Abstract
Changes in the expression of subunits of the GABA type A (GABAA) receptor are implicated in the development of ethanol tolerance and dependence as well as in the central hyperexcitability associated with ethanol withdrawal. The impact of such changes on GABAA receptor function and pharmacological sensitivity was investigated with cultured rat hippocampal neurons exposed to ethanol for 5 d and then subjected to ethanol withdrawal. Both ethanol treatment and withdrawal were associated with a marked decrease in the maximal density of GABA-evoked Cl- currents, whereas the potency of GABA was unaffected. Ethanol exposure also reduced the modulatory efficacy of the benzodiazepine receptor agonists lorazepam, zolpidem, and zaleplon as well as that of the inverse agonists Ro 15-4513 and FG 7142, effects that were associated with a reduced abundance of mRNAs encoding the receptor subunits α1, α3, γ2L, and γ2S. Ethanol withdrawal restored the efficacy of lorazepam, but not that of low concentrations of zolpidem or zaleplon, to control values. Flumazenil, which was ineffective in control neurons, and Ro 15-4513 each potentiated the GABA response after ethanol withdrawal. These effects of withdrawal were accompanied by upregulation of the α2, α3, and α4 subunit mRNAs as well as of the α4 protein. Diazepam or γ-hydroxybutyrate, but not baclofen, prevented the changes in both GABAA receptor pharmacology and subunit mRNA levels induced by ethanol withdrawal. Changes in GABAA receptor gene expression induced by prolonged exposure to and withdrawal of ethanol are thus associated with altered GABAA receptor function and pharmacological sensitivity.
Keywords: GABAA receptor, ethanol, tolerance, dependence, gene expression, hippocampal neurons, patch clamp, γ-hydroxybutyrate, diazepam
Introduction
Certain acute pharmacological actions of ethanol, including its anxiolytic, sedative, ataxic, anticonvulsant, and general anesthetic effects, may be exerted through facilitation of the function of GABA type A (GABAA) receptors in specific brain regions (Faingold et al., 1998; Grobin et al., 1998; Ueno et al., 2001; Aguayo et al., 2002; Chester and Cunningham, 2002). GABAA receptors are ligand-gated Cl- channels and mediate fast inhibitory transmission in the mammalian CNS (Mehta and Ticku, 1999; Vicini, 1999). They are heteromeric complexes of five subunits that belong to various classes: α1-6, β1-4, γ1-3, δ, ϵ, π, θ, and ρ1-3 (Barnard et al., 1998; Whiting et al., 1999; Sieghart and Sperk, 2002). These subunits are expressed in a region- and ontogeny-dependent manner in the brain and generate a large number of GABAA receptor subtypes that differ not only in subunit composition but in their physiological and pharmacological properties (Sieghart, 1995; McKernan and Whiting, 1996; Barnard et al., 1998; Hevers and Luddens, 1998).
Prolonged exposure to ethanol results in the development of dependence and of tolerance to its behavioral actions (Suwaki et al., 2001). Altered GABAA receptor function, characterized by a reduced responsiveness to GABA, tolerance to ethanol, cross-tolerance to benzodiazepines and barbiturates, as well as sensitization to neurosteroids and inverse agonists, is thought to underlie these chronic effects of ethanol (Ticku and Burch, 1980; Allan and Harris, 1987; Morrow et al., 1988; Sanna et al., 1993; Devaud et al., 1996). The molecular mechanisms responsible for adaptation of GABAA receptors to long-term ethanol exposure remain unclear but may involve changes in cell surface density (Ticku and Burch, 1980), in post-translational protein modification (Kumar et al., 2002), or in subunit expression (Mhatre et al., 1993; Devaud et al., 1995, 1997; Follesa et al., 2003).
Long-term ethanol treatment results in a decrease in the abundance of the α1 subunit mRNA (Montpied et al., 1991; Devaud et al., 1995; Follesa et al., 2003) and an increase in that of α4, α6, γ1, and γ2S mRNAs (Devaud et al., 1995) in the cerebral cortex and cerebellum. Changes in GABAA receptor subunit expression after chronic ethanol exposure were also demonstrated in the hippocampus, a region considered to be less sensitive with regard to the acute ethanol effect on GABAA receptor function. Chronic ethanol exposure decreased α1 subunit expression, increased that of the α4 subunit (Mahmoudi et al., 1997; Matthews et al., 1998; Cagetti et al., 2003), and did not alter that of the γ2 subunit (Matthews et al., 1998). This pattern of changes was different from that of the cerebral cortex (Matthews et al., 1998). Chronic intermittent ethanol treatment also downregulated δ subunit expression and upregulated that of the γ1 and γ2 subunits in the hippocampus (Cagetti et al., 2003).
These changes are associated in most cases with alterations in the amounts of subunit proteins (Devaud et al., 1997), but it has remained unclear whether they are directly correlated with the changes in GABAA receptor function or pharmacological sensitivity that result from chronic exposure to and subsequent withdrawal of ethanol.
With the use of rat hippocampal neurons in primary culture, we have further evaluated the effects of prolonged exposure to and abrupt withdrawal of ethanol on GABAA receptor function, expression, and responsiveness to ligands selective for different receptor subtypes. The use of cultured hippocampal neurons allowed us, at variance with laboratory animals, to overcome the difficulty of establishing the precise onset of ethanol withdrawal caused by the possibility of the rapid washout of ethanol.
Materials and Methods
Primary culture of hippocampal neurons. Animal care and handling throughout all experimental procedures were in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC). The experimental protocols were also approved by the Animal Ethics Committee of the University of Cagliari. Primary cultures of hippocampal neurons were prepared from Sprague Dawley rats on postnatal days 1-3 as described previously (Costa et al., 2000), with minor modifications. Pups were killed by decapitation, and the hippocampus was removed and transferred to a culture dish containing Neurobasal A medium (Invitrogen, San Diego, CA) supplemented with 10% heat-inactivated fetal bovine serum (Sigma, St. Louis, MO), 25 μm glutamate, 0.5 mm glutamine, penicillin (100 U/ml), streptomycin (0.1 mg/ml), and amphotericin B (0.25 μg/ml). The tissue was chopped with scissors, and the resulting fragments were transferred to a sterile tube and gently dissociated by repeated passage through a Pasteur pipette with an opening of 0.5 mm. The dissociated cells were plated either in 35 mm culture dishes (4 × 106 cells) that had been coated with poly-l-lysine hydrobromide (100 μg/ml; 30-70 kDa) (Sigma) for measurement of GABAA receptor subunit mRNAs or in multi-well dishes containing 12-mm-round glass coverslips coated with poly-l-lysine (6 × 105 cells) for electrophysiological recording or immunocytofluorescence analysis. Cells were cultured in a humidified incubator containing 5% CO2 at 37°C. Twenty-four hours after plating, fetal bovine serum was replaced by B-27 supplement (Invitrogen), and glutamate was removed from the medium after 3 d in culture.
Ethanol treatment. After 5 d in culture, cells were exposed continuously for the next 5 d to 100 mm ethanol according to the procedure of Smothers et al. (1997), with minor modifications. Ethanol was added directly to the culture medium, and to prevent daily fluctuations in its concentration caused by evaporation, culture dishes and multi-well dishes with coverslips were kept inside a sealed sterile plastic container (pre-equilibrated with 5% CO2/95% air) along with an isomolar concentration of ethanol in an open beaker. Control cultures were kept likewise in a sealed sterile plastic container not containing ethanol. To further ensure the consistency of the ethanol concentration, the culture medium was replaced daily for both ethanol-treated and control cells. Because daily replacement of medium could have potential effects on the network properties of cells, we ran preliminary experiments with untreated cells in which a culture condition with daily changes of medium, starting from day 5 to days 10-12, was compared with a condition of no medium replacement for the same period of days. The subsequent measurement of several parameters such as total amount of mRNA extracted, functional and pharmacological responses of GABAA receptors, morphology of neurons, and abundance of glial cells, revealed no apparent alteration produced by this procedure (results not shown). Because the daily culture medium replacement actually began on day 5 of culture, it is likely that at this stage hippocampal neurons may have already reached a sufficient degree of maturation and differentiation, becoming much less sensitive to this procedure.
The concentration of 100 mm ethanol used in our study to treat cultured hippocampal cells was chosen on the basis of its efficacy in both acutely potentiating GABAA receptor function and producing changes in GABAA receptor subunit gene expression, as determined in pilot experiments (results not shown). Such a concentration of ethanol has been used in previous studies in our laboratory (Follesa et al., 2003) as well as in other laboratories (Smothers et al., 1997; van Zundert et al., 2000).
To assess the effects of chronic ethanol treatment, cultured cells exposed to ethanol, as described above, were used immediately after the removal of ethanol from the culture medium (0 hr of withdrawal). In withdrawal experiments, the ethanol-containing medium was replaced after 5 d by ethanol-free medium containing (or not) diazepam (10 μm), baclofen (100 μm), or γ-hydroxybutyrate (GHB) (1, 10, or 100 mm) with or without SCH 50911 (100 μm), after which the cells were incubated for an additional 3-24 hr. Ethanol, GHB, baclofen, and SCH 50911 were dissolved in medium, whereas diazepam was dissolved in dimethyl sulfoxide and subsequently diluted to the desired concentration in culture medium. Control neurons were treated with the corresponding vehicle. All experimental groups were compared with control cells maintained in culture for the same number of days or hours during ethanol withdrawal. Thus, each experimental group had its respective control processed at the same time.
Riboprobe preparation. GABAA receptor subunit cDNAs were prepared as described previously (Follesa et al., 1998) by reverse transcription and PCR. In brief, cDNA prepared from rat brain (1-10 ng) was subjected to amplification with TaqDNA polymerase (2.5 U) (Perkin-Elmer/Cetus, Norwalk, CT) in 100 μl of standard buffer (100 mm Tris-HCl, pH 8.3, 500 mm KCl, 15 mm MgCl2, 0,01% gelatin) containing 1 μm each of specific sense and antisense primers and 200 μm of each deoxynucleoside triphosphate. The primer pairs for the various receptor subunits were designed to include cDNA sequences with the lowest degree of homology among the different subunits (Follesa et al., 1998). The reaction was performed in a thermal cycler (Eppendorf) for 30 cycles of 94°C for 45 sec and 60°C for 1 min, with a final extension at 72°C for 15 min. The reaction products were separated by electrophoresis, visualized by staining with ethidium bromide, excised from the gel, purified, and cloned into the pAMP 1 vector (Invitrogen). The resulting plasmids were introduced into Escherichia coli DH5α and subsequently purified from the bacterial cells, and the cDNA inserts were sequenced with a Sequenase DNA sequencing kit (USB, Cleveland, OH). The determined nucleotide sequences were 100% identical to the respective previously published sequences. Plasmids containing the cDNA fragments corresponding to the various GABAA receptor subunits were linearized with restriction enzymes (Follesa et al., 1998) and used as templates for the appropriate RNA polymerase (SP6 or T7) to generate [α-32P]UTP-labeled cRNA probes for RNase protection assays.
RNA extraction and measurement of GABAAreceptor subunit mRNAs. Total RNA was isolated from cultured hippocampal cells with an RTN kit (Sigma) and quantified by measurement of absorbance at 260 nm. An RNase protection assay for the semiquantitative measurement of the GABAA receptors α1, α2, α3, α4, α5, γ2L, and γ2S subunit mRNAs was performed as described (Follesa et al., 1998). In brief, 15 μg of total RNA was dissolved in 20 μl of hybridization solution containing 150,000 cpm of 32P-labeled cRNA probe for a specific GABAA receptor subunit (6 × 107 to 7 × 107 cpm/μg) and 15,000 cpm of 32P-labeled cyclophilin cRNA (1 × 106 cpm/μg). Cyclophilin is expressed widely among tissues, including the brain, and its gene is most likely regulated in an “on or off” manner; cyclophilin mRNA was thus used as an internal standard for our measurements (Follesa et al., 1998). The hybridization reaction mixtures were incubated at 50°C overnight and then subjected to digestion with RNase, after which RNA-RNA hybrids were detected by electrophoresis (on a sequencing gel containing 5% polyacrylamide and urea) and autoradiography. The amounts of GABAA receptor subunit mRNAs and cyclophilin mRNA were determined by measurement of the optical density of the corresponding bands on the autoradiogram with a densitometer (model GS-700; Bio-Rad, Hercules, CA), which was calibrated to detect saturated values so that all measurements were in the linear range. The data were normalized by dividing the optical density of the protected fragment for each receptor subunit mRNA by that of the respective protected fragment for cyclophilin mRNA. The amount of mRNA was therefore expressed in arbitrary units.
