Abstract
Activation of cannabinoid CB1 receptors reduces glutamatergic synaptic transmission in the rodent striatum and is involved in the normal control of motor function by the basal ganglia. Here we investigated CB1 receptor regulation of glutamate release and uptake and synaptic transmission in the rat striatum. We show that CB1 receptor activation reduces both the release and uptake of [3H]glutamate in striatal slices. We also demonstrate that both activation of CB1 receptors and inhibition of glutamate uptake reduce corticostriatal synaptic transmission in a mutually occlusive manner and that both forms of depression are dependent on metabotropic glutamate receptor (mGluR) activation. We propose that CB1 receptor activation in the striatum decreases glutamate transporter activity and that the resulting increase in synaptic cleft glutamate concentration causes the activation of presynaptic mGluRs, which then decrease glutamate release.
Keywords: cannabinoid, striatum, glutamate, uptake, release, mGluR, tetrahydrocannabinol, basal ganglia
Introduction
Activation of cannabinoid CB1 receptors induces a characteristic array of behavioral effects in animals that include profound alterations in locomotor activity (Pertwee, 1997). The striatum, a brain region showing dense expression of the CB1 receptor, is an important site mediating these locomotor effects (Rodriguez et al., 1998). CB1 receptors couple to Gi/o type G-proteins that negatively regulate adenylyl cyclase activity and modulate the activity of various ion conductances (Pertwee, 1997). Electrophysiological studies in the rat striatum indicate that CB1 receptor activation reduces glutamatergic synaptic transmission, in a manner consistent with presynaptic inhibition of neurotransmitter release (Gerdeman and Lovinger, 2001; Huang et al., 2001). Such an action is likely to be of great physiological significance because excitatory input from the cortex provides the main driving force for striatal output and, therefore, movement (Rodriguez et al., 1998). Furthermore, activation of CB1 receptors is known to be necessary for the expression of long-term depression (LTD) at corticostriatal synapses, a phenomenon believed to be involved in motor learning (Gerdeman et al., 2002). Although the presence of CB1 receptors on the terminals of glutamatergic neurons that innervate the striatum supports the concept that cannabinoids reduce striatal glutamate release (Rodriguez et al., 2001), such an action has not been shown directly. Furthermore, the effect of cannabinoids on glutamate reuptake has never been investigated. We addressed these issues by investigating the effects of the active cannabis constituent Δ9-tetrahydrocannabinol (THC) on the uptake and K+-stimulated release of [3H]glutamate from rat striatal slices and on corticostriatal synaptic transmission.
Materials and Methods
Slice preparation. Male Sprague Dawley rats (250-350 gm) were killed by cervical dislocation, and their brains were placed in 4°C artificial CSF (aCSF) (in mm: 118 NaCl, 4.8 KCl, 2.6 CaCl2, 1.2 MgSO4, 25 NaHCO3, 1.2 KH2PO4, 11 glucose, and 0.6 ascorbic acid) aerated with 95% O2-5% CO2. Coronal slices (400 μm) were prepared, and, for glutamate release and uptake experiments, the striatum was then dissected from the surrounding tissue using a tissue punch (2 mm in diameter). For electrophysiological experiments, hemislices containing the striatum and overlying white matter were used.
[3H]Glutamate release assay. Release of [3H]glutamate was assessed as described previously (Mitchell and Doggett, 1980) with minor modifications. Striatal tissue was incubated for 30 min in oxygenated aCSF at 37°C containing 1 μm [3H]glutamic acid (42.9 Ci/mmol; NEN, Boston, MA) and then placed in the individual chambers of a Brandel SF-12 superfusion system (Brandel Instruments, Gaithersburg, MD), sandwiched between two filter papers, and perfused at 0.5 ml/min with oxygenated aCSF at 35-37°C. After a 30 min stabilization period, the perfusate was collected in 5 min time bins. At the end of the experiment, striatal tissue was recovered, resuspended in 0.5 ml of aCSF, and disrupted by probe sonication. Radioactivity in perfusates and tissue samples was determined by liquid scintillation spectroscopy (TriCarb 1500 Liquid Scintillation Analyser; Packard Instruments, Downers Grove, IL).
K+-evoked efflux of [3H]glutamate was assessed by applying a 5 min pulse of aCSF containing 40 mm KCl and calculated by subtracting the fraction of glutamate released in the 5 min period before KCl addition from the 5 min period after. K+-evoked release was almost entirely Ca2+ dependent (K+-evoked release in Ca2+-free aCSF containing 2 mm CoCl2 and 1 mm EGTA was 5 ± 5% of control; n = 6). Basal release of isotope was unchanged in Ca2+-free solution (99 ± 5% of control; n = 6). THC was applied for 20 min before coapplication with the K+ pulse. In antagonist experiments, SR141716 was applied 5 min before the addition of THC. The effect of THC on K+-evoked release was expressed as a percentage of control values for each experiment and presented as the mean ± SEM of at least seven separate experiments performed in triplicate.
