Abstract
Contusion spinal cord injury (SCI) at T8 produces respiratory abnormalities in conscious rats breathing room air and challenged with CO2. In seeking ways to improve respiration after SCI, we tested drugs that stimulate serotonin 1A (5-HT1A) receptors, based on our previous findings that these agents can counteract respiratory depression produced by morphine overdose. Respiratory function was measured with a head-out plethysmograph system in conscious rats. T8 SCI rats (n = 5) showed decreased tidal volume (Vt; 0.90 ± 0.02-0.66 ± 0.03 ml; p < 0.05) and increased respiratory rate (f;91 ± 3.7-132 ± 5.7 breaths/min; p < 0.05) with room air ventilation at 24 hr after injury. They also exhibited a diminished response to the respiratory stimulating effect of 7% CO2; minute ventilation increased to 250 ± 17 ml/min before, but only to 162 ± 15 ml/min at 24 hr after SCI (p < 0.05). Respiratory deficits during room air ventilation were also observed at 7 d after injury (n = 3). Treatment with the 5-HT1A receptor agonist 8-hydroxy-2-(di-n-propylmino)tetralin (8-OH-DPAT; 250μg/kg, i.p.) at 24 hr (n = 5) or 7 d (n = 3) after injury normalized Vt, f, and the respiratory response to 7% CO2. Identical results were obtained with another 5-HT1A receptor agonist, buspirone (1.5 mg/kg, i.p.; n = 3). In contrast, intraperitoneal saline vehicle administration (n = 5) showed no beneficial effects on SCI-impaired respiration. Finally, pretreatment with a specific antagonist of 5-HT1A receptors, 4-iodo-N-[2-[4-(methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinyl-benzamide (3 mg/kg, i.p.; n = 3) given 20 min before 8-OH-DPAT, prevented 8-OH-DPAT from restoring respiration to normal. Our results demonstrate that drugs that stimulate 5-HT1A receptors counteract respiratory abnormalities in conscious rats after SCI.
Keywords: rat, 5-HT1A, 8-OH-DPAT, buspirone, p-MPPI, tidal volume, respiratory rate, minute ventilation, plethysmograph, spinal cord injury
Introduction
We have reported that incomplete contusion at T8 results in consistent and significant abnormalities in respiratory function (Teng et al., 1999). These abnormalities were documented in conscious rats using a head-out plethysmograph system to evaluate respiratory activity. At 24 hr after spinal cord injury (SCI), there was an abnormal pattern of respiration during room air breathing and a reduction in the ability of rats to respond appropriately to breathing higher than normal levels of CO2. The abnormal respiratory pattern of SCI rats consisted of a decreased tidal volume (Vt) and an increased respiratory rate (f), a pattern that is also found in patients with lower thoracic SCI (Prakash, 1989). The respiratory abnormalities seen in rats after SCI appear related to loss of motoneurons innervating muscles of respiration, because acute treatment with basic fibroblast growth factor increases survival of these motoneurons and prevents the respiratory deficits after contusion injury (Teng et al., 1999).
Loss of motoneurons occurs rapidly after SCI. In our injury model, approximately half of the ventral horn motoneurons that are lost chronically are already gone by 4 hr after injury, and the remainder are gone by 24 hr (Teng et al., 1998; Grossman et al., 2001). Thus, treatments to increase their preservation have a relatively limited therapeutic window. An alternative or additional strategy would be enhancing the function of surviving respiratory motoneurons. The goal of the present study was to evaluate whether a newly recognized group of respiratory stimulant drugs, 5-HT1A receptor agonists (Sahibzada et al., 2000), would exert a beneficial effect on SCI-induced respiratory abnormalities.
The prototype drug of the group is 8-hydroxy-2-(di-n-propylmino)tetralin (8-OH-DPAT), and this and a related agent, buspirone, have been shown to counteract respiratory disturbances (i.e., apneustic breathing) produced by hypoxia, pentobarbital, and antagonists of the NMDA receptor complex (Lalley et al., 1994; Wilken et al., 1997). These agents given systemically will reverse apnea produced by morphine and dizocilpine overdose in anesthetized rats (Sahibzada et al., 1999, 2000). Respiratory stimulant effects of 5-HT1A receptor agonists are also observed when these agents are administered under conditions where breathing is normal. For example, administration of buspirone intravenously to anesthetized cats increased f, tidal phrenic activity, and minute phrenic activity (Garner et al., 1989). Additionally, buspirone decreased the apneic threshold and shifted the CO2 response curve to the left of the control CO2 response curve. Mendelson et al. (1990) administered buspirone intraperitoneally to conscious rats and reported an increase in f, Vt, and minute ventilation (Ve). This group of investigators went on to study buspirone in five patients with obstructive sleep apnea (Mendelson et al., 1991) and found that buspirone decreased the number of apneas by one-third. Based on the above positive findings of 5-HT1A receptor agonists in stimulating respiratory function, we set out to determine whether these agents can restore breathing to normal in the rat model of SCI.
Materials and Methods
Spinal cord injury
Female Sprague Dawley rats (250-280 and 360-390 gm; Taconic, Germantown, NY) were anesthetized with 4% chloral hydrate (360 mg/kg, i.p.). An incomplete spinal cord contusion injury was produced at the T8 vertebral level with a weight drop device (10 gm × 2.5 cm) as previously described (Wrathall et al., 1985). After SCI, manual expression of bladders was performed twice daily until a reflex bladder was established. Animal care also included housing the rats in pairs to reduce isolation-induced stress, maintaining ambient temperature at 22-25°C, and using highly absorbent bedding. No prophylactic antibiotics were given.
Monitoring of respiratory parameters by plethysmograph
Experiments were conducted in unanesthetized, awake, spontaneously breathing rats at 24 hr before SCI, and at 24 hr and 1 week after injury.
Acclimation of the animals. We found that correct plethysmograph recording of respiratory parameters of conscious rats required animal training for acclimatization. Animals were placed in the body cylinder of the plethysmograph (Fig. 1 A) for 60 min/d for at least 5 d. This procedure led them to become used to the environment. After acclimatization, rats remained quietly in the cylinder, allowing for the acquisition of data without physical signs of stress (i.e., defecation, urination, and bloody secretions in the eyes and nose) and motion artifacts.
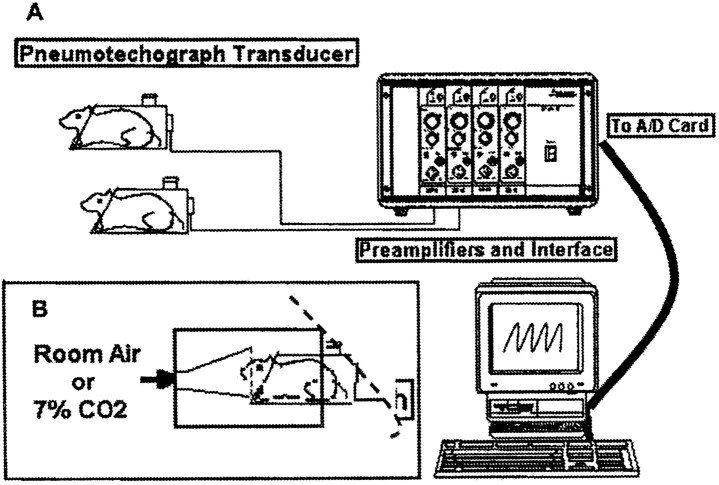
Figure 1.
