Abstract
Occulocutaneous albinism is caused by mutations in the gene encoding the enzyme tyrosinase. Individuals with this disorder are predisposed to visual system deficits. We determined the critical period during development when tyrosinase expression is essential for the appropriate pathfinding of ganglion cell axons from the retina to the dorsal lateral geniculate nucleus. We used a line of mice with a Tyrosinase transgene, the expression of which is regulatable with the lac operator-repressor system, to restrict tyrosinase activity to discrete periods of embryogenesis. When tyrosinase was expressed throughout the period of neuroblast divisions that produce the ipsilaterally projecting ganglion cells, axonal projections innervated the same volume of the ipsilateral dorsal lateral geniculate nucleus of the thalamus as in normal mice. If tyrosinase expression ceased before the end of neuroblast divisions, or was not initiated until after they had begun, the degree of ipsilateral innervation was smaller, as in albino mice. Tyrosinase expression was not required during the entire period of pathfinding itself or during final maturation of the retinogeniculate pathway. Thus, tyrosinase appears to set up a signal early in visual system development that determines the pathway taken later by ganglion cell axons.
Keywords: tyrosinase, albino, visual system development, lac repressor, gene regulation, occulocutaneous albinism
Introduction
Albino mammals, in addition to their external pigmentation deficits, have abnormalities of visual system development. Humans with occulocutaneous albinism type 1 are subject to low visual acuity, nystagmus (abnormal involuntary movements of the eyes), and strabismus (abnormal turning of the eyes) (Oetting and King, 1999). In albino animal models, three main cellular disorders have been identified: a reduction in the number of rod photoreceptors, underdevelopment of the central retinal specialization, and a misrouting of some temporal retinal ganglion cell axons (for review, see Guillery, 1986; Jeffery, 1997). Occulocutaneous albinism is caused by mutations at the albino (c) locus, which codes for tyrosinase, the initial enzyme in the melanin synthesis cascade. In melanocytes and the cells of the retinal pigment epithelium (RPE) where it is expressed, tyrosinase catalyzes the conversion both of tyrosine to DOPA and of DOPA to DOPAquinone (del Marmol and Beermann, 1996; Jeffery, 1997). Introduction of a functional Tyrosinase gene into albino animals completely rescues the albino phenotype (Jeffery et al., 1994, 1997), proving that both the pigmentation deficit and the visual system abnormalities are caused by the single gene defect in Tyrosinase. The precise role of tyrosinase during each phase of visual system development is not known, however, and experiments to determine the point in retinal development at which tyrosinase exerts its effects have been hindered by the limitations of previous animal models.
Ganglion cells, which are the output neurons from the eye, are produced early in retinal development. The initial organization of visual information in the retinogeniculate pathway depends on some retinal ganglion cell axons crossing at the optic chiasm to project to the contralateral dorsal lateral geniculate nucleus (dLGN) in the thalamus and some remaining ipsilateral. In albinos, fewer retinal ganglion cells project to the ipsilateral side of the brain, leading to a disruption of dLGN organization and to disorganization of visual information in the cortex. This disruption has been observed in many hypopigmented mammals including human albinos (Guillery et al., 1975) and Siamese cats, in which the abnormality can be precisely defined as a result of the well ordered feline dLGN (Guillery, 1986). In the mouse, where the dLGN is less ordered, this defect is manifest as a decrease in the extent of the dLGN innervated by ipsilateral projections (Jeffery et al., 1994).
To ensure that ganglion cells project to the dLGN on the appropriate side of the brain, tyrosinase activity could be required during any or all phases of ipsilateral retinogeniculate pathway development. In the RPE, tyrosinase is first expressed on embryonic day (E) 10 (Beermann et al., 1992), and pigment formation starts at E11 (Drager, 1985a). The onset of melanin formation is graded across the retina, with peripheral regions becoming pigmented first and melanin present in the entire RPE by E12.5 (Drager, 1985a). This occurs at the same time as the initial pattern of neuroblast divisions and cell cycle exit. Thus, the initiation of tyrosinase expression and the graded onset of pigment formation might be a developmental signal that sets up positional information in the retina, committing the ganglion cells produced from the neuroblasts to ipsilateral or contralateral projections at a later time. Ipsilaterally projecting retinal ganglion cells are produced between E11 and E16 (Drager, 1985a). Tyrosinase expression might be necessary throughout this period to ensure that neuroblasts properly divide and give rise to postmitotic cells. A third possibility is that tyrosinase might be necessary during ganglion cell pathfinding, which occurs between E12.5 and E18.5, as axons grow out of the eye, traverse the optic chiasm, and form their initial connections to the lateral geniculate nucleus (Silver, 1984; Guillery et al., 1995). After the ganglion cell axons reach the dLGN, there is a period of refinement of connections and ganglion cell death [E18 to postnatal day (P) 7] (Young, 1984; Stellwagen and Shatz, 2002). Thus, a final possibility is that tyrosinase expression in the eye is necessary to ensure that cells die or are maintained appropriately during this period.
