Abstract
Activation of P2Y receptors by released nucleotides subserves important autocrine-paracrine functions in various non-neural tissues. To investigate how P2Y receptors are activated in a neuronal environment, we used PC12 cells in which nucleotides were found to elicit increases in inositol phosphates via P2Y2 and decreases in cAMP via P2Y12 receptors. Depolarization of PC12 cells raised inositol phosphates, and blockade of voltage-gated Ca2+ channels by Cd2+ or degradation of extracellular nucleotides by apyrase prevented this effect. In nondepolarized cells, apyrase did not affect inositol phosphates. Depolarization of PC12 cells also reduced the A2A receptor-mediated synthesis of cAMP. This effect was again prevented by Cd2+ or apyrase, but apyrase enhanced the synthesis of cAMP even in nondepolarized cells. Overexpression of rat P2Y2 receptors increased the nucleotide-dependent inositol phosphate accumulation and enhanced the effect of K+ depolarization. Nevertheless, apyrase still failed to alter spontaneous inositol phosphate accumulation. Expression of rat P2Y1 receptors, in contrast, led to huge increases in spontaneous inositol phosphate accumulation, which was reduced by a receptor antagonist or by apyrase. This increased synthesis of inositol phosphates could not be further enhanced by depolarization or receptor agonists, but when endogenous nucleotides were removed by superfusion, recombinant P2Y1 receptors could be activated to mediate an inhibition of M-type K+ channels. These results indicate that nucleoside diphosphate-sensitive (P2Y12 and P2Y1) receptors are activated by spontaneous nucleotide release, whereas triphosphate-sensitive (P2Y2) receptors require an excess of depolarization-evoked release to become activated.
Keywords: P2Y receptors, inositol phosphates, cAMP, M-type K+ current, PC12 cells, nucleotide release
Introduction
Extracellular nucleotides regulate various physiological functions, including smooth muscle contraction, platelet aggregation, mucociliary clearance, cell proliferation, and neurotransmission. These actions are mediated by ionotropic P2X and G-protein-coupled P2Y receptors (Ralevic and Burnstock, 1998). At least seven different mammalian P2Y receptors have been identified: P2Y1 (Tokuyama et al., 1995), P2Y12 (Hollopeter et al., 2001), and P2Y13 (Communi et al., 2001) are preferentially activated by ADP, whereas P2Y6 is activated by UDP (Nicholas et al., 1996). P2Y11 prefers ATP as an agonist (Communi et al., 1997), whereas P2Y2 (Lustig et al., 1993) and P2Y4 (Communi et al., 1995; Kennedy et al., 2000) are equally sensitive to ATP and UTP. In rats, P2Y1, P2Y2, P2Y4, P2Y6, and P2Y12 have been detected (Ralevic and Burnstock, 1998; Hollopeter et al., 2001). All P2Y receptors of the rat but P2Y12 mediate increases in inositol phosphates (IPs) (Ralevic and Burnstock, 1998), and P2Y12 mediates an inhibition of adenylyl cyclase (Hollopeter et al., 2001).
ATP is released from various cells including fibroblast-like, epithelial, endothelial, glial, and neuronal cells (von Kugelgen et al., 1994; Schlosser et al., 1996; Wang et al., 1996; Lazarowski et al., 2000; Ostrom et al., 2000). ATP release from non-neural cells is Ca2+ independent and can be elicited by different stimuli such as hypotonic solutions (Wang et al., 1996), mechanical stimulation (Schlosser et al., 1996), or exchange of culture media (Lazarowski et al., 2000; Ostrom et al., 2000). In neurons, ATP is stored in vesicles and thus released by exocytosis (Zimmermann, 1994). Vesicle exocytosis occurs spontaneously at a slow rate and is accelerated when transmembrane Ca2+ entry is triggered by depolarization-induced opening of voltage-gated Ca2+ channels (Matthews, 1996). Therefore, neuronal ATP release is depolarization and Ca2+ dependent (von Kugelgen et al., 1994).
In non-neural tissues, such as liver, kidney, bones, and blood vessels, released nucleotides subserve autocrine-paracrine functions by activating certain P2Y receptors (Gerasimovskaya et al., 2002; Junankar et al., 2002; Schwiebert et al., 2002; Torres et al., 2002; You et al., 2002). In neurons, action potential- and Ca2+-dependent release of ATP contributes to synaptic transmission via P2X receptors (Robertson et al., 2001); however, much less is known about the activation of neuronal P2Y receptors by released nucleotides. One known example is the ATP-mediated autocrine inhibition of voltage-gated Ca2+ channels in bovine chromaffin cells, but the P2Y receptor that was involved remained unknown (Currie and Fox, 1996). To investigate how different P2Y receptors are activated by nucleotides released from neuronal sources, we used PC12 cells. These rat phaeochromocytoma cells are ontogenetically related to sympathetic neurons and release transmitters in an exocytotic manner (Greene and Tischler, 1976; Fisher and Burgoyne, 1999). Aside from P2X1 through P2X6, PC12 cells express RNA for P2Y2, P2Y4, P2Y6, and P2Y12 receptors (Arslan et al., 2000). Taking the synthesis of IPs and the reduction of cAMP accumulation as measures of receptor activation, we show that neuronal P2Y receptors are activated in an autocrine-paracrine manner as described previously for non-neural cells. However, diphosphate- and triphosphate-sensitive receptors are differentially activated by spontaneous and depolarization-evoked nucleotide release.
Materials and Methods
Materials. [2,8-3H]adenine (specific activity 32 Ci/mmol) and myo-[3H]inositol (74.7 Ci/mmol) were obtained from NEN (Vienna, Austria). Na-UTP, Na-UDP, Na2-ATP, Na-ADP, 4-(3-butoxy-4-methoxybenzyl) imidazoline-2-one (RO 20-1724), 3′,5′-cAMP, suramin, apyrase (grade VII, with an ∼1:1 ratio in ATPase and ADPase activity), 2-methylthio-AMP, 2-methylthio-ATP, pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonic acid tetrasodium (PPADS), and 2-p-(2-carboxyethyl)phenethylamino-5′-N-ethylcarboxamido-adenosine (CGS 21680) were purchased from Sigma (Vienna, Austria). 2-Chloro-N6-methyldeoxyadenosine 3′,5′-biphosphate (MRS 2216) was a gift of Dr. K. A. Jacobson (National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD).
Cell culture and transfection methods. PC12 cells were obtained from the European Collection of Animal Cell Cultures (Salisbury, UK), plated onto collagen-coated (Biomedical Technologies, Stoughton, MA) six-well culture dishes (NUNC, Roskilde, Denmark), and kept in OptiMEM (Invitrogen, Vienna, Austria) supplemented with 0.2 mm l-glutamine (HyClone, Aalst, Belgium), 25,000 IU/l-1 penicillin and 25 mg/l-1 streptomycin (Sigma, Vienna, Austria), 5% fetal calf serum, and 10% horse serum (both from Invitrogen). Once per week, cell cultures were split, and the medium was exchanged twice per week.
For the generation of PC12 cell clones stably expressing either the rat P2Y1 receptor linked to the green fluorescent protein (P2Y1-GFP) or the rat P2Y2 receptor, 15 μg of a rat P2Y1-enhanced GFP expression vector (kindly provided by Dr. G. Reiser, Magdeburg, Germany) (Vohringer et al., 2000) or 15 μg of the expression vector pcDNA3 containing the coding sequence for the rat P2Y2 receptor (kindly provided by Dr. T. Webb, Leicester, UK) was mixed with 50 μl of the TransFast transfection reagent (Promega, Mannheim, Germany) and added to semiconfluent PC12 cell cultures in serum-free medium. After a 1 hr incubation at 37°C, 2 vol of serum-free medium and the appropriate amount of serum (as above) were added. Forty-eight hours after transfection, this medium was exchanged for a medium supplemented with 500 μg/ml neomycin (G418) to allow for selection of drug resistance. This selection medium was replaced every 3-4 d until distinct islands of surviving cells were visible. Individual clones of antibiotic-resistant cells were transferred to 24-well plates and grown in medium containing 200 μg/ml neomycin.
Northern blot analysis. Northern blots were performed as described previously for P2Y receptors expressed in primary cultures of sympathetic neurons (Vartian et al., 2001). After extraction from PC12 cell cultures, 30 μg of RNA per lane was separated by electrophoresis through formaldehyde containing 1.5% agarose gels and transferred to nylon membranes. Subsequent to UV cross-linkage, membranes were hybridized overnight at 65°C in a hybridization solution containing 50 mm PIPES, pH 6.5, 100 mm NaCl, 50 mm sodium phosphate buffer, pH 7.0, 1 mm EDTA, pH 8.0, and 5% SDS. After hybridization, the blots were washed twice in 5% SDS, 1× SSC at 65°C for 15 min, and finally exposed to x-ray films. Thereafter, probes were removed from the membranes, and blots were subjected to further hybridization with additional P2Y receptor-specific probes.
