Abstract
The role of the ventral subiculum in cocaine- or cue-induced cocaine-seeking behavior was investigated in rats tested on a between-session reinstatement model. Rats were trained to self-administer cocaine (0.25 mg/infusion, i.v.) in a lever-pressing operant task in a daily 2 hr session. Responding was reinforced contingent on a modified fixed-ratio 5 schedule. Reinstatement tests began after the lever-pressing behavior was extinguished in the absence of cocaine and conditioned cues (light and tone). Bilateral microinjections of lidocaine (100 μg) into the ventral subiculum decreased cocaine- or cue-induced reinstatement of cocaine-seeking behavior compared with saline microinjections into the same area in another group of rats. Lidocaine microinjections, however, had no effect on cocaine self-administration behavior or food-maintained or food-reinstated responding. Collectively, these results suggest that the ventral subiculum plays an important role in cocaine-seeking behavior. Considering the role of this structure in context learning, our data suggest that the full expression of cocaine- or cue-induced reinstatement may depend on the context in which the cocaine experience occurred.
Keywords: cocaine, self-administration, reinstatement, context, conditioned stimuli, ventral subiculum, mesocorticolimbic circuitry
Introduction
A major difficulty in treating addiction to cocaine, amphetamine, and related stimulants is the high rate of relapse even after long periods of abstinence (Washton and Stone-Washton, 1990; O'Brien and McLellan, 1996). In fact, prevention of relapse has now been recognized as a central part of any successful treatment program. Relapse appears to be triggered by several factors, including stress (Kosten et al., 1986; Kreek and Koob, 1998; Sinha et al., 2000), conditioned stimuli (Childress et al., 1988, 1993; Carter and Tiffany, 1999), and re-exposure to drug (Jaffe et al., 1989). Understanding these factors and their underlying neural mechanisms is critical for developing effective treatments. Substantial progress has been made in identifying the neural circuitry responsible for cocaine-seeking behavior. For example, using neural imaging techniques such as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI), several clinical studies have shown that conditioned stimuli (CS) previously paired with cocaine consistently increase metabolic activity in anterior cingulate and orbitofrontal cortices and amygdala in cocaine addicts (Grant et al., 1996; Maas et al., 1998; Childress et al., 1999; Kilts et al., 2001). Moreover, although cocaine itself increases metabolic activity in many brain regions, the nucleus accumbens (NAcc), subcallosal cortex, right parahippocampus, and lateral prefrontal cortex seem to be involved in craving (Breiter et al., 1997). Taken together, these results suggest that the neural circuitry underlying cocaine-seeking behavior involves a limited number of brain structures.
This conclusion is consistent with the wealth of data available on the reinstatement of drug-seeking behavior in experimental animals. In the reinstatement paradigm, rats with a history of cocaine self-administration are assessed for resumption of extinguished lever pressing after re-exposure to the drug itself or to the cues previously associated with the drug. At the molecular level, reinstatement has been associated with changes in Fos, an immediate early gene, and γ protein kinase C, a plasticity-regulated gene, in anterior cingulate, basolateral amygdala (BLA), hippocampus, and NAcc core and shell (Neisewander et al., 2000; Ciccocioppo et al., 2001; Thomas and Everitt, 2001). All these structures receive dopamine (DA) innervation from the ventral tegmental area (VTA) and together form a complex motive circuit implicated in drug-seeking behavior (See, 2002; Shalev et al., 2002; Kalivas and McFarland, 2003). Manipulations of individual circuit components confirm this role. Inactivation of VTA, BLA, or prefrontal cortex, including anterior cingulate, for example, blocks either cue- or cocaine-induced reinstatement or both in rats (Grimm and See, 2000; Kruzich and See, 2001; McFarland and Kalivas, 2001; See, 2002).
Another likely component of the motive circuit is the ventral subiculum (vSUB), an extension of ventral hippocampus known to play a role in goal-directed behavior. Lesions of vSUB, for example, have been shown to block the potentiating effects of intra-NAcc amphetamine on operant responding reinforced by CS previously paired with sucrose reinforcement (Everitt et al., 2001). Electrical stimulation of vSUB, moreover, reinstates cocaine- or amphetamine-seeking behavior in rats (Vorel et al., 2001; Taepavarapruk and Phillips 2003). To assess the role of vSUB in reinstatement, we monitored cocaine- or CS-induced drug-seeking behavior in rats during temporary inactivation of vSUB by local injections of lidocaine.
Materials and Methods
Subjects. Male Sprague Dawley rats (330-500 gm), bred from animals supplied by Harlan Industries (Indianapolis, IN), were obtained from the departmental breeding colony. They were housed individually in hanging wire cages in a temperature- and humidity-controlled colony room on a 12 hr light/dark cycle (lights on at 7:30 A.M.). One week before operant training rats were placed on a restricted diet to reach ∼85% of free-feeding weight. After training, ad libitum access to food was available for 1 week before and after surgery to ensure presurgical and postsurgical health. Food restriction was then reinstated to maintain 85-90% of free-feeding weight throughout the experiments. Water was available ad libitum all the time. The experiments were conducted in the light cycle (between 9:00 A.M. and 9:00 P.M.). All procedures followed the National Institute of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the Indiana University Animal Care and Use Committee.
Test chambers. Animals were tested in four standard operant chambers (30 × 24 × 21 cm). Each was equipped with one lever, two cue lights (1 W), and a food cup on a side wall. The lever was located 9 cm above the grid floor in the middle of the wall. The cue lights were located 11 cm above the grid floor and were spaced 21 cm apart. The food cup was located below the lever. A house light (5 W) was installed on the back wall outside the chamber. A programmable audio speaker, food dispenser, and fluid pump were installed outside the chamber. Each chamber and its accessories were housed in sound- and light-attenuating cubicles equipped with a fan to mask extraneous noises (Med Associates Inc., East Fairfield, VT).
