Abstract
Fatigue refers to the decrement of response seen with repeated stimulation and is a prominent attribute of nociceptors. Whether fatigue in nociceptors involves transduction, spike initiation, or conduction mechanisms is unknown. We investigated systematically how electrical, mechanical, and heat conditioning stimuli (eCS, mCS, hCS) affected the subsequent response to a test-heat stimulus applied 5 sec later to the receptive field of cutaneous nociceptors. Standard teased-fiber techniques were used to record from mechano–heat-sensitive C-fiber afferents in the anesthetized monkey. The eCS was applied to the nerve trunk, whereas the hCS and mCS were applied to the heat-test site. For the eCS, the number of pulses rather than frequency of stimulation determined the level of fatigue. Fatigue varied inversely with the time interval between the eCS and the test stimulus. For comparable responses from the CS, the magnitude of fatigue was less after the mCS than after the eCS. The mCS (but not the eCS) sometimes evoked a paradoxical increase in response to the test-heat stimulus. Recovery from fatigue was significantly faster after the eCS and mCS than the hCS. The paradoxical enhancement after the mCS probably results from temporal summation of generator potentials produced by mechanical and heat stimulation and suggests that the time constant of the generator potential is on the order of seconds. Concurrent enhancement–fatigue effects may also explain why fatigue was less after the mCS than the eCS. The dependency of recovery from fatigue on the modality of the CS suggests that fatigue results from transduction–spike initiation mechanisms.
Keywords: pain, somatosensory system, primary afferents, adaptation, fatigue, sensitization, nociceptors
Introduction
Although much attention has been directed at nociceptor sensitization, whereby the response to a given stimulus increases with successive application of that stimulus, relatively little attention has been devoted to the reciprocal property, fatigue. Fatigue refers to a decrement in response to repeated stimuli applied to the receptive field of sensory cells. The term “desensitization” may be used interchangeably with fatigue or may be used as an umbrella term for fatigue, tachyphylaxis (decrement in response to repeated chemical stimuli), and adaptation (decrement in response to a sustained stimulus). Desensitization plays an important biological role in adjusting the response sensitivity to the appropriate stimulus range, thus extending the range of stimulus intensities over which a receptor system is sensitive. The property of fatigue is a particularly prominent property of nociceptors. For example, the mean response of C-fiber nociceptors to 3 sec heat stimuli applied to the hand with an interstimulus interval of 25 sec declines by 60% from the first to the second stimulus (LaMotte and Campbell, 1978). When a similar stimulus paradigm is applied to human subjects, a similar decline in the magnitude of pain (LaMotte and Campbell, 1978) and nociceptor response (Torebjörk et al., 1984) is observed.
Despite the profound influence fatigue has on pain sensation, little is known about the mechanisms. Furthermore, nociceptors have the distinctive property that they respond to multiple modalities, and little is known about cross-modality (heterologous) stimulus interaction effects. Insights into fatigue mechanisms could in principle be obtained from intracellular recordings. However, techniques for intracellular recordings from the terminals of nociceptors do not yet exist. Moreover, recordings directly from the dorsal root ganglia in dissociated cell culture have uncertain relations to in vivo physiology. In addition, cross-modal excitation of dorsal root ganglion cells with electrical, heat, and mechanical stimuli would form daunting technical challenges. To understand fatigue mechanisms better, we have undertaken the present study in a primate in vivo model.
Fatigue could be attributable to processes involved with stimulus transmission, stimulus transduction, spike initiation, or action potential conduction. To decipher the mechanisms of fatigue, we compared the effects of electrical stimulation of the nerve trunk with mechanical and heat stimulation of the receptive field. These conditioning stimuli (CS) were presented 5 sec before a test-heat stimulus. We varied the frequency and number of pulses systematically in the electrical CS. Fatigue was prominent with each stimulus modality, but differences were also evident. In addition, we uncovered a surprising enhancement effect of mechanical conditioning stimuli. Preliminary results have been reported previously (Peng et al., 1997; Meyer et al., 1997).
Materials and Methods
Neurophysiological preparation. A standard teased-fiber technique was used to record from single primary afferent nociceptors (Campbell and Meyer, 1983). Briefly, monkeys were sedated with ketamine, and then anesthetized to a level such that the corneal reflex was absent by intravenous administration of a mixture of sodium pentobarbital (3 mg · kg -1 · hr -1) and morphine sulfate (0.5 mg · kg -1 · hr -1). Animals were intubated, and peak expired CO2 was maintained at 35–40 mm Hg using mechanical ventilation. The electrocardiogram was monitored continuously, and rectal temperature was controlled at 38°C by means of a circulating-water heating pad. A solution of 5% dextrose in 0.9% normal saline was administered intravenously in the course of the experiments to maintain hydration.
The nerves used in this study were the medial antebrachial cutaneous and superficial radial nerves on the forelimb and the sural, saphenous, and superficial peroneal nerves on the hindlimb. None of the nerves had been used in previous studies, and the areas of the receptive fields were free of previous injury. A skin incision was made over the nerve of interest. The nerve was dissected free of connective tissue, and fascicles from the nerve were separated on a dissecting platform and placed on a silver electrode wire for extracellular recordings of action potentials from single nerve fibers. At the end of the recording session, the wound was irrigated repeatedly with saline solution and then sutured. Benzathine penicillin (450,000 U, i.m.) was injected at the beginning of each experiment. Animal housing conformed to federal regulations, and the facilities were accredited by the American Association for Accreditation of Laboratory Animal Care.
The analog action potential activity from the nerve filament was amplified and digitized at a 10 kHz rate. A real-time computer-based software program (DAPSYS, Brian Turnquist, Johns Hopkins University) provided window discriminators for real-time sorting of different action potential waveforms (for details, see http://www.dapsys.net). Waveforms passing a selectable threshold level were also saved for post hoc analysis. The recorded responses were time-indexed relative to stimulus delivery by the stimulus control software. The conduction velocity was determined by measuring the latency of response with stimulation at the nerve trunk electrode and the conduction distance between the stimulation and recording electrodes.
