Abstract
The extinction of the central fixated stimulus before the appearance of a new target stimulus reduces the latency of saccades and pursuit, a phenomenon known as the “gap effect.” The superior colliculus (SC) plays a prominent role in the gap effect for saccades, and recent data indicate that this structure also plays some role in the control of pursuit. We now show that the firing rate of buildup neurons in the rostral SC exhibits a gap effect during the initiation of both pursuit and saccadic eye movements to parafoveal targets. Most neurons exhibited an increase in tonic activity starting ∼100 msec after the offset of the fixation spot, regardless of whether the target later appeared inside or outside of the response field of the neuron. The subsequent appearance of the target in the response field evoked phasic increases in activity that were approximately twice as large as the effects on tonic activity. For both pursuit and saccades, the levels of tonic and phasic activity were inversely correlated with latency on a trial-by-trial basis. These changes in activity provide a neuronal correlate for the shared effects on latency observed previously with the gap paradigm for pursuit and saccades. Finally, the phasic activity at pursuit onset exhibited a gap effect just like the target-evoked response, whereas the burst activity at saccade onset was fixed in amplitude. These results suggest how SC neurons may coordinate the initiation of pursuit and saccades: buildup activity may gate the initiation of pursuit, whereas it indirectly triggers saccades by recruiting a saccade-related burst.
Keywords: pursuit, saccade, superior colliculus, monkey, gap effect, attention
Introduction
Humans and monkeys use a combination of smooth-pursuit and saccadic eye movements when visually examining objects in their environment. A basic feature shared by these two eye movements is that their initiation requires a break from fixation. In perhaps the simplest case, a single eccentric visual stimulus appears, and the initiation of pursuit and saccades involves a motor decision to break fixation and track the new stimulus. The extinction of the central fixated stimulus before the appearance of the new stimulus results in the well known “gap effect” for saccades, a general reduction in the latencies of saccades and the production of some saccades with very short latencies (∼100 msec) referred to as “express” saccades (Saslow, 1967; Fischer and Boch, 1983; Fischer and Ramsperger, 1984; Fischer et al., 1984; Krauzlis and Miles, 1996a,b). This reduction in latency is presumed to occur because the disappearance of the central stimulus causes an early release of fixation (Reuter-Lorenz et al., 1991; Kingstone and Klein, 1993; Sommer, 1994) or triggers the partial preparation of saccades (Becker, 1989; Kowler, 1990; Pare and Munoz, 1996). There is also a gap effect for pursuit (Merrison and Carpenter, 1995; Knox, 1996; Krauzlis and Miles, 1996a,b; Kimmig et al., 2002), suggesting that there are common signals that regulate the initiation of both pursuit and saccades (Krauzlis and Miles, 1996b).
The superior colliculus (SC) plays a pivotal role in the gap effect. Ablation of the SC eliminates express saccades and causes a general increase in saccade latencies (Schiller et al., 1980, 1987). Neurons in the rostral SC with fixation-related activity decrease their firing after the offset of the fixation stimulus on gap trials (Dorris and Munoz, 1995; Dorris et al., 1997). Conversely, many saccade-related neurons in the caudal SC exhibit preparatory activity that is elevated during the gap period (Dorris et al., 1997; Sparks et al., 2000). These changes in activity indicate a shift in the balance of activity across the SC in favor of those neurons with response fields including the upcoming target. This shift increases the likelihood that the subsequent target-evoked volley of activity will be sufficient to directly trigger the saccade (Edelman and Keller, 1996; Dorris et al., 1997; Sparks et al., 2000).
Neurons in the rostral SC modulate their activity during pursuit, as well as during fixation and small saccades (Krauzlis et al., 1997, 2000), and microstimulation and chemical microinjection in the rostral SC alter pursuit metrics (Basso et al., 2000), indicating a causal role. One explanation for these observations is that these rostral SC neurons specify the goal for eye movements involving parafoveal target locations, including pursuit (Krauzlis et al., 1997, 2000). If so, then the activity of these neurons might be expected to change during the gap effect for pursuit, as well as for saccades, concordant with the changes in eye movement latency. The aim of this study was to test these predictions. A brief report of some of these findings has been published recently (Krauzlis et al., 2002).
