Abstract
The chemokine receptor CXCR4 is expressed in the embryonic and mature CNS, yet its normal physiological function in neurons remains obscure. Here, we show that its cognate chemokine, stromal cell-derived factor-1 (SDF-1), promotes the survival of cultured embryonic retinal ganglion cell neurons even in the absence of other neurotrophic factors. This survival effect is mediated primarily through a cAMP-dependent pathway that acts through protein kinase A and MAP kinase. Addition of SDF-1 to a human neuronal cell line induces phosphorylation of p44/p42 MAP kinase and GSK3β. Mouse embryos lacking the CXCR4 receptor have a reduced number of retinal ganglion cells. The ligand of CXCR4, SDF-1, may therefore provide generalized trophic support to neurons during their development and maturation.
Keywords: SDF-1, slit-2, RGC, cAMP, survival, neurotrophic
Introduction
Neurons are overproduced early in development, and their excessive numbers are reduced as they compete for a limited supply of trophic factors in their environment (Hamburger and Levi-Montalcini, 1949). Access to sufficient trophic support is thought to prevent the activation of an inherent suicide program common to all cells (Meyer-Franke et al., 1998; Raff, 1998; Shen et al., 1999). It has been recognized for some time that elevating intracellular levels of cAMP can promote the survival of cultured neurons (Wakade et al., 1983; Rydel and Greene, 1988; Meyer-Franke et al., 1998). The survival-promoting effect of elevating cAMP is direct in some neurons, whereas in others, it is attributable to an increased sensitivity to trophic peptides (Wakade et al., 1983; Hanson et al., 1997; Meyer-Franke et al., 1998; Rydel and Greene, 1988).
Activation of seven transmembrane G-protein-coupled receptors is one way in which an elevation of cAMP can be achieved. In this study, we explored the possibility that activating a subfamily of G-protein-coupled receptors, the chemokine receptors, promotes neuronal survival. Chemokines are relatively short peptide hormones that were originally defined as chemoattractants for leukocytes but have since been found to have a broader spectrum of activities that includes triggering degranulation of leukocytes, cerebellar granule cell migration, angiogenesis, and T-cell differentiation (Luster, 1998; Nanki and Lipsky, 2000; Luther and Cyster, 2001; Mackay, 2001). There are ∼50 chemokines and 20 chemokine receptors identified to date (Luster, 1998; Murphy et al., 2000). Recently, the chemokine receptors CXCR4 and CCR5 have been shown to play an important role in the entry of HIV-1 into CD4+ T cells and macrophages (Alkhatib et al., 1996; Choe et al., 1996; Doranz et al., 1996; Dragic et al., 1996; Feng et al., 1996).
Chemokines are classified into four major families on the basis of the positions of structurally important cysteine residues. The CXC family contains the chemokine stromal cell-derived factor-1 (SDF-1), also named CXCL12 (Murphy et al., 2000). Unlike most other chemokines that activate multiple receptors, SDF-1 is thought to act exclusively through its receptor CXCR4. This is supported by the observation that both SDF-1 and CXCR4 knock-out mice die at approximately embryonic day 17 (E17) and are characterized by very similar defects in B-lymphopoiesis, myelopoiesis, cardiac ventricular septum formation, and vascular remodeling (Nagasawa et al., 1996; Ma et al., 1998; Tachibana et al., 1998; Zou et al., 1998).
CXCR4 is expressed abundantly on neurons and other cell types within the CNS (McGrath et al., 1999). Among the neural defects described in CXCR4 mutant embryos are misplaced cerebellar and dentate granule cells, leading to the suggestion that this receptor plays a role in neuronal cell migration (Zou et al., 1998; Bagri et al., 2002). Consistent with this hypothesis are the findings that SDF-1 acts as an attractant for cerebellar granule cells in vitro (Lu et al., 2001) and that ectopic expression of SDF-1 in slice cultures induces the mislocalization of migrating dentate granule neurons (Bagri et al., 2002). Our recent analysis of CXCR4 knock-outs has revealed that sensory axons expressing the neurotrophin receptor TrkA are misguided in the embryonic spinal cords of CXCR4 knock-outs. We have also shown recently that SDF-1 significantly reduces the responsiveness of multiple axons to several different repellent guidance cues in culture (Chalasani et al., 2003). Although these findings indicate that SDF-1 has an important role in the guidance of migrating cells and axons in the developing CNS, here, we demonstrate a very different additional function for SDF-1/CXCR4 signaling, the promotion of neuronal survival.
Materials and Methods
Cell culture and survival assay. Retinal ganglion cell (RGC) cultures were made by dissociating E6 chick neural retinas and plating them on poly-l-lysine-treated laminin-coated glass coverslips at a density of 1000 neurons per well (48 well dish, Costar, Cambridge, MA). The medium (F-12) was supplemented with 6 mg/ml glucose, 2 mm glutamine, 100 U/ml penicillin, 100 U/ml streptomycin, 5 ng/ml transferrin, and 5 ng/ml selenium along with different conditions as indicated. Chemokines were purchased from Peprotech (Rocky Hill, NJ). The cultures were fixed and stained with an antibody to islet-1 [39.4D5; Developmental Studies Hybridoma Bank (DSHB), Iowa City, IA]. Three coverslips were set up for each experiment, and 10 random fields were counted on each coverslip. The average number of islet-1-positive RGCs was compared between 72 and 24 hr. The data are the average of four experiments, with all the error bars representing an estimate of the SEM. Probability values were calculated using a two-tailed t test with different variances (heteroscedastic). Human teratocarcinoma (NT2N) cells were differentiated for 5 weeks in retinoic acid to induce neuronal properties (Pleasure et al., 1992). The cells were then treated with trypsin and plated on Matrigel-coated wells in 50% conditioned medium and 50% DMEM with 5% FBS, 1% penicillin-streptomycin, and the mitotic inhibitors FuDR, UR, and AraC overnight at a density of 30,000 cells per well (four-well dishes; Costar). The next day, the medium was removed, and serum-free medium with or without SDF-1 was added. These cultures were stained live using 1 μl/well of Syto16 (Molecular Probes, Eugene, OR), and living cells in 50 random fields were counted. Cells were counted in two wells for each condition, and data from four independent experiments are shown.
CREB phosphorylation. Neural retinas from E6 chicks were dissociated and plated in minimal medium as described above. After 24 hr, these cultures were stimulated for 30 min with SDF-1 with and without 20 μm AMD 3100, 100 ng/ml pertussis toxin (PTX), 200 nm PKI, or 20 μm PD98059. The cultures were then fixed for 20 min with 3.7% paraformaldehyde and stained with anti-islet-1 (1:200, 39.4D5, DSHB) and anti-phospho-specific CREB (ser-133, a kind gift from Dr. Judy Meinkoth). These antibodies were then detected by anti-mouse Alexa Fluor 488 and anti-rabbit Alexa Fluor 546 (Molecular Probes).
Immunohistochemistry. Frozen sections of E6 chick eyes were made at 30 μm and stained with antibodies to neurofilament (4H6, a gift from Dr. William Halfter, University of Pittsburgh) at 1:1000 and anti-islet-1 (39.4D5, DSHB) at 1:200. Similar sections were made of mouse embryos at E13.5 and stained with anti-neurofilament (2H3, DSHB) at 1:200 and anti-islet-1/2 (guinea pig polyclonal, a gift from Dr. Thomas Jessell, Columbia University) at 1:10,000. In the terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling (TUNEL) experiment, cultures were set up with or without SDF-1 and stained for islet-1 as described along with TUNEL reagent, TMR Red (Boehringer Mannheim, Indianapolis, IN). A total of 100 islet-positive nuclei were scored for each condition, and data from four independent experiments are shown. In the bromodeoxyuridine (BrdU) experiment, cultures were set up with or without SDF-1, and BrdU at 10 μm was added for 1 hr. These cultures were fixed 24 hr later, and BrdU-positive cells were detected using an anti-BrdU antibody conjugated to fluorescein (Boehringer Mannheim). An anti-islet-1 antibody detected RGC neurons. However, no RGC neurons were found to be BrdU-positive. For the TrkB experiment, cultures were set up in the manner described above. After 24 hr, they were left untreated or treated for 30 min with forskolin (10 μm) or SDF-1 (100 ng/ml). All the cultures were then stained live with an antibody to TrkB (a gift from Dr. Francis Lefcort, University of Montana) at 1:1000, washed, fixed, and then processed for islet-1 staining.
Inhibitor assays. RGC cultures were set up as described with or without SDF-1 and specific inhibitors. A protein kinase A inhibitor, PKI (Calbiochem, La Jolla, CA) at 200 nm; a PKG inhibitor, KT5823 (Calbiochem) at 1 μm; a cAMP antagonist, Rp-cAMPS (Sigma, St. Louis, MO) at 20 μm;a cGMP antagonist, Rp-cGMPS (Sigma) at 20 μm; a MAP kinase inhibitor, PD98059 (Calbiochem) at 20 μm; a PI-3 kinase inhibitor, LY294002 (Calbiochem) at 20 μm; an SDF-1 antagonist, AMD3100 (AIDS Research and Reference Reagent Program, National Institutes of Health, contributed by AnorMed, Langley, British Columbia, Canada) program, 24) at 20 μm; CXCR4- and CCR5-tropic glycoproteins, HxB-gp120 and JRFL-gp120 at 100 ng/ml, and PTX (Sigma) at 100 ng/ml were used. After 48 hr, these cultures were fixed and stained for islet-1 to identify RGCs. Data from four independent experiments are shown. The src family inhibitor PP1 was used at 1 μm with or without BDNF at 100 ng/ml and SDF-1 at 100 ng/ml. Cultures were fixed at 24 hr and another set at 72 hr, and the number of islet-1-positive cells counted in 10 random fields in each coverslip were compared. Data from four independent experiments are shown. These cultures were fixed at 48 hr, stained for islet-1, and counted in a similar manner as the rest of the inhibitor experiments. For slit-2 inhibition, protein was produced from transiently transfected 293T cells. The amount of protein was quantified by collapse assay on retinal growth cones. Equivalent volumes of slit-2 and mock-transfected supernatants were added to the cultures at the same time as SDF-1. These cultures were also processed in a similar manner as the rest of the inhibitor experiments.
