Abstract
At the vertebrate neuromuscular junction ATP is known to stabilize acetylcholine in the synaptic vesicles and to be co-released with it. We have shown previously that a nucleotide receptor, the P2Y1 receptor, is localized at the junction, and we propose that this mediates a trophic role for synaptic ATP there. Evidence in support of this and on its mechanism is given here. With the use of chick or mouse myotubes expressing promoter–reporter constructs from genes of acetylcholinesterase (AChE) or of the acetylcholine receptor subunits, P2Y1 receptor agonists were shown to stimulate the transcription of each of those genes. The pathway to activation of the AChE gene was shown to involve protein kinase C and intracellular Ca 2+ release. Application of dominant-negative or constitutively active mutants, or inhibitors of specific kinases, showed that it further proceeds via some of the known intermediates of extracellular signal-regulated kinase phosphorylation. In both chick and mouse myotubes this culminates in activation of the transcription factor Elk-1, confirmed by gel mobility shift assays and by the nuclear accumulation of phosphorylated Elk-1. All of the aforementioned activations by agonist were amplified when the content of P2Y1 receptors was boosted by transfection, and the activations were blocked by a P2Y1-selective antagonist. Two Elk-1 binding site sequences present in the AChE gene promoter were jointly sufficient to drive ATP-induced reporter gene transcription. Thus ATP regulates postsynaptic gene expression via a pathway to a selective transcription factor activation.
Keywords: acetylcholine receptor, acetylcholinesterase, ATP receptors, P2Y1receptor, neuromuscular junction, trophic factors, gene regulation, Elk-1
Introduction
In developing vertebrate neuromuscular junction (nmj), when a motor nerve terminal contacts a myotube, acetylcholine receptors (AChRs), acetylcholinesterase (AChE), and certain other proteins become localized and stabilized in a specialized postsynaptic apparatus. A few subsynaptic nuclei at each developing junction, it now is known, become specialized transcriptionally to sustain the local synthesis of those proteins, including the postsynaptic AChR and AChE (Sanes et al., 1991; Duclert and Changeux, 1995; Krejci et al., 1999; Rossi et al., 2000). In a parallel action, when the neural contacts are established, the evoked electrical activity selectively represses the transcription of AChR genes in the nonsynaptic nuclei (for review, see Duclert and Changeux, 1995). Trophic factors from the nerve have been deduced to initiate and/or maintain the postsynaptic specialization, notably agrin (Cohen et al., 1997; Fuhrer et al., 1999; Lin et al., 2001) and neuregulins (Fischbach and Rosen, 1997).
ATP is an additional potential such trophic factor at the nmj. In the synaptic vesicles in vertebrate skeletal muscles (Silinsky and Redman, 1996) or the related electroplaques (Israel and Dunant, 1998), ATP stabilizes acetylcholine (ACh) and is co-released quantally with it (in a ratio of ∼1 ATP to 5 ACh). Choi et al. (2001a) demonstrated that ATP can induce and sustain the expressions of AChE and AChR α-subunits in cultured myotubes and that the P2Y1 nucleotide receptor is localized at the nmj. We now have studied muscle P2Y1 receptor-induced expressions at the level of gene transcription. This involves the use of AChE promoter constructs and mutations. AChE is a polymorphic enzyme, and its localization at the nmjs occurs with different attachments (Massoulié et al., 1993; Grisaru et al., 1999; Legay et al., 1999). The promoter sequences of AChE genes are known at least in part and have been characterized functionally in Torpedo, mouse, rat, and human (for references, see Chan et al., 1999; Siow et al., 2002). We also show here that the expression of other subunits of the muscle AChR is enhanced via ATP action in parallel with that of the α-subunit.
We investigate here the route from the P2Y1 receptor to these gene activations. There is much evidence that the P2Y1 receptor is linked to the formation of inositol trisphosphate and diacylglycerol and to intracellular Ca 2+ mobilization (for references, see Sellers et al., 2001), and we have shown recently that this initial pathway occurs in the cultured chick myotubes treated with adenine nucleotides, blockable by a P2Y1-specific antagonist (Choi et al., 2001a; Tsim and Barnard, 2002). That pathway suggests the subsequent activation of protein kinase C (PKC), and we have tested for that case and for mitogen-activated protein (MAP) kinase activations that may ensue. In the only report so far of the downstream transductions of molecularly defined P2Y1 receptors (Sellers et al., 2001), activation of the recombinant human receptor stably expressed in human astrocytoma cells produced a sustained response of the extracellular signal-regulated kinase (ERK) isoforms. We examine whether this predicts the native response in the nucleotide-activated myotubes and identify some of the signaling intermediates and a transcription factor, Elk-1, linked to those gene activations.
Materials and Methods
Materials and purity of nucleotides. Materials not specified here were obtained as before (Choi et al., 2001a) or were from Sigma (St. Louis, MO). Cell culture medium and serum were from Invitrogen Life Technologies (Carlsbad, CA). 2-MeSADP stock solution (100 μm) was preincubated with 20 U/ml yeast hexokinase (Roche Biochemicals, Lewes, UK) in Buffer A (2.5 mm MgCl2/50 mm HEPES, pH 7.3) containing 25 mm glucose at 37°C for 30 min to remove all contaminating triphosphates, whereas ATP stock solution (100 μm) was pretreated in Buffer A with 20 U/ml creatine phosphokinase (CPK; Sigma) and 10 mm creatine phosphate (CP; Sigma) at room temperature for 90 min to remove all contaminating diphosphates (Choi et al., 2001a). Antibody sources are the following: anti-phospho-ERK, anti-ERK, anti-phospho-Elk-1, and anti-Elk-1 antibodies from New England Biolabs (Beverly, MA); anti-Raf antibody from Santa Cruz Biotechnology (Santa Cruz, CA); anti-α-tubulin antibody and others not stated from Sigma; peroxidase-or fluorescein-conjugated secondary antibodies from Cappel (Turnhout, Belgium).
Cell cultures. Primary chick myotubes were prepared from hindlimb muscles dissected from 11-d-old chick embryos and cultured at 37°Cina water-saturated 5% CO2 atmosphere, as described previously (Choi et al., 1998, 2001a). Myotubes were treated with a mitotic inhibitor (10 μm cytosine arabinoside) at day 3 after plating and were used on day 4. Undifferentiated mouse C2C12 myoblasts were maintained in DMEM supplemented with 20% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin and were incubated at 37°C in a water-saturated atmosphere of 95% air/5% CO2. We induced them to differentiate into myotubes by replacing the growth medium with DMEM supplemented with 2% heat-inactivated horse serum, 100 U/ml penicillin, and 100 μg/ml streptomycin, as described previously (Siow et al., 2002). Because skeletal muscle cells in culture can release some ATP into the medium and also can convert it there to ADP and because these agents over longer periods may give some desensitization of P2Y receptors, the cultures were pretreated in all cases with apyrase (2 U/ml) for 1 hr to eliminate all such free nucleotides followed by a gentle wash and drug application in apyrase-free medium. Where stated, for longer incubations with an agonist, significant loss thereof because of the ectonucleotidase on muscle cells was prevented by maintaining the appropriate enzymic regeneration system throughout (hexokinase/glucose or CPK/CP in Buffer A) as noted above, as well as by three changes of the agonist solution at approximately equal intervals.
Reporter gene constructs and cDNA transfections. The DNA (∼2.2 kb) encompassing the human AChE promoter (Ben Aziz-Aloya et al., 1993) was subcloned into pGL3 vector (BD Biosciences Clontech, Palo Alto, CA) immediately upstream of a luciferase gene, designated as pAChE-Luc (Choi et al., 2001a). Other promoters were tagged likewise with the luciferase reporter, which included an ∼930 bp chicken AChR α-subunit promoter region (Sanes et al., 1991) for pAChRα-Luc, a 550 bp rat AChR δ-subunit promoter region (Chahine et al., 1992) for pAChRδ-Luc, a 2 kb rat AChR ϵ-subunit promoter (Walke et al., 1994) for pAChRϵ-Luc, and a 2.1 kb mouse AChE promoter [generated by PCR via the published sequence (Li et al., 1993) as pAChEm-Luc]. The full-length cDNA encoding the chicken P2Y1 receptor (Webb et al., 1993) in the Invitrogen expression vector pcDNA3 (Invitrogen Life Technologies) also was used where stated. Raf cDNAs encoding the wild-type Raf-1 (RafWT), a constitutively active form of Raf (RafCAAX), and a dominant-negative Raf (RafS621A) were purchased from BD Biosciences Clontech. Human Elk-1 cDNA generated by RT-PCR from human fibroblasts was subcloned into pcDNA3, verified therein, and used for transfection. Two fragments from the human AChE promoter DNA from base pairs (bp) -1431 to -1412 or from -1102 to -1083, each covering an Elk-1 site identified in this work, were subcloned into pTA-Luc luciferase reporter vector (BD Biosciences Clontech). To enhance the promoter activity, we placed three copies of each of the above Elk-1 sites in tandem (without linkers) into the reporter vector to form pElk-1[1]-Luc and pElk-1[3]-Luc, respectively.
Myoblasts from 11 d chick embryos were cultured at 37°C for 2 d, transiently transfected with the plasmid constructs (2 μg of plasmid per 35 mm dish or 1 μg per 12-well plate) with the use of calcium phosphate, and then were allowed to fuse to myotubes for the treatments that have been stated, with methods as given in Choi et al. (2001a). In mouse C2C12 myoblasts the transfection was by treatment with Lipofectamine Plus (Invitrogen Life Technologies) (Siow et al., 2002). The transfection efficiency in both cases was determined with enzymatic staining from control cells cotransfected with β-galactosidase cDNA in the same vector; it was consistently ∼30%.
Gel mobility shift assay. A nuclear extract of myotubes was prepared as described by Choi et al. (2001b). Nuclear extract [2–5 μg of protein plus 2.5 μg of poly(dI-dC) per sample] was preincubated in binding buffer containing (in mm) 2.5 DTT, 5 MgCl2, 2.5 EDTA, 250 NaCl, and 50 Tris-HCl, pH 7.5, plus 20% glycerol for 20 min at room temperature. The samples were incubated further for 20 min with 32P-labeled double-stranded oligonucleotides (0.1 pmol) carrying the four putative Elk-1 binding sequences: 5′-CAC TCG TCC GGA ACT CTT CC-3′ (from -1431 to -1412 bp), 5′-GGG CCA CTG GAA GAC ACC CC-3′ (from -1287 to -1268 bp), 5′-GAG GCT CGG CGG AAG CCC CG-3′ (from -1102 to -1083 bp), and 5′-GGC CCA GTT CCG GGA AGA GG-3′ (from -322 to -302 bp). Competition assays were performed by incubating the unlabeled oligonucleotide probes (up to 4 pmol) similarly with nuclear extract in binding buffer. To obtain a supershift, we incubated the samples with anti-Elk-1 antibody (1: 100 dilution) in binding buffer for 20 min before the addition of radioactive probe. Finally, the reaction mixtures were separated on 4% polyacrylamide gel, which then was dried and subjected to autoradiography.
