Abstract
BDNF plays a critical role in activity-dependent neuroplasticity underlying learning and memory in the hippocampus. A frequent single nucleotide polymorphism in the targeting region of the human BDNF gene (val66met) has been associated with abnormal intracellular trafficking and regulated secretion of BDNF in cultured hippocampal neurons transfected with the met allele. In addition, the met allele has been associated with abnormal hippocampal neuronal function as well as impaired episodic memory in human subjects, but a direct effect of BDNF alleles on hippocampal processing of memory has not been demonstrated. We studied the relationship of the BDNF val66met genotype and hippocampal activity during episodic memory processing using blood oxygenation level-dependent functional magnetic resonance imaging and a declarative memory task in healthy individuals. Met carriers exhibited relatively diminished hippocampal engagement in comparison with val homozygotes during both encoding and retrieval processes. Remarkably, the interaction between the BDNF val66met genotype and the hippocampal response during encoding accounted for 25% of the total variation in recognition memory performance. These data implicate a specific genetic mechanism for substantial normal variation in human declarative memory and suggest that the basic effects of BDNF signaling on hippocampal function in experimental animals are important in humans.
Keywords: BDNF, hippocampus, human memory, gene, polymorphism, BOLD fMRI
Introduction
The molecular cascades governing the development and maturation of the CNS are highly conserved in adult organisms and contribute to complex experiential phenomena such as activity-dependent synaptic plasticity. A notable example is the neurotrophin BDNF, which not only regulates cell survival, proliferation, and synaptic growth in the developing CNS but also is a critical element in modulating synaptic changes, such as hippocampal long-term potentiation (LTP), associated with learning and adaptive behaviors in adult animals (Poo, 2001; Tyler et al., 2002). Thus, genetic and environmental influences on BDNF activity may contribute to alterations in hippocampal function and, subsequently, hippocampal-dependent learning and memory.
A frequent polymorphism producing a nonconservative amino acid substitution (valine to methionine) at codon 66 (val66met) has recently been identified in the human BDNF gene (dbSNP number rs6265). This sequence variant is located in the 5′ pro-BDNF sequence, which encodes the precursor peptide (pro-BDNF) that is proteolytically cleaved to form the mature protein (Seidah et al., 1996). Whereas this BDNF polymorphism does not affect mature BDNF protein function, it has recently been shown to dramatically alter the intracellular trafficking and packaging of pro-BDNF and, thus, the regulated secretion of the mature peptide. Specifically, rat hippocampal neurons transfected with the met allele exhibit abnormal intracellular trafficking and regulated secretion of BDNF in comparison with those transfected with the val allele (Egan et al., 2003). In healthy human subjects, the met allele is linked with diminished levels of hippocampal N-acetyl aspartate, a putative marker of neuronal integrity and synaptic abundance, and deficits in episodic memory (Egan et al., 2003). These findings suggest that genetically driven variation in BDNF secretion may significantly impact human hippocampal function and memory. However, the impact of this BDNF polymorphism on memory-related hippocampal activity has not been determined.
To directly assay the contribution of the BDNF val66met polymorphism to memory-related hippocampal activity, we studied healthy volunteers with blood oxygenation level-dependent functional magnetic resonance imaging (BOLD fMRI) while they performed a simple declarative memory task known to be dependent on the hippocampal formation (Gabrieli et al., 1998; Schacter and Wagner, 1999). The memory task involved the encoding and subsequent retrieval of complex, novel scenes. On the basis of the basic evidence that BDNF is important for memory-related hippocampal processes and the effect of the val66met polymorphism on BDNF secretion, we hypothesized that individuals homozygous for the val allele (val/val genotype), the variant associated with normal intracellular trafficking of BDNF and better episodic memory, would exhibit greater memory-related hippocampal activity than those carrying the met allele (val/met, met/met genotypes). We also predicted that these genotype-based differences would impact memory performance, with val homozygote individuals demonstrating better recognition accuracy than met carriers.
