Abstract
The amygdala is known to play an important role in conscious and unconscious processing of emotional and highly arousing stimuli. Neuroanatomical evidence suggests that the amygdala participates in the control of autonomic responses, such as skin conductance responses (SCRs), elicited by emotionally salient stimuli, but little is known regarding its functional role in such control.
We investigated this issue by showing emotional visual stimuli of varying arousal to patients with left (n = 12), right (n = 8), and bilateral (n = 3) amygdala damage and compared their results with those from 38 normal controls. Stimuli were presented both subliminally (using backward masking) and supraliminally under lateralized presentation to one visual hemifield. We collected SCRs as a physiological index of emotional responses. Subjects subsequently rated each stimulus on valence and arousal under free viewing conditions.
There were two key findings: (1) impaired overall SCR after right amygdala damage; and (2) impaired correlation of SCR with the rated arousal of the stimuli after left amygdala damage. The second finding was strengthened further by finding a positive correlation between the evoked SCR magnitude and postsurgery amygdala volume, indicating impaired autonomic responses with larger tissue damage. Bilateral amygdala damage resulted in severe impairments on both of the above measures.
Our results provide support for the hypothesis that the left and right amygdalae subserve different functions in emotion processing: the left may decode the arousal signaled by the specific stimulus, whereas the right may provide a global level of autonomic activation triggered automatically by any arousing stimulus.
Keywords: emotion, backward masking, amygdala, arousal, subliminal, temporal lobectomy, skin conductance
Introduction
Emotional stimuli are often characterized in terms of an underlying two-dimensional space of valence and arousal that is reflected in different physiological indices (Lang et al., 1993; Lang, 1995). Skin conductance responses (SCRs) are a measure of autonomic arousal and often used to index emotional processing (Lang et al., 1993; Bauer, 1998).
Evidence for the role of the amygdala in the expression of SCRs comes from both functional imaging studies (Furmark et al., 1997; Critchley et al., 2000, 2002) and lesion studies showing that it is essential for the acquisition of conditioned SCRs but not for declarative knowledge about the contingencies between the conditioned and unconditioned stimulus (Damasio, 1994; Bechara et al., 1995).
Functional imaging (Irwin et al., 1996; Morris et al., 1996) and lesion studies (Adolphs et al., 1994, 1995; Calder et al., 1996; Broks et al., 1998) suggest the amygdala is a key player in the processing of emotional stimuli, especially fear, but perhaps also other arousing emotions (Hamann et al., 2002). Studies investigating the role of the amygdala in valence and arousal (Adolphs et al., 1999; Taylor et al., 2000) report a disproportionate engagement of this structure in processing highly arousing aversive stimuli of biological relevance.
Hemispheric lateralization of emotional processing has focused on the valence dimension. Although Davidson and Irwin (1999) proposed a lateralization of positive emotions to the left and negative emotions to right prefrontal cortex, Borod (1993) reviewed studies that found right hemisphere dominance for emotional processing regardless of valence. However, both these frameworks are targeted at the cortical level only. Amygdala activation to emotional stimuli has been observed either bilaterally (Hariri et al., 2000; Liberzon et al., 2000; Taylor et al., 2000) or in the left (Morris et al., 1996; Lane et al., 1997, 1999; Blair et al., 1999; Dubois et al., 1999), whereas lesion studies instead implicate the right (Anderson et al., 2000; Adolphs et al., 2001), leaving this issue unresolved.
Several imaging studies have demonstrated amygdala involvement in unconscious emotional processing (Morris et al., 1998; Whalen et al., 1998; Rauch et al., 2000) using subliminal stimulus presentations, which are particularly useful in detecting amygdala involvement without further cortical engagement. These studies generally reported bilateral amygdala activation, although Morris et al. (1998) found a lateralization of amygdala activation depending on conscious or unconscious processing. Recently, Kubota et al. (2000) assessed SCRs to emotional pictures in unilateral temporal lobectomy patients and found reduced responses to negative pictures when presented to the lesioned hemisphere.
In the current study, we wanted to assess the effect of unilateral and bilateral amygdala damage on conscious and unconscious processing of visual emotional stimuli and to relate SCRs to overt rating performance. Specifically, we hypothesized that bilateral amygdala damage would produce the strongest deficit in the physiological responses (Bechara et al., 1995) and that right amygdala damage would result in more severe impairments than left amygdala damage (Adolphs et al., 2001; Anderson et al., 2000). Furthermore, we expected to find a dissociation between overt rating performance (a measure of conscious emotional processing) and physiological responses (a measure of unconscious processing): patients with amygdala damage should be more impaired in their autonomic responses but less in their cognitive ratings, as would be consistent with the role of amygdala in somatic response rather than cold cognition (Damasio, 1994). We tested these hypotheses with a design including the following factors: side of amygdala lesion (left, right, bilateral), arousal of the emotional stimulus (ranging from low to high), side of presentation of the emotional stimulus (left or right visual field), and mode of processing (subliminal or supraliminal presentation).
Materials and Methods
Subjects
We tested 12 subjects with left temporal damage (LTD), 8 with right temporal damage (RTD), and 3 with bilateral temporal damage (BTD). All patients had damage to the amygdala and were drawn from the patient registry of the Division of Cognitive Neuroscience and Behavioral Neurology (University of Iowa College of Medicine, Iowa City, IA). Causes for bilateral damage were Urbach-Wiethe disease resulting in selective calcifications of both amygdalae (one patient) and herpes simplex encephalitis resulting in complete, but nonselective, amygdala destruction (two patients). None of the patients with bilateral amygdala damage had any seizures or were taking antiepileptic medication.
All patients with unilateral damage underwent a partial resection of their anterior temporal lobe (including amygdala) because of intractable epilepsy. We used only patients in whom there was clear clinical evidence that: (1) seizures were localized to the anteromesial temporal lobe, as revealed through epilepsy monitoring and that was subsequently resected; (2) in all cases, there was a dramatic improvement on resection [class I and II according to the Engel classification (Engel et al., 1993)]; and (3) subjects were taking, at most, low doses of antiepileptic medication (seven LTD patients and five RTD patients). Statistical tests comparing those who were taking low doses of medication versus those who were medication free showed no differences between these two groups. In no case was there any indication of extratemporal involvement, an assessment that was based on neuropsychological testing, clinical EEG monitoring, and magnetic resonance (MR) imaging data in all of the subjects. Additionally, several subjects had clinical functional imaging performed that verified that regions of abnormal blood flow were confined to the medial temporal lobe subsequently resected. Tables 1 and 2 list the demographic and neuropsychological profile of each of the three patient groups.
Table 1.
Summary of demographics of all experimental groups
|
Group |
Sex (m/f) |
Age (years) |
Handedness (r/l) |
Education (years) |
|---|---|---|---|---|
| BTD | 2/1 | 51.3 (18.2) | 3/0 | 14.0 (2.8) |
| LTD | 6/6 | 39.0 (14.1) | 10/2 | 15.0 (1.4) |
| RTD | 6/2 | 36.3 (9.3) | 8/0 | 13.7 (2.1) |
| NC
|
20/18
|
33.6 (6.9)
|
35/3
|
16.2 (2.2)
|
Data are means ± SD. m/f, Male/female; r/l, right/left.