Northern blots. Total RNA was prepared by the LiCl method (Sambrook and Russel, 2001) from myotubes after the treatments as stated. All methods for Northern blotting, with detection by a ∼0.6 kb chicken AChE catalytic subunit cDNA 32P-labeled probe, were as stated by Siow et al. (2002). A loading of 20 μg of RNA per gel lane was used. The consistency of the RNA loading in every lane was confirmed by ethidium bromide staining of the ribosomal RNAs. Quantitations of that and of the 32P-labeled bands were made, using calibration curves made for the same gel (Choi et al., 2001a).
Immunoblotting and phosphorylation studies. For AChE analysis the cultured myotubes, after the treatments as stated, were homogenized, SDS-denatured at 100°C, electrophoresed, preblocked, and immunoblotted by using anti-AChE (chicken) catalytic subunit antibody (monoclonal, purified; Tsim et al., 1988) or anti-α-tubulin antibody, all as detailed by Choi et al. (2001a) or, for milk preblocking, by Pun and Tsim (1997). In phosphorylation studies myotube cultures (pretransfected or not) were starved of serum for 8 hr and incubated in serum-free culture medium with drugs as stated. Then the cultures were (with 1 mm Na3VO4 present) washed, extracted/SDS-denatured, electrophoresed, and electroblotted as described by Choi et al. (2001a). Blots were preblocked and probed (Sellers et al., 2001) with antiphospho-ERK (1:5000), antiphospho-Elk-1 (1:1000), or anti-Raf (1:1000) primary antibody dilutions. Blots from phosphorylation studies were reprobed with the appropriate phosphorylation state-independent ERK or Elk-1 antibodies (1: 1000). Then the blots were washed, incubated with the appropriate peroxidase-conjugated secondary antibodies in 1:1000 dilutions, and rewashed (Sellers et al., 2001). The immunocomplexes were visualized by the enhanced chemiluminescence (ECL) method (Amersham Biosciences, Piscataway, NJ). The intensities of the bands in the control and agonist-stimulated samples, run on the same gel and under strictly standardized ECL conditions, were compared on an image analyzer, using in each case a calibration plot constructed from a parallel gel with serial dilutions of one of those samples.
Immunocytochemical staining. Untreated C2C12 myotubes, or chick myotubes that were transfected transiently with pcDNA3/human Elk-1 cDNA (as above), were grown on 35 mm dishes. Confluent cells then were starved of serum for 8 hr before drug treatment. After three saline washes they were paraformaldehyde-fixed and reacted with antiphospho-Elk-1 antibody (1:1000; 16 hr at 4°C) and then with fluorescein-conjugated secondary antibody (1:1000) and viewed, with methods as used with other antibodies (Choi et al., 2001a).
Site-directed mutagenesis of Elk-1 sites. Two identified Elk-1 sites on the human AChE promoter described above were mutated. Overlapping PCR was performed with two PCR fragments containing a six-nucleotide site within Elk-1[-1431 to -1412] to be mutated (by the italic sequence). The first fragment was developed from the primer pair 5′-CGA GCT CGA GAT CCC ATT-3′ plus 5′-ACG GGG CGT GAG CAC TTA AGG AGA AGG GGC CG-3′, and the second was developed from the pair 5′-TGC CCC GCA CTC GTG AAT TCC TCT TCC CCG GC-3′ plus 5′-TCA TGG CTG CAG GGC AGG-3′, to give ΔElk-1[1]. For mutation within Elk-1[-1102 to -1083], overlapping PCR was performed similarly, using first 5′-CGA GCT CGA GAT CCC ATT-3′ plus 5′-CTT CCC CTC CGA GCC TTA AGC GGG GCT CAA TA-3′, and using second 5′-GAA GGG GAG GCT CGG AAT TCG CCC CGA GTT AT-3′ plus 5′-TCA TGG CTG CAG GGC AGG-3′, to give ΔElk-1[3]. For the double-mutant ΔElk-1[1,3], the mutated fragments from the above reactions were ligated via an internal KpnI site. All products were sequenced to confirm their integrity. All PCR reactions in this work were by a standard protocol (Pun and Tsim, 1997), except that for the mutagenesis Pfx polymerase was used at 60°C annealing temperature. Each mutated promoter fragment was inserted as before in the reporter gene to give the constructs pAChEΔElk-1[1]-Luc, etc.
Other procedures. AChE assays, luciferase assays, and protein concentration measurements (used to standardize the assay) were as specified by Choi et al. (2001a). PKC activity was measured with an assay kit (Promega, Madison, WI). Briefly, myotube cultures were treated for 1 hr with the appropriate drug and then lysed in 10 mm HEPES, 0.1% Triton X-100; the lysate was used to determine the PKC activity by phosphorylation of the specific peptide substrate. Statistical tests were made by the PRIMER program, version 1 (Glantz, 1988); differences from basal or control values (as shown in the plots) were classed as significant where *p < 0.01 and as highly significant where **p < 0.001.
Results
P2Y1 receptor-mediated AChE expression requires protein kinase C and cytosolic Ca 2+
We have found previously (Choi et al., 2001a) that activation by adenosine tri-and diphosphates of the P2Y1 receptors present in cultured chick myotubes mobilizes Ca 2+ from organellar stores and also leads to an increase in the expression of the AChE gene. It can be expected that one or more isoforms of PKC are involved in that pathway, and evidence for this was obtained here. The endogenous activity of PKC in the myotubes could be raised, in a positive control, by 12-O-tetradecanoylphorbol 13-acetate (TPA), the increase reaching 200% at 10 μm, but it also was raised by >100% by the application to the myotubes of agonists of the P2Y1 receptor, ATP or 2-MeSADP (Fig. 1A). The activation by these agonists was blocked by the P2Y1 receptor-specific antagonist (Boyer et al., 1996) adenosine 3′-phosphate 5′-phosphate (A3P5P), as illustrated for 2-MeSADP in Figure 1A. An increase in PKC in the myotubes was linked to the expression of AChE; the content of transcripts encoding the chicken AChE catalytic subunit, both the ∼4.8 and ∼6.0 kb isoforms, was raised highly significantly in this TPA treatment (Fig. 1B,C). The TPA-induced expression of the AChE transcripts was dose-dependent, and the induction was slightly higher for the ∼4.8 kb transcript, rising (at 50 nm TPA) to approximately fivefold of the level seen in the activator-free control culture (Fig. 1C).
Figure 1.
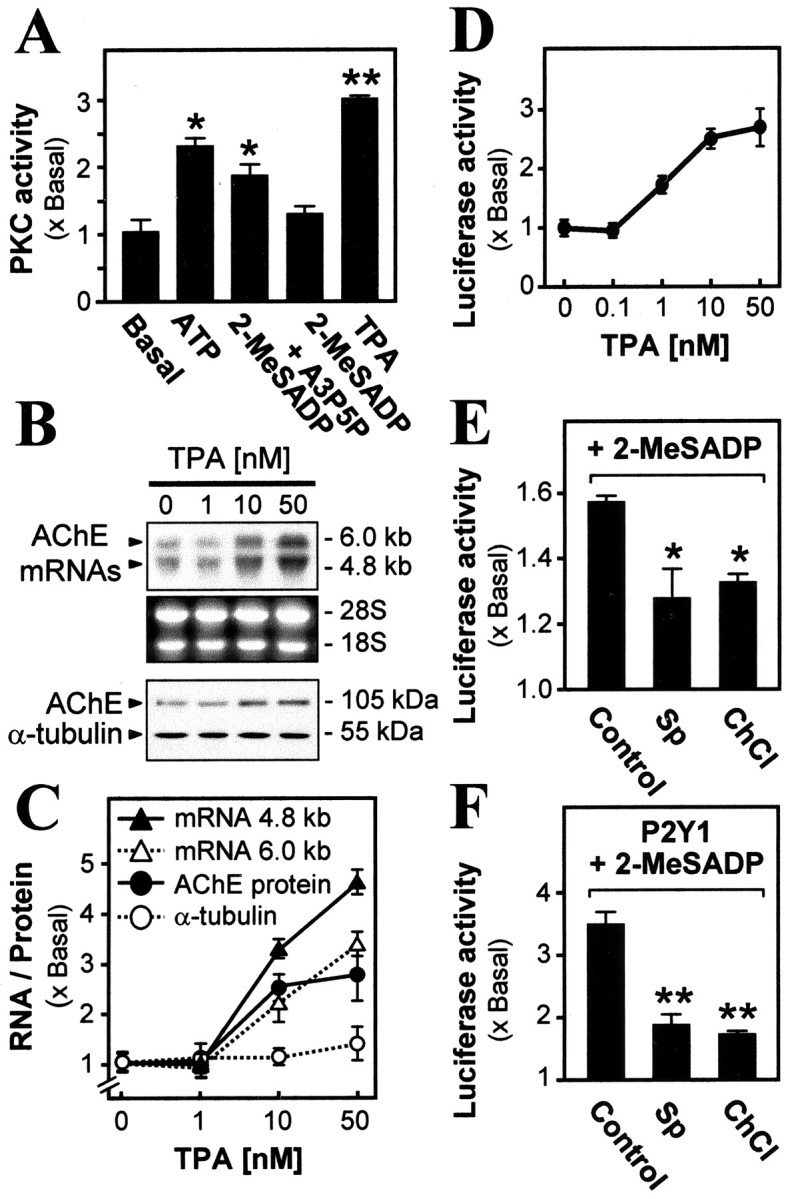
Activation of PKC via the P2Y 1 receptor induces AChEexpression.A, Myotubes were treated for 1 hr with ATP (50 μm), 2-MeSADP (50 μm), A3P5P (50 μm), or the PKC activator TPA (50 nm) and were assayed. B, Application of TPA induces AChE expression. Myotubes were treated for 16 hr with increasing concentrations of TPA. Increasing induction is seen of AChE transcripts (∼4.8 and ∼6.0 kb, recognized by a chicken cDNA probe) and (below) AChE subunit protein (at ∼105 kDa, recognized by its antibody). Total RNA (30 μg) or (below) protein (20 μg) was applied in all lanes. Ribosomal RNAs and α-tubulin are shown as loading controls. C, AChE transcripts and protein, with controlα-tubulin protein, produced by TPA treatments were quantitated by densitometry. D, Induction of AChE promoter activity by TPA application (50 nm for 16 hr) to myotubes pretransfected with pAChE-Luc. E, F, PKC inhibitors block the 2-MeSADP-induced AChE expression. Myotubes pretransfected with pAChE-Luc were exposed for 16 hr to 2-MeSADP (50 μm, with constant regeneration throughout; see Materials and Methods) and were assayed as before (Control). Where shown, staurosporine (Sp; 10 nm) or chelerythrine (ChCl; 1 μm) was also present. In F, the chicken P2Y1 receptor was overexpressed first in the myotubes. In this and further figures showing luciferase activity, the values are expressed as the ratio of the stimulated to the basal (transfected with pAChE-Luc alone, incubated in parallel) activity in a final lysate. *In this and other figures the difference from the control or basal level (except where noted) is significant (p < 0.01); **that difference is highly significant (p < 0.001). In all cases the values are mean ± SEM for five independent experiments, each with triplicate samples.
