Abstract
The proapoptotic Bcl-2 family members Bak and Bax play central and redundant roles in the regulation of apoptosis. In this study, we investigated the effect of loss of Bax and Bak in the CNS. The adult bax-/-bak-/- mice display masses of densely staining cells in the proliferative zones of the brain. These cells are shown to be a mix of neural progenitor cells and postmitotic cells at different stages of neural and glial differentiation. Both neural progenitor cells and mature neurons derived from bax-/-bak-/- mice were resistant to various apoptotic stimuli. Despite this resistance, postmitotic mature bax-/-bak-/- neurons remain as sensitive to excitoxic death as wild-type neurons. Thus, Bax and Bak play a critical role in regulating the number of neural progenitor cells in the adult brain but are not absolutely required for the initiation of neuronal cell death after neurotoxic injury.
Keywords: Bax, Bak, neural progenitor cells, proliferative zones, apoptosis, excitotoxicity
Introduction
Cell death plays important roles in normal brain development as well as in CNS disease (Oppenheim, 1991; Sadoul, 1998). Throughout development, induction of programmed cell death eliminates excess cells or cells that have failed to establish synaptic connections. Cell death is also implicated in the pathogenesis of many CNS disorders, including trauma, stroke, and neurodegenerative diseases. At least two mechanistically different forms of cell death have been described in the CNS. Factor deprivation-induced cell death is one mode of cell death that is found in brain development and disease (Purves et al., 1988). In contrast, excitotoxic cell death mediated by excess release of neurotransmittors involves an abnormal increase in signal transduction that leads to death (Sattler and Tymianski, 2001).
The Bcl-2 family of apoptotic regulatory proteins has been implicated in the control of neuronal cell survival. Disruption of the antiapoptotic bcl-xL gene leads to death at embryonic day 13, with embryos showing massive apoptosis in the developing spinal cord, brainstem, and dorsal root ganglia (Motoyama et al., 1995). Mice deficient for both bcl-xL and the proapoptotic gene bax still died at approximately embryonic day 13, but the massive apoptosis in the CNS was greatly reduced (Shindler et al., 1997). Single-deficient bax-/- mice examined at embryonic day 12 do not display a decrease in apoptosis in these areas of the brain compared with wild-type mice. Although neither bax-/- nor bak-/- mice display any overt neurological phenotype, numerous studies have investigated cell death in the CNS of these mice. Variable effects on dependence of NGF, excitotoxins, staurosporine, chemotherapeutic agents, and γ-irradiation have been reported previously (Deckwerth et al., 1996; Miller et al., 1997; Johnson et al., 1998; Xiang et al., 1998; Dargusch et al., 2001; Fannjiang et al., 2003). These data suggest a redundancy of bax and bak that was indeed discovered when mice deficient for both bax and bak were generated (Lindsten et al., 2000). The bax-/-bak-/- mice show a high perinatal mortality. Surviving mice show multiple defects in both developmental apoptosis and in the regulation of tissue homeostasis. In contrast to the single-deficient bax-/- or bak-/- mice, the bax-/-bak-/- mice do have an observable neural phenotype in that they are deaf and exhibit circling behavior. The brains of these mice displayed a normal gross anatomy; however, histological analysis of adult bax-/-bak-/- brains showed accumulations of densely staining small cells in the subventricular zone (SVZ).
In this study, we further investigated the effect of loss of Bax and Bak in the CNS. It is shown that the excess cells in the brains of adult bax-/-bak-/- mice are a mix of nestin-positive neural progenitor cells, neuronal-specific nuclear protein (NeuN)-, neural filament protein (NFP)-, and β-tubulin-immunoreactive neurons, and GFAP-reactive astrocytes. Neural progenitor cells derived from bax-/-bak-/- mice show resistance to apoptosis and are able to differentiate along neural, astrocytic, and oligodendroglial lines. In contrast, mature neurons present in cerebellar granule cell cultures from bax-/-bak-/- mice are as sensitive to excitotoxic cell death as wild-type cells, indicating that Bax and Bak are not required for initiation of this type of neuronal cell death.
Materials and Methods
Generation of mice. The breeding and genotyping of bax-/-bak-/- and single-deficient mice have been described previously (Lindsten et al., 2000). To study cell division in the brain, bromodeoxyuridine (BrdU) (Sigma, St. Louis, MO) was injected as described previously (van Praag et al., 1999). Briefly, bax-/-bak-/- and control mice 5-11 weeks old were injected with BrdU at a concentration of 50 μg/gm bodyweight, intraperitoneally. The injections were given daily for 12 d. Mice were killed 1 d, 12 d, or 4 weeks after the last injection, and brains were processed for histological analysis as outlined below.
Histological and immunohistochemical brain analyses. Mice were anesthetized and then perfused with saline followed by 4% paraformaldehyde. The brain was dissected and fixed overnight in the same fixative. Once fixed, the brains were processed through paraffin for microtome section at 4-5 μm. Adjacent sections were stained with either hematoxylin-eosin or with one of a panel of primary antibodies using immunoperoxidase or immunofluorescence as described previously (Kendler and Golden, 1996). The primary antibodies included anti-nestin (1:1000; kindly provided by Dr. J. Wolfe, Children's Hospital of Philadelphia, Philadelphia, PA), anti-NeuN (1:500; Chemicon, Temecula, CA), anti-β-tubulin (1:400; Covance, Princeton, NJ), anti-NFP (low molecular weight neurofilament at 1:1000; kindly provided by Dr. V. Lee, University of Pennsylvania, Philadelphia, PA), and anti-GFAP (1:500; kindly provided by Dr. V. Lee). Sections were visualized on a Nikon (Tokyo, Japan) E400 microscope equipped with a Nikon FDX35 camera.
BrdU and acetylated histone-3 detection. BrdU was injected into mice as described above. Brains were processed as outlined above. Sections were microwaved at high power in Antigen Unmasking Solution (H-3300; Vector Laboratories, Burlingame, CA) for 6 min followed by incubation in G3G4 (1:50; provided by the Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA) diluted in PBS containing 1% bovine serum albumin (BSA), 1% DMSO, 0.1% Triton X-100, and 10% goat serum at room temperature for 4 hr. Sections were next washed in PBS and incubated for 1 hr at room temperature in goat anti-mouse IgG at 1:100 conjugated to fluorescein isothioscyanate (FITC) (Jackson ImmunoResearch, West Grove, PA). Sections were then washed in PBS and incubated in histone-3 (rabbit IgG polyclonal antibody; Upstate Biotechnology, Lake Placid, NY) overnight at 4°C. After incubation in the second primary antibody, sections were washed in PBS and incubated at room temperature for 1 hr in goat anti-rabbit IgG conjugated to biotin (Jackson ImmunoResearch). After washes in PBS, sections were incubated in streptavidin conjugated to Cy3 (Jackson ImmunoResearch) for 15 min at room temperature. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) (Molecular Probes, Eugene, OR). Slides were coverslipped with GelMount (Fisher Scientific, Houston, TX) and visualized using a Leica (Nussloch, Germany) DMR microscope equipped with epifluorescence. Images were captured with a Hamamatsu (Hamamatsu City, Japan) three-chip digital camera.
