Abstract
ATP is a key extracellular messenger that mediates the propagation of Ca 2+ waves in astrocyte networks in various regions of the CNS. ATP-mediated Ca 2+ signals play critical roles in astrocyte proliferation and differentiation and in modulating neuronal activity. The actions of ATP on astrocytes are via two distinct subtypes of P2Y purinoceptors, P2Y1 and P2Y2 receptors (P2Y1Rs and P2Y2Rs), G-protein coupled receptors that stimulate mobilization of intracellular Ca 2+ ([Ca 2+]i) via the phospholipase Cβ-IP3 pathway. We report here that P2Y1R-mediated and P2Y2R-mediated Ca 2+ responses differentially show two forms of activity-dependent negative feedback. First, Ca 2+ responses mediated by either receptor exhibit slow depression that is independent of stimulation frequency. Second, responses mediated by P2Y1Rs, but not those mediated by P2Y2Rs, show rapid oscillations after high-frequency stimulation. We demonstrate that the oscillations are mediated by recruiting negative feedback by protein kinase C, and we map the site responsible for the effect of protein kinase C to Thr339 in the C terminus of P2Y1R. We propose that frequency-dependent changes in ATP-mediated Ca 2+ signaling pathways may modulate astrocyte function and astrocyte–neuron signaling in the CNS.
Keywords: P2Y1, P2Y2, PKC, frequency, astrocytes, ATP, Ca 2+signaling
Introduction
Astrocytes, the most abundant cell type in the CNS (Kuffler et al., 1984), form complex networks and are intimately associated with neurons, synapses (Araque et al., 1999; Haydon, 2001), and other non-neuronal cell types (Grafstein et al., 2000; Braet et al., 2001). Astrocytes respond to a variety of extracellular stimuli and produce regenerative intracellular signals by means of release of Ca 2+ from intracellular stores (Finkbeiner, 1993). Increased intracellular Ca 2+ ([Ca 2+]i) stimulates diverse processes within astrocytes, including proliferation (Neary et al., 1999), differentiation (Verkhratsky and Kettenmann, 1996), and secretion of chemical mediators, such as glutamate (Araque et al., 1998; Pasti et al., 2001), that regulate the activity of neurons (Parpura and Haydon, 2000). A rise in [Ca 2+]i localized to one part of an astrocyte may propagate within the rest of the cell, and Ca 2+ responses may be transmitted from one astrocyte to others leading to Ca 2+ waves that spread within astrocyte networks (Cornell-Bell et al., 1990).
A growing body of evidence indicates that a principal mechanism for the propagation of Ca 2+ waves between astrocytes is by release of ATP, which acts as a diffusible extracellular messenger (Haydon, 2001). Release of ATP from astrocytes during Ca 2+ wave propagation has been demonstrated by means of bioluminescence measurement (Guthrie et al., 1999). ATP is sufficient to stimulate Ca 2+ waves in networks of astrocytes. Moreover, the propagation of Ca 2+ waves is blocked by antagonists of P2Y purinoceptors (P2YRs), indicating that these receptors are required for Ca 2+ wave propagation (Cotrina et al., 1998; Guthrie et al., 1999; Fam et al., 2000). P2YRs comprise a multigene family of G-protein-coupled receptors activated by ATP and other nucleotides, in which seven bona fide subtypes (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, and P2Y13) have been identified (Ralevic and Burnstock, 1998; Nicholas, 2001). Using subtype-selective pharmacological tools, it has been demonstrated that transmission of astrocyte Ca 2+ waves is mediated by P2Y1R (Fam et al., 2000) and P2Y2R (Gallagher and Salter, 2000) subtypes of P2YR. Thus, the evidence indicates that ATP is the principal chemical “gliotransmitter” in the CNS, and that the main subtypes of gliotransmitter receptor are P2Y1R and P2Y2R.
P2YRs on astrocytes may be repeatedly stimulated in vivo by ATP released by neurons during synaptic activity (Fields and Stevens, 2000) or by neighboring astrocytes during Ca 2+ waves (Araque et al., 2001; Haydon, 2001). Thus, the frequency at which P2YRs on individual astrocytes are stimulated will be dependent on temporal variations in the activity within the neuronal and astrocyte networks. In the present study, we explored the frequency dependence of Ca 2+ responses initiated by stimulating P2Y1Rs or P2Y2Rs. We discovered that Ca 2+ responses mediated by these two receptors show use-dependent plasticity. Both receptors exhibit slow depression that, although use dependent, is frequency independent. Stimulating P2Y1Rs, but not P2Y2Rs, at high frequency recruits an additional negative feedback mechanism that causes oscillations of P2Y1R-mediated Ca 2+ signals. This negative feedback was found to be mediated by suppression of the Ca 2+ responses by protein kinase C (PKC), an effect that depends on a single threonine residue in the C terminus of P2Y1R.
Materials and Methods
Primary culture of dorsal spinal cord. Primary dissociated cultures of dorsal spinal cord were prepared from embryonic day 17 (E17)–E18 embryonic rats and maintained as described in detail previously (Salter and Hicks, 1994). Briefly, timed-pregnant Wistar rats were anesthetized, and embryos were removed surgically. The spinal cord was extracted from each embryo, and the dura was removed. Dorsal horn tissue was isolated according to the open-book technique (Peterson and Crain, 1982). The dorsal half of the cord was then incubated in 0.25% trypsin for 30 min, rinsed and mechanically dissociated by trituration, and then plated onto collagen-coated plastic disks affixed over holes in 35 mm culture dishes. Cells were maintained in DMEM (Invitrogen, Gaithersburg, MD) supplemented with 10% fetal bovine serum (FBS) and 10% horse serum for 1 week. After 1 week, the media was switched to DMEM plus 10% horse serum. Cells were used at 12–15 d in culture.
Generation and maintenance of 1321N1 human astrocytoma cells expressing wild-type and mutant P2Y1Rs. Rat P2Y1 purinoceptor (rP2Y1R) cDNA (GenBank accession number U22830) was excised from a P2Y1R-pGem 11-Z plasmid (from Dr. G. I. Bell, Howard Hughes Medical Institute, Chicago, IL) and subcloned into the BamHI–EcoRI restriction sites of the mammalian expression vector pcDNA3 (Invitrogen). rP2Y1R-pcDNA3 were grown in bacteria and purified. 1321N1 human astrocytoma cells were obtained from the European Collection of Cell Cultures. The upstream primer 5′-GAATTCATGTACCATACGACGTACCAGA-CTAC GCAATG ACCGAG GTG CCT-3′ was used to generate all wild-type and mutant rP2Y1 constructs. The downstream primers used to generate the stop335, stop338, stop342, and stop342/339A mutant constructs were 5′-GGATCCTCACAGTCTCCTTCTGAATGTATC-3′, 5′-GGATCCTCAGGCTCGGGACAGTCTCCTTCT-3′, 5′-GGATCC-TCAAGCTTTCCT-GGTGGCTCGGGA-3′, and 5′-GGATCCTCAAGCT-TTCCTTGCGGCTCGGGACAGTCT-3′, respectively. rP2Y1R–pcDNA3 was used in a PCR, along with the upstream primer and respective downstream primers. The full-length T339A mutant was generated using the QuickChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA) using the primers 5′-TCC CGA GCC GCC AGG AAA ACT-3′ and 5′-AGC TTT CCT GGC GGC TCG GGA-3′. PCR products were separated on a 1% agarose gel, purified, subcloned into the pCR-Blunt II-TOPO expression vector (Invitrogen), and grown and purified from bacteria. Purified constructs were then subcloned into pcDNA3. Cell lines of parental 1321N1 cells were maintained in DMEM (Invitrogen) supplemented with 10% FBS and 10% horse serum and split 1/12 every 3–4 d. When required for transfection, cells were split and plated onto culture dishes and were used within 2 d. Wild-type and mutant rP2Y1R-pcDNA3 was transiently transfected into 1321N1 cells using the calcium phosphate method. Transfected cells were used 2 d later for experiments.
