Abstract
Individual cerebellar cortical zones defined by the somatotopy of climbing fiber responses and by their olivo-cortico-nuclear connections located in the paramedian lobule and the copula pyramidis of the rat cerebellum were microinjected with cholera toxin B subunit. Collateral branches of climbing and mossy fibers were mapped and related to the pattern of zebrin-positive and -negative bands of Purkinje cells. Climbing fiber collaterals from the copula distribute to the anterior lobe: from the paramedian lobule mainly to lobulus simplex and rostral crus I. Climbing fibers terminating in particular zones (X, A2, C1, CX, C2, C3, D1, and D2) in the paramedian lobule or the copula collateralize to one or two corresponding zones in lobulus simplex, crus I and II, the paraflocculus, and/or the anterior lobe. These zones can be defined by their relationship to the pattern of zebrin banding. Collaterals from mossy fibers, labeled from the same injection sites in the copula and paramedian lobule, often distribute bilaterally in a symmetrical pattern of multiple but ill-defined longitudinal strips in the anterior lobe and/or lobulus simplex. One or more of these longitudinal aggregates of mossy fiber collaterals was always found subjacent to the strip(s) of labeled climbing fiber collaterals arising from the same locus in the paramedian lobule or the copula. Corticonuclear projections focused on the target nucleus of each zone, although a bilateral plexus of thinner axons, presumably of mossy fiber collateral origin, was sometimes also present in several other regions of the cerebellar nuclei. Overall, these results suggest that climbing fiber zones and zebrin banding reflect a common organizational scheme within the cerebellar cortex.
Keywords: cerebellum, cholera toxin B unit, climbing fiber collaterals, mossy fiber collaterals, zebrin pattern, zonal organization
Introduction
The role played by different parts of the cerebellum in motor control is thought to depend critically on differences in afferent and efferent connectivity. In particular, the inferior olive climbing fiber projection to the Purkinje cells of the cerebellar cortex is highly topographically organized, so that small groups of olive cells target different rostrocaudally oriented cortical zones. In turn, the Purkinje cells in each cortical zone are thought to project to specific cerebellar nuclei to influence different descending motor pathways (for review, see Brodal and Kawamura, 1980; Voogd and Bigaré, 1980; Buisseret-Delmas and Angaut, 1993; Voogd and Ruigrok, 1997; Garwicz, 2000). Evidence has also been obtained to suggest that the other major afferent input to the cerebellum, the mossy fibers, which arise from a variety of sources within the CNS, also terminate in the cerebellar cortex in parasagittal strips, although the pattern is generally more diffuse than that described for the climbing fiber afferent system (Voogd, 1969; Tolbert et al., 1993; Ji and Hawkes, 1994; Wu et al., 1999; Serapide et al., 2001).
However, perhaps the most striking evidence for a zonal compartmentation of the cerebellar cortex comes from studies of the distribution of biochemical markers. Most notable is the distribution of zebrin, which is confined to a subset of Purkinje cells, resulting in highly reproducible parasagittal bands of high and low immunoreactivity within the rodent cerebellar cortex (Hawkes and Leclerc, 1987).
To date, few attempts have been made to correlate the distribution of these different patterns of parasagittal organization, and none have attempted to correlate individual, functionally identified climbing fiber zones with the pattern of zebrin banding within the same experimental animal. It therefore remains unclear whether the rostrocaudal organization of climbing fiber (and mossy fiber) inputs and of zebrin banding reflect a common organizational scheme within the cerebellar cortex.
Within the cerebellar cortex, both climbing and mossy fiber afferents are known to collateralize (Armstrong et al., 1973a,b; Oscarsson and Sjȯlund, 1977a,b; Ekerot and Larson, 1982; Wiklund et al., 1984; Heckroth and Eisenman, 1988; Sugihara et al., 1999, 2001; Wu et al., 1999), and cholera toxin B subunit (CTb) has been found to be particularly effective at labeling these collaterals (Chen and Aston-Jones, 1998). In the present study, we therefore made small injections of CTb into selected climbing fiber cortical zones within the paramedian lobule (PMD) and copula pyramidis (COP) of the rat cerebellum and mapped the distribution of CTb-labeled climbing and mossy fiber collaterals. In all cases, we also used the parasagittal distribution of zebrin-positive (P+) and zebrin-negative (P-) Purkinje cells as a topographical marker (Hawkes and Leclerc, 1987).
Our study allows conclusions on the spatial distribution of climbing fiber and mossy fiber collaterals arising from different functionally defined zones and on the more general question of the relationship of these zones to the pattern of zebrin banding in the cerebellar cortex.
Materials and Methods
A total of 18 injections of CTb were made in 13 adult male Wistar rats (Table 1). For complete documentation of the cases indicated with an asterisk in Table 1, see our website: http://www.erasmusmc.nl/voogd-cfcollaterals. In seven animals, injections were made into an electrophysiologically identified climbing fiber zone in the PMD or COP. These experiments were performed at the Department of Physiology, University of Bristol in accordance with the UK Animals Scientific Procedures Act of 1986 and were approved by the institutional animal license advisory group. The injections in the remaining animals were performed at the Department of Neuroscience, Erasmus Medical Center of Rotterdam, using anatomical landmarks as a guide. For these experiments, permission was obtained from the local committee overseeing animal experiments.
Table 1.
Experiments used
|
|
|
Injection site localization |
Sections reacted for: |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case number
|
Injection type
|
Lobule
|
Zebrin
|
Physiology
|
CTb
|
CTb + zebrin
|
Zebrin
|
||||
| CTB5* | P | Copula | P4−(e) | Ipsi hindlimb | + | + | |||||
| CTB8* | P | Copula | P4− | Ipsi hindlimb | + | + | + | ||||
| 910-r | I | Copula | P4−/P5+ | − | + | + | |||||
| 911-r* | I | Copula | P4−/P5+ | − | + | + | |||||
| 924-I* | I | Copula | P5+/7+ | − | + | + | |||||
| 924-r* | I | Copula | P5+/7+ | − | + | + | |||||
| CTB1 | P | PMD | P5a−/P5+ | Ipsi forelimb | + | + | |||||
| CTB4* | I | PMD | P6+ | Ipsi forelimb | + | + | + | ||||
| CTB6 | P | PMD | P5+ | Ipsi and contra forelimb | + | + | + | ||||
| CTB7* | P | PMD | P4b+/P5+ | Ipsi forelimb | + | + | + | ||||
| CTB18* | I | PMD | P4b/P5a+ | Contra face | + | ||||||
| 864* | I | PMD | P5+ | − | + | + | |||||
| 889-I | I | PMD | P6+/7+ | − | + | ||||||
| 889-r | I | PMD | P5a−/P5 | − | + | ||||||
| 890-I* | I | PMD | P5− | − | + | ||||||
| 890-r* | I | PMD | P5a− | − | + | ||||||
| 910-I | I | PMD | P4b/5a+ | − | + | ||||||
| 911-I*
|
I
|
PMD
|
P4b/5a+
|
−
|
+
|
+
|
|
||||
Case numbers with -r and -I indicate experiments in which an injection was made on the right and left sides of the cerebellum in the same animal. P, Pressure injection; I, iontophoretic injection; Ipsi, ipsilateral; contra, contralateral. Cases indicated with an asterisk are included on our website (http://www.erasmusmc.nl/voogd-cfcollaterals).
Injections of CTb. The general procedures and electrophysiological techniques have been described in full in previous papers (Atkins and Apps, 1997; Pardoe and Apps, 2002). In brief, after a surgical level of anesthesia was established (sodium pentobarbitone, 60 mg/kg, i.p.), the rats were placed in a stereotaxic frame, and a small craniotomy was made to expose the left side of the posterior lobe of the cerebellum. The paramedian lobule and the copula pyramidis were selected for study because a detailed electrophysiological map of climbing fiber-evoked potentials is available for this region of the rat cerebellum (Fig. 1). Pairs of percutaneous needles were inserted into different body parts (ipsilateral and contralateral forelimbs, contralateral face, and ipsilateral hindlimb), and bipolar electrical stimulation of a particular body region was used (single or double 0.1 msec pulse, 1 kHz once every 1.5 sec) to evoke climbing fiber field potentials on the cerebellar surface. The responses were recorded with a tungsten-in-glass microelectrode (impedance of ∼50 kΩ) and relayed through a Humbug device (50–60 Hz noise eliminator; Quest Scientific, North Vancouver, British Columbia, Canada) before being bandpass filtered (30 Hz to 5 kHz).
Figure 1.

Posterior view of the right side of the rat cerebellum showing the location in the PMD and COP of climbing fiber zones identified by their peripheral input, according to the key shown at the bottom (based on Atkins and Apps, 1997). A2, C1, C2, C3, Climbing fiber zones A2, C1, C2, and C3; ipsi, ipsilateral; contra, contralateral.
