Abstract
Despite identification of >100 potassium channel subunits, relatively little is known about their roles in synaptic transmission. To address this issue we recorded presynaptic potassium currents (IPK) directly from the calyx of Held terminal in brainstem slices of rats. IPK was composed of a 4-aminopyridine (4-AP)-sensitive component and a smaller 4-AP-insensitive component composed of an iberiotoxin-sensitive current and an unidentified slowly activating potassium current. IPK could also be separated into a tetraethylammonium (TEA; 1 mm)-sensitive high-voltage-activated component and a margatoxin (10 nm)-sensitive low-voltage-activated component, which was also blocked by dendrotoxin-I (200 nm) and tityustoxin-Kα (100 nm). In outside-out patches excised from calyceal terminals, TEA (1 mm) consistently and to a large extent attenuated IPK, whereas margatoxin attenuated IPK only in a subset of patches (three of seven). Immunocytochemical examination using Kv subtype-specific antibodies indicated that multiple Kv1 and Kv3 subtypes were present at the calyceal terminal. In paired presynaptic and postsynaptic whole-cell recordings, TEA (1 mm) increased both the duration and peak amplitude of presynaptic action potentials and simultaneously potentiated EPSCs. Margatoxin alone had no such effect but reduced the amount of depolarization required for action potential generation, thereby inducing a burst of spikes when the nerve terminal was depolarized for a prolonged period. Thus, at the calyx of Held terminal, Kv3 channels directly regulate evoked transmitter release, whereas Kv1 channels reduce nerve terminal excitability, thereby preventing aberrant transmitter release. We conclude that both Kv3 and Kv1 channels contribute differentially to maintaining the fidelity of synaptic transmission at the calyx of Held.
Keywords: potassium channel, calyx of Held, transmitter release, Kv1, Kv3, TEA, margatoxin, HVA, LVA
Introduction
Potassium channel blockers increase transmitter release by broadening presynaptic action potentials, indicating that voltage-gated potassium channels in the nerve terminal play a regulatory role in transmitter release (Katz and Miledi, 1969; Augustine, 1990). Voltage-gated potassium currents in the nerve terminal can also mediate synaptic modulation by various signal molecules and drugs (Miura et al., 1992; Lupardus et al., 2000; Daniel and Crepel, 2001; Ishikawa and Takahashi, 2001; Lambe and Aghajanian, 2001). Although >100 different subunits of potassium channels have been identified (Coetzee et al., 1999), the functional roles of presynaptic potassium channel subtypes in regulating transmitter release remain essentially unknown.
Distinct types of potassium currents have been documented at the peripheral and central nerve terminals (Bielefeldt et al., 1992; Sakaba et al., 1997; Sun et al., 1999; Southan and Robertson, 2000). At the cerebellar basket cell nerve terminal, for example, potassium currents can be attenuated by dendrotoxin (DTX) or TEA, suggesting the presence of Kv1 and Kv3 channels in the terminal (Southan and Robertson, 2000); however, neither DTX nor TEA increases action potential-evoked Ca2+ influx into the nerve terminal, implying that they may not play a direct regulatory role in evoked transmitter release (Tan and Llano, 1999; Southan and Robertson, 2000). To clarify the role of a presynaptic potassium channel subtype in synaptic transmission, it is desirable to test the effect of type-specific blockers on both presynaptic potassium currents and postsynaptic responses at the same synapse. The calyx of Held seems ideal for this purpose because simultaneous presynaptic and postsynaptic whole-cell recordings are possible (Borst et al., 1995; Takahashi et al., 1996), and its large structure is also advantageous for immunocytochemical examination of presynaptic proteins (Kajikawa et al., 2001; Saitoh et al., 2001). Our results suggest that Kv3 and Kv1 channels play distinct roles in the regulation of transmitter release at this mammalian fast synapse.
Materials and Methods
Electrophysiology. All experiments were performed in accordance with the guidelines of the Physiological Society of Japan. Transverse brainstem slices (150-200 μm thick) containing the medial nucleus of the trapezoid body (MNTB) region were prepared from Wistar rats, 13-15 d old, killed by decapitation under halothane anesthesia (Forsythe and Barnes-Davies, 1993). MNTB principal cells and calyces were visually identified using a 60× water immersion lens (Olympus Optical, Tokyo, Japan) attached to an upright microscope (Axioskop; Zeiss, Oberkochen, Germany). Each slice was superfused with an artificial CSF (aCSF) containing (in mm): 125 NaCl, 2.5 KCl, 26 NaHCO3, 10 glucose, 1.25 NaH2PO4, 2 CaCl2, 1 MgCl2, 3 myo-inositol, 2 sodium pyruvate, 0.5 ascorbic acid, and 4 lactic acid, pH 7.4 with 95% O2 and 5% CO2. For recording EPSCs, the superfusate routinely contained bicuculline methiodide (10 μm; Sigma, St. Louis, MO) and strychnine hydrochloride (0.5 μm; Sigma) to block inhibitory synaptic responses. The patch pipette solution for postsynaptic recording contained (in mm): 110 CsF, 30 CsCl, 10 HEPES, 5 EGTA, and 1 MgCl2, pH adjusted to 7.3 with CsOH, 290-300 mOsm. N-(2,6-diethylphenylcarbamoylmethyl)-triethyl-ammonium chloride (QX314; 5 mm) was also included in the pipette solution to block action potential generation. The pipette solution for recording potassium currents from calyceal terminals or from outside-out patches contained (in mm): 97.5 potassium gluconate, 32.5 KCl, 10 HEPES, 5 EGTA, 1 MgCl2, 12 Na2 phosphocreatine, 2 ATP-Mg, and 0.5 GTP, pH adjusted to 7.3 with KOH, 290-300 mOsm. In experiments shown in Figure 2, the EGTA concentration was decreased to 0.2 mm. In experiments shown in Figure 4, potassium gluconate (97.5 mm) was replaced by equimolar N-methyl-d-glucamine gluconate to improve the voltage-clamp condition. Tetrodotoxin (TTX; 1 μm) (Wako, Osaka, Japan) was included in the aCSF during the whole-cell recording of potassium currents. For simultaneous presynaptic and postsynaptic whole-cell recordings, potassium glutamate (10 mm) was added to the presynaptic pipette solution by replacing equimolar potassium gluconate, and the EGTA concentration was lowered to 0.5 mm. Drugs were bath-applied by perfusion at 1.5-2 ml/min. TEA and 4-AP were purchased from Nacalai (Kyoto, Japan) and Wako, respectively. Synthetic peptide toxins, iberiotoxin (IbTX), margatoxin (MgTX), DTX-I, and tityustoxin-Kα (TsTX) were purchased from the Peptide Institute (Osaka, Japan).
Figure 2.