Single-cell [Ca2+]imeasurements and Ca2+imaging. The Ca 2+-sensitive fluorophore fura-2 (Molecular Probes, Eugene, OR) was used to measure [Ca 2+]i photometrically in single astrocytes. Single astrocytes were identified using criteria described previously (Salter and Hicks, 1994). Just before recording, cells were incubated at room temperature for 90 min in extracellular recording solution composed of (in mm): 140 NaCl, 5.4 KCl, 1.3 CaCl2, 25 HEPES, 33 glucose, and 0.5 μm tetrodotoxin (TTX), pH 7.35 and 315–320 mOsm, that had been supplemented with bovine serum albumin (BSA, 0.5%) and fura-2 AM (2 μm). Subsequently, the culture dish was thoroughly rinsed with extracellular solution lacking fura-2 AM and BSA and mounted on an inverted microscope (Diaphot-TMD; Nikon, Mississauga, Canada). To avoid neural–astrocyte signaling, the areas chosen were free of neurons. Cultures were viewed using a 40× epifluorescence Fluor objective lens. Recordings were made at room temperature (20–22°C).
Single-cell [Ca 2+]i measurement recording was done by means of single-photon counting from individual astrocytes (Salter and Hicks, 1994). In brief, light from a compact xenon arc lamp (75 W) was alternately guided through either a 340DF10 nm or a 380DF13 nm wave-length bandpass excitation filter (Omega Optical, Brattleboro, VT) by means of a mirrored chopper rotating at 50 or 60 Hz to the input of an inverted microscope (Diaphot-TMD; Nikon). Emitted light was sent to the side camera port of the microscope, where it entered a dual optical pass adapter (Nikon). Here, the light was directed through a 510DF20 nm bandpass filter by a DM 580 dichroic mirror, after which the light passed through a manually adjustable aperture and was detected by a photomultiplier tube in single-photon counting mode (Photon Technologies, London, Ontario, Canada). The output of the photomultiplier was sampled at a rate of 10 or 20 Hz by an IBM-compatible computer with hardware and software from Photon Technologies. All analysis was performed off-line. Using standardized Ca 2+ solutions ranging from 0 Ca 2+ to 40 μm, Rmin values were 0.3–0.5 and Rmax values were 2.0–3.0.
Drug application. The P2Y agonists 2-methylthio-ADP (2-MeSADP) and UTP were dissolved in extracellular solution. Agonists were applied to individual astrocytes by pressure ejection from a pipette located ∼20–40 μm from the cell being stimulated. We chose the concentrations of 2-MeSADP and UTP to evoke Ca 2+ responses of approximately equal amplitudes. All other drugs were dissolved in extracellular solution and applied directly to the bath.
Source of reagents. 2-MeSADP and UTP were obtained from Research Biochemicals (Natick, MA). Gö6850 and bisindolylmaleimide V (Bis. V) were supplied by Calbiochem (La Jolla, CA). All other reagents, except where indicated above, were from Sigma-Aldrich (Oakville, Ontario, Canada).
Results
We recorded fluorescence emission ratios using single-photon counting from individual astrocytes in primary cultures loaded with the ratiometric Ca 2+-sensitive fluorophore fura-2 (Salter and Hicks, 1994; Fam et al., 2000). To selectively activate P2Y1Rs or P2Y2Rs, we made brief applications (5–10 sec) of the P2YR subtype-selective agonists 2-MeSADP or UTP, respectively (Ho et al., 1995; Fam et al., 2000) from a micropipette positioned with the tip ∼10 μm from the cell under study. Each application evoked a transient Ca 2+ response in which [Ca 2+]i typically peaked within 5 sec after agonist application and returned to baseline within 60–90 sec. Ca 2+ responses evoked by 2-MeSADP or UTP are mediated by release of Ca 2+ from a common IP3-sensitive intracellular store (Idestrup and Salter, 1998).
Activity-dependent depression of P2Y1R or P2Y2R Ca 2+ responses by low-frequency receptor stimulation
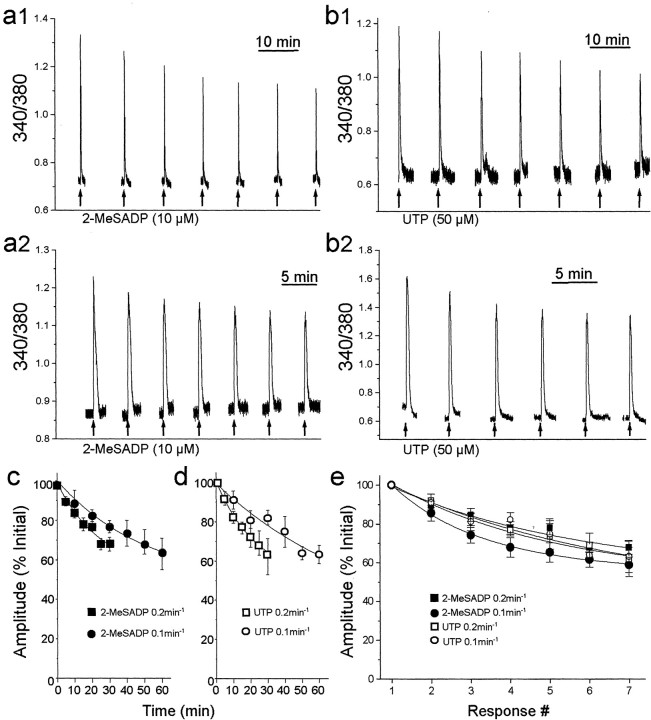
We began to study the frequency dependence of P2YR-evoked Ca 2+ responses by applying 2-MeSADP or UTP at low frequencies (0.1 or 0.2 min-1). We found that with a stimulation frequency of 0.1 min-1, the peak amplitude of Ca 2+ responses evoked by applying 2-MeSADP progressively declined to a stable level of 63 ± 5% (mean ± SEM) of the initial response (n = 9 cells) (Fig. 1a1). When we applied 2-MeSADP at 0.2 min-1, the peak of the Ca 2+ responses decreased to 68 ± 4% of the initial response (n = 10 cells) (Fig 1a2). Similarly, applying UTP at frequencies of 0.1 or 0.2 min-1 caused the peak amplitude of Ca 2+ responses to decline gradually to a stable level (Fig. 1b): 0.1 min-1, 63 ± 5% (n = 7 cells); 0.2 min-1, 62 ± 4% (n = 9 cells). We observed that the rate of decline of the Ca 2+ responses when applying either 2-MeSADP (Fig. 1c) or UTP (Fig. 1d) was approximately twice as fast with 0.2 min-1 stimulation compared with 0.1 min-1 stimulation. When analyzed in terms of number of responses, the rate of the decline of the peak Ca 2+ response with 0.1 min-1 stimulation was not different from that with 0.2 min-1 stimulation for Ca 2+ responses to 2-MeSADP or UTP (Fig. 1e). Thus, the decline in the Ca 2+ responses mediated by both receptor subtypes was not related to time but was related to the number of stimuli, which is a hallmark of a use-dependent process. Together, these results seem to indicate that when stimulated at low frequencies, both P2Y1Rs and P2Y2Rs in the astrocytes engage an activation-dependent mechanism that depresses the Ca 2+ responses.