Noninvasive measurements of respiratory function in conscious rats. A, Schematic presentation of the restrained head-out plethysmograph system for rodents. B, The animals breathe from a funnel fixed in the front wall of a box made of an opaque material. The box surrounds the front two-thirds of the body cylinder of the plethysmograph, and the rear outlet of the box is covered with a piece of bath towel (illustrated by a dashed line). The animals were exposed to room air for baseline recordings and then to a gas mixture containing 7% CO2 mixed with 60% O2 and 33% N2) for 5 min, and recording of respiratory activity was continued for another 2 min (a total recording duration of 7 min). After a new baseline was obtained by allowing the animals to breathe room air for 20 min, the rats were injected with drugs that affect 5-HT1A receptors, and the recordings were repeated as specified in each experiment (figure modified from Teng et al., 1999).
Noninvasive measurements off, Vt, and Ve. Noninvasive measurements of respiratory function in conscious rats were performed with a restrained head-out plethysmograph specially designed for rodents (BUXCO Electronics Inc., Sharon, CT) (Fig. 1A). The plethysmograph apparatus has a neck seal that prevents leakage of air from between the animal's neck and the plethysmograph opening. Displacement of the thoracic wall produced by the animal's respiratory movements causes changes in the cylinder pressure, which results in air flowing across a pneumotachograph located on the wall of the cylinder. The pressure drop across the pneumotachograph is measured with a pressure transducer and is proportional to the flow. This signal is amplified and integrated into volume. From measurements of volume and flow a computer and appropriate software provides respiratory parameters, such as f, Vt, and Ve. An additional opening on the wall of the box allows volume calibration by injecting and removing air from the box with a calibrated syringe.
The noise level in the laboratory was kept to a minimum to avoid startling the animals. Furthermore, the animals were visually isolated from the investigators by means of a chamber made of an opaque material that surrounded and covered the front end of the body plethysmograph (Fig. 1 B). This arrangement, although blocking the vision of the animal, allowed the experimental observer to continuously monitor the movements of the rat's body, i.e., observe the rat's body from the neck down, inside the transparent cylinder. In this way, the experimental observer could exclude any recorded indices of respiratory function caused by unexpected noise and by body movements unrelated to respiratory movements. As an additional safeguard against registering signals unrelated to respiratory movements, the plethysmograph software used rejects all recorded signals that are generated by air flow dynamics different from those of regular breathing triggered by thoracic and abdominal changes.
Measurement of ventilatory response to carbon dioxide. For measurement of the ventilatory response to CO2, animals were exposed to air containing 7% CO2 (mixed with 60% O2 and 33% N2) for 7 min with recording of respiratory activity during the last 2 min. Hyperoxia hypercapnia was used with the expectation that any changes in respiratory activity would be caused by the increased CO2 and not by any significant change in peripheral chemoreceptor activity caused by changes in oxygen.
Drug administration
The 5-HT1A receptor agonists 8-OH-DPAT and buspirone (both purchased from Research Biochemicals, Natick, MA) were dissolved in 0.9% saline (pH adjusted to 7.4). Both agonists were administered intraperitoneally in 0.5 ml of final injection volume per rat and in doses of 250 μg/kg for 8-OH-DPAT and 1.5 mg/kg for buspirone. The 5-HT1A receptor antagonist p-MPPI (Research Biochemicals) was also dissolved in 0.9% saline and given intraperitoneally at a dose of 3 mg/kg (pH 7.4; final volume, 0.5 ml). The doses used of the above drugs were based on data from an earlier study that demonstrated that 5-HT1A agonists could reverse morphine-induced respiratory depression (Sahibzada et al., 2000). Vehicle solution was 0.9% saline and was also injected intraperitoneally (pH 7.4; volume, 0.5 ml/rat).
Experimental protocol
SCI surgical procedures were performed only after animals finished at least 5 d of plethysmograph acclimatization (see above) and at 24 hr after plethysmograph data acquisition for preinjury respiratory parameters. Tests of functional deficits (Gale et al., 1985; Basso et al., 1995) were performed at 24 hr before SCI, and at 24 hr and 1 week afterwards to confirm that a proper degree of SCI was achieved.
Baseline respiratory function was measured with room air ventilation and after each animal was stabilized inside a body cylinder (Fig. 1 A,B) for 30 min at each time point analyzed before SCI and after injury. Immediately after the evaluation of baseline respiration, the animals were exposed to a gas mixture containing 7% CO2 for 7 min to monitor their ventilatory response to CO2 stimulus (Teng et al., 1999). For vehicle and 8-OH-DPAT studies, at 24 hr after injury, respiratory function of a SCI rat was first evaluated by plethysmograph for baseline performance as well as respiratory response to 7% CO2 challenge. Twenty-four minutes after the end of CO2 breathing and after a new baseline was recorded for 4 min starting at the 20 min time point, the rat was removed from the body cylinder (Fig. 1 A). The animal was then injected with saline (0.5 ml, i.p.) and immediately put back into the cylinder in a smooth manner for continuing respiratory monitoring (the injection procedure took ∼1.2 min on average). Baseline respiration (i.e., with room air ventilation) was examined for another 10 min, and at the end of this 10 min, ventilatory responses were evaluated when the animal was challenged by 7% CO2 for 7 min. Twenty-four minutes after the end of the CO2 stimulus (including a recording of a new baseline for 4 min), the rat was again taken out from the body cylinder. The animal was injected with 8-OH-DPAT (250 μg/kg in 0.5 ml, i.p., with the injection procedure requiring an average of 1.2 min) and immediately put back into the cylinder for continuing respiratory monitoring. After the drug administration, baseline respiration (i.e., with room ventilation) was examined continuously for another 23 min. At the end of the twenty-third minute, ventilatory response was evaluated once more when the rat was challenged with 7% CO2 for 7 min. A similar 8-OH-DPAT study was repeated at 7 d after SCI, except that no saline treatment was given.
In the time course study of the respiratory effect of 8-OH-DPAT, recordings of baseline respiratory function (for 4 min) and ventilatory response to 7% CO2 (for 7 min) were repeated hourly for up to 5 hr after the administration of 8-OH-DPAT. In experiments of p-MPPI antagonism of 8-OH-DPAT effects, p-MPPI (3 mg/kg in 0.5 ml/rat, i.p.) was given at 20 min before the administration of 8-OH-DPAT. Baseline respiratory function was examined beginning at 4 and 18 min after p-MPPI injection (each lasted for 2 min). Baseline recording was performed again at 4 and 8 min after intraperitoneal 8-OH-DPAT (each lasted for 2 min), and at the end of the tenth minute after 8-OH-DPAT, ventilatory response to breathing 7% CO2 was measured. For the study of buspirone effects, similar sequential procedures as those in the 8-OH-DPAT experiments were followed. However, the 7% CO2 challenge was given at 10 min after intraperitoneal injection of buspirone (1.5 mg/kg in 0.5 ml), because buspirone has a shorter duration of action than 8-OH-DPAT (Sahibzada et al., 2000). Neither a time course nor an antagonism study was performed for buspirone.
All experimental procedures were performed in strict accordance with the Laboratory Animal Welfare Act, Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD; DHEW Publication No. 78-23, Revised 1978) and after review and approval of our protocol by the Animal Care and Use Committee of Georgetown University. All animals survived the entire study.
Statistical analyses
Experimental data are expressed as mean ± SEM. Statistical significance was defined at the p < 0.05 level. Respiratory data were analyzed statistically using repeated measures ANOVA, followed by Tukey's or Dunn's test for multiple comparisons between groups as used in previous studies (Teng et al., 1999). The same statistical tests were used for analyzing respiratory data from drug treatment studies.