Operant training. Rats were first trained to press the lever contingent on a fixed-ratio 1 (FR 1) food reinforcement schedule. Each lever press resulted in delivery of one 45 mg sucrose pellet (Research Diet, New Brunswick, NJ) followed by a 10 sec time-out signaled by illumination of the house light. After lever-pressing behavior was established, the reinforcement schedule gradually increased to FR 5, and training continued until rats obtained 60 food pellets within 30 min.
Surgery. Rats were first given atropine (0.05 mg/kg, s.c.) and then anesthetized with ketamine (80 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.). For the cocaine self-administration group, a catheter constructed from PE10 and PE50 tubing (Fisher Scientific, Pittsburgh, PA) was inserted into the right jugular vein as described elsewhere (Caine and Koob, 1994). The PE50 end was inserted over metal tubing of a guide cannula (22 gauge; Plastics One, Roanoke, VA), which was bent into a right angle. The guide cannula was threaded under the skin and exited at the scapula of the skull. After catheterization, rats were fixed in a stereotaxic apparatus. Two guide cannulas (26 gauge; Plastics One) were bilaterally implanted 2 mm above the vSUB [vSUB coordinates: anteroposterior (AP): -6.3 mm, mediolateral (ML): ±5.2 mm, and dorsoventral (DV): -7.6 mm relative to bregma, midline and skull surface, respectively]. Four stainless steel screws were implanted in the skull for support. The cannulas and screws were held in place with dental cement. An obturator (Plastics One) was inserted into each guide cannula to prevent blockage. For the food self-administration group, the procedure for surgery was the same except that rats were not catheterized. After surgery, all rats were allowed to recover for 1 week during which 0.1 ml (10 mg/ml, i.v.) of gentamycin (Biowhitaker, Walkersville, MD) was given daily, and the catheter was flushed twice a day with heparinized physiological saline (30 U/ml heparin).
Cocaine and food self-administration training. For cocaine self-administration, rats were trained to press the lever for cocaine in a daily 2 hr session. The first response resulted in an infusion of 0.25 mg of cocaine in a volume of 0.05 ml accompanied by the CS, which consisted of two flashing stimulus lights and a tone for 6 sec. Delivery of the CS was followed by a 14 sec time-out period signaled by illumination of the house light. During cocaine infusions and time-outs responding was recorded but had no programmed consequences. After the first infusion, cocaine infusions and presentations of the CS were contingent on an FR 5 schedule. The session ended when 2 hr passed or 30 infusions of cocaine were delivered, whichever occurred first. The training continued until stable responding was established (the number of cocaine infusions varied by <20% in three consecutive training sessions). For food self-administration, rats were trained under an FR 5 reinforcement schedule in a daily 30 min session. Every fifth response was reinforced by one food pellet followed by a 10 sec time-out signaled by illumination of the house light. The training lasted until the response rate did not vary by >20% in three consecutive training sessions. Casual observations of the animals also were made during the self-administration, extinction, and reinstatement sessions to monitor motor behavior.
Extinction. Before extinction training, rats were tested for the effects of vSUB lidocaine or saline on cocaine or food self-administration behavior. After the test, self-administration training continued for 1 or 2 d, and thereafter, extinction training began in a daily 30 or 60 min session for food and cocaine self-administration groups, respectively. During each extinction session, responding was recorded but had no programmed consequences. Extinction training lasted 6 or 7 d, and by the end of the training, responding was ∼20% of the level observed in self-administration sessions.
Experiment 1: effects of lidocaine inactivation of vSUB on cocaine self-administration behavior. Two groups of rats received bilateral microinjections of either lidocaine (100 μg/0.5 μl/side) or physiological saline (0.5 μl/side). This dose of lidocaine has been shown to decrease cocaine- and cue-induced reinstatement when microinjected into amygdala (Kantak et al., 2002). Microinjections were delivered through 33 gauge injection cannulas that extended 2 mm below the guide cannula (Plastics One) by an infusion pump (Harvard Apparatus, Holliston, MA) over a 1 min period, and the injection cannula stayed in place for another minute to ensure drug diffusion. After the microinjections, rats were immediately put into the chambers, and the session started 1 min later. The reinforcement schedule was exactly the same as that in the cocaine self-administration training sessions.
Experiment 2: effects of lidocaine inactivation of vSUB on CS-induced reinstatement. After extinction training, rats that received lidocaine in experiment 1 were microinjected with saline (saline group), and the saline rats in experiment 1 received lidocaine in this and subsequent experiments (lidocaine group). The microinjection procedure and lidocaine dose were the same as in experiment 1. One minute after the microinjections the session started with a noncontingent presentation of the CS over a 6 sec period followed by a 14 sec time-out as in the cocaine self-administration training sessions. Responding was reinforced by the CS alone, contingent on an FR 5 schedule except that the first response was reinforced by the CS. The session lasted 60 min.
Experiment 3: effects of lidocaine inactivation of vSUB on cocaine-induced reinstatement. Nine of the 10 rats in experiment 2 plus another rat were tested for the effects of vSUB lidocaine on cocaine-induced reinstatement. Two reinstatement tests were conducted. In the first, rats received cocaine (10 mg/kg, i.p.) without any vSUB pretreatment. In the second, rats received microinjections of lidocaine (100 μg/0.5 μl/side) or saline (0.5 μl/side) into vSUB immediately before the cocaine injections. The sessions started 3 min after cocaine injections and lasted 60 min. Responding was reinforced only by the CS contingent on an FR 5 schedule. Extinction training continued for 2-3 d between each test.
Experiment 4: effects of lidocaine inactivation of vSUB on food self-administration behavior. Before the test session, rats received microinjections of the same dose of lidocaine or saline into vSUB as in experiments 1-3 (within-subject design). Each session began 3 min after the microinjection and lasted 30 min. The reinforcement contingency was the same as in the food self-administration training sessions. The order of microinjections of lidocaine and saline was counterbalanced among the rats. Food self-administration training continued for 2-3 d between each test.