In these incubations with TPA the AChE protein level was monitored also, using an antibody specific for chicken AChE (Tsim et al., 1988) in Western blotting (Fig. 1B). The content of the AChE catalytic subunit protein (apparent molecular mass, ∼105 kDa) was increased by TPA in a dose-dependent manner, up to approximately threefold of the control level with 50 nm TPA (Fig. 1C). As a control measure of general protein production the level of the α-tubulin protein (∼55 kDa) was not affected by the activator (Fig. 1B,C). This major increase in expression of the endogenous AChE transcript and protein mediated via a PKC pathway arises at the level of gene transcription. This was demonstrated by introducing into the myotubes thehuman AChE gene promoter coupled to luciferase in a reporter construct (pAChE-Luc). The application of TPA then induced the promoter-driven luciferase activity in a dose-dependent manner; the maximum induction at 50 nm TPA reached approximately threefold (Fig. 1D). In confirmation of the linkage of P2Y1 receptor activation in the muscle cells to PKC (Fig. 1A), the application of PKC inhibitors staurosporine (Sp) and chelerythrine chloride (ChCl) significantly reduced the P2Y1 receptor-mediated AChE gene activation. This blocking action was obtained both on the activation by endogenous P2Y1 receptors in myotubes (Fig. 1E) and also on a much greater AChE gene activation achieved by boosting their P2Y1 receptor content (Fig. 1F). That increased content, characterized previously in the myotubes (Choi et al., 2001a), was obtained by cotransfecting a chicken P2Y1 receptor-expressing plasmid (P2Y1/pcDNA 3) with the pAChE-Luc plasmid construct.
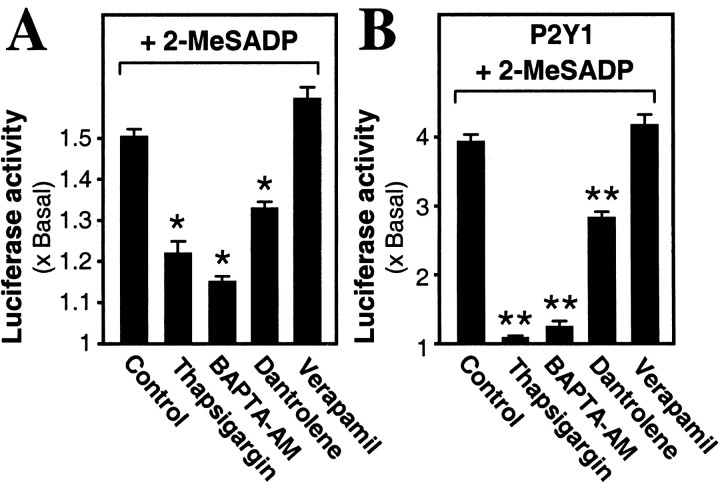
Linkage of the P2Y1 receptor-mediated intracellular Ca 2+ release to the AChE gene activation was probed also. Several blockers of Ca 2+ mobilization were tested, using pAChE-Luc activated via the P2Y1 receptor by its selective agonist 2-MeSADP. Thapsigargin (which depletes the Ca 2+ stores) and BAPTA-AM (which chelates released Ca 2+) both significantly reduced that promoter activity by up to 70% (Fig. 2A). Dantrolene (10 μm), which blocks the ryanodine receptor component of muscle intracellular Ca 2+ release, gave a partial but significant inhibition; in contrast, a blocker of external Ca 2+ entry into skeletal muscle cells, verapamil (10 μm), had no effect (Fig. 2A). Further, when the P2Y1 receptor level was boosted as above and stimulated with 2-MeSADP to produce a much higher pAChE-Luc activity, the same effects of these four agents were shown clearly, with thapsigargin and BAPTA-AM now giving essentially complete inhibition (Fig. 2B).
Figure 2.
The role of Ca 2+ in P2Y1 receptor-induced AChE expression. Myotubes pretransfected with pAChE-Luc were treated with thapsigargin (3 μm), BAPTA-AM (50 μm), dantrolene (10 μm), verapamil (10 μm), or vehicle (Control) for 2 hr before the exposure (as in Fig. 1) to 2-MeSADP (50 μm). Cultured chick myotubes without (A) or with (B) overexpression of the P2Y1 receptor are shown.
A Raf/MEK/ERK phosphorylation pathway is required for P2Y1 receptor-mediated AChE expression
The activation of ERK (see Introduction) was examined after the endogenous P2Y1 receptors were stimulated in myotubes. The antibodies to the mammalian ERK1 and phosphorylated ERK1 (ERK1-P) proteins readily detected those forms in chick myotubes, as in myotubes and other cell lines from mammals, all at ∼44 kDa (Fig. 3D). However, antibodies to the mammalian ∼42 kDa ERK2 and ERK2-P forms showed ample levels of those in the mammalian sources but detected very low amounts in the chick myotubes, only revealed after overexposure of the gels (Fig. 3D). Antibodies raised against chicken ERK subtypes are not available. However, because this difference was found similarly with two antibodies directed against entirely different epitopes (phospho- and nonphospho-) and also because a single antibody is used for ERK1-P and ERK2-P directed to the doubly phosphorylated Thr-Glu-Tyr epitope (Johnson et al., 1996), which is a fully conserved site responsible for their activated status, we conclude that there is a near-absence of ERK2 in these chicken cells. Hence ERK1 appears to be sufficient in mediating this pathway in the cultured chick myotubes, whereas both isoforms can operate in it in the mammalian muscle cells.
Figure 3.
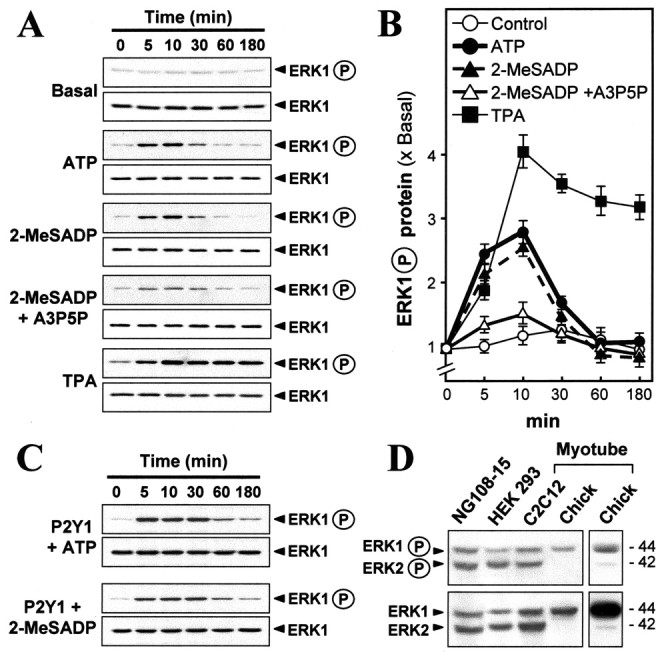
Activation of the muscle P2Y1 receptor induces phosphorylation of ERK. Myotubes were exposed to the agonists shown (50 μm for ATP, 2-MeSADP, and A3P5P; 50 nm for TPA) for the periods that are shown. Total ERK and phospho-ERK were monitored in blots, using their respective antibodies. A, P2Y1 receptor agonists increased the phosphorylation of ERK, blocked by A3P5P.B, Quantitation from a set of such blots by calibrated densitometry.Values are expressed as the ratio of the stimulated to the basal level (no drug treatment). C, Similar analyses in myotubes overexpressing the P2Y1 receptor. D, Only ERK1 expression is significant in chick myotubes in contrast to C2C12(mouse muscle line), HEK 293(human), and NG108–15(mouse/rat) cells. The far right lane is an overexposure of the gel to reveal the expression of ERK2 in chick myotube, although at very small amount.
In the chick myotubes ATP and 2-MeSADP induced a transient phosphorylation of ERK1, whereas the ERK1 total protein content was invariant; this activation is via P2Y1 receptors being blocked by the P2Y1 antagonist A3P5P (Fig. 3A). Plots of scanned data from four independent experiments of the type shown in Figure 3A showed the transient activation peaking at approximately three times the basal level and at 10 min of exposure (Fig. 3B). However, TPA induced a sustained phosphorylation of ERK1 (Fig. 3A,B). When the agonists were applied onto myotubes overexpressing P2Y1 receptors (as above), an activation of ERK1 of over sixfold of the basal level could be achieved (Fig. 3C; plot of those data not shown). Nevertheless, the induction was still transient.
Two effectors upstream of ERK have been tested, so far, for involvement in the P2Y1 receptor-mediated gene expression in muscle cells. In the testing of Raf-1 three cDNA constructs were used: wild-type (RafWT) or RafCAAX, a constitutively active, membrane-targeted mutant (Mineo et al., 1997) or RafS621A, a dominant-negative mutant (Mischak et al., 1996). After these cDNAs were cotransfected with pAChE-Luc into the myotubes, they all produced an overexpression of the Raf-1 protein (∼74 kDa; Fig. 4A). From quantitation of blots such as those illustrated in Figure 4A, overexpression of Raf-1 in myotubes did not change the expression level of ERK1, but the expression of RafWT and RafCAAX increased ERK1 phosphorylation by approximately twofold and approximately fivefold basal, respectively, whereas RafS621A reduced it by >50% as compared with cultures with no Raf transfection. Parallel to these changes in phosphorylation of ERK1, in the same cells the AChE gene promoter activity was stimulated by approximately sevenfold by the expression of constitutively active Raf or decreased by >40% by RafS621A (Fig. 4B). When P2Y1 receptors were overexpressed, 2-MeSADP stimulated the promoter activity by approximately fourfold (as before), a rise that was blocked significantly by the overexpression of the dominant-negative mutant of Raf. RafWT, serving as a control activator, potentiated the P2Y1 receptor-induced gene expression further (Fig. 4B).
Figure 4.