[3H]Glutamate uptake assay. [3H]Glutamate uptake was assessed using a method derived from Benjamin and Quastel (1976). Striatal tissue was incubated for 25 min at 37°C in oxygenated aCSF containing vehicle or test compounds. After 25 min, [3H]glutamic acid was added to the medium to give a final concentration of 250 nm, and the incubation continued for 30 min. At the end of the experiment, slices were rinsed in 5 ml of aCSF and sonicated, and the isotope content was quantified as described above. Aliquots of the homogenate were also used to determine protein content of the slices, according the method of Bradford (1976). Under these conditions, glutamate uptake increased linearly with time for up to 60 min and was not affected by an inhibitor of GABA transport (nipecotic acid; data not shown). Glutamate uptake was dependent on Na+ and temperature (9 ± 5% of control when NaCl was replaced with choline chloride; 4 ± 1% of control at 4°C; n = 6 for each) and was reduced to 33 ± 2% of control by the glutamate uptake inhibitor β-threo-hydroxy-aspartate (THA) (n = 6). The effects of THC on glutamate uptake are expressed as the mean ± SEM glutamate uptake (picomoles per milligram of protein) of at least six separate experiments performed in triplicate.
Electrophysiology. Slices were maintained at 30°C in a submerged recording chamber perfused with oxygenated aCSF (3-3.5 ml/min). Extracellular recordings were made using a glass microlectrode filled with aCSF placed in the dorsolateral striatum. Afferent fibers were stimulated by delivering monophasic constant voltage pulses to a bipolar nickel-chromium electrode placed in the white matter overlying the striatum at a frequency of 0.067 Hz. Data were filtered at 3 kHz and digitized at 10 kHz. Responses were recorded and analyzed online and offline using LTP software (Anderson and Collingridge, 2001). The amplitude of the population spike was used as a measure of synaptic transmission. After obtaining a stable baseline of at least 20 min, drugs were applied by addition to the perfusate.
Drugs. THC and SR141716 were stored as ethanol stock solutions (30 and 0.1 mm). AM251 and LY341495 were stored as DMSO stock solutions (10 and 0.5 mm). THA and (R)-baclofen were stored as stock solutions in one equivalent NaOH (100 and 10 μm). (RS)-3,5-Dihydroxyphenylglycine (DHPG), l-AP-4, and (2R,4 R)-4-aminopyrrolidene-2,4-dicarboxylate [(2R,4R)-APDC] were dissolved in dH2O (100, 30, and 50 mm). Dihydrokainate (DHK) was dissolved directly in aCSF as required. In all cases, application of vehicle did not affect synaptic responses (data not shown). THC and THA were purchased from Sigma (Poole, UK). SR141716 was a generous gift from Sanoffi-Synthelabo (Montpellier, France). All other drugs were purchased from Tocris Cookson (Bristol, UK).
Data analysis. All data are presented as mean ± SEM. Effects of cannabinoids on the release and uptake of glutamate were compared using one-way ANOVA followed by post hoc Tukey's test. Concentration-effect curves were fitted using the equation for a sigmoid dose-response curve (GraphPad Prism 3.0; GraphPad Software, San Diego, CA).
