Abstract
Mice lacking a synaptic isoform of glutamic acid decarboxylase (GAD65) do not exhibit ocular dominance plasticity unless an appropriate level of GABAergic transmission is restored by direct infusion of benzodiazepines into the brain. To better understand how intracortical inhibition triggers experience-dependent changes, we dissected the precise timing requirement for GABA function in the monocular deprivation (MD) paradigm.
Diazepam (DZ) or vehicle solution was infused daily before and/or during 4 d of MD in GAD65 knock-out mice. Extracellular single-unit recordings from the binocular zone of visual cortex were performed at the end of deprivation. We found that a minimum treatment of 2 d near the beginning of MD was sufficient to fully activate plasticity but did not need to overlap the deprivation per se. Extended delay after DZ infusion eventually led to loss of plasticity accompanied by improved intrinsic inhibitory circuit function. Two day DZ treatment just after eye opening similarly closed the critical period prematurely in wild-type mice.
Raising wild-type mice in complete darkness from birth delayed the peak sensitivity to MD as in other mammals. Interestingly, 2 d DZ infusion in the dark also closed the critical period, whereas equally brief light exposure during dark-rearing had no such effect. Thus, enhanced tonic signaling through GABAA receptors rapidly creates a milieu for plasticity within neocortex capable of triggering a critical period for ocular dominance independent of visual experience itself.
Keywords: GAD65, dark-rearing, GABA, diazepam, critical period, visual cortex
Introduction
The mammalian brain is shaped by experience during restricted “critical periods” in early life. Even briefly occluding one eye during this time causes a prominent shift of responsiveness [ocular dominance (OD)] in favor of the open eye in primary visual cortex. Monocular deprivation (MD) fails to induce significant changes later in adulthood, indicating an irreversible developmental process defines the transient sensitivity to MD. Typically plasticity is absent at eye opening, peaks at ∼4 weeks, and gradually declines over weeks (rodents: Fagiolini et al., 1994; Gordon and Stryker, 1996; Fagiolini and Hensch, 2000) to months (cat, primate: Daw, 1995). Yet, the critical period is not simply age-dependent, but rather a series of events itself controlled in a use-dependent manner. Rearing in complete darkness from birth leaves the cortex in a flexible state that can be altered by sensory perturbation even in adulthood (Cynader, 1983; Mower, 1991; Fagiolini et al., 1994; Daw, 1995).
Focus on cortical inhibition has recently provided major cellular insight into the critical period. Mice lacking a synaptic isoform of GABA-synthetic enzyme glutamic acid decarboxylase [GAD65 knock-out (KO) mice] exhibit reduced cortical GABA release with stimulation and do not respond to brief MD (Hensch et al., 1998). Importantly, functional enhancement of GABAergic transmission fully restores OD plasticity to GAD65 KO mice. Rescue is achieved by diazepam, one of the best-characterized benzodiazepine agonists, which selectively bind subsets of GABAA receptors to increase open probability and channel conductance in a use-dependent manner (Sieghart, 1995; Eghbali et al., 1997; Cherubini and Conti, 2001). Diazepam treatment notably induces robust plasticity throughout life in GAD65 KO but not adult wild-type (WT) mice (Fagiolini and Hensch, 2000). Thus, despite exposure to normal visual experience, sensitivity to MD is maintained into adulthood in GAD65 KO mice, similar to dark-reared WT animals (Mower, 1991). Indeed, dark rearing impairs inhibitory circuit maturation in visual cortex (Benevento et al., 1992, 1995; Chen et al., 2001; Morales et al., 2002).
These findings suggest that an evolving inhibitory-excitatory balance within cortex underlies the relatively late onset of the normal critical period. Direct enhancement of GABAergic transmission with diazepam prematurely reveals OD plasticity in young WT mice before their natural plastic period (Fagiolini and Hensch, 2000). Indirect manipulation of GABA cell growth by chronic overexpression of brain-derived neurotrophic factor (BDNF) similarly accelerates the beginning and end of plasticity (Hanover et al., 1999; Huang et al., 1999). Prolonged infusion of diazepam mimics a critical period to abolish latent plasticity in GAD65 KO mice (Fagiolini and Hensch, 2000). Taken together, these results represent the first direct manipulation of critical period timing in any system.
To better understand how inhibition induces plasticity in vivo, we determined the timing requirement for GABAergic transmission using the GAD65 KO mouse model and systematically varying the period of diazepam exposure before and/or during a saturating 4 d MD. We found an initial 2 d infusion to be sufficient for full OD shifts that strikingly did not need to overlap the time of deprivation itself. Moreover, brief infusion eventually closed the critical period in correlation with improved cortical inhibition not only in KO mice, but also in WT animals raised in complete darkness. Our findings reveal that tonic GABAA-mediated signaling (independent of phasic, visual input) rapidly triggers lasting cellular refinements that direct OD plasticity in visual cortex.
Materials and Methods
Animals. Mice carrying a functional disruption of GAD65 were generated as described previously (Asada et al., 1996). GAD65 KO mice were reared with normal visual experience (12 hr light/dark cycle). In Figures 1, 2, 3 monocular deprivations were started within the typical WT critical period from postnatal day 24 (P24) to P33. C57BL/6 mice were used in Figures 6 and 7. During dark rearing (DR), animals were kept in a darkroom, and feeding or cage cleaning was performed wearing an infrared visor. Some animals were removed to a 12 hr light/dark cycle around P30 for 2-4 d then returned to the dark. Fast photographic film was exposed in the darkroom for several days to monitor effectiveness of the light seal before use.
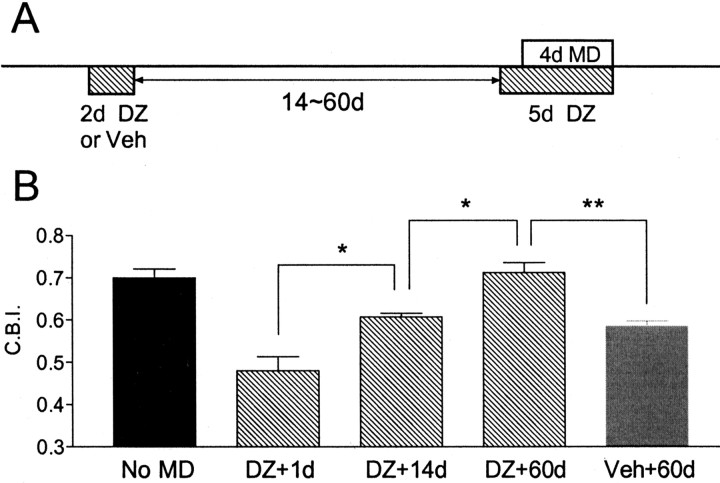
Figure 1.
