Abstract
Electrophysiological recording procedures were used to examine basolateral amygdala (BLA) cell firing during cocaine self-administration and relative to response-independent presentations of cocaine-associated stimuli. Of 72 neurons (n = 10 rats), 31 cells (43%) were classified as phasically active, exhibiting one of three types of patterned discharges relative to the drug-reinforced response, similar to that previously described for nucleus accumbens (Acb) neurons (Carelli, 2002). Briefly, neurons exhibited increased firing rates within seconds preceding the response [termed preresponse (PR)], increased activity within seconds after the response [termed reinforcement excitation (RFe)] or an inhibition in cell firing before and/or after the response for intravenous cocaine [termed reinforcement inhibition (RFi)]. To examine the responsiveness of these same neurons to cocaine-associated stimuli, the stimulus “probe” procedure was used. Specifically, probe trials (18-20) were presented in which the audiovisual (tone-house light) stimulus associated with intravenous cocaine delivery during self-administration was randomly presented by the computer, interspersed between reinforced lever press responses. Neurons classified as type PR or type RFi were not activated by the stimulus. In contrast, neurons that exhibited increased firing immediately after the response (type RFe neurons) were significantly activated by the audiovisual cue. These findings are discussed with respect to the role of the BLA in cocaine addiction as well as previous studies characterizing Acb cell firing during cocaine self-administration.
Keywords: addiction, amygdala, cocaine, self-administration, electrophysiology, behavior
Introduction
Numerous studies indicate that the basolateral amygdala (BLA) is a neural substrate critically involved in associative (conditioned) aspects of drug-seeking behavior. For example, the BLA is important for responding on a second-order schedule of cocaine reinforcement (Everitt et al., 1991; Whitelaw et al., 1996; Everitt and Robbins, 2000; Kantak et al., 2002) as well as for the establishment of cocaine-conditioned place preference (Fuchs et al., 2002). In addition, the BLA appears to be crucially involved in reinstatement of drug-taking behavior after extinction. For example, lesions of the BLA did not affect subsequent cocaine self-administration but decreased responding during extinction sessions and abolished the ability of drug-paired stimuli to reinstate lever pressing behavior for cocaine (Meil and See, 1997; See, 2002). Furthermore, electrical stimulation of the BLA is sufficient to reinstate cocaine self-administration after extinction (Hayes et al., 2003).
It has been reported that stimuli associated with cocaine-taking behavior, such as drug paraphernalia or environmental cues, are strong elicitors of drug “craving” and often lead to relapse after a period of drug abstinence in humans (Gawin, 1991; Dackis and O'Brien, 2001). Studies using brain metabolic imaging techniques such as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) revealed that specific brain regions, including the BLA and nucleus accumbens (Acb), are activated during reports of drug craving in cocaine addicts (Breiter et al., 1997; Childress et al., 1999; Bonson et al., 2002). Furthermore, anatomic studies have revealed that the BLA sends projections to the Acb (Groenewegen et al., 1991; Zahm and Brog, 1992; Brog et al., 1993; Wright et al., 1996). It is therefore possible that the BLA-Acb circuit plays an important role in mediating associative (conditioned) aspects of reward-related processing that may ultimately control drug-seeking behavior.
To date, the majority of electrophysiological studies completed in cocaine self-administering animals have focused on characterizing neuronal activity within the Acb (for review, see Carelli, 2002). Those studies have shown that Acb neurons exhibit four types of patterned discharges within seconds of the reinforced response for intravenous cocaine. Importantly, a subset of Acb neurons that encode goal-directed behaviors for cocaine are activated by cocaine-associated stimuli (Carelli, 2000). Given the importance of the amygdala (in particular, the basolateral subregion) in conditioned aspects of cocaine addiction (Everitt et al., 1991; Everitt and Robbins, 2000; See, 2002; Kalivas and McFarland, 2003) and the fact that this structure sends extensive projections to the Acb (Groenewegen et al., 1991; Zahm and Brog, 1992; Brog et al., 1993; Heimer et al., 1995, 1997; Wright et al., 1996), it is important to examine the activity of neurons in the BLA within these contexts. Therefore, the purpose of this study was to characterize BLA cell firing during cocaine self-administration and to examine the responsiveness of those neurons to cocaine-associated cues.
Materials and Methods
Cocaine self-administration. Animals (n = 10) were housed individually and maintained at no less than 85% of their preoperative body weight beginning 1 week after catheter implantation by regulation of food and water intake. Specifically, animals were given 10 ml of water (in addition to 1.0-1.5 ml of water consumed during the session) and 20 gm of Purina (St. Louis, MO) laboratory pellets each day for the duration of the experiment. Animals were surgically implanted with an intravenous catheter and trained to self-administer cocaine, as previously described (Carelli et al., 2000). Briefly, subjects were anesthetized with ketamine hydrochloride (100 mg/kg) and xylazine hydrochloride (20 mg/kg) and surgically implanted with a catheter into the jugular vein (Caine et al., 1993). The catheter was then routed subcutaneously to the back and attached to a coupling assembly. The fluid injection assembly (syringe pump) was connected to a swivel system in the experimental chambers that enabled intravenous infusion of cocaine during self-administration sessions.
One week after catheter implantation, rats were trained to self-administer cocaine during 2 hr experimental sessions. The beginning of the session was signaled by the onset of a cue light positioned 6.5 cm above the lever and extension of a retractable lever (Coulbourn Instruments, Allentown, PA). Lever depression on a fixed ratio 1 (FR1) schedule resulted in intravenous cocaine delivery (0.33 mg/infusion, dissolved in sterile heparinized saline vehicle) over a 6 sec period via a computer-controlled syringe pump (PHM-100; Med Associates, Inc., St. Albans, VT). Each drug infusion was signaled immediately by the onset of a tone-house light stimulus (65 dB, 2900 Hz, 100 mA) presented over a 20 sec interval (14 sec beyond the pump duration). During the 20 sec postresponse interval, lever press responding had no programmed consequences.
Electrophysiological recordings. Once behavioral responding was stable (2-3 weeks), animals were anesthetized with ketamine hydrochloride (100 mg/kg) and xylazine hydrochloride (20 mg/kg) and prepared for chronic extracellular recording in the amygdala. Electrodes were custom-designed and purchased from a commercial source (NB Labs, Denison, TX). Each array consisted of “bundles” of 4 microwires (50 μm diameter) arranged in two rows. Each row contained two wires with a tip separation of ∼0.5 mm. The entire array spanned an approximate distance of 1 mm anteroposterior and 1 mm mediolateral. Each array also contained a ground wire that was inserted 3-4 mm into the brain, ipsi-lateral to the array and ∼5 mm caudal to bregma. Arrays were permanently implanted bilaterally into the BLA (anteroposterior, -1.80 to -3.40 mm; mediolateral, 4.0-5.6 mm; and dorsoventral, 6.5-8.0 mm, relative to bregma, level skull).