Results
THC depresses glutamate release
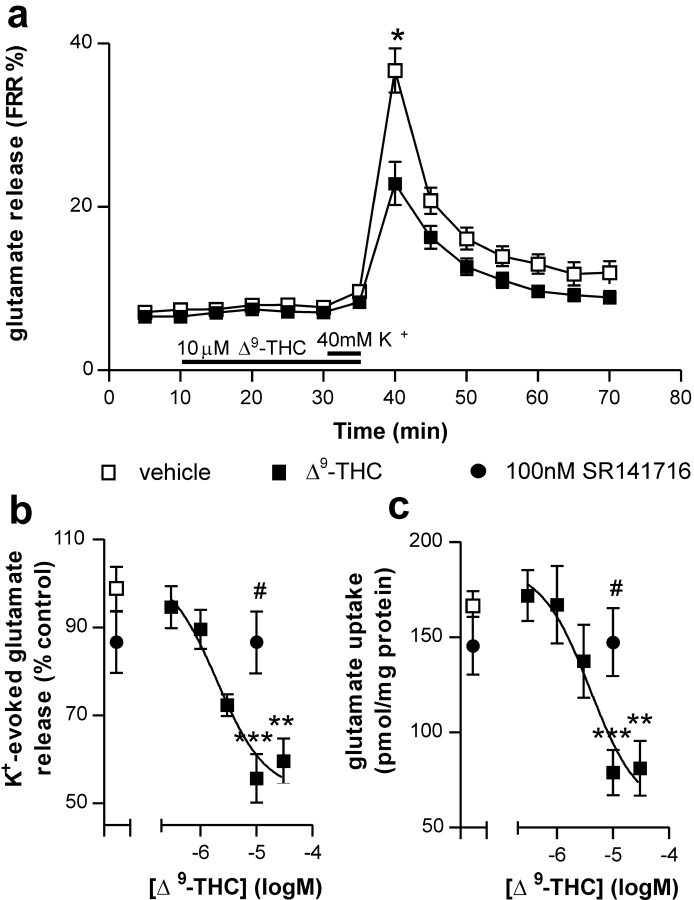
Basal release of [3H]glutamate was not affected by incubation with THC (Fig. 1a). THC dose dependently inhibited the K+-evoked release of [3H]glutamate from striatal slices (p < 0.001) with a calculated EC50 of 2.0 μm (Fig. 1a,b). The maximal effect of THC occurred at 10 μm (56 ± 6% of control; p < 0.001) and was blocked by 100 nm SR141716, a CB1 receptor antagonist (87 ± 9% of control values; p < 0.05 compared with 10 μm THC alone). SR141716 alone did not affect K+-evoked glutamate release (103 ± 11% of control values; p > 0.05).
Figure 1.
Effects of THC on [3H]glutamate release and uptake in rat striatal tissue. Application of 10 μm THC reduced K+-evoked glutamate release but did not effect basal efflux (a). The effects of THC on K+-evoked glutamate release were dose dependent and were blocked by the CB1 receptor antagonist SR141716 (b). THC also dose dependently reduced glutamate uptake in an SR141716-sensitive manner (c). Data points represent averages of six (a) and 6-10 (b, c) separate experiments performed in triplicate. Filled bars in a represent timing and duration of drug addition. Data were analyzed using one-way ANOVA followed by Tukey's post hoc test. **p < 0.01 and ***p <0.001 compared with vehicle; #p < 0.05 compared with THC alone.
THC depresses glutamate uptake
Incubation of striatal slices with THC resulted in a dose-dependent inhibition of [3H]glutamate uptake (p < 0.001) with a calculated EC50 of 3.9 μm (Fig. 1c). The maximal effect of THC on glutamate uptake, which occurred at a concentration of 10 μm (47 ± 7% of control; p < 0.001), was blocked by 100 nm SR141716 (89 ± 11% of control; p < 0.05 compared with 10 μm THC alone) and was completely occluded in the presence of an inhibitor of glutamate reuptake, 300 μm l-aspartate-β-hydroxamate (97 ± 5% of 10 μm THC alone; p > 0.05; data not shown). Incubation of striatal slices with SR141716 alone did not significantly affect glutamate uptake (88 ± 9% of control; p > 0.05).
THC depresses corticostriatal transmission
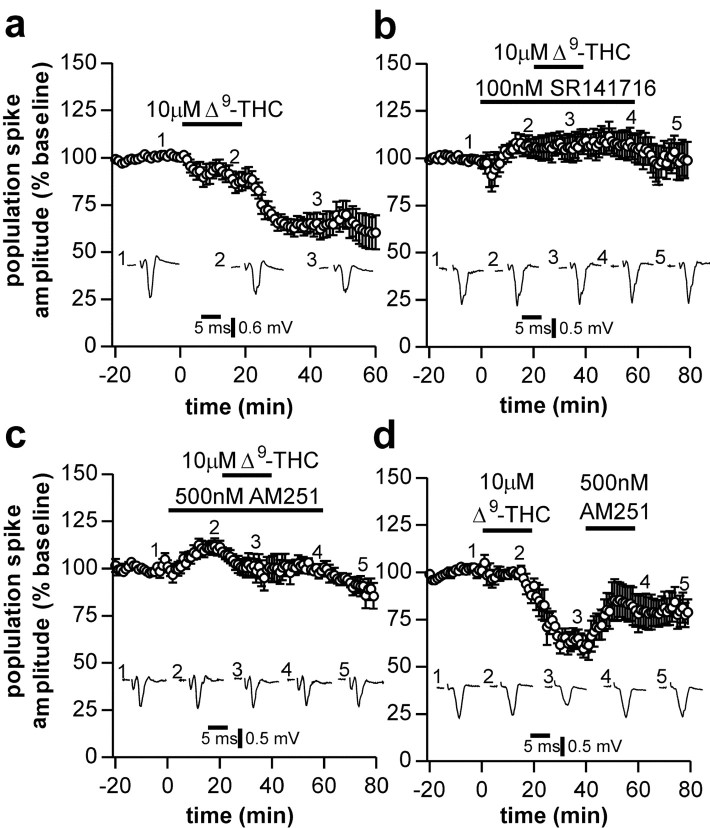