RGC counts in mice. Cryostat sections (30 μm) from wild-type and CXCR4 mutant E13.5 embryos were stained with islet-1 antibodies (guinea-pig polyclonal, a kind gift from Dr. Thomas Jessell). A single 2 μm optical section was reconstructed in every second 30 μm section for the whole eye with images obtained with a Leica confocal microscope (Leica, Nussloch, Germany). All islet-1-positive cells were counted in each optical section in which the lumen of the eye could be visualized. The number of sections in the table represents the number of alternate sections that were counted for each eye. The nonparametric Mann–Whitney test was used to test the hypothesis that sampled RGC counts from wild-type and mutant eyes were no different within litters.
RNA probes and in situ hybridization. Probes of length 1300, 1200, and 300 bp, representing the entire coding sequences of the chick CXCR4, mouse CXCR4, and mouse SDF-1, were made and used on frozen sections of E6 chick and E13.5 mouse embryos. The expression was detected using an anti-DIG-AP (Boehringer Mannheim). Representative sections of expression are shown. After the sections were developed for in situ hybridization, they were then processed for islet-1 expression to stain all of the RGC neurons.
Western blots. Cell lysates were made from NT2N cells that were plated at a density of 100,000 cells per well, serum starved for 6 hr, and treated with or without SDF-1 and the inhibitors for 20 min. Proteins were separated on 10% Bis-Tris Nupage gels (Invitrogen, San Diego, CA) and transferred to Immobilon-P membranes (Millipore, Molsheim, France). These were then probed with antibodies to phospho-GSK3β (New England Biolabs, Beverly, MA), stripped in 62.5 mm Tris at a pH of 6.7 with 100 μm βME and 2% SDS for 30 min at 60°C, and then reprobed with anti-GSK3β (Transduction Laboratories, Lexington, KY). Alternatively, blots were probed with antibodies to phospho-MAP kinase p44/42 (New England Biolabs) and then stripped and reprobed with anti-MAP kinase p44/42 (Santa Cruz Biotechnology, Santa Cruz, CA). A composite from two separate blots is shown in the figure. These results were confirmed with multiple blots.
Results
SDF-1 promotes the survival of cultured embryonic retinal ganglion neurons
RGCs provide a convenient model system in which to study neuronal survival. RGC neurons can be identified in cultures of dissociated E6 chick retinas by their expression of the transcription factor islet-1 (Fig. 1B,C) (Ericson et al., 1992; Halfter, 1998). RGCs die over the course of several days when cultured in serum-free medium without added neurotrophic factors. The adenylate cyclase activator forskolin promotes their survival, suggesting that signaling molecules that induce an elevation of cAMP could act as survival factors (Meyer-Franke et al., 1998; Shen et al., 1999) (see Fig. 3A, below). To test whether chemokines and their G-protein-coupled receptors might elevate cyclic nucleotide levels and thereby prevent neuronal death, we assayed a broad spectrum of chemokines for their ability to enhance RGC survival. Of those tested, only SDF-1 has a strong survival-enhancing effect.
Figure 1.
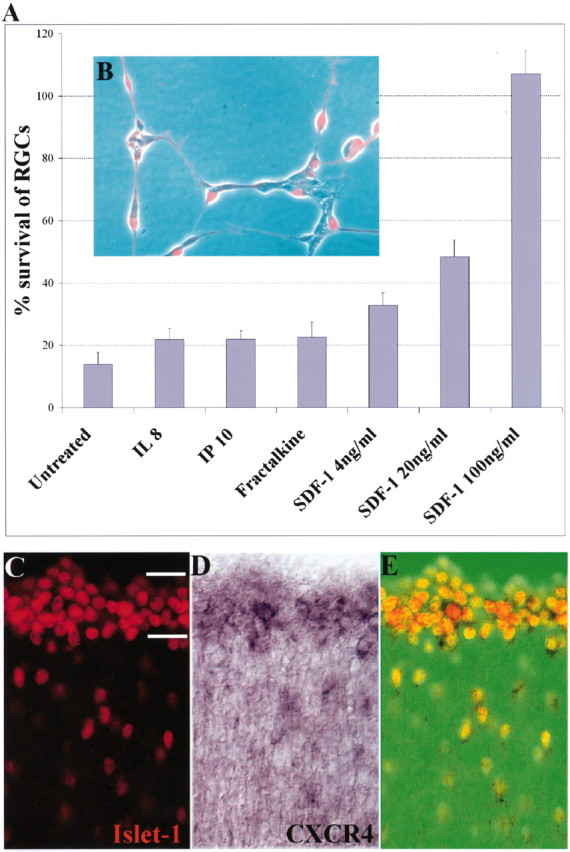
SDF-1 promotes the survival of cultured embryonic chick RGCs, and its receptor CXCR4 is expressed in the embryonic retina. A, SDF-1 promotes the survival of RGCs in a dose-dependent manner. Shown are the percentages of surviving RGCs at 72 compared with 24 hr in the presence of 100 ng/ml selected chemokines or of 4 (p = 0.028), 20 (p = 0.008), or 100 ng/ml (p = 0.001) SDF-1. p values were calculated by comparing each population with the untreated one using a two-tailed test with different variances. B, Identification of RGCs in culture with anti-islet-1. C, Visualization of RGC neurons with an antibody to islet-1 in a cross section of an E6 chick embryo. The hash marks represent the ganglion cell layer. D, Visualization of CXCR4-expressing cells in the RGC layer of this same section by in situ hybridization. E, Merged image showing CXCR4 in all islet-1-positive cells.
Figure 3.
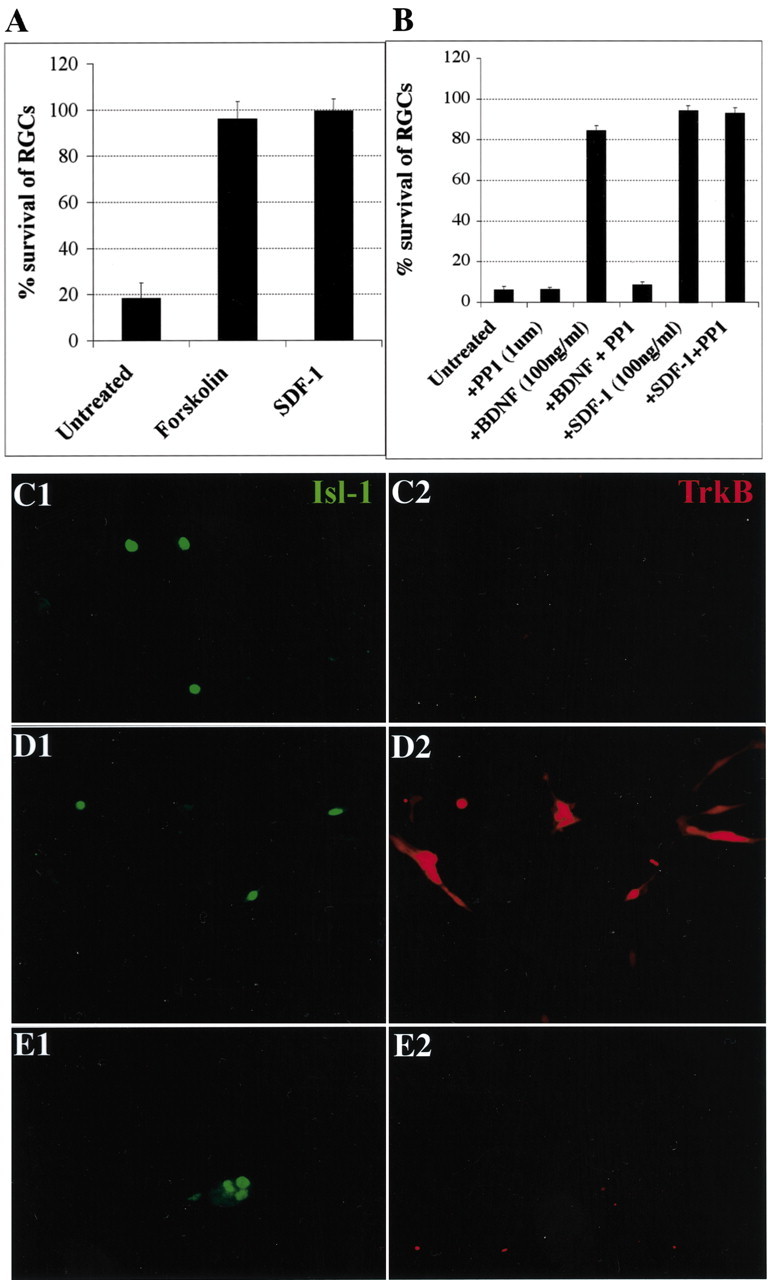
SDF promotes the survival of cultured RGCs without promoting the translocation of TrkB to the cell surface. A, RGC survival is enhanced by 10 μm forskolin (p = 0.002) to the same degree as by 100 ng/ml SDF-1 (p = 0.001). The average percentages of surviving RGCs at 72 hr compared with 24 hr are shown for three independent experiments. p values were calculated by comparing each population with the untreated one using a two-tailed test with different variances. B, SDF-1-promoted survival is not affected by the src family inhibitor PP1, whereas BDNF survival is completely blocked. C–E, Forskolin but not SDF-1 induces the translocation of TrkB to the surface of RGCs. After 24 hr, cultures were left untreated (C1, C2) or treated for 30 min with 10 μm forskolin (D1,D2) or 100 ng/ml SDF-1 (E1,E2). All cultures were then stained live with anti-rabbit TrkB antibody. RGCs are visualized in green with anti-islet-1 (C1–E1), and surface TrkB is visualized in the same fields in red (C2–E2) (20×). Although both forskolin and SDF-1 promote the survival of RGCs, forskolin induces the translocation of TrkB to the surface of retinal cells, whereas SDF-1 does not.