Areal calculations and cell counting. Calculations of the SVZ area were performed by obtaining a 50× image of the entire SVZ and surrounding image on a Leica DMR microscope equipped with a Hamamatsu three-chip digital camera. The image was imported into ImageJ (available at http://rsb.info.nih.gov/nih-image) where the SVZ was outlined and the area calculated with the analyze tool. The areal measurements were imported into Microsoft (Seattle, WA) Excel, and the mean ± SD for the areas was calculated for wild-type, bax-/-, bak-/-, and bax-/-bak-/- mice. At least three mice were used for each genotype. Comparisons between groups were first made by ANOVA, and then individual comparisons were made by Student's t test.
Cell counts in the corpus callosum were performed at the midline in sections that contained the mid-striatum. Three adjacent columns of cells, 250-μm-wide, were counted through the entire corpus callosum from dorsal to ventral. Counts were performed on 10 μm paraffin-embedded, hematoxylin-eosin-stained sections. The average cell density (cells per square millimeter) was determined for each brain using the three columns counted and then averaged across brains with the same genotype. Comparisons were made by Student's t tests.
BrdU counts were performed by counting labeled cells in three random fields of the SVZ each measuring 250 × 300 μm from three independent sections. Paraffin-embedded sections, 10-μm-thick, were used for these studies. The number of cells in this 750 × 300 μm area was then extrapolated to the entire SVZ using the areal calculations performed above for the same sections. All labeled cells were counted in brains in which the SVZ measured <250 × 300 μm. The total number of cells was then divided by the total area to obtain the final number of BrdU-labeled cells per unit area.
The percentage of differentiated cells in the SVZ was determined by counting the total number of nuclei (DAPI or hematoxylin stained) and the total number of cell-specific marker (GFAP, NeuN, or NFP; for antibody details, see above) labeled cells in three 250 × 300 μm fields from three independent sections. All sections were from paraffin-embedded tissue cut at either 4 or 10 μm. The average percentage of cells was then determined using the total number of nuclei in the field as the denominator. Statistical significance was determined by Student's t test.
Establishment of neural progenitor cell cultures. Neural progenitor cell (NPC) cultures were established from whole brains including cerebellum or cerebellum alone of bax-/-bak-/- and control mice, using established protocols (Ray et al., 1993; Heuer et al., 2001). Neural progenitor cell cultures could be successfully established from either whole brains including cerebellum or just the cerebellum. Briefly, brains were removed from newborn mice and minced with fine scissors followed by incubation with 0.05% trypsin-EDTA (Invitrogen, Carlsbad, CA) at 37°C for 20 min. Fetal calf serum (Cellgro, Herndon, VA) was added to inactivate the trypsin, followed by addition of DNase (Worthington, Freehold, NJ) at a final concentration of 0.5 mg/ml. DNase digestion proceeded for 10 min at 37°C. The cell suspension was then titruated to obtain a single cell suspension. Cells were resuspended in DMEM (Invitrogen), 10% FCS, 1% penicillin-streptomycin-fungizone (PSF) (Invitrogen), 1% N2 supplement (Invitrogen), and recombinant human fibroblast growth factor-2 (FGF-2) at 20 ng/ml (Promega, Madison, WI) and plated on poly-l-lysine (Sigma)-coated plates at 3 × 104 cells/cm2. One day later, the medium was replaced with the same medium containing only 1% FCS. The NPC cultures were passaged weekly after trypsin treatment. Cells could be frozen and thawed using standard techniques. For differentiation experiments, cells were plated on coverslips at 1 × 104 cells/cm2 in standard NPC medium. Cells were allowed to grow to semiconfluency, and then the medium was changed to DMEM, 1% FCS, N2, and PSF or to DMEM, 10% FCS, N2, PSF, and brain-derived neurotrophic factor (BDNF) (Promega) at 20 ng/ml. The medium was replaced with fresh aliquots of the same medium after 1 week and allowed to incubate for 1 additional week. Cells were fixed in acid alcohol, and immunolabeling was performed with the O1 (undiluted; kindly provided by Dr. J. Grinspan, University of Pennsylvania, Philadephia, PA), MAP2 (microtubule-associated protein 2) (1:100; Chemicon), and GFAP (1:1000; kindly provided by Dr. V. Lee) antibodies as described previously (Grinspan et al., 2000). Nuclear labeling with DAPI at 10 mg/ml was performed on all cultures after immunofluorescence. All studies were visualized on a Leica DMR microscope equipped with a Hamamatsu three-chip color digital camera. Images were acquired through Adobe Photoshop (Adobe Systems, San Jose, CA) on a PowerMac G3 (Apple Computers, Cupertino, CA).
Establishment of neuronal cell cultures. Cerebellar granule cell cultures were established from bax-/-bak-/- and control mice using previously described protocols (Miller and Johnson, 1996). Briefly, cerebella were dissected from 7-d-old [postnatal day 7 (P7)] mice, minced finely with scissors, resuspended in 0.05% trypsin-EDTA (Invitrogen), and incubated for 20 min at 37°C. Dialyzed fetal calf serum was added to inactivate the trypsin, and cells were resuspended and titruated in Basal Medium Eagle (Sigma) supplemented with 20 mm KCl, 100 U/ml penicillin, 100 μg/ml streptomycin, 10% dialyzed fetal calf serum (Invitrogen), and 4 mml-glutamine (Invitrogen). The cells were washed once and plated at 1.5-2.0 × 105/cm2 on poly-l-lysine-coated 12-well plates. One day later, aphidicholin was added to a final concentration of 3.3 μg/ml to prevent outgrowth of non-neuronal cells. Cells were incubated for 10 d before additional treatments.
Death assays. To investigate the sensitivity to apoptotic stimuli, NPC cultures of bax-/-bak-/- and control mice were plated at 3 × 104 cells/cm2 in poly-l-lysine-coated 24-well plates. The cells were grown to confluency and then subjected to the following treatments. For growth factor deprivation, the cultures were washed and the culture medium was changed to DMEM containing 0.35% BSA (Sigma) and PSF. The cells were harvested after 1, 2, and 3 d after growth factor deprivation by treatment with 0.05% trypsin-EDTA for 2 min at 37°C, and cell viability was determined by trypan blue exclusion. Cultures were also treated with the indicated amounts of staurosporine (Sigma) or etoposide (Sigma) for 24 hr. Cells were harvested, and viability was determined as outlined above.
