Abstract
Glycine acts as a necessary coagonist for glutamate at the NMDA receptor (NMDAR) complex by binding to the strychnine-insensitive glycine-B binding site on the NR1 subunit. The fact that glycine is normally found in the brain and spinal cord at concentrations that exceed those required to saturate this site has led to the speculation that glycine normally saturates NMDAR-containing synapses in vivo. However, additional lines of evidence suggest that synaptic glycine may be efficiently regulated in synaptic areas by the glycine transporter type 1 (GlyT1). The recent description of a potent and selective GlyT1 inhibitor (N-[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy)propyl]sarcosine [NFPS]) provides a tool for evaluation of the hypothesis that inhibition of GlyT1 may increase synaptic glycine and thereby potentiate NMDAR function in vivo. In the present study, we found that (+)-NFPS demonstrated >10-fold greater activity in an in vitro functional glycine reuptake assay relative to the racemic compound. In vivo, (+/-)-NFPS significantly enhanced long-term potentiation in the hippocampal dentate gyrus induced by high-frequency electrical stimulation of the afferent perforant pathway. Furthermore, (+)-NFPS induced a pattern of c-Fos immunoreactivity comparable with the atypical antipsychotic clozapine and enhanced prepulse inhibition of the acoustic startle response in DBA/2J mice, a strain with low basal levels of prepulse inhibition. Collectively, these data suggest that selective inhibition of GlyT1 can enhance NMDAR-sensitive activity in vivo and also support the idea that GlyT1 may represent a novel target for developing therapeutics to treat disorders associated with NMDAR hypofunction.
Keywords: glycine, schizophrenia, c-Fos, prepulse inhibition, long-term potentiation, DBA/2J mouse, NMDA
Introduction
Glycine acts as a necessary coagonist at the NMDA receptor (NMDAR) (Johnson and Ascher, 1987; Thomson et al., 1989) by binding to a strychnine-insensitive site on the NR1 NMDAR subunit as a necessary component for subsequent glutamate binding (Laube et al., 1997). The affinity of glycine for this binding site varies from 0.1 to 3.0 μm depending on the NR2 subunit makeup of the NMDAR complex (for review, see Danysz and Parsons, 1998). Because glycine is normally found at levels between 7 and 10 μm in the CSF (Ferraro and Hare, 1985), some investigators have suggested that glycine normally saturates NMDAR-associated synaptic regions. Recent evidence, however, indicates that glycine may normally exist at nonsaturating concentrations within these areas. Thus, a high-affinity glycine transporter type 1 (GlyT1) has been described with a distribution pattern that closely overlaps NMDAR localization (Smith et al., 1992). Additional support comes from studies in brainstem slices in which glycine potentiated NMDAR function, but only at concentrations in excess of 100 μm (Berger et al., 1998). Collectively, these results suggest that GlyT1 may tightly control glycine modulation of NMDAR function in the synaptic region. On the basis of these and similar studies, it has been proposed that inhibitors of GlyT1 may be useful for disease states associated with NMDAR hypo-function (e.g., schizophrenia). Inhibitors of GlyT1 are predicted to promote NMDAR function independent of toxic effects expected after administration of direct-acting NMDAR agonists. However, inhibitors of GlyT1 could only be effective if glycine does not saturate relevant NMDAR-associated glycine binding sites in vivo. Although slice studies (Berger et al., 1998) are encouraging, the relevance of such findings to NMDAR-dependent activity in vivo remains unclear. N-[3-(4′-Fluorophenyl)-3-(4′-phenylphenoxy)propyl]sarcosine (NFPS) has been described recently as a potent and selective human GlyT1 inhibitor (Atkinson et al., 2001; Aubrey and Vandenberg, 2001). Using whole-cell patch-clamp recordings from hippocampal pyramidal neurons, Bergeron et al. (1998) demonstrated that NFPS enhanced NMDAR-mediated current in the presence of glycine levels approximating those found in vivo. Using in vivo microdialysis, Atkinson et al. (2001) demonstrated that (R)-NFPS (ALX 5407) produced a modest, albeit significant, enhancement of extracellular glycine levels in the prefrontal cortex, suggesting that even greater increases in glycine may have occurred in the synaptic region. These findings, however, do not directly address the question of whether the glycine site on the NMDAR is saturated, or the role of the glycine transporter in regulating NMDAR function in glutamatergic synapses in vivo.
Accordingly, the present studies examined the role of NFPS administration on in vivo functional activity with known sensitivity to manipulation of NMDAR systems. An initial aim of this work was to characterize racemic NFPS and its component enantiomers in vitro. A second aim was to characterize the effect of NFPS administration in vivo. Specifically, we evaluated the effect of NFPS administration on the following: (1) regional expression of the immediate early gene c-Fos, (2) in vivo long-term potentiation (LTP), and (3) prepulse inhibition of the acoustic startle response (PPI) in a DBA/2J mouse strain.
Materials and Methods
In vitro uptake assay
Materials. [14C]Glycine (112.7 mCi/mmol) was obtained from PerkinElmer Life Sciences (Emeryville, CA). All of the chemicals were purchased from Sigma (St. Louis, MO).
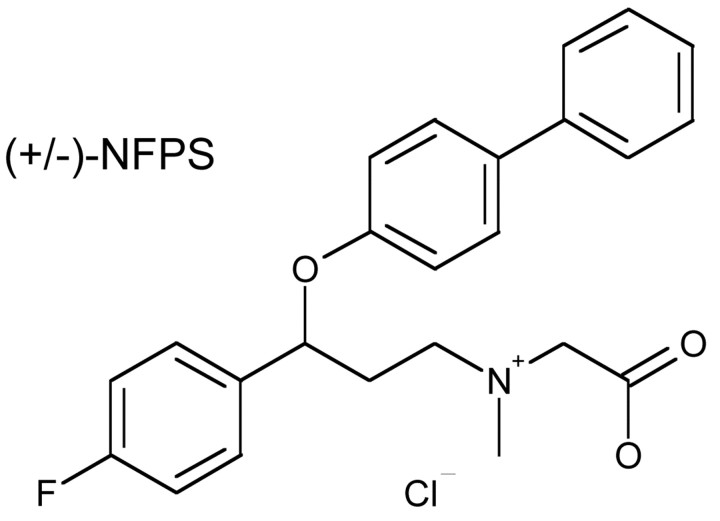
Compound synthesis. (+/-)-NFPS [(R, S)-NFPS] was synthesized at Merck Research Laboratories as a racemic mixture (chemical structure is depicted in Fig. 1). This mixture was resolved by chiral HPLC into its component enantiomers, (+)-NFPS and (-)-NFPS, respectively.