Immunoblot analysis. Hippocampal neurons were homogenized in a solution containing 10 mm Tris-HCl, pH 7.4, 0.32 m sucrose, 5 mm EDTA, and 0.1 mm phenylmethylsulfonyl fluoride, and the homogenate was centrifuged at 1000 × g for 10 min. The resulting supernatant was then centrifuged for 20 min at 12,000 × g, and the crude membrane pellet so obtained was washed three times with homogenization buffer and stored at -20°C until use. Portions of the crude membrane fraction (40 μg of protein) were incubated for 5 min at 95°C in 20 μl of SDS sample buffer and then subjected to SDS-PAGE on 10% minigels (Mini Protean II; Bio-Rad). The separated proteins were transferred electrophoretically to a nitrocellulose membrane and subjected to immunoblot analysis with goat polyclonal antibodies (1 μg/ml) generated in response to an extracellular epitope (peptide N1-19) of the rat GABAA receptor α4 subunit (Santa Cruz Biotechnology, Santa Cruz, CA). The membrane was incubated with the antibodies in the absence or presence of the N1-19 peptide (10 μg/μg of antibody) (Santa Cruz Biotechnology). Immune complexes were detected with an ECL detection kit (Amersham Biosciences, Little Chalfont, UK).
Immunocytofluorescence analysis. Cells cultured on coverslips were washed three times with PBS, fixed for 1 hr at room temperature with 4% paraformaldehyde in PBS, washed three times with TN buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl), and permeabilized for 1 hr at room temperature with TN-T buffer (0.1% Triton X-100 in TN buffer) containing 0.5% dried skim milk. Nonspecific binding sites for avidin and biotin were blocked by incubation of the cells for 15 min at room temperature, first with avidin D blocking solution and then with biotin blocking solution (Vector, Burlingame, CA). The cells were then incubated overnight at 4°C with the goat polyclonal antibodies (1:500 dilution in TN-T) to the GABAA receptor α4 subunit. After several washes with TN-T buffer, the cells were incubated for 1 hr at room temperature, first with biotin-conjugated donkey antibodies (1:200 in TN-T) to goat IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) and then with tetramethyl rhodamine isothiocyanate-conjugated streptavidin (Jackson ImmunoResearch Laboratories) diluted to a concentration of 2 μg/ml in TN-T. The cells were washed extensively with TN buffer, and a coverslip was then applied with permanent aqueous mounting medium (Sigma). Quantitative analysis was performed using a Leica four-dimensional confocal laser scanning microscope with an argon-krypton laser as described previously (Spiga et al., 2003). Confocal images were generated using PL Floutar 100× oil (numerical aperture = 1.3). Each frame was acquired eight times and then averaged to obtain noise-free images. Three-dimensional reconstructions of cells were obtained with the “maximum intensity” algorithm that was used on optical sections, usually at consecutive intervals of 0.5-1 μm, and were imaged through the depth of the labeled neurons and saved as image data set and processed with Scanware 4.2a Leica. All confocal images were white-labeled on a black background, in a gray scale ranging from 0 (black) to 255 (white). For morphometric and statistical analysis on three-dimensional reconstructed images, Bioscan Optimas version 6.5 software was used. The area of the body of the cell is obtained by marking its profile, excluding all dendritic trunks with a spline of 64 intervals. This yields the bounded area in calibrated square units (square micrometers). Perimeter values are evaluated similarly, and the total boundary length is expressed in micrometers. Major axis length is a real value, which is obtained from area objects giving the length of the major axis in calibrated units. Breadth is a real value, which is extracted from area objects giving the sum of the maximum distance of the boundary from either side of the major axis in calibrated units. Circularity is a real value of the ratio of the squared perimeter divided by the area (i.e., perimeter squared/area). This is a dimensionless number with a minimum value of 4π (12.57) achieved only for circular boundaries.
Whole-cell electrophysiological recording. Immediately before recording, coverslips were transferred to a perfusion chamber (Warner Instruments, Hampden, CT), and neurons were visualized with a Nikon upright microscope equipped with Nomarski optics. Large neurons with a pyramidal shape and well defined dendritic processes were selected for electrophysiological recording (see Fig. 1). The membrane potential was clamped at -60 mV with an Axopatch 200-B amplifier (Axon Instruments, Foster City, CA). The resting membrane potential for the studied neurons was approximately -60 mV. Recording pipettes (borosilicate capillaries with a filament; outer diameter, 1.5 mm) (Sutter Instruments, Novato, CA) were prepared with a two-step vertical puller (Sutter Instruments) and had resistances between 4 and 6 MΩ. Pipette capacitance and series resistance were compensated, the latter at 60%. Currents through the patch-clamp amplifier were filtered at 2 kHz and digitized at 5.5 kHz with commercial software (pClamp 8.1; Axon Instruments).
Figure 1.
Light micrographs of live rat hippocampal neurons in culture. Cells were cultured for 5 d in the absence (A) or presence (B) of 100 mm ethanol; some ethanol-treated cells were then incubated for an additional 6 hr in the absence of ethanol (C). Magnification, 200×.
The external solution contained (in mm): 130 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES-NaOH, pH 7.3, and 11 glucose. The internal solution contained (in m m): 140 CsCl, 2 MgCl2, 1 CaCl2, 10 EGTA, 10 HEPES-CsOH, pH 7.3, and 2 ATP (disodium salt). Drugs were applied with a fast-exchange flow-tube perfusion system driven by a motor (Warner Instruments). Agonists were applied at intervals of 30 sec. All experiments were performed at room temperature (23-25°C). Data were analyzed by pClampfit 8.01 software (Axon Instruments). Modulation of GABA-evoked Cl- currents by drugs is expressed as percentage change, [(I′/I) - 1] × 100%, where I is the average of control responses obtained before drug application and after drug washout, and I′ is the average of the agonist-induced responses obtained from the same cell in the presence of drug. Nonlinear regression analysis of GABA dose-response relations determined from the average peak current amplitude was performed with Prism software (version 4, Graphpad) according to the equation I = Imin ± (Imax - Imin)/(1 ± 10[log(EC50 - X)]nH), where Imin and Imax are the minimal and maximal responses to GABA, respectively, EC50 is the concentration of GABA that produces 50% of the maximal response, X is the test concentration of GABA, and nH is the Hill coefficient.
Statistical analysis. Data are presented as means ± SE. The statistical significance of differences was assessed by one-way ANOVA followed by Scheffe's test. A p value of <0.05 was considered statistically significant.
Results
Neither continuous exposure to 100 mm ethanol for 5 d nor subsequent withdrawal of ethanol for 6 hr appeared to affect the gross morphology of cultured rat hippocampal neurons (Fig. 1). This conclusion was supported by analysis of morphometric parameters by confocal laser-scanning microscopy (see Table 2). In addition, ethanol treatment and withdrawal did not affect the amount of total RNA in these cells (Table 1).
Table 2.
Morphometric parameters of rat hippocampal neurons subjected to ethanol withdrawal in the presence of diazepam, GHB, or baclofen
|
Experimental group |
Area (μm2) |
Perimeter (μm) |
Circularity |
Maximum axis length (μm) |
Breadth (μm) |
|---|---|---|---|---|---|
| Control (n = 33) | 173 ± 9 | 51.2 ± 1.3 | 15.6 ± 0.3 | 19.0 ± 0.5 | 13.7 ± 0.5 |
| Withdrawal (n = 41) | 171 ± 12 | 51.8 ± 2.0 | 16.3 ± 0.3 | 19.0 ± 0.8 | 13.6 ± 0.5 |
| Withdrawal + diazepam (n = 23) | 158 ± 9 | 47.7 ± 1.3 | 14.6 ± 0.2 | 17.4 ± 0.5 | 13.1 ± 0.4 |
| Withdrawal + GHB (n = 25) | 169 ± 10 | 51.1 ± 1.5 | 16.4 ± 0.3 | 18.5 ± 0.7 | 13.2 ± 0.4 |
| Withdrawal + baclofen (n = 22)
|
171 ± 11
|
50.5 ± 1.7
|
15.2 ± 0.2
|
18.0 ± 0.8
|
13.5 ± 0.5
|
Cells were cultured for 5 d in the absence (control) or presence of 100 mm ethanol, after which the ethanol-treated cells were incubated for an additional 6 hr in ethanol-free medium in the absence or presence of diazepam (10 μm), GHB (100 mm), or baclofen (100 μm). The cells were then examined by confocal laser-scanning microscopy for determination of the indicated morphometric parameters. Data are means ± SE of values determined from the indicated number (n) of cells.
Table 1.
Effects of chronic ethanol treatment and ethanol withdrawal on the total RNA content of hippocampal neurons
|
|
Total RNA (micrograms per dish) |
||||
|---|---|---|---|---|---|
| Experiment
|
Control
|
Chronic EtOH
|
EtOH withdrawal
|
||
| 1 | 14.41 ± 2.03 | 12.88 ± 2.32 | 15.40 ± 3.51 | ||
| 2 | 13.49 ± 3.02 | 15.93 ± 5.95 | 12.97 ± 5.65 | ||
| 3 | 16.85 ± 1.41 | 15.05 ± 2.66 | 16.25 ± 3.53 | ||
| 4 | 14.37 ± 2.15 | 16.25 ± 2.12 | 17.23 ± 2.79 | ||
| 5
|
18.69 ± 4.28
|
16.56 ± 1.15
|
15.36 ± 3.57
|
||
Cells were cultured in 35 mm dishes for 5 d in the absence (control) or presence (chronic EtOH) of 100 mm ethanol; some ethanol-treated cells were also incubated for an additional 6 hr in the absence of ethanol (EtOH withdrawal). Total RNA was isolated from the cells and quantitated. Data are expressed as micrograms of RNA per culture dish and are means ± SE of three dishes from five randomly selected experiments.
Effects of chronic ethanol treatment on GABAA receptor gene expression
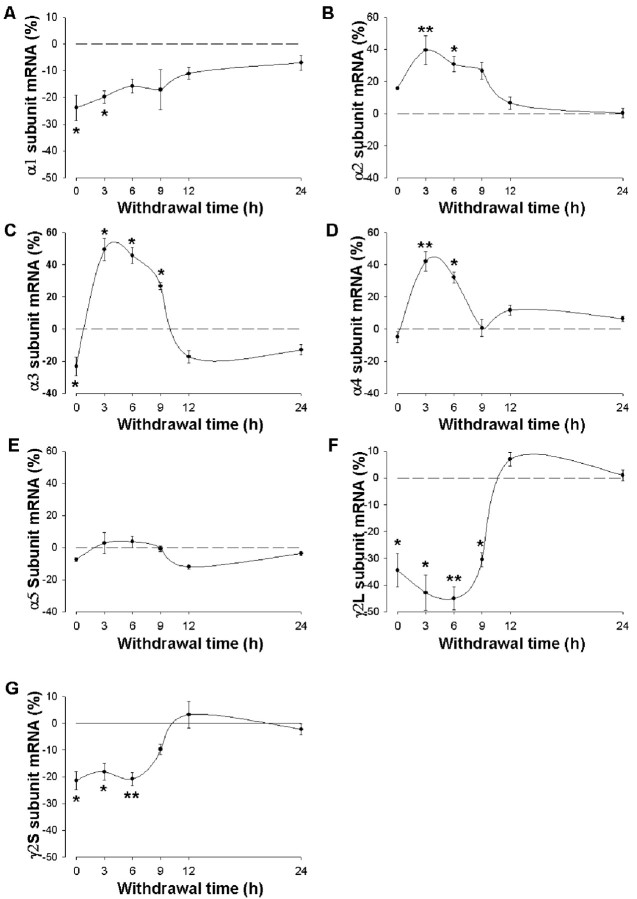
The abundance of GABAA receptors α1, α2, α3, α4, and α5 mRNAs as well as that of the mRNAs for the two splice variants of the γ2 subunits, γ2L and γ2S, were determined by RNase protection assay after continuous exposure of cultured hippocampal neurons to 100 mm ethanol for 5 d. Ethanol induced a significant decrease in the amount of α1, α3, γ2L, and γ2S subunit mRNAs relative to control values (Fig. 2A,C,F,G). ANOVA revealed a significant main effect of chronic ethanol treatment on mRNA levels for α1 (F(1,16) = 21.83; p < 0.001), α3 (F(1,20) = 9.48; p < 0.006), γ2L (F(1,11) = 17.31; p < 0.002), and γ2S (F(1,21) = 34.46; p < 0.001).
Figure 2.
Time course of the effects of ethanol withdrawal on the abundance of GABAA receptor subunit mRNAs in hippocampal cells. Cells were incubated first for 5 d with 100 mm ethanol and then for the indicated times in ethanol-free medium. The amounts of GABAA receptor α1 (A), α2 (B), α3 (C), α4 (D), α5 (E), γ2L (F), and γ2S (G) subunit mRNAs were then determined by RNase protection assay. Data are means ± SE of 6-13 values from three independent experiments and are expressed as a percentage of the corresponding value for control cultures incubated in the absence of ethanol for 5 d. *p < 0.05, **p < 0.001 versus control.
In contrast, chronic ethanol exposure did not significantly affect the abundance of α2(F(1,24) = 3.21; p < 0.067), α4(F(1,16) = 0.31; p < 0.588), and α5(F(1,24) = 4.26; p < 0.075) mRNAs (Fig. 2B,D,E).