Cloned fragments of P2Y1, P2Y2, P2Y4, and P2Y6 were used as probes for hybridization. The corresponding PCR fragments were amplified from cDNA obtained by reverse transcription of total RNA isolated from rat sympathetic neurons as described (Vartian et al., 2001). The PCR fragments were separated by electrophoresis through a 2% agarose gel, isolated, and cloned into pCR3.1 vector (Invitrogen). Plasmid DNA was isolated from positive clones and sequenced to verify the P2Y receptor identity. EcoRI fragments carrying the P2Y receptor sequences were labeled with [α-32P]deoxycytidine triphosphate by random priming using the Prime-a-Gene labeling system (Promega).
Determination of inositol phosphates. The method for determining IPs was adapted from that used previously for primary neuronal cell cultures (Bofill-Cardona et al., 2000) as follows. Cells were grown to confluence and incubated in serum-free and inositol-free DMEM supplemented with myo-[3H]inositol (2.5 μCi/ml) for 48 hr. Thereafter, the medium was exchanged for OptiMEM supplemented with 10 mm LiCl for 20 min to block inositol monophosphatase (Phiel and Klein, 2001). Antagonists or apyrase (1 U/ml) were added together with LiCl when appropriate. Subsequently, the cells were incubated for an additional 30 min (unless indicated otherwise) in a buffer (120 mm NaCl, 3 mm KCl, 2 mm MgCl2, 2 mm CaCl2, 20 mm glucose, 10 mm HEPES, 10 mm LiCl, adjusted to pH 7.4 with NaOH) containing one or more of the following: agonists, antagonists, apyrase, CdCl2 (1 mm), and/or 100 mm KCl (NaCl was reduced accordingly). Finally, cells were lysed in boiling EDTA (10 mm) solution. After centrifugation of samples, an aliquot of the supernatant was removed to estimate the total water-soluble radioactivity. The remainder was applied to anion exchange chromatography columns (Dowex AG 1-X8; Bio-Rad, Vienna, Austria) and washed three times with 3 ml of H2O. Columns were then washed with 10 ml of 50 mm ammonium formate, and finally inositol monophosphate (IP1) was eluted with 6 ml of 0.18 m ammonium formate and 0.1 m formic acid and quantitated by liquid scintillation counting (Bofill-Cardona et al., 2000). In some experiments, the radioactivity remaining in the pellet was also determined.
The radioactivity in the fraction of IP1 was expressed as percentage of the water-soluble radioactivity in the cells, which consists mainly of inositol (Bofill-Cardona et al., 2000). In initial experiments, the radioactivity in the IP1 fraction was also calculated as percentage of the radioactivity in the pellet; however, these values displayed large variations between experiments (see Fig. 1 A) and thus were not used routinely. Nucleotides or bradykinin (1 μm) reproducibly caused time-dependent increases in IP1 when compared with the values obtained in their absence (see Fig. 1 A). Nevertheless, the extent of basal and agonist-induced IP1 accumulation may vary between different preparations. Therefore, the IP1 values obtained after a 30 min incubation in the presence of nucleotides, bradykinin, apyrase, CdCl2, and KCl, respectively, were normalized to the values obtained after a 30 min incubation in their absence within the same preparation (normalized to basal).
Figure 1.

Enhancement of IP accumulation by nucleotides and bradykinin. A, After loading with myo-[3H]inositol, PC12 cells were preincubated in LiCl (10 mm) for 20 min. During subsequent incubation periods (5-45 min), LiCl alone (basal; ○), LiCl plus UTP (10 μm; □), or LiCl plus bradykinin (1 μm; ⋄) were present. The amount of radioactivity retrieved within the fraction of inositol monophosphate (IP1) after these incubation periods is shown as counts per minute (cpm; top panel), as percentage of the total radioactivity in the pellet (% pellet; middle panel), or as percentage of the total water-soluble radioactivity extracted from the cell cultures (% soluble; bottom panel). The results stem from two independent experiments, each performed in triplicate (i.e., n = 6). B, After loading with myo-[3H]inositol and preincubation in LiCl (10 mm), the cells were incubated for 30 min in LiCl alone or in LiCl plus the indicated concentrations of nucleotides. The amount of radioactivity retrieved within the fraction of inositol monophosphate (IP1) was calculated as percentage of the total water-soluble radioactivity extracted from the cell cultures, and values obtained in the presence of nucleotides were normalized to the data obtained in their absence (normalized to basal). In the absence of nucleotides, 3822.9 ± 222.0 cpm were retrieved in the IP1 fraction (n = 36).
Determination of cAMP. The accumulation of cAMP in PC12 cell cultures was determined as described previously (Unterberger et al., 2002). After labeling of cellular purines with tritiated adenine (2.5 μCi/ml for 12 hr), the medium was replaced by the buffer described above supplemented with 100 μm of the phosphodiesterase inhibitor RO 20-1724 and 1 U/ml adenosine deaminase. Dishes were then kept at room temperature for 105 min. During the last 15 min of this incubation period, the adenosine A2A receptor agonist CGS 21680, ADP (10 μm), apyrase (1 U/ml), CdCl2 (1 mm) and 100 mm KCl (NaCl was reduced accordingly) were also included in the medium. Where indicated, the P2Y12 receptor antagonist 2MesAMP (Hollopeter et al., 2001) was present for the last 25 min. The incubation was terminated by exchanging the buffer for 1 ml of 2.5% perchloric acid containing 100 μm nonlabeled cAMP followed by a 20 min incubation at 4°C. Subsequently, cAMP was separated from the other purines by a chromatographic procedure described previously (Unterberger et al., 2002). Finally, radioactivity within the samples obtained was determined by liquid scintillation counting.
The radioactivity in the fraction of cAMP was expressed as percentage of the total radioactivity extracted from the cells. Stimulation of PC12 cells with the adenosine A2A receptor agonist CGS 21680 caused a reproducible increase in these values of cAMP, but the extent of basal and stimulated cAMP synthesis may vary between different preparations (Unterberger et al., 2002). Therefore, the values of cAMP obtained in the presence of CGS 21680 were normalized to the values obtained in its absence within the same preparation (normalized to basal).
Electrophysiology. Currents through M-type K+ (KM) channels were determined as described previously for primary neuronal cell cultures (Scholze et al., 2002). Experiments were performed at room temperature (20-24°C) on isolated PC12 cells using the perforated-patch modification of the patch-clamp technique, which prevents rundown of M currents (IM). Patch pipettes were pulled (Flaming-Brown puller, Sutter Instruments, Novato, CA) from borosilicate glass capillaries (Science Products, Frankfurt/Main, Germany) and front-filled with a solution consisting of (in mm): 75 K2SO4, 55 KCl, 8 MgCl2, and 10 HEPES, adjusted to pH 7.3 with KOH. Then, electrodes were back-filled with the same solution containing 200 μg/ml amphotericin B (in 0.8% DMSO), which yielded tip resistencies of 1-3 MΩ. PC12 cells were submerged in and continuously superfused with (in mm): 140 NaCl, 6.0 KCl, 2.0 CaCl2, 2.0 MgCl2, 20 glucose, 10 HEPES, adjusted to pH 7.4 with NaOH. Tetrodotoxin (0.5 μm) was included to suppress voltage-activated Na+ currents. Superfusion was performed by the use of a DAD-12 drug application device (Adams & List, Westbury, NY). IM relaxations were evoked once every 20 sec by 1 sec hyperpolarizing voltage steps from -30 to -55 mV; the difference between current amplitudes 20 msec after the onset of hyperpolarizations and 20 msec before re-depolarization was taken as a measure for IM. Amplitudes obtained during the application of ADP (b) were compared with those measured before (a) and after (c) application of the nucleotide by calculating 100 - (200b/[a + c]) = % inhibition (Scholze et al., 2002).
Statistics. All data represent arithmetic means ± SEM; n represents number of culture dishes or of single cells in electrophysiological experiments. If error bars are not shown in Figures, they were smaller than the symbols. Concentration-response curves were fitted to experimentally obtained data by the ALLFIT program (De Lean et al., 1978). Significances of differences between single data points were evaluated by the nonparametric Mann-Whitney test, and p values <0.05 were accepted as indicators of statistically significant differences.
