Abstract
From an early age, musicians learn complex motor and auditory skills (e.g., the translation of visually perceived musical symbols into motor commands with simultaneous auditory monitoring of output), which they practice extensively from childhood throughout their entire careers. Using a voxel-by-voxel morphometric technique, we found gray matter volume differences in motor, auditory, and visual-spatial brain regions when comparing professional musicians (keyboard players) with a matched group of amateur musicians and non-musicians. Although some of these multiregional differences could be attributable to innate predisposition, we believe they may represent structural adaptations in response to long-term skill acquisition and the repetitive rehearsal of those skills. This hypothesis is supported by the strong association we found between structural differences, musician status, and practice intensity, as well as the wealth of supporting animal data showing structural changes in response to long-term motor training. However, only future experiments can determine the relative contribution of predisposition and practice.
Keywords: musician, brain, morphometry, motor training, sensorimotor, gray matter
Introduction
Musicians are skilled in performing complex physical and mental operations such as the translation of visually presented musical symbols into complex, sequential finger movements, improvisation, memorization of long musical phrases, and identification of tones without the use of a reference tone. Playing a musical instrument typically requires the simultaneous integration of multimodal sensory and motor information with multimodal sensory feedback mechanisms to monitor performance. Several behavioral, neurophysiological, and neuroimaging studies have explored these exceptional and highly specialized sensorimotor (Amunts, 1997; Hund-Georgiadis and Von Cramon, 1999), auditory (Altenmuller, 1986; Besson et al., 1994; Pantev et al., 1998; Zatorre et al., 1998; Keenan et al., 2001; Ohnishi et al., 2001), visual-spatial (Hetland, 2002), auditory-spatial (Munte et al., 2001), and memory (Chan et al., 1998) skills of musicians. Nevertheless, the neural correlates of musical skills are not fully understood, nor have firm associations between these skills and particular brain regions or characteristic brain anatomy been established. Several functional imaging studies have shown differences between musicians and non-musicians while performing motor, auditory, or somatosensory tasks (Elbert et al., 1995; Pantev et al., 1998; Schlaug, 2001). Similarly, structural brain differences between musicians and non-musicians were reported in a few a priori defined motor and auditory brain regions (Schlaug et al., 1995a,b; Amunts, 1997; Zatorre et al., 1998; Schlaug, 2001; Schneider et al., 2002; Hutchinson et al., 2003; Lee et al., 2003). However, no study has searched across the whole brain space for structural differences between musicians and non-musicians that could be linked to musicians' specialized skills and the extensive, long-term refinement of those skills.
The search for anatomical markers of extraordinary skills has fascinated researchers for many years (Schlaug et al., 1995b; Zatorre et al., 1998; Witelson et al., 1999; Maguire et al., 2000; Amidzic et al., 2001; Keenan et al., 2001; Munte et al., 2002). Investigations into the acquisition of new skills and the neural changes associated with mastering a skill (Karni et al., 1995; Pantev et al., 1998, 2001) represent one experimental model used to determine whether or not functional and anatomical markers of exceptional skills exist or develop. A common finding across most skill acquisition studies is the functional enlargement of the representative area that underlies that particular skill (Schlaug et al., 1994; Karni et al., 1995; Pascual-Leone et al., 1995; Toni et al., 1998). However, it remains unclear whether the continued practice or repetition of skills over a long period of time can also lead to the kinds of structural changes or even regional enlargement of the human brain that have been described in animal experiments (Black et al., 1990; Isaacs et al., 1992; Anderson et al., 1994; Kleim et al., 1996; van Praag et al., 1999). We applied an optimized method of voxel-based morphometry (VBM) (Ashburner and Friston, 2000; Good et al., 2001a,b) to explore whether structural brain differences exist between three matched groups of subjects (professional musicians, amateur musicians, and non-musicians) that differed in musician status and practice intensity.
Table 2.
Brain regions with positive correlation between gray matter and musician status
|
X |
Y |
Z |
t value |
Region (Brodmann area) |
|---|---|---|---|---|
| − 62 | − 40 | − 16 | 5.45 | Left inferior temporal gyrus (20) |
| 60 | − 40 | − 20 | 4.69 | Right inferior temporal gyrus (20) |
| − 28 | − 21 | 63 | 4.63 | Left precentral gyrus (4, 6) |
| 27 | − 50 | 60 | 4.55 | Right superior parietal cortex (5, 7) |
| 28 | − 22 | 62 | 4.32 | Right precentral gyrus (4, 6) |
| − 39 | − 28 | 3 | 4.32 | Left Heschl's gyrus (41) |
| − 42 | 50 | 0 | 4.10 | Left inferior frontal gyrus (46) |
| 10 | − 21 | 57 | 3.86 | Right medial frontal gyrus (6) |
| Left anterior cerebellar lobe | ||||
| − 32
|
− 56
|
− 30
|
3.58
|
(Larsell lobes HV/HVI)
|
p < 0.05 corrected for multiple comparisons; extent threshold, p < 0.1. Coordinates (given in millimeters for the maximum value in the cluster) refer to the template space and correspond only approximately to the space of the Talairach atlas.