Results
Effects of 8-OH-DPAT on T8 SCI-induced respiratory dysfunction
Respiratory function was evaluated both with the animals breathing room air and breathing a gas mixture containing 7% CO2, as described in Materials and Methods. Before SCI (n = 5), Vt, f, and Ve were 0.90 ± 0.02 ml, 91 ± 3.7 breaths/min and 82 ± 3.9 ml/min, respectively (Fig. 2). Exposure to 7% CO2 resulted in a statistically significant increase in all three indices of respiratory function (Fig. 2). As described in our previous study (Teng et al., 1999), 24 hr after injury there was a statistically significant decrease in Vt and a significant increase in f, resulting in a shallow and rapid breathing pattern (Fig. 2). There was also a reduced response to 7% CO2. After SCI the tidal volume increased from 0.66 ± 0.03 to 1.10 ± 0.03 ml (66%), as compared to an increase from 0.90 ± 0.02 to 1.6 ± 0.09 ml (82%) before SCI (Fig. 2). The changes in Ve elicited by 7% CO2 were also reduced. Before SCI, CO2 increased Ve from 82 ± 3.9 ml/min to 250 ± 17 ml/min (205%), whereas after SCI, Ve increased only from 86 ± 6.4 ml/min to 162 ± 15 ml/min (88%) (Fig. 2). Each of the five SCI animals then received vehicle for 8-OH-DPAT (i.e., intraperitoneal saline) followed by 8-OH-DPAT (250 μg/kg, i.p.). Figure 2 shows that vehicle treatment did not alter respiratory activity in animals breathing room air and had no effect on their response to CO2. In contrast, ∼24 min after treatment with 8-OH-DPAT, f and Vt values were restored to values not significantly different from the corresponding values before SCI. Most importantly, exposure to 7% CO2 now produced a ventilatory response that was identical to the normal response seen before SCI (Fig. 2).
Figure 2.
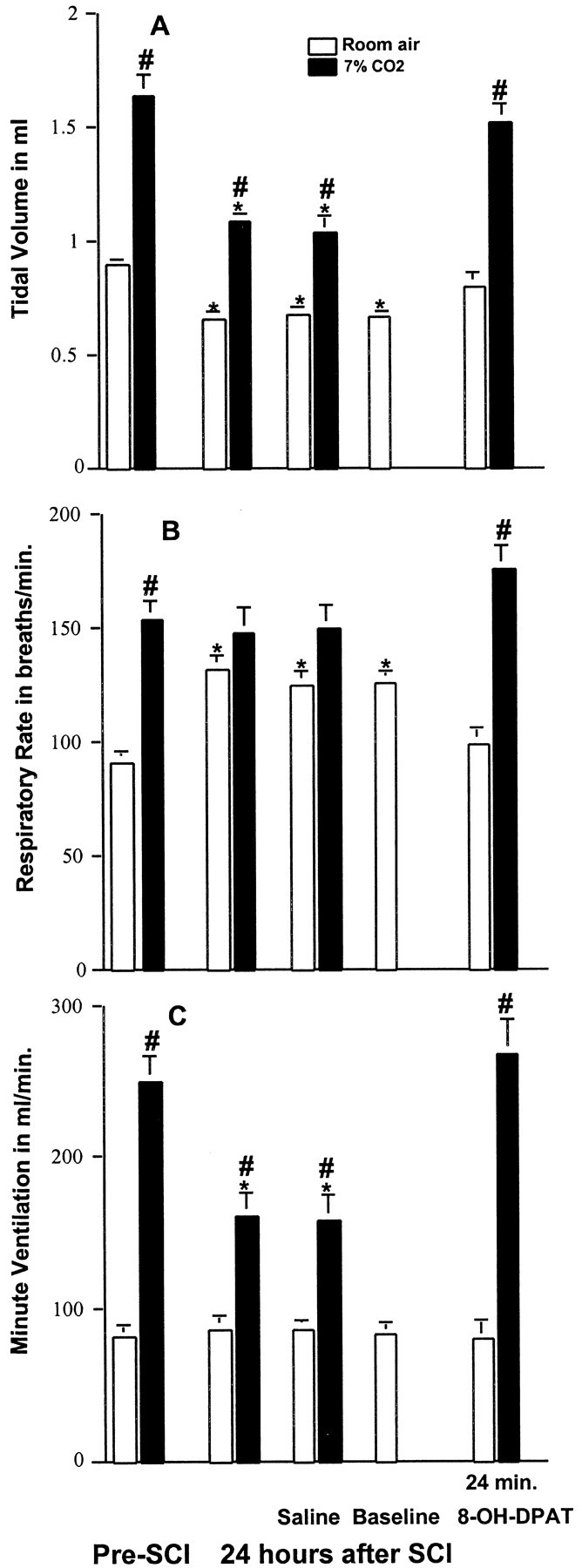
Effects of 8-OH-DPAT on SCI-induced respiratory dysfunction at 24 hr after injury. A, Vt is significantly decreased after SCI as compared with preinjury data for the same rats breathing room air or a gas mixture containing 7% CO2. In addition, the increase in Vt normally elicited by 7% CO2 is diminished. Administration of saline (vehicle for 8-OH-DPAT) had no effect on SCI-induced alterations in Vt. A baseline was re-established, and then 8-OH-DPAT (250 μg/kg, i.p.) was administered. Twenty-four minutes later, the Vt with room air and with 7% CO2 was normalized. B, The measurement of f under room air conditions was significantly increased at 24 hr after injury. There was no further increase in f in response to 7% CO2. Administration of saline had no effect. At 24 min after injection with 8-OH-DPAT, f with room air was reduced to preinjury levels and demonstrated a normal increase when respiration was stimulated with 7% CO2. C, Ve while breathing room air was not affected by SCI, but there was a decreased response to 7% CO2. Saline administration had no effect. The response to 7% CO2 returned to preinjury levels at 24 min after injection with 8-OH-DPAT. Data reported are means ± SEM for five rats and were analyzed by one-way ANOVA followed by Tukey's and Dunn's tests. *Significant difference from preinjury value obtained with the same breathing condition (room air or 7% CO2); #significant difference compared with value obtained breathing room air.
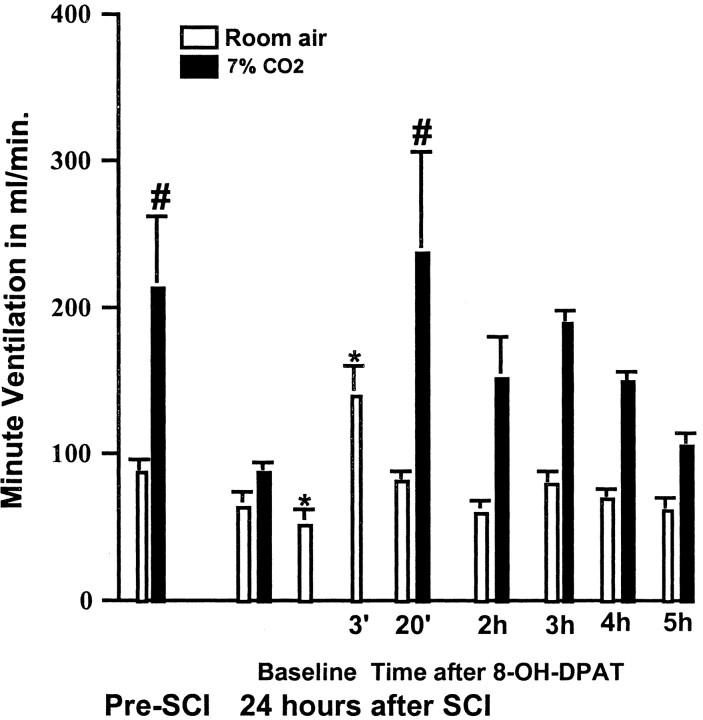
In another set of animals (n = 3), we studied the time course of the effect of 8-OH-DPAT. The stimulatory effect of 8-OH-DPAT on the ventilatory response to CO2 peaked at ∼20 min and remained near the normal range for up to 4 hr after the administration of the drug, as shown by the effect of 8-OH-DPAT on Ve (Fig. 3). After 5 hr the ventilatory response to CO2 was similar to the response observed 24 hr after SCI and before administering 8-OH-DPAT. There was also a transient stimulatory effect of 8-OH-DPAT on spontaneous respiration after SCI that was seen at 3 min after 8-OH-DPAT, and had, for the most part, disappeared by 20 min after administering the drug (Fig. 3).