Effects of ethanol withdrawal on GABAA receptor gene expression
To determine the effect of ethanol withdrawal on GABAA receptor gene expression, we incubated hippocampal neurons first with 100 mm ethanol for 5 d and then in the absence of ethanol for 3-24 hr. The abundance of the α1, γ2L, and γ2S subunit mRNAs, which was reduced after ethanol treatment, remained significantly decreased, relative to control values, 3 hr after removal of ethanol (Fig. 2A,F,G) (α1, F(5,36) = 5.29, p < 0.001; γ2L, F(5,18) = 18.11, p < 0.001; γ2S, F(5,42) = 12.13, p < 0.001). The amount of the α1 subunit mRNA had returned to control values 6 hr after ethanol removal (Fig. 2A). The amounts of the γ2L and γ2S subunit mRNAs remained significantly decreased 6 hr after ethanol withdrawal but had returned to control values by 9-12 hr (Fig. 2F,G). In contrast, the abundance of α2, α3, and α4 mRNAs was markedly increased, relative to control values, in response to ethanol withdrawal, with the maximal effects being apparent 3 hr after ethanol removal; the amounts of these mRNAs had returned to control values by 9-12 hr after ethanol withdrawal (Fig. 2B-D). ANOVA revealed a significant main effect of ethanol withdrawal on mRNA levels for α2 (F(5,52) = 7.97; p < 0.001), α3 (F(5,34) = 17.18; p < 0.001), and α4 (F(5,37) = 23.46; p < 0.001). Discontinuation of ethanol treatment had no effect (F(5,47) = 2.04; p < 0.088) on the abundance of the α5 subunit mRNA (Fig. 2E).
Effects of diazepam, GHB, and baclofen on ethanol withdrawal-induced changes in GABAA receptor gene expression
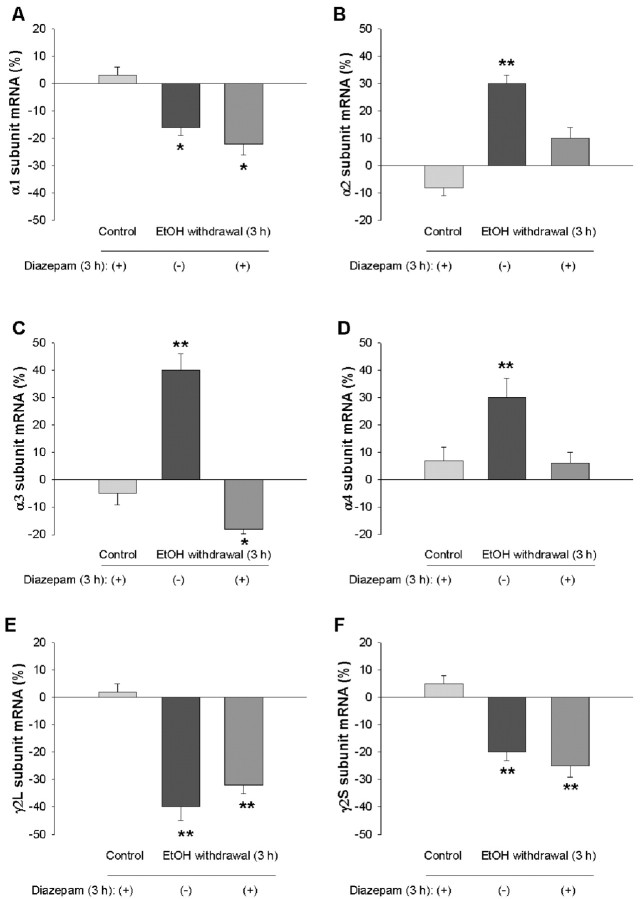
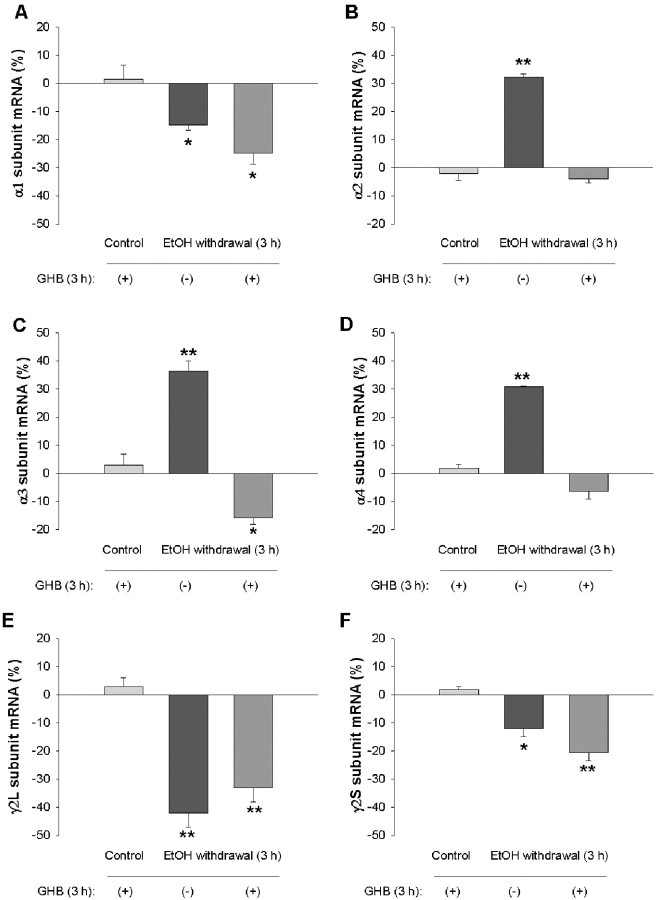
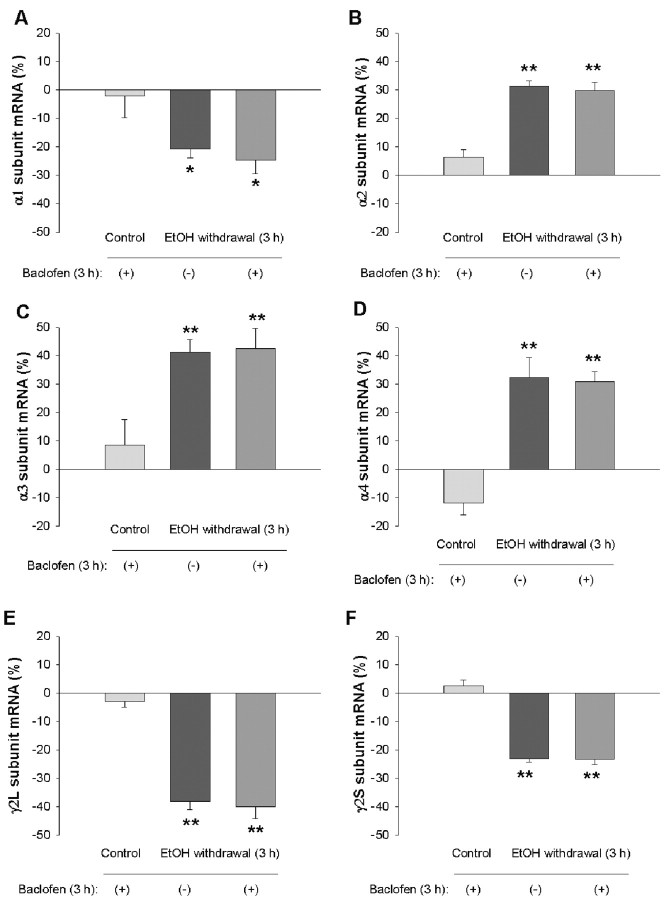
Benzodiazepines, GHB, and the GABAB receptor agonist baclofen reduce withdrawal symptoms and the craving for ethanol both in human alcoholics and in ethanol-dependent laboratory animals (Fadda et al., 1989; Gallimberti et al., 1989; Addolorato et al., 1996, 2002; Agabio et al., 1998; Lejoyeux et al., 1998; Colombo et al., 2000). We therefore examined the effects of diazepam, GHB, and baclofen on the changes in GABAA receptor gene expression observed during ethanol withdrawal. Hippocampal neurons were incubated first for 5 d with 100 mm ethanol and then for 3 hr in ethanol-free medium containing 10 μm diazepam, 1-100 mm GHB, or 100 μm baclofen. Diazepam prevented the changes in the abundance of the α2, α3, and α4 subunit mRNAs induced by ethanol withdrawal (Fig. 3). Similarly, GHB at 100 mm (Fig. 4), but not at 1 or 10 mm (data not shown), prevented the ethanol withdrawal-induced changes in subunit mRNA levels. In contrast, the amounts of α1, α2, α3, α4, γ2L, and γ2S mRNAs apparent 3 hr after ethanol withdrawal were not affected by the presence of baclofen (Fig. 5). Diazepam, GHB, or baclofen had no significant effect on the amounts of the various subunit mRNAs in neurons not exposed to ethanol (Figs. 3, 4, 5).
Figure 3.
Prevention by diazepam of changes in the abundance of GABAA receptor subunit mRNAs induced by ethanol withdrawal. Hippocampal neurons were incubated first for 5 d with 100 mm ethanol and then for 3 hr in ethanol-free medium in the absence or presence of 10 μm diazepam. Cells incubated for 5 d in the absence of ethanol were also exposed to diazepam for 3 hr. The abundance of GABAA receptor α1 (A), α2 (B), α3 (C), α4 (D), γ2L (E), and γ 2S (F) subunit mRNAs was then determined by RNase protection assay. Data are means ± SE of 9-12 values from three independent experiments and are expressed as a percentage of the corresponding value for control cultures incubated for 5 d in the absence of ethanol. *p < 0.05, **p < 0.001 versus control. EtOH, Ethanol.
Figure 4.
Prevention by GHB of changes in the abundance of GABAA receptor subunit mRNAs induced by ethanol withdrawal. Hippocampal neurons were incubated first for 5 d with 100 mm ethanol and then for 3 hr in ethanol-free medium in the absence or presence of 100 mm GHB. Cells incubated for 5 d in the absence of ethanol were also exposed to GHB for 3 hr. The abundance of GABAA receptor α1 (A), α2 (B), α3 (C), α4 (D), γ2L (E), and γ2S (F) subunit mRNAs was then determined by RNase protection assay. Data are means ± SE of 9-12 values from three independent experiments and are expressed as a percentage of the corresponding value for control cultures incubated for 5 d in the absence of ethanol. *p < 0.05, **p < 0.001 versus control.
Figure 5.
Lack of effect of baclofen on changes in the abundance of GABAA receptor subunit mRNAs induced by ethanol withdrawal. Hippocampal neurons were incubated first for 5 d with 100 mm ethanol and then for 3 hr in ethanol-free medium in the absence or presence of 100 μm baclofen. Cells incubated for 5 d in the absence of ethanol were also exposed to baclofen for 3 hr. The abundance of GABAA receptor α1 (A), α2 (B), α3 (C), α4 (D), γ2L (E), and γ2S (F) subunit mRNAs was then determined by RNase protection assay. Data are means ± SE of 9-12 values from three independent experiments, each performed in triplicate or quadruplicate, and are expressed as a percentage of the corresponding value for control cultures incubated for 5 d in the absence of ethanol. *p < 0.05, **p < 0.001 versus control.
Effect of ethanol withdrawal on α4 subunit abundance
Recent studies strongly suggested a pivotal role for the increased expression of the α4 subunit associated with withdrawal from ethanol (Devaud et al., 1995, 1997; Mahmoudi et al., 1997; Matthews et al., 1998; Cagetti et al., 2003; Follesa et al., 2003) as well as other positive allosteric modulators of the GABAA receptors (Smith et al., 1998b; Follesa et al., 2000). Therefore, to determine whether the increase in the abundance of the α4 subunit mRNA induced by discontinuation of ethanol exposure was associated with a similar increase in the amount of the encoded protein at the cell surface, we subjected hippocampal neurons to immunocytofluorescence analysis with a confocal laser-scanning microscope. The specific antibodies were generated in response to an extracellular epitope (peptide N1-19) of the rat α4 subunit and were characterized by immunoblot analysis of a crude membrane fraction of hippocampal neurons, in which they recognized a single protein of ∼70 kDa (Fig. 6).
Figure 6.