For long-term growth factor deprivation, cells were plated as above in multiple wells of a 24-well plate and allowed to grow for 4 d. After this period, the cultures were washed, the medium was replaced with DMEM containing 0.35% BSA, cells were harvested as outlined above at indicated time points, and cell size and cell concentration were measured with a Coulter (Krefeld, Germany) Z2 apparatus. To study excitotoxic death, cerebellar granule cell cultures were treated at day 10, as described previously (Miller et al., 1997). Briefly, the cell cultures were washed twice in Locke's solution (in mm: 154 NaCl, 5.6 KCl, 3.6 NaHCO3, 2.7 CaCl2, 5.6 glucose, and 5 HEPES, pH 7.4) followed by treatment for 30 min with the indicated doses of NMDA (Sigma) in Locke's solution supplemented with 3 μm glycine or NMDA and 150 nm (+)-5-methyl-10,11-dihydro-5H-dibenzo [a,d] cyclohepten-5,10-imine hydrogen maleate (MK-801) (Sigma). Cell cultures were washed again twice with Locke's solution, and the original medium was replaced. In some experiments, cell cultures were treated independently with thapsigargin (Sigma) at 1 μm final concentration. Twenty-four hours later, cell viability was determined using the Live/Dead Viability/Cytotoxicity kit (Molecular Probes), following the instructions of the manufacturer.
Flow cytometry. Neural progenitor cell cultures were treated with trypsin as above, and single-cell suspension was prepared. For intracellular staining with nestin (mouse anti-rat nestin; BD PharMingen, San Diego, CA), the cells were fixed with 4% paraformaldehyde and permeabilized with 0.3% saponin (Sigma). Secondary stain was with FITC-conjugated anti mouse IgG1 (BD PharMingen, San Diego, CA). Flow cytometry was performed on a LSR flow cytometer (BD PharMingen).
Results
Persistence of neural progenitor cells in the brains of adult bax-/-bak-/- mice
Adult mice deficient in both Bax and Bak have an increased population of cells in the SVZ of the cerebral hemispheres compared with wild-type mice (Fig. 1A,B). To quantitate this difference, we measured the SVZ area at the level of the mid-striatum in coronal sections. The cross-sectional area of the SVZ averaged 0.225 ± 0.085 mm2 for the bax-/-bak-/- brains, which was significantly greater than the cross-sectional area of the SVZ for wild-type brains (0.022 ± 0.002 mm2; p < 0.05). The mean areas for the single-deficient bak-/- (0.038 ± 0.011 mm2) (Fig. 1C) and bax-/- (0.099 ± 0.008 mm2) (Fig. 1D), although slightly increased, did not reach statistical difference from wild-type mice. The accumulation of cells seen in the SVZ zone of the bax-/-bak-/- brains was also above what would be expected for an additive effect of loss of Bax and Bak. Similar collections of cells are also present in the hippocampus, basal ganglia, and cerebellum (Fig. 1E,F and data not shown). These are areas where neural stem cells have been proposed to persist postnatally.
Figure 1.

Abnormal cells in the brains of adult bax-/-bak-/- mice are found in multiple areas. Hematoxylin and eosin-stained coronal sections at the level of the basal ganglia (BG) and hippocampus (hipp) from bax+/-bak+/- (A, E), bax+/+bak-/- (C), bax-/-bak+/+ (D), and bax-/-bak-/- (B, F) mice. Representative sections are shown. The surface area of the SVZ (*) and cell counts in the corpus callosum (co.ca.) were determined as outlined in Material and Methods and are shown in Results. E and F also include white matter (wm) and cerebral cortex (cc). Scale bar, 150 μm.
Increased cell numbers were also observed in areas in which neural progenitors are not thought to exist such, as the corpus callosum. To assess this increase, cell counts were also performed in the corpus callosum because its borders can be easily defined and compared between different samples. Counts performed in the mid-corpus callosum showed a doubling in the cell density present in the bax-/-bak-/- mice (mean of 4740 ± 860/mm2) compared with wild-type mice (mean of 2640 ± 340/mm2; p < 0.01). Single-deficient bax-/- and bak-/- mice have an intermediate cell count in the corpsus collosum, and neither were statistically different from wild-type mice (bax-/- mean of 3290 ± 410/mm2; bak-/- mean of 2900 ± 270/mm2). The inclusion of neuropil inherent in the corpus callosum sections potentially makes it more difficult to determine whether a gene dosage effect exists in this area.
Because the increase in the SVZ area appears to be the most novel phenotype of the bax-/-bak-/- mice, this area was subjected to additional analysis. First, a panel of antibodies was used to define the phenotype of the abnormal cell accumulations in the SVZ of the bax-/-bak-/- adult brain compared with wild-type, bax+/+bak+/+ mice (Fig. 2). Many cells in the bax-/-bak-/- brain expressed nestin, an intermediate filament expressed in neural progenitor cells (Lendahl et al., 1990). Histological sections stained with nestin are not amenable to precise quantitation because the antibody stains both soma and processes, but, compared with wild-type mice, there is a large increase in the number of nestin-positive cells in the bax-/-bak-/- mice. To determine whether these collections of cells also contain cells undergoing differentiation along the neural lineage, the expression of NeuN, a marker developmentally expressed in postmitotic neurons, and NFP specific for intermediate filaments found in mature neurons was examined. In addition, staining with GFAP was examined to evaluate the presence of glial differentiation. In the SVZ of bax-/-bak-/- brains, 16.9 ± 3.1% (mean ± SD) of cells were NeuN positive, whereas 11.2 ± 3.1% of cells stained positive for NFP and 13.4 ± 1.3% were positive for GFAP. In wild-type mice, the SVZ contained 13.7 ± 2.8% cells positive for NeuN, 7.3 ± 1.3% were positive for NFP, and 3.1 ± 0. 5% were positive for GFAP. Although all three cell types were more prevalent in the SVZ of bax-/-bak-/- brains, only the difference in the percentage of GFAP-positive cells was statistically significant (p < 0.001). However, because there was a significant increase in the area of the SVZ of bax-/-bak-/- mice compared with wild-type mice, the SVZ of bax-/-bak-/- mice contained a 10-fold increase in the absolute numbers of committed progenitors of both neural and glial lineages. Thus, the data indicate that the abnormal cell accumulations in the SVZ contain not only potential neuronal progenitors but also cells committed to both the glial and neuronal lineages.
Figure 2.