Figure 1.
Chemical structure of NFPS (N-[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy)propyl] sarcosine).
Uptake measurement. For uptake experiments, HEK-293 cells expressing rat GlyT1a or rat GlyT2 were cultured in 96-well scintillating Cytostar-T microplates (Amersham Biosciences, Arlington Heights, IL) (Mallorga et al., 2003). Culture medium was removed from the Cytostar plate, and cells were incubated with 30 μl of TB1A buffer (120 mm NaCl, 2mm KCl, 1 mm CaCl2,1mm MgCl2,10mm HEPES, 5 mm l-alanine, pH 7.5) with or without drug. Then, 30 μl of [14C]glycine diluted in TB1A was added to each well to give a final concentration of 10 μm unless otherwise specified. After incubation at room temperature for 3 hr, sealed 96-well Cytostar plates were counted on a Top Count (Packard, Meridian, CT). Nonspecific uptake of [14C]glycine was determined in the presence of 10 mm cold glycine. Uptake data represent the mean of at least triplicate determinations. Data were analyzed by nonlinear regression analysis using Prism software (Graph Pad, San Diego, CA).
c-Fos expression assay
Animals. Male Sprague Dawley rats (200-250 gm; Taconic, Germantown, NY) were housed in pairs with access to food and water ad libitum. Rats were acclimated to frequent handling before study onset to reduce handling stress to the animals during the experimental protocol. Intraperitoneal injections were performed with the following treatments: (+)-NFPS (10 mg/kg) in 20% β-cyclodextrine, pH 6-7, clozapine (20 mg/kg) in 2% lactic acid, pH 5, and control (20% β-cyclodextrine, pH 6-7). Two hours after the injection, animals were euthanized by CO2 inhalation and perfused with saline (250 ml) followed by cold, freshly prepared paraformaldehyde (4%). Brains were removed, postfixed in perfusion solution, and cryoprotected with 30-40% sucrose in phosphate buffer (0.1 m).
Immunocytochemistry. Coronal sections (40μm thick) were cut from each region of interest on a freezing microtome and collected in PBS. Sections were incubated in 10% normal donkey serum (Jackson ImmunoResearch, West Grove, PA) for 10 min, and subsequently washed with anti-c-Fos rabbit antibody (∼1 μg/ml; Santa Cruz Biotechnology, Santa Cruz, CA) diluted in PBS containing 0.1% Triton X-100 overnight at 4°C. Sections were rinsed with PBS and incubated with biotin-conjugated donkey anti-rabbit antibody (1/1000; Jackson ImmunoResearch) containing 1% normal donkey serum. Bound antibodies were detected using streptavidin conjugate Vector Elite ABC kit (Vector Laboratories, Burlingame, CA), and signal was visualized with diaminobenzidine (Sigma). Sections were dried, mounted on slides, and prepared for observation by microscope.
Counting of positive cells. Quantification of c-Fos-positive cells was performed in the prefrontal cortex, nucleus accumbens, and two regions of the striatum as reported previously (Robertson et al., 1994; Wan et al., 1995). The number of c-Fos-positive cells was computed within a 500 μm 2 surface area in each region. For each rat studied, c-Fos cells were counted in six consecutive sections of each brain region. A one-way ANOVA was performed, and if significant (p < 0.05), a Newman-Keuls multiple comparison test was carried out.
In vivo long-term potentiation
Animals. Male Sprague Dawley rats (Taconic) were used. All of the animals were allowed access to food and water ad libitum before testing. Animals were housed and tested in an Association for the Assessment and Accreditation of Laboratory Animal Care International (AAALAC)-accredited facility in strict compliance with all of the applicable regulations.
Procedure. Rats were anesthetized with 1.2-1.5 gm/kg urethane intraperitoneally (Sigma). Under urethane anesthesia, a polyethylene catheter was inserted into the jugular vein of the rats for the subsequent delivery of NFPS or vehicle (50% polyethylene glycol-20% polypropylene glycol-30% water). Rats were placed in a stereotaxic frame, and the skull was exposed. Using a steel burr and microdrill, small holes were stereotaxically placed over the site of the hippocampal dentate gyrus (anterioposterior, -4.0; lateral, +2.0; horizontal, -3.5) and the ipsilateral perforant path (anterioposterior, -7.5; lateral, +4.0; horizontal, -3.3) according to the atlas of Paxinos and Watson (1998). Electrical stimulation was delivered to the perforant path via a bipolar stimulating electrode (Rhodes Electrodes, Woodland Hills, CA) and recorded on a bipolar electrode constructed from Teflon-insulated stainless steel (A-M Systems, Carlsborg, WA). EPSP-population action potential (pop-spike) responses were evoked via a 0.1 msec electrical pulse delivered at a rate of 0.05 Hz using a Grass (West Warwick, RI) S88 stimulator and SIU5 stimulus isolation unit. Before the initiation of each experiment, an input-output relationship was established by increasing the voltage in a stepwise manner until the maximum EPSP response was obtained. The voltage required to produce ∼60% of the maximal EPSP slope was used for the remainder of the experiment. After a 30 min baseline period, the rat was injected with the vehicle or (+/-)-NFPS at a volume of 1 cc/kg. Test compound injections were infused by injection pump (Harvard Apparatus, Holliston, MA) at a rate of 0.05 ml/min. Immediately after the injection, the catheter was flushed with 0.5 cc of vehicle to ensure complete delivery of the targeted dose. A high-frequency tetanus (five trains; 80 msec in duration; 20 pulses/train; 0.1 msec/pulse; three times baseline stimulation voltage) was delivered 30 min after test compound administration to induce long-term potentiation. Recording continued for an additional 2 hr post-tetanus. EPSP slope was the primary measure used to evaluate the effect of drug treatment. Data were analyzed using repeated measures ANOVA. An effect was considered significant when p ≤ 0.05.