The surface of the cerebellum was then mapped from medial to lateral at 0.1 or 0.2 mm intervals, and each recording position was marked on a map of the cerebellar cortex. In five animals, a single pressure injection of 1–25 nl of 1% CTb (List Biologic, Campbell, CA) in 0.1 m phosphate buffer (PB) was made with a glass micropipette (20 μm tip diameter) into approximately the center of one of the cortical zones of climbing fiber-evoked responses in PMD or COP at a depth of 0.3–0.5 mm below the cerebellar cortical surface.
In two additional animals, electrophysiological techniques guided the injection, but iontophoresis was used to deliver the tracer through the glass micropipette (tip diameter, 10–14 μm) by passing anodal current pulses (4 μA, 7 sec on, 7 sec off) for a period of 10 min. Because this method can produce small, well defined injection sites of tracer material (Ruigrok et al., 1995; Teune et al., 1998), it was also used in the six additional animals in which injections were placed using anatomical landmarks and measurements as a guide. In five of these rats, injections were made bilaterally and, in one rat, unilaterally. All animals were monitored daily and recovered uneventfully.
Histology. After a survival period of 7 d, all animals were deeply reanesthetized with barbiturate and perfusion fixed (initial flush of 500 ml of 0.9% heparanised saline, followed by 1 l of 4% paraformaldehyde, 0.1% glutaraldehyde, and 4% sucrose). The brain was removed and postfixed in the perfusate for an additional 2–3 hr and subsequently was rinsed and stored overnight in 0.05 m PB, pH 7.4, containing 10% sucrose. The caudal brainstem and cerebellum were blocked and embedded in gelatin (10%), which was hardened for 3 hr in formaldehyde (4%) containing 30% sucrose, and then rinsed and stored overnight in 30% sucrose in PB (at 4°C).
Transverse sections of the gelatin blocks were cut at 40 μm on a freezing microtome and were serially collected in eight glass vials containing PB. Vials were selected for either CTb processing alone or CTb processing followed by zebrin immunostaining. For both procedures, the sections were incubated first in anti-CTb (raised in goat, 1:15,000; List Biologic) in Tris buffer containing 0.5 m NaCl and 0.5% Triton X-100, pH 8.6 (TBST), for 48 or 72 hr (at 4°C). The sections were then rinsed with TBST and incubated in biotinylated donkey anti-goat (1:2000 in TBST; List Biologic). After rinsing and overnight incubation (at 4°C) with the avidin–biotin complex (ABC Elite; Vector Laboratories, Burlingame, CA), the sections were thoroughly rinsed with PB before diaminobenzidine (DAB) incubation. Sections destined to be processed for zebrin II were incubated with DAB–cobalt (0.025% DAB, 0.01% CoCl2, and 0.005% H2O2 for 10–15 min), resulting in a black reaction product. The remaining sections were processed in the same DAB solution without cobalt ions but for a longer time period (up to 45 min). The CTb DAB–cobalt processed sections were incubated overnight with a monoclonal mouse antibody (zebrin II; 1:150 in TBST containing 2% normal horse serum; kindly provided by Dr. Richard Hawkes, Calgary, Canada). The sections were then rinsed and incubated with peroxidase-labeled rabbit anti-mouse (1:150 in TBST plus 2% normal horse serum) for 2 hr and thoroughly rinsed and incubated in DAB (see above), resulting in a brown staining of the zebrin bands (Hawkes and Leclerc, 1987; Voogd and Ruigrok, 1997). After final rinses in PB, sections from each vial were mounted serially, air dried, lightly counterstained with thionin, and coverslipped.
Plotting and reconstruction. Sections were digitized with a Minolta (Osaka, Japan) slide scanner, and labeled structures in every other section from each experiment were plotted by hand or by using ITRACER (Camino de la Costa, La Jolla, CA) to localize structures in the digitized sections. Outlines of zebrin-positive bands of Purkinje cells were checked by direct observation under the microscope and drawn on prints of the digitized sections. Graphical reconstructions of the cerebellum were made by stacking sections, using the floor of the fourth ventricle and the outline of the cerebellum as a guide. In these reconstructions, data on the zebrin pattern and on the localization of labeled climbing and mossy fibers were combined. To enhance their visibility, the width of the labeled strips of climbing fibers, which in many instances involved only one or a few climbing fibers, is generally exaggerated in these diagrams. In addition, only the most superficially located mossy fiber terminals are included to avoid overprojection. The reconstructions of mossy fiber terminals give, therefore, only an approximate impression of their localization. Reconstructions were made of the rostral and dorsal surfaces of the anterior lobe (ANT), of the rostral, dorsal, and caudal surfaces of lobulus simplex (SI), of the rostral surface of crus I, and of the dorsal and caudal surfaces of the posterior lobe. Retrogradely labeled cells in the inferior olive were plotted onto unfolded projections of the inferior olive and onto standard transverse diagrams, approximately corresponding to the levels illustrated in Figure 2 A.
Figure 2.

Corticonuclear and olivocerebellar projection zones of the rat cerebellum. The cortical zones (B), the subnuclei of the inferior olive that innervate particular zones (A), and their cerebellar and vestibular target nuclei (C) are indicated with the same symbols. The sections in A and C are viewed in the transverse plane, with sections 12 (A) and 8(C) being the most rostral. Redrawn from Buisseret-Delmas and Angaut (1993), with the addition of the A2 zone (asterisks)(Buisseret-Delmas, 1988a) and the X and CX zones (Buiseret-Delmas et al., 1993). A–D2, Zones A–D2; COP, copula pyramidis; CrI, crus I; CrII, crus II; DAOc, caudal part of dorsal accessory olive; DAOr, rostral part of dorsal accessoryolive; dl, dors all amina of the PO; DLH, dorsolateral hump; DLP, dorsolateral protuberance of the fastigial nucleus; DM, dorsomedial subnucleus of the PO; F, fastigial nucleus; FL, flocculus; IA, anterior interposed nucleus; IC, interstitial cell groups; IP, posterior interposed nucleus; I-X, lobules I–X; L, lateral cerebellar nucleus; LV, lateral vestibular nucleus; MAO, medial accessory olive; PFL, paraflocculus; PMD, paramedian lobule; PO, principal olive; SI, lobulus simplex; vl, ventral lamina of the PO; Y, group Y.
Results
Definition of zones
In the present study, individual, rostrocaudally oriented cerebellar cortical zones in the rat are defined by their inferior olive (climbing fiber) and Purkinje cell corticonuclear connections as reported by Buisseret-Delmas and coworkers (Buisseret-Delmas, 1988a,b; Buisseret-Delmas and Angaut, 1989, 1993; Buisseret-Delmas et al., 1993) (Fig. 2). Several differences from this scheme in the connections of the C and D zones are considered below (see also Discussion).
In some of the present experiments, climbing fiber-evoked responses on the cerebellar cortical surface were also charted to delineate the zones electrophysiologically (for additional details, see Materials and Methods). Because the use of climbing fiber responses to identify the zonal organization of the PMD and the COP in rats has already been described in detail by Atkins and Apps (1997) (see also Pardoe and Apps, 2002), additional details are not repeated here. In brief, however, zones A2, C1, C2, and C3 can be identified from medial to lateral in PMD and COP on the basis of differences in the peripheral stimulation that evokes the largest climbing fiber responses within each zone (Fig. 1).
In cats, zones C1 and C3 can be further subdivided into medial and lateral subzones (termed medial C1, Cx, and medial and lateral C3). These subzones are linked into pairs because of branching of axons of single olivary neurons. For example, medial C1 shares climbing fiber input with medial C3, whereas Cx shares climbing fiber input with zone X (Ekerot and Larson, 1979, 1982; Apps et al., 1991). This characteristic has not been substantiated in the rat. Zones X and CX share their electrophysiological properties with the C1 and C3 zones but differ from them anatomically because they receive climbing fibers from intermediate levels of medial accessory olive (MAO) instead of rostral dorsal accessory olive (DAO) (Campbell and Armstrong, 1985; Trott and Armstrong, 1987a,b; Buisseret-Delmas et al., 1993).
