestle in a glass homogenizer. The homogenate was centrifuged at 1000 × g at 4°C for 10 min to produce a pellet (P1) and a supernatant (S1). P1 was washed in homogenization buffer and centrifuged again to obtain P1′ and S1′. S1 and S1′ were combined and centrifuged at 12,500 × g for 30 min at 4°C to produce P2. The synaptosome-enriched P2 was washed once again, then resuspended in homogenization buffer, and layered carefully onto a 7.5-12% Ficoll gradient. The gradients were centrifuged at 68,000 × g at 4°C for 1 hr, and purified synaptosomes were recovered from the interface. Then the purified synaptosomes were removed to a beaker and stirred slowly while homogenization buffer was added drop-wise at 23°C to a final volume of 50 ml. The solution was centrifuged at 12,000 × g for 20 min, and the pellet was resuspended in immunoprecipitation buffer [containing (in mm) 50 Tris, pH 8.0, 1 MgCl2, 150 NaCl plus 1% NP40] along with protease inhibitors (1 mm aprotinin, 1 μg/ml leupeptin, 1 μg/ml antipain, 1 mm benzamidine).
For immunoprecipitation, 1.25 mg of synaptosome preparation was precleared with protein G-Sepharose (Pierce, Rockford, IL) for 30 min at room temperature (RT), followed by incubation with the polyclonal anti-α3 (α3-3209, Chemicon AB1948 or Chemicon AB1920, Temecula, CA), anti-α5 (Chemicon AB1928), anti-GluR1 (Chemicon AB1504), or anti-GluR2 (Chemicon AB1768) antisera for 1 hr at RT. Protein G-Sepharose was added, and the incubation continued for an additional hour. Then the mix was centrifuged and the pellet washed three times with 1× PBS (20 mm sodium phosphate, 150 mm NaCl, pH 8.0) plus 1% Triton X-100. The final pellet was resuspended in 2× SDS nonreducing sample buffer (100 mm Tris-HCl, pH 6.8, 10 mm EDTA, 4% SDS, 20% glycerol, 0.02% bromophenol blue).
For Western blotting, the final immunoprecipitated pellet or 25 μg of total forebrain synaptosomal extract was fractionated by 7.5% SDS-PAGE. The separated proteins were transferred to Immobilon-P membrane (Millipore, Bedford, MA). The membrane was incubated with a 1:1000 dilution of anti-β1 antibody (Chemicon MAB1997), 1:500 anti-α3 antibody (α3-3209), or 1:800 anti-syntaxin antibody (Stressgen Biotechnologies, San Diego, CA) for 1 hr at RT, washed three times, and then incubated for 30 min at RT with a horseradish peroxidaseconjugated secondary antibody (Jackson ImmunoResearch, West Grove, PA). The blot was developed with ECL Western Blotting Detection Reagent (Amersham Biosciences, Arlington Heights, IL).
Quantitation of signal that was detected in Western blots was performed with the Scion Image software (Scion, Frederick, MD). The net α3 integrin signal (total signal - background) in each animal was normalized against the corresponding syntaxin signal, and the normalized α3 level then was expressed as a percentage of that of the first wild-type animal.
Immunohistochemistry and histology
Immunohistochemical experiments were performed as described by Cherry and Davis (1995). Animals were anesthetized with isoflurane and perfused with 4% paraformaldehyde in 0.1 m phosphate buffer for 10 min. The dissected brain was separated into two hemispheres and postfixed in 4% paraformaldehyde for an additional 1 hr at 23°C. Paraffin sections of the brain were incubated in 7.5% goat serum in PBS containing 0.15% Triton X-100 (GS-PBST) for 1-2 hr at 23°C and then with 150 μl of diluted primary antisera [1:500 anti-α3 antisera (Chemicon AB1948) or 1:2000 anti-syntaxin antibody (Stressgen)] overnight at 4°C. Sections were washed twice at 23°C with PBST for 10 min and were incubated with a biotinylated secondary antibody for 1 hr. Then the sections were washed twice in PBST and finally incubated with Vectastain ABC reagent (Vector Laboratories, Burlingame, CA) with high salt (0.5 m NaCl) to reduce background staining. The immunostaining was visualized by reacting sections with 1 mg/ml diaminobenzidine/0.3% H2O2 for 5-10 min. After two washes in distilled water the sections were mounted on slides with 80% glycerol. For histological comparison between α3/+; α5/+;α8/+ heterozygotes and their wild-type siblings, frozen sections were stained with hematoxylin and eosin and mounted in Permount.
Electrophysiology
Hippocampal slices (400 μm) were bathed (1 ml/min) with artificial CSF in an interface chamber maintained at 30°C. The Schaffer collateral synapse was stimulated, and the population EPSP (pEPSP) was recorded in area CA1 stratum radiatum. Responses were monitored for 20 min before high-frequency stimulation (HFS) was given to insure a stable baseline. Measurements are shown as the average slope of the pEPSP from six individual traces and are standardized to the 20 min of baseline recordings. Baseline stimulus intensities were adjusted to produce a pEPSP at 50% of the maximal response. NMDA-dependent LTP was induced with one set of HFS consisting of two trains of 100 Hz stimulation for 1 sec, separated by 20 sec. Stimulus intensities used for the HFS were matched to those used in the baseline recordings. We minimized day-to-day variability in slice preparations and recordings by preparing mutant and wild-type hippocampal slices simultaneously and placed them side by side on the same recording chamber. Paired-pulse facilitation (PPF) was calculated as the average of the ratio of the second to the first response. Calculating PPF as the mean of the second response divided by the mean of the first response (Kim and Alger, 2001) did not affect the conclusions as to whether the control animals exhibited PPF or whether mutant animals were significantly different from the controls.
