Abstract
Early odor preference learning in rats is associated with increases of phosphorylated CREB (pCREB) in mitral cells of the olfactory bulb. In the present study, herpes simplex virus expressing CREB (HSV-CREB) and dominant-negative mutant CREB (HSV-mCREB) have been injected into the bulb to assess a causal role for CREB and pCREB in this model. Odor paired with stroking or with the β-adrenoceptor agonist isoproterenol produces odor approach 24 hr later. Isoproterenol-induced learning exhibits an inverted U curve dose-dependent learning relationship with both low and high doses failing to produce learning. pCREB increases have only been seen at the learning effective dose.
In the present study, injection of an HSV vector expressing mutant CREB into the olfactory bulb prevented learning induced by stroking. Control HSV expressing LacZ was without effect. Expression of mutant CREB shifted the dose–learning curve for isoproterenol to the right such that a higher dose was required to induce learning. Expression of CREB shifted the dose-learning curve for isoproterenol to the left, with a lower dose now producing learning. As expected from this shift, CREB overexpression interfered with learning induced by stroking.
When learning occurred, with either CREB or mutant CREB, pCREB was observed to be elevated relative to the nonlearning LacZ control groups. Unexpectedly, with odor plus stroking in the nonlearning CREB group, the level of pCREB was also higher than with odor plus stroking in LacZ controls that did learn.
The data demonstrate a causal role for CREB and pCREB in early mammalian odor preference learning, reinforcing CREB as a “universal” memory molecule. They support evidence that CREB overexpression can be deleterious and suggest the hypothesis of an optimal pCREB window for learning.
Keywords: herpes simplex virus, cAMP response element-binding protein, memory, pCREB assay, olfactory conditioning, isoproterenol
Introduction
Early odor preference learning offers a unique paradigm for the study of natural mammalian learning. In the neonate rat, neural circuitry changes that are critical for odor preference memory occur in the olfactory bulb (Wilson and Sullivan, 1994; Sullivan et al., 2000). We suggested that a change in the first synapse, the olfactory input to mitral cell connection, may underlie both the acquisition and expression of odor preference (Yuan et al., 2000, 2002). Phosphorylation of cAMP response element-binding protein (CREB), proposed as a universal “memory molecule” (Silva et al., 1998), is significantly increased in mitral cells after olfactory preference training (McLean et al., 1999). The present study asks whether phosphorylation of CREB in the olfactory bulb can be shown to be causal in early olfactory preference learning.
In neonate rats, a single 10 min session of tactile stimulation such as stroking, (unconditioned stimulus) paired with an odor (conditioned stimulus), typically peppermint, produces odor preference learning, seen as an approach to the odor 24 hr later (Sullivan and Leon, 1987; Wilson and Sullivan, 1994). Norepinephrine (NE) released from the locus ceruleus with tactile stimulation is known to act in the olfactory bulb via β-adrenoceptors (Wilson and Sullivan, 1994; Langdon et al., 1997; Yuan et al., 2000). The β-adrenoceptor agonist isoproterenol can substitute for stroking when given systemically or directly into the olfactory bulb to induce learning (Langdon et al., 1997; Sullivan et al., 2000; Yuan et al., 2000). NE and serotonin (5-HT) in the olfactory bulb have been shown to interact in early odor preference learning to promote increases in the cAMP-mediated CREB phosphorylation (Yuan et al., 2000). pCREB increases are transient and occur selectively in the peppermint-encoding area of the mitral cell layer in the olfactory bulb after peppermint conditioning (McLean et al., 1999). Both learning and increases in phosphorylated CREB (pCREB) occur when odor is paired with a moderate dose of isoproterenol (2 mg/kg) but not with lower (1 mg/kg) or higher (4 or 6 mg/kg) doses of isoproterenol (Sullivan et al., 1989a, 1991; Langdon et al., 1997; Yuan et al., 2000). The parallel inverted U curve profiles in both isoproterenol-induced learning and CREB phosphorylation can be shifted to the right by depleting 5-HT fibers in the olfactory bulb (Yuan et al., 2000), such that a higher dose of isoproterenol is now required in inducing learning and pCREB.
On the basis of these studies, we proposed a causal role for CREB in neonate rat odor preference learning, but the evidence was correlational (McLean et al., 1999; Yuan et al., 2000). Here we evaluate causality using a herpes simplex virus vector (HSV) to express additional CREB or dominant-negative mutant CREB (mCREB) (single point mutation at the phosphorylation site serine 133) in the neurons of rat pup olfactory bulbs. HSV-LacZ expressing Escherichia coli β-galactosidase was used to examine the expression of HSV-encoded proteins in the olfactory bulb and as a control to determine whether virus injection itself would affect odor preference learning.
In the present study, we ask whether additional CREB or mutant CREB in the olfactory bulb will alter normal odor preference learning and/or promote the occurrence of odor preference learning when sub-optimal or supra-optimal doses of isoproterenol are given. We also measure the levels of pCREB to assess the link between the substrate and the transcription factor.
