Abstract
This study was undertaken to provide a biophysical basis for the hypothesis that activity-dependent modulation of cadherin-mediated adhesion by transient changes of extracellular calcium ([Ca2+]e) is causally involved in coordination of synaptic plasticity. Characterization of homophilic N-cadherin binding by atomic force microscopy and laser tweezer trapping of N-cadherin-coated microbeads attached to the cell surface of cultured neuronal cells showed that adhesive activity of N-cadherin is effectively regulated between 0.3 and 0.8 mm [Ca2+]e. Furthermore, we show that an increase of [Ca2+]i, which is known to be essential for induction of synaptic plasticity, causes significant reduction of cadherin-mediated bead adhesion that could be completely suppressed by inhibition of actin depolymerization. The results of this study show that N-cadherin has ideal biophysical properties to serve as a Ca2+-dependent sensor for synaptic activity and, at the same time, is strategically located to control synaptic adhesion. A drop of [Ca2+]e and a concomitant increase of [Ca2+]i may act in concert to modulate N-cadherin-based adhesive contacts at synaptic sites.
Keywords: synaptic plasticity, long-term potentiation, actin, PC12 cells, cadherin 2, VE-cadherin
Introduction
Synapses are dynamic structures that change their efficacy and morphology in response to neural activities (Bi and Poo, 2001; Yuste and Bonhoeffer, 2001). Continuous shape changes of postsynaptic dendritic protrusions (dendritic spines) have been well documented in vivo and in vitro (Matus, 2000; Marrs et al., 2001). Activity-dependent remodeling of synaptic structure (synaptic plasticity) is considered to underlie certain aspects of learning and memory. A well studied form of synaptic plasticity is long-term potentiation (LTP) of the efficacy of synaptic transmission that can be elicited experimentally by particular patterns of high-frequency electrical stimulations (Malenka and Nicoll, 1999; Bi and Poo, 2001). LTP development is accompanied by the enlargement of both synaptic area and area of active synaptic zones as indicated by expansion of spine heads, duplication of postsynaptic densities (perforated synapses), and formation of new spines (Toni et al., 2001; Yuste and Bonhoeffer, 2001). A key event of LTP induction is the influx of Ca2+ through the presynaptic and postsynaptic membranes, which is accompanied by partial depletion of extracellular Ca2+ ([Ca2+]e). [Ca2+]e has been measured and calculated to reach values of 0.3-0.8 mm, depending on the frequency and duration of electrical depolarization (Nicholson et al., 1978; Krnjevic et al., 1980; Egelman and Montague, 1999).
There is increasing evidence that the Ca2+-dependent neural cell adhesion molecule N-cadherin plays an important role in certain steps of LTP generation (Murase and Schuman, 1999; Benson et al., 2000; Bruses, 2000; Huntley and Ernst, 2000). This view is primarily based on the observations that antibodies to N-cadherin and an inhibitory peptide sequence containing the histidine-alanine-valine (HAV) motif of the cadherin ectodomain have been shown to interfere with induction of LTP if present during conditioning electrical stimulation (Tang et al., 1998; Bozdagi et al., 2000). Interestingly, the HAV peptide was completely ineffective if [Ca2+]e was elevated to unphysiological high levels (5 mm) during stimulation.
The most straightforward interpretation of these observations suggests that a drop of [Ca2+]e in the synaptic cleft during electrical stimulation may be sufficient to cause separation of cadherin bonds to allow the HAV peptide to intercalate and prevent rebinding after cessation of stimulation. In crystals of the outermost external domain (EC1) of N-cadherin, the HAV motif was exposed to the adhesive surface (Shapiro et al., 1995), whereas recent studies on crystals of the entire external domain of C-cadherin indicate no involvement of HAV in transinteraction. Nevertheless, HAV-containing peptides have been shown in several independent studies to interfere with N-cadherin and E-cadherin function (Blaschuk et al., 1990; Lutz et al., 1995; Noe et al., 1999; Williams et al., 2000, 2001; Wanner and Wood, 2002). If separation of cadherin bonds do really occur during enhanced synaptic activity, one has to expect that both time constant (lifetime) and Ca2+ sensitivity of cadherin bonds are compatible with the conditions of electrical stimulation (e.g., several 1 sec episodes at 50-100 Hz) and the expected drop of [Ca2+]e to 0.3-0.8 mm. Furthermore, cadherin-mediated adhesion might be influenced by acute destabilization of the presynaptic and postsynaptic actin filament system caused by presynaptic and postsynaptic influx of Ca2+ (Furukawa et al., 1997; Bernstein et al., 1998; Halpain et al., 1998; Matus, 2000). Temporary weakening of cadherin-mediated adhesion may be sufficient to facilitate early events of synaptic remodeling.
In the present study, we combined atomic force microscopy (AFM) with laser tweezer studies to test the possibility that N-cadherin-mediated adhesion is effectively regulated at concentrations of extracellular Ca2+ known to occur during enhanced synaptic activity (0.3-0.8 mm). Moreover, we addressed the question whether an increase in [Ca2+]i may modify N-cadherin adhesion by destabilization of the neuronal actin filament system.
Materials and Methods
Recombinant cadherin-Fc. N-cadherin-Fc (chicken N-cadherin extracellular domain fused to Fc-portion of mouse IgG2) was a kind gift from Marc Mège (Institut du Fer à Moulin, Paris, France). Human embryonic kidney (HEK293) cells transfected with N-cadherin-Fc were cultured in DMEM (Invitrogen, Karlsruhe, Germany) containing 10% Ultroser serum supplement (Invitrogen) at 37°C in 5% CO2. N-cadherin-Fc was purified from the HEK293 supernatant by protein G-agarose (Sigma, Taufkirchen, Germany).
A vascular endothelial (VE)-cadherin-Fc fusion protein (mouse VE-cadherin extracellular domain fused to Fc-portion of human IgG1) was generated and purified as described previously (Moll and Vestweber, 1999; Baumgartner et al., 2000b). Chinese hamster ovary cells were stable transfected, and the secreted VE-cadherin-Fc was purified from the culture supernatants by affinity chromatography using protein A agarose (Oncogene, Cambridge, MA).