Figure 1.
Activation-dependent depression of P2Y1R or P2Y2R Ca 2+ responses by low-frequency receptor stimulation. a, Traces showing records of fura-2 emission ratios from two different astrocytes onto which 2-MeSADP (10 μm, 10 sec, arrows) was applied every 10 (a1) or 5 (a2) min. The gaps in the recordings in this figure, and in all others, indicate periods when fluorescence signals were not sampled to minimize photobleaching of fura-2. b, In two other astrocytes, UTP(50 μm, 10sec, arrows) was applied every 10 (b1) or 5(b2) min. The mean ± SEM amplitudes of responses evoked by applying 2-MeSADP (c) or UTP(d) at rates of 0.2 min-1 (squares) and 0.1 min-1 (circles) are plotted as a function of time. Response amplitudes are expressed as a percentage of the amplitude of the first response. The lines are the best fit to a single exponential decay for each data set. Separate samples of cells were used for the two stimulus frequencies. e, Mean amplitudes of responses evoked by applications of 2-MeSADP (black, 10 μm) or UTP (white, 50 μm) at rates of 0.2 min-1 (squares) or 0.1 min-1 (circles) plotted as a function of response number.
Oscillations of Ca 2+ responses of P2Y1Rs, but not of P2Y2Rs, with high-frequency receptor stimulation
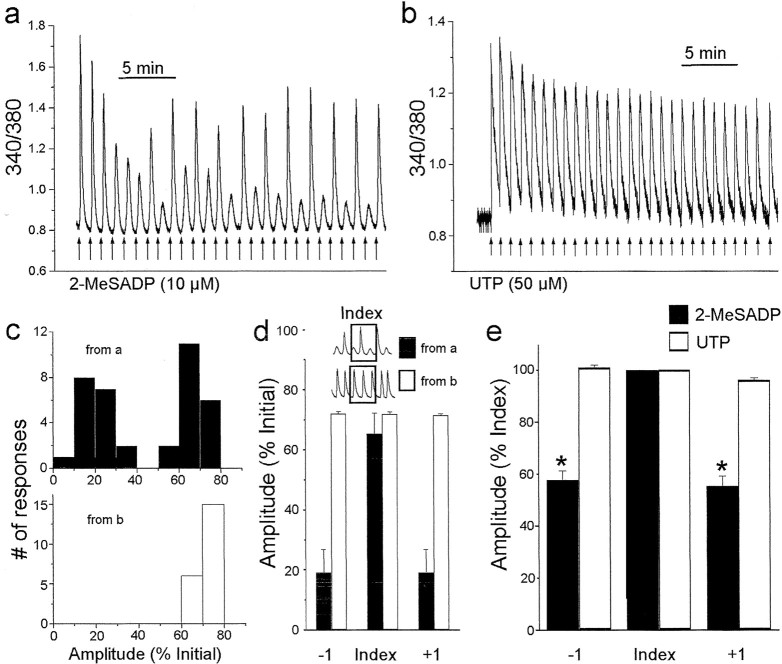
To investigate Ca 2+ responses when P2Y1Rs and P2Y2Rs were activated at a higher frequency, we applied 2-MeSADP or UTP once per minute, a frequency that produced minimal temporal overlap of successive responses (Fig. 2). With 2-MeSADP applications at this frequency, the Ca 2+ responses declined for several stimuli but then, strikingly, the responses began to oscillate in amplitude and the oscillation continued for the remainder of the stimulation train (Fig. 2a). At the end of the stimulation period, [Ca 2+]i returned to the baseline level and there were no spontaneous fluctuations of [Ca 2+]i (data not shown). In contrast to 2-MeSADP, applying UTP once per minute produced a progressive decline in the Ca 2+ responses (Fig. 2b). The UTP-evoked Ca 2+ responses did not oscillate; rather, the responses stabilized at 71 ± 3% of the initial level.
Figure 2.
Oscillations of Ca 2+ responses from P2Y1R but not P2Y2R activation with high-frequency stimulation. a, Continuous record of 340/380 emission ratio from an astrocyte onto which 2-MeSADP (10 μm, 5 sec, arrows) was applied at a rate of 1 min -1 (representative of the 9 cells studied). b, The 340/380 emission ratio from an astrocyte stimulated with UTP (50 μm, 5 sec, arrows) at a rate of 1 min -1 (representative of the 11 cells tested). In a and b, responses to the first 27 stimuli are shown. c, Histograms showing the distribution of Ca 2+ response amplitudes evoked in the experiments illustrated in a (black bars) or b (white bars). Only responses evoked after the initial decline were included, as described in Results. Response amplitudes are expressed as a percentage of the amplitude of the first response. d, Histogram showing the mean ± SEM amplitude of index, preceding (-1) and succeeding (+1) responses for the cell shown in a (black bars; n = 19 index responses) or b (white bars; n = 21 index responses). Amplitudes are expressed as a percentage of the amplitude of the first response. e, Average amplitudes of -1, index, and +1 responses from all astrocytes stimulated with either 2-MeSADP (10 μm; black bars; n = 98 index responses in 9 cells) or UTP (50 μm; white bars; n = 120 index responses in 11 cells) at a rate of 1 min -1. *p < 0.05; Student's t test.
To quantify the oscillations in responses to 2-MeSADP, we used a correlation method (Fig. 2c). For the cell tested with 2-MeSADP, Ca 2+ response amplitudes during the oscillatory part of the response train showed a bi-modal distribution with clearly separable peaks (Fig. 2c). We assigned each response in the larger amplitude group as an “index” response, and measured the amplitude of each index response, as well as those of the preceding (-1) and succeeding (+1) responses (Fig. 2d). Index response amplitude was 64 ± 7% of the initial response, and the amplitudes of the -1 and +1 responses were 19 ± 8 and 18 ± 8% of the initial response, respectively (n = 20 index responses). For the cell tested with UTP, the response amplitudes were distributed unimodally (Fig. 2c) during the part of the response train that corresponded to the oscillatory part for the cell tested with 2-MeSADP. Therefore, we considered each response in this group to be an index response. The amplitudes of the index, -1, and +1 responses were 72.1 ± 1, 72.8 ± 3, and 71.7 ± 3% of the initial response, respectively (n = 21 index responses).