The zebrin pattern
The molecular marker zebrin II is expressed in a subset of cerebellar Purkinje cells, which can be revealed by immunohistochemical techniques postmortem (see Materials and Methods). Rostrocaudally oriented bands of immunoreactive (zebrin-positive) Purkinje cells alternate with cells that are zebrin negative (see Fig. 6, left side). The nomenclature used in the present paper for these zebrin-positive and -negative bands was introduced by Hawkes and Leclerc (1987), and a total of seven zebrin-positive bands can be identified in the rat cerebellum (termed P1+ to P7+). Zebrin-negative bands P1- to P6-are indicated with the number of the next-medial zebrin-positive band. With the exception of the P3+ band in the anterior cerebellum, the zebrin-positive bands are distinct and clearly delineated, whereas some of the unnumbered “satellite” bands of Hawkes and Leclerc (1987) occur regularly and have been indicated in the present study with the letters a, b, d, and e. In the figures in the present report, only the zebrin-positive bands are indicated with their respective numbers.
Figure 6.
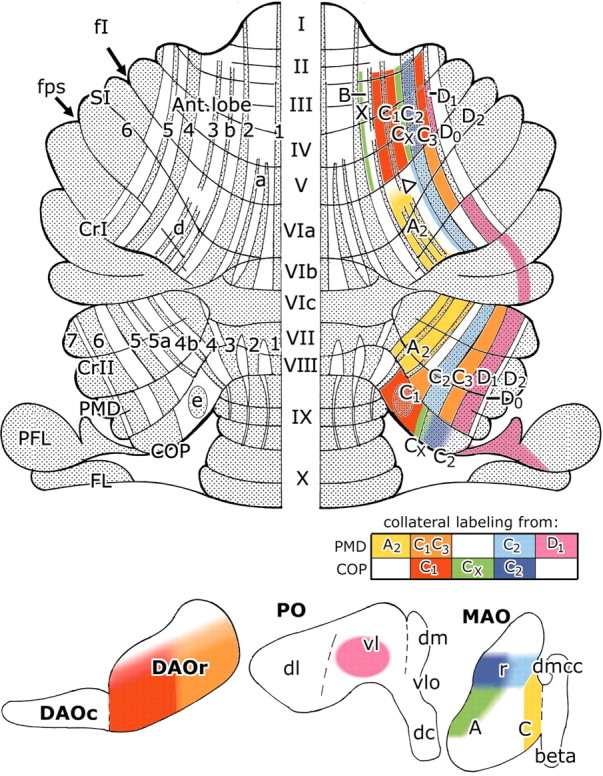
Summary of the present results and interpretation in relation to zebrin banding. Dorsal view of the flattened surface of the rat cerebellum, showing the zebrin nomenclature on the left and on the right the distribution of climbing fiber collaterals, arising from injections into different climbing fiber zones in the paramedian lobule and copula pyramidis, with their corresponding zonal identification. The block diagram contains the color code for the collateral projections for individual zones. The origin of these climbing fiber collaterals is shown in the horizontal projection diagrams of the contralateral inferior olive at the bottom. The putative CX zone in SI is indicated with an open arrowhead. Reasons for the localization of the DO and D2 zones in anterior P5- and P6+ and posterior P6- and P7+ bands, respectively, are given in Results and Discussion. 1–7, Zebrin-positive bands P1+ to P7+; A, subnucleus A of caudal MAO; A2, climbing fiber zone A2; a–e, zebrin satellite bands a, b, d, and e; ANT, anterior lobe; beta, subnucleusβ of caudal MAO; C, subnucleus C of caudal MAO; C1–C3, climbing fiber zones C1–C3; COP, copula pyramidis; II–VII, lobules VI–VII; CrI, crus I; CrII, crus II; CX, climbing fiber zone CX; D1, climbing fiber zone D1; dc, dorsal cap; dm, dorsomedial subnucleus of PO; DAOc, caudal dorsal accessory olive; DAOr, rostral dorsal accessory olive; dl, dorsal lamella of PO; dmcc, dorsomedial cell column; fI, primary fissure; fps, posterior superior fissure, MAO, medial accessory olive; PFL, paraflocculus; PMD, paramedian lobule; PO, principal olive; r, rostral; SI, lobulus simplex; vl, ventral lamella of PO; vlo, ventrolateral outgrowth; X, climbing fiber zone X.
In lobules VIb,c and VII, the nodulus, caudal crus I, the flocculus, and the paraflocculus, all Purkinje cells stain for zebrin and no bands can be distinguished. In crus II and PMD, two additional narrow zebrin-positive bands are located between P4+ and P5+ (indicated in the figures as bands P4b+ and P5a+), and bands P6+ and P7+ occupy the lateral part of crus II. In PMD, the pattern shifts laterally relative to crus II, and the P6+ and P7+ bands are often fused. This lateral shift is clearly visible in the oblique position of the bundle containing zebrin-stained axons of the P5+ band (see Fig. 4C). Fusion of P4b+ and P5a+ occurs in the ventral part of PMD and COP, and the latter contains an additional, ill-defined zebrin-positive patch (“e”).
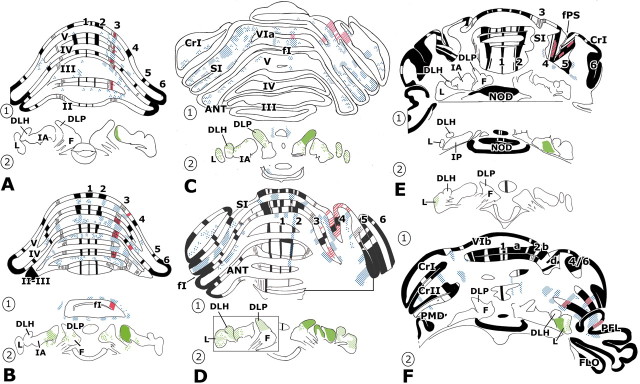
Figure 4.

A, Transverse section showing distribution of CTb-labeled mossy and climbing fiber collaterals in lobules IV and V. Note the regular and bilateral parcellation of mossy fiber terminals and two strips of labeled climbing fiber collaterals (asterisks), which convergeventrally. Case CTB8. Compare with Figure 5C. Scalebar, 500 μm.B, Transverse section showing CTb-labeled mossy and climbing fiber collaterals at higher power. Case CTB7, adjacent section to view shown in D. Scale bar, 50 μm. C, Oblique bundle of zebrin-immunoreactive axons belonging to zebrin-positive band P5+ in the lateral paramedian lobule. Case 925. Scale bar, 500 μm. D, Strips of CTb-labeled mossy and climbing fiber collaterals on facing surfaces of lobules V and VI, separated by the primary fissure, and located in zebrin-negative band P4-. The P4+ band is just visible in the top left corner. Case CTB7. Scale bar, 200 μm. E, Medial lobule of the paraflocculus containing a band of CTb-labeled mossy and climbing fiber collaterals. Case CTB4 (compare with Fig. 7F, panel 2). Scale bar, 500 μm. F, Collaterals of mossy and climbing fibers in the paraflocculus at higher power; for location, see box f in E. Case CTB4. Scale bar, 50 μm. G, Plexus of fine, CTb-labeled varicose axons in the dorsolateral protuberance of the fastigial nucleus, contralateral to the injection site. Case CTB18. Scale bar, 50 μm. H, Plexus of coarse, CTb-labeled varicose axons in the dorsolateral protuberance of the fastigial nucleus, ipsilateral to the injection site. Case CTB18. Same magnification as G. cf, Climbing fiber collaterals; m, midline; mf, mossy fiber collaterals; P4+, P5+, P5-, zebrin positive and negative bands P4+, P5+, and P5-; PFL, paraflocculus; V, VI, lobules V and VI.
The zebrin pattern, as described here, is highly reproducible. Small deviations from the sequence are sometimes visible in the reconstructions of individual cases, although such deviations are usually attributable to staining artifacts, missing sections, or errors in stacking of sections.
General features of CTb injection sites and labeling
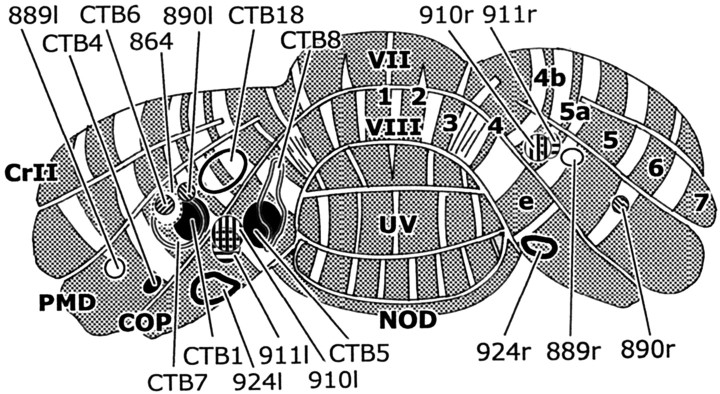
The CTb injection sites usually encompassed both the molecular layer and the underlying granular layer of the cerebellar cortex. Because most injection sites were situated in the apex of the lobules, they involved the white matter only to a limited extent. They were well circumscribed, although extensive labeling of parallel fibers and retrograde labeling of granule cells sometimes obscured their boundaries. Typically, iontophoresis resulted in small and discrete injection sites (range of 0.1–0.03 mm3), whereas pressure injections tended to produce larger injection sites (range of 0.4–0.04 mm3) and cause more extensive tissue damage. Figure 3 shows the location and extent of the individual injection sites as seen projected onto a posterior view of the rat cerebellum.