Figure 1A shows the survival of RGC neurons plated on poly-l-lysine-treated laminin-coated glass coverslips and cultured in defined medium along with representative CXC class chemokines. Fractalkine, a member of the CX3C family, was also tested because it has been reported to rescue cultured hippocampal neurons damaged by exposure to the HIV-1 envelope protein gp120 (Meucci et al., 2000). For this, 100 ng/ml SDF-1 (CXCL12), interleukin-8 (IL-8; CXCL8), MIP-3α (CCL20), eotaxin-1 (CCL11), RANTES (CCL5), GROα (CXCL1), Fractalkine (CX3CL1), IP-10 (CXCL10), or MIP-1β (CCL4) was added to these cultures. The four chemokines for which data are shown act through different receptors: IL-8 via CXCR1 and CXCR2, IP-10 via CXCR3, Fractalkine via CX3CR1, and SDF-1 via CXCR4. The percentage of RGCs surviving at 72 hr compared with those present at 24 hr is shown. Less than 20% of the RGCs present at 24 hr survive for 72 hr without any added chemokine. IL-8, IP-10, and Fractalkine exhibit little survival-promoting activity. In contrast, SDF-1 dramatically enhances neuronal survival, rescuing essentially all RGCs cultured under these conditions. Additional experiments demonstrated that SDF-1 also enhances the survival of cultured E8 chick sympathetic neurons (data not shown). The survival-promoting effect of SDF-1 is dose dependent in RGCs with a half-maximal effective concentration of ∼20 ng/ml. This concentration is similar to that needed to induce chemotaxis of activated T cells and also comparable with the measured Kd for the interaction of SDF-1 with the CXCR4 receptor (Hesselgesser et al., 1998b).
The known biological effects of SDF-1 are mediated through the activation of its receptor CXCR4 (Bleul et al., 1996; Feng et al., 1996). To determine whether CXCR4 is expressed in RGC neurons, we first cloned chick CXCR4 from a chick brain cDNA library using a mouse probe from a region of the receptor that is highly conserved among different species. The coding sequence we obtained is an exact match with the recent GenBank accession number AAG09054. This sequence was used to make a probe complementary to the full coding sequence of CXCR4 mRNA. Hybridization with this probe demonstrates that CXCR4 is expressed strongly within the RGC layer of E6 embryonic chick retinas (Fig. 1D). Immunohistochemical analysis revealed that all islet-positive cells in the retina express CXCR4 (Fig. 1E). Thus, RGC neurons express the receptor through which SDF-1 acts in other systems. As shown below, an antagonist of SDF-1 binding to CXCR4 interferes with the survival-enhancing effect of SDF-1. These results are consistent with CXCR4 serving as the SDF-1 receptor that promotes survival activity.
SDF-1 reduces the number of TUNEL-positive RGCs
The ability of SDF-1 to promote the survival of RGCs in culture could in principle be ascribed to the maintenance of already existing RGCs or to the enhanced proliferation or differentiation of progenitors that replace RGCs that die in culture. To distinguish between these two possibilities, we first determined whether SDF-1 reduces the rate of RGC death as determined by TUNEL staining (Fig. 2). As shown in Figure 2C, threefold fewer islet-1-expressing RGCs are TUNEL-positive when cultured with SDF-1 (Fig. 2C), suggesting that SDF-1 helps prevent or delay RGC death. To determine whether SDF-1 could also act as a mitogen that helps replenish dying RGCs from a pool of progenitors, SDF-1-treated cultures were pulsed with BrdU to detect dividing cells. BrdU at 10 μm was added for 1 hr to RGC cultures with or without SDF-1; 24 hr later, these cultures were fixed and processed to detect BrdU-labeled cells. No dividing cells were detected in our chick retinal cultures even in the presence of SDF-1. BrdU-labeled cells were easily detected in dividing cell lines cultured and processed in parallel (data not shown). We conclude that SDF-1 prevents or delays the death of already existing RGCs in culture.
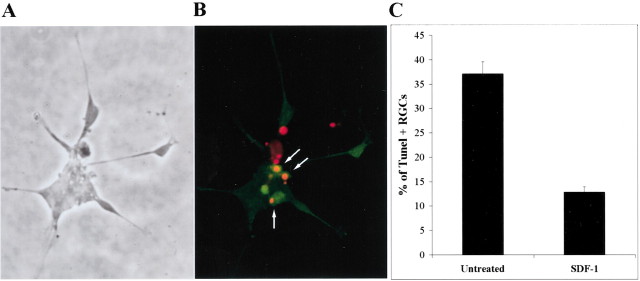
Figure 2.
SDF-1 reduces the percentage of TUNEL-positive RGCs. Dissociated E6 chick neural retinas were cultured for 48 hr, fixed, and processed for TUNEL staining (red) and islet-1 expression (green). A representative field is shown in phase-contrast optics (A) and in fluorescence (B). Some islet-1-expressing nuclei are also TUNEL-positive (arrows). C, The percentages of islet-1-positive cells with TUNEL staining were determined in the presence or the absence of 100 ng/ml SDF-1 (p = 0.002). The average percentages of TUNEL-positive RGCs are shown from four independent experiments. p values were calculated by comparing each population with the untreated one using a two-tailed test with different variances. Approximately threefold more TUNEL-positive RGCs were detected in the absence than in the presence of SDF-1.
Enhancement of RGC survival by SDF-1 does not depend on other neurotrophins
Previous work has shown that increasing cAMP levels with forskolin in cultured postnatal day 8 (P8) rat RGCs has only a small direct effect on their survival. Instead, elevated cAMP induces existing Trk receptors to move to the cell surface, thereby rendering RGCs more responsive to neurotrophins (Meyer-Franke et al., 1998; Shen et al., 1999). In contrast, our results indicate that SDF-1 promotes robust survival of embryonic chick RGCs even in defined medium that contains no neurotrophins (Figs. 1 A, 3A). Nevertheless, because forskolin treatment promotes the survival of RGCs to the same degree as SDF-1 (Fig. 3A), we addressed the question of whether SDF-1 activates Trk receptors even in the absence of neurotrophins (Lee and Chao, 2001). The src family inhibitor PP1 was tested for its ability to block the trophic effect of SDF-1. This inhibitor reduces baseline RGC survival at all time points in our assays and effectively blocks the survival effects of BDNF. However, PP1 has no effect on SDF-1-induced survival (Fig. 3B). We next examined cell surface expression of TrkB receptors in response to SDF-1. Although elevating cAMP levels with forskolin induces a dramatic translocation of TrkB to the surfaces of chick RGCs (Fig. 3D1,D2), no such translocation of TrkB is induced by SDF-1 (Fig. 3E1,E2). This implies that the two treatments are not identical. Perhaps forskolin elevates cAMP to a different degree than SDF-1, or perhaps SDF-1 activates additional parallel signaling pathways that are unaffected by forskolin. In either case, these results imply that SDF-1 promotes the survival of embryonic chick RGCs through a mechanism that is independent of TrkB redistribution or activation.
Enhancement of RGC survival by SDF-1 is mediated by the CXCR4 receptor
Selected inhibitors were used to begin characterizing the signaling pathway through which SDF-1 promotes RGC survival. We allowed cultures with or without SDF-1 and a variety of specific inhibitors to grow for 48 hr. In the absence of SDF-1, ∼50% of RGCs die, whereas death is negligible in its presence. The survival-promoting effects of SDF-1 are reduced by the small molecule CXCR4 specific antagonist AMD3100 (Gerlach et al., 2001) (Fig. 4A, compare columns 4 and 5). This, coupled with the expression of CXCR4 on RGCs (Fig. 1E), indicates that the survival-promoting activity of SDF-1 is mediated via CXCR4. Because CXCR4 is a seven-transmembrane G-protein-coupled receptor, PTX was used to test whether the survival effects of SDF-1 require a Gi/Go-type intermediary. We found that PTX blocks the survival-promoting effects of SDF-1 (Fig. 4A, compare columns 4 and 6). The HIV envelope (Env) glycoprotein HxB-gp120 has been reported to have toxic effects on cultured neurons and NT2N cells, although the mechanisms by which this occurs are not clear (Hesselgesser et al., 1997, 1998a; Kaul and Lipton, 1999). Hxb-gp120 can displace SDF-1 from the CXCR4 receptor (Staudinger et al., 2001). JRFL-gp120 is another HIV Env protein that binds CCR5 instead of CXCR4 and does not affect SDF-1 binding to CXCR4 (Berger et al., 1999). Purified HxB-gp120 but not JRFL-gp120 reduced the survival-promoting activity of SDF-1 (Fig. 4B, compare columns 4–6). Neither HxB-gp120 nor JRFL-gp120 has any detectable toxic activities of its own in this assay (Fig. 4B, compare columns 1–3). These results confirm that SDF-1 acts through CXCR4 to mediate its survival-promoting activity and also suggest that HxB-gp120-induced toxic effects could sometimes arise by its ability to block SDF-1-mediated neurotrophic action.
Figure 4.