After electrode implantation, presurgical behavioral performance was reestablished (typically within 1 d), and neuronal activity was characterized for each animal during a self-administration session and the next day during a self-administration session in which stimulus “probe” trials were presented (see below). Electrophysiological procedures have been described in detail previously (Carelli and Deadwyler, 1994; Carelli et al., 2000). Briefly, before the start of each session, the subject was connected to a flexible recording cable attached to a commutator (Med Associates, Inc.), which allowed virtually unrestrained movement within the chamber. The head stage of each recording cable contained 16 miniature unity-gain field effect transistors. BLA activity was recorded differentially between each active electrode and the inactive (reference) electrode from the permanently implanted microwires. The inactive electrode was examined before the start of the session to verify the absence of neuronal spike activity and served as the differential electrode for other electrodes with cell activity. Online isolation and discrimination of neuronal activity were accomplished using a commercially available neurophysiological system [multichannel acquisition processor (MAP) system; Plexon, Inc., Dallas, TX]. Multiple window discrimination modules and high-speed analog-to-digital signal processing in conjunction with computer software enabled isolation of neuronal signals on the basis of waveform analysis. The neurophysiological system incorporated an array of digital signal processors (DSPs) for continuous spike recognition. The DSPs provided a continuous parallel digital output of neuronal spike events to a Pentium computer. Another computer controlled behavioral events of the experiment (Med Associates, Inc.) and sent digital outputs corresponding to each event to the MAP box to be time-stamped along with the neural data. The neurophysiological system has the capability of recording up to four neurons per microwire using real-time discrimination of neuronal action potentials. However, in the present study, typically one or two neurons were recorded per microwire, as in previous reports (Chang et al., 1994; Nicolelis et al., 1997; Carelli et al., 2000). Criteria for identifying different neurons on a single wire have been described in detail elsewhere (Chang et al., 1994; Nicolelis et al., 1997; Nicolelis, 1999; Carelli et al., 2000). Briefly, discrimination of individual waveforms corresponding to a single cell was accomplished using template analysis procedures or time-voltage boxes provided by the neurophysiological software system (MAP system). The template analysis procedure involves taking a “sample” of the waveform and building a template of that extracellular waveform. Subsequent neurons that “match” this waveform are included as the same cell. When using time-voltage boxes, a sample of the waveform is taken, and then the experimenter superimposes two boxes onto it (typically one on the ascending limb and the other on the descending limb of the extracellular waveform). Subsequent sampled neurons are accepted as valid when they pass through both boxes. The parameters for isolation and discrimination of single-unit activity were determined and saved using the neurophysiological software and modified before each session as needed, for example, to discriminate “new” neurons that appeared on a given microwire electrode or to change the inactive electrode.
Stimulus probe trials. The responsiveness of BLA neurons to cocaine-associated stimuli was evaluated using the stimulus probe procedure previously used in our laboratory (Carelli, 2000, 2002). Specifically, response-independent presentations (12-18 per session) of the tone-house light stimulus (5 sec) was randomly presented by the computer, interspersed between reinforced lever press responses during self-administration sessions (typically within 1-4 min after a reinforced response).
Data analysis. Neural activity was characterized via raster displays and perievent histograms (PEHs) showing the activity of each cell during a 20 sec time interval that bracketed the cocaine-reinforced lever press response. BLA neurons were examined for changes (increases or decreases) in firing rates within four time epochs in each PEH (Carelli and Deadwyler, 1994). The four time epochs included (1) “baseline,” defined as the period (-10 to -7.5 sec) before the initiation of the reinforced lever press response; (2) “response,” defined as the period (-2.5 to 0 sec) immediately before and during the execution of the reinforced response; (3) “reinforcement,” defined as the period (0 to +2.5 sec) immediately after the response; and (4) “recovery,” defined as the period (+7.5 to +10 sec) after the reinforced response. Using this approach, we examined whether BLA neurons exhibited one of four types of neuronal firing patterns described previously for nucleus accumbens neurons [preresponse (PR), reinforcement excitation (RFe), reinforcement inhibition (RFi), and preresponse plus reinforcement (PR + RF) (Carelli and Dead-wyler, 1994; Carelli et al., 2000)]. Criteria for classifying each neuron into one of the four types of patterned discharges was based on 40% changes in cell firing within specified epochs compared with baseline rates, as described in detail previously (Carelli et al., 2000). Briefly, neurons were classified as type PR if they showed a ≥40% increase in the firing rate within a 1 sec period of maximal discharge during the response epoch only compared with its respective baseline activity. Type RFe cells displayed a ≥40% increase in cell firing within a 1 sec period of maximal discharge during the reinforcement phase only compared with its respective baseline activity. Neurons classified as type RFi had a ≥40% decrease in the firing rate within a 1 sec period during the response epoch, reinforcement epoch, or both compared with its respective baseline firing rate. Finally, type PR + RF cells displayed a ≥40% increase in activity during a 1 sec period within both the response and reinforcement epochs (but not the recovery phase) compared with its respective baseline rate, with an inhibition in activity between the two peak discharges. Statistical confirmation of the above cell type classification was accomplished using t test statistics for dependent samples that compared mean peak (types PR and RFe) or trough (type RFi) firing rates for all neurons of a given type with their respective baseline rates. “Nonphasic” neurons exhibited similar firing rates across the four time epochs without the 40% changes in activity characteristic of the four types of patterned discharges described above. Cell type classifications and behavioral responding were qualitatively similar during sessions involving probe trials versus those without probe trials. Therefore, to examine the responsiveness of specific types of neurons to cocaine-associated stimuli, data are presented here only for sessions involving probe trial presentations.
Activation of BLA neurons during stimulus probe trials was examined as follows. BLA neurons were examined for 40% changes (increases or decreases) in firing rates within 2.5 sec before versus 2.5 sec after cocaine-associated stimulus probe trials. t test statistics for dependent samples were also used to verify significant changes in cell firing during stimulus probe presentations by examining BLA cell firing rates 2.5 sec before versus 2.5 sec after probe trials. The latency to onset and duration of neuronal discharges for individual neurons were determined using established procedures (Carelli et al., 2000). Briefly, mean firing rates were examined within consecutive 80 msec periods (bins) during the epoch in which the cell exhibited its peak or trough changes in activity. The latency of onset was defined as the first of three consecutive 80 msec bins in which the firing rate consistently increased (for type PR and RFe cells) or decreased (for type RFi cells) by 40% compared with the respective baseline activity of each cell. The latency of offset was defined as the first of three consecutive 80 msec bins in which the firing rate subsequently returned to baseline levels. The duration of activation was determined by subtracting onset from offset values.
Histology. After the completion of the last experiment, animals were anesthetized with sodium pentobarbital (50 mg/kg), and a 10 μA current was passed for 6 sec through each recording electrode. The rat was perfused with 10% formalin, and the brain was removed, blocked, and sectioned (50 μm) throughout the rostrocaudal extent of the amygdala. Alternating sections were stained for either thionin or tyrosine hydroxylase. All sections were counterstained with Prussian blue to reveal a blue dot reaction product corresponding to the location of the marked electrode tip (Green, 1958; Carelli and Deadwyler, 1994). The procedure used to reconstruct electrode placements was as follows. Serial sections were examined under a light microscope, and the location of each electrode tip was plotted on coronal sections taken from the stereotaxic atlas of Paxinos and Watson (1997). Positions within the BLA and boundaries between this and adjacent regions were determined by examination of marked electrode tip locations relative to (1) the anatomic arrangement of the amygdala, as depicted in the stereotaxic atlas of Paxinos and Watson (1997); and (2) precise “landmarks” in the brain, for example, the position relative to the boundary of the caudal portion of the caudate-putamen (clearly visible on the tyrosine hydroxylase-stained sections).