Prepulse inhibition
Animals. Male DBA/2J (6-8 weeks of age; The Jackson Laboratory, Bar Harbor, ME), 129S6/SvEvTac, and C57BL/6 (5-10 weeks of age; Taconic) mice were used in the present studies. All of the mice were allowed access to food and water ad libitum before testing. Mice were housed on a reverse dark/light cycle (lights off at 6:00 A.M.) and tested in an AAALAC-accredited facility in strict compliance with all of the applicable regulations.
Procedure. SR-Lab (San Diego Instruments, San Diego, CA) acoustic startle chambers were used in the present studies. SR-Lab software controlled the delivery of all of the stimuli to the animals and recorded the response. Startle amplitude was measured as the mean value during a 65 msec period beginning at the onset of the startle-eliciting stimulus. Before the first session of any day, the chambers were calibrated for both movement, using equipment provided by SR-Lab, and for sound levels, using a Tandy sound level meter. In each session, animals were randomly assigned to an experimental group, received (+)-NFPS or vehicle (25% 2-hydroxypropyl-β-cyclodextrin-75% water, pH adjusted to ∼6 using 1N NaOH) by intraperitoneal injection, and were placed in the chambers 120 min postinjection. Clozapine-treated mice received 6 mg/kg clozapine intraperitoneally 20 min before placement in the testing chambers. The choice of a 20 min pretreatment time for clozapine was derived from previously reported studies using a similar paradigm (Olivier et al., 2001), whereas a 120 min pretreatment time was selected for NFPS because of the irreversible nature of binding to the GlyT1 site (Atkinson et al., 2001). Animals were given a 5 min acclimation period during which a 65 dB background noise was continuously present. This background noise remained present throughout the entire testing session. After the acclimation period, animals received a series of five 40 msec 118-120 dB bursts of white noise to partially habituate the animals to the startle-eliciting stimulus (Davis, 1988). After these five presentations, the test session, which consisted of 10 repetitions of trials, began. Six different trial types were presented during the session. These consisted of the following: a 10 msec prepulse at 70, 75, 80, or 85 dB (i.e., 5, 10, 15, and 20 dB above background noise) followed 100 msec later by the 118-120 dB 40 msec startle pulse (prepulse pulse conditions), the startle pulse alone (pulse alone), and a period during which no stimulus was presented. Previous studies in our laboratory had determined that these prepulse intensities were insufficient to induce a significant startle response independent of the startle stimulus. The stimuli were presented in random order with interstimulus intervals averaging 15 sec. Levels of prepulse inhibition were determined by the following formula: 100-[(prepulse pulse/pulse alone) × 100], and expressed as the percentage of prepulse inhibition ± SEM. Data were analyzed using repeated-measures ANOVA with the prepulse intensity as the within-group factor followed by analyses of simple main effects and, when appropriate, post hoc analysis using the Dunnett procedure. An effect was considered statistically significant when p ≤ 0.05.
Results
In vitro characterization
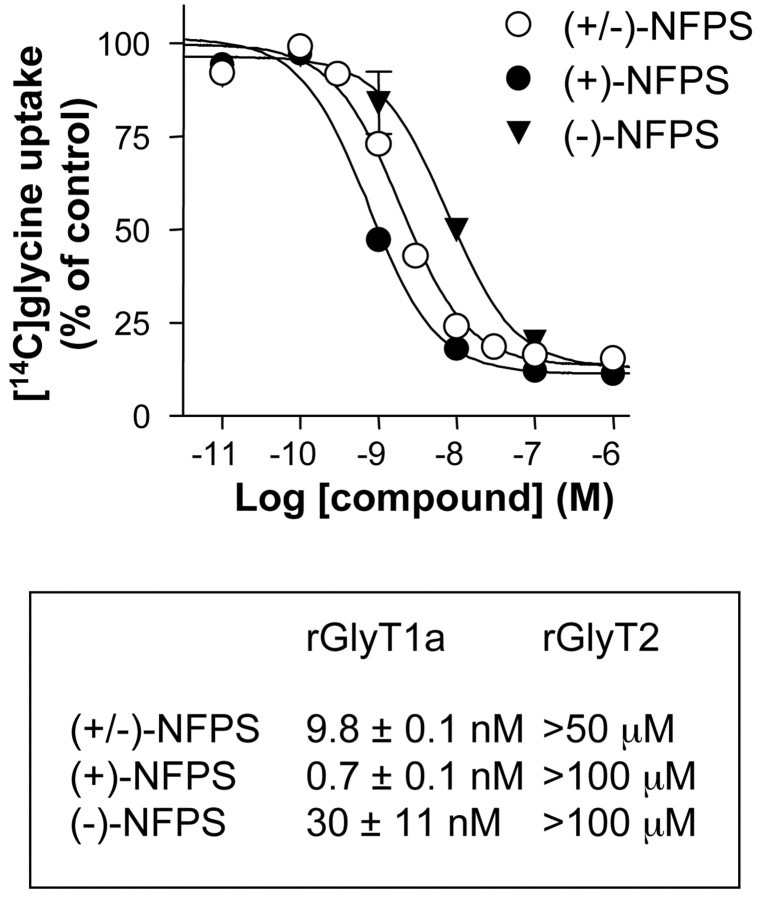
Consistent with a previous report (Atkinson et al., 2001), glycine uptake experiments performed on HEK-293 cells expressing recombinant rat GlyT1a and GlyT2 showed that (+/-)-NFPS is a potent and selective GlyT1a inhibitor. We extended this previous finding by separating the enantiomers of racemic NFPS to determine the activity of each enantiomer on glycine uptake. Interestingly, pharmacological analysis revealed that (+)-NFPS is the most potent enantiomer with a 0.7 nm IC50 and high selectivity for rGlyT1a versus rGlyT2 (Fig. 2). Both (+)-NFPS and (+/-)NFPS were used to perform the experiments reported below.
Figure 2.
