Abstract
Neurosteroids typified by 5α-pregnan-3α-ol-20-one (5α3α) have emerged as the most potent endogenous positive modulators of the GABAA receptor, the principal mediator of fast inhibitory transmission within the CNS. Neurosteroids can be synthesized de novo in the brain in levels sufficient to modulate GABAA receptor function and, thus, might play an important physiological-pathophysiological role. Indirect support for this proposal comes from the observation that neurosteroid action is region and neuron selective. However, the mechanism(s) that imparts specificity of action remains primarily elusive. Although neurosteroids are relatively promiscuous toward different GABAA receptor isoforms, the contribution of local neurosteroid metabolism has been relatively unexplored. Here, we investigate the role of neurosteroid metabolism by using electrophysiological techniques to compare the actions of 5α3α and its metabolically stable synthetic analog ganaxolone on inhibitory neurotransmission in CA1 and dentate gyrus neurons. Furthermore, we evaluate the contribution of a key enzyme in neurosteroid metabolism [i.e., 3α-hydroxysteroidoxidoreductase (3α-HSOR)] to the inactivation of endogenous, or exogenously applied 5α3α. We show that low concentrations of ganaxolone, but not of 5α3α, enhance inhibitory transmission in dentate gyrus, whereas both steroids are similarly effective in CA1 neurons. Furthermore, inhibition of 3α-HSOR by the contraceptive agent Provera results in enhanced synaptic and extrasynaptic GABAA receptor-mediated inhibition in the dentate gyrus but not in the CA1 region. Collectively, these findings advocate a crucial role for local steroid metabolism in shaping GABAA receptor-mediated inhibition in a regionally dependent manner and suggest a novel action by the contraceptive agent on inhibitory centers in the CNS.
Keywords: neurosteroid, inhibitory synaptic transmission, GABAA receptor, tonic current, hippocampus, patch-clamp
Introduction
A number of independent investigations have established that certain endogenous neurosteroids can potently and selectively enhance the action of the inhibitory neurotransmitter GABA at the GABAA receptor (Paul and Purdy, 1992; Lambert et al., 2001; Smith, 2002). Their behavioral repertoire is consistent with an enhancement of inhibition (i.e., they display anxiolytic, anticonvulsant, and analgesic activity) and, at higher doses, they are hypnotic and anesthetic (Belelli et al., 1990; Lambert et al., 1995). Neuroactive steroids might also be involved in the physiological and pathophysiological regulation of neuronal inhibition. Physiological levels of the most potent neurosteroid [i.e., 5α-pregnan-3α-ol-20-one (5α3α)] fall within the range of concentrations (≥10 nm) shown in vitro to enhance GABAA receptor function (Belelli et al., 1996a,b, 2002). Furthermore, neurosteroids can be synthesized de novo in the brain independent of peripheral sources both in glia and neurons. Thus, 5α3α can be synthesized from progesterone via the sequential action of 5α-reductase and 3α-hydroxysteroidoxidoreductase (3α-HSOR). Interestingly, in contrast to 5α-reductase, 3α-HSOR is involved in both the synthesis and degradation of 5α3α because, depending on whether the cytosolic or membrane-bound isoform prevails, it can either reduce 5α-dihydroprogesterone (5α-DHP) to 5α3α or oxidize 5α3α back to 5α-DHP, respectively (Mellon and Vaudry, 2001). The fact that the brain expression pattern and activity of the enzymes responsible for neurosteroid synthesis and degradation will selectively impact on GABAA receptor-mediated inhibition is supported by the following observations: (1) the activity of the enzymes involved in neurosteroid metabolism exhibits regional variations (Mellon and Vaudry, 2001), (2) administration of finasteride, an inhibitor of 5α-reductase, causes a dramatic decrease in rat brain 5α3α levels and concomitant changes in behavior consistent with the loss of an inhibitory tone (Matsumoto et al., 1999; Pinna et al., 2000), and (3) in animal models of psychiatric illness, inhibitory dysfunction appears to be associated with a loss of 5α-reductase activity (Dong et al., 2001). Findings from this and other laboratories have suggested that neurosteroid modulation of inhibitory transmission is neuron specific, a heterogeneity that might be conferred in part by the isoform of the GABAA receptor and post-translational modifications of the receptor, including phosphorylation (Cooper et al., 1999; Belelli et al., 2002; Harney et al., 2003; Koksma et al., 2003). However, the contribution of local neurosteroid metabolism to neurosteroid action on GABAA receptor-mediated neurotransmission has received little attention. Thus, we compared the actions of 5α3α and its metabolically stable synthetic analog ganaxolone (3β-methyl-3α-ol-5α-pregnan-20-one) in hippocampal CA1 pyramidal neurons and dentate gyrus (DG) granule cells. Additionally, we evaluated the contribution of 3α-HSOR activity to the inactivation of endogenous or exogenously applied 5α3α by using two structurally distinct inhibitors of 3α-HSOR, the anti-inflammatory agent indomethacin, and the commonly used contraceptive progestin, Provera. Interestingly, Provera is also effective in the treatment of catamenial epilepsy (Newmark and Penry, 1980). The results presented here advocate a crucial role for local steroid metabolism in shaping GABAA receptor-mediated inhibition in a regionally dependent manner. Furthermore, the findings with Provera imply that the contraceptive agent might alter the function of inhibitory centers in the CNS.
Materials and Methods
Recordings from rat hippocampal slices
Slice preparation. Hippocampal slices were prepared from Sprague Dawley rats of either sex [postnatal day (P) 16-P24] according to standard protocols described previously (Reynolds et al., 2003). Animals were killed by cervical dislocation in accordance with Schedule 1 of the United Kingdom Government Animals (Scientific Procedures) Act, 1986. The brain was rapidly removed and placed in oxygenated ice-cold artificial CSF (aCSF) solution containing the following (in mm): 225 sucrose, 2.95 KCl, 1.25 NaH2PO4, 26 NaHCO3, 0.5 CaCl2, 10 MgSO4, 10 Glucose, 1 ascorbic acid, and 3 pyruvic acid bubbled with 95% O2/5% CO2 to give a pH of 7.4 (330-340 mOsm). The tissue was maintained in ice-cold aCSF while 300 μm horizontal slices were cut using a Vibratome (Intracel, Royston, UK). The slices were placed in an incubation chamber, consisting of a beaker and a nylon mesh-covered plastic ring on which the slices lay, filled with circulating, oxygenated, extracellular solution containing the following (in mm): 126 NaCl, 2.95 KCl, 26 NaHCO3, 1.25 NaH2PO4, 2 CaCl2, 10 d-glucose, and 2 MgCl2, pH 7.4, when bubbled with 95% O2/5% CO2 (300-310 mOsm). Slices were incubated at 32°C for 1 hr and subsequently allowed to cool at room temperature before being used for recordings.
Electrophysiology. Whole-cell patch-clamp recordings were made at 35°C from hippocampal CA1 pyramidal neurons and dentate granule cells visually identified with a Zeiss 2FS (Zeiss, Welwyn Garden City, UK) or Olympus BX51 (Olympus, Southall, UK) microscope equipped with differential interference contrast-infrared optics. Patch pipettes were prepared from thick-walled borosilicate glass (Garner Glass, Claremont, CA) and had open tip resistances of 3-5 MΩ when filled with an intracellular solution that contained the following (in mm): 140 CsCl, 10 HEPES, 10 EGTA, 2 Mg-ATP, 1 CaCl2, 5 QX-314, pH 7.3, with CsOH, 295-305 mOsm. Miniature IPSCs (mIPSCs) were recorded using an Axopatch 1D or Axopatch 200B amplifier (Axon Instruments, Foster City, CA) at a holding potential of -60 mV in an extracellular recording solution (see above) also containing 2 mm kynurenic acid (Sigma, Poole, UK) and 0.5 μm tetrodotoxin (TTX; Tocris, Bristol, UK) to block ionotropic glutamate receptors and action potentials, respectively. To ensure a reliable detection of the tonic current in dentate granule cells, experiments investigating the influence of steroid or Provera were performed in the absence of TTX on slices previously incubated in the presence of the GABA transaminase inhibitor, 50 μm vigabatrin (Sigma) for 2-4 hr (Yeung et al., 2003). Series resistance ranged from 6-18 MΩ and was compensated up to 80%. In each case, currents were sampled at 10 kHz and filtered at 2 kHz using an 8-pole low-pass Bessel filter.
