Abstract
Serotonergic (5-HT) neurons in the brainstem modulate a wide range of physiological processes and behaviors. Two transcription factor genes, Pet-1 and Nkx2.2, are necessary but not sufficient to specify the 5-HT transmitter phenotype. Here we show that the Lim class homeobox gene Lmx1b is required for proper formation of the entire 5-HT system in the hindbrain, as indicated by the loss of expression of genes necessary for serotonin synthesis and transport in Lmx1b null mice. Lmx1b and Pet1 act downstream of Nkx2.2, and their expression is independently regulated at the time when 5-HT transmitter phenotype is specified. Ectopic expression of Lmx1b plus Pet-1 is able to induce formation of 5-HT cells in the most ventral spinal cord, where Nkx2.2 is normally expressed. Combined expression of all three genes, Lmx1b, Pet-1, and Nkx2.2, drives 5-HT differentiation in the dorsal spinal cord. Our studies therefore define a molecular pathway necessary and sufficient to specify the serotonergic neurotransmitter phenotype.
Keywords: Lmx1b, Pet-1, Nkx2.2, serotonergic, neurotransmitter specification, brainstem
Introduction
The serotonergic (5-HT) system contains nine small clusters of neurons localized along the midline of the hindbrain (Dahlstrom and Fuxe, 1964; Steinbusch, 1981; Tork, 1990; Jacobs and Azmitia, 1992). These neurons innervate throughout the brain and the spinal cord and modulate a wide array of physiological processes and behaviors (Steinbusch, 1981; Jacobs and Azmitia, 1992; Lucki, 1998). Dysfunction of the 5-HT system is therefore associated with many neurological and psychiatric disorders, including anxiety, depression, aggression, obsessive-compulsive disorder, and schizophrenia (Lucki, 1998; Davidson et al., 2000; Nelson and Chiavegatto, 2001).
5-HT neurons develop from the most ventral hindbrain in proximity to the floor plate (Lidov and Molliver, 1982; Wallace and Lauder, 1983; Hendricks et al., 1999). Signals derived from the floor plate, particularly sonic hedgehog, are required for 5-HT neuron formation (Matise et al., 1998; Ye et al., 1998; Hynes and Rosenthal, 1999; Goridis and Rohrer, 2002). Recent studies have led to the identification of several transcription factors necessary for 5-HT neuron development. The homeobox gene Nxk2.2 is expressed in the ventral precursor cells that first give rise to the visceral motor neurons (vMNs) and then to the 5-HT cells (Briscoe et al., 1999; Pattyn et al., 2003). In Nkx2.2 null mice, prospective 5-HT neurons are transformed into vMNs (Briscoe et al., 1999; Pattyn et al., 2003). Nkx2.2 promotes 5-HT differentiation primarily by suppressing the expression of Phox2b, a paired class homeobox gene (Pattyn et al., 2003). Pet-1, an ETS class transcription factor, is expressed specifically in postmitotic 5-HT neurons (Hendricks et al., 1999, 2003). In Pet-1 null mice, most 5-HT neurons fail to form, and the residual 5-HT neurons are also functionally compromised (Hendricks et al., 2003). Consequently, Pet-1 mutant mice show anxiety and aggressive behaviors (Hendricks et al., 2003). GATA3, a zinc finger gene, is also expressed in postmitotic 5-HT neurons (Van Doorninck et al., 1999); however, the function of GATA3 is still unclear, because the total number of 5-HT neurons is not changed in GATA3 mutant mice (Van Doorninck et al., 1999).
Despite this progress, the molecular pathway capable of the specification of the 5-HT transmitter phenotype remains unknown. For example, Nkx2.2 is also expressed in nonserotonergic neurons (Briscoe et al., 1999; Pattyn et al., 2003). As shown in this study, Pet-1 alone is also insufficient to induce 5-HT cell fate, suggesting the existence of additional transcription regulators for 5-HT differentiation.
The Lim class homeobox gene Lmx1b was initially characterized as a key regulator controlling dorsoventral patterning in the developing limbs (Johnson and Tabin, 1997). Mutation of this gene causes the nail patella syndrome, showing abnormalities in limb and kidney development (Chen et al., 1998). In the nervous system Lmx1b is expressed in restricted populations of neurons, including midbrain dopaminergic (DA) cells (Smidt et al., 2000). Lmx1b is required for the maintenance but not the initial specification of the DA transmitter phenotype (Smidt et al., 2000).
Here we show that Lmx1b is required for the formation of the entire 5-HT system in the hindbrain. Most significantly, we show that the combined function of Lmx1b, Pet-1, and Nkx2.2 is sufficient to induce 5-HT cell fate.
Materials and Methods
Animals. The generation of Lmx1b and Nkx2.2 mutant mice has been described previously (Chen et al., 1998; Sussel et al., 1998). The morning that vaginal plugs were observed was considered as embryonic day (E) 0.5. Wide-type Lmx1b allele was amplified with the following primers that produce a 0.27-kb product: 5′-GATAGGGCATTCAACCAGGACGAGCAAAGA-3′ and 5′-AAACAGAAGCCACAGAGAGCCAAGGAGAAG-3′. Wide-type Nkx2.2 allele was amplified with the following primers that produce a 0.37 kb product: 5′-GAAGCGCCGAGTGCTCTTCTCC-3′ and 5′-GCCGAGCTGTACTGGGCGTTGT-3′. Mutant Lmxb1 and Nkx2.2 alleles were amplified with primers derived from neo gene.