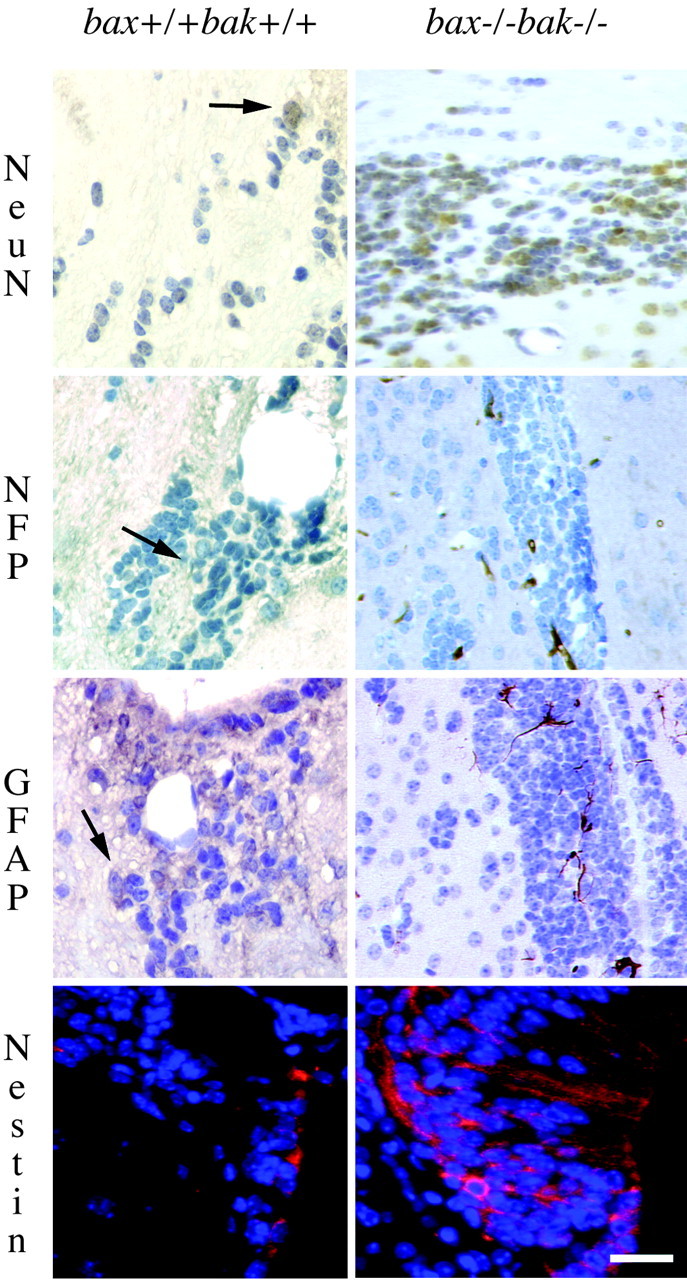
Phenotypic features of excess cells seen in the bax-/-bak-/- compared with wild-type mouse brains. Cells in the SVZ were phenotyped by immunohistochemistry. In the bax-/-bak-/- SVZ, 16.9 ± 3.1% of the cells labeled with the neuronal marker NeuN, labeling immature and mature neurons, whereas 11.2 ± 3.1% of the cells expressed the low molecular weight neurofilament proteins (NFP). Among the small immature cells, 13.4 ± 1.3% cells showed astrocytic differentiation (GFAP labeling). A total of 13.7 ± 2.8% NeuN-positive, 7.3 ± 1.3% NFP-positive, and 3.1 ± 0.5% GFAP-positive cells were present in the SVZ of wild-type mice. Immunofluorescence was used to demonstrate the expression of the intermediate filament nestin found in immature neurons within the SVZ (red; nuclear counter stain with DAPI, blue). Scale bar, 75 μm.
bax-/-bak-/- cells in the SVZ continue to proliferate
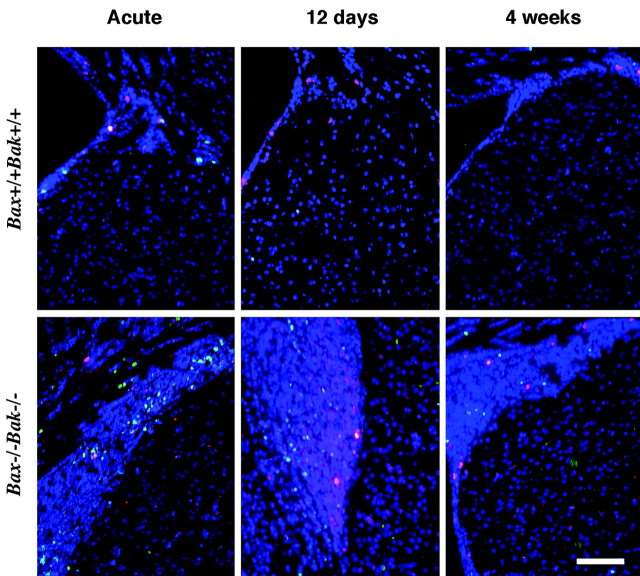
The above data suggests that bax-/-bak-/- brains contain increased numbers of progenitor cells. It was of interest to determine whether these cells are capable of proliferation and thus contribute to the increased size of the proliferative zones. To assess whether active cell division is taking place in the brains of bax-/-bak-/- mice, the S-phase substrate BrdU was combined with acetylated histone-3 labeling after injection with BrdU. bax-/-bak-/- and wild-type mice injected on 12 consecutive days with BrdU were killed 1 d, 12 d, and 4 weeks after the last injection. The number of BrdU-positive cells was determined per surface area. There were 400 ± 16 BrdU-positive cells/mm2 (mean ± SD) observed in the SVZ of bax-/-bak-/- mice killed acutely, 1 d after the last injection, whereas 318 ± 19 BrdU-positive cells/mm2 were observed in the wild-type mice (Fig. 3). This increase was statistically significant (p < 0.005). When corrected for the average differences in the surface area between bax-/-bak-/- and wild-type brains, the bax-/-bak-/- SVZ had 90 ± 4 BrdU-positive cells per section, whereas the wild-type SVZ had only 7 ± 1 BrdU-positive cells per section. The number of BrdU-labeled cells declines progressively at 12 d and 4 weeks post-BrdU injections (Fig. 3) (BrdU-positive cells at 12 d and 4 weeks were 35 ± 8 and 26 ± 10 cells/mm2 for wild-type and 57 ± 8 and 42 ± 10 cells/mm2 for bax-/-bak-/- mice, respectively). In contrast, on the basis of acetylated histone-3 immunolabeling, a marker of cells in the cell cycle, continued proliferation of SVZ cells at all time points was seen (Fig. 3). By day 12 after BrdU injections, occasional positive cells were observed outside the SVZ in the bax-/-bak-/- mice. To determine the cell lineage of the BrdU-positive cells, double labeling was performed with anti-BrdU and anti-β-tubulin for the neuronal lineage and with anti-GFAP antibodies for the astrocyte lineage (Fig. 4). In brain sections of mice killed acutely, the proportions of tubulin and GFAP-positive cells, within the BrdU-positive population, were similar for both bax-/-bak-/- and wild-type mice. This result indicates that there is an increase in the number of dividing cells present in the brains of adult bax-/-bak-/- mice compared with wild-type mice, but the percentage of dividing cells differentiating along the neural and astrocytic lineages was similar.
Figure 3.