Materials and Methods
Subjects. We compared 20 male professional musicians and 20 male amateur musicians to a matched control group of 40 male non-musicians (Table 1). The age range of all subjects was 18-40 years. To diminish any possible scanner effect, the right-handed male musicians were selected from a database of high-resolution anatomical magnetic resonance (MR) data sets of musicians and non-musicians acquired on the same MR scanner. All of the musicians were keyboard players. In addition, all subjects had undergone a brief test to assess their verbal intelligence quotient (IQ) using the Shipley-Hartford Vocabulary and Abstraction test, which correlates highly with the Wechsler Adult Intelligence Scale full-scale IQ (Paulson and Lin, 1970). For the purpose of this study, “Professional musicians” were defined as performing artists, full-time music teachers, or full-time conservatory students having an average daily practice time of at least 1 hr. Professional musicians with <1 hr of daily practice time were not included in this study. “Amateur musicians” were defined as those who played a musical instrument regularly but whose profession was outside the field of music. The professional musician groups' average daily practice time was approximately twice that of the amateur musicians (Table 1). Musicians were recruited through advertisements in newspapers and local music schools. All of the musicians (amateurs and professionals) had received formal training on a keyboard instrument; five of the musicians played stringed instruments in addition to being keyboard players. Both right-handed musician groups were matched for age and only showed a significant difference in average daily practice time. Therefore, the professional musician group is regarded as the high-practicing group, and the amateur musician group is regarded as the low-practicing group (Table 1). Age at commencement of musical training showed a huge overlap between both music groups, and no significant difference was found. Amateur musicians were slightly older than professional musicians at commencement of musical training. “Non-musicians” were defined as those who had never played a musical instrument. They were recruited from local universities and medical institutions and matched to the two musician groups for gender, age, and IQ score (Table 1). We included subjects of only one gender to exclude possible gender confound. Gender-related differences have been reported in numerous morphometric studies (Amunts et al., 2000; Nopoulos et al., 2000), and, more recently, pronounced gender effects were detected using voxel-based methods (Good et al., 2001b) that prompted another study examining musician effects to use only male subjects (Sluming et al., 2002).
Table 1.
Demographic characteristics of the samples
|
|
Professional musicians (n = 20) |
Amateur musicians (n = 20) |
Non-musicians (n = 40) |
|---|---|---|---|
| Age (year) | 23.05 (3.83) | 25.95 (5.61) | 26.92 (4.90) |
| Verbal IQ | 119.23 (7.06) | 122.57 (2.57) | 118.18 (5.08) |
| Age of commencement (year) | 6.00 (1.81) | 7.65 (4.17) | |
| Average practice time per day (hr) | 2.23 (0.91)* | 1.15 (1.0)* | |
| Average practice time per day × duration of practice (hr × year)
|
39.1 (21.24)*
|
17.88 (9.74)*
|
|
Data are mean (SD).
p < 0.001.
Informed consent was obtained from all subjects, and the study was approved by the Institutional Review Board of the Beth Israel Deaconess Medical Center.
Data acquisition and analysis. High-resolution anatomical images (voxel size, 1 mm3) of the whole brain were acquired on a 1.5 T Siemens Vision whole-body scanner (Erlangen, Germany) using a magnetization prepared rapid acquisition gradient echo sequence. Images were then analyzed using VBM, a fully automatic technique for computational analysis of differences in local gray matter volume. This method involves the following steps: (1) spatial normalization of all images to a standardized anatomical space by removing differences in overall size, position, and global shape; (2) extraction of gray and white matter from the normalized images; and (3) analysis of differences in local gray and white matter volume across the whole brain (Ashburner and Friston, 2000). We applied an optimized method of VBM (Ashburner and Friston, 2000; Good et al., 2001a,b) using the SPM99 package (Institute of Neurology, London, UK). The spatial normalization to the standard anatomical space was performed in a two-stage process. In the first step, we registered each image to the International Consortium for Brain Mapping template (Montreal Neurological Institute, Montreal, Canada), which approximates Talairach space. We applied a 12 parameter affine transformation to correct for image size and position. Regional volumes were preserved while corrections for global differences in whole brain volume were made. The normalized images of the non-musicians were averaged and smoothed with a Gaussian kernel of 8 mm full-width at half-maximum (FWHM) and then used as a new template with reduced scanner- and population-specific bias. In the second normalization step, we locally deformed each image of our entire group to the new template using a nonlinear spatial transformation. This accounts for the remaining shape differences between the images and the template and improves the overlap of corresponding anatomical structures. Finally, using a modified mixture model cluster analysis, normalized images were corrected for nonuniformities in signal intensity and partitioned into gray and white matter, CSF, and background. To remove unconnected nonbrain voxels (e.g., rims between brain surface and meninges), we applied a series of morphological erosions and dilations to the segmented images (Good et al., 2001a,b). The resulting gray and white matter images were smoothed with a Gaussian kernel of 12 mm FWHM. Voxel-by-voxel t tests using the general linear model were used to search for gray and white matter differences between professional musicians, amateur musicians, and non-musicians. We assessed the correlation between musician status and gray and white matter, respectively, by modeling the musician status as a three-level gradation. Professional musicians were assigned a value of 1, amateur musicians were assigned a value of 0.5, and non-musicians were assigned a value of 0.