Materials and Methods
Subjects. Sixty-four right-handed healthy subjects participated in this study according to the guidelines of the National Institute of Mental Health Institutional Review Board. Subjects were recruited from local advertisements and underwent extensive clinical examinations involving structured medical and psychiatric history questionnaires, neurocognitive test batteries, and diagnostic MRI scanning to rule out structural brain disease.
From this initial cohort, 28 subjects were selected who comprised two equal groups based on BDNF val 66met genotype (Val group, 14 val/val individuals; Met group, 12 val/met and two met/met individuals). Because of the population frequency of the met allele (∼0.19 in subjects of European ancestry), few met/met individuals are available (i.e., <4%) and, thus, we combined val/met and met/met genotypes. All subjects, except for one val/val homozygote (an African-American female) and two met carriers (an Asian-American female and male), were of European ancestry.
Importantly, the genotype groups were carefully matched for gender (six females and eight males in each group), age (mean ± SEM; Val group, 30.9 ± 1.3 years; Met group, 30.3 ± 1.6 years; F(1,26) = 0.09; p = 0.76), and mean intelligence quotient (IQ) (mean ± SEM; Val group, 110.7 ± 1.5; Met group, 108.1 ± 2.1; F(1,26) = 1.04; p = 0.32). All 28 subjects were also cleared of neurological, psychiatric, or substance abuse problems and had no history of other medical problems or medical treatment relevant to cerebral metabolism and blood flow. Thus, the potential for these various confounding factors to obscure the contribution of BDNF genetic variation to memory performance and hippocampal activity was minimized.
Furthermore, all subjects underwent genotyping of the apolipoprotein (APO) E gene ϵ alleles, because the ϵ4 allele has a dose-dependent effect on risk and age of onset for Alzheimer's disease (Corder et al., 1993) as well as an impact on memory-related brain activity in healthy, elderly subjects (Bookheimer et al., 2000). There was no significant difference in ϵ4 allele frequency between our two BDNF groups (Val group, four ϵ4 allele carriers; Met group, three ϵ4 allele carriers).
Genotyping. DNA was extracted using standard methods. BDNF val 66met and APO ϵ genotypes were determined using the Taqman 5′-exonuclease allelic discrimination assay (Corder et al., 1993). Data from a larger sample of subjects (Egan et al., 2003) has revealed that the frequencies of the BDNF val allele is 0.81 and that the genotype frequencies (val/val, 0.67; val/met, 0.28; met/met, 0.05) are in Hardy-Weinberg equilibrium.
Declarative memory paradigm. The fMRI paradigm consisted of the encoding and subsequent retrieval of novel, complex scenes, a task that has consistently been shown to produce activation of the hippocampal formation in human neuroimaging experiments (Stern et al., 1996; Gabrieli et al., 1997; Zeineh et al., 2000). Stimuli were presented in a blocked paradigm to maximize power and sensitivity for BOLD signal change in the hippocampal region (Birn et al., 2002). Four encoding blocks were followed by four retrieval blocks in an interleaved design with a passive rest condition, resulting in a total of 17 blocks. Each block was 20 sec long, producing a total scan time of 5 hr, 40 min. During encoding blocks, subjects viewed six images, presented serially for 3 sec each, and determined whether each image represented an “indoor” or “outdoor” scene. An equal number of “indoor” and “outdoor” scenes were presented in each encoding block. All scenes were of neutral emotional valence and were derived from the International Affective Picture System (Lang et al., 1997). During subsequent retrieval blocks, subjects again viewed six images, presented serially for 3 sec each, and determined whether each scene was “new” or “old.” In each retrieval block, half the scenes were “old” (i.e., presented during the encoding blocks) and half were “new” (i.e., not presented during the encoding blocks). The order of “indoor” and “outdoor” scenes as well as “new” and “old” scenes were randomly distributed throughout the encoding and retrieval blocks, respectively. During the interleaved rest blocks, subjects were instructed to fixate on a centrally presented cross-hair. Before the beginning of each block, subjects viewed a brief (2 sec) instruction: “Indoor or Outdoor?,” “New or Old?,” or “Rest.” During scanning, all subjects responded by button presses with their dominant hand, allowing for the determination of accuracy and reaction time.