In situ hybridization and immunostaining. Section in situ hybridization was performed as described (Ma et al., 1998), and detailed protocol is available on request. The following in situ probes, including SERT (0.78 kb) (Chang et al., 1996), TPH1 (0.63 kb) (Stoll et al., 1990), TPH2 (0.75 kb) (Walther et al., 2003), VMAT2 (0.75 kb) (GenBank accession number AJ555564), aromatic amino acid decarboxylase (AADC) (0.81 kb) (GenBank accession number NM_016672), Nkx2.2 (0.82 kb) (Price et al., 1992), Pet-1 (0.71 kb) (Pfaar et al., 2002), VGlUT3 (0.73 kb) (Schafer et al., 2002), and GATA3 (1.2 kb) (George et al., 1994) were PCR amplified with cDNA prepared from E13.5 or adult mouse brain. Standard immunostaining was performed with 5-HT antibody (ImmunoStar, Hudson, WI), TPH antibody (Sigma, St. Louis, MO), and Lmx1b antibody (kindly provided by Dr. Tom Jessell, University of Columbia).
In ovo electroporation. The cDNA fragments, which encode the full-length mouse Lmx1b (GenBank accession number NM_010725) and Pet1 (Pfaar et al., 2002) coding regions fused with the Myc epitope, were cloned into the RCASBP chick viral expression vector (Morgan and Fekete, 1996). The Nkx2.2 expression construct, in which the full-length mouse Nkx2.2 cDNA was cloned into the pcDNA3.1/Myc-His(B) (Invitrogen, San Diego, CA) vector, was provided by Dr. David Rowitch (Dana-Farber Cancer Institute). The purified plasmid DNAs were resuspended in sterile water. Hamburger and Hamilton (HH) stage 12 (E2) chick embryos were electroporated unilaterally (five 50 msec pulses at 25 V) with plasmid DNAs using an ECM830 electro-squareporator (BTX, San Diego, CA). After electroporation, the embryos were allowed to grow at 38°C for an additional 48-72 hr. For single plasmid electroporation, 3 μg/μl DNA was used. For Lmx1b plus Pet1 electroporation, 2 μg/μl of each plasmid was used. For triple injections, 1.7 μg/μl Pet1 plus 1.7 μg/μl Lmx1b plus 6.7 μg/μl Nkx2.2 were used.
Results
Lmx1b is expressed in developing serotonergic neurons
To determine whether Lmx1b is expressed in developing 5-HT neurons, we compared Lmx1b expression with that of Pet-1, the prospective 5-HT neuron marker (Hendricks et al., 1999, 2003). Neither Lmx1b nor Pet-1 is expressed in E10.5 ventral hindbrain (data not shown), consistent with the fact that 5-HT neurons develop after E10.75 (Pattyn et al., 2003). In rostral hindbrain of E11.5 embryos, Lmx1b and Pet-1 are expressed in two patches of cells adjacent to the floor plate (Fig. 1A,B, arrows), an area normally giving rise to 5-HT neurons (Lidov and Molliver, 1982; Wallace and Lauder, 1983; Hendricks et al., 1999). By E12.5, formation of 5-HT neurons extends to the caudal hindbrain. At this stage, Lmx1b and Pet-1 again show identical expression patterns in both the rostral (Fig. 1, compare C, D, arrows) and caudal (Fig. 1, compare E, F, arrows) hindbrain midline areas. Double immunostaining of Lmx1b and 5-HT confirmed that Lmx1b is expressed in all 5-HT neurons (Fig. 1G-I, arrows) (data not shown); however, a small subset of Lmx1b-positive neurons is 5-HT negative in E14.5 embryos (Fig. 1I, arrowhead), suggesting that Lmx1b may also be expressed in some nonserotonergic neurons within the raphe nuclei.
Figure 1.

Lmx1b is expressed in serotonergic neurons. Transverse sections through E11.5 pons (A, B), E12.5 caudal pons (C, D), E12.5 medulla (E, F), and E14.5 caudal pons (G-I) are shown. In situ hybridization was performed with the indicated probes (A-F). Lmx1b and Pet-1 are expressed in neurons derived from the ventral midline (A-F, arrows) that correspond to the primordial raphe nuclei. Lmx1b is also expressed weakly in the floor plate (A, arrowhead) and other areas in the medulla (A, star; E, arrowhead). G-H, Coronal sections through the E14.5 pons. Double immunostaining was performed with Lmx1b antibody (G, red) and 5-HT antibody (H, green). I is the superimposed image showing that Lmx1b is expressed in all 5-HT cells (with yellow nuclei) (I, arrow). A small subset of Lmx1b-positive cells is 5-HT negative (I, arrowhead).
Lmx1b is required for the proper specification of 5-HT transmitter phenotype
It has been reported that Pet-1 is essential for the development of the 5-HT transmitter phenotype (Hendricks et al., 1999, 2003). The stringent correlation of Lmx1b and Pet-1 expression suggests that Lmx1b might control 5-HT differentiation. To test this possibility, we examined the expression of a series of 5-HT markers in Lmx1b mutant embryos (Chen et al., 1998). Several enzymes are responsible for 5-HT synthesis. The tryptophan hydroxylases 1 and 2 (TPH1 and TPH2) convert tryptophan to 5-hydroxytryptophan (McGeer and McGeer, 1973; Walther et al., 2003); 5-hydroxytryptophan is then converted into 5-HT by AADC (McGeer and McGeer, 1973). At every stage examined (from E11.5 to P0), 5-HT immunostaining is entirely absent in the Lmx1b mutant hindbrain, including both the rostral areas (Fig. 2, compare A, B and C, D, arrows) and the caudal areas (Fig. 2, compare E, F, arrows). A complete loss of TPH immunostaining was also observed in Lmx1b mutants (data not shown). It should be pointed out that in wild-type embryos, TPH2 but not TPH1 is expressed prominently in 5-HT neurons (Fig. 2G, arrow) (data not shown). Consistent with the loss of 5-HT and TPH immunostaining, expression of TPH2 is absent in both the rostral (Fig. 2, compare G, H, arrow) and caudal (data not shown) hindbrain of Lmx1b mutants. Expression of AADC is also reduced significantly in the medulla area but is largely normal at the pons level (data not shown).
Figure 2.