Behavioral assays
Water maze. Mice were tested in a polypropylene pool with a diameter of 1.3 m. The platform for the hidden version of the task was 10 × 10 cm and made of Plexiglas; it was adjusted so that the platform was 2 cm below the water surface. The visible version of the task used a platform with a black 9 × 9 × 8 cm Plexiglas cube supported 18 cm above the surface of the platform. Spatial cues were placed on four sides of the room, and the swim pattern of the animals was tracked with a ceiling-mounted video camera connected to a digital tracking device (VP200, HVS Image, San Diego, CA). Data were processed by the HVS water maze software.
Before being trained in the water maze, the mice were handled extensively for 2 weeks. The animals were kept in individual cages during training and were allowed to acclimatize to the water maze room 1 hr before the start of the experiment each day. On the first day of training the mice were given a 60 sec practice swim and a practice climb onto the platform. Mice were placed into the water facing the wall of the maze and allowed to search for the platform. The trial ended when an animal climbed onto the platform or when a maximum of 60 sec had elapsed. The animal was allowed to stay on the platform for 20 sec before being returned to its cage. Four trials were performed each day with an inter-trial interval (ITI) of 60 min, and each trial began at either the 12, 3, 6, or 9 o'clock position of the pool. The platform location remained the same for any particular mouse for the duration of the training, but different animals were trained with the platform in different positions to avoid quadrant bias. Animals were trained for 7 d at the same time each day. At 1 hr after the last training trial a probe trial (or transfer test) was administered in which the platform was removed from the pool; animals were placed in a quadrant opposite to the location of the training platform and were allowed to swim for 60 sec. Both the time the mice spent searching for the platform in each quadrant and the number of times the mice entered the quadrant of the former platform location were measured. The visible platform task was performed 24 hr after the completion of the probe trial in which the location of the visible platform varied on each trial. Eight trials were administered in two blocks of four, with 1 hr ITI between blocks. The latency and the path length to reach the visible platform were measured.
Fear conditioning. The conditioning chamber (26 × 22 × 18 cm; San Diego Instruments, San Diego, CA) was made of Plexiglas and was equipped with a grid floor for delivery of the unconditioned stimulus (US) and photobeams to monitor activity. The conditioning chamber was placed inside a soundproof isolation cubicle. Training occurred in the presence of white light and background noise generated by a small fan. Each mouse was placed inside the conditioning chamber for 2 min before the onset of a conditioned stimulus (CS; an 85 dB tone), which lasted for 30 sec. A 2 sec US footshock (0.6 mA) was delivered immediately after the termination of the CS. Each mouse remained in the chamber for an additional 60 sec, followed by another CS-US pairing. Each mouse was returned to its home cage after another 30 sec. The test for contextual fear memory was performed 2 and 24 hr after training by measuring freezing behavior during a 5 min test in the conditioning chamber. Freezing was defined as a lack of movement in each 5 sec interval. Cued fear memory was tested in the presence of red light, coconut odor, and the absence of background noise. In addition, a triangular black box was put inside the conditioning chamber and the grid floor covered to present an altered context. Each mouse was placed in this novel context for 3 min at 24 hr after training, and they were exposed to the CS for another 3 min. Freezing behavior was recorded and processed by SDI Photobeam Activity System software (San Diego Instruments) throughout each testing session.
Results
Expression of integrins in the brain
We began our studies on the potential involvement of integrins in vertebrate learning by surveying the regional mRNA expression of 16 different integrin subunits (α1, α2, α3, α4, α5, α6, α7, α9, αV, αE, β1, β3, β4, β5, β6, and β7) in the adult mouse brain with RT-PCR (Table 1). Surprisingly, with the exception of α9 and αE, all of the subunits that were examined were expressed in most or all of these regions, with only modest or no preferential expression between these brain regions. The expression of integrin subunits α9 and αE was not detectable in the brain in our experiments. A representative RT-PCR result for α3 is illustrated in Figure 1A. In situ RNA hybridization experiments also have been reported that demonstrate widespread expression of many different integrin genes in the adult brain (Pinkstaff et al., 1999).
Table 1.
Regional mRNA expression of integrin subunits in the adult mouse brain with RT-PCR
|
|
α1 |
α2 |
α3 |
α4 |
α5 |
α6 |
α7 |
α9 |
αV |
αE |
β1 |
β3 |
β5 |
β6 |
β7 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cortex | ++ | ++ | +++ | +++ | +++ | +++ | + | − | +++ | − | +++ | ++ | +++ | + | ++ |
| Hippocampus | ++ | ++ | +++ | +++ | +++ | +++ | ++ | − | +++ | − | +++ | ++ | +++ | ++ | ++ |
| Cerebellum | + | ++ | +++ | + | +++ | +++ | +++ | − | +++ | − | +++ | + | +++ | ++ | ++ |
| Thalamus | ++ | ++ | +++ | ++ | +++ | +++ | + | − | + | − | +++ | ++ | +++ | ++ | ++ |
| Olfactory bulb
|
+
|
++
|
+++
|
++
|
+++
|
+++
|
+
|
−
|
+++
|
−
|
+++
|
++
|
+++
|
+
|
++
|
+++, Strong; ++, moderate; +, weak; −, undetectable.
Figure 1.
Analysis of integrinα3 expression in the adult brain. A, Expression in various regions of the brain as assayed by RT-PCR. Cx, Cortex; Hp, hippocampus; Cb, cerebellum; Th, thalamus; OB, olfactory bulb. B-E, Immunohistochemistry of the cortex (B), hippocampus (C, D), and cerebellum (E) with the use of an anti-α3 integrin antibody. Cellular staining was detected in all six layers of the cortex (I-VI), in the granule cells of the dentate gyrus (DG), in the pyramidal cells of the CA1 and CA3 regions, and in Purkinje neurons. At higher magnification (40 × objective), staining of the processes of the pyramidal cells (D) and Purkinje neurons (E) was also detectable (arrows).
