Abstract
Increasing evidence has suggested that the interaction between dopaminergic and glutamatergic systems in prefrontal cortex (PFC) plays an important role in normal mental functions and neuropsychiatric disorders. In this study, we examined the regulation of NMDA-type glutamate receptors by the PFC dopamine D4 receptor (one of the principal targets of antipsychotic drugs). Application of the D4 receptor agonist PD168077 caused a reversible decrease of the NMDA receptor (NMDAR)-mediated current in acutely isolated and cultured PFC pyramidal neurons, an effect that was blocked by selective D4 receptor antagonists. Furthermore, application of PD168077 produced a potent reduction of the amplitude (but not paired-pulse ratio) of evoked NMDAR EPSCs in PFC slices. The D4 modulation of NMDA receptors in PFC involved the inhibition of protein kinase A, activation of protein phosphatase 1 and the ensuing inhibition of active Ca2+–calmodulin-dependent kinase II (CaMKII). Moreover, PD168077 reduced the surface expression of NMDARs and triggered the internalization of NMDARs in a manner dependent on CaMKII activity. These results identify a mechanistic link between D4 and NMDA receptors in PFC pyramidal neurons, suggesting that D4 receptors may play an important role in modulating synaptic plasticity and thus cognitive and emotional processes in PFC circuits.
Keywords: dopamine receptors, NMDA receptor channels, NMDAR-EPSC, protein kinase A, protein phosphatase-1, Ca2+–calmodulin-dependent kinase II, NMDA receptor internalization
Introduction
Prefrontal cortex (PFC), which is highly associated with the control of cognition and emotion (Goldman-Rakic, 1995; Miller, 1999), is one of the most prominent brain regions affected by schizophrenia (Andreasen et al., 1997; Lewis and Lieberman, 2000). Schizophrenics often exhibit deficits in cognitive tasks requiring working memory subserved by PFC (Goldman-Rakic, 1994) and fail to show normal activation of PFC neurons when attempting to perform working memory tasks (Weinberger et al., 1986; Taylor, 1996). Despite the lack of a clear understanding of the etiology of schizophrenia, pharmacological therapies developed to alleviate clinical symptoms in patients implicate the aberrations of several neurotransmitter systems in this disorder. Among them, dysfunctions of the dopaminergic and glutamatergic systems in PFC are considered to be major contributing factors to the pathophysiology of schizophrenia (Carlsson et al., 2001).
PFC functions are highly influenced by the dopaminergic input from the ventral tegmental area (Brozoski et al., 1979; Berger et al., 1988). Among the five dopamine receptors, D4 receptor is expressed at a high level in PFC pyramidal principal neurons and GABAergic interneurons (Mrzljak et al., 1996; Wedzony et al., 2000). Several lines of evidence suggest that D4 receptor is critically involved in PFC functioning and neuropsychiatric disorders (Oak et al., 2000). Many of the “atypical” antipsychotic drugs (such as clozapine) have high affinities for D4 receptors (Van Tol et al., 1991; Kapur and Remington, 2001). Elevated D4 receptors have been found in PFC of schizophrenia patients (Seeman et al., 1993). D4 receptor antagonists can ameliorate cognitive deficits exhibited by monkeys after long-term treatment with the psychotomimetic drug phencyclidine (PCP) (Jentsch et al., 1997, 1999). Moreover, genetic ablation of D4 receptors in mice results in supersensitivity to psychomotor stimulants (Rubinstein et al., 1997), reduced exploration of novel stimuli (Dulawa et al., 1999), and cortical hyperexcitability (Rubinstein et al., 2001).
In addition to the well recognized dopaminergic dysfunction in schizophrenia, hypofunction of NMDA receptors has been implicated in schizophrenia (Tsai and Coyle, 2002). Administration of the noncompetitive NMDA receptor antagonists, such as PCP and MK-801, produces behavioral symptoms that remarkably resemble schizophrenia in humans and animals and exacerbates symptoms in schizophrenics (Javitt and Zukin, 1991; Jentsch and Roth, 1999). The connection of NMDA receptors to schizophrenia is further corroborated by a mouse model expressing 5% of the normal level of NR1 (Mohn et al., 1999). These mice exhibit a series of schizophrenia-like behaviors, and administration of antipsychotic drugs that target dopamine receptors ameliorates behavioral abnormalities in the NMDA receptor (NMDAR) mutant mice (Mohn et al., 1999).
In this study, we examined the interaction between D4 and NMDA receptors in PFC pyramidal neurons, in the hope of providing some clues on how the two prominent models of schizophrenia, hyperdopaminergia and hypoglutamatergia, may be mechanistically linked.
Materials and Methods
Acute-dissociation procedure. PFC neurons from young adult (3–5 weeks postnatal) rats or mice were acutely dissociated using procedures similar to those described previously (Yan and Surmeier, 1997; Feng et al., 2001). All experiments were performed with the approval of State University of New York at Buffalo Animal Care Committee. After incubation of brain slices in a NaHCO3-buffered saline, PFC was dissected and placed in an oxygenated Cell-Stir chamber (Wheaton, Inc., Millville, NJ) containing papain (0.4 mg/ml; Sigma, St. Louis, MO) in HEPES-buffered HBSS (Sigma) at 35°C. After 20–40 min of enzyme digestion, tissue was rinsed three times in the low Ca 2+, HEPES-buffered saline and mechanically dissociated with a graded series of fire-polished Pasteur pipettes. The cell suspension was then plated into a 35 mm Lux Petri dish that was then placed on the stage of a Nikon (Tokyo, Japan) inverted microscope.
Primary neuronal culture. Rat PFC cultures were prepared by modification of previously described methods (Goslin and Banker, 1991). Briefly, PFC was dissected from 18 d rat embryos, and cells were dissociated using trypsin and trituration through a Pasteur pipette. The neurons were plated on coverslips coated with poly-l-lysine in DMEM with 10% fetal calf serum at a density of 3000 cells/cm 2. When neurons attached to the coverslip within 24 hr, the medium was changed to Neurobasal with B27 supplement. Neurons were maintained in the same kind of media for 3 weeks before being used for recordings.
Whole-cell recordings. Whole-cell recordings of whole-cell ion channel currents used standard voltage-clamp techniques (Yan et al., 1999; Ma et al., 2003). The internal solution consisted of (in mm): 180 N-methyl-d-glucamine (NMG), 40 HEPES, 4 MgCl2, 0.1 BAPTA, 12 phosphocreatine, 2 Na2ATP, 0.2 Na3GTP, and 0.1 leupeptin, pH 7.2–7.3, 265–270 mOsm/l. The external solution consisted of (in mm): 127 NaCl, 20 CsCl, 10 HEPES, 1 CaCl2, 5 BaCl2, 12 glucose, 0.001 TTX, and 0.02 glycine, pH 7.3–7.4, 300–305 mOsm/l. Recordings were obtained with an Axon Instruments (Foster City, CA) 200B patch-clamp amplifier that was controlled and monitored with an IBM personal computer running pClamp (version 8) with a DigiData 1320 series interface (Axon Instruments). Electrode resistances were typically 2–4 MΩ in the bath. After seal rupture, series resistance (4–10 MΩ) was compensated (70–90%) and periodically monitored. The cell membrane potential was held at –60 mV. The application of NMDA (100 μm) evoked a partially desensitizing inward current. Peak values were measured for generating the plot as a function of time and drug application. NMDA was applied for 2 sec every 30 sec to minimize desensitization-induced decrease of current amplitude. Drugs were applied with a gravity-fed “sewer pipe” system. The array of application capillaries (∼150 μm inner diameter) was positioned a few hundred micrometers from the cell under study. Solution changes were effected by the SF-77B fast-step solution stimulus delivery device (Warner Instrument Co., Hamden, CT).
Dopamine receptor ligands PD168077 maleate (PD), l-741742 hydrochloride, l-745870 trihydrochloride (Tocris, Ballwin, MO), (RS)-(±)-sulpiride (Sigma), as well as second messenger reagents cpt-cAMP, myristoylated PKI[14–22], PKC19–36, PKI[5–24], U73122, microcystin, okadaic acid (OA), okadaic acid methyl ester (OAE), KN-93, KN-92, autocamtide-2 related inhibitory peptide (AIP), calmodulin, and calmidazolium (Calbiochem, San Diego, CA) were made up as concentrated stocks and stored at –20°C. The final DMSO concentration in all applied solutions was <0.1%. No change on NMDAR currents has been observed with this concentration of DMSO. Stocks were thawed and diluted immediately before use. The amino acid sequence for the phosphorylated inhibitor-1 (I-1) peptide p Thr35I-1[7–39] is: PRKIQFTVPLLEPHLDPEAAEQIRRRRP (pT)PATL. Mice with deficient α-CaMKII were created as described previously (Silva et al., 1992).
Data analyses were performed with AxoGraph (Axon Instruments, Union City, CA), Kaleidagraph (Albeck Software, Reading, PA), Origin 6 (OriginLab Co., Northampton, MA), and Statview (Abacus Concepts, Calabasas, CA). For analysis of statistical significance, Mann–Whitney U tests were performed to compare the current amplitudes in the presence or absence of agonists. ANOVA tests were performed to compare the differential degrees of current modulation between groups subjected to different treatment.
