Abstract
Peripheral nerve growth is regulated by the coordinated action of numerous external stimuli, including positively acting neurotrophin-derived growth cues and restrictive semaphorin cues. Here, we show that Semaphorin 3F (Sema 3F) can antagonize nerve growth factor (NGF)-stimulated TrkA (tyrosine receptor kinase A) signaling in sympathetic neurons, thereby apparently contributing to growth cone collapse. Sema 3F suppressed NGF-induced activation of the phosphatidylinositol 3 (PI3)-kinase-Akt and MEK (mitogen-activated protein kinase kinase)-ERK (extracellular signal-regulated kinase) pathways, both of which we show to be required to maintain growth cone structure. Sema 3F-induced growth cone collapse was partially reversed by sustained activation of the PI3-kinase and MEK pathways, which was achieved by overexpression of the Gab-1 (growth-associated binder 1) docking protein. These data indicate that a novel mechanism used by Sema 3F to collapse growth cones in sympathetic neurons is to dampen neurotrophin signaling, providing an intracellular mechanism for cross talk between positive and negative axon growth cues.
Keywords: growth cone, semaphorin 3F, nerve growth factor, PI3-kinase, MEK, TrkA, signal transduction
Introduction
During neuronal development, peripheral projections of sensory and sympathetic neurons are guided by patterned expression of both positive and negative growth cues in the local environment of extending growth cones. A major mediator of peripheral nerve growth is the neurotrophin nerve growth factor (NGF). NGF is required in vivo for peripheral axon projections by sensory neurons (Patel et al., 2000) and regulates target innervation by sympathetic neurons (Edwards et al., 1989). Furthermore, ectopic overexpression of neurotrophins in vivo can attract axons to inappropriate targets, leading to axonal misprojection (Edwards et al., 1989; Kawaja and Crutcher, 1997; Guidry et al., 1998). Conversely, chemorepellants of the class 3 subfamily of secreted semaphorins are among the most well characterized restrictive regulators of peripheral nerve growth. In vivo disruption of semaphorin function leads to aberrations in peripheral efferent projections, including misprojections and altered patterning of peripheral projections from doral root ganglion (DRG) neurons and disorganization of the sympathetic chain (Behar et al., 1996; Taniguchi et al., 1997). Peripheral neurons cultured in vitro also respond antagonistically to neurotrophins and semaphorins. For example, neurotrophins promote local axon growth (Campenot, 1977), act as chemoattractants (Gundersen and Barrett, 1979; Gallo et al., 1997), and promote filopodial protrusion and actin polymerization (Paves and Saarma, 1997; Gallo and Letourneau, 1998) in peripheral neurons. In contrast, semaphorins repel peripheral axons (Puschel et al., 1995; Chen et al., 1998) and collapse peripheral growth cones by depolymerizing actin (Fan et al., 1993; Renzi et al., 1999).
Because peripheral axons are simultaneously exposed to neurotrophins and semaphorins during development in vivo, antagonistic regulation of actin and other cytoskeletal components by neurotrophins and semaphorins is likely a critical determinant of growth cone behavior and axon growth in peripheral neurons. Tuttle and O'Leary (1998) demonstrated that neurotrophins can either increase or decrease the sensitivity of cultured chick sensory neurons to semaphorin-mediated collapse. However, it is not known how the growth cone integrates the antagonistic effects of these factors. Recent studies demonstrated the existence of cross-talk pathways for mediating antagonist actions of Slit and Eph proteins on chemoattractants, such as netrins and chemokines (Lu et al., 2001; Stein and Tessier-Lavigne, 2001; Wu et al., 2001). We explored whether inhibitory actions of semaphorins are mediated by antagonizing neurotrophin signaling by studying cross talk in sympathetic neurons. We report that Semaphorin 3F (Sema 3F) suppresses NGF-mediated phosphatidylinositol-3 (PI3)kinase and MEK (mitogen-activated protein kinase kinase) activation, two pathways previously known to be critical for neurotrophin regulation of cytoskeletal dynamics (Rodriguez-Viciana et al., 1997; Veeranna et al., 1998; Atwal et al., 2000; Sanchez et al., 2000) and shown here to be directly involved in maintenance of growth cone structure. We also demonstrate that suppression of NGF signaling contributes to the ability of Sema 3F to collapse growth cones in sympathetic axons.
Materials and Methods
Cell culture. Superior cervical ganglia were dissected from postnatal day (P) 1 Sprague Dawley rat pups and cultured in either mass or compartmented Campenot cultures as described previously (Atwal et al., 2000). Cells were routinely maintained in 10 ng/ml of NGF in all compartments. For adenovirus infection experiments, cells were plated in side compartments, and axons were grown outside the compartment to facilitate viral infection. Cells were infected with 100 multiplicities of infection (MOI) of virus overnight and assayed 48 hr after infection. The green fluorescent protein (GFP) and growth-associated binder 1 (Gab-1) viruses have been described previously (Korhonen et al., 1999). Sema 3F was collected from conditioned medium of COS cells transiently transfected with pSecTagmycSema 3F and concentrated using Amicon Centriprep-30 filters (Millipore, Bedford, MA). Sema 3F was used at a 1:10 dilution in assays unless otherwise noted. A 1:10 dilution resulted in a final concentration of ∼10 ng/ml of Sema 3F, as estimated by quantitating the amount of Sema 3F protein on a polyacrylamide gel relative to a known amount of protein. Sema 3F production was confirmed and normalized from batch to batch by Western blot analysis. Control cells were treated with a similarly prepared supernatant from vector-transfected COS cells. Anti-NGF from Sigma (St. Louis, MO) was diluted 1:1000. K252a (Calbiochem, La Jolla, CA), LY294002, PD98059 (Biomol, Plymouth Meeting, PA), and U0126 (Promega, Madison, WI) stock solutions were prepared in DMSO and stored at -20°C. 8-Br-cGMP and Y27632 (Calbiochem) stocks were prepared in water and stored at -20°C.
Growth cone collapse assay. Four to five days after plating, axons in Campenot cultures growing at 37°C and 5% CO2 were treated for 30 min at 37°C and 5% CO2 with experimental media and then quickly washed with PBS, fixed with 4% paraformaldehyde, and labeled with rhodaminephalloidin (Molecular Probes, Eugene, OR) to visualize growth cones. A Zeiss (Thornwood, NY) Axioscop-2 fitted with a 100× objective was used for analysis, and digital images were captured using a Sony (Tokyo, Japan) CCD videocamera and Northern Eclipse imaging software (Empix, Mississauga, Ontario, Canada). Growth cones from five to seven fields per dish were examined. Intact growth cones were scored by the presence of a flattened lamellipodium, extending at least three micrometers and containing at least two filopodial extensions. Collapsed growth cones exhibited no flattened lamellipodium. Experiments were performed in duplicate and repeated at least three times, with the exception of the 8-Br-cGMP experiment, which was repeated twice.
Immunostaining. Cells were fixed and permeabilized as described above, blocked with 1% BSA (Calbiochem) for 30 min, and incubated with primary antibodies against TrkA (tyrosine receptor kinase A) [rat TrkA (RTA); gift from L. Reichardt, University of California, San Francisco, San Francisco, CA], Npn-2 (neuropilin-2) (Chen et al., 1998), or Gab-1 (Korhonen et al., 1999) overnight at 4°C. Cells were then washed, incubated with FITC-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA) and rhodamine-phalloidin, washed again, and then mounted.