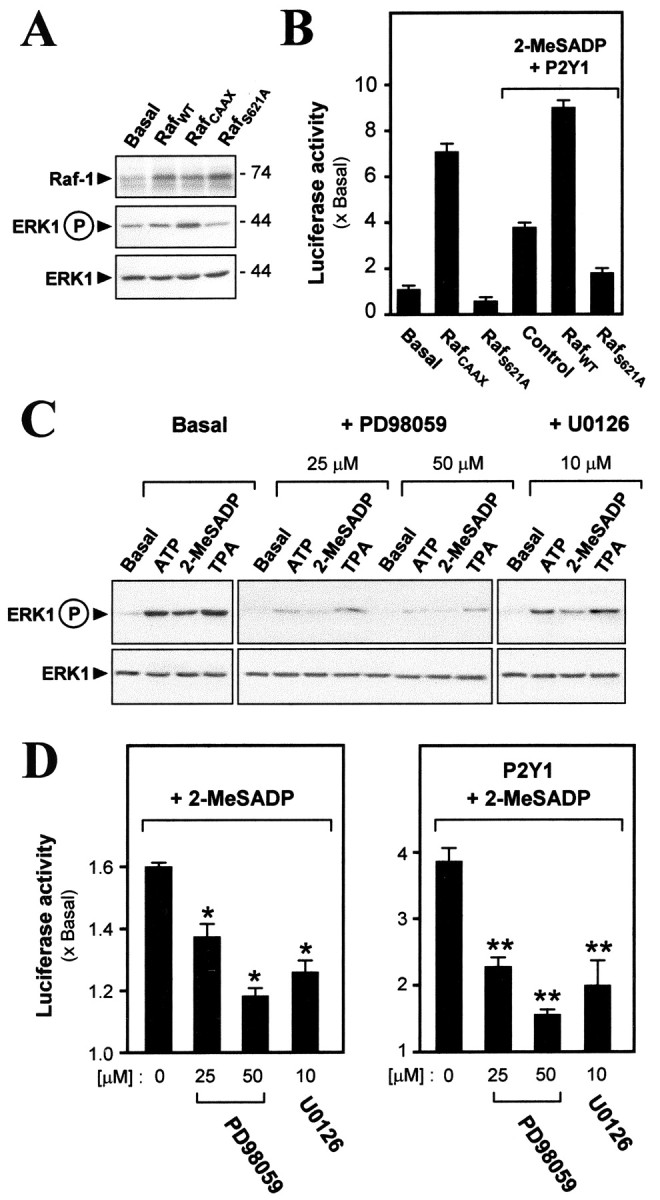
Involvement of Raf/MEK signaling in P2Y1-mediated activations. A, In a control study the expression vectors for Raf-1 [wild type (RafWT) or a constitutively active mutant (RafCAAX) or a dominant-negative mutant (RafS621A)] were cotransfected with pAChE-Luc. Analyses are shown at 4d after transfection. All three Raf cDNA constructs are seen to produce an overexpression of the Raf-1 protein (∼74 kDa). RafCAAX induces ERK1 phosphorylation, but RafS621A decreases it. B, Cotransfections of Raf (as in A) and pAChE-Luc in myotubes, either overexpressing P2Y1 receptors and exposed to 2-MeSADP (as in Fig. 1) or not, as shown, followed by reporter activity measurement. C, Myotubes were exposed for 10 min to the agents that are shown (50 μm for ATP, 2-MeSADP, and A3P5P; 10 nm for TPA) alone or together with MEK inhibitor (given a 10 min preincubation) PD98059 or U0126. Phospho-ERK1 and total ERK were determined as before. D, On pAChE-Luc-transfected myotubes 50 μm 2-MeSADP was applied alone or together with PD98059 or U0126 (preincubated 10 min) for 16 hr in myotubes without or with P2Y1 receptor overexpression, followed by a luciferase assay. For 25 μm PD 98059 + 2-MeSADP, p < 0.05; all others are as defined for Figure 1.
Two inhibitors of MEK, PD98059 and U0126, were applied to the myotubes, each acting on both the MEK1 and the MEK2 isoforms (Dudley et al., 1995; Favata et al., 1998). Each inhibitor blocked the P2Y1 receptor-mediated ERK1 phosphorylation as induced by ATP or 2-MeSADP and also that induced by TPA (Fig. 4C). In all cases the total content of ERK1 protein did not change. The concentrations that were effective here are in the range found to be needed in other cases to inhibit MEK strongly when applied to intact cells (Dudley et al., 1995; Favata et al., 1998). These inhibitors likewise reduced the 2-MeSADP-induced gene expression of AChE (Fig. 4D). The blocking effect of the inhibitors was also very strong in the myotubes overexpressing the P2Y1 receptor (Fig. 4D). These results demonstrate the inclusion of Raf-1, MEK (1 and/or 2), and ERK1 in the pathway from P2Y1 receptor activity to the activation of the AChE gene in the muscle cells.
Elk-1 mediates the promoter activity of human AChE
Elk-1 is one of the transcription factors that can be phosphorylated via ERK-P; this produces Elk-1P, the transcriptionally active form doubly phosphorylated on two specific serines in a conserved sequence near its C terminus [Hodge et al. (1998), and references cited therein]. Elk-1 was the first to be examined here because it has been shown recently that activation of the P2Y1 receptor in astrocytoma cells leads to phosphorylation of Elk-1, and not any of several other transcription factors that are potential ERK targets (Sellers et al., 2001). Myotubes were transfected with a plasmid construct of human Elk-1 cDNA; antibodies against human Elk-1 or against its phospho-form were used then in immunoblotting extracts. Elk-1 protein was detected strongly therein, plus a constant low basal level of Elk-1P (∼62 kDa; Fig. 5A). In fact, tests on nontransfected myotubes showed that these antibodies also recognized the chicken Elk-1 proteins of that size. In the transfected myotubes the application of P2Y1 receptor agonists ATP or 2-MeSADP induced the phosphorylation of Elk-1 to ∼2.7 times basal, declining after 30 min (Fig. 5A, right). The PKC activator TPA stimulated the phosphorylation similarly but rose earlier. When the P2Y1 receptor was overexpressed in the myotubes in conjunction with increased Elk-1 expression, the application of 2-MeSADP stimulated the promoter-driven luciferase activity significantly, showing a clear Elk-1 concentration dependence (Fig. 5B). In control cells treated likewise but having only the endogenous P2Y1 level, significant increases with Elk-1 concentration also were seen, but on a smaller scale (Fig. 5B). The activation of P2Y1 receptors not only induced the phosphorylation of Elk-1, but, further, it increased the presence of the activated Elk-1P in the muscle cell nucleus. The phosphorylated Elk-1 was detectable by immunofluorescence in the nuclei of the control myotubes, but this was enhanced greatly after the application of ATP or 2-MeSADP or of TPA (Fig. 5C).
Figure 5.
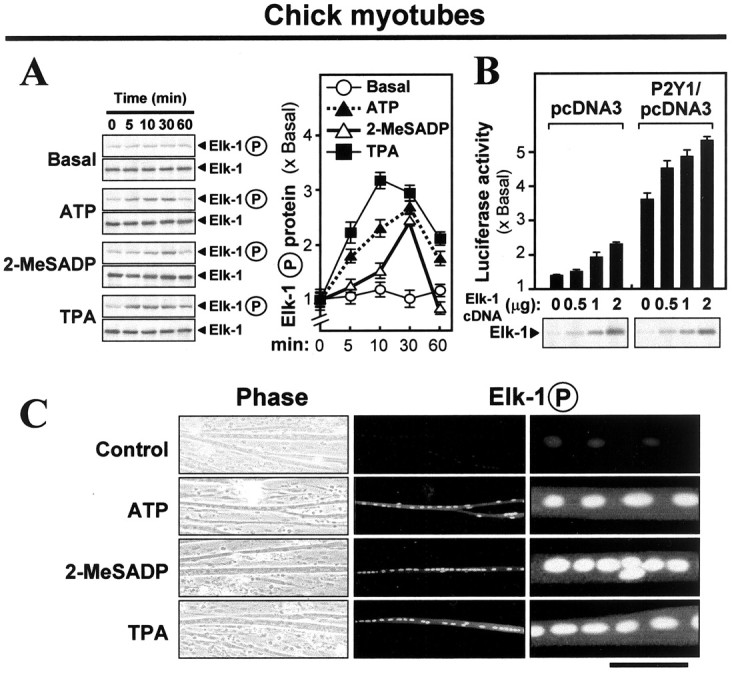
Phosphorylation of Elk-1 is required for P2Y1 receptor-mediated gene activation. A, Chick myotubes pretransfected with human Elk-1 plasmid were exposed to the drugs (50 μm for ATP and 2-MeSADP; 10 nm for TPA) for the periods that are shown. Total Elk-1 and phospho-Elk-1 were monitored in blots by using their respective antibodies. Right, Quantitation from a set of such blots by calibrated densitometry, expressed relative to the basal level (no drug treatment). B, Chick myotubes were pretransfected jointly with human Elk-1 plasmid (with the amounts per 12-well plate as shown) and pAChE-Luc plus either P2Y1 receptor plasmid or its empty pcDNA3 vector. The Elk-1 protein content was verified in the samples as shown in the blots below. 2-MeSADP (50 μm) was applied (with regeneration) for 16 hr in all cases, and then luciferase activity was assayed. C, Chick myotubes pretransfected with human Elk-1 cDNA plasmid (2 μg per plate) were exposed for 20 min to the agents as shown (50 μm for ATP and 2-MeSADP; 10 nm for TPA). Then the myotubes were fixed and stained with anti-phospho-Elk-1 antibody (1:1000), followed by fluorescent secondary antibody. Left to right, Phase-contrast, low power, high power, all of the same field; the scale bar then represents 250, 250, or 50 μm. The row of myonuclei contains most of the phospho-Elk-1.
This P2Y1-linked phosphorylation was revealed also in the endogenous Elk-1 protein by using mouse myotubes (C2C12 cells) in which the stronger recognition by the mammalian antibodies of the mouse Elk-1 and Elk-1P proteins improved the sensitivity. As in chick myotube studies reported previously (Choi et al., 2001a), the P2Y1 agonists ATP or 2-MeSADP stimulated the AChE promoter activity two-to threefold in pAChE-Luc-transfected mouse myotubes. That induction was blocked by A3P5P, a specific inhibitor of P2Y1 receptors (Fig. 6A). With the application of ATP or 2-MeSADP or TPA onto these mouse myotubes cultured without introduction of P2Y1 receptors, phosphorylation of Elk-1 was induced to approximately three times the basal level (Fig. 6B). As with the chick myotubes (Fig. 5A) the induction here declined after 30 min. With the use of staining by the anti-Elk-1P antibody the untransfected mouse myotubes again showed weak but definite reaction; with P2Y1-agonist or TPA treatment a robust increase in activated endogenous Elk-1P was found in the muscle cell nuclei (Fig. 6C). In both mouse and chick myotubes a minority of the nuclei consistently showed little or no Elk-1P (Figs. 5C, 6C), attributed to nonsynchronous maturation.
Figure 6.
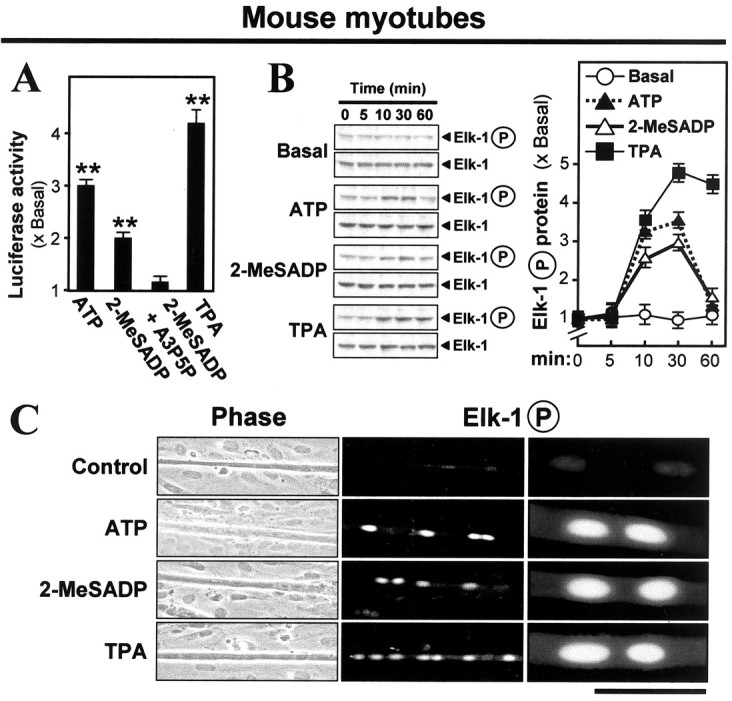
Phosphorylation of endogenous Elk-1 by activation of P2Y1 receptors in mouse C2C12 myotubes. A, C2C12 myotubes pretransfected with pAChE-Luc were exposed for 16 hr to ATP (50 μm), 2-MeSADP (50 μm), A3P5P (50 μm), or the PKC activator TPA (10 nm) and were assayed. B, Untransfected C2C12 myotubes were exposed to the indicated drugs (concentrations used as in A) for the periods that are shown. Total Elk-1 and phospho-Elk-1 were monitored in blots as in Figure 5A. C, Mouse C2C12 myotubes were exposed for 20 min to the agents shown (concentrations used as in A). Then the myotubes were fixed and stained with anti-phospho-Elk-1 antibody (1:1000), followed by fluorescent secondary antibody. Left to right, Phase-contrast, low power, high power, all of the same field; the scale bar then represents 250, 250, or 50 μm. The row of myonuclei contains most of the phospho-Elk-1.