Results
Activation of endogenous P2Y2 receptors by added nucleotides
In nondifferentiated PC12 cells, ATP and UTP have been found either to stimulate an accumulation of IPs (Murrin and Boarder, 1992) or to leave IP3 unchanged (Arslan et al., 2000). To verify whether the present PC12 cell clone was capable of synthesizing IPs in response to nucleotides, PC12 cells were labeled with tritiated myo-inositol and preincubated in 10 mm LiCl for 20 min to block inositol phosphatases. Thereafter, the cells were incubated for various periods of time in 10 μm UTP, in 1 μm bradykinin for comparison, or in buffer lacking agonists. As shown in Figure 1A, both UTP and bradykinin caused time-dependent increases in the radioactivity retrieved in the fraction of IP1, which hardly changed in the absence of agonists.
When UTP was applied for 30 min at different concentrations, its effect was concentration-dependent, with half-maximal stimulation between 1 and 10 μm. At concentrations up to 100 μm, ATP was equi-effective to UTP in stimulating IP1 synthesis, but at higher concentrations, ATP was much more effective than UTP. In contrast, ADP, UDP, and 2MeSATP at up to 100 μm did not cause significant changes in IP1 accumulation (Fig. 1B).
To further elucidate which P2 receptors mediated these effects, two P2 receptor antagonists, suramin and PPADS, were used. Suramin, at 10-100 μm, reduced the IP1 stimulating effect of 10 μm UTP in a concentration-dependent manner, whereas PPADS (100 μm) had no such effect. PPADS also failed to attenuate the effect of 10 μm ATP (Fig. 2A) but reduced the effect of 1 mm ATP; in its presence, ATP was equipotent and equi-effective to UTP applied alone in stimulating IP1 synthesis: half-maximal effects occurred at 3.2 ± 1.2 μm UTP and at 4.7 ± 1.7 μm ATP (plus 100 μm PPADS), and the maximum with both nucleotides was a 2.5-fold stimulation over basal (Fig. 2B). PPADS (100 μm) entirely blocks P2X receptor-mediated events in PC12 cells (Vartian and Boehm, 2001). Therefore, these results suggest that ATP concentrations above 100 μm enhance IP1 accumulation via activation of both P2Y and P2X receptors. UTP, in contrast, does not activate P2X receptors (Ralevic and Burnstock, 1998) and thus stimulated IP1 synthesis only via P2Y receptors. Among the P2Y receptors expressed in the rat, P2Y2 and P2Y4 receptors are activated by both ATP and UTP (Kennedy et al., 2000). Previously, rat P2Y4 receptors were found to be blocked by PPADS (Suarez-Huerta et al., 2001) but not by suramin (Bogdanov et al., 1998), exactly the opposite of the present findings. Therefore, the present results are compatible with P2Y2 receptors mediating the nucleotide-dependent IP synthesis.
Figure 2.
Characterization of the receptor mediating the nucleotide-dependent synthesis of IPs. After loading with myo-[3H]inositol and preincubation in LiCl (10 mm), the cells were incubated for 30 min in LiCl alone or in LiCl plus nucleotides. The amount of radioactivity retrieved within the fraction of inositol monophosphate (IP1) was calculated as percentage of the total water-soluble radioactivity extracted from the cell cultures, and values obtained in the presence of nucleotides were normalized to the data obtained in their absence (normalized to basal). A, Cultures were exposed to the indicated concentrations of either UTP (open bars) or ATP (filled bars) in the absence or presence of the indicated concentrations of suramin or PPADS. n = 6-9. *p < 0.05 and ***p < 0.001 versus the value obtained in the presence of UTP only; n.s. indicates no significant difference. In the absence of nucleotides or antagonists, 3580.3 ± 363.4 cpm were retrieved in the IP1 fraction (n = 9). B, Cultures were exposed to the indicated concentrations of either UTP alone or ATP plus 100 μm PPADS. n = 4-9. In the absence of nucleotides or antagonists, 3023.8 ± 311.5 cpm were retrieved in the IP1 fraction (n = 16). C, Northern blot analysis performed with total RNA (30 μg per lane) isolated from either PC12 cell cultures or from rat superior cervical ganglion (SCG) cultures. PC12 cells had been treated with nerve growth factor (+NGF) (50 ng/ml for 5 d) before RNA extraction or remained untreated (-NGF). The blot was probed consecutively with [α32P]deoxycytidine triphosphate-labeled cDNA probes specific for P2Y1, P2Y2, P2Y4, and P2Y6, among which the P2Y4-specific probe gave no signal (data not shown). The bottom panel shows total RNA stained with methylene blue. D, PC12 cells had been treated with nerve growth factor (+NGF) (50 ng/ml for 5 d) or remained untreated (-NGF). The cultures were then exposed to the indicated concentrations of UDP (squares) or UTP (circles). n = 3-9. *p < 0.05 and ***p < 0.01 versus the value obtained in cells not treated with nerve growth factor. In the absence of nucleotides, 2929.7 ± 332.6 cpm were retrieved in the IP1 fraction of NGF-treated cells (n = 7), and 3784.3 ± 264.8 cpm were retrieved in the IP1 fraction of untreated cells (n = 7; p = 0.12 vs NGF-treated cells).
To investigate in further detail which P2Y receptor subtypes might be involved in the nucleotide-dependent synthesis of IP1 as described above, we performed Northern blots to check for their expression level. Total RNA was isolated from either untreated or NGF-differentiated PC12 cells and compared with RNA from primary cultures of rat superior cervical ganglia (Vartian et al., 2001). After separation by agarose gel electrophoresis and blotting, the extracted RNA was analyzed with probes specific for P2Y1, P2Y2, P2Y4, and P2Y6. As shown in Figure 2C, considerable amounts of RNA coding for P2Y2, but none for P2Y1, P2Y4 (data not shown), or P2Y6, were detected in nondifferentiated PC12 cells. RNA isolated from primary cultures of rat superior cervical ganglia gave positive signals for P2Y1, P2Y2, and P2Y6 (Fig. 2C), but not for P2Y4 (data not shown). Previously, transcripts for P2Y4 and P2Y6 receptors, but none for P2Y1 receptors, had been detected in PC12 cells by RT-PCR (Arslan et al., 2000; Unterberger et al., 2002). Thus, nondifferentiated PC12 cells express high levels of P2Y2, but of no other phospholipase C-linked P2Y receptor.
After differentiation of PC12 cells with recombinant human β-nerve growth factor (50 ng/ml for 5 d), P2Y2 receptor-specific RNA was slightly decreased, and the expression of P2Y6 receptors was induced (Fig. 2C). In parallel, the UTP-dependent IP1 accumulation was reduced in nerve growth factor-treated cells as compared with nontreated PC12 cells, and the P2Y6 receptor agonist UDP still failed to significantly alter IP1 (Fig. 2D). This corroborates that the P2Y2 but no other P2Y receptor subtype mediates the nucleotide-dependent IP1 accumulation in PC12 cells.
Autocrine-paracrine activation of endogenous P2Y2 receptors
In non-neural cells, such as Madin-Darby canine kidney cells, endogenous P2Y receptors were found to be activated by endogenous nucleotides released, for instance, in response to the exchange of culture media (Ostrom et al., 2000). To reveal whether endogenous P2Y receptors of PC12 cells may become activated under the present experimental conditions even in the absence of added nucleotides, cultures were incubated in LiCl (10 mm) in the presence of apyrase (1 U/ml); however, the degradation of nucleotides by apyrase did not alter the values of basal IP1 accumulation, although the enzyme did abolish the IP1-stimulating effect of 10 μm ATP (Fig. 3). To certify that this inhibitory action of apyrase was not caused by some unspecific effect on IP accumulation or phospholipase C activity, the enzyme was also used together with bradykinin; however, apyrase left the IP synthesis in the presence of this peptide unchanged (Fig. 3). Depolarization of PC12 cells by 100 mm K+ enhanced the IP1 accumulation twofold, i.e., to almost the same extent as the exogenous application of 10 μm ATP. This effect of depolarizing K+ concentrations was abolished in the presence of apyrase, which indicates that it was caused by the release of endogenous nucleotides. In addition, the effect was also attenuated in the presence of 1 mm Cd2+ (Fig. 3), which prevents Ca2+ entry via voltage-gated Ca2+ channels and thereby suppresses depolarization-evoked transmitter release (Boehm, 1999). Thus, the stimulation of IP1 synthesis by endogenously released nucleotides required depolarization-evoked release.
Figure 3.