Neurotrophin inductions and biochemistry. Residual NGF was removed from cultures by washing cells with NGF-free, serum-free medium for 4 hr. Cells were then incubated for 30 min with different stimulation media, as detailed in Results. Cells were lysed as described previously (Atwal et al., 2000). Immunoprecipitation and Western blotting protocols have been described previously (Vaillant et al., 1999; Atwal et al., 2000). Antibodies used include the following: Shc (Upstate Biotechnology, Waltham, MA), PLC-γ1 (Upstate Biotechnology), panTrk (203), TrkA (RTA), phosphotyrosine (4G10; Upstate Biotechnology), phospho-(Ser473)-Akt (New England Biolabs, Beverly, MA), phospho-(Thr183/Tyr185)-ERK (Promega), Akt (New England Biolabs), and ERK1 (Santa Cruz Biotechnology, Santa Cruz, CA). Densitometry was performed using NIH Image 1.62 software.
Results
Sema 3F collapses sympathetic neuron growth cones and reduces axonal growth in the presence of NGF
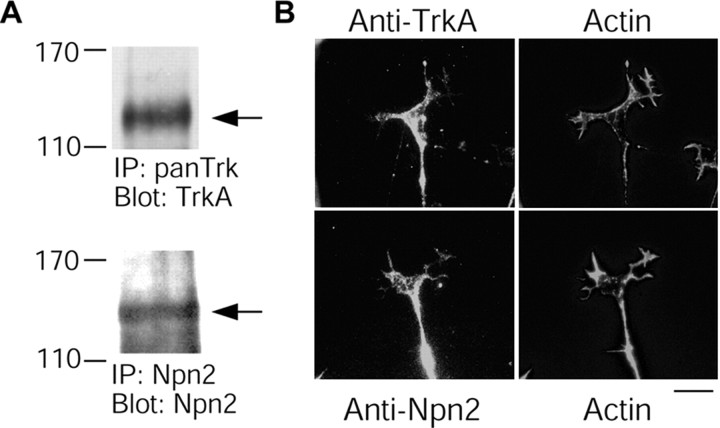
To examine potential interactions between NGF and the semaphorins, we first confirmed that cultured neonatal rat sympathetic neurons expressed the NGF receptor TrkA and the Sema 3F coreceptor Npn-2 (Chen et al., 1997; Giger et al., 1998) in axonal growth cones. Western blot analysis confirmed that both proteins were expressed (Fig. 1A). Furthermore, immunostaining combined with rhodamine-phalloidin labeling of F-actin to visualize growth cones revealed that TrkA and Npn-2 were localized to growth cones (Fig. 1B). Thirty-minute treatment with Sema3A has been shown previously to efficiently collapse chick sympathetic neurons (Renzi et al., 1999). Thus, we next confirmed that similar treatment with Sema 3F led to the collapse of rat sympathetic growth cones in the presence of NGF. Neurons were cultured in compartmented chambers (Campenot, 1977) in 10 ng/ml of NGF (Fig. 2A), conditions under which growth cones were clearly visible at the leading edge of virtually all axons growing unidirectionally in side chambers. Axons on one side were maintained in 10 ng/ml of NGF, whereas axons on the other side were treated with 10 ng/ml of NGF plus Sema 3F. Thirty minutes of Sema 3F treatment collapsed nearly all (99 ± 3%) growth cones (Fig. 2B). Consistent with previous reports (Fan et al., 1993), analysis at earlier time points revealed partial collapse (21 ± 10% at 5 min; 44 ± 8% at 15 min). Similar results were obtained when axons were grown in the presence of a saturating concentration of NGF (50 ng/ml; data not shown).
Figure 1.
Postnatal sympathetic neurons express TrkA and Npn-2 in growth cones. A, Pan-Trk or Npn-2 antibodies were used to immunoprecipitate (IP) proteins from sympathetic neuron lysates. Western blotting (Blot) was then performed to show the presence of both TrkA (anti-RTA) and Npn-2. Arrows indicate bands of the appropriate size. B, TrkA and Npn-2 expression in growth cones was examined by comparing immunoreactive regions with actin-rich growth cones, as detected by rhodamine-phalloidin costaining. Both TrkA and Npn-2 expression are found within growth cones. Scale bar, 5 μm.
Figure 2.
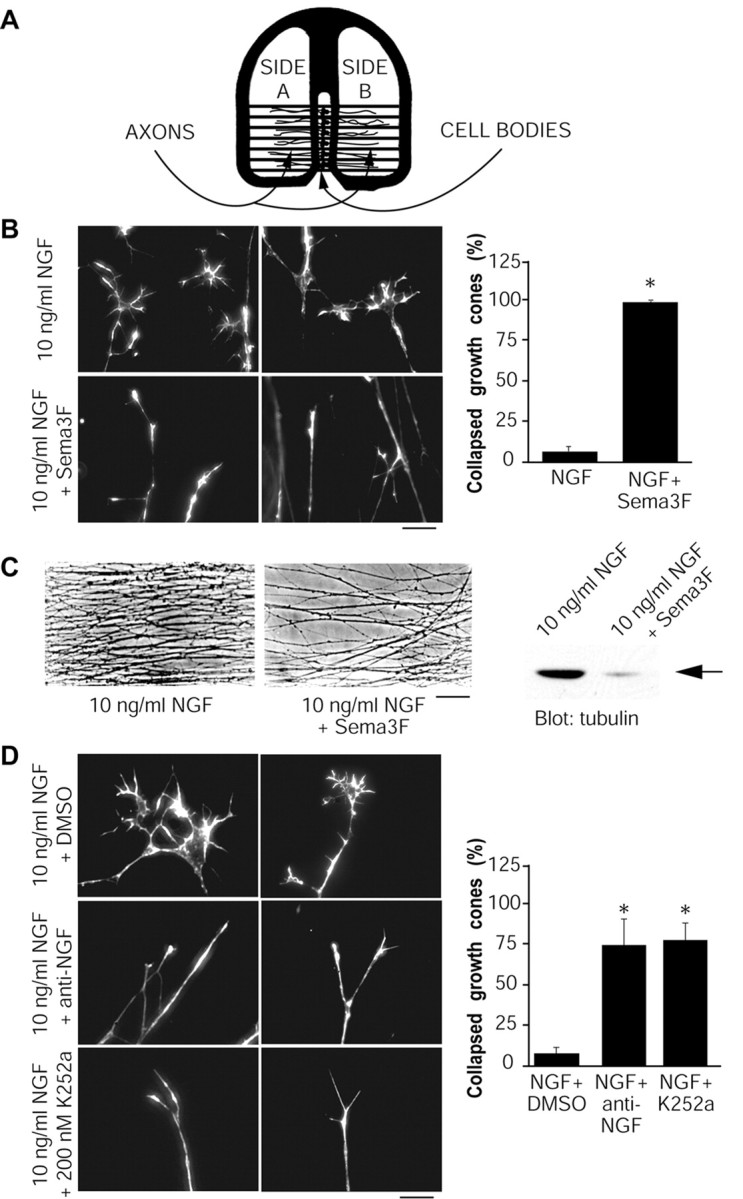
Growth cone collapse is caused by Sema 3F and inhibition of NGF-TrkA signaling. A, Schematic diagram of Campenot chambers, in which cells are plated in the center compartment, and axons grow into side compartments. In experiments described here, side compartments contained 10 ng/ml of NGF, axons were acutely treated for 30 min at 37°C under different conditions as indicated, and growth cones were analyzed by rhodamine-phalloidin staining. B, Sema 3F collapses NGF-induced growth cones. Left, Representative micrographs of rhodamine-phalloidin-stained growth cones treated with 10 ng/ml of NGF or 10 ng/ml of NGF plus Sema 3F. The graph indicates the fraction of collapsed growth cones for the two conditions (n = 4). C, Sema 3F inhibits long-term axonal growth. Left, Representative micrographs showing axonal density in compartments treated for 2 d with 10 ng/ml of NGF plus or minus Sema 3F. Right, A Western blot for total α-tubulin in compartments treated under these two conditions. The arrow indicates a band of the appropriate size. D, Inhibition of NGF or TrkA causes growth cone collapse. Photographs are representative micrographs of rhodamine-phalloidin-stained growth cones treated with anti-NGF (1:1000), K252a (200 nm), or DMSO as a control. The graph indicates the fraction of collapsed growth cones for each condition (n = 3). Scale bars: B, D, 10 μm; C, 80 μm. *p < 0.001; t test comparing treatment to NGF (B) or NGF plus DMSO (D).