Competition experiments revealed that (+/-)-NFPS and its enantiomers fully antagonized [14C]glycine (10 μm) uptake in HEK-293 cells recombinantly expressing rat GlyT1a with IC50 values shown in Table 1. Similar experiments with rat GlyT2 revealed the subtype selectivity of these compounds. Data are the mean IC50 values ± SEMs from three experiments. Nonspecific uptake was determined in the presence of 10 mm cold glycine. Error bars represent SEMs.
c-Fos expression
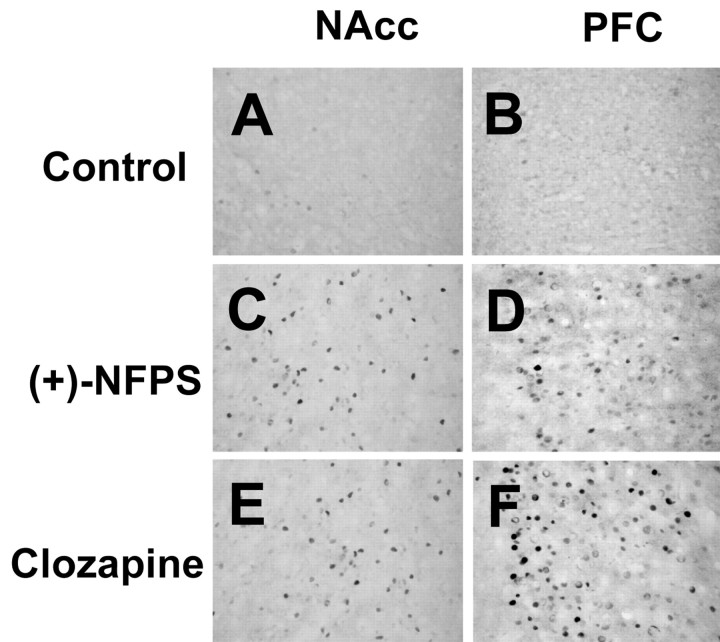
As illustrated in Figure 3, (+)-NFPS and clozapine produced a marked increase in the number of cells displaying c-Fos immunoreactivity in the nucleus accumbens. Quantitative analysis revealed a significant fourfold to fivefold increase in c-Fos cells 2 hr after (+)NFPS and clozapine treatment (Table 1). Similar threefold and sixfold increases in c-Fos in the prefrontal cortex were found in rats receiving (+)-NFPS and clozapine, respectively (Fig. 3, Table 1). In the striatum, (+)-NFPS and clozapine produced a similar and specific pattern of c-Fos expression. Neither drug induced a significant increase in c-Fos expression in cells located in the dorsolateral striatum, whereas in the medial portion of this brain structure, a significantly higher number of c-Fos-positive cells was observed after clozapine treatment (Table 1).
Figure 3.
Photomicrographs illustrating the expression of c-Fos immunoreactivity in nucleus accumbens (NAcc) (left column) and prefrontal cortex (PFC) (right column) of rats treated with vehicle (A, B), (+)-NFPS (10 mg/kg, i.p.) (C, D), and clozapine (20 mg/kg, i.p.) (E, F).
Table 1.
Summary of group data for c-Fos studies
|
|
PFC |
NAcc |
M Str |
DL Str |
|---|---|---|---|---|
| Control | 18 ± 4 | 19 ± 4 | 9 ± 1 | 8 ± 2 |
| (+)-NFPS | 52 ± 10* | 80 ± 12** | 18 ± 5 | 13 ± 2 |
| Clozapine
|
112 ± 12***
|
104 ± 10***
|
29 ± 2**
|
14 ± 1
|
PFC, Prefrontal cortex; NAcc, nucleus accumbens; M Str, medial striatum; DL Str, dorsolateral striatum.
Data are means ± SEMs from 24 sections per area and 12 sections per area for control and (+)-NFPS rats (n = 4) and clozapine rats (n = 3), respectively.
ANOVA (p < 0.05) followed by Newman-Keuls multiple comparison test. *p < 0.05; **p < 0.01; ***p < 0.001 versus control.
Long-term potentiation
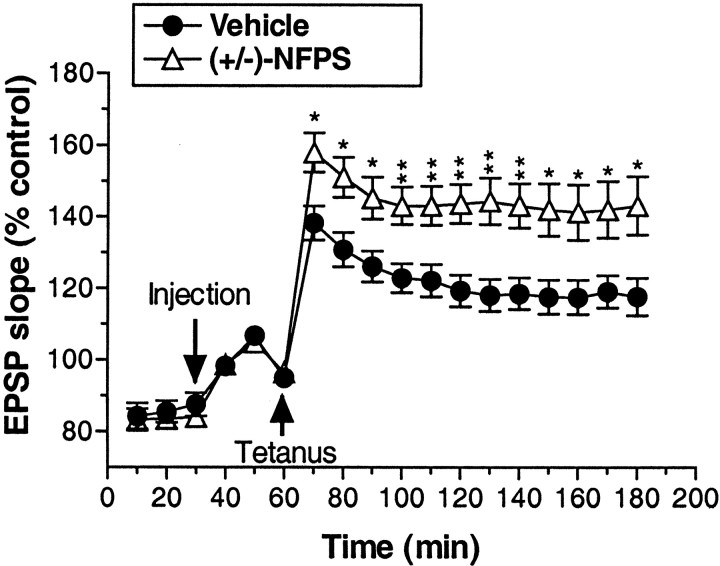
As depicted in Figure 4, application of high-frequency tetanic stimulation induced a long-lasting enhancement of dentate gyrus EPSP slope (LTP) after administration of both vehicle (∼20% increase over baseline) and (+/-)-NFPS (∼50% increase over baseline). LTP persisted throughout the 2 hr posttetanus data collection period. Treatment with (+/-)-NFPS (3 mg/kg, i.v.) significantly enhanced LTP relative to vehicle treatment as reflected by a significant main effect of treatment (F(1,18) = 8.81; p < 0.009) and a significant treatment by time interaction (F(17,306) = 6.1; p < 0.004). Additional analysis comparing only the time points before tetanus revealed a lack of basal difference between treatment groups (p > 0.61), suggesting that the overall treatment effects were not attributable to baseline differences between groups. This was further confirmed by the finding of a significant main effect of treatment (F(1,18) = 9.96; p < 0.006), but no significant treatment by time interaction (p > 0.56) when only the data from the time points after the tetanus were included in the analysis.