fMRI acquisition parameters. Each subject was scanned using a GE Signa 3T scanner with a real-time functional imaging upgrade (General Electric, Milwaukee, WI). An automated shim procedure was applied to minimize possible magnetic field inhomogeneities. BOLD functional images were acquired with a gradient echo planar imaging (EPI) sequence and covered 24 axial slices (4 mm thick, 1 mm gap) that began at the cerebral vertex and encompassed the entire cerebrum and the majority of the cerebellum (repetition time/echo time, 2000/28 msec; field of view, 24 cm; matrix, 64 × 64). All scanning parameters were selected to optimize the quality of the BOLD signal while maintaining a sufficient number of slices to acquire whole-brain data. Before the collection of fMRI data for each subject, we acquired a reference EPI scan and visually inspected it for artifacts (i.e., ghosting) as well as for good signal across the entire volume of acquisition, including the medial temporal lobes. The fMRI data from all 28 subjects included in this study were cleared of such problems.
Image analysis. Analysis of the fMRI data were completed using statistical parametric mapping (SPM99; http://www.fil.ion.ucl.ac.uk/spm). Images for each subject were realigned to the first volume in the time series to correct for head motion, spatially normalized into a standard stereotactic space (Montreal Neurological Institute template) using a 12 parameter affine model and smoothed to minimize noise and residual differences in gyral anatomy with a Gaussian filter, set at 8 mm full-width at half-maximum. Voxel-wise signal intensities were ratio normalized to the whole-brain global mean.
Predetermined condition effects at each voxel within an anatomically defined region of interest that included the bilateral hippocampi and parahippocampal cortices (Giedd et al., 1996) were calculated using a t-statistic, producing a statistical image for the contrasts of encoding versus rest and retrieval versus rest for each subject. These individual contrast images were then used in second-level random effects models, which account for both scan-to-scan and subject-to-subject variability, to determine task-specific regional responses at the group level for the entire sample (main effects of task) and paired t tests (direct comparisons between groups). Because of our strong a priori hypothesis regarding the differential response of the hippocampus and our use of a rigorous random effects statistical model, a statistical threshold of p < 0.05, with a small volume correction for multiple comparisons, was used to identify significant responses for all comparisons.
Whole-brain image analyses for all predetermined condition effects were also calculated using second-level random effects models. Because we did not have a priori hypotheses regarding the activity of brain regions outside of the hippocampal formation, we used a statistical threshold of p < 0.05, corrected for multiple comparisons across all supra-threshold voxels, for these whole-brain comparisons.
Regression analysis. We examined the relationship between BDNF genotype, mean BOLD responses in the hippocampus, and memory performance (i.e., recognition accuracy) using a hierarchical multiple regression in which variables are entered (or removed) from the linear equation on the basis of their ability to improve R2 at each successive step in the model (PROC REG MAXR; SAS Institute, Inc., Cary, NC). Mean BOLD percentage signal change for all 28 subjects was extracted from both the left and right hippocampal clusters that demonstrated a significant main effect for encoding and for retrieval (Fig. 1). Importantly, these clusters were selected in an unbiased manner (i.e., they were not chosen on the basis of their sensitivity to either BDNF genotype or a priori correlation with performance). In addition to BDNF genotype and mean BOLD signal changes, we created interaction terms representing the potential contribution of BDNF genotype to left and right hippocampal activity during both encoding and retrieval (e.g., BDNF genotype x left hippocampal encoding activity). Gender and IQ were also entered as variables.
Figure 1.