Figure 3.
Location of cerebellar cortical injection sites in the 18 cases used in the present study. Sites of injection projected onto the surface of a reconstruction of the caudal surface of the rat cerebellum. For additional details, see Table 1. Zebrin-positive bands are shaded and indicated with their numerals. COP, Copula pyramidis; CrII, crus II; NOD, nodulus; PMD, paramedian lobule; UV, uvula; VII, VIII, lobules VII and VIII.
In all cases, a bundle of faintly stained axons was evident that could be followed from the injection site into the white matter in the direction of the cerebellar nuclei. Within the cerebellar nuclei, these gave rise to one or more plexuses of intensely stained, fairly coarse, varicose axons (Fig. 4H). Climbing fibers labeled as a result of axonal collateral–collateral transport of CTb (referred to as climbing fiber collaterals in the present study) could be present near the injection site and/or in other parts of the cerebellum. In our transversely sectioned material, they usually appeared as two thin parallel lines of varicose axonal fragments, delineating the dendrites of Purkinje cells (Fig. 4B). Only in the paraflocculus was the complete arborization of individual climbing fibers observed (Fig. 4E,F), whereas the parent axons of labeled climbing fiber collaterals were only at best weakly labeled. In addition, in all but one experiment mossy fiber collaterals were labeled with CTb, although only in terminal rosettes (Fig. 4A,B,D,F); mossy fiber axons were never found stained.
Climbing fiber collateral labeling from CTb injections in the COP
Two cases with injections in COP are illustrated in Figure 5. In case CTB8, a large pressure injection was made into the medial half of COP, at a site where climbing fiber potentials were evoked by stimulation of the ipsilateral hindlimb. The injection site was located between the P4+ band medially and the lateral, zebrin-positive pole (P5+/P7+) of COP and included the zebrin-positive patch e (Fig. 5A). Retrograde labeling in the contralateral inferior olive was present mainly in caudolateral portions of the rostral half of the DAO. A few labeled cells were also present in the lateral pole of the MAO (Fig. 5D,E) and more caudally in subnucleus B of caudal MAO (data not shown). On the basis of its electrophysiological characteristics and connections with DAO, this region of the cerebellar cortex was considered as part of the C1 zone by Buisseret-Delmas (1988b) and Atkins and Apps (1997). In experiment 924, two small, iontophoretic injections were made, one on either side of the cerebellum into the lateral zebrin-positive pole (P5+/7+) of COP (in Fig. 5F–J, only the injection site on the right hand side of the cerebellum is shown, case 924-r). The pattern of retrograde labeling in rostral MAO indicated that the two injections in this experiment were both confined to the C2 zone (compare Fig. 5I,J with Fig. 2).
Figure 5.
Example injections into the copula pyramidis. In A and F, the caudal surface of the cerebellum has been reconstructed for two cases (CTB8 and 924-r, respectively). B, G and C,H are reconstructions of dorsal and rostral views of the anterior lobe. Zebrin-positive (P) bands are indicated in black and shown with their corresponding P numbers. The injection site and labeled parallel fibers are indicated in orange, and labeled mossy fiber rosettes are depicted as blue dots. Red contours indicate the position of labeled climbing fiber collaterals. For case CTB8, B′ and C′ show the right side of the cerebellum as depicted in B and C but without the mossy fiber labeling to show more clearly the relationship between the pattern of zebrin banding and the distribution of climbing fiber collateral labeling (indicated with red hatching). For both cases, the localization of retrogradely labeled neurons in the contralateral inferior olive is indicated in purple on an unfolded horizontal projection of DAO (E) and MAO (J) and on transverse sections through the olive (D, I). Scattered labeled cells are indicated with dots. The level of the transverse sections in D and I are indicated with a black horizontal line in E and J (numbers refer to levels depicted in Fig. 2 A). The broken vertical line in E indicates the junction between the dorsal and the ventral folds of DAO. 1–7, Zebrin-positive bands P1 + to P7 +; a, b, e, zebrin-positive satellite bands a, b, and e; BETA, subnucleus β of caudal MAO; COP, copulapyramidis; II–VII, lobules II–VII; CrI, crusI; CrII, crusII; DAOc, caudal part of DAO; DAOr, rostral part of DAO; DMCC, dorsomedial cell column; i, injection site; MAO, medial accessory olive; med, medial; NOD, nodulus; PMD, paramedian lobule; PO, principal olive; UV, uvula.
The collateral labeling of climbing fibers in these and all other cases with injections in COP was restricted to the ANT. The injection into the C1 zone (case CTB8) resulted in two strips of extensive collateral labeling: one medial and one lateral to the P4+ band in lobules III–V (Figs. 4A, 5B′,C′). The medial strip overlapped the P3+ band and was delimited medially by Purkinje cells of zebrin-positive satellite band b (Fig. 5C′). A few climbing fiber collaterals were also present in the P4+ band in two of the sections. The lateral strip was located within the P4-band.
In comparison, collateral labeling of climbing fibers after the injection into the C2 zone (case 924-r) was almost entirely restricted to the zebrin-positive P4+ band, with some climbing fibers in the zebrin-negative region immediately medial to it in lobules III–V of ANT (Fig. 5F–H). The labeling in this zebrin-negative region may have arisen because of diffusion of tracer material medially to involve the P4-band in the copula. Similar observations were made in case CTB5, with a small injection in the C1 zone, limited to the zebrin-positive patch e of the COP, and in case 924-l, with an injection of its zebrin-positive lateral pole (for complete documentation of these and other cases, see our website: http://www.erasmusmc.nl/voogd-cfcollaterals).
The results on the collateral climbing fiber projection of COP are summarized in Figure 6. We interpret the collateral climbing fiber labeling in the anterior lobe P2-, P3+, and P3-zebrin bands as corresponding to the C1 zone (Fig. 6, red) and the labeling in the lateral P4-band as the C3 zone (Fig. 6, red). The intervening P4+ band in the anterior lobe, which receives climbing fibers from the lateral, zebrin-positive pole of the COP, therefore, corresponds to the C2 zone (Fig. 6, dark blue).
Injections located at the medial border of the zebrin-positive pole of COP (Fig. 3, cases 910l, 911l) (see also our website) resulted in a different pattern of labeling with climbing fiber collaterals occupying two narrow strips, one immediately medial to P4+ and the other lateral to P2+ in lobules III–V of ANT (Fig. 6, green). Retrograde labeling in the olive was present laterally in the contralateral MAO at intermediate levels, extending caudally into subnucleus A (Fig. 6, green). Together with the anterograde labeling in a region corresponding to the interstitial cell groups of Buisseret-Delmas et al. (1993) (compare with Fig. 2), these results therefore suggest that the injection site in these cases was centered on the CX zone. The same pattern of climbing fiber branching in the anterior lobe has also been observed in studies of the projection of the inferior olive after injections of the anterograde tracer Phaseolus vulgaris lectin at intermediate levels of MAO (Voogd et al., 1993; Voogd and Ruigrok, 1997). Branching of climbing fibers from similar regions of MAO to the vermal X zone and the CX zone has also been observed in ANT by using double-retrograde axonal tracer techniques in the cat (Apps et al., 1991). Thus, the zebrin-negative strips medial to P4+ and lateral to P2+ in ANT of the rat cerebellum may be identified, respectively, as the CX and X zones, receiving climbing fiber collaterals from a presumed CX zone located at the border between the C1 and C2 zones in COP. The empty strip between the X and C1 zones corresponds to the B zone, where climbing fibers could be labeled from injections of caudal DAO (Voogd and Ruigrok, 1997) (Fig. 2).
Climbing fiber collateral labeling from CTb injections in the PMD
Three injections in PMD were centered on the medial region, containing the narrow, zebrin-positive bands P4b+ and P5a+ (Fig. 3, cases CTB18, 910r, 911r), the zebrin-positive P5+ band (cases CTB6 and 864), the zebrin-negative bands P5a- and P5-, which border on either side of P5+ (cases 890l and 890r), and the zebrin-positive bands P6+ and P7+ in the lateral part of the lobule (cases CTB4 and 889l). These, and some other cases with injections including more than one zebrin-positive or negative band, are fully documented at our website. Climbing fiber collateral labeling in cases with injections in PMD was mostly restricted to the SI and the adjoining crus I in the caudal bank of the posterior superior fissure (Fig. 6).