Cell proliferation occurs in the SVZ of the adult bax-/-bak-/- brain. After 12 daily BrdU injections, mice were killed 1 d (acute), 12 d, or 4 weeks later. Histological sections were stained with anti-BrdU (green) and with an anti-histone-3 antibody (red). BrdU-incorporating cells were observed in the bax-/-bak-/- mice at increased numbers when compared with the wild-type mice (for quantitation, see Results). In addition, the bax-/-bak-/- mice show a relatively constant (over time) number of cells in the cell cycle as visualized by antibody to acetylated histone-3. Scale bar, 150 μm.
Figure 4.
SVZ cells show BrdU incorporation and labeling with neuronal markers. Dual-color fluorescent labeling of sections from bax+/+bak+/+ and bax-/-bak-/- mice showed many cells with BrdU incorporation (red nuclear label) and β-tubulin (green cytoplasmic label). Double-labeled cells (arrows in β-tubulin/BrdU images) are seen in both the wild-type and bax-/-bak-/- mice. Although more double-labeled cells are present in the bax-/-bak-/- mice, similar percentages of double-labeled cells were seen in the bax+/+bak+/+ and bax-/-bak-/- brains (62.5 vs 61.3%, respectively). No GFAP/BrdU double-labeled cells were seen in the sections from the bax+/+bak+/+ mice, and only a few were seen in the bax-/-bak-/- mice (6 per 100 μm2) (arrowhead identified GFAP labeling in a BrdU-positive nucleus identified by the arrow). Scale bar, 25 μm.
Establishment of neural progenitor cell cultures from bax-/-bak-/- and control mice
NPC cultures established from neonatal mice have proven a useful tool for functional assays (Heuer et al., 2001). Because the high perinatal lethality limits the number of bax-/-bak-/- mice available for experiments, cell lines established from these mice are particularly useful for elucidating the effects of loss of Bax and Bak. Therefore, NPC cultures were established from bax-/-bak-/- and control mice to further characterize these cells. The cultures were established following standard protocols in the presence of N2 supplement and FGF-2 (Ray et al., 1993; Heuer et al., 2001). The cultures contained a high percentage of nestin-positive cells (89% for bax+/+bak+/+, 87% for bax-/-bak+/+, 73% for bax+/-bak-/-, and 88% for bax-/-bak-/- cultures), confirming that the cultures were enriched for neural progenitor cells.
To test the differentiation potential of the NPC culture established from bax-/-bak-/- mice, the cells were subjected to various conditions known to induce differentiation (Palmer et al., 1997). Cells grown in the presence of N2 and FGF-2, the standard NPC medium, showed no staining for mature astrocytes (GFAP) or neurons (MAP2) (Fig. 5). Forty-seven percent of the cells stained with O1, which labels oligodendrocytes (Sommer and Schachner, 1981); however, the cellular processes were blunt and immature, indicating that the cells were not mature oligodendrocytes. In NPC cultures growing in the presence of N2 but in the absence of FGF-2 for 2 weeks, dramatic changes were observed. Phase-contrast microscopy showed marked changes in the cellular morphology (Fig. 5). The cell bodies were larger, and they exhibited longer and more elaborate processes. Eighty-three percent of the cells stained positive for the neuronal marker MAP2 (Fig. 5). Eleven percent of cells also stained positive for O1 with the morphology of mature oligodendrocytes (Fig. 5). No GFAP-positive cells were observed. However, when the NPC cultures were subjected to treatment for 2 weeks in high concentration of FCS (10%) and BDNF, differentiation to GFAP staining astrocytes (26%) was observed, but minimal or no neuronal or oligodendrocyte differentiation was observed (Fig. 5). Cultures from wild-type mice showed similar profiles; in the presence of complete medium, there were 40% O1-positive cells, in N2 alone, there were 71% MAP2-positive cells and 17% O1-positive cells, and, in the presence of FCS and BDNF, there was 29% GFAP-positive cells.
Figure 5.
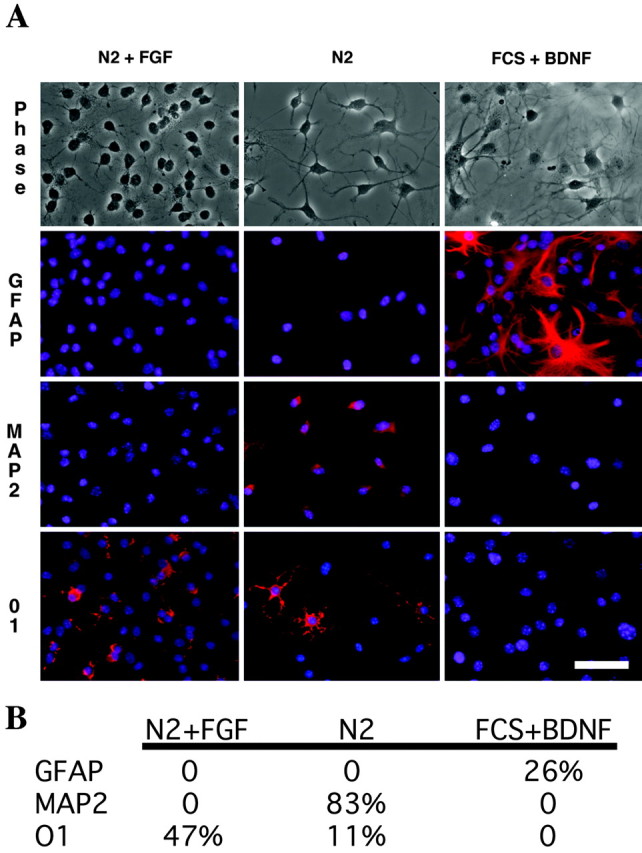
bax-/-bak-/- NPC cultures are able to differentiate along neural and glial lineages. NPC cultures were grown in the presence of N2 supplement, 1% FCS and FGF-2, in N2 supplement and 1% FCS, or in the presence of 10% FCS and BDNF. After 2 weeks in differentiation medium, cells were stained with antibodies against O1 (oligodendral marker), MAP2 (neural marker), and GFAP (astrocyte marker) (A), and quantitation is shown in B. Culture in N2, 1% FCS, and FGF-2 resulted in small cells with relatively short blunt processes that occasionally labeled with O1 but not with GFAP or MAP2. The morphology and O1 staining indicate that some of these cells are immature oligodendrocytes. In N2 and 1% FCS media, the cells become large and elaborate extensive processes. These cells show differentiation along the lines of mature neurons (MAP2 labeling) and mature oligodendrocytes (compare the complex morphology of the O1 labeled cells with N2 alone vs those with N2 and FGF) (B). No astrocytes were seen with the N2 media alone. FCS and BDNF, conditions known to differentiate astrocytes from NPCs, exhibit the expected result with large flat cells showing strong GFAP immunoreactivity. Under these conditions, no neurons or oligodendrocytes were observed. For percentages of differentiated cells from wild-type mice, see Results. Scale bar, 25 μm.