Receptive fields were located by squeezing the skin innervated by the nerve under study. Calibrated nylon monofilaments (von Frey filaments, Stoelting aesthesiometer set; Stoelting, Wood Dale, IL) were used to identify sensitive spots in the receptive field and to determine response thresholds. The mechanical threshold was defined as the pressure produced by the von Frey hair that reliably produced at least one action potential using the technique of ascending method of limits. The boundary of the receptive field was determined with suprathreshold von Frey filaments. Radiant heat was used to test for thermal sensitivity. This study was restricted to C-fibers (conduction velocity <2 m/sec) responsive to mechanical and heat stimuli (CMHs).
Interaction studies. The recording configuration and test paradigm used to study interaction effects are illustrated in Figure 1, A and B. The tripolar nerve trunk electrode was placed between the recording electrode and the receptive field. This electrode was used to provide the electrical conditioning stimuli, as will be described.
Figure 1.
A, Experimental configuration. Heat-test stimuli (47°C, 2 sec) were applied to the receptive field via a CO2 laser over an area 8 mm in diameter. The electrical conditioning stimulus (CS) was delivered at the nerve trunk electrode (NTE). The mechanical CS was delivered with a von Frey hair at different “hot” spots (○) within the receptive field, inside or outside the heat-tested area. The heat CS was delivered to the receptive field with the laser. RE, recording electrode. Shaded area, receptive field. B, Sequence of stimuli. The test-heat stimuli (typically 47°C, 2 sec) were delivered every 60 sec. Trial A was not applied until after the response to the repeated test-heat stimuli stabilized (Fig. 2). The CS was completed 5 sec (typically) before the onset of the test stimulus. To combine data across fibers, the response after the CS was normalized by dividing the response at D by the average response to the two trials before the CS [D/(B + C)/2].
The test stimulus used in nearly all of the studies was a 47°C, 2 sec heat pulse delivered every 60 sec (58 sec interstimulus interval) with a laser thermal stimulator (Meyer et al., 1976) (Fig. 1 B). Once the response to the test stimulus reached a stable level (i.e., after trial 5) (Fig. 2), the effects of a CS were assessed. The CS consisted of electrical pulses delivered to the nerve trunk or natural stimuli (mechanical and heat) delivered to the receptive field (Fig. 1). In general, the interval between the end of the CS and the start of the test stimulus was 5 sec, but this was varied in some experiments. At least four test stimuli separated each presentation of a CS.
Figure 2.
Normalized response of the C-fiber mechano–heat nociceptors (CMHs) to the first five presentations of the test-heat stimulus (n = 46; interstimulus interval = 58 sec). The response decreased between trials 1 and 2 and then stabilized to ∼60% of the response to the first heat stimulus. The response of a given fiber was normalized by dividing by the response of that fiber to the first trial (error bars, ±SEM). Fibers were divided into two groups on the basis of whether the response to the first heat stimulus was above (high responders) or below (low responders) the median response. The high responders exhibited more fatigue.
Electrical–heat interaction studies. Electrical conditioning stimulation was applied at the nerve trunk between the recording electrode and the receptive field. The electrical threshold at the nerve trunk electrode was determined for each fiber, and intensities of two times threshold were applied. The number of pulses applied (5, 10, 20, or 40) and the frequencies of stimulation (5, 10, 20, and 40 Hz) were varied systematically for each fiber. Thus, the first frequency selected was 5 Hz, and 5, 10, 20, and 40 pulses were applied at this frequency. Subsequently, the frequency was increased to 10 Hz, and so forth. Given that the duration of the CS varied depending on the above parameters, the time of onset of the electrical stimulus was adjusted so that the time from the end of the CS to the start of the test stimulus was 5 sec.
Mechanical–heat and heat–heat interaction studies. The effects of the electrical CS were compared with the effects of a mechanical CS and a heat CS. Mechanical stimuli were delivered with a hand-held von Frey probe. Stimuli were applied to one of the punctate areas of mechanical sensitivity within the heat-stimulated region, using the protocol illustrated in Figure 1 B. The number of action potentials evoked by the mechanical CS varied widely with each trial and could not be controlled precisely. A spectrum of evoked response was obtained, however, over multiple trials. The heat CS consisted of a 2 sec constant temperature suprathreshold stimulus (typically 47°C) applied with the laser thermal stimulator over the same area irradiated with test-heat stimuli. As with the electrical stimuli, the time from the end of the CS to the start of the test stimulus was always 5 sec.
Data analysis. Because the response to the test-heat stimulus varied from fiber to fiber, the response to the test-heat stimulus was normalized according to the following formula (see Fig. 1 for nomenclature): D/[(B + C)/2]. The effects of electrical stimulation were analyzed using a two-factor (frequency and number of electrical pulses) repeated measures ANOVA. Means ± SEM are given unless otherwise stated.
Results
A total of 46 CMHs were recorded. The conduction velocities ranged from 0.43 to 1.4 m/sec, with a mean of 0.87 ± 0.03 m/sec. All receptive fields were on the hairy skin. The mechanical receptive field size ranged from 8 to 340 mm2, with an average of 43 ± 8 mm2. The mechanical thresholds measured with von Frey hairs ranged from 0.5 to 8.2 bar, with a mean of 2.5 ± 0.2 bar. The electrical stimulation threshold at the nerve trunk (0.1 msec duration stimulus) ranged from 9.4 to 65 V, with a mean of 36 ± 4 V (n = 17). The electrical stimulation threshold at the receptive field (1.0 msec duration stimulus) ranged from 2 to 114 V, with a mean of 29 ± 9 V (n = 14).
Initial fatigue to the repeated test-heat stimulus
As can be seen in Figure 2, responses during repetitive testing with the test-heat stimulus decreased for the first few trials. The mean response to trial 2 was 66 ± 3% of the response to trial 1 (p < 0.001; paired t test). Subsequently, a slight decline in response was evident over the next three trials. Because of this initial fatigue to repeated applications of the test-heat stimulus, the effects of a CS were always determined after at least five presentations of the test stimulus, at which point, responses to the test stimulus were relatively constant.
The amount of fatigue in the heat response was found to be dependent on the initial heat response of the fiber. The CMHs were separated into two groups on the basis of whether the response to the first heat stimulus (i.e., trial 1) was greater or less than the median response. The mean response of the high responders to the first heat stimulus (33.1 ± 6.5 action potentials) was thus greater than for the low responders (10.6 ± 0.6 action potentials). As shown in Figure 2, the response to the second heat stimulus was 58 ± 3% of the response to the first stimulus for the high responders and 74 ± 4% (p < 0.01) for the low responders. Thus, the high responders showed more fatigue than the low responders. This probably reflects the fact that the stimulus in trial 1 (which is the conditioning stimulus for trial 2) elicited more action potentials in the high responders than in the low responders.
