Abstract
Relay neurons of the lateral geniculate nucleus innervate visual cortex, but they also provide axonal collaterals to neurons in the thalamic reticular nucleus, and these thalamic reticular neurons provide feedback inhibition to relay cells. An alternative source of inhibitory inputs onto geniculate relay neurons arises from intralaminar interneurons that provide feedforward inhibition via retinogeniculate innervation, and perhaps feedback inhibition via the corticothalamic pathway, analogous to that involving thalamic reticular neurons. Several reports indicate that relay neurons may also give rise to axonal collaterals within the lateral geniculate nucleus, constituting another route for feedback or local integration. We now provide new data indicating that collaterals from geniculate relay neurons provide excitatory input to local intralaminar interneurons and that this pathway may serve as a previously unknown means of local feedback inhibition. This circuitry could prove important in such activities as surround inhibition of receptive fields or increasing signal gain over noise.
Keywords: lateral geniculate nucleus, thalamus, inhibition, axon collaterals, relay neurons, interneurons
Introduction
Thalamic relay cells project to cortex, but en route they provide collateral innervation within the thalamus. One clear example is the collateral innervation of the GABAergic neurons in the thalamic reticular nucleus. These thalamic reticular neurons primarily innervate relay neurons and thereby provide a route of feedback inhibition (Scheibel and Scheibel, 1966; Jones, 1985; Yen et al., 1985b; Sherman and Guillery, 2001).
In addition to collaterals within the thalamic reticular nucleus, relay neurons of the lateral geniculate nucleus also give rise to local collaterals within the main geniculate laminae (Ferster and LeVay, 1978; Friedlander et al., 1981; Stanford et al., 1983). The targets of these intrageniculate collaterals are unknown. Plausible candidates are other relay cells or local interneurons, of which two types exist: one is located within the main laminae (intralaminar interneurons), and the other is located in the zones between laminae (interlaminar interneurons) (Jones, 1985; Montero, 1989). Interlaminar interneurons are thought to be displaced cells of the thalamic reticular nucleus, thus receiving input from relay cell collaterals and providing feedback inhibition to relay cells (Sanchez-Vives et al., 1996). In contrast, intralaminar interneurons are innervated by retinal axons and are, thus, thought to be involved mainly in feedforward inhibition to relay cells. However, we provide new evidence that the intrageniculate collaterals of relay cells provide synaptic input onto these intralaminar interneurons and that this pathway provides another, previously unappreciated, route for inhibitory feedback onto the relay neurons.
Materials and Methods
Thalamic slices were obtained from young cats (4-8 weeks of age), using methods similar to those described previously (Cox et al., 1998; Cox and Sherman, 2000). Briefly, cats were deeply anesthetized with ketamine (25 mg/kg) and xylazine (1 mg/kg), a craniotomy was made, and a block of tissue containing the lateral geniculate nucleus was quickly removed and placed into cold, oxygenated slicing medium (∼4°C) containing (in mm): 2.5 KCl, 1.25 NaH2PO4, 7.0 MgCl2, 0.5 CaCl2, 25.0 NaHCO3, 25.0 glucose, 110.0 choline chloride, 11.6 ascorbic acid, and 3.1 pyruvic acid. Tissue slices (300-400 μm) were typically cut in a coronal plane, transferred to a holding chamber containing oxygenated physiological saline maintained at 30°C, and incubated for at least 2 hr before recording. The physiological saline contained (in mm): 126.0 NaCl, 2.5 KCl, 1.25 NaH2PO4, 2.0 MgCl2, 2.0 CaCl2, 26.0 NaHCO3, and 10.0 glucose. These solutions were gassed with 95% O2/5% CO2 to a final pH of 7.4. Individual slices were then transferred to a submersion-type recording chamber on a modified microscope stage, maintained at 30 ± 1°C, and superfused with oxygenated physiological solution at a rate of 3-4 ml/min.