Thus, the bax-/-bak-/- NPC cultures are capable of differentation along both neural and glial lineages, as shown previously for similar cultures from normal mice and rats (Palmer et al., 1997; Heuer et al., 2001). These results confirm the data obtained from in vivo staining presented in Figures 2, 3, and 4 and indicate that the process of neural and glial differentiation is not dependent on Bax and Bak.
NPC cultures from bax-/-bak-/- mice are resistant to apoptotic stimuli
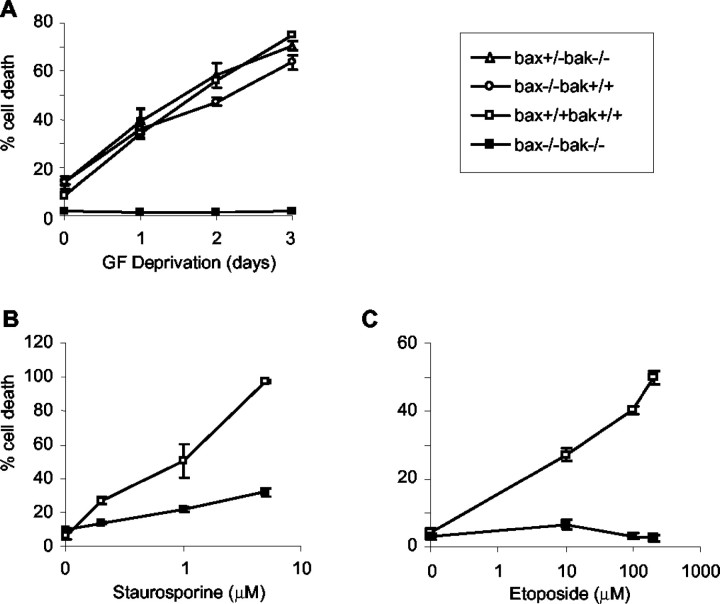
To test the hypothesis that resistance to apoptotic signals contributes to the persistence of neural progenitor cells in the brains of bax-/-bak-/- mice, NPC cultures from bax-/-bak-/- mice and control genotypes were tested for sensitivity to different apoptotic stimuli. The NPC cultures are suitable for these type of experiments because these cultures have been shown to be absolutely dependent on the growth supplements present in FCS, N2, and FGF-2 for their survival (Ray et al., 1993). It has been shown in mouse embryo fibroblasts and hematopoietic cells that growth factor deprivation induces apoptotic cell death (Lindsten et al., 2000; Wei et al., 2001). Thus, growth factor deprivation of NPC cultures represents a physiological test to assess the sensitivity to apoptotic signals. Figure 6A shows that, when NPC cultures from single-deficient and wild-type mice were deprived of the growth factors FCS, N2, and FGF-2, the wild-type and single-deficient cells die rapidly over the course of 3 d. However, bax-/-bak-/- NPC cultures were resistant to this treatment. NPC cultures from bax-/-bak-/- and wild-type mice were also treated with two drugs often used in apoptosis research, staurosporine and etoposide, at indicated doses, and cell death was determined 24 hr later (Fig. 6B,C). Again, the bax-/-bak-/- NPC cultures showed resistance to these two apoptotic stimuli, whereas the majority of wild-type cells die. NPC cultures derived from single-deficient bax or bak mice showed no significant difference in cell death after treatment with staurosporine or etoposide, compared with cultures from wild-type mice. Thus, at 5 μm staurosporine, the percentage of dead cells for wild-type cells was 90.9 ± 4.7%, for bax-/- cells was 84.7 ± 3.6%, and for bak-/- cells was 87.5 ± 5.0%. Similarly, after treatment with 200 μm etoposide, the percentage of dead cells in wild-type cultures was 35.4 ± 2.1%, for bax-/- cells was 30.1 ± 4.3%, and for bak-/- cells was 45.8 ± 3.0%.
Figure 6.
NPC cultures from bax-/-bak-/- mice are resistant to apoptotic stimuli. NPC cultures from bax-/-bak-/- and control genotypes were grown without N2 supplement, 1% FCS, and FGF-2 for 3 d (A), treated with staurosporine for 24 hr (B), or treated with etoposide for 24 hr (C). Cells were harvested, and viability was determined by trypan blue exclusion. The mean ± SD of triplicate samples are shown. GF, Growth factor.
The persistence of increased NPC pools lasts throughout adult life in bax-/-bak-/- mice. Therefore, the effects of long-term withdrawal of growth factors were investigated in bax-/-bak-/- and wild-type NPC cultures deprived of FCS, N2 supplement, and FGF-2 for 31 d (Fig. 7). The bax-/-bak-/- NPCs stopped proliferating, but, even after 31 d without growth factors, the viability of the bax-/-bak-/- NPCs was 91%. However, the absence of Bax and Bak does not protect NPC from the metabolic changes that accompanies growth factor withdrawal as the bax-/-bak-/- NPC became progressively smaller during the period of nutrient deprivation. There was a 54% decrease in size over the 31 d starvation period. Morphologically, the cells also showed shortened processes, and the nestin median staining intensity determined by flow cytometry analysis showed an approximate fourfold decrease (data not shown). Readdition of growth factor led to increase in cell size close to that of unmanipulated cells. Cell proliferation was observed at 2 weeks after readdition of growth factor (data not shown). In contrast, the wild-type cells rapidly died within the first week of culture. Thus, the small size of starved bax-/-bak-/- NPC observed in vitro after long-term growth factor deprivation may reflect the situation in vivo in which these cells have accumulated in excess of the trophic signals that are normally required to maintain NPC survival when Bax and Bak are present.
Figure 7.
Effects of long-term growth factor deprivation in bax-/-bak-/- NPC cultures. bax-/-bak-/- (filled squares) and wild-type (open squares) NPC cultures were grown to confluency in standard NPC medium and then deprived of N2 supplement, 1% FCS, and FGF-2 for 31 d. At day 31, growth factors were readded. Relative cell number compared with the number of cells present at the onset of the experiment (A) and cell size (B) are shown at indicated time points. The majority of the wild-type cells died within 1 week and was not available for additional sampling. The viability of the bax-/-bak-/- cells was 91% at day 31. A representative experiment is shown.