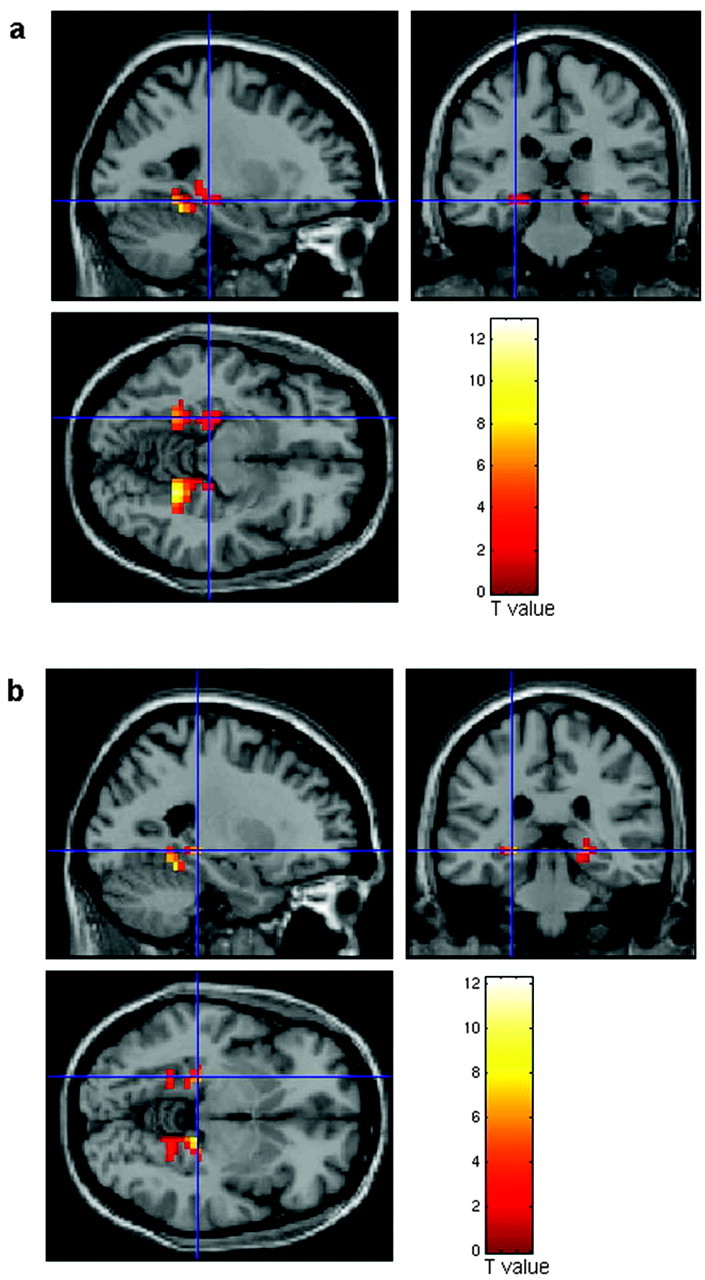
Statistical parametric maps showing significant engagement of the hippocampal formation (hippocampus and parahippocampal gyrus) during encoding and retrieval. BOLD fMRI responses in the posterior hippocampal formation are shown overlaid onto averaged structural MRIs in the axial, coronal, and sagittal plane. Blue cross-hairs are centered on the left hippocampal formation clusters during encoding and retrieval that contributed maximally to the observed variance in memory performance (see Regression analysis in Materials and Methods). a, Talairach coordinates and voxel-level statistics for the maximal voxel in the left and right hippocampal formation during encoding are x =-25 mm; y =-44 mm; z = -10 mm; cluster size = 42 voxels; voxel-level corrected p < 0.001; Z score = 5.59; and x = 22 mm; y = -48 mm; z = -10 mm; cluster size = 60 voxels; voxel-level corrected p < 0.001; Z score = 7.24, respectively. b, Talairach coordinates and voxel-level statistics for the maximal voxel in the left and right HF during retrieval are x = -25 mm; y = -44 mm; z = -10 mm; cluster size = 36 voxels; voxel-level corrected p < 0.001; Z score = 6.32; and x = 22 mm; y =-48 mm; z = -10 mm; cluster size = 64 voxels; voxel-level corrected p < 0.001; Z score = 7.08, respectively.