Drug application. 5α3α, ganaxolone, indomethacin, and Provera were prepared as concentrated (1000×) stock solutions in DMSO while pentobarbital and bicuculline methobromide (10-2m) were dissolved in water. These stock solutions were diluted in the extracellular solution to the desired concentration. The final maximum DMSO concentration (0.2%) had no effect on any of the mIPSC parameters or the tonic current. Preliminary time course experiments revealed that the steroid and 3α-HSOR inhibitors effect was maximum after 5-8 min of exposure and stable afterward up to 30 min. Thus, all modulatory agents were applied via the perfusion system (2-4 ml/min) and allowed to infiltrate the slice for a minimum of 10 min before recordings were acquired. With the exception of 5α3α and ganaxolone, which were generous gifts from Dr. K. Gee (University of California, Irvine, CA), all drugs tested were obtained from Sigma.
Data analysis. Data were recorded onto a digital audiotape (DAT) using a Bio-Logic (Claix, France) DTR 1200 recorder and analyzed off-line using the Strathclyde Electrophysiology Software, WinEDR/WinWCP (courtesy of Dr. J. Dempster, University of Strathclyde, Glasgow, UK). Individual mIPSCs were detected using a -4 pA amplitude threshold detection algorithm and visually inspected for validity. Accepted events were analyzed with respect to peak amplitude, 10-90% rise time, charge transfer, and time for events to decay by 50% (T50) and 90% (T90). To minimize the contribution of dendritically generated currents, which are subject to the unquantifiable effects of cable filtering, analysis was limited to those events that fell within the limits of a Gaussian distribution that describes the peak of the rise-time histogram. Analysis of rise-time histograms generally revealed a skewed distribution with a clear peak below 1 msec. Miniature IPSCs were fitted with either one or two exponentials given by the equations: y(t) = A · e(-t/τ), for mono-exponential mIPSCs; and y(t) = A1 · e(-t/τ1) + A2 · e(-t/τ2) for bi-exponential mIPSCs, where A is amplitude, t is time, and τ is the decay time constant. An improvement of the fit by two exponentials compared with one resulted in a reduction in the SD of the residuals and was confirmed by use of the F-test. To compare mono-exponentially versus bi-exponentially decaying events recorded from each cell, a weighted decay time constant (τw) was also calculated for those mIPSCs exhibiting a bi-exponential decay (τw = τ1 · P1 + τ2 · P2, where τ1 and τ2 are the decay time constants of the first and second exponential functions, and P1 and P2 are the proportions of the current amplitude described by each component.). Individual τ values derived from mono-exponentially decaying events and τw values were averaged together to give a cumulative τ value. Tonic current amplitude was calculated as the difference between the holding current before and after application of 30 μm bicuculline methobromide (Brickley et al., 1996).
All results are reported as the arithmetic mean ± SEM. The large sample approximation of the Kolmogorov-Smirnoff (KS) test (SPSS software; SPSS, Chicago, IL) was used to compare the distribution of the mIPSCs parameters. Statistical significance of mean data was assessed with the unpaired Student's t test or repeated measures ANOVA post hoc followed by the Newman-Keuls test as appropriate, using the SigmaStat (SPSS) software package.
Recordings from Xenopus laevis oocytes. Xenopus laevis oocytes (stage V-VI) were isolated as described previously (Belelli et al., 1996a) and injected into their cytoplasm with 20-40 nl of cRNA transcripts (1 mg/ml) prepared from linearized human GABAA α1, β3, and γ2 cDNA according to standard protocols. Two to twelve days after injection, recordings were performed on such oocytes voltage-clamped at -60 mV with an Axoclamp 2A (Axon Instruments) in the twin-electrode voltage-clamp mode. The voltage and current-passing electrodes were filled with 3 m KCl and had resistances of 0.8-2 MΩ when measured in the recording solution (frog Ringer). The oocytes were continually superfused with frog Ringer containing the following (in mm): 120 NaCl, 1.8 CaCl2, 2 KCl, and 5 HEPES, pH 7.4, with NaOH, at the rate of 7-10 ml/min. Membrane current responses were low-pass filtered at 200 Hz, recorded onto DATs via a Bio-Logic DAT recorder (DTR1200), and simultaneously displayed on a Lectromed (Letchworth, UK) multitrace two-pen recorder. All drugs were applied via the superfusion system. The effects of indomethacin and Provera were assessed by using a concentration of GABA producing a half-maximal response (i.e., EC50). Indomethacin and Provera were prepared as concentrated stock (1000×) in DMSO and diluted to the desired concentration in frog Ringer. The final maximum DMSO concentration (0.1%) had no effect on the control GABA-evoked response. Drugs were preapplied for 40-60 sec before being coapplied with the appropriate GABA concentration. Data were expressed as a percentage of the control response and statistically analyzed by repeated measures ANOVA.
Results
As a prelude to investigate the role of neurosteroid metabolism in shaping GABAergic synaptic transmission, we first characterized the properties of GABAA receptor-mediated mIPSCs recorded at 35°C from hippocampal pyramidal CA1 and DG neurons. Such currents were reversibly blocked by the competitive GABAA receptor antagonist bicuculline (30 μm; data not shown). As illustrated in Table 1, CA1 and DG mIPSC parameters were similar and in agreement with previous studies on rats of similar age (Hollrigel and Soltesz, 1997; Harney et al., 2003).
Table 1.
Summary of mIPSC parameters at 35°C from 62 DG and 57 CA1 neurons
|
|
DG (n = 62) |
CA1 (n = 57) |
|---|---|---|
| Peak amplitude (pA) | 71.57 ± 1.82 | 70.35 ± 1.8 |
| Rise time (msec) | 0.46 ± 0.01 | 0.47 ± 0.01 |
| T50 (msec) | 6.24 ± 0.67 | 5.63 ± 0.18 |
| T90 (msec) | 13.48 ± 0.34* | 12.11 ± 0.34* |
| τ (msec) | 7.54 ± 0.21 | 7.45 ± 0.23 |
| τ1 (msec) | 1.38 ± 0.17 | 1.1 ± 0.05 |
| τ2 (msec) | 11.49 ± 0.38 | 11.08 ± 0.37 |
| Cumulative τ (msec)
|
7.46 ± 0.21
|
7.32 ± 0.22
|
p < 0.05; CA1 versus DG; unpaired t test.