Brief DZ infusion is sufficient to rescue MD effects in GAD65 KO mice. A, OD histogram of nondeprived GAD65 KO mice exhibits a typical contralateral (cont.) eye bias. Number of animals and cells as indicated. B, DZ infusion the day before and on the first day of a 4 d MD shift OD toward the open, ipsilateral (ipsi.) eye in GAD65 KO mice, yielding a balanced distribution (p < 0.0001 vs no MD; χ2 test). C, D, WT mice during the critical period (C) and GAD65 KO mice infused for 5 d (D) display similar OD distributions in response to 4 d MD. Neither is significantly different from that in B (p > 0.2 and p > 0.08, respectively; χ2 test). CBI value in top right corner of each cumulative histogram indicates bias in favor of the contralateral eye.
Figure 2.
Initial 2 d DZ treatment at MD onset is minimum effective requirement. A, Each symbol represents CBI per animal. Filled and open symbols indicate GAD65 KO and WT mice, respectively. Whereas DZ infusion just the day before 4 d MD (Pre 1d) slightly reduces the CBI (0.63 ± 0.04; p > 0.08 vs no MD; t test), a further 1, 2, or 4 d of treatment into brief MD shifts the OD to a similarly significant extent (0.55 ± 0.03, 0.54 ± 0.02, 0.51 ± 0.03 for first 2 d, 3 d, full 5 d, respectively; p < 0.002 vs no MD; t test). These values are identical to WT mice deprived during the critical period (0.54 ± 0.01; p > 0.4; t test). B, DZ infusion for the last 2 d during a 4 d MD (Last 2 d) is ineffective (0.66 ± 0.02, n = 5; p < 0.04 vs first 2 d DZ; t test). Shaded region indicates range of nondeprived CBIs for GAD65 KO mice. *p < 0.05, **p < 0.01, t test.
Figure 3.

Two day DZ pretreatment produces a plastic milieu independent of MD. A, Vehicle or DZ infusion for 2 d was completed 1 d before brief MD in GAD65 KO mice (Pre 2d Veh; Pre 2d DZ). B, Pre 2d DZ induces a significant reduction of CBI values (0.48 ± 0.03, n = 6 vs 0.71 ± 0.02, n = 3 for Pre 2d Veh; p < 0.003; t test). C, D, GAD65 KO mice with Pre 2d DZ (D) exhibit prominent OD shifts toward the open, ipsilateral eye [p < 0.0001 vs Pre 2d Veh (C); χ2 test]. **p < 0.01, t test.
Figure 6.

Brief DZ prematurely closes, and DR delays critical period onset in WT mice. A-C, MD 10 d after 2 d of DZ infusion at P16 (A, P16 DZ + 10d) produces little or no ocular dominance shift, compared with MD during the same period without pretreatment (B, P28 LR; p < 0.002, χ2 test). Complete darkness from birth to the peak of the natural critical period (C, P28 DR) fails to activate plasticity (p < 0.0001 vs P28 LR; χ2test). Data for P28 LR (B) adapted from Fagiolini et al. (2003). D, Both P28 DR and P16 DZ + 10d abolish the prominent plasticity seen at P28 with LR. CBI in response to MD for P28 LR (0.50 ± 0.01; n = 10) was significantly different from that of P28 DR (0.66 ± 0.01; n = 4; p < 0.001) and P16 DZ + 10d (0.63 ± 0.02; n = 3; p < 0.001). ***p < 0.001, t test.
Figure 7.
Brief DZ (but not light exposure) closes the critical period during DR in WT mice. A, WT mice reared in complete darkness from birth, except for 2-4 d light exposure at P30, underwent MD as adults (>P50). B, Whereas WT mice reared normally hardly respond to brief MD in adulthood (Adult+MD, CBI = 0.69 ± 0.02; n = 3), DR mice shift significantly (Adult DR+MD, CBI = 0.57 ± 0.03; n = 9; p < 0.03 vs Adult+MD; t test). Light exposure for 2-4 d during DR has little effect on the delayed OD plasticity (2d light+MD, CBI = 0.60 ± 0.02; n = 9; p < 0.03 vs Adult+MD; t test). C, Vehicle (Veh) or DZ-treated (2 d at P30) DR mice were later subjected to MD as adults (>P50). D, DZ pretreatment in the dark eliminates the typical delay of OD plasticity (2d DZ+MD, CBI = 0.71 ± 0.01; n = 8), whereas vehicle infusion does not (2d Veh+MD, 0.61 ± 0.02, n = 6, p < 0.004 vs 2d DZ+MD; t test; Welch's correction). Shaded region indicates range of nondeprived CBIs for DR mice (0.75 ± 0.02; n = 6) that was significantly different from that of DR+MD, 2d light+MD, or 2d Veh+MD (p < 0.001) but not from Adult+MD or 2d DZ+MD (p > 0.09). **p < 0.03.
Monocular deprivation and drug infusion. MD and drug infusion were performed as described previously (Hensch et al., 1998). Eyelid margins were trimmed and sutured under halothane anesthesia for 4 (brief deprivation) or 15 d (long-term deprivation). Sutures were checked daily to make sure the eyes remained closed. All recordings were obtained contralateral to the deprived eye. In drug infusion experiments, diazepam (2 mg/ml; Wako Pure Chemical, Osaka, Japan) or vehicle solution (50% propylene glycol; Wako) were injected daily into both lateral ventricles (1.5 μl/hemisphere). Each injection was completed within 10 min under halothane anesthesia. A >100-fold saturating dose of diazepam was previously determined by recording GABAA responses in visual cortical slices and infused to ensure adequate drug diffusion in vivo (Hensch et al., 1998). Some infusions were conducted blind to solution.