The statistical model described above assumes that the gray and white matter values of the amateur musicians fall between those of the professional musicians and the non-musicians. Taking into account the possibility that we may not find strong voxel-by-voxel correlations between musician status and gray matter if the gray matter group differences did not follow this pattern, we performed a supplementary analysis by comparing professional musicians directly with the non-musicians.
To avoid possible edge effects around the border between gray and white matter and to include only relatively homogeneous voxels, we excluded all voxels with a gray or white matter volume value of <0.2 (of a maximum value of 1). All statistical images were thresholded at p < 0.05 and corrected for multiple comparisons (Benjamini and Hochberg, 1995). Corresponding to a spatial extent threshold of p < 0.1, only clusters with a minimum of 225 voxels are reported.
Results
In comparing these three groups (professional musicians, amateur musicians, and non-musicians), areas with a significant positive correlation between musician status and increase in gray matter volume were found in perirolandic regions including primary motor and somatosensory areas, premotor areas, anterior superior parietal areas, and in the inferior temporal gyrus bilaterally (Figs. 1, 2). A positive correlation means that the gray matter volume is highest in professional musicians, intermediate in amateur musicians, and lowest in non-musicians. Additional positive correlations with musician status were seen in the left cerebellum (Fig. 3), left Heschl's gyrus (Fig. 1), and left inferior frontal gyrus. When the spatial extent threshold was lowered (225-190 voxels), gray matter volume in both Heschl's gyri was found to be positively correlated with musician status. No significant effects were seen in the planum temporale (PT). There were no areas showing a significant decrease in gray matter volume in relation to musician status. These results partly corroborate and greatly expand on our previous data obtained with traditional morphometric techniques in a priori defined anatomical regions, which showed differences in a marker of primary motor cortex size and cerebellar volume between musicians and non-musicians (Amunts, 1997; Schlaug, 2001; Hutchinson et al., 2003).
Figure 1.
Brain regions with gray matter differences between professional musicians, amateur musicians, and non-musicians. The musician status was modeled as a three-level gradation in which professional musicians were ranked highest, amateur musicians were intermediate, and non-musicians were ranked lowest (see Materials and Methods for details). Only those voxels with a significant positive correlation between musician status and increase in gray matter volume are shown (p < 0.05; corrected for multiple comparisons). Only clusters of voxels consisting of at least 225 voxels are displayed, corresponding to a spatial extent threshold of p < 0.1. These clusters were overlaid on the rendered cortex surface of a selected single subject. Yellow lines indicate selected cuts through this brain, and the corresponding axial slices are shown in the left and right panels. These axial slices show the overlay of the results onto the average of all 80 single anatomical images.
Figure 2.
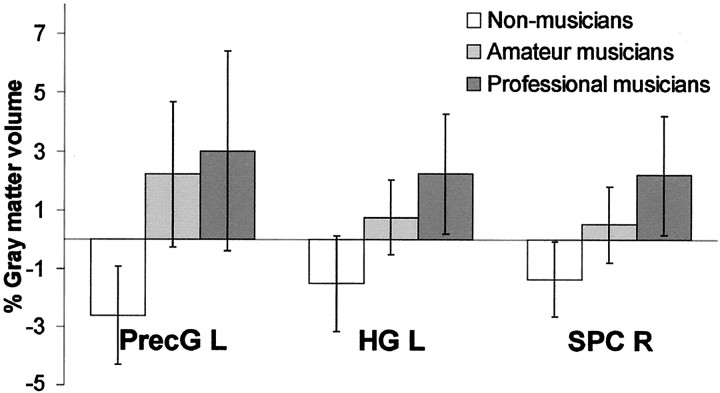
Relative differences in gray matter volume (mean and SD) between professional musicians, amateur musicians, and non-musicians in three selected regions. Regional differences in the left precentral gyrus (PrecG L), left Heschl's gyrus (HG L), and right superior parietal cortex (SPC R) using a spherical region of interest with a radius of 8 mm centered at the local maximal difference are shown.