We studied the expression pattern of selected integrin proteins, including α3, by immunohistochemistry to complement previous studies of α5, α8, and αV (Einheber et al., 1996; Nishimura et al., 1998; Bi et al., 2001; King et al., 2001). The α3 integrin was detected throughout the brain (Fig. 1B-E), consistent with the RT-PCR results. Within the neocortex α3-immunoreactive cells were detected in all six layers of the cortex. Pyramidal neurons of the CA1 and CA3 regions of the hippocampus were labeled along with granule neurons of the dentate gyrus and Purkinje neurons of the cerebellum (Fig. 1B-E). The processes that emanate from many of these neurons also were found to contain α3-immunoreactive material (Fig. 1D,E). These observations along with previous studies reveal that several integrin subunits, including α3, α5, αV, and α8, are expressed in the adult mouse brain and are associated with neurites and synapses.
We prepared antisera against the cytoplasmic domain of mouse α3 integrin and tested the antisera by Western blotting. The antisera recognized a 150 kDa protein from the cortex after nonreducing gel electrophoresis, consistent with the predicted size of the native α3 integrin subunit (Fig. 2A). The antisera also recognized a 180 kDa protein in adults, which could be a larger form of α3 integrin in adult animals or a cross-reacting protein specific to the adult stage. The specificity of the antisera was tested by probing extracts from fetuses of homozygous α3 knock-outs collected before the time of lethality. Control fetuses contained immunoreactive protein that migrated as a broad band of ∼150 kDa that was not detected in the homozygous mutant fetuses (Fig. 2A).
Figure 2.

Synaptosome localization of α3 and α5 integrins in cortex and hippocampus. A, Immunoblot of integrin α3 protein in total forebrain extracts of α3 integrin heterozygous (α3/+) adults and in wild-type (+/+) and homozygous (α3/α3) mutant fetuses. The full-length α3 integrin subunit is identified; the asterisk identifies a larger integrin that is specific to the adult. Synaptosomes are shown from the following: lane 1, an α3/α3 homozygous fetus; lanes 2, 4, two individual wild-type fetuses; lanes 3, 5, two individual α3/+ heterozygous adults. B, β1 integrin is coimmunoprecipitated by anti-α3 and anti-α5 antibodies. Lane 1, Immunoblot of β1 integrin protein in total synaptosomal extract; lanes 2-9, proteins from total synaptosomal extract were immunoprecipitated with no antibody (lane 2), preimmune serum for α3-3209 (lane 3), anti-α3 integrin α3-3209 (lane 4), AB1948 (lane 5), AB1920 (lane 6), anti-α5 integrin (lane 7), anti-GluR1 (lane 8), or anti-GluR2 (lane 9) antibodies, followed by immunoblotting with an anti-β1 antibody. C, Immunoblot to compare the level of integrin α3 protein in total forebrain extracts of wild-type and heterozygous integrin adults. Two individual adult animals were used to represent each genotype. The full-length α3 integrin (top) and syntaxin (control; middle) protein are indicated. In the bottom panel the normalized level of α3 protein in each animal was expressed as a percentage relative to the level detected in the first wild-type animal (wt1; see Materials and Methods).
To explore the subcellular localization of α3 integrin, we prepared synaptosomes from homogenates of the cortex and hippocampus and assayed these for the presence of α3. Our anti-α3 antisera as well as two additional commercial antibodies immunoprecipitated a protein of 110 kDa that was detected by an anti-β1 antibody from crude synaptosomes (data not shown) or synaptosomes purified on a Ficoll gradient (Fig. 2B). β1 integrin is the only β-integrin known to associate with α3. Parallel experiments that used an anti-α5 antibody produced a similar conclusion. An anti-α5 antibody also immunoprecipitated β1-integrin from crude and enriched synaptosomes (Fig. 2B). Other antisera [anti-GluR1, anti-GluR2, anti-syntaxin (data not shown), and anti-SNAP-25 (data not shown)] failed to immunoprecipitate β1-integrin, confirming the specificity of the interaction. These results along with previous observations strongly suggest that several different integrins, including α3/β1, α5/β1, and α8/β1, are localized at synapses in the adult brain.
Synaptic transmission and plasticity in integrin-deficient mice
To determine whether α3 integrin participates in synaptic transmission and plasticity, we measured the field EPSPs (fEPSPs) in field CA1 of hippocampal slices prepared from the α3 heterozygous mutants along with control siblings. Homozygous α3 mutants die within a few hours of birth (Kreidberg et al., 1996), so this approach assumes that a 50% reduction in integrin level in heterozygous adults, which we confirmed as shown in Figure 2C, may produce phenotypic effects. We observed no difference in basal synaptic transmission in α3 mutants as compared with their control littermates by comparing stimulus intensity versus fEPSP or fiber volley versus fEPSP graphs (data not shown). However, the α3 heterozygotes did exhibit a more rapidly decaying NMDA receptor-dependent LTP induced with two 100 Hz stimuli (Fig. 3A,B). The magnitude of LTP induced in the heterozygotes was not significantly different from control animals immediately after the tetanus, but it decayed rapidly so that a significant difference in magnitude was detected at 60 min. The rapidly decaying LTP was observed in α3 heterozygotes that were out-crossed repeatedly into two different genetic backgrounds (C57BL/6 and 129SvEv). Therefore, the LTP deficit maps genetically to the α3 knock-out allele.