To distinguish between these possibilities, we used a regulatable Tyrosinase mouse model to limit tyrosinase expression to discrete periods during development. Regulation of tyrosinase expression in this model is based on control with the lac operator-repressor gene regulatory system. The endogenous lac regulatory system of Escherichia coli controls the expression of the gene products responsible for lactose metabolism. In the absence of lactose, the lac repressor protein (encoded by the lacI gene) occupies the lac operator sequences (lacO) in the promoter, blocking transcription of the downstream coding sequences. When lactose, or a lactose analog such as isopropyl-thio-β-d-galactoside (IPTG), binds to the repressor, it causes the repressor to lose its affinity for the operators, thereby relieving repression and allowing transcription to proceed. As we have shown (Cronin et al., 2001), this system has been adapted successfully to control expression from the mouse genome.
Experimental control of tyrosinase expression is achieved by the introduction of two transgenes onto an albino background strain of mice. The first transgene codes for ubiquitous expression of the lac repressor protein. The target transgene used in these studies was generated by introducing lac operator DNA sequences into the murine Tyrosinase promoter and then using the modified promoter to drive the expression of a wild-type Tyrosinase cDNA. As described previously, the Tyrosinase transgene confers a pigmented phenotype on the albino recipient strain (Cronin et al., 2001), and because a cloned Tyrosinase promoter is used, the transgene is expressed in the same tissues and with the same developmental time course as the endogenous gene. In animals transgenic for both the regulatable Tyrosinase transgene and the lac repressor transgene, expression of the introduced functional Tyrosinase gene is repressed, and the phenotype is albino. When double-transgenic animals are exposed to IPTG in their drinking water, repression of the Tyrosinase transgene is relieved, and the mice are pigmented. To investigate the role of tyrosinase in retinogeniculate pathway formation, females pregnant with double-transgenic pups were fed IPTG during discrete periods of visual system development, and the degree of ipsilateral retinogeniculate innervation in the dLGN of the pups once they had reached adulthood was examined.
Materials and Methods
Preparation of mice and IPTG treatment. The Tyrosinase and lacI transgenes were introduced into the albino ICR strain of mice (Harlan Sprague Dawley, Indianapolis, IN) [for details on transgene construction, see Cronin et al. (2001)], and albino control animals used for this study were also from the ICR strain. The wild-type pigmented animals used for comparison were generated from a DBA × ICR F1 intercross. Transgenic animals were hemizygous for the indicated transgene(s). The Tyrosinase transgenic animals were from the TyrlacO-43 line (Cronin et al., 2001). Some ICR mice were found to have retinal degeneration; therefore all mice used for these experiments were genotyped with the diagnostic PCR (Pittler and Baehr, 1991), and only those mice either heterozygous or homozygous wild-type at the rd locus were used for the studies. Pregnancies were timed by observation of a vaginal plug on day E0. When indicated, pregnant or nursing females had their drinking water replaced with a 10 mm solution of IPTG (Inalco) that was changed at least every 4 d. Mice were kept on a 14 hr light/10 hr dark cycle and were between 1.5 and 7 months of age at the time of analysis. Animal housing and treatment were in accordance with institutional and National Institutes of Health guidelines.
Determining the size of the ipsilateral retinogeniculate pathway. Mice were anesthetized with 2.5% avertin (0.017 cc/g, i.p.), and 4 μl of peroxidase-conjugated wheat germ agglutinin (40 mg/ml; Vector Laboratories) was injected into the right eye. One day later, mice were given a lethal dose of Nembutal sodium and perfused transcardially (1.25% paraformaldehyde, 1.5% gluteraldehyde, in 0.1 m phosphate, pH 7.4). Brains were removed and postfixed in the perfusate for an additional 2-4 hr, cryoprotected in 20% sucrose in 0.1 m phosphate, pH 7.4, overnight, and frozen on dry ice. Floating sections (50 μm) through the entire dLGN were collected in 1% Triton X-100/PBS, and reacted for peroxidase activity with 3,3′,5,5′-tetramethylbenzidine (TMB) (TMB substrate kit; Vector Laboratories), and the reaction was stopped in water acidified to pH 3 with acetic acid. Sections were mounted onto gelatinized slides, air dried, and coverslipped in DPX. Only those animals with uniform labeling of the contralateral dLGN and low background were used for the analysis (n = 5-12 animals per group). Sections were analyzed using a digital imaging system (MCID, Imaging Research), and the area of label in the ipsilateral dLGN for each section was determined. Total volume of the ipsilateral projection to the dLGN was calculated for each animal from the sum of the areas of label in each of the sections through the dLGN multiplied by the thickness of the sections, and an average for each group of animals was obtained.
Determining the levels of ocular pigmentation. Pregnant females were killed by cervical dislocation, and E16.5 embryos were surgically removed from the uterine horns. Embryos were immersed in 10% formalin for ∼2 weeks. The eyes were then enucleated, the cornea, lens, and nuclear layer of the retina were removed, and the perioptic mesenchyme (future choroid and sclera) was microdissected from the RPE. The samples were then mounted in glycerin on Superfrost Plus slides and coverslipped. The slides were imaged using an Olympus BH-2 microscope equipped with a digital camera. RPE from the different groups was captured in succession to minimize any possible variation resulting from changing input light levels. Images of comparable regions of each RPE were analyzed using the Image Pro Plus image processing package to determine the percentage of pigmented area of each image. All images were filtered using the Hi-Gauss and Erode options of Image Pro Plus, and albino background levels were subtracted from each of the other groups. Each group contained from 7 to 27 animals.