Cerebellar granule cells cultures from bax-/-bak-/- mice are sensitive to excitotoxic cell death
Given the persistent proliferation and differentiation observed in the bax-/-bak-/- NPC pool, it was surprising that the size of the brain was not affected to a greater extent. Part of this may be that the sizes of bax-/-bak-/- cells are still under the control of trophic signals as documented above. In addition, postmitotic neurons are subjected to stresses that may lead to their elimination by mechanisms not affected by deficiency of Bax and Bak. Excitotoxic death is one such stress to which postmitotic neurons are exposed. The treatment of cerebellar granule cell cultures with excitotoxins such as NMDA is a well studied model for excitotoxic cell death of mature neurons (Miller and Johnson, 1996). Cerebellar granule cell cultures were established from P7 wild-type, single-deficient bak-/-and bax-/-, and bax-/-bak-/- mice. After 10 d in vitro, the cultures were exposed to a 30 min pulse of NMDA, and cell death was determined 24 hr later. Figure 8A shows that all genotypes tested, including the bax-/-bak-/-, were sensitive to NMDA-induced cell death. The cell death was blocked in the presence of MK-801, a noncompetitive NMDA receptor antagonist. To compare the sensitivity of the cerebellar granule cultures to apoptotic stimuli versus NMDA-induced cell death, parallel cultures were treated with thapsigargin (an endoplasmic reticulum stress agent) or NMDA. Figure 8 B shows that, whereas both wild-type and bax-/-bak-/- cultures died in the presence of NMDA, the bax-/-bak-/- cultures were resistant to thapsigargin treatment. Thus, whereas Bax and Bak appear to play a crucial role in neural development, these proapoptotic proteins are not involved in the regulation of excitotoxic death.
Figure 8.
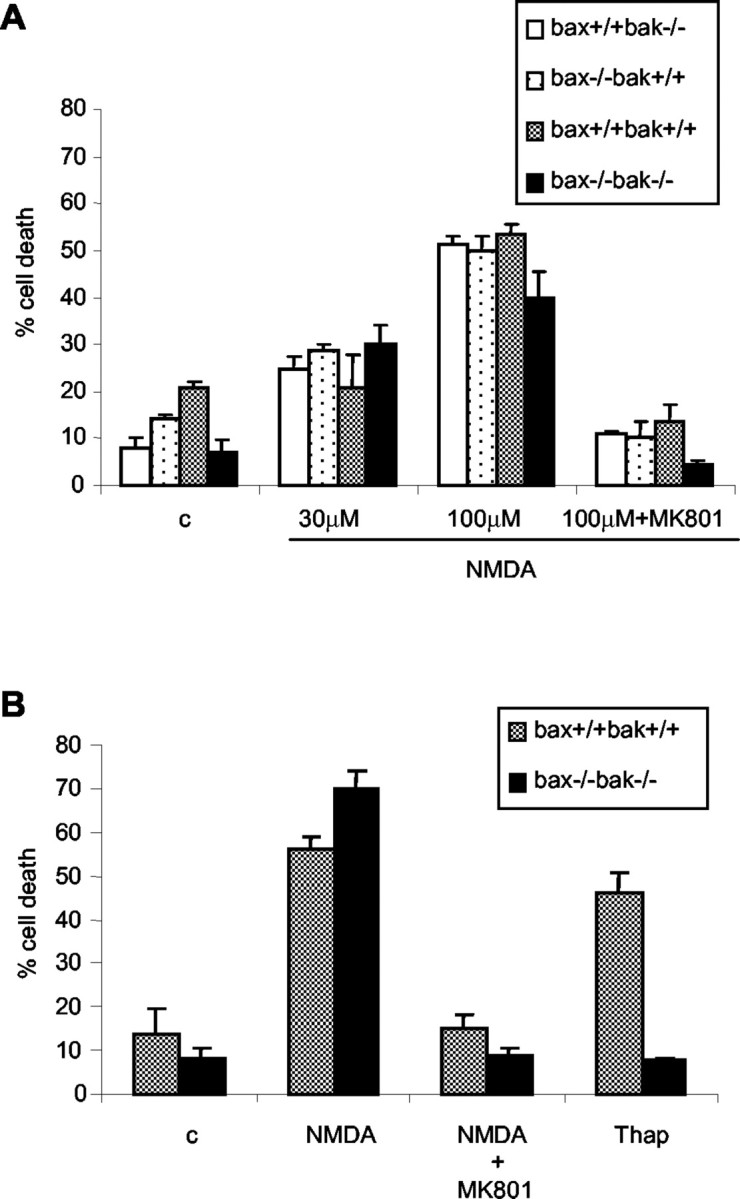
bax-/-bak-/- cerebellar granule cells are sensitive to NMDA-induced excitotoxic death. Cerebellar granule cell cultures were established from bax-/-bak-/- and control mice. Cells were treated with NMDA at indicated concentrations in the absence or presence of MK-801 (A) and treated with NMDA with or without MK-801 or thapsigargin (B; Thap). Viablity was determined by calcein AM-ethidium bromide staining. The mean ± SD of triplicates are shown from a representative experiment. c, No treatment control.
Discussion
The persistence of neural progenitor cells is now established in a wide variety of vertebrate species, including man. However, the factors that regulate their accumulation and persistence in vivo remain to be established. In the above studies, we demonstrate that adult bax-/-bak-/- mice display a large expansion in the numbers of neural progenitor cells and early postmitotic neurons that persist in the proliferative zones of the brain. These accumulated NPCs are resistant to a wide variety of apoptotic stimuli when tested in vitro. Whereas some accumulations of cells were found in bax and bak single-deficient mice, NPC cultures derived from these mice were sensitive to apoptotic stimuli.
The bax-/-bak-/- NPC have the capability in vitro to respond to trophic signals and to differentiate along both neural and glial lineages, suggesting that the accumulation of cells is not attributable to an inherent deficiency in differentiative capability. In fact, there is a slightly higher percentage of differentiated cells present in the bax-/-bak-/- SVZ compared with that seen in wild-type mice. However, differentiated cells only account for a portion of the cells present in the SVZ. This may be attributable to the fact that, although the NPCs of the bax-/-bak-/- mice are resistant to apoptosis, which accounts for the accumulation of cells, and are able to differentiate, the availability of growth factors may limit their further expansion past the early stages of differentiation and seeding into the brain. This is illustrated in the long-term growth factor deprivation experiments, in which bax-/-bak-/- cells stayed alive for 31 d without growth factors but showed significant loss of cell volume. The experiments in this study do not address the possibility of deficiency in cell migration as a factor contributing to the accumulation of cells.
Although it is clear that Bax and Bak play an important role in early neuronal development, these proapoptotic proteins do not seem to be involved in excitotoxic cell death, which is the underlying cause of cell death seen in many neurological disorders. This may in part be attributable to the fact that it has been suggested that excitotoxic cell death has both necrotic as well as apoptotic features (Ankarcrona et al., 1995). Some excitotoxins induce nitric oxide production, and nitric oxide can induce cell death with apoptotic features (Brune, 2003). Interestingly, postnatal cortical cultures from bax-deficient mice showed partial resistance to cell death induced by the nitric oxide donor sodium nitroprusside (Ghatan et al., 2000). Future studies will indicate whether cells from bax-/-bak-/- mice will display further resistance to treatment with nitric oxide donors.
Cerebellar granule cell cultures established from bax-/-bak-/- mice may prove useful in elucidating the molecular pathways of excitotoxic cell death in the absence of the classical Bax/Bak-dependent mitochondrial death pathway. As another example of Bax/Bak-independent neuronal degeneration, we showed recently that Wallerian degeneration in the bax-/-bak-/- mice is identical to that found in wild-type mice after axotomy of the optic as well as the sciatic nerve (Whitmore et al., 2003).