Figure 3.
The time course of the effect of 8-OH-DPAT on Ve in rats at 24 hr after SCI. Bars represent the average Ve ± SEM for rats (n = 3) before and after SCI, the baseline measure before 8-OH-DPAT administration, and at specified times after the drug injection (250 μg/kg, i.p.) at 24 hr after injury. In this group of rats, SCI resulted in a small drop in baseline Ve when breathing room air, and greatly diminished the increase in Ve in response to 7% CO2 challenge. 8-OH-DPAT treatment produced a transient increase in Ve with room air conditions at the 3 min point. At 20 min after treatment, Ve returned to a preinjury level, and the increase in Ve in response to 7% CO2 was similar to that seen before SCI. Data reported are means ± SEM and were analyzed by repeated measures ANOVA followed by Tukey's test. *Significant difference from preinjury value obtained with the same breathing condition (room air or 7% CO2); #significant difference compared with value obtained breathing room air.
We also examined the respiratory function and the response to 8-OH-DPAT of these same SCI animals at 7 d after injury. These results are summarized in Figure 4. SCI-induced respiratory dysfunction was still present; that is, during room air breathing, Vt was significantly reduced (p < 0.05) from values before SCI, and f was significantly increased (p < 0.05) as compared to preinjury values. These animals still exhibited a breathing pattern that was more shallow and rapid than before injury. However, the ventilatory response to 7% CO2 of these animals was not significantly different from that observed before SCI (96 ± 5.1 to 254 ± 41 ml/min vs 82 ± 3.9 to 250 ± 17 ml/min; p < 0.05). The effect of treatment with 8-OH-DPAT on respiration 7 d after SCI is also shown in Figure 4. The most striking effect of 8-OH-DPAT was on CO2-induced increases in Ve (Fig. 4C). Seven percent CO2 was a powerful stimulant of respiration in these 8-OH-DPAT-treated rats. The increase in Ve was even greater than that before SCI. A similar enhancement in f by 8-OH-DPAT can also be noted (Fig. 4B). In contrast, injection of the saline vehicle had no effect on respiration.
Figure 4.
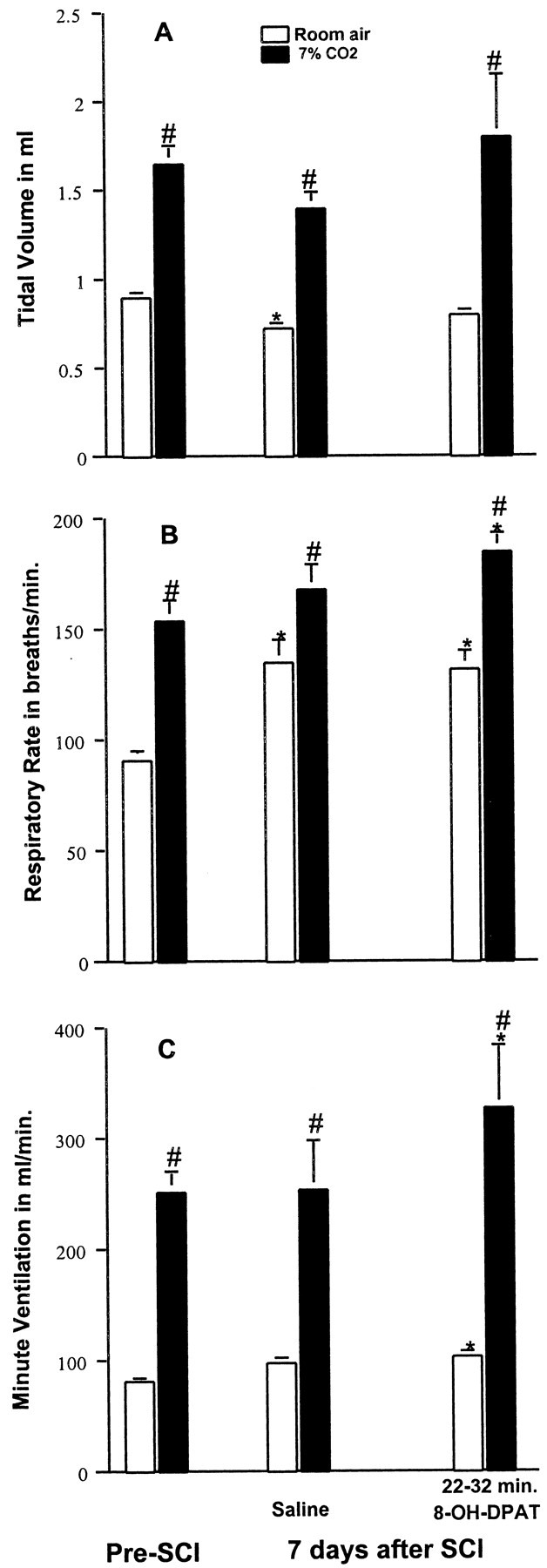
Respiratory response to 8-OH-DPAT of SCI animals at 7 d after injury. Tidal volume (A) was significantly decreased, and respiratory rate (B) significantly increased at 7 d after SCI as compared with preinjury data for the same rats, whereas Ve (C) was similar to that before injury. Stimulation with a gas mixture containing 7% CO2 increased Vt, f, and Ve, similar to that seen before injury. Saline injection has no effect. Administration of 8-OH-DPAT (250 μg/kg, i.p.) normalized the Vt with room air conditions, but f and Ve were significantly greater than that before injury with both room air and 7% CO2 conditions. Data reported were means ± SEM for three rats and were analyzed by one-way ANOVA followed by Tukey's and Dunn's tests. *Significant difference from preinjury value obtained with the same breathing condition (room air or 7% CO2); #significant difference compared with values obtained breathing room air.
Effects of 8-OH-DPAT on respiratory function of normal rats
Three uninjured animals were studied for the purpose of determining the effect of 8-OH-DPAT (250 μg/kg, i.p.) on their respiratory function. The time points chosen for analyzing an effect of 8-OH-DPAT were the same, i.e., at 4 and ∼20-30 min after administering the drug. Data are presented in Figure 5 and indicate that at 4 min after administering 8-OH-DPAT, there were statistically significant increases in Vt, f, and Ve. The significant increases in f and Ve, but not the increase in Vt, persisted until 22 min after administering the drug. However, unlike the effect on SCI animals, the ventilatory response to 7% CO2 was not significantly changed by 8-OH-DPAT administration in normal rats.
Figure 5.