Statistical analysis. Data for each group were averaged, and SE of the mean was calculated. A one-way ANOVA showed a significant effect of either genotype or IPTG treatment conditions in each set of experiments (p < 0.005). Individual groups were compared by t test with Bonferroni correction at a significance level of p < 0.05.
Results
As described previously (Cronin et al., 2001), embryonic expression of tyrosinase follows the normal developmental time course in the TyrlacO-43 line of regulatable Tyrosinase transgenic mice, with pigment first appearing at E11.5. As a probable consequence of insertion site effects on the Tyrosinase transgene, the TyrlacO-43 transgenic animals do not achieve the levels of pigmentation seen in wild-type mice. To determine the degree of RPE pigmentation more precisely, images of E16.5 RPE were compared from the different experimental groups. The Tyrosinase transgenic animals, although significantly darker than albinos (Fig. 1A, Tyr), are not as pigmented as wild-type animals (22.3 ± 0.9 vs 49.2 ± 0.8% pigmented area, as assessed by densitometry). Nevertheless, these Tyrosinase transgenic animals provide a highly regulatable model for control of pigment levels during development, as shown in Figure 1, B and C. In animals doubly transgenic for the Tyrosinase transgene and the lac repressor, in which tyrosinase expression was repressed, the RPE was essentially unpigmented. Continuous administration of the lactose analog IPTG to females pregnant with double-transgenic embryos allowed for expression of tyrosinase, resulting in RPE pigmentation at E11.5 (data not shown), as occurs for wild-type animals. Restricting IPTG treatment to discrete periods of development differentially affected pigmentation. Discontinuing IPTG treatment on E13.5 resulted in significantly less pigment than continuing treatment through E16.5; however, delaying the onset of IPTG treatment until E12.5 did not produce pigment levels significantly different from treatment starting at E0.
Figure 1.
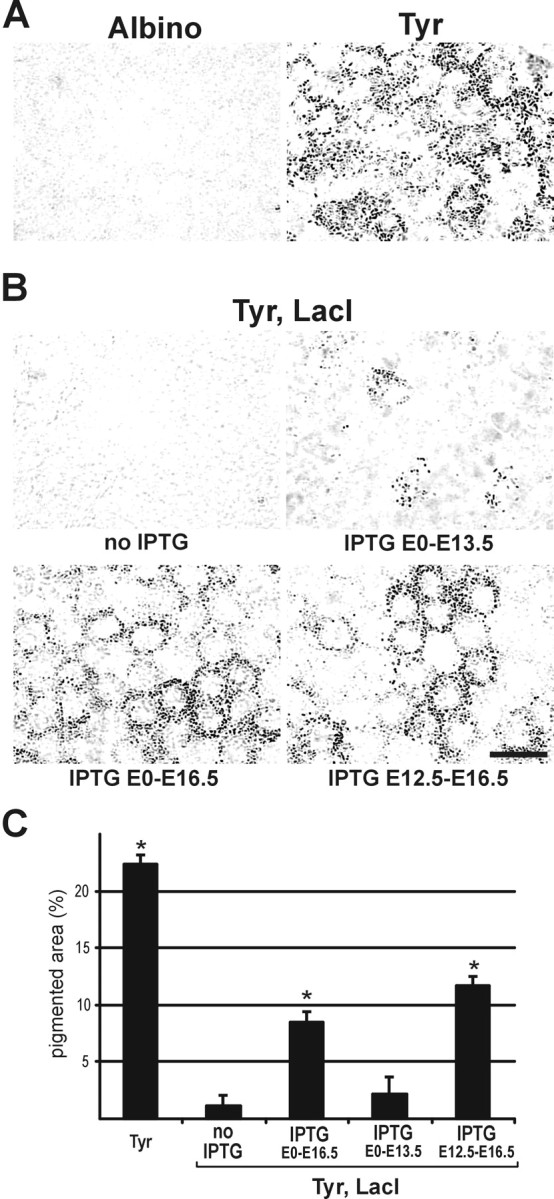
Induction of RPE pigmentation by treatment with the lactose analog IPTG in mouse embryos doubly transgenic for a regulatable Tyrosinase transgene and the lac repressor. A, Photomicrographs of the RPE from E16.5 embryos show that Tyrosinase transgenic animals are highly pigmented relative to albino control animals. B, For Tyr, LacI double-transgenic animals, treatment with IPTG during development allows for differential control of the level of pigmentation achieved. C, The percentage of area containing detectable pigment (as assessed by densitometry; see Materials and Methods) was determined from images of the RPE from E16.5 animals. Repression of the Tyrosinase transgene in the Tyr, LacI double-transgenic animals results in an essentially unpigmented RPE. Treatment of double-transgenic animals with IPTG derepresses the Tyrosinase transgene, leading to pigmentation of the RPE (beginning at E11.5, as in wild-type animals). Treatment up to the time of the assay (E0 -E16.5) and a delayed treatment (E12.5-E16.5) both lead to increased pigmentation, whereas termination of treatment at E13.5 yields a level of pigmentation close to that of the untreated animals. Asterisk indicates significantly different from both the albino baseline and the untreated Tyr, LacI group. Scale bar, 20 μm.