Cases with injections into the P4b+/5a+ region in the medial part of PMD resulted in retrograde labeling restricted to subnucleus C of caudal MAO (Fig. 6, yellow) and a plexus of coarse axons labeled in the dorsolateral protuberance of the fastigial nucleus (Fig. 7C, panel 2, right nuclei). These injections were therefore situated in the A2 zone (compare with Fig. 2). Collateral climbing fiber labeling occurred in the P4-, P4b+, and P5a+ bands in medial crus II and in the region of the d stripes, medial to P4+ in caudal SI and the rostral aspect of crus I (Fig. 6, yellow). These regions are therefore considered to represent extensions of the A2 zone.
Figure 7.
Transverse sections containing mossy fiber and climbing fiber collateral and cerebellar nuclear labeling in six cases with unilateral injections in COP or PMD. Injection sites and other particulars are documented at our website. A, CTB5; B, CTB8; C, CTB18; D, CTB7; E, 864; F, CTB4. Boxed area in D, panel 2, is taken from a more rostral section than the nuclei on the right side. Regions in the cerebellar nuclei containing labeled coarse varicose fibers are indicated with solid green, and labeled thin varicose fibers are stippled in green. Other conventions as in Figure 5. 1–7, Zebrin-positive bands P1+ to P7+; a, zebrin-positive satellite band a; ANT, anterior lobe; b, zebrin-positive satellite band b; CrI, crus I; CrII, crus II; d, zebrin-positive satellite bands d; DLH, dorsolateral hump; DLP, dorsolateral protuberance; F, fastigial nucleus; FI, primary fissure; FLO, flocculus; FPS, posterior superior fissure; IA, anterior interposed nucleus; II–VI, lobules II–VI; L, lateral cerebellar nucleus; NOD, nodulus; PFL, paraflocculus; PMD, paramedian lobule; SI, simplex lobule.
In cases with injections limited to the P5+ band in PMD (CTB6 and 864), retrograde labeling in the inferior olive was present medially in rostral MAO (Fig. 6, light blue). The injections, therefore, were situated in the C2 zone. Collateral climbing fiber labeling was restricted to P5+ in ventral crus II and to P4+ in caudal SI and in crus I in the rostral bank of the posterior superior fissure, with a few labeled fibers in the medially adjoining zebrin-negative P4-area (Fig. 6, arrowhead). The latter may be attributable to diffusion of the tracer to zebrin-negative regions neighboring on P5+, which, by analogy with our findings on ANT, may represent the CX zone (cf. Pardoe and Apps, 2002). The P4+ zebrin band in SI and crus I is therefore interpreted as corresponding to the C2 zone (Fig. 6, light blue).
In experiment 890, two small iontophoretic injections were made in PMD into the zebrin-negative areas on either side of P5+ (i.e., medial and lateral to the C2 zone). Both injections labeled cells in rostromedial DAO (Fig. 6, orange). The medial (case 890-r) and lateral (case 890-l) injection sites were confined, therefore, to the C1 and C3 zone, respectively (Fig. 3). In case 890-r, collateral labeling of climbing fibers extended into the P4b-band of the crus II. On both sides of the cerebellum, several narrow strips of one or two climbing fiber collaterals were located in the zebrin-negative P4-area in rostral SI, extending into ANT. This region is located lateral to P4+ (which corresponds to the C2 zone) and is likely, therefore, to correspond to the C3 zone in SI (Fig. 6, orange) (cf. Pardoe and Apps, 2002). Collateral climbing fiber projections from the C1 or C3 zones in PMD to a presumed C1 zone in SI (i.e., to a region medial to the P4+ band) were not found in these cases. However, a collateral projection to SI may be present in other experiments in which injections involved the C1 zone in PMD (cases CTB1, CTB7, and 889-r), although in such cases the labeling could not be definitely attributed to branching from this zone because the injection sites also involved the neighboring A2 and/or CX zones. The location of a presumed C1 zone in SI is therefore left blank in Figure 6.
Two injections were located laterally in PMD. In case CTB4, with an injection medially in the fused zebrin-positive P6+and P7+ bands of PMD, retrograde labeling in the olive was located mainly in the ventral lamella of the principal olive (PO) (Fig. 6, pink). Collateral labeling of climbing fibers was widespread, with labeling present in the P5+ band in ANT, in rostral crus I, and also in the P6+ band in PMD and crus II. Caudally, the collateral climbing fiber labeling extended into a strip in the dorsal paraflocculus (Figs. 4E,F, 6, pink). Similar observations were also made in case 889-l, with a more laterally located injection in PMD (Fig. 3). Retrograde labeling in the olive was found mainly in the dorsal lamella and the bend of PO, and collateral labeling extended into the P6+ band in ANT and SI and into the P7+ band in crus I, II, and PMD, and also included the paraflocculus.
In PMD, the fused P6+ and P7+ bands receive their climbing fiber projections from PO and therefore represent the D1 and D2 zones. Because the more medial injection site in case CTB4 resulted in retrograde labeling entirely within the ventral lamella of PO, it seems likely that the injection site in this case was confined to the D1 zone. Climbing fiber collaterals were found in the P6+ band in crus II and the P5+ band in crus I and ANT, which therefore represent the D1 zone in these lobules (Fig. 6, pink). By inference, the anterior P6+ and posterior P7+ bands therefore correspond to the D2 zone. A D0 zone, receiving climbing fibers from the dorsomedial subnucleus of PO (DM) and projecting to the dorsolateral hump (Buisseret-Delmas and Angaut, 1989) (Fig. 2), could not be identified in our experiments. This may have been because our investigation was confined to a study of collateralization from PMD and COP. In a previous report, the D0 zone was found to be located in the zebrin-negative P5-band in ANT and in the P6-band in crus II (Voogd et al., 1993). In PMD, P6+ and P7+ usually fuse and P6-is absent.
Correspondence between electrophysiologically defined climbing fiber zones and the zebrin pattern
In all cases with injections into electrophysiologically identified climbing fiber zones in COP and PMD (summarized in Table 1), the observations of Atkins and Apps (1997) could be confirmed on their cortical location and the pattern of retrograde olivary labeling from these zones (Fig. 1) (see also our website). Injections into the face-receiving A2 zone in medial PMD (i.e., a medial region of cortex containing climbing fiber potentials evoked by contralateral face stimulation) were located in the region of the narrow P4b+ and P5a+ zebrin bands. Injections into the hindlimb-receiving part of the C1 zone in the medial half of COP (i.e., a region of cortex just lateral to the A2 zone containing climbing fiber potentials evoked at short latency by ipsilateral hindlimb stimulation) were located within the zebrin-negative P4-area, containing the zebrin-positive patch e. Injections into the corresponding forelimb-receiving part of the C1 zone in medial PMD included the zebrin-negative P4b-band. Injections targeted at a more lateral region of PMD, which receives climbing fiber input from both forelimbs and, therefore, corresponds to the C2 zone, were located in zebrin band P5+. Finally, the location of the electrophysiologically defined C3 zone in relation to the zebrin bands could not be directly demonstrated but is likely to correspond to the P5-band. In case CTB4, the injection was targeted at this zone (i.e., at a narrow lateral region of PMD containing climbing fiber potentials evoked at short latency by ipsilateral forelimb stimulation), but the injection pipette deviated laterally and ended up in the neighboring P6+ band.
Mossy fiber collateral labeling resulting from injections in COP and PMD
Most, if not all, of the injections were not confined to the molecular layer but penetrated into the granular layer, resulting in labeling of mossy fiber collaterals. These were usually present bilaterally in the lobule containing the injection site (Fig. 5A). Similar to the distribution of climbing fiber collateral labeling, mossy fiber collaterals were mainly restricted to ANT in cases with injections in COP and to lobules of the posterior lobe in cases with injections in PMD. However, in contrast to the climbing fiber collateral labeling, mossy fiber collaterals were often distributed bilaterally, in patterns of multiple, symmetrically arranged, but ill-defined strips or patches. This pattern was most evident in cases with large injections centered on the A2, C1, or D zones (Figs. 4A, 5A,B, 7) (see also our website). One highly consistent feature in these cases was the topographical correspondence between the labeled climbing fiber strips, with a strip of labeled mossy fiber collaterals in the subjacent granular layer (Figs. 4A,B,D,F, 5B,C, 7A–F). This was also evident in experiments with smaller injections, although the mossy fiber collateral labeling was usually sparse (Fig. 7A,E). Cases with injections into the C2 zone produced little (924, 864) or no (CTB6) collateral mossy fiber labeling, which, in case 864, was confined to multiple patches on the ipsilateral side. In experiments with bilateral injections, similar patterns of mossy fiber labeling were observed on both sides of the cerebellum. Clearly, the injection site origin of the patterns of mossy fiber collaterals could not be established in such cases. However, it is noteworthy that, even for these results, the mossy fiber collateral labeling was consistently observed subjacent to strips of labeled climbing fibers.