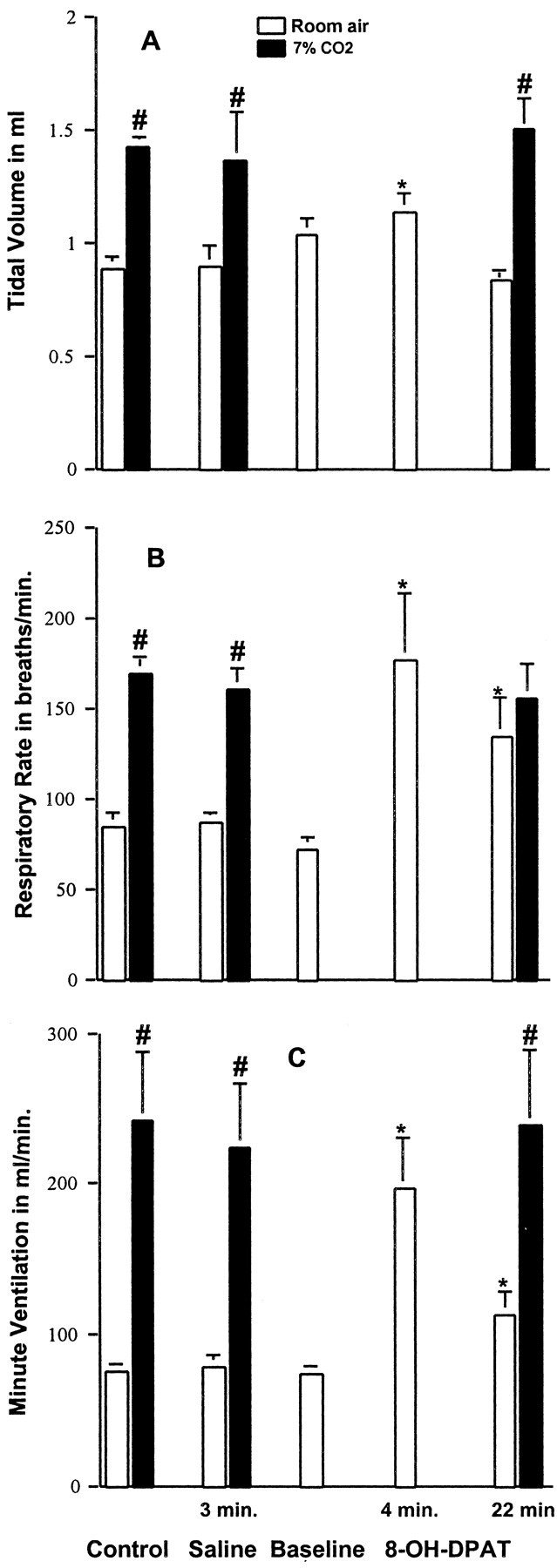
Effects of 8-OH-DPAT on respiratory function of normal rats. Normal rats breathing room air exhibited a transitory increase in Vt (A) at 4 min and a longer lasting increase in f (B) and Ve (C) at 4 and at 22 min after administration of 8-OH-DPAT (250 μg/kg, i.p.). The values for Vt, f, and Ve obtained with 7% CO2 conditions were not affected by administration of 8-OH-DPAT. Data reported are means ± SEM for three rats and were analyzed by one-way ANOVA followed by Dunn's tests. *Significant difference from preinjury value obtained with the same breathing condition (room air or 7% CO2); #significant difference compared with values obtained breathing room air. Administration of saline at the 3 min time point had no effect.
Effects of buspirone on T8 SCI-induced respiratory dysfunction
The effects of a second 5-HT1A agonist, buspirone, were studied in a similar manner to that described for 8-OH-DPAT. The data are presented in Figure 6. In this group of animals (n = 3), at 24 hr after SCI, respiration also became more shallow and rapid, and a striking decrease in the ventilatory response to CO2 was observed. Eight to 15 min after administration of buspirone (1.5 mg/kg, i.p.), there was a nonsignificant tendency toward increased Ve, and both f and Vt values were restored to values not significantly different from corresponding values present before SCI (Fig. 6). Most importantly, the ventilatory response to 7% CO2 became normal.
Figure 6.
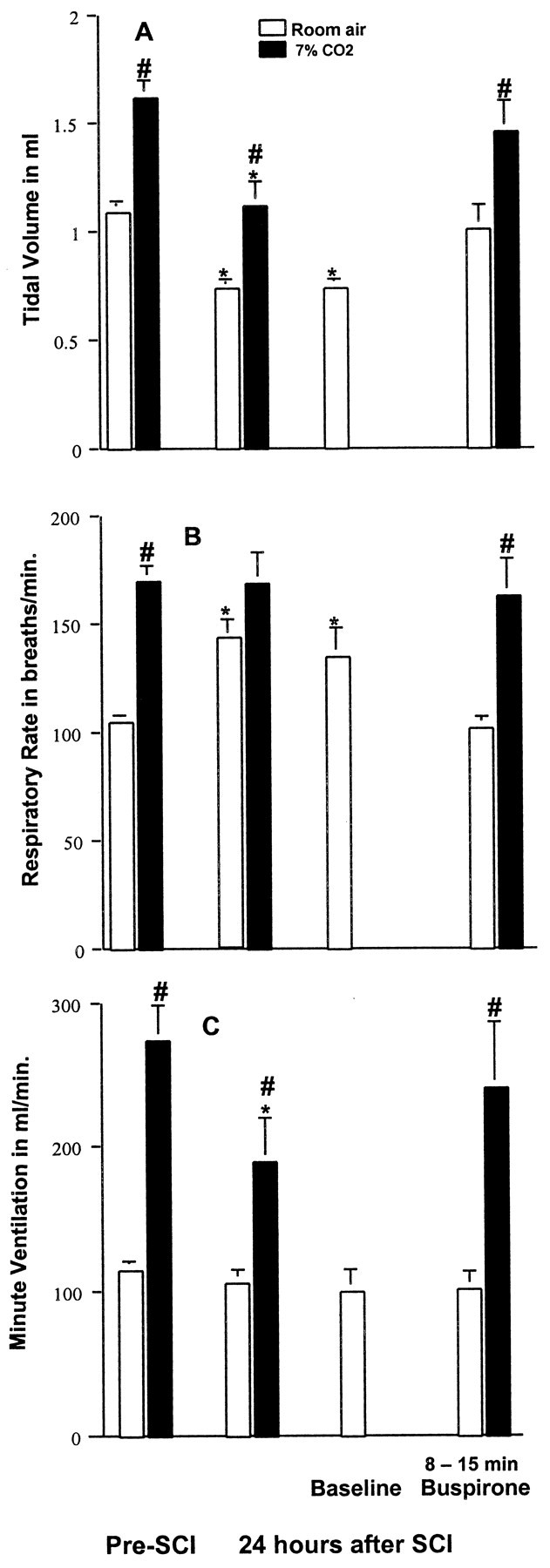
Effects of buspirone on SCI-induced respiratory dysfunction. At 24 hr after SCI, rats demonstrated reduced Vt (A) and increased f (B) with room air breathing conditions, although Ve (C) was similar to that obtained before injury. Stimulation with 7% CO2 increased Ve to a lesser extent than before injury. A baseline was re-established, and then buspirone (1.5 mg/kg, i.p.) was administered. Buspirone normalized Vt and f with room air breathing conditions and increased the response of Ve to 7%CO2 to equal that seen before SCI. Data reported are mean ± SEM for three rats and were analyzed by one-way ANOVA followed by Tukey's and Dunn's tests. *Significant difference from preinjury value obtained with the same breathing condition (room air or 7% CO2); #significant difference compared with values obtained breathing room air.
Effects of treatment with 8-OH-DPAT on T8 SCI-induced respiratory dysfunction occurring at 24 hr in animals pretreated with an antagonist (p-MPPI) of the 5-HT1A receptor
Twenty-four hours after SCI, animals (n = 3) exhibited the shallow, rapid breathing pattern (Fig. 7) previously described. At this time, they were given p-MPPI (3 mg/kg, i.p.), a specific antagonist of the 5-HT1A receptor (Kung et al., 1994). Treatment with p-MPPI did not significantly (p > 0.05) alter respiratory function of animals breathing room air (data not shown). The animals were given 8-OH-DPAT (250 μg/kg, i.p.) ∼9-18 min after administration of p-MPPI. The antagonist, MPPI, blocked the transient early stimulatory effects of 8-OH-DPAT respiration (data not shown). Furthermore, 8-OH-DPAT failed to restore the ventilatory response to CO2 to normal, in contrast to the powerful effect observed in SCI animals not pretreated with p-MPPI (compare data in Fig. 7 with data in Fig. 2). Indeed, in animals not pretreated with p-MPPI, Ve increased from 81 ± 11 to 268 ± 22 ml/min, an increase of more than threefold (Fig. 2). However, in animals that were treated with p-MPPI before receiving 8-OH-DPAT, Ve increased only from 88 ± 13 ml/min to 143 ± 28 ml/min (Fig. 7), an increase similar to that seen at 24 hr after SCI in animals receiving no treatment (87 ± 6.3 to 162 ± 15 ml/min) (Fig. 2).