Inward and outward currents remaining after 4-AP application. A, Currents evoked by the command pulses (bottom) in the presence of 4-AP (5 mm) (i), 4-AP and IbTX (200 nm) (ii), and 4-AP, IbTX, and CdCl2 (100 μm) (iii). The IbTX-sensitive outward currents (i-ii) and the Cd2+-sensitive inward Ca2+ currents (ii-iii) were obtained by subtraction. EGTA concentration in the presynaptic pipette solution was 0.2 mm in these experiments. B, The I-V relationships of IbTX-sensitive currents (circles) and Ca2+ currents (diamonds) with 0.2 mm (open symbols; n = 5) or 5 mm (filled symbols; n = 6) EGTA in presynaptic pipettes. The current amplitudes are normalized to the total IPK amplitude at +20 mV. C, The effect of TEA (1, 10, 30 mm) on the slow outward currents (iii) remaining in the presence of 4-AP, IbTX, and Cd2+. Sample traces (superimposed) are evoked by a depolarizing pulse to +20 mV. Data points in the I-V relationships are obtained from four experiments.
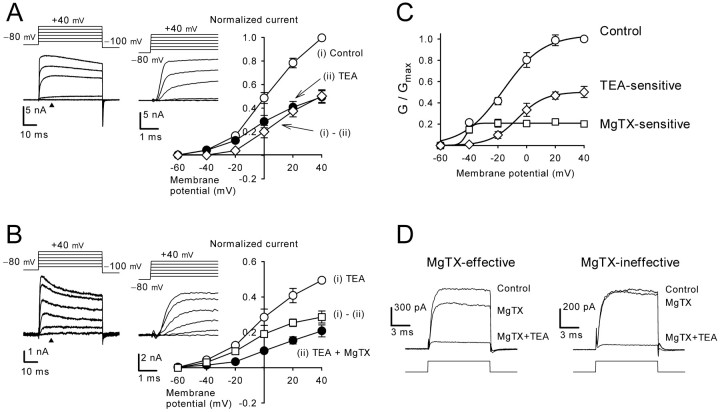
Figure 4.
Potassium currents recorded from calyceal whole terminal with reduced intracellular potassium concentration and those recorded from outside-out patches excised from calyces.A,I-V relationships before (i) and after (ii) application of TEA (1 mm) and TEA-sensitive difference currents (i-ii) in the presence of Cd2+ (100 μm). Sample records of TEA-sensitive currents are shown in the insets at two different time scales. B, I-V relationships before (i) and after (ii) application of MgTX (10 nm) and MgTX-sensitive currents (i-ii) in the presence of TEA (1 mm) and Cd2+ (100 μm). Sample records of MgTX-sensitive currents are shown in the insets at two different time scales. C, Whole-terminal chord conductance of TEA- and MgTX-sensitive currents both normalized to that of the total presynaptic potassium conductance (Control). Fitting curves are drawn according to the Boltzmann equation G = Gmax /{1+exp[(V - V1/2)/k]}, where Gmax was 1.0 for the total current, 0.50 for the TEA-sensitive current, and 0.21 for the MgTX-sensitive current. The half-activation potential (V1/2) was -16 mV for the total current, -6.5 mV for the TEA-sensitive current, and -42 mV for the MgTX-sensitive current. The apparent slope factor (k) was 14 mV for the total current, 9.6 mV for the TEA-sensitive current, and 2.3 mV for the MgTX-sensitive current. D, Potassium currents recorded from outside-out patches excised from calyceal terminals, before and after application of MgTX and addition of TEA (superimposed). MgTX attenuated outward currents in one patch (left) but had no effect in another patch (right). Voltage step was from -80 to 0 mV.
EPSCs were evoked at 0.1 Hz by extracellular stimulation of the presynaptic trapezoid fibers using a tungsten bipolar electrode positioned half-way between the midline and the MNTB, or by presynaptic action potentials elicited directly by a whole-cell pipette under current-clamp mode. Postsynaptic MNTB principal cells were voltage-clamped at the holding potential of -70 mV. EPSCs derived from the calyx of Held synapse were identified as those evoked in an all-or-none manner for graded stimulus intensity and having amplitudes larger than 1 nA at -70 mV (Forsythe and Barnes-Davies, 1993). For recording IPK, calyces or outside-out patches excised from calyceal terminals were voltage-clamped at a holding potential of -80 mV, and depolarizing voltage steps were applied every 15 sec in the presence of TTX (Forsythe, 1994). The access resistance of postsynaptic recordings was 3-15 MΩ (electrode resistance, 1.5-4 MΩ) and compensated by 80%. The presynaptic access resistance was 6-18 MΩ (electrode resistance, 4-6 MΩ) and compensated by 60-80%. Leak currents in whole-cell recordings were subtracted by the scaled pulse (P/8) protocol. Current recordings with a single pipette were made using a patch-clamp amplifier (Axopatch-1D or Axopatch-200B; Axon Instruments, Foster City, CA). Simultaneous presynaptic and postsynaptic recordings were made using a MultiClamp 700A amplifier (Axon Instruments) equipped with a voltage follower having high input impedance. In conventional patch-clamp amplifiers, in current-clamp mode, action potentials can be distorted in shape or associated with artificial potential changes (Magistretti et al., 1996). The records were low-pass filtered at 5-6 kHz and digitized at 20-50 kHz by an analog-to-digital converter (Digidata 1200A or Digidata 1320A) with pClamp8 software (Axon Instruments). The liquid junction potentials between the pipette solution and the aCSF, which were not corrected for, were +10 mV for potassium gluconate solution and -2 mV for N-methyl-d-glucamine gluconate solution (see Fig. 4). All experiments were made at room temperature (23-29°C). Values in the text and figures are given as means ± SEM, and statistical comparisons were made using the Student's paired two-tailed t test unless noted otherwise. Differences were considered significant as p < 0.05.