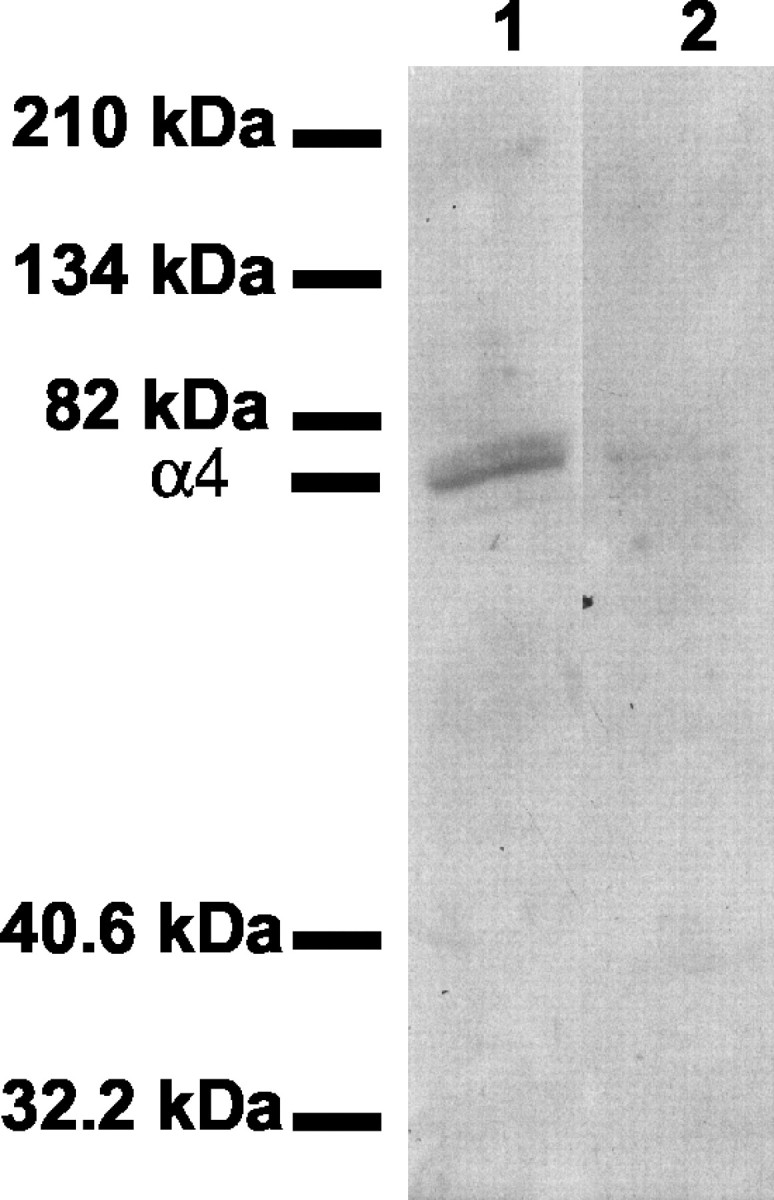
Immunoblot analysis of the GABAA receptor α4 subunit in hippocampal neurons. A crude membrane fraction (40 μg of protein) of cultured hippocampal neurons was subjected to immunoblot analysis with antibodies specific for the GABAA receptor α4 subunit in either the absence (lane 1) or presence (lane 2) of the α4 peptide N1-19. The positions of molecular size standards and of the α4 protein are indicated.
A low level of α4 subunit immunoreactivity was detected in control hippocampal neurons, as expected (Pirker et al., 2000); it was localized mostly in the perinuclear region, at the cell membrane, and in association with dendrites and synapses (Fig. 7A). In neurons subjected to chronic exposure to ethanol and subsequent ethanol withdrawal for 6 hr, the abundance of the α4 subunit was markedly increased (F(1,78) = 37.57; p < 0.001) compared with that apparent in control cells (Fig. 7B,G). This effect was most pronounced for the α4 protein localized at the cell membrane and in association with dendrites and synapses. Replacement of ethanol with 10 μm diazepam (Fig. 7C,G) or 100 mm GHB (Fig. 7D,G), but not with 100 μm baclofen (Fig. 7E,G), prevented the increase in α4 subunit expression induced by ethanol withdrawal. No immunoreactivity was detected on incubation of neurons with the antibodies to α4 together with the N1-19 peptide antigen (Fig. 7F).
Figure 7.
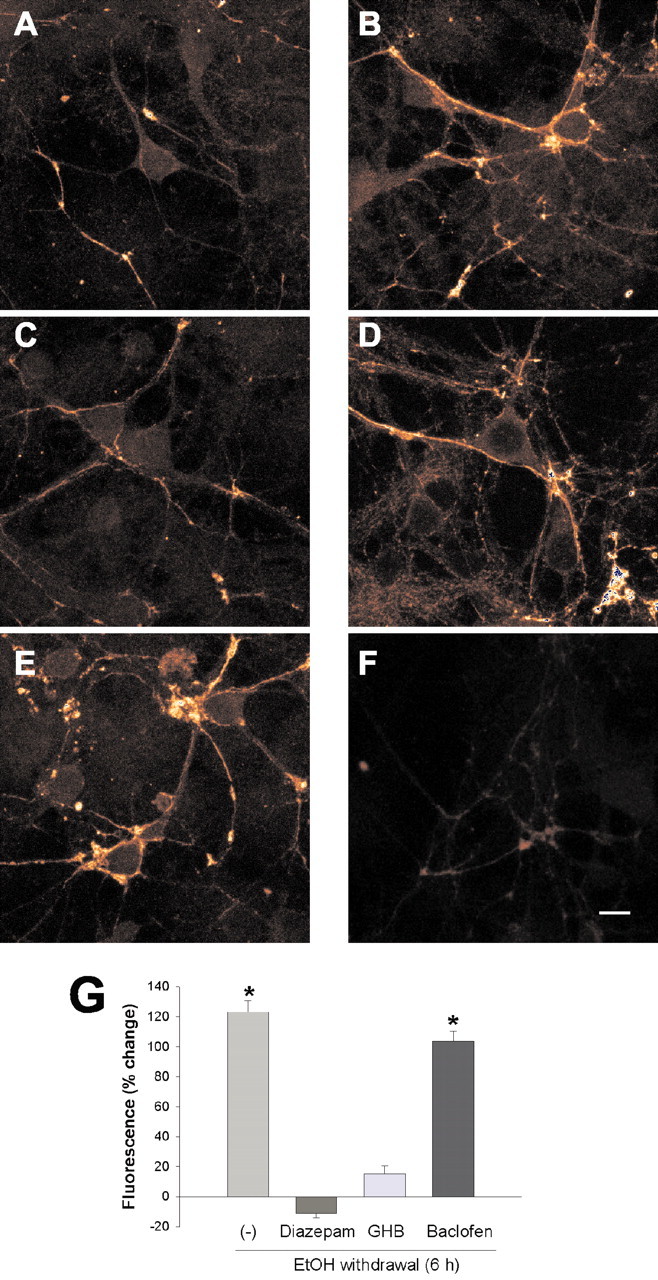
Ethanol withdrawal-induced increase in the abundance of the GABAA receptor α4 subunit in hippocampal neurons and its inhibition by diazepam or GHB. Cells were cultured for 5 d in the absence (A) or presence of 100 mm ethanol; the ethanol-treated cells were subsequently incubated for an additional 6 hr in ethanol-free medium in the absence (B) or presence of 10 μm diazepam (C), 100 mm GHB (D), or 100 μm baclofen (E). All cells were then subjected to immunocytofluorescence analysis with antibodies to an extracellular epitope (peptide N1-19) of the α4 subunit. F, Control cells were also subjected to analysis with the specific antibodies in the presence of the N1-19 peptide (10 μg/ml). Representative images are shown. Scale bar, 10 μm. G, Semiquantitative determination of the abundance of the α4 subunit as determined by image analysis of the immunocytofluorescence data. Results are expressed as percentage change in fluorescence intensity relative to control cells and are means ± SE of values for at least 40 randomly selected cells for each experimental group and in three independent experiments. *p < 0.001 versus control neurons.
Effect of chronic ethanol withdrawal on morphometric parameters
The use of confocal microscopy allowed us to also carefully evaluate the effect of ethanol withdrawal on cell morphology. The morphometric parameters (area, perimeter, circularity, length, and breadth) measured in cultured hippocampal neurons from control and ethanol withdrawal groups, the latter either in the absence or presence of diazepam (10 μm), GHB (100 mm), or baclofen (100 μm), did not show any statistically significant variation (Table 2).
Effects of chronic exposure to and withdrawal of ethanol on GABA-evoked Cl- currents
We next examined whether the changes in GABAA receptor gene expression induced in hippocampal neurons by chronic exposure to and subsequent withdrawal of ethanol result in alterations in GABAA receptor function or sensitivity to various modulatory drugs. For these electrophysiological experiments, the effects of withdrawal were evaluated 6 hr after discontinuation of ethanol exposure (5 d, 100 mm) because preliminary data revealed that the studied changes were maximal at this time. We first determined the dose-response relation for GABA-evoked Cl- currents recorded from hippocampal neurons in the whole-cell patch-clamp configuration (Fig. 8A,B). Data were normalized with respect to the maximal current induced by 300 μm GABA. Calculation of EC50 values revealed that chronic exposure to (6.8 ± 1.1 μm) or subsequent withdrawal of (9.0 ± 1.2 μm) ethanol had no significant effect (F(2,21) = 1.88; p < 0.177) on the apparent potency of GABA compared with the value for control cells (6.1 ± 1.1 μm). The Hill coefficients calculated for control neurons and cells subjected to chronic ethanol treatment or to ethanol withdrawal were 1.3, 1.0, and 1.1, respectively. The maximal density of GABAA receptor-mediated current (current/capacitance) was reduced significantly (F(2,58) = 6.08; p < 0.004) by 33 or 28% after chronic ethanol treatment or ethanol withdrawal, respectively (Fig. 8C).
Figure 8.
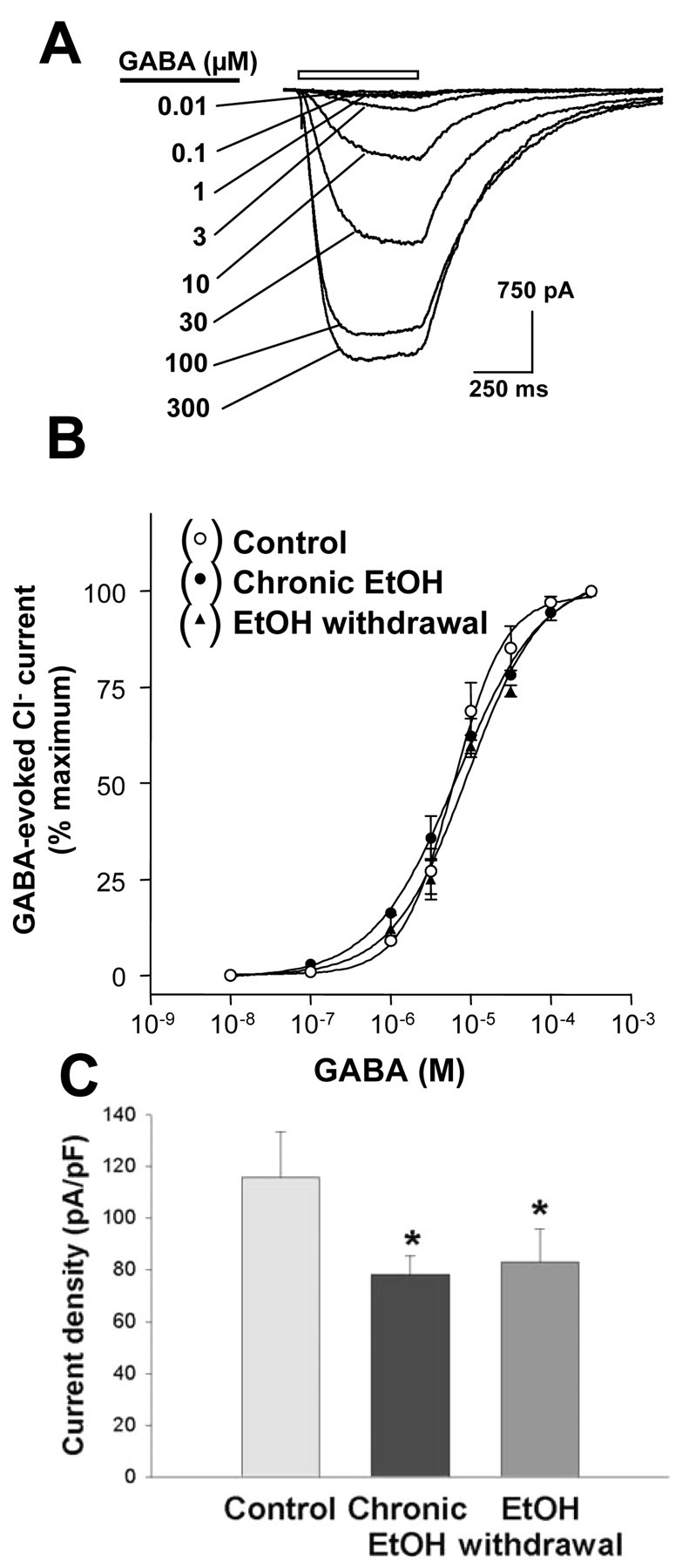
Effects of chronic exposure to and subsequent withdrawal of ethanol on GABA-induced Cl- currents in cultured hippocampal neurons in the whole-cell patch-clamp configuration. A, Representative tracings recorded from a control cell showing Cl- currents evoked by GABA at concentrations of 0.01-300 μm. The period of GABA application is indicated by the horizontal bar. B, Dose-response curves for GABA-evoked Cl- currents in control cells, cells exposed to 100 mm ethanol for 5 d, and cells subjected to ethanol withdrawal for 6 hr. Data were normalized with respect to the maximal current amplitude apparent at 300 μm GABA and are means ± SE of values from 6-10 neurons. C, Maximal current density for GABAA receptor-mediated currents in neurons of the three experimental groups described in B. Data are means ± SE of values from 18-23 neurons. *p < 0.05 versus control neurons.