Electrophysiological recordings in slices. To evaluate the regulation of NMDAR-mediated EPSCs by D4 receptors in PFC slices, the whole-cell technique (Wang et al., 2002; Zhong et al., 2003) was used for voltage-clamp recordings using patch electrodes (5–9MΩ) filled with the following internal solution (in mm): 130 Cs-methanesulfonate, 10 CsCl, 4 NaCl, 10 HEPES, 1 MgCl2, 5 EGTA, 2.2 QX-314, 12 phosphocreatine, 5 MgATP, 0.2 Na3GTP, and 0.1 leupeptin, pH 7.2–7.3, 265–270 mOsm/l. The slice (300 μm) was placed in a perfusion chamber attached to the fixed-stage of an upright microscope (Olympus, Tokyo, Japan) and submerged in continuously flowing oxygenated artificial CSF (ACSF). Cells were visualized with a 40× water-immersion lens and illuminated with near infrared (IR) light, and the image was detected with an IR-sensitive CCD camera. A Multiclamp 700A amplifier was used for these recordings. Tight seals (2–10 GΩ) from visualized pyramidal neurons were obtained by applying negative pressure. The membrane was disrupted with additional suction, and the whole-cell configuration was obtained. The access resistances ranged from 13–18 MΩ and were compensated 50–70%.
For the recording of NMDAR-mediated EPSCs, cells were bathed in ACSF containing CNQX (20 μm) and bicuculline (10 μm) to block AMPA–kainate receptors and GABAA receptors. Evoked currents were generated with a 50 μsec pulse from a stimulation isolation unit controlled by a S48 pulse generator (Astro-Med, Inc., West Warwick, RI). A bipolar stimulating electrode (FHC, Inc., Bowdoinham, ME) was positioned ∼100 μm from the neuron under recording. Before stimulation, cells (voltage-clamped at –70 mV) were depolarized to +60 mV for 3 sec to fully relieve the voltage-dependent Mg 2+ block of NMDAR channels (Hestrin et al., 1990). Clampfit Program (Axon Instruments) was used to analyze evoked synaptic activity. The amplitude of EPSC was calculated by taking the mean of a 2–4 msec window around the peak and comparing with the mean of a 4–8 msec window immediately before the stimulation artifact.
Western blot analysis. PFC slices were prepared as previously described (Gu et al., 2003). After incubation in a NaHCO3-buffered saline for 1 hr, PFC slices were treated with various agents for 30 min as described in the text, followed by a 10 min treatment with PD168077. Equal amounts of protein from PFC slice homogenates were separated on 7.5% acrylamide gels and transferred to nitrocellulose membranes. The blots were blocked with 5% nonfat dry milk for 1 hr at room temperature. Then the blots were incubated with the anti-Thr 286-phosphorylated CaMKII antibody (1:2000; Promega, Madison, WI) for 1 hr at room temperature. After being rinsed, the blots were incubated with horseradish peroxidase-conjugated anti-rabbit antibodies (1:2000; Amersham Biosciences, Arlington Heights, IL) for 1 hr at room temperature. After three washes, the blots were exposed to the enhanced chemiluminescence substrate. Then the blots were stripped for 1 hr at 50°C followed by saturation in 5% nonfat dry milk and incubated with an anti-CaMKII antibody (1:2000; Upstate Biotechnology, Lake Placid, NY) for the detection of the total CaMKII. Quantitation was obtained from densitometric measurements of immunoreactive bands on films.
Biochemical measurement of surface-expressed receptors. After treatment, PFC slices were incubated with ACSF containing 1 mg/ml Sulfo-NHS-LC-Biotin (Pierce, Rockford, IL) for 20 min on ice. The slices were then rinsed three times in TBS to quench the biotin reaction, followed by homogenization in 300 μl of modified radioimmunoprecipitation assay buffer (1% Triton X-100, 0.1% SDS, 0.5% deoxycholic acid, 50 mm NaPO4, 150 mm NaCl, 2 mm EDTA, 50 mm NaF, 10 mm sodium pyrophosphate, 1 mm sodium orthovanadate, 1 mm PMSF, and 1 mg/ml leupeptin). The homogenates were centrifuged at 14,000 × g for 15 min at 4°C. Fifteen micrograms of protein were removed to measure total NR1. For surface protein, 150 μg of protein was incubated with 100 μlof 50% Neutravidin Agarose (Pierce) for 2 hr at 4°C, and bound proteins were resuspended in 25 μl of SDS sample buffer and boiled. Quantitative Western blots were performed on both total and biotinylated (surface) proteins using anti-NR1 (1:1000; Upstate Biotechnology).
Immunocytochemical measurement of internalized receptors. Surface NMDARs were labeled in live cultured PFC neurons (2–3 weeks in vitro) by 15 min incubation with an antibody directed at the N terminus of the NR1 subunit (1:50; Chemicon, Temecula, CA). After washout of the antibody, cells were treated with different agents as described in the text. After the treatment, cells were chilled on ice and surface-stripped with an acidic solution (0.5 m NaCl, 0.2 N acetic acid) for 3 min. Cells were then fixed in 4% PFA, permeabilized in 0.1% Triton X-100 and stained with a fluorescein-conjugated secondary antibody (1:200; Sigma) for 50 min at room temperature. After washing in PBS for three times, the coverslips were mounted on slides with VECTASHIELD mounting media (Vector Laboratories, Burlingame, CA).
Labeled cells were imaged using a 100× objective with a cooled CCD camera mounted on a Nikon microscope. All specimens were imaged under identical conditions and analyzed using identical parameters. Images were first thresholded to subtract the average background fluorescence, then the level of internalized NMDAR immunoreactivity on the same length of dendrites and the same area of somas in treated versus untreated cells was compared. Four independent experiments were performed, and each time two coverslips for each of the four treatments were observed. On each coverslip, the immunofluorescence intensity of three or four neurons was quantified. For each neuron, the immunoreactivity of soma (within a 15 × 15 μm area) and four neurites (100 μm each) were measured. Quantitative analyses were conducted blindly (without knowledge of experimental treatment).
Results
Activation of D4 receptors reduces NMDA-evoked currents in dissociated PFC pyramidal neurons
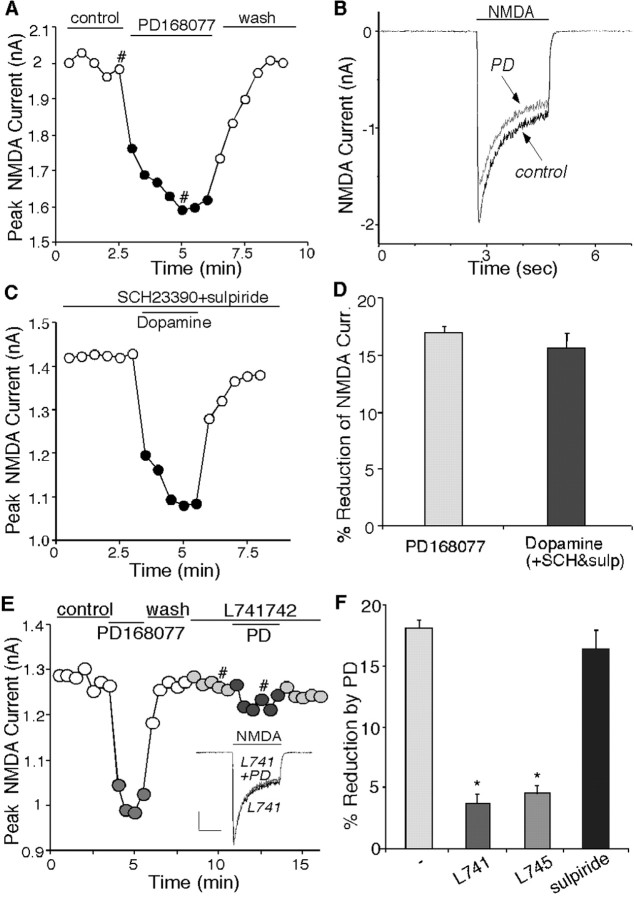
To test the potential impact of D4 receptors on NMDA signaling, we first examined the effect of PD168077, a potent and highly selective D4 receptor agonist (Glase et al., 1997; Wang et al., 2002), on NMDA receptor-mediated currents in acutely isolated PFC pyramidal neurons. Application of NMDA (100 μm) evoked a partially desensitizing inward current that was completely blocked by the NMDA receptor antagonist d-APV (50 μm), confirming mediation by the NMDA receptor. Application of PD168077 (20–40 μm) caused a significant reduction in the amplitude of NMDAR currents in isolated PFC pyramidal neurons. The time course and current traces from a representative cell is shown in Figure 1, A and B. The PD168077-induced reduction of NMDAR currents was reversible and had fast-onset kinetics, taking 1–2 min to stabilize. After recovery from the first application, a second application of PD168077 resulted in a similar response (92.1 ± 2.5% of first response; n = 54). To further confirm the involvement of D4 receptors in the regulation of NMDAR currents, we also tested the effect of dopamine (50 μm) on NMDAR currents in the presence of D1/D5 antagonist SCH23390 (10 μm) and D2/D3 antagonist sulpiride (5 μm). As shown in Figure 1C, when D1/D5 and D2/D3 receptors were blocked, dopamine produced a reversible inhibition on NMDAR currents, similar to the PD168077 effect, suggesting that dopamine released on PFC pyramidal neurons could indeed modulate NMDA receptors via the activation of D4 receptors. As summarized in Figure 1D, in a sample of acutely dissociated PFC pyramidal neurons, the D4 receptor agonist PD168077 produced a 17.2 ± 0.3% (n = 156; p < 0.01; Mann–Whitney U test) reduction of NMDAR currents. This effect was mimicked by application of dopamine in the presence of D1/D5 and D2/D3 antagonists (15.3 ± 1.3%; n = 14; p < 0.01; Mann–Whitney U test) (Fig. 1D).