Electrical–heat interaction studies
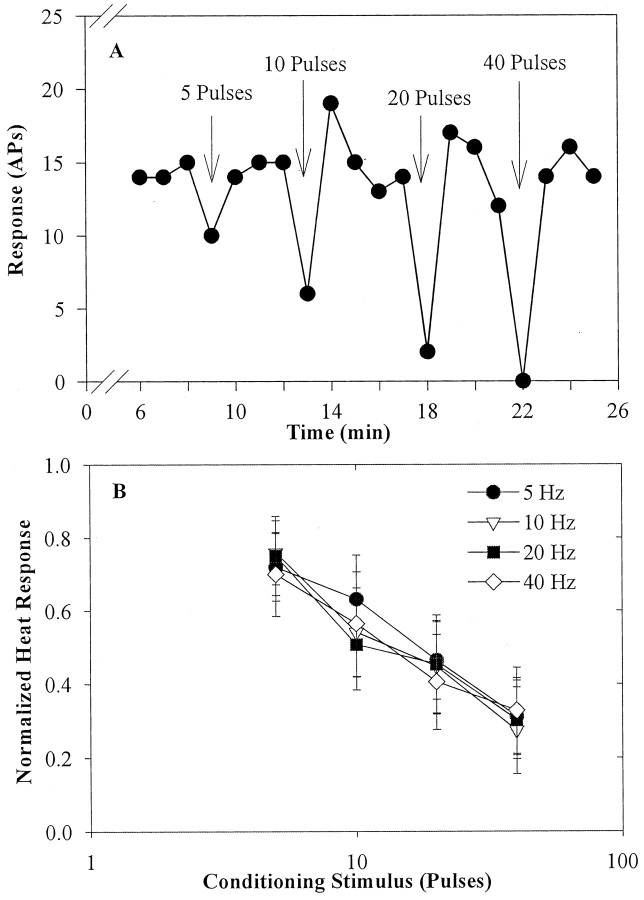
Figure 3A illustrates the effects of electrical stimulation with 5–40 pulses at 5 Hz on the heat response of a typical fiber. The response to the test stimulus immediately after the CS (Fig. 1B, stimulus D) decreased for all stimulus conditions. The degree of fatigue varied directly with the number of pulses delivered to the nerve trunk. The response returned to the baseline by trial E and remained at this level until delivery of the next CS.
Figure 3.
Fatigue after an electrical conditioning stimulus. A, Fatigue in a CMH after an electrical CS applied to the nerve trunk. The response to the 47°C, 2 sec heat-test stimulus is plotted as a function of time. The electrical CS (arrow) ended 5 sec before the next test stimulus and consisted of a 5 Hz pulse train containing 5, 10, 20, and 40 pulses. The amount of fatigue increased as the number of pulses in the CS increased. B, Response fatigue after an electrical CS depends on the number of conditioning pulses. Each fiber was exposed to all combinations of four different pulse counts and four stimulation frequencies. The mean ± SEM normalized response of eight CMHs to trial D is plotted as a function of the number of pulses in the CS. The frequency of stimulation had no effect on the degree of fatigue. See Figure 1 for normalization procedure. APs, action potentials.
In eight CMHs, we obtained a complete set of data at the four different pulse numbers (i.e., 5, 10, 20, and 40 pulses) and at the four different frequencies (i.e., 5, 10, 20, and 40 Hz). As shown in Figure 3B, the fatigue in trial D produced by the CS varied directly with the number of pulses (p < 0.001) but was independent of stimulus frequency (p > 0.4). The response to trial E was not significantly different from the response before the CS (p > 0.5). There was no correlation between conduction velocity and the degree of fatigue.
Effects of a mechanical conditioning stimulus
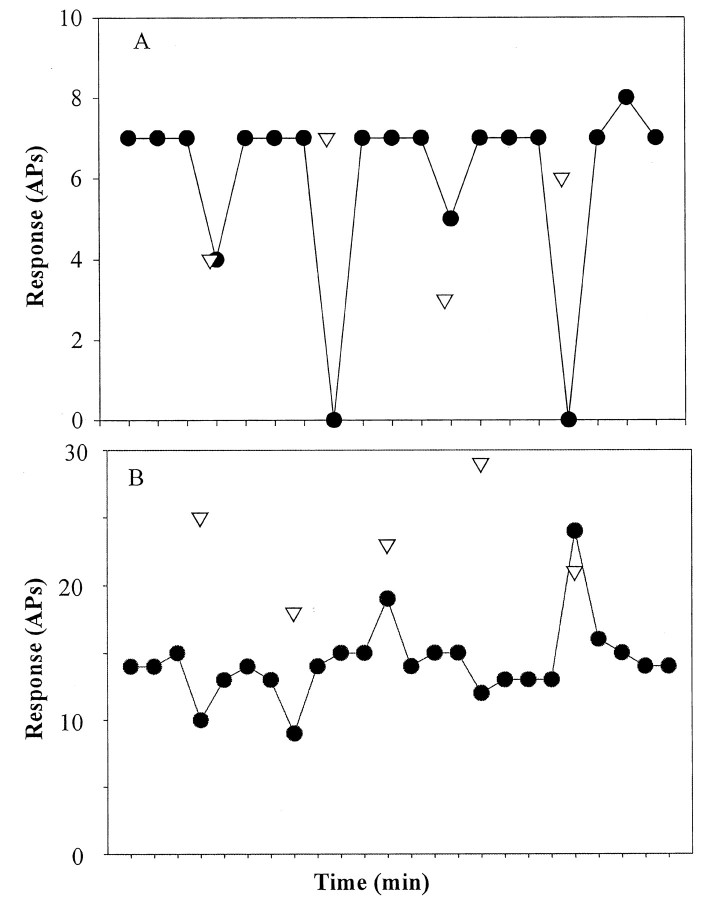
Having established that the number of pulses applied and not the frequency of electrical stimulation was the critical parameter of the CS, we then investigated the changes in heat response after mechanical stimuli applied within the heat-stimulated area of the receptive field. An example of one such study in one fiber is shown in Figure 4A. The magnitude of the response to the mechanical CS varied from 3 to 7 in this fiber (Fig. 4, triangles). The magnitude of fatigue increased with the magnitude of the response to the CS.