Application of 10 μm THC for 20 min elicited a depression in the population spike amplitude that persisted for the duration of the recording (Fig. 2a). Ten to 20 min after commencing washout of THC, responses were 64 ± 4% of control (n = 7; p < 0.05). This depression was prevented by application of the CB1 receptor antagonists SR141716 (100 nm; 102 ± 3%; n = 4; p < 0.05 compared with THC alone) (Fig. 2b) and AM251 (500 nm; 91 ± 4%; n = 4; p < 0.05 compared with THC alone) (Fig. 2c). Application of 500 nm AM251 20-40 min after washout of THC elicited a partial reversal in the depression to 90 ± 9% of control (n = 4; p < 0.05 compared with pre-AM251) (Fig. 2d), indicating that the persistent effect observed with THC may be attributable to slow washout of the drug consistent with the high lipid solubility of cannabinoids. This high lipid solubility may also explain the slow onset of the depression observed with THC application (Fig. 2a). The synthetic cannabinoid WIN 55,212-2 (3 μm) also depressed synaptic transmission to a similar degree as THC with a similar time delay (responses 46 ± 11% of control; n = 6; data not shown).
Figure 2.
Effects of THC on corticostriatal neurotransmission. Application of 10 μm THC induced a slowly developing, long-lasting depression in population spike amplitude (a). When applied in the presence of CB1 receptor antagonists, 100 nmSR141716 (b) or 500 nm AM251 (c), THC no longer depressed population spike amplitude. The persistent depression induced by application of THC was partially restored by subsequent application of AM251 (d). In this and subsequent figures, inset traces (1-5) represent the average of four individual responses from a typical experiment at the indicated time points, and data points represent the average response each minute from four to seven separate experiments.
Blockade of glutamate uptake mimics the actions of THC
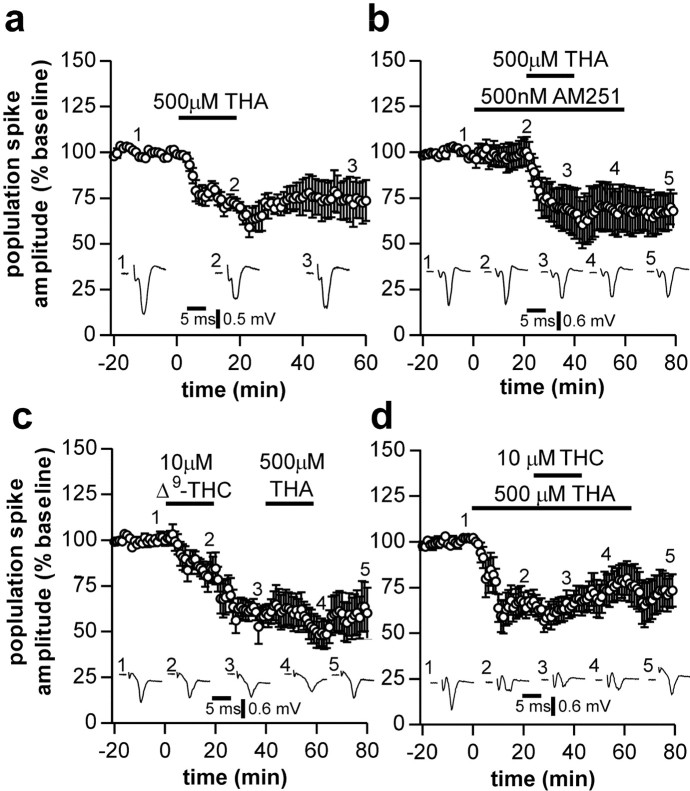
Because we showed here that CB1 receptor activation reduces glutamate uptake as well as glutamate release, we were interested to see what effect blockade of glutamate uptake had on corticostriatal transmission. Application of 500 μm THA (a transportable inhibitor of glutamate transporters) for 20 min produced a depression in population spike amplitude (0-10 min after washout of THA responses were 66 ± 5% of control; n = 8; p < 0.05) (Fig. 3a). The effect of THA was not antagonized by AM251 (500 nm; responses were 62 ± 10% of control; n = 8) (Fig. 3b). We also investigated the effects of the nontransportable inhibitor DHK. Application of DHK (300 μm, 20 min) also depressed synaptic transmission (responses 51 ± 7% of control; n = 4; p < 0.05; data not shown).