The effect of 5α3α and ganaxolone on mIPSCs recorded from CA1 and DG neurons
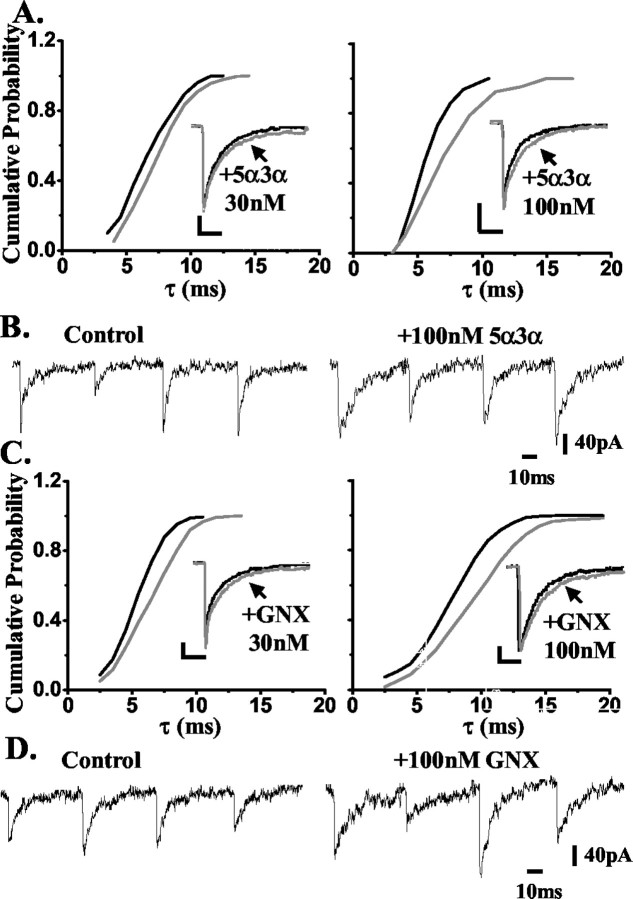
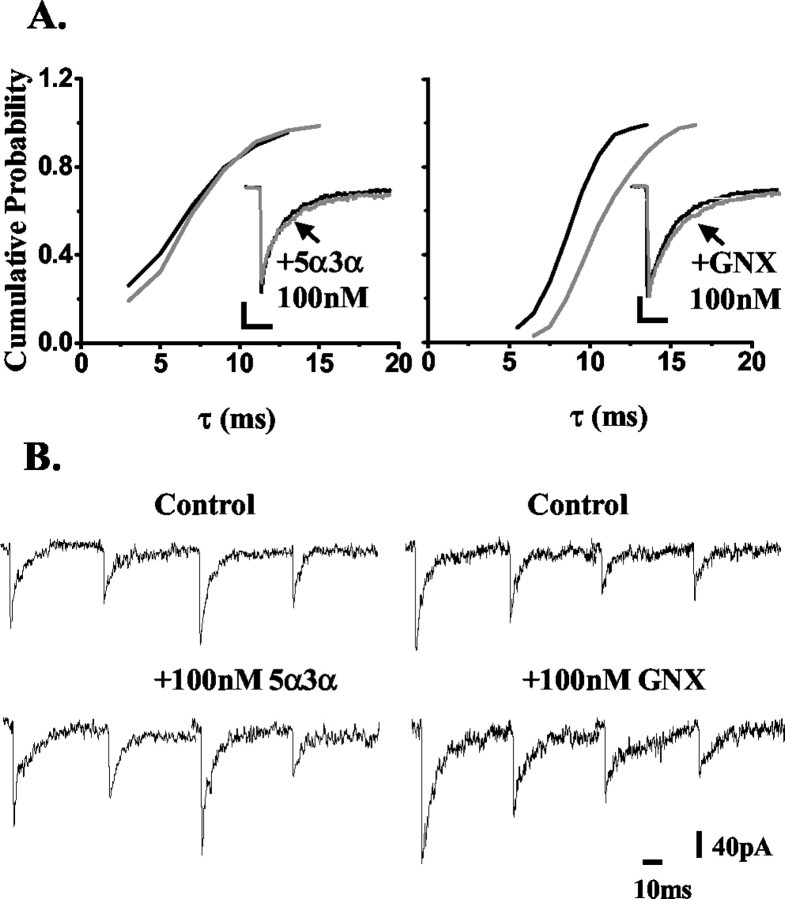
Previous investigations have suggested that GABAA receptors mediating synaptic transmission in hippocampal DG but not CA1 neurons are insensitive to the action of low (≤100 nm) concentrations of certain neurosteroids [e.g., 5α-tetrahydro-deoxycorticosterone (5α-THDOC) or 5β-pregnan-3α-ol-20-one], although higher steroid concentrations did enhance inhibitory transmission (Cooper et al., 1999; Harney et al., 2003). We, and others, have shown that the subunit composition and phosphorylation of the GABAA receptor or associated proteins influence neurosteroid action (Puia et al., 1993; Fancsick et al., 2000; Belelli et al., 2002; Harney et al., 2003; Koksma et al., 2003). However, these are unlikely to fully account for the differential neurosteroid sensitivity of dentate granule cells compared with CA1 neurons (see Discussion). An alternative, yet not mutually exclusive, explanation might lie in local steroid metabolism, which could blunt the steroid action in the dentate gyrus but not in the CA1 region. To test this hypothesis, we investigated and compared the actions of the most potent neurosteroid 5α3α and its metabolically stable synthetic analog ganaxolone on GABAA receptor-mediated mIPSCs recorded from CA1 and DG neurons. As shown in Figure 1, A and B, low concentrations (≤100 nm) of 5α3α significantly enhanced inhibitory transmission in CA1 neurons (i.e., the steroid prolonged the synaptic current decay of GABAA receptor-mediated mIPSCs in all cells tested; p < 0.05; KS test). Thus, 30 and 100 nm 5α3α significantly increased τ by 11 ± 3% (n = 5; p < 0.05) and 25 ± 5% (n = 7; p < 0.01), respectively (Table 2), whereas no significant effect was observed on the peak amplitude (data not shown). The synthetic derivative ganaxolone was similarly effective in CA1 neurons (Fig. 1C,D), with 30 and 100 nm of the steroid producing a significant increase of τ (p < 0.01 for both steroid concentrations) (Table 2) and no significant effect on the peak amplitude (data not shown). In contrast, when 5α3α action was investigated in the dentate gyrus, only 1 of 7 and 3 of 7 cells were sensitive to 30 and 100 nm of the steroid, respectively (KS test; p < 0.05). Overall, the changes in τ values were not significantly different from control for either concentration (Fig. 2A,B, left; Table 2) (p > 0.05 for both concentrations). However, 30 and 100 nm ganaxolone prolonged the mIPSCs synaptic current decay of every DG neuron tested (n = 6-7; p < 0.05; KS test) (Fig. 2A,B, right; Table 2).
Figure 1.
The effect of 5α3α and ganaxolone (GNX) on the decay of mIPSCs recorded from CA1 neurons. A, A cumulative probability plot of the decay of all mIPSCs, expressed as the cumulative time constant τ (see Materials and Methods), recorded from an exemplar CA1 neuron before and after the application of 30 (left) and 100 nm (right) 5α3α. The rightward shift of this relationship induced by either steroid concentration indicates that all mIPSCs recorded from this cell were sensitive to the neurosteroid. The insets illustrate the normalized ensemble average of all mIPSCs from the same CA1 neurons before and after application of 30 (left) and 100 nm (right) 5α3α. Calibration: 20 pA (left), 10 pA (right), 10 msec. B, Examples of individual mIPSC traces recorded from the same CA1 neuron before and after the application of 100 nm 5α3α. C, A cumulative probability plot of the decay of all mIPSCs, expressed as the cumulative time constant τ (see Materials and Methods), recorded from an exemplar CA1 neuron before and after the application of 30 (left) and 100 nm (right) ganaxolone. The rightward shift of this relationship induced by either steroid concentration indicates that all mIPSCs recorded from this cell were sensitive to this steroid. The insets illustrate the normalized ensemble average of all mIPSCs from the same CA1 neurons before and after application of 30 (left) and 100 nm (right) ganaxolone. Calibration: 10 pA, 10 msec. D, Examples of individual mIPSC traces recorded from the same CA1 neuron before and after the application of 100 nm ganaxolone.
Table 2.
The effect of 5α3α and ganaxolone on the decay time constant τ of mIPSCs recorded from CA1 and DG neurons
|
|
CA1 |
Dentate gyrus |
||||
|---|---|---|---|---|---|---|
| τ Percentage increase
|
5α3α
|
Ganaxolone
|
5α3α
|
Ganaxolone
|
||
| 30 nm | 11 ± 3%* | 20 ± 3%**,*** | 4 ± 3% | 22 ± 4%**,*** | ||
| n = 5 | n = 5 | n = 7 | n = 7 | |||
| 100 nm | 25 ± 5%** | 25 ± 6%** | 9 ± 4% | 29 ± 9%**,*** | ||
|
|
n = 7
|
n = 6
|
n = 7
|
n = 6
|
||
Relative changes are expressed as percentage increase of control values. *p < 0.05 versus control; **p < 0.01 versus control; ***p < 0.05 versus same concentration of 5α3α for a given neuronal type; repeated-measures ANOVA; n, number of neurons.
Figure 2.
The effect of 5α3α and ganaxolone (GNX) on the decay of mIPSCs recorded from DG neurons. A, A cumulative probability plot of the decay of all mIPSCs, expressed as the cumulative time constant τ (see Materials and Methods), recorded from an exemplar DG neuron before and after the application of 100 nm 5α3α (left) and 100 nm ganaxolone (right). The rightward shift of this relationship induced by ganaxolone indicates that all mIPSCs recorded from this cell were sensitive to this steroid. In contrast, the lack of the rightward shift induced by 100 nm 5α3α indicates that all mIPSCs recorded from this cell were insensitive to this neurosteroid. The insets illustrate the normalized ensemble average of all mIPSCs from the same DG neurons before and after application of 100 nm 5α3α (left) and 100 nm ganaxolone (right). Calibration: 20 pA, 10 msec. B, Examples of individual mIPSC traces recorded from the same DG neurons before and after the application of 100 nm 5α3α (left) and 100 nm ganaxolone (right).