To determine the effect of Sema 3F-mediated growth cone collapse on long-term axonal growth, we also performed experiments in which axons were exposed to Sema 3F continuously for 2 d in compartmented cultures. One side compartment contained NGF plus Sema 3F, whereas the other contained NGF alone; this long-term treatment had no detrimental effects on neuronal survival in the center compartment. Axonal density was greatly reduced in the continuous presence of Sema 3F, relative to axons of the same neurons growing into NGF alone in the other side compartment (Fig. 2C). Consistent with these morphological data, Western blot analysis revealed that total axonal tubulin was greatly decreased in side compartments cultured in NGF plus Sema 3F (Fig. 2C), confirming reduced axon growth. Interestingly, the axons that did grow in the presence of Sema 3F displayed elaborated growth cones after 24 hr in culture (data not shown), suggesting that at least some axons may desensitize when in the constant presence of Sema 3F. Thus, Sema 3F acutely induces sympathetic growth cone collapse in the presence of NGF and decreases long-term axonal growth.
Sema 3F antagonizes TrkA-mediated MEK and PI3-kinase activation, which are necessary for NGF to promote growth cone formation
We hypothesized that Sema 3F could collapse growth cones, at least in part by antagonizing the growth-promoting effects of NGF. Whereas local NGF stimulation is known to be required for axon growth in sympathetic neurons (Campenot, 1977), the contribution of NGF-TrkA signaling to the maintenance of growth cone integrity in these cells is not known. Thus, we first determined whether inhibition of NGF-mediated TrkA activation would itself cause growth cone collapse. Axons on one side were maintained in 10 ng/ml of NGF, whereas axons on the other side were treated with 10 ng/ml of NGF plus either a function-blocking NGF antibody or a selective inhibitor of TrkA, K252a, at concentrations that block TrkA activation in sympathetic neurons (Vaillant et al., 1999). Rhodamine-phalloidin staining after 30 min of treatment revealed that both anti-NGF and K252a collapsed ∼75% of the growth cones (Fig. 2D). Under either of these experimental conditions, Trk signaling was inhibited by ∼75%. Treatment of axons with anti-NGF for a slightly longer time (45 min) collapsed virtually all growth cones (data not shown). Thus, in sympathetic neurons, sustained activation of TrkA by NGF is required for growth cone maintenance, and inhibition of NGF-mediated TrkA activation is sufficient to cause growth cone collapse.
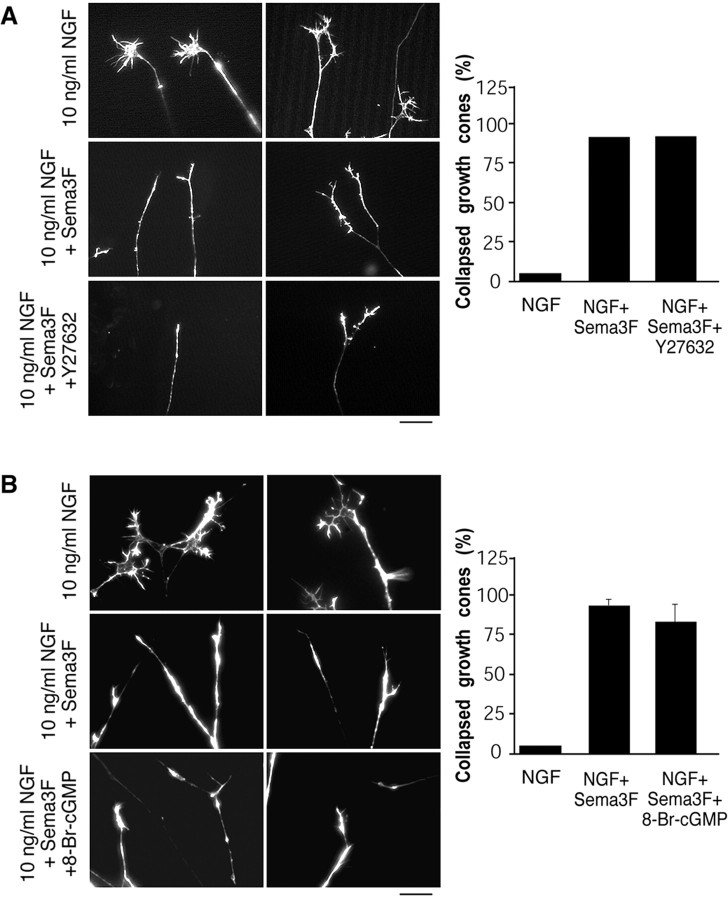
To determine how Sema 3F might antagonize TrkA-mediated growth cone formation and maintenance, we first considered two pathways that have been reported previously to be important for the effects of semaphorin, signaling via Rho-kinase (Dontchev and Letourneau, 2002) or cGMP (Song et al., 1998). Initially, to examine a potential role for Rho-kinase, we performed experiments with 10 ng/ml of NGF and Sema 3F in one side compartment and 10 ng/ml of NGF, Sema 3F, and 10 μm Y27632 (a selective Rho-kinase inhibitor) in the other. The presence of Y27632 had no effect on Sema 3F-promoted collapse (Fig. 3A), despite the fact that it promoted the growth of rat P7 cerebellar neurons both basally and on a myelin substrate (data not shown). Similarly, elevation of cGMP with 5 mm of the pharmacological agent 8-Br-cGMP, a concentration that has been shown previously to block semaphorin-mediated collapse of sensory neurons (Song et al., 1998), had no effect on sympathetic neuron growth cone collapse in response to Sema 3F (Fig. 3B). Thus, Sema 3F may be acting through a previously undescribed mechanism to antagonize NGF-promoted sympathetic neuron growth cone formation.
Figure 3.
Sema 3F-mediated collapse of sympathetic growth cones is not antagonized by inhibition of Rho-kinase or elevation of cGMP. A, B, Axonal side compartments from compartmented cultures of sympathetic neurons were treated for 30 min with 10 ng/ml of NGF, 10 ng/ml of NGF plus Sema 3F, or 10 ng/ml of NGF plus Sema 3F with either 10 μm Y26732 (A) or 5 mm 8-Br-cGMP (B). Photographs are representative micrographs of rhodamine-phalloidin-stained growth cones after the various treatments, showing that neither Y27632 nor 8-Br-cGMP could inhibit the Sema 3F-mediated collapse. The graphs indicate the fraction of collapsed growth cones for each condition (n = 3 for Y27632; n = 2 for 8-Br-cGMP). Scale bars, 10 μm.