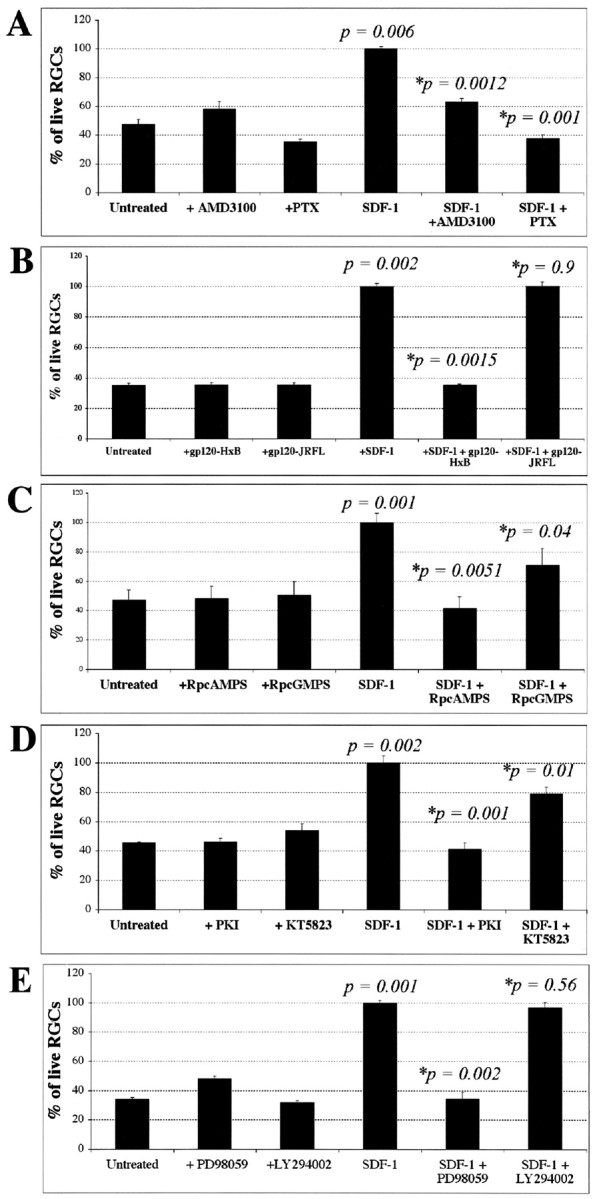
Signaling pathways required for SDF-1-mediated RGC survival. The average number of RGCs per field 48 hr after plating is shown for each condition in four independent experiments. Each inhibitor was tested in the presence or absence of 100 ng/ml SDF-1. A, AMD3100 (20 μm), an antagonist of SDF-1 binding to CXCR4, does not affect RGC survival by itself but blocks the survival-enhancing effect of SDF-1. The Gi inhibitor PTX (100 ng/ml) does not affect RGC survival by itself but blocks the survival-enhancing effect of SDF-1. B, HxB-gp120 (100 ng/ml), anantagonist of SDF-1 binding to CXCR4, does not affect RGC survival by itself but blocks the survival-enhancing effect of SDF-1. JRFL-gp120 (100 ng/ml) does not antagonize SDF-1 binding and does not block its survival effect C, The cAMP antagonist Rp-cAMPS (20 μm) does not affect RGC survival by itself but blocks the survival-enhancing effect of SDF-1. The cGMP antagonist Rp-cGMPs (20 μm) has a lesser effect on SDF-1 activity than the cAMP antagonist. D, The PKA inhibitor PKI (200 nm) does not affect RGC survival by itself but blocks the survival-enhancing effect of SDF-1, whereas a 1 μm concentration of the PKG inhibitor KT5823 is less effective in blocking the effect of SDF-1. E, The survival effect of SDF-1 is also blocked by a 20 μm concentration of the MAP kinase inhibitor PD98059. In contrast, a 20 μm concentration of the PI-3 kinase inhibitor LY294002 does not reduce the effect of SDF-1. p values were calculated by comparing each population with the untreated one, and those with an asterisk were obtained comparing each population with the SDF-1-treated one using a two-tailed test with different variances.
Enhancement of RGC survival by SDF-1 is mediated by a cAMP-dependent pathway
The survival-promoting effect of SDF-1 is completely blocked by an antagonist of cAMP, Rp-cAMPS (Fig. 4C, compare columns 4 and 5) and an inhibitor of PKA, PKI (Fig. 4D, compare columns 4 and 5). An antagonist of cGMP, Rp-cGMPS (Fig. 4C, compare columns 4 and 6) or an inhibitor of PKG, KT58230 (Fig. 4D, compare columns 4 and 6) only partially reduced the effectiveness of SDF-1. A MAP kinase inhibitor, PD98059 (Fig. 4E, compare columns 4 and 5) significantly reduces the SDF-1-induced survival effect, whereas a PI3 kinase inhibitor, LY 294002, (Fig. 4E, compare columns 4 and 6) does not. None of these agents affected RGC survival in the absence of SDF-1, indicating that nonspecific and toxic effects of these agents are minimal as used. These results are consistent with the hypothesis that SDF-1 induces most of its survival-enhancing effects in RGC neurons by binding to the CXCR4 receptor and that activation of the receptor stimulates a cAMP-mediated signaling cascade ultimately promoting RGC survival.
SDF-1 induces the phosphorylation of CREB
A convenient readout for the elevation of cAMP is cAMP-stimulated phosphorylation of CREB and the translocation of phospho-CREB from the cytoplasm into nuclei (Gonzalez and Montminy, 1989; Hagiwara et al., 1993). An antibody specific to the phosphorylated form can identify activated CREB. Retinal neurons were plated in fully defined minimal medium for 24 hr. RGC neurons were identified by staining with an islet-1 antibody. There is almost no phosphorylated CREB in RGC nuclei under these baseline conditions (Fig. 5, compare A1 and A2). In contrast, phospho-CREB is clearly detected in RGC nuclei after exposure to 100 ng/ml SDF-1 for 30 min (Fig. 5, compare B1 and B2). SDF-1-dependent phosphorylation of CREB is blocked by the SDF-1 antagonist AMD3100 (Fig. 5, compare C1 and C2), the Gi/Go inhibitor PTX (Fig. 5, compare D1 and D2) and the PKA inhibitor PKI (Fig. 5, compare E1 and E2). In some instances, CREB can be activated in a PKA-independent manner via MAP kinase (Grewal et al., 2000). The MAP kinase inhibitor PD98059 does not block SDF-1-induced CREB phosphorylation in RGCs (Fig. 5, compare F1 and F2), although it can block SDF-1-mediated cell survival (Fig. 4E), indicating that this alternative pathway for CREB activation is not activated by SDF-1. These results support a pathway in which SDF-1 activates its receptor CXCR4, acts through a Gi/Go intermediary, and induces an elevation in cAMP.
Figure 5.
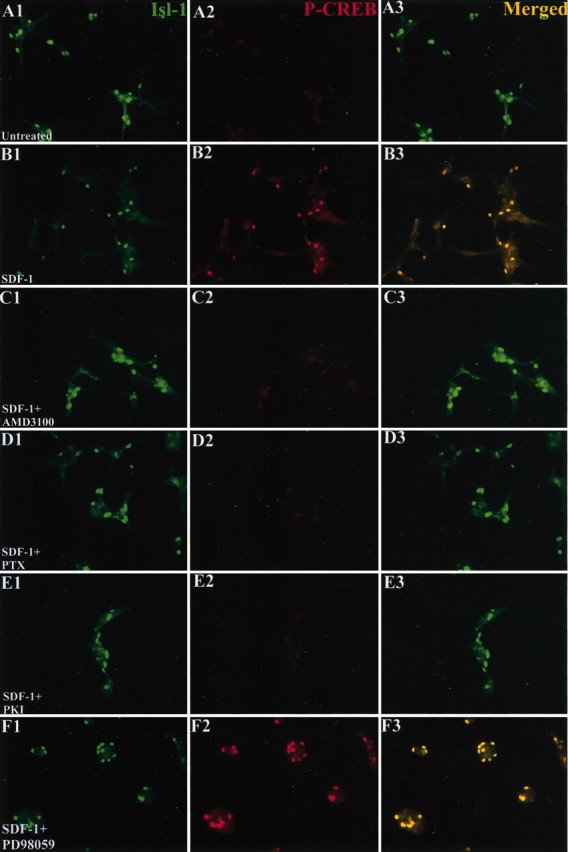
SDF-1 induces phosphorylation and translocation of CREB into the nucleus. E6 retinal neurons were cultured in defined minimal medium for 24 hr (A1–A3) and then exposed to 100 ng/ml SDF-1 (B1–B3), SDF-1 plus a 20 μm concentration of the CXCR4 antagonist AMD3100 (C1–C3), SDF-1 plus 100 ng/ml PTX (D1–D3), SDF-1 plus a 200 nm concentration of the PKA inhibitor PKI (E1–E3), or SDF-1 plus a 20 μm concentration of MAP kinase inhibitor PD98059 (F1–F3). After 30 min, the cultures were fixed and stained for islet-1 (A1–F1) (green) and phosphorylated CREB (A2–F2) (red). Merged images are shown in A3–F3. SDF-1 induces translocation of phosphorylated CREB into the nuclei of retinal neurons that is blocked by all three pharmacological agents.
Enhancement of NT2N survival by SDF-1 is associated with phosphorylation of MAP kinase and GSK3b
To better define the signaling pathway through which SDF-1 promotes neuronal survival, it was necessary to identify a neuronal cell line whose survival is similarly enhanced. NT2 neurons (NT2N) are differentiated teratocarcinoma cells that have neuronal properties that include axonal and dendritic process outgrowth, synapse formation, and the ability to integrate into neural tissues in vivo (Pleasure et al., 1992; Hartley et al., 1999; Philips et al., 1999). NT2N cells have also been shown to express CXCR4 and respond to SDF-1 by activating strong calcium transients (Coughlan et al., 2000). When cultured in serum-free medium, they begin to die within the first day of culture. Very high concentrations of SDF-1 have been reported to induce cell death in NT2N cells (Hesselgesser et al., 1998a). In contrast, we find that lower concentrations of SDF-1 promote NT2N survival. Roughly 40% of NT2N cells die during a 24 hr time period, but many of these dying cells are rescued by SDF-1 (Fig. 6A). To investigate the signaling pathway affected by SDF-1, Western blots of NT2N cell lysates were made from cultures without SDF-1, with SDF-1, and with SDF-1 plus selected inhibitors. These blots were then probed for the phosphorylated and nonphosphorylated forms of two kinases reported to be in the downstream pathway of CXCR4 activation: Akt and MAP kinase p44/42 (Ganju et al., 1998). The phosphorylation of GSK3β was also monitored because both it and MAP kinase have been reported to be activated by forskolin and have been proposed to promote cell survival in other systems. Although GSK3β is normally considered a downstream effector of Akt, MAP kinase has also been reported to directly phosphorylate GSK3β in cortical neurons (Li et al., 2000).
Figure 6.