AFM measurements. Cadherin-cadherin transinteractions were characterized by force-distance measurements of cadherins coupled via flexible linkers to the tip and substrate of a Bioscope AFM driven by a Nanoscope III controler (Digital Instruments, Santa Barbara, CA). N-cadherin-Fc was linked covalently to the Si3N4 tips of the cantilever (Park Scientific, Sunnyvale, CA) and freshly cleaved mica plates (Wacker, Burghausen, Germany) using polyethylene glycol (PEG) spacers containing an amino-reactive cross-linker group [N-hydroxy-succinimide (NHS) ester] at one end and a thiol-reactive group (2-[pyridyldithio]propionate) at the other end, as described previously in detail (Hinterdorfer et al., 1996). The NHS group served to link PEG to free amino groups of both the Si3N4 tip and the SiOH plate introduced by treatment of the tip and plate with 2-aminoethanol HCl (Sigma). The average labeling density of N-cadherin-Fc assayed by ELISA (Hinterdorfer et al., 1996) was calculated to be in the range of ∼400-800 molecules/μm2 surface. Force measurements were performed in buffer A (5 mm HEPES, 150 mm NaCl, adjusted with NaOH to pH 7.4, various Ca2+ concentrations by the addition of CaCl2). Unbinding forces between molecules coupled to the tip and plate of the AFM were monitored by force-distance cycles at amplitudes of 300 nm and at frequencies ranging from 0.5-10 Hz. Force-distance cycles were performed either at constant lateral positions or with lateral shifts of 1 nm/sec. Spring constant of the cantilever in force measurements was 0.03 N/m (determined by thermal noise analysis). Force-distance cycles were analyzed as described previously in detail (Baumgartner et al., 2000a; Baumgartner and Drenckhahn, 2002a).
Coating of polystyrene beads. N-cadherin-Fc was coupled to tosyl-activated superparamagnetic polystyrene microbeads (Dynabeads; diameter, 2.8 μm; Dynal, Oslo, Norway). After extensive vortexing, 2 × 107 beads were washed in PBS. Washing was performed by sedimenting the beads via application of a magnetic field for ∼1 min using a magnetic tube holder (MPC-E-1; Dynal). Washed beads were resuspended in 100 μl of coupling buffer [0.1 m borate buffer, pH 9.5, 0.1% bovine serum albunim (BSA)] and allowed to react with 10 μg of N-cadherin-Fc under gentle overhead rotation for 24 hr at 37°C. Beads were sedimented, washed with PBS, and incubated in blocking buffer (0.2 m Tris, pH 8.5, 0.1% BSA) for 4 hr at 37°C. After final washing in PBS, beads were resuspended in 100 μl of HBSS (Invitrogen) containing 0.1% BSA and 0.02% sodium azide.
VE-cadherin was coupled to protein A-coated superparamagnetic polystyrene microbeads (Dynabeads; diameter, 2.8 μm; Dynal) as described recently (Baumgartner et al., 2003). Beads were stored for up to 7 d in HBSS containing 0.1% BSA and 0.02% sodium azide at 4°C at slow overhead rotation to avoid sedimentation. Concentration of N-cadherin-Fc and VE-cadherin-Fc bound to the bead surface (24.6 μm2/bead) was determined by ELISA as described previously (Baumgartner et al., 2000b, 2003), yielding approximately equal surface densities for N-cadherin and VE-cadherin (500-800 molecules/μm2).
Cell cultures. PC12 rat pheochromocytoma cells (Greene and Tischler, 1976), endogenously expressing N-cadherin, were cultured in DMEM containing 5% FCS and 10% horse serum (Biochrom, Berlin, Germany) at 37°C. Chicken sympathetic neurons were cultured as described previously (Wiese et al., 1999). In brief, sympathetic chain ganglia were isolated from chicken embryos (embryonic day 12), incubated for 30 min in PBS containing 0.1% trypsin (Sigma), and seeded onto an uncoated culture dish to allow settling of non-neuronal cells. After 3 hr, the nonadherent neuronal cells were removed and seeded onto laminin-coated coverslips for experiments. Cells were grown in Ham's F14 medium (Invitrogen) containing 10% horse serum, NGF, and CNTF (10 ng/ml for both; Sigma).
A microvascular endothelial cell line was generated from mouse myocardium (MyEnd) and characterized as described previously (Golenhofen et al., 2002). MyEnd cells were cultured in DMEM containing 10% FCS at 37°C in humidified atmosphere (5% CO2). For experiments, cells were grown on coverslips, coated for PC12 cells with polyornithine (0.01%), and for MyEnd cells with gelatin, cross-linked with glutaraldehyde (Schnittler et al., 1993).
Laser tweezer. The home-built laser tweezer setup consisted of an Nd:YAG laser (1064 nm), the beam of which was expanded to fill the back aperture of a high numerical aperture objective (100×, 1.3 oil; Zeiss, Oberkochen, Germany), coupled through the epi-illumination port of an Axiovert 135 microscope (Zeiss) and reflected to the objective by a dicroic mirror (FT510; Zeiss). Beads were probed with laser intensities ranging from 20-200 mW. Beads resisting displacement at 20 mW always resisted detachment at higher laser intensities (up to 200 mW tested). For experimental convenience, we decided to perform all tweezer experiments at 30 mW. Protein-coated polystyrene microbeads were allowed to interact with the cell surface of monolayers for 15 min. Next, 100-300 beads were probed during the following 5-10 min by the laser tweezer. All tweezer experiments were performed in medium containing decomplemented FCS. Decomplementation was performed at 56°C for 60 min.
For determination of the Ca2+ dependency of bead binding, [Ca2+]e was adjusted by an addition of various concentrations of EGTA to the culture medium. Free [Ca2+] was measured using a Ca2+-sensitive macroelectrode (Ingold, Steinbach, Germany). To rule out unspecific EGTA effects, some experiments were performed using Ca2+-free DMEM medium (Invitrogen) supplemented with varying concentrations of CaCl2. Cytochalasin D, A23187 (both from Sigma), and jasplakinolide (Calbiochem, Bad Soden, Germany) were used at 10 μm in culture medium. Jasplakinolide was applied for 60 min, cytochalasin D for 50 min, and A23187 for 30 min under cell culture conditions as described above. Depolarization of PC12 cells was performed by adjusting the culture medium to a final concentration of 50 mm K+.
Specificity of binding of N-cadherin-Fc-coated microbeads was investigated by the addition of 3 mm EGTA to the culture medium during the experiment. In addition, beads and cells were preincubated with 0.2 mm of the inhibitory peptide AHAVD (Pineda, Berlin, Germany) for 15 min and subsequently probed for cadherin interaction in the presence of the peptide by the laser tweezer. Specificity of VE-cadherin-Fc binding was investigated by Ca2+ depletion of the culture medium (addition of 3 mm EGTA) or by the addition of a monoclonal antibody against the extracellular domain of VE-cadherin [monoclonal antibody (mAb) 11D4.1; hybridoma supernatant] (Gotsch et al., 1997). Furthermore, control beads were coated with human Fc fragment only, and these beads were applied to the MyEnd cells and probed by the laser tweezer.