The effect of inhibitors of 3α-hydroxysteroidoxidoreductase on mIPSCs recorded from CA1 and DG neurons
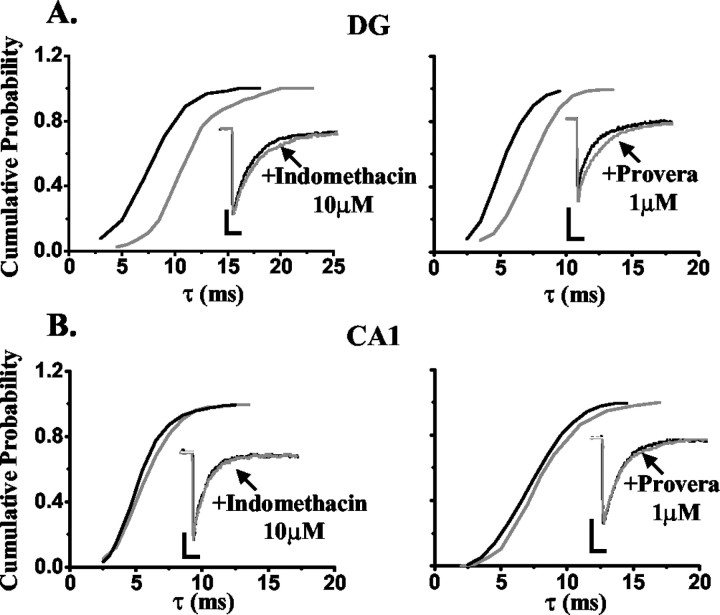
It is clearly established that although the reduction of progesterone to 5α-DHP by 5α-reductase is irreversible, the reduction of the latter to 5α3α by 3α-HSOR can reverse to the inactive precursor (i.e., 5α-DHP) (Mellon and Vaudry, 2001). Thus, we hypothesized that the differential sensitivity of DG granule cells to the action of 5α3α versus ganaxolone may be accounted for by local steroid metabolism, that is, the endogenous progesterone metabolite would be oxidized back to the inactive precursor 5α-dihydroprogesterone, whereas ganaxolone, by virtue of the methyl group in the 3β position, would not (Carter et al., 1997). Thus, to test this hypothesis and investigate the contribution of local steroid metabolism to the selectivity of neurosteroid action, we used indomethacin (10 μm) to inhibit the activity of 3α-HSOR. This concentration equates to five times the calculated IC50 for indomethacin inhibition of rat brain 3α-HSOR and, thus, is expected to completely block the activity of this enzyme (Penning et al., 1985). In a series of preliminary experiments, 10 μm indomethacin was devoid of action at recombinant α1β3γ2 GABAA receptors expressed in Xenopus laevis oocytes (response, 92 ± 3% of control; n = 4; data not shown). However, when the enzyme inhibitor was tested on mIPSCs recorded from DG neurons, the synaptic current decay (i.e., τ) was significantly prolonged by 20 ± 4% (n = 7; p < 0.05) (Fig. 3A, left; Table 3). To validate the specificity of this effect, we also used the structurally distinct contraceptive agent Provera, which is reported to inhibit rat brain 3α-HSOR with an ∼10-fold greater potency than indomethacin (Penning et al., 1985). In common with indomethacin, Provera (1 μm) was devoid of action at recombinant α1β3γ2 GABAA receptors expressed in Xenopus laevis oocytes (response, 90 ± 5% of control; n = 4; data not shown) but significantly prolonged the synaptic current decay of mIPSCs recorded from DG neurons by 24 ± 5% (n = 7; p < 0.05) (Fig. 3A, right; Table 3). The increase in τ produced by 1 μm Provera was not significantly different from that caused by 10 μm indomethacin (p > 0.05) (Table 3). Furthermore, in the presence of 1 μm Provera, 100 nm 5α3α produced a significant increase of τ in addition to that produced by Provera or 5α3α alone (p < 0.05) (Table 3). Therefore, these results suggest that when metabolic inactivation is prevented, an endogenous neurosteroid tone is revealed, which is sufficient to modulate synaptic inhibition. Thus, these findings are consistent with the view that neurosteroid metabolism influences GABAA receptor-mediated synaptic transmission in the dentate gyrus and, in addition, the selectivity of action of administrated neuroactive steroids.
Figure 3.
The effect of indomethacin and Provera on the decay of mIPSCs recorded from DG and CA1 neurons. A, A cumulative probability plot of the decay of all mIPSCs, expressed as the cumulative time constant τ (see Materials and Methods), recorded from an exemplar DG neuron before and after the application of 10 μm indomethacin (left) and 1 μm Provera (right). The rightward shift of this relationship induced by either indomethacin or Provera indicates that all mIPSCs recorded from these cells were sensitive to the 3α-HSOR inhibitor. The insets illustrate the normalized ensemble average of all mIPSCs from the same DG neurons before and after application of 10 μm indomethacin (left) and 1 μm Provera (right). B, A cumulative probability plot of the decay of all mIPSCs, expressed as the cumulative time constant τ (see Materials and Methods), recorded from an exemplar CA1 neuron before and after the application of 10 μm indomethacin (left) and 1 μm Provera (right). The modest rightward shift of this relationship induced by either indomethacin or Provera indicates that all mIPSCs recorded from these cells were marginally sensitive to the 3α-HSOR inhibitor. Note that the magnitude of the shift induced by either 3α-HSOR inhibitor in the CA1 neuron is much smaller compared with that observed for a DG neuron. The insets illustrate the normalized ensemble average of all mIPSCs from the same CA1 neurons before and after application of 10 μm indomethacin (left) and 1 μm Provera (right). Calibration: 20 pA, 10 msec.
Table 3.
The effect of indomethacin and Provera on the decay time constant τ of mIPSCs recorded from CA1 and DG neurons
|
|
Control |
+ Indomethacin (10 μm) |
Control |
+ Provera (1 μm) |
+ Provera (1 μm) + 5α3α (100 nm) |
Control |
+ 5α3α (100 nm) |
|---|---|---|---|---|---|---|---|
| Dentate gyrus | |||||||
| τ (msec) | 7.8 ± 0.5 | 9.3 ± 0.7 | 7.1 ± 0.5 | 8.8 ± 0.6 | 9.7 ± 0.9 | 8.8 ± 1 | 9.5 ± 1 |
| n = 7 | n = 7 | n = 7 | n = 7 | n = 5 | n = 7 | n = 7 | |
| Percentage increase | 20 ± 4*,** | 24 ± 5*,** | 35 ± 6* | 9 ± 4** | |||
| CA1 | |||||||
| τ (msec) | 6.5 ± 0.5 | 6.8 ± 0.3 | 8.1 ± 0.5 | 9 ± 0.6 | |||
| n = 5 | n = 5 | n = 8 | n = 8 | ||||
| Percentage increase
|
|
7 ± 4***
|
|
8 ± 2*,***
|
|
|
|
Relative changes are expressed as percentage increase of control values. *p < 0.001 versus control; **p < 0.05 versus plus 1 μm Provera plus 100 nm 5α3α; ***p < 0.05 CA1 versus dentate gyrus; repeated-measures ANOVA; n, number of neurons.
The observation that, in contrast to the situation of the dentate gyrus, 5α3α and ganaxolone are similarly effective at prolonging GABAA receptor-mediated mIPSCs in CA1 neurons suggests that 3α-HSOR activity might display regional differences. To test this hypothesis, we evaluated and compared the actions of indomethacin and Provera on mIPSCs recorded from CA1 neurons. As illustrated in Table 3 and Figure 3B, 10 μm indomethacin had no significant effect (increase, 7 ± 4%; n = 5; p > 0.05) on CA1 mIPSCs, and 1 μm Provera produced only a modest but significant increase of τ (increase, 8 ± 2%; n = 8; p < 0.05). Furthermore, the effects of these 3α-HSOR inhibitors in CA1 were significantly different from those observed in the DG (p < 0.05 for both indomethacin and Provera in CA1 vs dentate gyrus) (Table 3). These results imply that 3α-HSOR activity differs between CA1 and dentate gyrus and, hence, influences the ability of administrated neuroactive steroids to modulate GABAA receptor-mediated neurotransmission in a manner that is neuron selective (see Discussion).