Results
Self-administration behavior
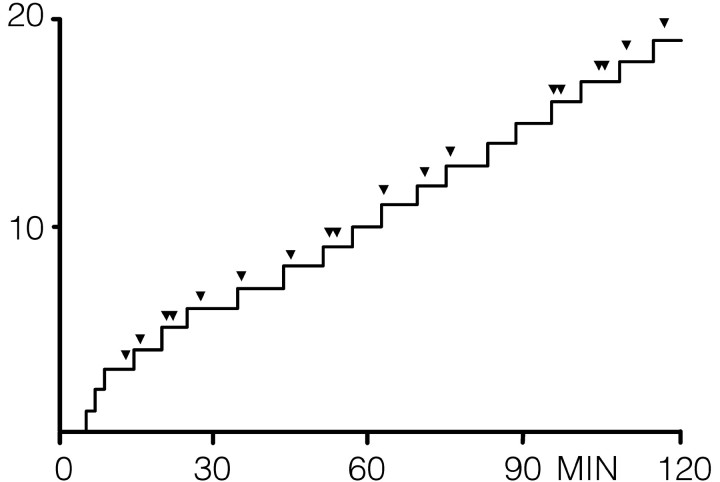
The cumulative record in Figure 1 shows the behavioral response pattern for one representative animal during a cocaine self-administration session in which stimulus probe trials (indicated by arrowheads, 5 sec duration each) were interspersed between reinforced responses. During the session, the animal exhibited an initial “burst” of 3 responses (termed “load-up” behavior) followed by 16 regularly spaced responses with a mean interinfusion interval (INT) of 6.10 ± 0.28 min. Across all 10 animals, the mean number of responses was 24 ± 1.78 with a mean INT of 5.81 ± 0.24 min. t tests for dependent samples indicated no significant difference in the number of responses (p > 0.05) or INT (p > 0.5) during sessions in which probe trials were not given (previous day) compared with sessions including probe trials, indicating that behavioral responding was not altered by stimulus probe presentations.
Figure 1.
Cumulative records showing the behavioral (lever press) response pattern for a single animal during a cocaine (0.33 mg/infusion) self-administration session in which stimulus probe trials (5 sec) were randomly presented (indicated by arrowheads). Each upward deflection indicates a reinforced response (FR1). y-Axis, Number of lever presses.
BLA neurons exhibit patterned discharges relative to operant responding for intravenous self-administration of cocaine
A total of 72 amygdala neurons were recorded during cocaine self-administration sessions. The major finding of this study was that 31 cells (43%) exhibited one of three types of neuronal discharge patterns similar to those described previously for Acb neurons (Carelli and Deadwyler, 1994; Carelli et al., 2000; Carelli, 2002). This classification was based on PEH inspection, quantification, and statistical analysis (Table 1). Briefly, an increase in the firing rate immediately before the reinforced lever press response designated some neurons as PR cells. Other types of neurons exhibited excitation (type RFe) or inhibition (type RFi) of the firing rate within seconds after the cocaine-reinforced response. The remaining 41 neurons (57%) exhibited no change in the firing rate (increase or decrease) relative to the operant response for cocaine (nonphasic type).
Table 1.
Mean ± SEM of BLA neurons across four time epochs showing three types of patterned discharges relative to reinforced response for cocaine during self-administration sessions
|
Epoch (sec) |
Type PR (n = 16) |
Type RFe (n = 10) |
Type RFi (n = 5) |
|---|---|---|---|
| Baseline (-10 to -7.5) | 1.10 ± 0.42 | 0.56 ± 0.12 | 3.05 ± 1.23 |
| Before response (-2.5 to 0) | 3.73 ± 1.07a* | 0.86 ± 0.18 | 1.51 ± 0.61a |
| After response (0 to 2.5) | 1.79 ± 0.59 | 2.46 ± 0.62a* | 1.64 ± 0.76a |
| Recovery (7.5 to 10)
|
1.18 ± 0.39
|
1.19 ± 0.31
|
2.87 ± 1.27
|
A ≥ 40% change in firing rate relative to baseline in each column.
p < 0.05 compared with baseline in each column.
Preresponse cell firing
Of 31 phasically active neurons, 16 cells (52%) exhibited increased firing rates within seconds before the reinforced response for cocaine and were classified as type PR neurons. Across all type PR neurons (n = 16), there was a significant increase in the firing rate within 2.5 sec preceding the reinforced response (3.73 ± 1.07 Hz), compared with baseline rates (1.10 ± 0.42 Hz; t = 3.72; p < 0.01; Table 1). The mean latency to onset across all type PR cells was 5.27 ± 0.63 before the reinforced response.
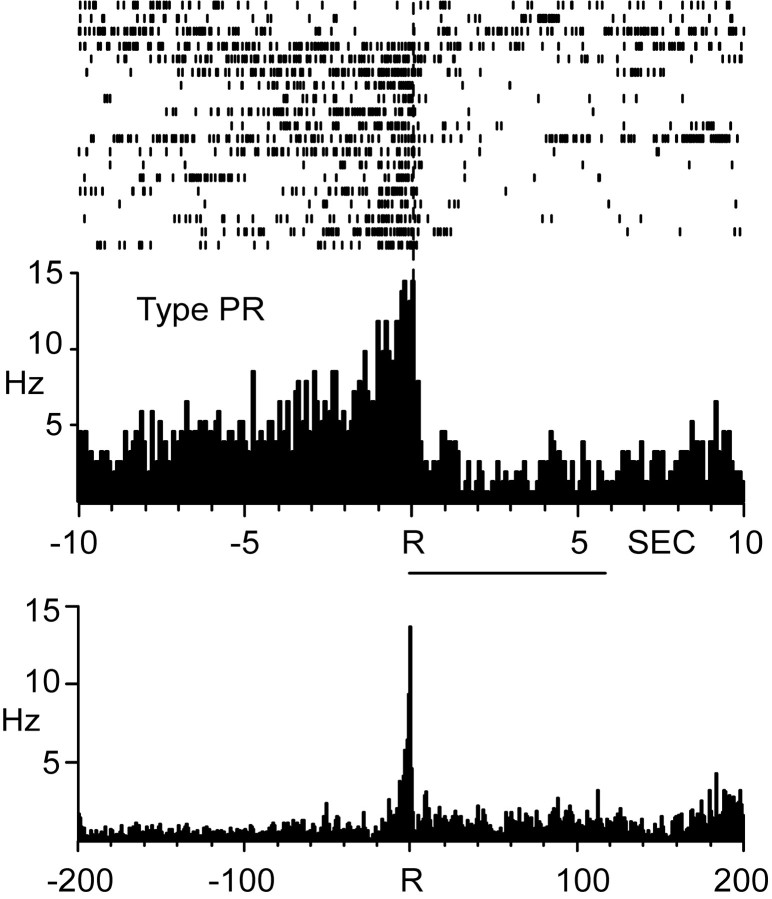
An example of one PR neuron is illustrated in Figure 2. The top raster plot and PEH show the activity of the cell within the 20 sec analysis interval (10 sec before and 10 sec after the response). The neuron displayed a mean baseline firing rate of 2.99 Hz with a peak discharge rate of ∼15 Hz beginning 3.52 sec before the reinforced response for intravenous cocaine. The cell exhibited a slight decline in the firing rate within ∼4 sec after the response, followed by a return to baseline levels. Figure 2, bottom PEH, shows the activity of the same BLA cell across a longer interval encompassing the majority of the interinfusion interval (i.e., 200 sec before and 200 sec after the response). This PEH illustrates that the increase in cell firing was not long-lasting but was confined primarily to the 5 sec interval preceding the reinforced response for cocaine. Inspection of PEHs across the longer interval encompassing the majority of the interinfusion interval indicated that this was the case for all type PR neurons.