The expression of α5 and α8 integrins overlaps with α3 in the hippocampus, with all subunits being expressed in the dentate gyrus and in the CA1 and CA3 fields of the hippocampus (data not shown) (Pinkstaff et al., 1999). Furthermore, because all three integrins dimerize with β1, we wondered whether these integrins might compensate for each other functionally. To test this possibility, we generated α3/+;α5/+ double, α3/+;α8/+ double, α5/+;α8/+ double, and α3/+;α5/+;α8/+ triple heterozygotes and assayed each genotype for activity-dependent synaptic plasticity. No difference in LTP was detected in α5/+ or α8/+ animals when compared with control siblings (Fig. 3C,D). Similarly, no significant deficit was found in α5/+;α8/+ double mutants (Fig. 3E). In contrast, double heterozygotes of α5/+ or α8/+ made in combination with α3/+ both showed a deficit in LTP (Fig. 3F,G). The magnitude of the deficit at 60 min in α3/+;α5/+ and α3/+;α8/+ was similar to that observed in α3/+ heterozygotes alone (Fig. 3A,F,G). However, the double heterozygote α3/+;α8/+ produced a more severe deficiency immediately after the tetanus (Fig. 3G). The triple heterozygotes, in contrast, nearly completely eliminated LTP when measured at 60 min (Fig. 3H). These data obtained with mutant combinations confirm the primary role of α3 in promoting the stability of LTP. They further suggest some redundancy and a role for α8 for the induction or stability of LTP within seconds or minutes after tetanus when α3 also is limiting, and for α5 on the magnitude of LTP that can be generated when both α3 and α8 are limiting.
We next examined PPF in single, double, and triple heterozygotes. No significant difference was detected at interpulse intervals (IPIs) from 10 to 400 msec between controls and any single or double heterozygote combination except for α3/+;α5/+ (Fig. 4A-F),
Figure 4.

Impaired paired-pulse facilitation (PPF) in integrin mutant heterozygotes. PPF was used to assay short-term synaptic plasticity and presynaptic function. Insets, Representative pEPSP traces from PPF experiments with the use of an interpulse interval of 100 msec (mean of 6 successive EPSPs). The first response (dotted line) and second response (solid line) for mutant (a) and wild-type (b) animals are shown. Calibration: 1 mV, 10 msec. Because PPF varies with the size of the first response, the stimulation was adjusted so that the slope of the pEPSP from the first stimulus for all slices was the same. Asterisks indicate a value significantly different from controls (p < 0.05, Student's t test). A-E, PPF was unaffected for the genotypes α3/+ (n = 10; WT, n = 8), α5/+ (n = 7; WT, n = 8), α8/+ (n = 9; WT, n = 13), α5/+; α8/+ (n = 8; WT, n = 9), and α3/+; α8/+ (n = 11; WT, n = 8). F, G, Defective PPF was observed in α3/+; α5/+ (n = 11; WT, n = 10) at interpulse intervals of 75, 100, 150, and 200 msec and in α3/+; α5/+; α8/+ mutants (n = 14; WT, n = 8) at interpulse intervals of 20, 30, 40, 50, 75, 100, and 150 msec.
which was defective at IPIs of 75-200 msec. The triple heterozygote also exhibited a deficiency in PPF that is similar or perhaps slightly more extreme than that observed in α3/+;α5/+ heterozygotes (Fig. 4G). These data therefore indicate that wild-type levels of α3 and α5 integrins are required for normal presynaptic plasticity, as assayed by PPF.
Behavioral analyses of integrin-deficient mice
We tested all single, double, and triple heterozygotes in a series of neurological and behavioral tests to uncover the potential roles for integrins in behavior and to correlate behavioral deficits with detectable physiological deficits. No deficiencies were detected in general health and neurological properties, including body weight, grooming behavior, walking, mating, reflex behaviors (eye blink, ear twitch, pupil constriction, body righting, and whisker touch), and responses to approaching objects or a visual cliff. Moreover, all seven genotypes were indistinguishable from controls in performance on the accelerating rotarod, for locomotor activity in an open field, and thigmotaxis (data not shown). All seven genotypes and their littermate controls also were tested for spatial memory in the hidden platform version of the water maze test. In this test, which requires normal hippocampal function, animals learn to escape to a hidden platform in a pool by using spatial cues (Morris et al., 1982). None of the single heterozygotes or double heterozygotes performed differently from their respective controls in this task for spatial memory. The triple heterozygotes, however, were discovered to be deficient in spatial memory ability. They exhibited a normally improving escape latency over 7 d of training that was comparable to control siblings (Fig. 5A). Subsequent probe tests, however, demonstrated that the triple heterozygotes spent less time in the quadrant that previously contained the hidden platform (Fig. 5B) and failed to cross the virtual platform position as frequently as controls (Fig. 5C). Nevertheless, these animals exhibited a normal speed of swimming and learned as well as controls in the visible platform version of this task (Fig. 5E), which does not require normal hippocampal function (Morris et al., 1982). Therefore, the simultaneous reduction in expression of α3, β5, and α8 together produces an inability to learn spatial information to the same extent as control animals. This observation is consistent with the possibility that the integrins have some overlapping functions in the CNS.
Figure 5.

Performance of α3/+;α5/+;α8/+ triple heterozygotes on the water maze task. A, Escape latency during the acquisition phase in the hidden platform task. No significant difference was detected between the mutant heterozygotes (n = 18) and wild-type controls (n = 18) (F(1,27) = 0.72; p = 0.85, ANOVA). B, Percentage of time spent in each quadrant during a probe trial performed 1 hr after the last training trial on day 7. Controls (n = 18) spent significantly more time in the trained quadrant than the triple heterozygotes (n = 18; p < 0.003, Scheffé's post hoc). ANOVA with repeated measures showed further that the controls spent significantly more time in the trained quadrant than in the other quadrants (F(3,68) = 32.53; p < 0.00001), but the triple heterozygotes spent a similar amount of time in all quadrants (F(3,68) = 2.49; p = 0.067). C, Number of platform crossings during the probe trial. Control mice crossed the virtual platform position in the trained quadrant more than the mutants (p < 0.01, Scheffé's post hoc) and crossed this position in the trained quadrant more often than the corresponding position in other quadrants (F(3,68) = 0.32; p = 0.81). D, E, Swim speed and mean escape latency versus trial number during the visible platform task. No significant difference for either measure was detected between control and the triple heterozygotes (F(1,112) = 0.67 and p = 0.697 for swim speed; F(1,112) = 0.35 and p = 0.93 for escape latency). Asterisk indicates a significant difference.