Quantification of F-actin. For determination of the relative F-actin contents, PC12 cells were fixed at room temperature with 3% formaldehyde in PBS for 15 min and then permeabilized with 0.1% (v/v) Triton X-100 in PBS for 5 min. Afterward, each coverslip was incubated with 500 μl (1 μg/ml) of phalloidin covalently labeled with tetramethyl-rhodamine isothiocyanate (TRITC) for 1 hr at 37°C (Faulstich et al., 1983). Series of experiments with changing concentrations of TRITC-phalloidin (0.1-10 μm) were conducted to show that the conditions chosen allowed saturation of binding. After washing three times for 5 min in PBS, TRITC-phalloidin was extracted from the cells by two subsequent 1 hr incubation steps with 1 ml of methanol at 37°C. Methanol supernatants were pooled, centrifuged at 100,000 × g for 20 min, and extracted TRITC-phalloidin was quantified spectrophotometrically at an excitation wavelength of 540 nm and an emission wavelength of 563 nm.
Results
Ca2+ dependency of cadherin binding
Ca2+ dependency of cadherin transinteraction was determined by two approaches, single-molecule AFM and laser tweezer assay. Although AFM allows determination of binding activity of single purified molecules, laser tweezer studies are designed to study transinteraction of recombinant cadherins with endogenous cadherins expressed on the surface of cultured cell lines.
AFM
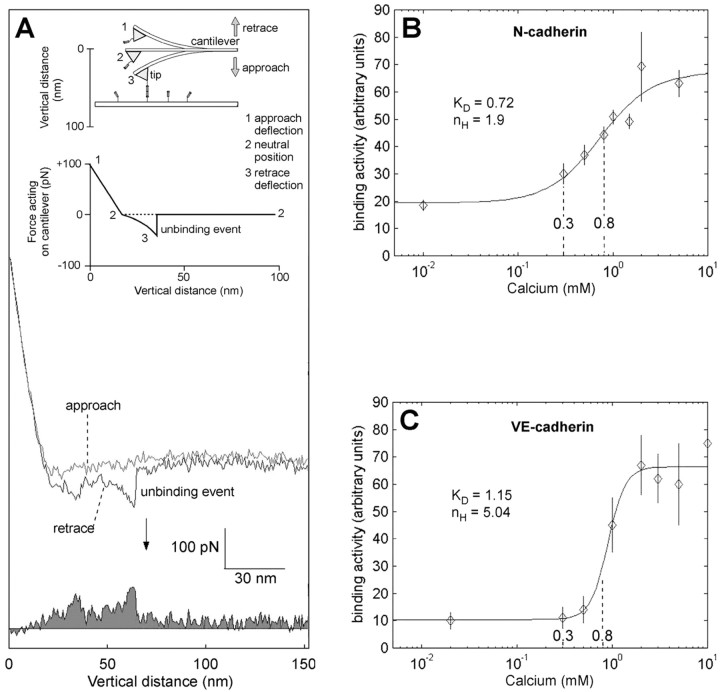
The general principle of determination of unbinding forces and lifetime of bonds of transinteracting recombinant cadherin dimers is illustrated in Figure 1A. Cadherins covalently attached to the tip of the cantilever and surface of the plate are brought into interaction and separation by cyclic upward and downward movements of the tip at a frequency of 1-2 Hz (force-distance cycles). During downward movement (approach), the tip of the cantilever will eventually hit the plate and, during further downward movement, will gradually be deflected upwards until the end of the approach (defined as zero position of the cycle). During the following retrace movement, the cantilever bends back with the same linear force slope until the tip separates from the plate to reach the unbent neutral position. During further progression of the retrace movement, the cantilever will remain in the neutral position if no interaction occurs between tip- and plate-bound cadherins. Interaction between cadherins can proceed throughout the entire period during which plate and tip are in contact. If transinteraction has taken place, the cantilever will be pulled down below the neutral line until a critical force is reached (unbinding force) at which the cadherin bond breaks (unbinding is followed by an abrupt jump of the cantilever to the neutral position). The length of the unbinding jump depends on the spring constant of the cantilever and is directly proportional to the unbinding force. Because the cadherins are attached to the plate and tip by flexible PEG-linkers, molecules can freely diffuse within the radius of the length of the linkers (∼8 nm), allowing them to undergo unimpaired encounter reactions (Hinterdorfer et al., 1996).
Figure 1.
Ca2+ dependency of N-cadherin and VE-cadherin binding probed with AFM. A-C, Binding activity between tip- and substrate-attached N-cadherin-Fc (A, B) and VE-cadherin-Fc (C) at various Ca2+ concentrations was measured with the AFM. A, General working principle of force measurements. Chimeric N-cadherin-Fc is covalently attached by PEG-linkers to a substrate and the cantilever tip of the AFM. Molecules are brought into contact by downward movement of the tip. During upward movement, a downward deflection of the cantilever will occur if tip- and substrate-bound molecules undergo binding. Retrace (arrow indicates an unbinding event of ∼100 pN) and approach were subtracted, and the area below the resulting curve (gray) was integrated and taken as a measure for the average binding activity. B, Ca2+ dependency of homophilic N-cadherin-Fc binding. C, Ca2+ dependency of homophilic VE-cadherin-Fc binding. All measurements were obtained by stepwise increasing the Ca2+ concentration by fluid exchange. Each point is the average of at least 400 force-distance cycles. Note a moderate cooperativity of N-cadherin binding, with a Hill coeficient of 1.9 at an apparent KD of 0.72 mm, and pronounced cooperativity of VE-cadherin binding, with a Hill coefficient of 5.04 and an apparent KD of 1.15 mm. The range of the values of [Ca2+]e determined during high-frequency stimulation (0.3-0.8 mm) is indicated.
Approach-retrace cycles at vr = 800 nm/sec and 0.1 sec encounter time were performed at different Ca2+ concentrations (0-10 mm). The total area between the force curve and the neutral line was taken as measure for binding activity (Fig. 1A), as outlined in detail in a previous study (Baumgartner et al., 2000b). Each point of the plot shown in Figure 1, B (N-cadherin) and C (VE-cadherin), represents the mean activity of at least 600 retrace cycles using one AFM tip. Four different tips were used, and all yielded equal results. Ca2+ dependency of binding activity (transinteraction between tip- and substrate-bound cadherins) of N-cadherin showed half-maximal adhesion (apparent KD) of 0.72 mm Ca2+ with a moderate degree of cooperativity (Hill coefficient, nh = 1.9). The Ca2+ dependency of VE-cadherin was determined recently (Baumgartner et al., 2000b) and has been repeated for comparison. Most interestingly, VE-cadherin-Fc required a considerably higher [Ca2+]e for half-maximal transinteraction (KD = 1.15 mm Ca2+) and displayed very high cooperativity (nh = 5.0). Thus, VE-cadherin displays nearly an “all-or-nothing” transition close to the physiological Ca2+ level (Fig. 1C), whereas N-cadherin is less sensitive to [Ca2+]e and displayed a moderate degree of cooperativity, which would allow a graded response of adhesive activity over a broader range of [Ca2+]e.