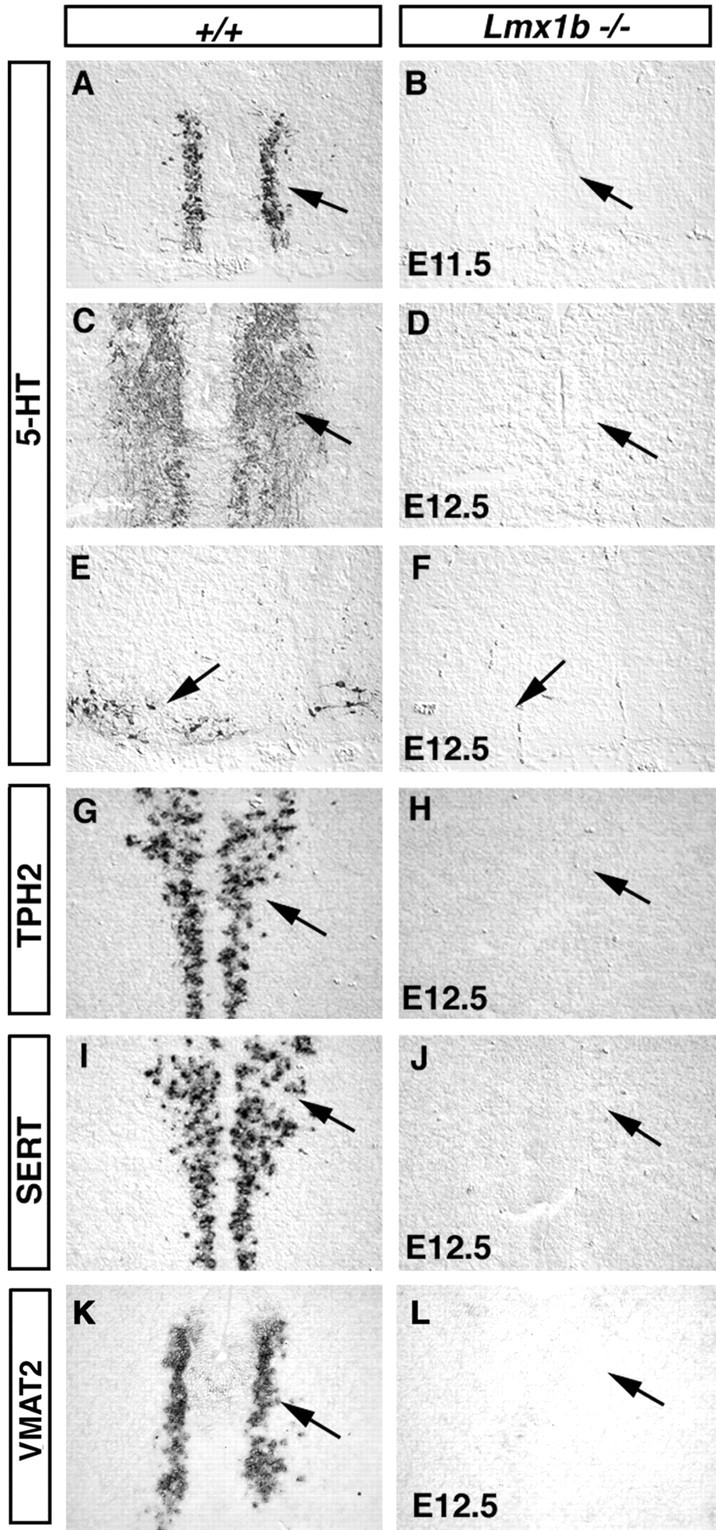
Development of 5-HT neurons is compromised in Lmx1b mutants. Transverse sections through the pons (A-D, G-J) and the medulla (E, F, K, L) of embryos with indicated stages and genotypes are shown. 5-HT immunostaining (A-F) and in situ hybridization (G-L) were performed with the indicated probes (G-L).
Proper function of 5-HT neurons also requires two transporter proteins, SERT and VMAT2 (Weihe and Eiden, 2000). SERT (plasma membrane serotonin transporter) is located in the presynaptic terminals and is responsible for the reuptake of 5-HT after synaptic release (Blakely et al., 1991). VMAT2 (the vesicular monoamine transporter) packages 5-HT into synaptic vesicles (Weihe and Eiden, 2000). In Lmx1b mutants, expression of both SERT (Fig. 2, compare I, J, arrows) and VMAT2 (Fig. 2, compare K, L, arrows) in prospective 5-HT cells is absent. Therefore Lmx1b regulates a set of genes necessary for 5-HT synthesis and transport.
Lmx1b is also weakly expressed in the floor plate (Fig. 1A, arrowhead). To rule out the possibility that the defect of 5-HT neuron development is caused by a patterning change in the ventral hindbrain, we examined a series of markers expressed in the floor plate or ventral precursors. At the medulla level, expression of sonic hedgehog in the floor plate, which plays a central role in patterning the ventral neural tube (Hynes and Rosenthal, 1999), is not affected in Lmx1b mutants (data not shown). Expression of Nkx2.2, which is required for 5-HT differentiation, is also normal in Lmx1b mutant embryos at stages E9.5-E11.5 (Fig. 3, compare A, B, arrows) (data not shown). Consistent with a lack of a patterning defect, prospective 5-HT neurons are formed, as indicated by a normal expression of Pet-1 (Fig. 3, compare C, D, arrows) and GATA3 (Fig. 3, compare E, F, arrows) in E11.5 mutant embryos. A significant amount of Pet-1 expression is also detected in E12.5 mutant embryos, although Pet-1 expression is eventually reduced at prenatal stages (data not shown). 5-HT neurons also release another neurotransmitter (glutamate), and they express VGLUT3, the vesicular glutamate transporter (Gras et al., 2002). Expression of VGLUT3 in prospective 5-HT neurons is largely unaffected in E11.5 and E12.5 Lmx1b mutants (Fig. 3, compare G, H, arrows) (data not shown). The normal expression of a set of markers for prospective 5-HT neurons suggests that Lmx1b is required neither for neurogenesis nor for an early neuronal survival. Lmx1b therefore specifically controls the development of the 5-HT transmitter phenotype.