Experiment 5: effects of lidocaine inactivation of vSUB on food-induced reinstatement. The role of the vSUB in food-induced reinstatement was investigated after rats in experiment 4 went through extinction training. The same dose of lidocaine or saline was microinjected into vSUB using the same procedures as above, and again the order of microinjections was counterbalanced among the rats. Extinction training was conducted between the reinstatement sessions (2-3 d). The reinstatement session started with a noncontingent delivery of a train of three food pellets spaced 10 sec apart, during which the house light was illuminated. Thereafter, the same train was delivered every 5 min. Responding had no programmed consequences during the session, which lasted 30 min.
Histology. After the experiments, rats were deeply anesthetized with chloropent (3.3 ml/kg) and were transcardially perfused with saline followed by formalin (10%). The brains were removed, soaked in formalin for at least 24 hr, sliced at 60 μm thickness, mounted on gelatin-coated slides, and stained with cresyl violet. The positions of injection cannula were inspected under a light microscope.
Drugs. Cocaine hydrochloride was obtained from the National Institute on Drug Abuse (Bethesda, MD). Lidocaine hydrochloride was purchased from Sigma (St. Louis, MO). Both drugs were dissolved in physiological saline. The drug concentrations (salt) for cocaine and lidocaine were 5 and 200 mg/ml, respectively.
Statistics. Responding was recorded during the self-administration, extinction, and reinstatement sessions. Response rates were calculated as responses per hour for the cocaine- or CS-induced reinstatement for the cocaine group and as responses per minute for the food group. For the drug self-administration group, a non-repeated two-way ANOVA (treatments × test sessions) was conducted because of missing values in either of the treatment groups. The group means were then compared with Bonferroni post-test. For the food group, a repeated one-way ANOVA was used for data analysis; mean responding rates for different treatments were compared with Tukey's test.
Results
Histology
The positions of all microinjection sites are shown schematically in Figure 1. All placements were either entirely within the vSUB or on the border immediately adjacent to the CA1 region of hippocampus.
Figure 1.
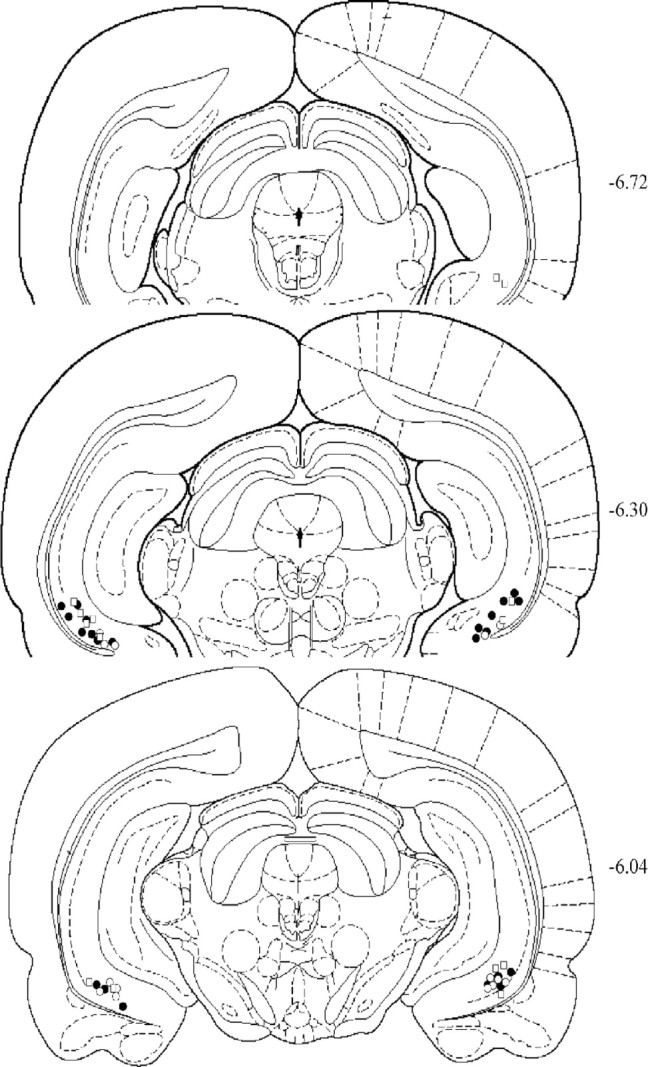
Schematic representations of injection sites in vSUB. Coronal brain section images were adapted from the atlas of Paxinos and Watson (1998). Filled circles and open circles represent the injection sites in saline and lidocaine groups, respectively. The injection sites for the food-training group are represented by open squares.
Experiment 1: effects of lidocaine inactivation of vSUB on cocaine self-administration behavior
After cocaine self-administration behavior stabilized, we assessed the effects of vSUB microinjections of lidocaine or saline on this behavior. As shown in Table 1, pre- and post-microinjection responding was similar in both groups. After a lever press, animals showed a rapid burst of grooming (face washing) and often engaged in repetitive head bobbing in one corner of the cage until they returned to the lever for the next press.
Table 1.
Effects of lidocaine inactivation of vSUB on cocaine self-administration behavior
|
|
Responding (responses/2 hr)
|
||
|---|---|---|---|
| Group
|
Control
|
Pretreatment
|
|
| Saline (n = 7) | 94 ± 4 | 71 ± 13 | |
| Lidocaine (n = 6)
|
113 ± 11
|
105 ± 14
|
|
Data are group mean ± SEM. Control data (no vSUB infusions) were from the cocaine self-administration sessions before the pretreatment sessions. Responding was reinforced contingent on an FR 5 schedule except that the first cocaine infusion was delivered after the first response. The two groups showed similar baseline responding. The responding after vSUB lidocaine (100 μg/side) was similar to that after vSUB saline (Tukey's test; p > 0.05).