Pavlovian fear conditioning of contextual cues requires the normal functioning of both the hippocampus and the amygdala, whereas conditioning to tones only (cued) requires normal functioning of the amygdala, but not the hippocampus (Kim and Fanselow, 1992). We again examined the performance of all seven genotypes and their controls after both contextual and cued fear conditioning.
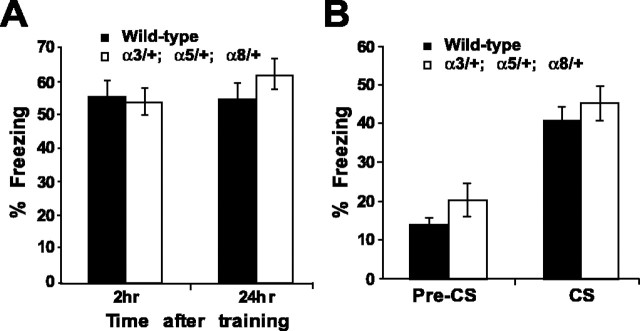
During training the animals were presented with two presentations of tone paired with a footshock inside a soundproof chamber. Then memory of contextual fear conditioning was measured by placing the animals inside the same chamber in which they had received the training and recording the percentage of time that they exhibited a fear response (freezing) over a 5 min interval. The triple heterozygotes (Fig. 6A) as well as all single and double heterozygotes (data not shown) exhibited normal memory at both 2 and 24 hr after training. To measure memory of the tone, we placed the animals into a chamber with different contextual cues and measured their fear response to the tone. The percentage of time that the controls and the triple heterozygotes spent freezing before the tone onset during the memory test (Pre-CS) was not significantly different (Fig. 6B), nor was there any difference in freezing responses between groups during the presentation of the tone (CS). Therefore, although the triple heterozygotes exhibit a deficiency in spatial memory in the water maze, this deficiency is not generalizable to other hippocampal-based memory tasks.
Figure 6.
Performance of α3/+;α5/+;α8/+ triple heterozygotes in fear-conditioning assays. A, Performance after contextual fear conditioning at 2 and 24 hr after two CS-US pairings. No significant difference in conditioned responding was observed between wild-type (n = 18) and mutant (n = 18) animals at either time point (F(1,34) = 0.405 and p = 0.81 for 2 hr; F(1,34) = 0.54 and p = 0.71 for 24 hr). B, Performance at 24 hr after cued fear conditioning with two CS-US pairings. Both wild-type and triple heterozygous mutants exhibited conditioned responding during the 3 min presentation of the CS (p < 0.000001 for wild-type; p < 0.0003 for the triple heterozygotes). No difference was observed between the two groups either before (p = 0.17) or during (p = 0.42) the presentation of the tone.
Brain anatomy of integrin-deficient mice
To determine whether the deficits in LTP, PPF, and spatial memory observed in the triple heterozygotes were attributable to developmental defects of the CNS and particularly the hippocampus, we compared their brain anatomy with normal siblings. A complete loss of α3 function (homozygous knock-out) has been reported to disrupt cortical delamination (Dulabon et al., 2000). Nissl staining of paraffin brain sections failed to uncover any detectable difference in the laminar organization of the neocortex of the mutant heterozygotes (Fig. 7A,B). We found no obvious difference in the number of cells or their organization in each layer in the sensory and motor cortex and in the various subfields of the hippocampus and dentate gyrus (Fig. 7C,D). In addition, we detected no obvious difference in the size or organization of hippocampal neuropil regions as revealed by staining with an anti-syntaxin antibody (Fig. 7E,F). Therefore, these results, combined with behavioral results that test for normal hippocampal function (Fig. 6), suggest that the behavioral and physiological deficits of the triple heterozygotes are attributable to a physiological rather than a developmental requirement for integrins.
Figure 7.

Normal neuroanatomy in integrin heterozygotes. A-D, Nissl staining of parasagittal sections of sensory cortex (A, B) and hippocampus (C, D) in controls (A, C) and α3/+;α5/+;α8/+ triple heterozygotes (B, D). Layers I-VI in the cortex are indicated. E, F, Immunohistochemical staining of control (E) and α3/+;α5/+;α8/+ (F) hippocampal sections with an anti-syntaxin antibody. DG, Dentate gyrus; CC corpus callosum; Slu, stratum lucidum.
Discussion
As reported here, we have examined the role for integrins in synaptic plasticity and behavior by assaying animals heterozygous for several different α-integrin genes. We adopted the approach of examining dominant phenotypes for two reasons. First, integrin knock-out alleles, in general, are homozygous lethal. This is attributable to a requirement for integrins for some developmental processes (Bouvard et al., 2001). The second reason is that behavioral phenotypes are quite sensitive to gene dosage. For example, Drosophila learning mutants (dunce, rutabaga, turnip, cabbage, Volado, and PP1) and circadian rhythm mutants (clock, cycle, lark, timeless, period, and doubletime) have behavioral phenotypes that are dominant or semidominant (Davis, 1996; Grotewiel et al., 1998) (see www.flybase.org). Moreover, the few mouse learning mutants tested as heterozygotes (NF1, NGF, BDNF) are also dominant or semidominant (Chen et al., 1997; Linnarsson et al., 1997; Costa et al., 2002). The sensitivity of behavior to gene dosage is poorly appreciated, yet it offers an approach to screen for and study mutants that have developmental roles in addition to roles in the function of the adult CNS.