Materials and Methods
Animal preparation. We collected data from two adult rhesus monkeys (Macaca mulatta) that were 5 years of age and weighed 9–12 kg. All experimental protocols for the monkeys were approved by the Institutional Animal Care and Use Committee and complied with United States Public Health Service policy on the humane care and use of laboratory animals. The monkeys were under the care of the Institute veterinarian. Under isoflurane anesthesia and aseptic conditions, we attached a head-holder using dental acrylic and titanium screws. The head-holder allowed us to fix the head in the standard stereotaxic position during experiments. During the same surgery, we also implanted a search coil around each eye (Judge et al., 1980). The coils were used to monitor eye position with the electromagnetic induction technique (Fuchs and Robinson, 1966). After initial behavioral training, we affixed a recording chamber for SC single-neuron recording to the skull in a second surgical procedure. The chamber was angled 38o to the posterior of vertical and directed at the midline, 15 mm above and 1 mm posterior to the inter-aural line.
Recording procedures. Extracellular potentials were recorded from neurons in the intermediate and deep layers of the SC (1–3.5 mm below the collicular surface). During the experimental sessions, monkeys sat in a standard primate chair and faced a video monitor (Eizo FX-E7) at a viewing distance of 41 cm that was used to present visual stimuli under computer control (Vision Research Graphics, Durham, NH). The discharge of single neurons was recorded using tungsten microelectrodes (Frederick Haer Company, Bowdoinham, ME) with impedances of 0.7–1.5 MΩ measured at 1 kHz. Electrodes were advanced through stainless steel guide tubes (23 gauge) with a microdrive mounted on top of the recording chamber. The guide tubes were held fixed in the chamber with a delrin grid system (Crist et al., 1988). Spikes from single neurons were isolated and converted to timing pulses using standard electronics (BAK Electronics, Germantown, MD) and a template algorithm (Alpha Omega Engineering, Atlanta, GA).
The presentation of stimuli and the acquisition, display, and storage of data were controlled by a personal computer using the Tempo software package (Reflective Computing, St. Louis, MO). A second personal computer, equipped with a high-speed graphics card (VSG2/3; Cambridge Research Systems, Rochester, UK) and VisionWorks software (Swift et al., 1997) acted as a server device for presenting the visual stimuli and received instructions from the Tempo computer via its serial and parallel ports. The AC voltages induced in the eye coils were routed to a phase-detector circuit that provided separate DC voltage outputs proportional to horizontal and vertical eye position (Riverbend Instruments, Birmingham, AL). The outputs were low-pass filtered (six pole Bessel filter; −3 dB at 180 Hz) and then sampled at 1 kHz (A/D converter; Computer-Boards, Middleboro, MA). The coil output voltages were calibrated with respect to eye position by having the animal fixate small spot stimuli at known eccentricities. All neuronal spike data, eye movement data, and events related to the onset of stimuli were stored on disk during the experiment and later transferred to a Free BSD Linux-based system for subsequent off-line analysis.
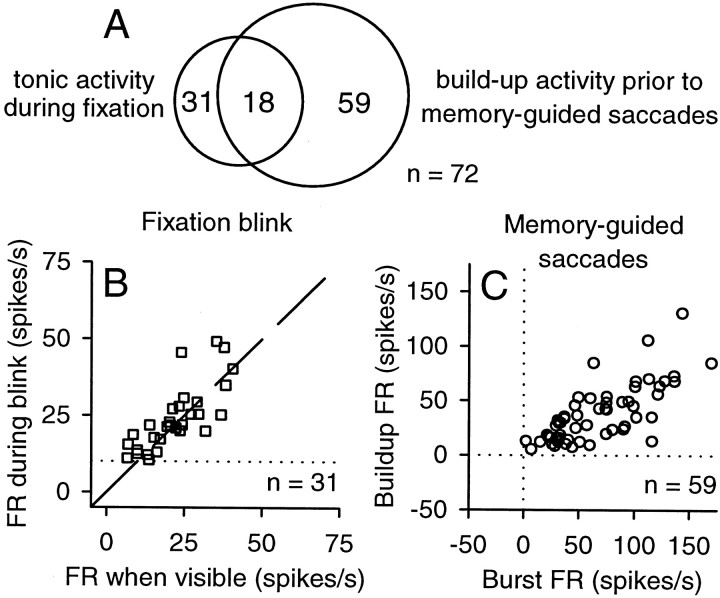
Neuron classification. After initial mapping of the response fields using visually guided saccades, we tested neurons [n = 80 (55 and 25 for monkeys W and A, respectively)] using the fixation blink paradigm (Munoz and Wurtz, 1993) and memory-guided saccades (Hikosaka and Wurtz, 1983) in separate blocks of trials. As summarized in Figure 1 A, we focused our study on neurons (n = 72) that had the same functional properties as those described previously for “rostral buildup neurons” (Krauzlis et al., 2000). The remaining neurons were active only during the motor execution of saccades (n = 5) or during the presentation of visual stimuli (n = 3).