Figure 3.
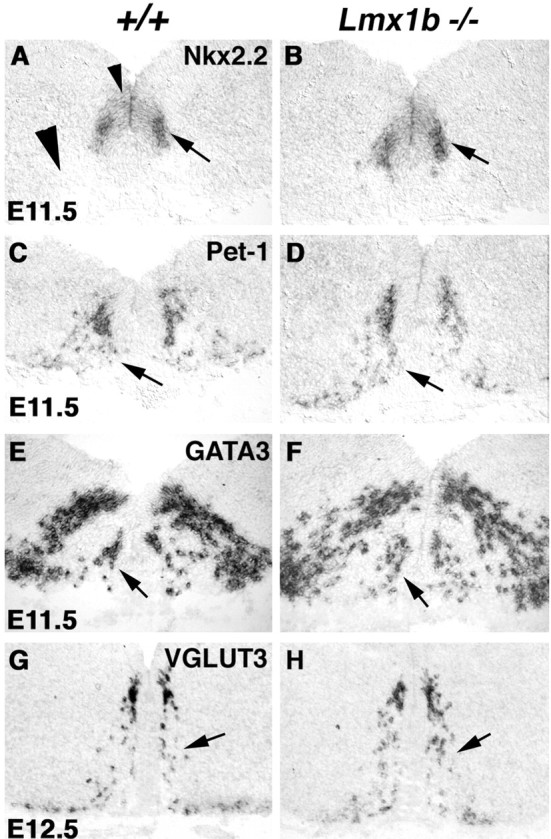
Prospective 5-HT neurons are formed in Lmx1b null embryos. Transverse sections through E11.5 medulla (A-D) and E12.5 pons (E-H) are shown. In situ hybridization was performed with the indicated probes.
Lmx1b and Pet-1 act downstream of Nkx2.2
Pet-1 and Nkx2.2 are also necessary for the specification of 5-HT cell fate (Briscoe et al., 1999; Pattyn et al., 2003). We next examined the functional relationship among these three genes. Nkx2.2 is expressed in the precursor cells (Fig. 4A, small arrowhead) (Briscoe et al., 1999; Pattyn et al., 2003) and possibly in newly formed postmitotic 5-HT neurons in E11.5 medulla (Fig. 4A, arrow). By the time developing 5-HT neurons migrate to a more ventral position, however, Nkx2.2 expression is turned off (Fig. 4A, large arrowhead). By contrast, Lmx1b and Pet-1 are expressed in postmitotic cells, and their expression is maintained in mature 5-HT neurons (Fig. 1) (data not shown) (Hendricks et al., 1999). In line with this temporal order, expression of Lmx1b and Pet-1 is absent in Nkx2.2 mutant hindbrain, from caudal pons to the medulla (Fig. 4, compare A, B; C, D, arrows) (data not shown); however, Lmx1b and Pet-1 expression in the most rostral 5-HT neurons is not affected in E11.75 Nkx2.2 mutants (data not shown), consistent with the fact that development of the most rostral 5-HT neurons is independent of Nkx2.2 (Briscoe et al., 1999). In addition, expression of the GATA3 zinc finger gene, which is normally detected in 5-HT cells (Fig. 4E, arrow) (Van Doorninck et al., 1999), is also absent in E11.75 Nkx2.2 mutants (Fig. 4, compare E, F, arrows), suggesting that Lmx1b, Pet-1, and GATA3 all act downstream of Nkx2.2 during caudal 5-HT neuron development.
Figure 4.
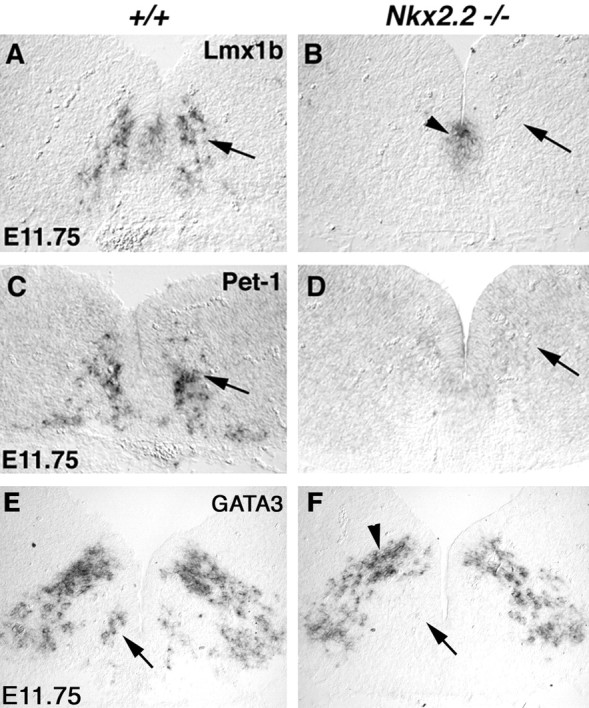
Nkx2.2 is necessary for the expression of Lmx1b, Pet-1, and GATA3. Transverse sections through medulla of E11.75 wild-type (A, C, D) and Nkx2.2 mutants (B, D, F) are shown. In situ hybridization was performed with the indicated probes. Expression of Lmx1b, Pet-1, and GATA3 in prospective 5-HT neurons is lost in Nkx2.2 null medulla (B, D, F, arrows). Residual Lmx1b expression in the floor plate is still detected in Nkx2.2 mutants (B, arrowhead). Also, GATA3 expression in the more dorsally localized neurons is also unaffected in the mutants (F, arrowhead).