The effect of 5α3α, ganaxolone, and Provera on the extrasynaptic GABAA receptor-mediated tonic conductance
A number of reports have provided compelling evidence for the presence in the dentate gyrus of an extrasynaptic GABAA receptor-mediated tonic conductance, which has been suggested to be mediated by receptors composed of α4,βx and δ subunits (Mody, 2001; Nusser and Mody, 2002; Stell and Mody, 2002). Recently, we and others have shown that this receptor combination is exquisitively sensitive to the actions of neurosteroids and to those of a number of therapeutically used drugs, which include the anesthetic agents etomidate and pentobarbital but not benzodiazepines (Belelli et al., 2002; Brown et al., 2002; Wohlfarth et al., 2002). Thus, we reasoned that if 3α-HSOR enzymatic activity in the DG prevents modulation of synaptic GABAA receptors by exogenously applied 5α3α, this may additionally hinder modulation of the extrasynaptic GABAA receptor-mediated tonic conductance by 5α3α but not by ganaxolone. Thus, in agreement with previous findings (Stell and Mody, 2002), application of bicuculline (30 μm) unmasked a tonic current of 32 ± 4 pA (n = 17). More importantly, 100 nm ganaxolone enhanced the tonic current in four of five cells tested from 21 ± 4 to 47 ± 7 pA (increase, 136 ± 28%; p < 0.05) (Fig. 4). In contrast, the tonic current from only one of five cells was sensitive to 5α3α action with an increase of 147%. Overall, the tonic current in the presence of 100 nm 5α3α was not significantly different from that calculated in its absence (control, 32 ± 8 pA; plus 100 nm 5α3α, 39 ± 8 pA; p > 0.05; n = 5) (Fig. 5). However, block of 3α-HSOR by 1 μm Provera resulted in a significant increase of the tonic current from 40 ± 9 to 72 ± 18 pA (increase, 74 ± 8; p < 0.05; n = 5) (Fig. 6). The action of this contraceptive was blocked by the subsequent application of 30 μm bicuculline, thus demonstrating that the effects of Provera are GABAA receptor-mediated. Collectively, these results demonstrate that metabolic inactivation of the endogenous neurosteroid tone in the dentate gyrus modulates inhibitory neurotransmission at both synaptic and extrasynaptic loci.
Figure 4.
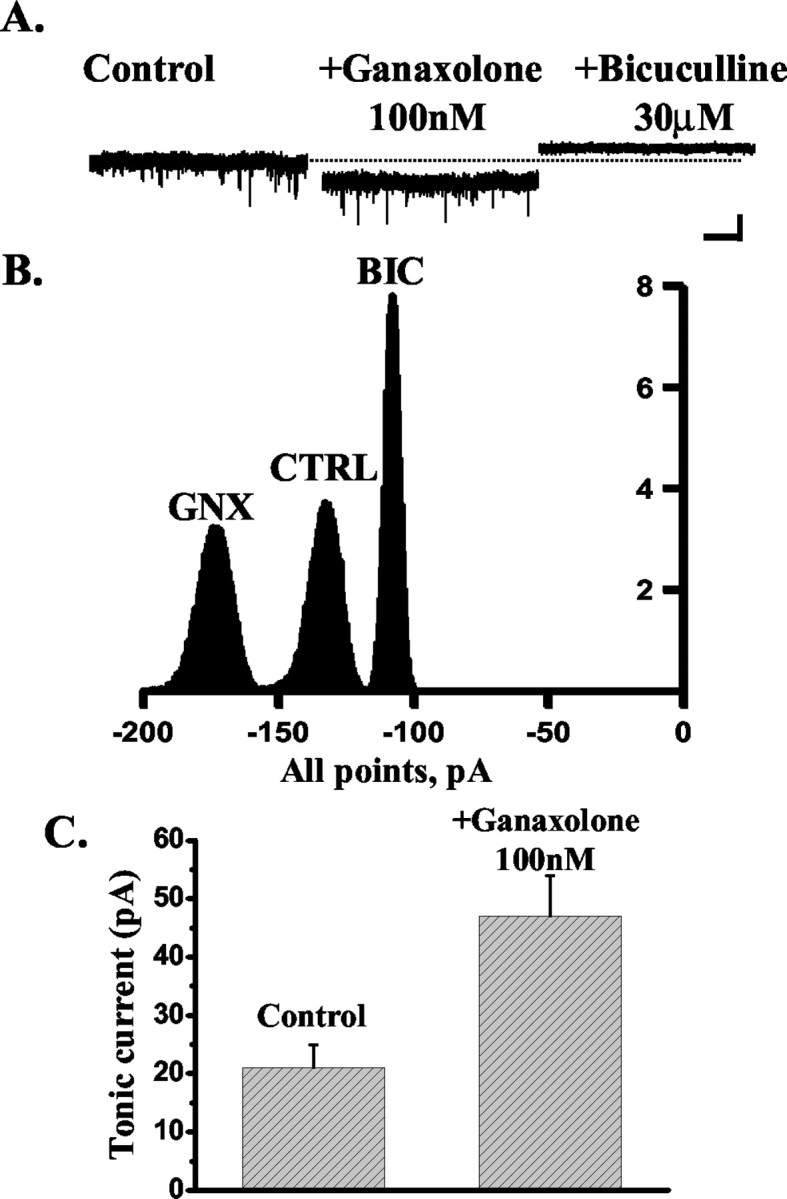
The effect of ganaxolone on the tonic current recorded from DG neurons. A, The tonic current, calculated as the difference between the holding current in the presence and absence of 30 μm bicuculline (see Materials and Methods), recorded from an exemplar DG neuron is enhanced by the application of 100 nm ganaxolone, and the steroid effect is blocked by the subsequent application of 30 μm bicuculline. The dashed line indicates the holding current (pA) under control conditions. Calibration: 50 pA, 5 sec. B, Corresponding all-point histograms illustrate the amplitude of the holding current under control conditions (CTRL) in the presence of 100 nm ganaxolone (GNX) and after the application of 30 μm bicuculline (BIC). C, Bar graph summarizing the enhancement of the tonic current induced by 100 nm ganaxolone in five DG neurons.
Figure 5.
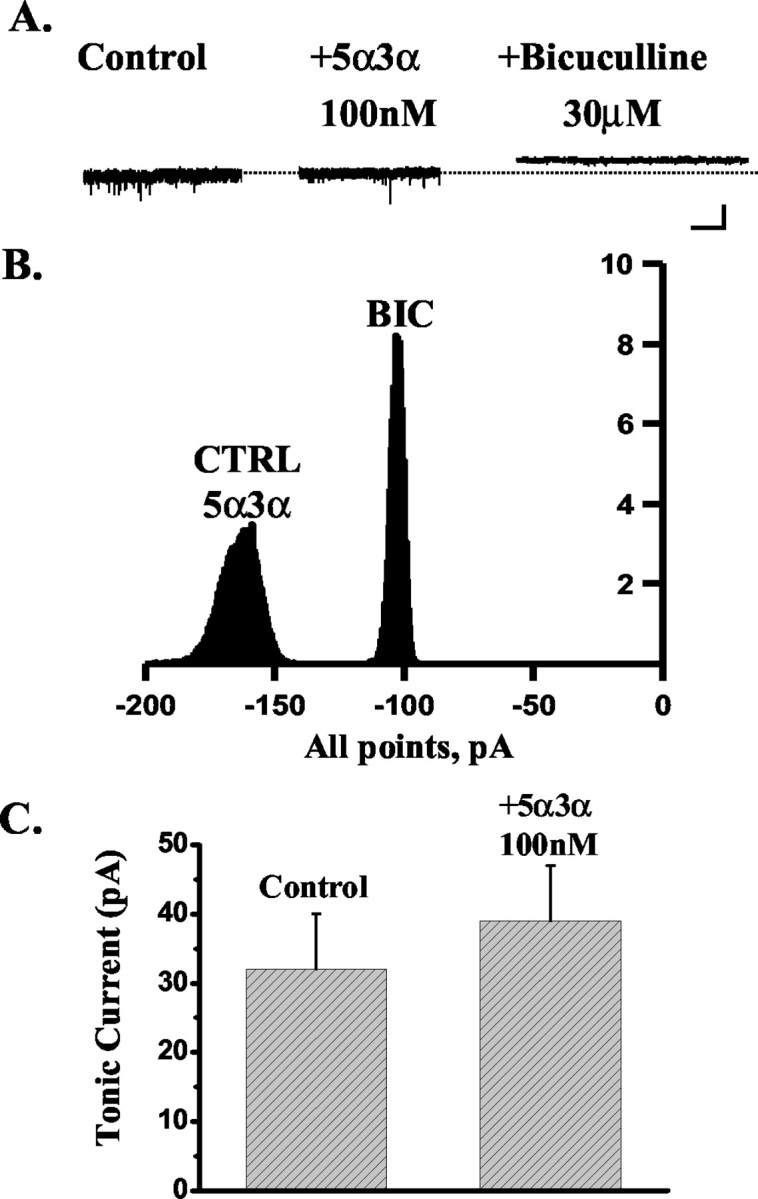
The effect of 5α3α on the tonic current recorded from DG neurons. A, The tonic current, calculated as the difference between the holding current (pA) in the presence and absence of 30 μm bicuculline (see Materials and Methods), recorded from an exemplar DG neuron is insensitive to the application of 100 nm 5α3α. The dashed line indicates the holding current under control conditions. Calibration: 50 pA, 5 sec. B, Corresponding all-point histograms illustrate the amplitude of the holding current under control conditions (CTRL) in the presence of 100 nm 5α3α and after the application of 30 μm bicuculline (BIC). Note that all-point histograms illustrating the amplitude of the holding current under control conditions and in the presence of 100 nm 5α3α overlap, thus indicating the lack of sensitivity of the tonic current recorded from this cell to 5α3α action. C, Bar graph summarizing the lack of a significant effect by 100 nm 5α3α on the tonic current recorded from five DG neurons.