Figure 4.
Heat responses after a mechanical conditioning stimulus applied within the heat-stimulated area. A, For this CMH, the heat response decreased after each mechanical CS, and the amount of fatigue varied directly with the response to the mechanical stimulus. B, For this CMH, the heat response sometimes increased after the mechanical stimulus. Both enhanced and fatigued heat responses occurred without apparent relationship to the number of action potentials evoked by the CS. The response to heat is plotted as a function of time during the run. The response to the mechanical CS is indicated by the inverted triangle. The mechanical CS was delivered 5 sec before the test-heat stimulus. APs, action potentials.
Frequently, however, an altogether different effect of the mechanical CS was encountered. In the example shown in Figure 4B, a mechanical CS to the same locus caused fatigue in certain trials and enhancement (an increase in response) in other trials. Also, in this example, the fatigue associated with the mechanical stimulus that evoked 18 action potentials was actually greater than the fatigue associated with the mechanical stimulus that evoked 29 action potentials. Thus, unlike with electrical stimulation, the amount of fatigue did not always vary systematically with the number of action potentials produced by the mechanical CS.
Variability of heat response
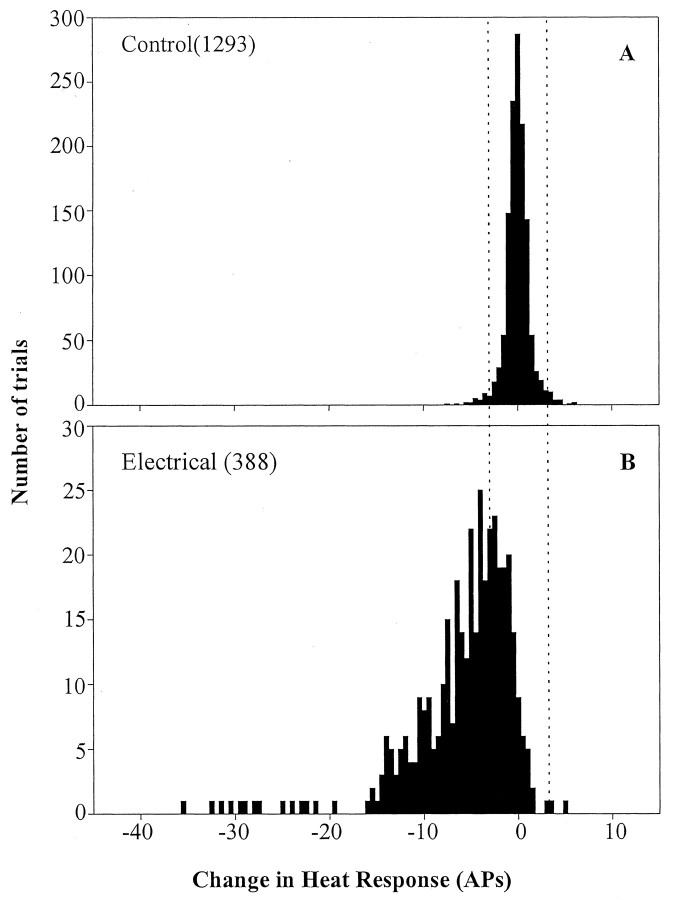
To develop a statistical criterion by which to judge whether a response in a particular trial was significantly different from the baseline response, the variability without a CS was determined. Referring to Figure 1B, this variability was calculated by subtracting the average response to test stimuli A and B from the response to test stimulus C. The variability of this difference fits a Gaussian distribution, which is shown in Figure 5A, and the mean ± SD of this distribution was 0 ± 1 action potential. Thus, any difference that exceeds three action potentials is >3 SD from the mean and is considered significant for purposes of the subsequent analysis.
Figure 5.
Variability of test-heat evoked response. A, To establish the variability in heat response without a CS, a control data point was determined for each CS. The change in response during the three test stimuli before the CS was computed [C - (A + B)/2; see Fig. 1 for nomenclature]. In the absence of a CS, the distribution had a mean of zero and an SD of 1 action potential. A significant change after a CS was defined as any change that was greater than three SDs from the mean (i.e., ±3 action potentials) of the control data. This criterion is illustrated by the dotted vertical lines. B, Variability of heat response after the electrical CS. The response to the test stimulus after the CS was subtracted from the average response to the two test stimuli before the CS [D - (B + C)/2; Fig. 1]. Response fatigue was common and manifest by a shift to the left of the distribution. APs, action potentials.
In Figure 5B, the change in response after the electrical CS for all trials is plotted. The electrical CS often produced a significant decrease in the response but resulted in a response that was greater than three action potentials above the mean in only 3 of 388 trials.
Fatigue and enhancement after a mechanical conditioning stimulus
For each of the 46 fibers studied, the change in response after the mechanical CS was computed as D - (B + C)/2 (Fig. 1) and plotted as a function of the number of action potentials evoked by the mechanical CS. Three types of interaction effects were seen. For many of the fibers (19 of 46), only fatigue was observed. An example of one such fiber is shown in Figure 6A. For this particular fiber, the amount of fatigue increased as the response to the CS increased. In these fibers, the fatigue effects were present regardless of the spot stimulated within the receptive field. Also, for these fibers, the fatigue effects were observed over a spectrum of responses to the mechanical stimulus. This variability in response to the mechanical CS arose in part from use of different stimulus intensities and the variability in sensitivity from spot to spot within the receptive field.
Figure 6.

Examples of the effects of the mechanical conditioning stimulus on the response to the test-heat stimulus in three different nociceptors. Change in heat response after the CS is plotted as a function of the magnitude of response to the mechanical stimulus. Responses outside the dashed horizontal lines exceed control responses by 3 SDs, and this difference is considered significant (Fig. 5A). A, This CMH exhibited a decrease in heat response (or no significant change) after the mechanical CS regardless of the location stimulated or number of action potentials induced by the mechanical CS. B, This CMH displayed both response fatigue and response enhancement after the mechanical CS. Response enhancement was especially common for one particular location with in the receptive field (open circles) but was also seen for mechanical stimuli delivered to other areas (closed circles). C, An example of a CMH that displayed response enhancement but no response fatigue. APs, action potentials.