Immunocytochemistry. Wistar rats (13-15 d old) were anesthetized with Nembutal and transcardially perfused with a fixative (4% paraformaldehyde and 0.1% picric acid in 0.1 m sodium phosphate, pH 7.4). After fixation, rats were decapitated, and tissue blocks of the brainstem including the MNTB region were removed for postfixation for 1 d at 4°C. The fixed tissues were cryoprotected at 4°C in 0.1 m sodium phosphate with sucrose of graded concentrations: in 4% sucrose for 30 min, 10% for 2 hr, 15% for 2 hr, and 20% for overnight. Transverse slices (30 μm in thickness) were cut using a cryostat (CM3050; Leica, Nussloch, Germany) at -21 or -22°C. The sections were then processed for immunocytochemistry as follows: (1) blocking and permeablization in PBS containing 4% skim milk and 0.3% (w/v) Triton X-100 overnight at 4°C, (2) application of primary antibodies in PBS containing 1% (w/v) bovine serum albumin and 0.02% Triton X-100 for 2 d at 4°C, (3) application of secondary antibodies in the same buffer as primary antibodies for 2 d at 4°C, and (4) mounting with ProLong antifade kit (Molecular Probes, Eugene, OR). Kv channels were detected with mouse monoclonal anti-Kv1.1, anti-Kv1.2, and anti-Kv1.6 (Upstate Biotechnology, Lake Placid, NY; diluted 1:100), rabbit polyclonal anti-Kv1.3, anti-Kv1.5, anti-Kv2.1, anti-Kv3.2, and anti-Kv3.4 (Alomone, Jerusalem, Israel; diluted 1:100), or rabbit polyclonal anti-Kv1.4 and anti-Kv3.1b antibodies (Chemicon, Temecula, CA; diluted 1:200). The specificity of the antibodies was confirmed by Western blot analysis using a crude sample of brain homogenate. To define presynaptic terminals, we used rabbit anti-synaptophysin antibody (Zymed, South San Francisco, CA; diluted 1:100) or mouse anti-synaptophysin antibody (Chemicon; diluted 1:200). To visualize the Kv channels and synaptophysin, we used the goat secondary antibodies conjugated with Alexa fluor 488 and Alexa fluor 568 (Molecular Probes; diluted 1:200), respectively. The specificities of Kv channel primary antibodies were evaluated by preabsorption with corresponding antigens (Alomone or Chemicon) for 1 hr at 30°C. Stained sections were viewed with a 100× oil-immersion objective (numerical aperture 1.35) using a confocal laser scanning microscope (Fluoview FV300; Olympus Optical). Emission wavelengths were 510-530 nm (for green) and >610 nm (for red). All of the immunocytochemical procedures were performed at room temperature (22-27°C) unless noted otherwise.
Results
4-AP-sensitive and -insensitive potassium currents at the calyx of Held terminal
In whole-cell voltage-clamp recording from calyceal nerve terminals, sustained outward currents were evoked by voltage steps from a holding potential of -80 mV in the presence of TTX (Fig. 1A). These currents were detectable on depolarization positive to -40 mV, and their amplitude increased steeply for larger voltage steps. These currents had fast activation kinetics with a 10-90% rise time of 0.68 ± 0.15 msec (at +20 mV, n = 6). The potassium channel blocker 4-AP at 5 mm blocked most of these currents (Fig. 1A), as reported previously (Forsythe, 1994; Ishikawa and Takahashi, 2001). Although 4-AP can undergo use-dependent unblock (Campbell et al., 1993), this is unlikely to contribute significantly to our measurements made 10 msec after the onset of depolarization. The effect of 4-AP was concentration dependent, and the IC50 estimated for the currents evoked by a voltage step to -20 mV was 17 μm (Fig. 1B).
Figure 1.
4-AP blocks presynaptic potassium currents. A, Top, IPK evoked by command pulses from -80 mV to various potentials by 20 mV steps (from -60 to +20 mV, top) before (left) and during (right) 4-AP (5 mm) application in the presence of TTX. Bottom, The current-voltage (I-V) relationships of IPK before and during 4-AP application (n = 6). Current amplitudes were measured at 10 msec from the onset of a command pulse (arrowheads). B, Concentration-response relationship for the inhibitory effect of 4-AP on IPK (evoked by depolarization to -20 mV). 4-AP was applied incrementally from low to high concentrations at three to four calyces. A curve fitted to data points was drawn according to the equation y = 1 - (maximal inhibition)/[1 + (IC50 /x)n], where the maximal inhibition was 1.03, IC50 was 17 μm, and the apparent Hill coefficient (n) was 0.66. Ordinate indicates the amplitude of IPK and normalized to control before 4-AP application. Error bars indicate SEMs.
After most of IPK were blocked by 4-AP (5 mm), small currents having inward and outward components remained (Fig. 2A). IbTX (100-200 nm), a large-conductance Ca2+-activated potassium (BK) channel-specific blocker, attenuated these outward currents. The IbTX-sensitive currents obtained by subtraction had a mean rise time (10-90%) of 2.2 ± 0.4 msec (n = 5, at +20 mV with 0.2 mm EGTA in the pipette) (Fig. 2A) and comprised 12 ± 4% (n = 5) of total IPK at +20 mV (Fig. 2B). When the EGTA concentration in the pipette was higher (5 mm), the IbTX-sensitive current was smaller (7 ± 2%; n = 6) (Fig. 2B) and slower (10-90% rise time; 3.9 ± 0.7 msec; n = 6), being consistent with the Ca2+-activated nature of the current. In the presence of 4-AP and IbTX, inward and outward currents still remained. Cd2+ (100 μm) abolished these inward currents, indicating that they were high-voltage-activated (HVA) Ca2+ currents. The maximal amplitude of Ca2+ currents (at 0 mV) corresponded to 8 ± 1% (n = 5) of total IPK at the same voltage (Fig. 2B). In the presence of 4-AP (5 mm), IbTX (100-200 nm), and Cd2+ (100 μm), slowly activating outward currents were discerned above -40 mV. This component comprised 7 ± 1% (with 0.2 mm EGTA, n = 6; or 8 ± 1% in 5 mm EGTA, n = 5) of total IPK at +20 mV (Fig. 2A, iii). The slow activation kinetics of the outward currents (10-90% rise time; 42 ± 2 msec at +20 mV; n = 6) suggests that these derive from channels distinct from those comprising the main component of IPK. TEA attenuated this component in a concentration-dependent manner (Fig. 2C). Thus the voltage-dependent potassium currents at the calyx of Held terminal are composed of a 4-AP-sensitive main component and a much smaller 4-AP-insensitive component comprising BK channel currents and an unidentified slowly activating potassium current.
TEA- and MgTX-sensitive potassium currents
A low concentration of TEA (1 mm) attenuated IPK at the membrane potential above -20 mV (Fig. 3A), suggesting that HVA currents predominate the TEA-sensitive currents. The effect of TEA was reversible after washout (data not shown), and the TEA block was concentration dependent (Fig. 3A). The 4-AP-insensitive BK currents were sensitive to TEA, and slowly activating potassium currents were also partially blocked by 1 mm TEA (Fig. 2). Therefore, TEA-sensitive currents are likely to include these 4-AP-insensitive currents as a minor component. The main TEA-sensitive component has an HVA nature, fast activation kinetics (Fig. 4A), and was sensitive to 4-AP, suggesting that it arises from Kv3 channels (Coetzee et al., 1999).
Figure 3.