Figure 6.
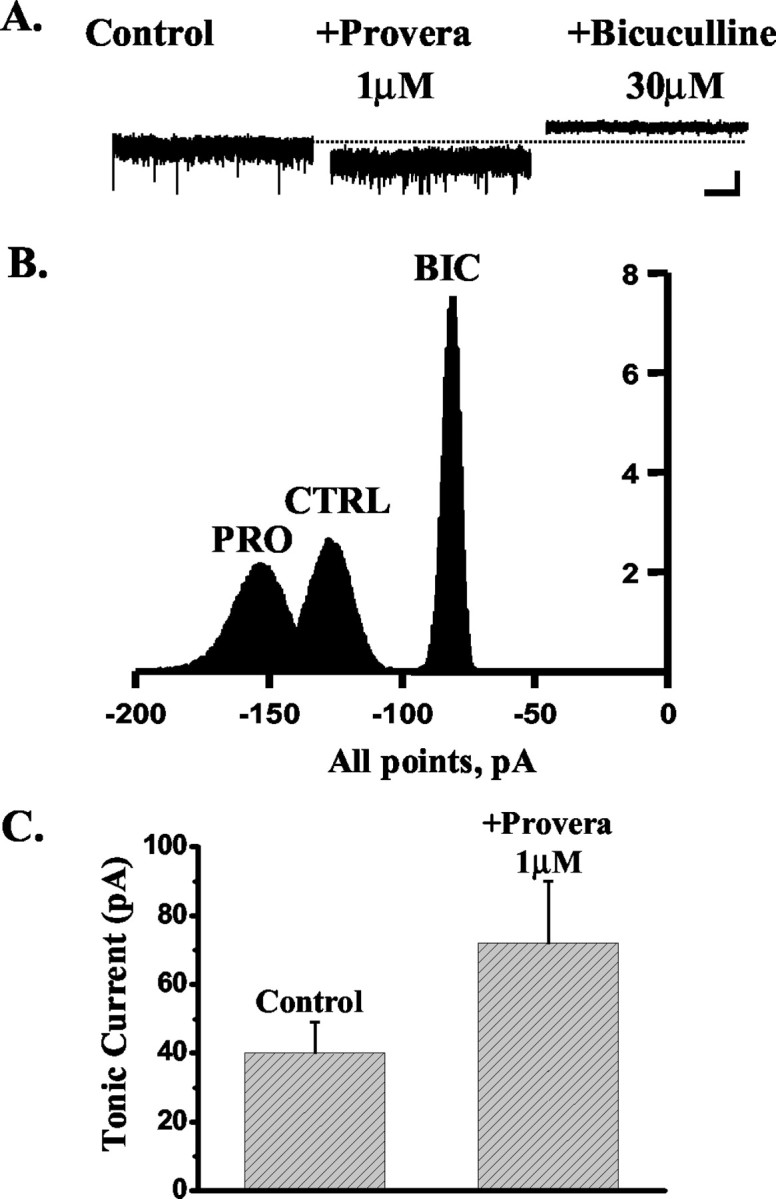
The effect of Provera on the tonic current recorded from DG neurons. A, The tonic current, calculated as the difference between the holding current (pA) in the presence and absence of 30 μm bicuculline (see Materials and Methods), recorded from an exemplar DG neuron is enhanced by the application of 1 μm Provera, and this effect is blocked by the subsequent application of 30 μm bicuculline. The dashed line indicates the holding current under control conditions. Calibration: 50 pA, 5 sec. B, Corresponding all-point histograms illustrate the amplitude of the holding current under control conditions (CTRL) in the presence of 1 μm Provera (PRO) and after the application of 30 μm bicuculline (BIC) C, Bar graph summarizing the enhancement of the tonic current induced by 1 μm Provera in five DG neurons.
Discussion
The action of 5α3α and ganaxolone on GABAA receptor-mediated inhibition in CA1 and dentate gyrus
The aim of the present investigation was twofold: (1) to evaluate the contribution of neurosteroid metabolism to the differential steroid sensitivity of neuronal GABAA receptors, and (2) to assess whether physiologically neurosteroid metabolism exerts a permissive role to shape GABAA receptor-mediated neurotransmission in a regionally dependent manner. To address the first objective, we compared and contrasted the action of the potent endogenously occurring progesterone metabolite 5α3α and the metabolically stable 5α3α analog, ganaxolone, on GABAA receptor-mediated mIPSCs recorded from CA1 and dentate granule cells. The findings presented here show that low concentrations of both 5α3α and ganaxolone are similarly effective at prolonging mIPSCs recorded from CA1 neurons. These results are consistent with the observation that 5α3α and ganaxolone are both equally effective and potent in enhancing the function of both recombinant and native GABAA receptors (Belelli et al., 1996a, b; Carter et al., 1997). In contrast, in the dentate gyrus, both synaptic and extrasynaptic GABAA receptors are sensitive to low concentrations of the synthetic analog ganaxolone but not to 5α3α. The relative insensitivity of DG synapses to 5α3α is in agreement with previous reports on low concentrations (≤100 nm) of either the 5β-isomer or 5α-THDOC in brain slices prepared from rats of comparable age (Cooper et al., 1999; Harney et al., 2003). Proposed reasons for this relative insensitivity include receptor subunit composition and phosphorylation. Of the subunits expressed in dentate gyrus and CA1 that may impact on neurosteroid action (Nusser et al., 1996; Brooks-Kayal et al., 2001), the α1 subunit and the α4 and δ-containing receptor assembly emerge as putative candidates to impart increased neurosteroid sensitivity. In contrast, receptors incorporating the α2 subunit are significantly less sensitive to physiologically relevant concentrations (≤100 nm) of 5α3α (Belelli et al., 2002; Brown et al., 2002). However, recombinant GABAA receptors containing any of these subunits do not discriminate between 5α3α and ganaxolone (Carter et al., 1997; Mascia et al., 2002; D. Belelli, unpublished observations). In addition, α4 and δ-containing recombinant receptors are exquisitively sensitive to steroid action.