We applied this analytic approach to all cells tested with 2-MeSADP or UTP at a frequency of 1 min-1 (Fig. 2e). For 2-MeSADP (n = 9 cells), the -1 and +1 responses were significantly smaller than the index responses but were not different from each other. In contrast, for UTP (n = 11 cells), there were no differences in the amplitudes of the -1, index, or +1 responses (Fig. 2e). To determine whether the difference between P2Y1R-mediated and P2Y2-mediated responses was related to potential differences in degree of receptor activation, we decreased the concentrations of 2-MeSADP and UTP to below the respective EC50 concentrations and found that for 2-MeSADP (20 nm, 1 min-1), the -1 and +1 responses were significantly smaller than index responses (n = 6 cells; see below), whereas for UTP (50 nm, 1 min-1), there were no differences in the amplitudes of the -1, index, or +1 responses (n = 7 cells; data not shown). Together, these results indicate that with high-frequency activation, Ca 2+ responses mediated by P2Y1Rs, but not those mediated by P2Y2Rs, show rapid oscillations. These oscillations are in addition to the slow depression of Ca 2+ responses observed with activating either receptor subtype. Because P2Y1Rs and P2Y2Rs access a common intracellular pool of Ca 2+ in astrocytes (Idestrup and Salter, 1998), there is no difference in the releasable store of Ca 2+ that might account for the selective oscillations of P2Y1R-mediated Ca 2+ responses. Rather, the present results imply that it is the signaling itself that is differentially regulated, and that high-frequency activation of P2Y1Rs engages a negative-feedback mechanism that is transient and recurrent and not engaged by P2Y2Rs.
Depression of P2Y1R-mediated Ca 2+ responses by PKC is required for oscillations
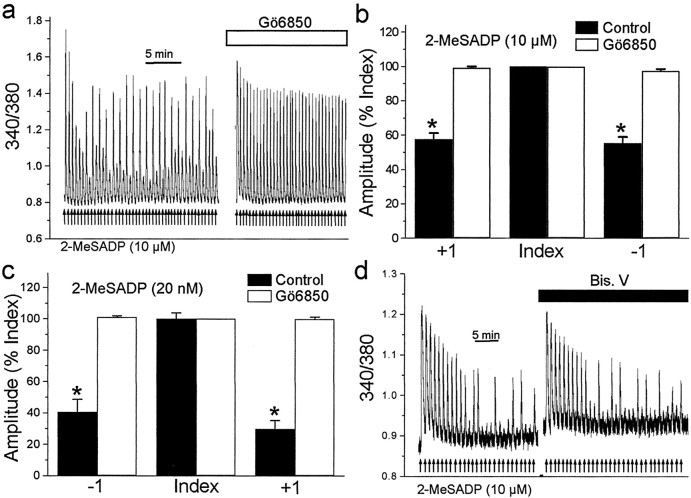
We wondered whether the negative feedback on P2Y1R Ca 2+ responses that causes the oscillations might be mediated by PKC, which is activated in parallel with IP3 generation (Berridge, 1993). In this case, direct activation of PKC should depress 2-MeSADP-evoked Ca 2+ responses. We therefore investigated the effect of the PKC activator, phorbol 12, 13-myristate acetate (PMA) (Macfarlane and Manzel, 1994), which was found to nearly abolish Ca 2+ responses evoked by 2-MeSADP (Fig. 3). The effect of PMA was prevented by the PKC inhibitor Gö6850 (Toullec et al., 1991), but was not affected by Bis. V, an analog to Gö6850 that does not inhibit PKC (Davis et al., 1992). Thus, activation of PKC is sufficient to suppress P2Y1R-mediated Ca 2+ responses.
Figure 3.
Activating protein kinase C suppresses P2Y1R-mediated Ca 2+ responses. 2-MeSADP (arrows) was applied as indicated before and during bath administration of the following: PMA alone (1 μm; black bar) (a), Gö6850 (1 μm; white bar) and then PMA plus Gö6850 (b), or Bis. V (1 μm; white bar) and then Bis. V plus PMA (c). In all cases in this and all subsequent figures, administration of PMA, Gö6850, or Bis. V began 20 min before the subsequent application of agonist. The histogram in d shows mean amplitude of responses evoked by 2-MeSADP in the presence of PMA (n = 8 cells), Gö6850 (n = 10 cells), PMA plus Gö6850 (n = 10 cells), Bis. V (n = 3 cells), or PMA plus Bis. V (n = 3 cells) expressed as a percentage of the control response immediately before the treatment. *p < 0.05; Student's t test.
To determine whether PKC activity is required for the oscillations of P2Y1R-evoked Ca 2+ responses, we examined the effect of Gö6850, which was administered by bath before and during a train of 2-MeSADP applications. We found that Ca 2+ responses evoked by 2-MeSADP and applied at a rate of 1 min-1 declined progressively to a stable level, but the responses did not oscillate during Gö6850 administration (Fig. 4a). This effect of Gö6850 to prevent the oscillation was observed in all cells tested (n = 11). On average, the amplitudes of the -1 and +1 responses during the train were not different from that of the index responses during administration of Gö6850 (Fig. 4b,c). In contrast, bath application of Bis. V did not prevent either the progressive decline or the subsequent oscillations in the amplitude of Ca 2+ responses evoked by 1 min-1 applications of 2-MeSADP in any of the cells tested (n = 3) (Fig. 4d).
Figure 4.
Oscillations of P2Y1R-mediated Ca 2+ responses are prevented by inhibiting protein kinase C. a, Record of fura-2 emission ratios from a single cell. 2-MeSADP (10 μm, 5 sec, arrows) was applied at a rate of 1 min -1 before or during bath application of the Gö6850 (1 μm; white bar). The application of Gö6850 began 20 min before the start of the train of 2-MeSADP shown on the right. b, Histogram showing the average amplitude of -1, index, and +1 responses for 2-MeSADP-evoked responses without (black bars; n = 9 cells) or with bath-applied Gö6850 (white bars; n = 5 cells).*p < 0.01; Student's t test. c, Histogram showing the average amplitude of -1, index, and +1 responses for P2Y1R-mediated responses evoked at a rate of 1 min -1 by submaximal concentrations of 2-MeSADP (20 nm) without (black bars; n = 25 index responses from 6 cells) or with bath-applied Gö6850 (white bars; n = 57 index responses from 4 cells).*p < 0.01; Student's t test. d, 2-MeSADP applications (10 μm, 5 sec, 1 min -1, arrows) made before or during bath administration of Bis. V (1 μm) are shown for another astrocyte (representative of the 3 cells tested).
Together, these results indicate that PKC is required for the oscillations in P2Y1R Ca 2+ responses. The amplitude of the first response in the train of 2-MeSADP application was unaffected by Gö6850, as were Ca 2+ responses at lower stimulation frequencies (data not shown), and therefore, P2Y1R-mediated Ca 2+ responses are not tonically inhibited by basal PKC activity. Thus, we conclude that high-frequency activation of P2Y1Rs stimulates PKC, which then feeds back to depress the Ca 2+ responses.