Some of the fibers (12 of 46) showed both fatigue and enhancement, an example of which is shown in Figure 6B. Even when stimulation was confined to one punctate area (indicated by the open symbols), fatigue, enhancement, or no significant effects were observed over a wide range of responses to the CS.
Uncommonly (3 of 46 fibers), enhancement but not fatigue was observed. An example is shown in Figure 6C. The rest of the fibers (12 of 46) showed no significant change in response after the mechanical CS.
Comparison of fatigue after electrical and mechanical conditioning stimulus
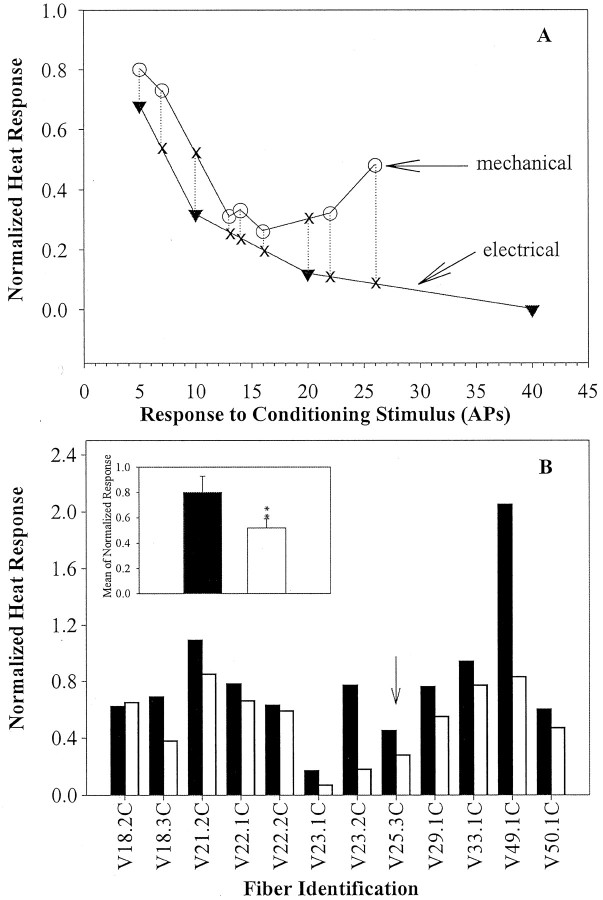
For 12 fibers, a systematic comparison was made between the effects of electrical and mechanical stimulation. The electrical stimulation protocol outlined previously was used to study each fiber. Different forces of von Frey hairs were applied to evoke responses that would span the number of action potentials produced by electrical stimulation. The mechanical stimuli were applied to one or more spots in the receptive field. The data for a typical fiber are shown in Figure 7A. The mean normalized heat response after the conditioning electrical stimuli was averaged across the different stimulation frequencies and plotted as a function of the number of pulses in the CS. The normalized heat response after seven mechanical CSs of varying intensity applied to different spots is also shown in Figure 7A. There was only one instance for this fiber in which the number of action potentials produced by the mechanical CS corresponded to the number of pulses from the electrical CS (i.e., at five action potentials, five pulses). Thus, to compare the effects of the electrical versus the mechanical CS, interpolation points were determined, as shown by the dotted and solid lines in Figure 7A. For this fiber, nine points of comparison were available for analysis. The mean of these nine values for the mechanical CS was compared with the mean of the corresponding nine values for the electrical CS. The results of this computation for this fiber and the 11 other fibers are shown in Figure 7B. As shown by the inset in Figure 7B, there was significantly less fatigue for the mechanical CS than for the electrical CS (p < 0.01; paired t test).
Figure 7.
Comparison of fatigue after electrical and mechanical conditioning stimuli. A, Normalized heat response after mechanical and electrical CSs for a representative CMH. Normalized response to the test-heat stimulus is plotted as a function of action potentials evoked by the CS. The electrical CS (inverted triangles) was applied to the nerve trunk, and the mechanical CS (open circles) was applied to the receptive field. Comparisons were made for all data points over the range of condition stimuli where the two curves overlap. Because most data points for mechanical stimulation did not have an exact match for data points for electrical stimulation (and vice versa), we did a linear interpolation between data points (X = interpolation points used in the statistical comparison). B, Comparison of the average normalized response after the CS for each of 12 CMHs studied with both mechanical (filled bars) and electrical (open bars) CSs. For all but one fiber (V 18.2 C), the mean fatigue induced by the mechanical CS was less than the mean fatigue induced by the electrical CS. The arrow points to the data from the CMH shown in A. The inset shows the mean ± SEM fatigue for the 12 fibers. The fatigue was significantly greater with the electrical CS (**p < 0.01). APs, action potentials.
Mechanical stimulation inside versus outside the heat-stimulated area
For 10 fibers, the laser heat stimulus was positioned so that only part of the receptive field was heated. This allowed us to investigate the effects of mechanical stimulation within and outside the area that was heat stimulated. We reasoned that mechanical stimulation outside the heat-stimulated area would produce fatigue by inducing antidromically conducted action potentials in a manner similar to the effects of electrical stimulation. Using a type of analysis similar to that described for Figure 7, we found that mechanical stimulation outside the heat-stimulated area led to a significantly greater decrement in the test-heat response (4.9 ± 0.8 action potentials for outside and 1.6 ± 1.3 action potentials for inside; p < 0.03; paired t test). For six of these fibers, the effects of electrical stimulation at the nerve trunk were compared with mechanical stimulation of the receptive field outside the heat-stimulated area. The magnitude of fatigue did not differ (6.6 ± 2.8 action potentials for mechanical stimulation outside and 6.0 ± 1.8 action potentials for electrical stimulation; p > 0.5).