Whole-cell recordings were obtained using a visualized slice preparation, as described previously (Edwards et al., 1989; Stuart et al., 1993). Recording pipettes were pulled from 1.5 mm outer diameter capillary tubing with tip resistances of 4-6 MΩ when filled with the intracellular solution containing (in mm): 117 K-gluconate, 13 KCl, 1.0 MgCl2, 0.07 CaCl2, 0.1 EGTA, 10.0 HEPES, 2.0 Na2-ATP, 0.4 Na-GTP, and 0.5% biocytin. In some recordings, Cs + was substituted for K+ in the intracellular solution. The pH of the solution was adjusted to 7.3 using KOH (or CsOH), and osmolality was adjusted to 290 mOsm. The use of this intracellular solution results in a ∼10 mV junction potential that is corrected in the voltage measures. After the recording, slices were placed in 4% paraformaldehyde for overnight fixation, and standard protocols were used to reveal the biocytin and assess the axon collaterals of labeled cells with the light microscope (Horikawa and Armstrong, 1988; Cox and Sherman, 2000).
Concentrated stock solution of (±)-1-aminocyclopentane-trans-1,3-dicarboxylic acid (ACPD) was prepared in 0.1 m NaOH and diluted in physiological solution to a final concentration of 100-500μm. ACPD was applied to the tissue by a bolus injection (20-60 sec duration) into the input line of the chamber using a motorized syringe pump, or in a few noted cases, bath applied. On the basis of the rate of agonist injection into the input line and the chamber perfusion rate, the final bath concentration of ACPD agents was estimated to be about one-fourth of the initial concentration in the injection line. All antagonists were diluted to final concentration in physiological solution just before use and bath applied. All excitatory amino acid agonists and antagonists were purchased from Tocris Cookson (St. Louis, MO), and remaining chemicals were purchased from Sigma (St. Louis, MO).
Results
Our analyses are based on intracellular recordings from intralaminar interneurons and a subset of relay neurons for which we were able to identify a well stained axon that projected hundreds of micrometers toward or into the thalamic reticular nucleus. In 13 of 24 relay cells, we found intrageniculate collaterals apparently innervating lamina A or A1 of the lateral geniculate nucleus. Figure 1 shows two examples of such intrageniculate axon collaterals. These collaterals were exceedingly thin, had few branches, and we could not follow them over long distances (<50 μm). However, the delicate nature of these collaterals made them particularly difficult to find (and, perhaps, to label), so it is not clear whether or not the failure to find such collaterals for all relay cells represents a false negative. Such intrageniculate collaterals have been described previously from other preparations (Friedlander et al., 1981; Stanford et al., 1983).
Figure 1.
Intrageniculate axonal collaterals arising from lateral geniculate nucleus relay neurons. Ai, A digital image of a biocytin-filled lateral geniculate nucleus relay neuron with associated axon (arrow). Aii, Higher magnification of outlined area in Ai shows that the main axon gives rise to axon collateral (arrowhead). B, Different relay neuron with main axon that gives rise to axon collaterals in lateral geniculate nucleus. Bi, Low-power photomicrograph of relay neuron and its main axon (arrow). Bii, Increased magnification of main axon with two identified axon collaterals (outlined areas). Biii,Biv, Details of the two collaterals (arrowheads) outlined in Bii.
To investigate whether these intrageniculate axon collaterals innervated intralaminar interneurons, we took advantage of the differential actions of metabotropic glutamate receptor (mGluR) agonists on relay cells and interneurons. The mGluR agonist (ACPD) strongly depolarizes thalamic relay neurons, often producing action potential discharge by suppressing a potassium conductance (McCormick and von Krosigk, 1992; Cox et al., 1998; Cox and Sherman, 1999), but has little or no direct effect on the membrane potential of interneurons, as indicated from somatic recordings (Pape and Mc-Cormick, 1995; Cox et al., 1998). The interneurons from which recordings were made were identified as intralaminar on the basis of their location during recording (i.e., within a laminae rather than inter-laminar), and this was verified by locating a subpopulation of 14 of these cells after biocytin labeling (Fig. 2). Every one of these cells had morphological characteristics of intralaminar interneurons, including small somata; long, richly branched, axoniform dendrites with terminal boutons; and a dendritic arbor oriented perpendicular to laminar borders (Guillery, 1966; Hamos et al., 1985; Pape and Mc-Cormick, 1995). These cells were also physiologically identified by their robust depolarizing sag in response to hyperpolarizing current pulses, which seems to result from evoking the hyperpolarization-activated cation conductance (Ih) and the apparent lack of a low threshold calcium spike (Fig. 3A). These interneurons had an average resting membrane potential of -65 ± 5 (SD) mV (n = 21) and an apparent input resistance that averaged 224 ± 101 MΩ.