Figure 2.
Single BLA neuron showing an increase in firing rate within seconds preceding the reinforced response for cocaine, characteristic of type PR firing. Top, Single-trial raster plot and PEH of BLA cell firing within a 20 sec analysis interval relative to the lever press response. The duration of drug infusion is indicated by horizontal line below the PEH. Bottom, PEH showing the activity of the same BLA neuron displayed above across a longer interval encompassing a large portion of the interinfusion interval. The PEH excludes activity in which lever presses occurred within intervals on either side of the response. Each PEH contains 250 bins in all figures. R at dashed vertical line, Reinforced responses in each PEH here and in subsequent figures.
Postresponse activity
Of the remaining 15 phasically active cells, 10 neurons exhibited a significant increase in the firing rate within 2.5 sec after the reinforced response for intravenous cocaine, characteristic of type RFe activity. Across all type RFe neurons, there was a significant increase in the firing rate within 2.5 sec after the response (2.46 ± 0.62 Hz) compared with baseline activity (0.56 ± 0.12 Hz; t = 3.42; p < 0.01; Table 1).
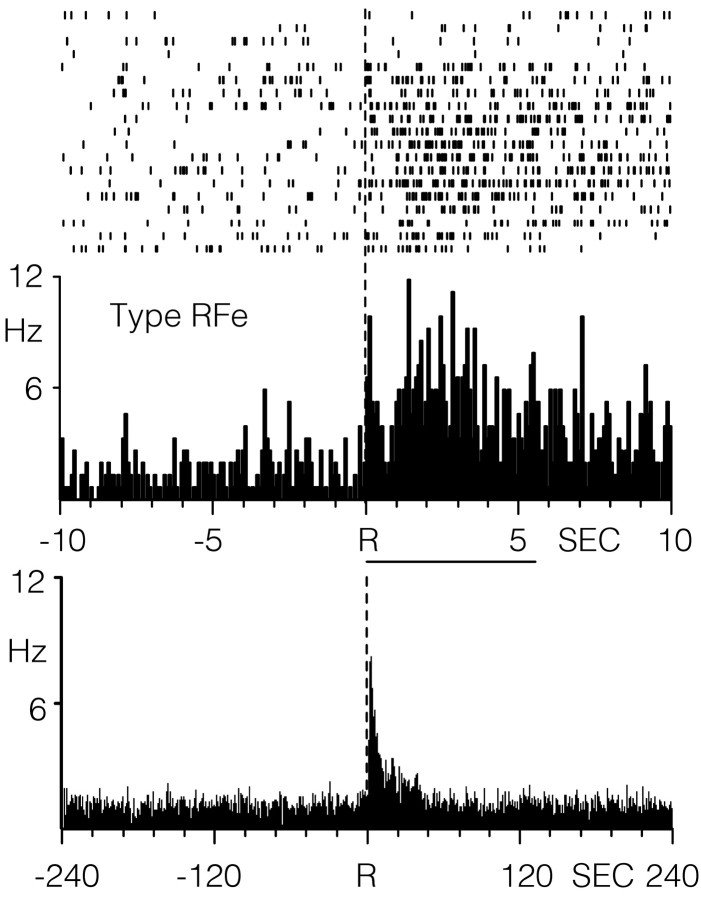
An example of an RFe cell is shown in the raster plot and PEH in Figure 3. In this case, the neuron displayed a baseline firing rate of 0.78 Hz with an increase in activity immediately after the lever press response to ∼12 Hz. The PEH in Figure 2, bottom, shows the activity of the same neuron across a longer interval spanning the interinfusion interval (i.e., 4 min before and 4 min after the response). Note that the peak increase in activity was confined to ∼12 sec after the response, although rates remained slightly elevated for an additional 30 sec. Across all type RFe cells, the mean latency to onset was 416 ± 150 msec after the response. For six type RFe cells, the mean offset latency (i.e., time in which the cell firing rates returned to baseline activity) was 3.87 ± 1.03 sec after the response. The remaining four RFe cells had a prolonged duration that extended slightly beyond the 20 sec analysis interval, as illustrated for one neuron in Figure 3, bottom.
Figure 3.
Single BLA neuron showing an increase in firing rate within seconds after the reinforced response for cocaine, characteristic of type RFe activity. Top, Single-trial raster plot and PEH of BLA cell firing within a 20 sec analysis interval relative to the lever press response. The duration of drug infusion is indicated by the horizontal line below the PEH. Bottom, PEH showing the activity of the same BLA neuron displayed across a longer interval encompassing a large portion of the interinfusion interval. The PEH excludes activity in which lever presses occurred within intervals on either side of the response.
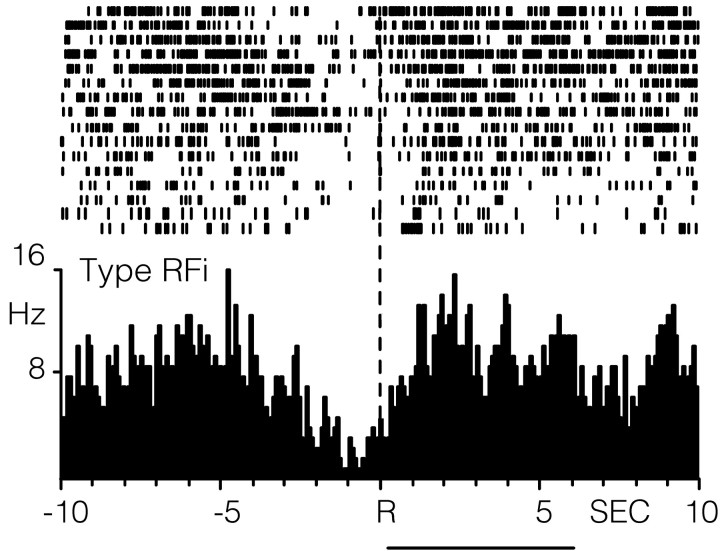
The remaining five BLA cells exhibited an inhibition in cell firing, relative to baseline activity, within 2.5 sec before the reinforced response, after the response, or both, classified as type RFi neurons. An example of a type RFi neuron is shown in Figure 4. For this cell, there was a marked decline in the firing rate from a baseline rate of 6.66 Hz beginning 2.24 sec before the reinforced response and continuing for 1.36 after response completion. Across all RFi cells, the onset to the inhibition in cell firing began 864 ± 440 msec before the response and extended 1.68 ± 0.49 sec after response completion. Within the 20 sec analysis interval, all type RFi cells showed a 40% decline in cell firing within 2.5 sec before the response, after the response, or both (Table 1).
Figure 4.
A single BLA neuron showing an inhibition from background firing rates seconds before and after the reinforced response for intravenous cocaine. The raster plot and PEH show the activity of the BLA neuron within the 20 sec analysis interval. The duration of drug infusion is indicated by the horizontal line below the PEH.