Figure 4.
Group data depicting the effect of (+/-)-NFPS (3 mg/kg, i.v.) administration on LTP in the hippocampal dentate gyrus produced by delivery of a high-frequency electrical stimulation of the perforant path in anesthetized rats. An injection of vehicle or (+/-)-NFPS was administered 30 min after the initiation of each experiment and 30 min before the delivery of the tetanic stimulation. After stimulation of the perforant pathway, NFPS-treated rats displayed a significantly greater magnitude of LTP that was maintained for the duration of the testing period. Data are grouped in 10 min bins, and asterisks represent a significant difference from vehicle-treated rats: *p < 0.05, **p < 0.01. Error bars represent SEMs; n = 10 per group.
Prepulse inhibition
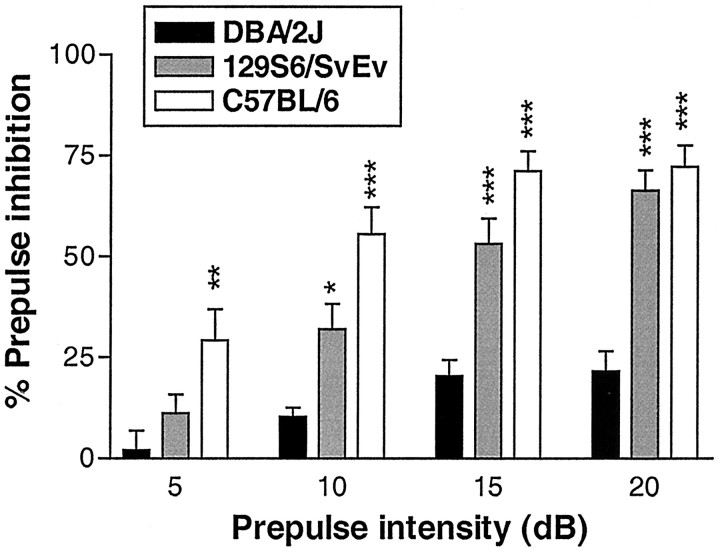
An initial study was conducted wherein DBA/2J mice were compared with two additional strains commonly used in the research environment (129S6 and C57BL/6). As depicted in Figure 5, both 129S6 and C57BL/6 mice displayed significantly higher levels of PPI across multiple prepulse intensities relative to DBA/2J mice. This was confirmed by the finding of a significant main effect of mouse strain (F(2,23) = 27.2; p < 0.001) and a significant strain × prepulse intensity interaction (F(6,69) = 5.7; p < 0.001).
Figure 5.
Group data depicting basal PPI in three mouse strains at four prepulse intensities (5-20 dB above background). DBA/2J mice showed significantly lower levels of PPI relative to the 129S6 and C57BL/6 strains. Asterisks represent a significant difference from DBA/2J mice: *p < 0.05, **p < 0.01, ***p < 0.001. Error bars represent SEMs; n = 8 per group.
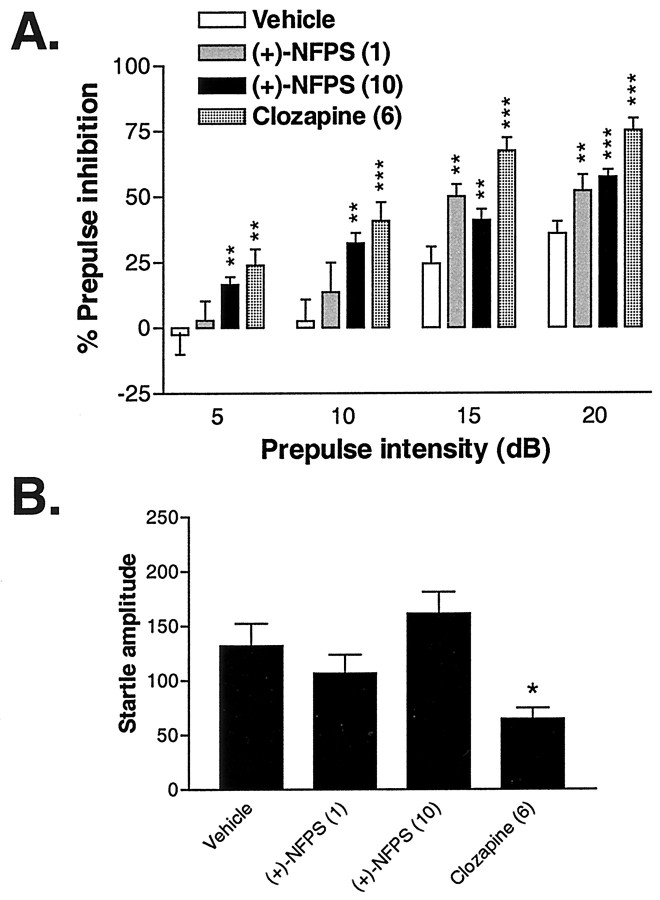
DBA/2J mice were further examined after administration of vehicle, (+)-NFPS (1 and 10 mg/kg, i.p.), and clozapine (6 mg/kg, i.p.). Although not systematically examined in the current studies, NFPS administration was generally well tolerated during the relatively short time frame of these acute studies. Thus, no enhanced mortality or overt adverse behavioral effects were noted. As depicted in Figure 6, both NFPS and clozapine enhanced PPI in this strain of mouse. Interestingly, NFPS (10 mg/kg) and clozapine were effective in enhancing PPI at all of the prepulse intensities examined, whereas the lower dose of NFPS (1 mg/kg) only enhanced PPI at the two highest prepulse intensities examined (i.e., 15 and 20 dB above background). These results were confirmed by the finding of a significant main effect of treatment (F(3,65) = 11.36; p < 0.001). Furthermore, the lack of a significant treatment × prepulse intensity interaction (p > 0.09) suggests that the effects of these treatments enhance PPI in these mice regardless of the prepulse intensity examined. It is noteworthy that the changes in PPI that occurred after NFPS treatment occurred independent of any significant change in basal startle amplitude as assessed by the response of the mice to the pulse-alone condition (Fig. 6B). In contrast, clozapine at this dose did significantly impair basal startle amplitude (overall effect, F(3,65) = 4.9; p < 0.005) (see Fig. 6 for the result of post hoc analyses).