The progressive decline in P2Y1R responses was not prevented by Gö6850, and moreover, the stable level reached in Gö6850 matched the amplitude of the larger group responses during the oscillations before Gö6850 (Fig. 4a). In addition, the responses to 2-MeSADP at 1 min-1 during administration of Gö6850 declined e-fold in 3.6 ± 0.8 responses (n = 5 cells), which was similar to the decline with stimulation at 0.1 and 0.2 min-1 (compare with Fig. 1e). This finding implies that 1 min-1 activation of P2Y1Rs engages the use-dependent depression mechanism observed at the lower frequencies, and this depression is independent of PKC.
PKC-dependent negative feedback is not engaged by P2Y2R activation
That P2Y2R-mediated Ca 2+ responses do not show oscillations when activated at 1 min-1 does not exclude the possibility that they might be susceptible to PKC-mediated negative feedback. We therefore examined the effect of PKC activators and inhibitors on Ca 2+ responses evoked by UTP. We found that UTP-evoked Ca 2+ responses were greatly depressed by bath-applied PMA (Fig. 5a), and that the effect of PMA was prevented by Gö6850 but not Bis. V (Fig. 5b). Therefore, activation of PKC is sufficient to suppress Ca 2+ responses mediated by P2Y2Rs. However, we found that the decline of Ca 2+ responses evoked by applying UTP at a rate of 1 min-1 persisted when Gö6850 was administered (Fig. 5c), and that there was no statistically significant effect of Gö6850 on the rate or extent of the decline (Fig. 5d). To determine whether P2Y2-mediated responses might be depressed by PKC mobilized by high-frequency activation of P2Y1Rs, we examined two UTP-evoked responses spaced ∼10 min apart with or without intervening stimulation of P2Y1Rs at 1 min-1. The second UTP application was made after a large response to 2-MeSADP. We found that with intervening P2Y1R activation, the second UTP-evoked response was 85 ± 18% of the first response (n = 10 cells), and with no intervening P2Y1R activation, the second UTP-evoked response was 91 ± 9% of the first (n = 11 cells; p > 0.3). Thus, although both P2Y1R- and P2Y2R-mediated Ca 2+ responses can be downregulated by PKC, only P2Y1R responses show PKC-mediated negative feedback with high-frequency receptor activation.
Figure 5.
PKC-dependent negative feedback is not engaged by P2Y2R activation. a, UTP (50 μm; 10 sec) was applied as indicated (arrows) before and during application of PMA (1 μm; black bar). b, The mean amplitudes of UTP-evoked responses in the presence of PMA (n = 15 cells), PMA plus Gö6850 (1 μm; n = 13 cells), or PMA plus Bis. V (1 μm; n = 6 cells) are plotted as a percentage of control responses before the treatments. *p < 0.01; Student's t test. c, Record of fura-2 emission ratio from an astrocyte onto which UTP (50 μm, 5 sec) was applied at a rate of 1 min -1 in the presence of Gö6850. d, Histogram showing mean amplitudes of responses evoked by applications of UTP at a rate of 1 min -1 without (black bars; n = 11 cells) or with bath-applied Gö6850 (1 μm; white bars; n = 7 cells). Response amplitudes are expressed as a percentage of the amplitude of the first response. e, Record of fura-2 emission ratios from a single cell. 2-MeSADP (10 μm, 5 sec) was applied at a rate of 1 min -1 over 10 min, and then UTP (50 μm, 5 sec) was applied at a similar rate. Gö6850 (1 μm, 5 sec) was bath-applied 20 min before the first 2-MeSADP application (representative of 7 cells). f, On the left, the histogram shows mean amplitudes of the seventh response evoked by applications of 2-MeSADP (10 μm, 5 sec) at a rate of 1 min -1 without (black bars; n = 9 cells) or with preapplication of UTP (white bars; n = 6 cells). The right part of the graph shows the mean amplitudes of the seventh response evoked by applications of UTP (50 μm, 5 sec) at a rate of 1 min -1 without (black bars; n = 7 cells) or with preapplication of 2-MeSADP (white bars; n = 7 cells). Response amplitudes are expressed as a percentage of the amplitude of the first response.
PKC-independent negative feedback does not cross between P2YR subtypes
High-frequency activation of either P2Y1Rs or P2Y2Rs, however, engages a use-dependent depression mechanism observed at the lower frequencies and that is independent of PKC. To determine whether the PKC-independent negative feedback crosses between P2Y1Rs and P2Y2Rs, we repeatedly stimulated one receptor subtype at high frequency to produce the negative feedback, and then probed the responses mediated by the other receptor subtype (Fig. 5e,f). These experiments were performed in the presence of bath-applied Gö6850 to isolate the PKC-independent negative feedback. If the PKC-independent feedback from stimulating the first receptor subtype had crossed to the second receptor subtype, it would be predicted that this would occlude the PKC-independent feedback of the second subtype, and thus, the responses to stimulating that subtype would be stable. However, in experiments in which we first repeatedly applied 2-MeSADP until response amplitude stabilized and then applied UTP (Fig. 5e), we found that the UTP-evoked responses progressively declined to a stable level, and that this level was not statistically significantly different from that in experiments in which P2Y2Rs were stimulated without previous P2Y1Rs stimulation (Student's t test; p > 0.1) (Fig. 5f). Similarly, there was no difference in the level to which P2Y1R-mediated responses declined regardless of whether P2Y2Rs were or were not prestimulated (Student's t test; p > 0.1) (Fig. 5f). We therefore conclude that PKC-independent depression of one P2YR subtype does not occlude subsequent PKC-independent depression of the other P2YR subtype. This implies that PKC-independent negative feedback does not cross between the two subtypes of P2YRs.
Thr339 in the C terminus of P2Y1R is necessary for downregulation by PKC
The deduced amino acid sequence of P2Y1R contains multiple serine and threonine residues among its four intracellular domains (Tokuyama et al., 1995). Four of these residues (Thr330, Ser336, Thr339, and Ser343) are clustered in the C terminus of P2Y1R, and are, in consensus, phosphorylation sequences for PKC (Yaffe et al., 2001). To determine whether any of these Ser or Thr residues are required for PKC-mediated depression of P2Y1R-evoked Ca 2+ responses, we expressed wild-type and mutant rat P2Y1Rs in 1321N1 cells (Fig. 6). The parent 1321N1 cells do not endogenously express any type of P2 purinoceptor and are, therefore, ideal for studying recombinant P2 receptors (Lazarowski et al., 1997). In 1321N1 cells transfected with full-length, wild-type P2Y1R, applying 2-MeSADP evoked transient Ca 2+ responses that were reversibly blocked by the selective P2Y1R antagonist adenosine-3′-phosphate-5′-phosphosulfate (A3P5PS) (Boyer et al., 1996) and nearly abolished by bath-applying PMA (Fig. 6a). Thus, Ca 2+ responses of recombinant P2Y1R were pharmacologically similar to responses of astrocytes to 2-MeSADP and, like those responses, were suppressed by PKC.
Figure 6.