Figure 2.
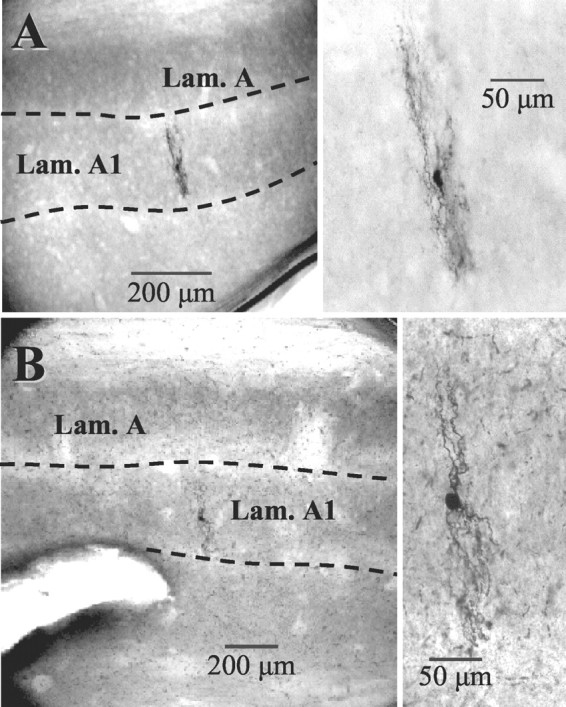
Intralaminar interneurons from lateral geniculate nucleus. A, B, Left panels show location of two interneurons studied in the geniculate lamina. The dendritic trees of the cells were always perpendicular to the long axis of lamina A1 (Lam. A1). A, B, Right panels show higher magnification of interneurons. Note the small soma size and complicated dendritic architecture typical for this class of neurons.
Figure 3.
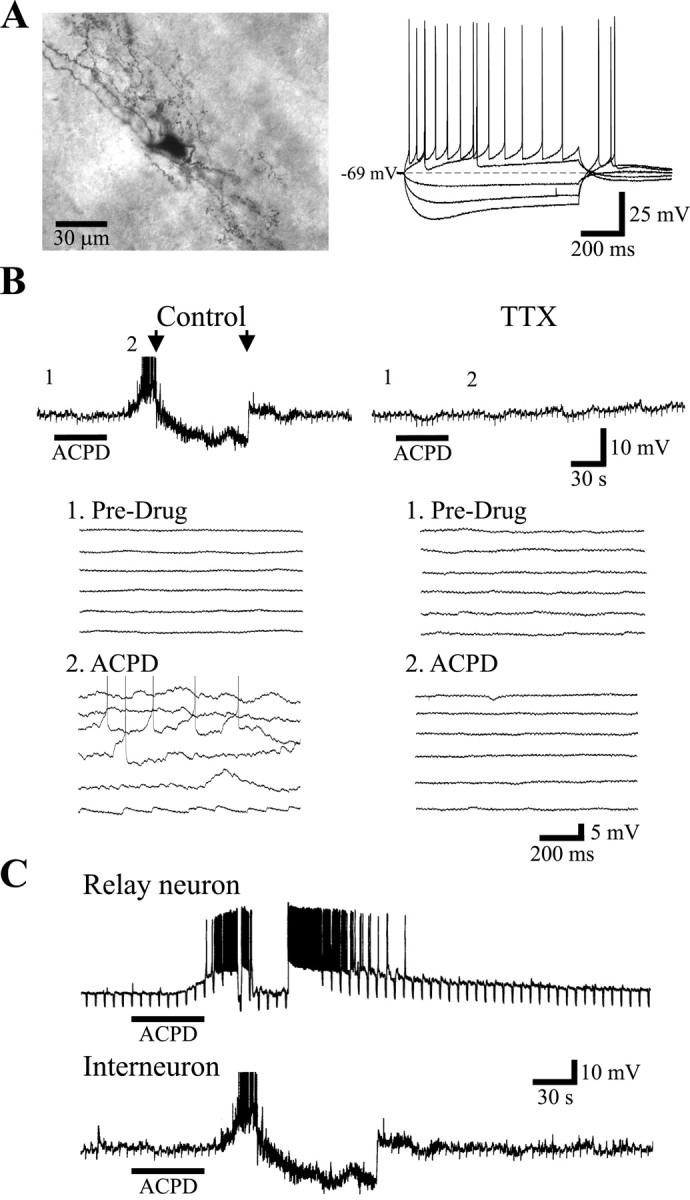
ACPD increases synaptic activity in a TTX-dependent manner. A, Digital image of typical lateral geniculate nucleus interneuron. Note the extensive dendritic architecture and thin axon-like dendrites. Right, Intracellular recording from neuron on left indicating membrane response to current injection. Note the depolarizing sag in response to hyperpolarizing current pulses, as well as the lack of burst discharge after offset of the hyperpolarizing current steps. Depolarizing current only evokes tonic action potential discharge. B, The mGluR agonist ACPD (125 μm) produces a robust increase in spontaneous baseline activity. Bottom, Same recording at a faster time base. In control conditions, there is little spontaneous activity (1); however, after ACPD application, there is a robust increase in spontaneous depolarizations (2). Right, After bath application of TTX (1μm), ACPD no longer produces any obvious changes in baseline activity. Note the lack of apparent effect at the faster time base (1 vs 2). C, In a current-clamp recording from a relay neuron, ACPD produces a robust depolarization that evokes action potential discharge (spikes truncated). The repolarization of the membrane potential during spike discharge is because of a manual clamping of the membrane potential to resting level. The time course of action potential discharge is similar to that of the increased activity recorded in interneurons (cf. interneuron recording from B at same time calibration).