Although the precise time relationship between IPTG treatment and expression of tyrosinase has not been determined, the fact that there was a clear difference in RPE pigment levels when IPTG was stopped at E13.5 versus those animals in which it was continued up to E16.5 suggests that the effect of IPTG on tyrosinase activity is rapid. This is not surprising given that tyrosinase mRNA and protein both have relatively short half-lives [4 and 3.5 hr, respectively (Burchill et al., 1988; Rungta et al., 1996)]. Also, it has been shown previously that there is a rapid uptake of IPTG into mouse tissues (Wyborski and Short, 1991) and rapid IPTG derepression of gene expression (Lee et al., 1997; Wu et al., 1997), with detectable expression at 4 hr after injection. Taken together, these data show that the time from addition or withdrawal of the IPTG to a change in the levels of tyrosinase in our transgenic model is short. The TyrlacO-43 model system, therefore, can be used to regulate the timing of tyrosinase expression, and thus the degree of pigmentation during development. We used this regulatability to analyze the effects of limiting tyrosinase expression to discrete periods of development on the formation of the ipsilateral retinogeniculate projection.
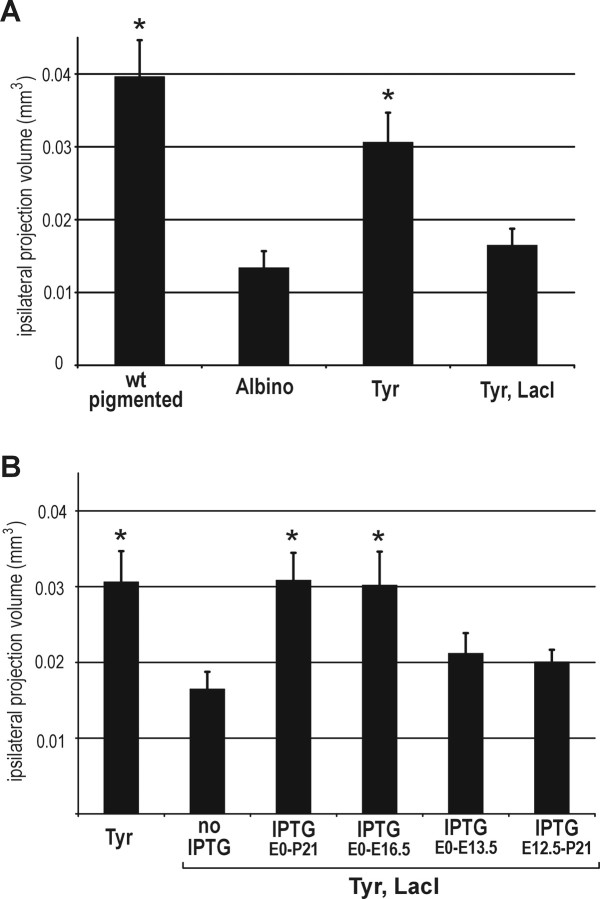
We compared retinal ganglion cell projections to the dLGN in transgenic and control animals (an example of the staining in the dLGN is shown in Fig. 2). In agreement with previous results (Jeffery et al., 1994), albino mice had a smaller ipsilateral retinogeniculate projection than wild-type pigmented animals. In the Tyrosinase transgenic group, the volume of the ipsilateral projection was similar to wild-type, indicating that the full wild-type level of tyrosinase activity and pigmentation are not necessary for development of the retinogeniculate pathway. When the Tyrosinase transgene was repressed in the Tyr, LacI double-transgenic group, the volume of the ipsilateral projection was reduced to albino levels (Fig. 3A). These data demonstrate that the albino abnormality in ganglion cell pathfinding is rescued by the Tyrosinase transgene and that repression of transgene expression with the lac repressor is tight enough to reverse this phenotype.