Figure 7.
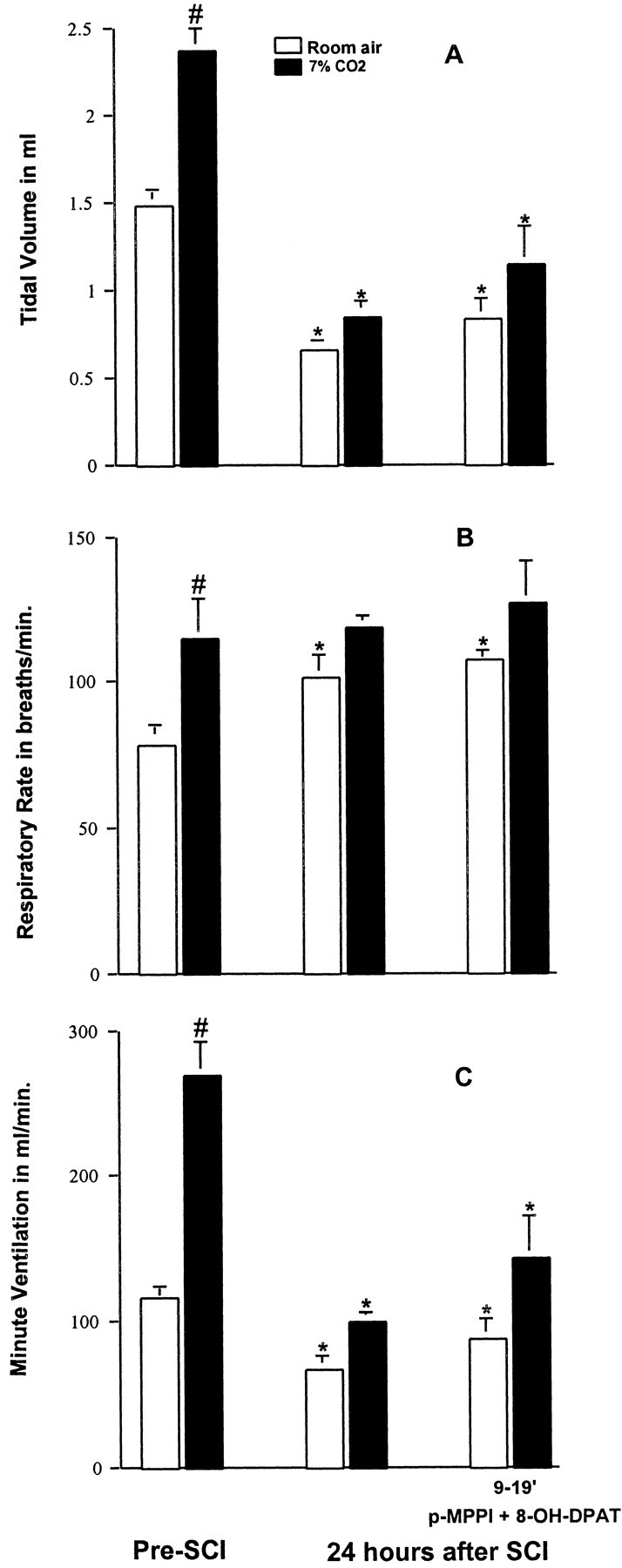
Effects of 8-OH-DPAT on SCI-induced respiratory dysfunction at 24 hr in animals treated with the 5-HT1A receptor antagonist p-MPPI. Twenty-four hours after SCI, animals exhibited decreased Vt (A), increased f (B), and decreased Ve (C) with room air breathing conditions as well as a reduced response to 7% CO2. Treatment with p-MPPI (3 mg/kg, i.p.) followed by 8-OH-DPAT (250 μg/kg, i.p.) blocked the normalization of respiration previously seen with 8-OH-DPAT (Fig. 2). Data reported are means ± SEM for three rats and were analyzed by one-way ANOVA followed by Tukey's and Dunn's tests. *Significant difference from preinjury value obtained with the same breathing condition (room air or 7% CO2); #significant difference compared with values obtained breathing room air.
Discussion
Our data confirm and extend previous results (Teng et al., 1999) demonstrating that incomplete spinal cord contusion injury at T8 in rats results in significant disturbances in respiratory function at 24 hr and 7 d after injury. The disturbances in respiratory function consisted of a decrease in Vt and an increase in f during room air breathing, as well as a reduction in the ventilatory response to breathing 7% CO2. Although Ve was normal in the SCI animals during room air breathing, the combination of depressed Vt and increased f may impair gas exchange and cause respiratory failure (Rochester, 1993) and is found in patients with lower thoracic SCI (Prakash, 1989), with respiratory muscle weakness (Gibson et al., 1977) and with muscular dystrophy (Bégin et al., 1980). Administering the 5-HT1A receptor agonist drug 8-OH-DPAT intraperitoneally to these rats at 24 hr after injury fully counteracted the respiratory disturbances created by SCI while rats were breathing room air. At both time points, 8-OH-DPAT was found to restore Vt and f values to the normal range. Furthermore, administering 8-OH-DPAT at 24 hr after injury fully restored the ventilatory response to CO2.
Two lines of evidence suggest that the beneficial effects of 8-OH-DPAT on respiratory function after SCI were mediated through 5-HT1A receptors. First, although the bulk of our data were obtained with 8-OH-DPAT, we observed that buspirone, a partial agonist at 5-HT1A receptors (Taylor, 1988), also reversed the respiratory disturbances. Second, pretreatment of animals with p-MPPI, a selective antagonist of the 5-HT1A receptor (Kung et al., 1994), prevented 8-OH-DPAT from counteracting SCI-induced impairment of respiratory function. Based on the totality of the data obtained with 8-OH-DPAT, buspirone, and p-MPPI, we conclude that activation of 5-HT1A receptors is an effective way of reversing disturbed respiratory function after incomplete contusion at T8. Additional studies will be needed to determine whether serotonin agonists will be effective with the more severe respiratory deficits expected from an upper thoracic or cervical SCI.
The reason that 5-HT1A receptor stimulation with drugs such as 8-OH-DPAT and buspirone benefits our respiratory impaired SCI rats is unclear. In seeking clues regarding the possible site of action of these drugs, it is important to consider what causes respiratory dysfunction after contusive SCI at T8. Data on cell loss over the first 24 hr in this model (Grossman et al., 2001) show that loss of ventral motoneurons at specified distances rostral and caudal to the injury epicenter progressed symmetrically with time. At 24 hr, tissue destruction was so severe that ventral motoneurons were completely absent at the epicenter and 2 mm in either direction, thus, a 4 mm length of cord was devoid. Further, at 4 mm rostral and caudal only ∼44% of ventral motoneurons survived. In addition, glia were significantly reduced at the lesion epicenter and at distances of up to ±4 mm distal from it. With such tissue destruction, functional innervation of both intercostal and abdominal muscles (motoneurons at T5-L3) (Holstege, 1991) would be significantly impaired causing the respiratory dysfunction seen at 24 hr after injury in this and our earlier study (Teng et al., 1999). Drugs that activate the 5-HT1A receptor eliminated the respiratory defects caused by SCI in the present study. We suggest that 5-HT1A receptor activation might increase the excitability of those ventral motoneurons that have survived injury. The basis for this suggested mechanism is that recent findings indicate that 5-HT1A receptors do exist on these neurons (Kheck et al., 1995), and when activated, amplify their excitatory output (Takahashi and Berger, 1990).