As reported previously (Pape and McCormick, 1995; Cox et al., 1998), ACPD produced little change in the membrane potential of these intralaminar interneurons, as seen from somatic recordings. However, we observed a robust increase in the spontaneous baseline activity in all but one (20 of 21) of these neurons (Fig. 3B). Closer examination revealed that the agonist produced an apparent increase in spontaneous depolarizing potentials (Fig. 3B1 vs 3B2). In neurons with relatively depolarized membrane potentials, the robust increase in short depolarizing potentials often evoked action potential discharge (Figs. 3B,4A). In recordings from relay neurons, similar ACPD application resulted in a strong depolarization that could produce action potential discharge (n = 25; Fig. 3C) (Cox and Sherman, 1999). Assuming the increase in depolarizing potentials in the interneurons may be because of suprathreshold excitation of excitatory neurons that innervate the recorded cell, we next applied the voltage-dependent Na+-channel blocker TTX to eliminate action potentials in the presynaptic cells. In TTX, ACPD did not produce an increase in spontaneous activity in any of the 12 cells tested (Fig. 3B). These data suggest that the increase in the short depolarizing potentials is, indeed, a result of excitation of neurons that synaptically innervate the recorded interneurons and this is not because of an action on presynaptic terminals of excitatory neurons or a direct effect of ACPD on the recorded cells.
Figure 4.