Figure 1.
Activation of D4 receptors reversibly reduced NMDA receptor currents in acutely dissociated PFC pyramidal neurons. A, Plot of peak NMDAR current showing that the D4 agonist PD168077 (20 μm) decreased NMDA (100 μm)-evoked currents in the cell. B, Representative current traces taken from the records used to construct A (at time points denoted by #). C, Plot of peak NMDAR current showing that dopamine (50 μm) decreased NMDAR currents in the presence of the D1/D5 antagonist SCH23390 (10μm) and the D2/D3 antagonist sulpiride (5μm). D, Cumulative data (mean ± SEM) showing the percentage reduction of NMDAR currents by different agonists: PD168077 (n = 156) and dopamine (in the presence of SCH23390 and sulpiride; n = 14). E, Plot of peak NMDAR current showing that the selective D4 antagonist l-741742 (10 μm) blocked PD168077-induced reduction of NMDAR currents. Inset, Representative current traces (at time points denoted by #). Calibration: 0.25 nA, 1 sec. F, Cumulative data (mean ± SEM) showing the percentage reduction of NMDAR currents by PD168077 in the absence (n = 37) or presence of l-745870 (10 μm; n = 22), l-741742 (10 μm; n = 9), or sulpiride (5 μm; n = 10) (*p < 0.005; ANOVA).
The effect of D4 receptors on NMDAR currents was also tested in cultured PFC pyramidal neurons. PD168077 or dopamine (co-applied with D1/D5 and D2/D3 antagonists) decreased NMDAR currents by 15.8 ± 0.9% (n = 19; p < 0.01; Mann–Whitney U test) or 18.9 ± 2.5% (n = 11; p < 0.01; Mann–Whitney U test), respectively, consistent with the results obtained in acutely dissociated neurons.
To verify that D4 receptors were mediating the modulation seen with PD168077, we examined the ability of selective dopamine receptor antagonists to prevent the action of PD168077. As shown in Figure 1E, PD168077 (20 μm) produced a reversible reduction of NMDAR currents in the dissociated PFC neuron, and this effect was blocked by l-741742 (10 μm), a highly selective D4 antagonist (Rowley et al., 1996). As summarized in Figure 1F, PD168077 had little effect on NMDAR currents in the presence of l-741742 (3.6 ± 0.8%; n = 9; p > 0.05; Mann–Whitney U test), which was significantly (p < 0.005; ANOVA) different from the PD168077 effect in control neurons (18.0 ± 0.8%; n = 37; p < 0.01; Mann–Whitney U test). Similarly, in the presence of l-745870 (10 μm), another highly selective D4 antagonist (Patel et al., 1997), PD168077 failed to modulate NMDAR currents (4.4 ± 0.7%; n = 22; p > 0.05; Mann–Whitney U test) (Fig. 1F). In contrast, the PD168077-induced reduction of NMDAR currents was intact in the presence of the D2/D3 receptor antagonist sulpiride (5 μm; 16.4 ± 1.5%; n = 10; p < 0.01; Mann–Whitney U test) (Fig. 1F). Cultured PFC neurons gave similar results. PD168077 or dopamine (coapplied with D1/D5 and D2/D3 antagonists) failed to produce a significant effect on NMDAR currents in the presence of l-741742 (PD168077: 1.7 ± 0.8%, n = 6; dopamine: 3.3 ± 0.6%, n = 6; p > 0.05, Mann–Whitney U test). The pharmacological profile of these responses thus identifies D4 as the receptor underlying the PD168077-induced inhibition of NMDAR currents.
Activation of D4 receptors decreases NMDAR-mediated EPSCs in PFC slices
To understand the impact of D4 receptors on NMDAR-mediated synaptic transmission, we examined the effect of PD168077 on NMDAR-EPSCs in PFC slices. Cells were bathed in ACSF containing the non-NMDA receptor antagonist CNQX (20 μm) and GABAA receptor antagonist bicuculline (10 μm). NMDAR-EPSCs were evoked in PFC pyramidal neurons depolarized to +60 mV to relieve the Mg2+ block. Application of the NMDAR antagonist d-APV (50 μm) blocked the EPSCs (n = 6), indicating that these synaptic currents were indeed mediated by NMDA receptors. Application of PD168077 induced a potent and long-lasting reduction in the amplitude of NMDAR EPSCs. A representative experiment is shown in Figure 2, A and B. In parallel control measurements in which no PD168077 was administrated, NMDAR EPSCs remained stable throughout the length of the recording (Fig. 2A), indicating that the inhibition of NMDAR EPSCs produced by PD168077 did not result from an agonist-independent run-down of the evoked responses. In a sample of PFC pyramidal neurons we examined, PD168077 decreased the mean amplitude of NMDAR EPSCs by 42.3 ± 3.2% (n = 13; p < 0.001; Mann–Whitney U test). The bigger effect of PD168077 found in slices than in dissociated neurons could be attributed to the more intact D4 receptors localized at dendritic processes that were not truncated in slices. Alternatively, D4 receptors may preferably affect synaptic NMDA receptors. Thus, PD168077 had a larger impact on NMDAR EPSCs that were evoked by stimulation of synaptic NMDA receptors than on NMDAR currents in isolated neurons in which both extrasynaptic and synaptic NMDA receptors were stimulated.
Figure 2.
Activation of D4 receptors reduced the amplitude of NMDAR-mediated EPSCs in PFC slices but did not change the paired-pulse ratio of NMDAR EPSCs. A, Plot of peak evoked NMDAR EPSCs as a function of time and agonist application. NMDAR EPSCs were decreased by PD168077 (20μm) but remained stable in the control cell not subjected to the D4 agonist. Each point represents the average peak (mean ± SEM) of three consecutive NMDAR EPSCs. B, Representative current traces (average of 3 trials) taken from the records used to construct A (at time points denoted by #). Calibration: 50 pA, 100 msec. Stimulus artifacts were blanked. C, The ratio of paired-pulse facilitation (PPF) of NMDAR EPSCs (interstimuli interval: 100 msec) under control conditions and after application of PD168077. The filled squares (and error bars) indicate the mean (± SEM) of the ratio of PPF (n = 9). D, Representative NMDAR EPSCs (average of 3 trials) evoked by double pulses from a PFC neuron before (control) and after PD168077 application. Calibration: 50 pA, 100 msec.
To determine whether the D4 regulation of NMDAR EPSCs involves changes in the probability of transmitter release (Pr), we examined the responses to paired pulses, a measure that is exquisitely sensitive to changes in Pr (Manabe et al., 1993; Debanne et al., 1996). When double pulses with 100 msec intervals were delivered to PFC neurons, the second NMDAR EPSC showed a larger amplitude than the first one, but the ratio of this paired-pulse facilitation (PPR) remained primarily unaltered after PD168077 application (Fig. 2C) (PPR in control: 1.69 ± 0.1, PPR in PD168077: 1.75 ± 0.1, n = 9; p > 0.05, ANOVA). The NMDAR EPSC traces evoked by double pulses from a representative neuron is shown in Figure 2D. This result suggests that activation of D4 receptors may not cause a significant change in glutamate release, and the reduction of NMDAR EPSC amplitudes by PD168077 is likely to be attributed to the D4-induced change in postsynaptic NMDA receptors.