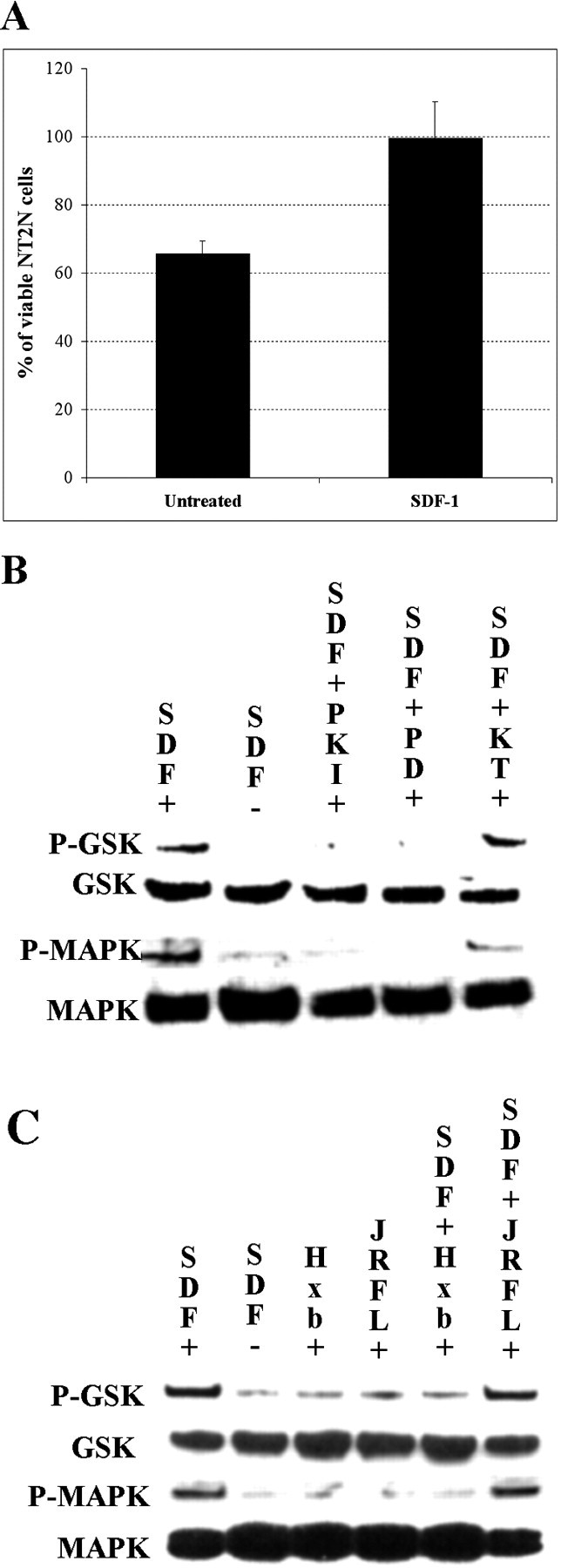
SDF-1 enhances survival and induces phosphorylation of MAP kinase and GSK3β in NT2N cells. A, SDF-1 100 ng/ml (p = 0.028) increases the number of NT2N cells after 24 hr in serum-free medium. p values were calculated by comparing each population with the untreated one using a two-tailed test with different variances. B, SDF-1 enhances phosphorylation of GSK3β and MAP kinase 44/42. The PKA inhibitor PKI at 20 μm blocks SDF-1-induced phosphorylation of GSK3β and MAP kinase. The MAP kinase inhibitor PD98059 at 20 μm also blocks the phosphorylation of both GSK3β and MAP kinase. The PKG antagonist KT5823 at 1 μm does not block SDF-1-induced phosphorylation of GSK3β and MAP kinase. C, Neither 100 ng/ml HxB-gp120 nor JRFL-gp120 affects GSK3β or MAP kinase phosphorylation on their own. HxB-gp120 but not JRFL-gp120 blocks SDF-1-induced phosphorylation of GSK3β and MAP kinase.
SDF-1 induces the phosphorylation of both GSK3β and MAP kinase (Fig. 6B, compare columns 1 and 2 in rows 1 and 3), consistent with the ability of the MAP kinase inhibitor PD98059 to block the SDF survival effect (Fig. 4E). Indeed, phosphorylation of both MAP kinase and GSK3β was greatly inhibited by the same inhibitors of PKA and MAP kinase that blocked the survival-promoting effects of SDF-1 on chick RGCs (Fig. 6B, compare column 1 with columns 3 and 4 in rows 1 and 3). No phosphorylation of Akt was detected on our blots (data not shown). MAP kinase appears to be upstream from GSK3β, because inhibiting MAP kinase activity prevents SDF-1 from inducing GSK3β phosphorylation. A PKG inhibitor did not block phosphorylation of either MAP kinase or GSK3β (Fig. 6B, compare columns 1 and 5 in rows 1 and 3). These results support the hypothesis that SDF-1 activates a cAMP-triggered signaling cascade that promotes neuronal survival through MAP kinase and GSK3β. Under similar conditions, however, we were unable to detect a significant increase in cAMP levels using a radioimmunoassay in NT2N cells treated with SDF-1. The CXCR4-tropic glycoprotein HxB-gp120 prevents SDF-1-induced phosphorylation of MAP kinase and GSK3β in NT2N cells, whereas the CCR5-tropic glycoprotein JRFL-gp120 had no such effect (Fig. 6C, compare column 1 with columns 5 and 6 in rows 1 and 3). Neither of the two glycoproteins alone have any effect on the phosphorylation of either GSK3β or MAP kinase (Fig. 6C, compare column 1 with columns 3 and 4 in rows 1 and 3). These results are consistent with the idea that Hxb-gp120 blocks SDF-1 neurotrophic effects by preventing SDF-1 from binding and activating CXCR4.
SDF-1-induced survival can be blocked by slit-2
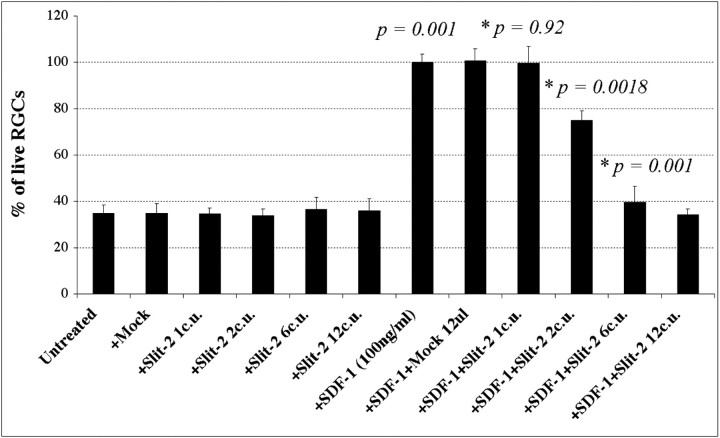
We then analyzed the effects of another molecule, slit-2, on SDF-1-induced RGC survival. Slit-2 was originally described in vertebrates as a branching factor, promoting branching of DRG axons, and a chemorepellent for migrating cells in the CNS (Wang et al., 1996; Hu, 1999). Since then, it has been shown to repel a variety of axons, including retinal axons, olfactory bulb axons, and fore-brain axons (Li et al., 1999; Nguyen Ba-Charvet et al., 1999; Niclou et al., 2000). It has been shown recently that the chemotaxis of T cells toward a source of SDF-1 in a Boyden chamber assay is reduced in the presence of slit-2 (Wu et al., 2001). Similarly, we find that slit-2 antagonizes the trophic effect of SDF-1 on RGCs. Approximately six collapsing units of slit-2 reverse the survival-promoting activity of SDF-1 (Fig. 7, compare columns 7 and 11). A collapsing unit is defined as the amount of protein needed to cause 50% of embryonic chick retinal growth cones to collapse in a collapse assay. The half-maximal dose for slit-2 reversal of SDF-1 activity is approximately three collapsing units. These results demonstrate that slit-2 reverses the survival-promoting effects of SDF-1 when applied at doses somewhat higher than those required to obtain growth cone collapse.
Figure 7.
SDF-1-induced survival is blocked by slit-2. Increasing concentrations of slit-2 (≥6 collapsing units) can block SDF-1-induced survival. p values were calculated by comparing each population with the untreated one using a two-tailed test with different variances.
RGC number is reduced in the CXCR4 knock-out mice
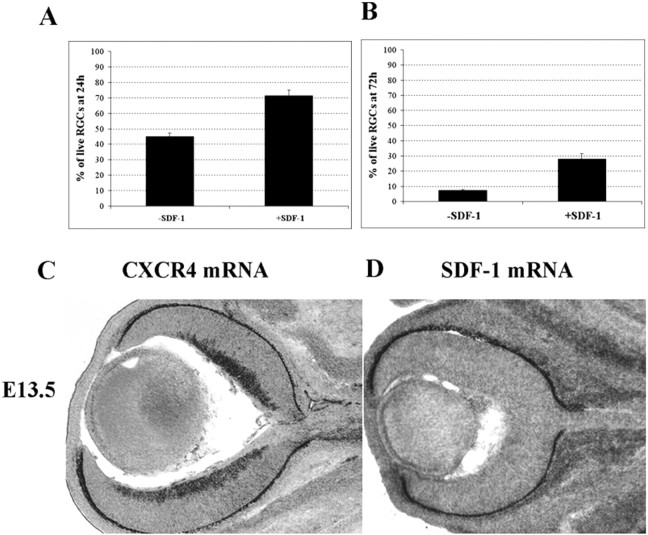
If SDF-1 promotes neuronal survival through the activation of CXCR4 in vivo, then the absence of CXCR4 should lead to increased neuronal loss during development. To investigate this possibility, the numbers of RGCs in the retinas of wild-type and CXCR4 knock-out embryonic mouse littermates were compared. First, however, we confirmed that SDF-1 promotes the survival of cultured E16.5 mouse RGCs without the addition of any other trophic factors. SDF-1 at 100 ng/ml increases the proportion of surviving RGCs from <50 to 70% after 24 hr in culture (Fig. 8A) and from ∼10 to 30% after 72 hr in culture (Fig. 8B). CXCR4 mRNA is expressed in the E13.5 mouse RGC layer (Fig. 8C), and SDF-1 is expressed outside the eye in tissues surrounding the optic nerve (Fig. 8D). Thus, as in chick, CXCR4 is expressed in mouse RGCs, and SDF-1 promotes their survival in vitro.
Figure 8.
SDF-1 enhances the survival of embryonic mouse RGCs. After 24 (A; p = 0.001) or 72 (B; p = 0.005) hr in culture, an increased percentage of RGCs survive in the presence of 100 ng/ml SDF-1. p values were calculated by comparing each population with the untreated one using a two-tailed test with different variances. C, CXCR4 mRNA is expressed in the RGC cell layer of E13.5 mouse retina. D, At the same embryonic stage, SDF-1 mRNA is expressed in connective tissues surrounding the eye.