Fatigue after a heat-conditioning stimulus
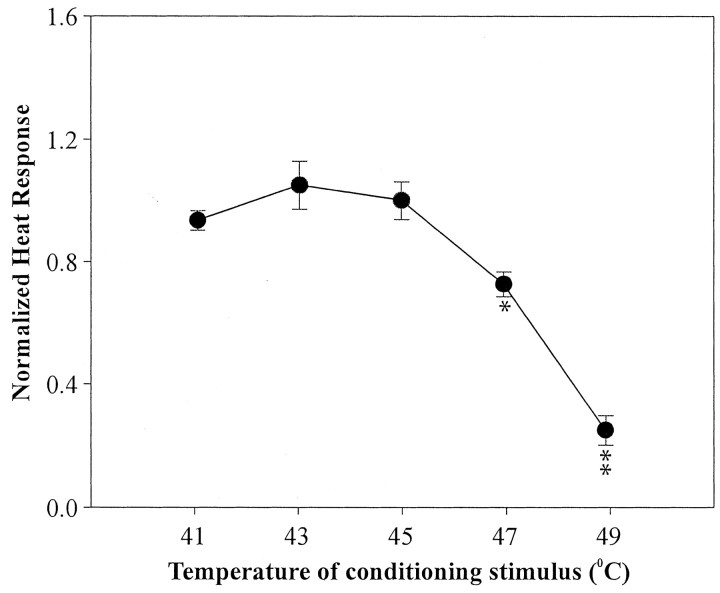
We also studied the effects of a suprathreshold heat CS on the response to the subsequent test–heat stimulus for 36 fibers. The heat CS produced fatigue that was dependent on the temperature of the conditioning stimulus, as shown in Figure 8.
Figure 8.
Fatigue after a heat CS. The normalized response to the test-heat stimulus is plotted as a function of the temperature of the heat CS. Fatigue increased as the temperature (hence evoked response) of the CS increased. (*p < 0.05 and **p < 0.001 compared with response for lower CS temperatures)
Time course of recovery from fatigue depends on the conditioning stimulus
For six fibers, the time between the electrical CS and the test-heat stimulus was varied systematically. As shown by the response of one fiber in Figure 9A, the amount of fatigue varied inversely with the time between the conditioning and test stimulus but directly with the number of pulses. Even with a time interval of 40 sec, there was still more than 20% fatigue after a 40 pulse electrical CS. The response of the six fibers increased exponentially with time between the conditioning and the test stimulus. The mean time constant for recovery was 28 ± 5 sec.
Figure 9.
Recovery from fatigue. A, Fatigue decreased as the time between the electrical CS and test-heat stimulus increased. The normalized response of one CMH to the test-heat stimulus is plotted as a function of the time between the conditioning and test stimulus. Each line corresponds to a different number of pulses in the electrical CS. B, Recovery took longer after a heat CS compared with electrical and mechanical CS. Normalized response is plotted for the three trials that follow the CS. Trials D, E, and F refer to the nomenclature used in Figure 1. At the second trial after the CS (trial E), the response returned to baseline levels after the electrical and mechanical CS (0.99 ± 0.04 and 0.98 ± 0.02, respectively) but was still significantly reduced after the heat CS (0.86 ± 0.02; p < 0.001). Only fibers exposed to electrical, mechanical, and heat CSs were included (n = 15). Only trials with at least a 20% decrease in response in trial D were included.
As another way to investigate the time course for recovery from fatigue for each of the CS modalities, the normalized response [response divided by (B + C)/2] to the first, second, and third test stimuli after the CS (i.e., trials D, E, and F) was computed (Fig. 9B). We included only trials in which the response immediately after the CS decreased by at least 20%. The initial fatigue (∼60%) was comparable for all three modalities. For the electrical and mechanical CSs, the response recovered to baseline by trial E. However, the heat CS produced fatigue that was still present in trial E. Thus, recovery after the heat CS took longer than after the mechanical or electrical CS.
Effects of subthreshold stimulation
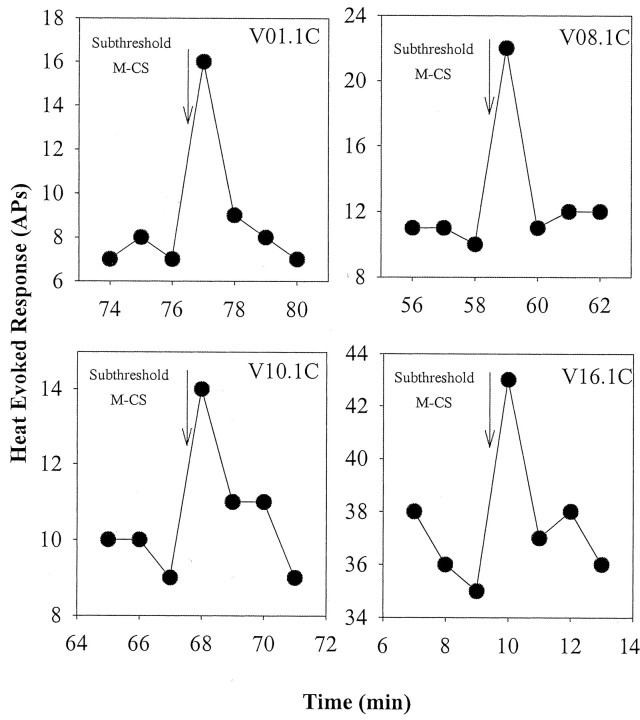
A subthreshold mechanical CS usually had no effect on the subsequent heat response. However, on several occasions (10 of 177 trials in 29 fibers), a prominent enhancement was seen. Examples of this in four fibers are shown in Figure 10. The enhancement effect was erratic in the sense that the effect was often not repeatable within the same fiber.
Figure 10.
An enhanced response to the test-heat stimulus was seen occasionally after a mechanical stimulus that was subthreshold for evoking a response. Response to the heat stimulus is plotted as a function of time for four different CMHs that were exposed to a subthreshold mechanical stimulus at the time indicated by the arrow (the interval from the CS to the test-heat response was 5 sec).
In one CMH, a subthreshold electrical stimulation was applied across the receptive field. The stimulus was applied at 10 Hz for 2 sec (20 pulses) with a pulse width of 1 msec. The voltage applied across the receptive field was just below the threshold for eliciting an action potential. When this electrical CS was applied 5 or 2 sec before the subsequent test–heat stimulus, there was no alteration in the heat response. However, when this subthreshold electrical stimulation was delivered concurrently with the test-heat stimulus, the response increased markedly from 8.7 ± 0.4 to 14.2 ± 1.1 action potentials (p < 0.01; paired t test; 12 trials).
Discussion
We confirm here that fatigue effects in nociceptors are profound (LaMotte and Campbell, 1978; Belmonte and Giraldez, 1981; LaMotte et al., 1984; Slugg et al., 2000) and provide new evidence regarding mechanism. Nerve trunk electrical stimulation produced prominent fatigue of the subsequent heat-evoked response, and this fatigue was proportional to the number of pulses but not the frequency of stimulation. Fatigue was heterologous, but variably so. For the same evoked response, the electrical CS produced more fatigue than mechanical stimulation at the heat-tested region. Also, the recovery of the heat response after an electrical or mechanical CS was faster than after a heat CS. A surprising finding was that mechanical stimulation of the receptive field may lead to an enhancement of the response to the subsequent heat stimulus.