The D4 reduction of NMDAR currents is dependent on the inhibition of protein kinase A and activation of protein phosphatase 1
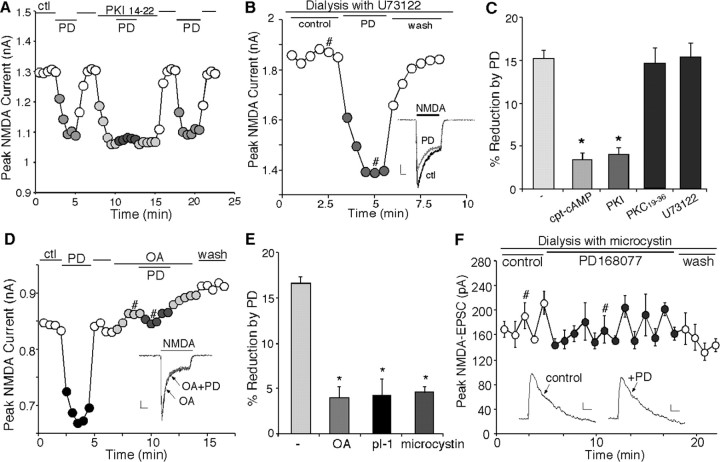
We next examined the signal transduction pathways mediating the reduction of NMDAR currents by D4 receptors. Activation of D4 receptors inhibits adenylate cyclase and cAMP formation in transfected cell lines (Chio et al., 1994), whereas protein kinase A (PKA) phosphorylation of NMDAR subunits changes the channel activity (Raman et al., 1996). This led us to test whether the D4 reduction of NMDAR currents was through the inhibition of PKA. As shown in Figure 3A, inhibiting PKA activity by application of the membrane-permeable PKA inhibitory peptide, myristoylated PKI[14–22] (1 μm), produced a depressing effect on NMDAR currents, mimicking the effect of PD168077. Moreover, PKI[14–22] occluded the ability of PD168077 to reduce NMDAR currents, and washing off the inhibitor restored the effect of PD168077 (Fig. 3A). In contrast, dialysis with the phospholipase C (PLC) inhibitor U73122 (5 μm) failed to block the PD168077-induced decrease of NMDAR currents (Fig. 3B). As summarized in Figure 3C, PD168077 had little effect on NMDAR currents when PKA was activated by application of the membrane-permeable cAMP analog cpt-cAMP (50 μm; 3.4 ± 0.9%; n = 9; p > 0.05; Mann–Whitney U test). The PD168077-induced reduction of NMDAR currents was also widely abolished in neurons dialyzed with the specific PKA inhibitory peptide PKI[5–24] (20 μm) or in the presence of myristoylated PKI[14–22] (3.9 ± 0.8%; n = 20; data pooled together; p > 0.05; Mann–Whitney U test) (Fig. 3C), but was almost intact in neurons loaded with the PKC inhibitory peptide PKC19–36 (20 μm; 14.5 ± 1.8%; n = 8; p < 0.01; Mann–Whitney U test) (Fig. 3C) or U73122 (15.3 ± 1.7%; n = 12; p < 0.01; Mann–Whitney U test) (Fig. 3C). These results suggest that the PD168077 reduction of NMDAR currents depends on the inhibition of PKA and does not require activation of PLC or PKC.
Figure 3.
The effect of PD168077 on NMDAR currents was dependent on PKA–PP1, but not PKC or PLC. A, Plot of peak NMDAR currents showing that application of the membrane-permeable myristoylated PKA inhibitor PKI[14–22] (1μm) reduced NMDAR currents and occluded the effect of subsequent application of PD168077 (20 μm). B, Plot of peak NMDAR currents showing that dialysis with the PLC inhibitor U73122 (5 μm) did not prevent the PD168077-induced reduction of NMDAR currents. Inset, Representative current traces (at time points denoted by #). Calibration: 0.25 nA, 0.5 sec. C, Cumulative data (mean ± SEM) showing the percentage reduction of NMDAR currents by PD168077 in the absence (n = 8) or presence of cpt-cAMP (50μm; n = 9), PKI (PKI[5–24]: 20μm, PKI[14–22]: 1μm; n = 20), PKC19–36 (20μm; n = 8), or U73122 (n = 12) (*p < 0.005; ANOVA). D, Plot of peak NMDAR currents showing that the membrane-permeable PP1–2A inhibitor OA (0.5 μm) blocked the ability of PD168077 (20μm) to reduce NMDAR currents. Inset, Representative current traces (at time points denoted by #). Calibration: 0.1 nA, 0.5 sec. E, Cumulative data (mean ± SEM) showing the percentage modulation of NMDAR currents by PD168077 in the absence (n = 17) or presence of OA (0.5 μm; n = 15), pThr35I-1[7–39] (40 μm; n = 17), or microcystin (5 μm; n = 14) (*p < 0.005; ANOVA). F, Plot of peak evoked NMDAR EPSCs as a function of time and agonist application in a neuron dialyzed with the PP1–2A inhibitor microcystin (20μm). Each point represents the average peak (mean ± SEM) of three consecutive NMDAR EPSCs. Microcystin markedly diminished the PD168077 (20μm)-induced reduction of NMDAR EPSCs. Inset, Representative current traces (average of 3 trials) taken from the records used to construct F (at time points denoted by #). Calibration: 20 pA, 40 msec.
The D4-induced inhibition of PKA could directly modulate NMDAR currents through decreased phosphorylation of NR1 subunits on the PKA sites (Tingley et al., 1997). Alternatively, the inhibition of PKA could cause the disinhibition of protein phosphatase 1 (PP1) via decreased phosphorylation of the inhibitory protein I-1 (Ingebritsen and Cohen, 1983), leading to the increased dephosphorylation of NMDAR subunits and downregulation of NMDAR currents. To test which is the potential signaling mechanism, we examined the effect of PD168077 on NMDA receptors in the presence of phosphatase inhibitors.
As shown in Figure 3D, bath application of the PP1–2A inhibitor OA (0.5 μm) markedly attenuated the ability of PD168077 to inhibit NMDAR currents (4.1 ± 0.9%; n = 15; p > 0.05; Mann– Whitney U test). On the contrary, application of OAE, a compound with a similar structure as OA but lacking the ability to inhibit PP1–2A, did not affect the PD168077-induced inhibition of NMDAR currents (data not shown), suggesting the involvement of PP1 or PP2A. To test the role of PP1 in D4 modulation of NMDAR currents, we dialyzed PFC pyramidal neurons with the phosphorylated inhibitor-1 (I-1) peptide pThr35I-1[7–39], derived from the PP1 interaction region. Biochemical analysis demonstrated that the phospho-I-1 peptide pThr35I-1[7–39] potently inhibited PP1 catalytic activity with an IC50 at the nanomolar range (Foulkes et al., 1983). Comparing with the PD168077 effect in control cells (16.7 ± 0.7%; n = 17; p < 0.01; Mann–Whitney U test) (Fig. 3E), the ability of PD168077 to modulate NMDAR currents was significantly (p < 0.005; ANOVA) diminished by dialysis with the pThr35I-1[7–39] peptide (40 μm; 4.2 ± 1.8%; n = 17; p > 0.05; Mann–Whitney U test) (Fig. 3E). Dialysis with microcystin (5 μm), another structurally different and potent PP1–2A inhibitor, also significantly (p < 0.005; ANOVA) attenuated the effect of PD168077 (4.8 ± 0.7%; n = 14; p > 0.05; Mann–Whitney U test) (Fig. 3E). These results indicate that activation of PP1 is required to link D4 receptors to the reduction of NMDAR currents.
We then examined the involvement of phosphatases in D4 modulation of NMDAR EPSC amplitudes in pyramidal neurons recorded in PFC slices. Neurons were dialyzed with microcystin (20 μm), and the effect of PD168077 on NMDAR-EPSCs was examined. As shown in Figure 3F, in the microcystin-loaded neuron, PD168077 (20 μm) failed to inhibit the amplitude of NMDAR EPSCs. In a sample of PFC pyramidal neurons tested, PD168077 caused little reduction in the mean amplitude of NMDAR EPSCs in cells dialyzed with microcystin (9.8 ± 3.2%; mean ± SEM; n = 7; p > 0.05; Mann–Whitney U test), which was significantly (p < 0.005; ANOVA) different from the effect of PD168077 in control cells (42.3 ± 3.2%; n = 13; p < 0.001; Mann–Whitney U test). These results indicate that the D4 modulation of NMDAR EPSCs also requires the activation of PP1.
The D4 modulation of NMDA receptors depends on the inhibition of active CaMKII
The D4-induced activation of PP1 could lead to the downregulation of NMDAR currents by two potential mechanisms. One is through the increased PP1 dephosphorylation of NMDA receptor NR1 subunit directly (Westphal et al., 1999). The other is through the decreased CaMKII regulation of NMDA receptors, because activated PP1 could reduce CaMKII Thr286 autophosphorylation and its consequent Ca2+-independent kinase activity (Shields et al., 1985; Miller and Kennedy, 1986). To test which signaling mechanism is involved, we examined the D4 effect on NMDAR currents in PFC pyramidal neurons in which CaMKII was inhibited.
As shown in Figure 4A, bath application of the selective CaMKII inhibitor KN-93 (10 μm) prevented PD168077 from decreasing the NMDAR current amplitude. Washing off KN-93 restored the ability of PD168077 to modulate NMDA receptors. Dialysis with the highly specific and potent inhibitor of CaMKII, AIP peptide (10 μm; Ishida et al., 1995), also prevented the PD168077-induced suppression of NMDAR currents (Fig. 4B). In contrast, inclusion of calmodulin (10 μm) in the patch electrode failed to block the depressing effect of PD168077 (Fig. 4B). As summarized in Figure 4C, PD168077 had little effect on NMDAR currents in the presence of KN-93 (3.3 ± 0.7%; n = 9; p > 0.05; Mann–Whitney U test) or AIP peptide (4.0 ± 0.9%; n = 11; p > 0.05; Mann–Whitney U test), which was significantly (p < 0.005; ANOVA) different from the effect of PD168077 in the absence of these CaMKII inhibitors (17.3 ± 1.1%; n = 22; p < 0.01; Mann–Whitney U test) or in the presence of KN-92 (10 μm), the inactive analog of KN-93 (13.4 ± 1.5%; n = 9; p < 0.01; Mann–Whitney U test). Dialysis with calmodulin or the calmodulin antagonist calmidazolium (20 μm) was without effect on the PD168077-induced decrease of NMDAR currents (calmodulin: 13.3 ± 1.6%, n = 8; calmidazolium: 15.9 ± 1.5%, n = 13, p < 0.01, Mann–Whitney U test). These results argue against the involvement of Ca2+–calmodulin-dependent inactivation of NMDARs (Ehlers et al., 1996; Zhang et al., 1998) in the D4 modulation of NMDAR currents in PFC pyramidal neurons.