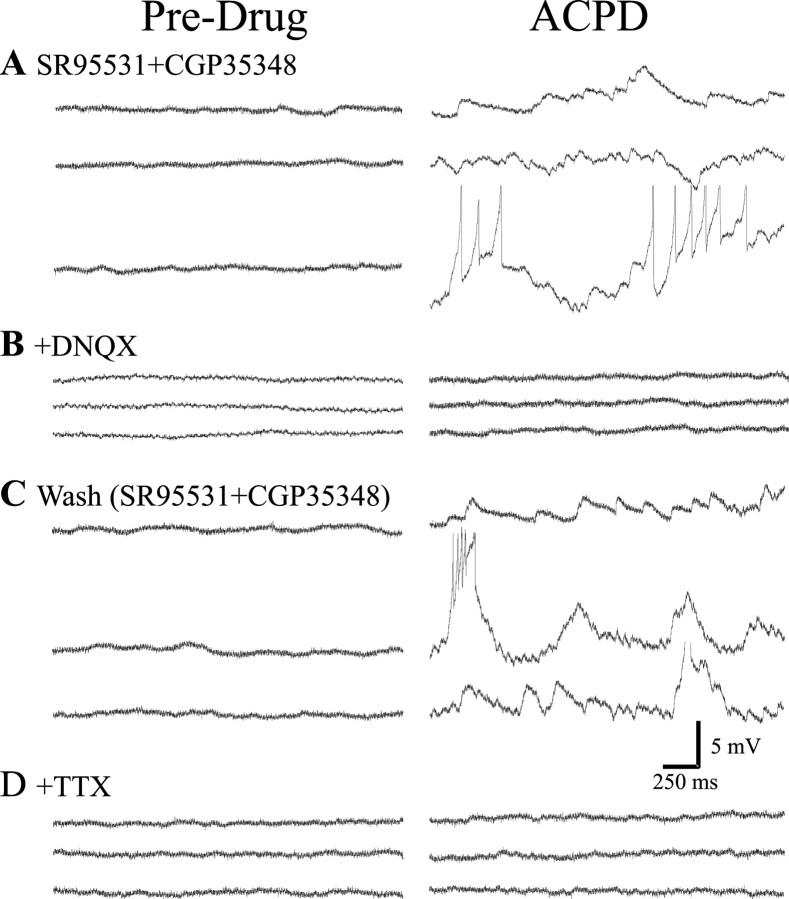
ACPD increases spontaneous EPSPs in interneurons. A, In a recording from geniculate interneuron, excitatory spontaneous activity was isolated by antagonizing GABA receptors with SR-95531 (20 μm) and CGP35348 (200 μm). Under these conditions, application of ACPD (250 μm) produced a robust increase in spontaneous membrane depolarizations. This increased activity could produce spike discharge (truncated), depending on the resting membrane potential of the neuron. B, After the addition of the non-NMDA glutamate receptor antagonist DNQX (30 μm), ACPD produced no apparent change in baseline activity, indicating that the depolarizations were likely EPSPs. C, After washout of DNQX, the ACPD-mediated increase in spontaneous EPSPs was similar to that observed in A. D, Synaptic activity was then attenuated by TTX (1 μm), and, once again, the ACPD-mediated increase in spontaneous EPSP activity was completely attenuated.
The source of these apparent synaptic inputs to the recorded interneurons was unclear. Aside from the axon collaterals from thalamic relay neurons, excitatory innervation of interneurons arises from retinal, cortical, and possibly brainstem axons; furthermore, possible inhibitory inputs emanate from local inter-neurons, nearby neurons of the thalamic reticular nucleus, and brainstem sources (for review, see Jones, 1985). In the in vitro thalamic slice preparation, somata of excitatory inputs from retinal, cortical, and brainstem sources (i.e., all but relay cells) are absent, and, thus, the only plausible excitatory innervation with intact somata would be from relay neuron axon collaterals. In contrast, much of the possible inhibitory innervation from other interneurons and thalamic reticular cells remains intact within the slice.