Figure 1.
Classification of neurons reported in this study. A, A Venn diagram illustrating the numbers of neurons identified with fixation-related activity (n = 31) and buildup activity (n = 59). A subset of these neurons (n = 18) exhibited both fixation and buildup activity, for a total of 72 neurons. B, Activity of rostral SC neurons identified as fixation cells during the fixation blink paradigm. The firing rate (FR) during fixation with the target blinked off (a 100 msec interval starting 100 msec after the onset of the blink) is plotted against the firing rate during fixation with the target visible (a 100 msec interval starting 100 msec before the onset of the blink). The dashed line indicates unity slope; the dotted horizontal line indicates 10 spikes per second, which is the criterion minimum firing rate for fixation cells (Munoz and Wurtz, 1993). C, Activity of rostral SC neurons identified as buildup neurons during memory-guided saccades. The buildup firing rate (average activity during a 75 msec interval starting 100 msec before saccade onset) is plotted against the burst firing rate (average activity during an interval spanning 8 msec before saccade onset until 8 msec before saccade end).
Many of the rostral buildup neurons we selected for study (n = 31 of 72) met the criteria defined previously for “fixation cells” (Munoz and Wurtz, 1993): they maintained a firing rate of at least 10 spikes per second during blinks imposed during fixation. We documented this maintained activity by comparing the firing rate in a 100 msec blink interval (starting 100 msec after the onset of the blink) with the firing rate in a 100 msec visible interval (starting 100 msec before the onset of the blink), as shown in Figure 1 B and described previously in more detail (Krauzlis, 2001). For most of the rostral buildup neurons in our sample (n = 59 of 72), we observed an increase in activity before and during small memory-guided saccades. For these neurons, we tested activity during saccades to remembered stimuli placed at near eccentricities (3–5o) along the horizontal meridian in the contralateral hemifield (Fig. 1C). As found for buildup neurons at more caudal locations in the SC (Munoz and Wurtz, 1995), the “burst” activity for these neurons (defined as the average firing rate in an interval from 8 msec before saccade onset until 8 msec before saccade end) was generally preceded by lesser “buildup” activity (defined as the average firing rate in a 75 msec interval starting 100 msec before saccade onset). These saccades did not always produce large increases in activity, because the endpoints were chosen to be large enough to elicit reliable memory-guided saccades and match the stimulus locations used in the “gap” paradigm (described below). Thus, the endpoints did not always fall in the center of the response field of the neuron. However, to be classified as a buildup neuron, the buildup activity had to be significantly greater (Wilcoxon rank sum test; p < 0.05) than the “baseline” activity during fixation (defined as the average firing rate in a 100 msec interval starting 100 msec before fixation point offset). Of the neurons with tonic activity during fixation (n = 31 of 72, most also exhibited increased activity during memory-guided saccades (18 of 72); the remainder (13 of 72) had response fields that did not extend far enough from 0o to be tested with memory-guided saccades.
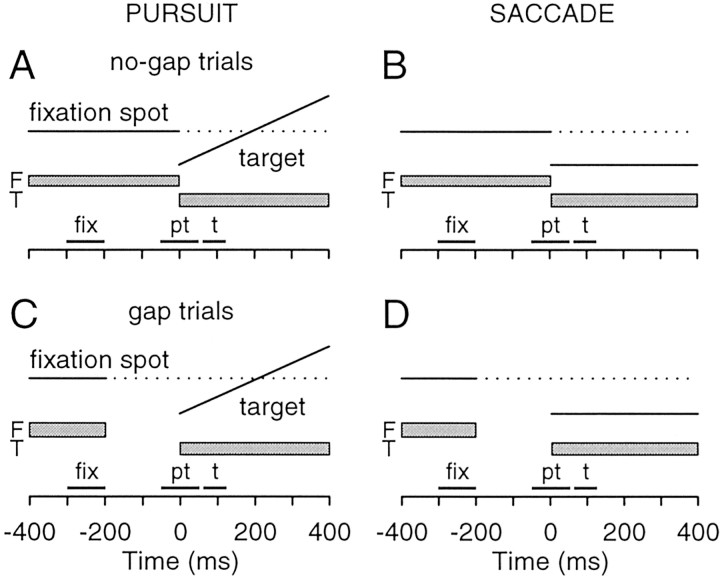
Behavioral paradigms. Neurons identified as rostral buildup neurons were then studied with the gap paradigm illustrated in Figure 2. At the beginning of each experimental trial, the monkey fixated a small spot stimulus (0.2o diameter) that appeared at the center of the display. During this fixation period, which had a randomized duration of 500–1000 msec, the monkey was required to remain within 2o of the central target; otherwise, the fixation spot was extinguished and the paradigm reverted to the fixation period after a 2500 msec timeout. At the end of the fixation period, the central spot was extinguished and a second small spot stimulus appeared at a slightly eccentric location along the horizontal meridian. On pursuit trials, this second stimulus appeared at ∼2o and moved horizontally toward and through the center of the display at a constant speed of 15o/sec. The second stimulus appeared either immediately after the offset of the fixation