Besides determination of Ca2+ dependency of transinteraction, AFM studies allowed also to determine two additional important parameters of N-cadherin function, namely strength of binding (unbinding force) of ∼40 pN at separation velocity of 400 nm/sec and lifetime of interaction τ ∼ 1.1 sec (corresponding to koff = τ-1 ∼ 0.9 sec-1). Both parameters were in the range determined previously by our group for VE-cadherin (Baumgartner et al., 2000b), namely an unbinding force of ∼40 pN at separation velocity of 400 nm/sec and lifetime of bonds at zero force of τ ∼ 600 msec (corresponding to koff = 1.8 sec-1).
Laser tweezer
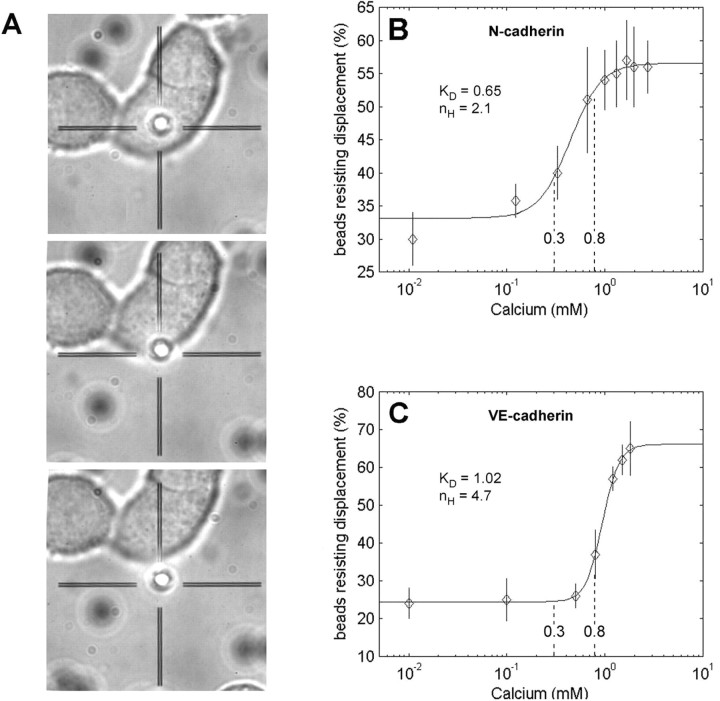
Cadherin-Fc-coated beads were allowed to settle for 15 min on the cell surface of PC12 cells (for N-cadherin-Fc) or MyEnd cells (VE-cadherin-Fc). As recently shown by immunostaining with antibodies to cadherin and β-catenin, as well as by staining with TRITC-phalloidin, typical adherens-like junctions formed between the majority of beads (60-80%) and the cell surface (Lambert et al., 2000; Baumgartner et al., 2003).
Typically, ∼70% of the beads suspended in DMEM (containing 1.8 mm Ca2+) were tightly bound to the cell surface and resisted displacement (detachment) by laser tweezers (Fig. 2). Specificity of binding of VE-cadherin-coated beads to the cell surface was confirmed by the following control experiments: beads coated with the Fc fragment only, instead of VE-cadherin-Fc, displayed strongly reduced frequency of binding with only 5-12% of beads resisting detachment by laser tweezer. The addition of mAb 11D4.1 (hybridoma supernatant) directed against the extracellular domain of VE-cadherin reduced bead-cell adhesion by ∼60% for VE-cadherin. Specificity of binding of beads coated with N-cadherin-Fc was tested by the addition of 3 mm EGTA to the culture medium, as well as by the addition of 0.2 mm of the inhibitory peptide AHAVD corresponding to amino acid residues 79-83 (Blaschuk et al., 1990; Tang et al., 1998), which resulted in ∼80% reduction of bead-cell adhesion to PC12 cells. The nonsense peptide HADAV did not effect bead binding significantly (Table 1).
Figure 2.
Ca2+ dependency of N-cadherin and VE-cadherin binding probed by laser tweezer. A-C, Beads coated with N-cadherin-Fc (A, B) and VE-cadherin-Fc (C) settled on dorsal surface of PC12 (A, B) and MyEnd (C) monolayers were probed with laser tweezer at various extracellular Ca2+ concentrations of [Ca2+]e. A, Time series of a typical control experiment. At 0 mm [Ca2+]e, the majority of beads can be removed by laser trapping from the dorsal cell surface. B, C, Each point represents the percentage of beads (average of ≥200 beads per point) resisting displacement by laser tweezer of three to six experiments (different batches of cell cultures). Ca2+ dependency of binding of N-cadherin-Fc-coated beads displayed an apparent KD of 0.65 mm with a moderate Hill coefficient of nh = 2.1, whereas VE-cadherin-Fc-coated beads displayed an apparent KD of 1.02 mm Ca2+ and high cooperativity with a Hill coefficient of nh = 4.7. The range of the values of [Ca2+]e determined during high-frequency stimulation (0.3-0.8 mm) is indicated.
Table 1.
Influence of [Ca2+]i and the actin filament system on N-cadherin binding
|
|
Percentage of beads resisting displacement |
||
|---|---|---|---|
| Conditions (DMEM, 1.8 mm Ca2+)
|
Absolute values
|
Normalized values
|
|
| Control | 72.5 ± 1.5% (n = 17) | 100% | |
| 3 mm EGTA | 28.0 ± 1.4% (n = 14) | 0% | |
| Peptide AHAVD 0.2 mm | 36.8 ± 2.2% (n = 4) | 19.7% | |
| Peptide HADAV 0.2 mm | 69.9 ± 1.3% (n = 3) | 94.2% | |
| A23187 | 27.7 ± 1.3% (n = 4) | −0.7% | |
| Jasplakinolide plus A23187 | 75.6 ± 1.9% (n = 4) | 107.0% | |
| Cytochalasin D | 31.7 ± 1.8% (n = 4) | 8.3% | |
| Jasplakinolide plus cytochalasin D | 65.1 ± 1.7% (n = 4) | 83.4% | |
| Depolarization with 50 mm K+
|
35.0 ± 2.0% (n = 4)
|
9.7%
|
|
Percentage of beads coated with N-cadherin-Fc tightly bound to the cell surface of PC12 cells. Binding was determined by laser tweezer at various conditions. Beads were allowed to settle on the cell surface 15 min before drug treatment. Drugs were applied at a concentration of 10 μm, and the inhibitory AHAVD peptide and the nonsense peptide HADAV were applied at 20μm. Each experiment represents at least 100 individual beads probed by laser tweezer.