Thus, in our next series of experiments, we determined whether the depolarizing potentials were actually excitatory or inhibitory synaptic potentials. To do so, we applied GABA antagonists, either bicuculline methiodide (30 μm) or SR-95531 (20 μm) and CGP35348 (200 μm) or CGP46381 (200 μm) to attenuate GABAA and GABAB receptors, respectively. Under these conditions, ACPD still evoked a robust increase in spontaneous depolarizations (n = 15; Fig. 4A). Furthermore, after the addition of the AMPA receptor antagonist DNQX (30 μm), ACPD no longer produced an increase in depolarizing potentials (Fig. 4B; n = 4). The ACPD-mediated increase in spontaneous depolarizations returned after washout of DNQX (Fig. 4C). The ACPD-mediated increase in baseline activity was also attenuated by TTX (Fig. 4D). Our data, thus, indicate that ACPD produces an increase in EPSPs in interneurons in a TTX-sensitive manner, and the most plausible explanation for this is that ACPD strongly depolarizes neighboring relay neurons that synapse onto the recorded interneuron.
Discussion
Our results suggest that axons of many relay cells give rise to local collaterals within the main laminae of the lateral geniculate nucleus and that these form excitatory synapses onto intralaminar interneurons. Such an arrangement provides an additional pathway of inhibitory feedback to relay neurons that is independent of thalamic reticular neurons. A previous study reported a rhythmic excitatory activity in relay neurons that originated from excitatory innervation from other relay cells (Soltesz and Crunelli, 1992), presumably via the intrageniculate axon collaterals from relay cells, as described here. Our study, by focusing on intralaminar interneurons and their potential inhibitory influence on relay neurons within the lateral geniculate nucleus, extends this observation of functional axonal collaterals from relay neurons.
It is worth noting that an attempt to find local collaterals of relay cell axons in the ventrobasal complex of cats after intracellular filling of these cells with horseradish peroxidase has failed to do so (Yen and Jones, 1983; Yen et al., 1985a). Similarly, intrageniculate axon collaterals have not been identified within rat ventral lateral nucleus (Sawyer et al., 1994). There could be several reasons for this, including failure to fill or visualize delicate collaterals and simple variation between species or thalamic nuclei. For instance, the rat thalamus, except for the lateral geniculate nucleus, has virtually no interneurons (Arcelli et al., 1997), and because we found these to be a target of intrageniculate relay axon collaterals, the absence of interneurons in the rat ventral lateral nucleus could result in a concomitant absence of these collaterals.
Earlier studies noted the presence of these intrageniculate collaterals (Ferster and LeVay, 1978; Friedlander et al., 1981; Stanford et al., 1983), and it was suggested that they innervated GABAergic thalamic reticular cells or interlaminar interneurons (Montero, 1989; Sanchez-Vives et al., 1996; Steriade et al., 1997). Interlaminar interneurons share many features of thalamic reticular neurons such as intrinsic membrane properties as well as sensitivity to various neuromodulators that differ significantly from analogous properties in intralaminar interneurons (Sanchez-Vives et al., 1996). The interlaminar interneurons also appear to be innervated by relay neuron collaterals, and it has been suggested that these behave as displaced thalamic reticular neurons. In the present study, recordings were limited to intralaminar interneurons as distinguished both by their location and intrinsic membrane properties (Figs. 2, 3). Our results indicate that axon collaterals from thalamic relay neurons form functional excitatory glutamatergic synapses onto inhibitory intralaminar interneurons within the lateral geniculate nucleus, and because these neurons provide inhibitory input to relay cells, this indicates a previously unappreciated inhibitory control of relay neurons.