Materials and Methods
Animals
Sprague Dawley rat pups of both sexes were used in this study. Litters were culled to 12 pups per litter on postnatal day 1 (P1) (the day of birth is considered P0). The dams were maintained under a 12 hr light/dark cycle, with ad libitum access to food and water. All experimental procedures were approved by the Memorial University Institutional Animal Care Committee.
Virus vector
HSV-LacZ, HSV-CREB (overexpression of CREB), and HSV-mCREB (overexpression of a dominant-negative mutant CREB) were used in this study with the average titer of the recombinant virus stocks at 4.0 × 107 infectious U/ml (for viral vector preparation, see Carlezon et al., 1998; Neve and Geller, 1999).
Virus injection
On P4, rat pups were anesthetized under hypothermia on ice and placed in a stereotaxic frame. The skull over the central region of each olfactory bulb was carefully removed by a dental drill. A total of 1 μl of virus per bulb was injected at four levels from ventral to dorsal into each bulb over a 2 min time course using a 27 gauge Hamilton syringe. Pups were warmed up and returned to dams after recovery.
Odor conditioning
The procedure for odor conditioning for natural learning has been described previously (Sullivan et al., 1989b, 1991; McLean et al., 1993). Briefly, on P6, rat pups were removed from the dam and placed on fresh bedding 10 min before odor exposure. In the odor plus stroking (O/S) group, pups were placed on peppermint-scented bedding (0.3 ml of peppermint per 500 ml of normal bedding) and stroked vigorously on the hind region using a sable brush every other 30 sec for 30 sec over a 10 min period. In the odor only (O/O) group, the pups were only exposed to the peppermint scented-bedding without being stroked. The naive pups were placed on fresh bedding for a 10 min period. Immediately after these conditions, the pups were returned to the dams.
The procedure for odor conditioning using isoproterenol has been described previously (Langdon et al., 1997; Yuan et al., 2000). Briefly, on P6, saline or (±) isoproterenol (1, 2, or 4 mg/kg; Sigma, St. Louis, MO) was injected subcutaneously into pups 40 min before exposure to the peppermint odor. Each pup was removed from the dam 30 min after injection and placed on fresh bedding. Ten minutes later, pups were placed on peppermint-scented bedding for 10 min. After odor exposure, pups were returned to their dams.
Odor preference test
On P7, pups were subjected to odor preference testing. A stainless steel test box (30 × 20 × 18 cm) was placed on two training boxes that were separated by a 2 cm neutral zone. One box contained fresh bedding; the other contained peppermint-scented bedding. Each pup was removed from the dam and placed in the neutral zone of the test box. The amount of time the pup spent on either peppermint-scented bedding or normal bedding was recorded for five 1 min trials. The percentage of time the pup spent on peppermint-scented bedding over the 5 min period was calculated.
5-Bromo-4chloro-3-indolyl-β-d-galactoside histochemistry
The LacZ marker gene produces β-galactosidase, which can be visualized by 5-bromo-4chloro-3-indolyl-β-d-galactoside (X-gal) histochemical staining. X-gal histochemistry was performed on the olfactory bulbs of HSV-LacZ-injected pups. Expression of the virus has been reported to be maximal at 2–4 d after injection (Carlezon et al., 1998). Thus, 2 or 3 d after viral injection, pups were given an overdose of sodium pentobarbital (80 mg/kg) and perfused transcardially with an ice-cold saline solution, followed by a fixative solution (0.5% paraformaldehyde plus 2% glutaraldehyde in 0.1 m phosphate buffer, pH 7.4). Brains were removed from the skull, postfixed in the same solution for 1 hr, and then transferred to a 30% sucrose solution overnight.
Coronal sections (40 μm) were cut in a cryostat the next day. Sections were mounted onto slides and air dried at room temperature. Alternate sections were collected for X-gal staining and Nissl staining. Slides containing olfactory bulb sections for X-gal staining were then incubated overnight with a solution containing 3.1 mm potassium ferricyanide, 3.1 mm potassium ferrocyanide, 0.15 m NaCl, 1 mm MgCl2, 0.01% sodium deoxycholate, 0.02% NP-40, and 0.2 mg/ml X-gal (Invitrogen, Grand Island, NY) (dissolved in N, N′-dimethyl formamide) in 10 mm phosphate buffer, pH 7.4. An insoluble blue color indicated β-galactosidase activity. After a brief rinse in PBS, all slides were dehydrated and coverslipped with Permount (Sigma, Fair Lawn, NJ). Possible cytoarchitectural damage attributable to virus injections was examined in Nisslstained sections.