Here, however, we demonstrate that extrasynaptic GABAA receptors in the dentate gyrus are relatively insensitive to 5α3α and, yet, such receptors are thought to comprise the α4 and δ subunits (Mody, 2001). Thus, it seems highly unlikely that the receptor subunit composition might explain the differential sensitivity of DG cells to 5α3α and ganaxolone. Recently, phosphorylation has been shown to impact on neurosteroid enhancement of synaptic inhibition (Fancsick et al., 2000; Vicini et al., 2002; Harney et al., 2003; Koksma et al., 2003). Although the precise mechanism by which such regulation occurs remains to be determined, it appears improbable that phosphorylation might selectively influence the actions of 5α3α but not those of ganaxolone. Collectively, these observations strongly implicate local metabolism in the differential sensitivity of DG cells to the endogenous and synthetic steroid. In rat brain, 5α3α can be metabolized by the following different pathways: (1) it can be reduced at the 20 position to the potent GABAA receptor-active neurosteroid 5α-pregnan-3α,20α-diol (Belelli et al., 1996b), (2) it can be sulfated at the 3α-OH group, and (3) it can be oxidized back to the 5α3α precursor (i.e., 5α-DHP). The latter two metabolites are devoid of action at GABAA receptors with the conversion of 5α3α to 5α-DHP being the most important pathway at least in the brain (Korneyev et al., 1993; Dong et al., 2001). In contrast, ganaxolone, by virtue of the methyl group in the 3β position, potentially can only be converted to the 20 hydroxy GABAA receptor-acting metabolite or possibly the 3α-sulfate ester. Contrary to 5α3α, the anticonvulsant action of ganaxolone in both animals and humans is relatively long lasting (Carter et al., 1997; Monaghan et al., 1997). Thus, although a detailed pharmacokinetic profile of ganaxolone in the rodent brain is not available, either the steroid is in the main metabolically stable or the primary metabolites must be active at the GABAA receptor.
The role of 3α-HSOR
The findings discussed above suggest that local neurosteroid metabolism can impart selectivity to the action of administrated steroids at the GABAA receptor. Furthermore, they imply that local metabolism of endogenous neurosteroids influences GABAA receptor-mediated inhibition in a neuron-specific manner. To validate this hypothesis, we blocked the activity of 3α-HSOR in both CA1 and DG neurons with indomethacin and confirmed the specificity of this effect with a more potent, structurally diverse inhibitor of 3α-HSOR (i.e., Provera). Although both ligands display additional pharmacological actions (e.g., indomethacin inhibits arachidonate cyclo-oxygenase, whereas Provera is active at cytosolic progesterone receptors) (Goodman et al., 2001), such actions are unlikely to account for the rapid prolongation of the mIPSC decay or enhancement of GABAA receptor-mediated extrasynaptic tonic conductance observed in dentate gyrus. In addition, neither agent exhibits a direct positive modulatory action on recombinant GABAA receptors.
The reaction catalyzing the conversion of 5α-DHP to 5α3α is established as reversible (Mellon and Vaudry, 2001). Specifically, Li et al. (1997) have identified in rat brain two isoforms of 3α-HSOR: one subtype that is cytosolic and primarily catalyzes the reaction in the reductive direction (i.e., leading to the formation of 5α3α), and another isoform that is membrane-bound and catalyzes the reaction preferentially in the oxidative direction (i.e., leading to the formation of 5α-DHP). Interestingly, when these two enzymatic activities were examined across the rodent brain, a differential regional distribution became apparent, and the dentate gyrus in particular was shown to have a high activity of the membrane-bound 3α-HSOR isoform (Li et al., 1997). We speculate that in the dentate gyrus, the membrane-bound isoform of 3α-HSOR regulates the endogenous levels of 5α3α. This proposal is consistent with previous reports showing that conversion of nanomolar concentrations of 5α3α and 5α-THDOC to 5α-DHP and 5α-deoxycorticosterone, respectively, occurs in rat brain (Purdy et al., 1991). Furthermore, 3H-5α3α is rapidly metabolized to 5α-DHP in a neuroblastoma cell line, and this conversion can be blocked by indomethacin (Rupprecht et al., 1993). Whether the locus of enzyme action is intracellular remains to be determined. From the evidence presented here, it is argued that 3α-HSOR activity in dentate gyrus is sufficient to prevent modulation of neuronal synaptic and extrasynaptic inhibition by relatively low concentrations of exogenously applied 5α3α. Furthermore, the results with either indomethacin or Provera suggest that if not for metabolic inactivation, endogenous dentate gyrus levels of 5α3α would be high enough to enhance both synaptic and extrasynaptic GABAA receptor-mediated inhibition. Interestingly, the magnitude of the steroid effect on the decay of mIPSCs recorded from the dentate gyrus when 3α-HSOR activity is blocked is similar to that produced by the same steroid concentration at CA1 synapses in the absence of inhibition of 3α-HSOR. These findings, coupled with the observation that inhibition of 3α-HSOR activity in CA1 has little if any effect on synaptic inhibition, suggest that the naturally occurring levels of 5α3α are low in both CA1 and dentate gyrus but for different reasons. In the dentate gyrus, local 5α3α is metabolically inactivated by 3α-HSOR, whereas in CA1, the steroid levels are maintained low by other means, possibly because of a reduced synthesis.
To our knowledge, these results provide the first demonstration that 3α-HSOR may shape neuronal inhibition mediated by both synaptic and extrasynaptic GABAA receptors in a neuron-specific manner. In addition, this enzyme influences the action of exogenously applied 5α3α. Such findings are consistent with the recent report that IPSCs recorded from cortical neurons of in vitro brain slices obtained from mice injected systemically with a 5α-reductase inhibitor (i.e., SKF 105111) display faster decay kinetics than their vehicle-injected controls (Puia et al., 2003). Collectively, these observations imply that physiological and pathophysiological alterations of neurosteroid anabolic or catabolic machinery might selectively alter neuronal inhibition. In support of this view, in a mouse model of psychiatric disorders, abnormally low levels of 5α3α are positively correlated with a reduced 5α-reductase activity (Dong et al., 2001). It is tempting to speculate that some human neurological and psychiatric illnesses associated with inhibitory dysfunction and abnormal levels of GABAA receptor-acting neurosteroids (e.g., catamenial epilepsy and postpartum dysphoria) might also be characterized by regionally dependent alterations of the steroid metabolic machinery.
In summary, we demonstrated that selectivity of neurosteroid action at inhibitory GABAergic synaptic and extrasynaptic sites is influenced by local steroid metabolism in a neuron-specific manner. In this regard, we speculate that the ability of the contraceptive agent Provera to inhibit 3α-HSOR might have important neurological implications on the long-term use of this progestin and the action of androgen and progesterone derivatives on inhibitory centers in the CNS. It is intriguing that administration of medroxyprogesterone (i.e., Provera) has long been known to be therapeutically beneficial for the treatment of catamenial seizures but not of noncatamenial epilepsy (Newmark and Penry, 1980). These observations highlight 3α-HSOR as a potential novel target for the development of new therapeutics in the treatment of catamenial epilepsy. Importantly, these discoveries provide the starting point to investigate the role played by specific enzymes involved in neurosteroid metabolism in pathophysiological conditions associated with inhibitory dysfunction.
Footnotes
This work was supported by the Medical Research Council (MRC), Epilepsy Research Foundation, Anonymous Trust, and Commission of the European Communities Programme Quality of Life and Management of Living Resources, QLK1-CT-2000-00179. D.B. is an MRC Senior Fellow. We thank Dr. John Dempster for help and useful comments on the miniature IPSCs analysis.
Correspondence should be addressed to Delia Belelli, Department of Pharmacology and Neuroscience, University of Dundee, Ninewells Hospital and Medical School, Ninewells Hospital, Ninewells Avenue, Dundee, DD1 9SY United Kingdom. E-mail: d.belelli@dundee.ac.uk.