Figure 3.
Location of cerebellar gray matter effects. The significant gray matter differences in the cerebellum in a selected coronal and axial section are shown. Orientation of these sections has been performed according to the coordinating system described by Grodd et al. (2001). The cluster in the cerebellar region corresponds to the area of the cerebellar finger-hand representation, as shown in functional imaging studies, and is located in the lobes HV/HVI according to the classification of Larsell and Jansen (1971).
As found in the correlational approach, the direct comparison between the professional musician and non-musician groups revealed similar regions, whereas the location of the maximum voxels, and the extent of the clusters, varied slightly from the main analysis (Fig. 4). The cerebellar region did not become significant in the direct comparison of professional musicians with non-musicians because the cluster size did not exceed the spatial extent threshold of p < 0.1.
Figure 4.
Overlap between different types of statistical analyses. This figure shows the results of the direct comparison between professional musicians and non-musicians (green) and the correlation with musician status (red) using the same statistical thresholds as in Figure 1. The overlap between both results is shown in yellow (as a result of mixing red and green). Results are displayed as maximum intensity projections (“glass brain”), which show highest values within each orientation.
The use of statistical cutoffs similar to those used in the gray matter analysis revealed no significant correlations between white matter volume and musician status.
Discussion
Our results suggest a pattern of differences in the gray matter distribution between professional musicians, amateur musicians, and non-musicians that involve motor, auditory, and visual regions. Motor-related regions such as the premotor and cerebellar cortex have been shown to play a critical role in the planning, preparation, execution, and control of bimanual sequential finger movements. Furthermore, functional changes in the movement representation pattern have been demonstrated during motor learning in these regions (Karni et al., 1995). The cluster of cerebellar gray matter differences in our study is located in the lobes HV/HVI according to the classification of Larsell and Jansen (1971) and to regions V and VI in the new three-dimensional MR imaging atlas of the cerebellum by Schmahmann et al. (1999). This region may correspond to the area of the cerebellar finger-hand representation as shown in some functional imaging studies (Grodd et al., 2001), although other studies have also found activation in this area with auditory-musical tasks that are being performed repeatedly by musicians (Griffiths et al., 1999; Gaab et al., 2003). Several studies have implicated the cerebellum in cognitive skill learning (Kim et al., 1994; Parsons, 2001) as well as in music processing (Griffiths et al., 1999; Parsons, 2001; Gaab et al., 2003). These aspects of music training could also contribute to the structural differences in the cerebellum. The structural differences found in the left Heschl's gyrus (Figs. 1, 2) support the results of a recent study showing higher gray matter volume in this region in musicians, which was associated with neurophysiological source activity differences between professional musicians, amateur musicians, and non-musicians while listening to tones (Schneider et al., 2002). No differences were found in the planum temporale in this study. This is in agreement with our previous studies (Schlaug et al., 1995a; Keenan et al., 2001). These previous studies reported anatomical PT differences only between musicians with absolute pitch (AP) and those without AP, but we did not find differences between musicians without absolute pitch and non-musicians in PT size or right-left asymmetry. Five of 20 individuals in the professional musician group had AP. This may not be enough subjects to lead to gray matter differences in our study. A recent voxel-by-voxel analysis (Luders et al., 2003) comparing male AP musicians with male non-AP musicians replicated the PT asymmetry effect observed in studies using traditional morphometric techniques (Schlaug et al., 1995a; Keenan et al., 2001).
The finding in the superior parietal region is of particular interest considering the existing literature on visual-spatial processing differences in groups of subjects with and without musical training (Hetland, 2002). This region is known to play an important role in integrating multimodal sensory information (e.g., visual, auditory, and somatosensory) and providing guidance for motor operations through intense reciprocal connections with the premotor cortex (Friedman and Goldman-Rakic, 1994; Andersen et al., 1997; Bushara et al., 1999). Both functions are of enormous importance to the performing musicians. In addition, the superior parietal lobe was also found to play an important role in sight-reading, a musical task that depends on the fast integration of multimodal sensory information and motor preparation (Sergent et al., 1992). Throughout their entire musical life, musicians repetitively practice this visual-spatial to motor transformation by reading musical notation and translating it into motor plans accompanied by simultaneous auditory feedback that aids the matching of the visual patterns to the motor program.
Our study also showed a strong increase in gray matter volume related to musician status in the inferior temporal gyrus, most probably including anatomical regions involved in the ventral visual stream. The interpretation of this result is aided by functional neuroimaging studies that have shown learning-related increases in functional activity in the inferotemporal cortex and associated increases in the ventral prefrontal cortex, into which the inferotemporal cortex projects when subjects learn to choose actions prompted by visual stimuli (Passingham and Toni, 2001), a process in which musicians are continuously engaged while playing their instrument.