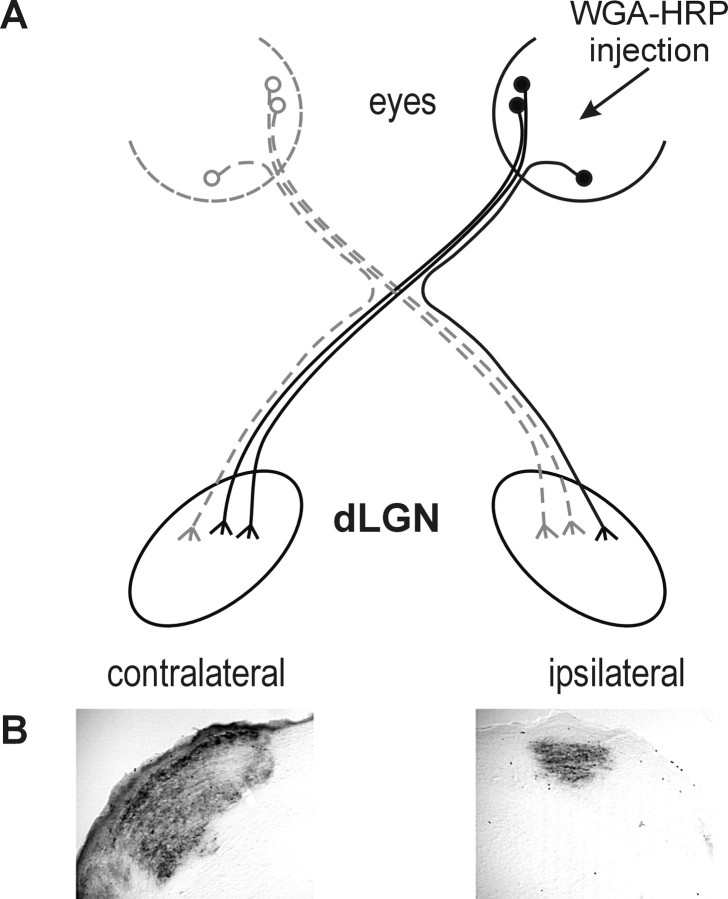
Figure 2.
Anterograde labeling of the dLGN after unilateral eye injection of peroxidase-conjugated wheat germ agglutinin (WGA-HRP). A, A model of the retinogeniculate pathway is shown, highlighting ganglion cells from one eye labeled after injection of the neuronal tracer WGA-HRP into the eye. B, A brain section showing the pattern of labeling in the ipsilateral and contralateral dLGN after the injection of WGA-HRP into one eye. Brains were sectioned and reacted for peroxidase activity to determine the volume of the dLGN containing projections on the ipsilateral side. This section is from approximately the middle of the nucleus of a wild-type pigmented mouse.
Figure 3.
Derepression of tyrosinase expression at specific time points in development rescues a defect in ipsilateral dLGN projection. The ipsilateral projection volume of the dLGN is graphed. A, Albino mice have a deficit in ipsilateral projection volume in the dLGN. The Tyrosinase transgene rescues this deficit, and the rescue is reversed in the Tyr, LacI double-transgenic animals, in which expression of the Tyrosinase transgene is repressed. B, IPTG treatment throughout embryogenesis and until weaning at P21 rescues the albino deficit, as does treatment E0 -E16.5. Treatment E0 -E13.5 and E12.5-P21 fail to rescue the deficit. Asterisk indicates significantly different from both the albino and the Tyr, LacI groups.
Next, we restricted tyrosinase expression to discrete periods of development and analyzed its effect on the development of the ipsilateral retinogeniculate pathway (Fig. 3B). Tyrosinase was derepressed in Tyr, LacI double-transgenic animals by adding the lactose analog IPTG to the mother's drinking water. As shown in Figure 1, this treatment results in pigmentation of the embryonic RPE. When IPTG was administered throughout the entire period of embryogenesis and until weaning at P21, ipsilateral projection volumes were the same as those in the unrepressed Tyr single-transgenic animals. This result is in agreement with the idea that the albino abnormalities are determined developmentally and shows that continued tyrosinase expression after P21 is not necessary for the normal ipsilateral projection to be present in the adult. The result also suggests that there may be a threshold level of tyrosinase activity necessary for correct development of the ipsilateral retinogeniculate pathway, because treatment of Tyr, LacI double-transgenic animals with IPTG did not result in the same level of pigmentation seen in Tyr single-transgenic animals (Fig. 1C).
Animals treated from the beginning of embryogenesis to E16.5, throughout the phase of neuroblast divisions that produce the ipsilateral ganglion cells, also had ipsilateral retinogeniculate projection volumes like those of the unrepressed Tyr animals; however, a shorter early treatment, from E0 through E13.5, resulted in a smaller ipsilateral projection volume that was not statistically different from the repressed Tyr, LacI animals that had received no IPTG. Similarly, treatment with IPTG starting later than tyrosinase normally begins to be expressed in the RPE, from E12.5 to weaning, resulted in a smaller ipsilateral projection volume that was not statistically different from the repressed Tyr, LacI animals (Fig. 3B). Thus, treatment from E0 to E16.5 was necessary for correct formation of the ipsilateral retinogeniculate pathway, but treatment for a shorter period (E0 -E13.5) or only during a later period (E12.5-P21) was insufficient.
Discussion
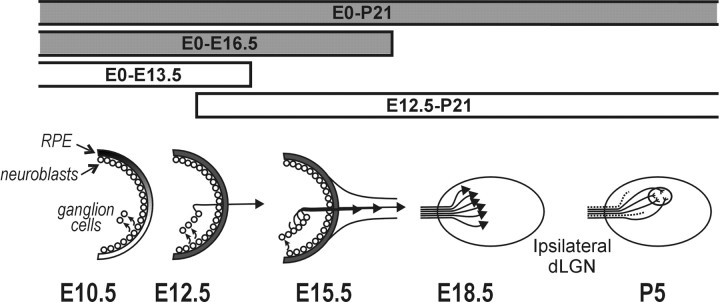
These studies define a small window of time during which treatment with IPTG changes the developmental phenotype of the ipsilateral retinogeniculate pathway in the mouse. Tyrosinase, which normally begins to be expressed at E10, must be expressed until after E13 but before E16 for correct development of this pathway. As depicted schematically in Figure 4, E11.5-E16.5 is the time during which neuroblasts are dividing and giving rise to prospective ipsilaterally projecting ganglion cells. Thus, tyrosinase might be required for correct neuroblast divisions or for some developmental event that occurs as the newly formed ganglion cells leave the cell cycle.