Ca2+ dependency of VE-cadherin and N-cadherin interaction was determined by the percentage of tightly bound cadherin-Fc-coated beads (resisting detachment after 15 min of settling) as a function of [Ca2+]e. The results of these experiments for N-cadherin and VE-cadherin are plotted in Figure 2, in which each data point represents the mean percentage of beads resisting detachment at a given [Ca2+]e. Each point represents at least three experiments (different cell batches) counting at least 200 beads. Ca2+ dependency of the binding of beads coated with N-cadherin-Fc displayed an apparent KD of 0.65 mm (Hill coefficient, nh = 2.1), whereas beads coated with VE-cadherin-Fc displayed an apparent KD of 1.02 mm Ca2+ and high cooperativity with a Hill coefficient of nh = 4.7. Maximum binding of beads was reached at [Ca2+]e ≥ 1.1 mm for both cadherins. [Ca2+]e, higher than that depicted in Figure 2, led to cell damage and could therefore not be investigated with accuracy. Because the laser tweezer studies on N-cadherin adhesion relied on a heterologous system (chicken N-cadherin, rat PC12 cells), we performed some confirmatory experiments with cultured chicken sympathetic neurons (homologous systems). In these experiments, adhesion was also inhibited by excess EGTA and the AHAVD peptide, and the KD for [Ca2+] was in the range of the values obtained for PC12 cells (0.6-0.7 mm).
In addition to determination of cadherin binding in dependency to changes of [Ca2+]e, laser tweezer experiments also allowed us to study the effects of bead binding in response to changes of [Ca2+]i. Synaptic activity has been shown to cause presynaptic and postsynaptic increases of [Ca2+]i by opening of voltage-gated Ca2+ channels (presynaptic and postsynaptic) and ionotropic transmitter receptors such as the NMDA receptor (postsynaptic) (Regehr and Tank, 1990; Perkel et al., 1993; Malenka and Nicoll, 1999). The latter has been shown to be required for LTP induction of glutamatergic synapses. To address the question whether transinteraction of N-cadherin might also be influenced by [Ca2+]i and the actin filament system, PC12 cells were treated with 10 μm A23187 or 10 μm of the actin-fragmenting compound cytochalasin D in the absence and presence of 10 μm jasplakinolide, which stabilizes actin filaments (Scott et al., 1988; Bubb et al., 1994; Kawamura et al., 2003). An increase of [Ca2+]i induced by the application of A23187 caused a significant reduction of cellular F-actin content by ∼30% (Table 2), a phenomenon observed in many other cell types and is generally believed to be caused by activation of the Ca2+-dependent protein gelsolin, which inhibits actin polymerization (Yin and Stossel, 1979; Kuhne et al., 1993). Application of cytochalasin D reduced the F-actin content of PC12 cells to a similar degree (40%) (Table 2). Both A23187 and cytochalasin D caused significant reduction of beads bound on PC12 cells to the baseline level seen with zero [Ca2+]e, suggesting a significant role of the actin filament system in regulation of N-cadherin-mediated adhesion. When the F-actin-stabilizing compound jasplakinolide was applied before the addition of A23187 or cytochalasin D, bead adhesion was not attenuated in response to either A23187 or cytochalasin D (Table 1). These experiments show that reduction of bead adhesion, resulting from elevation of [Ca2+]i by A23187, is causally related to depolymerization of F-actin. In an additional experiment, PC12 cells were depolarized by adjusting the culture medium to 50 mm K+. Under these conditions, [Ca2+]i has been shown in PC12 cells to rise to ∼0.5 μm (Shafer and Atchison, 1991; Masuda et al., 2002). As to be expected from the A23187 experiments, K+ depolarization caused a similar degree of reduction of both F-actin content and bead adhesion, as seen in response to A23187 and cytochalasin D, respectively (Tables 1, 2).
Table 2.
Relative amount of F-actin content of PC12 cells determined by TRITC-phalloidin extraction
|
|
Relative content of F-actin |
|---|---|
| Control | 100 ± 3.4% (n = 6) |
| Cytochalasin D | 61.9 ± 6.2% (n = 6)* |
| A23187 | 70.2 ± 3.9% (n = 6)* |
| 50 mm K+
|
82.4 ± 6.3% (n = 5)*
|
Average content of untreated cells was normalized to 100%. Note the significant reduction of F-actin content in cultures treated with cytochalasin D, A23187, and depolarization with K+, respectively. *p < 0.01 compared with control (t test).
Discussion
The present study provides a biophysical basis for supporting the challenging observation of Tang et al. (1998), implying that activity-induced modulation of cadherin-dependent adhesion by transient changes of [Ca2+]e is causally involved in coordination of synaptic plasticity. The data of our study suggest that N-cadherin may serve as both sensor for synaptic activity and effector of synaptic remodeling. These functions strongly depend on activity-induced depletion of [Ca2+]e and a concomitant increase in [Ca2+]i.
Ca2+ sensitivity of N-cadherin
Measurements of [Ca2+]e in rat cerebellar and hippocampal cortex by microelectrodes indicate a stimulation-evoked drop of [Ca2+]e to 0.5-0.8 mm and, under certain conditions, even to lower values (Nicholson et al., 1978; Krnjevic et al., 1980). These measurements are supported by theoretical studies dealing with [Ca2+]e in response to opening of voltage-gated Ca2+ channels and Ca2+-permeable ionotropic transmitter receptors such as NMDA receptor (Egelman and Montague, 1999; Rusakov, 2001).
Both our single-molecule AFM measurements and laser tweezer studies on cultured neuronal cells show that adhesive activity of N-cadherin is regulated exactly between 0.3 and 0.8 mm [Ca2+]e, with a KD of 0.65 mm (tweezer) and 0.72 mm (AFM) Ca2+. By this property, N-cadherin differs profoundly from VE-cadherin (Baumgartner et al., 2000b; this study) and E-cadherin (Pertz et al., 1999), both of which were shown to require almost physiological levels of [Ca2+]e for maximal adhesion (KD, ∼1 mm Ca2+) and display high cooperativity with almost all-or-nothing behavior of adhesiveness if [Ca2+]e drops below 1 mm (Baumgartner et al., 2000b). It is interesting to mention that N-cadherin and E-cadherin display mutually exclusive synaptic distribution (Fannon and Colman, 1996). Whereas N-cadherin has been reported to be associated with excitatory synaptic sites (Benson et al., 2000), E-cadherin appears to be primarily confined to inhibitory synapses, which may not undergo activity-dependent reduction of [Ca2+]e to levels occurring in glutamatergic excitatory synapses. Differences in Ca2+ sensitivity of cadherins might also be the key for understanding why another synaptic cadherin, cadherin 11 (which has not been biophysically characterized so far), appears to suppress synaptic plasticity rather than support it (Manabe et al., 2000).