Figure 4.
The D4 reduction of NMDA receptors required the inhibition of CaMKII activity. A, Plot of peak NMDAR currents showing that the CaMKII inhibitor KN-93 (10 μm) prevented PD168077 (40 μm) from reducing NMDAR currents. B, Plot of peak NMDAR currents as a function of time and agonist application in neurons dialyzed with the CaMKII inhibitory peptide AIP (10μm) or the purified calmodulin (10 μm). C, Cumulative data (mean ± SEM) showing the percentage reduction of NMDAR currents by PD168077 in the absence (n = 22) or presence of KN-93 (10μm; n = 9), KN-92 (10μm; n = 9), AIP (n = 11), calmodulin (n = 8), or calmidazolium (20 μm; n = 13) (*p < 0.005; ANOVA). D, E, Plot of peak NMDAR currents (D) or evoked NMDAR EPSCs (E) as a function of time and drug application in neurons from wild-type versus α-CaMKII +/– mice. Each point (E) represents the average peak (mean ± SEM) of three consecutive NMDAR EPSCs. The effect of PD168077 (20 μm) on NMDA receptors was markedly attenuated in the mutant cell. D, Inset, Immunoblots of total CaMKII and Thr286 autophosphorylated CaMKII in PFC slices from wild-type versus α-CaMKII heterozygous mice. F, Representative current traces (average of 3 trials) taken from the records used to construct E (at time points denoted by #). Calibration: 20 pA, 100 msec.
To further confirm the involvement of CaMKII in D4 modulation of NMDA receptors, we took advantage of the mutant mice deficient for α-CaMKII (Silva et al., 1992). α-CaMKII knock-out mice, especially the heterozygotes, exhibit a spectrum of behavioral abnormalities, including a decreased fear response and an increase in defensive aggression, and therefore provide a model for studying the molecular basis underlying emotional disorders (Chen et al., 1994). Thus, we examined the effect of PD168077 on NMDAR currents in these mutant mice. We first compared the expression of total CaMKII and active CaMKII (Thr286-autophosphorylated) in PFC slices from wild-type mice versus α-CaMKII heterozygotes. As shown in Figure 4D (inset), the total CaMKII protein level was markedly reduced, and the active CaMKII was almost abolished in α-CaMKII heterozygotes. Application of PD168077 (40 μm) failed to produce any significant effect on NMDAR currents in PFC neurons isolated from these mutant mice (3.2 ± 1.2%; n = 14; p > 0.05; Mann–Whitney U test), which was significantly (p < 0.005; ANOVA) different from the effect of PD168077 in wild-type control cells (13.8 ± 2.1%; n = 12; p < 0.01; Mann–Whitney U test). A representative example is shown in Figure 4D. These lines of evidence show that the D4 reduction of NMDAR currents depends on the inhibition of CaMKII activity.
To test the involvement of CaMKII in D4 modulation of NMDAR-mediated synaptic transmission, we examined the effect of PD168077 on NMDAR-EPSCs in α-CaMKII+/– mice. A representative example is shown in Figure 4, E and F. Application of PD168077 (20 μm) had a significantly (p < 0.005; ANOVA) smaller effect on the amplitude of NMDAR EPSCs in PFC neurons from the α-CaMKII heterozygotes, compared with wild-type mice (wild-type: 43.2 ± 3.0%, n = 5; heterozygote: 18.3 ± 3.1%, n = 11). These results suggest that changes in CaMKII activity are required for the D4 modulation of NMDAR transmission in PFC slices.
D4 receptors decrease CaMKII activity in a PKA–PP1-dependent manner
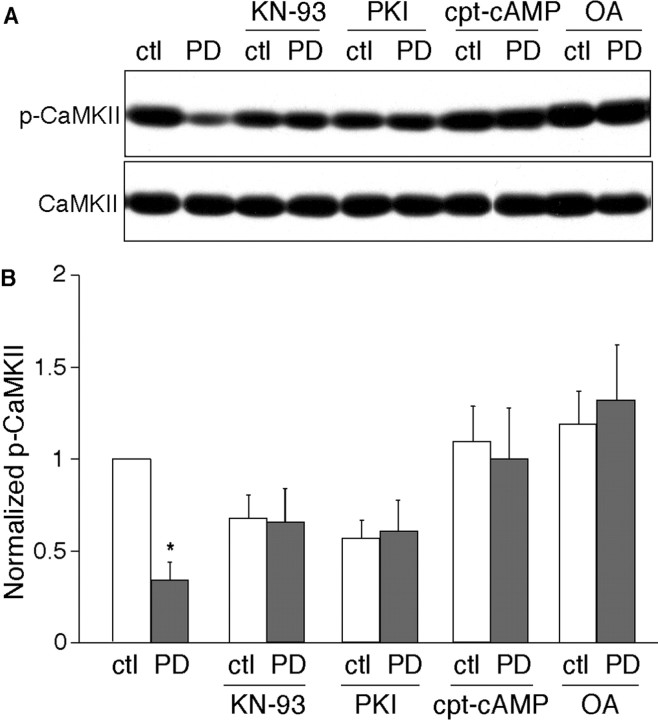
We then examined whether activation of D4 receptors can indeed inhibit CaMKII activity. The Thr286 autophosphorylation state of CaMKII determines its Ca2+-independent activity (Miller and Kennedy, 1986; Miller et al., 1988), therefore, we compared the phosphorylation of CaMKII at Thr286 in PFC slices treated with or without PD168077. A representative example is shown in Figure 5A. A short (10 min) treatment of PD168077 (40 μm) potently reduced the Thr286 phosphorylation of CaMKII. This ability of PD168077 to reduce CaMKII activity was prevented by pretreatment with KN-93 (10 μm). When PKA activity was suppressed by the inhibitor, myristoylated PKI[14–22] (1 μm), subsequent treatment with PD168077 had no further effect on CaMKII autophosphorylation. In the presence of the PKA activator cpt-cAMP (100 μm), the PD168077 reduction of CaMKII autophosphorylation was blocked. Application of the PP1 inhibitor OA (0.3 μm) also eliminated the PD168077 effect on CaMKII activity. The expression levels of total CaMKII were not changed by any of these treatments (Fig. 5A).
Figure 5.
Activation of D4 receptors reduced CaMKII activity in PFC slices, which was blocked by activation of PKA or inhibition of PP1. A, Immunoblots of autophosphorylated CaMKII and total CaMKII. PFC slices were pretreated without or with KN-93 (10 μm), PKI[14–22] (1 μm), cpt-cAMP (100 μm), or OA (0.3 μm) for 30 min, followed by incubation without or with PD168077 (40 μm) for 10 min. After treatment, slice lysates were blotted with an antibody specific for Thr286-phosphorylated CaMKII. After stripping out signals, membranes were reblotted with an antibody recognizing the total CaMKII. B, Quantitation of Thr286-phosphorylated CaMKII with various treatments (n = 5–8 for each condition; *p < 0.001; ANOVA).
Quantification of the data are summarized in Figure 5B. Under control conditions, PD168077 decreased CaMKII Thr286 phosphorylation to 35 ± 9% of the basal level (n = 8). Pretreatment with KN-93 abolished the ability of PD168077 to reduce CaMKII autophosphorylation (96 ± 11%; n = 6). In PFC slices in which PKA was inhibited by PKI[14–22] or activated by cpt-cAMP, subsequent application of PD168077 failed to produce a significant inhibition of CaMKII autophosphorylation (in the presence of PKI[14–22]: 105 ± 10%, n = 5; in the presence of cpt-cAMP: 85 ± 18%, n = 6). When PP1 was inhibited by OA, PD168077 also lost the ability to reduce CaMKII Thr286 phosphorylation (110 ± 20%; n = 8). These results suggest that D4 receptors can suppress the kinase activity of CaMKII through PP1 that is activated by PKA inhibition.