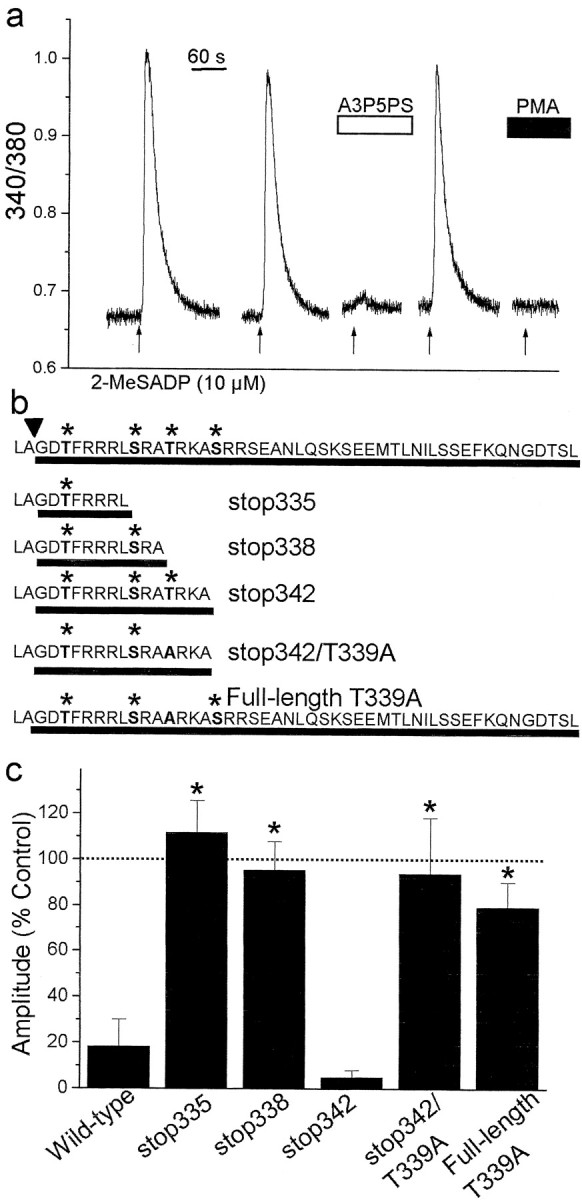
Thr 339 in the C terminus of P2Y1R is necessary for suppression of Ca 2+ responses by PKC. a, Record of the fura-2 emission ratio from a 1321N1 cell expressing full-length rP2Y1R; 2-MeSADP (10 μm, 5 sec, arrows) was applied as indicated. The P2Y1R-selective antagonist A3P5PS (100 μm) was bath-applied as indicated by the white bar. PMA (1 μm) was applied before and during the last application of 2-MeSADP. b, Primary amino acid sequence of the C terminus of the P2Y1R constructs used. The full-length, wild-type rP2Y1R sequence is shown at the top. The arrowhead indicates the end of the predicted Tm7, and the lines show the intracellular region of the C terminus for each construct. P2Y1Rs were truncated after Leu335 (stop335), Ala338 (stop338), or Ala342 (stop342). In stop342/T339A, Thr 339 was replaced by Ala. The asterisks indicate Ser or Thr residues in consensus PKC phosphorylation sequences. Also shown is the sequence of a full-length P2Y1R in which Thr339 was replaced by Ala (full-length T339A). c, The histogram shows mean amplitude of 2-MeSADP-evoked responses in the presence of PMA (1 μm) in 1321N1 cells expressing wild-type P2Y1R(n = 11 cells), stop335 (n = 5 cells), stop338 (n = 11 cells), stop342 (n = 4 cells), stop342/T339A (n = 6 cells), or full-length T339A (n = 9 cells). Response amplitudes evoked in the presence of PMA are expressed as a percentage of the amplitude of the response before applying PMA. *p < 0.01; Student's t test. Dotted line indicates 100% level.
To investigate the role of residues in the C terminus of P2Y1R in the suppression of Ca 2+ responses by PKC, we generated a series of mutant receptors lacking one or more of the consensus PKC phosphorylation sequences (Fig. 6b). In cells expressing all of the mutant receptors truncated at or beyond Leu335, we found that applying 2-MeSADP evoked transient Ca 2+ responses that were reversibly blocked by A3P5PS. When we eliminated the entire sequence just after the end of the predicted Tm7, no functional receptors were expressed, and therefore, we restricted our studies to longer P2Y1R constructs. In cells expressing P2Y1Rs truncated at amino acid 335 (stop335), which included only the first of the consensus PKC sites, functional receptors were produced, but the Ca 2+ responses were not suppressed by PMA (Fig. 6c). Similarly, PMA did not affect Ca 2+ responses mediated by P2Y1Rs truncated at amino acid 338 (stop338). However, with P2Y1Rs in which the C terminus beyond amino acid 342 (stop342) was deleted, PMA dramatically depressed the Ca 2+ responses to 2-MeSADP. Thus, P2Y1Rstop342 contains all of the sequence information for suppression of Ca 2+ responses by PKC. The sequence added in P2Y1Rstop342 includes Thr339, a consensus phosphorylation sequence for PKC. We found that mutating Thr339 to Ala in either truncated (stop342/T339A) or full-length (full-length T339A) P2Y1R eliminated the depression of 2-MeSADP-evoked Ca 2+ responses by PMA (Fig. 6c). Together, these results suggest that Thr339 is required for PKC-dependent downregulation of Ca 2+ responses mediated by P2Y1Rs.
Discussion
In the present study, we have identified two forms of activation-dependent negative feedback that differentially regulate P2YR-evoked Ca 2+ signaling in astrocytes: a slow depression of Ca 2+ responses and a rapid oscillation of the responses. The slow depression is shared by Ca 2+ responses mediated by P2Y1Rs and by P2Y2Rs, occurs over the range of stimulation frequencies studied, and is independent of PKC. However, the rapid oscillation is selectively expressed with P2Y1R-mediated Ca 2+ responses and is only observed with high-frequency stimulation. The rapid oscillation is dependent on PKC, which causes negative feedback inhibition that requires Thr339 in the C terminus of P2Y1R.
The slow depression of Ca 2+ responses mediated by either P2Y1Rs or P2Y2Rs was use-dependent and frequency-independent and was not affected by the PKC inhibitor Gö6850. We interpret these findings as indicating that activating P2Y1Rs or P2Y2Rs engages a PKC-independent negative-feedback mechanism. A common negative-feedback mechanism for G-protein-coupled receptors is through phosphorylation of activated receptors by G-protein receptor kinases (GRKs), leading to recruitment of the adapter protein β-arrestin, which acts to uncouple the receptor from its cognate G-protein and initiate clathrin-dependent internalization (Ferguson, 2001). Prolonged application of UTP has been shown to cause internalization of recombinant epitope-tagged P2Y2Rs (Sromek and Harden, 1998), indicating that such a PKC-independent feedback mechanism may act on P2YRs. Whether the slow depression of P2Y1Rs or P2Y2Rs reported here may be because of negative feedback by GRK-β-arrestin signaling or by non-β-arrestin, negative regulatory mechanisms described for some G-protein-coupled receptors (Smyth et al., 2000; Olivares-Reyes et al., 2001) remain to be established.