Figure 4.
Tyrosinase expression is necessary during the early development of the ipsilateral retinogeniculate pathway. Tyrosinase is first expressed at E10.5. By E11.5, there is graded pigmentation in the RPE, and the first ipsilateral ganglion cells are produced from the neuroblasts. At E12.5 these first cells begin sending out axons. At E16.5 the last ipsilateral ganglion cells are produced. By E18.5 all of the ipsilaterally projecting axons have traversed the optic chiasm. E18-P5 is the period of refinement of connections and ganglion cell death, which produces the final form of the retinogeniculate pathway. Periods of IPTG treatment in Tyr, LacI double-transgenic animals that rescue the albino abnormality are represented by shaded bars. Tyrosinase is necessary throughout the period of neuroblast divisions that produce ipsilaterally projecting ganglion cells.
There is controversy in the literature based on studies using different pigment mutants as to whether the degree of pigmentation correlates with the severity of visual system abnormalities (Sanderson et al., 1974; LaVail et al., 1978; Mangini et al., 1985; Balkema and Drager, 1990; Donatien and Jeffery, 2002; Donatien et al., 2002; Rachel et al., 2002b). The Tyrosinase transgenic animals used in our studies had less RPE pigmentation than wild type, but there was no significant difference between the volumes of the respective ipsilateral retinogeniculate projections (although it is possible that there was a small difference between these groups that did not reach statistical significance in our studies). Similarly, delaying IPTG treatment until E12.5 did not affect the degree of pigmentation achieved at E16.5 but did cause a decrease in the amount of ipsilateral dLGN innervation. This suggests that, at least for our Tyr transgenic animals, it is the timing of tyrosinase expression or pigment formation, or both, and not the total amount of pigmentation that is the important developmental determinant of this effect.
Our result that tyrosinase is necessary during the period of neuroblast divisions is consistent with an earlier finding that the retina is the site of action for the tyrosinase effect on development of the ipsilateral retinogeniculate pathway (Marcus et al., 1996). When cultured with midline chiasmatic cells from either wild-type or albino animals, ganglion cell axons from the ventrotemporal area of wild-type retinas, which project ipsilaterally, grew shorter neurites than axons from “crossed” parts of the retina, which project contralaterally and grow more freely. This response was found to be independent of the source of the chiasm cells. In contrast, axons from albino ventrotemporal retinas grown on either wild-type or albino chiasm cells grew longer neurites, as if they emanated from the crossed part of the retina. This experiment led to the conclusion that the albino mutation has its effect in the retina, and not at the chiasm, and causes the abnormally small ipsilateral retinogeniculate projection that is seen in the adult albino visual system. Our studies on intact developing animals correlate with the early culture experiments and further define the developmental time during which tyrosinase exerts its effect on ganglion cell development.
The necessity for tyrosinase during neuroblast divisions is consistent with the observation that early phases of neurogenesis and cell cycle kinetics are disrupted in albinos (Ilia and Jeffery, 2000; Rachel et al., 2002a). It remains to be determined how changes in tyrosinase expression and pigment formation in the RPE affect the neuroblast divisions in the adjacent cell layer. There is evidence for an effect of altered levels of DOPA, a melanin precursor with known effects on cell cycle (Wick, 1977; Akeo et al., 1994; Ilia and Jeffery, 1999). It has also been suggested that melanin could alter calcium levels in the retina by acting as a calcium sink (Drager, 1985b), with resultant effects on the cell cycle. It could also be a combination of these mechanisms with some as yet unidentified molecular signal that leads to perturbed neurogenesis in the albino retina.
Neuroblast divisions in the retina and subsequent cell fate determination have been studied for some time (for review, see Livesey and Cepko, 2001). It appears that both factors intrinsic to the neuroblast and environmental cues after cell birth are important in the determination of cell fate. This has been studied in relation to whether particular neuroblasts give rise to neurons of a certain class, for instance, amacrine cells versus cone photoreceptors (Belliveau and Cepko, 1999). The results presented here suggest that for ganglion cells, ipsilateral versus contralateral projection is also a cell fate determination that is made during the period of neuroblast divisions.