Experiment 2: effects of lidocaine inactivation of vSUB on CS-induced reinstatement
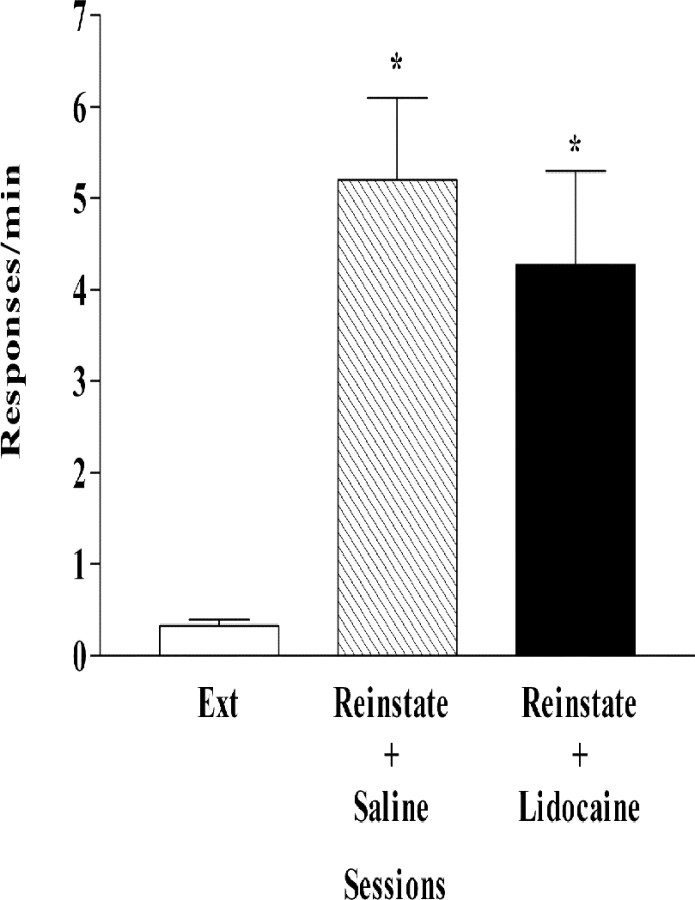
One week after extinction training, CS-induced reinstatement tests were conducted in rats pretreated with either saline or lidocaine. The mean response rates on the last day of self-administration and extinction training for the saline and lidocaine groups were comparable, but as shown in Figure 2, the response rate after vSUB lidocaine was significantly lower than the response rate for rats pretreated with vSUB saline. The pattern of responding for representative rats in each group is shown in Figure 3.
Figure 2.
Effects of bilateral microinjections of lidocaine (100 μg/side) or saline (0.5 μl/side) into vSUB on CS-reinstated responding. The session began with a noncontingent delivery of the CS followed by a time-out as in the cocaine self-administration training sessions. Responding was reinforced only by the CS contingent on an FR 5 schedule in a 1 hr session, except for the first response, which was reinforced by CS. Open and closed bars indicate data obtained from rats assigned to the saline and lidocaine groups, respectively. SA indicates the last day of the cocaine self-administration. Ext indicates the extinction session before reinstatement. Reinstate+Microinjection indicates the reinstatement session in which saline or lidocaine was microinjected into vSUB. Data are represented as mean ± SEM responses per hour. Reinstatement responding was significantly decreased by lidocaine (n = 10) compared with the saline microinjections (n = 6) (Bonferroni test; p < 0.05).
Figure 3.
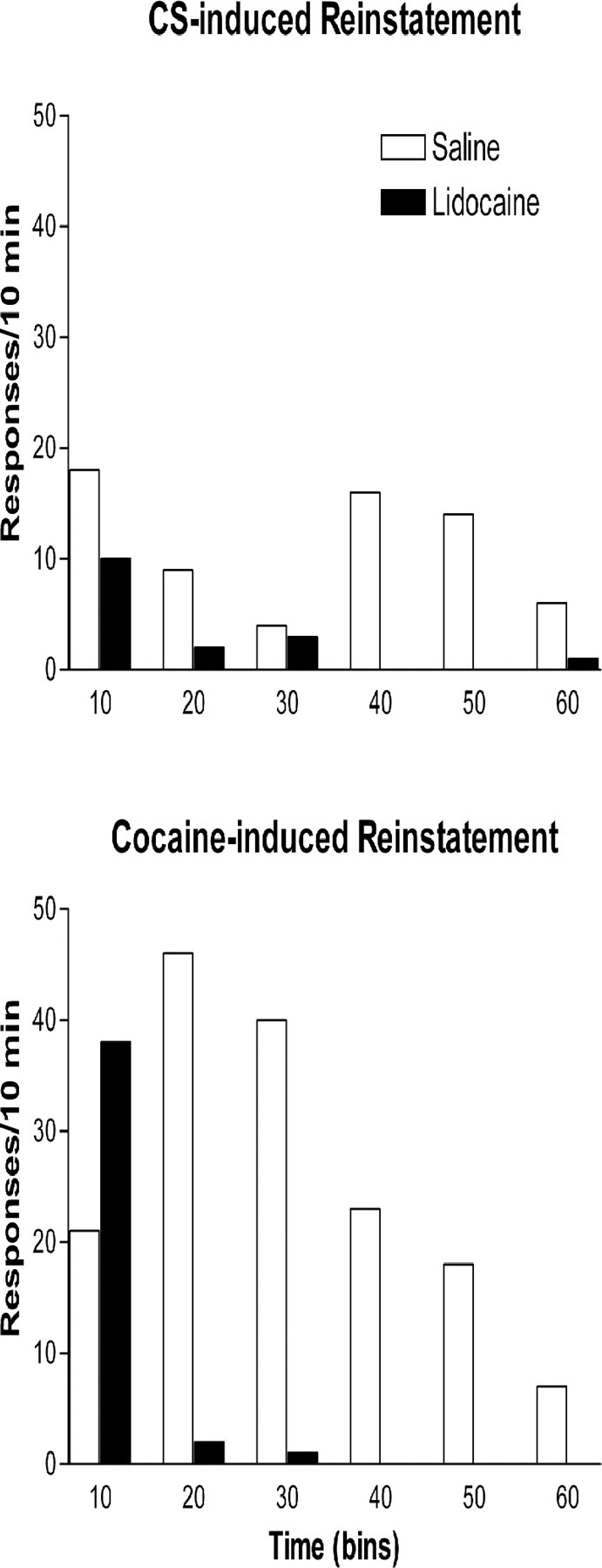
Representative records of lever presses by individual rats during the CS (top) or cocaine reinstatement session (bottom) after vSUB microinfusion of saline or lidocaine as indicated in the legend. The 1 hr reinstatement session was divided into six 10 min bins. In both sessions, responding was reinforced only by the CS. Note that reduced responding to vSUB lidocaine was evident in both sessions by the second bin.