The corticonuclear projection of COP and PML
One or more foci of labeled coarse, varicose fibers were present in the cerebellar nuclei in all experiments (Figs. 4 H, 7) (see also our website). The localization of these plexuses was in general accordance with Buisseret-Delmas' description of the target nuclei of the corticonuclear projection zones in rats (Fig. 2). The A2 zone projected to the dorsolateral protuberance of the fastigial nucleus (Fig. 7C, panel 2, right cerebellar nuclei); the C1 and C3 zones targeted the anterior interposed nucleus (Fig. 7 A, B, panel 2, right nuclei); the C2 zone targeted the posterior interposed nucleus (Fig. 7E, panel 2); and the D1 and D2 zones targeted the lateral cerebellar nucleus (Fig. 7F). Cases with climbing fiber collateral labeling of the X and CX zones displayed terminal labeling in the interstitial cell groups (cases 910-r, 911-r; see our website). However, with respect to the projections to the anterior and posterior interposed nuclei, our observations differed from the scheme proposed by Buisseret-Delmas (1988b): the C1 and C2 zones never projected to both nuclei but always found their exclusive target in either the anterior interposed nucleus (in the case of the C1 zone) or the posterior interposed nucleus (in the case of the C2 zone). In experiments with coarse terminal labeling in both interposed nuclei, the injection site always included both C zones (Fig. 7D, panel 2, right nuclei).
In several cases, an additional plexus of labeled fine, varicose fibers was distributed bilaterally and symmetrically over one or more of the cerebellar nuclei (Figs. 4G, 7B–D,F). Bilateral labeling of the cerebellar nuclei was always found in combination with a strong bilateral distribution of mossy fiber collaterals.
Discussion
In the present study, collaterals of olivocerebellar fibers terminating in PMD and COP were found to distribute in a climbing fiber zone-specific longitudinal pattern. This confirms the principle, established in the original electrophysiological studies in cats by Armstrong et al. (1973a,b) and by Oscarsson and Sjȯlund (1977a,b), that climbing fiber collateralization remains confined to particular olivocerebellar zones.
With a few exceptions, to be discussed below, olivocerebellar fibers from rostral DAO innervate the zebrin-negative territory of the C1 and C3 zones, whereas rostral MAO and PO innervate respectively the zebrin-positive territory of the C2 and D zones. The A2 zone corresponds to the region of the P4b+ and P5a+ bands in medial PMD and SI. In comparison, mossy fibers terminating in PMD and COP collateralize in an often bilateral pattern of longitudinal strips and patches. At least one of these longitudinal aggregates of mossy fiber terminals is always found directly underneath a strip of climbing fiber collaterals labeled from the same injection site.
Methods and interpretations
Our study confirms the observations of Chen and Aston-Jones (1998) on the ability of CTb to label climbing and mossy fiber collaterals. Although extensive labeling of climbing fiber collaterals was observed in cases with large injections (Figs. 4A, 5B,C, case CTB8), the labeling in cases with small injections generally consisted of one or more longitudinal strips with a width of one or a few climbing fibers. These observations are in accordance with the spatial distribution of individual, biotinylated dextran amine (BDA)-labeled olivocerebellar fibers (Sugihara et al., 2001).
Mossy and climbing fiber collaterals both terminate in the cerebellar nuclei. In the present experiments, it was not possible, however, to identify climbing fiber collateral projections to the nuclei, because these overlap with the Purkinje cell corticonuclear projection (Ruigrok and Voogd, 2000). On the other hand, mossy fiber collaterals are likely to be responsible for the multinuclear, bilateral plexus of thin-diameter axons in many of our cases. Such a bilateral nuclear collateral projection has been demonstrated for the lateral reticular nucleus, the nucleus reticularis tegmenti pontis, and the spinal cord (Mihailoff, 1994; Ruigrok et al., 1995; Wu et al., 1999; Matsushita and Xiong, 2001). In contrast, Panto et al. (2001) proposed the Purkinje cells as the origin of the multiple foci of axonal labeling in different cerebellar nuclei, observed after their injections of BDA into individual cerebellar cortical zones in rats. However, the possibility of a mossy fiber collateral projection was not considered by these authors, and no mention was made of the presence of contralateral labeling. The latter is one of the main reasons in favor of this type of nuclear labeling being attributable to collateralization of mossy fibers.
Climbing fiber zones and the zebrin pattern
The pattern of zebrin banding is caused by the alternation of zebrin-positive and -negative Purkinje cells (Hawkes and Leclerc, 1987), whereas the longitudinal pattern in the olivocerebellar projection is made up of climbing fiber zones innervated by different olivary subnuclei. Previous studies have found a correlation between the position of anterogradely labeled longitudinal strips of climbing fibers and the zebrin pattern but were unable to show a precise correspondence of specific zebrin-positive or -negative bands with the olivocerebellar projection arising from individual olivary subnuclei (Gravel et al., 1987; Wassef et al., 1992). The present results therefore significantly extend these previous findings by showing that, to some degree, the two cortical patterns of zebrin bands and climbing fiber zones appear to coincide.
This conclusion would seem to be at odds with the results of Voogd and Ruigrok (1997), who found that the borders of some climbing fiber zones in the uvula of the rat are located in the middle of zebrin-positive bands. The discrepancy may have arisen because different regions of the cerebellum were investigated in the two studies and/or because of differences in the resolution of the techniques used. However, in several instances, discrete climbing fiber zones were found to share a single zebrin-negative band (i.e., X and B within P2- and C1 and CX within P3-).
The distribution of olivocerebellar fibers from rostral DAO to the zebrin-negative zones C1 and C3 confirms previous observations on the termination of climbing fibers after discrete injections into different subnuclei of the olive (Voogd et al., 1993; Voogd and Ruigrok, 1997). These previous reports also provided evidence that caudal DAO projects to the zebrin-negative P2-band, which therefore, at least partially, corresponds to the B zone. In contrast, rostral MAO and PO project to zebrin-positive territories. Rostral MAO projects to anterior P4+ and posterior P5+ (corresponding to the C2 zone), whereas cells at intermediate levels of the MAO project to the junction of the P4- and P5+ bands in COP and to two narrow zebrin-negative strips in ANT, located lateral to P2+ and medial to P4+ (corresponding to the X and Cx zones, respectively).
Regarding the lateral D zones, the present findings differ from the original description of these zones in the rat by Azizi and Woodward (1987) and by Buisseret-Delmas and Angaut (1989) (Fig. 2). Voogd et al. (1993) found the DM-innervated D0 zone to target the zebrin-negative anterior P5- and posterior P6-bands, whereas in the present study, we found the D1 zone to project to the more medially located anterior P5+ and posterior P6+ bands. By inference, the D2 zone corresponds to anterior P6+ and posterior P7+. Thus, the medial to lateral sequence of the D zones as proposed by us (D1,D0, and D2) and their innervation by the ventral and dorsal lamellae of the PO differ from the original data.
Overall, the zebrin pattern of the rat cerebellar hemisphere can be considered, therefore, to be the result of zebrin-negative zones innervated by DAO, DM, and intermediate MAO, interdigitating with zebrin-positive zones innervated by rostral MAO and PO. In crus I and the paraflocculus, in which zebrin-negative zones are not represented, the Purkinje cells are uniformly zebrin positive. Thus, the shift in numbering of the zebrin bands, corresponding to particular climbing fiber zones, between the anterior and posterior cerebellum, can be explained by the absence of zebrin-negative DAO and DM-innervated zones in crus I. The uniform zebrin-positive appearance of crus I prevented Hawkes and Leclerc (1987) from establishing their continuity. It remains to be determined whether this distinction between zebrin-negative and -positive zones is linked to the fact that subnuclei of the olive innervating the former all receive a somatotopically organized input from the periphery (Molinari et al., 1996; Yatim et al., 1996), whereas olivary subnuclei innervating the latter are dominated by afferent connections from higher levels of the brainstem.
Collateralization of mossy fibers and their relationship to climbing fiber collaterals
In cases with large unilateral injections, mossy fiber collaterals were found to be distributed in a bilateral symmetrical pattern with an ipsilateral predominance, occupying ill-defined parasagittal compartments. A similar, multiple zonal distribution of mossy fiber collaterals was demonstrated in the rat by Heckroth and Eisenman (1988). In the present experiments, the nuclear origin of labeled mossy fiber collaterals could not be studied in any detail, although retrograde cell labeling was noted in many brainstem structures known to provide mossy fiber projections to the cerebellum (e.g., the vestibular, trigeminal, lateral reticular, pontine, and reticular tegmental nuclei).