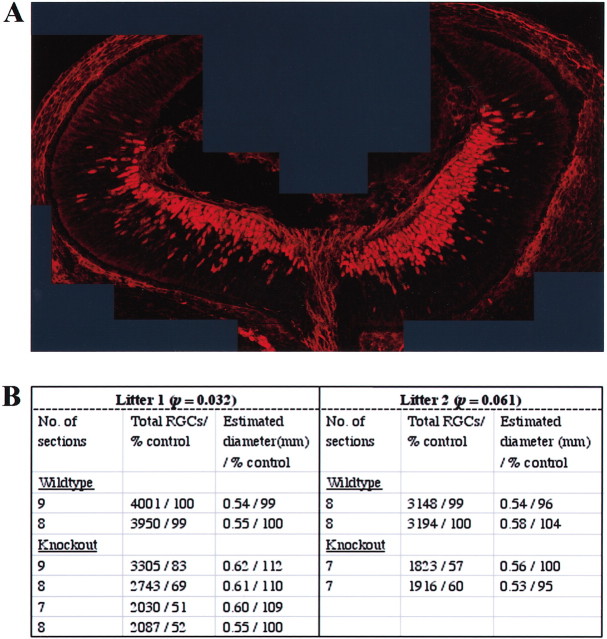
We used islet-1 as a marker for RGC neurons in the embryonic mouse retina (Erskine et al., 2000). Although islet-1 has been shown to stain both RGCs and displaced amacrine cells at late embryonic ages (E21.5), at the earlier embryonic age used for our analysis it is a specific marker for RGCs (Galli-Resta et al., 1997; Erskine et al., 2000). The numbers of RGCs were compared in wild-type and CXCR4 knock-out littermates. RGCs were counted in thin optical sections (∼2 μm) made with a confocal microscope from alternate 30 μm serial cryostat sections through entire wild-type and mutant eyes. This provides a sample count proportional to the total number of RGCs throughout each eye that does not depend on the precise thickness of individual sections or require correction for nuclei spanning more than one section. A representative reconstruction of a confocal section from an E13.5 wild-type eye is shown in Figure 9A. The relative numbers of RGCs were estimated in four mutant and two wild-type eyes from one litter and from two mutant and two wild-type eyes in a second litter (Fig. 9B, table). There is an ∼35% reduction in the number of sampled RGCs in CXCR4 mutant compared with wild-type littermates. A parameter-free distribution was assumed, and a Mann–Whitney test was used to estimate that there are 3.2 and 6.1% chances that there are no differences in RGC numbers between wild-type and mutant embryos for each of the two litters. The weights of mutant and wild-type embryos were not significantly different from one another (data not shown), nor were the diameters of their eyes (Fig. 9B). Our data show that eyes in E13.5 CXCR4 mutants are of normal size but contain reduced numbers of RGCs. We also compared the number of islet-1/2-positive neurons at E11.5 wild-type and mutant littermates, a time when the first-born RGC neurons begin extending axons in the retina (Young 1985; Cepko et al., 1996). At this early time point, we find no difference in the number of neurons between two knock-outs and four wild-type littermates. These observations are consistent with the hypothesis that SDF-1 helps to promote the survival of RGCs after E11.5; however, they do not eliminate the possibility that RGC production is reduced in CXCR4 mutant embryos.
Figure 9.
Mice lacking CXCR4 have fewer RGCs. A, Confocal reconstruction of a representative 2 μm optical section from an E13.5 wild-type eye. B, Table comparing the number of islet-1-positive RGCs (see Materials and Methods) in wild-type and CXCR4 mutant eyes. The number of RGCs in mutant embryos is estimated to be ∼65% of those in wild-type littermates.
Discussion
Neuronal survival is known to be enhanced in many neuronal types by the elevation of cAMP, but ligands that promote neuronal survival in vivo through this mechanism have been hard to identify. Our results suggest that SDF-1 can promote the survival of RGC neurons through the activation of CXCR4 and the stimulation of a cAMP-mediated signaling pathway. This is the first time that SDF-1 has been proposed to have a neurotrophic role during normal development.
Our results are consistent with the hypothesis that SDF-1 acts directly on RGC neurons. These are the only neurons within the retina that express SDF-1 receptor, CXCR4. Moreover, the survival-promoting effect of SDF-1 does not require the presence of other more traditional neurotrophic factors, nor is it mediated by the src kinases common to neurotrophin actions. However, it is impossible to rule out the possibility that SDF-1 promotes neuronal survival by inducing the synthesis or release of another neurotrophic factor.
That SDF-1 exerts its survival effects through the elevation of cAMP is somewhat unexpected. This survival effect is clearly blocked by PTX, yet PTX-sensitive pathways generally act through a Gi intermediary that inhibits adenylate cyclase. However, a PTX-sensitive elevation of cAMP like that seen in the survival-promoting pathway of SDF-1 is not without precedent. For example, the metabotropic glutamate receptor mGluR1 can activate adenylate cyclase through a PTX-sensitive Gi intermediary (Conn and Pin, 1997). SDF-1 may also activate an additional cGMP-mediated pathway in primary embryonic neurons, because an antagonist of cGMP and an inhibitor of PKG each partially reduce the survival-enhancing effects of SDF-1. Similarly, the chemotactic effect of SDF-1 on T cells is mediated through PTX-sensitive activation of both PKG and PKA (Jinquan et al., 2000), and SDF-1 activates a cAMP-dependent pathway to promote an anti-inflammatory reaction in peripheral blood mononuclear cells (Damas et al., 2002). The cAMP-activated pathway we describe seems to play the predominant role in promoting neuronal survival, whereas a cGMP-mediated pathway appears to be activated in parallel and enhances survival to a lesser extent.
The CXCR4-tropic HIV coat glycoprotein HxB-gp120 has been reported previously to have toxic effects on cultured neurons and NT2N cells (Hesselgesser et al., 1998a; Kaul and Lipton, 1999). HIV isolates that bind CXCR4 are toxic to neurons, whereas those that bind CCR5 appear to be less toxic (Ohagen et al., 1999). It has therefore been argued that HxB-gp120 produced by HIV-infected tissue might have a directly toxic effect on nearby cells in the CNS (Ohagen et al., 1999). This apparent toxic effect of HxB-gp120 is also detected in our studies by its reduction in the survival of RGCs but only when SDF-1 is present in the medium. Recent studies have indicated that Hxb-gp120 can cause toxicity to neurons by two different pathways, a CXCR4-dependent PTX-sensitive pathway and a second PTX-independent pathway (Zheng et al., 1999). Our experiments suggest that some of this glycoprotein toxicity could be a result of its ability to block the binding of SDF-1 to CXCR4 (Staudinger et al., 2001), thereby preventing SDF-1 from activating cAMP-mediated survival pathways.
In contrast to our finding that SDF-1 promotes the survival of embryonic chick and mouse RGC neurons, other studies have reported that SDF-1 has neurotoxic effects on E15–E17 rat cerebrocortical cultures (Kaul and Lipton, 1999) and on 13–16 week human fetal neurons (Zheng et al., 1999). One possible explanation for this discrepancy is that different neuron types might behave dissimilarly. For example, SDF-1 might act as a trophic factor early in embryogenesis and have different effects later in development. We have not examined the effects of SDF-1 on older RGC neurons. In at least one instance, SDF-1 has been shown to cause neurotoxicity indirectly via the release of tumor necrosis factor from astrocytes in mixed cultures (Bezzi et al., 2001). Astrocytes have not yet differentiated in the retinas from which we prepare cultured RGCs (Young, 1985; Cepko et al., 1996). This and the fact that CXCR4 is expressed on only RGCs at these early developmental times may have enabled us to detect a direct trophic effect of SDF-1 on RGC neurons in our cultures. Finally, a recent study has concluded that SDF-1 activates three separate signaling pathways in CD4 T cells, one being a PTX-sensitive prosurvival pathway, whereas another is a competing PTX-insensitive apoptotic pathway (Vlahakis et al., 2002). Our results suggest that in our culture conditions, the PTX-sensitive prosurvival pathway predominates in embryonic RGCs.
Slit-2, a known axonal repellent, has been shown recently to interfere with the chemotactic activity of leukocytes toward SDF-1 (Wu et al., 2001), and here we show that slit-2 also interferes with the survival-promoting activity of SDF-1 on RGC neurons. Conversely, SDF-1 reduces the efficacy of multiple repellents on several neuronal types (Chalasani et al., 2003). For example, concentrations of SDF-1 that promote RGC survival reduce the sensitivity of RGC axons to slit-2 by ∼10-fold. The first steps of the signaling pathway by which SDF-1 opposes repellent action are identical to those through which it promotes RGC survival. Together, these results suggest that the slit-2- and SDF-1-activated signaling pathways act in opposition to one another. A more general antagonism between survival and axon repellents is hinted at by reports that sema3A can have neurotoxic effects (Fankhauser et al., 1999; Shirvan et al., 1999). We speculate that neurons with misdirected axons that are in contact with repellents for long periods of time may become resistant to survival-promoting signals. This effect, combined with the better known loss of target-specific trophic signals (Purves et al., 1988; Barde, 1989; Bernstein and Lichtman, 1999), would help remove neurons whose axons were irretrievably lost. Conversely, correctly routed axons with access to target-specific trophic factors may be better able to ignore incidental or weak repellent cues.
The SDF-1 receptor CXCR4 is expressed in a wide variety of neuronal tissues, including RGCs, sympathetic ganglia, dorsal root sensory ganglia, spinal cord, hindbrain, midbrain, olfactory bulb, and the external granule cell layer of the cerebellum (McGrath et al., 1999, and our results). SDF-1 therefore has the potential to enhance neuronal survival among a wide variety of central and peripheral neurons. SDF-1 itself is expressed primarily outside the developing CNS during embryogenesis (McGrath et al., 1999). Thus, embryonic RGCs and other neurons will most often encounter SDF-1 as their axons grow through non-neuronal tissues. Our in vitro results suggest that SDF-1 provides trophic support to embryonic retinal neurons after retinal axons first exit the eye at E11.5 (Young, 1985; Cepko et al., 1996). This expectation is supported by the reduced numbers of E13.5 RGCs in CXCR4 knock-outs compared with wild-type littermates. Other trophic factors such as BDNF, NGF, and CNTF have been shown to promote RGC survival in vitro (Lehwalder et al., 1989; Mansour-Robaey et al., 1994), and it is therefore not surprising that many RGCs persist within the embryo even in the absence of SDF-1/CXCR4 signaling. Unfortunately, it is not practical to determine whether RGC numbers are reduced further over time during late embryonic and postnatal life, because loss of CXCR4 has an embryonic lethal phenotype. This issue can be examined in greater detail when eye-specific conditional knock-outs become available.