Table 2.
Summary of neuropsychological indices
|
Group |
PIQ |
VIQ |
Benton faces |
BDI |
|---|---|---|---|---|
| BTD | 102.0 (12.1) | 98.0 (17.0) | 42.0 (2.6) | 0.0 (0.0) |
| LTD | 116.0 (16.8) | 109.0 (19.8) | 42.0 (4.2) | 3.5 (2.1) |
| RTD
|
103.0 (21.9)
|
98.3 (8.7)
|
40.3 (3.1)
|
4.6 (5.1)
|
Data are means ± SD. All patients were tested individually in the neuropsychological tests, and all values were within the normal range. Control subjects were not tested on neuropsychological measures. PIQ and VIQ are performance and verbal intelligence quotient measures, respectively, from the Wechsler Adult Intelligence Test III. Benton Faces measures the ability to visually discriminate faces (raw scores), and BDI is the score from the Beck Depression Inventory.
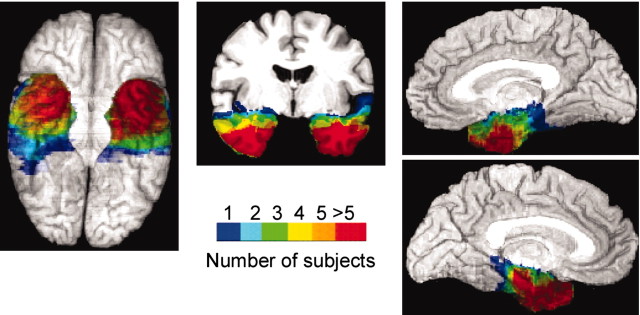
To quantify the volumetric extent of amygdala damage in subjects with unilateral amygdala damage, we traced the amygdala on presurgical MR scans of the subjects' brains and compared the reconstructed volume of the amygdala on the presurgical scans to those derived from the corresponding regions on the postsurgical scan. After alignment of brain volumes, the boundaries of the amygdala and hippocampus were ascertained from the atlas of Duvernoy (1991). Guide traces were drawn initially in the sagittal view to identify the alveus of the hippocampus, which serves as the posterior border of the amygdala. The amygdala boundaries were then defined anteriorly using the white matter of the parahippocampal gyrus as the anterior, lateral, and inferior borders. Posteriorly, the amygdala was bounded by the temporal horn of the lateral ventricle, the basal ganglia provided the superior border, the uncus the medial border, the white matter of the temporal lobe the lateral border, and the alveus of the hippocampus the posterior border. Detailed postsurgery volumes of the amygdala, the hippocampus, and the total brain volume are shown in Table 3 for patients with unilateral temporal lobectomy. Figure 1 depicts the overlap of temporal lesions in the patient groups.
Table 3.
Postsurgery amygdala, hippocampus, and total brain volumes [means (range)] and time elapsed since surgery for patients with unilateral temporal damage
|
|
Amygdala volume (mm3) |
Hippocampus volume (mm3) |
||||
|---|---|---|---|---|---|---|
| Group
|
Intact
|
Lesioned
|
Intact
|
Lesioned
|
||
| LTD | 1799.4 (1176.2 - 2325.9) | 1023.8 (164.8 - 1768.6) | 4298.7 (3671.5 - 5065.8) | 1935.1 (492.7 - 4367.1) | ||
| RTD
|
2194.2 (1604.4 - 2889.6)
|
986.2 (0 - 1736.6)
|
4003.8 (3235.8 - 5027.2)
|
1421.3 (650.7 - 2611.5)
|
||
| Group
|
Total brain volume (mm3)
|
Years since surgery
|
|---|---|---|
| LTD | 1132325.3 (1017004.4 - 1411006.6) | 5.92 (0 - 16) |
| RTD
|
181528.1 (1013430.1 - 1294299.6)
|
4.38 (1 - 10)
|
The volumes were computed by comparing reconstructed presurgical with postsurgical MR scans. The amygdala and hippocampus were defined by anatomical landmarks (see Materials and Methods).
Figure 1.
Schematic display of lesion overlap in patients with unilateral and bilateral temporal damage. The number of subjects whose lesion included a given voxel is encoded by color (compare scale).
In addition, we tested 38 neurologically and psychiatrically normal controls (NCs) of similar age, who were recruited through local hospital advertisement. These subjects were paid for their participation in the experiment. All subjects gave informed written consent as approved by the local Institutional Review Board.
Stimuli
All stimuli were drawn from the International Affective Picture Series (IAPS), a well-normed emotional picture set that has been used extensively in emotion research, including studies of physiological reactions to the pictures and normative ratings for valence and arousal (Lang et al., 1988). Lang et al. (1993) reported previously that skin conductance responses correlated significantly with arousal ratings for these stimuli in normal subjects.
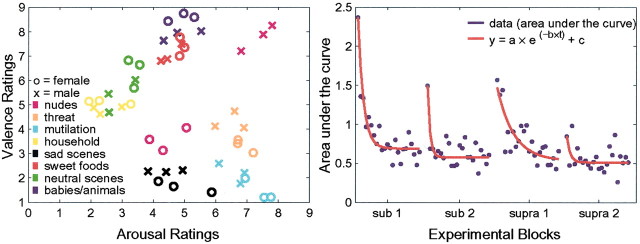
We selected the stimuli to cover the full range of the arousal and valence dimension (Fig. 2, left). To sample for a variety of different emotional content, we included pictures of babies, sweet food items, neutral social scenes, mutilation, threatening animals, household objects, and sad scenes. Lang et al. (1993) described a U-shaped relationship between valence and arousal ratings, indicating that pictures of extreme valence also scored high on arousal whereas neutral picture obtain only low arousal ratings. However, we also selected pictures that do not fit this relationship (e.g., babies/animal: positive valence, medium arousal) to fully cover both dimensions. This selection procedure allowed for an analysis of the independent contributions of valence and of arousal.
Figure 2.
Normative arousal and valence ratings and within-block habituation. Left, Scatterplot of normative valence and arousal ratings for both sexes of the stimuli used in this study, as provided by data from Lang et al. (1988). Emotion categories are color-coded. Right, Example of within-block habituation of skin conductance responses in the normal controls. SCR data (mean response in normal controls) of each block are shown in blue, and the exponential regression equation for each block is shown in red.
We also measured the luminance of the stimuli and equated the different emotion categories with regard to their mean luminance. Statistical analyses revealed that stimulus luminance had no effect on the results.
We preselected 64 stimuli, presented them to five normal subjects in a pilot experiment, and selected 24 stimuli for the final set based on the reliability of their autonomic responses to these stimuli. For the subliminal presentation, we used a backward masking procedure (see below) and selected a neutral landscape picture as the mask.
Experimental procedure
The entire experiment consisted of six parts in the following fixed order: two subliminal presentation blocks, a surprise recognition task, two supraliminal presentation blocks, and a final (supraliminal and free viewing) rating task. All 24 stimuli were presented once in a fully randomized order during each presentation block, half of them to each visual field with a lateralized presentation. In the second block of each processing mode (subliminal, supraliminal), the stimuli were presented into the other visual field, respectively. Thus, we obtained data from each stimulus presented to each visual field under both subliminal and supraliminal processing.