Stimulation of IP synthesis by released nucleotides. After loading with myo-[3H]inositol and preincubation in LiCl (10 mm), the cells were incubated for 30 min in LiCl alone or LiCl plus the indicated concentrations of apyrase, ATP, bradykinin (Bk), KCl (NaCl was reduced accordingly), or CdCl2. The amount of radioactivity retrieved within the fraction of inositol monophosphate (IP1) was calculated as percentage of the total water-soluble radioactivity extracted from the cell cultures, and values obtained in the presence of the above agents were normalized to the data obtained in their absence (normalized to basal); n = 5-14. **p < 0.01 versus the value obtained in the presence of LiCl only; ++p < 0.01 versus the value obtained in the presence of LiCl plus ATP; ##p < 0.01 versus the value obtained in the presence of LiCl plus 100 mm K+. In the presence of LiCl only, 2968.6 ± 200.5 cpm were retrieved in the IP1 fraction (n = 16).
Autocrine-paracrine activation of endogenous P2Y12 receptors
Apart from P2Y2 receptors, as shown above, PC12 cells express another endogenous P2Y receptor: this is a P2Y12 receptor, activation of which causes an inhibition of adenylyl cyclase (Unterberger et al., 2002). In contrast to P2Y2 receptors, P2Y12 receptors are activated by ADP rather than ATP (Hollopeter et al., 2001). To investigate whether this latter receptor might show a different pattern of activation, we measured the formation of cAMP under various conditions. After labeling of PC12 cells with [3H]adenine, 339.7 ± 23.3 cpm (n = 23) were retrieved within the fraction of cAMP, which corresponded to 0.060 ± 0.007% of the total radioactivity extracted from the cultures. These and all subsequent values were obtained in the presence of the phosphodiesterase inhibitor RO 20-1724 (100 μm). Therefore, any drug-induced alteration will reflect changes in adenylyl cyclase activity rather than alterations in cAMP degradation (Unterberger et al., 2002). Exposure of PC12 cells to 1 μm of the A2A adenosine receptor agonist CGS 21680 for 15 min increased cellular cAMP by ∼20-fold (Fig. 4A). In the presence of 10 μm ADP, this CGS 21680-induced accumulation of cAMP was markedly reduced. The P2Y12 receptor antagonist 2-MeSAMP (10-100 μm) (Hollopeter et al., 2001) abolished this inhibitory effect of ADP (Fig. 4C). This corroborates previous results indicating that P2Y12 receptors mediate an inhibition of adenylyl cyclase in PC12 cells (Kubista et al., 2003).
Figure 4.

Inhibition of cAMP synthesis by added and released nucleotides. After loading with [3H]adenine, PC12 cells were incubated in RO 20-1724 (100 μm) for 105 min. During the last 15 min of this incubation period, 1 μm CGS 21680 was present either alone or together with the indicated concentrations of additional agents. The amount of radioactivity retrieved within the fraction of cAMP was calculated as percentage of the total radioactivity extracted from the cell cultures, and values obtained in the presence of CGS 21680 were normalized to the data obtained in its absence (normalized to basal). A, Cells were incubated in the presence (open bar) or absence (filled bar) of 1 μm CGS 21680; n = 41. B, Cells were incubated in the presence of the indicated concentrations (micromolar) of CGS 21680 or 2MeSAMP, or both; n = 6-9; **p < 0.01 versus the value obtained in the presence of CGS 21680 only. C, Cells were incubated in the presence of the indicated concentrations (micromolar) of CGS 21680 and ADP or 2MeSAMP, or both; n=6-9; ***p < 0.001 versus the value obtained in the presence of CGS 21680 only; n.s. indicates no significant difference. D, Cells were incubated in the presence of the indicated concentrations of CGS 21680, apyrase, and KCl (NaCl was reduced accordingly) or CdCl2, or both; n = 6-9; **p < 0.01 and ***p < 0.001, respectively, versus the value obtained in the presence of CGS 21680 only; n.s. indicates no significant difference.
When 10 or 100 μm 2-MeSAMP was applied together with CGS 21680, but in the absence of ADP, the stimulation of cAMP synthesis by the A2A receptor agonist was enhanced significantly (Fig. 4B). Furthermore, application of apyrase (1 U/ml) together with CGS 21680 caused a similar increase in cAMP accumulation as the P2Y12 receptor antagonist (Fig. 4D). Thus, there was a spontaneous activation of the P2Y receptor that is negatively linked to adenylyl cyclase. When PC12 cells were depolarized by 100 mm K+, the CGS 21680-induced cAMP accumulation was reduced to approximately the same extent as by 10 μm ADP. This inhibition attributable to K+ depolarization was attenuated by the addition of apyrase and thus was mediated by released nucleotides. Finally, the inhibitory effect of 100 mm K+ was prevented by 1 mm Cd2+ (Fig. 4D), which indicates a role of Ca2+-dependent vesicle exocytosis. Cd2+ (1 mm) per se did not alter the CGS 21680-induced cAMP accumulation. Taken together, these results suggest that P2Y12 receptors endogenously expressed in PC12 cells are activated to some extent by spontaneously released nucleotides and can be activated further by nucleotides released by K+ depolarization.
Autocrine-paracrine activation of overexpressed P2Y2 receptors
One of the reasons for the lack of spontaneous P2Y2 receptor activation as opposed to the spontaneous activation of P2Y12 receptors might be a low number of endogenously expressed P2Y2 receptors. Therefore, PC12 cell clones stably overexpressing rat P2Y2 receptors (PC12-P2Y2) were generated, and three clones (clones 12, 14, and 17) were tested for their capability to synthesize IP1 in the absence and presence of UTP. As shown in Figure 5A, in clones 12 and 17, the spontaneous accumulation of IP1 was not different from that in wild-type PC12 cells, whereas in clone 14 it was enhanced by a factor of 3.5. In these three clones, the increases in IP1 synthesis caused by 10 μm UTP were 6.3 ± 0.5 (clone 12), 6.9 ± 0.8 (clone 14), and 8.9 ± 0.6 (clone 17)-fold, respectively. In wild-type PC12 cells, in contrast, this UTP-induced increase was only 2.2 ± 0.15-fold (Fig. 5A). Thus, overexpression increased the efficacy of the agonists. In addition, in clone 14 of PC12-P2Y2 cells, not only the efficacies but also the potencies of both ATP and UTP to stimulate IP1 synthesis were enhanced as compared with wild-type PC12 cells; half-maximal effects were observed at 1.4 ± 0.1 μm (ATP) and 1.6 ± 0.4 μm(UTP), respectively, and the maximum was a ninefold stimulation over basal (Fig. 5B).
Figure 5.
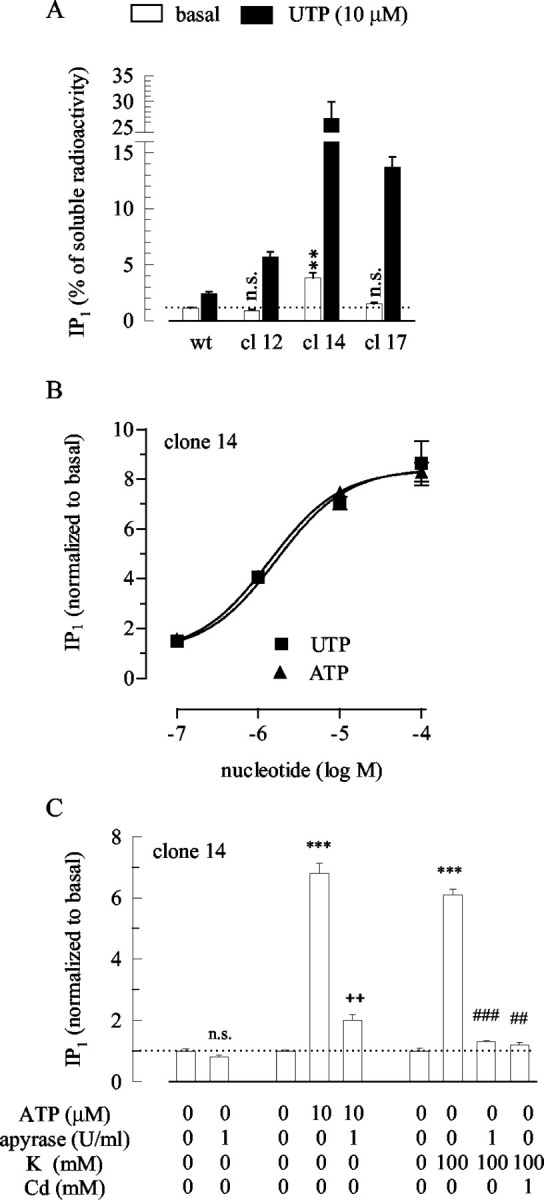
Stimulation of IP synthesis in P2Y2 receptor-overexpressing cells. After loading with myo-[3H]inositol and preincubation in LiCl (10 mm), the cells were incubated for 30 min in LiCl alone or LiCl plus the indicated concentrations of apyrase, ATP, UTP, KCl (NaCl was reduced accordingly), or CdCl2. The amount of radioactivity retrieved within the fraction of inositol monophosphate (IP1) was calculated as percentage of the total water-soluble radioactivity extracted from the cell cultures (A), and values obtained in the presence of the above agents were normalized to the data obtained in their absence (normalized to basal) (B, C). A, Either wild-type PC12 cells or three clones (clones 12, 14, or 17) of P2Y2 receptor-overexpressing cells were exposed to LiCl (open bars) or to LiCl plus 10 μm UTP (filled bars); n = 6; **p < 0.01 for the difference between the value obtained in the presence LiCl in P2Y2 receptor-overexpressing cells versus the value in wild-type cells; n.s. indicates no significant difference. B, PC12-P2Y2 cells of clone 14 were exposed to the indicated concentrations of ATP or UTP; n = 6-9. C, PC12-P2Y2 cells of clone 14 were exposed to the indicated concentrations of one or more of the following: ATP, apyrase, KCl, and CdCl2; n = 6-9; ***p < 0.001 versus the value obtained in the presence of LiCl only; ++p < 0.01 versus the value obtained in the presence of LiCl plus ATP; ##p < 0.01 and ###p < 0.001 versus the value obtained in the presence of LiCl plus KCl. In the absence of nucleotides or other agents, 2210.0 ± 92.1 cpm were retrieved in the IP1 fraction of wild-type PC12 cells (n = 6), 1595.7 ± 62.4 cpm in clone 12 (n = 6; p < 0.01 vs wild type), 5143.6 ± 390.1 in clone 14 (n = 24; p < 0.001 vs wild type), and 2744.5 ± 226.9 cpm in clone 17 (n = 6; p > 0.1 vs wild type).