D4 receptors reduce the NMDAR surface expression and increase the NMDAR internalization in a CaMKII-dependent manner
To test whether the PD168077 reduction of NMDAR-mediated ionic and synaptic currents can be accounted for by decreased NMDARs on the cell membrane, we performed surface biotinylation to measure levels of surface NR1 in PFC slices. Surface proteins were first labeled with sulfo-NHS-LC-biotin, and then biotinylated surface proteins were separated from nonlabeled intracellular proteins by reaction with Neutravidin beads. Surface and total proteins were subjected to electrophoresis and probed with an antibody against the NR1 subunit. As shown in Figure 6A, treatment of PFC slices with PD168077 (40 μm, 10 min) reduced the level of surface NR1, with no change in the total NR1 protein. Furthermore, the PD168077 effect on the surface expression of NR1 was blocked by pretreatment with the CaMKII inhibitor KN-93 (5 μm, 30 min). Analysis of band intensities in a sample of experiments indicated that PD168077 decreased the level of surface NR1 to 60 ± 12% of control (Fig. 6B)(n = 6;p < 0.01; ANOVA), but failed to do so in the presence of the CaMKII inhibitor (94 ± 13%; n = 5; p > 0.05; ANOVA) (Fig. 6B).
Figure 6.

Activation of D4 receptors reduced the NMDAR surface expression and increased the NMDAR internalization in a CaMKII-dependent manner. A, Immunoblots showing the surface NR1 and total NR1 in PFC slices under different treatment conditions. PD168077 (40 μm; 10 min) decreased the level of surface NR1, and this effect was abolished by the pretreatment with KN-93 (5 μm; 30 min). B, Quantitation of the NR1 surface level with various treatments (n = 5–6 for each condition; *p < 0.01; ANOVA). C, Examples of internalized NMDAR immunoreactivity in cultured PFC pyramidal neurons under different treatment conditions. The untreated cell showed little NMDAR internalization, whereas the cell treated with PD168077 (40 μm; 5 min) showed significant staining for NMDARs internalized from the plasma membrane. Pretreatment with KN-93 (5 μm; 10 min) blocked the PD168077-induced NMDAR internalization. D, Quantitation of the internalized NR1 with various treatments (n = 24–32 cells per group from four cultures; *p < 0.001, ANOVA).
We also performed immunocytochemical experiments to detect NMDAR internalization in cultured PFC pyramidal neurons. Surface NMDARs were first stained with an antibody to the extracellular region of NR1 subunit, and then after the treatment with various agents, surface-bound antibodies were stripped away so that only internalized NMDARs were visualized. As shown in Figure 6C, application of PD168077 (40 μm, 5 min) triggered a rapid internalization of NMDARs. To test the role of CaMKII in the D4-regulated NMDAR internalization, we pre-treated PFC cultures with KN-93 (5 μm, 10 min). This CaMKII inhibitor abolished the ability of PD168077 to induce NMDAR internalization (Fig. 6C). Quantification of fluorescently labeled, internalized NMDARs in a sample of cells indicates that PD168077 increased NMDAR internalization by 3.2 ± 0.6-fold (Fig. 6D)(n = 20; p < 0.001; ANOVA), and this effect was lost in the presence of the CaMKII inhibitor (1.14 ± 0.4-fold, n = 15; p > 0.05; ANOVA) (Fig. 6D). Taken together, these results suggest that PD168077 regulates the trafficking of NMDARs in a CaMKII-dependent manner.
Discussion
Interactions between dopaminergic and glutamatergic systems in PFC
Most of the antipsychotic drugs act on the dopamine system (Creese et al., 1976; Seeman et al., 1976). Their significant efficacy forms the corner stone for the “dopamine hypothesis of schizophrenia” (Davis et al., 1991). The enrichment of D4 receptors in PFC (Mrzljak et al., 1996; Ariano et al., 1997) and its high affinity for many atypical antipsychotic drugs (Van Tol et al., 1991; Kapur and Remington, 2001) have led the speculation that D4 receptors play a key role in regulating PFC functions and are highly involved in the pathophysiology of neuropsychiatric disorders. Cortical hyperexcitability exhibited in D4 receptor knock-out mice (Rubinstein et al., 2001) suggests that D4 receptors may function as an inhibitory modulator of glutamate activity in the PFC. However, it is still unclear how D4 receptors interact with the glutamate system in PFC pyramidal neurons. In this study, we demonstrated that activation of D4 receptors significantly reduced the currents through the NMDA-type glutamate receptor channels in acutely dissociated and cultured PFC pyramidal neurons. Moreover, activation of D4 receptors produced a potent reduction of the NMDAR-mediated EPSCs in PFC slices. It suggests that the postsynaptic NMDA receptor is one of the key targets of D4 receptors in PFC neurons. Because the schizophrenia-like behaviors induced by administration of NMDAR antagonists (Javitt and Zukin, 1991; Jentsch and Roth, 1999) or NMDAR knock-down (Mohn et al., 1999) have implicated NMDAR hypofunction in mental disorders, the D4 interaction with NMDAR channels in PFC could play a significant role in regulating the cognitive and emotional status.
Dopamine, by activating different receptors and their downstream second-messenger cascades, can regulate NMDAR channels in a complex manner. It has been reported that NMDAR-mediated responses in PFC are potentiated by D1 receptors and are suppressed or unchanged by D2 receptors (Zheng et al., 1999; Seamans et al., 2001; Gonzalez-Islas and Hablitz, 2003). In this study, we found that when D1 and D2 receptors were blocked, dopamine inhibited NMDAR currents in PFC pyramidal neurons, an effect that was blocked by D4 antagonists, indicating the mediation by D4 receptors. These results suggest that activation of different dopamine receptors may have differential functional consequences on NMDAR channels in PFC neurons.
Mechanisms for the D4 regulation of NMDA receptors in PFC
The NMDAR channel activity can be regulated by protein phosphorylation–dephosphorylation via a variety of protein kinases–phosphatases (Lieberman and Mody, 1994; Raman et al., 1996; Westphal et al., 1999). Our results show that the D4 modulation of NMDAR currents or NMDAR EPSCs in PFC pyramidal neurons is mediated by a signaling cascade involving the inhibition of PKA and the subsequent activation of PP1. Consistent with this, a PKA–PP1-mediated pathway has been implicated in the D1 regulation of NMDA receptors in striatum (Snyder et al., 1998; Flores-Hernandez et al., 2000). Experiments with various CaMKII inhibitors and CaMKII+/– mice further show that the D4-mediated suppression of NMDA receptor functions in PFC pyramidal neurons was dependent on the inhibition of CaMKII activity, which was downstream of PKA inhibition and PP1 activation. Moreover, the D4 regulation of NMDA receptors in PFC was not dependent on PLC stimulation or calmodulin activation. A different mechanism that is independent of PKA but involves the transactivation of a receptor tyrosine kinase and the enhanced Ca2+–calmodulin-dependent inactivation of NMDA receptors has been proposed to explain the irreversible inhibition of NMDA receptors by D2-class receptors in hippocampus (Kotecha et al., 2002). It suggests that dopamine receptors could be coupled to distinct signaling pathways to regulate NMDAR transmission in different types of neurons.
In the brain, NMDA receptors are found both in the cytoplasm of neurons and at excitatory synapses (Petralia et al., 1994). At the postsynaptic membrane, NMDA receptors interact with postsynaptic density (PSD) components, a macromolecular complex containing anchoring and signaling elements (Sheng and Pak, 2000). The subcellular distribution of NMDA receptors to postsynaptic sites can be regulated by neuronal activity, PKA, and PKC (Rao and Craig, 1997; Crump et al., 2001; Lan et al., 2001). In this study, we found that activation of D4 receptors reduced the surface expression of NMDA receptors in PFC neurons, which is opposite to the D1-induced increase of NMDA receptors at the postsynaptic membrane in striatal neurons (Dunah and Standaert, 2001). Interestingly, the opposing effects of D4 versus D1 receptors on the NMDAR targeting are consistent with the PFC D4-induced suppression (this study) versus the striatal D1-induced enhancement of NMDAR currents (Flores-Hernandez et al., 2000). Because PKA activity has been implicated as a mediator of enhanced synaptic transport or stabilization of NMDA receptors (Crump et al., 2001), the inhibitory or stimulatory effect on PKA activity produced by D4 or D1 receptors might be associated with their differing effects on the NMDAR trafficking.
CaMKII is an important downstream target of the PKA-gated PP1 in PSDs of glutamatergic synapses in which NMDARs are concentrated. D4 receptor activation in PFC reduced the NMDAR surface expression and increased NMDAR internalization in a CaMKII-dependent manner (Fig. 6). Several possible mechanisms could account for this finding. First, activation and Thr286 autophosphorylation of CaMKII have been shown to induce high-affinity binding to NMDA receptor subunits (Strack and Colbran, 1998; Leonard et al., 1999). Thus, the D4-induced inhibition of CaMKII activity may reduce the interaction between CaMKII and NMDARs, causing the dispersal of NMDARs from postsynaptic membrane, leading to the reduced NMDA response. Second, previous studies found that the calcineurin-dependent dephosphorylation of endocytic proteins can trigger endocytosis of synaptic vesicles in nerve terminals (Slepnev et al., 1998). Therefore, the D4 inhibition of CaMKII activity may change the Ca2+-dependent phosphorylation–dephosphorylation of proteins that are part of the endocytic complex, which in turn enhances endocytosis by promoting the assembly and function of the complex.