Combined expression of Lmx1b, Pet-1, and Nkx2.2 is sufficient to specify 5-HT transmitter phenotype
We then asked whether Nkx2.2, Lmx1b, and Pet-1 together are sufficient to specify the 5-HT transmitter phenotype, by ectopically expressing these genes in chick spinal cord. To do this, we cloned the mouse full-length Lmx1b and Pet-1 cDNAs into the RCASBP viral expression vector. We also used an Nkx2.2 expression construct driven by the cytomegalovirus (CMV) promoter, which is functional in chick neural tube (Sun et al., 2001). These plasmids, singly or in combination, were electroporated into E2 chick neural tube, and the embryos were analyzed 2 or 3 d later. Not surprisingly, singular ectopic expression of Lmx1b, Pet-1, or Nkx2.2 is not sufficient to induce the formation of 5-HT neurons in the spinal cord (Fig. 5A-D) (data not shown). Because endogenous Nkx2.2 is expressed in the most ventral precursor cells (Ericson et al., 1997), the lack of 5-HT induction after ectopic expression of Lmx1b or Pet-1 also suggests that Lmx1b or Pet-1 plus endogenous Nkx2.2 is not sufficient to induce the 5-HT cell fate.
Figure 5.
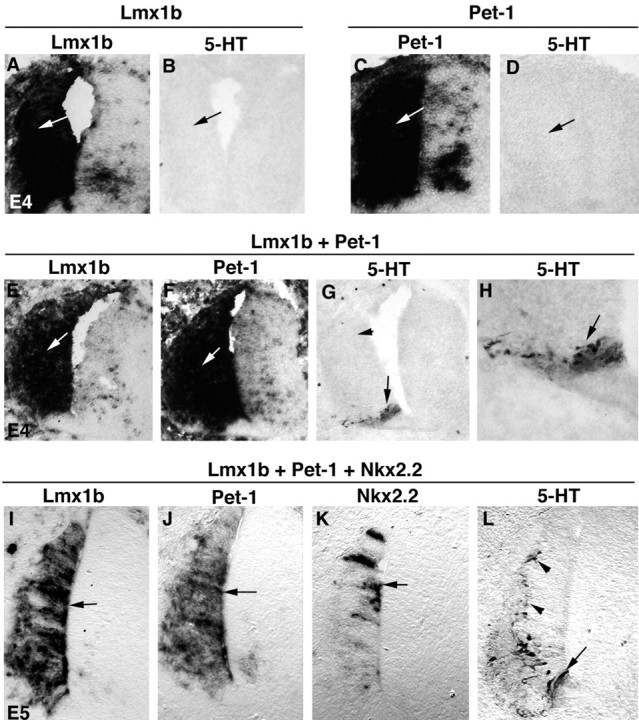
Lmx1b, Pet-1, and Nkx2.2 in combination are sufficient to induce 5-HT cell fate in the chick spinal cord. Transverse sections through the spinal cord are shown. Plasmids were electroporated at E2 and analyzed at E4 (A-H) or E5 (I-L). The plasmids used for electroporation are shown above the lines. Expression of the transgenes (the names of which are shown at the top of the panels and below the lines) was detected by in situ hybridization. Also, 5-HT immunostaining was performed (B, D, G, H, L). H is the high magnification of the positive area shown in G.
Remarkably, co-electroporation of Lmx1b plus Pet-1 is sufficient to induce the formation of 5-HT cells in the most ventral spinal cord (Fig. 5G,H, arrows) but not in the dorsal spinal cord (Fig. 5G, arrowhead), despite the fact that the exogenous Lmx1b and Pet-1 genes are expressed throughout the dorsal ventral axis (Fig. 5E,F, arrows). As mentioned above, Nkx2.2 is endogenously expressed in the ventral precursors in the spinal cord. We then asked whether Nkx2.2 confers the competence to allow Lmx1b and Pet-1 to drive the 5-HT cell fate. We therefore electroporated all three genes, Lmx1b, Pet-1 plus Nkx2.2, into E2 chick neural tubes. We note that Nkx2.2 driven by the CMV promoter is expressed in fewer cells than Lmx1b and Pet-1 driven by the RCAS viral promoter (Fig. 5, compare K, I, J, arrows). Nonetheless, with this combination, ectopic 5-HT neurons appear in both the ventral (Fig. 5L, arrow) and dorsal (Fig. 5L, arrowheads) spinal cord, indicating that combinatorial expression of Nkx2.2, Pet-1, and Lmx1b is sufficient to specify the 5-HT cell fate in the spinal cord.
Discussion
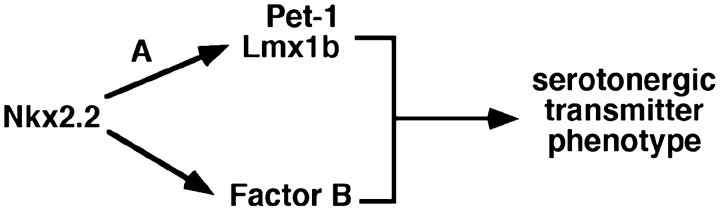
In this study, we demonstrated that Lmx1b is the third gene (besides Pet-1 and Nkx2.2) identified thus far that is required for 5-HT cell fate specification. Lmx1b controls a set of molecules essential for serotonin synthesis (tryptophan hydroxylase), vesicular transport (VMAT2), and reuptake after synaptic release (SERT). Figure 6 summarizes a model regarding how 5-HT neurons are formed in the caudal hindbrain. Nkx2.2 is required for the expression of Lmx1b, Pet-1, and an unknown target gene. These downstream genes then work coordinately to drive the specification of the 5-HT transmitter phenotype (Fig. 6).
Figure 6.
A molecular pathway controlling 5-HT cell fate specification. Nkx2.2 plus an unknown cofactor (A) directly or indirectly activate the expression of Pet-1 and Lmx1b in prospective 5-HT neurons. Nkx2.2 might need a different cofactor to activate another downstream factor (B), or it removes an unknown inhibitor (data not shown) to allow Lmx1b and Pet-1 to promote 5-HT cell fate.