Figure 3.
Effects of glutamate uptake inhibition on corticostriatal neurotransmission. Application of 500 μm THA induced a long-lasting depression in population spike amplitude (a) that was not prevented by the CB1 receptor antagonist AM251 (b). Application of THA no longer depressed population spike amplitude when applied after THC (c). Similarly, THC no longer depressed population spike amplitude when applied in the presence of THA (d).
The effect of THA on corticostriatal transmission coupled to the observation that THC decreases glutamate uptake into striatal slices raises the possibility that the primary effect of CB1 activation is to decrease glutamate uptake. Therefore, we next investigated whether the activation of CB1 receptors and inhibition of glutamate uptake depress glutamate transmission via a common mechanism. After application of 10 μm THC (responses depressed to 60 ± 5% of control), subsequent application of 500 μm THA failed to further depress transmission (responses 54 ± 10% of control; n = 6; p > 0.05 compared with THC alone) (Fig. 3c). Similarly, DHK also failed to further depress synaptic transmission after application of THC (responses 47 ± 7% of control after THC, 47 ± 15% of control after subsequent application of DHK; n = 4; p > 0.05; data not shown). In experiments in which THA was applied for 60 min and THC was coapplied for the middle 20 min of this application, the depression induced by THA was not increased during addition of THC (responses before and after application of THC were 64 ± 8% of control and 74 ± 9% of control, respectively; n = 6; p > 0.05) (Fig. 3d). This suggests that blockade of glutamate transport and activation of CB1 receptors act to depress glutamate transmission by a similar mechanism.
Metabotropic glutamate receptors are involved in the cannabinoid regulation of corticostriatal transmission
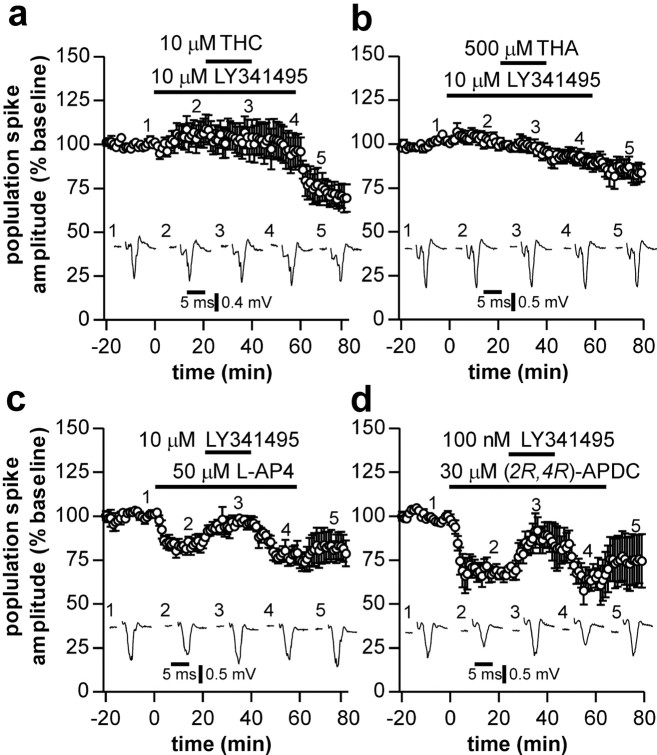
A consequence of reducing glutamate uptake will be to increase the extracellular glutamate concentration. This increase in glutamate may then activate presynaptic metabotropic glutamate receptors (mGluRs) to decrease glutamate release. Therefore, we next investigated whether the synaptic depression induced by both THC and THA involves activation of mGluRs. The broad-spectrum mGluR antagonist LY341495 (10 μm) abolished the depression induced by THC (responses 93 ± 4% of control measured 10-20 min after washout of THC; n = 8) (Fig. 4a). After commencing washout of LY341495, responses rapidly decreased in amplitude, which may again reflect the poor clearance rate of THC from brain tissue compared with the rapid clearance of LY341495. The depression induced by THA was also antagonized by 10 μmLY341495 (responses 91 ± 4% of control; n = 7) (Fig. 4b). In contrast, the reduction in synaptic transmission resulting from GABAB receptor activation by 1 μm (R)-baclofen was not reduced by LY341495 (32 ± 11 vs 33 ± 9% of control; n = 5 and 6, respectively; p > 0.05; data not shown), ruling out a nonspecific effect of LY341495 on G-protein function in the presynaptic terminal.