In summary, we have demonstrated that activation of D4 receptors in PFC pyramidal neurons causes the inhibition of PKA, leading to the disinhibition of PP1 caused by the decreased phosphorylation of inhibitor-1. Activated PP1 reduces the autophosphorylation and autonomous activity of CaMKII, resulting in the internalization of NMDA receptors and the reduction of NMDAR-mediated currents. Given the key role of D4 and NMDA receptors in schizophrenia and other neuropsychiatric disorders, the present results provide a possible cellular mechanism that could underlie the D4 regulation of cognitive functions associated with PFC.
Footnotes
This work was supported by National Institutes of Health Grants MH63128 and AG21923 (Z.Y.), National Science Foundation Grant IBN-0117026 (Z.Y.), and Howard Hughes Medical Institute Biomedical Research Support Program Grant 53000261 (State University of New York at Buffalo). We thank Xiaoqing Chen for her technical support.
Correspondence should be addressed to Dr. Zhen Yan, Department of Physiology and Biophysics, State University of New York at Buffalo, 124 Sherman Hall, Buffalo, NY 14214. E-mail: zhenyan@buffalo.edu.
Copyright © 2003 Society for Neuroscience 0270-6474/03/239852-10$15.00/0
References
- Andreasen NC, O'Leary DS, Flaum M, Nopoulos P, Watkins GL, Boles Ponto LL, Hichwa RD ( 1997) Hypofrontality in schizophrenia: distributed dysfunctional circuits in neuroleptic-naive patients. Lancet 349: 1730–1734. [DOI] [PubMed] [Google Scholar]
- Ariano MA, Wang J, Noblett KL, Larson ER, Sibley DR ( 1997) Cellular distribution of the rat D4 dopamine receptor protein in the CNS using anti-receptor antisera. Brain Res 752: 26–34. [DOI] [PubMed] [Google Scholar]
- Berger BS, Trottier C, Verney P, Gaspar P, Alvarez C ( 1988) Regional and laminar distribution of the dopamine and serotonin innervation in the macaque cerebral cortex: a radioautographic study. J Comp Neurol 273: 99–119. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Brown RM, Rosvold HE, Goldman PS ( 1979) Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science 205: 929–932. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Waters N, Holm-Waters S, Tedroff J, Nilsson M, Carlsson ML ( 2001) Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annu Rev Pharmacol Toxicol 41: 237–260. [DOI] [PubMed] [Google Scholar]
- Chen C, Rainnie DG, Greene RW, Tonegawa S ( 1994) Abnormal fear response and aggressive behavior in mutant mice deficient for alpha-calcium-calmodulin kinase II. Science 266: 291–294. [DOI] [PubMed] [Google Scholar]
- Chio CL, Drong RF, Riley DT, Gill GS, Slightom JL, Huff RM ( 1994) D4 dopamine receptor-mediated signaling events determined in transfected Chinese hamster ovary cells. J Biol Chem 269: 11813–11819. [PubMed] [Google Scholar]
- Creese I, Burt DR, Snyder SH ( 1976) Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science 192: 481–483. [DOI] [PubMed] [Google Scholar]
- Crump FT, Dillman KS, Craig AM ( 2001) cAMP-dependent protein kinase mediates activity-regulated synaptic targeting of NMDA receptors. J Neurosci 21: 5079–5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KL, Kahn RS, Ko G, Davidson M ( 1991) Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry 148: 1474–1486. [DOI] [PubMed] [Google Scholar]
- Debanne D, Guerineau NC, Gahwiler BH, Thompson SM ( 1996) Paired-pulse facilitation and depression at unitary synapses in rat hippocampus: quantal fluctuation affects subsequent release. J Physiol (Lond) 491: 163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulawa SC, Grandy DK, Low MJ, Paulus MP, Geyer MA ( 1999) Dopamine D4 receptor-knock-out mice exhibit reduced exploration of novel stimuli. J Neurosci 19: 9550–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunah AW, Standaert DG ( 2001) Dopamine D1 receptor-dependent trafficking of striatal NMDA glutamate receptors to the postsynaptic membrane. J Neurosci 21: 5546–5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD, Zhang S, Bernhadt JP, Huganir RL ( 1996) Inactivation of NMDA receptors by direct interaction of calmodulin with the NR1 subunit. Cell 84: 745–755. [DOI] [PubMed] [Google Scholar]
- Feng J, Cai X, Zhao JH, Yan Z ( 2001) Serotonin receptors modulate GABAA receptor channels through activation of anchored protein kinase C in prefrontal cortical neurons. J Neurosci 21: 6502–6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Hernandez J, Hernandez S, Snyder GL, Yan Z, Fienberg AA, Moss SJ, Greengard P, Surmeier DJ ( 2000) D(1) dopamine receptor activation reduces GABA(A) receptor currents in neostriatal neurons through a PKA/DARPP-32/PP1 signaling cascade. J Neurophysiol 83: 2996–3004. [DOI] [PubMed] [Google Scholar]
- Foulkes JG, Strada SJ, Henderson PJ, Cohen P ( 1983) A kinetic analysis of the effects of inhibitor-1 and inhibitor-2 on the activity of protein phosphatase-1. Eur J Biochem 132: 309–313. [DOI] [PubMed] [Google Scholar]
- Glase SA, Akunne HC, Georgic LM, Heffner TG, MacKenzie RG, Manley PJ, Pugsley TA, Wise LD ( 1997) Substituted [(4-phenylpiperazinyl)-methyl]benzamides: selective dopamine D4 agonists. J Med Chem 40: 1771–1772. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS ( 1994) Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci 6: 348–357. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS ( 1995) Cellular basis of working memory. Neuron 14: 477–485. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Islas C, Hablitz JJ ( 2003) Dopamine enhances EPSCs in layer II-III pyramidal neurons in rat prefrontal cortex. J Neurosci 23: 867–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslin K, Banker G ( 1991) Rat hippocampal neurons in low density culture. In: Culturing nerve cells (Banker G, Golsin K, eds), pp 251–282. Cambridge, MA: MIT.
- Gu Z, Zhong P, Yan Z ( 2003) Activation of muscarinic receptors inhibits β-amyloid peptide-induced signaling in cortical slices. J Biol Chem 278: 17546–17556. [DOI] [PubMed] [Google Scholar]
- Hestrin S, Nicoll RA, Perkel DJ, Sah P ( 1990) Analysis of excitatory synaptic action in pyramidal cells using whole-cell recording from rat hippocampal slices. J Physiol (Lond) 422: 203–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingebritsen TS, Cohen P ( 1983) Protein phosphatases: properties and role in cellular regulation. Science 221: 331–338. [DOI] [PubMed] [Google Scholar]
- Ishida A, Kameshita I, Okuno S, Kitani T, Fujisawa H ( 1995) A novel highly specific and potent inhibitor of calmodulin-dependent protein kinase II. Biochem Biophys Res Commun 212: 806–812. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR ( 1991) Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 148: 1301–1308. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH ( 1999) The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology 20: 201–225. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Redmond Jr DE, Elsworth JD, Taylor JR, Youngren KD, Roth RH ( 1997) Enduring cognitive deficits and cortical dopamine dysfunction in monkeys after long-term administration of phencyclidine. Science 277: 953–955. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR, Redmond Jr DE, Elsworth JD, Youngren KD, Roth RH ( 1999) Dopamine D4 receptor antagonist reversal of subchronic phencyclidine-induced object retrieval/detour deficits in monkeys. Psychopharmacology 142: 78–84. [DOI] [PubMed] [Google Scholar]
- Kapur S, Remington G ( 2001) Atypical antipsychotics: new directions and new challenges in the treatment of schizophrenia. Annu Rev Med 52: 503–517. [DOI] [PubMed] [Google Scholar]
- Kotecha SA, Oak JN, Jackson MF, Perez Y, Orser BA, Van Tol HH, MacDonald JF ( 2002) A D2 class dopamine receptor transactivates a receptor tyrosine kinase to inhibit NMDA receptor transmission. Neuron 35: 1111–1122. [DOI] [PubMed] [Google Scholar]
- Lan JY, Skeberdis VA, Jover T, Grooms SY, Lin Y, Araneda RC, Zheng X, Bennett MV, Zukin RS ( 2001) Protein kinase C modulates NMDA receptor trafficking and gating. Nat Neurosci 4: 382–390. [DOI] [PubMed] [Google Scholar]
- Leonard AS, Lim IA, Hemsworth DE, Horne MC, Hell JW ( 1999) Calcium/calmodulin-dependent protein kinase II is associated with the N-methyl-d-aspartate receptor. Proc Natl Acad Sci USA 96: 3239–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Lieberman JA ( 2000) Catching up on schizophrenia: natural history and neurobiology. Neuron 28: 325–334. [DOI] [PubMed] [Google Scholar]