Promoter sites controlling AChE gene expression in chick muscle cells
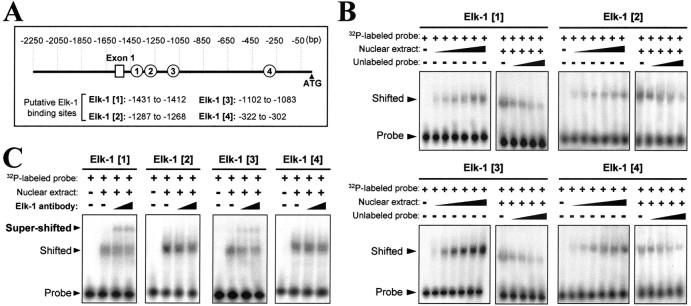
A search in the promoter region of the human AChE gene sequence for potential binding sequences for Elk-1 revealed four possible sites downstream of the 5′-untranslated exon 1. These are located in the first intron at -1431 to -1412 bp, -1287 to -1268 bp, -1102 to -1083 bp, and -322 to -302 bp upstream of the ATG start site, designated as Elk-1[1] to Elk-1[4], respectively, as shown in Figure 7A. However, the regulatory elements that actually function with Elk-1 in this promoter are not known. Also, there is a need to confirm that these sites apply to the chicken Elk-1. These questions were addressed by performing gel mobility shift assays, probing a nuclear extract from cultured chick myotubes with [32P]-labeled double-stranded oligonucleotides synthesized according to the sequences of the four putative Elk-1 binding sites in the human AChE promoter. Binding occurred from the nuclear extract in a concentration-dependent manner of all four of those oligonucleotide probes, as shown by the shifts in their mobilities (Fig. 7B). Nonspecific binding was avoided by (1) always including a large excess of a nonspecific competitor, double-stranded poly(dI-dC), and (2) demonstrating a progressive block of the binding by increasing amounts of the unlabeled specific probe, although this was less effective for Elk-1[2] (Fig. 7B). However, the mobility shifts still may not be definitive here, because Elk-1 is in the Ets family of transcription factors (Macleod et al., 1992), and in the sequences that have been tested there are inevitably similarities (although not full identity) to known binding sites of some other Ets members. Therefore, an antibody specific for the Elk-1 protein (phosphorylated or not) also was applied. It bound in the complex, as manifested by the slower migration (“super-shift”) of its band, but only two of the putative binding sequences, Elk-1[1] and Elk-1[3], were validated thus as specific to Elk-1 binding (Fig. 7C). These lie in the 5′-half of the first intron (Fig. 7A), in agreement with the finding (Chan et al., 1999) that this region is essential for AChE gene expression in rat muscle.
Figure 7.
Identification of Elk-1 binding sites on human AChE promoter. A, Potential binding sites of the transcription factor Elk-1, found by consensus sequence search, in the ∼2.2 kb human AChE promoter DNA. They are numbered from 1 to 4, located in the first intron, at the stated positions upstream of the ATG start site. B, A nuclear extract of chick myotubes was used with 32P-labeled double-stranded oligonucleotides (0.1 pmol per sample) covering, in turn, the potential Elk-1 sites 1–4 in gel mobility shift assays as described in Materials and Methods. Competition assays were performed by including in the incubation increasing amounts (as indicated above the gels) of the nuclear extract (from 1 to 6 μg of protein) or of the same oligonucleotide unlabeled (up to 40-fold excess). The positions of the free probe and of the bound (shifted) probe are indicated by arrowheads. C, Anti-Elk-1 antibody (1:100 dilution) was preincubated before the assay as in B, applying the top level of nuclear extract used in B to shift the specific complexes further.
Association of these Elk-1 binding sites on the AChE gene promoter with P2Y1 receptor activation was demonstrated further. The response elements Elk-1[1] and Elk-1[3] identified above were inserted in the luciferase reporter vector and transfected into the myotubes. Each showed promoter activation by ATP and 2-MeSADP, and this response was increased further when the P2Y1 receptor was overexpressed in these myotubes (Fig. 8A,B). Stimulation of the promoter activity via these two sites also was produced by overexpression of Raf and was produced greatly by the expression instead of the constitutively active mutant of Raf (Fig. 8A,B). The involvement of PKC in the same system also was confirmed by demonstrating parallel activation by TPA (Fig. 8).
Figure 8.
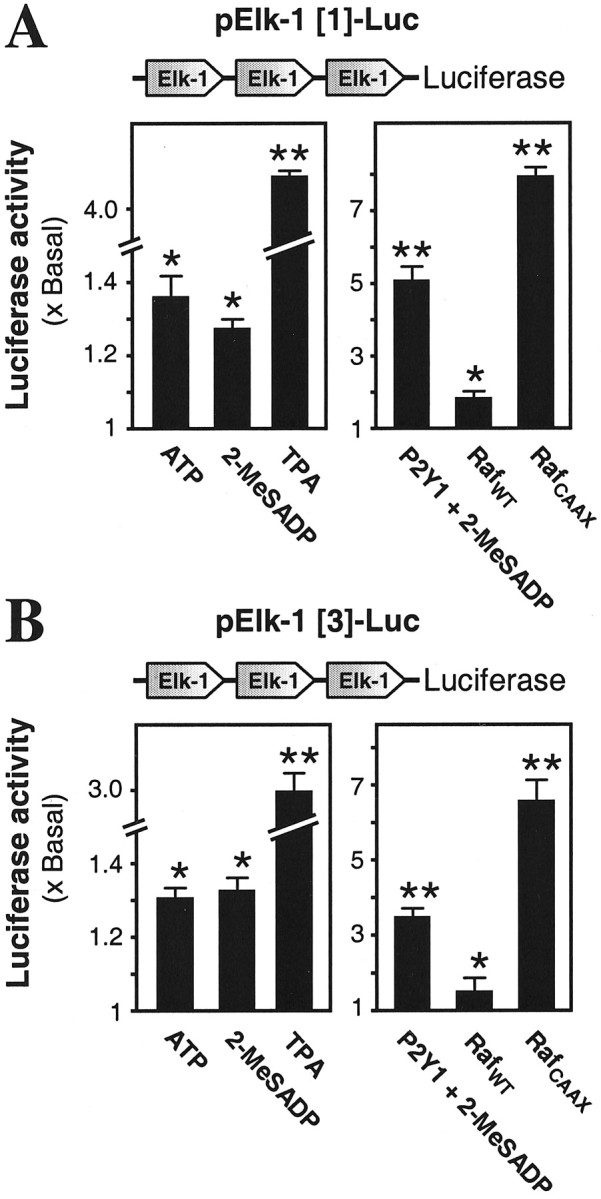
The two identified Elk-1 binding sites exert promoter activity and respond to activation of the P2Y1 receptor or Raf. Reporter gene constructs of Elk-1[1] (A) or Elk-1[3] (B), each having three consecutive copies of one of the identified Elk-1 binding sites from the AChE promoter, were pretransfected into myotubes. Cultures were exposed for 16 hr to the agents that are shown (50 μm for ATP and 2-MeSADP, with regeneration; 10 nm for TPA). In other cases that pretransfection was made jointly with wild-type Raf-1 (RafWT) or constitutively active Raf-1 mutant (RafCAAX) plasmids or with the P2Y1 receptor plasmid; in the latter case only, 50 μm 2-MeSADP was applied finally as before.In all cases the luciferase assay was performed after the ligand (or medium only) incubation.
To test the role of these two Elk-1 binding sites in the AChE gene activation, we performed mutations to inactivate the Ets-type consensus sequences lying within them. The entire 2.2 kb upstream sequence of the human AChE gene, which includes the promoter region (Ben Aziz-Aloya et al., 1993), was mutated at its Elk-1[1] and Elk-1[3] elements singly or together to change six consecutive nucleotides in each, as specified in Figure 9A. The Elk-1[1] mutant or the Elk-1[3] mutant each has totally lost the ability to give the gel mobility shift with myotube nuclear extract (Fig. 9A). These mutant promoters then were tagged downstream with the luciferase reporter to give the constructs: pAChEΔ Elk[1]-Luc, pAChEΔ Elk[3]-Luc showed the least activity. These activity losses were seen even more strongly when the wild-type promoter response to the agonists was raised by boosting the P2Y1 receptor content (Fig. 9B, right). The same relative effects of the mutant series were produced when the wild-type promoter response was elicited weakly by boosting the content of Raf, and they were seen strongly when activation was by the constitutively active mutant of Raf (Fig. 9B, right).
Figure 9.