Although our main hypothesis was focused on the detection of effects in gray matter, we also wondered about the lack of a finding in the white matter. There are at least two explanations for this. Either most of the presumed plastic changes do occur in the cerebral gray matter or the VBM method is insensitive to white matter differences most likely caused by low-intensity contrast differences between two groups (Ashburner and Friston, 2000; Bookstein, 2001). This latter interpretation is the more likely explanation for the lack of a white matter effect. This may be one reason that we were not able to replicate our own findings with this method (Schlaug et al., 1995a; Lee et al., 2003) or those of other groups (Ozturk et al., 2002) who found corpus callosum differences between musicians and non-musicians. Another reason might be the obvious differences in the methodology, because the corpus callosum is typically measured in one mid-sagittal section and areal group differences are small, up to a few percentages according to a few reports. Other, newer imaging techniques such as diffusion tensor imaging (DTI) might be more sensitive to differences in white matter tracts than VBM-based methods, and a recent study found differences between musicians and non-musicians using DTI (Schmithorst and Wilke, 2002).
A direct comparison between the professional musicians and the non-musicians revealed results similar to those in our main analysis, showing only subtle differences in the extent and maximum signal values. In this subanalysis, only the cerebellar region did not reach the spatial extent threshold. The similarity between the results of both analyses further supports our hypothesis of a monotonic relationship between musician status and gray matter volumes and shows that the cerebellum may be particularly sensitive for showing a monotonic effect. This is supported by an independent analysis of cerebellar volume comparing musicians with non-musicians using traditional volumetric methods (Hutchinson et al., 2003). This finding has recently been replicated by a different group (Sluming et al., 2003).
Our study examined these effects in only one gender. The reasons for this are manifold. First, there is a growing body of literature on gender interaction in traditional morphometric studies (Foundas et al., 1999; Amunts et al., 2000; Kansaku et al., 2000; Nopoulos et al., 2000). Second, a recent VBM study reported huge gender effects (Good et al., 2001b) that are further supported by studies showing histological differences between males and females (Witelson et al., 1995). Third, experimental studies have shown that microstructural changes co-vary with the menstrual cycle (Woolley and McEwen, 1992), which could potentially mask a musician effect in our study. Therefore, we chose to use only male subjects in this study. However, one should exercise caution when generalizing a single-gender finding to the whole population.
Overall, the results of our study and other studies (Elbert et al., 1995; Zatorre et al., 1998; Keenan et al., 2001; Pantev et al., 2001; Schneider et al., 2002) provide strong links between specialized skills and particular brain structures. It is certainly possible that some of the structural differences may be attributable to innate predisposition. However, similar to what has been described in animal studies (Black et al., 1990; Isaacs et al., 1992; Zilles, 1992; Anderson et al., 1994; Zheng and Purves, 1995; Kleim et al., 1996; Anderson et al., 2002), it is also possible that the explanation for our findings and those of others is that neural plasticity in humans may lead to use-dependent regional growth and structural adaptation in cerebral gray matter in response to intense environmental demands during a critical period of brain maturation. The strong association between gray matter differences and musician status in our study lends further support to the proposal that the brains of musicians show use-dependent structural changes. Amateur musicians showed an intermediate increase in gray matter volume when compared with non-musicians and professional musicians (Fig. 2). Additional support for the proposal of structural plasticity comes from animal experiments showing microstructural changes in the cerebellum, primary motor cortex, and hippocampus related to motor skill learning and continuous motor activity (Anderson et al., 1994). Compared with a voluntary exercise control group, acrobatic motor training in rats resulted in an increase in the number of synapses per neuron and a greater number of glial cells and increased glial volume per Purkinje cell in the cerebellar cortex. The forced routine exercise group had a predominant increase in capillary density, as well as smaller changes in synapse and glial cell density. The sum of these microstructural changes has been reported to lead to volume differences detectable on a macroscopic level in several animal experiments (Pysh and Weiss, 1979; Anderson et al., 1994; Passingham and Toni, 2001; Anderson et al., 2002). Also of interest is the fact that these experimental animal studies do not report any white matter effects that correspond to the results reported here.