Nuclear cell extract and CREB–pCREB assay
Pups used for the CREB–pCREB assay were anesthetized with CO2 and immediately killed by decapitation. Both olfactory bulbs were collected immediately on dry ice and stored in microcentrifuge tubes at -70°C. Olfactory bulb tissue was homogenized using 100 μl/sample of buffer A containing 10 mm HEPES, pH 7.9, 1.5 mm MgCl2, 10 mm KCl, 1 mm dithoithreitol (DTT), 1 mm PMSF, and 0.1% NP-40. The samples were incubated on ice for 15 min and then centrifuged at 1000 × g at 4°C for 10 min. The supernatant was discarded. The pellet was resuspended in 500 μl of buffer A without NP-40. Again, the samples were centrifuged at 1000 × g for 10 min, and the supernatant was discarded. The pellet was resuspended in 100 μl of TransAm lysis buffer (Active Motif, Carlsbad, CA) containing DTT and a protease inhibitor mixture. The samples were rocked at 4°C for 30 min and then centrifuged for 10 min at 14,000 × g at 4°C in a microcentrifuge. The supernatant (nuclear extract) was collected. Protein determination was performed by a BCA protein assay kit (Pierce, Rockford, IL).
CREB–pCREB protein content was determined using CREB–pCREB assays (Active Motif) according to the instructions of the manufacturer. Briefly, 20 μl of sample nuclear extract containing 10 μg of total protein was loaded into each well of a 96-well plate, which was coated by an oligonucleotide containing a cAMP-responsive element (CRE). CREB contained in the nuclear cell extracts binds specifically to this oligonucleotide. After incubations in a primary antibody against CREB (1:1000) or pCREB (1:500) and in a secondary antibody conjugated to horseradish peroxidase, CREB–pCREB was visualized and quantified by a colorimetric reaction and read by a spectrophotometer at 450 nm. Forskolin-stimulated WI-38 cell extract (2.5 μg) was used as a positive control.
Experimental procedures
Expression of HSV-LacZ in the olfactory bulb and its effect on odor preference learning. To determine whether transgenes are well expressed in olfactory bulb neurons and whether virus injection itself affects odor preference learning, 43 rat pups of both sexes from eight litters were divided into six groups: two injection conditions (HSV-LacZ and saline) × three training conditions (O/S, O/O, and naive).
X-gal histochemistry and Nissl staining were performed on the olfactory bulbs of HSV-LacZ-injected pups after they were tested for odor preference.
The causality of CREB in natural odor preference learning. Eighty-seven pups from 10 litters were divided into nine groups: three injection conditions (HSV-CREB, HSV-mCREB, and HSV-lacZ) × three training conditions (O/S, O/O, and naive). In each litter, no more than one pup was assigned to each group. Odor preference learning and testing were performed as described above.
To confirm the increase of CREB expression in the olfactory bulb at the time of learning after the viral injection, eighteen pups from three litters were injected bilaterally into olfactory bulbs with either HSV-CREB or HSV-LacZ on P4. On P6, the pups were killed by decapitation. Both olfactory bulbs were collected in dry ice and stored in microcentrifuge tubes at -70°C until a CREB assay was performed.
To test whether increased CREB substrate results in an enhanced pCREB expression after conditioning, further delineating the effects of CREB–pCREB levels on odor preference learning, a pCREB assay was performed on the olfactory bulbs of the rats from the O/S groups injected with either HSV-LacZ or HSV-CREB. Eighteen pups from three litters were used in this experiment. Previous work showed that pCREB increases maximally at 10 min after odor conditioning (McLean et al., 1999). Therefore, 10 min after being taken away from the peppermint bedding, the pups were killed by decapitation. Both olfactory bulbs were collected for a pCREB assay.
The effects of CREB levels on isoproterenol-induced odor preference learning. Experiments were performed to determine whether additional wild-type CREB (by HSV-CREB injection) or dominant-negative CREB (mCREB binds to the DNA but does not promote downstream transcription because it is not phosphorylated) changes the sensitivity of the system to the unconditioned stimulus. The hypothesis is that the change in sensitivity would shift the isoproterenol-effective inverted U curve. Ninety-eight pups from nine litters were used. Twelve groups were created in this experiment: three virus injection conditions (HSV-lacZ, HSV-CREB, and HSV-mCREB) × four drug–saline injection conditions (saline, 1 mg/kg isoproterenol, 2 mg/kg isoproterenol, and 4 mg/kg isoproterenol). No more than one male or female pup from the same litter was assigned to the same group.
CREB phosphorylation has been proposed as a critical step in the acquisition of long-term odor preference memory (McLean et al., 1999). pCREB assays were performed on the olfactory bulbs of the rats from the groups that exhibited learning in the first set of experiments: the 1 mg/kg HSV-CREB group or the 4 mg/kg HSV-mCREB group and their corresponding nonlearning control groups (the 1 mg/kg HSV-LacZ and the 4 mg/kg HSV-LacZ groups). Thirty-six pups from nine litters were used in this experiment.
Statistical analyses
Paired two-tailed t tests with litter as the matching variable were used to evaluate two group comparisons. One-way ANOVAs were used to assess groups of behavioral data. Post hoc comparisons using the Dunnett's multiple comparison test had p set at < 0.05.
Results
Expression of HSV-LacZ in the olfactory bulb and its effect on odor preference learning
Insoluble dark blue cells were seen in all layers of the olfactory bulb using X-gal staining 3 d after HSV-LacZ injection (Fig. 1). The area around the injection site was heavily stained and HSV-LacZ spread well along the rostrocaudal axis. Control pups with saline injections did not show any X-gal staining. Microinjection of HSV-LacZ caused minimal damage to tissue structures evaluated by Nissl staining (data not shown).