The results presented here provide direct evidence that integrins are involved in activity-dependent synaptic plasticity and in spatial memory. The phenotypes we observed in the mutant animals map to the relevant integrin loci because we have out-crossed all of the mutants to the wild-type genetic backgrounds for at least six generations, thereby replacing >98% of the original genome. In addition, the detected phenotypes in the triple heterozygotes are not likely attributable to structural defects in the CNS because we have failed to find any gross neuroanatomical abnormalities in the mutant brains. However, we cannot rule out the possibility that there may be subtle subcellular structural defects that exceed our level of detection. Further support for a physiological versus a structural basis of the mutant phenotypes is that the mutant animals perform indistinguishably from wild-type controls in a battery of neurological and behavioral tests, including eye blink reflex, ear twitch, whisker touch, pupil constriction to light, righting response, rotarod motor learning, open field exploration, and both cued and contextual fear conditioning. In other words, the mutants are indistinguishable from controls in all tests except for the spatial version of the Morris water maze.
We found that heterozygosity for the α3 locus reduces the magnitude of LTP to ∼50% of its normal level when measured at 1 hr after HFS. LTP immediately after tetanus in these animals also was depressed, but this depression failed to reach statistical significance. These findings, along with the normal basal synaptic transmission, normal neuroanatomy, and normal learning (in many assays) of animals heterozygous for α3 (and/or other integrin genes), argue that the α3 integrin is required for experience-dependent synaptic change.
How might this integrin be involved in LTP? Integrins are involved in many different processes, and there are consequently many different ways in which the α3 integrin might influence LTP. The integrins are known to stimulate changes in intracellular calcium (Sjaastad and Nelson, 1997), so a role in LTP may be via the regulation of this molecule. An alternative is that the integrins may be involved in some way in the sensitivity of neurotransmitter receptor function. A switch in NMDA receptor subunit composition during the maturation of synapses is under the control of certain integrins (Chavis and Westbrook, 2001), and it is easy to envision analogous mechanisms underlying LTP. A third possibility is that tetanus may induce integrin clustering or a switch of the integrin into a high-affinity state, one that is sufficient to produce a structural change at the synapse and an associated potentiation of function.
The integrins, however, not only are involved in long-term synaptic change but also are involved in short-term plasticity. Animals heterozygous for α3 and α5 or for α3, α5, and α8 have defective PPF. PPF in α3/+;α5/+ double heterozygotes, for instance, forms in parallel to PPF in control animals at short inter-stimulus intervals and reaches a similar magnitude but is dampened at longer interstimulus intervals. PPF is attributable to an enhanced probability of synaptic vesicle release, with the second stimulus caused by residual calcium remaining from the first (Wu and Saggau, 1994). The presence of the PPF deficit only in animals heterozygous for both α3 and α5, but not either allele alone, suggests that these integrins function together to influence release probability, perhaps by regulating the presynaptic level of calcium. As mentioned above, several different integrins, including α5β1, are involved in regulating intracellular calcium levels after their activation (Sjaastad and Nelson, 1997). It may be that PPF requires an integrin-dependent elevation or maintenance of presynaptic calcium after the first pulse. Alternatively, because integrins function via an intimate association with the cytoskeleton, they may be involved in directly regulating release probability via a mechanical mechanism. The compounding effects of α8 in synapses from α3/+;α5/+;α8/+ triple heterozygotes is likely attributable to a postsynaptic role for α8, because the expression of this integrin has been found only postsynaptically (Einheber et al., 1996). Postsynaptic neurons also may play a significant role in modulating transmitter release at the presynaptic terminals (Davis and Murphey, 1993; Markram et al., 1998; Reyes et al., 1998; Scanziani et al., 1998).
Although animals heterozygous for the α3 null allele have a clear deficit in LTP, we failed to find any associated behavioral deficit in these animals. Indeed, a clear phenotype in water maze performance was detected only in animals simultaneously heterozygous for α3, α5, and α8. Table 2 summarizes the correspondence in physiological and behavioral phenotypes of the seven genotypes that were studied. Some redundancy of integrin function is to be expected, given that all three of these integrins share β1 as a partner and that all three interact with some of the same ligands (van der Flier and Sonnenberg, 2001). It is interesting, however, that these heterozygotes perform normally in contextual fear conditioning, which is also a hippocampal-dependent task. It is likely that at least some different molecular mechanisms govern performance in the two different hippocampal-dependent tasks or that perhaps they require a different threshold of integrin function for normal performance. The dissociation between LTP and spatial learning that was observed is possibly also attributable to different threshold requirements for integrin function, or it is possible that NMDA receptor-dependent LTP in area CA1 is a permissive but dispensable physiological component of normal spatial learning.
Table 2.
Behavioral and physiological phenotypes of integrin heterozygotes
|
Genotype |
LTP |
PPF |
Spatial memory |
Fear conditioning |
|---|---|---|---|---|
| α3/+ | — | Normal | Normal | Normal |
| α5/+ | Normal | Normal | Normal | Normal |
| α3/+ | Normal | Normal | Normal | Normal |
| α3/+; α5/+ | — | — | Normal | Normal |
| α3/+; α8/+ | — | Normal | Normal | Normal |
| α5/+; α8/+ | Normal | Normal | Normal | Normal |
| α3/+; α5/+; α8/+
|
—
|
—
|
—
|
Normal
|
A myriad of other molecules is involved in integrin interactions and integrin function. This complexity makes difficult any precise assignment for how integrins regulate synaptic plasticity and behavior. One attractive possibility, beyond those mentioned above, is that the integrins function in synaptic plasticity and behavior by mediating the activation of the MAPK pathway. The pathway that leads to ERK activation frequently has been implicated in synaptic change and behavior (Adams and Sweatt, 2002). The activation of integrins causes activation of the tyrosine kinases FAK, FYN, and SRC. The phosphorylation of FAK, in particular, by SRC produces a binding site for the adapter protein, GRB2, which is linked via the activation of RAS to the MAPK cascade (Giancotti and Ruoslahti, 1999). Another attractive model mentioned above is that the synaptic integrins function in an inside-to-out signaling such that second messenger cascade activation in neurons modifies the affinity of integrins at the cell surface for their ligands (Grotewiel et al., 1998). This could lead in principle to a fairly rapid but subtle morphological change in the structure of the synapse such that synaptic transmission would be altered.