The lateralized presentation was achieved by instructing subjects to fixate on a centrally located fixation cross on the screen; the stimuli were then presented in the perifoveal area left or right of the fixation cross (1.9° off foveal vision). Fixation was validated with two separate measures: subjectively, through verbal reports after each trial, and objectively, through a lateral electrooculogram (EOG) that controlled for left and right eye movements.
Subjects were also informed about the backward masking procedure before the experiment. Target recognition during the subliminal presentation was checked with two independent measures: subjective reports after each trial and a surprise forced-choice recognition task applied immediately after the second subliminal presentation block. Based on their verbal reports, we excluded all trials from the analyses in which subjects correctly recognized the target in the subliminal presentation. Merikle et al. (2001) argued that subjective measures are more appropriate in establishing stimulus awareness, because objective measures (such as a forced-choice recognition task) are only valid if subjects fail to perform above chance level on the task; correct performance could be based on fleeting stimulus features without conscious perception of the entire stimulus.
The following descriptions provide the details regarding each part of the experiment.
Subliminal presentation. Stimuli were presented from a Macintosh computer (version 8.6) on a calibrated Mitsubishi Diamond Pro monitor operating at a 100 Hz refresh rate, using Psyscope 1.1 presentation software (http://psyscope.psy.cmu.edu). During the subliminal presentation, subjects were cued with a 1000 Hz tone for 500 msec to indicate the beginning of a trial. After a preparation pause of 1000 msec, targets were shown for 30 msec, immediately followed by the masking stimulus for 2000 msec. After an additional data collection period of 6000 msec after the offset of the masking stimulus, two questions were shown on the screen for 2000 msec, each with a gap of 1000 msec between them. The two questions asked subjects to indicate verbally whether they broke fixation and whether they recognized the target stimulus. A final intertrial interval (ITI) of 3000 msec concluded each trial. Thus, each subliminal presentation block lasted for ∼7.5 min, and SCR data were collected during the entire block.
Recognition task. After the two subliminal presentations, subjects were confronted with an unannounced forced-choice recognition task. Each target was presented side by side with a distractor stimulus. We chose distractors from the same emotional categories as the targets, but a target was never paired with a distractor of the same category. Subjects simply had to indicate which of the two pictures they thought they had seen in the subliminal presentation. There was no time limit. We calculated probabilities for hits and misses from the recognition task to judge the overall recognition performance.
Supraliminal presentation. The supraliminal presentation was very similar to the subliminal presentation, the main difference being that the target was presented for 2000 msec with no masking stimulus afterward (all other presentation timings remained the same). After each target, subjects only had to answer a single question: whether they had broken fixation (2000 msec question, 1000 msec answer time, 3000 msec ITI). Each supraliminal block lasted for ∼6.2 min.
Ratings task. Finally, the subjects had to rate each target on four different dimensions (arousal, valence, novelty, and visual complexity) using nine-point Likert scales. We chose the latter two dimensions to control for possibly confounding aspects of stimulus processing that would not be related to emotional processing. For this task, stimuli were shown again in free field, and there was no time limit.
Data acquisition
Physiological data (SCR and EOG) were acquired on a P122 Grass Instruments (Warwick, RI) polygraph with a microsiemens scaling interface (model SCA1A; Grass Instruments). The analog signal was digitized using the MP100WS digital converter (Biopac Systems, Goleta, CA) and was fed into AcqKnowledge 3.2.4 recording software (Biopac Systems) on a Macintosh G3 powerbook. We converted the time series from millivolts to microsiemens post hoc and extracted SCRs. Meditrace 530 Ag/AgCl electrodes and Neuroline 71008-k electrodes were used to acquire the physiological signal at the thenar and hypothenar site for SCR on both hands and for the EOG at the lateral and medial rectus. Both SCR and EOG data were sampled at 100 Hz. Data were transformed and analyzed using Excel 97 (Microsoft, Seattle, WA), SPSS version 10.0.5 (SPSS Inc., Chicago, IL), and Matlab 5.3 (Mathworks, Sherborn, MS).
Skin conductance data transformation
Before extraction of data for skin conductance responses, we smoothed and detrended the original time series to remove high-frequency noise and drift. For smoothing, we averaged data points using a moving window of 100 adjacent data points. For detrending, we applied a difference algorithm to the time series that removed the global baseline decay on the skin conductance signal. This algorithm calculated the differences between two selected data points and divided it by the number of intermittent data points. We then extracted the area under the curve in a 5 sec interval starting with the presentation of each target. These measures of the skin conductance responses and data transformations have been used in previous studies (Bechara et al., 1995).
These derived data displayed typical and pronounced within-block habituation (Sokolov, 1963), but with large interindividual differences in the habituation rate. To remove the effect of habituation, we fitted an exponential regression to the mean responses in each group (y = a × e(-b × t) + c, with t being the position of the stimulus within a block). This regression model conforms to the previously reported exponential decay of the skin conductance signal (Groves and Thompson, 1970). We chose a three-parameter model to account for the initial value, the slope of the function, and an asymptote for differences in habituation between the groups. Figure 2 (right) shows our derived skin conductance response measure (area under the curve) fitted with the respective regression function for the normal controls.
In a second step, we calculated the individual stimulus responses as the residuals of the data with respect to the exponential regression model. Finally, we added the group constant c from the exponential regression to each individual residual to account for differences in the overall response habituation.
The obtained SCR residuals represent the individual deviations from the predicted response curve and carry the variance because of emotional processing of our experimental manipulations after the nuisance variance of within-block habituation has been partialed out. These residuals were subsequently used in all analyses we report here.
Statistical analyses
We recognize that our data were collected in continuous variables, which suggests the use of parametric statistics. In contrast, the small sample sizes of the patient groups and non-normal distribution of data indicate the use of nonparametric statistics. We, hence, chose a dual approach using both parametric and nonparametric statistics. We present linear regression results in the figures to preserve the continuity of the underlying variables. These parametric results are complemented and cross-validated by nonparametric rank-ordered statistics (Spearman's R) that we provide below.
Results
Manipulation check
Eye movement
We chose the EOG criterion as the more objective and conservative measure of loss of fixation. Across all groups, subjects gave verbal subjective responses that were 95.3% accurate given the EOG data; in only 2.6% of the responses, subjects reported not having moved their eyes where the EOG shows that they did. These results confirm a high degree of accuracy of the verbal reports. Nevertheless, we excluded data points based on the EOG responses rather than on the verbal reports.
Target recognition
We assessed performance on the recognition task by calculating the mean frequencies of correct and false responses for each group. If these frequencies were at chance level (i.e., 12 correct and 12 false responses), subjects would be classified as not having recognized the target during the subliminal presentation. We could not calculate these indices for the subjects with bilateral amygdala damage because of the small sample size and missing data (one subject was severely amnesic). For the remaining groups, one-sample t tests revealed no significant departure from chance level (NCcorr = 12.68, NCfalse = 11.32, t = 1.41, p > 0.15; LTDcorr = 12.36, LTDfalse = 11.64, t = 0.59, p > 0.55; RTDcorr = 11.00, RTDfalse = 13.00, t =-1.43, p > 0.15).