Results
Consistent with prior reports (Gabrieli et al., 1998; Schacter and Wagner, 1999), we found significant bilateral activation of the posterior hippocampal formation (hippocampus and parahippocampal gyrus) during both encoding and retrieval in all subjects (Fig. 1). In addition, both encoding and retrieval were associated with significant bilateral activations in the inferotemporal, parietal, and frontal cortices, a distributed network critical for visuospatial information processing (Ungerleider and Haxby, 1994). A conjunction analysis revealed a significant degree of overlap in both hippocampal and cortical clusters during encoding and retrieval, a finding consistent with a conservancy of circuits underlying the initial formation and subsequent recall of specific episodic information (Persson and Nyberg, 2000). The posterior localization of the observed hippocampal activity may reflect the visual nature of the stimuli used and the processing of this information within object-sensitive fusiform and parahippocampal regions (Ungerleider and Haxby, 1994) and adjacent, interconnected hippocampal structures (Small et al., 2001).
As hypothesized, direct group comparisons revealed that memory-related hippocampal activity was greater, during both encoding and retrieval, in subjects homozygous for the BDNF val allele, the relatively normal functional variant (Fig. 2). The BDNF val66met polymorphism, however, had no impact on activity within the distributed cortical network (e.g., inferotemporal, parietal and frontal locales) involved with general visuospatial information processing. The specificity of this BDNF effect on hippocampal activity is consistent with the expression pattern of BDNF in the brain, which is highest in the hippocampus (Murer et al., 2001), as well as the critical role of BDNF in hippocampal processes, particularly activity-dependent synaptic plasticity, mediating learning and memory (Poo, 2001; Tyler et al., 2002).
Figure 2.
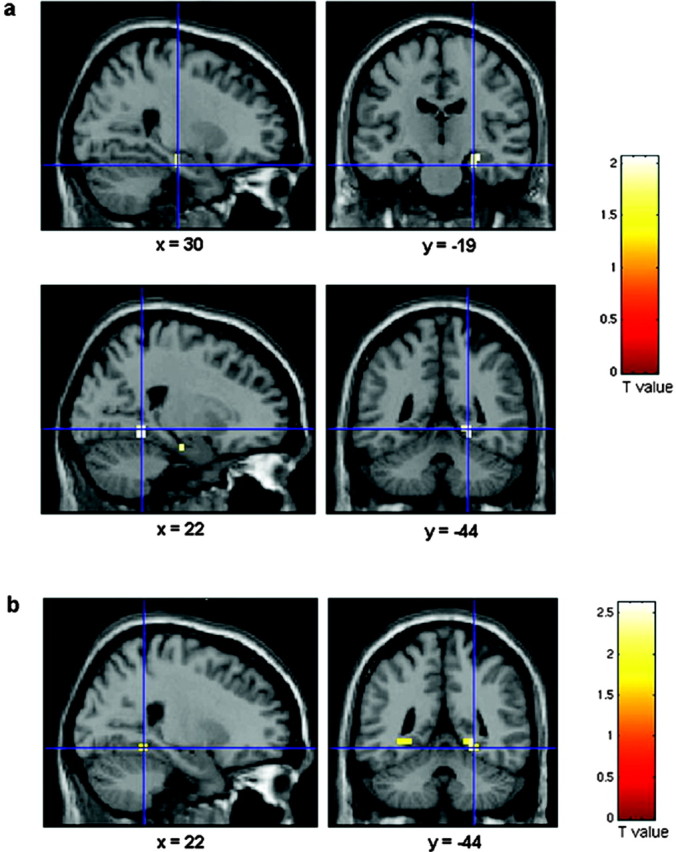
Genotype-based parametric comparisons showing significantly greater hippocampal activity in the Val group versus the Met group during both encoding and retrieval. BOLD fMRI responses in the posterior hippocampal formation are shown overlaid onto averaged structural MRIs in the coronal and sagittal plane. a, Talairach coordinates and voxel-level statistics for the maximal voxels in the right hippocampus and parahippocampal gyrus exhibiting Val greater than Met activity during encoding: x = 30 mm; y =-19 mm; z = -12 mm; cluster size = 5 voxels; voxel-level corrected p = 0.047; Z score = 1.91; and x = 22 mm; y = -44 mm; z = -6 mm; cluster size = 6 voxels; voxel-level corrected p = 0.043; Z score = 1.97, respectively. b, Talairach coordinates and voxel-level statistics for the maximal voxels in the left and right parahippocampal gyrus exhibiting Val greater than Met activity during retrieval: x = -30 mm; y = -44 mm; z = -2 mm; cluster size = 3 voxels; voxel-level corrected p = 0.046; Z score = 1.87; and x = 22 mm; y =-48 mm; z = -6 mm; cluster size = 7 voxels; voxel-level corrected p = 0.035; Z score = 2.45, respectively.