Taken together, these results suggest that the choice between ipsilateral and contralateral pathways is specified by ganglion cells while their axons are still in the retina and that tyrosinase expression during the period of neuroblast divisions alters this specification. Although a number of signaling molecules have been identified that influence retinal ganglion cell pathfinding (Snow et al., 1991; Deiner et al., 1997; Erskine et al., 2000; Niclou et al., 2000; Ringstedt et al., 2000), none have been shown to affect ipsilateral and contralateral cells differentially. Thus, it is not known what signals are disrupted to cause the inappropriate crossing of some temporal retinal ganglion cell axons in albino mammals. Our results suggest that whatever the guidance cues may be, tyrosinase expression early in development affects the ability of some ganglion cells to express or recognize the appropriate guidance cues once they reach the chiasm.
Footnotes
This work was supported by the National Center for Research Resources and National Institutes of Health Grant RR11102 (H.S.), and National Research Service Award predoctoral fellowship MH12406 (C.A.C.). We thank W. Gluba and B. Bernier for excellent technical support and D. Bayliss and M. Harrison for access to equipment.
Correspondence should be addressed to Dr. Heidi Scrable, University of Virginia, Department of Neuroscience, P.O. Box 801392, Charlottesville, VA 22908-1392. E-mail: hs2n@virginia.edu.
Copyright © 2003 Society for Neuroscience 0270-6474/03/2311692-06$15.00/0
References
- Akeo K, Tanaka Y, Okisaka S ( 1994) A comparison between melanotic and amelanotic retinal pigment epithelial cells in vitro concerning the effects of l-dopa and oxygen on cell cycle. Pigment Cell Res 7: 145-151. [DOI] [PubMed] [Google Scholar]
- Balkema GW, Drager UC ( 1990) Origins of uncrossed retinofugal projections in normal and hypopigmented mice. Vis Neurosci 4: 595-604. [DOI] [PubMed] [Google Scholar]
- Beermann F, Schmid E, Schutz G ( 1992) Expression of the mouse tyrosinase gene during embryonic development: recapitulation of the temporal regulation in transgenic mice. Proc Natl Acad Sci USA 89: 2809-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belliveau MJ, Cepko CL ( 1999) Extrinsic and intrinsic factors control the genesis of amacrine and cone cells in the rat retina. Development 126: 555-566. [DOI] [PubMed] [Google Scholar]
- Burchill SA, Virden R, Fuller BB, Thody AJ ( 1988) Regulation of tyrosinase synthesis by alpha-melanocyte-stimulating hormone in hair follicular melanocytes of the mouse. J Endocrinol 116: 17-23. [DOI] [PubMed] [Google Scholar]
- Cronin CA, Gluba W, Scrable H ( 2001) The lac operator-repressor system is functional in the mouse. Genes Dev 15: 1506-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiner MS, Kennedy TE, Fazeli A, Serafini T, Tessier-Lavigne M, Sretavan DW ( 1997) Netrin-1 and DCC mediate axon guidance locally at the optic disc: loss of function leads to optic nerve hypoplasia. Neuron 19: 575-589. [DOI] [PubMed] [Google Scholar]
- del Marmol V, Beermann F ( 1996) Tyrosinase and related proteins in mammalian pigmentation. FEBS Lett 381: 165-168. [DOI] [PubMed] [Google Scholar]
- Donatien P, Jeffery G ( 2002) Correlation between rod photoreceptor numbers and levels of ocular pigmentation. Invest Ophthalmol Vis Sci 43: 1198-1203. [PubMed] [Google Scholar]
- Donatien P, Aigner B, Jeffery G ( 2002) Variations in cell density in the ganglion cell layer of the retina as a function of ocular pigmentation. Eur J Neurosci 15: 1597-1602. [DOI] [PubMed] [Google Scholar]
- Drager UC ( 1985a) Birth dates of retinal ganglion cells giving rise to the crossed and uncrossed optic projections in the mouse. Proc R Soc Lond B Biol Sci 224: 57-77. [DOI] [PubMed] [Google Scholar]
- Drager UC ( 1985b) Calcium binding in pigmented and albino eyes. Proc Natl Acad Sci USA 82: 6716-6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erskine L, Williams SE, Brose K, Kidd T, Rachel RA, Goodman CS, Tessier-Lavigne M, Mason CA ( 2000) Retinal ganglion cell axon guidance in the mouse optic chiasm: expression and function of robos and slits. J Neurosci 20: 4975-4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillery RW ( 1986) Neural abnormalities of albinos. Trends Neurosci 9: 364-367. [Google Scholar]
- Guillery RW, Okoro AN, Witkop Jr CJ ( 1975) Abnormal visual pathways in the brain of a human albino. Brain Res 96: 373-377. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Mason CA, Taylor JSH ( 1995) Developmental determinants at the mammalian optic chiasm. J Neurosci 15: 4727-4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilia M, Jeffery G ( 1999) Retinal mitosis is regulated by dopa, a melanin precursor that may influence the time at which cells exit the cell cycle: analysis of patterns of cell production in pigmented and albino retinae. J Comp Neurol 405: 394-405. [DOI] [PubMed] [Google Scholar]