Novelty and complexity
Subjects rated all stimuli with regard to their novelty and visual complexity to control for basic visuoperceptual differences between the stimuli. Subjects completed the novelty rating by referring to occurrences of the stimulus in their autobiographical past. These ratings are quite different than the arousal and valence ratings because the latter were made by referring to the current emotional state. Thus, both ratings are based on different time frames.
We found a significant positive correlation between novelty and arousal (r = 0.469; p < 0.05) and a significant negative correlation between novelty and valence (r = -0.734; p < .001). Correlations between visual complexity and arousal and valence did not reach statistical significance (arousal: r = 0.160, p > 0.45; valence: r = -0.207, p > 0.30).
Global SCRs to emotional pictures
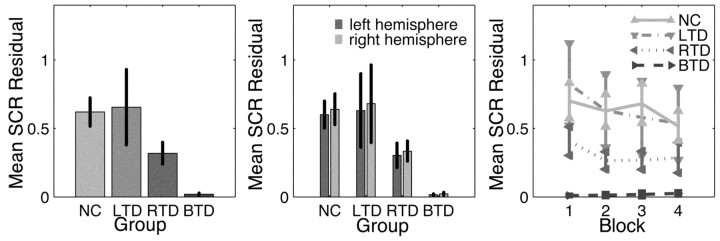
We calculated the group mean SCR by averaging the residuals from the habituation regression. Data are shown in Figure 3 (left). Because of the heterogeneity of group variance, we refrained from performing omnibus tests for assessing group differences and used contrasts that compared each of the patient groups with the normal controls. These contrasts incorporated a correction for unequal variances between the groups (Welch test) (Welch, 1938; Maxwell and Delaney, 2000). We found significant differences between normal controls and patients with RTD (F(1,35.85) = 5.728; p < 0.025) and patients with BTD (F(1,37.33) = 31.06; p < 2.5 × 10-6) but not between normal controls and patients with LTD (F(1,14.72) = .005; p > 0.90).
Figure 3.
Group SCR responses to experimental stimuli (means ± SEM). Left, Group SCR response to all emotional stimuli (residuals from group-wise habituation model) across all recording blocks (two subliminal and two supraliminal) for all experimental groups. Middle, Decomposition of the overall SCR response shown in the left panel with regard to hemispheric stimulation. No significant differences were found between the hemispheres. Right, Mean SCR response for all experimental groups in all recording blocks. This is a decomposition of the data in the left panel. Normal controls and unilateral temporal patients show a slight (nonsignificant) decrease in mean responsiveness in the course of the experiment.
To check for differential responses caused by lateralized presentation of the stimuli, we broke down responses for each hemisphere to which the stimuli were presented (Fig. 3, middle) but found no significant differences in either group (all p > 0.30). This analysis, thus, confirms that the averaging procedure does not conceal differential responsiveness because of the side of stimulus presentation.
Subsequently, we were also interested in whether the overall responsiveness to emotional stimuli (after partialling the within-block habituation) would still attenuate in the course of the experiment. Figure 3 (right) shows the data for each recording block. Normal controls and unilateral temporal patients showed a slight, but nonsignificant, decrease in response magnitude as they advanced from block 1 (subliminal 1) to block 4 (supraliminal 2). This was confirmed by two-factorial repeated measures multivariate ANOVA (MANOVA) on the factors block and group, which yielded a nonsignificant main effect for block (Wilk's Λ = 0.959; F(3,56) = 0.789; p > 0.50) and an equally nonsignificant block × group interaction effect (Wilk's Λ= 0.948; F(9,136.4) = 0.338; p > 0.95).
Finally, we investigated whether impaired responsiveness might be correlated with the volumetric extent of amygdala damage. We calculated these indices only for subjects with unilateral amygdala damage. There were no significant correlations for subjects with either left or right amygdala damage (all p > 0.12). Similarly, we correlated SCRs with the time elapsed since surgery but found no significant association in either group (all p > 0.25).
Correlation of SCRs with normative ratings
In a second step, we were interested in the relationship between autonomic responses and normative ratings of arousal and valence reported by Lang et al. (1988). By choosing the normative ratings as a uniform scale in the comparison, differences between groups can be assessed directly. We, therefore, submitted the SCR residuals to a group-wise linear regression and assessed whether the SCRs under the different experimental conditions (mode of processing, visual field) predicted the normative ratings of arousal and valence. Because of differences in group variances, we transformed the SCR residuals to Z values and used them in the analyses. The results are shown in Figures 4 and 5.
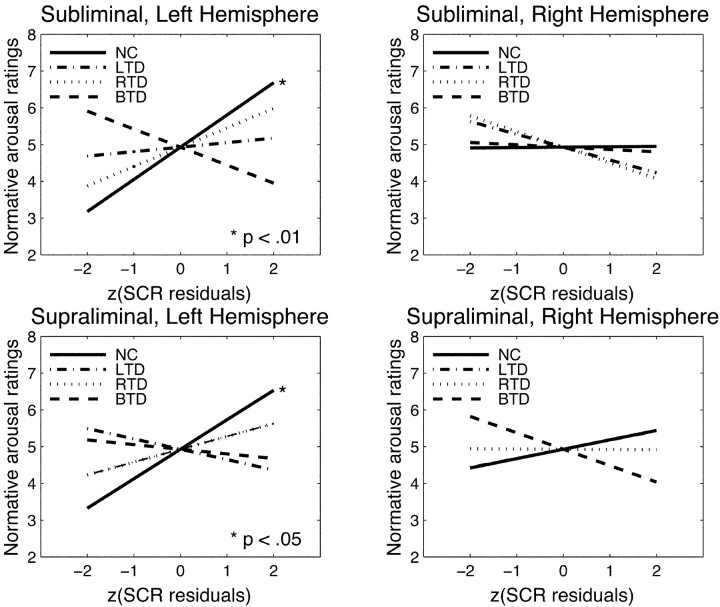
Figure 4.
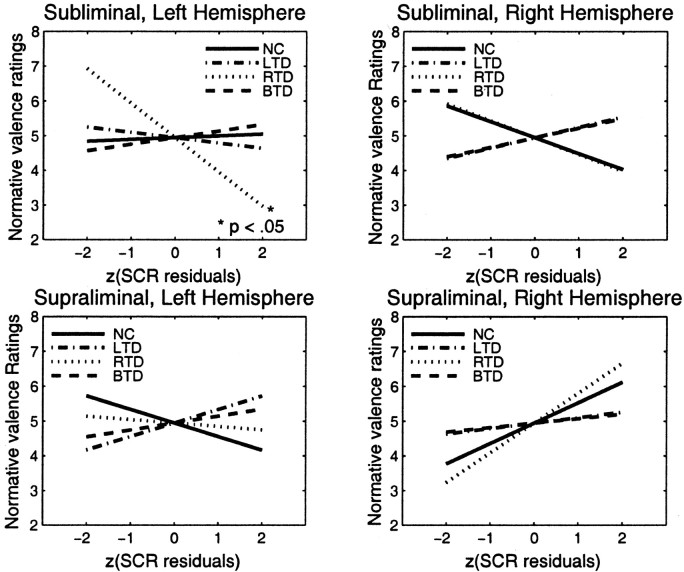
Graphs of the regression lines for normative arousal ratings versus group SCRs for different experimental conditions (mode of processing, stimulated hemisphere). Z-transformed values are used for these plots because of different variances in the experimental groups, thus allowing a visual comparison of the plots on a uniform scale. The SCRs of normal controls significantly predict normative arousal ratings when the stimuli are presented into the left hemisphere but not into the right. None of the patient groups' SCRs show a significant relationship, although right temporal patients perform better (nonsignificant) under left hemispheric stimulation than left temporal patients. Right hemisphere stimulation does not lead to any significant relationship between SCRs and normative ratings. (Normal controls and bilateral temporal patients have the same regression line in the bottom right graph, and, thus, data from normals conceal those from the bilateral patients.)