Figure 4.
Effects of mGluR activation on corticostriatal neurotransmission. Application of THC (a) or THA (b) no longer reduced population spike amplitude when applied in the presence of the broad-spectrum mGluR antagonist LY341495. Depressions in population spike amplitude induced by agonists of group III, l-AP-4 (c), and group II, (2R,4R)-APDC (d), mGluRs were also prevented by LY341495.
Finally, we investigated the effects of activation of mGluRs on corticostriatal transmission. The group I-specific mGluR agonist DHPG (100 μm) failed to affect synaptic transmission (n = 4; data not shown). However, activation of group II mGluRs with 2R,4R-APDC (30 μm) or group III mGluRs with l-AP-4 (50 μm) elicited depressions in population spike amplitude (responses depressed to 63 ± 5 and 82 ± 3% of control by APDC and l-AP-4, respectively), which were reversed by LY341495 (Fig. 4c,d) but were insensitive to application of AM251 (data not shown).
Discussion
We showed here that activation of CB1 receptors reduces both the uptake and release of glutamate from striatal slices. Furthermore, both activation of CB1 receptors and inhibition of glutamate uptake elicit a depression in corticostriatal synaptic transmission. The depression observed under these two conditions is mutually occlusive, suggesting that they act via a common mechanism. As the depression induced by both CB1 receptor activation and inhibition of glutamate uptake are blocked by an antagonist of mGluRs, we believe that activation of CB1 receptors reduces glutamate uptake, which leads to activation of presynaptic mGluRs attributable to increased concentrations of glutamate in the synaptic cleft.
Previously, it has been demonstrated that the reduction in corticostriatal synaptic transmission seen during CB1 receptor activation is mediated by a presynaptic decrease in glutamate release (Gerdeman and Lovinger, 2001; Huang et al., 2001) that is dependent on a reduction in N-type Ca2+ channel conductance (Huang et al., 2001). To date, the assumption has been that presynaptic CB1 receptors directly mediate this inhibition of Ca2+ conductance and thus the reduction in glutamate release. Our data are consistent with the depression in glutamate transmission being mediated by a presynaptic effect involving a reduction in Ca2+ conductance, but we believe that activation of presynaptic group II-III mGluRs rather than CB1 receptors is responsible for this modulation. Group II and III mGluRs are known to negatively couple to Ca2+ conductances and to decrease transmitter release in numerous brain regions (Anwyl, 1999).
CB1 receptors reduce glutamate release and uptake
Although electrophysiology studies have suggested that CB1 receptor activation inhibits the release of glutamate in the striatum (Gerdeman and Lovinger, 2001; Huang et al., 2001), ours is the first study to show this by measuring the release of [3H]glutamate. Activation of CB1 receptors has also been shown to inhibit glutamate release in the hippocampus (Wang, 2003). However, this is the first study to show that activation of CB1 receptors also decreases glutamate uptake in the CNS. It is unlikely that this decrease in uptake is a result of altered glutamate efflux because THC has no effect on basal glutamate release and release was not stimulated by application of high K+ during the uptake experiments. Activation of CB1 receptors has also been shown to reduce the uptake of GABA in the basal ganglia (Maneuf et al., 1996), but the mechanism involved is unknown.
Our data show that the depressions in synaptic transmission induced by activation of CB1 receptors and by inhibition of glutamate uptake are mutually occlusive. Occlusion experiments can be difficult to interpret and, under some circumstances, may reflect that two independent pathways converge onto a common final target. However, given that the depressions induced by both THA and THC are blocked by the mGluR antagonist LY341495, whereas only the THC depression is sensitive to CB1 receptor antagonists, we are confident that these results show that the occlusion occurs at the level of mGluR activation (i.e., THC increases glutamate levels to activate mGluRs) rather than at some downstream regulator of transmitter release (e.g., inhibition of N-type Ca2+ channels).