Recent studies show that Nkx2.2 promotes 5-HT cell fate primarily by repressing the expression of Phox2b, a paired class homeobox gene (Pattyn et al., 2003). Thus, it remains unclear whether Nkx2.2 directly or indirectly regulates the expression of Lmx1b and Pet-1. Regardless, Nkx2.2 needs a cofactor (Fig. 6, factor A) expressed in the 5-HT precursor cells to activate Lmx1b and Pet-1 expression, because Lmx1b and Pet-1 are not expressed in all cells derived from Nkx2.2-positive precursor cells, for instance, the visceral motor neurons in the hindbrain and the V3 interneurons in the spinal cord (Hendricks et al., 1999; Qian et al., 2002; Pattyn et al., 2003) (data not shown).
Lmx1b and Pet-1 are sufficient to induce formation of 5-HT-positive cells in the most ventral spinal cord, where Nkx2.2 is expressed, but they need ectopic Nkx2.2 expression to do so in the dorsal spinal cord (Fig. 6). Nkx2.2 either activates another downstream factor (Fig. 6, Factor B) or removes an unknown inhibitor to allow Lmx1b and Pet-1 to specify the 5-HT transmitter phenotype. The putative factor B remains to be identified; however, the fact that 5-HT transmitter phenotype can be induced in the spinal cord suggests that factor B might be expressed throughout the ventral hindbrain and spinal cord. This would be in contrast to the restriction of Lmx1b and Pet-1 expression to the ventral hindbrain. In other words, although Nkx2.2 needs a hindbrain-specific coregulator (Fig. 6, factor A) to activate Lmx1b and Pet-1, it may use a more generic coactivator to regulate factor B. Such model would explain why Nkx2.2 alone or Lmx1b plus Pet1 are insufficient to specify 5-HT cell fate in the spinal cord, whereas a combination of all three genes can. Previously, the GATA3 zinc finger gene has been suggested to be necessary for 5-HT neuron development in the caudal hindbrain (Van Doorninck et al., 1999); however, although the raphe nuclei are disorganized, the total number of 5-HT neurons is not changed in GATA3 null mutants (Van Doorninck et al., 1999). Also, GATA3 is not expressed in the most ventral spinal cord (Pata et al., 1999). Thus, GATA3 is an unlikely candidate for the missing factor B, although it remains a formal possibility that a GATA3-like family member might be involved in 5-HT cell fate specification.
It is noteworthy that although Lmx1b is required for the specification of the 5-HT transmitter phenotype throughout the hindbrain, the most rostral cluster of 5-HT neurons still develops in Nkx2.2 mutants, and residual 5-HT cells are also formed in Pet-1 mutants (Briscoe et al., 1999; Hendricks et al., 2003). Thus, besides working together with Pet-1 and Nkx2.2 to specify the vast majority of 5-HT neurons, Lmx1b may act with other factors to control the formation of the remaining subset of 5-HT neurons. Lmx1b likely acts autonomously in the caudal hindbrain, because Lmx1b is expressed in postmitotic 5-HT neurons (Fig. 1), and no patterning defect is detected in Lmx1b mutant medulla (Fig. 3); however, Lmx1b is essential for proper formation of the isthmic organizer in the junction of midbrain and hindbrain (Adams et al., 2000; Matsunaga et al., 2002). Thus, it remains a formal possibility that Lmx1b might nonautonomously control the formation of the most rostral clusters of 5-HT neurons. Indeed, other structures adjacent to the isthmic organizer fail to develop properly in Lmx1b mutants, including the locus ceruleus noradrenergic center (indicated by loss of expression of β-dopamine hydroxylase) and motor nuclei III and IV (indicated by loss of Phox2b expression) (data not shown).
While this manuscript was being reviewed, Ding and colleagues (2003) reported an independent analysis of Lmx1b mutants, and they also showed an essential role of Lmx1b in 5-HT specification; however, the model that they proposed is quite different from ours (Fig. 6). In their model (Ding et al., 2003), Pet-1 (and GATA3) acts downstream of Lmx1b, whereas in our model Pet-1 and Lmx1b act in combination to specify 5-HT cell fate (Fig. 6). The discrepancy most likely arrived from the stages chosen for their analysis. For instance, they analyzed E14.5 mutant embryos and showed a significant reduction of Pet-1 expression at this stage. We found, however, that Pet1 expression is not affected in E11.5 Lmx1b mutants (Fig. 3), and significant Pet1 expression is also observed at E12.5 (data not shown). In the case of GATA3, although we show a normal expression in E11.5 Lmx1b mutants (Fig. 3), Ding et al. (2003) reported a small reduction at E11. Because caudal 5-HT neurons just start to form at E11 (Pattyn et al., 2003), subtle difference of developmental stages among littermates might cause small variability of GATA3 expression. Thus, our data suggest that at the time when 5-HT neurotransmitter phenotype is specified, expression of Pet-1 (and GATA3) is largely independent of Lmx1b. Interestingly, Lmx1b expression is also unaffected in Pet-1 null mice (Ding et al., 2003). Therefore, Lmx1b and Pet-1 act in combination, rather than in series to promote 5-HT cell fate (Fig. 6). Our model is also consistent with the finding that only combined (but not singular) expression of Lmx1b and Pet-1 is able to induce formation of 5-HT neurons in the ventral spinal cord (Fig. 5); however, the loss of Pet-1 expression in Lmx1b null embryos at E14.5 (Ding et al., 2003) (data not shown) suggests a possible role of Lmx1b in maintaining Pet-1 expression at late embryonic stages.
Lmx1b is also required for proper development of the midbrain DA neurons (Smidt et al., 2000); however, the roles of Lmx1b in these two monoaminergic systems are different. Although Lmx1b is required for the specification of 5-HT transmitter phenotype, Lmx1b is necessary only for the maintenance but not the initial specification of the DA transmitter phenotype (Van Doorninck et al., 1999). Ontogenetically, both DA and 5-HT neurons develop from the ventral precursors adjacent to the floor plate, and their development is regulated by some common signals from the floor plate, such as sonic hedgehog (Hynes and Rosenthal, 1999; Goridis and Rohrer, 2002). Thus, it is not surprising that DA and 5-HT neurons share some overlapping molecular pathways. It will be interesting to determine whether Lmx1b acts in combination with DA-specific transcription factors, such as Nurr1 (Zetterstrom et al., 1997) and Ptx3 (Asbreuk et al., 2002), to control DA neuron development. Lmx1b may also control some common features between DA and 5-HT neurons. Indeed, we found that in Lmx1b mutants, expression of VMAT2, the vesicular transporter for both DA and 5-HT (Weihe and Eiden, 2000), is apparently lost both in prospective 5-HT neurons (Fig. 2) and in midbrain DA neurons (data not shown).