Although we speculate that 5-HT1A receptor activation could restore SCI-induced respiratory dysfunction to normal by affecting motoneurons, 5-HT1A receptors on other neurons could also be involved. The spinal cord regions most adversely affected by the contusion injury at T8 are the dorsal horns (Noble and Wrathall, 1985, 1989) that are involved in processing sensory input. Most importantly, intercostal and abdominal muscle afferents (including group II afferent fibers) influence supraspinal respiratory group neurons in the brainstem and motor output to skeletal muscles responsible for breathing (Shannon, 1980; Shannon and Lindsey, 1983). Indeed, removing some of this afferent input by performing a thoracic dorsal rhizotomy has been shown to decrease Vt, increase f, and reduce the ability of CO2 to stimulate breathing (Gautier, 1973). This profile of respiratory effects produced by dorsal rhizotomy mimics the profile of respiratory changes that we observed in our SCI rats. The densest population of 5-HT1A receptors in the spinal cord is in the dorsal horn (Thor et al., 1993) in laminae I-IV, and particularly in lamina II (Thor et al., 1993), suggesting a role in processing sensory inputs. A significant proportion of 5-HT1A receptor sites are located on the terminals of primary afferent neurons (Daval et al., 1987), whereas others are postsynaptic (Wikberg and Hajos, 1987) and located on neurons intrinsic to the dorsal horn (Pompeiano et al., 1992). In addition, confocal and electron microscopic study of contacts between serotonin-immunoreactive fibers and interneurons in the dorsal horn reveals that axodendritic synaptic contacts exist between 5-HT fibers and interneurons in pathways from muscle afferents with dorsal horn group II interneurons (Jankowska et al., 1997). Furthermore, 5-HT axons contact spinal interneurons that project to motor nuclei and are activated by muscle afferents (Maxwell et al., 2000). Locally applied 5-HT tested on extracellularly recorded responses of spinal interneurons evoked by group II muscle spindle afferents exerts a modulatory action (Jankowska et al., 1997). Jankowska et al. (1997) suggest that transfer of information from group II muscle afferents to supraspinal centers may be gated by descending serotonergic pathways to adjust to the requirements of a specific behavioral situation. Because the neural pathways in the spinal cord responsible for transferring afferent information from intercostal muscles to supraspinal centers are influenced by serotonin, presumably via 5-HT1A receptors, we speculate that drugs such as 8-OH-DPAT and buspirone might act in the dorsal horn of SCI rats to restore respiratory function to normal by acting on these pathways. Consistent with this speculation, Remmers (1970) found that sustained stimulation of chest wall mechanoreceptors provokes a slowing of the breathing rate, a response that we also observed with 5-HT1A receptor activation in rats after SCI.
The possibility of a supraspinal site of action of 5-HT1A receptor agonists to counteract respiratory dysfunction created by SCI seems less likely. 8-OH-DPAT has shown little or no effect on output from key brainstem respiratory centers (Johnson et al., 1996, 2001). Further studies will be needed to examine various possible sites of action of 5-HT1A receptor agonists in reducing the respiratory deficit after SCI.
Our data obtained with CO2 challenge 7 d after SCI injury indicated that animals no longer exhibited a depressed respiratory response while they continued to exhibit respiratory dysfunction when breathing room air. Furthermore, when animals at 7 d after SCI received 8-OH-DPAT, and their room air breathing was restored to normal, CO2 challenge now evoked a significantly greater response on Ve than was noted before injuring the spinal cord. This is evidence of plasticity by 7 d after injury in circuits involved in controlling respiratory function, specifically those involved in eliciting the respiratory changes evoked by CO2.
5-HT1A receptors may be involved in the functional plasticity of these respiratory pathways. Serotonin appears to activate a latent pathway used for recovery of ipsilateral phrenic nerve activity in a C2 hemisection model (Zhou and Goshgarian, 2000). Giroux et al. (1999) demonstrated that 5-HT1A receptors labeled with radioactive 8-OH-DPAT significantly increased in laminae II, III, and X of lumbar segments at 15 d after spinal cord transection. Upregulation of 5-HT1A receptors was suggested to be attributable to denervation supersensitivity, specifically postsynaptic hypersensitivity in response to loss of descending input. Kinkead et al. (1998) showed that cervical dorsal rhizotomy enhanced serotonin innervation of phrenic motor neurons. Baker-Herman and Mitchell (2002) reported that respiratory long-term facilitation of phrenic amplitude (i.e., long-lasting increase in respiratory amplitude after repeated hypoxic episodes) requires spinal serotonin receptor activation. Similar studies at 7 d after contusion SCI will be needed to determine whether upregulation of 5-HT1A receptors also occurs in our model and serves to explain the difference in effect of serotonin agonists administered at 1 and 7 d after SCI. However, others have reported proliferation of 5-HT-containing terminals in lamina II following chronic SCI in rats (Zhang et al., 1993), consistent with our finding full recovery of respiratory function by 35 d after SCI (Teng et al., 1999).
In summary, our data suggest that drugs that stimulate 5-HT1A receptors such as 8-OH-DPAT and buspirone are effective in restoring disturbances in respiratory function after SCI, specifically, incomplete spinal cord contusion injury produced at T8. Additional data of ours also show that these drugs will reverse morphine-induced apnea and dizocilpine-induced apnea (Sahibzada et al., 1999, 2000). Others have reported that these drugs will also reverse apneustic type breathing (Lalley et al., 1994; Wilken et al., 1997). Thus, the positive effect of 5-HT1A receptor agonists on disturbed respiratory function may be a general phenomenon and not limited to SCI.
Footnotes
This work was supported by National Institutes of Health Grants P01-NS-28130 and R01-NS-35647.
Correspondence should be addressed to Dr. Jean R. Wrathall, Department of Neuroscience, Georgetown University Medical Center, 3970 Reservoir Road Northwest, Washington, DC 20057. E-mail: wrathalj@georgetown.edu.
Y. D. Teng's present address: Departments of Neurosurgery and Neurology, Harvard Medical School, Children's Hospital, Boston, MA 02115 or Neurosurgical Research, Veterans Affairs Healthcare Boston System, West Roxbury, MA 02132.
A. M. Taveira-DaSilva's present address: Pulmonary Critical Care Medicine Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD 20892.