Experiment 3: effects of lidocaine inactivation of vSUB on cocaine-induced reinstatement
In the drug-induced reinstatement test, rats first received injections of cocaine (10 mg/kg, i.p.) without vSUB microinjections. Response rates under these conditions were comparable in the two groups, exceeding 160 responses/hr in each case. With vSUB microinjections, however, lidocaine significantly lowered cocaine-induced responding relative to saline. These results are summarized in Figure 4. The lidocaine effect on lever pressing was most apparent by 10 min after the start of the session (Fig. 3), although stereotyped behavioral responses (e.g., corner head bobbing) remained.
Figure 4.

Effects of bilateral microinjections of lidocaine (100 μg/side) or saline (0.5 μl/side) into vSUB on cocaine-reinstated responding. Data are presented as in Figure 2, which also shows performance on the last day of cocaine self-administration. Responding was reinforced only by the CS in the reinstatement session contingent on an FR 5 schedule. Ext indicates the extinction session before reinstatement. Reinstate indicates the reinstatement session in which the rats received cocaine (10 mg/kg, i.p.) but did not have any vSUB pretreatment. Reinstate+Microinjection indicates the reinstatement session in which the rats received microinjections of saline or lidocaine into vSUB before cocaine (10 mg/kg, i.p.). Responding was reinforced only by the CS contingent on an FR 5 schedule in a 1 hr session. There was a significant difference between the lidocaine (n = 10) and saline (n = 7) groups (Bonferroni post test; p < 0.05).
Experiment 4: effects of lidocaine inactivation of vSUB on food self-administration
Using a within-subject design, we assessed the effects of vSUB lidocaine or saline on food-maintained responding in a separate group of rats. As shown in Table 2, there was no significant difference in responding between saline and lidocaine microinjections
Table 2.
Effects of lidocaine inactivation of vSUB on food-reinforced responding
|
Pretreatment |
Responding (responses/min) |
|---|---|
| Control | 25 ± 3 |
| Saline | 25 ± 5 |
| Lidocaine
|
24 ± 2
|
Data are mean ± SEM (n = 7). Control data for food-reinforced responding were from the food self-administration before the pretreatment sessions. vSUB lidocaine (100 μg/side) did not significantly decrease food-reinforced responding compared with either saline pretreatment or control (Tukey's test; p > 0.05).
Experiment 5: effects of lidocaine inactivation of vSUB on food-induced reinstatement
One week after extinction training, food-induced reinstatement tests were conducted. There was no significant difference in responding between vSUB lidocaine and saline as shown in Figure 5. Note that food-reinstated responding after either type of vSUB pretreatment was significantly higher than that in the extinction control sessions.
Figure 5.
Effects of bilateral microinjections of lidocaine (100 μg/side) or saline (0.5 μl/side) into vSUB on food-reinstated responding. Thirty minute reinstatement sessions (Reinstate) started with a noncontingent delivery of a train of three food pellets; another train was delivered every 5 min thereafter. Responding had no programmed consequences. Ext indicates the extinction session before reinstatement. Pretreatments with saline or lidocaine were counterbalanced among rats. There was no significant difference in responding between saline and lidocaine microinjections (n = 7), but responding after either type of pretreatment was significantly higher than during extinction (Tukey's test; p < 0.05). Baseline data on food self-administration is presented in Table 2.
Discussion
The reinstatement model of drug-seeking behavior in experimental animals has gained increasing attention as a way of studying the neural substrates of craving and relapse (Shalev et al., 2002; Kalivas and McFarland, 2003). In light of evidence implicating vSUB in cocaine-seeking behavior (Vorel et al., 2001), we used this model to assess the effects of temporarily inactivating vSUB on the resumption of extinguished lever pressing in rats induced by intraperitoneal cocaine or cocaine-related cues. Our results indicate that vSUB inactivation significantly decreases both cocaine and cue-induced reinstatement, providing direct support for vSUB in cocaine-seeking behavior.