Electrophysiological recording and analysis. Surgical and electrophysiological procedures were as described in detail elsewhere (Gordon and Stryker, 1996; Hensch et al., 1998; Fagiolini et al., 2003). Electrophysiological recordings were performed under Nembutal (50 mg/kg; Abbott Labs, North Chicago, IL)/chlorprothixene (0.2 mg; Sigma, St. Louis, MO) anesthesia. For each animal, 5-8 single units (>70 μm apart) were recorded in each of 4-6 vertical penetrations spaced evenly (>200 μm intervals) across the mediolateral extent of primary visual cortex to map the monocular and binocular zones and avoid sampling bias. Cells were assigned ocular dominance scores using a 7-point classification scheme of Hubel and Wiesel (Daw, 1995). For each binocular zone, a contralateral bias index (CBI) was calculated according to the formula: CBI = [(n1 - n7) + (2/3)(n2 - n6) + (1/3)(n3 - n5) + N]/2 N, where N = total number of cells, and nx = number of cells of ocular dominance score equal to x. This weighted average of the bias toward one eye or the other takes values from 0 to 1 for complete ipsilateral or contralateral eye dominance, respectively.
Results
Two day diazepam treatment fully rescues plasticity in GAD65 KO mice
Postsynaptic enhancement of GABAergic transmission by DZ concurrent with brief MD can completely restore OD plasticity to mice whose GABA release is functionally compromised. Both local cortical infusion through an osmotic minipump system and global treatment by repeated intraventricular injection are similarly effective (Hensch et al., 1998; Fagiolini and Hensch, 2000). Because DZ is rapidly broken down in vivo (Berrueta et al., 1992; Hensch et al., 1998), one can systematically probe when GABAergic transmission is required for OD plasticity by daily DZ infusions at discrete times with respect to MD in GAD65 KO mice (Asada et al., 1996).
We performed extracellular single-unit recordings in the binocular zone of primary visual cortex, and evaluated every cell on a seven-point OD scale (see Materials and Methods) (Daw, 1995; Gordon and Stryker, 1996; Hensch et al., 1998). Each distribution was further represented as a single parameter, the CBI, which is an indicator of the degree to which the contralateral eye dominates cortical responses. The OD histogram of nondeprived mutants displayed the typical bias toward contralateral eye input (Fig. 1A) (Gordon and Stryker, 1996).
When intraventricular DZ injections were initiated the day before and continued throughout a brief 4 d period of MD, a prominent shift of OD in favor of the open eye was produced, confirming earlier results from an independent line of GAD65 KO mice (Hensch et al., 1998). Rescue was significant both for cumulative distributions (Fig. 1D) (p < 0.0001 vs No MD, χ2 test) as well as across individual animals (Fig. 2A) (p < 0.001 vs No MD; t test). When the latter half of the MD period was not exposed to DZ, the 3 d infusion protocol also yielded a similarly robust OD plasticity (Fig. 2A) (p < 0.001 vs No MD; t test).
Further shortening DZ infusion for just the initial 2 d, 1 d before and the first day of MD, still produced a significant OD shift (Fig. 1B) (p < 0.0001 vs No MD; χ2 test) (Fig. 2A) (p < 0.002 vs No MD; t test). The magnitude of this plasticity with initial 2 d infusion was similar to that of fully deprived WT mice (Fig. 2A)(p > 0.6 vs WT + MD; t test), as well as that of KO mice with 5 d infusions (Fig. 2A) (p > 0.3 vs full 5d DZ; t test). Thus, brief enhancement of GABAergic transmission at the onset of MD is sufficient for full OD shifts to occur in GAD65 KO mice.
On the other hand, DZ treatment during the latter half of MD was ineffective. Little OD plasticity was observed in GAD65 KO mice infused only during the last 2 d of MD (Fig. 2B)(p > 0.1 vs no MD; p < 0.04 vs first 2 d DZ + MD; t test). GABA circuits must, therefore, play a critical role in starting experience-dependent changes that are eventually consolidated by other factors.
Brief diazepam exposure triggers a limited sensitive period for MD
Whereas initial 2 d infusion had a prominent effect on OD shifts, preinjection of DZ just the day before MD yielded only slight, variable plasticity (Fig. 2A) (p > 0.08 vs no MD; t test). This suggests that optimal GABAergic transmission during the first day of MD may be critical for full OD plasticity. An appropriate inhibitory-excitatory balance may actively detect perturbations in sensory input from the two eyes. Alternatively, pretreatment might effectively establish a plastic environment over a 2 d period because OD did shift in some cases with just 1 d of preinfusion (Fig. 2A).
To distinguish between these possibilities, brief MD for 4 d was started 1 d after 2 d of DZ treatment (Fig. 3A, Pre 2d DZ). This insured that the drug had washed out completely before MD (Berrueta et al., 1992; Hensch et al., 1998). A strikingly clear OD shift was detected under this experimental paradigm (Fig 3B,3D), the magnitude of which was significantly larger than that for 1 d preinfusion (Fig. 2A vs Fig. 3B) (p < 0.01, t test). Moreover, the CBI tended to be lower than even that for first 2 d DZ (Fig. 2A vs Fig. 3B) but did not reach statistical significance (p > 0.1, t test). On the other hand, preinfusion of vehicle for the same time window did not reveal any plasticity (Fig. 3C). A 2 d interval after just 1 d of DZ injection produced a variable and overall modest plasticity as for 1 d of preinfusion (Fig. 2A).
Thus, 2 d DZ treatment is the minimum requirement for full OD plasticity to occur in GAD65 KO mice and need not overlap the time of monocular occlusion. We then explored whether this sensitivity to MD is eventually lost, as occurs normally in WT animals. Previously, it was shown that GAD65 KO mice retain the potential for OD plasticity throughout life, because both brief MD concurrent with DZ and long-term MD alone produce significant shifts even at adult stages (Fagiolini and Hensch, 2000). Consistent with the earlier results, vehicle pretreatment did not abolish the potential for plasticity, and brief MD with DZ continued to yield OD shifts even at 3 months of age (Fig. 4) (p < 0.009 vs adult no MD; t test).