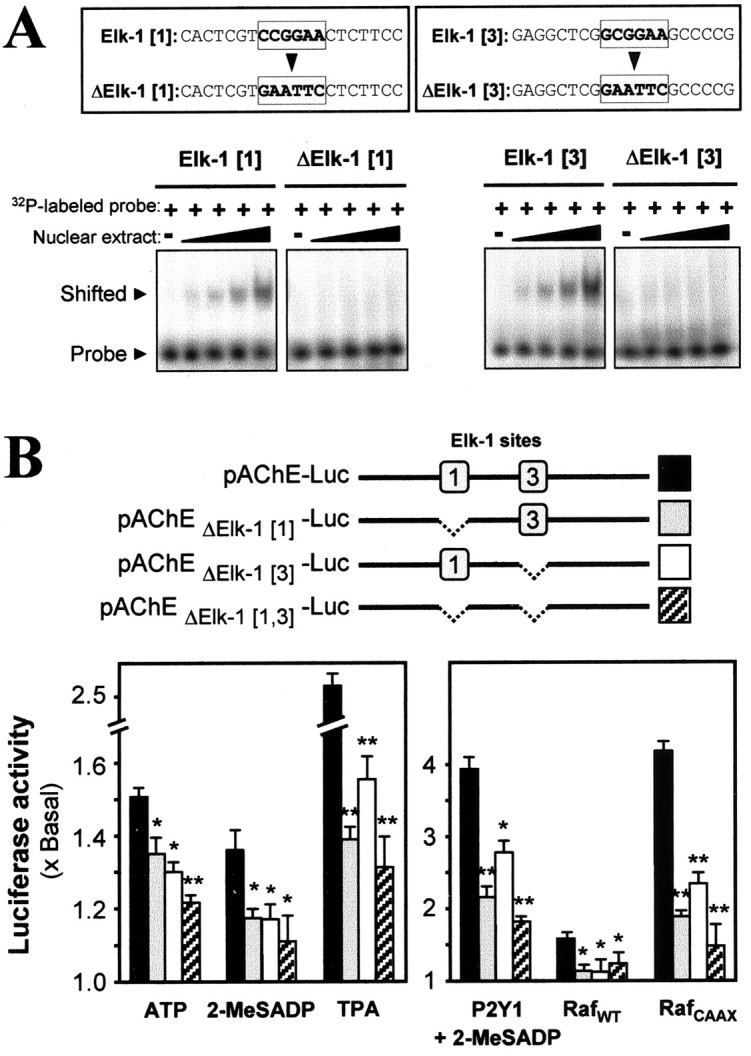
Mutation of the Elk-1 site of the AChE promoter blocks its response to P2Y1 receptor or Raf-1 activation. A, B, The two identified Elk-1 binding sites on the AChE promoter were mutated as shown in A. To confirm the loss of the Elk-1 binding, we performed gel shift assays as for Figure 7, using oligonucleotide probes for the mutant (Δ) or wild-type sequences. The amount of nuclear extractthat was used increased stepwise from 2 to 5 μg of protein. The ∼2.2 kb AChE promoter DNA thus was mutated at either or both of its Elk-1 binding sites sequences and was tagged with luciferase reporter gene, as shown in B. Myotubes pretransfected with one of those mutant (or wild-type) AChE gene expression constructs were exposed for 16 hr (as for Fig. 8) to the agents shown (left). Such pretransfected myotubes also were pretransfected jointly with P2Y1 receptor or RafWT or RafCAAX plasmids. These were incubated (16 hr, as before) with 50 μm 2-MeSADP for the P2Y1 overexpression case or with medium alone for the Raf cases. Luciferase assays were performed finally for all. Significance here is marked relative to the wild type: *p < 0.05 for the second ATP bar; all others are as defined for Figure 1.
The AChE promoter activity also was induced by the TPA activation of PKC (Fig. 9B, left) and again was strongest when both of the Elk-1 sites were present. Because activation of this receptor in the muscle cells both stimulates PKC (Fig. 1A) and produces some of the series of downstream activations that TPA does (Figs. 3B, 4C, 5A, 6A, 9B), we deduce that PKC acts in a signaling pathway from the P2Y1 receptor to activation of the AChE gene. However, P2Y1 receptor activity in muscle is not thought to produce a generalized PKC action, because a specific pathway is evoked and because in vivo this PKC action will be confined by the localization of the P2Y1 receptors at the nmj (Choi et al., 2001a), combined with the local release of ATP there. All of these tests in which gene activation was initiated at different points on its pathway from the P2Y1 receptor were consistent in implicating both of the Elk-1[1] and Elk-1[3] elements of the native promoter. They also indicate that the two sites operate additively.
P2Y1 receptor activation of genes of AChR subunits
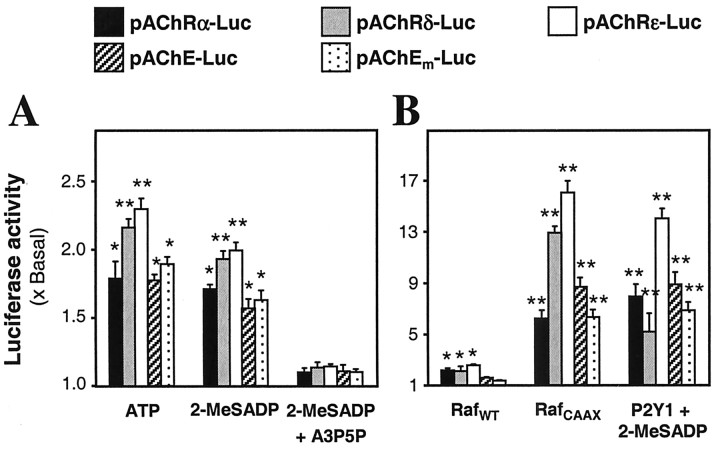
There is evidence that the expression and clustering of muscle AChE and AChR have some, but not all, of their control mechanisms in common (Sanes and Lichtman, 1999). The ATP regulation has appeared to act on both. Thus we have reported (Choi et al., 2001a) that activation by ATP or 2-MeSADP of the P2Y1 receptor in chick myotubes also induces the mRNA encoding the α-subunit of the endogenous AChR. Further, the promoter region (∼930 bp) of the chicken AChR α-subunit, when driving expression in the myotubes of a luciferase reporter gene (pAChRα-Luc), responds to P2Y1 receptor activation with approximately the same increase (maximum ∼1.7-fold) as is seen here with human pAChE-Luc (compare Fig. 1E). We have, therefore, extended that study to compare those inductions via P2Y1 receptors with parallel activations of mammalian genes for AChE and two other AChR subunits. Promoter-containing regions of the rat δ (550 bp) and rat ϵ (2 kb) AChR subunit genes were tagged downstream with the luciferase gene to form the constructs pAChRδ-Luc and pAChRϵ-Luc and were transfected into chick myotubes. The application of ATP or 2-MeSADP then stimulated 1.5-to 2.3-fold the basal promoter activity (Fig. 10A). The mouse AChE promoter (pAChEm-Luc) showed a response similar to that of the other promoter constructs and the corresponding human AChE construct, after the challenges with P2Y1 receptor agonists (Fig. 10A). In all cases the P2Y1-induced promoter activities were blocked by A3P5P. In addition, the content of P2Y1 receptors in chick myotubes was boosted by transfection, as in the AChE studies above; each AChR gene activation then was induced more markedly by incubation with 2-MeSADP. The maximum response reached ∼14 times the basal activity in the highest case (where, although this might be coincidental, the longest promoter region was used), the ϵ-subunit of AChR (Fig. 10B).
Figure 10.
The P2Y1-induced gene expressions are found in promoter/reporter constructs of three AChR subunits (chicken pAChRα-Luc, rat pAChRδ-Luc, or rat pAChRϵ-Luc) and of human or mouse AChE (pAChE-Luc, pAChEm-Luc). Note that in chicken AChR a single subunit corresponds to mammalian γand ϵ subunits. A, Each construct was pretransfected into chick myotubes and was exposed for 16 hr to ATP (50 μm), 2-MeSADP (50 μm), or 2-MeSADP with A3P5P (50 μm for both) or to medium alone (basal level) and was assayed. B, The promoter constructs, either alone (basal) or jointly with plasmids expressing RafWT or RafCAAX or the chicken P2Y1 receptor, were transfected into cultured chick myotubes. For the latter, exposure to 50 μm 2-MeSADP for 16 hr, as before, was given. Luciferase assays were performed finally for all.
The MAP kinase signaling pathway was involved again in this response; the AChR promoter activities, which were raised slightly by overexpression of wild-type Raf, were stimulated highly by the introduction of mutant constitutively active Raf, in both cases without agonist (Fig. 10B). Again the ϵ-subunit gene was the most sensitive, its activation reaching 16-fold. In treatments made in parallel that used the promoters of the mouse or the human AChE gene, the same effects, to seven- to ninefold activation, were found with the two (Fig. 10B).
Discussion
The results support the concept of a novel function of ATP in the regulation of expression of AChE and AChR in developing skeletal muscle, mediated by the P2Y1 receptor. Thus the promoter of the AChE catalytic subunit gene (Figs. 1E,F, 2, 8, 10) and the gene promoters of the AChR subunits (testing here 3 of 4; Fig. 10) all can be activated by external application to the muscle cells of ATP or a specific P2Y1 agonist and can be blocked by a P2Y1-selective antagonist. The extent of this activation can be remarkable; thus the 14-fold increase observed with it for the ϵ-subunit gene of the AChR (Fig. 10B) should be corrected to ∼46-fold, because the P2Y1 transfection efficiency in these cells was consistently ∼30%.
In that and some other cases the effect of boosting the P2Y1 receptor content by transfection was tested. In these cases as a safeguard a parallel plot is presented showing that the same effect is obtained with the endogenous receptor but at a lower magnitude. That endogenous set always showed a rise above the level (basal) seen without agonist, which was significant at p < 0.01 (or p < 0.05 where marked in Figs. 8B, 10B). The large increases beyond this, which are shown with the boosting, support the identification of the P2Y1 receptor as the initial mediator. However, in several of the parallel studies without added receptor ATP is seen to give larger activations than 2-MeSADP (which is much more selective than ATP for P2Y1 in the Gq-linked P2Y receptors). Hence there is a suggestion that another P2Y receptor also may contribute to a lesser degree.
Initially, we have studied here ATP-mediated gene expressions in muscle cells organized into myotubes at the stage just before neurons would make contact with the muscle in vivo. However, when that contact occurs and presynaptically released ATP bombards the postsynaptic membrane, then (Choi et al., 2001a) in vivo the expression of muscle P2Y1 receptors greatly increases. In both chicken and rat muscles the P2Y1 receptors subsequently become colocalized with AChRs at the nmjs; further, the P2Y1 receptor expression is lost on muscle denervation and restored on reinnervation (Choi et al., 2001a). Molecular studies on innervated muscles now will be needed to establish the proposed P2Y1 signaling to the subsynaptic nuclei.
We should note that the P2Y1 receptor is, exceptionally for P2Y receptors, widely expressed on brain neurons (Moore et al., 2000) and that ATP is known generally to be co-released at central and peripheral cholinergic and bioaminergic [and even some GABAergic (Jo and Schlichter, 1999)] neuronal synapses. Further, an ionotropic ATP receptor, P2X7, has been reported to be widespread at brain excitatory presynaptic terminals (Deuchars et al., 2001), suggesting ATP cotransmission there. Hence further investigation is indicated as to whether the postsynaptic actions of the P2Y1 receptor now being uncovered at the nmj have a wider relevance at such central synapses.
Gene regulations linked to the muscle P2Y1 receptor
Agonist stimulation of the P2Y1 receptors on the muscle cells strongly activates the transcription factor Elk-1 by its phosphorylation and binding in the nucleus (Figs. 5, 6). Further, the activation of the AChE catalytic subunit gene promoter, when initiated by treating the cells with a P2Y1 agonist (Figs. 5B, 6B, 10), was Elk-1 concentration-dependent. Two candidate Elk-1-responsive elements in the AChE gene promoter were confirmed as such because they bind specifically to native Elk-1 protein (Fig. 7). Two 20 bp elements separated by 310 bp in the AChE gene upstream sequence are each sufficient for P2Y1-mediated promoter activity but also show synergy (Figs. 8, 9). As just noted above, the P2Y1 receptor is linked also to activation of the promoters in AChR subunits; the responsive element or elements acting therein have not been studied as yet, although putative Elk-1 consensus sequences can be found there.