- Lieberman DN, Mody I ( 1994) Regulation of NMDA channel function by endogenous Ca(2+)-dependent phosphatase. Nature 369: 235–239. [DOI] [PubMed] [Google Scholar]
- Ma XH, Zhong P, Gu Z, Feng J, Yan Z ( 2003) Muscarinic Potentiation of GABAA receptor currents is gated by insulin signaling in prefrontal cortex. J Neurosci 23: 1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe T, Wyllie DJ, Perkel DJ, Nicoll RA ( 1993) Modulation of synaptic transmission and long-term potentiation: effects on paired pulse facilitation and EPSC variance in the CA1 region of the hippocampus. J Neurophysiol 70: 1451–1459. [DOI] [PubMed] [Google Scholar]
- Miller EK ( 1999) The prefrontal cortex: complex neural properties for complex behavior. Neuron 22: 15–17. [DOI] [PubMed] [Google Scholar]
- Miller SG, Kennedy MB ( 1986) Regulation of brain type II Ca 2+/calmodulin-dependent protein kinase by autophosphorylation: a Ca 2+-triggered molecular switch. Cell 44: 861–870. [DOI] [PubMed] [Google Scholar]
- Miller SG, Patton BL, Kennedy MB ( 1988) Sequences of autophosphorylation sites in neuronal type II CaM kinase that control Ca2(+)-independent activity. Neuron 1: 593–604. [DOI] [PubMed] [Google Scholar]
- Mohn AR, Gainetdinov RR, Caron MG, Koller BH ( 1999) Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell 98: 427–436. [DOI] [PubMed] [Google Scholar]
- Mrzljak L, Bergson C, Pappy M, Huff R, Levenson R, Goldman-Rakic PS ( 1996) Localization of dopamine D4 receptors in GABAergic neurons of the primate brain. Nature 381: 245–248. [DOI] [PubMed] [Google Scholar]
- Oak JN, Oldenhof J, Van Tol HH ( 2000) The dopamine D(4) receptor: one decade of research. Eur J Pharmacol 405: 303–327. [DOI] [PubMed] [Google Scholar]
- Patel S, Freedman S, Chapman KL, Emms F, Fletcher AE, Knowles M, Marwood R, Mcallister G, Myers J, Curtis N, Kulagowski JJ, Leeson PD, Ridgill M, Graham M, Matheson S, Rathbone D, Watt AP, Bristow LJ, Rupniak NM, Baskin E, et al. ( 1997) Biological profile of L-745, 870, a selective antagonist with high affinity for the dopamine D4 receptor. J Pharmacol Exp Ther 283: 636–647. [PubMed] [Google Scholar]
- Petralia RS, Yokotani N, Wenthold RJ ( 1994) Light and electron microscope distribution of the NMDA receptor subunit NMDAR1 in the rat nervous system using a selective anti-peptide antibody. J Neurosci 14: 667–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Tong G, Jahr CE ( 1996) Beta-adrenergic regulation of synaptic NMDA receptors by cAMP-dependent protein kinase. Neuron 16: 415–421. [DOI] [PubMed] [Google Scholar]
- Rao A, Craig AM ( 1997) Activity regulates the synaptic localization of the NMDA receptor in hippocampal neurons. Neuron 19: 801–812. [DOI] [PubMed] [Google Scholar]
- Rowley M, Broughton HB, Collins I, Baker R, Emms F, Marwood R, Patel S, Patel S, Ragan CI, Freedman SB, Leeson PD ( 1996) 5-(4-Chlorophenyl)-4-methyl-3-(1-(2-phenylethyl)piperidin-4-yl)isoxazole: a potent, selective antagonist at human cloned dopamine D4 receptors. J Med Chem 39: 1943–1945. [DOI] [PubMed] [Google Scholar]
- Rubinstein M, Phillips TJ, Bunzow JR, Falzone TL, Dziewczapolski G, Zhang G, Fang Y, Larson JL, McDougall JA, Chester JA, Saez C, Pugsley TA, Gershanik O, Low MJ, Grandy DK ( 1997) Mice lacking dopamine D4 receptors are supersensitive to ethanol, cocaine, and methamphetamine. Cell 90: 991–1001. [DOI] [PubMed] [Google Scholar]
- Rubinstein M, Cepeda C, Hurst RS, Flores-Hernandez J, Ariano MA, Falzone TL, Kozell LB, Meshul CK, Bunzow JR, Low MJ, Levine MS, Grandy DK ( 2001) Dopamine D4 receptor-deficient mice display cortical hyperexcitability. J Neurosci 21: 3756–3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Durstewitz D, Christie BR, Stevens CF, Sejnowski TJ ( 2001) Dopamine D1–D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proc Natl Acad Sci USA 98: 301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P, Lee T, Chau-Wong M, Wong K ( 1976) Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature 261: 717–719. [DOI] [PubMed] [Google Scholar]
- Seeman P, Guan HC, Van Tol HH ( 1993) Dopamine D4 receptors elevated in schizophrenia. Nature 365: 441–445. [DOI] [PubMed] [Google Scholar]
- Sheng M, Pak DT ( 2000) Ligand-gated ion channel interactions with cytoskeletal and signaling proteins. Annu Rev Physiol 62: 755–778. [DOI] [PubMed] [Google Scholar]
- Shields SM, Ingebritsen TS, Kelly PT ( 1985) Identification of protein phosphatase 1 in synaptic junctions: dephosphorylation of endogenous calmodulin-dependent kinase II and synapse-enriched phosphoproteins. J Neurosci 5: 3414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Stevens CF, Tonegawa S, Wang Y ( 1992) Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science 257: 201–206. [DOI] [PubMed] [Google Scholar]
- Slepnev VI, Ochoa GC, Butler MH, Grabs D, Camilli PD ( 1998) Role of phosphorylation in regulation of the assembly of endocytic coat complexes. Science 281: 821–824. [DOI] [PubMed] [Google Scholar]
- Snyder GL, Fienberg AA, Huganir RL, Greengard P ( 1998) A dopamine/D1 receptor/protein kinase A/dopamine- and cAMP-regulated phosphoprotein (Mr 32 kDa)/protein phosphatase-1 pathway regulates dephosphorylation of the NMDA receptor. J Neurosci 18: 10297–10303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack S, Colbran RJ ( 1998) Autophosphorylation-dependent targeting of calcium/calmodulin-dependent protein kinase II by the NR2B subunit of the NMDA receptor. J Biol Chem 273: 20689–20692. [DOI] [PubMed] [Google Scholar]
- Taylor SF ( 1996) Cerebral blood flow activation and functional lesions in schizophrenia. Schizophr Res 19: 129–140. [DOI] [PubMed] [Google Scholar]
- Tingley WG, Ehlers MD, Kameyama K, Doherty C, Ptak JB, Riley CT, Huganir RL ( 1997) Characterization of protein kinase A and protein kinase C phosphorylation of the N-methyl-d-aspartate receptor NR1 subunit using phosphorylation site-specific antibodies. J Biol Chem 272: 5157–5166. [DOI] [PubMed] [Google Scholar]
- Tsai G, Coyle JT ( 2002) Glutamatergic mechanisms in schizophrenia. Annu Rev Pharmacol Toxicol 42: 165–79. [DOI] [PubMed] [Google Scholar]
- Van Tol HH, Bunzow JR, Guan HC, Sunahara RK, Seeman P, Niznik HB, Civelli O ( 1991) Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature 350: 610–614. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhong P, Yan Z ( 2002) Dopamine D4 receptors modulate GABAergic signaling in pyramidal neurons of prefrontal cortex. J Neurosci 22: 9185–9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedzony K, Chocyk A, Mackowiak M, Fijal K, Czyrak A ( 2000) Cortical localization of dopamine D4 receptors in the rat brain–immunocytochemical study. J Physiol Pharmacol 51: 205–221. [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Sec RF ( 1986) Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. Arch Gen Psychiatry 43: 114–124. [DOI] [PubMed] [Google Scholar]
- Westphal RS, Tavalin SJ, Lin JW, Alto NM, Fraser ID, Langeberg LK, Sheng M, Scott JD ( 1999) Regulation of NMDA receptors by an associated phosphatase-kinase signaling complex. Science 285: 93–96. [DOI] [PubMed] [Google Scholar]
- Yan Z, Surmeier DJ ( 1997) D5 dopamine receptors enhance Zn 2+-sensitive GABA(A) currents in striatal cholinergic interneurons through a PKA/PP1 cascade. Neuron 19: 1115–1126. [DOI] [PubMed] [Google Scholar]
- Yan Z, Hsieh-Wilson L, Feng J, Tomizawa K, Allen PB, Fienberg AA, Nairn AC, Greengard P ( 1999) Protein phosphatase 1 modulation of neostriatal AMPA channels: regulation by DARPP-32 and spinophilin. Nat Neurosci 2: 13–17. [DOI] [PubMed] [Google Scholar]
- Zhang S, Ehlers MD, Bernhardt JP, Su CT, Huganir RL ( 1998) Calmodulin mediates calcium-dependent inactivation of N-methyl-d-aspartate receptors. Neuron 21: 443–453. [DOI] [PubMed] [Google Scholar]
- Zheng P, Zhang XX, Bunney BS, Shi WX ( 1999) Opposite modulation of cortical N-methyl-d-aspartate receptor-mediated responses by low and high concentrations of dopamine. Neuroscience 91: 527–535. [DOI] [PubMed] [Google Scholar]
- Zhong P, Gu Z, Wang X, Jiang H, Feng J, Yan Z ( 2003) Impaired modulation of GABAergic transmission by muscarinic receptors in a mouse transgenic model of Alzheimer's disease. J Biol Chem 278: 26888–26896. [DOI] [PubMed] [Google Scholar]