Regulation of N-cadherin adhesion by actin
The cytoplasmic domain of cadherins is tethered via catenin-type adaptor molecules to the actin filament cytoskeleton, which has been shown to be important for regulation of adhesion (Adams and Nelson, 1998; Gumbiner, 2000; Baumgartner and Drenckhahn, 2002b). Several studies including the present experiments with PC12 cells have shown depolymerization of presynaptic and postsynaptic actin filaments (F-actin) and collapse, as well as displacement of F-actin assemblies in response to depolarization of the membrane potential and stimulation of NMDA receptors, respectively (Bernstein and Bamburg, 1985; Bernstein et al., 1998; Colicos et al., 2001). Both conditions cause a rise of [Ca2+]i at levels sufficient to induce fragmentation and depolymerization of F-actin by a calcineurin- and gelsolin-dependent mechanism (Furukawa et al., 1997; Halpain et al., 1998). In line with this notion, hippocampal neurons of mice lacking gelsolin exhibited markedly decreased F-actin depolymerization despite enhanced [Ca2+]i during exposure to glutamate (Furukawa et al., 1997).
In the present study, we show that the state of actin polymerization of PC12 cells is strikingly influenced by [Ca2+]i (treatment with Ca2+-ionophore A23187 and depolarization with 50 mm K+). Reduction of F-actin induced by either A23187 or K+ depolarization (∼30% at 10 μm A23187 within 30 min) (Table 2) was accompanied by attenuation of adhesion of N-cadherin-coated beads close to baseline values obtained in the absence of [Ca2+]e (EGTA control). Similar results were obtained by cytochalasin D treatment of these cells (Tables 1, 2). Under these conditions, no significant weakening of bead adhesion was observed if F-actin was stabilized by pretreatment with jasplakinolide. These findings are in line with protection of glutamate-induced loss of F-actin and collapse of spine-head morphology by pretreatment of cultures with jasplakinolide (Halpain et al., 1998).
Functional implications
The results of this study allow us to conclude that N-cadherin-mediated adhesion is compromised by two mechanisms during enhanced synaptic activity, namely by the drop of [Ca2+]e and concomitant increase of [Ca2+]i. Both mechanisms act in concert and correlate positively with duration and strength of synaptic activity. Because the lifetime of N-cadherin transinteraction at normal [Ca2+]e is only 1.1 sec (koff = 0.9 sec-1), the bulk of cadherins will dissociate at least once during any stimulus that lasts longer than 1 sec. The capacity of dissociated cadherins to rebind across the synaptic cleft will be strongly reduced as long as [Ca2+]e is <0.8 mm. This view is in full agreement with the observation of Tang et al. (1998), who found that the HAV peptide interferes with establishment of LTP only if present during conditional stimulation. Rebinding of N-cadherin at physiological [Ca2+]e is probably too rapid to allow HAV peptide to compete with the HAV-binding site of N-cadherin. This is supported by the observation that HAV peptides had no effect on the development of LTP when [Ca2+]e is kept high (5 mm) during the conditioning stimulation.
If the cytodomain of cadherins remains tethered to the actin filament system, most of the externally detached cadherins will be kept in place and, hence, be able to rebind after cessation of the stimulus and recovery of [Ca2+]e to values >0.8 mm. [Ca2+]i can be expected to remain for a longer period at elevated levels and stimulate actin depolymerization by binding to gelsolin and calcineurin (Furukawa et al., 1997; Halpain et al., 1998). Local depolymerization of F-actin can be expected to proceed until the arrival of the next burst of stimulated transmitter release with concomitant depletion of [Ca2+]e and rise of [Ca2+]i. This has been elegantly shown by reversible collapse and displacement of presynaptic and postsynaptic green fluorescent protein-tagged actin within 0.5-3 min after application of a single tetanus train (50 Hz lasting 30 sec) and irreversible distributional changes after repetitive spaced tetani (Colicos et al., 2001). During additional episodes of enhanced synaptic activity, a fraction of externally detached cadherins can be assumed no longer immobilized by the actin cytoskeleton. These untethered cadherins will become prone to lateral diffusion in the plane of the presynaptic and postsynaptic plasma membranes. This view is supported by the experiments by Tanaka et al. (2000), who observed rapid dispersion of presynaptic N-cadherin within 1 min after high K+ treatment. Note that most of the dispersed N-cadherin did not colocalize with β-catenin, indicating no linkage to the actin cytoskeleton. Those cadherins that diffuse centrifugally to the periphery of the synaptic area will gradually be exposed to normal [Ca2+]e, allowing them to transinteract with full capacity with cadherins of the opposing synaptic cell surface. These transinteracting cadherins may be subsequently stabilized by the linkage of their cytodomains to actin filaments, which can be expected to be more stable at the synaptic periphery where [Ca2+]i levels may be lower and recover earlier than underneath the active synaptic zone. Rapid recruitment of β-catenin into spine heads by phosphorylation-dependent signaling has been demonstrated recently (Murase et al., 2002), and it appears reasonable to assume that synaptically recruited active (dephosphorylated) β-catenin may serve to stabilize newly formed cadherin bonds at the margins of the synaptic contacts. These bonds will also be less likely challenged by activity-dependent [Ca2+]e depletion than cadherin bonds remaining close to the active synaptic zone. A fraction of these cadherins may be stabilized as dimers by still unknown mechanisms that make them resistant against proteases and denaturation by urea (Tanaka et al., 2000).
Additional repetitions of synaptic stimulation may finally drive all cadherins away from the active synaptic zone toward the synaptic periphery. This might allow active zones to spread laterally and separate into perforated synapses (Toni et al., 2001; Yuste and Bonhoeffer, 2001). The important role of actin dynamics in generation of LTP has been demonstrated by inhibition of both development and maintenance of early LTP by a variety of actin-modulating compounds such as lantrunculin, cytochalasin D, and phalloidin (Kim and Lisman, 1999; Krucker et al., 2000).
Footnotes
This work was supported by Deutsche Forschungsgemeinschaft Grants SFB 487 TP B5 and SFB 581 TP B16. We thank R. M. Mège and M. Lambert for providing the N-cadherin-Fc-expressing cells. We are grateful to Agnes Weth and Heike Arthen for skillful technical assistance.
Correspondence should be addressed to Dr. Detlev Drenckhahn, Institute of Anatomy and Cell Biology, Julius-Maximilians-University, Koellikerstrasse 6, D-97070 Würzburg, Germany. E-mail: anat015@mail.uni-wuerzburg.de.
Copyright © 2003 Society for Neuroscience 0270-6474/03/2311008-07$15.00/0
W.B. and N.G. contributed equally to this work.