The vSUB is well known to modulate locomotor behavior. Microinjections of NMDA into vSUB, for example, increase locomotion in rats (Yang and Mogenson, 1987; Wu and Brudzynski, 1995; Legault and Wise, 1999). Excitotoxic lesions of vSUB, on the other hand, decrease spontaneous locomotion and the locomotor-stimulating effects of either systemic or intra-NAcc amphetamine (Burns et al., 1993, 1996; Caine et al., 2001). One could argue, therefore, that the effect of inactivating vSUB reflects a loss of motor activity rather than specific modulation of cocaine- or CS-induced reinstatement. This explanation seems unlikely, however, given our finding that neither food-maintained responding nor food-induced reinstatement was affected by vSUB inactivation. Another issue to consider is the stereotyped pattern of motor activation induced by cocaine. It is conceivable, for example, that during cocaine self-administration, lever pressing becomes part of a stereotyped motor response. Thus, vSUB inactivation may decrease cocaine-induced perseverative responding rather than drug-seeking behavior. Although we cannot completely rule out this possibility, it seems unlikely for two reasons. First, stereotyped activity during both cocaine self-administration and cocaine-induced reinstatement occurred away from the lever and was manifested as burst grooming and head bobbing. In fact, animals broke away from this pattern to approach and press the lever. Second, if lever pressing were driven by motor perseveration, then vSUB inactivation should disrupt this response during both the self-administration and reinstatement sessions. That only the reinstatement session was affected argues against motor perseveration. Moreover, the failure of vSUB inactivation to block self-administration responding indicates that this manipulation does not decrease the reinforcing effects of cocaine. Consistent with this view, behavioral responding maintained by cocaine persists even after excitotoxic lesions of vSUB (Caine et al., 2001).
It is possible, however, that the effects of lidocaine are attenuated by cocaine because they both compete for membrane sodium channels (Liu et al., 1996), and in fact, cocaine appears to have higher affinity for these sites than lidocaine (Wilcox et al., 2001). Consistent with this reasoning, Kantak et al. (2002) reported that 100 μg but not 10 μg of lidocaine microinjected into BLA blocked cocaine-induced reinstatement, although the lower dose was able to block cue-induced reinstatement. To ensure lidocaine-induced inactivation of vSUB even in the presence of cocaine, we microinjected a relatively high lidocaine dose (100 μg). Moreover, we microinjected lidocaine into the vSUB 1 min before intraperitoneal injections of cocaine in our reinstatement test and 1 min before the onset of the cocaine self-administration session. It is unlikely, therefore, that our results with vSUB lidocaine can be explained by a pharmacodynamic interaction with cocaine. Our data suggest, instead, that the factors governing cocaine-seeking behavior depend, at least in part, on the mechanisms underlying vSUB function.
Although these mechanisms remain to be established, the hippocampus, including vSUB, has long been known to play a critical role in context learning and memory (Holland and Bouton, 1999; Rolls, 2000). In fact, episodic memory retrieval in humans depends on contextual cues (Maguire et al., 1997). Consistent with this information, lesions of vSUB in rats decrease context-conditioned freezing behavior and disrupt contextual retrieval of fear memory (Maren, 1999; Corcoran and Maren, 2001). The association formed between contextual cues and cocaine experiences could contribute to cocaine-seeking behavior. A role for contextual cues in relapse to cocaine seeking is indicated by recent evidence that lever-pressing behavior is reinstated when rats are reintroduced to a drug self-administration environment after responding is extinguished in a different environment (Crombag and Shaham, 2002). Thus, because of its role in context learning, vSUB is likely to modulate the drug-seeking response. Strong support for this view comes from evidence that electrical stimulation of vSUB reinstates cocaine-seeking behavior after responding for the drug in a self-administration environment is extinguished (Vorel et al., 2001). Similar results have been found for amphetamine-seeking behavior (Taepavarapruk and Phillips, 2003). It appears, therefore, that vSUB mediates context-dependent reinstatement. If this is the case, then inactivation of vSUB should decrease cocaine- or CS-induced reinstatement of drug-seeking by decreasing the contribution of contextual cues. It could be argued, however, that in our reinstatement tests contextual cues lose their motivational value because extinction training occurs in the drug self-administration environment. Under these conditions, neither cocaine- or CS-induced reinstatement should be affected by vSUB inactivation. To explain our results implicating vSUB in both types of reinstatement, it is important to note that we conducted extinction training in the absence of both the CS and cocaine. Thus, any associations between context and CS or cocaine are likely to be retained. It is likely, therefore, that the full expression of cocaine- or CS-induced reinstatement depends on context. This view is consistent with ample evidence in the fear-conditioning literature, which indicates that the magnitude of the conditioned fear response depends on interactions between context and the discrete CS (Bouton and Bolles, 1979; Corcoran and Maren, 2001; Bouton, 2002).
These interactions are further supported by the anatomical and functional connections known to exist between vSUB and the other components of the mesocorticolimbic circuit critical to cocaine- or CS-induced reinstatement (See, 2002; Shalev et al., 2002; Kalivas and McFarland, 2003). The subiculum, for example, projects directly to NAcc, BLA, and prefrontal cortex (PFC) and receives input from BLA and PFC (Groenewegen et al., 1990; Witter and Groenewegen, 1990; Jay and Witter, 1991; Pennartz et al., 1994; French and Totterdell, 2003). Chemical and electrical stimulation of the subiculum, moreover, increases extracellular DA levels in NAcc (Blaha et al., 1997; Brudzynski and Gibson, 1997; Legault and Wise, 1999; Legault et al., 2000) and excites NAcc neurons (Lopes da Silva et al., 1984; Boeijinga et al., 1990). In addition, electrical stimulation of vSUB induces excitatory responses in PFC neurons (Jay et al., 1995a,b). It is also interesting that reciprocal connections between amygdala and subiculum allow for mutual modulation of synaptic plasticity (Maren and Fanselow, 1995; Akirav and Richter-Levin, 2002). Although there is no anatomical evidence for a direct vSUB-VTA connection, chemical stimulation of vSUB has been reported to increase VTA DA release and the activity of VTA DA neurons (Legault and Wise, 1999; Todd and Grace, 1999; Legault et al., 2000). It is likely, therefore, that through interactions involving vSUB, NAcc, BLA, and PFC contextual cues act in combination with discrete CS and cocaine to contribute to cocaine-seeking behavior.