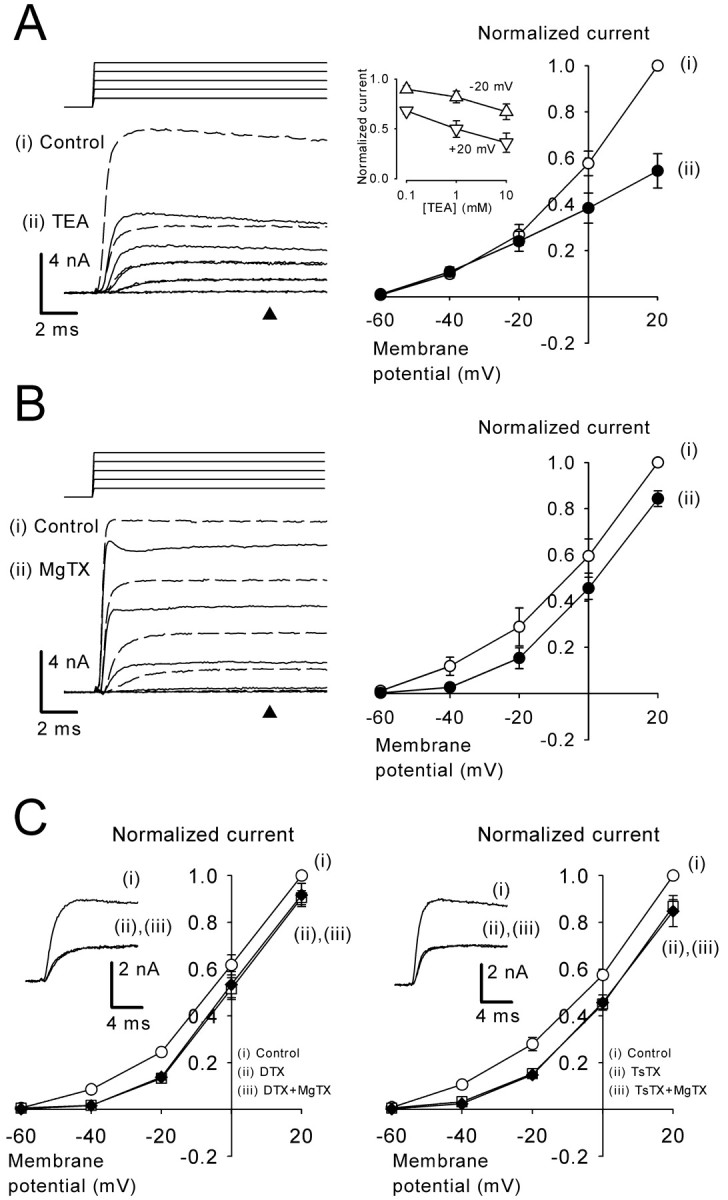
The effect of TEA and Kv1-blocking toxins on presynaptic potassium currents. A, TEA (1 mm) attenuated IPK. Left, sample records of IPK before (i, dashed lines) and after (ii, continuous lines) TEA application. Right, The I-V relationships of IPK (n = 5) before (i) and during (ii) TEA application. Inset graph shows IPK at +20 mV and -20 mV (normalized to control at each potential) at three different concentrations of TEA (logarithmic plot in abscissa). Data are derived from four cells. The current amplitude was measured at 10 msec from the onset of the command pulse (A, B, arrowheads). B, MgTX (10 nm) attenuated IPK. Left, Sample records of IPK before (i, dashed lines) and during (ii, continuous lines) MgTX application. Right, The I-V relationships of IPK (n = 4) before (i) and during (ii) MgTX application. C, DTX-I (200 nm; n = 3) (left panel) or TsTX (100 nm; n = 4) (right panel) occluded the IPK-blocking effect of MgTX. Presynaptic potassium currents before (i) and after (ii, red) application of DTX-I (left panel) or TsTX (right panel) and subsequent additions of MgTX (iii) at -20 mV are shown in the insets (superimposed) and in I-V relationships.
The scorpion peptide MgTX blocks Kv1.3 channels at picomolar concentrations and Kv1.6 channels at nanomolar concentrations (Garcia-Calvo et al., 1993). MgTX may also block Kv1.2 channels given its binding activity to Kv1.2 in rat brain synaptosomes (Knaus et al., 1995). MgTX (10 nm) attenuated IPK (Fig. 3B), with its effect not reversible for at least 30 min after washout. The MgTX-sensitive currents had a low-voltage-activated (LVA) nature, with a clear activation at -40 mV. Similar to MgTX, DTX-I (200 nm), which blocks Kv1.1, 1.2, and 1.6 (Harvey, 1997), also attenuated IPK (Fig. 3C). In addition, TsTX (100 nm), which specifically blocks Kv1.2 (Hopkins, 1998), attenuated LVA IPK, as reported recently (Dodson et al., 2003). In the presence of DTX-I or TsTX, MgTX no longer affected IPK. Because all of these toxins can block Kv1.2 channels, these results suggest that the toxin-sensitive LVA potassium currents may derive from either all homomeric Kv1.2 channels or heteromeric channels containing Kv1.2. Given that Kv1 channels are generally sensitive to 4-AP (Coetzee et al., 1999), these channels are likely to comprise the 4-AP-sensitive main component of IPK, particularly at the low voltage range.
Contribution of Kv3 and Kv1 components to the presynaptic potassium currents
We next examined the relative contribution of Kv3 and Kv1 channels to total IPK after improving the voltage-clamp condition by reducing the potassium concentration in the presynaptic pipette by 75% (see Materials and Methods). We first applied Cd2+ (100 μm) and blocked both Ca2+ channels and Ca2+-activated potassium channels. In the presence of Cd2+, we then applied TEA (1 mm) (Fig. 4A) followed by MgTX (10 nm) in addition to TEA (Fig. 4B). The TEA-sensitive current, which was obtained by subtracting currents in the presence of TEA from control (Fig. 4A), had a fast rise time (10-90%; 0.54 ± 0.05 msec at +20 mV; n = 5) compared with the MgTX-sensitive current (Fig. 4B) (10-90% rise time; 1.2 ± 0.1 msec at +20 mV; n = 4). The activation curve of the TEA-sensitive current (deduced from chord conductance) had an HVA nature with a maximal conductance corresponding to 50 ± 5% of total IPK conductance (at +40 mV; n = 5) (Fig. 4C). In contrast, the MgTX-sensitive current had an LVA nature, with its maximal conductance corresponding to 20 ± 5% (n = 4) of total IPK conductance (Fig. 4C). To examine whether there is a current component sensitive to both TEA and MgTX, in another set of experiments we reversed the order by applying MgTX first, followed by application of TEA and MgTX. The TEA-sensitive conductance measured in the presence of MgTX was 51 ± 9% (at +40 mV; n = 4) of total IPK conductance, which was similar to that obtained in the absence of MgTX (50 ± 5%), suggesting that there is little overlap between the TEA- and the MgTX-sensitive currents.