The rodent striatum is known to express the GLT1 (glutamate transporter 1), GLAST (glutamate-aspartate transporter), and EAAC (excitatory amino acid carrier) glutamate transporters (Danbolt, 2001). Although THA is a transportable inhibitor of all glutamate transporters, DHK, a nontransportable inhibitor of uptake, is selective for the GLT1 transporter that is thought to be the major transporter of glutamate in most brain regions and is predominantly expressed by glia (Danbolt, 2001). However, recent studies have shown that DHK blocks ∼70% of total transporter activity in neuronal preparations (Suchak et al., 2003), and a DHK-sensitive variant of GLT1 that is expressed by neurons has been identified (Chen et al., 2002). Our results therefore suggest that the GLT1 transporter is involved in the CB1 receptor-mediated depression of transmission, but we cannot at this stage rule out the involvement of additional transporter types. Given that striatal CB1 receptors are expressed on presynaptic and postsynaptic neurons and on glia (Rodriguez et al., 2001) and that GLT1 variants are expressed by both neurons and glia, it is not yet possible to identify the location of the transporters involved. Similarly, how CB1 receptor activation modulates transporter function is unknown, although it may involve regulation of transporter protein phosphorylation. For example, PKA activation is known to increase striatal glutamate transporter function (Pisano et al., 1996), and thus, by reducing cAMP levels via a Gi/o G-protein, CB1 receptor activation could alter transporter phosphorylation and function.
Role of mGluRs
LY341495 is an antagonist of all subtypes of mGluRs (Kingston et al., 1998) but does not antagonize CB1 receptor effects per se (Kreitzer and Regehr, 2001; Sjöström et al., 2003). However, this compound shows some selectivity depending on dose: at the concentration we used (10 μm), LY341495 will effectively antagonize group II (mGlu2 and mGlu3) and group III (mGlu7 and mGlu8) receptors but not group I receptors (Kingston et al., 1998). Thus, it is likely that group II and/or group III mGluRs are involved in the depression of glutamate transmission observed here. This is consistent with our and previous (Rouse et al., 2000) observations that agonists of group II and III, but not group I, mGluRs depress transmission and with the known presynaptic localization of both group II and II mGluRs in the striatum.
Inhibition of glutamate uptake would also be expected to facilitate activation of postsynaptic group I mGluRs, as has been demonstrated in the cerebellum (Brasnjo and Otis, 2001). However, we do not believe that activation of group I receptors is involved in the depression observed by CB1 receptor activation, because (1) group I mGluR agonists do not depress synaptic transmission in the striatum and (2) LY341495 is not an effective antagonist of group I receptors at the concentration we used. We cannot rule out that activation of group I receptors does occur after activation of CB1 receptors and inhibition of glutamate transporter function, but such activation probably produces changes in postsynaptic neurons that are not detected using extracellular recording techniques. Interestingly, group I mGluRs are required for induction of LTD at corticostriatal synapses (Gubellini et al., 2001; Sung et al., 2001) as are CB1 receptors (Gerdeman et al., 2002). We do not yet know whether the inhibition of glutamate uptake mechanisms by CB1 receptors is involved in LTD induction, but it is possible that, during induction of LTD, activation of CB1 receptors may facilitate activation of group I mGluRs.
Conclusion
We showed that activation of CB1 receptors reduces glutamate release in the striatum via an indirect effect involving presynaptic mGluRs. Activation of mGluRs is achieved by a CB1 receptor-dependent reduction in glutamate transporter function that raises the extracellular glutamate concentration. These findings add unexpected complexity to the effects of cannabinoids on synaptic transmission. Given that cannabinoids are known to reduce synaptic transmission in numerous brain regions, it will be interesting to see whether CB1 receptor regulation of transporter function also occurs at other synapses and to identify the mechanisms involved.
Footnotes
This work was funded by the Biotechnology and Biological Sciences Research Council.
Correspondence should be addressed to Dr. Stephen M. Fitzjohn, School of Biological Sciences, University of Manchester, 1.124 Stopford Building, Oxford Road, Manchester, M13 9PT, UK. E-mail: stephen.fitzjohn@man.ac.uk.
J. M. Brotchie's present address: Toronto Western Research Institute, Division of Applied and Interventional Research, McLaughlin Wing, Room 11-419, 399 Bathurst Street, Toronto, Ontario, M5T 2S8, Canada.