Responsiveness of BLA neurons to cocaine-associated stimuli
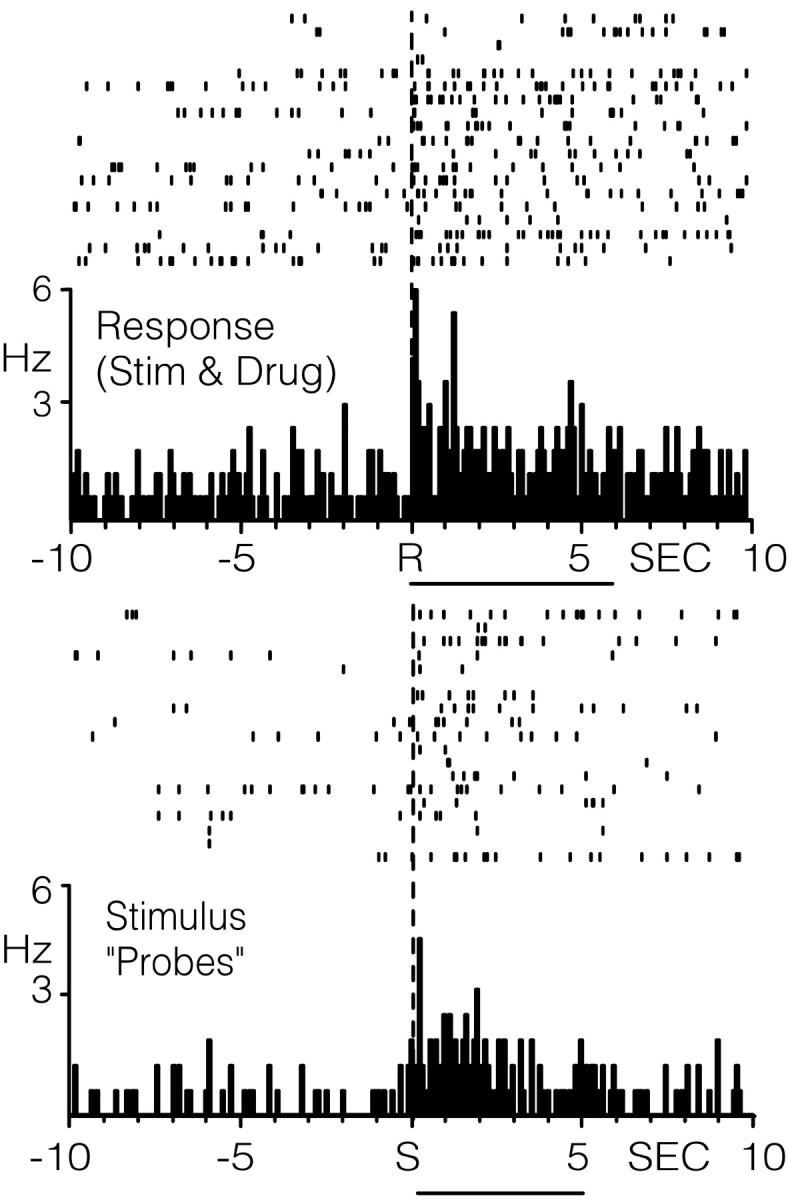
An important issue addressed in this study was whether BLA neurons are activated by presentations of the tone-house light stimulus associated with cocaine delivery during the self-administration task. The results of this analysis are summarized across all cell types in Table 2. As illustrated for one neuron in Figure 5, type PR cells were not responsive to presentations of the stimulus probes. Figure 5, top PEH, shows the activity of a single neuron relative to lever press responding for intravenous cocaine. The cell exhibited a robust increase in activity within 2 sec preceding the response, characteristic of type PR firing. However, the same neuron showed no change in the firing rate relative to presentations of the stimulus probes (bottom PEH). Across all type PR cells, mean firing 2.5 sec before stimulus probe presentations (0.93 ± 0.34 Hz) was not significantly different from mean firing rates 2.5 sec after stimulus probe onset (1.28 ± 0.45 Hz; t = 1.52; p > 0.05; Table 1).
Table 2.
Mean ± SEM of BLA neurons exhibiting one of four types of patterned discharges relative to stimulus probe trials
|
Epoch (sec) |
Type PR (n = 16) |
Type RFe (n = 10) |
Type RFi (n = 5) |
|---|---|---|---|
| Before probe (-2.5 to 0) | 0.93 ± 0.34 | 0.66 ± 0.25 | 2.91 ± 1.02 |
| After probe (0 to 2.5)
|
1.28 ± 0.45
|
1.85 ± 0.59a*
|
2.18 ± 1.10
|
A ≥40% change in firing rate relative to before probe in each column.
p < 0.05 compared with before probe in each column.
Figure 5.
Raster plot and PEHs showing the activity of a single type PR neuron relative to the cocaine-reinforced response (top) or stimulus-only probes (bottom). R at dashed vertical line at time 0 in top PEH, Reinforced response; S at dashed line in bottom PEH, stimulus-only probes. The horizontal line below the top PEH indicates cocaine delivery (0.33 mg/infusion, 6 sec). The horizontal line below bottom the PEH indicates tone-house light stimulus probe duration (5 sec).
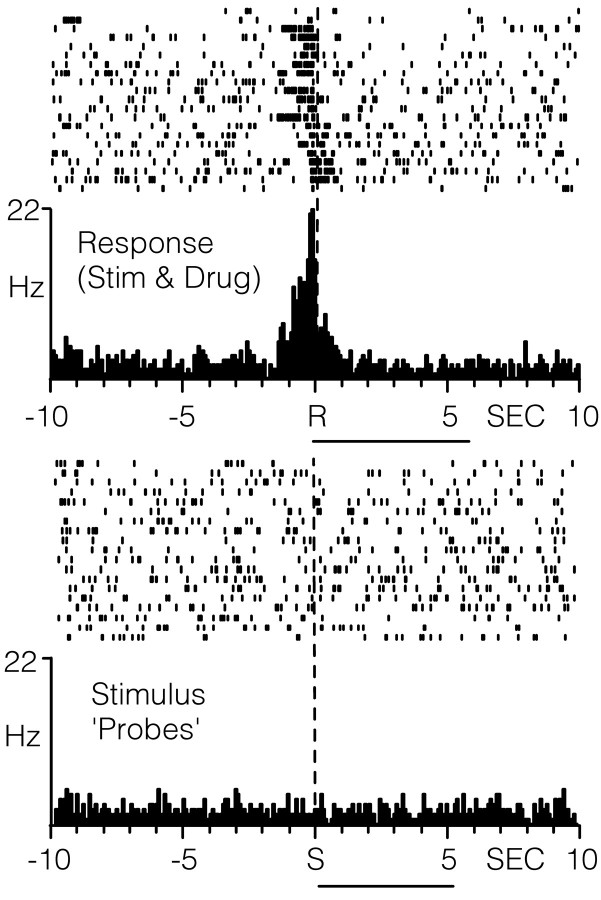
In contrast, neurons that exhibited an increase in the firing rate immediately after the lever press response for cocaine (type RFe cells) were activated by stimulus probes. An example of one such neuron is illustrated in Figure 6. Relative to reinforced response (top PEH), the RFe cell showed an increase in firing from a baseline rate of 0.60 Hz to a peak of ∼6 Hz. This same neuron was also activated during stimulus probe presentations (bottom PEH) similar in magnitude and duration to that observed after the reinforced response (top PEH). Across all RFe cells, there was a significant increase in cell firing within 2.5 sec after probe onset (1.85 ± 0.59 Hz) compared with 2.5 sec immediately before probe onset (0.66 ± 0.25 Hz; t = 3.01; p < 0.05; Table 2). The mean onset latency after probe presentations was 340 ± 110 msec, with a mean duration of 3.69 ± 0.55 sec.