Figure 5.
Graphs of regression lines relating normative valence ratings and group SCRs. None of the linear regressions yield significant correlation coefficients, except for right temporal patients in the left hemisphere under subliminal processing (top left). Because of similar regression lines, bilateral temporal patients conceal those of left temporal patients under right hemispheric processing (right graphs), and right temporal patients are concealing those of normal controls for subliminal, right hemisphere stimulation (top right).
Only normal controls under left hemispheric stimulation under both subliminal and supraliminal processing displayed a significant relationship between their SCR residuals and normative arousal ratings (subliminal: r = 0.592, p < 0.01; supraliminal: r = 0.486, p < 0.05). No such correlations were observed for the right hemisphere (subliminal: r = 0.050, p > 0.80; supraliminal: r = 0.109, p > 0.60). In none of the patient groups, the correlation between SCR residuals and normative arousal ratings rose to a significant level (all p > 0.15).
The correlations between SCR residuals and normative valence ratings were all nonsignificant (Fig. 5), except for one significant negative relationship in patients with RTD when stimuli were shown subliminally to the left hemisphere (r = -0.417; p < 0.05). We found no significant differences between recordings from both hands (F(1,57 = 0.118; p > 0.70). Therefore, we report pooled results from both hands. The following two sections will look at these findings in more detail.
Arousal dimension
As reported above, normal controls showed significant relationships when stimuli were presented under both modes of processing (subliminal as well as supraliminal) to the left hemisphere (right visual field) but no such relationships when they were presented to the right hemisphere (left visual field). In none of the patient groups were SCR residuals correlated significantly with normative arousal ratings. However, on closer inspection of the patient data under left hemispheric stimulation, in which normals displayed significant correlations, we found that residuals from subjects with right amygdala damage correlated better with normative arousal ratings than did residuals from subjects with left amygdala damage (subliminal, left hemisphere: LTD: r = 0.069, p > 0.70; RTD: r = 0.293, p > 0.15; supraliminal, left hemisphere: LTD: r = -0.069, p > 0.70; RTD: r = 0.186, p > 0.35). Of all subject groups, the one with bilateral amygdala damage always showed negative correlations between SCR residuals and normative arousal ratings in all stimulation conditions, yielding the poorest relationship with the normative ratings (all p > 0.09; the significance test for correlations is nondirectional, leading to trends of significance also for negative correlations).
Subsequently, we wanted to assess whether these nonparametric correlation coefficients differed significantly between the groups. This is done by applying a Fisher Z transformation to the correlation coefficients and calculating the differences between the groups. These difference scores can be then submitted to a Z test for significant departure from a normal distribution. The only significant differences occurred between normal controls and left temporal patients when stimuli were presented to the left hemisphere in the supraliminal presentation (ZNC,LTD = 2.675; p < 0.01), whereas in the subliminal presentation this index only approached significance (ZNC,LTD = 1.937; p < 0.06).
Comparisons between normal controls and right temporal patients under these conditions showed no significant difference between correlation coefficients (subliminal: ZNC,RTD = 0.588, p > 0.25; supraliminal: ZNC,RTD = 0.706, p > 0.20). This supports the observation that right temporal patients performed better than left temporal patients when stimuli were presented to the left hemisphere. However, the direct comparison between right and left temporal patients under left hemispheric processing did not reach a significant threshold (subliminal: ZNC,RTD = 0.485, p > 0.30; supraliminal: ZNC,RTD = 0.732, p > 0.20). Comparisons to bilateral temporal patients could not be applied because of the small sample size of that group.
In a final step, we asked whether the impairments found in the patient group related to the extent of amygdala damage; we omitted bilateral temporal patients from this analysis and used again nonparametric correlations to assess this relationship.
Although none of the correlation coefficients reached significance, we found a noticeable positive relationship between physiological arousal magnitude and postsurgery amygdala volume in left temporal patients in the supraliminal condition (Rsupra = 0.527; p > 0.09); for the subliminal condition, we found an inverse relationship (Rsub = -0.245; p > 0.45). In right temporal patients, the relationships were less pronounced (Rsupra = 0.0; p > 0.95; Rsub = 0.107; p > 0.80). Thus, only in the supraliminal condition was a decreasing physiological response related to smaller amygdala volume. Relating the physiological arousal to the hippocampus volume in the lesioned hemisphere, we found an inverse relationship in left temporal patients in the subliminal condition (Rsub = -0.409; p > 0.20) and a nonsignificant positive correlation in the supraliminal condition (Rsupra = 0.209; p > 0.50). Right temporal patients showed a similar, but more pronounced, pattern (Rsub = -0.75; p < 0.06; Rsupra = 0.25; p > 0.55). Correlation of physiological arousal of stimuli presented to the lesioned hemisphere, and total brain volume revealed nonsignificant negative relationships in patients with left temporal damage (Rsub =-0.200; p > 0.55; Rsupra =-0.200; p > 0.55) and differential correlations in right temporal patients (Rsub = 0.750; p < 0.06; Rsupra = -0.286; p > 0.50).
A separate analysis without the explicitly sexual stimuli confirmed that the effects of arousal were general and not attributable solely the sexual stimuli. This was true for all subject groups, even those in which unbalanced gender proportions might have been of concern (e.g., the RTD group).
Valence dimension
The results of the group-wise regression analyses of normative valence ratings and SCR residuals are shown in Figure 5. Here, only one analysis yielded a significant relationship: subliminal SCR residuals after left hemispheric stimulation in right temporal patients displayed an inverse relationship with the normative valence ratings. All other analyses did not reach statistical significance. These findings on the two dimensions substantiate previous findings by Lang et al. (1993), who claimed that SCR is an index of emotional arousal but not of valence.
Using the same Z test for testing differences between correlation coefficients as above, we found no significant differences between normal controls and left and right temporal patients. Comparisons with bilateral temporal patients could not be applied because of the small sample size of that group. Treating the arousal and valence dimension as binary by confining the statistical test to only the very high and very low arousal stimuli and the very positive and very negative valence stimuli confirmed the results shown here.