Effects of chronic exposure to and withdrawal of ethanol on acute ethanol modulation of GABAA receptor function
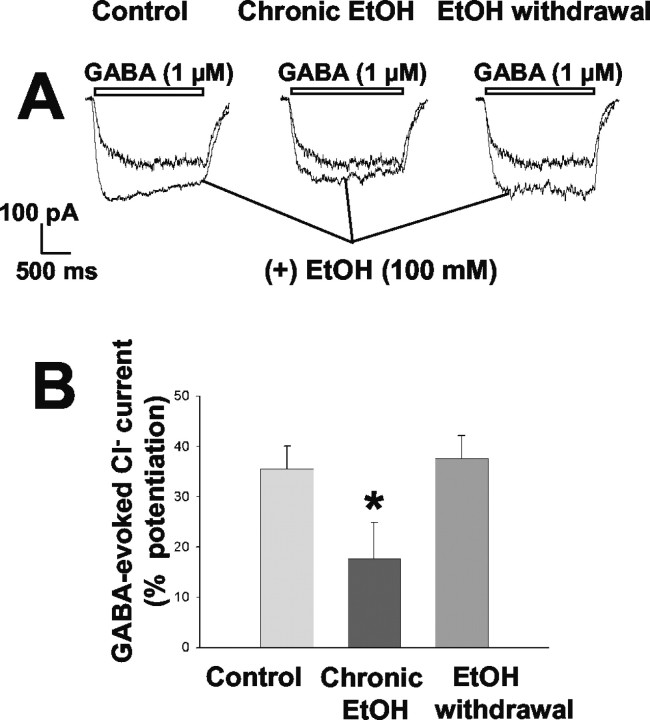
Previous electrophysiological studies have shown that ethanol enhances GABAA receptor function in hippocampal neurons (Reynolds et al., 1992; Aguayo and Pancetti, 1994; Poelchen et al., 2000), whereas others reported no significant ethanol potentiating effect (Proctor et al., 1992a,b; Soldo et al., 1994). Thus, we next measured the acute effect of ethanol on GABA responses in cultured hippocampal neurons from the control, chronic ethanol exposure, and ethanol withdrawal groups. Acute application of 100 mm ethanol potentiated Cl- currents induced by 1 μm GABA (∼EC10) in control neurons by 36 ± 5% (Fig. 9). This acute effect of ethanol was inhibited significantly in cells chronically exposed to ethanol (18 ± 8% potentiation) but not in those subjected to ethanol withdrawal (38 ± 5% potentiation) (F(2,28) = 6.46; p < 0.005).
Figure 9.
Acute modulatory action of ethanol on GABAA receptor function in hippocampal neurons subjected to chronic exposure to or withdrawal of ethanol. A, Representative tracings of Cl- currents induced by 1 μm GABA in a control cell, a cell exposed to 100 mm ethanol for 5 d, and a cell subjected to ethanol withdrawal for 6 hr. GABA was administered in the absence or presence of 100 mm ethanol. B, Quantitation of the acute effects of 100 mm ethanol on GABA-evoked Cl- currents in the three experimental groups. Data are expressed as percentage potentiation of the GABA response and are means ± SE of values from 8-13 neurons. *p < 0.05 versus value for control cells.
Effects of chronic exposure to and withdrawal of ethanol on GABAA receptor sensitivity to benzodiazepine receptor ligands
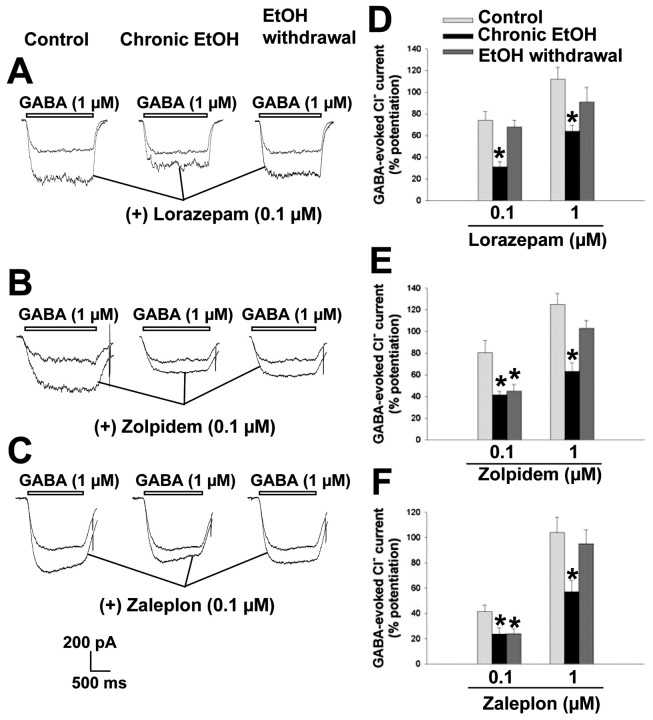
Given that the pharmacology of benzodiazepine receptor ligands is dependent on the subunit composition of GABAA receptors, especially with regard to the α and γ subunits (Pritchett et al., 1989; Barnard et al., 1998), we next examined the impact of the changes in subunit mRNA abundance induced by chronic exposure to and withdrawal of ethanol on benzodiazepine receptor pharmacology in hippocampal neurons. We evaluated the effects of positive (lorazepam, zolpidem, zaleplon) and negative (Ro 15-4513, FG 4172) modulators of GABAA receptors as well as of flumazenil, a competitive antagonist of the benzodiazepine receptor, on control neurons as well as on those subjected to long-term treatment with or withdrawal of ethanol.
The benzodiazepine lorazepam markedly potentiated (74 ± 8 and 112 ± 11% at 0.1 and 1 μm, respectively) the Cl- current induced by 1 μm GABA in control neurons (Fig. 10A,D). The efficacy of this benzodiazepine was reduced significantly (∼50%) in neurons subjected to chronic ethanol treatment compared with that in control neurons. In neurons subjected to ethanol withdrawal, however, the modulatory efficacy of lorazepam was restored to a level similar to that apparent in control neurons (0.1 μm lorazepam, F(2,41) = 14.72, p < 0.001; 1 μm lorazepam, F(2,52) = 9.52, p < 0.001). The imidazopyridine zolpidem (Fig. 10B,E) and the pyrazolopyrimidine zaleplon (Fig. 10C,F), both of which are selective for GABAA receptors containing the α1 subunit (Sanna et al., 2002), also potentiated GABA-evoked Cl- currents in a concentration-dependent manner in control neurons. Consistent with the lower receptor affinity and modulatory potency of zaleplon compared with zolpidem (Damgen, 1999; Sanna et al., 2002), the potentiating effect of zaleplon in control neurons was smaller than that of zolpidem at a concentration of 0.1 μm but not at 1 μm. The potentiating effects of both compounds at both concentrations tested were significantly reduced (∼50%) in neurons subjected to chronic ethanol treatment. In neurons subjected to ethanol withdrawal, whereas the effects of zolpidem and zaleplon at 0.1 μm remained reduced at levels similar to those apparent after chronic ethanol exposure, at the higher concentration (1 μm) both drugs potentiated the GABA response to an extent similar to that observed in control neurons (0.1 μm zolpidem, F(2,30) = 11.90, p < 0.001; 1 μm zolpidem, F(2,31) = 14.75, p < 0.001; 0.1 μm zaleplon, F(2,27) = 4.99, p < 0.014; 1 μm zaleplon, F(2,27) = 5.58, p < 0.009).
Figure 10.
Modulatory action of benzodiazepine receptor agonists on GABAA receptor function in hippocampal neurons subjected to chronic exposure to or withdrawal of ethanol. A-C, Representative tracings of Cl- currents induced by 1 μm GABA in control cells, cells exposed to 100 mm ethanol for 5 d, and cells subjected to ethanol withdrawal for 6 hr. GABA was administered in the absence or presence of lorazepam (A), zolpidem (B), or zaleplon (C), each at a concentration of 0.1 μm. D-F, Quantitation of the respective modulatory effects of the three test drugs (0.1 or 1 μm) on GABA-evoked Cl- currents in the three experimental groups. Data are expressed as percentage potentiation of the GABA response and are means ± SE of values from 9-23 neurons. *p < 0.05 versus value for control cells.
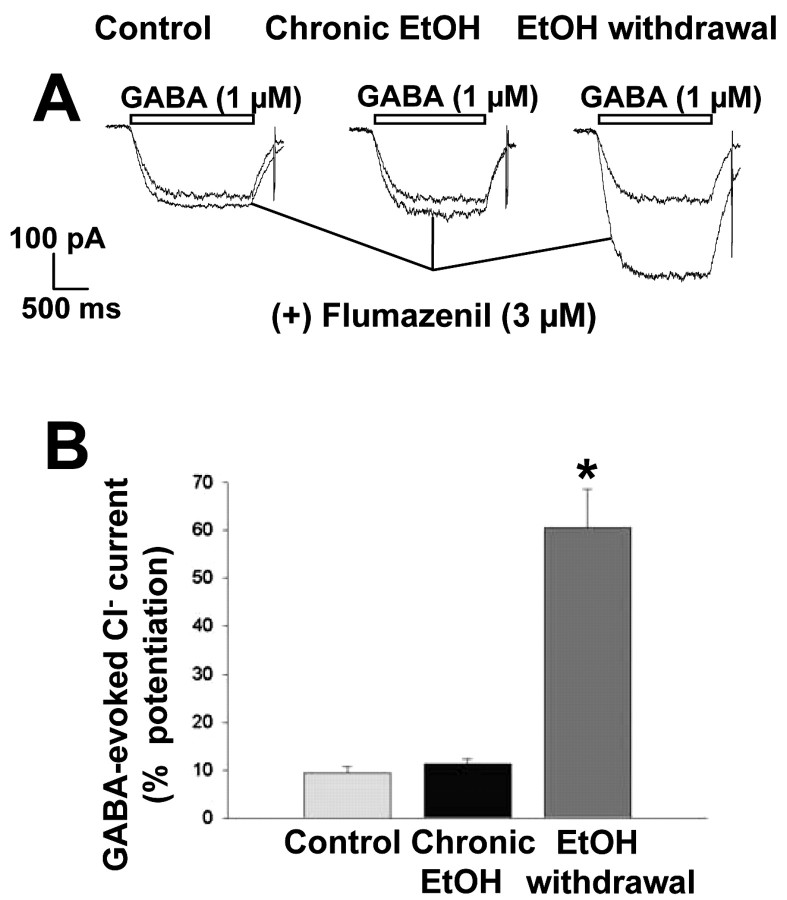
Consistent with its pharmacological profile of a pure antagonist devoid of intrinsic activity, flumazenil (3 μm) did not significantly affect GABA-evoked Cl- currents in either control neurons or neurons subjected to long-term treatment with ethanol (Fig. 11). Flumazenil, however, markedly potentiated (60 ± 8%) the GABA response in neurons subjected to ethanol withdrawal (F(2,40) = 33.68; p < 0.001).
Figure 11.
Agonist-like action of the benzodiazepine receptor antagonist flumazenil in hippocampal neurons subjected to ethanol withdrawal. A, Representative tracings of Cl- currents induced by 1 μm GABA in a control cell, a cell exposed to 100 mm ethanol for 5 d, and a cell subjected to ethanol withdrawal for 6 hr. GABA was administered in the absence or presence of flumazenil (3 μm). B, Quantitation of the modulatory effect of flumazenil on GABA-evoked Cl- currents in the three experimental groups. Data are expressed as percentage potentiation of the GABA response and are means ± SE of values from 8-25 neurons. *p < 0.01 versus value for control cells.
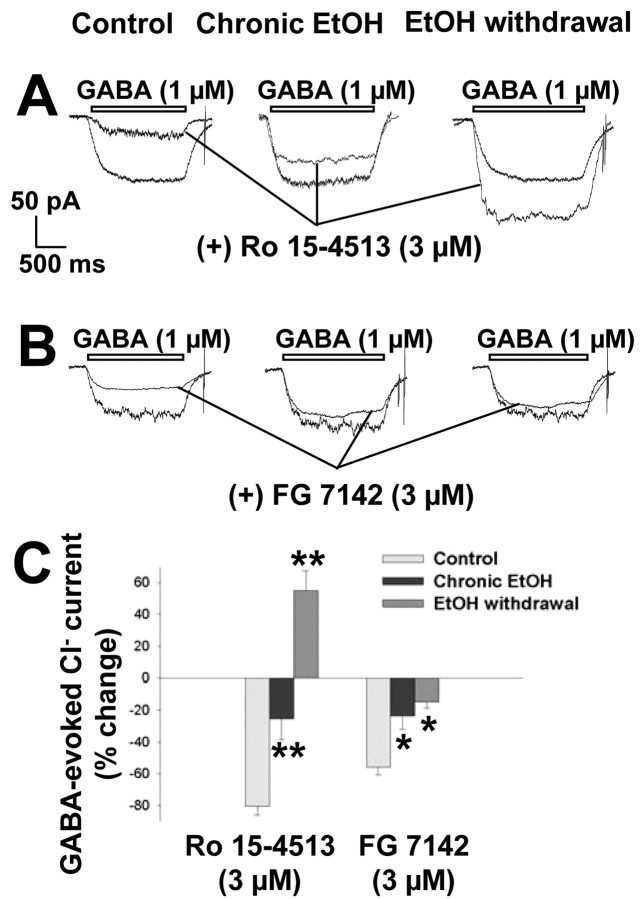
We next investigated the effects of two inverse agonists of the benzodiazepine receptor, the benzodiazepine derivative Ro 15-4513 and the β-carboline FG 7142. Ro 15-4513 (3 μm) markedly inhibited (80 ± 5%) GABA-evoked Cl- currents in control neurons (Fig. 12A,C). This inhibitory effect was significantly reduced (25 ± 13%) in neurons subjected to chronic ethanol exposure, and Ro 15-4513 potentiated the GABA response (55 ± 13%) after ethanol withdrawal (F(2,24) = 81.25; p < 0.001), again consistent with the notion that discontinuation of long-term ethanol treatment induces an increase in the density of α4-containing GABAA receptors. FG 7142 (3 μm) inhibited (56 ± 5%) GABA-evoked Cl- currents in control neurons (Fig. 12B,C). This effect was significantly reduced in neurons subjected to chronic ethanol treatment or to subsequent withdrawal (F(2,20) = 11.12; p < 0.001).