The location of mossy fiber collateral labeling in the granular layer directly underneath the labeled climbing fiber collaterals was observed in all cases but one. Recent studies of tactile projections to crus II of the rat cerebellum (Brown and Bower, 2001) revealed a similarity in the peripheral receptive field organization of Purkinje cell complex spikes (generated by activity in climbing fibers) and the immediately subjacent granule cells (which receive their extracerebellar input from mossy fibers). Although the patchy distribution of mossy and climbing fibers described by Brown and Bower (2001) clearly differs from our observations of a longitudinal zonal arrangement, there is agreement nonetheless on the congruence in the spatial organization of these two cerebellar afferent systems (cf. Eccles et al., 1972; Ekerot and Larson, 1973, 1980; Garwicz et al., 1998).
Functional considerations
The presence of two regions in the cerebellum, one in the anterior lobe and the other in the posterior lobe, with a similar zonally organized input from climbing and mossy fiber afferents that send branches to both regions, a corresponding zebrin pattern, and projections to the same cerebellar nuclei, appears to be a redundant feature of the cerebellum. However, some observations may mitigate this negative view. For example, Apps (2000) used double-retrograde tracing techniques in cats and found that only ∼7% of the total population of olivary neurons innervating the forelimb-receiving parts of the C1 zone in ANT and PMD have axons that branch to supply climbing fibers to both regions of cortex. Thus, at least different rostrocaudal parts of the C1 zone appear to have primarily independent climbing fiber input, and similar conclusions may also apply to their mossy fiber connections (Herrero et al., 2002).
In addition, the anterior and posterior lobes of the rat cerebellum differ significantly in the relative mediolateral widths of individual zones. Zebrin-negative zones are wide anteriorly, whereas zebrin-positive zones dominate the posterior cerebellum. The output of a mossy fiber signal, transmitted by the parallel fibers to the Purkinje cells in different zebrin-positive and -negative zones may differ therefore for the anterior and posterior cerebellum. Nevertheless, a unifying hypothesis on the functional significance of the features that distinguish zebrin-positive and -negative Purkinje cells remains to be determined (for review, see Voogd et al., 1996; Hawkes and Eisenman, 1997; Dehnes et al., 1998; Fritschy et al., 1999).
Footnotes
This work was supported by the Medical Research Council (United Kingdom) and the European Economic Community. We thank Rachel Bissett, Clare Everard, and Erika Sabel-Goedknegt for histological processing of the material, Eddy Dalm for his help in the processing of the illustrations, Dr. Richard Hawkes for making the Zebrin II antibody available, and the Benjamin Meaker Foundation, which supported J. V. during his appointment as a visiting professor at the University of Bristol. The website http://www.erasmusmc.nl/voogd-cfcollaterals was prepared by Bram Schaareman, Erasmus MC Rotterdam.
Correspondence should be addressed to J. Voogd, Department of Neuroscience, Erasmus MC Rotterdam, Box 1738, 3000 DR Rotterdam, The Netherlands. E-mail: j.voogd@erasmusmc.nl.
Copyright © 2003 Society for Neuroscience 0270-6474/03/234645-12$15.00/0
References
- Apps R ( 2000) Rostrocaudal branching within the climbing fibre projection to forelimb-receiving areas of the cerebellar cortical C1 zone. J Comp Neurol 419: 193–204. [DOI] [PubMed] [Google Scholar]
- Apps R, Trott JR, Dietrichs E ( 1991) A study of branching in the projection from the inferior olive to the x and lateral c1 zones of the cat cerebellum using a combined electrophysiological and retrograde fluorescent double-labelling technique. Exp Brain Res 871: 141–152. [DOI] [PubMed] [Google Scholar]
- Armstrong DM, Harvey RJ, Schild RF ( 1973a) The spatial organisation of climbing fibre branching in the cat cerebellum. Exp Brain Res 18: 40–58. [DOI] [PubMed] [Google Scholar]
- Armstrong DM, Harvey RJ, Schild RF ( 1973b) Branching of inferior olivary axons to terminate in different folia, lobules or lobes of the cerebellum. Brain Res 54: 365–371. [DOI] [PubMed] [Google Scholar]
- Atkins MJ, Apps R ( 1997) Somatotopical organisation within the climbing fibre projection to paramedian lobule and copula pyramidis of the rat cerebellum. J Comp Neurol 389: 249–263. [PubMed] [Google Scholar]
- Azizi SA, Woodward DJ ( 1987) Inferior olivary nuclear complex of the rat: morphology and comments on the principles of organization within the olivocerebellar system. J Comp Neurol 263: 467–484. [DOI] [PubMed] [Google Scholar]
- Brodal A, Kawamura K ( 1980) Olivocerebellar projection: a review. Adv Anat Embryol Cell Biol 64: 1–137. [PubMed] [Google Scholar]
- Brown IE, Bower JM ( 2001) Congruence of mossy fibre and climbing fibre tactile projections in the lateral hemispheres of the rat cerebellum. J Comp Neurol 429: 59–70. [DOI] [PubMed] [Google Scholar]
- Buisseret-Delmas C ( 1988a) Sagittal organization of the olivocerebellonuclear pathway in the rat. I. Connections with the nucleus fastigii and the nucleus vestibularis lateralis. Neurosci Res 5: 475–493. [DOI] [PubMed] [Google Scholar]
- Buisseret-Delmas C ( 1988b) Sagittal organization of the olivocerebellonuclear pathway in the rat. II. Connections with the nucleus interpositus. Neurosci Res 5: 494–512. [DOI] [PubMed] [Google Scholar]
- Buisseret-Delmas C, Angaut P ( 1989) Sagittal organisation of the olivocerebellonuclear pathway in the rat. III. Connections with the nucleus dentatus. Neurosci Res 72: 131–143. [DOI] [PubMed] [Google Scholar]
- Buisseret-Delmas C, Angaut P ( 1993) The cerebellar olivo-corticonuclear connections in the rat. Prog Neurobiol 40: 63–87. [DOI] [PubMed] [Google Scholar]
- Buisseret-Delmas C, Yatim N, Buisseret P, Angaut P ( 1993) The X zone and CX subzone of the cerebellum in the rat. Neurosci Res 16: 195–207. [DOI] [PubMed] [Google Scholar]
- Campbell NC, Armstrong DM ( 1985) Origin in the medial accessory olive of climbing fibres to the x and lateral c1 zones of the cat cerebellum: a combined electrophysiological/WGA-HRP investigation. Exp Brain Res 58: 520–531. [DOI] [PubMed] [Google Scholar]
- Chen S, Aston-Jones G ( 1998) Axonal collateral-collateral transport of tract tracers in brain neurons: false anterograde labelling and useful tool. Neuroscience 82: 1151–1163. [DOI] [PubMed] [Google Scholar]
- Dehnes Y, Chaudhry FA, Ullensvang K, Lehre KP, Storm-Mathisen J, Danbolt NC ( 1998) The glutamate transporter EAAT4 in rat cerebellar Purkinje cells: a glutamate-gated chloride channel concentrated near the synapse in parts of the dendritic membrane facing astroglia. J Neurosci 18: 3606–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Sabah NH, Schmidt RF, Táboøiková H ( 1972) Integration by Purkyneĕ cells of mossy and climbing fibres inputs from cutaneous mechanoreceptors. Exp Brain Res 15: 498–520. [DOI] [PubMed] [Google Scholar]
- Ekerot C-F, Larson B ( 1973) Correlation between sagittal projection zones of climbing and mossy fibre paths in cat cerebellar anterior lobe. Brain Res 64: 446–450. [DOI] [PubMed] [Google Scholar]
- Ekerot CF, Larson B ( 1979) The dorsal spino-olivocerebellar system in the cat. I. Functional organization and termination in anterior lobe. Exp Brain Res 36: 201–217. [DOI] [PubMed] [Google Scholar]
- Ekerot CF, Larson B ( 1980) Termination in overlapping sagittal zones in cerebellar anterior lobe of mossy and climbing fibres paths activated from dorsal funiculus. Exp Brain Res 38: 163–172. [DOI] [PubMed] [Google Scholar]