These intralaminar interneurons are a rather interesting and unique cell type in thalamus, because, in addition to synaptic outputs via conventional axons, they also possess efferent synaptic terminals emanating from distal dendrites. These dendritic terminals serve two roles: they are presynaptic to relay cells and postsynaptic, mainly in the lateral geniculate nucleus, to retinal or brainstem inputs (Erisir et al., 1997; Cox and Sherman, 2000). It has been suggested that input/output activities of these distal dendrites are electrotonically isolated not only from each other but also from the activity of more proximal dendritic circuitry that controls axonal output, thereby allowing these interneurons to multiplex several input/output relationships independently. We have previously shown that the dendritic terminals of these interneurons contain mGluRs that, when activated by ACPD, increase transmitter release, suggesting (but not proving) that ACPD depolarizes these terminals (Cox et al., 1998; Cox and Sherman, 2000). Yet, ACPD in the presence of TTX has little or no discernable effect on somatic recordings of these interneurons (Fig. 3), indicating that any membrane voltage changes caused by ACPD in the dendritic terminals are effectively isolated from the soma. This, in turn, suggests that present evidence of EPSPs from relay cell inputs recorded at the somata of interneurons is likely because of synaptic inputs on relatively proximal dendritic locations. Thus, this relay cell input can effectively control the axonal output of interneurons, but not the dendritic output, because relay cells do not appear to innervate dendritic terminals of interneurons (Erisir et al., 1997).
Interneurons have previously been granted a mainly feedforward inhibitory role with respect to relay cells, and any feedback role was thought to require innervation of interneurons from cortex. Our findings could have interesting implications, given that thalamic reticular neurons and intralaminar interneurons have different sensitivity and responsiveness to various putative transmitters and neuromodulators. For example, activation of mGluRs on interneurons has no appreciable action at the somatic level (Pape and McCormick, 1995; Cox et al., 1998), but activation of specific mGluRs on thalamic reticular neurons may produce either excitatory or inhibitory actions (Cox and Sherman, 1999). Thus, excitatory output of glutamatergic relay neurons could have a variety of possible feedback actions. In addition, modulators, such as noradrenaline and serotonin, have differential actions on interneurons and thalamic reticular neurons (McCormick and Wang, 1991; Pape and McCormick, 1995), implying that the excitability state of these inhibitory neurons could be differentially regulated, thereby differentially affecting relay cell activity. The presence of functional relay cell innervation of the interneurons places these interneurons as part of a fast, disynaptic inhibitory feedback circuit requiring neither a cortical nor thalamic reticular route.
Footnotes
This work was supported by National Eye Institute Grant EY03038 (National Institutes of Health). We gratefully thank Susan Van Horn for excellent technical assistance.
Correspondence should be addressed to Dr. Charles L. Cox, Department of Molecular and Integrative Physiology, University of Illinois, Urbana-Champaign, 524 Burrill Hall, 407 South Goodwin Avenue, Urbana, IL 61801. E-mail: clcox@life.uiuc.edu.
Copyright © 2003 Society for Neuroscience 0270-6474/03/237642-05$15.00/0
References
- Arcelli P, Frassoni C, Regondi MC, De Biasi S, Spreafico R ( 1997) GABAergic neurons in mammalian thalamus: a marker of thalamic complexity? Brain Res Bull 42: 27-37. [DOI] [PubMed] [Google Scholar]
- Cox CL, Sherman SM ( 1999) Glutamate inhibits thalamic reticular neurons. J Neurosci 19: 6694-6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CL, Sherman SM ( 2000) Control of dendritic outputs of inhibitory interneurons in the lateral geniculate nucleus. Neuron 27: 597-610. [DOI] [PubMed] [Google Scholar]
- Cox CL, Zhou Q, Sherman SM ( 1998) Glutamate locally activates dendritic outputs of thalamic interneurons. Nature 394: 478-482. [DOI] [PubMed] [Google Scholar]
- Edwards FA, Konnerth A, Sakmann B, Takahashi T ( 1989) A thin slice preparation for patch clamp recordings from neurons of the mammalian central nervous system. Pflugers Arch 414: 600-612. [DOI] [PubMed] [Google Scholar]
- Erisir A, Van Horn SC, Bickford ME, Sherman SM ( 1997) Immunocytochemistry and distribution of parabrachial terminals in the lateral geniculate nucleus of the cat: a comparison with corticogeniculate terminals. J Comp Neurol 377: 535-549. [PubMed] [Google Scholar]
- Ferster D, LeVay S ( 1978) The axonal arborizations of lateral geniculate neurons in the striate cortex of the cat. J Comp Neurol 182: 923-944. [DOI] [PubMed] [Google Scholar]
- Friedlander MJ, Lin C-S, Stanford LR, Sherman SM ( 1981) Morphology of functionally identified neurons in lateral geniculate nucleus of the cat. J Neurophysiol 46: 80-129. [DOI] [PubMed] [Google Scholar]
- Guillery RW ( 1966) A study of Golgi preparations from the dorsal lateral geniculate nuceleus of the adult cat. J Comp Neurol 128: 21-50. [DOI] [PubMed] [Google Scholar]
- Hamos JE, Van Horn SC, Raczkowski D, Uhlrich DJ, Sherman SM ( 1985) Synaptic connectivity of a local circuit neurone in lateral geniculate nucleus of the cat. Nature 317: 618-621. [DOI] [PubMed] [Google Scholar]
- Horikawa K, Armstrong WE ( 1988) A versatile means of intracellular labeling: injection of biocytin and its detection with avidin conjugates. J Neurosci Methods 25: 1-11. [DOI] [PubMed] [Google Scholar]
- Jones EG ( 1985) The thalamus. New York: Plenum.