Although the TEA-sensitive conductance contains a small portion of 4-AP-insensitive, slow-activating currents (Fig. 2C), the above results suggest that ∼50% of IPK derives from Kv3 channels. In outside-out patches excised from the calyceal terminals, TEA attenuated outward currents in all six patches examined (Fig. 4D) on average by 83 ± 3% (at 0 mV), suggesting that Kv 3 is the main potassium channel expressed on the nonsynaptic surface of the calyces. In this regard, it has been reported recently that Kv3.1b-specific immunogold particles are localized on the nonsynaptic side of the calyx of Held terminal (Elezgarai et al., 2003). MgTX (10 nm) attenuated outward currents in three patches by 27 ± 4% on average but had no effect in four other patches (Fig. 4D), suggesting that the density of Kv1 channels at the calyceal terminal is low. Given the substantial contribution of the MgTX-sensitive current to IPK (Fig. 4B), Kv1 channels may also be expressed at other regions, such as the presynaptic axon, as suggested recently (Dodson et al., 2003).
Immunocytochemical examination of potassium channel subunits at the calyx of Held terminal
Using subunit-specific antibodies, we examined which subtypes of Kv1 and Kv3 channels were expressed at the calyceal terminal. To distinguish Kv channel immunofluorescence from nonspecific background signals, the primary antibodies were preabsorbed with corresponding antigens (Fig. 5, bottom panels). Fluorescence intensity was measured using densitometry after background subtraction. Presynaptically localized Kv channels were identified by an overlap with synaptophysin immunofluorescence (Fig. 5) (Kajikawa et al., 2001; Saitoh et al., 2001). At the calyx of Held, clear signals were observed for Kv3.1b and Kv 3.4 (Fig. 5). Although less clear, positive signals were also discerned for Kv1.1, Kv1.2, and Kv1.3. Signals for Kv1.4, Kv1.5, Kv1.6, and Kv 3.2 were marginal, and there was no Kv2.1 immunoreactivity at the terminal. All Kv channels examined were more or less expressed in the somatic region of MNTB neurons. Although the fluorescence intensity depends partially on the quality of antibodies, these results provide a rough idea regarding Kv channel subtypes expressed at the calyx of Held synapse.
Figure 5.

Immunoreactivity of Kv channels at the calyx of Held. Each bottom panel shows the background after absorbing the primary antibodies of Kv channels with corresponding antigens. Ordinate (S/N) in the bar graphs (bottom) indicates the background-subtracted signal intensity divided by the background intensity. Immunofluorescence signals of Kv channels that overlapped with synaptophysin signals were taken as presynaptic signals, and their averaged intensities were determined by densitometric measurements (filled columns). Intensities of somatic signals (open columns) were estimated from signals of Kv channels surrounded by synaptophysin signals excluding nucleus. Each data derived from 16 to 24 cells in three slices.
Effects of potassium channel blockers on EPSCs
We examined whether Kv1 and Kv3 channels play regulatory roles in synaptic transmission by testing the effect of potassium channel blockers on EPSCs. As reported previously (Ishikawa and Takahashi, 2001), 4-AP increased the EPSC amplitude in a concentration-dependent manner (Fig. 6A,B), with a nearly threefold increase at 100 μm (potentiation by 188 ± 35%; n = 9). Above 100 μm, 4-AP caused presynaptic action potential firing (data not shown), which induced EPSC bursts and synaptic depression (Fig. 6A). Similar to 4-AP, TEA potentiated EPSCs in a concentration-dependent manner (Fig. 6C,D), with a maximal potentiation being 99 ± 7% at 1-10 mm (n = 16). Unlike 4-AP, TEA did not cause a burst of EPSCs even at its maximal concentration. Despite the presence of IbTX-sensitive currents in the nerve terminal (Fig. 2), IbTX (200-300 nm) had no effect on EPSCs (data not shown).
Figure 6.
The facilitatory effects of 4-AP and TEA on EPSCs. EPSCs evoked by fiber stimulation were recorded from MNTB neurons at the holding potential of -70 mV. A, Three concentrations of 4-AP (1, 10, 100 μm) were applied incrementally. Note that 100 μm 4-AP caused a synaptic depression during application. Sample records are averaged EPSCs before (a) and during applications of 1 μm (b), 10 μm (c), and 100 μm (d) 4-AP (superimposed). B, The concentration-response relationship for the facilitatory effect of 4-AP on EPSCs (n = 7-11). C, Five concentrations of TEA (0.01-10 mm) were applied incrementally. Sample records are averaged EPSCs during application of 0.01 mm (a), 0.1 mm (b), 3 mm (c), and 10 mm (d) TEA (superimposed). D, The concentration-response relationship for the facilitatory effect of TEA on EPSCs. Data from 6-16 experiments at each concentration are normalized to the EPSC amplitude before TEA application. A curve fitted to data points was drawn according to the equation y = 1 + (maximal increase)/[1 + (EC50 /x)n], where the maximal increase was 1.11, EC50 was 0.22 mm, and the apparent Hill coefficient (n) was 1.13.
To study the mechanism underlying synaptic potentiation by TEA, we made simultaneous presynaptic and postsynaptic recordings and evoked EPSCs by presynaptic action potentials (elicited using 1 msec depolarizing pulses) (Fig. 7). After TEA application (1 mm), presynaptic action potentials became broader, with half-width increasing by 68 ± 3% (n = 5; p < 0.001), and became larger in peak amplitude (by 10 ± 2%; n = 5; p < 0.05). There was a sublinear relationship between the half-width of presynaptic action potential and the amplitude of EPSCs (Fig. 7). TEA had no effect on the presynaptic resting potential (change by 2 ± 1%; n = 5).
Figure 7.
The effect of TEA on presynaptic action potentials and EPSCs in a simultaneous presynaptic and postsynaptic whole-cell recording. Presynaptic action potentials were evoked by a depolarizing pulse of 1 msec in duration. TEA prolonged presynaptic action potential duration and potentiated EPSCs. The half-width of action potential was measured as a duration between 50% rise time and 50% decay time (measured from baseline) of action potentials. Sample records are averaged presynaptic action potentials and EPSCs before (a) and during (b) application of TEA (1 mm) (superimposed). Presynaptic resting potential was -70 mV. Right bottom, The relationship between the half-width of presynaptic action potential and the amplitude of EPSC during TEA application.