In addition to the effect of the BDNF val66met polymorphism on memory-related hippocampal activity, and consistent with the prior finding of impaired episodic memory in met carriers (Egan et al., 2003), val homozygotes in the present study were significantly more accurate at recognizing both “new” and “old” (i.e., encoded) scenes during retrieval (percentage correct ± SEM; Val group, 91.6 ± 1.5; Met group, 84.5 ± 2.6; F(1,26) = 5.69; p = 0.02). The increased number of recognition errors in met carriers was equally distributed across misses (i.e., not recognizing encoded scenes as “old”) and false alarms (i.e., recognizing novel scenes as “old”). Importantly, this difference in memory performance did not simply reflect the differential ability of subjects from each group to accurately encode these stimuli, because there was no difference in encoding accuracy between groups (percentage correct ± SEM; Val group, 94.9 ± 0.6; Met group, 93.2 ± 1.2; F(1,26) = 1.71; p > 0.20). Furthermore, the absence of systematic group differences in reaction time during either encoding (msec ± SEM; Val group, 1316.4 ± 44.9; Met group,1314.5 ± 56.8; F(1,26) = 0.001; p = 0.98) or retrieval (msec ± SEM; Val group, 1554.1 ± 43.5; Met group, 1613.33 ± 41.5; F(1,26) = 0.97; p = 0.33) suggests that the observed disparity in memory performance is unlikely to be driven by differential attention during the tasks.
We used a modified hierarchical stepwise regression analysis to explore the relationship between the observed influence of the BDNF val66met polymorphism on memory-related hippocampal activity and recognition accuracy. This approach allows for the unbiased determination of the contribution of independent variables as well as the interaction of specific variables to the observed variation in memory performance. Only two variables entered the model significantly as determined by scree inspection and “F to enter”: the interaction term of BDNF val66met genotype and mean left hippocampal activity during encoding (F(1,26) = 9.44; R2 = 0.25; p = 0.005), and mean left hippocampal activity during retrieval (F(1,26) = 4.99; R2 = 0.04; p = 0.035). Together, ∼30% of the total variation in recognition memory performance was accounted for by these two variables. It is striking that an interaction term reflecting BDNF genotype modulation of hippocampal engagement during the encoding of novel scenes accounted for the majority of the explained variance (25%), followed only by simple activation of the hippocampus during recognition itself.