Figure 4.
Early 2 d DZ treatment abolishes adult plasticity in GAD65 KO mice. A, GAD65 KO mice were preinfused with DZ or vehicle for 2 d, followed 14 or >60 d later by 4 d MD concurrent with DZ infusion. B, Whereas vehicle infusion leaves the mutant sensitive to MD into adulthood even 60 d later (Veh + 60d, CBI = 0.58 ± 0.01; n = 3), DZ pretreatment abolishes this plastic potential (DZ + 60d, CBI = 0.71 ± 0.02, n = 3, p < 0.01 vs Veh + 60d; t test), identical to that of nondeprived adult mice (no MD, CBI = 0.70 ± 0.02; n = 4). Just 14 d after DZ treatment, plasticity partially remains (DZ + 14d, CBI = 0.61 ± 0.01, n= 3, p < 0.03 vs DZ + 60d, t test) but is already significantly reduced compared with the maximal shift at 1 d after DZ (p < 0.03 vs DZ + 1d; t test). *p < 0.05, **p < 0.01, t test.
However, when DZ was infused for 2 d around P30, 2 months later (at P90) neither 4 d MD concurrent with 5 d DZ treatment (Fig. 4) (p > 0.7 vs adult no MD; t test) nor long-term MD (p > 0.4 vs adult no MD; t test) produced any OD shift. An intermediate time interval of 2 weeks after brief DZ infusion still showed a residual plasticity that was, however, significantly weaker than the maximal shift induced shortly after drug treatment. Two day DZ exposure in GAD65 KO mice is, therefore, sufficient to gradually eliminate sensitivity to MD with a time course similar to the natural critical period in mice (Gordon and Stryker, 1996; Fagiolini and Hensch, 2000).
Brief diazepam triggers progressive maturation of intracortical inhibition
Whereas OD shifts were induced immediately after brief DZ treatment, this plasticity was abolished later, suggesting that DZ triggered irreversible changes of intracortical circuitry to limit the plastic period. To address the cellular basis of this effect, we examined neuronal spiking behavior after visual stimulation. Prolonged discharge is an hyperexcitability of a single neuron when light-bar stimuli exit the receptive field of the cell (Hensch et al., 1998) (Fig. 5A). Although nearly abolished during the critical period in WT animals, it was prevalent and regulated by DZ in GAD65 KO mice throughout life (Fagiolini and Hensch, 2000) (Fig. 5B), indicating that prolonged discharge reflects the strength of cortical inhibition.
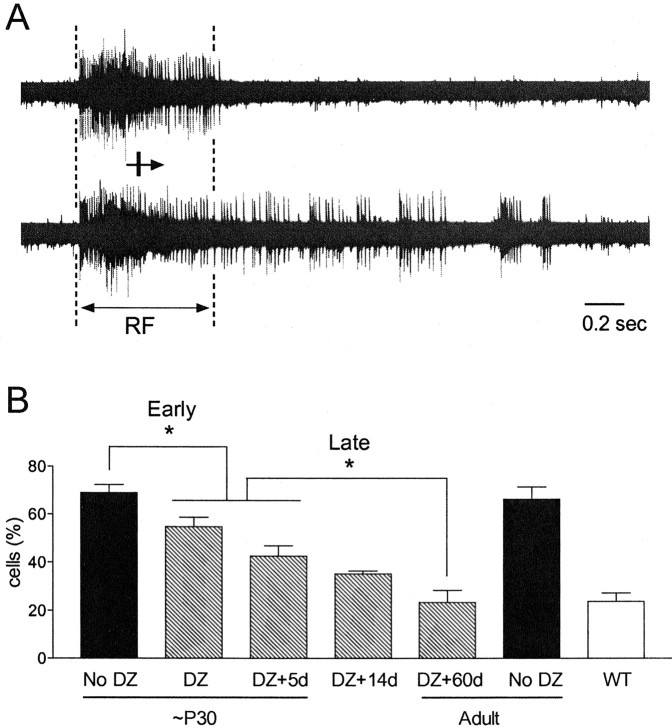
Figure 5.
Brief DZ treatment progressively reduces prolonged discharge in GAD65 KO. A, Examples of neuronal responses showing normal (top trace) or prolonged discharge (bottom trace). Excess spikes in bottom trace continue even after light-bar stimuli have exited the receptive field (RF). B, Visual cortical neurons in GAD65 KO mice exhibit prolonged discharge throughout life (∼P30: 69 ± 3%, 135 cells, 6 mice; adult: 66 ± 5%, 59 cells, 3 mice). Immediately (55 ± 4%, 124 cells, 5 mice, p < 0.03, t test) or 5 d after 2 d DZ infusion at approximately P30 (43 ± 4%, 122 cells, 6 mice, p < 0.001), hyperexcitability is significantly reduced compared with naive GAD65 KO mice. In addition to such early effects, 60 d after DZ infusion, further reduction (23 ± 5%, 62 cells, 3 mice, p < 0.05 vs DZ + 5d) is comparable to mature WT levels (24 ± 4%, 150 cells, 6 mice P28-P67 combined) and correlates with a gradual end to the critical period. *p < 0.05, t test.
We infused DZ for 2 d into GAD65 KO mice and waited for several periods before counting the proportion of neurons displaying prolonged discharge. Immediately after treatment, prolonged discharge was slightly but significantly reduced (Fig. 5B) (p < 0.03 vs no DZ; t test) consistent with the acute action of DZ on postsynaptic responses at active GABAergic synapses (Sieghart, 1995; Eghbali et al., 1997). Five or 14 d after the brief DZ exposure, a more prominent decrement of prolonged discharge was observed, despite the absence of drug caused by earlier degradation (Fig. 5B)(p < 0.001 vs no DZ; t test). Finally, at 60 d after DZ, prolonged discharge was further reduced (Fig. 5B)(p < 0.05 vs DZ + 5d; p < 0.005 vs adult no DZ) to levels that were indistinguishable from mature WT (Fig. 5B) (p > 0.3).