Figure 1.
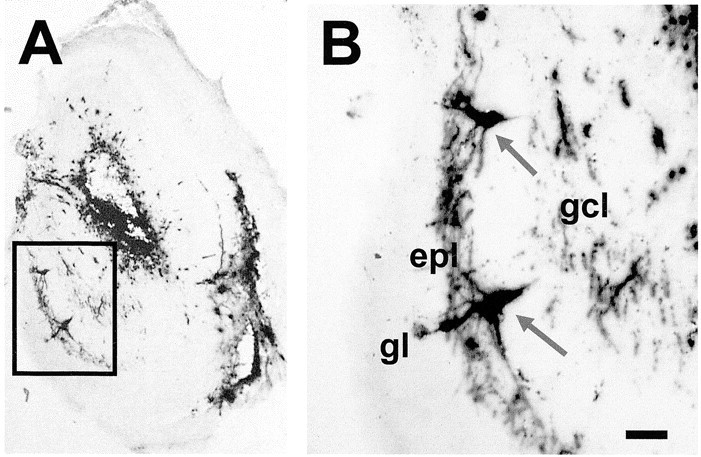
Immunohistology of β-galactosidase by X-gal staining showing expression of LacZ in many cells throughout the ol factory bulb. Labeled cells in A are shown a thigher magnification in B. Mitral cells are indicated by arrows. Scale bar, 50 μm.epl, External plexiform layer; gcl, granule cell layer; gl, glomerular layer.
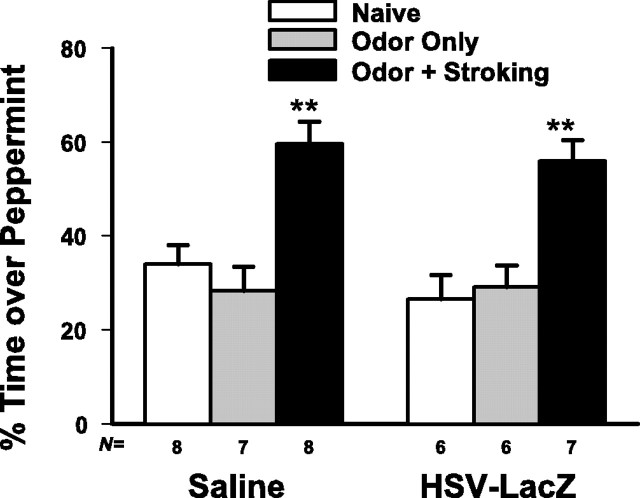
HSV-LacZ did not affect the animals' behavioral performance. As shown in Figure 2, both saline-injected (F(2,20) = 13.38; p < 0.001) and HSV-LacZ-injected (F(2,16) = 12.34; p < 0.001) pups demonstrated significant training effects. Post hoc Dunnett's comparisons showed the pups subjected to stroking paired with peppermint odor (O/S) during training spent significantly more time over peppermint odor at the time of testing the next day compared with the naive control group (p < 0.01).
Figure 2.
Odor preference test showing that HSV-LacZ injection itself does not affect odor preference learning. HSV-LacZ-injected pups in the paired odor plus stroking group demonstrate significant preference learning when compared with those in the naive group. The same pattern applies to the saline-injected pups. **p < 0.01.
The causality of CREB in natural odor preference learning
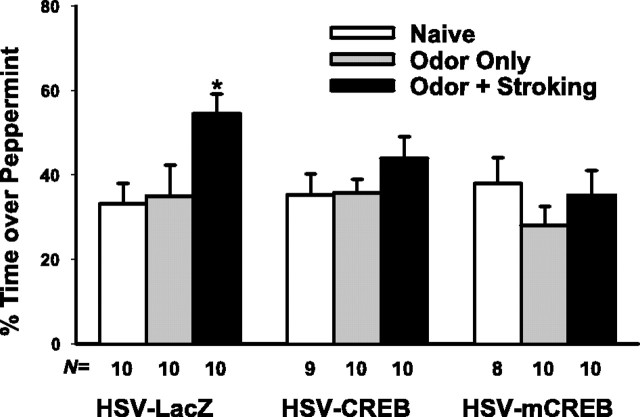
HSV-mCREB injection prevented learning as shown in Figure 3. HSV-mCREB-injected animals in the O/S group did not show a preference for peppermint after training (F(2,25) = 0.85; p = 0.44), whereas the control littermate HSV-LacZ-injected pups showed training effects (F(2,27) = 4.32; p < 0.05). Post hoc comparisons showed that the O/S group learned (p < 0.05) compared with the naive group. Interestingly, HSV-CREB injection did not improve preference learning in the O/S group; rather, the opposite occurred (F(2,26) = 1.22; p = 0.31). There was no significant learning in this group. Additional CREB impaired the ability of animals to learn the odor, suggesting that there is a window for CREB and implying an optimal window for pCREB functioning given that pCREB is the critical mediator for CREB pathway activation.