- Ekerot CF, Larson B ( 1982) Branching of olivary axons to innervate pairs of sagittal zones in the cerebellar anterior lobe of the cat. Exp Brain Res 48: 185–198. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Meskenaite V, Weinmann O, Honer M, Benke D, Mohler H ( 1999) GABAB-receptor splice variants GB1a and GB1b in rat brain: developmental regulation, cellular distribution and extrasynaptic localization. Eur J Neurosci 11: 761–768. [DOI] [PubMed] [Google Scholar]
- Garwicz M ( 2000) Micro-organisation of cerebellar modules controlling forelimb movements. Prog Brain Res 124: 187–199. [DOI] [PubMed] [Google Scholar]
- Garwicz M, Jȯrntell H, Ekerot CF ( 1998) Cutaneous receptive fields and topography of mossy fibres and climbing fibres projecting to cat cerebellar C3 zone. J Physiol (Lond) 512: 277–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravel C, Eisenman L, Sasseville R, Hawkes R ( 1987) Parasagittal organization of the rat cerebellar cortex: a direct correlation between antigenic bands revealed by mabQ113 and the topography of the olivocerebellar projection. J Comp Neurol 265: 294–310. [DOI] [PubMed] [Google Scholar]
- Hawkes R, Eisenman LM ( 1997) Stripes and zones: the origins of regionalization of the adult cerebellum. Perspect Dev Neurobiol 5: 95–105. [PubMed] [Google Scholar]
- Hawkes R, Leclerc N ( 1987) Antigenic map of the rat cerebellar cortex: the distribution of parasagittal bands as revealed by monoclonal antiPurkinje cell antibody mabQ113. J Comp Neurol 256: 29–41. [DOI] [PubMed] [Google Scholar]
- Heckroth JA, Eisenman LM ( 1988) Parasagittal organization of mossy fibre collaterals in the cerebellum of the mouse. J Comp Neurol 270: 385–394. [DOI] [PubMed] [Google Scholar]
- Herrero L, Pardoe J, Apps R ( 2002) Pontine and lateral reticular projections to the c1 zone in lobulus simplex and paramedian lobule of the rat cerebellar cortex. Cerebellum 1: 185–199. [DOI] [PubMed] [Google Scholar]
- Ji Z, Hawkes R ( 1994) Topography of Purkinje cell compartments and mossy fiber terminal fields in lobules II and III of the rat cerebellar cortex: spinocerebellar and cuneocerebellar projections. Neuroscience 61: 935–954. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Xiong G ( 2001) Uncrossed and crossed projections from the upper cervical spinal cord to the cerebellar nuclei in the rat, studied by anterograde axonal tracing. J Comp Neurol 432: 101–118. [DOI] [PubMed] [Google Scholar]
- Mihailoff GA ( 1994) Identification of pontocerebellar axon collateral synaptic boutons in the rat cerebellar nuclei. Brain Res 648: 313–318. [DOI] [PubMed] [Google Scholar]
- Molinari HH, Schultze KE, Strominger NL ( 1996) Gracile, cuneate, and spinal trigeminal projections to inferior olive in rat and monkey. J Comp Neurol 375: 467–480. [DOI] [PubMed] [Google Scholar]
- Oscarsson O, Sjȯlund B ( 1977a) The ventral spino-olivocerebellar system in the cat. I. Identification of five paths and their termination in the cerebellar anterior lobe. Exp Brain Res 28: 469–486. [DOI] [PubMed] [Google Scholar]
- Oscarsson O, Sjȯlund B ( 1977b) The ventral spino-olivocerebellar system in the cat. II. Termination zones in the cerebellar posterior lobe. Exp Brain Res 28: 487–503. [DOI] [PubMed] [Google Scholar]
- Panto MR, Zappala A, Parenti R, Serapide MF, Cicirata F ( 2001) Corticonuclear projections of the cerebellum preserve both anteroposterior and mediolateral pairing patterns. Eur J Neurosci 13: 694–708. [DOI] [PubMed] [Google Scholar]
- Pardoe J, Apps R ( 2002) Structure-function relations of two somatotopically corresponding regions of the rat cerebellar cortex: olivo-corticonuclear connections. Cerebellum 1: 165–184. [DOI] [PubMed] [Google Scholar]
- Ruigrok TJ, Voogd J ( 2000) Organization of projections from the inferior olive to the cerebellar nuclei in the rat. J Comp Neurol 426: 209–228. [DOI] [PubMed] [Google Scholar]
- Ruigrok TJ, Cella F, Voogd J ( 1995) Connections of the lateral reticular nucleus to the lateral vestibular nucleus in the rat. An anterograde tracing study with Phaseolus vulgaris leucoagglutinin. Eur J Neurosci 7: 1410–1413. [DOI] [PubMed] [Google Scholar]
- Serapide MF, Panto MR, Parenti R, Zappala A, Cicirata F ( 2001) Multiple zonal projections of the basilar pontine nuclei to the cerebellar cortex of the rat. J Comp Neurol 430: 471–484. [DOI] [PubMed] [Google Scholar]
- Sugihara I, Wu H, Shinoda Y ( 1999) Morphology of single olivocerebellar axons labeled with biotinylated dextran amine in the rat. J Comp Neurol 414: 131–148. [PubMed] [Google Scholar]
- Sugihara I, Wu HS, Shinoda Y ( 2001) The entire trajectories of single olivocerebellar axons in the cerebellar cortex and their contribution to cerebellar compartmentalization. J Neurosci 21: 7715–7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teune TM, van der Burg J, de Zeeuw CI, Voogd J, Ruigrok TJ ( 1998) Single Purkinje cells can innervate multiple classes of projection neurons in the cerebellar nuclei of the rat: a light microscopic and ultrastructural triple-tracer study in the rat. J Comp Neurol 392: 164–178. [DOI] [PubMed] [Google Scholar]
- Tolbert DL, Alisky JM, Clark BR ( 1993) Lower thoracic-upper lumbar spinocerebellar projections in rats: a complex topography revealed in computer reconstructions of the unfolded anterior lobe. Neuroscience 55: 755–774. [DOI] [PubMed] [Google Scholar]
- Trott JR, Armstrong DM ( 1987a) The cerebellar corticonuclear projection from lobule Vb/c of the cat anterior lobe: a combined electrophysiological and autoradiographic study. I. projections from the intermediate region. Exp Brain Res 66: 318–338. [DOI] [PubMed] [Google Scholar]
- Trott JR, Armstrong DM ( 1987b) The cerebellar corticonuclear projection from lobule Vb/c of the cat anterior lobe: a combined electrophysiological and autoradiographic study. II. projections from the vermis. Exp Brain Res 68: 339–354. [DOI] [PubMed] [Google Scholar]
- Voogd J ( 1969) The importance of fibre connections in the comparative anatomy of the mammalian cerebellum. In: Neurobiology of cerebellar evolution and development (Llinas R ed), pp 493–514. Chicago: AMAERF Institute for Biomedical Research.
- Voogd J, Bigaré F ( 1980) Topographical distribution of olivary and corticonuclear fibres in the cerebellum: a review. In: The olivary nucleus. Anatomy and physiology (de Montigny C, Courville J, eds) pp 207–234. New York: Raven.
- Voogd J, Ruigrok TJ ( 1997) Transverse and longitudinal patterns in the mammalian cerebellum. Prog Brain Res 114: 21–37. [DOI] [PubMed] [Google Scholar]
- Voogd J, Eisenman LM, Ruigrok TJH ( 1993) Relation of olivocerebellar projection zones to zebrin pattern in rat cerebellum. Soc Neurosci Abstr 19: 1216. [Google Scholar]
- Voogd J, Jaarsma D, Marani E ( 1996) The cerebellum, chemoarchitecture, and anatomy. In: Handbook of chemical neuroanatomy, Vol 12, Integrated systems of the CNS, Pt III, Cerebellum, basal ganglia, olfactory system. (Swanson LW, Bjȯrklund A, Hȯkfelt T, eds), pp 1–369. Amsterdam: Elsevier.
- Wassef M, Angaut P, Arsenio-Nunes L, Bourrat F, Sotelo C ( 1992) Purkinje cell heterogeneity: its role in organizing the topography of the cerebellar cortex connections. In: The cerebellum revisited (Llinas R, Sotelo C, eds), pp 5–21. New York: Springer.
- Wiklund L, Toggenburger G, Cuénod M ( 1984) Selective retrograde labelling of the rat olivocerebellar climbing fibre system with d-[3H]aspartate. Neuroscience 13: 441–468. [DOI] [PubMed] [Google Scholar]
- Wu HS, Sugihara I, Shinoda Y ( 1999) Projection patterns of single mossy fibres originating from the lateral reticular nucleus in the rat cerebellar cortex and nuclei. J Comp Neurol 411: 97–118. [DOI] [PubMed] [Google Scholar]
- Yatim N, Billig I, Compoint C, Buisseret P, Buisseret-Delmas C ( 1996) Trigeminocerebellar and trigemino-olivary projections in rats. Neurosci Res 25: 267–283. [DOI] [PubMed] [Google Scholar]