Copyright © 2003 Society for Neuroscience 0270-6474/03/2310013-08$15.00/0
References
- Belelli D, Lan NC, Gee KW ( 1990) Anticonvulsant steroids and the GABA/benzodiazepine receptor-chloride ionophore complex. Neurosci Biobehav Rev 14: 315-322. [DOI] [PubMed] [Google Scholar]
- Belelli D, Callachan H, Hill-Venning C, Peters JA, Lambert JJ ( 1996a) Interaction of positive allosteric modulators with human and Drosophila recombinant GABA receptors expressed in Xenopus laevis oocytes. Br J Pharmacol 118: 563-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ, Peters JA, Gee KW, Lan NC ( 1996b) Modulation of human recombinant GABAA receptors by pregnanediols. Neuropharmacology 35: 1223-1231. [DOI] [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ ( 2002) The influence of subunit composition on the interaction of neurosteroids with GABAA receptors. Neuropharmacology 43: 651-661. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M ( 1996) Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol (Lond) 497: 753-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Kelly ME, Coulter DA ( 2001) γ-Aminobutyric acidA receptor subunit expression predicts functional changes in hippocampal dentate granule cells during postnatal development. J Neurochem 77: 1266-1278. [DOI] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA ( 2002) Pharmacological characterization of a novel cell line expressing human α4β3δ GABAA receptors. Br J Pharmacol 136: 965-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RB, Wood PL, Wieland S, Hawkinson JE, Belelli D, Lambert JJ, White HS, Wolf HH, Mirsadeghi S, Tahir SH, Bolger MB, Lan NC, Gee KW ( 1997) Characterization of the anticonvulsant properties of ganaxolone (CCD 1042; 3α-hydroxy-3β-methyl-5α-pregnan-20-one), a selective, high-affinity, steroid modulator of the γ-aminobutyric acidA receptor. J Pharmacol Exp Ther 280: 1284-1295. [PubMed] [Google Scholar]
- Cooper EJ, Johnston GA, Edwards FA ( 1999) Effects of a naturally occurring neurosteroid on GABAA IPSCs during development in rat hippocampal or cerebellar slices. J Physiol (Lond) 521: 437-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E, Matsumoto K, Uzunova V, Sugaya I, Takahata H, Nomura H, Watanabe H, Costa E, Guidotti A ( 2001) Brain 5α-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc Natl Acad Sci USA 98: 2849-2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancsik A, Linn DM, Tasker JG ( 2000) Neurosteroid modulation of GABA IPSCs is phosphorylation dependent. J Neurosci 20: 3067-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman LS, Gilman AG, Limbird LE, Hardman JG ( 2001) The pharmacological basis of therapeutics, Ed 10 (Goodman LS, Gilman AG, eds). New York: McGraw-Hill.
- Harney S, Frenguelli BG, Lambert JJ ( 2003) Phosphorylation influences neurosteroid modulation of synaptic GABAA receptors in rat CA1 and DG neurons. Neuropharmacology 45: 873-883. [DOI] [PubMed] [Google Scholar]
- Hollrigel GS, Soltesz I ( 1997) Slow kinetics of miniature IPSCs during early postnatal development in granule cells of the dentate gyrus. J Neurosci 17: 5119-5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koksma JJ, van Kesteren RE, Rosahl TW, Zwart R, Smit AB, Luddens H, Brussaard AB ( 2003) Oxytocin regulates neurosteroid modulation of GABAA receptors in supraoptic nucleus around parturition. J Neurosci 23: 788-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korneyev A, Guidotti A, Costa E ( 1993) Regional and interspecies differences in brain progesterone metabolism. J Neurochem 61: 2041-2047. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Hill-Venning C, Peters JA ( 1995) Neurosteroids and GABAA receptor function. Trends Pharmacol Sci 16: 295-303. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Harney SC, Peters JA, Frenguelli BG ( 2001) Modulation of native and recombinant GABAA receptors by endogenous and synthetic neuroactive steroids. Brain Res Brain Res Rev 37: 68-80. [DOI] [PubMed] [Google Scholar]
- Li X, Bertics PJ, Karavolas HJ ( 1997) Regional distribution of cytosolic and particulate 5α-dihydroprogesterone 3α-hydroxysteroid oxidoreductases in female rat brain. J Steroid Biochem Mol Biol 60: 311-318. [DOI] [PubMed] [Google Scholar]
- Mascia MP, Biggio F, Mancuso L, Cabras S, Cocco PL, Gorini G, Manca A, Marra C, Purdy RH, Follesa P, Biggio G ( 2002) Changes in GABAA receptor gene expression induced by withdrawal of, but not by long-term exposure to, ganaxolone in cultured rat cerebellar granule cells. J Pharmacol Exp Ther 303: 1014-1020. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Uzunova V, Pinna G, Taki K, Uzunov DP, Watanabe H, Mienville JM, Guidotti A, Costa E ( 1999) Permissive role of brain allopregnanolone content in the regulation of pentobarbital-induced righting reflex loss. Neuropharmacology 38: 955-963. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Vaudry H ( 2001) Biosynthesis of neurosteroids and regulation of their synthesis. Int Rev Neurobiol 46: 33-78. [DOI] [PubMed] [Google Scholar]
- Mody I ( 2001) Distinguishing between GABAA receptors responsible for tonic and phasic conductances. Neurochem Res 26: 907-913. [DOI] [PubMed] [Google Scholar]
- Monaghan EP, Navalta LA, Shum L, Ashbrook DW, Lee DA ( 1997) Initial human experience with ganaxolone, a neuroactive steroid with antiepileptic activity. Epilepsia 38: 1026-1031. [DOI] [PubMed] [Google Scholar]
- Newmark ME, Penry JK ( 1980) Catamenial epilepsy: a review. Epilepsia 21: 281-300. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Mody I ( 2002) Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol 87: 2624-2628. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P ( 1996) Differential synaptic localization of two major γ-aminobutyric acid type A receptor α subunits on hyppocampal pyramidal cells. Proc Natl Acad Sci USA 93: 11939-11944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul SM, Purdy RH ( 1992) Neuroactive steroids. FASEB J 6: 2311-2322. [PubMed] [Google Scholar]
- Penning TM, Sharp RB, Krieger NR ( 1985) Purification and properties of 3 α-hydroxysteroid dehydrogenase from rat brain cytosol. Inhibition by nonsteroidal anti-inflammatory drugs and progestins. J Biol Chem 260: 15266-15272. [PubMed] [Google Scholar]
- Pinna G, Uzunova V, Matsumoto K, Puia G, Mienville JM, Costa E, Guidotti A ( 2000) Brain allopregnanolone regulates the potency of the GABAA receptor agonist muscimol. Neuropharmacology 39: 440-448. [DOI] [PubMed] [Google Scholar]
- Puia G, Ducic I, Vicini S, Costa E ( 1993) Does neurosteroid modulatory efficacy depend on GABAA receptor subunit composition? Receptors Channels 1: 135-142. [PubMed] [Google Scholar]
- Puia G, Mienville JM, Matsumoto K, Takahata H, Watanabe H, Costa E, Guidotti A ( 2003) On the putative physiological role of allopregnanolone on GABAA receptor function. Neuropharmacology 44: 49-55. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore Jr PH, Paul SM ( 1991) Stress-induced elevations of γ-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci USA 88: 4553-4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds D, Rosahl TW, Cirone J, O'Meara GF, Haythornthwaite A, Newman RJ, Myers J, Sur C, Howell O, Atack J, Macaulay AJ, Hadingham KL, Hutson PH, Belelli D, Lambert JJ, Dawson GR, McKernan R, Whiting PJ, Wafford KA ( 2003) Sedation and anesthesia mediated by distinct GABAA receptor isoforms. J Neurosci 23: 8608-8617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht R, Reul JM, Trapp T, van Steensel B, Wetzel C, Damm K, Zieglgansberger W, Holsboer F ( 1993) Progesterone receptor-mediated effects of neuroactive steroids. Neuron 11: 523-530. [DOI] [PubMed] [Google Scholar]
- Smith SS ( 2002) Withdrawal properties of a neuroactive steroid: implications for GABAA receptor gene regulation in the brain and anxiety behavior. Steroids 67: 519-528. [DOI] [PubMed] [Google Scholar]
- Stell BM, Mody I ( 2002) Receptors with different affinities mediate phasic and tonic GABAA conductances in hippocampal neurons. J Neurosci 22: RC223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicini S, Losi G, Homanics GE ( 2002) GABAA receptor δ subunit deletion prevents neurosteroid modulation of inhibitory synaptic currents in cerebellar neurons. Neuropharmacology 43: 646-650. [DOI] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL ( 2002) Enhanced neurosteroid potentiation of ternary GABAA receptors containing the δ subunit. J Neurosci 22: 1541-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung JY, Canning KJ, Zhu G, Pennefather P, MacDonald JF, Orser BA ( 2003) Tonically activated GABAA receptors in hippocampal neurons are high-affinity, low-conductance sensors for extracellular GABA. Mol Pharmacol 63: 2-8. [DOI] [PubMed] [Google Scholar]