Figure 3.
Odor preference test showing that CREB and mCREB injections block odor preference learning in a learning-effective paradigm (i.e., peppermint odor paired with stroking). Both HSV-CREB-and HSV-mCREB-injected pups show deficient odor preference learning in contrast to the HSV-LacZ control group. *p < 0.05.
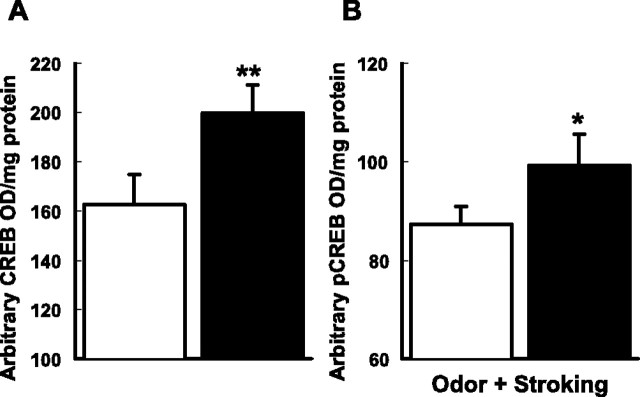
To confirm further the increase of CREB expression at the time of learning, a CREB assay was performed on the olfactory bulbs of the HSV-CREB-injected pups that were killed on the day of training (2 d after HSV-CREB injection). The HSV-CREB injection group showed a 22.9% increase in CREB expression (Fig. 4A) in the optical density per milligram of protein of CREB in olfactory bulb tissue relative to that of the HSV-LacZ control group (t(8) = 4.46; p < 0.01).
Figure 4.
A, CREB assay showing that CREB in the olfactory bulb is increased 2 d after HSV-CREB injection. CREB content is presented as arbitrary optical density (OD) per milligram of protein. **p < 0.01. B, pCREB assay showing that pCREB is significantly increased in the olfactory bulbs of the HSV-CREB-injected (nonlearning) group compared with those of the HSV-LacZ (learning) group 10 min after they are subjected to odor paired with stroking preference training on P6. pCREB content is presented as arbitrary optical density per milligram of protein. *p< 0.05. For both A and B, white bars indicate HSV-LacZ injections; black bars indicate HSV-CREB injections. n = 9 for all columns.
pCREB expression 10 min after O/S conditioning was measured by a pCREB assay to test whether increasing CREB substrate increased pCREB after learning. Figure 4B shows that there was a significant 13.8% increase (t(8) = 2.48; p < 0.05) in the optical density per milligram of protein of pCREB in the HSV-CREB-injected group compared with the HSV-LacZ group. This suggests that increasing CREB substrate by HSV-CREB injection enhances pCREB levels correspondingly and that increasing CREB–pCREB beyond an optimal level interferes with learning.
The effects of CREB levels on isoproterenol-induced odor preference learning
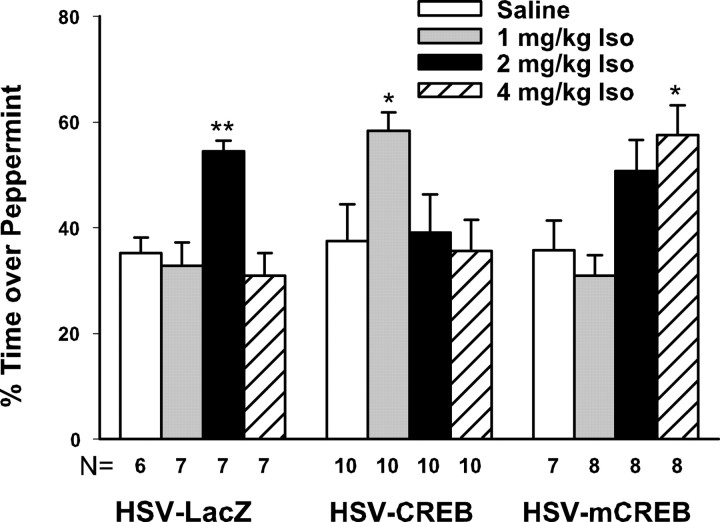
Figure 5 demonstrates that providing HSV-CREB or HSV-mCREB shifted the isoproterenol-induced learning curve to the left or right, respectively. The control group (HSV-LacZ) shows the typical inverted U curve learning effect of varying concentrations of isoproterenol (F(3,23) = 9.33; p < 0.001). Post hoc Dunnett's comparisons showed that 2 mg/kg isoproterenol paired with odor provided significant learning compared with the saline controls (p < 0.01), whereas the 1 or 4 mg/kg injections of isoproterenol did not. In contrast, increasing CREB expression by HSV-CREB injection enhanced the sensitivity of the system to the unconditioned stimulus (F(3,36) = 3.09; p < 0.05). Post hoc Dunnett's comparisons demonstrated that, an originally ineffective learning dose, 1 mg/kg isoproterenol now induced significant learning when paired with odor. No learning was seen, relative to the saline control, with either the normally optimal dose, 2 mg/kg, or the higher dose, 4 mg/kg, of isoproterenol, in the HSV-CREB-injected groups. Thus, CREB shifted the isoproterenol dose–response relationship to the left. This is consistent with the results in the previous experiment that a critical CREB–pCREB window occurs and that too much, as well as too little, CREB–pCREB can prevent learning. Surprisingly, HSV-mCREB-injected pups developed odor preferences (F(3,27) = 5.63; p < 0.01) when the higher dose of isoproterenol, 4 mg/kg, was used. The 4 mg/kg group showed significant learning when compared with the saline group (p < 0.05), whereas the other groups did not differ from the saline control group. Thus, it appears that mCREB shifts the inverted U curve dose–learning relationship for isoproterenol to the right.