Figure 12.
Modulatory effects of the benzodiazepine receptor inverse agonists Ro 15-4513 and FG 7142 on GABAA receptor function in hippocampal neurons subjected to chronic exposure to or withdrawal of ethanol. A, B, Representative tracings of Cl- currents induced by 1 μm GABA in control cells, cells exposed to 100 mm ethanol for 5 d, and cells subjected to ethanol withdrawal for 6 hr. GABA was administered in the absence or presence of Ro 15-4513 (A) or FG 7142 (B), each at a concentration of 3 μm. C, Quantitation of the modulatory effects of Ro 15-4513 and FG 7142 on GABA-evoked Cl- currents in the three experimental groups. Data are expressed as percentage change in the GABA response and are means ± SE of values from 5-12 neurons. *p < 0.05, **p < 0.01 versus value for control cells.
Effects of diazepam, GHB, and baclofen on GABAA receptor pharmacology during ethanol withdrawal
Finally, we examined whether diazepam, GHB, and baclofen were able to block the changes in pharmacological sensitivity of GABAA receptors induced by ethanol withdrawal. Incubation of neurons with either 10 μm diazepam (Fig. 13) or 100 mm GHB (Table 3) during the 6 hr period of ethanol withdrawal resulted in a significant inhibition of the modulatory efficacy of 1 μm lorazepam and reversed the agonist-like actions of 3 μm flumazenil and 3 μm Ro 15-4513 (1 μm lorazepam, F(1,23) = 50.73, p < 0.001; 3 μm flumazenil, F(1,20) = 39.14, p < 0.001; 3 μm Ro 15-4513, F(1,20) = 118.57, p < 0.001).
Figure 13.
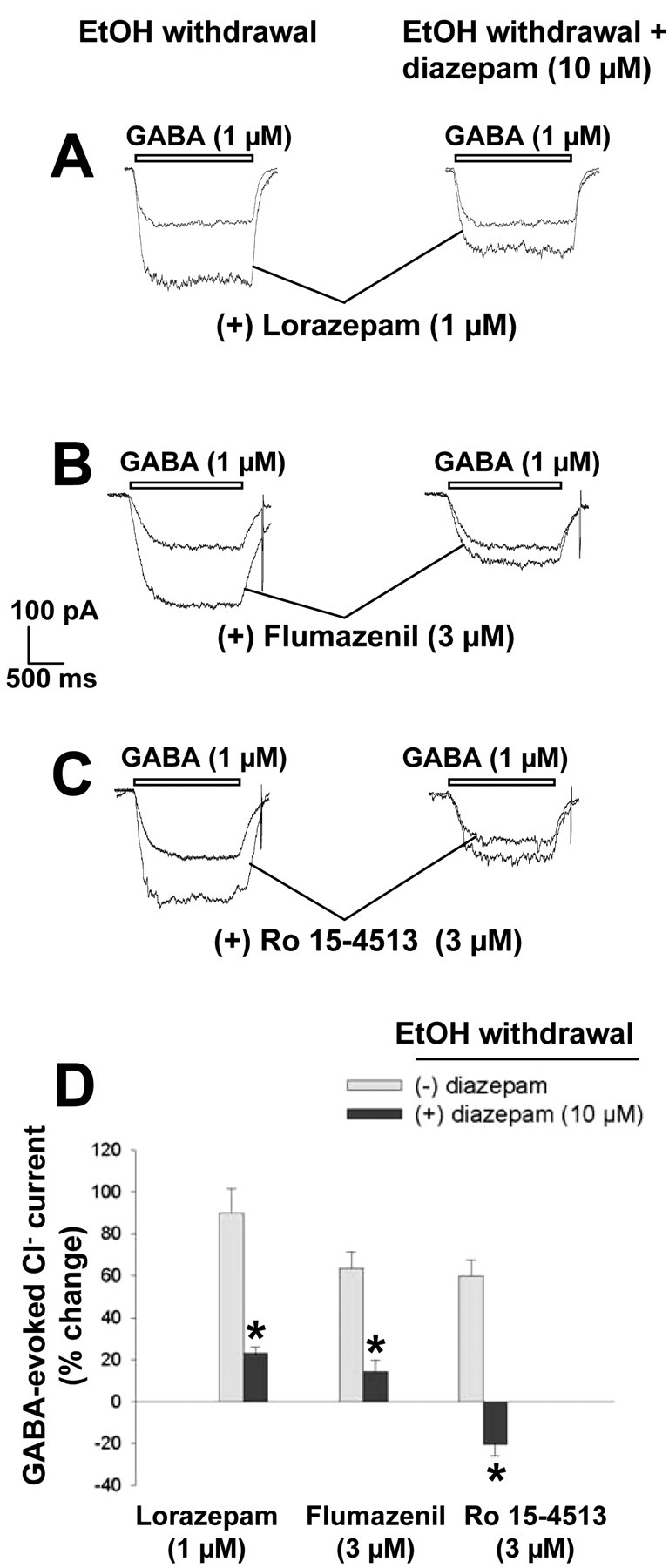
Effects of diazepam on the changes in GABAA receptor sensitivity to lorazepam, flumazenil, or Ro 15-4513 induced by ethanol withdrawal. A-C, Representative traces of Cl- currents induced by 1 μm GABA in hippocampal neurons that had been incubated first for 5 d with 100 mm ethanol and then for 6 hr in ethanol-free medium in the absence or presence of 10 μm diazepam. GABA was applied in the absence or presence of 1 μm lorazepam (A), 3 μm flumazenil (B), or 3 μm Ro 15-4513 (C). D, Quantitation of the modulatory effects of lorazepam, flumazenil, and Ro 15-4513 on GABA-evoked Cl- currents in the two experimental groups. Data are expressed as percentage change in the GABA response and are means ± SE of values from 9-16 neurons. *p < 0.01 versus the corresponding value for cells subjected to ethanol withdrawal in the absence of diazepam.
Table 3.
Effects of GHB and GABAb receptor-specific drugs on ethanol withdrawal-induced changes in GABAa receptor sensitivity to benzodiazepine receptor ligands
|
|
GABA-evoked Cl− current (% change) |
||||||
|---|---|---|---|---|---|---|---|
| Drug
|
Vehicle
|
GHB
|
GHB + SCH 50911
|
Baclofen
|
|||
| Lorazepam | 91 ± 14 | 23 ± 3* | 37 ± 5* | 109 ± 14 | |||
| Flumazenil | 63 ± 8 | 14 ± 5* | 10 ± 3* | 46 ± 5 | |||
| Ro 15-4513
|
59 ± 8
|
−20 ± 5*
|
−27 ± 6*
|
54 ± 8
|
|||
Currents induced by 1 μm GABA were measured in hippocampal neurons that had been incubated first for 5 d with 100 mm ethanol and then for 6 hr in ethanol-free medium in the absence or presence of 100 mm GHB (with or without 100 μm SCH 50911) or 100 μm baclofen. GABA was applied in the absence or presence of 1 μm lorazepam, 3 μmM flumazenil, or 3 μm Ro 15-4513. Data are expressed as percentage change in the GABA response and are means ± SE of values from 12-22 neurons. *p < 0.05 versus the corresponding value for ethanol withdrawal in the absence or drug (vehicle).
To determine whether the action of GHB might involve an interaction with GABAB receptors, we exposed neurons to both 100 mm GHB and 100 μm SCH 50911, a competitive antagonist of the GABAB receptor, during ethanol withdrawal. SCH 50911 failed to antagonize the inhibitory effect of GHB on the changes in GABAA receptor sensitivity to lorazepam, flumazenil, or Ro 15-4513 induced by ethanol withdrawal (Table 3). Furthermore, baclofen (100 μm) did not mimic this action of GHB (Table 3).
Discussion
A correlation of alterations in GABAA receptor function and pharmacological sensitivity with specific changes in receptor gene expression after prolonged ethanol treatment has been explored previously (Devaud et al., 1996; Kang et al., 1998; Cagetti et al., 2003; Follesa et al., 2003).
We have shown further that chronic ethanol exposure and its subsequent discontinuation induce marked changes in GABAA receptor function and pharmacological responsiveness to both nonselective and selective benzodiazepine receptor ligands. Furthermore, these changes may result from alterations in the abundance of mRNAs encoding α1-4 and γ2 subunits as well as in that of the α4 protein. The pattern of changes in GABAA receptor subunit mRNA levels observed in our study differs to some extent from that of other previous reports, particularly regarding the γ2 subunit, the expression of which decreased in our study after prolonged ethanol exposure and withdrawal, was unchanged after 14 or 40 d of ethanol treatment (Matthews et al., 1998), or increased in chronic intermittent ethanol-treated rats (Cagetti et al., 2003).
GABAA receptor function after prolonged ethanol exposure and its discontinuation
Exposure of hippocampal neurons to ethanol induced a marked reduction in the maximal Cl- current density attributable to GABAA receptors. This effect was still apparent 6 hr after discontinuation of ethanol exposure. Given that the potency of GABA was not significantly affected by either treatment, this reduction in current density appears to be caused by a decrease in the cell surface density of GABAA receptors. This conclusion is consistent with our observation that the abundance of α1, γ2L, and γ2S subunit mRNAs was reduced after chronic ethanol exposure and its subsequent discontinuation and is in line with recently published data showing that ethanol exposure reduces cell surface expression of α1 subunit-containing GABAA receptor subtypes (Kumar et al., 2003). Indeed, a marked decrease in the efficiency of α1βγ2 receptor assembly has been demonstrated in the α1 subunit knock-out mice (Sur et al., 2001; Kralic et al., 2002). Furthermore, given that ethanol withdrawal was also associated with an increased abundance of α2, α3, and α4 subunit mRNAs, such treatment, in addition to maintaining a reduction in GABAA receptor density, likely results in a shift in the pattern of receptor subunit assembly.
Differential effects of nonselective and selective benzodiazepine receptor ligands after prolonged ethanol exposure and its abrupt discontinuation
The reduction in the abundance of the α1, α3, γ2L, and γ2S subunit mRNAs induced by prolonged ethanol exposure may be responsible for the associated functional uncoupling between the neurotransmitter binding site and the modulatory benzodiazepine recognition site of the GABAA receptor, as is apparent from the loss of pharmacological efficacy of benzodiazepine receptor ligands. Consistent with previous evidence (Morrow et al., 1988; Buck and Harris, 1990; Sanna et al., 1993), the efficacy of lorazepam, zolpidem, and zaleplon was significantly reduced after prolonged exposure of hippocampal neurons to ethanol. The negative modulatory efficacy of the benzodiazepine receptor inverse agonists Ro 15-4513 and FG 7142 was also reduced in the ethanol-treated cells.
Discontinuation of chronic ethanol exposure induced distinct changes in the sensitivity of GABAA receptors to the various benzodiazepine recognition site ligands. These changes appear to be dependent on the alterations in GABAA receptor subunit expression triggered by such treatment. Potentiation of GABA-evoked Cl- currents by lorazepam was restored to control levels 6 hr after ethanol withdrawal. Lorazepam is a classic benzodiazepine derivative that binds with similar high affinities and modulates GABA-evoked Cl- currents with similar potencies at GABAA receptors containing α1-3 or α5 subunits in combination with β and γ2 subunits; it does not exhibit substantial affinity for receptors containing α4 or α6 subunits (Sieghart, 1995; Barnard et al., 1998). Restoration of the modulatory efficacy of lorazepam to control values after discontinuation of ethanol exposure may thus result from increased expression of the α2 and α3 subunits.