- Ilia M, Jeffery G ( 2000) Retinal cell addition and rod production depend on early stages of ocular melanin synthesis. J Comp Neurol 420: 437-444. [PubMed] [Google Scholar]
- Jeffery G ( 1997) The albino retina: an abnormality that provides insight into normal retinal development. Trends Neurosci 20: 165-169. [DOI] [PubMed] [Google Scholar]
- Jeffery G, Schutz G, Montoliu L ( 1994) Correction of abnormal retinal pathways found with albinism by introduction of a functional tyrosinase gene in transgenic mice. Dev Biol 166: 460-464. [DOI] [PubMed] [Google Scholar]
- Jeffery G, Brem G, Montoliu L ( 1997) Correction of retinal abnormalities found in albinism by introduction of a functional tyrosinase gene in transgenic mice and rabbits. Dev Brain Res 99: 95-102. [DOI] [PubMed] [Google Scholar]
- LaVail JH, Nixon RA, Sidman RL ( 1978) Genetic control of retinal ganglion cell projections. J Comp Neurol 182: 399-421. [DOI] [PubMed] [Google Scholar]
- Lee AV, Weng CN, McGuire SE, Wolf DM, Yee D ( 1997) Lac repressor inducible gene expression in human breast cancer cells in vitro and in a xenograft tumor. Biotechniques 23: 1062-1068. [DOI] [PubMed] [Google Scholar]
- Livesey FJ, Cepko CL ( 2001) Vertebrate neural cell-fate determination: lessons from the retina. Nat Rev Neurosci 2: 109-118. [DOI] [PubMed] [Google Scholar]
- Mangini NJ, Vanable Jr JW, Williams MA, Pinto LH ( 1985) The optokinetic nystagmus and ocular pigmentation of hypopigmented mouse mutants. J Comp Neurol 241: 191-209. [DOI] [PubMed] [Google Scholar]
- Marcus RC, Wang LC, Mason CA ( 1996) Retinal axon divergence in the optic chiasm: midline cells are unaffected by the albino mutation. Development 122: 859-868. [DOI] [PubMed] [Google Scholar]
- Niclou SP, Jia L, Raper JA ( 2000) Slit2 is a repellent for retinal ganglion cell axons. J Neurosci 20: 4962-4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oetting WS, King RA ( 1999) Molecular basis of albinism: mutations and polymorphisms of pigmentation genes associated with albinism. Hum Mutat 13: 99-115. [DOI] [PubMed] [Google Scholar]
- Pittler SJ, Baehr W ( 1991) Identification of a nonsense mutation in the rod photoreceptor cGMP phosphodiesterase beta-subunit gene of the rd mouse. Proc Natl Acad Sci USA 88: 8322-8326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachel RA, Dolen G, Hayes NL, Lu A, Erskine L, Nowakowski RS, Mason CA ( 2002a) Spatiotemporal features of early neuronogenesis differ in wild-type and albino mouse retina. J Neurosci 22: 4249-4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachel RA, Mason CA, Beermann F ( 2002b) Influence of tyrosinase levels on pigment accumulation in the retinal pigment epithelium and on the uncrossed retinal projection. Pigment Cell Res 15: 273-281. [DOI] [PubMed] [Google Scholar]
- Ringstedt T, Braisted JE, Brose K, Kidd T, Goodman C, Tessier-Lavigne M, O'Leary DD ( 2000) Slit inhibition of retinal axon growth and its role in retinal axon pathfinding and innervation patterns in the diencephalon. J Neurosci 20: 4983-4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rungta D, Corn TD, Fuller BB ( 1996) Regulation of tyrosinase mRNA in mouse melanoma cells by alpha-melanocyte-stimulating hormone. J Invest Dermatol 107: 689-693. [DOI] [PubMed] [Google Scholar]
- Sanderson KJ, Guillery RW, Shackelford RM ( 1974) Congenitally abnormal visual pathways in mink (Mustela vision) with reduced retinal pigment. J Comp Neurol 154: 225-248. [DOI] [PubMed] [Google Scholar]
- Silver J ( 1984) Studies on the factors that govern directionality of axonal growth in the embryonic optic nerve and at the chiasm of mice. J Comp Neurol 223: 238-251. [DOI] [PubMed] [Google Scholar]
- Snow DM, Watanabe M, Letourneau PC, Silver J ( 1991) A chondroitin sulfate proteoglycan may influence the direction of retinal ganglion cell outgrowth. Development 113: 1473-1485. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Shatz CJ ( 2002) An instructive role for retinal waves in the development of retinogeniculate connectivity. Neuron 33: 357-367. [DOI] [PubMed] [Google Scholar]
- Wick MM ( 1977) l-Dopa methyl ester as a new antitumour agent. Nature 269: 512-513. [DOI] [PubMed] [Google Scholar]
- Wu JD, Hsueh HC, Huang WT, Liu HS, Leung HW, Ho YR, Lin MT, Lai MD ( 1997) The inducible lactose operator-repressor system is functional in the whole animal. DNA Cell Biol 16: 17-22. [DOI] [PubMed] [Google Scholar]
- Wyborski DL, Short JM ( 1991) Analysis of inducers of the E. coli lac repressor system in mammalian cells and whole animals. Nucleic Acids Res 19: 4647-4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW ( 1984) Cell death during differentiation of the retina in the mouse. J Comp Neurol 229: 362-373. [DOI] [PubMed] [Google Scholar]