Figure 5.
Odor preference test showing that CREB and mCREB injections shift the isoproterenol inverted U curve to the left and right, respectively. For the HSV-LacZ-injected control group, 2 mg/kg isoproterenol induces odor preference learning when paired with peppermint odor, whereas a lower or higher dose does not. In contrast, the HSV-CREB-injected group shows that a lower dose of isoproterenol (1 mg/kg) produces learning, whereas for the HSV-mCREB-injected group, a higher dose of isoproterenol (4 mg/kg) produces learning. *p < 0.05; **p < 0.01. Iso, Isoproterenol.
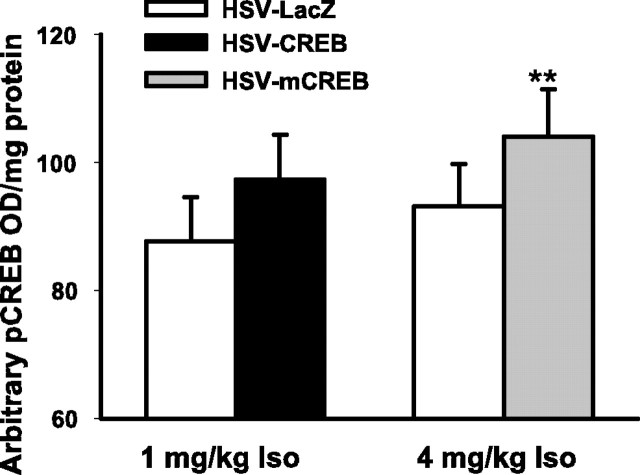
More important than the CREB increase itself is the phosphorylation of CREB, because pCREB is the initial step for CREB activation (Bito et al., 1996; Deisseroth et al., 1996) and the CRE-induced gene expression that underlies long-term synaptic plasticity and memory formation (Silva et al., 1998). Again, we compared the pCREB levels in both learning groups with their nonlearning controls. We were particularly interested to know whether 4 mg/kg isoproterenol increased pCREB in HSV-mCREB-injected pups, which would be consistent with our behavioral results. As seen in Figure 6, we found that the learning group, HSV-CREB plus 1 mg/kg isoproterenol, produced an 11.1% increase in the optical density per milligram of protein of pCREB over that of the nonlearning control group, HSV-LacZ plus 1 mg/kg isoproterenol, although this increase was not significant (t(8) = 1.04; p < .33). The HSV-mCREB plus 4 mg/kg isoproterenol learning group showed a similar 11.7% increase in optical density per milligram of protein compared with the nonlearning control group (HSV-LacZ plus 4 mg/kg isoproterenol). This difference was significant (t(8) = 3.09; p < 0.05). Thus, both learning groups showed similar percentage increases in pCREB compared with corresponding nonlearning groups, although only one comparison was significant.
Figure 6.
pCREB assay showing that pCREB is increased in the olfactory bulbs of the learning groups relative to nonlearning groups. HSV-CREB plus 1 mg/kg isoproterenol and HSV-mCREB plus 4 mg/kg isoproterenol learning groups demonstrate increased pCREB expression after training relative to their LacZ control groups. pCREB content is presented by arbitrary optical density (OD) per milligram of protein. **p < 0.01. Iso, Isoproterenol. n = 9 for all columns.
Discussion
Natural, stroking-induced odor learning or 2 mg/kg isoproterenol-induced odor learning was prevented by infusion of an HSV-mCREB into the olfactory bulb. This result implicates CREB as a mediator of early odor preference learning. Because the serine 133 site is the only one not available for phosphorylation (Josselyn et al., 2002), it also suggests phosphorylation of serine 133 may be critical in early odor preference learning.
Unexpectedly, a bi-directional effect of mCREB was observed in these experiments. The pairing of odor and a 4 mg/kg dose of isoproterenol, which normally does not produce learning, was a successful learning inducer if HSV-mCREB was previously infused into the olfactory bulb. pCREB assay of this novel effective learning condition revealed higher levels of pCREB, as seen previously with learning, than those seen in rat pups receiving LacZ infusions. We hypothesized previously that the failure of high doses of isoproterenol to produce learning and/or increased pCREB might be related to enhanced protein kinase A (PKA) activation of phosphatases (Yuan et al., 2000). Because mCREB would provide a “false” target for both kinase and phosphatase activity, it could alter the balance of enzyme activities in infected neurons to decrease, or increase, the likelihood of pCREB expression depending on the enzyme levels induced by training. Thus, with optimal enzyme levels (odor plus stroking or odor plus 2 mg/kg isoproterenol), mCREB is deleterious, but with an excess of phosphatase activity (odor plus 4 mg/kg isoproterenol), for example, it could be beneficial.