Despite these current unknowns, the available data suggest that the integrin family of cell adhesion receptors is likely to have a very profound role in regulating synaptic structure, signaling, and plasticity. There is now evidence indicating that a minimum of eight different integrins resides at hippocampal synapses, including α3, α5, αV, α8, β1, β3, β5, and β8 (Einheber et al., 1996; Nishimura et al., 1998; this study). These expression data offer intriguing possibilities for integrin participation in synaptic function. One general possibility is that the coexpressed integrins may be functionally redundant and participate in the same synaptic process. Alternatively, they may have distinct functions, with some being required for maintaining synaptic organization and integrity, for instance, and others involved in different aspects of synaptic plasticity. The differential sensitivity of LTP and PPF to integrin gene dosage shown above is consistent with the idea that the different integrins may have distinct synaptic functions. A second general possibility is that the integrins may be expressed at synapses in a combinatorial manner such that different synapses may express a different combination of integrin receptors. Electron immunomicrographs that show differential α8 expression at two adjacent synapses of the same CA1 neuron (Einheber et al., 1996) are consistent with the hypothesis of combinatorial expression patterns. Such expression patterns would provide marked flexibility of synapses and neuronal circuits via the cell adhesion and signaling functions offered by the integrin family of receptors.
Footnotes
We thank Dr. Richard Paylor (Baylor College of Medicine) for significant help in establishing mouse behavioral assays in the laboratory. We also thank the Jackson Laboratories, Jordan Kreidberg (Harvard Medical School), and Lou Reichardt (University of California, San Francisco) for supplying theα5,α3, andα8 knock-out animals, respectively. This work was supported by Grant MH60420 from the National Institute of Mental Health to R.L.D. and the Baylor College of Medicine Mental Retardation Research Center (Grant HD24064). R.L.D. is the recipient of the R. P. Doherty-Welch Chair in Science at the Baylor College of Medicine.
Correspondence should be addressed to Ronald L. Davis, Department of Molecular and Cellular Biology, Baylor College of Medicine, Houston, TX 77030. E-mail: rdavis@bcm.tmc.edu.
Copyright © 2003 Society for Neuroscience 0270-6474/03/237107-10$15.00/0
References
- Adams JP, Sweatt JD ( 2002) Molecular psychology: roles for the ERK MAP kinase cascade in memory. Annu Rev Pharmacol Toxicol 42: 135-163. [DOI] [PubMed] [Google Scholar]
- Bahr BA, Staubli U, Xiao P, Chun D, Ji ZX, Esteban ET, Lynch G ( 1997) Arg-Gly-Asp-Ser-selective adhesion and the stabilization of long-term potentiation: pharmacological studies and the characterization of a candidate matrix receptor. J Neurosci 17: 1320-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi X, Lynch G, Zhou J, Gall CM ( 2001) Polarized distribution of α5 integrin in dendrites of hippocampal and cortical neurons. J Comp Neurol 435: 184-193. [DOI] [PubMed] [Google Scholar]
- Bouvard D, Brakebusch C, Gustafsson E, Aszodi A, Bengtsson T, Berna A, Fassler R ( 2001) Functional consequences of integrin gene mutations in mice. Circ Res 89: 211-223. [DOI] [PubMed] [Google Scholar]
- Chang HP, Lindberg FP, Wang HL, Huang AM, Lee EH ( 1999) Impaired memory retention and decreased long-term potentiation in integrin-associated protein-deficient mice. Learn Mem 6: 448-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HP, Ma YL, Wan FJ, Tsai LY, Lindberg FP, Lee EH ( 2001) Functional blocking of integrin-associated protein impairs memory retention and decreases glutamate release from the hippocampus. Neuroscience 102: 289-296. [DOI] [PubMed] [Google Scholar]
- Chavis P, Westbrook G ( 2001) Integrins mediate functional pre- and postsynaptic maturation at a hippocampal synapse. Nature 411: 317-321. [DOI] [PubMed] [Google Scholar]
- Chen KS, Nishimura MC, Armanini MP, Crowley C, Spencer SD, Phillips HS ( 1997) Disruption of a single allele of the nerve growth factor gene results in atrophy of basal forebrain cholinergic neurons and memory deficits. J Neurosci 17: 7288-7296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry JA, Davis RL ( 1995) A mouse homolog of dunce, a gene important for learning and memory in Drosophila, is preferentially expressed in olfactory receptor neurons. J Neurobiol 28: 102-113. [DOI] [PubMed] [Google Scholar]
- Chun D, Gall CM, Bi X, Lynch G ( 2001) Evidence that integrins contribute to multiple stages in the consolidation of long-term potentiation in rat hippocampus. Neuroscience 105: 815-829. [DOI] [PubMed] [Google Scholar]
- Costa RM, Federov NB, Kogan JH, Murphy GG, Stern J, Ohno M, Kucherlapati R, Jacks T, Silva AJ ( 2002) Mechanism for the learning deficits in a mouse model of neurofibromatosis type 1. Nature 415: 526-528. [DOI] [PubMed] [Google Scholar]