Figure 6.
Raster plot and PEHs showing the activity of a single type RFe cell relative to the cocaine-reinforced response (top) or stimulus-only probes (bottom). R at dashed vertical line at time 0 in top PEH, Reinforced response; S at dashed line in bottom PEH, stimulus-only probes. The horizontal line below the top PEH indicates cocaine delivery (0.33 mg/infusion, 6 sec). The horizontal line below the bottom PEH indicates tone-house light stimulus probe duration (5 sec).
Neurons that exhibited an inhibition in cell firing after the reinforced response for intravenous cocaine (type RFi cells) were not activated by the stimulus probes (t = 2.42; p > 0.05; Table 2). Likewise, neurons classified as nonphasic (i.e., exhibiting no significant change in the firing rate relative to the response for cocaine) were also not activated during stimulus probe presentations (t = 2.01; p > 0.05).
Histology
Inspection of the brains of all 10 animals revealed that of 80 microwires chronically implanted, 52 wires were histologically verified to be in the amygdala. Only neurons recorded from wires positioned in the amygdala were included in this report. Of the 52 wires, the majority (45 wires) were positioned in the basolateral region of the amygdala, as defined by Paxinos and Watson (1997). The remaining seven wires were located within the lateral amygdala (n = 3), basomedial amygdala (n = 1), or central nucleus (n = 3). Across all animals, bilateral electrode placements in the amygdala ranged from -1.80 to -3.14 posterior to bregma and from 4.2 to 5.6 mm lateral to the midline. Figure 7 shows the distribution of marked electrode placements located within the amygdala across all animals on coronal sections of the stereotaxic atlas of Paxinos and Watson (1997).
Figure 7.
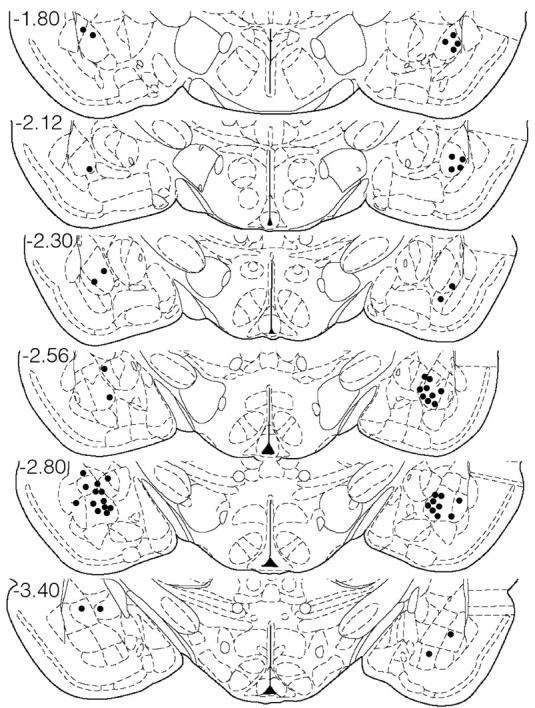
Coronal diagrams showing electrode tip placement of marked wires across all 10 animals. Filled circles, Electrode locations that were marked by the presence of a blue dot reaction product (Prussian blue) corresponding to the location of a single electrode tip. Numbers to the left, Anteroposterior coordinates (in millimeters) caudal to bregma. Diagrams were taken from the stereotaxic atlas of Paxinos and Watson (1997).
Discussion
The findings presented here represent the first detailed characterization of BLA cell firing during cocaine self-administration in rats. The results indicate that a subset of BLA neurons exhibit one of three types of patterned discharges within seconds of the operant response for intravenous cocaine. Neuronal firing patterns encompass the important aspects of drug-seeking behavior, including response initiation, execution, and completion, and are similar to those described for Acb neurons (Carelli, 2002). This study also revealed that a subset of BLA neurons are activated by stimuli previously paired with the intravenous delivery of cocaine during self-administration sessions, also similar to that reported for Acb neurons (Carelli, 2000).
Characterization of BLA activity during cocaine self-administration
Despite similarities in the activity of Acb and BLA neurons, one noticeable difference is the absence of a fourth type of neuronal firing pattern (termed PR + RF) previously reported for Acb neurons only during cocaine self-administration and not water reinforcement sessions. Interestingly, type PR + RF cell firing consists of the same three features of response-related activity exhibited by the other cell types. The absence of this fourth type of neuronal firing pattern by BLA neurons supports the view that PR + RF activity may represent a form of Acb cell firing related uniquely to cocaine reinforcement (Carelli and Deadwyler, 1994).
Of the BLA neurons classified as phasically active, patterned discharges were confined primarily within seconds of the reinforced response for cocaine. This differs from previous Acb studies showing that a subset of Acb neurons exhibit long-term cyclic alterations in firing that span the entire interinfusion interval of the self-administration session (Peoples and West, 1996; Carelli, 2002). It has been speculated that these long-term changes may be related to changes in tonic levels of dopamine that are known to fluctuate relative to lever press responding during cocaine self-administration sessions (Peoples and West, 1996; Nicola and Deadwyler, 2000). Our findings indicate that the long-term changes in Acb cell firing are likely not influenced by the population of BLA neurons characterized in this report but may reflect inputs from other cortical or subcortical regions (e.g., prefrontal cortex or hippocampus) onto Acb neurons (see below).
It is also not known whether the BLA neurons that are activated during cocaine self-administration are the same cells that respond during water reinforcement because this study was limited to cocaine. This is an important issue because we have previously reported that different populations of Acb neurons discharge during a multiple schedule for water and cocaine (Carelli et al., 2000). Whether the differential firing patterns exhibited by distinct populations of Acb neurons during a water-cocaine multiple schedule represents an influence from particular sub-populations of BLA neurons remains to be determined.
Responsiveness of BLA neurons to cocaine-associated stimuli
The results reported here reveal that stimuli that have been paired with the intravenous infusion of cocaine during self-administration are capable of activating discrete subsets of BLA cells and support the view that the BLA is important in stimulus-reward associations (Everitt et al., 1991; See, 2002). Here, after the establishment of stable self-administration behavior, probe trials of the audiovisual stimulus only (no drug) were given randomly by the experimenter during the interinfusion interval. Neurons that displayed an increase in firing rate immediately after the reinforced response (type RFe cells) were significantly activated by the audiovisual cue. These findings are consistent with our previous reports showing activation of Acb type RFe neurons relative to cocaine-associated stimuli (Carelli, 2000) and may indicate that this activation is driven, in part, by discrete populations of BLA neurons.
In humans, stimuli associated with drug-taking behavior (e.g., drug-taking paraphernalia) have the ability to elicit strong drug “craving,” which often leads to relapse after a period of drug abstinence (Gawin, 1991; O'Brien et al., 1992). It is believed that associative learning (in particular, Pavlovian conditioning) plays an important role in this process (Stewart et al., 1984; Ehrman et al., 1992; O'Brien et al., 1992; Childress et al., 1999; Dackis and O'Brien, 2001; See, 2002). Studies using brain metabolic imaging techniques such as PET and fMRI have implicated a potentially crucial role of the BLA, Acb, and associated brain regions in this process (Breiter et al., 1997; Maas et al., 1998; Childress et al., 1999; Garavan et al., 2000). The present study extends those reports by showing that a subset of BLA neurons are activated by stimuli previously associated with cocaine delivery during self-administration sessions in rats.