Fatigue to heat stimuli probably relates to effects on transduction and spike initiation mechanisms
Effects on stimulus transmission, transduction, spike initiation, and propagation could in principle account for fatigue. Transmission effects seem unlikely, because this would not account for the prominent fatigue induced by electrical stimulation of the nerve trunk. Fatigue is unlikely to arise from propagation failure, because we recorded in these experiments an entrained response during the 40 Hz electrical CS applied to the nerve trunk. Also, fatigue increased with electrical stimulus frequency up to 40 Hz, suggesting that the axon and its terminals could conduct at least up to this frequency. Moreover, the response to the test-heat stimulus was typically at rates substantially lower than 40 Hz. Recent experiments in our laboratory also indicate that nociceptor terminals are able to track frequencies of 40 Hz (Wu et al., 2000). The capacity for enhancement (see below) also argues against conduction failure as a mechanism.
Fatigue most likely represents effects on transduction and/or spike initiation. Recent evidence indicates that action potentials conduct to the most distal parts of the nociceptive terminals in the cornea via TTX-resistant channels (Brock et al., 2001). Thus, it is likely that action potentials propagate to the regions responsible for transduction. In addition, the generator potential seems to be TTX resistant (Lowenstein et al., 1963; Brock et al., 2001). Antidromically conducted action potentials might deactivate TTX-resistant voltage-gated sodium channels at the terminal and thus interfere with action potential initiation. Effects could also be mediated by changes in membrane polarization (e.g., prolonged hyperpolarization)
Fatigue effects are prominent in one of the known transduction channels in nociceptors. The TRPV1 channel (VR1), sensitive to heat, protons, and other ligands, has been studied extensively. The capsaicin-induced current is subject to fatigue effects similar to those observed with mechanical and heat stimulation of in vivo nociceptors. Calcium-dependent dephosphorylation of the VR1 channel has been suggested as a mechanism for this desensitization (Docherty et al., 1996; Koplas et al., 1997; Piper et al., 1999). Phosphorylation is regulated through a cascade of molecules including protein kinase A, cAMP, and cyclic nucleotides (Bhave et al., 2002). Heat stimuli induce an inward current in certain small, isolated DRG cells (Schwarz et al., 2000). These currents decrease by ∼50% with brief heat pulses presented every 30 sec. This is remarkably similar to the decrease in heat response observed in the present study (Fig. 1). Surprisingly, the effects on DRG currents are found to be independent of both internal and external [Ca 2+]. This could suggest that the major transducer for the heat responses of these nociceptors is a non-VR1 channel, because dephosphorylation of the VR1 channel seems to be calcium dependent. Another possibility is that the VR1 channel has separate modes of activation for heat and capsaicin and that the mechanism for fatigue is calcium independent for heat but not capsaicin (Tominaga et al., 1998). However, Tominaga et al. (1998) also found heat-induced cross-desensitization to capsaicin even in the absence of Ca 2+.
The time course of recovery depends on the conditioning stimulus
The time constant for recovery for repeated heat stimuli (Tillman, 1992) or repeated mechanical stimuli (Slugg et al., 2000) is ∼2.5 min (homologous desensitization). In contrast, when the conditioning stimulus was mechanical (or electrical) and the test stimulus was heat, we found that the recovery time constant was ∼0.5 min (heterologous desensitization). Thus, the direct activation of the heat transduction pathways induces longer-lasting fatigue than what is obtained from antidromically conducted action potentials. One way to account for this is that depolarization induces fatigue on the basis of effects that are downstream from heat transduction. For example, depolarization could inactivate voltage-dependent sodium channels responsible for spike initiation and affect transduction minimally, whereas transduction could engage a desensitizing effect on both transduction (channel dephosphorylation) and spike initiation.
Effects of mechanical stimulation: paradoxical enhancement
Nociceptors in primates have multiple points of sensitivity in the skin to mechanical stimuli. In this study, the heat stimulus was applied over an area 8 mm in diameter; thus, multiple fiber endings belonging to the same nociceptive fiber were activated. Whether the heat response is dominated by one terminal or shared among multiple terminals is unknown. In the case of mechanical stimuli, only one of these punctate regions of sensitivity was activated at any one time. Therefore, the effects of the mechanical CS on heat response are probably primarily the result of antidromic stimulation. Thus, the effects of the mechanical and electrical CS should be the same. However, a surprise finding in this study was the discovery that the mechanical CS may at times lead to an enhanced response to the test-heat stimulus. Even with fibers in which only fatigue was observed in response to the mechanical CS, a greater decrement in response was produced by the electrical CS (Fig. 7). A likely explanation is that the net heat response after a mechanical CS reflects the competition between fatigue and enhancement mechanisms.
One possible mechanism for the enhancement is that the mechanical stimulus somehow enhances heat transmission. For example, the mechanical stimulus could momentarily reduce the distance between the skin surface and the terminal membrane. Although this possibility cannot be completely discounted, it seems unlikely, because enhancement was often observed for weak mechanical stimuli that did not leave any visible signs of tissue displacement. Release of sensitizing chemicals from the terminals after antidromic invasion is also unlikely, because the electrical CS did not lead to an enhanced heat response.
In an anecdotal study of one nociceptor, we noted that electrical stimulation within the receptive field led to enhancement only when applied concurrently with the heat stimulus. In contrast, enhancement from mechanical stimuli was observed even when the CS was presented 5 sec before the heat stimulus. Thus, depolarization by itself probably does not lead to enhancement. Two possibilities remain plausible. One is that the mechanical CS produces a generator potential with a long time constant of decay. This generator potential adds together with the generator potential from the heat stimulus to produce an enhanced heat response. Summation of generator potentials may also have been at play when enhancement by subthreshold stimuli was observed. Another possibility is that mechanical stimulation (but not simply depolarization, as with antidromic conduction action potentials from electrical stimulation) may lead to effects on second-messenger systems. For example, a mechanical transduction-related influx of calcium could lead to activation of protein kinases that could lead to channel phosphorylation and thus augment heat transduction.