These results indicate that Bax and Bak play a crucial role in neural stem cell persistence. However, the postmitotic neurons derived from bax-/-bak-/- cells do not appear to be protected from effects of excitotoxins.
Footnotes
This work was supported in part by grants from the National Cancer Institute (C.B.T) and National Institutes of Health Grant HD26979 (J.A.G). W.-X.Z. is supported by a postdoctoral fellowship from the Cancer Research Institute. The anti-BrdU antibody G3G4 was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by The University of Iowa (Department of Biological Sciences, Iowa City, IA). We thank Dr. Judith Grinspan and Elizabeth Hirshorn for assistance with immunolabeling.
Correspondence should be addressed to Dr. Craig B. Thompson, Abramson Family Cancer Research Institute, University of Pennsylvania, 421 Curie Boulevard, Philadelphia, PA 19104. E-mail: craig@mail.med.upenn.edu.
Copyright © 2003 Society for Neuroscience 0270-6474/03/2311112-08$15.00/0
T.L. and J.A.G. contributed equally to this work.
References
- Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P ( 1995) Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron 15: 961-973. [DOI] [PubMed] [Google Scholar]
- Brune B ( 2003) Nitric oxide: NO apoptosis or turning it ON? Cell Death Differ 10: 864-869. [DOI] [PubMed] [Google Scholar]
- Dargusch R, Piasecki D, Tan S, Liu Y, Schubert D ( 2001) The role of Bax in glutamate-induced nerve cell death. J Neurochem 76: 295-301. [DOI] [PubMed] [Google Scholar]
- Deckwerth TL, Elliott JL, Knudson CM, Johnson Jr EM, Snider WD, Korsmeyer SJ ( 1996) BAX is required for neuronal death after trophic factor deprivation and during development. Neuron 17: 401-411. [DOI] [PubMed] [Google Scholar]
- Fannjiang Y, Kim CH, Huganir RL, Zou S, Lindsten T, Thompson CB, Mito T, Traystman RJ, Larsen T, Griffin DE, Mandir AS, Dawson TM, Dike S, Sappington AL, Kerr DA, Jonas EA, Kaczmarek LK, Hardwick JM ( 2003) BAK alters neuronal excitability and can switch from anti- to pro-death function during postnatal development. Dev Cell 4: 575-585. [DOI] [PubMed] [Google Scholar]
- Ghatan S, Larner S, Kinoshita Y, Hetman M, Patel L, Xia Z, Youle RJ, Morrison RS ( 2000) p38 MAP kinase mediates bax translocation in nitric oxide-induced apoptosis in neurons. J Cell Biol 150: 335-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinspan JB, Edell E, Carpio DF, Beesley JS, Lavy L, Pleasure D, Golden JA ( 2000) Stage-specific effects of bone morphogenetic proteins on the oligodendrocyte lineage. J Neurobiol 43: 1-17. [PubMed] [Google Scholar]
- Heuer GG, Skorupa AF, Prasad Alur RK, Jiang K, Wolfe JH ( 2001) Accumulation of abnormal amounts of glycosaminoglycans in murine mucopolysaccharidosis type VII neural progenitor cells does not alter the growth rate or efficiency of differentiation into neurons. Mol Cell Neurosci 17: 167-178. [DOI] [PubMed] [Google Scholar]
- Johnson MD, Xiang H, London S, Kinoshita Y, Knudson M, Mayberg M, Korsmeyer SJ, Morrison RS ( 1998) Evidence for involvement of Bax and p53, but not caspases, in radiation-induced cell death of cultured postnatal hippocampal neurons. J Neurosci Res 54: 721-733. [DOI] [PubMed] [Google Scholar]
- Kendler A, Golden JA ( 1996) Progenitor cell proliferation outside the ventricular and subventricular zones during human brain development. J Neuropathol Exp Neurol 55: 1253-1258. [DOI] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, McKay RD ( 1990) CNS stem cells express a new class of intermediate filament protein. Cell 60: 585-595. [DOI] [PubMed] [Google Scholar]
- Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, Chen Y, Wei M, Eng VM, Adelman DM, Simon MC, Ma A, Golden JA, Evan G, Korsmeyer SJ, MacGregor GR, Thompson CB ( 2000) The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell 6: 1389-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TM, Johnson Jr EM ( 1996) Metabolic and genetic analyses of apoptosis in potassium/serum-deprived rat cerebellar granule cells. J Neurosci 16: 7487-7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TM, Moulder KL, Knudson CM, Creedon DJ, Deshmukh M, Korsmeyer SJ, Johnson Jr EM ( 1997) Bax deletion further orders the cell death pathway in cerebellar granule cells and suggests a caspase-independent pathway to cell death. J Cell Biol 139: 205-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoyama N, Wang F, Roth KA, Sawa H, Nakayama K, Negishi I, Senju S, Zhang Q, Fujii S, et al ( 1995) Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science 267: 1506-1510. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW ( 1991) Cell death during development of the nervous system. Annu Rev Neurosci 14: 453-501. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Takahashi J, Gage FH ( 1997) The adult rat hippocampus contains primordial neural stem cells. Mol Cell Neurosci 8: 389-404. [DOI] [PubMed] [Google Scholar]
- Purves D, Snider WD, Voyvodic JT ( 1988) Trophic regulation of nerve cell morphology and innervation in the autonomic nervous system. Nature 336: 123-128. [DOI] [PubMed] [Google Scholar]
- Ray J, Peterson DA, Schinstine M, Gage FH ( 1993) Proliferation, differentiation, and long-term culture of primary hippocampal neurons. Proc Natl Acad Sci USA 90: 3602-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoul R ( 1998) Bcl-2 family members in the development and degenerative pathologies of the nervous system. Cell Death Differ 5: 805-815. [DOI] [PubMed] [Google Scholar]
- Sattler R, Tymianski M ( 2001) Molecular mechanisms of glutamate receptor-mediated excitotoxic neuronal cell death. Mol Neurobiol 24: 107-129. [DOI] [PubMed] [Google Scholar]
- Shindler KS, Latham CB, Roth KA ( 1997) Bax deficiency prevents the increased cell death of immature neurons in bcl-x-deficient mice. J Neurosci 17: 3112-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer I, Schachner M ( 1981) Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev Biol 83: 311-327. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH ( 1999) Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA 96: 13427-13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ ( 2001) Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292: 727-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmore AV, Lindsten T, Raff MC, Thompson CB ( 2003) The proapoptotic proteins Bax and Bak are not involved in Wallerian degeneration. Cell Death Differ 10: 260-261. [DOI] [PubMed] [Google Scholar]
- Xiang H, Kinoshita Y, Knudson CM, Korsmeyer SJ, Schwartzkroin PA, Morrison RS ( 1998) Bax involvement in p53-mediated neuronal cell death. J Neurosci 18: 1363-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]