Copyright © 2003 Society for Neuroscience 0270-6474/03/234182-08$15.00/0
References
- Baker-Herman TL, Mitchell GS ( 2002) Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci 22: 6239–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC ( 1995) A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12: 1–21. [DOI] [PubMed] [Google Scholar]
- Bégin R, Bureau MA, Lupien L, Lemieux B ( 1980) Control of breathing in Duchenne's muscular dystrophy. Am J Med 69: 227–234. [DOI] [PubMed] [Google Scholar]
- Daval G, Verge D, Basbaum AI, Bourgoin S, Hamon M ( 1987) Autoradiographic evidence of serotonin 1 binding sites on primary afferent fibres in the dorsal horn of the rat spinal cord. Neurosci Lett 83: 71–76. [DOI] [PubMed] [Google Scholar]
- Gale K, Kerasidis H, Wrathall JR ( 1985) Spinal cord contusion in the rat: behavioral analysis of functional neurological impairment. Exp Neurol 88: 123–134. [DOI] [PubMed] [Google Scholar]
- Garner SJ, Eldridge FL, Wagner PG, Dowell RT ( 1989) Buspirone, an anxiolytic drug that stimulates respiration. Am Rev Respir Dis 139: 946–950. [DOI] [PubMed] [Google Scholar]
- Gautier H ( 1973) Respiratory responses of the anesthetized rabbit to vagotomy and thoracic dorsal rhizotomy. Resp Physiol 17: 238–247. [DOI] [PubMed] [Google Scholar]
- Gibson JG, Pride NB, Newsom Davis J, Loh LC ( 1977) Pulmonary mechanics in patients with respiratory muscle weakness. Am Rev Respir Dis 115: 389–395. [DOI] [PubMed] [Google Scholar]
- Giroux N, Rossignol S, Reader TA ( 1999) Autoradiographic study of alpha 1-and alpha 2-noradrenergic and serotonin 1A receptors in the spinal cord of normal and chronically transected cats. J Comp Neurol 406: 402–414. [PubMed] [Google Scholar]
- Grossman SD, Rosenberg LJ, Wrathall JR ( 2001) Temporal-spatial pattern of acute neuronal and glial loss after spinal cord contusion. Exp Neurol 168: 273–282. [DOI] [PubMed] [Google Scholar]
- Holstege G ( 1991) Descending motor pathways and the spinal motor system: limbic and nonlimbic components. Prog Brain Res 87: 307–421. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Djouhri L, Heden C, Szabo-Lackberg Z, Yin XK ( 1997) Modulation of responses of four types of feline ascending tract neurons by serotonin and noradrenaline. Eur J Neurosci 9: 1375–1387. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Smith JC, Feldman JL ( 1996) Modulation of respiratory rhythm in vitro: role of Gi/O protein-mediated mechanisms. J Appl Physiol 80: 2120–2133. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Wilkerson JER, Henderson DR, Wenninger MR, Mitchell GS ( 2001) Serotonin elicits long-lasting enhancement of rhythmic respiratory activity in turtle brain stems in vitro J Appl Physiol 91: 2703–2712. [DOI] [PubMed] [Google Scholar]
- Kheck NM, Gannon PJ, Azmitia EC ( 1995) 5-HT1A receptor localization on the axon hillock of cervical spinal motoneurons in primates. J Comp Neurol 355: 211–220. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Zhan WZ, Prakash YS, Bach KB, Sieck GC, Mitchell GS ( 1998) Cervical dorsal rhizotomy enhances serotonergic innervation of phrenic motoneurons and serotonin-dependent long-term facilitation of respiratory motor output in rats. J Neurosci 18: 8436–8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung H, Kung MP, Clarke W, Maayani S, Zhuang ZP ( 1994) A potential 5-HT1A receptor antagonist: p-MPPI. Life Sci 55: 1459–1462. [DOI] [PubMed] [Google Scholar]
- Lalley PM, Bischoff AM, Richter DW ( 1994) Serotonin 1A receptor activation suppresses respiratory apneusis in the cat. Neurosci Lett 172: 59–62. [DOI] [PubMed] [Google Scholar]
- Maxwell DJ, Riddell JS, Jankowska E ( 2000) Serotonergic and noradrenergic axonal contacts associated with premotor interneurons in spinal pathways from group II muscle afferents. Eur J Neurosci 12: 1271–1280. [DOI] [PubMed] [Google Scholar]
- Mendelson WB, Martin JV, Rapoport DM ( 1990) Effects of buspirone on sleep and respiration. Am Rev Respir Dis 141: 1527–1530. [DOI] [PubMed] [Google Scholar]
- Mendelson WB, Maczaj M, Holt J ( 1991) Buspirone administration to sleep apnea patients. J Clin Psychopharmacol 11: 71–72. [DOI] [PubMed] [Google Scholar]
- Noble LJ, Wrathall JR ( 1985) Spinal cord contusion in the rat: morphometric analyses of alterations in the spinal cord. Exp Neurol 88: 135–149. [DOI] [PubMed] [Google Scholar]
- Noble LJ, Wrathall JR ( 1989) Correlative analysis of lesion development and functional status after graded spinal cord contusive injuries in the rat. Exp Neurol 103: 34–40. [DOI] [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G ( 1992) Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain: correlation with receptor binding. J Neurosci 12: 440–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash UBS ( 1989) Neurologic diseases. In: Textbook of pulmonary diseases, Vol II, Ed 4 (Baum GL, Wolinsky E, eds), pp 1409–1436. Boston: Little, Brown. [Google Scholar]
- Remmers JE ( 1970) Inhibition of inspiratory activity by intercostals muscle afferents. Respir Physiol 10: 358–383. [DOI] [PubMed] [Google Scholar]
- Rochester DF ( 1993) Respiratory muscles and ventilatory failure: 1993 perspective. Am J Med Sci 305: 394–402. [DOI] [PubMed] [Google Scholar]
- Sahibzada N, Ferreira M, Wasserman AM, Dretchen KL, Gillis RA ( 1999) 5-HT1A receptor activation reverses apnea induced by NMDA receptor blockade in the rat. Soc Neurosci Abstr 25: 936. [Google Scholar]
- Sahibzada N, Ferreira M, Wasserman AM, Taveira-DaSilva AM, Gillis RA ( 2000) Reversal of morphine-induced apnea in the anesthetized rat by drugs that activate 5-hydroxytryptamine (1A) receptors. J Pharmacol Exp Ther 292: 704–713. [PubMed] [Google Scholar]
- Shannon R ( 1980) Intercostal and abdominal muscle afferent influence on medullary dorsal respiratory group neurons. Respir Physiol 39: 73–94. [DOI] [PubMed] [Google Scholar]
- Shannon R, Lindsey BG ( 1983) Intercostal and abdominal muscle afferent influence on pneumotaxic center respiratory neurons. Respir Physiol 52: 85–98. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Berger AJ ( 1990) Direct excitation of rat spinal motoneurones by serotonin. J Physiol (Lond) 423: 63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DP ( 1988) Buspirone, a new approach to the treatment of anxiety. FASEB J 2: 2445–2452. [DOI] [PubMed] [Google Scholar]
- Teng YD, Mocchetti I, Wrathall JR ( 1998) Basic and acidic fibroblast growth factors protect spinal motor neurons in vivo after experimental spinal cord injury. Eur J Neurosci 10: 798–802. [DOI] [PubMed] [Google Scholar]
- Teng YD, Mocchetti I, Taveira-DaSilva AM, Gillis RA, Wrathall JR ( 1999) Basic fibroblast growth factor increases long-term survival of spinal motor neurons and improves respiratory function after experimental spinal cord injury. J Neurosci 19: 7037–7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor KB, Nickolaus S, Helke CJ ( 1993) Autoradiographic localization of 5-hydroxytryptamine 1A, 5-hydroxytryptamine 1B and 5-hydroxytryptamine 1C binding sites in the rat spinal cord. Neuroscience 55: 235–252. [DOI] [PubMed] [Google Scholar]
- Wikberg JE, Hajos M ( 1987) Spinal cord alpha 2-adrenoreceptors may be located postsynaptically with respect to primary sensory neurons: destruction of primary C-afferents with neonatal capsaicin does not affect the number of [3H]clonidine binding sites in mice. Neurosci Lett 76: 63–68. [DOI] [PubMed] [Google Scholar]
- Wilken B, Lalley P, Bischoff AM, Christen HJ, Behnke J, Hanefeld F, Richter DW ( 1997) Treatment of apneustic respiratory disturbance with a serotonin-receptor agonist. J Pediatr 130: 89–94. [DOI] [PubMed] [Google Scholar]
- Wrathall JR, Pettegrew R, Harvey F ( 1985) Spinal cord contusion in the rat: production of graded, reproducible injury groups. Exp Neurol 88: 108–122. [DOI] [PubMed] [Google Scholar]
- Zhang B, Goldberger ME, Murray M ( 1993) Proliferation of SP- and 5-HT-containing terminals in lamina II of rat spinal cord following dorsal rhizotomy: quantitative EM-immunocytochemical studies. Exp Neurol 123: 51–63. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Goshgarian HG ( 2000) 5-Hydroxytryptophan-induced respiratory recovery after cervical spinal cord hemisection in rats. J Appl Physiol 89: 1528–1536. [DOI] [PubMed] [Google Scholar]