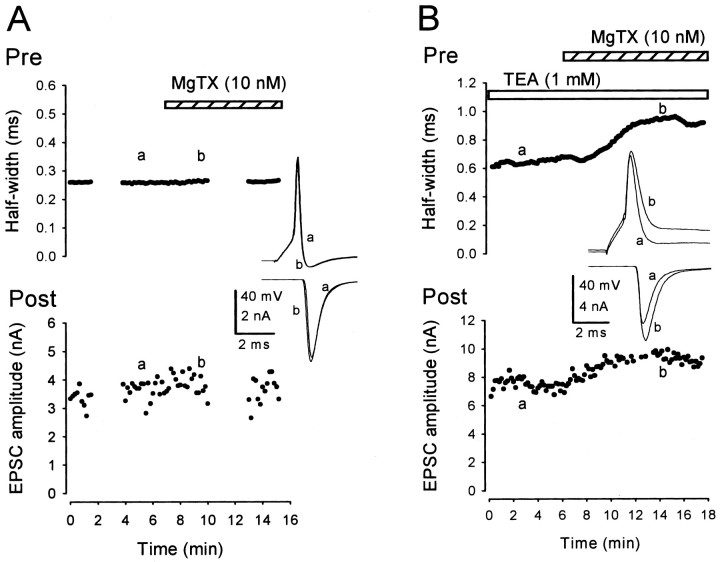
We next examined the effect of MgTX in simultaneous presynaptic and postsynaptic recordings. In contrast to TEA, MgTX had no effect on the amplitude or waveform of presynaptic action potentials or on the EPSC amplitude in simultaneous recordings (change by 3 ± 3%; n = 3) (Fig. 8A), as well as in single postsynaptic recordings (by 3 ± 2%; n = 4). MgTX also had no effect on the presynaptic resting potential (0 ± 1%; n = 5). The lack of effect of MgTX on EPSCs evoked by a presynaptic action potential might arise from the relatively slow activation kinetics of the MgTX-sensitive channel (10-90% rise time 1.2 ± 0.1 msec) (Fig. 4). Because presynaptic action potentials last no longer than 1 msec, the activation time of the MgTX-sensitive channels may not be fast enough to affect presynaptic action potentials. MgTX might then have prolonged EPSCs if presynaptic action potentials had been broadened. This was tested by broadening presynaptic action potentials by TEA (1 mm) (Fig. 8B). MgTX, applied in the presence of TEA, further prolonged presynaptic action potential duration (half-width, by 36 ± 11%; n = 5) and potentiated EPSCs (by 21 ± 7%; n = 5) (Fig. 8 B). MgTX had no effect on the action potential amplitude (2 ± 4%; n = 5). These results suggest that Kv1 channels can regulate transmitter release only when the action potential is broadened.
Figure 8.
The effect of MgTX on the presynaptic action potential and EPSC. A, MgTX (10 nm) had no effect on presynaptic action potential (sample records on top panel) or EPSCs (bottom panel). Presynaptic resting potential was -71 mV. Gaps in the time plot are caused by experiments for Figure 9. B, MgTX (10 nm) applied in the presence of TEA (1 mm) prolonged presynaptic action potential duration and potentiated EPSCs. Presynaptic resting potential was -78 mV. Sample records in A and B are averaged presynaptic action potentials (top panels) and EPSCs (bottom panels) before (a) and during (b) application of MgTX (superimposed).
A prominent effect of MgTX was observed when the nerve terminal was depolarized for a sustained period (Fig. 9). Suprathreshold presynaptic depolarization by a long depolarizing pulse (100 msec) normally evokes a single action potential (Fig. 9A), with the number of action potentials increasing to 3.6 on average (n = 5) by further depolarization (with injection currents up to 240 pA). In the presence of MgTX, however, a sustained presynaptic depolarization induced a burst of action potentials associated with a train of EPSCs of decreasing amplitude (Fig. 9A). MgTX significantly lowered the minimal current required for generating action potential (by 49 ± 5%; n = 5; p < 0.005) (Fig. 9B) and increased the maximum number of action potentials (by 858 ± 362%; n = 5). In contrast to MgTX, TEA (1 mm) did not induce a burst of spikes on prolonged depolarization but slightly reduced the minimal current required for spike generation (by 23 ± 4%; n = 3). These results suggest that the MgTX-sensitive LVA channels play a stabilizing role in the nerve terminal.
Figure 9.
MgTX caused a burst of presynaptic action potentials during sustained depolarization. A, Presynaptic action potentials evoked by a long depolarizing pulse (100 msec) accompanied by EPSCs before (left panel) and during (right panel) application of MgTX (10 nm). Bottom panel, Number of action potentials (ordinate) evoked by depolarizing currents of various intensities (abscissa). Presynaptic resting potential was -71 mV. B, Left panel, The minimal current amplitude to evoke presynaptic action potentials before and during MgTX application. Right panel, The maximum number of action potentials elicited by a depolarizing pulse (100 msec) increased up to 240 pA before and during MgTX application. Data in B derived from three paired recordings and two single presynaptic recordings.
Discussion
Identification of potassium channels expressed at the calyx of Held terminal
At the calyx of Held terminal the voltage-dependent potassium currents have two main components. One is the HVA current sensitive to both 4-AP and TEA. The other is the LVA current sensitive to 4-AP, MgTX, DTX-I, and TsTX. In addition, IPK contained a minor 4-AP-insensitive component including IbTX-sensitive currents and slowly activating potassium currents. Pharmacological properties of the 4-AP-sensitive HVA and LVA currents suggest that they derive mainly from the Kv3 and Kv1 channels, respectively (Coetzee et al., 1999). The results of occlusion experiments suggest that most of the LVA channels are homomeric Kv 1.2 channels or heteromeric channels containing Kv1.2 subunits. In this regard, it has been suggested recently that Kv1.2 homomers coexist with Kv1.1/1.2 heteromers at the presynaptic site of the calyx of Held synapse (Dodson et al., 2003). Recombinant homomeric Kv1.1 has a moderate TEA sensitivity (IC50, 0.3 mm), whereas Kv1.1/1.2 heteromer is >10 times less sensitive to TEA (Hopkins, 1998). This is consistent with our results indicating little overlap between TEA- and MgTX-sensitive currents; however, given multiple Kv1 subunits expressed at the calyceal terminal (Fig. 5), the exact composition of Kv1 channels remains to be determined.
Transcripts for Kv3.1 and 3.3 are expressed in the anterior ventral cochlear nucleus, where the cell body that gives rise to the calyx of Held is located (Weiser et al., 1994; Rudy et al., 1999). Among recombinant channels composed of Kv3 channels, those comprising Kv3.1 have the highest 4-AP sensitivity, with an IC50 of 20-600 μm (Mathie et al., 1998; Coetzee et al., 1999), comparable with that for IPK recorded from the calyx of Held. Although the presence of Kv3.3 is not testable because of a lack of specific antibody, the calyx terminal showed clear immunoreactivities against Kv3.1 and Kv3.4. Thus these channels may together contribute to the TEA-sensitive HVA currents at the calyx of Held presynaptic terminal.