Analysis of ratings
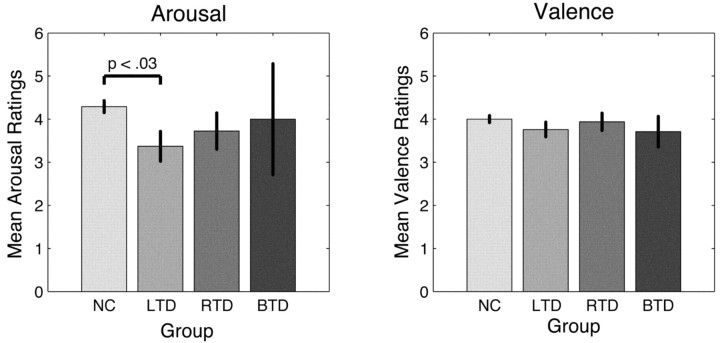
We next analyzed the overt ratings from our subjects. Initially, we compared the mean group ratings of valence and arousal of all three patient groups to the mean ratings of the normal controls. These data are shown in Figure 6. Using the same Welsh test for unequal group variances as in the SCR analysis, we found one significant difference between NC and LTD ratings of arousal (F(1,14.73 = 6.13; p < 0.03).
Figure 6.
Mean group ratings of the stimuli (SEM) on the arousal and valence dimension. Arousal ratings of patients with left temporal damage are significantly lower than those of normal controls.
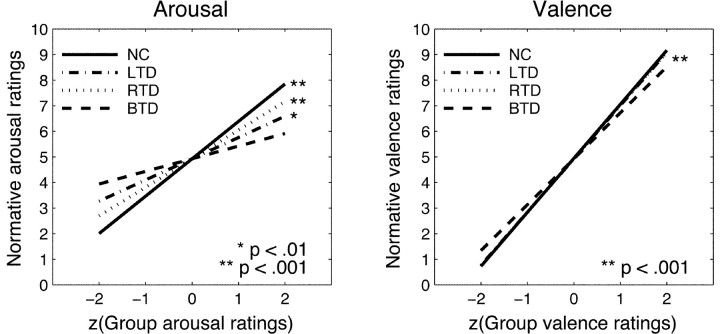
In a second step, we related the group ratings to the normative ratings to see whether we could find impairments in their rating performance that might parallel the finding from the physiological responses. We chose a similar analysis strategy as for the skin conductance responses and regressed the normative ratings of valence and arousal onto the respective mean group ratings. Because of potential gender differences for some of the stimuli, we first analyzed the data separately for each gender but found no significant difference between males and females. Thus, we report collapsed group data here. Results of the group-wise linear regressions are shown in Figure 7. We chose a display in Z scores because it aids the visual evaluation of the graphs by focusing merely on the slope of the regression lines and excluding the constant term.
Figure 7.
Graphs of regression lines relating normative arousal and valence ratings to group arousal and valence ratings. Arousal ratings of normal controls show the closest relationship to normative ratings. Data from unilateral patients still significantly predict normative ratings, but both correlation coefficients are smaller than those in normal controls. Additionally, arousal ratings from right temporal patients relate better to normative ratings than those from left temporal patients who are especially impaired in the high arousal range. Ratings from bilateral temporal patients are most impaired and do not show a significant relationship with normative ratings. The valence ratings of all groups correlate highly significantly with the normative valence ratings, and regression lines, thus, overlap.
Arousal ratings of normal controls showed the closest correspondence to the normative ratings (Fig. 7, left). R coefficients for both unilateral patient groups were smaller than for normals but still significant (NC: r = 0.939, p < 0.001; LTD: r = 0.391, p < 0.05; RTD: r = 0.673, p < 0.001); bilateral temporal patients did not show a significant relationship to the normative arousal ratings (r = 0.252, p > 0.20).
We used the same Z test (after applying Fisher's Z transformation) as in the SCR analysis to test for significant differences between the groups in these nonparametric R coefficients. Only the contrasts between normal controls and left temporal patients yielded significant differences in the coefficients (ZNC,LTD = 2.886; p < 0.01), and the difference between normal controls and right temporal patients approached significance (ZNC,RTD = 1.624; p < 0.06). All other contrasts failed to reach significance, especially the interesting contrast between left and right temporal patients that by visual inspection indicates an advantage for the right temporal patients (r = 0.673 vs r = 0.391 for LTD; ZNC,RTD = 0.541; p > 0.25).
Relating the arousal ratings of unilateral patients to their postsurgery amygdala volume revealed a positive (but nonsignificant) correlation for left temporal patients (r = 0.291; p > 0.35) and a negative correlation for right temporal patients that was approaching significance (r = -0.750; p < 0.06). Similarly, correlations of arousal ratings with postsurgery hippocampus volume were positive but nonsignificant (all p > 0.15), whereas correlations with total brain volume were all negative but nonsignificant (all p > 0.07). Finally, correlations between ratings and time since neurosurgery revealed a positive trend for RTD (r = 0.699; p < 0.06) and equally positive, but nonsignificant, results for LTD (r = 0.174; p > 0.55).
In contrast, there were no impairments in the valence ratings (Fig. 7, right) in any of the patient groups compared with the normative ratings (all R > 0.85; all p < .001).
Taken together, the electrodermal activity and ratings analysis support the idea that skin conductance responses are a measure of stimulus arousal but not valence and that amygdala damage leads to dual impairments in autonomic and cognitive discrimination of stimulus arousal.
Discussion
Summary of findings
We investigated the effect of unilateral and bilateral amygdala damage on SCRs to emotionally arousing stimuli and the cognitive evaluation of stimulus arousal. First, we found a significant decrease in overall SCRs in RTD and BTD patients but normal responsiveness in LTD patients. This suggests an important role for the right amygdala for the production of a general arousal level and is corroborated by Davidson et al. (1992), who found hypoarousability in RTD patients in an arousal/habituation study. However, our data also suggest that additional damage to the left amygdala (as in the BTD patients) exacerbates this impairment.
Second, SCRs in normal controls were significantly predictive of normative ratings of arousal when they were presented subliminally or supraliminally to the left hemisphere (right visual field) but not when presented to the right hemisphere. None of the patient groups showed such a correlation, but, interestingly, patients with an intact left amygdala (RTD patients), like normal controls, showed noticeably better autonomic discrimination of arousal than LTD patients when stimuli were presented to the left hemisphere.
For the valence dimension, we did not find any conclusive patterns in the physiological data, as would be expected given previous findings that SCRs are a measure of arousal, not of valence (Lang et al., 1993).
Lang et al. (1993) also reported gender differences in both ratings and physiological responses. We did not find any gender effects in our study, likely because of small sample sizes and unbalanced gender ratios. However, we verified that the effects we report were not driven by the stimulus class that would be expected to show the largest gender differences, sexually explicit pictures; the findings held even when this category of stimuli was removed from the analysis.
In a third analysis, we investigated cognitive ratings of arousal to the stimuli. We compared the mean arousal and valence ratings of our patients with those of the normal controls and found a significant reduction in arousal ratings in LTD patients. Comparing the group ratings with the normative ratings, we also found that arousal ratings of normal controls were highly correlated with normative ratings, whereas such a correlation was reduced but still significant in patients with unilateral amygdala damage and essentially absent in bilateral amygdala patients. Interestingly, ratings given by RTD patients correlated better than those given by left LTD patients. This matches the reduction in overall arousal ratings in this group compared with normal controls. Impaired cognitive ratings of arousal, thus, depend more on the left amygdala than they do on the right amygdala, exactly the converse of our findings for autonomic measures of arousal described above.
We did not find any differences between the groups in their valence ratings, which were all highly correlated with normative ratings. Thus, amygdala damage does not affect the cognitive evaluation of stimulus valence. We also did not find any correlation between any task measures and the time elapsed since neurosurgery. However, we provide some evidence of a correlation between autonomic arousal and volumetric extent of amygdala resection. These findings reinforce the interpretation that the results we report in subjects with unilateral temporal damage are indeed caused, at least in part, by their amygdala damage.