The above results reveal that in PC12-P2Y2 cells, the ability of P2Y2 receptors to mediate nucleotide-dependent IP1 synthesis was markedly enhanced. To evaluate whether overexpressed P2Y2 receptors might become activated by spontaneously released nucleotides, we further used clone 14, which displayed the highest levels of spontaneous as well as UTP-dependent IP1 synthesis (Fig. 5A). Nevertheless, apyrase (1 U/ml) failed to significantly reduce the accumulation of IP1 in the absence of exogenous nucleotides, although the enzyme did reduce the stimulating effect of ATP. Depolarization of PC12-P2Y2 cells by 100 mm K+ enhanced the IP1 accumulation to almost the same extent as 10 μm ATP (Fig. 5C), as described above for untransfected PC12 cells (Fig. 3). Again, in PC12-P2Y2 cells the IP1-increasing action of 100 mm K+ was abolished in the presence of apyrase and by the Ca2+ channel blockade by Cd2+ (Fig. 5C), as it was in untransfected PC12 cells. Thus, overexpression of P2Y2 receptors was not sufficient to allow for a stimulation of IP1 synthesis by released nucleotides in the absence of depolarizing stimuli.
Autocrine-paracrine activation of heterologously expressed P2Y1 receptors
P2Y2 receptors are equipotently activated by ATP and UTP, but they are insensitive to nucleoside diphosphates (Nicholas et al., 1996). P2Y12 receptors, in contrast, are potently activated by ADP (Hollopeter et al., 2001). To learn whether another P2Y receptor that accepts diphosphates as agonists might also be activated by spontaneously released nucleotides, PC12 cell lines permanently expressing rat P2Y1 receptors linked to eGFP (PC12-P2Y1) were generated. Previously, this fusion protein was found to mediate a stimulation of IP synthesis about as efficiently as nontagged rat P2Y1 receptors (Vohringer et al., 2000). In the three PC12-P2Y1 clones that were tested for IP1 accumulation (clones 4, 5, and 8), its synthesis in the absence of added nucleotides was increased by factors of up to 45. Addition of 1 μm of the P2Y1 receptor antagonist MRS 2216 (Nandanan et al., 1999) significantly reduced these values of spontaneous IP1 accumulation in these three PC12-P2Y1 clones but had no effect in untransfected PC12 cells (Fig. 6A). This action of MRS 2216 was concentration dependent, with half-maximal effects at 0.37 ± 0.13 μm (Fig. 6B). This value is in reasonable agreement with the half-maximal concentrations of MRS 2216 (0.2 μm) required to antagonize the stimulation of IP synthesis via P2Y1 receptors in turkey erythrocytes (Nandanan et al., 1999). Addition of apyrase (1 U/ml) also reduced the spontaneous accumulation of IP1 in the three PC12-P2Y1 clones tested by 75% to >90% (Fig. 6C).
Figure 6.
Stimulation of IP synthesis in P2Y1 receptor-expressing cells. After loading with myo-[3H]inositol and preincubation in LiCl (10 mm), the cells were incubated for 30 min in LiCl alone or LiCl plus the indicated concentrations of MRS 2216, apyrase, 2-methylthio ATP (2MeSATP), KCl (NaCl was reduced accordingly), or CdCl2. The amount of radioactivity retrieved within the fraction of inositol monophosphate (IP1) was calculated as percentage of the total water-soluble radioactivity extracted from the cell cultures (A), and values obtained in the presence of the above agents were normalized to the data obtained in their absence (normalized to basal) (B[en]D). A, Either wild-type PC12 cells or three clones (clones 4, 5, and 8) of P2Y1 receptor-expressing cells were exposed to LiCl (open bars) or LiCl plus 1 μm MRS 2216 (filled bars); n = 6-12. The p values shown above the bars are related to the differences between the values obtained in the presence of LiCl in the P2Y1 receptor-expressing cells versus the same value in wild-type PC12 cells; *p < 0.05, **p < 0.01, and ***p < 0.001 versus the value obtained in the absence of MRS 2216, respectively. B, PC12-P2Y1 cells of clone 5 were incubated in the indicated concentrations of MRS 2216; n = 4-6. C, Three clones (clones 4, 5, and 8) of P2Y1 receptor-expressing cells were exposed to LiCl (open bars) or LiCl plus apyrase (filled bars); n = 6-12; **p < 0.01 and ***p < 0.001 versus the value obtained in the absence of apyrase, respectively. D, Two clones (clones 5 and 8) of P2Y1 receptor-expressing cells were exposed to the indicated concentrations of one or more of the following: 2MeSATP, apyrase, KCl, and CdCl2; n = 6-12; ***p < 0.001 versus the value obtained in the presence of LiCl only; n.s. indicates no significant difference. In the absence of nucleotides or other agents, 3178.6 ± 153.6 cpm were retrieved in the IP1 fraction of wild-type PC12 cells (n = 6), 119973.8 ± 9125.1 cpm in clone 4 (n = 9; p < 0.001 vs wild type), 306452.8 ± 25304.4 in clone 5 (n = 24; p < 0.001 vs wild type), and 13004.3 ± 1974.7 cpm in clone 8 (n = 15; p < 0.001 vs wild type).
The data shown above indicate that the P2Y1 receptors were highly activated under our routine experimental conditions. This conclusion is supported further by the fact that addition of 2MeSATP (10 μm), which potently activates the P2Y1-eGFP fusion protein in human embryonic kidney (HEK) 293 cells (Vohringer et al., 2000), either failed to significantly enhance IP1 accumulation or caused an increase by only 13 ± 3% (Fig. 6D). In untransfected PC12 cells, for comparison, this P2Y1 agonist also failed to cause significant changes in IP1 synthesis (Fig. 1B). Depolarization of PC12-P2Y1 cells by 100 mm K+ did not enhance the accumulation of IP1, nor did the concomitant application of 1 mm Cd2+ cause any further change. In the presence of 100 mm K+ plus 1 U/ml apyrase, the IP1 values obtained with PC12-P2Y1 cells were significantly lower than those obtained under control conditions and about the same as those obtained in the presence of apyrase only (Fig. 6D). Thus, P2Y1 receptors heterologously expressed in PC12 cells are spontaneously activated to an extent that renders further activation by either exogenously added or endogenously released nucleotides virtually impossible.
Activation of heterologously expressed P2Y1 receptors by added nucleotides
From the data presented above it was not possible to estimate at which nucleotide concentrations the P2Y1-GFP receptors were activated when expressed in PC12 cells. To be able to do so, we searched for a measure of receptor activation that could be determined in the absence of released nucleotides. Single PC12 cells can be investigated by electrophysiological recording techniques under continuous superfusion to remove released nucleotides (Vartian and Boehm, 2001). P2Y1 receptors, when expressed in rat superior cervical ganglion neurons, mediate an inhibition of KM channels in the presence of nanomolar ADP concentrations (Brown et al., 2000). We therefore hypothesized that this effect might also be seen in PC12 cells and determined IM under continuous superfusion. In wild-type PC12 cells, ADP failed to cause significant changes in IP1 accumulation (Fig. 1B) and left IM amplitudes unchanged (Fig. 7A). In PC12-P2Y1 cells, however, ADP reduced IM in a
Figure 7.