Figure 6.
A, The effect of vehicle, two doses of NFPS (1 and 10 mg/kg, i.p.), and clozapine (6 mg/kg, i.p.) on PPI in DBA/2J mice at four prepulse intensities (5-20 dB above background). Vehicle and NFPS were administered 120 min before placement in the testing apparatus, whereas clozapine was administered 20 min before testing. Asterisks represent a significant difference from the vehicle group: **p < 0.01, ***p < 0.001. Error bars represent SEMs. B, The effect of vehicle, NFPS, and clozapine on startle amplitude during pulse-alone trials in the same mice represented in A. The asterisk represents a significant difference from the vehicle group: *p < 0.05. Error bars represent SEMs.
Discussion
The results of the present study confirm that NFPS is a potent and selective inhibitor of rat GlyT1a. The results also demonstrate that the administration of this compound in vivo produces activity consistent with those expected after potentiation of glycine-NMDAR function and further suggest an antipsychotic and/or procognitive profile of this compound in these preclinical animal studies.
The suggestion that potentiation of NMDAR function may be useful for the treatment of schizophrenia is derived from the corollary observation that NMDAR hypofunction may be critically involved in the etiology or symptoms associated with this disease. Thus, NMDAR antagonists, such as phencyclidine (PCP) and ketamine, induce psychotic states in normal human volunteers and exacerbate existing symptomatology in schizophrenic patients (Olney et al., 1999). Furthermore, increases in NMDAR density has been reported in a variety of brain regions of schizophrenic patients (Ishimaru et al., 1992, 1994). In specific regard to a role for glycine in the treatment of schizophrenia, recent genetic evidence suggests that a polymorphism in a primate-specific gene (G72) may be linked to schizophrenia in Canadian and Russian populations (Chumakov et al., 2002). Interestingly, it has been demonstrated that the protein coded by G72 positively modulates d-amino acid oxidase, which in turn metabolizes d-serine (Chumakov et al., 2002). Because d-serine is a known agonist at the NMDAR-associated glycine binding site (for review, see Hashimoto and Oka, 1997), these data raise the possibility of a primary deficiency of the NMDAR-dependent glycine system in the schizophrenic condition. Consistent with this suggestion, administration of agonists at this allosteric glycine binding site results in a significant symptomatic improvement in schizophrenic patients (Javitt et al., 1994; Goff et al., 1995; Heresco-Levy et al., 1996, 1999, 2002; Tsai et al., 1998).
The ability of NFPS to selectively enhance c-Fos immunoreactivity in the nucleus accumbens and prefrontal cortex, but not in the dorsolateral striatum, is similar to results produced by a wide variety of atypical antipsychotic drugs (Robertson and Fibiger, 1992; Robertson et al., 1994) and clozapine in this study. In addition, the atypical index of (+)-NFPS as defined by Robertson et al. (1994) is positive (+56), suggesting that (+)-NFPS might be devoid of extrapyramidal side effects that characterize typical antipsychotic drugs such as haloperidol (Robertson et al., 1994; Deutch et al., 1996). Although not reaching statistical significance, the small activation in the medial striatum by (+)NFPS paralleled the effect of clozapine and is consistent with activation of the prefrontal cortex (Robertson et al., 1994). The present results demonstrate that NFPS-induced c-Fos expression patterns resemble those of clozapine, suggesting that GlyT1 inhibitors may share an atypical antipsychotic profile. Although both typical and atypical antipsychotic-induced c-Fos expression appears to be dependent on intact NMDA receptor function (Leveque et al., 2000), the cellular and molecular mechanisms involved likely differ between NFPS and typical antipsychotics such as haloperidol. It has been proposed that haloperidol stimulates NMDA receptor activity in the striatum and induces c-Fos expression through disinhibition of protein kinase A and subsequent phosphorylation of a serine residue on the NMDAR NR1 subunit (Leveque et al., 2000). In contrast to this indirect effect on NMDAR function, the stimulating effect of (+)-NFPS on c-Fos expression may result from an increased availability of synaptic glycine and subsequent potentiation of NMDAR synaptic activity. Clozapine may have a direct, albeit mechanistically distinct, action on NMDAR function in that clozapine administration results in a release of excitatory amino acids and enhances electrically evoked EPSPs through a potentiation of NMDA receptors in the rat prefrontal cortex (Daly and Moghaddam, 1993; Arvanov et al., 1997).
It is well established that LTP in the dentate gyrus region of the hippocampus is reliant on activity-dependent NMDAR function in vivo (Morris et al., 1986; Abraham and Mason, 1988). Thus, this measure provided a means to assess the impact of increased glycine levels on synaptic NMDAR function. We hypothesized that, if glycine is normally maintained at subsaturating concentrations within these synapses in vivo, administration of the GlyT1 inhibitor NFPS should enhance LTP. Consistent with this hypothesis, we showed that NFPS administration resulted in significantly greater enhancement of LTP relative to vehicle treatment. This finding extends previous reports in vitro, in which glycine enhanced LTP (Tauck and Ashbeck 1990; Watanabe et al., 1992), to an in vivo preparation. Given the postulated role of LTP as a molecular mechanism underlying memory formation (Lynch, 1998), these results further allow for the possibility that selective inhibitors of GlyT1 may enhance learning and memory processes.