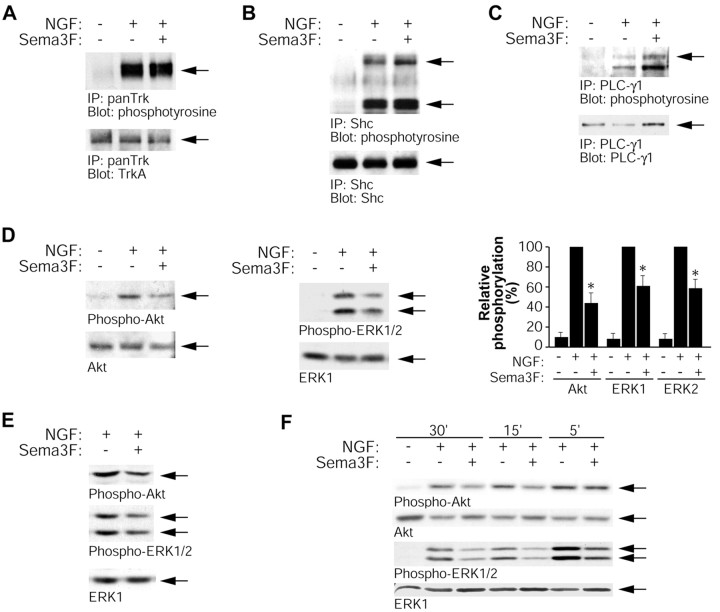
To determine whether Sema 3F-induced growth cone collapse could be explained by direct Sema 3F-dependent inhibition of TrkA signaling, we characterized TrkA signaling pathways in mass cultures of sympathetic neurons treated with 50 ng/ml of NGF in the presence or absence of Sema 3F for 30 min. The NGF-induced tyrosine phosphorylation of TrkA and two major direct TrkA substrates, Shc and PLC-γ1, was not affected by Sema 3F (Fig. 4A-C). However, Sema 3F treatment decreased the levels of activation of several well characterized proteins that act downstream of TrkA, the PI3-kinase effector Akt, and MAP kinases ERK1 and ERK2. Western blot analysis with antibodies specific to phosphorylated activated forms of Akt and ERKs revealed that Sema 3F treatment decreased the NGF-induced phosphorylation of Akt to 43 ± 11%, ERK1 to 60 ± 10%, and ERK2 to 58 ± 9% of control in five separate experiments (Fig. 4D). Decreases in Akt and ERK activity were confirmed by in vitro kinase assays of immunoprecipitated Akt or ERK1/2 (M. Boudreau, J. Atwal, and D. Kaplan, unpublished data). Similar experiments in compartmented cultures indicated that Sema 3F downregulated NGF-induced PI3-kinase and ERK signaling specifically in axons (Fig. 4E). Downregulation of ERK activation was seen as early as 5 min after Sema 3F treatment (the earliest time point examined), and peak downregulation of both Akt and ERK could be observed by 15 min (Fig. 4F). Because collapse was only partial at 15 min, this suggests that Akt and ERK downregulation precedes collapse. We also observed downregulation of NGF-dependent Akt and ERK phosphorylation but not TrkA, Shc, or PLC-γ1 phosphorylation by another semaphorin that collapses sympathetic axons (Sema3A) (data not shown).
Figure 4.
Sema 3F treatment affects downstream TrkA-signaling pathways. Mass cultures of sympathetic neurons were stimulated for 30 min with medium alone, 50 ng/ml of NGF, or 50 ng/ml of NGF plus Sema 3F, and cells were lysed and analyzed for activation of proteins in the Trk-signaling pathway. A-C, Tyrosine phosphorylation of TrkA (A), Shc (B), and PLC-γ1 (C) was assessed by immunoprecipitating with antibodies specific to each protein and probing Western blots with anti-phosphotyrosine. Arrows indicate TrkA (A), multiple Shc isoforms (B), or PLC-γ1 (C). Lower blots in each panel are reprobes of immunoprecipitated proteins. D, Total cell lysates were analyzed for Akt or ERK phosphorylation by Western blot, probing with phospho-Akt or phospho-ERK antibodies, and then reprobing for total Akt or ERK1. The graph shows the amount of Akt or ERK phosphorylation relative to the maximal phosphorylation seen with 50 ng/ml of NGF in the same experiment, averaged over five independent experiments. *p < 0.001; t test comparing NGF plus Sema 3F to NGF alone. E, Western blot analysis for phospho-Akt, phospho-ERK, or ERK1 reprobe in lysates from axons only in side compartments treated with 50 ng/ml of NGF or 50 ng/ml of NGF plus Sema 3F for 30 min. F, Time course of Sema 3F downregulation of Akt and ERK phosphorylation. Cells were washed and stimulated as described above and lysed after 5, 15, or 30 min of stimulation. Western blot analysis for phospho-Akt and phospho-ERK shows downregulation of ERK phosphorylation as early as 5 min after stimulation and downregulation of Akt phosphorylation by 15 min.
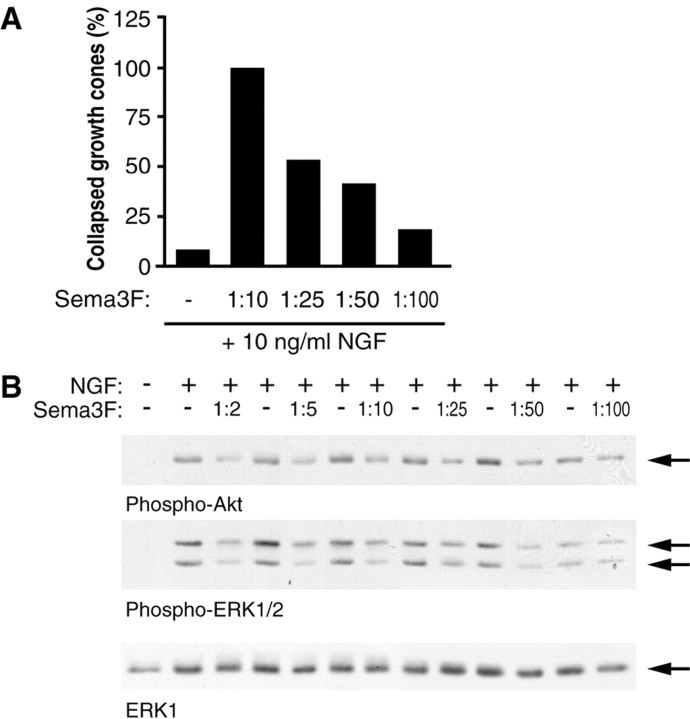
The above experiments were all performed using a 1:10 dilution of Sema 3F-conditioned medium. At this dose, growth cone collapse of sympathetic axons is complete, whereas negative regulation of PI3-kinase and ERK signaling is partial. To confirm that our observations were occurring within a dosage range of Sema 3F that is functionally relevant and to see whether we could detect greater biochemical effects at higher concentrations of Sema 3F, we performed dose-response analysis. When diluted 1:100, Sema 3F stimulated growth cone collapse in a small percentage of neurons (Fig. 5A). Collapse increased in a dose-dependent manner, reaching 100% collapse at a 1:10 dilution. Western blot analysis of mass cultures of sympathetic neurons cultured in 50 ng/ml of NGF revealed that Sema 3F-dependent downregulation of phospho-Akt and phospho-ERK signals was detectable when Sema 3F was present in as low as a 1:50 dilution, corresponding to ∼40% collapse (Fig. 5B). Interestingly, Sema 3F doses as high as a 1:2 dilution did not reduce phospho-Akt and phospho-ERK to basal levels. This suggests that Sema 3F cannot completely shut down NGF-induced activation of the PI3-kinase and ERK pathways.
Figure 5.
Dose-response curve of Sema 3F-mediated growth cone collapse and inhibition of Akt and ERK phosphorylation. A, Axons in compartmented cultures were treated with 10 ng/ml of NGF plus or minus varying dilutions of Sema 3F-conditioned medium and the percentage of collapsed growth cones quantitated by rhodamine-phalloidin staining after 30 min. B, Mass cultures of sympathetic neurons were treated with 50 ng/ml of NGF plus or minus varying dilutions of Sema 3F-conditioned medium, and Western blot analysis was used to quantitate the extent of Akt and ERK phosphorylation using phosphorylation-specific antibodies. The blots were then reprobed for total ERK1 to evaluate protein levels. Similar results were obtained in two independent experiments. Arrows indicate bands of appropriate sizes.