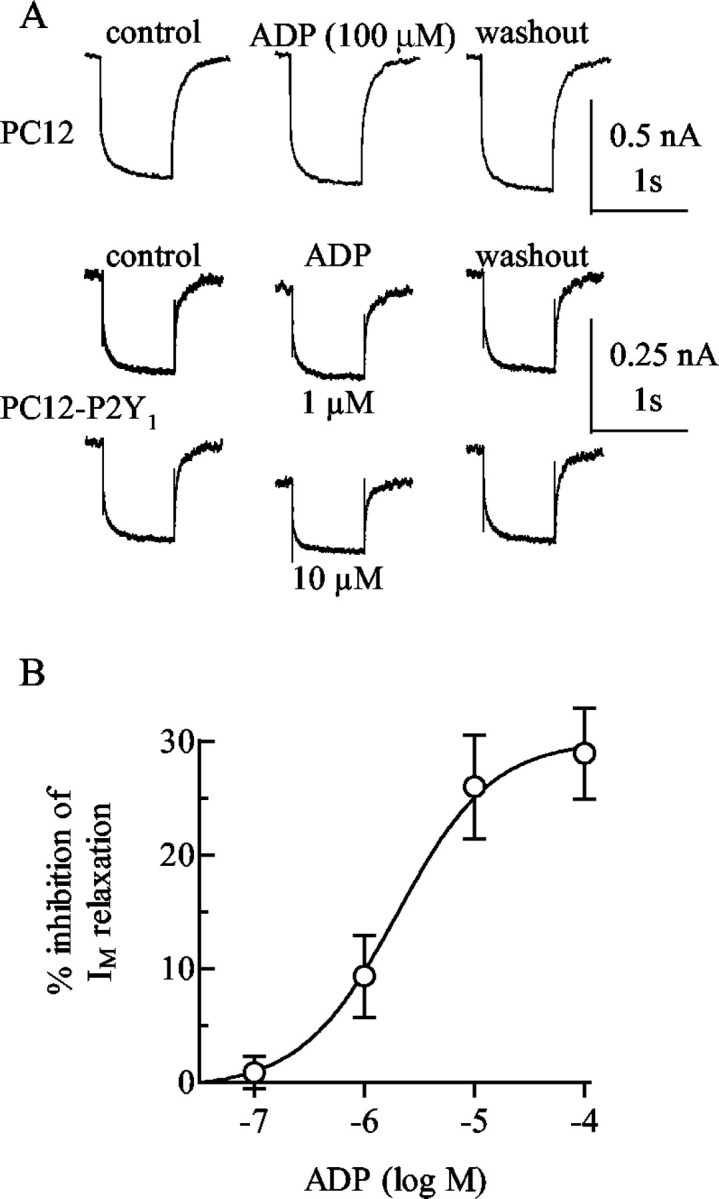
Inhibition of IM in P2Y1 receptor expressing cells. A, IM was measured by the amphotericin B-perforated patch technique in a wild-type PC12 cell and in a PC12 cell stably expressing P2Y1 receptors (PC12-P2Y1). The current traces shown were obtained by clamping the cell at -30 mV and by applying 1 sec hyperpolarizing voltage steps to -55 mV; the recordings were performed before (control), during, and after (washout) the application of the indicated concentrations of ADP. B, Four to six PC12-P2Y1 cells were exposed to the indicated concentrations of ADP, and the graph shows the concentration dependence of the inhibition of IM relaxation amplitudes, determined as shown in A.
Discussion
P2X receptors mediate neurotransmission when ATP is released by presynaptic action potentials (Robertson et al., 2001). In contrast, the mechanisms of activation and the functions of neuronal P2Y receptors are essentially unknown (Boehm, 2003). When P2Y receptors are heterologously expressed in neurons, their activation leads to an inhibition of KM and voltage-gated Ca2+ channels (Simon et al., 2002; Filippov et al., 2003). Evidence has been presented that depolarization of chromaffin cells leads to an autocrine-paracrine inhibition of Ca2+ channels via opioid and P2Y receptors (Currie and Fox, 1996). In non-neural cells, in contrast, the autocrine-paracrine activation of P2Y receptors does not require depolarizing stimuli, and a simple exchange of culture media was found sufficient (Lazarowski et al., 2000; Ostrom et al., 2000). By using PC12 cells, the present results reveal that neuronal P2Y receptors are differentially activated by spontaneous and depolarization-evoked nucleotide release.
The accumulation of IPs and the inhibition of cAMP synthesis in the presence of added nucleotides revealed that PC12 cells express two types of functional P2Y receptors. The one coupled to IP synthesis is a P2Y2 receptor as revealed by the following results: (1) it was equipotently activated by ATP and UTP and thus could be P2Y2 or P2Y4 (Kennedy et al., 2000); (2) P2Y2 receptors are expressed at higher levels than P2Y4; (3) the receptor was blocked by suramin but not by PPADS, and the reverse is true for P2Y4 (Bogdanov et al., 1998; Suarez-Huerta et al., 2001); (4) differentiation of PC12 cells reduced the expression of P2Y2 and diminished the IP synthesis in response to UTP. The reduction of cAMP, in contrast, is induced by ADP and ATP, but not UDP or UTP, and is antagonized by the P2Y12 antagonists 2MeSAMP and AR-C69931MX (Unterberger et al., 2002; Kubista et al., 2003), thus indicating a role of this latter receptor.
Degradation of nucleotides by apyrase did not alter IP accumulation in the absence of added nucleotides but enhanced the cAMP synthesis triggered by A2A receptor activation. Thus, P2Y12 but not P2Y2 receptors were activated spontaneously. Depolarization of PC12 cells further decreased the cAMP synthesis and increased the IP accumulation. Both effects were abolished by apyrase and by the blockade of voltage-gated Ca2+ channels by Cd2+. Hence, both types of P2Y receptors expressed in PC12 cells can be activated by depolarization-evoked nucleotide release.
One reason for the differential sensitivities for endogenously released nucleotides could be differences in agonist potencies of nucleotides at the receptors involved; however, overexpression of P2Y2 receptors did not allow for spontaneous receptor activation, although the potencies and efficacies of added nucleotides to induce IP synthesis were increased. In contrast, the heterologous expression of P2Y1 receptors led to huge increases in spontaneous IP accumulation, which was hardly enhanced by added nucleotides or depolarization of the cells, but mostly reduced by apyrase or a P2Y1 receptor antagonist. Nevertheless, these P2Y1 receptors could be activated by adding ADP when released nucleotides were removed by continuous superfusion. Furthermore, similar concentrations of added nucleotides were required to cause half-maximal activation of overexpressed P2Y2 receptors (1.4 μm ATP) and heterologously expressed P2Y1 receptors (1.9 μm ADP). Thus, there were no significant differences in the potencies of agonistic nucleotides at these two receptors expressed in PC12 cells. One major difference between P2Y1 and P2Y2 receptors is their distinct nucleotide sensitivity: P2Y1 but not P2Y2 receptors are activated not only by ATP but also by ADP (Ralevic and Burnstock, 1998). P2Y12 receptors also accept ADP as an agonist (Hollopeter et al., 2001). Hence, the propensity of recombinant P2Y1 and native P2Y12 receptors to be activated by spontaneous nucleotide release is related to their sensitivity for nucleoside diphosphates rather than to higher receptor densities or higher nucleotide potencies.
This leads to the question of species and quantities of nucleotides released from PC12 cells. Large and small dense-core vesicles contain millimolar concentrations of adenine nucleotides. Among these, one can find ATP, ADP, and AMP at a 1:0.14:0.04 ratio (Zimmermann, 1994). Therefore, the main nucleotide released from PC12 cells is ATP. We did not try to determine concentrations of ATP or ADP in the culture medium, because concentrations of nucleotides in solutions in which cells under investigation are incubated do not reflect the concentrations close to the plasma membrane, where receptors are activated (Beigi et al., 1999). Nevertheless, our results with endogenous and overexpressed P2Y2 receptors provide an indication of the periplasmalemmal ATP concentration yielded by K+ depolarization: 100 mm K+ stimulated IP synthesis somewhat less than 10 μm but definitely more than 1 μm ATP. Similarly, the K+ depolarization reduced cAMP accumulation to almost the same extent as 10 μm ADP, which is equipotent and equi-effective to ATP in inhibiting adenylyl cyclase in PC12 cells (Unterberger et al., 2002). Therefore, the concentration of endogenous ATP surrounding PC12 cells during K+ depolarization must be in the low micromolar range. This estimate is similar to the concentration of ATP surrounding platelets when stimulated by thrombin, which lies between 5 and 20 μm (Beigi et al., 1999). Because neither endogenous nor overexpressed P2Y2 receptors were activated under basal conditions, the periplasmalemmal ATP concentration in the absence of depolarizing stimuli must be below the 100 nm of added ATP that was required to cause minimal stimulation of IP synthesis (Fig. 5B).