Discussion
The results of this study have several important implications. First, unbiased selection of hippocampal regions engaged during encoding or retrieval proved to be significant predictors of behavioral variance. Thus, hippocampal activity across individuals, as measured by BOLD fMRI, could be linked directly with memory performance, although the magnitude of the effect is relatively small. This finding is consistent with earlier reports (Gabrieli et al., 1998; Schacter and Wagner, 1999). Second, the finding that the interaction of BDNF genotype and hippocampal activity during encoding accounts for a substantial proportion of the behavioral variance during retrieval (25%) suggests that BDNF modulation of hippocampal engagement is a key process in the initial acquisition of information and is consistent with the known role of BDNF in activity-dependent plasticity and hippocampal LTP, processes that are thought to underlie the formation of new learning and memory (Poo, 2001; Tyler et al., 2002). Third, the contribution of hippocampal activity during retrieval to variation in memory performance was not modulated by BDNF, suggesting that accurate judgments about recognition may, in part, reflect the simple engagement of hippocampal subregions. However, this retrieval-related activity also seems to be a less robust predictor of performance than BDNF-modulated hippocampal activity during encoding. Our finding of a preeminent contribution of BDNF-modulated hippocampal activity during encoding to subsequent memory performance is consistent with studies indicating that the strength of the hippocampal trace during encoding is the most robust predictor of subsequent memory accuracy (Wagner et al., 1999; Fell et al., 2001). Finally, our data highlight a laterality effect during the information processing associated with our paradigm, because hippocampal activity in the left hemisphere, during both encoding and retrieval, was the predictor of performance. This effect suggests that scenes from the real world that undergo further semantic encoding (during “indoor” or “outdoor” judgments) and that engage the left hippocampal formation as a consequence are subsequently better remembered.
These results suggest that the BDNF val66met polymorphism has a dramatic and regionally specific impact on memory-related brain activity that may contribute significantly to human variation in normal memory ability (Wechsler, 1997). This effect may be mediated through alterations in activity-dependent hippocampal processes requiring BDNF-regulated secretion. Specifically, it is conceivable that the abnormal intracellular trafficking and regulated secretion of BDNF in met carriers may result in impaired hippocampal LTP or analogous synaptic events that may underlie encoding, reflected in their relatively diminished hippocampal BOLD fMRI responses, and a subsequently weakened hippocampal trace. The poorer recall and recognition of previously encoded items in these same met carriers may, thus, reflect the weakness of this initial trace. Alternatively, given the importance of BDNF in neuronal survival and synaptic proliferation, our observed BDNF effect on memory-related hippocampal activity and recognition accuracy may reflect abnormalities in met carriers in the development of the extended brain circuitry, centered on the hippocampus, which is critical for mediating consolidation of episodic information. Additional studies are needed to determine the contribution of these and potentially other mechanisms to the observed modulation of memory-related hippocampal activity and memory performance by the BDNF val66met polymorphism.
Our data implicate a genetic mechanism for variation in normal human declarative memory. A common functional polymorphism in the gene encoding BDNF, a protein critical for hippocampal synaptic plasticity involved in learning and memory in lower animals, has a significant impact on the activity of the human hippocampus during declarative memory processing. In turn, this BDNF-driven variation in hippocampal activity strongly predicts how accurately information is remembered. The BDNF val66met polymorphism may impact on the expression of human conditions that affect hippocampal function (e.g., aging, trauma, degenerative disease), and BDNF signaling may be a propitious target for interventions to enhance declarative memory. More generally, our current findings along with those of other recent studies (Bookheimer et al., 2000; Egan et al., 2001; Hariri et al., 2002), highlight the potential of functional neuroimaging as an approach for exploring the biological impact of genetic variation on information processing within distinct brain regions and circuits in relatively small samples of healthy subjects (Hariri and Weinberger, 2003).
Footnotes
This study was supported by the National Institute of Mental Health Intramural Research Program. We thank A. Gopal, K. Munoz, S. Sust, and D. Goldsmith for technical assistance.
Correspondence should be addressed to Dr. Daniel R. Weinberger, Clinical Brain Disorders Branch, National Institute of Mental Health, 10 Center Drive, Room 4S235, Bethesda, MD 20892-1384. E-mail: weinberd@intra.nimh.nih.gov.
Copyright © 2003 Society for Neuroscience 0270-6474/03/236690-05$15.00/0
T.E.G. and V.S.M. contributed equally to this work.