Consistent with this line of reasoning, the efficacies of zolpidem and zaleplon at 0.1 μm, a concentration at which both drugs in vitro modulate selectively GABA responses mediated by α1-containing receptors (Sanna et al., 2002), remained reduced after ethanol withdrawal at a level similar to that observed during chronic ethanol exposure. At a higher concentration (1 μm), at which these drugs, like lorazepam, act nonselectively at α1-, α2-, or α3-containing receptors (Sanna et al., 2002), the efficacies of zolpidem and zaleplon were restored to control levels after ethanol withdrawal. The inhibitory action of FG 7142, a β-carboline with preferential affinity for α1-containing receptors (Sieghart, 1995; Barnard et al., 1998), was also reduced by chronic ethanol exposure and remained so after ethanol withdrawal.
Given that GABAA receptor subtypes containing α1 or α2 subunits mediate the sedative and anxiolytic effects of benzodiazepines, respectively (Mohler et al., 2002), our results are consistent with the reduced sedative efficacy of benzodiazepines in human alcoholics as well as with the ability of these drugs to reduce the anxiogenic effect of ethanol withdrawal (Sellers et al., 1983; Lejoyeux et al., 1998).
Upregulation of the α4 subunit during ethanol withdrawal
Consistent with previous observations in the cerebral cortex, hippocampus, and cerebellar granule cells (Devaud et al., 1995; Devaud et al., 1997; Mahmoudi et al., 1997; Matthews et al., 1998; Cagetti et al., 2003; Follesa et al., 2003), we have shown that ethanol withdrawal resulted in a marked increase in the abundance of the α4 subunit mRNA in cultured hippocampal neurons. This effect was accompanied by a pronounced increase in the amount of the α4 protein that was apparent predominantly at the cell membrane and in association with dendrites and synapses.
Upregulation of the α4 subunit is also induced by withdrawal of benzodiazepine receptor ligands (Follesa et al., 2001, 2002) or neurosteroids (Smith et al., 1998a,b; Follesa et al., 2000), suggesting that it might play an important role in the cellular hyperexcitability and anxiety-like behavior apparent in both animals and humans during withdrawal from these positive allosteric modulators of GABAA receptors.
GABA exhibits a high affinity but relatively low efficacy at GABAA receptors containing the α4 subunit; these receptors are also insensitive to classic benzodiazepines and possess high affinity for flumazenil and Ro 15-4513 (Wafford et al., 1996; Whittemore et al., 1996). Consistent with recent data showing an increased sensitivity to Ro 15-4513 associated with an enhanced expression of the α4 subunit after chronic ethanol exposure in chronic intermittent ethanol-treated rats (Cagetti et al., 2003), we showed that this compound and flumazenil, a partial inverse agonist and antagonist, respectively, in control cells, both acted as positive modulators of GABA responses after discontinuation of prolonged ethanol treatment, consistent with the notion that ethanol withdrawal increases the abundance of GABAA receptors containing the α4 subunit. Flumazenil ameliorates ethanol withdrawal symptoms such as anxiety and hyperexcitability in animals and human alcoholics (File et al., 1989; Buck et al., 1991; Gerra et al., 1991; Nutt et al., 1993; Moy et al., 1997), an effect that has been proposed to result from blockade of the action of a putative endogenous benzodiazepine receptor ligand endowed with inverse agonist activity. Our data now suggest that this effect of flumazenil might also be attributable to an increase in the density of α4-containing receptors associated with ethanol withdrawal.
Prevention of ethanol withdrawal-induced molecular and functional effects by diazepam or GHB
We have now shown that the presence of diazepam or GHB, drugs used to treat ethanol dependence, craving, and withdrawal syndrome (Addolorato et al., 1996; Lejoyeux et al., 1998) during the period of ethanol withdrawal, prevented the increases in the abundance of α2, α3, and α4 subunit mRNAs, the upregulation of the α4 protein, the restoration of lorazepam efficacy, and the conferment of positive modulatory action on both flumazenil and Ro 15-4513 induced by such withdrawal. Neither drug, however, altered the molecular and functional changes associated with prolonged exposure to ethanol, including the reduced abundance of α1, α3, γ2L, and γ2S subunit mRNAs and the reduced efficacy of lorazepam and other GABAergic modulators.
The inhibitory effects of diazepam during ethanol withdrawal are consistent with its mechanism of action at the GABAA receptor, whereas the mechanism by which GHB elicits its effects is not clear. Despite its similarities to GABA and GABAergic drugs in terms of chemical structure and pharmacological profile, GHB does not possess activity at GABAA receptors (Serra et al., 1991; Feigenbaum and Howard, 1996; Follesa et al., 2003). GHB has been suggested to exert its central depressant effects by increasing the synthesis and extracellular concentration of GABA in specific brain regions (Gobaille et al., 1999). Furthermore, administration of GHB, like that of ethanol (Morrow et al., 2001), has been shown to increase the formation of neuroactive steroids in rats (Barbaccia et al., 2002), an effect mediated by GABAB receptors. The accumulation of neuroactive steroids in the brain would be expected to result in an increased GABAergic tone; however, the GABAB receptor antagonist SCH 50911 failed to inhibit the action of GHB during ethanol withdrawal, and the GABAB receptor agonist baclofen did not mimic GHB action, suggesting that GABAB receptors do not contribute to the effects of GHB in our experimental model.
Conclusions
We have shown that prolonged exposure to and subsequent withdrawal of ethanol are associated with marked, specific, and opposite changes in GABAA receptor subunit gene expression as well as in receptor function and pharmacological sensitivity in cultured rat hippocampal neurons. Downregulation of GABAA receptors and a reduction in the efficacy of various benzodiazepine receptor ligands induced by prolonged ethanol treatment are associated with a reduced expression of α1, α3, γ2L, and γ2S subunits. In contrast, an increase in the abundance of α4-containing receptors induced by ethanol withdrawal may be an important determinant of withdrawal syndrome and is blocked by drugs that are effective in the treatment of ethanol dependence.
Footnotes
This work was supported by Grant CE00042735 from Ministero dell'Istruzione dell'Università e della Ricerca, Project Center of Excellence for the Neurobioloy of Dependence, D.M. 21 January 2001, and in part by the GIO.I.A. Foundation. We thank C. Fernando Valenzuela and Mario Carta for helpful discussions.
Correspondence should be addressed to Dr. Enrico Sanna, Department of Experimental Biology, Section of Neuroscience, University of Cagliari, Cittadella Universitaria, SS 554, km 4500, 09042 Monserrato (CA), Italy. E-mail: esanna@unica.it.
Copyright © 2003 Society for Neuroscience 0270-6474/03/2311711-14$15.00/0
References
- Addolorato G, Castelli E, Stefanini GF, Casella G, Caputo F, Marsigli L, Bernardi M, Gasbarrini G ( 1996) An open multicentric study evaluating 4-hydroxybutyric acid sodium salt in the medium-term treatment of 179 alcohol dependent subjects. GHB Study Group. Alcohol Alcohol 31: 341-345. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Caputo F, Capristo E, Janiri L, Bernardi M, Agabio R, Colombo G, Gessa GL, Gasbarrini G ( 2002) Rapid suppression of alcohol withdrawal syndrome by baclofen. Am J Med 112: 226-229. [DOI] [PubMed] [Google Scholar]
- Agabio R, Colombo G, Loche A, Lobina C, Pani ML, Reali R, Gessa GL ( 1998) Gamma-hydroxybutyric acid reducing effect on ethanol intake: evidence in favour of a substitution mechanism. Alcohol Alcohol 33: 465-474. [DOI] [PubMed] [Google Scholar]
- Aguayo LG, Pancetti FC ( 1994) Ethanol modulation of the gamma-aminobutyric acidA- and glycine-activated Cl- current in cultured mouse neurons. J Pharmacol Exp Ther 270: 61-69. [PubMed] [Google Scholar]
- Aguayo LG, Peoples RW, Yeh HH, Yevenes GE ( 2002) GABA(A) receptors as molecular sites of ethanol action. Direct or indirect actions? Curr Top Med Chem 2: 869-885. [DOI] [PubMed] [Google Scholar]
- Allan AM, Harris RA ( 1987) Acute and chronic ethanol treatments alter GABA receptor-operated chloride channels. Pharmacol Biochem Behav 27: 665-670. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Colombo G, Affricano D, Carai MA, Vacca G, Melis S, Purdy RH, Gessa GL ( 2002) GABA(B) receptor-mediated increase of neurosteroids by gamma-hydroxybutyric acid. Neuropharmacology 42: 782-791. [DOI] [PubMed] [Google Scholar]
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ ( 1998) International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev 50: 291-313. [PubMed] [Google Scholar]
- Buck KJ, Harris RA ( 1990) Benzodiazepine agonist and inverse agonist actions on GABAA receptor-operated chloride channels. II. Chronic effects of ethanol. J Pharmacol Exp Ther 253: 713-719. [PubMed] [Google Scholar]
- Buck KJ, Heim H, Harris RA ( 1991) Reversal of alcohol dependence and tolerance by a single administration of flumazenil. J Pharmacol Exp Ther 257: 984-989. [PubMed] [Google Scholar]
- Cagetti E, Liang J, Spigelman I, Olsen RW ( 2003) Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol 63: 53-64. [DOI] [PubMed] [Google Scholar]
- Chester JA, Cunningham CL ( 2002) GABA(A) receptor modulation of the rewarding and aversive effects of ethanol. Alcohol 26: 131-143. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Carai MA, Lobina C, Pani M, Reali R, Addolorato G, Gessa GL ( 2000) Ability of baclofen in reducing alcohol intake and withdrawal severity: I. Preclinical evidence. Alcohol Clin Exp Res 24: 58-66. [PubMed] [Google Scholar]
- Costa ET, Soto EE, Cardoso RA, Olivera DS, Valenzuela CF ( 2000) Acute effects of ethanol on kainate receptors in cultured hippocampal neurons. Alcohol Clin Exp Res 24: 220-225. [PubMed] [Google Scholar]
- Damgen KLH ( 1999) Zaleplon displays a selectivity to recombinant GABAA receptors different from zolpidem, zopiclone and benzodiazepines. Neurosci Res Commun 25: 139-148. [Google Scholar]
- Devaud LL, Smith FD, Grayson DR, Morrow AL ( 1995) Chronic ethanol consumption differentially alters the expression of gamma-aminobutyric acidA receptor subunit mRNAs in rat cerebral cortex: competitive, quantitative reverse transcriptase-polymerase chain reaction analysis. Mol Pharmacol 48: 861-868. [PubMed] [Google Scholar]
- Devaud LL, Purdy RH, Finn DA, Morrow AL ( 1996) Sensitization of gamma-aminobutyric acidA receptors to neuroactive steroids in rats during ethanol withdrawal. J Pharmacol Exp Ther 278: 510-517. [PubMed] [Google Scholar]
- Devaud LL, Fritschy JM, Sieghart W, Morrow AL ( 1997) Bidirectional alterations of GABA(A) receptor subunit peptide levels in rat cortex during chronic ethanol consumption and withdrawal. J Neurochem 69: 126-130. [DOI] [PubMed] [Google Scholar]
- Fadda F, Colombo G, Mosca E, Gessa GL ( 1989) Suppression by gamma-hydroxybutyric acid of ethanol withdrawal syndrome in rats. Alcohol Alcohol 24: 447-451. [PubMed] [Google Scholar]
- Faingold CL, N′Gouemo P, Riaz A ( 1998) Ethanol and neurotransmitter interactions-from molecular to integrative effects. Prog Neurobiol 55: 509-535. [DOI] [PubMed] [Google Scholar]
- Feigenbaum JJ, Howard SG ( 1996) Gamma hydroxybutyrate is not a GABA agonist. Prog Neurobiol 50: 1-7. [DOI] [PubMed] [Google Scholar]
- File SE, Baldwin HA, Hitchcott PK ( 1989) Flumazenil but not nitrendipine reverses the increased anxiety during ethanol withdrawal in the rat. Psychopharmacology (Berl) 98: 262-264. [DOI] [PubMed] [Google Scholar]