Lidocaine inactivation of vSUB failed to attenuate responding for food reinforcement. Because food is available in the home environment as well as in the operant test chamber, food is unlikely to convey context-specific information to the same extent as cocaine. It should be mentioned, however, that we did not study the effects of vSUB lidocaine on CS-induced food-seeking behavior, because the CS in this case did not elicit sufficient responding to detect a decrease (Sun and Rebec, 2002).
In summary, our results show that functional inactivation of vSUB can decrease cocaine- or CS-induced cocaine-seeking behavior without affecting behavior maintained or reinstated by food. These data suggest that vSUB is an important component in the circuitry that underlies cocaine-seeking behavior.
Footnotes
This work was supported by National Institutes of Health Grant DA02451. We are grateful to Dr. Eliot L. Gardner (National Institute on Drug Abuse) for valuable comments and suggestions. We also thank Paul Langley and Faye Caylor for technical and administrative support.
Correspondence should be addressed to George V. Rebec, Program in Neural Science, Department of Psychology, Indiana University, 1101 East Tenth Street, Bloomington, IN 47405. E-mail: rebec@indiana.edu.
Copyright © 2003 Society for Neuroscience 0270-6474/03/2310258-07$15.00/0
References
- Akirav I, Richter-Levin G ( 2002) Mechanisms of amygdala modulation of hippocampal plasticity. J Neurosci 22: 9912-9921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaha CD, Yang CR, Floresco SB, Barr AM, Phillips AG ( 1997) Stimulation of the ventral subiculum of the hippocampus evokes glutamate receptor-mediated changes in dopamine efflux in the rat nucleus accumbens. Eur J Neurosci 9: 902-911. [DOI] [PubMed] [Google Scholar]
- Boeijinga PH, Pennartz CM, Lopes da Silva FH ( 1990) Paired-pulse facilitation in the nucleus accumbens following stimulation of subicular inputs in the rat. Neuroscience 35: 301-11. [DOI] [PubMed] [Google Scholar]
- Bouton ME ( 2002) Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry 52: 976-986. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC ( 1979) Role of conditioned contextual stimuli in reinstatement of extinguished fear. J Exp Psychol Anim Behav Process 5: 368-378. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE ( 1997) Acute effects of cocaine on human brain activity and emotion. Neuron 19: 591-611. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM, Gibson CJ ( 1997) Release of dopamine in the nucleus accumbens caused by stimulation of the subiculum in freely moving rats. Brain Res Bull 42: 303-308. [DOI] [PubMed] [Google Scholar]
- Burns LH, Robbins TW, Everitt BJ ( 1993) Differential effects of excitotoxic lesions of the basolateral amygdala, ventral subiculum and medial prefrontal cortex on responding with conditioned reinforcement and locomotor activity potentiated by intra-accumbens infusions of d-amphetamine. Behav Brain Res 55: 167-183. [DOI] [PubMed] [Google Scholar]
- Burns LH, Annett L, Kelley AE, Everitt BJ, Robbins TW ( 1996) Effects of lesions to amygdala, ventral subiculum, medial prefrontal cortex, and nucleus accumbens on the reaction to novelty: implication for limbic-striatal interactions. Behav Neurosci 110: 60-73. [DOI] [PubMed] [Google Scholar]
- Caine SB, Koob GF ( 1994) Effects of dopamine D-1 and D-2 antagonists on cocaine self-administration under different schedules of reinforcement in the rat. J Pharmacol Exp Ther 270: 209-218. [PubMed] [Google Scholar]
- Caine SB, Humby T, Robbins TW, Everitt BJ ( 2001) Behavioral effects of psychomotor stimulants in rats with dorsal or ventral subiculum lesions: locomotion, cocaine self-administration, and prepulse inhibition of startle. Behav Neurosci 115: 880-894. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST ( 1999) Meta-analysis of cue-reactivity in addiction research. Addiction 94: 327-340. [PubMed] [Google Scholar]
- Childress AR, McLellan AT, Ehrman R, O'Brien CP ( 1988) Classically conditioned responses in opioid and cocaine dependence: a role in relapse? NIDA Res Monogr 84:25-43. [PubMed]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O'Brien CP ( 1993) Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr 137: 73-95. [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP ( 1999) Limbic activation during cue-induced cocaine craving. Am J Psychiatry 156: 11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F ( 2001) Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc Natl Acad Sci USA 98: 1976-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Maren S ( 2001) Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. J Neurosci 21: 1720-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Shaham Y ( 2002) Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci 116: 169-173. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW ( 2001) The neuropsychological basis of addictive behaviour. Brain Res Brain Res Rev 36: 129-138. [DOI] [PubMed] [Google Scholar]
- French SJ, Totterdell S ( 2003) Individual nucleus accumbens-projection neurons receive both basolateral amygdala and ventral subicular afferents in rats. Neuroscience 119: 19-31. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A ( 1996) Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci USA 93: 12040-12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, See RE ( 2000) Dissociation of primary and secondary reward-relevant limbic nuclei in an animal model of relapse. Neuropsychopharmacology 22: 473-479. [DOI] [PubMed] [Google Scholar]
- Groenewegen, HJ, Berendse, HW, Wolters, JG, Lohman AH ( 1990) The anatomical relationship of the prefrontal cortex with the striatopallidal system, the thalamus and the amygdala: evidence for a parallel organization. Prog Brain Res 85: 95-116; discussion 116-118. [DOI] [PubMed] [Google Scholar]
- Holland PC, Bouton ME ( 1999) Hippocampus and context in classical conditioning. Curr Opin Neurobiol 9: 195-202. [DOI] [PubMed] [Google Scholar]
- Jaffe JH, Cascella NG, Kumor KM, Sherer MA ( 1989) Cocaine-induced cocaine craving. Psychopharmacology 97: 59-64. [DOI] [PubMed] [Google Scholar]
- Jay TM, Witter MP ( 1991) Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J Comp Neurol 313: 574-586. [DOI] [PubMed] [Google Scholar]