To determine whether the effects of Sema 3F are selective for signaling through the NGF-TrkA pathway, we tested the ability of Sema 3F to downregulate Akt and ERK when activated by a stimulus other than NGF. KCl stimulation, which promotes neuronal survival, has been shown to activate both the PI3-kinase-Akt and MEK-ERK pathways in sympathetic neurons (Vaillant et al., 1999). To perform these experiments, sympathetic neurons were washed free of neurotrophins and then switched into 50 mm KCl plus or minus Sema 3F for 30 min. Western blot analysis revealed that Sema 3F decreased KCl-mediated Akt phosphorylation but had no effect on ERK phosphorylation (Fig. 6A). To further elucidate whether or not Sema 3F could decrease PI3-kinase and ERK signaling independently of TrkA, we pretreated mass cultures in the presence or absence of Sema 3F for 25 min and then stimulated neurons with 50 ng/ml of NGF alone for 5 min. Pretreatment with Sema 3F reduced the amount of ERK activation detected after NGF stimulation but had no effect on Akt activation (Fig. 6B). Together, these data suggest that down-regulation of the PI3-kinase-Akt and ERK pathways by Sema 3F can occur independently of NGF-TrkA signaling in sympathetic neurons. In addition, the effects of Sema 3F on Akt appear to be dissociable from the effects on ERK and vice versa.
Figure 6.
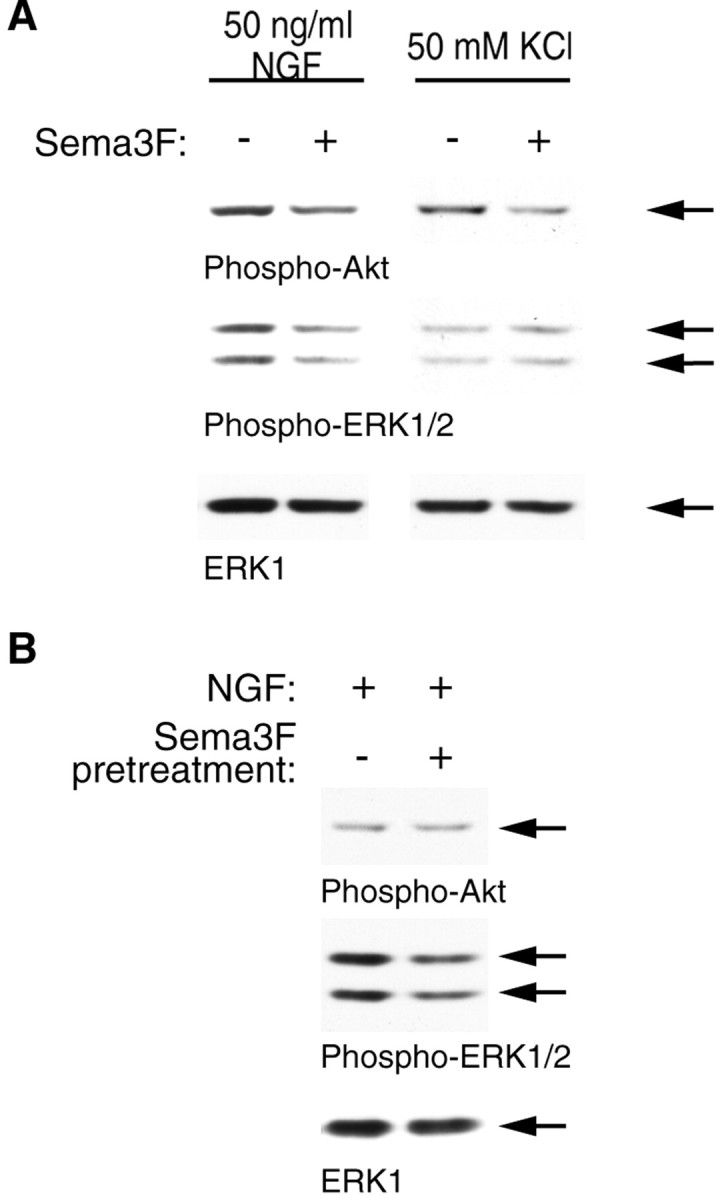
Sema 3F can inhibit downstream Akt and ERK phosphorylation in a TrkA-independent manner. A, Mass cultures of sympathetic neurons were established in NGF and then switched to 50 mm KCl plus or minus 1:10 Sema 3F for 30 min. Alternatively, mass cultures of neurons were washed free of NGF and then switched to 1:10 Sema 3F for 25 min, washed again, and stimulated with 50 ng/ml of NGF alone for 5 min. Lysates were then analyzed by Western blot analysis for phospho-Akt, phospho-ERK, or total ERK1 protein. Similar results were obtained in two independent experiments. Arrows indicate bands of appropriate sizes.
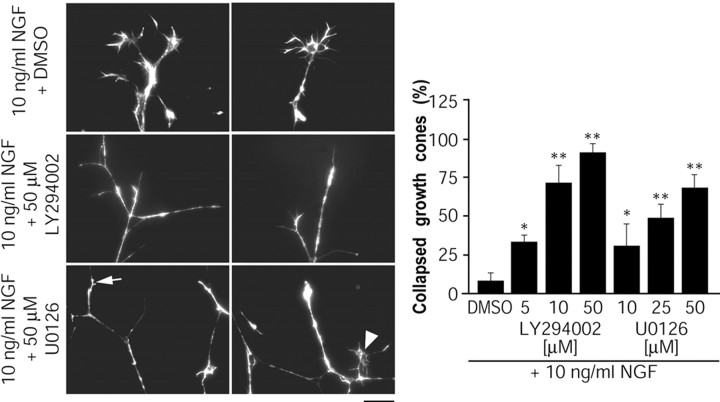
We have shown previously that PI3-kinase and MEK-ERK are essential for axon extension in response to local neurotrophin application in sympathetic neurons (Atwal et al., 2000), but the role these pathways play in growth cone formation in sympathetic neurons has not been examined. To determine whether reductions in PI3-kinase and MEK activity such as those seen during Sema 3F treatment of sympathetic neurons could directly contribute to growth cone collapse, we asked whether inhibition of PI3-kinase or MEK activity would be sufficient to cause growth cone collapse in the presence of NGF. Compartmented cultures were treated for 30 min with 10 ng/ml of NGF on one side and 10 ng/ml of NGF plus LY294002 or U0126 (selective inhibitors of PI3-kinase and MEK, respectively) on the other side. The concentrations of inhibitors used here specifically suppress PI3-kinase or MEK in sympathetic neurons grown in compartmented chambers (Atwal et al., 2000). Inhibition of PI3-kinase by LY294002 collapsed up to 90% of all growth cones in a dose-dependent manner (Fig. 7). Inhibition of MEK with U0126 collapsed growth cones, although to a lesser extent, up to 70% with 50 μm U0126 (Fig. 7). Another MEK inhibitor, PD98059, also collapsed ∼45% of growth cones (data not shown). Thus, TrkA-mediated activation of the PI3-kinase-Akt and MEK-ERK pathways is essential for NGF to promote growth cone formation, and inhibition of these pathways by Sema 3F could cause growth cone collapse.
Figure 7.
Inhibition of the PI3-kinase-Akt or MEK-ERK pathways is sufficient to induce growth cone collapse in sympathetic axons. Axons in side compartments were treated for 30 min with 10 ng/ml of NGF plus or minus LY294002 (5, 10, or 50 μm) to inhibit PI3-kinase, U0126 (10, 25, or 50 μm) to inhibit MEK, or DMSO as a control. Representative photomicrographs of rhodamine-phalloidin-stained growth cones are shown on the left. Quantification of growth cone collapse by the inhibitors is shown in the graph on the right (n = 4). *p < 0.05; **p < 0.001; t test comparing treatments to NGF plus DMSO.