It is possible that SDF-1 could provide trophic support to neurons in the mature nervous system. Both CXCR4 and SDF-1 are found in the postnatal CNS (Gabuzda and Wang, 1999; McGrath et al., 1999; Zheng et al., 1999; Tham et al., 2001). SDF-1 is expressed in the cerebellum, olfactory bulb, cortex, dentate gyrus of the hippocampus, and some thalamic nuclei. CXCR4 is widely expressed in CNS neurons, microglia, and astrocytes. Again, it may be possible to determine whether SDF-1 provides trophic support to these cells in mature animals when conditional knock-outs or more potent CXCR4 antagonists become available. There is some evidence to suggest that increased expression of CXCR4 is correlated with neuropathogenesis induced by HIV-1 or by other forms of injury, including trauma and stroke (Gabuzda and Wang, 1999; Klein et al., 1999; Ohagen et al., 1999; Zheng et al., 1999). Moreover, a chemokine like SDF-1 is expected to be expressed in damaged or inflamed tissues (Gonzalo et al., 2000; Evert et al., 2001). We therefore hypothesize that SDF-1 activation of CXCR4 not only provides generalized trophic support to embryonic and mature neurons but also might help support neurons damaged by injury or inflammation.
Footnotes
This research was supported by National Institutes of Health Grant RO1-NS26527 to J.A.R. We gratefully acknowledge Pete Bannerman and Ashleigh Hanna for help with the confocal microscope. We also thank Connie Page for providing NT2N cells, Darlene Ghavimi for making the HIV glycoproteins, Drs. Kimberly Sabelko and Andrea Webber for help with the mice, Cynthia Ito and Radhia Ben-Mohamed for technical help, and Cynthia Ito and Thomas Kreibich for their help in data analysis. We thank Dr. Morris Brinbaum and Eileen Whiteman for the GSK, phospho-GSK, and AKT antibodies; Dr. Thomas Jessell for islet-1/2 antibodies; Dr. Francis Lefcort for TrkB antibodies; and Dr. William Halfter for neurofilament antibodies. We thank Drs. David Manning and Judy Meinkoth for their advice with the signaling pathway and LiJia, Thomas Kreibich, and Drs. Kimberly Sabelko and Andrea Webber for their help with this manuscript.
Correspondence should be addressed to Jonathan A. Raper, University of Pennsylvania School of Medicine, 1115 BRB II/III, 421 Curie Boulevard, Philadelphia, PA 19104. E-mail: raperj@mail.med.upenn.edu.
C. M. Coughlan's present address: Biological Sciences Department, 257 Crawford Hall, University of Pittsburgh, Pittsburgh, PA 15260.
Copyright © 2003 Society for Neuroscience 0270-6474/03/234601-12$15.00/0
References
- Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA ( 1996) CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272: 1955–1958. [DOI] [PubMed] [Google Scholar]
- Bagri A, Gurney T, He X, Zou YR, Littman DR, Tessier-Lavigne M, Pleasure SJ ( 2002) The chemokine SDF1 regulates migration of dentate granule cells. Development 129: 4249–4260. [DOI] [PubMed] [Google Scholar]
- Barde Y-A ( 1989) Trophic factors and neuronal survival. Neuron 2: 1525–1534. [DOI] [PubMed] [Google Scholar]
- Berger EA, Murphy PM, Farber JM ( 1999) Chemokine receptors as HIV-1 co-receptors: roles in viral entry, tropism and disease. Annu Rev Immunol 17: 657–700. [DOI] [PubMed] [Google Scholar]
- Bernstein M, Lichtman JW ( 1999) Axonal atrophy: the retraction reaction. Curr Opin Neurobiol 9: 364–370. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A ( 2001) CXCR4-activated astrocyte glutamate release via TNFα: amplification by microglia triggers neurotoxicity Nat Neurosci 4: 702–710. [DOI] [PubMed] [Google Scholar]
- Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer TA ( 1996) The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature 382: 829–833. [DOI] [PubMed] [Google Scholar]
- Cepko CL, Austin CP, Yang X, Alexiades M, Ezzeddine D ( 1996) Cell fate determination in the vertebrate retina. Proc Natl Acad Sci USA 93: 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalasani SH, Sabelko KA, Sunshine MJ, Littman DR, Raper JA ( 2003) A chemokine, SDF-1, reduces the effectiveness of multiple axon repellants and is required for normal axon pathfinding. J Neurosci 23: 1360–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath PD, Wu L, Mackay CR, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J ( 1996) The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell 85: 1135–1148. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin J-P ( 1997) Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol 37: 205–237. [DOI] [PubMed] [Google Scholar]
- Coughlan CM, McManus CM, Sharron M, Gao Z, Murphy D, Jaffer S, Choe W, Chen W, Hesselgesser J, Gaylord H, Kalyuzhny A, Lee VM, Wolf B, Doms RW, Kolson DL ( 2000) Expression of multiple functional chemokine receptors and monocyte chemoattractant protein-1 in human neurons. Neuroscience 97: 591–600. [DOI] [PubMed] [Google Scholar]
- Damas JK, Waehre T, Yndestad A, Ueland T, Muller F, Eiken HG, Holm AM, Halvorsen B, Froland SS, Gullestad L, Aukrust P ( 2002) Stromal cell-derived factor-1α in unstable angina: potential anti-inflammatory and matrix-stabilizing effects. Circulation 106: 36–42. [DOI] [PubMed] [Google Scholar]
- Doranz BJ, Rucker J, Yi Y, Smyth RJ, Samson M, Peiper SC, Paramentier M, Collman RG, Doms RW ( 1996) A dual-tropic HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-3 and CKR-2b as fusion cofactors. Cell 85: 1149–1158. [DOI] [PubMed] [Google Scholar]
- Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, Paxton WA ( 1996) HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381: 667–673. [DOI] [PubMed] [Google Scholar]
- Ericson J, Thor S, Edlund T, Jessell TM, Yamada T ( 1992) Early stages of motor neuron differentiation revealed by expression of homeobox islet-1. Science 256: 1555–1560. [DOI] [PubMed] [Google Scholar]
- Erskine L, Williams SE, Brose K, Kidd T, Rachel RA, Goodman CS, Tessier-Lavigne M, Mason CA ( 2000) Retinal ganglion cell axon guidance in the mouse optic chiasm: expression and function of Robos and Slits. J Neurosci 20: 4975–4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evert BO, Vogt IR, Kindermann C, Ozimek L, de Vos RA, Brunt ER, Schmitt I, Klockgether T, Wullner U ( 2001) Inflammatory genes are upregulated in expanded ataxin-3-expressing cell lines and spinocerebellar ataxia type 3 brains. J Neurosci 21: 5389–5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser C, Friedlander RM, Gagliardini V ( 1999) Prevention of nuclear localization of activated caspases correlates with inhibition of apoptosis. Apoptosis 5: 117–132. [DOI] [PubMed] [Google Scholar]
- Feng Y, Broder CJ, Kennedy PE, Berger EA ( 1996) HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 272: 872–877. [DOI] [PubMed] [Google Scholar]
- Gabuzda D, Wang J ( 1999) Chemokine receptors and virus entry in the central nervous system. J Neurovirol 6: 643–658. [DOI] [PubMed] [Google Scholar]
- Galli-Resta L, Resta G, Tan SS, Reese BE ( 1997) Mosaics of islet-1-expressing amacrine cells assembled by short-range cellular interactions. J Neurosci 17: 7831–7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganju RK, Brubaker SA, Meyer J, Dutt P, Yang Y, Qin S, Newman W, Groopman JE ( 1998) The α-chemokine, stromal cell-derived factor-1α, binds to the transmembrane G-protein-coupled CXCR-4 receptor and activates multiple signal transduction pathways. J Biol Chem 273: 23169–23175. [DOI] [PubMed] [Google Scholar]
- Gerlach LO, Skerlj RT, Bridger GJ, Schwartz TW ( 2001) Molecular interactions of cyclam and bicyclam non-peptide antagonists with the CXCR4 chemokine receptor. J Biol Chem 276: 14153–14160. [DOI] [PubMed] [Google Scholar]
- Gonzalo JA, Lloyd CM, Peled A, Delaney T, Coyle AJ, Gutierrez-Ramos JC ( 2000) Critical involvement of the chemotactic axis CXCR4/stromal cell-derived factor-1α in the inflammatory component of allergic airway disease. J Immunol 165: 499–508. [DOI] [PubMed] [Google Scholar]
- Gonzalez GA, Montminy MR ( 1989) Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell 59: 675–680. [DOI] [PubMed] [Google Scholar]
- Grewal SS, Fass DM, Yao H, Ellig CL, Goodman RH, Stork PJS ( 2000) Calcium and cAMP signals differentially regulate cAMP-responsive element-binding protein function via a RAP1-extracellular signal-regulated kinase pathway. J Biol Chem 275: 34433–34441. [DOI] [PubMed] [Google Scholar]
- Hagiwara M, Brindle P, Harootunian A, Armstrong R, Rivier J, Vale W, Tsien R, Montminy MR ( 1993) Coupling of hormonal stimulation and transcription via cyclic AMP-responsive factor CREB is rate limited by nuclear entry of protein kinase A. Mol Cell Biol 13: 4852–4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfter W ( 1998) Disruption of the retinal basal lamina during early embryonic development leads to a retraction of the vitreal end feet, an increased number of ganglion cells and aberrant axonal outgrowth. J Comp Neurol 397: 89–104. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Levi-Montalcini R ( 1949) Proliferation, differentiation and degeneration in the spinal ganglia of the chick embryo under normal and experimental conditions. J Exp Zool 111: 457–502. [DOI] [PubMed] [Google Scholar]
- Hanson Jr MG, Shen S, Wiemelt AP, McMorris FA, Barres BA ( 1997) Cyclic AMP elevation is sufficient to promote survival of spinal motor neurons in vitro. J Neurosci 18: 7361–7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley RS, Margulis M, Fishman PS, Lee VMY, Tang CM ( 1999) Functional synapses are formed between human Ntera2 (NT2N, hNT) neurons grown on astrocytes. J Comp Neurol 407: 1–10. [DOI] [PubMed] [Google Scholar]