Thus, once enhanced, GABAergic transmission had long-lasting effects on itself even after DZ was removed, triggering a progressive functional maturation of inhibitory circuits in parallel with the loss of plasticity. To examine such durable effects of 2 d DZ treatment even in WT animals, we infused young precritical period mice at P16 and recorded the response to MD 10 d later at the usual peak of plasticity. The OD shift was greatly attenuated and significantly different from normally reared animals deprived at this age (Fig. 6A,B) (p < 0.002; χ2 test), showing accelerated closure of the critical period.
Brief diazepam closes the critical period in dark-reared WT mice
Sensory input is believed to be essential for the functional refinement of neuronal circuits in the mammalian visual system. We thus examined whether the action of DZ depends on visual experience using WT animals. DR from birth maintains the visual cortex in an immature state, prolonging sensitivity to MD into adulthood (Cynader, 1983; Fagiolini et al., 1994; Daw, 1995). More specifically, it is known that DR slows the entire profile of the critical period, such that at 6 weeks of age, normal cats are more plastic than DR cats, whereas at 16 weeks, DR cats are more plastic (Mower, 1991; Beaver et al., 2001).
In other words, the onset of the critical period is delayed by DR. To first confirm this concept in mice, we recorded the response to MD after brief DR just to the usual peak of plasticity (Fig. 6C). Little or no OD shift was observed in comparison with light-reared controls at this age (Fig. 6B,D). In contrast, adult animals raised with normal visual experience exhibited little or no OD plasticity, whereas mice reared in complete darkness to adulthood (>P55) clearly retained this plastic capacity (Fig. 7B) (Fagiolini et al., 2003). The maturational profile for OD in the dark was quite distinct from that of orientation preference, which first appeared normally at P30 (DR: 22 ± 4%, 106 cells, four mice vs LR: 21 ± 2%, 82 cells, three mice; p = 0.9) then was degraded by continued deprivation (>P55; DR: 5 ± 2%, 90 cells, three mice vs LR: 37 ± 3%, 373 cells, 13 mice; p < 0.0002) (Fagiolini et al., 2003) as shown previously in cats (Crair et al., 1998).
In cat visual cortex, very brief visual experience (6 hr) during DR is reported to trigger a developmental process that eventually eliminates OD plasticity (Mower et al., 1983). We examined the effects of brief light exposure (Fig. 7A) on DR mice and found that 2-4 d of visual input near P30 failed to prevent plasticity by MD after removal from the dark 20 d later (Fig. 7B)(p < 0.001 vs DR no MD; t test). There was no significant difference in CBI from adult DR + MD group (Fig. 7B) (p > 0.3; t test). Thus, several days of visual experience during DR had little effect on delaying the critical period by DR in mice. We attribute this difference from the cat to the timing of light exposure and generally slower plasticity processes in mice, as exemplified by their slower time course of response to MD (Gordon and Stryker, 1996).
In comparison, we injected DZ for 2 d into DR mice at approximately P30. A total of 20 min in the light under a dissecting microscope was required to complete these injections into mice anesthetized in the darkroom. This amount of light exposure is negligible given that several days were ineffective (Fig. 7B) and that plasticity mechanisms are precluded under anesthesia (Imamura and Kasamatsu, 1991). More than 20 d after treatment, MD was experienced immediately after removal from the dark (Fig. 7C).
Remarkably, OD shifts could not be detected by MD in DR adult mice previously exposed to DZ (Fig. 7D) (p > 0.09 vs DR no MD; t test). As expected, vehicle treatment under the same conditions did not abolish the plasticity delayed by DR (Fig. 7B,D) (p < 0.001 vs DR no MD, p < 0.004 vs DR 2d DZ, t test). Moreover, the CBI for DR 2d DZ + MD was identical to that of light-reared adults + MD (p > 0.2; t test). Thus, 2 d DZ treatment eventually closes the critical period in complete darkness, replacing even longer periods of normal visual experience.
This supports the above results from GAD65 KO mice (Figs. 3, 4) suggesting that brief enhancement of inhibition may potently induce a plastic milieu independent of the sensory deprivation itself, which runs its course within 20 d after the treatment. In DR adult WT mice, a much lower proportion of prolonged discharge (23 ± 2%, 321 cells, 13 mice) was observed than in adult GAD65 mutants. Thus, DR produces a more subtle alteration of inhibition than the uniform reduction of GABA release caused by GAD65 deletion (Tsumoto and Freeman, 1987; Morales et al., 2002). Nevertheless, a similar tendency toward reduced prolonged discharge was seen 20 d after brief DZ exposure in the dark, which did not reach significance because of the initially weaker and variable phenotype (16 ± 4%, 185 cells, eight mice; p > 0.08). In contrast, degradation of orientation preference by DR was not rescued by 2d DZ treatment in the dark (100 cells, 4 mice; p > 0.3 vs DR + vehicle; p < 0.01 vs LR adult) consistent with separable mechanisms underlying maturation of individual receptive field properties (Fagiolini et al., 2003).
Discussion
Our parametric study has demonstrated that brief DZ infusion can trigger a “critical period” independent of visual experience in developing neocortex. Moreover, the consequences far outlast the initial exposure, because MD is effective even without the continued presence of drug. The switch is thrown rapidly within 24-48 hr, and thus does not reflect a chronic response to drug treatment, such as withdrawal or tolerance after several days at high dose (Gallager et al., 1984; Tehrani and Barnes, 1997). This may bear serious clinical implications for even the brief use of benzodiazepines in human infants. Since the well known action of DZ is mediated through particular GABAA receptor subunits actively binding GABA (Sieghart, 1995), our findings identify fundamental cellular events underlying experience-dependent plasticity.
Critical period activation by brief enhancement of inhibition
We have briefly manipulated two conditions under which the onset of OD plasticity is delayed. Mice lacking GAD65 harbor plasticity machinery that lies dormant throughout life unless an appropriate level of GABAergic transmission is exogenously supplied. Dark-reared mice similarly fail to activate plasticity with a normal time course. A common misconception about DR is that critical period onset is normal but then prolonged in duration. Both the evidence from cats (Mower, 1991; Beaver et al., 2001) and our recordings of mice dark-reared just to the typical peak of MD sensitivity (P28) (Fig. 6C,D) demonstrate that in fact critical period onset is delayed in the dark. These findings are entirely consistent with the GAD65 KO mouse phenotype, because DR impairs inhibitory circuit maturation in visual cortex (Benevento et al., 1992, 1995; Chen et al., 2001; Morales et al., 2002).