Enhancement of Akt and ERK activation by overexpression of Gab-1 is sufficient to partially rescue Sema 3F-mediated growth cone collapse
These results suggest that Sema 3F can induce growth cone collapse, at least in part by its ability to dampen NGF-induced PI3-kinase and MEK signaling, which are both required for growth cone maintenance. One prediction of this model is that hyperactivation of these pathways would antagonize a Sema 3F-induced collapse. To test this prediction, we used recombinant adenovirus to overexpress Gab-1, an adaptor protein recruited by TrkA to activate both PI3-kinase and MEK (Korhonen et al., 1999), in sympathetic neurons. Gab-1 has the advantage that it increases both endogenous PI3-kinase and MEK activities to levels similar to those observed in NGF-treated cells. Moreover, immunocytochemistry for Gab-1 revealed that the overexpressed protein was distributed throughout axons of infected neurons and, importantly, was expressed well in growth cone tips where it colocalized with F-actin (Fig. 8A). Control cells infected with GFP adenovirus were also readily detected by GFP fluorescence, again present in growth cones (Fig. 8A). Western blot analysis confirmed that overexpression of Gab-1 in sympathetic neurons elevated phospho-Akt and phospho-ERK levels in response to NGF stimulation, and that Sema 3F treatment no longer repressed phospho-Akt or phospho-ERK in Gab-1-overexpressing cells (Fig. 8B).
Figure 8.
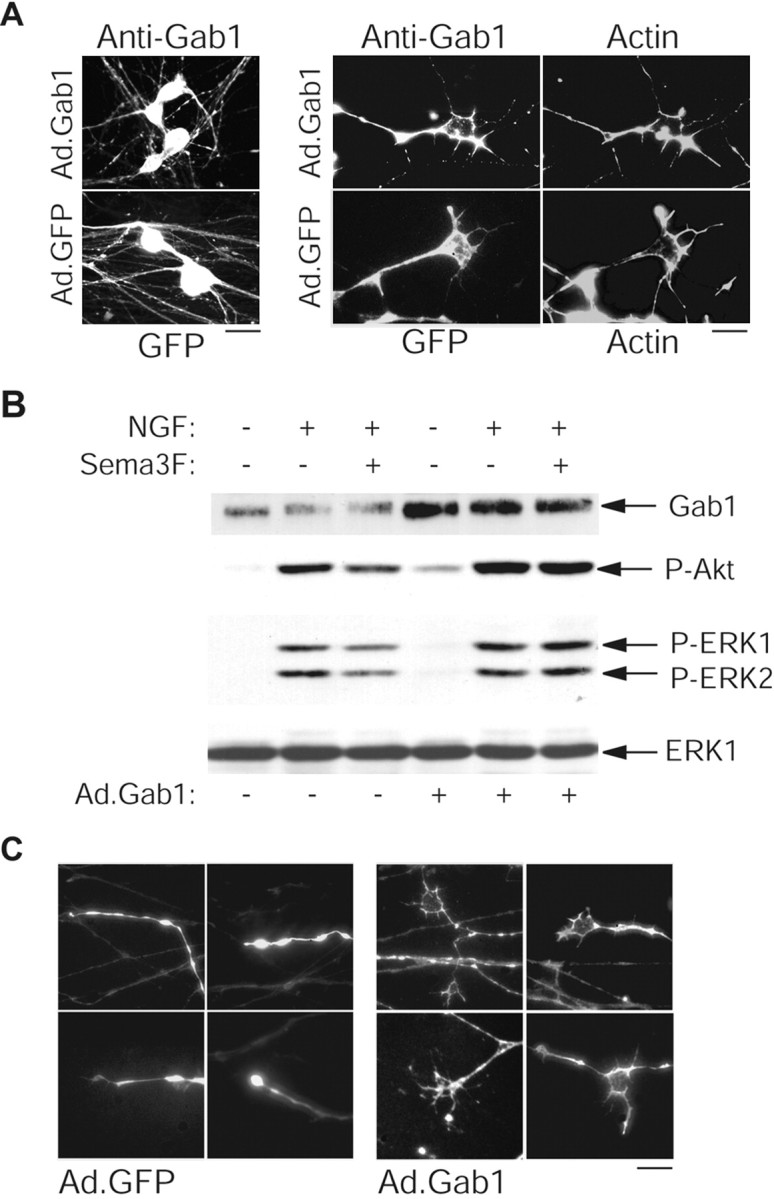
Sustained activation of the PI3-kinase-Akt and MEK-ERK pathways by overexpressing Gab-1 partially rescues Sema 3F-induced growth cone collapse. Neurons were infected with 100 MOI recombinant adenovirus expressing wild-type Gab-1 (Ad.Gab-1) or GFP (Ad.GFP) overnight and analyzed 2 d after infection. A, Photomicrographs showing that GTP expression and Gab-1 immunoreactivity were detected in both cell bodies and axons (left) and could be visualized in growth cones, as seen by costaining with rhodamine-phalloidin-labeled actin (right). Endogenous Gab-1 was below the level of detectability by immunostaining. B, Overexpression of Gab-1 enhances PI3-kinase and ERK activation. Infected neurons were stimulated as described in Figure 3, and lysates were analyzed for Gab-1, phospho-Akt, phospho-Erk, or ERK1 levels. Arrows indicate bands of appropriate sizes. C, Representative photomicrographs of growth cones from Gab-1-expressing neurons (visualized by Gab-1 immunostaining) or GFP-expressing neurons (visualized directly) that were treated with 10 ng/ml of NGF plus Sema 3F for 30 min at 48 hr after infection. Scale bars: A, 15 μm (left), 5 μm (right); C, 10 μm.
We then asked whether this biochemical rescue resulted in a rescue of Sema 3F-mediated growth cone collapse. Gab-1 overexpression enhanced axonal extension in NGF-treated compartmented cultures relative to GFP-infected neurons (data not shown), as it does in PC12 cells (Korhonen et al., 1999). When GFP-infected axons were treated for 30 min with Sema 3F, virtually all growth cones collapsed. In contrast, when Gab-1-infected axons were treated with Sema 3F, ∼32 ± 7% of axons exhibited elaborated growth cones similar to controls (Fig. 8C), whereas another 15 ± 10% displayed growth cones that were partially rescued from collapse (n = 3). Such growth cones were never observed in GFP-infected or uninfected axons in the presence of Sema 3F. We confirmed that Gab-1-overexpressing neurons expressed normal levels of Npn-2, as assessed by Western blotting (data not shown), suggesting that they should bind and recognize Sema 3F normally. Thus, a biochemical rescue of the PI3-kinase and MEK-ERK activities results in a partial morphological rescue of Sema 3F-induced growth cone collapse. We do not know whether the incompleteness of the rescue is attributable to an inability of Gab-1 overexpression to completely prevent the effect of Sema 3F on NGF signaling in all cells or whether there is an additional NGF-independent pathway for the effects of Sema 3F.
Discussion
Together, these data provide evidence for a novel mechanism used by Sema 3F to induce growth cone collapse in sympathetic neurons by suppressing NGF-dependent activation of PI3-kinase and MEK. Data presented here thus define a previously undescribed mechanism for cross talk between semaphorins and receptor tyrosine kinase-signaling pathways and identify a direct role of Trk, PI3-kinase, and MEK in maintaining growth cone structure. We propose that PI3-kinase and MEK are novel targets of semaphorin action and that Sema 3F-dependent inhibition of NGF-induced PI3-kinase and MEK activity is involved in growth cone collapse.
A number of other signaling proteins have been implicated previously in semaphorin-mediated collapse or repulsion of different neurons, including the Rho GTPase and cGMP. The collapse of growth cones by semaphorin can be reversed by inhibition of components of the Rho-signaling pathway, such as Rhokinase (Dontchev and Letourneau, 2002) or suppressors of LIM (the three gene products Lin-11, Isl-1, and Mec-3) kinase or cofilin function (Aizawa et al., 2001), or by pharmacologically increasing cGMP (Song et al., 1998). However, we did not observe a rescue of semaphorin-induced growth cone collapse by inhibiting Rho-kinase activity or increasing cGMP levels. Sympathetic neurons may use either a unique set of intracellular signaling proteins to maintain growth cones or multiple signals in addition to PI3-kinase and MEK. Our results showing that Gab-1 expression partially rescues semaphorin-mediated growth cone collapse while elevating PI3-kinase and MEK activities suggests that other signaling pathways are indeed involved. Such pathways may include Rac1 (Jin and Strittmatter, 1997), molecule interacting with CasL (Terman et al., 2002), or protein kinase A (Gallo and Letourneau, 1998).