Taken together, our findings suggest that amygdala damage impairs both the physiological response and the cognitive evaluation to an arousing emotional stimulus. The finding is in line with previous reports that the amygdala plays a critical role in regulating autonomic arousal and in cognitive processing of emotionally arousing stimuli. For instance, Adolphs et al. (1999) found impaired recognition of emotional arousal from facial expressions, words, and sentences but intact recognition of emotional valence. Our data further suggests a predominant role of the left amygdala in the ability to detect stimulus arousal.
Caveats
Our study is limited by the relatively small sample sizes of subjects, a limitation dictated by the rarity of patients with such lesions and the time-intensive nature of our task. In addition to future replication with additional lesion patients, functional imaging studies of the amygdala in normal individuals will be important to corroborate the present findings.
Second, the area under the curve in a specific time window is a measure that potentially combines several different response fluctuations, making it vulnerable to nonspecific and spontaneous responses. However, we recorded 96 responses from each subject altogether (24 stimuli in four blocks), making it unlikely that nonspecific fluctuations would produce any systematic effect.
Third, our stimuli covaried in terms of their perceived arousal and novelty, making it possible that SCRs might, in part, reflect orienting responses driven by the novelty of the stimuli rather than emotional responses driven by their arousal. If novelty would have a systematic effect, we should have observed a significant decrease in responsiveness in the course of the experiment because of multiple stimulus exposures. However, our MANOVA analysis of block order yielded neither a significant main effect of block order nor a significant interaction effect (block × group), thus making it unlikely that our results are merely an orienting response to novel stimuli.
Finally, it is important to point out that the history of epilepsy in subjects with unilateral amygdala damage complicates the interpretation of their performances. In particular, there may be dysfunctions in structures extending beyond those resected during surgery. Nonetheless, the fact that we found a positive correlation between amygdala volume and SCRs in LTD subjects, together with the impairments found in subjects with bilateral amygdala damage (who do not have epilepsy), supports the interpretation that the findings we report are attributable, at least in part, to amygdala damage.
An explanatory model
The specific patterns of impairments in the different patient groups suggest different and complementary roles for the left and right amygdala in the processing of emotional stimuli. Whereas the right amygdala seems to provide an overall level of physiological arousal in response to stimuli (Davidson et al., 1992), the left amygdala provides the better discrimination between different magnitudes of arousal. Our finding for a more prominent role for the left amygdala in processing arousal is consistent with the larger number of imaging studies reporting left rather than right amygdala activation to emotionally arousing stimuli such as facial expressions of fear (Morris et al., 1996; Lane et al., 1997, 1999; Phillips et al., 1998).
Our findings support the following model of emotional information processing. There might be an initial, perhaps automatic and relatively undifferentiated emotional, reaction that is mediated by the right amygdala, followed by a more differentiated emotional reaction that discriminates differences in arousal magnitude mediated by the left amygdala. Such a mechanism would be consistent with the findings of Morris et al. (1998), who reported enhanced activation of the right amygdala after subliminal presentations of emotional facial expression in a conditioning paradigm, whereas the left amygdala showed increased activation when these stimuli were presented supraliminally. Additional support for this model comes from studies investigating habituation in the amygdala (Whalen et al., 1998; Phillips et al., 2001; Wright et al., 2001). These studies demonstrate greater habituation in the right amygdala and more sustained activation in the left amygdala, possibly because of more elaborate linguistic processing (but see Breiter et al., 1996; Wright et al., 2003).
Interestingly, the effects of unilateral and bilateral amygdala damage pertain also to the cognitive evaluation of emotional stimuli. Although the cognitive ratings of stimulus arousal are impaired in bilateral temporal patients (Adolphs et al., 1999), they are largely preserved in patients with unilateral amygdala damage. Consistently, we found evidence for a graded effect that is just like the converse of the physiological findings: patients with RTD performed better on arousal ratings than patients with LTD. This is consistent with a recent imaging study by Phelps et al. (2001), who found that left amygdala activation correlated with SCRs to a cognitive representation of fear. However, other lesions studies have found impairments in the recognition of fear after right, rather than left, amygdala damage (Anderson et al., 2000; Adolphs et al., 2001). Parts of these conflicting results might be explained by task-specific amygdala activation, as recently explored by Hariri et al. (2002). The authors suggest that more complex stimuli (such as IAPS pictures) call for more cognitive/linguistic elaboration and, thus, draw on a left hemisphere network involving the amygdala and cortical language areas. Another explanation might be the nature of the rating task: patients in the studies of Anderson et al. (2000) and Adolphs et al. (2001) were asked to rate the actual emotion in the facial stimulus while we instructed our subjects to focus on arousal and valence on emotional stimuli. Although activated when a cognitive representation of fear elicits SCRs, the left amygdala is evidently not sufficient to mediate such responses in the absence of the right amygdala, pointing to what will likely turn out to be a complex interplay of information processing between the two amygdalae. Future studies that combine functional imaging in patients with unilateral damage may be especially informative in dissecting this architecture.
Our findings may suggest that humans use their internal physiological arousal response, in part to guide their cognitive assessment of these stimuli (Damasio, 1994). The extensive connections between the amygdala and association neocortices (Amaral et al., 1992) might permit such an interplay between emotional autonomic reaction and cognitive evaluation of the stimuli. Our findings suggest that the left amygdala is particularly involved in the representation of emotional arousal of a stimulus, which can then be used to guide cognition and behavior.
Footnotes
This work was supported in part by grants from the National Institutes of Health and the Klingenstein Fund to R.A. We thank M. Karafin for help with testing, T. W. Buchanan for help with data analysis, and D. Tranel for providing neuropsychological evaluation of the subjects.
Correspondence should be addressed to Dr. Jan Gläscher, Neuroimage Nord, Department of Neurology, Building S10, University Hospital Hamburg-Eppendorf, Martinistrasse 52, D-20246 Hamburg, Germany. E-mail: glaescher@uke.uni-hamburg.de.
Copyright © 2003 Society for Neuroscience 0270-6474/03/2310274-09$15.00/0
References
- Adolphs R, Tranel D, Damasio H, Damasio A ( 1994) Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 372: 613-614. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio A ( 1995) Fear and the human amygdala. J Neurosci 15: 5879-5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Russell JA, Tranel D ( 1999) A role for the human amygdala in recognizing emotional arousal from unpleasant stimuli. Psychol Sci 10: 167-171. [Google Scholar]
- Adolphs R, Tranel D, Damasio H ( 2001) Emotion recognition from faces and prosody following temporal lobectomy. Neuropsychology 15: 396-404. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkänen A, Carmichael ST ( 1992) Anatomical organization of the primate amygdaloid complex. In: The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction (Aggleton JP, ed), pp 1-66. New York: Wiley-Liss.