The signaling route from the P2Y1 receptor
In signaling from the P2Y1 receptor when expressed in astrocytoma cells (Sellers et al., 2001), again Elk-1, and not several other common transcription factors that were tested, became activated when P2Y1 agonists were applied, this reaction again being blocked by a P2Y1-selective antagonist. Elk-1 activation there involved a well known route for a Gq-coupled receptor transduction via phospholipase C-β, PKC, PI3-kinase, MEK1, and ERK 1/2. To achieve the Elk-1 outcome, we sustained the activation of ERK1/2 by its dual phosphorylation and nuclear translocation (>2 hr), whereas Src, for example, was involved only in a transient (∼15 min) ERK response (Sellers et al., 2001). Indeed, it long has been a general conclusion that ERK activation must be sustained for it to affect gene transcription, and a general mechanism for this control by signal duration has been described recently (Murphy et al., 2002). In the myotubes, however, the P2Y1-mediated ERK activation was of the transient class (Fig. 3), yet that produced Elk-1 phosphorylation (Figs. 5, 6) and gene activations (Figs. 4, 5, 10). The route to this involves PKC (as discussed below); it also involves intracellular Ca 2+ release, which was observed directly as a fast response to ATP, 2-MeSADP, and 2-MeSATP (Choi et al., 2001a), and is required for the AChE promoter activation (Figs. 1, 2, 4). Those two intermediates could operate in parallel if one of the Ca 2+-dependent forms of PKC acts, or both may be needed for an activation of Ras (Cullen and Lockyer, 2002). The pathway here also includes Raf-1 (Figs. 4, 8, 10), which requires Ras both for its membrane translocation and for its activation (Mineo et al., 1997). Raf action then is commonly through MEK to MAP kinases, and this action was found here.
The delayed response at the gene level to the P2Y1-elicited transient ERK signaling in the myotube can be understood in the light of a dual status of Elk-1. This also can function as one of the transcription factors for the c-fos gene in immediate early gene expression (Hodge et al., 1998), an initial response to many stimuli, which can be activated strongly by ERK1/2 and which in turn activates many genes. The Elk-1 protein has a docking domain for ERK (and another for p38-MAPK) and a DNA-binding site, when phosphorylated, for the Ets-type Elk-1-responsive element. That element is present in the promoter of the c-fos gene but also of a number of later-transcribed genes for signaling proteins, including, as we have shown here, the AChE gene. In one or two cases other receptors have shown this dual signaling of Elk-1. Thus growth hormone applied to cells (native or transfected) expressing its receptor produces strong ERK1/2 phosphorylation in 5 min in a transient response and produces c-fos mRNA and phospho-Elk-1 strongly within 30 min; hormone-stimulated Elk-1 binding site/luciferase activity appears, but only slowly, after some hours (Hodge et al., 1998).
Comparing the regulation of the AChR subunits by neuregulin, it is known that in chick (Altiok et al., 1997) and in mouse C2C12 (Si and Mei, 1999) myotubes this also depends on ERK activation. Unusually for a stimulus leading to a transcriptional outcome, only the transient phase of the ERK response was induced by neuregulin, whereas a lag of ∼10 hr occurred before the AChR ϵ-subunit mRNAs began to increase, to a maximum at ∼24 hr (Si et al., 1999). Here those properties are found also in the regulation by adenine nucleotides; the transient ERK activations plus the lag and the time to a plateau of the rise of the mRNAs of AChR and AChE (Fig. 3B) (Choi et al., 2001a) are in each case very similar to those seen (Si et al., 1999) in the AChR response to neuregulin. In the latter case the pathway from ERK-P includes c-jun gene activation and JUN kinase (Si et al., 1999), but it merits exploration now whether the signaling downstream from ERK may have some elements in common in the responses to nucleotide and to neuregulin. We also should note that in the P2Y1 receptor-mediated pathway Elk-1 is not thought to act as an intermediate transcription factor needed to express a final activator, because its promoter site in the AChE gene acted directly as an ATP-linked transgene (Fig. 9).
Differences in the regulation of AChE and of AChR
Activation of PKC by the phorbol ester TPA alone led to a clear enhancement of the human AChE promoter activity (Figs. 1, 5A, 6A, 9), whereas the reverse effect is known with AChR. Thus TPA in similar conditions suppresses the mRNA and promoter activity of the AChR α-subunit gene in chick myotubes (Laufer et al., 1991). In rat myotubes in which PKC was activated, either constitutively or by limited phorbol ester treatment, the same effects were shown on α-subunit and δ-subunit genes (Macpherson et al., 2002), whereas similar phorbol ester stimulation of PKC decreases AChR membrane insertion at mouse nmjs (Lanuza et al., 2000).
The P2Y1 receptor is coupled to Gq, and its activation is known to stimulate PKC in other cell types [Sellers et al. (2001) and references cited therein]. A specific pathway could be directed by a compartmentalization in the nmj region of PKC, which is selective among its 12 isozymes, because isozyme-specific anchors generally act thus for PKC (Dempsey et al., 2000). Indeed, at the nmj an uncommon isozyme PKCθ, Ca 2+-independent and neurally regulated, is localized in muscle postsynaptically (Hilgenberg et al., 1996). Also, the Ca 2+-dependent isozyme PKCβ has been located on the nmj postsynaptic membrane; this is colocalized there with the scaffolding protein gravin, which has binding sites for that isozyme and for some G-protein-coupled receptors (range yet unknown) (Perkins et al., 2001). PKCθ and PKCβ are both phorbol-stimulated, and either or both could account in principle for the parallel effect of TPA and P2Y1 receptor activation on AChE expression. For AChR regulation the relationship to PKC isozymes appears to be different; PKCα (phorbol-stimulated) has been reported to block an initial step of the AChR expression pathway in myotubes (Altiok et al., 1997), whereas PKCζ (a phorbol-insensitive isozyme) is also prominent therein and appears to affect the AChR expression there (Altiok and Changeux, 2001). Hence opposite effects on AChR gene expression could arise from the activation of P2Y1 receptors or from phorbol ester treatment, dependent on the relative availabilities and locations of the PKC isozymes there.
Although the production of the AChE protein was stimulated by the TPA treatment (Fig. 1C), it should be noted that the AChE enzymatic activity remained unchanged at all of the TPA concentrations that were applied (data not shown). That phenomenon occurred likewise in the stimulation of the myotubes by P2Y1 receptor agonists as shown by Choi et al. (2001a), for which several possible reasons were considered. Pools of active and inactive AChE are known to occur in native muscles, the latter pool being much higher in noninnervated muscle cells; thus ∼80% of the AChE protein in untreated chick myotubes does not mature to the active form (Rotundo, 1988). The lack in myotubes of the collagentail subunit of AChE (ColQ), which is needed for assembly and stabilization of the final multimeric form of AChE at the nmj (Legay et al., 1999), seems to be likely to contribute to this inactivity of the catalytic subunits. An exactly parallel case to the present one is known, i.e., the AChE protein increase elicited in myotubes by another nerve-derived regulator, calcitonin gene-related peptide, shows the same behavior (Choi et al., 1998).
For AChR genes another Ets transcription factor, GABP, acts in their neuregulin activation, binding at a 6 bp N-box element (Schaeffer et al., 2001). For muscle AChE a DNA sequence, including an N-box in its first intron, acts as an enhancer element for AChE synthesis and aggregation at the nmj, also via GABP activation (Briguet and Ruegg, 2000; Angus et al., 2001). The mediators can differ for those two regulations, because in chick myotubes treated with ATP we see a strong stimulation at the AChE and AChR genes, but with neuregulin (Pun and Tsim, 1995) we see none with AChE. The N-box and the Elk-1 sites in the AChE gene occur at very different locations, and any interaction is unknown as yet.
Footnotes
This work was supported by the Research Grants Council of Hong Kong (Grants 6112/00M, 6098/02M, and 2/99C to K.W.K.T.) and by the Wellcome Trust (to E.A.B.). R.C.Y.C. was supported by a postdoctoral matching fund from Hong Kong University of Science and Technology. We thank Professor H. Soreq (Hebrew University of Jerusalem) for providing human AChE promoter DNA and Dr. D. Goldman (University of Michigan) for providing rat AChR subunitδ and ϵ promoter DNAs.
Correspondence should be addressed to Dr. Karl W. K. Tsim, Department of Biology, The Hong Kong University of Science and Technology, Clear Water Bay Road, Hong Kong SAR, China. E-mail: botsim@ust.hk.
Copyright © 2003 Society for Neuroscience 0270-6474/03/234445-12$15.00/0
References
- Altiok N, Changeux J-P ( 2001) Electrical activity regulates AChR gene expression via JNK, PKC, and Sp1 in skeletal chick muscle. FEBS Lett 487: 333–338. [DOI] [PubMed] [Google Scholar]
- Altiok N, Altiok S, Changeux J-P ( 1997) Heregulin-stimulated acetylcholine receptor gene expression in muscle: requirement for MAP kinase and evidence for a parallel inhibitory pathway independent of electrical activity. EMBO J 16: 717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angus LM, Chan RYY, Jasmin BJ ( 2001) Role of intronic E- and N-box motifs in the transcriptional induction of the acetylcholinesterase gene during myogenic differentiation. J Biol Chem 276: 17603–17609. [DOI] [PubMed] [Google Scholar]
- Ben Aziz-Aloya R, Seidman S, Timberg R, Sternfeld M, Zakut H, Soreq H ( 1993) Expression of a human acetylcholinesterase promoter–reporter construct in developing neuromuscular junctions of Xenopus embryos. Proc Natl Acad Sci USA 90: 2471–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JL, Romero-Avila T, Schachter JB, Harden TK ( 1996) Identification of competitive antagonists of the P2Y1 receptor. Mol Pharmacol 50: 1323–1329. [PubMed] [Google Scholar]
- Briguet A, Ruegg MA ( 2000) The Ets transcription factor GABP is required for postsynaptic differentiation in vivo J Neurosci 20: 5989–5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahine KG, Walke W, Goldman D ( 1992) A 102 base pair sequence of the nicotinic acetylcholine receptor δ-subunit gene confers regulation by muscle electrical activity. Development 115: 213–219. [DOI] [PubMed] [Google Scholar]
- Chan RYY, Boudreau-Lariviere C, Angus LM, Mankal FA, Jasmin BJ ( 1999) An intronic enhancer containing an N-box motif is required for synapse-and tissue-specific expression of the acetylcholinesterase gene in skeletal muscle fibers. Proc Natl Acad Sci USA 96: 4627–4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi RCY, Yung LY, Dong TTX, Wan DCC, Wong YH, Tsim KWK ( 1998) The calcitonin gene-related peptide-induced acetylcholinesterase synthesis in cultured chick myotubes is mediated by cyclic AMP. J Neurochem 71: 152–160. [DOI] [PubMed] [Google Scholar]
- Choi RCY, Man MLS, Ling KKY, Ip NY, Simon J, Barnard EA, Tsim KWK ( 2001a) Expression of the P2Y1 nucleotide receptor in chick muscle: its functional role in the regulation of acetylcholinesterase and acetylcholine receptor. J Neurosci 21: 9224–9234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi RCY, Siow NL, Zhu SQ, Wan DCC, Wong YH, Tsim KWK ( 2001b) The cyclic AMP-mediated expression of acetylcholinesterase in myotubes shows contrasting activation and repression between avian and mammalian enzymes. Mol Cell Neurosci 17: 732–745. [DOI] [PubMed] [Google Scholar]
- Cohen I, Rimer M, Lomo T, McMahan UJ ( 1997) Agrin-induced postsynaptic-like apparatus in skeletal muscle fibers in vivo Mol Cell Neurosci 9: 237–253. [DOI] [PubMed] [Google Scholar]
- Cullen PJ, Lockyer PJ ( 2002) Integration of calcium and Ras signaling. Nat Rev Mol Cell Biol 3: 339–348. [DOI] [PubMed] [Google Scholar]
- Dempsey EC, Newton AC, Mochly-Rosen D, Fields AP, Reyland ME, Insel PA, Messing RO ( 2000) Protein kinase C isozymes and the regulation of diverse cell responses. Am J Physiol Lung Cell Mol Physiol 279: L429–L438. [DOI] [PubMed] [Google Scholar]
- Deuchars SA, Atkinson L, Brooke RE, Musa H, Milligan CJ, Batten TF, Buckley NJ, Parson SH, Deuchars J ( 2001) Neuronal P2X7 receptors are targeted to presynaptic terminals in the central and peripheral nervous systems. J Neurosci 21: 7143–7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclert A, Changeux J-P ( 1995) Acetylcholine receptor gene expression at the developing neuromuscular junction. Physiol Rev 75: 339–368. [DOI] [PubMed] [Google Scholar]
- Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR ( 1995) A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA 92: 7686–7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM ( 1998) Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem 273: 18623–18632. [DOI] [PubMed] [Google Scholar]
- Fischbach GD, Rosen KM ( 1997) ARIA: a neuromuscular junction neuregulin. Annu Rev Neurosci 20: 429–458. [DOI] [PubMed] [Google Scholar]
- Fuhrer C, Gautam M, Sugiyama JE, Hall ZW ( 1999) Roles of rapsyn and agrin in interaction of postsynaptic proteins with acetylcholine receptors. J Neurosci 19: 6405–6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz SA ( 1988) Primer of biostatistics. New York: McGraw-Hill.