A number of previous studies have also examined interactions between neurotrophins and semaphorins, revealing potential interactions in addition to those defined here. For example, Dontchev and Letourneau (2002) recently examined embryonic chick DRG neurons and found that pretreatment with NGF or BDNF conferred resistance against Sema 3A-mediated collapse by a mechanism involving PKA but not PI3-kinase activity. Furthermore, they found that the cGMP-dependent protein kinase (PKG) and Rho-kinase pathways contributed to Sema 3A-mediated collapse, although Arimura et al. (2000) had reported previously that inhibition of Rho-kinase did not inhibit Sema 3A-mediated collapse of the same neurons. In a second report involving chick DRG neurons, Eickholt et al. (2002) showed that activation of glycogen synthase kinase-3 (GSK-3) activity was required for semaphorin 3A-mediated growth cone collapse, but that suppression of PI3-kinase activity had no effect. One explanation for differences between these results and those reported here may simply be differences between chick sensory neurons and rat sympathetic neurons. In fact, there appear to be differences even between chick versus rat sensory neurons; inhibiting PKG in chick DRG neurons prevents collapse (Dontchev and Letourneau, 2002), whereas activating PKG by elevating cGMP levels in rat DRG neurons prevents collapse (Song et al., 1998). A second explanation derives from the fact that postnatal sympathetic neurons are dependent on NGF for local growth, whereas embryonic DRG neurons exhibit neurotrophin-independent growth. Thus, the relative importance of the different pathways that are recruited by semaphorins to cause growth cone collapse may differ as a function of the predominant growth-promoting cues encountered by the navigating axons in vivo.
It is not known precisely how PI3-kinase and MEK-ERK regulate growth cone structure. PI3-kinase has been shown to alter actin dynamics (Rodriguez-Viciana et al., 1997), nucleate new actin filaments (Derman et al., 1997; Niggli, 2000), and implicated in mediating chemotaxis in different cellular contexts (Funamoto et al., 2001). Thus PI3-kinase may target the actin network in the growth cone either directly or via the effector Rac (Yasui et al., 2001) or perhaps through GSK-3 inhibition (Eickholt et al., 2002). The ERKs can regulate microtubule function by phosphorylating microtubule-associated or neurofilament proteins (Veeranna et al., 1998; Sanchez et al., 2000), which may also lead to altered growth cone dynamics. Interestingly, EphB2-mediated neurite retraction of NG108 neuroblastoma cells has recently been shown to involve downregulation of the Ras-MEK-ERK pathway (Elowe et al., 2001), whereas netrin-mediated neurite outgrowth of commissural neurons involves ERK activation by deleted in colorectal cancer (Forcet et al., 2002). Indeed, both the PI3-kinase and MEK pathways may represent common molecular points of convergence for different growth cone-regulating factors.
Although the molecular mechanism underlying cross talk between semaphorin and neurotrophin signaling pathways is unknown, one potential component is the neuropilin-interacting protein RGS-GAIP interacting protein (GIPC) (Cai and Reed, 1999). GIPC also interacts with TrkA and, when overexpressed, decreases NGF-dependent ERK activation in PC12 cells (Lou et al., 2001). Alternatively, plexins have been reported to contain sequence homology to RasGAPs. Downregulation of Ras activity by a RasGAP could in principle explain downregulation of PI3-kinase and MEK activation by Sema 3F, although functional Ras-GAP activity remains to be demonstrated for plexin receptors. In conclusion, our results suggest a model whereby semaphorin collapses developing sympathetic axons at least in part by suppressing Trk-mediated positive growth and guidance activities. Because neurotrophin-induced regeneration of injured neurons may be counteracted by chemorepulsive signals (Pasterkamp and Verhaagen, 2001), elucidating this cross-talk pathway should provide useful entry points for therapies to assist axonal repair in the adult nervous system.
Footnotes
This work was supported by grants from the Canadian Institutes of Health Research (CIHR) to F.D.M. and D.R.K. J.K.A. was supported by a McGill Majors studentship. D.R.K. is a recipient of the Harold Johns and Canadian Cancer Society Research Scientist Award and a Canada Research chair, and F.D.M. is a CIHR senior scientist. M.T.L. is an investigator at the Howard Hughes Medical Institute. We thank S. Carbonetto, T. Kennedy, and W. Sossin for helpful discussions and H. Chen for reagents.
Correspondence should be addressed to either of the following: David R. Kaplan or Freda D. Miller, The Hospital for Sick Children, 555 University Avenue, Toronto, Ontario, Canada M5G 1X8. E-mail: dkaplan@sickkids.ca or fredam@sickkids.ca.
J. K. Atwal's present address: Department of Biological Sciences, Stanford University, Stanford, CA 94305-5020.
Copyright © 2003 Society for Neuroscience 0270-6474/03/237602-08$15.00/0
References
- Aizawa H, Wakatsuki S, Ishii A, Moriyama K, Sasaki Y, Ohashi K, Sekine-Aizawa Y, Sehara-Fujisawa A, Mizuno K, Goshima Y, Yahara I ( 2001) Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A-induced growth cone collapse. Nat Neurosci 4: 367-373. [DOI] [PubMed] [Google Scholar]
- Arimura N, Inagaki N, Chihara K, Menager C, Nakamura N, Amano M, Iwamatsu A, Goshimia Y, Kaibuchi K ( 2000) Phosphorylation of collapsin response mediator protein-2 by Rho-kinase. Evidence for two separate signaling pathways for growth cone collapse. J Biol Chem 275: 23973-27980. [DOI] [PubMed] [Google Scholar]
- Atwal JK, Massie B, Miller FD, Kaplan DR ( 2000) The TrkB-Shc site signals neuronal survival and local axon growth via MEK and P13-kinase. Neuron 27: 265-277. [DOI] [PubMed] [Google Scholar]
- Behar O, Golden JA, Mashimo H, Schoen FJ, Fishman MC ( 1996) Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature 383: 525-528. [DOI] [PubMed] [Google Scholar]
- Cai H, Reed RR ( 1999) Cloning and characterization of neuropilin-1-interacting protein: a PSD-95/Dlg/ZO-1 domain-containing protein that interacts with the cytoplasmic domain of neuropilin-1. J Neurosci 19: 6519-6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campenot RB ( 1977) Local control of neurite development by nerve growth factor. Proc Natl Acad Sci USA 74: 4516-4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Chedotal A, He Z, Goodman CS, Tessier-Lavigne M ( 1997) Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron 19: 547-559. [DOI] [PubMed] [Google Scholar]
- Chen H, He Z, Bagri A, Tessier-Lavigne M ( 1998) Semaphorin-neuropilin interactions underlying sympathetic axon responses to class III semaphorins. Neuron 21: 1283-1290. [DOI] [PubMed] [Google Scholar]
- Derman MP, Toker A, Hartwig JH, Spokes K, Falck JR, Chen CS, Cantley LC, Cantley LG ( 1997) The lipid products of phosphoinositide 3-kinase increase cell motility through protein kinase C. J Biol Chem 272: 6465-6470. [DOI] [PubMed] [Google Scholar]