One reason for the misconception about the effects of DR is the finding that orientation preference emerges even in the absence of visual experience (Crair et al., 1998; Chapman et al., 1999). Here, we confirmed that orientation preference appears normally after 28 d in the dark, but then degrades with prolonged lack of visual input just as in cats (Crair et al., 1998). This profile differed strikingly from that of sensitivity to MD and failed to be rescued by DZ treatment in the dark. Recent evidence indeed supports a dissociation of molecular substrates underlying these distinct receptive field properties (Fagiolini et al., 2003).
How can transiently enhanced GABAergic transmission replace visual experience to trigger the critical period for OD? Homeostatic mechanisms in the dark would act to reduce miniature IPSC amplitudes by decreasing the number of postsynaptic GABAA receptors clustered at neocortical synapses because of activity deprivation (Kilman et al., 2002). Somehow brief DZ exposure must override this effect. Although rapid insertion of GABA receptors is possible under physiological stimulation (Nusser et al., 1998a), to date only the opposite (internalization) has been reported for DZ after prolonged exposure (Gallager et al., 1984; Tehrani and Barnes, 1997).
We propose a testable two-step process of inhibitory circuit refinement after DZ treatment as a basis for further study. Initially, acute benzodiazepine action may potentiate GABAergic transmission at individual synapses. Brief, augmented stimulation triggers long-term potentiation of inhibitory transmission in visual cortex that is induced postsynaptically (Komatsu, 1994). At the same time, DZ binding would focus GABAA receptors to the synapse by promoting removal of extrasynaptic receptors through internalization (Tehrani and Barnes, 1997). Exocytosis and endocytosis occur at extrasynaptic or perisynaptic sites, so that rapid receptor accumulation or removal from the postsynaptic domain likely results from lateral diffusion in the plane of the membrane (Rosenberg et al., 2001; Choquet and Triller, 2003).
In the second stage, morphological changes may reinforce and stabilize relevant inhibitory connections. Formation of novel GABA synapses occurs with as little as 24 hr of whisker stimulation in barrel cortex (Knott et al., 2002). As in our DZ findings, such alterations in synaptic connectivity last well beyond the period of increased sensory experience, persisting for days after the stimulation. Two molecules in particular may contribute to the growth and expansion of inhibitory connections. The extracellular protease tissue-type plasminogen activator (tPA) is maximally activated in visual cortex 2 d after sensory deprivation, but intriguingly not in GAD65 KO mice (Mataga et al., 2002). In the visual cortex of DR animals, BDNF is paradoxically elevated at the protein level and may facilitate a morphological maturation induced by pulsed action of DZ (Pollock et al., 2001). Transplanted Schwann cells acting as biological minipumps also allow normal development of visual cortex in the dark (Fagiolini et al., 1997), possibly through neurotrophin release acting on inhibitory interneuron maturation (Huang et al., 1999).
Specific GABA circuits may control critical period plasticity
The diverse array of neocortical interneurons is in fact precisely organized with respect to domains of synaptic contact onto target cells (Somogyi et al., 1998). Increasing inhibition at the axon initial segment (e.g., Chandelier cells) or on the soma (e.g., Large Basket cells) would ideally regulate spike generation or back-propagation, respectively. Chandelier cell contacts may be particularly plastic, because they contain PSA-NCAM (Arellano et al., 2002), a known substrate of proteases like tPA (Endo et al., 1999). Although this could enable appropriate spike timing-dependent plasticity (Feldman, 2000; Bi and Poo, 2001), it is unclear why progressive improvement of neural coding after DZ treatment should close the critical period. Evolving excitatory-inhibitory balance may alternately release, then ultimately limit, downstream factors permissive for consolidating structural plasticity such as transcription factors (Pham et al., 1999), proteases (Mataga et al., 2002), or extracellular matrix components (Pizzorusso et al., 2002). Critical period duration (<20 d) likely reflects a continuum of these plastic events once triggered by GABAergic transmission.
Central neurons receive a continuous barrage of GABAergic input (Mody, 2001; Nusser and Mody, 2002). Remarkably, benzodiazepines effectively tap into this spontaneous activity in complete darkness to induce the critical period, whereas similar intervals of light-evoked phasic inhibition are inadequate (Fig. 7). Consistent with this result is the DZ rescue of mice lacking GAD65, a synaptic, apo-form engaged mainly by phasic levels of activity (Hensch et al., 1998; Tian et al., 1999), but retaining a normal complement of GAD67 required for GABA synthesis under tonic or base level firing conditions (Asada et al., 1996; Soghomonian and Martin, 1998). Where its organization has been finely dissected, tonic GABA release occurs preferentially, or exclusively, at sites close to the action potential initiation site (Soltesz et al., 1995).
Actively released GABA acting on postsynaptic GABAA receptors is believed to mediate “phasic” inhibition, whereas “tonic” currents also result from the persistent activation of extrasynaptic receptors by ambient GABA. In granule cells of hippocampus and cerebellum, the latter contain α4 or α6 subunits, respectively (Nusser et al., 1998b; Stell and Mody, 2002; Nusser and Mody, 2002), and so are insensitive to benzodiazepine agonists (Sieghart, 1995). Critical period induction by DZ in cortical pyramidal cells would instead require extrasynaptic GABAA receptors containing α5 (Crestani et al., 2002) or α1, 2, 3 subunits activated by random, spontaneous synaptic events generated by basket or axo-axonic cells. Because α subunits are preferentially targeted to these different domains (Nusser et al., 1996; Nyiri et al., 2001; Klausberger et al., 2002), future work can dissect relevant GABA circuits by immunolocalization (Cherubini and Conti, 2001; Chen et al., 2001) and selective genetic disruption of their benzodiazepine sensitivity (Rudolph et al., 2001).