- Anderson AK, Spencer DD, Fulbright RK, Phelps EA ( 2000) Contribution of the anteromedial temporal lobes to the evaluation of facial emotion. Neuropsychology 14: 526-536. [DOI] [PubMed] [Google Scholar]
- Bauer RM ( 1998) Physiological measures of emotion. J Clin Neurophysiol 15: 388-396. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR ( 1995) Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science 269: 1115-1118. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Morris JS, Frith CD, Perrett DI, Dolan RJ ( 1999) Dissociable neural responses to facial expressions of sadness and anger. Brain 122: 883-893. [DOI] [PubMed] [Google Scholar]
- Borod JC ( 1993) Cerebral mechanisms underlying facial, prosodic, and lexical emotional expression: a review of neuropsychological studies and methodological issues. Neuropsychology 7: 445-463. [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BE ( 1996) Response and habituation of the human amygdala during visual processing of facial expression. Neuron 17: 875-887. [DOI] [PubMed] [Google Scholar]
- Broks P, Young AW, Maratos EJ, Coffey PJ, Calder AJ, Isaac CL, Mayes AR, Hodges JR, Montaldi D, Cezayirli E, Roberts N, Hadley D ( 1998) Face processing impairments after encephalitis: amygdala damage and recognition of fear. Neuropsychologia 36: 59-70. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Young AW, Rowland D, Perrett DI, Hodges JR, Etcoff NL ( 1996) Facial emotion recognition after bilateral amygdala damage: differentially severe impairment of fear. Cognit Neuropsychol 13: 699-745. [Google Scholar]
- Critchley HD, Elliott R, Mathias CJ, Dolan RJ ( 2000) Neural activity relating to generation and representation of galvanic skin conductance responses: a functional magnetic resonance imaging study. J Neurosci 20: 3033-3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ ( 2002) Fear conditioning in humans: the influence of awareness and autonomic arousal on functional neuroanatomy. Neuron 33: 653-663. [DOI] [PubMed] [Google Scholar]
- Damasio AR ( 1994) Descartes' error. New York: Putnam.
- Davidson RA, Fedio P, Smith BD, Aureille E, Martin A ( 1992) Lateralized mediation of arousal and habituation: differential bilateral electrodermal activity in unilateral temporal lobectomy patients. Neuropsychologia 30: 1053-1063. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W ( 1999) The functional neuroanatomy of emotion and affective style. Trends Cogn Sci 3: 11-21. [DOI] [PubMed] [Google Scholar]
- Dubois S, Rossion B, Schiltz C, Bodart JM, Michel C, Bruyer R, Crommelinck M ( 1999) Effect of familiarity on the processing of human faces. NeuroImage 9: 278-289. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM ( 1991) The human brain: surface, three-dimensional sectional anatomy with MRI, and blood supply. New York: Springer.
- Engel JJ, Van Ness PC, Rasmussen TB, Ojemann LM ( 1993) Outcome with respect to epileptic seizures. In: Surgical treatment of epilepsies (Engel JJ, ed), pp 609-621. New York: Raven.
- Furmark T, Fischer H, Wik G, Larsson M, Fredrikson M ( 1997) The amygdala and individual differences in human fear conditioning. NeuroReport 8: 3957-3960. [DOI] [PubMed] [Google Scholar]
- Groves PM, Thompson RF ( 1970) Habituation: a dual-process theory. Psychol Rev 77: 419-450. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Hoffman JM, Kilts CD ( 2002) Ecstasy and agony: activation of the human amygdala in positive and negative emotion. Psychol Sci 13: 135-141. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC ( 2000) Modulating emotional responses: effects of a neocortical network on the limbic system. NeuroReport 11: 43-48. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR ( 2002) The amygdala response to emotion stimuli: a comparison of faces and scenes. NeuroImage 17: 317-323. [DOI] [PubMed] [Google Scholar]
- Irwin W, Davidson RJ, Lowe MJ, Mock BJ, Sorenson JA, Turski PA ( 1996) Human amygdala activation detected with echo-planar functional magnetic resonance imaging. NeuroReport 7: 1765-1769. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Sato W, Murai T, Toichi M, Ikeda A, Sengoku A ( 2000) Emotional cognition without awareness after unilateral temporal lobectomy in humans. J Neurosci 20: RC97(1-5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Bradley MM, Lang PJ, Ahern GL, Davidson RJ, Schwartz GE ( 1997) Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia 35: 1437-1444. [DOI] [PubMed] [Google Scholar]
- Lane RD, Chua PM, Dolan RJ ( 1999) Common effects of emotional valence, arousal and attention on neural activation during visual processing of pictures. Neuropsychologia 37: 989-997. [DOI] [PubMed] [Google Scholar]
- Lang PJ ( 1995) The emotion probe. Studies of motivation and attention. Am Psychol 50: 372-385. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Öhman A, Vaitl D ( 1988) The international affective picture set (photographic slides). Gainesville, FL: Center for Research in Psycholophysiology, University of Florida.
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO ( 1993) Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology 30: 261-273. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Taylor SF, Fig LM, Decker LR, Koeppe RA, Minoshima S ( 2000) Limbic activation and psychophysiologic responses to aversive visual stimuli. Interaction with cognitive task. Neuropsychopharmacology 23: 508-516. [DOI] [PubMed] [Google Scholar]
- Maxwell SE, Delaney HD ( 2000) Designing experiments and analyzing data: a model comparison perspective. Mahwah, NJ: Lawrences Erlbaum.
- Merikle PM, Smilek D, Eastwood JD ( 2001) Perception without awareness: perspectives from cognitive psychology. Cognition 79: 115-134. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ ( 1996) A differential neural response in the human amygdala to fearful and happy facial expressions. Nature 383: 812-815. [DOI] [PubMed] [Google Scholar]
- Morris JS, Öhman A, Dolan RJ ( 1998) Conscious and unconscious emotional learning in the human amygdala. Nature 393: 467-470. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O'Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M ( 2001) Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci 4: 437-441. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Scott SK, Calder AJ, Andrew C, Giampietro V, Williams SC, Bullmore ET, Brammer M, Gray JA ( 1998) Neural responses to facial and vocal expressions of fear and disgust. Proc R Soc Lond B Biol Sci 265: 1809-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Medford N, Young AW, Williams L, Williams SC, Bullmore ET, Gray JA, Brammer MJ ( 2001) Time course of left and right amygdalar responses to fearful facial expressions. Hum Brain Mapp 12: 193-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK ( 2000) Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry 47: 769-776. [DOI] [PubMed] [Google Scholar]
- Sokolov EN ( 1963) Perception and the conditioned reflex. Oxford: Pergamon.
- Taylor SF, Liberzon I, Koeppe RA ( 2000) The effect of graded aversive stimuli on limbic and visual activation. Neuropsychologia 38: 1415-1425. [DOI] [PubMed] [Google Scholar]
- Welch BL ( 1938) The significance of the difference between two mean when the population variances are unequal. Biometrika 29: 350-362. [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA ( 1998) Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci 18: 411-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Fischer H, Whalen PJ, McInerney SC, Shin LM, Rauch SL ( 2001) Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. NeuroReport 12: 379-383. [DOI] [PubMed] [Google Scholar]
- Wright CI, Martis B, Schwartz CE, Shin LM, Fischer H, McMullin K, Rauch SL ( 2003) Novelty responses and differential effects of order in the amygdala, substantia nigra, and inferior temporal cortex. NeuroImage 18: 660-669. [DOI] [PubMed] [Google Scholar]