The enhancement to heat produced by a mechanical stimulus recovered quickly; the response was back to baseline within 1 min. In contrast, sensitization to heat produced by injury, capsaicin, or protons lasts at least tens of minutes (LaMotte et al., 1982; Guenther et al., 1999). Thus, the enhancement observed here and the sensitization associated with tissue injury probably result from different mechanisms. Whereas heat sensitization appears to involve increases in the concentration of intracellular calcium ions (Guenther et al., 1999), this may not be the case for the heat enhancement after mechanical stimulation.
The amount of fatigue or enhancement that was observed for a given mechanical CS was quite variable (Fig. 6B). This variability could reflect the spatial separation of heat and mechanical transduction channels, a short space constant of the generator potential (or second-messenger effects), imprecise positioning of the mechanical stimulus with the hand-held probe (unlikely, because the same evoked response from the mechanical stimulus was associated with variable enhancement–fatigue effects), and the competition between fatigue and enhancement mechanisms.
Enhancement data might provide estimates of the time and space constants of generator potentials
The generator potential decays exponentially with time. If summed generator potentials account for enhancement, the time constant of this decay might be estimated by examining the likelihood of enhancement as a function of the conditioning-test interval. Our results suggest that this time constant is on the order of seconds. We also determined that enhancement did not occur for mechanical stimuli within the receptive field immediately adjacent to the heat-tested area. This suggests that the space constant of the generator potential is in the submillimeter range.
Footnotes
This research was supported by National Institutes of Health Grant NS-14447. We appreciate the technical assistance of Timothy Hartke and Sylvia Horasek.
Correspondence should be addressed to Dr. James N. Campbell, Room 5-109, Meyer Building, Department of Neurosurgery, Johns Hopkins University School of Medicine, 600 North Wolfe Street, Baltimore, MD 21287. E-mail: jcampbel@jhmi.edu.
Y. B. Peng's present address: Department of Psychology, University of Texas at Arlington, Arlington, TX 76019.
Copyright © 2003 Society for Neuroscience 0270-6474/03/234766-09$15.00/0
References
- Belmonte C, Giraldez F ( 1981) Responses of cat corneal sensory receptors to mechanical and thermal stimulation. J Physiol (Lond) 321: 355–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW ( 2002) cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron 35: 721–731. [DOI] [PubMed] [Google Scholar]
- Brock JA, Pianova S, Belmonte C ( 2001) Differences between nerve terminal impulses of polymodal nociceptors and cold sensory receptors of the guinea-pig cornea. J Physiol (Lond) 533: 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JN, Meyer RA ( 1983) Sensitization of unmyelinated nociceptive afferents in the monkey varies with skin type. J Neurophysiol 49: 98–110. [DOI] [PubMed] [Google Scholar]
- Docherty RJ, Yeats JC, Bevan S, Boddeke HW ( 1996) Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurones from adult rats. Pflügers Arch 431: 828–837. [DOI] [PubMed] [Google Scholar]
- Guenther S, Reeh PW, Kress M ( 1999) Rises in [Ca 2+]i mediate capsaicin and proton-induced heat sensitization of rat primary nociceptive neurons. Eur J Neurosci 11: 3143–3150. [DOI] [PubMed] [Google Scholar]
- Koplas PA, Rosenberg RL, Oxford GS ( 1997) The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J Neurosci 17: 3525–3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte RH, Campbell JN ( 1978) Comparison of responses of warm and nociceptive C-fiber afferents in monkey with human judgements of thermal pain. J Neurophysiol 41: 509–528. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Thalhammer JG, Torebjörk HE, Robinson CJ ( 1982) Peripheral neural mechanisms of cutaneous hyperalgesia following mild injury by heat. J Neurosci 2: 765–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte RH, Torebjörk HE, Robinson CJ, Thalhammer JG ( 1984) Time-intensity profiles of cutaneous pain in normal and hyperalgesic skin: a comparison with C-fiber nociceptor activities in monkey and human. J Neurophysiol 51: 1434–1450. [DOI] [PubMed] [Google Scholar]
- Lowenstein WR, Terzuolo CA, Washizu Y ( 1963) Separation of transducer and impulse-generating processes in sensory receptors. Science 142: 1180–1181. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Walker RE, Mountcastle VB ( 1976) A laser stimulator for the study of cutaneous thermal pain sensation. IEEE Trans Biomed Eng 23: 54–60. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Peng YB, Campbell JN ( 1997) Mechanical stimulation can lead to a short-term enhancement of the response to heat in C-fiber nociceptors of monkey. Soc Neurosci Abstr 23: 1257. [Google Scholar]
- Peng YB, Campbell JN, Meyer RA ( 1997) Antidromic activation of C-fiber nociceptors in monkey produces a short-term suppression of the response to heat stimuli. Soc Neurosci Abstr 23: 1257. [Google Scholar]
- Piper AS, Yeats JC, Bevan S, Docherty RJ ( 1999) A study of the voltage dependence of capsaicin-activated membrane currents in rat sensory neurones before and after acute desensitization. J Physiol (Lond) 518: 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz S, Greffrath W, Busselberg D, Treede RD ( 2000) Inactivation and tachyphylaxis of heat-evoked inward currents in nociceptive primary sensory neurones of rats. J Physiol (Lond) 528: 539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slugg RM, Meyer RA, Campbell JN ( 2000) Response of cutaneous A- and C-fiber nociceptors in the monkey to controlled-force stimuli. J Neurophysiol 83: 2179–2191. [DOI] [PubMed] [Google Scholar]
- Tillman DB ( 1992) Heat response properties of unmyelinated nociceptors. PhD dissertation, The Johns Hopkins University.
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D ( 1998) The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21: 531–543. [DOI] [PubMed] [Google Scholar]
- Torebjörk HE, LaMotte RH, Robinson CJ ( 1984) Peripheral neural correlates of magnitude of cutaneous pain and hyperalgesia: simultaneous recordings in humans of sensory judgments of pain and evoked responses in nociceptors with C-fibers. J Neurophysiol 51: 325–339. [DOI] [PubMed] [Google Scholar]
- Wu G, Ringkamp M, Meyer RA ( 2000) The terminal arbor of cutaneous nociceptors in monkey follows high frequency electrical stimuli. Soc Neurosci Abstr 26: 1697. [Google Scholar]