Previous published studies (Toth and Lajtha, 1986; Javitt et al., 1997, 1999) examining the behavioral effects of glycine agonists or GlyT inhibitors have been limited to the demonstration that glycine, the glycine uptake inhibitor glycyldodecylamide, and several novel glycineamide derivatives specifically inhibit PCP-but not amphetamine-induced hyperlocomotion in mice. In an effort to extend these findings using NFPS, we examined the role of GlyT1 inhibition on PPI in DBA/2J mice. PPI is a well characterized measure of sensorimotor gating that is deficient in schizophrenic patients and in animals after treatment with NMDAR antagonists (for review, see Geyer et al., 2001). The DBA/2J strain of mice displays low levels of prepulse inhibition relative to alternate strains of mice (Olivier et al., 2001) (see also Results). PPI is significantly enhanced in these mice after treatment with clozapine, risperidone, haloperidol, and raclopride (McCaughran et al., 1997; Olivier et al., 2001), leading to the suggestion that this strain of mouse may provide a model system for the examination of novel antipsychotic drug agents (Olivier et al., 2001). In the present study, we confirmed that DBA/2J mice display low levels of basal PPI relative to 129S6 and C57BL/6 mouse strains. Furthermore, we demonstrated that NFPS enhances PPI in this strain of mouse with a level of efficacy comparable with that of clozapine. Interestingly, the enhancement of PPI after administration of NFPS was observed independent of any change in baseline startle amplitude.
In summary, the present results confirm that NFPS represents a selective and potent inhibitor of GlyT1. Functional in vivo studies using c-Fos immunoreactivity, in vivo LTP, and PPI behavioral measures add additional support to the suggestion that enhancement of synaptic glycine via blockade of GlyT1 results in augmentation of NMDAR-sensitive functional activity. Collectively, these data support the suggestion that glycine is normally maintained at subsaturating concentrations synaptically and that inhibition of GlyT1 may provide a novel treatment approach for schizophrenia, psychosis, cognitive dysfunction, and related disorders.
Footnotes
Correspondence should be addressed to Dr. Gene G. Kinney, Department of Neuroscience, Merck Research Laboratories, WP46-300, P.O. Box 4, 770 Sumneytown Pike, West Point, PA 19486. E-mail: gene_kinney@merck.com.
P. J. Conn's present address: Department of Pharmacology, Vanderbilt University Medical Center, Twenty-Third Avenue South at Pierce, 452-B Preston Research Building, Nashville, TN 37232-6600.
Copyright © 2003 Society for Neuroscience 0270-6474/03/237586-06$15.00/0
G.G.K. and C.S. contributed equally to this work.
References
- Abraham WC, Mason SE ( 1988) Effects of the NMDA receptor/channel antagonist CPP and MK801 on hippocampal field potentials and long-term potentiation in anesthetized rats. Brain Res 462: 40-46. [DOI] [PubMed] [Google Scholar]
- Arvanov VL, Liang X, Schwartz J, Grossman S, Wang RY ( 1997) Clozapine and haloperidol modulate N-methyl-d-aspartate- and non-N-methyl-d-aspartate receptor-mediated neurotransmission in rat prefrontal cortical neurons in vitro J Pharmacol Exp Ther 283: 226-234. [PubMed] [Google Scholar]
- Atkinson BN, Bell SC, De Vivo M, Kowalski LR, Sechner SM, Ognyanov VI, Tham C-S, Tsai C, Jia J, Ashton D, Klitenick MA ( 2001) ALX 5407: a potent, selective inhibitor of the hGlyT1 glycine transporter. Mol Pharmacol 60: 1414-1420. [DOI] [PubMed] [Google Scholar]
- Aubrey KR, Vandenberg RJ ( 2001) N[3-(4′-Fluorophenyl)-3-(4′-phenylphenoxy)propyl]sarcosine (NFPS) is a selective persistent inhibitor of glycine transport. Br J Pharmacol 134: 1429-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger AJ, Dieudonne S, Ascher P ( 1998) Glycine uptake governs glycine site occupancy at NMDA receptors of excitatory synapses. J Neurophysiol 80: 3336-3340. [DOI] [PubMed] [Google Scholar]
- Bergeron R, Meyer TM, Coyle JT, Greene RW ( 1998) Modulation of N-methyl-d-aspartate receptor function by glycine transport. Proc Natl Acad Sci USA 95: 15730-15734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumakov I, Blumenfeld M, Guerassimenko O, Cavarec L, Palicio M, Abderrahim H, Bougueleret L, Barry C, Tanaka H, La Rosa P, Puech A, Tahri N, Cohen-Akenine A, Delabrosse S, Lissarrague S, Picard F-P, Maurice K, Essioux L, Millasseau P, Grel P, et al. ( 2002) Genetic and physiological data implicating the new human gene G72 and the gene for d-amino acid oxidase in schizophrenia. Proc Natl Acad Sci USA 99: 13675-13680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly DA, Moghaddam B ( 1993) Actions of haloperidol on the extracellular levels of excitatory amino acids in the prefrontal cortex and striatum of conscious rats. Neurosci Lett 152: 61-64. [DOI] [PubMed] [Google Scholar]
- Danysz W, Parsons AC ( 1998) Glycine and N-methyl-d-aspartate receptors: physiological significance and possible therapeutic applications. Pharmacol Rev 50: 597-664. [PubMed] [Google Scholar]
- Davis M ( 1988) Apomorphine, d-amphetamine, strychnine and yohimbine do not alter prepulse inhibition of the acoustic startle reflex. Psychopharmacology (Berl) 95: 151-156. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Lewis DA, Whitehead RE, Elsworth JD, Iadarola MJ, Redmond DE, Roth RH ( 1996) Effects of D2 dopamine receptor antagonists on Fos protein expression in the striatal complex and entorhinal cortex of the nonhuman primate. Synapse 23: 182-191. [DOI] [PubMed] [Google Scholar]
- Ferraro TN, Hare TA ( 1985) Free and conjugated amino acids in human CSF: influence of age and sex. Brain Res 572: 154-163. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR ( 2001) Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 156: 117-154. [DOI] [PubMed] [Google Scholar]