The BLA is also implicated in reinstatement of cocaine-seeking behaviors after extinction (Meil and See, 1997; Grimm and See, 2000; Ciccocioppo et al., 2001; Kantak et al., 2002; See, 2002; Kalivas and McFarland, 2003). For example, lesions of the BLA did not affect subsequent cocaine self-administration but decreased responding during extinction sessions and abolished the ability of drug-paired stimuli to reinstate lever pressing behavior for cocaine (Meil and See, 1997; See, 2002). Although we did not examine BLA cell firing during extinction and reinstatement of cocaine self-administration behavior, our findings showing activation of BLA neurons during presentation of cocaine-associated cues are consistent with other studies showing increased fos expression in the BLA during exposure to cocaine-associated cues (Neisewander et al., 2000; Ciccocioppo et al., 2001). It should be noted, however, that distinct subregions of the BLA may subserve different aspects of maintenance versus reinstatement of cocaine-seeking behaviors (Kruzich and See, 2001; Kantak et al., 2002; See, 2002).
It has been speculated that the ability of cocaine-associated stimuli to elicit cocaine-seeking behavior may involve activation of dopamine transmission in the Acb and amygdala (Wilson et al., 1994; Di Chiara, 1995; Weiss et al., 2000). For example, Weiss et al. (2000) showed dopamine levels significantly increased in the Acb and amygdala (measured via microdialysis) during exposure to cocaine-associated stimuli. We have recently shown rapid increases in dopamine in the Acb during presentations of the same cocaine-associated audiovisual stimuli used in the present study (Phillips et al., 2003). Furthermore, dopamine has been shown to modulate prefrontal cortical activation of BLA neurons in a classical conditioning paradigm (Grace and Rosenkranz, 2002; Rosenkranz and Grace, 2002). The relative contribution of rapid (phasic) versus tonic changes in dopamine (Wightman and Robinson, 2002) within the BLA, especially with respect to conditioned drug effects, remains to be determined.
The BLA-Acb pathway: one part of a larger “brain reward” circuit
The present findings show that BLA neurons encode information about cocaine-seeking behavior and cocaine-associated cues in a manner similar to that of Acb neurons. Because the BLA sends extensive projections to the Acb (Groenewegen et al., 1991; Zahm and Brog, 1992; Brog et al., 1993; Groenewegen et al., 1996; Wright et al., 1996), these findings support the view that the BLA may influence the activity of distinct populations of Acb neurons during drug-seeking behaviors (Robbins et al., 1989; Pennartz et al., 1994; Carelli, 2002). However, it is important to note that the BLA-Acb pathway is just one component of a larger brain reward circuit. For example, extensive studies completed in rodents show that the Acb receives afferent projections from a variety of cortical and subcortical structures, including not only the basolateral amygdala but also the prefrontal cortex (McGeorge and Faull, 1989; Zahm and Brog, 1992; Brog et al., 1993), the subiculum of the hippocampus (Groenewegen et al., 1991; Zahm and Brog, 1992; Brog et al., 1993), and the ventral tegmental area (Zahm and Brog, 1992). Indeed, patterned discharges associated with goal-directed behavior for drug reward have also been observed in the prefrontal cortex (Chang et al., 1997, 2000). Thus, the similarity in neuronal firing patterns observed by BLA and Acb neurons may reflect a widely distributed organization across different neural structures of the brain reward pathway. Whether the neuronal firing patterns reported here are ubiquitous across the brain (e.g., in cortical or subcortical structures not intimately linked with the Acb) or limited to a discrete reinforcement pathway remains to be elucidated.
Conclusions
Numerous studies indicate that the BLA is a neural substrate critically involved in associative (conditioned) aspects of drug-seeking behavior as well as “natural” rewards. Our findings are consistent with this view by showing that distinct populations of BLA neurons encode the important features of cocaine-seeking behaviors and are activated by stimuli previously paired with the intravenous delivery of cocaine during self-administration sessions. These results are consistent with the view that the BLA is one component of a much larger integrated neural system involved in mediating and controlling goal-directed behaviors. Although informative, additional studies are needed to explore a number of relevant issues with respect to the role of the BLA in the brain reward system. For example, it will be important to determine the contribution of operant versus classical conditioning to the patterned activation of BLA neurons, as previously examined for Acb cells (Carelli, 2002). Likewise, examination of BLA cell firing during responding for secondary reinforcement as well as during extinction and reinstatement of cocaine self-administration will provide important insight into factors underlying the activation of the BLA during goal-directed behaviors.
Footnotes
This research was supported by National Institute on Drug Abuse (NIDA) Grant DA14339 (R.M.C.), The Whitehall Foundation (R.M.C.), and NIDA Training Grant DA07244 (J.A.H.). We thank Stephanie Ijames and Susan Brooks for technical assistance and Paul Phillips for helpful suggestions on this manuscript.
Correspondence should be addressed to Dr. Regina M. Carelli, Department of Psychology, The University of North Carolina at Chapel Hill, Campus Box 3270, Davie Hall, Chapel Hill, NC 27599-3270. E-mail: rcarelli@unc.edu.
Copyright © 2003 Society for Neuroscience 0270-6474/03/238204-08$15.00/0
References
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew T, Rosen BR, Hyman SE ( 1997) Acute effects of cocaine on human brain activity and emotion. Neuron 19 : 591-611. [DOI] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED ( 2002) Neural systems and cue-induced cocaine craving. Neuropsychopharmacology 26 : 376-386. [DOI] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS ( 1993) The patterns of afferent innervation of the core and shell in the “accumbens” part of the ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol 338 : 255-278. [DOI] [PubMed] [Google Scholar]
- Caine SB, Lintz R, Koob GF ( 1993) Intravenous drug self-administration techniques in animals. In: Behavioral neuroscience: a practical approach (Sahgal A, ed), pp 117-143. Oxford: Oxford UP.