The rapid oscillations were an emergent characteristic that appeared with high-frequency activation of P2Y1Rs. Although the oscillations were prevented by inhibiting PKC, there was no basal suppression of P2Y1R-mediated Ca 2+ responses by this kinase. Therefore, the suppression of the oscillations by inhibiting PKC and the observation that the oscillations develop only after a number of stimuli imply that PKC-mediated negative feedback becomes engaged gradually during the high-frequency train of P2Y1R responses. Once engaged, it is possible that the oscillations in the amplitude of the Ca 2+ responses are caused by oscillations of PKC activation (Dale et al., 2001). Alternatively, PKC activity might be relatively constant, but the activity of the opposing phosphatase might oscillate in response to repeated activation of P2Y1Rs. Either of these cyclical mechanisms would be suppressed by inhibiting PKC. In contrast to P2Y1Rs, high-frequency activation of P2Y2Rs does not engage PKC-mediated negative feedback, although P2Y2R-mediated Ca 2+ responses are inhibited by activating PKC. Stimulation of P2Y2Rs leads to activation of the phospholipase Cβ-IP3 cascade, as does stimulating P2Y1Rs (Idestrup and Salter, 1998), which implies that PKC is activated by P2Y2R stimulation. Therefore, it may be that activated P2Y2Rs are protected from feedback inhibition by PKC, whereas P2Y1Rs are not protected.
The differential frequency-dependent regulation of the two main gliotransmitter receptor subtypes has important implications for Ca 2+-dependent downstream signal transduction within individual astrocytes and for the propagation of Ca 2+ waves within networks of astrocytes. The different frequency-response characteristics of the Ca 2+ responses during repetitive activity suggest that the two receptors may have distinct roles in downstream signal transduction. Such distinct signaling roles may explain why astrocytes express two subtypes of receptor that are so closely related: the receptors are both activated equipotently by the same endogenous ligand, ATP (Ralevic and Burnstock, 1998), and both participate in the propagation of intercellular Ca 2+ waves (Fam et al., 2000). Because P2Y1Rs are expressed in nearly all astrocytes, whereas P2Y2Rs are only expressed in a subpopulation of astrocytes (Ho et al., 1995), the differential feedback regulation is predicted to introduce frequency-dependent gating of the propagation of Ca 2+ waves. That is, at low frequencies, Ca 2+ waves would be transmitted with equal fidelity to cells expressing P2Y1Rs alone or P2Y1Rs plus P2Y2Rs. But at high frequency, the fidelity of wave transmission would be greater to astrocytes expressing P2Y2Rs and there would be decreased transmission through astrocytes expressing P2Y1Rs alone.
In the CNS, ATP is released by activity of neurons as well as astrocytes (Burnstock, 1997; Fields and Stevens, 2000; Jo and Role, 2002). Individual astrocytes thereby use extracellular ATP in decoding and responding to the activity of neuronal and astrocyte networks of which they are a part, with the level of extracellular ATP being decoded through P2Y1Rs and P2Y2Rs. Our present results indicate that decoding the temporal profile of extracellular ATP into Ca 2+ signals in astrocytes depends on the type of P2YR that is activated and the frequency of activation. Low-frequency ATP signals will be similarly decoded by activating either P2Y1Rs or P2Y2Rs, whereas high-frequency ATP signals will be decoded by P2Y1Rs into Ca 2+ responses that are effectively half of those decoded by P2Y2Rs. The rise in Ca 2+ in astrocytes and other types of nonexcitable cells is not an end in and of itself, but is an initiator of pleotropic downstream signaling cascades that are highly sensitive to the frequency as well as the amplitude and duration of the Ca 2+ signals (Dolmetsch et al., 1997, 1998; Li et al., 1998). Therefore, the profile of downstream Ca 2+-dependent effectors engaged by a given pattern of ATP signals will depend on whether this pattern is transduced through P2Y1Rs or P2Y2Rs. Thus, astrocytes expressing P2Y1Rs alone may respond differently than those expressing P2Y1Rs and P2Y2Rs. Moreover, because the level of expression of these receptors is known to change depending on other signals received by the astrocytes (John et al., 1999; Zhu and Kimelberg, 2001), the responses of a given cell to activity in the neuronal and astrocyte networks will be conditional based on its past history.
In summary, we have described two mechanistically distinct forms of activity-dependent negative feedback of Ca 2+ responses by P2Y1Rs and P2Y2Rs in astrocytes. These receptors may mediate neuron–astrocyte and astrocyte–astrocyte communication in many regions of the CNS. These forms of cell–cell communication are emerging as potentially having critical roles in the functioning of the CNS under physiological and pathological conditions. Thus, our finding of frequency-dependent changes in ATP-mediated Ca 2+ signaling may have important implications for CNS function in health and disease.
Footnotes
This work was supported by the Canadian Institutes of Health Research (CIHR) and the Nicole Feldman Memorial Fund. C.J.G. is a clinician/scientist trainee of the Hospital for Sick Children. S.R.F. and L.V.K. are supported by CIHR. M.W.S. is a CIHR investigator. We thank Janice L. Hicks and David Wong for preparing and maintaining dorsal horn cultures.
Correspondence should be addressed to Michael W. Salter, Program in Brain and Behavior, The Hospital for Sick Children, 555 University Avenue, Toronto, Ontario, Canada, M5G 1X8. E-mail: mike.salter@utoronto.ca.