- Grisaru D, Sternfeld M, Eldor A, Glick D, Soreq H ( 1999) Structural roles of acetylcholinesterase variants in biology and pathology. Eur J Biochem 264: 672–686. [DOI] [PubMed] [Google Scholar]
- Hilgenberg L, Yearwood S, Milstein S, Miles K ( 1996) Neural influence on protein kinase C isoform expression in skeletal muscle. J Neurosci 16: 4994–5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge C, Liao J, Stofega M, Guan K, Carter-Su C, Schwartz J ( 1998) Growth hormone stimulates phosphorylation and activation of Elk-1 and expression of c-fos, egr-1, and junB through activation of extracellular signal-regulated kinases 1 and 2. J Biol Chem 273: 31327–31336. [DOI] [PubMed] [Google Scholar]
- Israel M, Dunant Y ( 1998) Acetylcholine release and the cholinergic genomic locus. Mol Neurobiol 16: 1–20. [DOI] [PubMed] [Google Scholar]
- Jo YH, Schlichter R ( 1999) Synaptic co-release of ATP and GABA in cultured spinal neurons. Nat Neurosci 2: 241–245. [DOI] [PubMed] [Google Scholar]
- Johnson LN, Noble ME, Owen DJ ( 1996) Active and inactive protein kinases: structural basis for regulation. Cell 85: 149–158. [DOI] [PubMed] [Google Scholar]
- Krejci E, Legay C, Thomine S, Sketelj J, Massoulié J ( 1999) Differences in expression of acetylcholinesterase and collagen Q control the distribution and oligomerization of the collagen-tailed forms in fast and slow muscles. J Neurosci 19: 10672–10679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanuza MA, Li MX, Jia M, Kim S, Davenport R, Dunlap V, Nelson PG ( 2000) Protein kinase C-mediated changes in synaptic efficacy at the neuromuscular junction in vitro: the role of postsynaptic acetylcholine receptors. J Neurosci Res 61: 616–625. [DOI] [PubMed] [Google Scholar]
- Laufer R, Klarsfeld A, Changeux J-P ( 1991) Phorbol esters inhibit the activity of the chicken acetylcholine receptor α-subunit gene promoter. Role of myogenic regulators. Eur J Biochem 202: 813–818. [DOI] [PubMed] [Google Scholar]
- Legay C, Mankal FA, Massoulié J, Jasmin BJ ( 1999) Stability and secretion of acetylcholinesterase forms in skeletal muscle cells. J Neurosci 19: 8252–8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Camp S, Rachinsky TL, Bongiorno C, Taylor P ( 1993) Promoter elements and transcriptional control of the mouse acetylcholinesterase gene. J Biol Chem 268: 3563–3572. [PubMed] [Google Scholar]
- Lin W, Burgess RW, Dominguez B, Pfaff SL, Sanes JR, Lee KF ( 2001) Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature 410: 1057–1064. [DOI] [PubMed] [Google Scholar]
- Macleod K, Leprince D, Stehelin D ( 1992) The Ets family. Trends Biochem Sci 17: 251–256. [DOI] [PubMed] [Google Scholar]
- Macpherson P, Kostrominova T, Tang H, Goldman D ( 2002) Protein kinase C and calcium/calmodulin-activated protein kinase II (CaMKII) suppress nicotinic acetylcholine receptor gene expression in mammalian muscle. J Biol Chem 277: 15638–15646. [DOI] [PubMed] [Google Scholar]
- Massoulié J, Pezzementi L, Bon S, Krejci E, Vallette F-M ( 1993) Molecular and cellular biology of cholinesterases. Prog Neurobiol 41: 31–91. [DOI] [PubMed] [Google Scholar]
- Mineo C, Anderson RG, White MA ( 1997) Physical association with Ras enhances activation of membrane-bound Raf (RafCAAX). J Biol Chem 272: 10345–10348. [DOI] [PubMed] [Google Scholar]
- Mischak H, Seitz T, Janosch P, Eulitz M, Steen H, Schellerer M, Philipp A, Kolch W ( 1996) Negative regulation of Raf-1 by phosphorylation of serine 621. Mol Cell Biol 16: 5409–5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D, Chambers J, Waldvogel H, Faull R, Emson P ( 2000) Regional and cellular distribution of the P2Y1 purinergic receptor: striking neuronal localisation. J Comp Neurol 421: 374–384. [DOI] [PubMed] [Google Scholar]
- Murphy LO, Smith S, Chen RH, Fingar DC, Blenis J ( 2002) Molecular interpretation of ERK signal duration by immediate early gene products. Nat Cell Biol 4: 556–564. [DOI] [PubMed] [Google Scholar]
- Perkins GA, Wang L, Huang LJ, Humphries K, Yao VJ, Martone M, Deerinck TJ, Barraclough DM, Violin JD, Smith D, Newton A, Scott JD, Taylor SS, Ellisman MH ( 2001) PKA, PKC, and AKAP localization in and around the neuromuscular junction. BMC Neurosci 2: 17–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pun S, Tsim KWK ( 1995) Truncated form of pro-acetylcholine receptor-inducing activity (ARIA) induces AChR α-subunit but not AChE transcripts in cultured chick myotubes. Neurosci Lett 198: 107–110. [DOI] [PubMed] [Google Scholar]
- Pun S, Tsim KWK ( 1997) Antisense agrin cDNA transfection blocks neuroblastoma cell-induced acetylcholine receptor aggregation when co-cultured with myotubes. Mol Cell Neurosci 10: 87–99. [DOI] [PubMed] [Google Scholar]
- Rossi SG, Vazquez AE, Rotundo RL ( 2000) Local control of acetylcholinesterase gene expression in multinucleated skeletal muscle fibers: individual nuclei respond to signals from the overlying plasma membrane. J Neurosci 20: 919–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotundo RL ( 1988) Biogenesis of acetylcholinesterase molecular forms in muscle. J Biol Chem 263: 19398–19406. [PubMed] [Google Scholar]
- Sambrook J, Russel DW ( 2001) Molecular cloning: a laboratory manual, 3rd Ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory.
- Sanes JR, Lichtman JW ( 1999) Development of the vertebrate neuromuscular junction. Annu Rev Neurosci 22: 389–442. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Johnson YR, Kotzbauer PT, Mudd J, Hanley T, Martinou JC, Merlie JP ( 1991) Selective expression of an acetylcholine receptor-lacZ transgene in synaptic nuclei of adult muscle fibers. Development 113: 1181–1191. [DOI] [PubMed] [Google Scholar]
- Schaeffer L, de Kerchove d'Exaerde A, Changeux J-P ( 2001) Targeting transcription to the neuromuscular synapse. Neuron 31: 15–22. [DOI] [PubMed] [Google Scholar]
- Sellers LA, Simon J, Lundahl TS, Cousens DJ, Humphrey PP, Barnard EA ( 2001) Adenosine nucleotides acting at the human P2Y1 receptor stimulate mitogen-activated protein kinases and induce apoptosis. J Biol Chem 276: 16379–16390. [DOI] [PubMed] [Google Scholar]
- Si J, Mei L ( 1999) ERK MAP kinase activation is required for ARIA-induced increase in all five AChR subunit mRNAs as well as synapse-specific expression of the AChR ϵ-transgene. Mol Brain Res 67: 18–27. [DOI] [PubMed] [Google Scholar]
- Si J, Wang Q, Mei L ( 1999) Essential roles of c-JUN and c-JUN N-terminal kinase (JNK) in neuregulin-increased expression of the acetylcholine receptor ϵ-subunit. J Neurosci 19: 8498–8508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky EM, Redman RS ( 1996) Synchronous release of ATP and neuro-transmitter within milliseconds of a motor nerve impulse in the frog. J Physiol (Lond) 492: 815–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siow NL, Choi RCY, Cheng AWM, Jiang JXS, Wan DCC, Zhu SQ, Tsim KWK ( 2002) A cyclic AMP-dependent pathway regulates the expression of acetylcholinesterase during myogenic differentiation of C2C12 cells. J Biol Chem 277: 36129–36136. [DOI] [PubMed] [Google Scholar]
- Tsim KWK, Barnard EA ( 2002) The signaling pathways mediated by P2Y nucleotide receptors in the formation and maintenance of the skeletal neuromuscular junction. Neurosignals 11: 58–64. [DOI] [PubMed] [Google Scholar]
- Tsim KWK, Randall WR, Barnard EA ( 1988) Monoclonal antibodies specific for the different subunits of asymmetric acetylcholinesterase from chick muscle. J Neurochem 51: 95–104. [DOI] [PubMed] [Google Scholar]
- Walke W, Staple J, Adams L, Gnegy M, Chahine K, Goldman D ( 1994) Calcium-dependent regulation of rat and chick muscle nicotinic acetylcholine receptor (nAChR) gene expression. J Biol Chem 269: 19447–19456. [PubMed] [Google Scholar]
- Webb TE, Simon J, Krishek BJ, Bateson AN, Smart TG, King BF, Burnstock G, Barnard EA ( 1993) Cloning and functional expression of a brain G-protein-coupled ATP receptor. FEBS Lett 324: 219–225. [DOI] [PubMed] [Google Scholar]