- Hesselgesser J, Halks-Miller M, DelVecchio V, Peiper SC, Hoxie J, Kolson DL, Taub D, Horuk R ( 1997) CD4-independent association between HIVgp120 and CXCR4: functional chemokine receptors are expressed in human neurons. Curr Biol 7: 112–121. [DOI] [PubMed] [Google Scholar]
- Hesselgesser J, Taub D, Baskar P, Greenberg M, Hoxie J, Kolson DL, Horuk R ( 1998a) Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1α is mediated by the chemokine receptor CXCR4. Curr Biol 8: 595–598. [DOI] [PubMed] [Google Scholar]
- Hesselgesser J, Liang M, Hoxie J, Greenberg M, Brass LF, Orsini MJ, Taub D, Horuk R ( 1998b) Identification and characterization of the CXCR4 chemokine receptor in human T cell lines: ligand binding, biological activity and HIV-1 infectivity. J Immunol 160: 877–883. [PubMed] [Google Scholar]
- Hu H ( 1999) Chemorepulsion of neuronal migration by Slit2 in the developing mammalian forebrain. Neuron 23: 703–711. [DOI] [PubMed] [Google Scholar]
- Jinquan T, Quan S, Jacobi HH, Madsen HO, Glue C, Skov PS, Malling HJ, Poulsen LK ( 2000) CXC chemokine receptor 4 expression and stromal cell-derived factor-1α-induced chemotaxis in CD+ T lymphocytes are regulated by interleukin-4 and interleukin-10. Immunology 99: 402–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Lipton SA ( 1999) Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc Natl Acad Sci USA 96: 8212–8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RS, Williams KC, Alvarez-Hernandez X, Westmoreland S, Force T, Lackner AA, Luster AD ( 1999) Chemokine receptor expression and signaling in macaque and human fetal neurons and astrocytes: implications for the neuropathogenesis of AIDS. J Immunol 163: 1636–1646. [PubMed] [Google Scholar]
- Lee FS, Chao MV ( 2001) Activation of Trk neurotrophin receptors in the absence of neurotrophins. Proc Natl Acad Sci USA 98: 3555–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehwalder D, Jeffrey PL, Unsicker K ( 1989) Survival of purified embryonic chick retinal ganglion cells in the presence of neurotrophic factors. J Neurosci Res 24: 329–337. [DOI] [PubMed] [Google Scholar]
- Li HS, Chen JH, Wu W, Fagaly T, Zhou L, Yuan W, Dupuis S, Jiang ZH, Nash W, Gick C, Ornitz DM, Wu JY, Rao Y ( 1999) Vertebrate slit, a secreted ligand for the transmembrane protein roundabout, is a repellant for olfactory bulb axons. Cell 96: 807–818. [DOI] [PubMed] [Google Scholar]
- Li M, Wang X, Meintzer MK, Laessig T, Brinbaum MJ, Heidenreich KA ( 2000) Cyclic AMP promotes neuronal survival by phosphorylation of glycogen synthase kinase 3β. Mol Cell Biol 20: 9356–9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Sun EE, Klein RS, Flanagan JG ( 2001) Ephrin-B reverse signaling is mediated by a novel PDZ-RGS protein and selectively inhibits G protein-coupled chemoattraction. Cell 105: 69–79. [DOI] [PubMed] [Google Scholar]
- Luster AD ( 1998) Mechanisms of disease: chemokines-chemotactic cytokines that mediate inflammation. N Engl J Med 338: 436–445. [DOI] [PubMed] [Google Scholar]
- Luther SA, Cyster JG ( 2001) Chemokines as regulators of T cell differentiation. Nat Immunol 2: 102–107. [DOI] [PubMed] [Google Scholar]
- Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA ( 1998) Impaired B-lymphopoiesis, myelopoiesis and derailed cerebellar neuron migration in CXCr4-and SDF-1-deficient mice. Proc Natl Acad Sci USA 95: 9448–9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay CR ( 2001) Chemokines: immunology's high impact factors. Nat Immunol 2: 95–101. [DOI] [PubMed] [Google Scholar]
- Mansour-Robaey S, Clarke DB, Wang YC, Bray GM, Aguayo AJ ( 1994) Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc Natl Acad Sci USA 91: 1632–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath KE, Koniski AD, Maltgy KM, McGann JK, Palis J ( 1999) Embryonic expression and function of chemokine SDF-1 and its receptor, CXCR4. Dev Biol 213: 442–456. [DOI] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AS, Miller RJ ( 2000) Expression of CX3CR1 chemokine receptors on neurons and their role in neuronal survival. Proc Natl Acad Sci USA 97: 8075–8080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Franke A, Wilkinson GA, Kruttgen A, Hu M, Munro E, Hanson MG, Reichardt LF, Barres BA ( 1998) Depolarization and cAMP elevation rapidly recruit TrkB to the plasma membrane of CNS neurons. Neuron 21: 681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA ( 2000) International Union of Pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev 52: 145–176. [PubMed] [Google Scholar]
- Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T ( 1996) Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 382: 635–638. [DOI] [PubMed] [Google Scholar]
- Nanki T, Lipsky PE ( 2000) Cutting edge: stromal cell-derived factor-1 is a co-stimulator for CD4+ T cell activation. J Immunol 164: 5010–5014. [DOI] [PubMed] [Google Scholar]
- Nguyen Ba-Charvet KT, Brose K, Marillat V, Kidd T, Goodman CS, Tessier-Lavigne M, Sotelo C, Chedotal A ( 1999) Slit-2 mediated chemorepulsion and collapse of developing forebrain axons. Neuron 22: 463–473. [DOI] [PubMed] [Google Scholar]
- Niclou SP, Jia L, Raper JA ( 2000) Slit2 is a repellant for retinal ganglion cell axons. J Neurosci 20: 4962–4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohagen A, Ghosh S, He J, Huang K, Chen Y, Yuan M, Osathanondh R, Gartner S, Shi B, Shaw G, Gabuzda D ( 1999) Apoptosis induced by infection of primary brain cultures with diverse human immunodeficiency virus type I isolates: evidence for the role of the envelope. J Virol 73: 897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips MF, Muir JK, Saatman KE, Raghupathi R, Lee VMY, Trojanowski JQ, McIntosh TK ( 1999) Survival and integration of transplanted postmitotic human neurons following experimental brain injury in immunocompetent rats. J Neurosurg 90: 116–124. [DOI] [PubMed] [Google Scholar]
- Pleasure SJ, Page C, Lee VMY ( 1992) Pure, postmitotic polarized human neurons derived from NTera2N cells provide a system for expressing exogenous proteins in differentiated neurons. J Neurosci 12: 1802–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D, Snider WD, Voyvodic JT ( 1988) Trophic regulation of nerve cell morphology and innervation in the autonomic nervous system. Nature 336: 123–128. [DOI] [PubMed] [Google Scholar]
- Raff M ( 1998) Cell suicide for beginners. Nature 396: 119–122. [DOI] [PubMed] [Google Scholar]
- Rydel RE, Greene LA ( 1988) cAMP analogs promote survival and neurite outgrowth in cultures of rat sympathetic and sensory neurons independently of NGF. Proc Natl Acad Sci USA 85: 1257–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Wiemelt AP, McMorris FA, Barres BA ( 1999) Retinal ganglion cells lose trophic responsiveness after axotomy. Neuron 23: 285–295. [DOI] [PubMed] [Google Scholar]
- Shirvan A, Ziv I, Fleminger G, Shina R, He Z, Brudo I, Melamed E, Barzilai A ( 1999) Semaphorins as mediators of neuronal apoptosis. J Neurochem 73: 961–971. [DOI] [PubMed] [Google Scholar]
- Staudinger R, Wang X, Brandés JC ( 2001) HIV-1 envelope is a neutral antagonist to CXCR4 in T cells. Biochem Biophys Res Commun 280: 1003–1007. [DOI] [PubMed] [Google Scholar]
- Tachibana K, Hirota S, Lizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsishima K, Yoshida N, Nishikawa S, Kishimoto T, Nagasawa T ( 1998) The chemokine receptor CXCR4 is essential for the vascularization of the gastrointestinal tract. Nature 393: 591–594. [DOI] [PubMed] [Google Scholar]
- Tham TN, Lazarini F, Franceschini IA, Amara A, Dubois-Dalcq M ( 2001) Developmental pattern of expression of the α chemokine stromal cell-derived factor 1 in the rat central nervous system. Eur J Neurosci 13: 845–856. [DOI] [PubMed] [Google Scholar]
- Vlahakis SR, Villasis-Keever A, Gomez T, Vanegas M, Vlahakis N, Paya CV ( 2002) G protein-coupled chemokine receptors induce both survival and apoptotic signaling pathways. J Immunol 169: 5546–5554. [DOI] [PubMed] [Google Scholar]
- Wakade AR, Edgar D, Thoenen H ( 1983) Both nerve growth factor and high K + concentrations support survival of chick embryo sympathetic neurons. Exp Cell Res 144: 377–384. [DOI] [PubMed] [Google Scholar]
- Wang KH, Brose K, Arnott D, Kidd T, Goodman CS, Henzel W, Tessier-Lavigne M ( 1996) Biochemical purification of a mammalian slit protein as a positive regulator of sensory axon elongation and branching. Cell 96: 771–784. [DOI] [PubMed] [Google Scholar]
- Wu JY, Feng L, Park HT, Havlioglu N, Wen L, Tang H, Bacon KB, Jiang ZH, Zhang XC, Rao Y ( 2001) The neuronal repellant Slit inhibits leukocyte chemotaxis induced by chemotactic factors. Nature 410: 948–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW ( 1985) Cell differentiation in the retina of the mouse. Anat Rec 212: 199–205. [DOI] [PubMed] [Google Scholar]
- Zheng J, Thylin MR, Ghorpade A, Xiong H, Persidsky Y, Cotter R, Niemann D, Che M, Zeng Y, Gelbard HA, Shepard RB, Swartz JM, Gendelman HE ( 1999) Intracellular CXCR4 signaling, neuronal apoptosis and neuropathogenic mechanisms of HIV-1 associated dementia. J Neuroimmunol 98: 185–200. [DOI] [PubMed] [Google Scholar]
- Zou Y, Kottmann AH, Kuroda M, Taniuchi I, Littman DR ( 1998) Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 393: 595–599. [DOI] [PubMed] [Google Scholar]