In contrast to P2Y2 receptors, endogenous P2Y12 and recombinant P2Y1 receptors were activated spontaneously. At rat P2Y12 receptors, ADP is 10-fold more potent than ATP (Simon et al., 2002), and at the rat P2Y1-GFP fusion protein, ADP is 100-fold more potent than ATP (Vohringer et al., 2000). Thus, the spontaneous activation of P2Y12 and P2Y1 receptors was mediated by ADP rather than ATP. The spontaneous activation of P2Y12 receptors limited the stimulation of cAMP synthesis by the A2A receptor agonist CGS 21680. In the absence of added nucleotides, CGS 21680 enhanced cAMP accumulation 20-fold; in the presence of apyrase or the P2Y12 antagonist 2MeSAMP, however, this stimulation was increased to ≥50-fold, and added ADP reduced this stimulation down to 5-fold over basal. Thus, the value obtained in the absence of agonists and antagonists was close to the arithmetic mean of these two extremes, which indicates that the receptors were approximately half-activated. In rat endothelial cells, inhibition of adenylyl cyclase via P2Y12 receptors is half-maximal at 3 μm ADP (Simon et al., 2002). P2Y1-GFP receptors were completely activated under conditions of spontaneous nucleotide release. When released nucleotides were removed by superfusion, the P2Y1-GFP receptor was activated at 1-10 μm ADP, as determined by the inhibition of IM. In HEK 293 cells expressing the P2Y1-GFP receptor, ∼1 μm ADP causes maximal IP accumulation (Vohringer et al., 2000). Taken together, the above considerations suggest that the basal periplasmalemmal ADP concentration in PC12 cell cultures was in the range of 1-10 μm.
These data indicate that in PC12 cell cultures, ADP concentrations are higher than those of ATP, although more ATP is released (Zimmermann, 1994). The explanation for this discrepancy is the fact that PC12 cells express ectonucleotidases that degrade ATP four times more rapidly than ADP. As a consequence, extracellular ADP accumulates (Vollmayer et al., 2001), and this is the basis for the spontaneous activation of nucleoside diphosphate-sensitive (P2Y12 and P2Y1) receptors and the lack of spontaneous activity of (P2Y2) receptors that are sensitive only to nucleoside triphosphates. Nevertheless, these latter receptors become activated when the release of ATP is increased by K+ depolarization and exceeds its degradation to ADP. Thus, the endowment of PC12 cells with ectonucleotidases and P2Y receptors provides specific sensors for spontaneous and stimulation-evoked vesicle exocytosis. Accordingly, in PC12 cells, levels of second messengers linked to P2Y receptors will correlate with the electrical activity of the cells.
The present as well as previous results show that in a given cell, the autocrine-paracrine regulation via released nucleotides depends on the presence of both P2Y receptors and nucleotidases. In hepatoma cells (Junankar et al., 2002), for example, released ATP is degraded to ADP to stimulate P2Y1 receptors. In epithelial (Torres et al., 2002) and osteoblastic (You et al., 2002) cells, in contrast, released ATP itself activates P2Y11 and P2Y2 receptors, respectively. In PC12 cells, both types of autocrine-paracrine regulation occur under resting conditions and during depolarization and involve P2Y12 and P2Y2 receptors, respectively. In sympathetic neurons that are ontogenetically related to PC12 cells, nucleotide release occurs at a low rate that is mostly enhanced by depolarization (von Kugelgen et al., 1994). Together with nucleotides, sympathetic neurons release soluble nucleotidases that degrade ATP twice as rapidly as ADP (Mihaylova-Todorova et al., 2002), and therefore ADP accumulates rather than ATP (Todorov et al., 1997). Because sympathetic neurons, like PC12 cells, express several P2Y receptors linked to different signaling cascades (Fig. 2C), the phenomena described above for PC12 cells can be expected to apply to the sympathetic and possibly to other pathways within the nervous system.
Footnotes
This work was supported by European Commission Grant QLRT-2000-00929 and by grants from the Austrian Science Fund (Fonds zur Foerderung der Wissenschaftlichen Forschung; P14951 and P15797) and from the Virologie Fonds of the Medical Faculty of the University of Vienna. We are indebted to Dr. M. Freissmuth for carefully reading this manuscript.
Correspondence should be addressed to Stefan Boehm, Institute of Pharmacology, University of Vienna, Waehringerstrasse 13a, A-1090 Vienna, Austria. E-mail: stefan.boehm@univie.ac.at.
Copyright © 2003 Society for Neuroscience 0270-6474/03/237479-10$15.00/0
References
- Arslan G, Filipeanu CM, Irenius E, Kull B, Clementi E, Allgaier C, Erlinge D, Fredholm BB ( 2000) P2Y receptors contribute to ATP-induced increases in intracellular calcium in differentiated but not undifferentiated PC12 cells. Neuropharmacology 39: 482-496. [DOI] [PubMed] [Google Scholar]
- Beigi R, Kobatake E, Aizawa M, Dubyak GR ( 1999) Detection of local ATP release from activated platelets using cell surface-attached firefly luciferase. Am J Physiol 276: C267-C278. [DOI] [PubMed] [Google Scholar]
- Boehm S ( 1999) ATP stimulates sympathetic transmitter release via presynaptic P2X purinoceptors. J Neurosci 19: 737-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm S ( 2003) P2Ys go neuronal: modulation of Ca2+ and K+ channels by recombinant receptors. Br J Pharmacol 138: 1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bofill-Cardona E, Vartian N, Nanoff C, Freissmuth M, Boehm S ( 2000) Two different signaling mechanisms involved in the excitation of rat sympathetic neurons by uridine nucleotides. Mol Pharmacol 57: 1165-1172. [PubMed] [Google Scholar]
- Bogdanov YD, Wildman SS, Clements MP, King BF, Burnstock G ( 1998) Molecular cloning and characterization of rat P2Y4 nucleotide receptor. Br J Pharmacol 124: 428-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Filippov AK, Barnard EA ( 2000) Inhibition of potassium and calcium currents in neurones by molecularly-defined P2Y receptors. J Auton Nerv Syst 81: 31-36. [DOI] [PubMed] [Google Scholar]
- Communi D, Pirotton S, Parmentier M, Boeynaems JM ( 1995) Cloning and functional expression of a human uridine nucleotide receptor. J Biol Chem 270: 30849-30852. [DOI] [PubMed] [Google Scholar]
- Communi D, Govaerts C, Parmentier M, Boeynaems JM ( 1997) Cloning of a human purinergic receptor coupled to phospholipase C and adenylyl cyclase. J Biol Chem 51: 31969-31973. [DOI] [PubMed] [Google Scholar]
- Communi D, Gonzales NS, Detheux M, Brezillon S, Lannoy V, Parmentier M, Boeynaems JM ( 2001) Identification of a novel human ADP receptor coupled to Gi. J Biol Chem 276: 41479-41485. [DOI] [PubMed] [Google Scholar]
- Currie KPM, Fox AP ( 1996) ATP serves as a negative feedback inhibitor of voltage-gated Ca2+ channel currents in bovine adrenal chromaffin cells. Neuron 16: 1027-1036. [DOI] [PubMed] [Google Scholar]
- De Lean A, Munson PJ, Rodbard D ( 1978) Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol 235: 97-102. [DOI] [PubMed] [Google Scholar]
- Filippov AK, Simon J, Barnard EA, Brown DA ( 2003) Coupling of the nucleotide P2Y4 receptor to neuronal ion channels. Br J Pharmacol 138: 400-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RJ, Burgoyne RD ( 1999) The effect of transfection with Botukinum neurotoxin C1 light chain on exocytosis measured in cell populations and by single-cell amperometry in PC12 cells. Pflügers Arch 437: 754-762. [DOI] [PubMed] [Google Scholar]