Identification of potassium channels regulating transmitter release at the calyx of Held synapse
Bath application of 4-AP or TEA caused a marked potentiation of EPSCs via broadening presynaptic action potentials (Wang and Kaczmarek, 1998; Ishikawa and Takahashi, 2001). In contrast to the calyx of Held in mice (Wang and Kaczmarek, 1998), TEA significantly increased the peak amplitude of presynaptic action potential, suggesting that the activation of Kv3 channels is fast enough to attenuate the overshoot of presynaptic action potentials in the rat calyceal terminal. MgTX blocked the TEA-insensitive IPK but had no direct effect on basal synaptic transmission. Although it has been reported that recently TsTX causes aberrant action potentials in presynaptic recordings (Dodson et al., 2003), we never saw MgTX alone causing aberrant presynaptic action potentials or aberrant burst of EPSCs, either in the paired recordings or in single presynaptic or postsynaptic recordings. Only after presynaptic action potentials were broadened by TEA did MgTX further prolong their duration and enhance EPSCs. These results suggest that the activation kinetics of Kv1 channels is too slow to affect presynaptic action potentials in a normal condition. Even in the presence of both MgTX and TEA, no aberrant presynaptic firings were observed. Although Kv1 channels do not normally contribute to action potential duration or maintaining resting potential, they raise the threshold level of current required for presynaptic action potential generation. Thus, during a small but sustained depolarization of nerve terminals, the LVA channels are activated, thereby stabilizing the nerve terminal.
The IbTX-sensitive BK channels are expressed in amphibian motor nerve terminals (Robitaille et al., 1993; Yazejian et al., 2000), in chick autonomic nerve terminals (Sun et al., 1999), in fish retinal bipolar cells (Sakaba et al., 1997), in mammalian pituitary cells (Bielefeldt et al., 1992), and at the calyx of Held terminal of rats (Fig. 2). At the amphibian motor nerve terminal, BK channels are activated during Ca2+ entry through nearby voltage-dependent Ca2+ channels, thereby effectively regulating transmitter release (Robitaille et al., 1993). In contrast, at the calyx of Held terminal, the BK channel blocker IbTX had no effect on EPSCs. Because the activation kinetics of presynaptic BK currents (10-90% rise time; 2.2 ± 0.4 msec) is even slower than that of the MgTX-sensitive currents (1.2 ± 0.1 msec), BK channels cannot prolong action potential duration at this mammalian synapse. Similarly, at the hippocampal synapse, IbTX potentiates EPSCs only in the presence of 4-AP (Hu et al., 2001). The faster activation kinetics of BK channel currents at the amphibian neuromuscular junction (Yazejian et al., 2000) and in retinal bipolar cells (Sakaba et al., 1997) might arise from distinct β subunits (Weiger et al., 2000). Thus, in contrast to the amphibian neuromuscular junction, at the mammalian central synapse there is no evidence that BK channels play a regulatory role in transmitter release.
Physiological roles of presynaptic potassium channels at the calyx of Held synapse
HVA Kv3 channels with slow inactivation kinetics ensure reproducible shortenings of presynaptic action potentials. As proposed for the postsynaptic MNTB neurons (Brew and Forsythe, 1995), this is essential for the high-fidelity transmission at the calyx of Held synapse required for the binaural processing of sound-source localization. This contrasts with the pituitary nerve terminals (Jackson et al., 1991) or hippocampal mossy fiber terminal (Geiger and Jonas, 2000), in which fast-inactivating potassium channels broaden action potential duration in an activity-dependent manner, thereby facilitating transmitter release.
The LVA Kv1 channels also contribute to the high-fidelity transmission by preventing nerve terminals from aberrant firings as suggested for the postsynaptic MNTB neurons (Brew and Forsythe, 1995). When nerve terminals are depolarized for a prolonged period, for example during anoxia, Kv1 channels will be activated and stabilize presynaptic terminals, thereby preventing generation of aberrant firings. It remains to be seen whether Kv1 channels shorten presynaptic action potentials in immature animals, which have broader presynaptic action potentials (Taschenberger and von Gersdorff, 2000).
Footnotes
This study was supported by a Grant-in-Aid for Specially Promoted Research from the Ministry of Education, Culture, Sports, Science and Technology. We thank Ian Forsythe and Tetsuhiro Tsujimoto for comments on this manuscript and Peter Strong and Maria Garcia for advice on K channel blockers.
Correspondence should be addressed to Tomoyuki Takahashi, Department of Neurophysiology, University of Tokyo Graduate School of Medicine, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan. E-mail: ttakahas-tky@umin.ac.jp.
W.-B.Li's present address: Department of Pathophysiology, Institute of Basic Medicine, Hebei Medical University, Shijiazhuang 050017, People's Republic of China.
Copyright © 2003 Society for Neuroscience 0270-6474/03/2310445-09$15.00/0
References
- Augustine GJ ( 1990) Regulation of transmitter release at the squid giant synapse by presynaptic delayed rectifier potassium current. J Physiol (Lond) 431: 343-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielefeldt K, Rotter JL, Jackson MB ( 1992) Three potassium channels in rat posterior pituitary nerve terminals. J Physiol (Lond) 458: 41-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst JGG, Helmchen F, Sakmann B ( 1995) Pre- and postsynaptic whole-cell recordings in the medial nucleus of the trapezoid body of the rat. J Physiol (Lond) 489: 825-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew HM, Forsythe ID ( 1995) Two voltage-dependent K+ conductances with complementary functions in postsynaptic integration at a central auditory synapse. J Neurosci 15: 8011-8022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DL, Qu Y, Rasmusson RL, Strauss HC ( 1993) The calcium-independent transient outward potassium current in isolated ferret right ventricular myocytes. II. Closed state reverse use-dependent block by 4-aminopyridine. J Gen Physiol 101: 603-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de Miera E, Rudy B ( 1999) Molecular diversity of K+ channels. Ann NY Acad Sci 868: 233-285. [DOI] [PubMed] [Google Scholar]
- Daniel H, Crepel F ( 2001) Control of Ca2+ influx by cannabinoid and metabotropic glutamate receptors in rat cerebellar cortex requires K+ channels. J Physiol (Lond) 537: 793-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson PD, Billups B, Rusznak Z, Szucs G, Barker MC, Forsythe ID ( 2003) Presynaptic Kv1.2 channels suppress synaptic terminal hyperexcitability following action potential invasion. J Physiol (Lond) 550: 27-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elezgarai I, Diez J, Puente N, Azkue JJ, Benitez R, Bilbao A, Knopfel T, Donate-Oliver F, Grandes P ( 2003) Subcellular localization of the voltage-dependent potassium channel Kv3.1b in postnatal and adult rat medial nucleus of the trapezoid body. Neuroscience 118: 889-898. [DOI] [PubMed] [Google Scholar]