Copyright © 2003 Society for Neuroscience 0270-6474/03/234437-08$15.00/0
References
- Araque A, Parpura V, Sanzgiri RP, Haydon PG ( 1998) Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur J Neurosci 10: 2129–2142. [DOI] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG ( 1999) Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci 22: 208–215. [DOI] [PubMed] [Google Scholar]
- Araque A, Carmignoto G, Haydon PG ( 2001) Dynamic signaling between astrocytes and neurons. Annu Rev Physiol 63: 795–813. [DOI] [PubMed] [Google Scholar]
- Berridge MJ ( 1993) Inositol trisphosphate and calcium signaling. Nature 361: 315–325. [DOI] [PubMed] [Google Scholar]
- Boyer JL, Romero-Avila T, Schachter JB, Harden TK ( 1996) Identification of competitive antagonists of the P2Y1 receptor. Mol Pharmacol 50: 1323–1329. [PubMed] [Google Scholar]
- Braet K, Paemeleire K, D'Herde K, Sanderson MJ, Leybaert L ( 2001) Astrocyte-endothelial cell calcium signals conveyed by two signalling pathways. Eur J Neurosci 13: 79–91. [PubMed] [Google Scholar]
- Burnstock G ( 1997) The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology 36: 1127–1139. [DOI] [PubMed] [Google Scholar]
- Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ ( 1990) Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science 247: 470–473. [DOI] [PubMed] [Google Scholar]
- Cotrina ML, Lin JH, Nedergaard M ( 1998) Cytoskeletal assembly and ATP release regulate astrocytic calcium signaling. J Neurosci 18: 8794–8804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale LB, Babwah AV, Bhattacharya M, Kelvin DJ, Ferguson SS ( 2001) Spatial-temporal patterning of metabotropic glutamate receptor-mediated inositol 1,4,5-triphosphate, calcium, and protein kinase C oscillations: protein kinase C-dependent receptor phosphorylation is not required. J Biol Chem 276: 35900–35908. [DOI] [PubMed] [Google Scholar]
- Davis PD, Hill CH, Lawton G, Nixon JS, Wilkinson SE, Hurst SA, Keech E, Turner SE ( 1992) Inhibitors of protein kinase C: 1,2,3-bisarylmaleimides. J Med Chem 35: 177–184. [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI ( 1997) Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature 386: 855–858. [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Xu K, Lewis RS ( 1998) Calcium oscillations increase the efficiency and specificity of gene expression. Nature 392: 933–936. [DOI] [PubMed] [Google Scholar]
- Fam SR, Gallagher CJ, Salter MW ( 2000) P2Y1 purinoceptor-mediated Ca 2+ signaling and Ca 2+ wave propagation in dorsal spinal cord astrocytes. J Neurosci 20: 2800–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SS ( 2001) Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev 53: 1–24. [PubMed] [Google Scholar]
- Fields RD, Stevens B ( 2000) ATP: an extracellular signaling molecule between neurons and glia. Trends Neurosci 23: 625–633. [DOI] [PubMed] [Google Scholar]
- Finkbeiner SM ( 1993) Glial calcium. Glia 9: 83–104. [DOI] [PubMed] [Google Scholar]
- Gallagher CJ, Salter MW ( 2000) P2Y1 and P2Y2 purinoceptors support astrocyte Ca 2+ waves at different rates of propagation. Soc Neurosci Abstr 26: 707.3. [Google Scholar]
- Grafstein B, Liu S, Cotrina ML, Goldman SA, Nedergaard M ( 2000) Meningeal cells can communicate with astrocytes by calcium signaling. Ann Neurol 47: 18–25. [PubMed] [Google Scholar]
- Guthrie PB, Knappenberger J, Segal M, Bennett MV, Charles AC, Kater SB ( 1999) ATP released from astrocytes mediates glial calcium waves. J Neurosci 19: 520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG ( 2001) Glia: listening and talking to the synapse. Nat Rev Neurosci 2: 185–193. [DOI] [PubMed] [Google Scholar]
- Ho C, Hicks J, Salter MW ( 1995) A novel P2-purinoceptor expressed by a subpopulation of astrocytes from the dorsal spinal cord of the rat. Br J Pharmacol 116: 2909–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idestrup CP, Salter MW ( 1998) P2Y and P2U receptors differentially release intracellular Ca2+ via the phospholipase c/inositol 1,4,5-triphosphate pathway in astrocytes from the dorsal spinal cord. Neuroscience 86: 913–923. [DOI] [PubMed] [Google Scholar]
- Jo YH, Role LW ( 2002) Coordinate release of ATP and GABA at in vitro synapses of lateral hypothalamic neurons. J Neurosci 22: 4794–4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John GR, Scemes E, Suadicani SO, Liu JS, Charles PC, Lee SC, Spray DC, Brosnan CF ( 1999) IL-1beta differentially regulates calcium wave propagation between primary human fetal astrocytes via pathways involving P2 receptors and gap junction channels. Proc Natl Acad Sci USA 96: 11613–11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler SW, Nicholls JW, Martin AR ( 1984) From neuron to brain. Sunder-land, MA: Sinauer.
- Lazarowski ER, Homolya L, Boucher RC, Harden TK ( 1997) Direct demonstration of mechanically induced release of cellular UTP and its implication for uridine nucleotide receptor activation. J Biol Chem 272: 24348–24354. [DOI] [PubMed] [Google Scholar]
- Li W, Llopis J, Whitney M, Zlokarnik G, Tsien RY ( 1998) Cell-permeant caged InsP3 ester shows that Ca 2+ spike frequency can optimize gene expression. Nature 392: 936–941. [DOI] [PubMed] [Google Scholar]
- Macfarlane DE, Manzel L ( 1994) Activation of beta-isozyme of protein kinase C (PKC beta) is necessary and sufficient for phorbol ester-induced differentiation of HL-60 promyelocytes: studies with PKC beta-defective PET mutant. J Biol Chem 269: 4327–4331. [PubMed] [Google Scholar]
- Neary JT, Kang Y, Bu Y, Yu E, Akong K, Peters CM ( 1999) Mitogenic signaling by ATP/P2Y purinergic receptors in astrocytes: involvement of a calcium-independent protein kinase C, extracellular signal-regulated protein kinase pathway distinct from the phosphatidylinositol-specific phospholipase C/calcium pathway. J Neurosci 19: 4211–4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas RA ( 2001) Identification of the P2Y(12) receptor: a novel member of the P2Y family of receptors activated by extracellular nucleotides. Mol Pharmacol 60: 416–420. [PubMed] [Google Scholar]
- Olivares-Reyes JA, Smith RD, Hunyady L, Shah BH, Catt KJ ( 2001) Agonist-induced signaling, desensitization, and internalization of a phosphorylation-deficient AT1A angiotensin receptor. J Biol Chem 276: 37761–37768. [DOI] [PubMed] [Google Scholar]
- Parpura V, Haydon PG ( 2000) Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc Natl Acad Sci USA 97: 8629–8634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasti L, Zonta M, Pozzan T, Vicini S, Carmignoto G ( 2001) Cytosolic calcium oscillations in astrocytes may regulate exocytotic release of glutamate. J Neurosci 21: 477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson ER, Crain SM ( 1982) Nerve growth factor attenuates neurotoxic effects of taxol on spinal cord-ganglion explants from fetal mice. Science 217: 377–379. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G ( 1998) Receptors for purines and pyrimidines. Pharmacol Rev 50: 413–492. [PubMed] [Google Scholar]
- Salter MW, Hicks JL ( 1994) ATP-evoked increases in intracellular calcium in neurons and glia from the dorsal spinal cord. J Neurosci 14: 1563–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth EM, Austin SC, Reilly MP, FitzGerald GA ( 2000) Internalization and sequestration of the human prostacyclin receptor. J Biol Chem 275: 32037–32045. [DOI] [PubMed] [Google Scholar]
- Sromek SM, Harden TK ( 1998) Agonist-induced internalization of the P2Y2 receptor. Mol Pharmacol 54: 485–494. [DOI] [PubMed] [Google Scholar]
- Tokuyama Y, Hara M, Jones EM, Fan Z, Bell GI ( 1995) Cloning of rat and mouse P2Y purinoceptors. Biochem Biophys Res Commun 211: 211–218. [DOI] [PubMed] [Google Scholar]
- Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, Duhame L, Charon D, Kirilovsky J ( 1991) The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem 266: 15771–15781. [PubMed] [Google Scholar]
- Verkhratsky A, Kettenmann H ( 1996) Calcium signalling in glial cells. Trends Neurosci 19: 346–352. [DOI] [PubMed] [Google Scholar]
- Yaffe MB, Leparc GG, Lai J, Obata T, Volinia S, Cantley LC ( 2001) A motif-based profile scanning approach for genome-wide prediction of signaling pathways. Nat Biotechnol 19: 348–353. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Kimelberg HK ( 2001) Developmental expression of metabotropic P2Y(1) and P2Y(2) receptors in freshly isolated astrocytes from rat hippocampus. J Neurochem 77: 530–541. [DOI] [PubMed] [Google Scholar]