Tonic inhibitory input increases the electrotonic length of the dendritic tree and consequently increases attenuation of dendritic inputs as they propagate into the soma and axon (Hausser and Clark, 1997). Although a reduction in membrane time constant increases temporal precision of synaptic input coding, tonic inhibition also increases jitter in the timing of output spikes. Feedforward inhibition at the soma then enforces temporal fidelity and precise coincidence detection (Pouille and Scanziani, 2001). Interestingly, the ultimate outcome of brief DZ treatment is the maturation of this type of inhibition important in regulating the input-output relations of central neurons, such as prolonged discharge (Fig. 5B). Tonic GABA release may, thus, trigger a critical period for proper neural coding by optimizing phasic inhibitory circuits.
Footnotes
This work was supported in part by the Special Postdoctoral Researchers Program at The Institute of Physical and Chemical Research (RIKEN) (Y.I.) and Special Coordination Funds for Promoting Science and Technology from Japan Science and Technology Corporation (T.K.H.). We thank S. Fujishima and Y. Mizuguchi for genotyping and maintenance of the GAD65 KO mouse colony.
Correspondence should be addressed to Takao Kurt Hensch, Laboratory for Neuronal Circuit Development, The Institute of Physical and Chemical Research (RIKEN) Brain Science Institute, 2-1 Hirosawa, Wako-shi, Saitama 351-0198, Japan. E-mail: hensch@postman.riken.go.jp.
Copyright © 2003 Society for Neuroscience 0270-6474/03/236695-08$15.00/0
References
- Arellano JI, DeFelipe J, Munoz A ( 2002) PSA-NCAM immunoreactivity in chandelier cell axon terminals of the human temporal cortex. Cereb Cortex 12: 617-624. [DOI] [PubMed] [Google Scholar]
- Asada H, Kawamura Y, Maruyama K, Kume H, Ding R, Ji FY, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K ( 1996) Mice lacking the 65 kDa isoform of glutamic acid decarboxylase (GAD65) maintain normal levels of GAD67 and GABA in their brains but are susceptible to seizures. Biochem Biophys Res Commun 229: 891-895. [DOI] [PubMed] [Google Scholar]
- Beaver CJ, Ji Q-H, Daw NW ( 2001) Layer differences in the effect of monocular vision in light and dark reared kittens. Vis Neurosci 18: 811-820. [DOI] [PubMed] [Google Scholar]
- Benevento LA, Bakkum BW, Port JD, Cohen RS ( 1992) The effects of dark-rearing on the electrophysiology of the rat visual cortex. Brain Res 572: 198-207. [DOI] [PubMed] [Google Scholar]
- Benevento LA, Bakkum BW, Cohen RS ( 1995) gamma-Aminobutyric acid and somatostatin immunoreactivity in the visual cortex of normal and dark-reared rats. Brain Res 689: 172-182. [DOI] [PubMed] [Google Scholar]
- Berrueta LA, Gallo B, Vicente F ( 1992) Biopharmacological data and high-performance liquid chromatographic analysis of 1, 4-benzodiazepines in biological fluids: a review. J Pharm Biomed Anal 10: 109-136. [DOI] [PubMed] [Google Scholar]
- Bi G, Poo M ( 2001) Synaptic modification by correlated activity: Hebb's postulate revisited. Annu Rev Neurosci 24: 139-166. [DOI] [PubMed] [Google Scholar]
- Chapman B, Godecke I, Bonhoeffer T ( 1999) Development of orientation preference in the mammalian visual cortex. J Neurobiol 41: 18-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Yang C, Mower GD ( 2001) Developmental changes in the expression of GABA(A) receptor subunits (alpha(1), alpha(2), alpha(3)) in the cat visual cortex and the effects of dark rearing. Mol Brain Res 88: 135-143. [DOI] [PubMed] [Google Scholar]
- Cherubini E, Conti F ( 2001) Generating diversity at GABAergic synapses. Trends Neurosci 24: 155-162. [DOI] [PubMed] [Google Scholar]
- Choquet D, Triller A ( 2003) The role of receptor diffusion in the organization of the postsynaptic membrane. Nat Rev Neurosci 4: 251-265. [DOI] [PubMed] [Google Scholar]
- Crair MC, Gillespie DC, Stryker MP ( 1998) The role of visual experience in the development of columns in cat visual cortex. Science 279: 566-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Bluthmann H, Mohler H, Rudolph U ( 2002) Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors. Proc Natl Acad Sci USA 99: 8980-8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynader M ( 1983) Prolonged sensitivity to monocular deprivation in dark-reared cats: effects of age and visual exposure. Brain Res 284: 155-164. [DOI] [PubMed] [Google Scholar]
- Daw N ( 1995) Visual development. New York: Plenum.