References
- Adams CL, Nelson WJ ( 1998) Cytomechanics of cadherin-mediated cell-cell adhesion. Curr Opin Cell Biol 10: 572-577. [DOI] [PubMed] [Google Scholar]
- Baumgartner W, Drenckhahn D ( 2002a) An expectation-maximisation algorithm for the deconvolution of the intrinsic distribution of single molecule's parameters. Comput Chem 26: 321-326. [DOI] [PubMed] [Google Scholar]
- Baumgartner W, Drenckhahn D ( 2002b) Transmembrane cooperative linkage in cellular adhesion. Eur J Cell Biol 81: 161-168. [DOI] [PubMed] [Google Scholar]
- Baumgartner W, Hinterdorfer P, Schindler H ( 2000a) Data analysis of interaction forces measured with the atomic force microscope. Ultramicroscopy 82: 85-95. [DOI] [PubMed] [Google Scholar]
- Baumgartner W, Hinterdorfer P, Ness W, Raab A, Vestweber D, Schindler H, Drenckhahn D ( 2000b) Cadherin interaction probed by atomic force microscopy. Proc Natl Acad Sci USA 97: 4005-4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner W, Schütz GJ, Wiegand J, Golenhofen N, Drenckhahn D ( 2003) Cadherin function probed by laser tweezer and single molecule fluorescence in vascular endothelial cells. J Cell Sci 116: 1001-1011. [DOI] [PubMed] [Google Scholar]
- Benson DL, Schnapp LM, Shapiro L, Huntley GW ( 2000) Making memories stick: cell-adhesion molecules in synaptic plasticity. Trends Cell Biol 10: 473-482. [DOI] [PubMed] [Google Scholar]
- Bernstein BW, Bamburg JR ( 1985) Reorganization of actin in depolarized synaptosomes. J Neurosci 5: 2565-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BW, DeWit M, Bamburg JR ( 1998) Actin disassembles reversibly during electrically induced recycling of synaptic vesicles in cultured neurons. Brain Res Mol Brain Res 53: 236-251. [DOI] [PubMed] [Google Scholar]
- Bi G, Poo M ( 2001) Synaptic modification by correlated activity: Hebb's postulate revisited. Annu Rev Neurosci 24: 139-166. [DOI] [PubMed] [Google Scholar]
- Blaschuk OW, Sullivan R, David S, Pouliot Y ( 1990) Identification of a cadherin cell adhesion recognition sequence. Dev Biol 139: 227-229. [DOI] [PubMed] [Google Scholar]
- Bozdagi O, Shan W, Tanaka H, Benson DL, Huntley GW ( 2000) Increasing numbers of synaptic puncta during late-phase LTP: N-cadherin is synthesized, recruited to synaptic sites, and required for potentiation. Neuron 28: 245-259. [DOI] [PubMed] [Google Scholar]
- Bruses JL ( 2000) Cadherin-mediated adhesion at the interneuronal synapse. Curr Opin Cell Biol 12: 593-597. [DOI] [PubMed] [Google Scholar]
- Bubb MR, Senderowicz AM, Sausville EA, Duncan KL, Korn ED ( 1994) Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J Biol Chem 269: 14869-14871. [PubMed] [Google Scholar]
- Colicos MA, Collins BE, Sailor MJ, Goda Y ( 2001) Remodeling of synaptic actin induced by photoconductive stimulation. Cell 107: 605-616. [DOI] [PubMed] [Google Scholar]
- Egelman DM, Montague PR ( 1999) Calcium dynamics in the extracellular space of mammalian neural tissue. Biophys J 76: 1856-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fannon AM, Colman DR ( 1996) A model for central synaptic junctional complex formation based on the differential adhesive specificities of the cadherins. Neuron 17: 423-434. [DOI] [PubMed] [Google Scholar]
- Faulstich H, Trischmann H, Mayer D ( 1983) Preparation of tetramethylrhodaminyl-phalloidin and uptake of the toxin into short-term cultured hepatocytes by endocytosis. Exp Cell Res 144: 73-82. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Fu W, Li Y, Witke W, Kwiatkowski DJ, Mattson MP ( 1997) The actin-severing protein gelsolin modulates calcium channel and NMDA receptor activities and vulnerability to excitotoxicity in hippocampal neurons. J Neurosci 17: 8178-8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golenhofen N, Ness W, Wawrousek EF, Drenckhahn D ( 2002) Expression and induction of the stress protein alpha-B-crystallin in vascular endothelial cells. Histochem Cell Biol 117: 203-209. [DOI] [PubMed] [Google Scholar]
- Gotsch U, Borges E, Bosse R, Boggemeyer E, Simon M, Mossmann H, Vestweber D ( 1997) VE-cadherin antibody accelerates neutrophil recruitment in vivo J Cell Sci 110: 583-588. [DOI] [PubMed] [Google Scholar]
- Greene LA, Tischler AS ( 1976) Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA 73: 2424-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM ( 2000) Regulation of cadherin adhesive activity. J Cell Biol 148: 399-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpain S, Hipolito A, Saffer L ( 1998) Regulation of F-actin stability in dendritic spines by glutamate receptors and calcineurin. J Neurosci 18: 9835-9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinterdorfer P, Baumgartner W, Gruber HJ, Schilcher K, Schindler H ( 1996) Detection and localization of individual antibody-antigen recognition events by atomic force microscopy. Proc Natl Acad Sci USA 93: 3477-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley A, Ernst E ( 2000) Complementary and alternative therapies for treating multiple sclerosis symptoms: a systematic review. Complement Ther Med 8: 97-105. [DOI] [PubMed] [Google Scholar]
- Kawamura S, Miyamoto S, Brown JH ( 2003) Initiation and transduction of stretch-induced RhoA and Rac1 activation through caveolae: cytoskeletal regulation of ERK translocation. J Biol Chem 278: 31111-31117. [DOI] [PubMed] [Google Scholar]
- Kim CH, Lisman JE ( 1999) A role of actin filament in synaptic transmission and long-term potentiation. J Neurosci 19: 4314-4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjevic K, Morris ME, Reiffenstein RJ ( 1980) Changes in extracellular Ca2+ and K+ activity accompanying hippocampal discharges. Can J Physiol Pharmacol 58: 579-582. [DOI] [PubMed] [Google Scholar]
- Krucker T, Siggins GR, Halpain S ( 2000) Dynamic actin filaments are required for stable long-term potentiation (LTP) in area CA1 of the hippocampus. Proc Natl Acad Sci USA 97: 6856-6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhne W, Besselmann M, Noll T, Muhs A, Watanabe H, Piper HM ( 1993) Disintegration of cytoskeletal structure of actin filaments in energy-depleted endothelial cells. Am J Physiol 264: H1599-H1608. [DOI] [PubMed] [Google Scholar]