- McCormick DA, von Krosigk M ( 1992) Corticothalamic activation modulates thalamic firing through glutamate “metabotropic” receptors. Proc Natl Acad Sci USA 89: 2774-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Wang Z ( 1991) Serotonin and noradrenaline excite GABAergic neurones of the guinea pig and cat thalamic reticular nucleus. J Physiol (Lond) 442: 235-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero VM ( 1989) The GABA-immunoreactive neurons in the interlaminar regions of the cat lateral geniculate nucleus: light and electron microscopic observations. Exp Brain Res 75: 497-512. [DOI] [PubMed] [Google Scholar]
- Pape H-C, McCormick DA ( 1995) Electrophysiological and pharmacological properties of interneurons in the cat dorsal lateral geniculate nucleus. Neuroscience 68: 1105-1125. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vives MV, Bal T, Kim U, von Krosigk M, McCormick DA ( 1996) Are the interlaminar zones of the ferret dorsal lateral geniculate nucleus actually part of the perigeniculate nucleus? J Neurosci 16: 5923-5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer SF, Young SJ, Groves PM, Tepper JM ( 1994) Cerebellar-responsive neurons in the thalamic ventroanterior-ventrolateral complex of rats: in vivo electrophysiology. Neuroscience 63: 711-724. [DOI] [PubMed] [Google Scholar]
- Scheibel ME, Scheibel AB ( 1966) The organization of the nucleus reticularis thalami: a golgi study. Brain Res 1: 43-62. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW ( 2001) Exploring the thalamus. San Diego: Academic.
- Soltesz I, Crunelli V ( 1992) A role for low-frequency, rhythmic synaptic potentials in the synchronization of cat thalamocortical cells. J Physiol (Lond) 457: 257-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford LR, Friedlander MJ, Sherman SM ( 1983) Morphological and physiological properties of geniculate W-cells of the cat: a comparison with X- and Y-cells. J Neurophysiol 50: 582-608. [DOI] [PubMed] [Google Scholar]
- Steriade M, Jones EG, McCormick DA ( 1997) Thalamus: organization and function, Vol 1. New York: Elsevier. [Google Scholar]
- Stuart GJ, Dodt HU, Sakmann B ( 1993) Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Eur J Physiol 423: 511-518. [DOI] [PubMed] [Google Scholar]
- Yen CT, Jones EG ( 1983) Intracellular staining of physiologically identified neurons and axons in the somatosensory thalamus of the cat. Brain Res 280: 148-154. [DOI] [PubMed] [Google Scholar]
- Yen CT, Conley M, Jones EG ( 1985a) Morphological and functional types of neurons in cat ventral posterior thalamic nucleus. J Neurosci 5: 1316-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen CT, Conley M, Hendry SHC, Jones EG ( 1985b) The morphology of physiologically identified GABAergic neurons in the somatic sensory part of the thalamic reticular nucleus in the cat. J Neurosci 5: 2254-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]