The ability of HSV-mCREB to alter downstream transcription and behavioral outcomes has been demonstrated previously in nucleus accumbens (Carlezon et al., 1998, 2000). No effect of HSV-mCREB, however, was seen in a previous study of long-term memory when it was infused into the amygdala (Josselyn et al., 2002).
HSV-CREB infusions lowered the threshold for isoproterenol-induced odor learning. Infusion of HSV-CREB in the olfactory bulb produced learning in rat pups given odor plus 1 mg/kg isoproterenol, normally an ineffective pairing for learning. The ability of CREB to lower the threshold for isoproterenol-induced odor learning further supports our hypothesis that CREB has a causal role in early odor preference learning. pCREB was also significantly increased in this novel learning condition compared with rat pups receiving HSV-LacZ infusions. The ability of HSV-CREB to alter downstream genomic expression and behavior has been demonstrated previously in the nucleus accumbens (Carlezon et al., 1998, 2000). HSV-CREB has also been shown to convert short-term memory to long-term memory in the amygdala, although it did not render a weak unconditioned stimulus more effective (Josselyn et al., 2002) as seen here.
A bi-directional effect of CREB appeared when HSV-CREB was infused before normally effective learning conditions. Thus, rat pups given odor plus stroking or odor plus 2 mg/kg isoproterenol did not learn whether HSV-CREB was infused before training. This suggests that CREB function has a strict window such that too much CREB interferes with normal odor preference learning in rat pups.
Overexpression of CREB has been shown to interfere with learning in other paradigms (Guzowski and McGaugh, 1997). The present pattern of results is consistent with that literature. However, here, pCREB levels were also assessed in the odor plus stroking group given CREB infusion. This group had higher pCREB levels than the LacZ-infused controls that successfully learned the odor preference. This outcome supports the hypothesis of an optimal window for pCREB level in initiating the development of odor memory. It has been shown that the duration of pCREB activation critically influences downstream gene expression (Bito et al., 1996), and it has been proposed that overactivation of CREB might lead to increased repressor activity (Silva et al., 1998); however, this is the first study to demonstrate a negative effect of higher pCREB levels on learning and memory.
CREB is a target of the cAMP–PKA pathway. We showed that cAMP is increased in the olfactory bulbs by stroking and by isoproterenol (Yuan et al., 2003). Interaction of NE and 5-HT changed the cAMP expression in mitral cells. We suggested a model of early odor preference learning in which the locus ceruleus input activates β-adrenoceptors on mitral cells to trigger a cAMP increase (Yuan et al., 2003). This increase is hypothesized to interact with calcium currents activated by the odor input to mitral cells to enhance pCREB in those same cells. Other evidence, however, demonstrates CREB phosphorylation at the serine 133 site through a variety of protein kinases, including those activated by calcium (Silva et al., 1998). An alternative model of odor preference learning suggests that NE-induced disinhibition of mitral cells by granule cells (Wilson and Sullivan, 1994), could enhance NMDA currents from odor input onto mitral cells. This model would also predict pCREB increases in mitral cells. Both mechanisms are likely to contribute to early odor preference learning.
Our data from optical imaging during memory retrieval (Yuan et al., 2002) and from electrophysiological measurements of olfactory nerve-evoked potentials during acquisition (Yuan et al., 2000) suggest that a critical change is the potentiation of odor input to the mitral cells. An input potentiation model is also supported by previous evidence of enhanced 2-DG (Woo et al., 1987; Johnson and Leon, 1996) and c-Fos (Johnson et al., 1995) during memory retrieval. Potentiation of odor input after appetitive olfactory conditioning has also been reported for the honeybee (Faber et al., 1999) and the sheep (Kendrick et al., 1992).
CREB and pCREB were first shown to have causal roles in the encoding of sensory memory in Aplysia. Using genetic tools, CREB has been shown to be causal in olfactory learning in Drosophila. This is the first report that CREB and pCREB have causal roles in mammalian olfactory learning.
Footnotes
This work was supported by Canadian Institutes of Health Research (Grant ROP-53761) and Canadian Economic Development Agreement (Grant ARJ-4037988-1) Regional Partnership to J.H.M. and C.W.H. Q.Y. holds a Canadian Institutes of Health Research studentship. Special thanks to Dr. Eric Nestler for the HSV-CREB and HSV-mCREB amplicon constructions.
Correspondence should be addressed to Dr. John H. McLean, Division of Basic Medical Sciences, Memorial University of Newfoundland, St. John's, Newfoundland, Canada A1B 3V6. E-mail: mclean@mun.ca.