- Goff DC, Tsai G, Manoach DS, Coyle JT ( 1995) Dose-finding trial of d-cycloserine added to neuroleptics for negative symptoms in schizophrenia. Am J Psychiatry 152: 1213-1215. [DOI] [PubMed] [Google Scholar]
- Hashimoto A, Oka T ( 1997) Free d-aspartate and d-serine in the mammalian brain and periphery. Prog Neurobiol 52: 325-353. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Javitt D, Ermilov M, Mordel C, Horowitz A, Kelly D ( 1996) Double-blind, placebo-controlled, crossover trial of glycine adjuvant therapy for treatment-resistant schizophrenia. Br J Psychiatry 169: 610-617. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Javitt D, Ermilov M, Mordel C, Silipo G, Lichenstein M ( 1999) Efficacy of high-dose glycine in the treatment of enduring negative symptoms of schizophrenia. Arch Gen Psychiatry 56: 29-36. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Ermilov M, Shimoni J, Shapira B, Silipo G, Javitt DC ( 2002) Placebo-controlled trial of d-cycloserine added to conventional neuroleptics, olanzapine, or risperidone in schizophrenia. Am J Psychiatry 159: 480-482. [DOI] [PubMed] [Google Scholar]
- Ishimaru M, Kurumaji A, Toru M ( 1992) NMDA-associated glycine binding site increases in schizophrenic brains. Biol Psychiatry 32: 379-381. [DOI] [PubMed] [Google Scholar]
- Ishimaru M, Kurumaji A, Toru M ( 1994) Increases in strychnine-insensitive glycine binding sites in cerebral cortex of chronic schizophrenics: evidence for glutamate hypothesis. Biol Psychiatry 35: 84-95. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zylberman I, Zukin SR, Heresco-Levy U, Lindenmayer JP ( 1994) Amelioration of negative symptoms in schizophrenia by glycine. Am J Psychiatry 151: 1234-1236. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Sershen H, Hashim A, Lajtha A ( 1997) Reversal of phencyclidine-induced hyperactivity by glycine and the glycine uptake inhibitor glycyldodecylamide. Neuropsychopharmacology 17: 202-204. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Balla A, Sershen H, Lajtha A ( 1999) Reversal of phencyclidine-induced effects by glycine and glycine transport inhibitors. Biol Psychiatry 45: 668-679. [DOI] [PubMed] [Google Scholar]
- Johnson JW, Ascher P ( 1987) Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature 325: 529-531. [DOI] [PubMed] [Google Scholar]
- Laube B, Hirai H, Sturgess M, Betz H, Kuhse J ( 1997) Molecular determinants of agonist discrimination by NMDA receptor subunits: analysis of the glutamate binding site on the NR2B subunit. Neuron 18: 493-503. [DOI] [PubMed] [Google Scholar]
- Leveque JC, Macias W, Rajadhyaksha A, Carlson RR, Barczak A, Kang S, Li XM, Coyle JT, Huganir RL, Heckers S, Konradi C ( 2000) Intracellular modulation of NMDA receptor function by antipsychotic drugs. J Neurosci 20: 4011-4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G ( 1998) Memory and the brain: unexpected chemistries and a new pharmacology. Neurobiol Learn Mem 70: 82-100. [DOI] [PubMed] [Google Scholar]
- Mallorga PJ, Williams JB, Jacobson M, Marques R, Chaudary A, Conn J, Pettibone D, Sur C 2003. Pharmacology and expression analysis of glycine transporter GlyT1 with [3H]-(N-[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy)propyl])sarcosine. Neuropharmacology, in press. [DOI] [PubMed]
- McCaughran J, Mahjubi E, Decena E, Hitzemann R ( 1997) Genetics, haloperidol-induced catalepsy and haloperidol-induced changes in acoustic startle and prepulse inhibition. Psychopharmacology (Berl) 134: 131-139. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Anderson E, Lynch G, Baudry M ( 1986) Selective impairment of learning and blockade of LTP by NMDA receptor antagonist, AP5. Nature 319: 774-776. [DOI] [PubMed] [Google Scholar]
- Olivier B, Leahy C, Mullen T, Paylor R, Groppi VE, Sarnyai Z, Brunner D ( 2001) The DBA/2J strain and prepulse inhibition of startle: a model system to test antipsychotics? Psychopharmacology (Berl) 156: 284-290. [DOI] [PubMed] [Google Scholar]
- Olney JW, Newcomer JW, Farber NB ( 1999) NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res 33: 523-533. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C ( 1998) The rat brain in stereotaxic coordinates, Ed 4. San Diego: Academic.
- Robertson GS, Fibiger HC ( 1992) Neuroleptics increase c-fos expression in the forebrain: contrasting effects of haloperidol and clozapine. Neuroscience 46: 315-328. [DOI] [PubMed] [Google Scholar]
- Robertson GS, Matsumura H, Fibiger HC ( 1994) Induction patterns of Fos-like immunoreactivity in the forebrain as predictors of atypical antipsychotic activity. J Pharmacol Exp Ther 271: 1058-1066. [PubMed] [Google Scholar]
- Smith KE, Borden LA, Hartig PR, Branchek T, Weinshank RL ( 1992) Cloning and expression of a glycine transporter reveal colocalization with NMDA receptors. Neuron 8: 927-935. [DOI] [PubMed] [Google Scholar]
- Tauck DL, Ashbeck GA ( 1990) Glycine synergistically potentiates the enhancement of LTP induced by a sulfhydryl reducing agent. Brain Res 519: 129-132. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Walker VE, Flynn DM ( 1989) Glycine enhances NMDA-receptor mediated synaptic potentials in neocortical slices. Nature 338: 422-424. [DOI] [PubMed] [Google Scholar]
- Toth E, Lajtha A ( 1986) Antagonism of phencyclidine-induced hyperactivity by glycine in mice. Neurochem Res 11: 393-400. [DOI] [PubMed] [Google Scholar]
- Tsai G, Yang P, Chung L-C, Lange N, Coyle J ( 1998) d-Serine added to antipsychotics for the treatment of schizophrenia. Biol Psychiatry 44: 1081-1089. [DOI] [PubMed] [Google Scholar]
- Wan W, Ennulat DJ, Cohen BM ( 1995) Acute administration of typical and atypical antipsychotic drugs induces distinctive patterns of Fos expression in the rat forebrain. Brain Res 688: 95-104. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Saito H, Abe K ( 1992) Effects of glycine and structurally related amino acids on generation of long-term potentiation in rat hippocampal slices. Eur J Pharmacol 223: 179-184. [DOI] [PubMed] [Google Scholar]