In summary, our studies show that Lmx1b plays an essential role in the specification of the 5-HT transmitter phenotype. Dysfunction of the 5-HT system has been associated with a wide range of neurological and psychiatric disorders (Lucki, 1998; Davidson et al., 2000; Nelson and Chiavegatto, 2001). The demonstration of the sufficiency of Lmx1b, Pet-1, and Nkx2.2 to specify the 5-HT transmitter phenotype might eventually pave the way for in vitro preparation of 5-HT neurons that could be used for cell transplantation-based therapy.
Footnotes
This work was supported by a Medical Foundation Fellowship to C.-L.C. and by National Institutes of Health Grant 1 R01 DE13843-01 to Q.M. Q.M. is a Claudia Adams Barr Scholar and a Pew Scholar in Biomedical Sciences. We thank Dr. John Rubenstein for providing the Nkx2.2 null mice. We thank Paul A. Gray, David Rowitch, Ronald Puettmann-Holgado, Chuck Stiles, and Jeremy Green for critical discussion and comments. We also thank Dr. Tom Jessell for the Lmx1b antibody.
Correspondence should be addressed to Qiufu Ma, SM1022B, Dana-Farber Cancer Institute, One Jimmy Fund Way, Boston, MA 02115. E-mail: Qiufu_Ma@dfci.harvard.edu.
Copyright © 2003 Society for Neuroscience 0270-6474/03/239961-07$15.00/0
L.C. and C.-L.C. contributed equally to this work.
References
- Adams KA, Maida JM, Golden JA, Riddle RD ( 2000) The transcription factor Lmx1b maintains Wnt1 expression within the isthmic organizer. Development 127: 1857-1867. [DOI] [PubMed] [Google Scholar]
- Asbreuk CH, Vogelaar CF, Hellemons A, Smidt MP, Burbach JP ( 2002) CNS expression pattern of Lmx1b and coexpression with ptx genes suggest functional cooperativity in the development of forebrain motor control systems. Mol Cell Neurosci 21: 410-420. [DOI] [PubMed] [Google Scholar]
- Blakely RD, Berson HE, Fremeau RTJ, Caron MG, Peek MM, Prince HK, Bradley CC ( 1991) Cloning and expression of a functional serotonin transporter from rat brain. Nature 354: 66-70. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Sussel L, Serup P, Hartigan-O'Connor D, Jessell TM, Rubenstein J, Ericson J ( 1999) Homeobox gene Nkx2.2 and specification of neuronal identity by graded Sonic hedgehog signalling. Nature 398: 622-627. [DOI] [PubMed] [Google Scholar]
- Chang AS, Chang SM, Starnes DM, Schroeter S, Bauman AL, Blakely RD ( 1996) Cloning and expression of the mouse serotonin transporter. Brain Res Mol Brain Res 43: 185-192. [DOI] [PubMed] [Google Scholar]
- Chen H, Lun Y, Ovchinnikov D, Kokubo H, Oberg KC, Pepicelli CV, Gan L, Lee B, Johnson RL ( 1998) Limb and kidney defects in Lmx1b mutant mice suggest an involvement of LMX1B in human nail patella syndrome. Nat Genet 19: 51-55. [DOI] [PubMed] [Google Scholar]
- Dahlstrom A, Fuxe K ( 1964) Localization of monoamines in the lower brain stem. Experientia 20: 398-399. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL ( 2000) Dysfunction in the neural circuitry of emotion regulation—a possible prelude to violence. Science 289: 591-594. [DOI] [PubMed] [Google Scholar]
- Ding YQ, Marklund U, Yuan W, Yin J, Wegman L, Ericson J, Deneris E, Johnson RL, Chen Z ( 2003) Lmx1b is essential for the development of serotonergic neurons. Nat Neurosci 6: 933-938. [DOI] [PubMed] [Google Scholar]
- Ericson J, Rashbass P, Schedl A, Brenner-Morton S, Kawakami A, Van Heynigen V, Jessell TM, Briscoe J ( 1997) Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell 90: 169-180. [DOI] [PubMed] [Google Scholar]
- George KM, Leonard MW, Roth ME, Lieuw KH, Kioussis D, Grosveld F, Engel JD ( 1994) Embryonic expression and cloning of the murine GATA-3 gene. Development 120: 2673-2686. [DOI] [PubMed] [Google Scholar]
- Goridis C, Rohrer H ( 2002) Specification of catecholaminergic and serotonergic neurons. Nat Rev Neurosci 3: 531-541. [DOI] [PubMed] [Google Scholar]
- Gras C, Herzog E, Bellenchi GC, Bernard V, Ravassard P, Pohl M, Gasnier B, Giros B, El Mestikawy S ( 2002) A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci 22: 5442-5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks T, Francis N, Fyodorov D, Deneris ES ( 1999) The ETS domain factor Pet-1 is an early and precise marker of central serotonin neurons and interacts with a conserved element in serotonergic genes. J Neurosci 19: 10348-10356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES ( 2003) Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron 37: 233-247. [DOI] [PubMed] [Google Scholar]
- Hynes M, Rosenthal A ( 1999) Specification of dopaminergic and serotonergic neurons in the vertebrate CNS. Curr Opin Neurobiol 9: 26-36. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC ( 1992) Structure and function of the brain serotonin system. Physiol Rev 72: 165-229. [DOI] [PubMed] [Google Scholar]
- Johnson RL, Tabin CJ ( 1997) Molecular models for vertebrate limb development. Cell 90: 979-990. [DOI] [PubMed] [Google Scholar]
- Lidov HG, Molliver ME ( 1982) An immunohistochemical study of serotonin neuron development in the rat: ascending pathways and terminal fields. Brain Res Bull 8: 389-430. [DOI] [PubMed] [Google Scholar]