- Jay TM, Burette F, Laroche S ( 1995a) NMDA receptor-dependent long-term potentiation in the hippocampal afferent fibre system to the prefrontal cortex in the rat. Eur J Neurosci 7: 247-250. [DOI] [PubMed] [Google Scholar]
- Jay TM, Glowinski J, Thierry AM ( 1995b) Inhibition of hippocampoprefrontal cortex excitatory responses by the mesocortical DA system. NeuroReport 6: 1845-1848. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K ( 2003) Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology 168: 44-56. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB ( 2002) Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J Neurosci 22: 1126-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP ( 2001) Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry 58: 334-341. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Rounsaville BJ, Kleber HD ( 1986) A 2.5-year follow-up of depression, life crises, and treatment effects on abstinence among opioid addicts. Arch Gen Psychiatry 43: 733-738. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF ( 1998) Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend 51: 23-47. [DOI] [PubMed] [Google Scholar]
- Kruzich PJ, See RE ( 2001) Differential contributions of the basolateral and central amygdala in the acquisition and expression of conditioned relapse to cocaine-seeking behavior. J Neurosci 21: RC155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legault M, Wise RA ( 1999) Injections of N-methyl-d-aspartate into the ventral hippocampus increase extracellular dopamine in the ventral tegmental area and nucleus accumbens. Synapse 31: 241-249. [DOI] [PubMed] [Google Scholar]
- Legault M, Rompre PP, Wise RA ( 2000) Chemical stimulation of the ventral hippocampus elevates nucleus accumbens dopamine by activating dopaminergic neurons of the ventral tegmental area. J Neurosci 20: 1635-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Hariman RJ, Bauman JL ( 1996) Cocaine concentration-effect relationship in the presence and absence of lidocaine: evidence of competitive binding between cocaine and lidocaine. J Pharmacol Exp Ther 276: 568-577. [PubMed] [Google Scholar]
- Lopes da Silva FH, Arnolds DE, Neijt HC ( 1984) A functional link between the limbic cortex and ventral striatum: physiology of the subiculum accumbens pathway. Exp Brain Res 55: 205-214. [DOI] [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, Kukes TJ, Renshaw PF ( 1998) Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry 155: 124-126. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Frackowiak RS, Frith CD ( 1997) Recalling routes around London: activation of the right hippocampus in taxi drivers. J Neurosci 17: 7103-7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S ( 1999) Neurotoxic or electrolytic lesions of the ventral subiculum produce deficits in the acquisition and expression of Pavlovian fear conditioning in rats. Behav Neurosci 113: 283-290. [DOI] [PubMed] [Google Scholar]
- Maren S, Fanselow MS ( 1995) Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. J Neurosci 15: 7548-7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW ( 2001) The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 21: 8655-8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF ( 2000) Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci 20: 798-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien CP, McLellan AT ( 1996) Myths about the treatment of addiction. Lancet 347: 237-240. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C ( 1998) The rat brain in stereotaxic coordinates, 4th Edition. San Diego: Academic.
- Pennartz CM, Groenewegen HJ, Lopes da Silva FH ( 1994) The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog Neurobiol 42: 719-61. [DOI] [PubMed] [Google Scholar]
- Rolls ET ( 2000) Memory systems in the brain. Annu Rev Psychol 51: 599-630. [DOI] [PubMed] [Google Scholar]
- See RE ( 2002) Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav 71: 517-529. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y ( 2002) Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev 54: 1-42. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O'Malley SS ( 2000) Psychological stress, drug-related cues and cocaine craving. Psychopharmacology 152: 140-148. [DOI] [PubMed] [Google Scholar]
- Sun W, Rebec GV ( 2002) Roles of the ventral subiculum in cocaine-induced reinstatement of cocaine-seeking behavior in the rat. Soc Neurosci Abstr 28: 499.5. [Google Scholar]
- Taepavarapruk P, Phillips AG ( 2003) Neurochemical correlates of relapse to d-amphetamine self-administration by rats induced by stimulation of the ventral subiculum. Psychopharmacology 168: 99-108. [DOI] [PubMed] [Google Scholar]
- Thomas KL, Everitt BJ ( 2001) Limbic-cortical-ventral striatal activation during retrieval of a discrete cocaine-associated stimulus: a cellular imaging study with gamma protein kinase C expression. J Neurosci 21: 2526-2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd CL, Grace AA ( 1999) Modulation of ventral tegmental area dopamine cell activity by the ventral subiculum and entorhinal cortex. Ann NY Acad Sci 877: 688-690. [DOI] [PubMed] [Google Scholar]
- Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL ( 2001) Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science 292: 1175-1178. [DOI] [PubMed] [Google Scholar]
- Washton AM, Stone-Washton N ( 1990) Abstinence and relapse in outpatient cocaine addicts. J Psychoactive Drugs 22: 135-147. [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Paul IA, Ordway GA, Woolverton WL ( 2001) Role of the dopamine transporter and the sodium channel in the cocaine-like discriminative stimulus effects of local anesthetics in rats. Psychopharmacology 157: 260-268. [DOI] [PubMed] [Google Scholar]
- Witter MP, Groenewegen HJ ( 1990) The subiculum: cytoarchitectonically a simple structure, but hodologically complex. Prog Brain Res 83: 47-58. [DOI] [PubMed] [Google Scholar]
- Wu M, Brudzynski SM ( 1995) Mesolimbic dopamine terminals and locomotor activity induced from the subiculum. NeuroReport 6: 1601-1604. [DOI] [PubMed] [Google Scholar]
- Yang CR, Mogenson GJ ( 1987) Hippocampal signal transmission to the pedunculopontine nucleus and its regulation by dopamine D2 receptors in the nucleus accumbens: an electrophysiological and behavioural study. Neuroscience 23: 1041-1055. [DOI] [PubMed] [Google Scholar]