- Follesa P, Floris S, Tuligi G, Mostallino MC, Concas A, Biggio G ( 1998) Molecular and functional adaptation of the GABA(A) receptor complex during pregnancy and after delivery in the rat brain. Eur J Neurosci 10: 2905-2912. [DOI] [PubMed] [Google Scholar]
- Follesa P, Serra M, Cagetti E, Pisu MG, Porta S, Floris S, Massa F, Sanna E, Biggio G ( 2000) Allopregnanolone synthesis in cerebellar granule cells: roles in regulation of GABA(A) receptor expression and function during progesterone treatment and withdrawal. Mol Pharmacol 57: 1262-1270. [PubMed] [Google Scholar]
- Follesa P, Cagetti E, Mancuso L, Biggio F, Manca A, Maciocco E, Massa F, Desole MS, Carta M, Busonero F, Sanna E, Biggio G ( 2001) Increase in expression of the GABA(A) receptor alpha(4) subunit gene induced by withdrawal of, but not by long-term treatment with, benzodiazepine full or partial agonists. Brain Res Mol Brain Res 92: 138-148. [DOI] [PubMed] [Google Scholar]
- Follesa P, Mancuso L, Biggio F, Cagetti E, Franco M, Trapani G, Biggio G ( 2002) Changes in GABA(A) receptor gene expression induced by withdrawal of, but not by long-term exposure to, zaleplon or zolpidem. Neuropharmacology 42: 191-198. [DOI] [PubMed] [Google Scholar]
- Follesa P, Mancuso L, Biggio F, Mostallino MC, Manca A, Mascia MP, Busonero F, Talani G, Sanna E, Biggio G ( 2003) Gamma-hydroxybutyric acid and diazepam antagonize a rapid increase in GABA(A) receptors alpha(4) subunit mRNA abundance induced by ethanol withdrawal in cerebellar granule cells. Mol Pharmacol 63: 896-907. [DOI] [PubMed] [Google Scholar]
- Gallimberti L, Canton G, Gentile N, Ferri M, Cibin M, Ferrara SD, Fadda F, Gessa GL ( 1989) Gamma-hydroxybutyric acid for treatment of alcohol withdrawal syndrome. Lancet 2: 787-789. [DOI] [PubMed] [Google Scholar]
- Gerra GCR, Volpi R, Maninetti L, Delsignore R, Coiro V ( 1991) Effectiveness of flumazenil in the treatment of alcohol withdrawal. Curr Ther Res 50: 62-66. [Google Scholar]
- Gobaille S, Hechler V, Andriamampandry C, Kemmel V, Maitre M ( 1999) gamma-Hydroxybutyrate modulates synthesis and extracellular concentration of gamma-aminobutyric acid in discrete rat brain regions in vivo. J Pharmacol Exp Ther 290: 303-309. [PubMed] [Google Scholar]
- Grobin AC, Matthews DB, Devaud LL, Morrow AL ( 1998) The role of GABA(A) receptors in the acute and chronic effects of ethanol. Psychopharmacology (Berl) 139: 2-19. [DOI] [PubMed] [Google Scholar]
- Hevers W, Luddens H ( 1998) The diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol Neurobiol 18: 35-86. [DOI] [PubMed] [Google Scholar]
- Kang MH, Spigelman I, Olsen RW ( 1998) Alteration in the sensitivity of GABA(A) receptors to allosteric modulatory drugs in rat hippocampus after chronic intermittent ethanol treatment. Alcohol Clin Exp Res 22: 2165-2173. [PubMed] [Google Scholar]
- Kralic JE, O'Buckley TK, Khisti RT, Hodge CW, Homanics GE, Morrow AL ( 2002) GABA(A) receptor alpha-1 subunit deletion alters receptor subtype assembly, pharmacological and behavioral responses to benzodiazepines and zolpidem. Neuropharmacology 43: 685-694. [DOI] [PubMed] [Google Scholar]
- Kumar S, Sieghart W, Morrow AL ( 2002) Association of protein kinase C with GABA(A) receptors containing alpha1 and alpha4 subunits in the cerebral cortex: selective effects of chronic ethanol consumption. J Neurochem 82: 110-117. [DOI] [PubMed] [Google Scholar]
- Kumar S, Kralic JE, O'Buckley TK, Grobin AC, Morrow AL ( 2003) Chronic ethanol consumption enhances internalization of alpha1 subunit-containing GABAA receptors in cerebral cortex. J Neurochem 86: 700-708. [DOI] [PubMed] [Google Scholar]
- Lejoyeux M, Solomon J, Ades J ( 1998) Benzodiazepine treatment for alcohol-dependent patients. Alcohol Alcohol 33: 563-575. [DOI] [PubMed] [Google Scholar]
- Mahmoudi M, Kang MH, Tillakaratne N, Tobin AJ, Olsen RW ( 1997) Chronic intermittent ethanol treatment in rats increases GABA(A) receptor alpha4-subunit expression: possible relevance to alcohol dependence. J Neurochem 68: 2485-2492. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Devaud LL, Fritschy JM, Sieghart W, Morrow AL ( 1998) Differential regulation of GABA(A) receptor gene expression by ethanol in the rat hippocampus versus cerebral cortex. J Neurochem 70: 1160-1166. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ ( 1996) Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci 19: 139-143. [DOI] [PubMed] [Google Scholar]
- Mehta AK, Ticku MK ( 1999) An update on GABAA receptors. Brain Res Brain Res Rev 29: 196-217. [DOI] [PubMed] [Google Scholar]
- Mhatre MC, Pena G, Sieghart W, Ticku MK ( 1993) Antibodies specific for GABAA receptor alpha subunits reveal that chronic alcohol treatment down-regulates alpha-subunit expression in rat brain regions. J Neurochem 61: 1620-1625. [DOI] [PubMed] [Google Scholar]
- Mohler H, Fritschy JM, Rudolph U ( 2002) A new benzodiazepine pharmacology. J Pharmacol Exp Ther 300: 2-8. [DOI] [PubMed] [Google Scholar]
- Montpied P, Morrow AL, Karanian JW, Ginns EI, Martin BM, Paul SM ( 1991) Prolonged ethanol inhalation decreases gamma-aminobutyric acidA receptor alpha subunit mRNAs in the rat cerebral cortex. Mol Pharmacol 39: 157-163. [PubMed] [Google Scholar]
- Morrow AL, Suzdak PD, Karanian JW, Paul SM ( 1988) Chronic ethanol administration alters gamma-aminobutyric acid, pentobarbital and ethanol-mediated 36Cl- uptake in cerebral cortical synaptoneurosomes. J Pharmacol Exp Ther 246: 158-164. [PubMed] [Google Scholar]
- Morrow AL, VanDoren MJ, Penland SN, Matthews DB ( 2001) The role of GABAergic neuroactive steroids in ethanol action, tolerance and dependence. Brain Res Brain Res Rev 37: 98-109. [DOI] [PubMed] [Google Scholar]
- Moy SS, Knapp DJ, Criswell HE, Breese GR ( 1997) Flumazenil blockade of anxiety following ethanol withdrawal in rats. Psychopharmacology (Berl) 131: 354-360. [DOI] [PubMed] [Google Scholar]
- Nutt D, Glue P, Wilson S, Groves S, Coupland N, Bailey J ( 1993) Flumazenil in alcohol withdrawal. Alcohol Alcohol [Suppl] 2: 337-341. [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G ( 2000) GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 101: 815-850. [DOI] [PubMed] [Google Scholar]
- Poelchen W, Proctor WR, Dunwiddie TV ( 2000) The in vitro ethanol sensitivity of hippocampal synaptic gamma-aminobutyric acid(A) responses differs in lines of mice and rats genetically selected for behavioral sensitivity or insensitivity to ethanol. J Pharmacol Exp Ther 295: 741-746. [PubMed] [Google Scholar]
- Pritchett DB, Sontheimer H, Shivers BD, Ymer S, Kettenmann H, Schofield PR, Seeburg PH ( 1989) Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature 338: 582-585. [DOI] [PubMed] [Google Scholar]
- Proctor WR, Allan AM, Dunwiddie TV ( 1992a) Brain region-dependent sensitivity of GABAA receptor-mediated responses to modulation by ethanol. Alcohol Clin Exp Res 16: 480-489. [DOI] [PubMed] [Google Scholar]
- Proctor WR, Soldo BL, Allan AM, Dunwiddie TV ( 1992b) Ethanol enhances synaptically evoked GABAA receptor-mediated responses in cerebral cortical neurons in rat brain slices. Brain Res 595: 220-227. [DOI] [PubMed] [Google Scholar]
- Reynolds JN, Prasad A, MacDonald JF ( 1992) Ethanol modulation of GABA receptor-activated Cl- currents in neurons of the chick, rat and mouse central nervous system. Eur J Pharmacol 224: 173-181. [DOI] [PubMed] [Google Scholar]
- Sanna E, Serra M, Cossu A, Colombo G, Follesa P, Cuccheddu T, Concas A, Biggio G ( 1993) Chronic ethanol intoxication induces differential effects on GABAA and NMDA receptor function in the rat brain. Alcohol Clin Exp Res 17: 115-123. [DOI] [PubMed] [Google Scholar]
- Sanna E, Busonero F, Talani G, Carta M, Massa F, Peis M, Maciocco E, Biggio G ( 2002) Comparison of the effects of zaleplon, zolpidem, and triazolam at various GABA(A) receptor subtypes. Eur J Pharmacol 451: 103-110. [DOI] [PubMed] [Google Scholar]
- Sellers EM, Naranjo CA, Harrison M, Devenyi P, Roach C, Sykora K ( 1983) Diazepam loading: simplified treatment of alcohol withdrawal. Clin Pharmacol Ther 34: 822-826. [DOI] [PubMed] [Google Scholar]
- Serra M, Sanna E, Foddi C, Concas A, Biggio G ( 1991) Failure of gamma-hydroxybutyrate to alter the function of the GABAA receptor complex in the rat cerebral cortex. Psychopharmacology (Berl) 104: 351-355. [DOI] [PubMed] [Google Scholar]
- Sieghart W ( 1995) Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol Rev 47: 181-234. [PubMed] [Google Scholar]
- Sieghart W, Sperk G ( 2002) Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr Top Med Chem 2: 795-816. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen JM, Li X ( 1998a) GABA(A) receptor alpha4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature 392: 926-930. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Li X, Moran MH, Bitran D, Frye CA, Hsu FC ( 1998b) Withdrawal from 3α-OH-5α-pregnan-20-one using a pseudopregnancy model alters the kinetics of hippocampal GABAA-gated current and increases the GABAA receptor α4 subunit in association with increased anxiety. J Neurosci 18: 5275-5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smothers CT, Mrotek JJ, Lovinger DM ( 1997) Chronic ethanol exposure leads to a selective enhancement of N-methyl-d-aspartate receptor function in cultured hippocampal neurons. J Pharmacol Exp Ther 283: 1214-1222. [PubMed] [Google Scholar]
- Soldo BL, Proctor WR, Dunwiddie TV ( 1994) Ethanol differentially modulates GABAA receptor-mediated chloride currents in hippocampal, cortical, and septal neurons in rat brain slices. Synapse 18: 94-103. [DOI] [PubMed] [Google Scholar]
- Spiga S, Serra GP, Puddu MC, Foddai M, Diana M ( 2003) Morphine withdrawal-induced abnormalities in the VTA: confocal laser scanning microscopy. Eur J Neurosci 17: 605-612. [DOI] [PubMed] [Google Scholar]
- Sur C, Wafford KA, Reynolds DS, Hadingham KL, Bromidge F, Macaulay A, Collinson N, O'Meara G, Howell O, Newman R, Myers J, Atack JR, Dawson GR, McKernan RM, Whiting PJ, Rosahl TW ( 2001) Loss of the major GABA(A) receptor subtype in the brain is not lethal in mice. J Neurosci 21: 3409-3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwaki H, Kalant H, Higuchi S, Crabbe JC, Ohkuma S, Katsura M, Yoshimura M, Stewart RC, Li TK, Weiss F ( 2001) Recent research on alcohol tolerance and dependence. Alcohol Clin Exp Res 25: 189S-196S. [DOI] [PubMed] [Google Scholar]
- Ticku MK, Burch T ( 1980) Alterations in gamma-aminobutyric acid receptor sensitivity following acute and chronic ethanol treatments. J Neurochem 34: 417-423. [DOI] [PubMed] [Google Scholar]
- Ueno S, Harris RA, Messing RO, Sanchez-Perez AM, Hodge CW, McMahon T, Wang D, Mehmert KK, Kelley SP, Haywood A, Olive MF, Buck KJ, Hood HM, Blednov Y, Findlay G, Mascia MP ( 2001) Alcohol actions on GABA(A) receptors: from protein structure to mouse behavior. Alcohol Clin Exp Res 25: 76S-81S. [DOI] [PubMed] [Google Scholar]
- van Zundert B, Albarran FA, Aguayo LG ( 2000) Effects of chronic ethanol treatment on gamma-aminobutyric acid(A) and glycine receptors in mouse glycinergic spinal neurons. J Pharmacol Exp Ther 295: 423-429. [PubMed] [Google Scholar]
- Vicini S ( 1999) New perspectives in the functional role of GABA(A) channel heterogeneity. Mol Neurobiol 19: 97-110. [DOI] [PubMed] [Google Scholar]
- Wafford KA, Thompson SA, Thomas D, Sikela J, Wilcox AS, Whiting PJ ( 1996) Functional characterization of human gamma-aminobutyric acidA receptors containing the alpha 4 subunit. Mol Pharmacol 50: 670-678. [PubMed] [Google Scholar]
- Whiting PJ, Bonnert TP, McKernan RM, Farrar S, Le Bourdelles B, Heavens RP, Smith DW, Hewson L, Rigby MR, Sirinathsinghji DJ, Thompson SA, Wafford KA ( 1999) Molecular and functional diversity of the expanding GABA-A receptor gene family. Ann NY Acad Sci 868: 645-653. [DOI] [PubMed] [Google Scholar]
- Whittemore ER, Yang W, Drewe JA, Woodward RM ( 1996) Pharmacology of the human gamma-aminobutyric acidA receptor alpha 4 subunit expressed in Xenopus laevis oocytes. Mol Pharmacol 50: 1364-1375. [PubMed] [Google Scholar]