- Lucki I ( 1998) The spectrum of behaviors influenced by serotonin. Biol Psychiatry 44: 151-162. [DOI] [PubMed] [Google Scholar]
- Ma Q, Chen ZF, Barrantes IB, de la Pompa JL, Anderson DJ ( 1998) Neurogenin 1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron 20: 469-482. [DOI] [PubMed] [Google Scholar]
- Matsunaga E, Katahira T, Nakamura H ( 2002) Role of Lmx1b and Wnt1 in mesencephalon and metencephalon development. Development 129: 5269-5277. [DOI] [PubMed] [Google Scholar]
- Matise MP, Epstein DJ, Park HL, Platt KA, Joyner AL ( 1998) Gli2 is required for induction of floor plate and adjacent cells, but not most ventral neurons in the mouse central nervous system. Development 125: 2759-2770. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG ( 1973) Neurotransmitter synthetic enzymes. Prog Neurobiol 2: 69-117. [DOI] [PubMed] [Google Scholar]
- Morgan BA, Fekete DM ( 1996) Manipulating gene expression with replication-competent retroviruses. In: Methods in avian embryology, Vol 51 (Bronner-Fraser ME, ed), pp 185-218. San Diego: Academic. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Chiavegatto S ( 2001) Molecular basis of aggression. Trends Neurosci 24: 713-719. [DOI] [PubMed] [Google Scholar]
- Pata I, Studer M, van Doorninck JH, Briscoe J, Kuuse S, Engel JD, Grosveld F, Karis A ( 1999) The transcription factor GATA3 is a downstream effector of Hoxb1 specification in rhombomere 4. Development 126: 5523-5531. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Vallstedt A, Dias JM, Samad OA, Krumlauf R, Rijli FM, Brunet JF, Ericson J ( 2003) Coordinated temporal and spatial control of motor neuron and serotonergic neuron generation from a common pool of CNS progenitors. Genes Dev 15: 729-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaar H, von Holst A, Vogt Weisenhorn DM, Brodski C, Guimera J, Wurst W ( 2002) mPet-1, a mouse ETS-domain transcription factor, is expressed in central serotonergic neurons. Dev Genes Evol 212: 43-46. [DOI] [PubMed] [Google Scholar]
- Price M, Lazzaro D, Pohl T, Mattei MG, Ruther U, Olivo JC, Duboule D, Di Lauro R ( 1992) Regional expression of the homeobox gene Nkx-2.2 in the developing mammalian forebrain. Neuron 8: 241-255. [DOI] [PubMed] [Google Scholar]
- Qian Y, Shirasawa S, Chen CL, Cheng L, Ma Q ( 2002) Proper development of relay somatic sensory neurons and D2/D4 interneurons requires homeobox genes Rnx/Tlx-3 and Tlx-1. Genes Dev 16: 1220-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer MK, Varoqui H, Defamie N, Weihe E, Erickson JD ( 2002) Molecular cloning and functional identification of mouse vesicular glutamate transporter 3 and its expression in subsets of novel excitatory neurons. J Biol Chem 277: 50734-50748. [DOI] [PubMed] [Google Scholar]
- Smidt MP, Asbreuk CH, Cox JJ, Chen H, Johnson RL, Burbach JP ( 2000) A second independent pathway for development of mesencephalic dopaminergic neurons requires Lmx1b. Nat Neurosci 3: 337-341. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW ( 1981) Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience 6: 557-618. [DOI] [PubMed] [Google Scholar]
- Stoll J, Kozak CA, Goldman D ( 1990) Characterization and chromosomal mapping of a cDNA encoding tryptophan hydroxylase from a mouse mastocytoma cell line. Genomics 7: 88-96. [DOI] [PubMed] [Google Scholar]
- Sun T, Echelard Y, Lu R, Yuk DI, Kaing S, Stiles CD, Rowitch DH ( 2001) Olig bHLH proteins interact with homeodomain proteins to regulate cell fate acquisition in progenitors of the ventral neural tube. Curr Biol 11: 1413-1420. [DOI] [PubMed] [Google Scholar]
- Sussel L, Kalamaras J, Hartigan-O'Connor DJ, Meneses JJ, Pedersen RA, Rubenstein JL, German MS ( 1998) Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic beta cells. Development 125: 2213-2221. [DOI] [PubMed] [Google Scholar]
- Tork I ( 1990) Anatomy of the serotonergic system. Ann NY Acad Sci 600: 9-34. [DOI] [PubMed] [Google Scholar]
- Van Doorninck JH, van Der Wees J, Karis A, Goedknegt E, Engel JD, Coesmans M, Rutteman M, Grosveld F, De Zeeuw CI ( 1999) GATA-3 is involved in the development of serotonergic neurons in the caudal raphe nuclei. J Neurosci 19: RC12(1-8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JA, Lauder JM ( 1983) Development of the serotonergic system in the rat embryo: an immunocytochemical study. Brain Res Bull 10: 459-479. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Peter JU, Bashammakh S, Hortnagl H, Voits M, Fink H, Bader M ( 2003) Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 3: 76. [DOI] [PubMed] [Google Scholar]
- Weihe E, Eiden LE ( 2000) Chemical neuroanatomy of the vesicular amine transporters. FASEB J 14: 2435-2449. [DOI] [PubMed] [Google Scholar]
- Ye W, Shimamura K, Rubenstein JL, Hynes MA, Rosenthal A ( 1998) FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell 93: 755-766. [DOI] [PubMed] [Google Scholar]
- Zetterstrom RH, Solomin L, Jansson L, Hoffer BJ, Olson L, Perlmann T ( 1997) Dopamine neuron agenesis in Nurr1-deficient mice. Science 276: 248-250. [DOI] [PubMed] [Google Scholar]