Copyright © 2003 Society for Neuroscience 0270-6474/03/2311073-05$15.00/0
References
- Anderson WW, Collingridge GL ( 2001) The LTP Program: a data acquisition program for on-line analysis of long-term potentiation and other synaptic events. J Neurosci Methods 108: 71-83. [DOI] [PubMed] [Google Scholar]
- Anwyl R ( 1999) Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Brain Res Rev 29: 83-120. [DOI] [PubMed] [Google Scholar]
- Benjamin AM, Quastel JH ( 1976) Cerebral uptakes and exchange diffusion in vitro of l- and d-glutamates. J Neurochem 26: 431-441. [DOI] [PubMed] [Google Scholar]
- Bradford MM ( 1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254. [DOI] [PubMed] [Google Scholar]
- Brasnjo G, Otis TS ( 2001) Neuronal glutamate transporters control activation of postsynaptic metabotropic glutamate receptors and influence cerebellar long-term depression. Neuron 31: 607-616. [DOI] [PubMed] [Google Scholar]
- Chen W, Aoki C, Mahadomrongkul V, Gruber CE, Wang GJ, Blitzblau R, Irwin N, Rosenberg PA ( 2002) Expression of a variant form of the glutamate transporter GLT1 in neuronal cultures and in neurons and astrocytes in the rat brain. J Neurosci 22: 2142-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC ( 2001) Glutamate uptake. Prog Neurobiol 65: 1-105. [DOI] [PubMed] [Google Scholar]
- Gerdeman G, Lovinger DM ( 2001) CB1 cannabinoid receptor inhibits synaptic release of glutamate in rat dorsolateral striatum. J Neurophysiol 85: 468-471. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM ( 2002) Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci 5: 446-451. [DOI] [PubMed] [Google Scholar]
- Gubellini P, Saulle E, Centonze D, Bonsi P, Pisani A, Bernardi G, Conquet F, Calabresi P ( 2001) Selective involvement of mGlu1 receptors in corticostriatal LTD. Neuropharmacology 40: 839-846. [DOI] [PubMed] [Google Scholar]
- Huang CC, Lo SW, Hsu KS ( 2001) Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons. J Physiol (Lond) 532: 731-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston AE, Ornstein PL, Wright RA, Johnson BG, Mayne NG, Burnett JP, Belagaje R, Wu S, Schoepp DD ( 1998) LY341495 is a nanomolar potent and selective antagonist of group II metabotropic glutamate receptors. Neuropharmacology 37: 1-12. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG ( 2001) Cerebellar depolarization-induced suppression of inhibition is mediated by endogenous cannabinoids. J Neurosci 21: RC174(1-5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneuf YP, Nash JE, Crossman AR, Brotchie JM ( 1996) Activation of the cannabinoid receptor by delta 9-tetrahydrocannabinol reduces gamma-aminobutyric acid uptake in the globus pallidus. Eur J Pharmacol 308: 161-164. [DOI] [PubMed] [Google Scholar]
- Mitchell PR, Doggett NS ( 1980) Modulation of striatal [3H]-glutamic acid release by dopaminergic drugs. Life Sci 26: 2073-2081. [DOI] [PubMed] [Google Scholar]
- Pertwee RG ( 1997) Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther 74: 129-180. [DOI] [PubMed] [Google Scholar]
- Pisano P, Samuel D, Nieoullon A, Kerkerian-Le Goff L ( 1996) Activation of the adenylate cyclase-dependent protein kinase pathway increases high affinity glutamate uptake into rat striatal synaptosomes. Neuropharmacology 35: 541-547. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Del Arco I, Martin-Calderon JL, Gorriti MA, Navarro M ( 1998) Role of the endogenous cannabinoid system in the regulation of motor activity. Neurobiol Dis 5: 483-501. [DOI] [PubMed] [Google Scholar]
- Rodriguez JJ, Mackie K, Pickel VM ( 2001) Ultrastructural localization of the CB1 cannabinoid receptor in mu-opioid receptor patches of the rat caudate putamen nucleus. J Neurosci 21: 823-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse ST, Marino MJ, Bradley SR, Awad H, Wittmann M, Conn PJ ( 2000) Distribution and roles of metabotropic glutamate receptors in the basal ganglia motor circuit: implications for treatment of Parkinson's disease and related disorders. Pharmacol Ther 88: 427-435. [DOI] [PubMed] [Google Scholar]
- Sjöström PJ, Turrigiano GG, Nelson SB ( 2003) Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron 39: 641-654. [DOI] [PubMed] [Google Scholar]
- Suchak SK, Baloyianni NV, Perkinton MS, Williams RJ, Meldrum BS, Rattray M ( 2003) The “glial” glutamate transporter, EAAT2 (Glt-1) accounts for high affinity glutamate uptake into adult rodent nerve endings. J Neurochem 84: 522-532. [DOI] [PubMed] [Google Scholar]
- Sung KW, Choi S, Lovinger DM ( 2001) Activation of group I mGluRs is necessary for induction of long-term depression at striatal synapses. J Neurophysiol 86: 2405-2412. [DOI] [PubMed] [Google Scholar]
- Wang SJ ( 2003) Cannabinoid CB(1) receptor-mediated inhibition of glutamate release from rat hippocampal synaptosomes. Eur J Pharmacol 469: 47-55. [DOI] [PubMed] [Google Scholar]