Alternatively, it is also possible that extremes or particular patterns of normal anatomical variability foster the development of extraordinary abilities, in which case such special anatomy would be a prerequisite for advanced skill acquisition rather than its consequence. If these structural differences are innate, individuals exhibiting such differences in brain anatomy might be drawn to becoming musicians and, therefore, face fewer obstacles in mastering a musical instrument because they are equipped with the necessary brain anatomy. Although self-selection for musicianship by individuals with innate brain structural differences cannot be completely ruled out, the strong relationship between structural differences and musician status, as well as a wealth of supporting data from animal experiments examining structural brain effects of skill acquisition and long-term motor training, would support the proposal that volumetric structural differences seen in musicians might actually be adaptations to long-term musical training. Furthermore, finding differences between musicians and non-musicians in several anatomically distinct brain regions makes it less likely that these differences are innate and determine whether someone succeeds in becoming a musician. Only future experiments can determine the relative contribution of predisposition and practice; however, we believe the results of our study establish a base for future studies probing more directly causal relationships between long-term training and related structural changes in specific brain regions.
Footnotes
This work was made possible by the generous support of the German Academic Exchange Service (Deutscher Akademischer Austausch Dienst), the National Science Foundation (Grant BCS-0132508), and the International Foundation for Music Research. G.S. is supported by a Clinical Investigator Development Award from the Doris Duke Charitable Foundation and a grant from the Dana Clinical Hypothesis Program in Imaging. We gratefully acknowledge Drs. Albert Galaburda and Michael Charness for reading a previous version of this manuscript and providing helpful comments.
Correspondence should be addressed to Dr. Gottfried Schlaug, Department of Neurology, Beth Israel Deaconess Medical Center and Harvard Medical School, 330 Brookline Avenue, Boston, MA 02215. E-mail: gschlaug@bidmc.harvard.edu.
Copyright © 2003 Society for Neuroscience 0270-6474/03/239240-06$15.00/0
References
- Altenmuller E ( 1986) Brain electrical correlates of cerebral music processing in the human. Eur Arch Psychiatry Neurol Sci 235: 342-354. [DOI] [PubMed] [Google Scholar]
- Amidzic O, Riehle HJ, Fehr T, Wienbruch C, Elbert T ( 2001) Pattern of focal gamma-bursts in chess players. Nature 412: 603. [DOI] [PubMed] [Google Scholar]
- Amunts K ( 1997) Motor cortex and hand motor skills: Structural compliance in the human brain. Human Brain Mapp 5: 206-215. [DOI] [PubMed] [Google Scholar]
- Amunts K, Jancke L, Mohlberg H, Steinmetz H, Zilles K ( 2000) Interhemispheric asymmetry of the human motor cortex related to handedness and gender. Neuropsychologia 38: 304-312. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Snyder LH, Bradley DC, Xing J ( 1997) Multimodal representation of space in the posterior parietal cortex and its use in planning movements. Annu Rev Neurosci 20: 303-330. [DOI] [PubMed] [Google Scholar]
- Anderson BJ, Li X, Alcantara AA, Isaacs KR, Black JE, Greenough WT ( 1994) Glial hypertrophy is associated with synaptogenesis following motor-skill learning, but not with angiogenesis following exercise. Glia 11: 73-80. [DOI] [PubMed] [Google Scholar]
- Anderson BJ, Eckburg PB, Relucio KI ( 2002) Alterations in the thickness of motor cortical subregions after motor-skill learning and exercise. Learn Mem 9: 1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ ( 2000) Voxel-based morphometry-the methods. NeuroImage 11: 805-821. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y ( 1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 57: 289-300. [Google Scholar]
- Besson M, Faita F, Requin J ( 1994) Brain waves associated with musical incongruities differ for musicians and non-musicians. Neurosci Lett 168: 101-105. [DOI] [PubMed] [Google Scholar]
- Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT ( 1990) Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci USA 87: 5568-5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookstein FL ( 2001) “Voxel-based morphometry” should not be used with imperfectly registered images. NeuroImage 14: 1454-1462. [DOI] [PubMed] [Google Scholar]
- Bushara KO, Weeks RA, Ishii K, Catalan MJ, Tian B, Rauschecker JP, Hallett M ( 1999) Modality-specific frontal and parietal areas for auditory and visual spatial localization in humans. Nat Neurosci 2: 759-766. [DOI] [PubMed] [Google Scholar]
- Chan AS, Ho YC, Cheung MC ( 1998) Music training improves verbal memory. Nature 396: 128. [DOI] [PubMed] [Google Scholar]