- Forsythe ID ( 1994) Direct patch recording from identified presynaptic terminals mediating glutamatergic EPSCs in the rat CNS, in vitro. J Physiol (Lond) 479: 381-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe ID, Barnes-Davies M ( 1993) The binaural auditory pathway: excitatory amino acid receptors mediate dual time-course excitatory postsynaptic currents in the rat medial nucleus of the trapezoid body. Proc R Soc Lond B Biol Sci 251: 151-157. [DOI] [PubMed] [Google Scholar]
- Garcia-Calvo M, Leonard RJ, Novick J, Stevens SP, Schmalhofer W, Kaczorowski GJ, Garcia ML ( 1993) Purification, characterization, and biosynthesis of margatoxin, a component of Centruroides margaritatus venom that selectively inhibits voltage-dependent potassium channels. J Biol Chem 268: 18866-18874. [PubMed] [Google Scholar]
- Geiger JPR, Jonas P ( 2000) Dynamic control of presynaptic Ca2+ inflow by fast-inactivating K+ channels in hippocampal mossy fiber boutons. Neuron 28: 927-939. [DOI] [PubMed] [Google Scholar]
- Harvey AL ( 1997) Recent studies on dendrotoxins and potassium ion channels. Gen Pharmacol 28: 7-12. [DOI] [PubMed] [Google Scholar]
- Hopkins WF ( 1998) Toxin and subunit specificity of blocking affinity of three peptide toxins for heteromultimeric, voltage-gated potassium channels expressed in Xenopus oocytes. J Pharmacol Exp Ther 285: 1051-1060. [PubMed] [Google Scholar]
- Hu H, Shao L-R, Chavoshy S, Gu N, Trieb M, Behrens R, Laake P, Pongs O, Knaus HG, Ottersen OP, Storm JF ( 2001) Presynaptic Ca2+-activated K+ channels in glutamatergic hippocampal terminals and their role in spike repolarization and regulation of transmitter release. J Neurosci 21: 9585-9597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Takahashi T ( 2001) Mechanisms underlying presynaptic facilitatory effect of cyclothiazide at the calyx of Held of juvenile rats. J Physiol (Lond) 533: 423-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB, Konnerth A, Augustine GJ ( 1991) Action potential broadening and frequency-dependent facilitation of calcium signals in pituitary nerve terminals. Proc Natl Acad Sci USA 88: 380-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajikawa Y, Saitoh N, Takahashi T ( 2001) GTP-binding protein βγ subunits mediate presynaptic calcium current inhibition by GABAB receptor. Proc Natl Acad Sci USA 98: 8054-8058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Miledi R ( 1969) Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol (Lond) 203: 459-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus H-G, Koch ROA, Eberhart A, Kaczrowski GJ, Garcia ML, Slaughter RS ( 1995) [125I]Margatoxin, an extraordinarily high affinity ligand for voltage-gated potassium channels in mammalian brain. Biochemistry 34: 13627-13634. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Aghajanian GK ( 2001) The role of Kv1.2-containing potassium channels in serotonin-induced glutamate release from thalamocortical terminals in rat frontal cortex. J Neurosci 21: 9955-9963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupardus PJ, Wilke RA, Aydar E, Palmer CP, Chen Y, Ruoho AE, Jackson MB ( 2000) Membrane-delimited coupling between sigma receptors and K+ channels in rat neurohypophysial terminals requires neither G-protein nor ATP. J Physiol (Lond) 526: 527-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti J, Mantegazza M, Guatteo E, Wanke E ( 1996) Action potentials recorded with patch-clamp amplifiers: are they genuine? Trends Neurosci 19: 530-534. [DOI] [PubMed] [Google Scholar]
- Mathie A, Wooltorton JRA, Watkins CS ( 1998) Voltage-activated potassium channels in mammalian neurons and their block by novel pharmacological agents. Gen Pharmacol 30: 13-24. [DOI] [PubMed] [Google Scholar]
- Miura M, Belvisi MG, Stretton CD, Yacoub MH, Barnes PJ ( 1992) Role of K+ channels in the modulation of cholinergic neural responses in guinea-pig and human airways. J Physiol (Lond) 455: 1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille R, Garcia ML, Kaczorowski GJ, Charlton MP ( 1993) Functional colocalization of calcium and calcium-gated potassium channels in control of transmitter release. Neuron 11: 645-655. [DOI] [PubMed] [Google Scholar]
- Rudy B, Chow A, Lau D, Amarillo Y, Ozaita A, Saganich M, Moreno H, Nadal MS, Hernandez-Pineda R, Hernandez-Cruz A, Erisir A, Leonard C, Vega-Saenz de Miera E ( 1999) Contributions of Kv3 channels to neuronal excitability. Ann NY Acad Sci 868: 304-343. [DOI] [PubMed] [Google Scholar]
- Saitoh N, Hori T, Takahashi T ( 2001) Activation of the epsilon isoform of protein kinase C in the mammalian nerve terminal. Proc Natl Acad Sci USA 98: 14017-14021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaba T, Ishikane H, Tachibana M ( 1997) Ca2+-activated K+ current at presynaptic terminals of goldfish retinal bipolar cells. Neurosci Res 27: 219-228. [DOI] [PubMed] [Google Scholar]
- Southan AP, Robertson B ( 2000) Electrophysiological characterization of voltage-gated K+ currents in cerebellar basket and Purkinje cells: Kv1 and Kv3 channel subfamilies are present in basket cell nerve terminals. J Neurosci 20: 114-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X-P, Schlichter LC, Stanley EF ( 1999) Single-channel properties of BK-type calcium-activated potassium channels at a cholinergic presynaptic nerve terminal. J Physiol (Lond) 518: 639-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Forsythe ID, Tsujimoto T, Barnes-Davies M, Onodera K ( 1996) Presynaptic calcium current modulation by a metabotropic glutamate receptor. Science 274: 594-597. [DOI] [PubMed] [Google Scholar]
- Tan YP, Llano I ( 1999) Modulation by K+ channels of action potential-evoked intracellular Ca2+ concentration rises in rat cerebellar basket cell axons. J Physiol (Lond) 520: 65-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschenberger H, von Gersdorff H ( 2000) Fine-tuning an auditory synapse for speed and fidelity: developmental changes in presynaptic waveform, EPSC kinetics, and synaptic plasticity. J Neurosci 20: 9162-9173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L-Y, Kaczmarek LK ( 1998) High-frequency firing helps replenish the readily releasable pool of synaptic vesicles. Nature 394: 384-388. [DOI] [PubMed] [Google Scholar]
- Weiger TM, Holmqvist MH, Levitan IB, Clark FT, Sprague S, Huang W-J, Ge P, Wang C, Lawson D, Jurman ME, Glucksmann MA, Silos-Santiago I, DiStefano PS, Curtis R ( 2000) A novel nervous system β subunit that downregulates human large conductance calcium-dependent potassium channels. J Neurosci 20: 3563-3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser M, Vega-Saenz de Miera E, Kentros C, Moreno H, Franzen L, Hillman D, Baker H, Rudy B ( 1994) Differential expression of Shaw-related K+ channels in the rat central nervous system. J Neurosci 14: 949-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazejian B, Sun X-P, Grinnell AD ( 2000) Tracking presynaptic Ca2+ dynamics during neurotransmitter release with Ca2+-activated K+ channels. Nat Neurosci 3: 566-571. [DOI] [PubMed] [Google Scholar]