- Davis GW, Murphey RK ( 1993) A role for postsynaptic neurons in determining presynaptic release properties in the cricket CNS: evidence for retrograde control of facilitation. J Neurosci 13: 3827-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL ( 1996) Physiology and biochemistry of Drosophila learning mutants. Physiol Rev 76: 299-317. [DOI] [PubMed] [Google Scholar]
- Dulabon L, Olson EC, Taglienti MG, Eisenhuth S, McGrath B, Walsh CA, Kreidberg JA, Anton ES ( 2000) Reelin binds α3β1 integrin and inhibits neuronal migration. Neuron 27: 33-44. [DOI] [PubMed] [Google Scholar]
- Einheber S, Schnapp LM, Salzer JL, Cappiello ZB, Milner TA ( 1996) Regional and ultrastructural distribution of the α8 integrin subunit in developing and adult rat brain suggests a role in synaptic function. J Comp Neurol 370: 105-134. [DOI] [PubMed] [Google Scholar]
- Garner CC, Nash J, Huganir RL ( 2000) PDZ domains in synapse assembly and signaling. Trends Cell Biol 10: 274-280. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E ( 1999) Integrin signaling. Science 285: 1028-1032. [DOI] [PubMed] [Google Scholar]
- Greengard P ( 2001) The neurobiology of slow synaptic transmission. Science 295: 1024-1030. [DOI] [PubMed] [Google Scholar]
- Grotewiel MS, Beck CD, Wu KH, Zhu XR, Davis RL ( 1998) Integrin-mediated short-term memory in Drosophila Nature 391: 455-460. [DOI] [PubMed] [Google Scholar]
- Huang AM, Wang HL, Tang YP, Lee EH ( 1998) Expression of integrin-associated protein gene associated with memory formation in rats. J Neurosci 18: 4305-4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MJ ( 2000) Integrin structure. Biochem Soc Trans 28: 311-339. [PubMed] [Google Scholar]
- Kennedy MB ( 2000) Signal-processing machines at the postsynaptic density. Science 290: 750-754. [DOI] [PubMed] [Google Scholar]
- Kim J, Alger BE ( 2001) Random response fluctuations lead to spurious paired-pulse facilitation. J Neurosci 21: 9608-9618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS ( 1992) Modality-specific retrograde amnesia of fear. Science 256: 675-677. [DOI] [PubMed] [Google Scholar]
- King VR, McBride A, Priestley JV ( 2001) Immunohistochemical expression of the α5 integrin subunit in the normal adult rat central nervous system. J Neurocytol 30: 243-252. [DOI] [PubMed] [Google Scholar]
- Kramar EA, Bernard JA, Gall CM, Lynch G ( 2002) α3 Integrin receptors contribute to the consolidation of long-term potentiation. Neuroscience 110: 29-39. [DOI] [PubMed] [Google Scholar]
- Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, Jaenisch R ( 1996) α3β1 Integrin has a crucial role in kidney and lung organogenesis. Development 122: 3537-3547. [DOI] [PubMed] [Google Scholar]
- Linnarsson S, Bjorklund A, Ernfors P ( 1997) Learning deficit in BDNF mutant mice. Eur J Neurosci 9: 2581-2587. [DOI] [PubMed] [Google Scholar]
- Markram H, Wang Y, Tsodyks M ( 1998) Differential signaling via the same axon of neocortical pyramidal neurons. Proc Natl Acad Sci USA 95: 5323-5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TPJ ( 2002) Prime movers of synaptic vesicle exocytosis. Neuron 34: 9-12. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J ( 1982) Place navigation impaired in rats with hippocampal lesions. Nature 297: 681-683. [DOI] [PubMed] [Google Scholar]
- Nishimura SL, Boylen KP, Einheber S, Milner TA, Ramos DM, Pytela R ( 1998) Synaptic and glial localization of the integrin αvβ8 in mouse and rat brain. Brain Res 791: 271-282. [DOI] [PubMed] [Google Scholar]
- Pinkstaff JK, Detterich J, Lynch G, Gall C ( 1999) Integrin subunit gene expression is regionally differentiated in adult brain. J Neurosci 19: 1541-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop A ( 1999) Integrating bits and pieces: synapse structure and formation in Drosophila embryos. Cell Tissue Res 297: 169-186. [DOI] [PubMed] [Google Scholar]
- Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P, Sakmann B ( 1998) Target cell-specific facilitation and depression in neocortical circuits. Nat Neurosci 1: 279-285. [DOI] [PubMed] [Google Scholar]
- Scanziani M, Gahwiler BH, Charpak S ( 1998) Target cell-specific modulation of transmitter release at terminals from a single axon. Proc Natl Acad Sci USA 95: 12004-12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M ( 2001) Molecular organization of the postsynaptic specialization. Proc Natl Acad Sci USA 98: 7058-7061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjaastad MD, Nelson WJ ( 1997) Integrin-mediated calcium signaling and regulation of cell adhesion by intracellular calcium. BioEssays 19: 47-55. [DOI] [PubMed] [Google Scholar]
- Staubli U, Chun D, Lynch G ( 1998) Time-dependent reversal of long-term potentiation by an integrin antagonist. J Neurosci 18: 3460-3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC ( 2000) The synaptic vesicle cycle revisited. Neuron 28: 317-320. [DOI] [PubMed] [Google Scholar]
- van der Flier A, Sonnenberg A ( 2001) Function and interactions of integrins. Cell Tissue Res 305: 285-298. [DOI] [PubMed] [Google Scholar]
- Wu LG, Saggau P ( 1994) Presynaptic calcium is increased during normal synaptic transmission and paired-pulse facilitation, but not in long-term potentiation in area CA1 of hippocampus. J Neurosci 14: 645-654. [DOI] [PMC free article] [PubMed] [Google Scholar]