- Elbert T, Pantev C, Wienbruch C, Rockstroh B, Taub E ( 1995) Increased cortical representation of the fingers of the left hand in string players. Science 270: 305-307. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Faulhaber JR, Kulynych JJ, Browning CA, Weinberger DR ( 1999) Hemispheric and sex-linked differences in Sylvian fissure morphology: a quantitative approach using volumetric magnetic resonance imaging. Neuropsychiatry Neuropsychol Behav Neurol 12: 1-10. [PubMed] [Google Scholar]
- Friedman HR, Goldman-Rakic PS ( 1994) Coactivation of prefrontal cortex and inferior parietal cortex in working memory tasks revealed by 2DG functional mapping in the rhesus monkey. J Neurosci 14: 2775-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaab N, Gaser C, Zaehle T, Jancke L, Schlaug G ( 2003) Functional anatomy of pitch memory - an fMRI study with sparse temporal sampling. NeuroImage 19: 1417-1426. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS ( 2001a) A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage 14: 21-36. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS ( 2001b) Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. NeuroImage 14: 685-700. [DOI] [PubMed] [Google Scholar]
- Griffiths TD, Johnsrude I, Dean JL, Green GG ( 1999) A common neural substrate for the analysis of pitch and duration pattern in segmented sound? NeuroReport 10: 3825-3830. [DOI] [PubMed] [Google Scholar]
- Grodd W, Hulsmann E, Lotze M, Wildgruber D, Erb M ( 2001) Sensorimotor mapping of the human cerebellum: fMRI evidence of somatotopic organization. Hum Brain Mapp 13: 55-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetland L ( 2002) Learning to make music enhances spatial reasoning. J Aesthet Educ 34: 179-238. [Google Scholar]
- Hund-Georgiadis M, Von Cramon DY ( 1999) Motor-learning-related changes in piano players and non-musicians revealed by functional magnetic-resonance signals. Exp Brain Res 125: 417-425. [DOI] [PubMed] [Google Scholar]
- Hutchinson S, Lee LHL, Gaab N, Schlaug G ( 2003) Cerebellar volume of musicians. Cereb Cortex 13: 943-949. [DOI] [PubMed] [Google Scholar]
- Isaacs KR, Anderson BJ, Alcantara AA, Black JE, Greenough WT ( 1992) Exercise and the brain: angiogenesis in the adult rat cerebellum after vigorous physical activity and motor skill learning. J Cereb Blood Flow Metab 12: 110-119. [DOI] [PubMed] [Google Scholar]
- Kansaku K, Yamaura A, Kitazawa S ( 2000) Sex differences in lateralization revealed in the posterior language areas. Cereb Cortex 10: 866-872. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG ( 1995) Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature 377: 155-158. [DOI] [PubMed] [Google Scholar]
- Keenan JP, Thangaraj V, Halpern AR, Schlaug G ( 2001) Absolute pitch and planum temporale. NeuroImage 14: 1402-1408. [DOI] [PubMed] [Google Scholar]
- Kim SG, Ugurbil K, Strick PL ( 1994) Activation of a cerebellar output nucleus during cognitive processing. Science 265: 949-951. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Lussnig E, Schwarz ER, Comery TA, Greenough WT ( 1996) Synaptogenesis and Fos expression in the motor cortex of the adult rat after motor skill learning. J Neurosci 16: 4529-4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsell J, Jansen J ( 1971) The comparative anatomy and histology of the cerebellum, Vol 3. Minneapolis: University of Minnesota. [Google Scholar]
- Lee DJ, Chen Y, Schlaug G ( 2003) Corpus callosum: musician and gender effects. NeuroReport 14: 205-209. [DOI] [PubMed] [Google Scholar]
- Luders E, Gaser C, Jancke L, Schlaug G ( 2003) Structural asymmetries in the musician's brain: a whole-brain voxel-based approach. Presented at the Ninth International Conference on Functional Mapping of the Human Brain, June 19-22, 2003, New York. Available on CD-Rom in NeuroImage, Vol. 19, No. 2. [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD ( 2000) Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci USA 97: 4398-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munte TF, Kohlmetz C, Nager W, Altenmuller E ( 2001) Neuroperception. Superior auditory spatial tuning in conductors. Nature 409: 580. [DOI] [PubMed] [Google Scholar]
- Munte TF, Altenmuller E, Jancke L ( 2002) The musician's brain as a model of neuroplasticity. Nat Rev Neurosci 3: 473-478. [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Flaum M, O'Leary D, Andreasen NC ( 2000) Sexual dimorphism in the human brain: evaluation of tissue volume, tissue composition and surface anatomy using magnetic resonance imaging. Psychiatry Res 98: 1-13. [DOI] [PubMed] [Google Scholar]
- Ohnishi T, Matsuda H, Asada T, Aruga M, Hirakata M, Nishikawa M, Katoh A, Imabayashi E ( 2001) Functional anatomy of musical perception in musicians. Cereb Cortex 11: 754-760. [DOI] [PubMed] [Google Scholar]