Copyright © 2003 Society for Neuroscience 0270-6474/03/234760-06$15.00/0
References
- Bito H, Deisseroth K, Tsien RW ( 1996) CREB phosphorylation and dephosphorylation: a Ca 2+- and stimulus duration-dependent switch for hippocampal gene expression. Cell 87: 1203–1214. [DOI] [PubMed] [Google Scholar]
- Carlezon Jr WA, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, Duman RS, Neve RL, Nestler EJ ( 1998) Regulation of cocaine reward by CREB. Science 282: 2272–2275. [DOI] [PubMed] [Google Scholar]
- Carlezon Jr WA, Haile CN, Coppersmith R, Hayashi Y, Malinow R, Neve RL, Nestler EJ ( 2000) Distinct sites of opiate reward and aversion within the midbrain identified using a herpes simplex virus vector expressing GluR1. J Neurosci 20: RC62(1–5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K, Bito H, Tsien RW ( 1996) Signaling from synapse to nucleus: postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron 16: 89–101. [DOI] [PubMed] [Google Scholar]
- Faber T, Joerges J, Menzel R ( 1999) Associative learning modifies neural representations of odors in the insect brain. Nat Neurosci 2: 74–78. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, McGaugh JL ( 1997) Antisense oligodeoxynucleotide-mediated disruption of hippocampal cAMP response element binding protein levels impairs consolidation of memory for water maze training. Proc Natl Acad Sci USA 94: 2693–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Leon M ( 1996) Spatial distribution of [14C]2-deoxyglucose uptake in the glomerular layer of the rat olfactory bulb following early odor preference learning. J Comp Neurol 376: 557–566. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Woo CC, Duong H, Nguyen V, Leon M ( 1995) A learned odor evokes an enhanced Fos-like glomerular response in the olfactory bulb of young rats. Brain Res 699: 192–200. [DOI] [PubMed] [Google Scholar]
- Josselyn SA, Shi C, Carlezon Jr WA, Neve RL, Nestler EJ, Davis M ( 2002) Long-term memory is facilitated by cAMP response element-binding protein overexpression in the amygdala. J Neurosci 21: 2404–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick KM, Lévy F, Keverne EB, Levy F ( 1992) Changes in the sensory processing of olfactory signals induced by birth in sheep. Science 256: 833–836. [DOI] [PubMed] [Google Scholar]
- Langdon PE, Harley CW, McLean JH ( 1997) Increased β adrenoceptor activation overcomes conditioned olfactory learning deficits induced by serotonin depletion. Dev Brain Res 102: 291–293. [DOI] [PubMed] [Google Scholar]
- McLean JH, Darby-King A, Sullivan RM, King SR ( 1993) Serotonergic influence on olfactory learning in the neonate rat. Behav Neural Biol 60: 152–162. [DOI] [PubMed] [Google Scholar]
- McLean JH, Harley CW, Darby-King A, Yuan Q ( 1999) pCREB in the neonate rat olfactory bulb is selectively and transiently increased by odor preference-conditioned training. Learn Mem 6: 608–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve RL, Geller AI ( 1999) Genetic analysis of neuronal physiology with defective herpes simplex virus vectors. Adv Neurol 79: 1027–1032. [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S ( 1998) CREB and memory. Annu Rev Neurosci 21: 127–148. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Leon M ( 1987) One-trial olfactory learning enhances olfactory bulb responses to an appetitive conditioned odor in 7-day-old rats. Dev Brain Res 35: 307–311. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA, Leon M ( 1989a) Norepinephrine and learning-induced plasticity in infant rat olfactory sytem. J Neurosci 9: 3998–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA, Leon M ( 1989b) Associative processes in early olfactory preference acquisition: neural and behavioral consequences. Psychobiology 17: 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, McGaugh JL, Leon M ( 1991) Norepinephrine-induced plasticity and one-trial olfactory learning in neonatal rats. Dev Brain Res 60: 219–228. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Stackenwalt G, Nasr F, Lemon C, Wilson DA ( 2000) Association of an odor with activation of olfactory bulb noradrenergic beta-receptors or locus coeruleus stimulation is sufficient to produce learned approach responses to that odor in neonatal rats. Behav Neurosci 114: 957–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM ( 1994) Neurobiology of associative learning in the neonate: early olfactory learning. Behav Neural Biol 61: 1–18. [DOI] [PubMed] [Google Scholar]
- Woo CC, Coopersmith R, Leon M ( 1987) Localized changes in olfactory bulb morphology associated with early olfactory learning. J Comp Neurol 263: 113–125. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Harley CW, Bruce AJ, Darby-King A, McLean JH ( 2000) Isoproterenol increases CREB phosphorylation and olfactory nerve-evoked potentials in normal and 5-HT-depleted olfactory bulbs in rat pups only at doses that produce odor preference learning. Learn Mem 7: 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Harley CW, McLean JH, Knopfel T ( 2002) Optical imaging of odor preference memory in the rat olfactory bulb. J Neurophysiol 87: 3156–3159. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Harley CW, McLean JH ( 2003) Mitral cell beta(1) and 5-HT(2A) receptor colocalization and cAMP coregulation: a new model of norepinephrine-induced learning in the olfactory bulb. Learn Mem 10: 5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]