- Eghbali M, Curmi JP, Birnir B, Gage PW ( 1997) Hippocampal GABAA channel conductance increased by diazepam. Nature 388: 71-75. [DOI] [PubMed] [Google Scholar]
- Endo A, Nagai N, Urano T, Takada Y, Hashimoto K, Takada A ( 1999) Proteolysis of neuronal cell adhesion molecule by the tissue plasminogen activator-plasmin system after kainate injection in the mouse hippocampus. Neurosci Res 33: 1-8. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Hensch TK ( 2000) Inhibitory threshold for critical-period activation in primary visual cortex. Nature 404: 183-186. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Pizzorusso T, Berardi N, Domenici L, Maffei L ( 1994) Functional postnatal development of the rat primary visual cortex and the role of visual experience: dark rearing and monocular deprivation. Vision Res 34: 709-720. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Pizzorusso T, Porciatti V, Cenni M, Maffei L ( 1997) Transplant of Schwann cells allows normal development of the visual cortex of dark-reared rats. Eur J Neurosci 9: 102-112. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Katagiri H, Miyamoto H, Mori H, Grant SGN, Mishina M, Hensch TK ( 2003) Separable features of visual cortical plasticity revealed by N-methyl-daspartate receptor 2A signaling. Proc Natl Acad Sci USA 100: 2854-2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE ( 2000) Inhibition and plasticity. Nat Neurosci 3: 303-304. [DOI] [PubMed] [Google Scholar]
- Gallager DW, Lakoski JM, Gonsalves SF, Rauch SL ( 1984) Chronic benzodiazepine treatment decreases postsynaptic GABA sensitivity. Nature 308: 74-77. [DOI] [PubMed] [Google Scholar]
- Gordon JA, Stryker MP ( 1996) Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J Neurosci 16: 3274-3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanover JL, Huang ZJ, Tonegawa S, Stryker MP ( 1999) Brain-derived neurotrophic factor overexpression induces precocious critical period in mouse visual cortex. J Neurosci 19: RC40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausser M, Clark BA ( 1997) Tonic synaptic inhibition modulates neuronal output pattern and spatio-temporal synaptic integration. Neuron 19: 665-678. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF ( 1998) Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science 282: 1504-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S ( 1999) BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 98: 739-755. [DOI] [PubMed] [Google Scholar]
- Imamura K, Kasamatsu T ( 1991) Ocular dominance plasticity: usefulness of anesthetized and paralyzed preparations. Jpn J Physiol 41: 521-549. [DOI] [PubMed] [Google Scholar]
- Kilman V, van Rossum MC, Turrigiano GG ( 2002) Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABA(A) receptors clustered at neocortical synapses. J Neurosci 22: 1328-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Roberts JD, Somogyi P ( 2002) Cell type- and input-specific differences in the number and subtypes of synaptic GABA(A) receptors in the hippocampus. J Neurosci 22: 2513-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott GW, Quairiaux C, Genoud C, Welker E ( 2002) Formation of dendritic spines with GABAergic synapses induced by whisker stimulation in adult mice. Neuron 34: 265-273. [DOI] [PubMed] [Google Scholar]
- Komatsu Y ( 1994) Age-dependent long-term potentiation of inhibitory synaptic transmission in rat visual cortex. J Neurosci 14: 6488-6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mataga N, Nagai N, Hensch TK ( 2002) Permissive proteolytic activity for visual cortical plasticity. Proc Natl Acad Sci USA 99: 7717-7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I ( 2001) Distinguishing between GABA(A) receptors responsible for tonic and phasic conductances. Neurochem Res 26: 907-913. [DOI] [PubMed] [Google Scholar]
- Morales B, Choi SY, Kirkwood A ( 2002) Dark rearing alters the development of GABAergic transmission in visual cortex. J Neurosci 22: 8084-8090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mower GD ( 1991) The effect of dark rearing on the time course of the critical period in cat visual cortex. Dev Brain Res 58: 151-158. [DOI] [PubMed] [Google Scholar]
- Mower GD, Christen WG, Caplan CJ ( 1983) Very brief visual experience eliminates plasticity in the cat visual cortex. Science 221: 178-180. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Mody I ( 2002) Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol 87: 2624-2628. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Benke D, Fritschy JM, Somogyi P ( 1996) Differential synaptic localization of two major gamma-aminobutyric acid type A receptor alpha subunits on hippocampal pyramidal cells. Proc Natl Acad Sci USA 93: 11939-11944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Hajos N, Somogyi P, Mody I ( 1998a) Increased number of synaptic GABA(A) receptors underlies potentiation at hippocampal inhibitory synapses. Nature 395: 172-177. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P ( 1998b) Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci 18: 1693-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyiri G, Freund TF, Somogyi P ( 2001) Input-dependent synaptic targeting of alpha(2)-subunit-containing GABA(A) receptors in synapses of hippocampal pyramidal cells of the rat. Eur J Neurosci 13: 428-442. [DOI] [PubMed] [Google Scholar]
- Pham TA, Impey S, Storm DR, Stryker MP ( 1999) CRE-mediated gene transcription in neocortical neuronal plasticity during the developmental critical period. Neuron 22: 63-72. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L ( 2002) Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298: 1248-1251. [DOI] [PubMed] [Google Scholar]
- Pollock GS, Vernon E, Forbes ME, Yan Q, Ma YT, Hsieh T, Robichon R, Frost DO, Johnson JE ( 2001) Effects of early visual experience and diurnal rhythms on BDNF mRNA and protein levels in the visual system, hippocampus, and cerebellum. J Neurosci 21: 3923-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouille F, Scanziani M ( 2001) Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science 293: 1159-1163. [DOI] [PubMed] [Google Scholar]
- Rosenberg M, Meier J, Triller A, Vannier C ( 2001) Dynamics of glycine receptor insertion in the neuronal plasma membrane. J Neurosci 21: 5036-5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Mohler H ( 2001) GABA(A) receptor subtypes: dissecting their pharmacological functions. Trends Pharmacol Sci 22: 188-194. [DOI] [PubMed] [Google Scholar]
- Sieghart W ( 1995) Structure and pharmacology of γ-aminobutyric acidA receptor subtypes. Pharmacol Rev 47: 181-234. [PubMed] [Google Scholar]
- Soghomonian JJ, Martin DL ( 1998) Two isoforms of glutamate decarboxylase: why? Trends Pharmacol 19: 500-505. [DOI] [PubMed] [Google Scholar]
- Soltesz I, Smetters DK, Mody I ( 1995) Tonic inhibition originates from synapses close to the soma. Neuron 14: 1273-1283. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Tamas G, Lujan R, Buhl EH ( 1998) Salient features of synaptic organisation in the cerebral cortex. Brain Res Rev 26: 113-135. [DOI] [PubMed] [Google Scholar]
- Stell BM, Mody I ( 2002) Receptors with different affinities mediate phasic and tonic GABAA conductances in hippocampal neurons. J Neurosci 22: RC223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehrani MHJ, Barnes EM ( 1997) Sequestration of γ-Aminobutyric AcidA receptors on clathrin-coated vesicles during chronic benzodiazepine administration in vivo. J. Pharmacol Exp Ther 283: 384-390. [PubMed] [Google Scholar]
- Tian N, Petersen C, Kash S, Baekkeskov S, Copenhagen D, Nicoll R ( 1999) The role of the synthetic enzyme GAD65 in the control of neuronal gamma-aminobutyric acid release. Proc Natl Acad Sci USA 96: 12911-12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumoto T, Freeman RD ( 1987) Dark-reared cats: responsivity of cortical cells influenced pharmacologically by an inhibitory antagonist. Exp Brain Res 65: 666-672. [DOI] [PubMed] [Google Scholar]