- Ozturk AH, Tascioglu B, Aktekin M, Kurtoglu Z, Erden I ( 2002) Morphometric comparison of the human corpus callosum in professional musicians and non-musicians by using in vivo magnetic resonance imaging. J Neuroradiol 29: 29-34. [PubMed] [Google Scholar]
- Pantev C, Oostenveld R, Engelien A, Ross B, Roberts LE, Hoke M ( 1998) Increased auditory cortical representation in musicians. Nature 392: 811-814. [DOI] [PubMed] [Google Scholar]
- Pantev C, Roberts LE, Schulz M, Engelien A, Ross B ( 2001) Timbre-specific enhancement of auditory cortical representations in musicians. NeuroReport 12: 169-174. [DOI] [PubMed] [Google Scholar]
- Parsons LM ( 2001) Exploring the functional neuroanatomy of music performance, perception, and comprehension. Ann NY Acad Sci 930: 211-231. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Nguyet D, Cohen LG, Brasil-Neto JP, Cammarota A, Hallett M ( 1995) Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol 74: 1037-1045. [DOI] [PubMed] [Google Scholar]
- Passingham RE, Toni I ( 2001) Contrasting the dorsal and ventral visual systems: guidance of movement versus decision making. NeuroImage 14: S125-S131. [DOI] [PubMed] [Google Scholar]
- Paulson MJ, Lin T ( 1970) Predicting WAIS IQ from Shipley-Hartford scores. J Clin Psychol 26: 453-461. [DOI] [PubMed] [Google Scholar]
- Pysh JJ, Weiss GM ( 1979) Exercise during development induces an increase in Purkinje cell dendritic tree size. Science 206: 230-232. [DOI] [PubMed] [Google Scholar]
- Schlaug G ( 2001) The brain of musicians. A model for functional and structural adaptation. Ann NY Acad Sci 930: 281-299. [PubMed] [Google Scholar]
- Schlaug G, Knorr U, Seitz R ( 1994) Inter-subject variability of cerebral activations in acquiring a motor skill: a study with positron emission tomography. Exp Brain Res 98: 523-534. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Jancke L, Huang Y, Staiger JF, Steinmetz H ( 1995a) Increased corpus callosum size in musicians. Neuropsychologia 33: 1047-1055. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Jancke L, Huang Y, Steinmetz H ( 1995b) In vivo evidence of structural brain asymmetry in musicians. Science 267: 699-701. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, McDonald D, Holmes C, Lavoie K, Hurwitz AS, Kabani N, Toga A, Evans A, Petrides M ( 1999) Three-dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. NeuroImage 10: 233-260. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M ( 2002) Differences in white matter architecture between musicians and non-musicians: a diffusion tensor imaging study. Neurosci Lett 321: 57-60. [DOI] [PubMed] [Google Scholar]
- Schneider P, Scherg M, Dosch HG, Specht HJ, Gutschalk A, Rupp A ( 2002) Morphology of Heschl's gyrus reflects enhanced activation in the auditory cortex of musicians. Nat Neurosci 5: 688-694. [DOI] [PubMed] [Google Scholar]
- Sergent J, Zuck E, Terriah S, MacDonald B ( 1992) Distributed neural network underlying musical sight-reading and keyboard performance. Science 257: 106-109. [DOI] [PubMed] [Google Scholar]
- Sluming V, Barrick T, Howard M, Cezayirli E, Mayes A, Roberts N ( 2002) Voxel-based morphometry reveals increased gray matter density in Broca's area in male symphony orchestra musicians. NeuroImage 17: 1613-1622. [DOI] [PubMed] [Google Scholar]
- Sluming V, Taylor J, Keller S, Cezayirli E, Roberts N ( 2003) Cerebellar volume and its association with musical performance in symphony orchestra musicians. Presented at the Ninth International Conference on Functional Mapping of the Human Brain, June 19-22, 2003, New York. Available on CD-Rom in NeuroImage, Vol. 19, No. 2. [Google Scholar]
- Toni I, Krams M, Turner R, Passingham RE ( 1998) The time course of changes during motor sequence learning: a whole-brain fMRI study. NeuroImage 8: 50-61. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH ( 1999) Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA 96: 13427-13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witelson SF, Glezer II, Kigar DL ( 1995) Women have greater density of neurons in posterior temporal cortex. J Neurosci 15: 3418-3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witelson SF, Kigar DL, Harvey T ( 1999) The exceptional brain of Albert Einstein. Lancet 353: 2149-2153. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS ( 1992) Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci 12: 2549-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Perry DW, Beckett CA, Westbury CF, Evans AC ( 1998) Functional anatomy of musical processing in listeners with absolute pitch and relative pitch. Proc Natl Acad Sci USA 95: 3172-3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D, Purves D ( 1995) Effects of increased neural activity on brain growth. Proc Natl Acad Sci USA 92: 1802-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K ( 1992) Neuronal plasticity as an adaptive property of the central nervous system. Anat Anz 174: 383-391. [DOI] [PubMed] [Google Scholar]