- Carelli RM ( 2000) Activation of accumbens cell firing by stimuli associated with cocaine delivery during self-administration, Synapse 35 : 238-242. [DOI] [PubMed] [Google Scholar]
- Carelli RM ( 2002) The nucleus accumbens and reward: Neurophysiological investigations in behaving animals. Behav Cogn Neurosci Rev 1 : 281-296. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Deadwyler SA ( 1994) A comparison of nucleus accumbens neuronal firing patterns during cocaine self-administration and water reinforcement in rats. J Neurosci 14 : 7735-7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, Ijames S, Crumling A ( 2000) Evidence that separate neural circuits in the nucleus accumbens encode cocaine versus “natural” (water and food) reward. J Neurosci 20 : 4255-4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JY, Sawyer SF, Lee RS, Woodward DJ ( 1994) Electrophysiological and pharmacological evidence for the role of the nucleus accumbens in cocaine self-administration in freely moving rats. J Neurosci 14 : 1224-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JY, Zhang L, Janak PH, Woodward DJ ( 1997) Neuronal responses in prefrontal cortex and nucleus accumbens during heroin self-administration in freely moving rats. Brain Res 754 : 12-20. [DOI] [PubMed] [Google Scholar]
- Chang JY, Janak PH, Woodward DJ ( 2000) Neuronal and behavioral correlations in the medial prefrontal cortex and nucleus accumbens during cocaine self-administration by rats. Neuroscience 99 : 433-443. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP ( 1999) Limbic activation during cue-induced cocaine craving. Am J Psychiatry 156 (1): 11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F ( 2001) Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D1 antagonists. Proc Natl Acad Sci USA 98 : 1976-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis CA, O'Brien CP ( 2001) Cocaine dependence: a disease of the brain's reward centers. J Subst Abuse Treat 21 (3): 111-117. [DOI] [PubMed] [Google Scholar]
- Di Chiara G ( 1995) The role of dopamine in drug abuse viewed from the perspective of its role in motivation. Drug Alcohol Depend 38 : 95-137. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, O'Brien CP ( 1992) Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology 107 : 523-529. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW ( 2000) Second-order schedules of drug reinforcement in rats and monkeys: measurement of reinforcing efficacy and drug-seeking behaviour. Psychopharmacology 153 : 17-30. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Morris KA, O'Brien A, Robbins TW ( 1991) The basolateral amygdala-ventral striatal system and conditioned place preference: further evidence of limbic-striatal interactions underlying reward-related processes. Neuroscience 42 : 1-18. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Weber SM, Rice HJ, Neisewander JL ( 2002) Effects of excitotoxic lesions of the basolateral amygdala on cocaine-seeking behavior and cocaine conditioned place preference in rats. Brain Res 929 : 15-25. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA ( 2000) Cue-induced cocaine craving: neuroanatomical specificity for drug uses and drug stimuli. Am J Psychiatry 157 : 1789-1798. [DOI] [PubMed] [Google Scholar]
- Gawin FH ( 1991) Cocaine addiction: psychology and neurophysiology. Science 251 : 1580-1585. [DOI] [PubMed] [Google Scholar]
- Grace AA, Rosenkranz JA ( 2002) Regulation of conditioned responses of basolateral amygdala neurons. Physiol Behav 77 : 489-493. [DOI] [PubMed] [Google Scholar]
- Green JD ( 1958) A simple microelectrode for recording from the central nervous system. Nature 182 : 962. [DOI] [PubMed] [Google Scholar]
- Grimm JW, See RE ( 2000) Dissociation of primary and secondary reward-relevant limbic nuclei in an animal model of relapse. Neuropsychopharmacology 22 : 473-479. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW, Meredith GE, Haber SN, Voorn P, Walters JG, Lohman AHM ( 1991) Functional anatomy of the ventral, limbic system-innervated striatum. In: The mesolimbic dopamine system: from motivation to action (Willner P, Scheel-Kruger J, eds), pp 19-59. New York: Wiley.
- Groenewegen HJ, Wright CI, Beijer AV ( 1996) The nucleus accumbens: gateway for limbic structures to reach the motor system? Prog Brain Res 107 : 485-511. [DOI] [PubMed] [Google Scholar]
- Hayes RJ, Vorel SR, Spector J, Liu X, Gardner EL ( 2003) Electrical and chemical stimulation of the basolateral complex of the amygdala reinstates cocaine-seeking behavior in the rat. Psychopharmacology 168 : 75-83. [DOI] [PubMed] [Google Scholar]
- Heimer L, Zahm DS, Alheid GF ( 1995) Basal ganglia. In: The rat nervous system, Ed 2 (Paxinos G, ed), pp 579-628. San Diego: Academic.
- Heimer L, Alheid GF, de Olmos JS, Groenewegen HJ, Haber SN, Harlan RE, Zahm DS ( 1997) The accumbens: beyond the core-shell dichotomy. J Neuropsychiatry Clin Neurosci 9 : 354-381. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K ( 2003) Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology 168 : 44-56. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB ( 2002) Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J Neurosci 22 : 1126-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruzich PJ, See RE ( 2001) Differential contributions of the basolateral and central amygdala in the acquisition and expression of conditioned relapse to cocaine-seeking behavior. J Neurosci 21 : RC155(1-5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, Kukes TJ, Renshaw PF ( 1998) Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry 155 : 124-126. [DOI] [PubMed] [Google Scholar]
- Meil WM, See RE ( 1997) Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behav Brain Res 87 : 139-148. [DOI] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RLM ( 1989) The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience 29 : 503-537. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF ( 2000) Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci 20 : 798-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Deadwyler SA ( 2000) Firing rate of nucleus accumbens neurons is dopamine-dependent and reflects the timing of cocaine-seeking behavior in rats on a progressive ratio schedule of reinforcement. J Neurosci 20 : 5526-5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolelis MAL ( 1999) Methods for neural ensemble recordings. Boca Raton, FL: CRC. [PubMed]
- Nicolelis MAL, Ghazanfar AA, Faggin BM, Votaw S, Oliveira LMO ( 1997) Reconstructing the engram: simultaneous, multisite, many single neuron recordings. Neuron 18 : 529-537. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, McLellan AT, Ehrman R ( 1992) Classical conditioning in drug-dependent humans. Ann NY Acad Sci 654 : 400-415. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C ( 1997) The rat brain in stereotaxic coordinates, Compact Ed 3. San Diego: Academic.
- Pennartz CM, Groenewegen HJ, Lopes da Silva FH ( 1994) The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioral, electrophysiological and anatomical data. Prog Neurobiol 42 : 719-761. [DOI] [PubMed] [Google Scholar]
- Peoples LL, West MO ( 1996) Phasic firing of single neurons in the rat nucleus accumbens correlated with the timing of intravenous cocaine self-administration. J Neurosci 16 : 3459-3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PEM, Stuber GD, Heien MLAV, Wightman RM, Carelli RM ( 2003) Subsecond dopamine release promotes cocaine seeking. Nature 422 : 614-618. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Cador M, Taylor JR, Everitt BJ ( 1989) Limbic-striatal interactions in reward-related processes. Neurosci Biobehav Rev 13 : 155-162. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA ( 2002) Dopamine-mediated modulation of odour-evoked amygdala potentials during pavlovian conditioning. Nature 417 : 282-287. [DOI] [PubMed] [Google Scholar]
- See RE ( 2002) Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav 71 : 517-529. [DOI] [PubMed] [Google Scholar]
- Stewart J, deWit H, Eikelboom R ( 1984) Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev 91 : 251-268. [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O ( 2000) Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extra-cellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci USA 97 : 4321-4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw RB, Markou A, Robbins TW, Everitt BJ ( 1996) Excitotoxic lesions of the basolateral amygdala impair the acquisition of cocaine-seeking behaviour under a second-order schedule of reinforcement. Psychopharmacology 127 : 213-224. [PubMed] [Google Scholar]
- Wightman RM, Robinson DL ( 2002) Transient changes in mesolimbic dopamine and their association with “reward.” J Neurochem 82 : 721-735. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Nobrega JN, Corigall WA, Coen KM, Shannak K, Kish SJ ( 1994) Amygdala dopamine levels are markedly elevated after self-but not passive administration of cocaine. Brain Res 668 : 39-45. [DOI] [PubMed] [Google Scholar]
- Wright CI, Beijer VJ, Groenewegen HJ ( 1996) Basal amygdaloid complex afferents to the rat nucleus accumbens are compartmentally organized. J Neurosci 16 : 1877-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS, Brog JS ( 1992) Commentary: on the significance of subterritories in the “accumbens” part of the rat ventral striatum. Neuroscience 50 : 751-767. [DOI] [PubMed] [Google Scholar]