- Gerasimovskaya EV, Ahmad S, White CW, Jones PL, Carpenter TC, Stenmark KR ( 2002) Extracellular ATP is an autocrine-paracrine regulator of hypoxia-induced adventitial fibroblast growth. J Biol Chem 277: 44638-44650. [DOI] [PubMed] [Google Scholar]
- Greene LA, Tischler AS ( 1976) Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA 73: 2424-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollopeter G, Jantzen HM, Vincent D, Li G, England L, Ramakrishnan V, Yang RB, Nurden A, Julius D, Conley PB ( 2001) Identification of the plateleted ADP receptor targeted by antithrombotic drugs. Nature 409: 202-207. [DOI] [PubMed] [Google Scholar]
- Junankar PR, Karjalainen A, Kirk K ( 2002) The role of P2Y1 purinergic receptors and cytosolic Ca2+ in hypotonically activated osmolyte efflux from a rat hepatoma cell line. J Biol Chem 277: 40324-40334. [DOI] [PubMed] [Google Scholar]
- Kennedy C, Qi AD, Herold CL, Harden TK, Nicholas RA ( 2000) ATP, an agonist at the rat P2Y(4) receptor, is an antagonist at the human P2Y4 receptor. Mol Pharmacol 57: 926-931. [PubMed] [Google Scholar]
- Kubista H, Lechner SG, Wolf AM, Boehm S ( 2003) Attenuation of the P2Y receptor-mediated control of neuronal Ca2+ channels in PC12 cell by antithrombotic drugs. Br J Pharmacol 138: 343-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski ER, Boucher RC, Harden TK ( 2000) Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. J Biol Chem 275: 31061-31068. [DOI] [PubMed] [Google Scholar]
- Lustig KD, Shiau AK, Brake AJ, Julius D ( 1993) Expression cloning of an ATP receptor from mouse neuroblastoma cells. Proc Natl Acad Sci USA 90: 5113-5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G ( 1996) Transmitter release. Annu Rev Neurosci 19: 219-233. [DOI] [PubMed] [Google Scholar]
- Mihaylova-Todorova ST, Todorov LD, Westfall DP ( 2002) Enzyme kinetics and pharmacological characterization of nucleotidases released from the guinea pig isolated vas deferens during nerve stimulation: evidence for a soluble ecto-nucleoside triphosphate diphosphohydrolase-like ATPase and a soluble ecto-5′-nucleotidase-like AMPase. J Pharmacol Exp Ther 302: 992-1001. [DOI] [PubMed] [Google Scholar]
- Murrin JA, Boarder MR ( 1992) Neuronal nucleotide receptor linked to phospholipase C and phospholipase D? Stimulation of PC12 cells by ATP analogues and UTP. Mol Pharmacol 41: 561-568. [PubMed] [Google Scholar]
- Nandanan E, Camaioni E, Jang SY, Kim YC, Cristalli G, Herdewijn P, Secrist JA, Tiwari KN, Mohanram A, Harden TK, Boyer JL, Jacobson KA ( 1999) Structure-activity relationships of biphosphate nucleotide derivatives as P2Y1 receptor antagonists and partial agonists. J Med Chem 42: 1625-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas RA, Watt WC, Lazarowski ER, Li Q, Harden TK ( 1996) Uridine nucleotide selectivity of three phospholipase C-activating P2 receptors: identification of a UDP-selective, a UTP-selective, and an ATP- and UTP-specific receptor. Mol Pharmacol 50: 224-229. [PubMed] [Google Scholar]
- Ostrom RS, Gregorian C, Insel PA ( 2000) Cellular release of and response to ATP as key determinants of the set-point of signal transduction pathways. J Biol Chem 275: 11735-11739. [DOI] [PubMed] [Google Scholar]
- Phiel CJ, Klein PS ( 2001) Molecular targets of lithium action. Annu Rev Pharmacol Toxicol 41: 789-813. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G ( 1998) Receptors for purines and pyrimidines. Pharmacol Rev 50: 413-492. [PubMed] [Google Scholar]
- Robertson SJ, Ennion SJ, Evans RJ, Edwards FA ( 2001) Synaptic P2X receptors. Curr Opin Neurobiol 11: 378-386. [DOI] [PubMed] [Google Scholar]
- Schlosser SF, Burgstahler AD, Nathanson MH ( 1996) Isolated rat hepatocytes can signal to other hepatocytes and bile duct cells by release of nucleotides. Proc Natl Acad Sci USA 93: 9948-9953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholze T, Moskvina E, Mayer M, Just H, Kubista H, Boehm S ( 2002) Sympathoexcitation by bradykinin involves Ca2+-independent protein kinase C. J Neurosci 22: 5823-5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwiebert EM, Wallace DP, Braunstein GM, King SR, Peti-Peterdi J, Hanaoka K, Guggino WB, Guay-Woodford LM, Bell BD, Suillivan LP, Grantham JJ, Taylor AL ( 2002) Autocrine extracellular purinergic signaling in epithelial cells derived from polycystic kidneys. Am J Physiol 282: F763-F775. [DOI] [PubMed] [Google Scholar]
- Simon J, Filippov AK, Goransson S, Wong YH, Frelin C, Michel AD, Brown DA, Barnard EA ( 2002) Characterization and channel coupling of the P2Y12 nucleotide receptor of brain capillary endothelial cells. J Biol Chem 277: 31390-31400. [DOI] [PubMed] [Google Scholar]
- Suarez-Huerta N, Pouillon V, Boeynaems JM, Robaye B ( 2001) Molecular cloning and characterization of the mouse P2Y4 receptor. Eur J Pharmacol 416: 197-202. [DOI] [PubMed] [Google Scholar]
- Tokuyama Y, Hara M, Jones EMC, Fan Z, Bell GI ( 1995) Cloning of rat and mouse P2Y purinoceptors. Biochem Biophys Res Commun 211: 211-218. [DOI] [PubMed] [Google Scholar]
- Todorov LD, Mihaylova-Todorova S, Westfall TD, Sneddon P, Kennedy C, Bjur RA, Westfall DP ( 1997) Neuronal release of soluble nucleotidases and their role in neurotransmitter inactivation. Nature 387: 76-79. [DOI] [PubMed] [Google Scholar]
- Torres B, Zambon AC, Insel PA ( 2002) P2Y11 receptors activate adenylyl cyclase and contribute to nucleotide-promoted cAMP formation in MDCK-D1 cells. J Biol Chem 277: 7761-7765. [DOI] [PubMed] [Google Scholar]
- Unterberger U, Moskvina E, Scholze T, Freissmuth M, Boehm S ( 2002) Inhibition of adenylyl cyclase by neuronal P2Y receptors. Br J Pharmacol 135: 673-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartian N, Boehms S ( 2001) P2Y receptor-mediated inhibition of voltage-activated Ca2+ currents in PC12 cells. Eur J Neurosci 13: 899-908. [DOI] [PubMed] [Google Scholar]
- Vartian N, Moskvina E, Scholze T, Unterberger U, Allgaier C, Boehm S ( 2001) UTP evokes noradrenaline release from rat sympathetic neurons by activation of protein kinase C. J Neurochem 77: 876-885. [DOI] [PubMed] [Google Scholar]
- Vohringer C, Schafer R, Reiser G ( 2000) A chimeric rat brain P2Y1 receptor tagged with green fluorescent protein: high affinity ligand recognition of adenosine diphosphates and triphosphates and selectivity identical to that of the wild-type receptor. Biochem Pharmacol 59: 791-800. [DOI] [PubMed] [Google Scholar]
- Vollmayer P, Koch M, Braun N, Heine P, Servos J, Israr E, Kegel B, Zimmermann H ( 2001) Multiple ectonucleotidases in PC12 cells: identification and cellular distribution after heterologous expression. J Neurochem 78: 1019-1028. [DOI] [PubMed] [Google Scholar]
- von Kugelgen I, Allgaier C, Schobert A, Starke K ( 1994) Co-release of noradrenaline and ATP from cultured sympathetic neurons. Neuroscience 61: 199-202. [DOI] [PubMed] [Google Scholar]
- Wang Y, Roman R, Lidofsky SD, Fitz JG ( 1996) Autocrine signaling through ATP release represents a novel mechanism for cell volume regulation. Proc Natl Acad Sci USA 93: 12020-12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You J, Jacobs CR, Steinberg TH, Donahue HJ ( 2002) P2Y purinoceptors are responsible for oscillatory fluid flow-induced intracellular Ca2+ mobilization in osteoblastic cells. J Biol Chem 277: 48724-48729. [DOI] [PubMed] [Google Scholar]
- Zimmermann H ( 1994) Signaling via ATP in the nervous system. Trends Neurosci 17: 420-426. [DOI] [PubMed] [Google Scholar]