References
- Birn RM, Cox RW, Bandettini PA ( 2002) Detection versus estimation in event-related fMRI: choosing the optimal stimulus timing. NeuroImage 15: 252-264. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW ( 2000) Patterns of brain activation in people at risk for Alzheimer's disease. N Engl J Med 343: 450-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA ( 1993) Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 261: 921-923. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR ( 2001) Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA 98: 6917-6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR ( 2003) The BDNF val 66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112: 257-269. [DOI] [PubMed] [Google Scholar]
- Fell J, Klaver P, Lehnertz K, Grunwald T, Schaller C, Elger CE, Fernandez G ( 2001) Human memory formation is accompanied by rhinalhippocampal coupling and decoupling. Nat Neurosci 4: 1259-1264. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD, Brewer JB, Desmond JE, Glover GH ( 1997) Separate neural bases of two fundamental memory processes in the human medial temporal lobe. Science 276: 264-266. [DOI] [PubMed] [Google Scholar]
- Gabrieli JDE, Brewer JB, Poldrack RA ( 1998) Images of medial temporal lobe functions in human learning and memory. Neurobiol Learn Mem 70: 275-283. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL ( 1996) Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4-18 years. J Comp Neurol 366: 223-230. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Weinberger DR ( 2003) Imaging genomics. Br Med Bull 65: 237-248. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR ( 2002) Serotonin transporter genetic variation and the response of the human amygdala. Science 297: 400-403. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN ( 1997) International Affective Picture System (IAPS): technical manual and affective ratings. NIMH Center for the Study of Emotion and Attention, University of Florida, Gainesville, FL.
- Murer MG, Yan Q, Raisman-Vozari R ( 2001) Brain-derived neurotrophic factor in the control human brain, and in Alzheimer's disease and Parkinson's disease. Prog Neurobiol 63: 71-124. [DOI] [PubMed] [Google Scholar]
- Persson J, Nyberg L ( 2000) Conjunction analysis of cortical activations common to encoding and retrieval. Microsc Res Tech 51: 39-44. [DOI] [PubMed] [Google Scholar]
- Poo MM ( 2001) Neurotrophins as synaptic modulators. Nat Rev Neurosci 2: 24-32. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wagner AD ( 1999) Perspectives: neuroscience. Remembrance of things past. Science 285: 1503-1504. [DOI] [PubMed] [Google Scholar]
- Seidah NG, Benjannet S, Pareek S, Chretien M, Murphy RA ( 1996) Cellular processing of the neurotrophin precursors of NT3 and BDNF by the mammalian proprotein convertases. FEBS Lett 379: 247-250. [DOI] [PubMed] [Google Scholar]
- Small SA, Nava AS, Perera GM, DeLaPaz R, Mayeux R, Stern Y ( 2001) Circuit mechanisms underlying memory encoding and retrieval in the long axis of the hippocampal formation. Nat Neurosci 4: 442-449. [DOI] [PubMed] [Google Scholar]
- Stern CE, Corkin S, Gonzalez RG, Guimaraes AR, Baker JR, Jennings PJ, Carr CA, Sugiura RM, Vedantham V, Rosen BR ( 1996) The hippocampal formation participates in novel picture encoding: evidence from functional magnetic resonance imaging. Proc Natl Acad Sci USA 93: 8660-8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Alonso M, Bramham CR, Pozzo-Miller LD ( 2002) From acquisition to consolidation: on the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn Mem 9: 224-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Haxby JV ( 1994) 'What' and 'where' in the human brain. Curr Opin Neurobiol 4: 157-165. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Koutstaal W, Schacter DL ( 1999) When encoding yields remembering: insights from event-related neuroimaging. Philos Trans R Soc Lond B Biol Sci 354: 1307-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D ( 1997) Wechsler memory scale, Ed 3. San Antonio: The Psychological Corporation.
- Zeineh MM, Engel SA, Bookheimer SY ( 2000) Application of cortical unfolding techniques to functional MRI of the human hippocampal region. NeuroImage 11: 668-683. [DOI] [PubMed] [Google Scholar]