- Dontchev VD, Letourneau PC ( 2002) Nerve growth factor and semaphorin 3A signaling pathways interact in regulating sensory neuronal growth cone motility. J Neurosci 22: 6659-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RH, Rutter WJ, Hanahan D ( 1989) Directed expression of NGF to pancreatic beta cells in transgenic mice leads to selective hyperinnervation of the islets. Cell 58: 161-170. [DOI] [PubMed] [Google Scholar]
- Eickolt BJ, Walsh FS, Doherty P ( 2002) An inactive pool of GSK-3 at the leading edge of growth cones is implicated in Semaphorin 3A signaling. J Cell Biol 157: 211-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elowe S, Holland SJ, Kulkarni S, Pawson T ( 2001) Downregulation of the ras-mitogen-activated protein kinase pathway by the ephb2 receptor tyrosine kinase is required for ephrin-induced neurite retraction. Mol Cell Biol 21: 7429-7441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Mansfield SG, Redmond T, Gordon-Weeks PR, Raper JA ( 1993) The organization of F-actin and microtubules in growth cones exposed to a brain-derived collapsing factor. J Cell Biol 121: 867-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcet C, Stein E, Pays L, Corset F, Llambi F, Tessier-Lavigne M, Mehlen P ( 2002) Netrin-1-mediated axon outgrowth requires deleted in colorectal cancer-dependent MAPK activation. Nature 417: 443-447. [DOI] [PubMed] [Google Scholar]
- Funamoto S, Milan K, Meili R, Firtel RA ( 2001) Role of phosphatidylinositol 3′ kinase and a downstream pleckstrin homology domain-containing protein in controlling chemotaxis in dictyostelium. J Cell Biol 153: 795-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G, Letourneau PC ( 1998) Localized sources of neurotrophins initiate axon collateral sprouting. J Neurosci 18: 5403-5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G, Lefcort FB, Letourneau PC ( 1997) The trkA receptor mediates growth cone turning toward a localized source of nerve growth factor. J Neurosci 17: 5445-5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giger RJ, Urquhart ER, Gillespie SK, Levengood DV, Ginty DD, Kolodkin AL ( 1998) Neuropilin-2 is a receptor for semaphorin IV: insight into the structural basis of receptor function and specificity. Neuron 21: 1079-1092. [DOI] [PubMed] [Google Scholar]
- Guidry G, Landis SC, Davis BM, Albers KM ( 1998) Overexpression of nerve growth factor in epidermis disrupts the distribution and properties of sympathetic innervation in footpads. J Comp Neurol 393: 231-243. [DOI] [PubMed] [Google Scholar]
- Gundersen RW, Barrett JN ( 1979) Neuronal chemotaxis: chick dorsal-root axons turn toward high concentrations of nerve growth factor. Science 206: 1079-1080. [DOI] [PubMed] [Google Scholar]
- Jin Z, Strittmatter SM ( 1997) Rac1 mediates collapsin-1-induced growth cone collapse. J Neurosci 17: 6256-6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaja MD, Crutcher KA ( 1997) Sympathetic axons invade the brains of mice overexpressing nerve growth factor. J Comp Neurol 383: 60-72. [PubMed] [Google Scholar]
- Korhonen JM, Said FA, Wong AJ, Kaplan DR ( 1999) Gab-1 mediates neurite outgrowth, DNA synthesis, and survival in PC12 cells. J Biol Chem 274: 37307-37314. [DOI] [PubMed] [Google Scholar]
- Lou X, Yano H, Lee F, Chao MV, Farquhar MG ( 2001) GIPC and GAIP form a complex with TrkA: a putative link between G protein and receptor tyrosine kinase pathways. Mol Biol Cell 12: 615-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Sun EE, Klein RS, Flanagan JG ( 2001) Ephrin-B reverse signaling is mediated by a novel PDZ-RGS protein and selectively inhibits G protein-coupled chemoattraction. Cell 105: 69-79. [DOI] [PubMed] [Google Scholar]
- Niggli V ( 2000) A membrane-permeant ester of phosphatidylinositol 3, 4, 5-trisphosphate (PIP(3)) is an activator of human neutrophil migration. FEBS Lett 473: 217-221. [DOI] [PubMed] [Google Scholar]
- Pasterkamp RJ, Verhaagen J ( 2001) Emerging roles for semaphorins in neural regeneration. Brain Res Brain Res Rev 35: 36-54. [DOI] [PubMed] [Google Scholar]
- Patel TD, Jackman A, Rice FL, Kucera J, Snider WD ( 2000) Development of sensory neurons in the absence of NGF/TrkA signaling in vivo Neuron 25: 345-357. [DOI] [PubMed] [Google Scholar]
- Paves H, Saarma M ( 1997) Neurotrophins as in vitro growth cone guidance molecules for embryonic sensory neurons. Cell Tissue Res 290: 285-297. [DOI] [PubMed] [Google Scholar]
- Puschel AW, Adams RH, Betz H ( 1995) Murine semaphorin D/collapsin is a member of a diverse gene family and creates domains inhibitory for axonal extension. Neuron 14: 941-948. [DOI] [PubMed] [Google Scholar]
- Renzi MJ, Feiner L, Koppel AM, Raper JA ( 1999) A dominant negative receptor for specific secreted semaphorins is generated by deleting an extracellular domain from neuropilin-1. J Neurosci 19: 7870-7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Warne PH, Khwaja A, Marte BM, Pappin D, Das P, Waterfield MD, Ridley A, Downward J ( 1997) Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell 89: 457-467. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Diaz-Nido J, Avila J ( 2000) Phosphorylation of microtubule-associated protein 2 (MAP2) and its relevance for the regulation of the neuronal cytoskeleton function. Prog Neurobiol 61: 133-168. [DOI] [PubMed] [Google Scholar]
- Song H, Ming G, He Z, Lehmann M, McKerracher L, Tessier-Lavigne M, Poo M ( 1998) Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science 281: 1515-1518. [DOI] [PubMed] [Google Scholar]
- Stein E, Tessier-Lavigne M ( 2001) Hierarchical organization of guidance receptors: silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science 291: 1928-1938. [DOI] [PubMed] [Google Scholar]
- Taniguchi M, Yuasa S, Fujisawa H, Naruse I, Saga S, Mishina M, Yagi T ( 1997) Disruption of semaphorin III/D gene causes severe abnormality in peripheral nerve projection. Neuron 19: 519-530. [DOI] [PubMed] [Google Scholar]
- Terman JR, Mao T, Pasterkamp RJ, Yu HH, Kolodkin AL ( 2002) MICALs, a family of conserved flavoprotein oxidoreductases, function in plexin-mediated axonal repulsion. Cell 109: 887-900. [DOI] [PubMed] [Google Scholar]
- Tuttle R, O'Leary DD ( 1998) Neurotrophins rapidly modulate growth cone response to the axon guidance molecule, collapsin-1. Mol Cell Neurosci 11: 1-8. [DOI] [PubMed] [Google Scholar]
- Vaillant AR, Mazzoni I, Tudan C, Boudreau M, Kaplan DR, Miller FD ( 1999) Depolarization and neurotrophins converge on the phosphatidylinositol 3-kinase-Akt pathway to synergistically regulate neuronal survival. J Cell Biol 146: 955-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeranna, Amin ND, Ahn NG, Jaffe H, Winters CA, Grant P, Pant HC ( 1998) Mitogen-activated protein kinases (Erk1, 2) phosphorylate Lys-Ser-Pro (KSP) repeats in neurofilament proteins NF-H and NF-M. J Neurosci 18: 4008-4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JY, Feng L, Park HT, Havlioglu N, Wen L, Tang H, Bacon KB, Jiang Z, Zhang X, Rao Y ( 2001) The neuronal repellent Slit inhibits leukocyte chemotaxis induced by chemotactic factors. Nature 410: 948-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui H, Katoh H, Yamaguchi Y, Aoki J, Fujita H, Mori K, Negishi M ( 2001) Differential responses to nerve growth factor and epidermal growth factor in neurite outgrowth of PC12 cells are determined by Rac1 activation systems. J Biol Chem 276: 15298-15305. [DOI] [PubMed] [Google Scholar]