- Lambert M, Padilla F, Mège RM ( 2000) Immobilized dimers of N-cadherin-Fc chimera mimic cadherin-mediated cell contact formation: contribution of both outside-in and inside-out signals. J Cell Sci 113: 2207-2219. [DOI] [PubMed] [Google Scholar]
- Lutz KL, Jois SD, Siahaan TJ ( 1995) Secondary structure of the HAV peptide which regulates cadherin-cadherin interaction. J Biomol Struct Dyn 13: 447-455. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA ( 1999) Long-term potentiation-a decade of progress? Science 285: 1870-1874. [DOI] [PubMed] [Google Scholar]
- Manabe T, Togashi H, Uchida N, Suzuki SC, Hayakawa Y, Yamamoto M, Yoda H, Miyakawa T, Takeichi M, Chisaka O ( 2000) Loss of cadherin-11 adhesion receptor enhances plastic changes in hippocampal synapses and modifies behavioral responses. Mol Cell Neurosci 15: 534-546. [DOI] [PubMed] [Google Scholar]
- Marrs GS, Green SH, Dailey ME ( 2001) Rapid formation and remodeling of postsynaptic densities in developing dendrites. Nat Neurosci 4: 1006-1013. [DOI] [PubMed] [Google Scholar]
- Masuda Y, Oguma T, Kimura A ( 2002) Biphasic effects of oxethazaine, a topical anesthetic, on the intracellular Ca(2+) concentration of PC12 cells. Biochem Pharmacol 64: 677-687. [DOI] [PubMed] [Google Scholar]
- Matus A ( 2000) Actin-based plasticity in dendritic spines. Science 290: 754-758. [DOI] [PubMed] [Google Scholar]
- Moll T, Vestweber D ( 1999) Construction and purification of adhesion molecule immunoglobulin chimeric proteins. Methods Mol Biol 96: 77-84. [DOI] [PubMed] [Google Scholar]
- Murase S, Schuman EM ( 1999) The role of cell adhesion molecules in synaptic plasticity and memory. Curr Opin Cell Biol 11: 549-553. [DOI] [PubMed] [Google Scholar]
- Murase S, Mosser E, Schuman EM ( 2002) Depolarization drives beta-Catenin into neuronal spines promoting changes in synaptic structure and function. Neuron 35: 91-105. [DOI] [PubMed] [Google Scholar]
- Nicholson C, ten Bruggencate G, Stockle H, Steinberg R ( 1978) Calcium and potassium changes in extracellular microenvironment of cat cerebellar cortex. J Neurophysiol 41: 1026-1039. [DOI] [PubMed] [Google Scholar]
- Noe V, Chastre E, Bruyneel E, Gespach C, Mareel M ( 1999) Extracellular regulation of cancer invasion: the E-cadherin-catenin and other pathways. Biochem Soc Symp 65: 43-62. [PubMed] [Google Scholar]
- Perkel DJ, Petrozzino JJ, Nicoll RA, Connor JA ( 1993) The role of Ca2+ entry via synaptically activated NMDA receptors in the induction of long-term potentiation. Neuron 11: 817-823. [DOI] [PubMed] [Google Scholar]
- Pertz O, Bozic D, Koch AW, Fauser C, Brancaccio A, Engel J ( 1999) A new crystal structure, Ca2+ dependence and mutational analysis reveal molecular details of E-cadherin homoassociation. EMBO J 18: 1738-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr WG, Tank DW ( 1990) Postsynaptic NMDA receptor-mediated calcium accumulation in hippocampal CA1 pyramidal cell dendrites. Nature 345: 807-810. [DOI] [PubMed] [Google Scholar]
- Rusakov DA ( 2001) The role of perisynaptic glial sheaths in glutamate spillover and extracellular Ca(2+) depletion. Biophys J 81: 1947-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittler HJ, Franke RP, Akbay U, Mrowietz C, Drenckhahn D ( 1993) Improved in vitro rheological system for studying the effect of fluid shear stress on cultured cells. Am J Physiol 265: C289-C298. [DOI] [PubMed] [Google Scholar]
- Scott VR, Boehme R, Matthews TR ( 1988) New class of antifungal agents: jasplakinolide, a cyclodepsipeptide from the marine sponge, Jaspis species. Antimicrob Agents Chemother 32: 1154-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer TJ, Atchison WD( 1991) Transmitter, ion channel and receptor properties of pheochromocytoma (PC12) cells: a model for neurotoxicological studies. Neurotoxicology 12: 473-492. [PubMed] [Google Scholar]
- Shapiro L, Fannon AM, Kwong PD, Thompson A, Lehmann MS, Grubel G, Legrand JF, Als-Nielsen J, Colman DR, Hendrickson WA ( 1995) Structural basis of cell-cell adhesion by cadherins. Nature 374: 327-337. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Shan W, Phillips GR, Arndt K, Bozdagi O, Shapiro L, Huntley GW, Benson DL, Colman DR ( 2000) Molecular modification of N-cadherin in response to synaptic activity. Neuron 25: 93-107. [DOI] [PubMed] [Google Scholar]
- Tang L, Hung CP, Schuman EM ( 1998) A role for the cadherin family of cell adhesion molecules in hippocampal long-term potentiation. Neuron 20: 1165-1175. [DOI] [PubMed] [Google Scholar]
- Toni N, Buchs PA, Nikonenko I, Povilaitite P, Parisi L, Muller D ( 2001) Remodeling of synaptic membranes after induction of long-term potentiation. J Neurosci 21: 6245-6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner IB, Wood PM ( 2002) N-cadherin mediates axon-aligned process growth and cell-cell interaction in rat Schwann cells. J Neurosci 22: 4066-4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese S, Digby MR, Gunnersen JM, Gotz R, Pei G, Holtmann B, Lowenthal J, Sendtner M ( 1999) The anti-apoptotic protein ITA is essential for NGF-mediated survival of embryonic chick neurons. Nat Neurosci 2: 978-983. [DOI] [PubMed] [Google Scholar]
- Williams E, Williams G, Gour BJ, Blaschuk OW, Doherty P ( 2000) A novel family of cyclic peptide antagonists suggests that N-cadherin specificity is determined by amino acids that flank the HAV motif. J Biol Chem 275: 4007-4012. [DOI] [PubMed] [Google Scholar]
- Williams EJ, Williams G, Howell FV, Skaper SD, Walsh FS, Doherty P ( 2001) Identification of an N-cadherin motif that can interact with the fibroblast growth factor receptor and is required for axonal growth. J Biol Chem 276: 43879-43886. [DOI] [PubMed] [Google Scholar]
- Yin HL, Stossel TP ( 1979) Control of cytoplasmic actin gel-sol transformation by gelsolin, a calcium-dependent regulatory protein. Nature 281: 583-586. [DOI] [PubMed] [Google Scholar]
- Yuste R, Bonhoeffer T ( 2001) Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu Rev Neurosci 24: 1071-1089. [DOI] [PubMed] [Google Scholar]