spot (no-gap trials) (Fig. 2 A) or after a delay of 200 msec (gap trials) (Fig. 2C). The small offset in the position of the target stimulus allowed us to elicit pursuit with few or no accompanying saccades (Rashbass, 1961), which would have otherwise confounded our analysis of pursuit-related activity. Any pursuit trials containing a saccade in a 600 msec interval beginning 100 msec before target onset were excluded from analysis. The target stimulus always moved horizontally toward the center of the display, and its starting location was always either inside the response field of the neuron under study or in the opposite hemifield. On saccade trials (Fig. 2 B,D), the second stimulus appeared at ∼3.5o and remained stationary. Once the second stimulus appeared, the monkey was allowed 500 msec to get its eyes within 3o of the stimulus position and was required to stay within 3o of the target for the remainder of the trial. The monkey was given a liquid reward at the end of each trial performed correctly. The target on saccade trials was placed at slightly more eccentric locations than on pursuit trials to avoid the increase in saccade latencies and the interference with the gap effect, observed with target eccentricities of ≤2° (Weber et al., 1992; Krauzlis and Miles, 1996b). As on pursuit trials, the target stimulus was located either within the response field of the neuron under study or in the opposite hemifield.
Figure 2.
Schematic diagrams of the experimental conditions. On pursuit no-gap trials (A), monkeys initially fixated a central target (F) that was extinguished at the same time that an eccentric target (t) appeared, moving at a constant speed back toward the center of the display. Saccade no-gap trials (B) were similar, except that the eccentric target appeared and remained stationary. On pursuit gap trials (C) and saccade gap trials (D), the target appeared 200 msec after the extinction of the fixation spot. Black horizontal bars in C and D indicate three measurement intervals. Visual fixation and pretarget activity were defined as the average firing rates in the 100 msec intervals labeled fix and pt, respectively. The target-aligned peak activity was defined as the highest firing rate in the 60 msec interval labeled t. F, Fixation stimulus; T, target stimulus.
The combination of three experimental factors [gap vs no-gap, pursuit vs saccades, and stimulus inside response field (i.e., contralateral) vs outside response field (i.e., ipsilateral)] produced a total of eight unique conditions. We presented these experimental conditions in two different modes: (1) all eight conditions fully interleaved, which we will refer to as the no-gap and gap conditions, and (2) the four gap conditions (pursuit vs saccades, inside vs outside of response field) run sequentially in separate blocks of trials, which we will refer to as the blocked-gap conditions. Thus, under blocked-gap conditions, the monkey could predict both the direction and the type of eye movement that would be required, although we excluded from analysis any trials with anticipatory responses (defined as eye movements with latencies <50 msec with respect to stimulus onset).
Data analysis. To document the changes in neuronal activity associated with the gap paradigm, we measured the firing rate in four epochs. For two of these epochs, we measured the average firing rate activity within a fixed temporal window. (1)We defined the “visual fixation” activity (Fig. 2, black bar labeled fix) as either the final 100 msec before the fixation spot was extinguished (gap trials) or a matching 100 msec interval starting 300 msec before the fixation spot was extinguished (no-gap trials). The average firing rate during this epoch provided a measure of the baseline activity of the neuron. (2) We defined the “pretarget” activity (Fig. 2, black bar labeled pt) as 50 msec before target appearance to 50 msec after target appearance. The average firing rate during this epoch provided a measure of the activity just before any changes caused by the appearance of the target stimulus and was chosen to match the measurements provided in previous reports (Dorris et al., 1997). For the remaining two epochs, we measured the peak, rather than the average firing rate. (3) We defined the “target-aligned peak” as the highest activity within the interval 60–120 msec after the appearance of the target (Fig. 2, black bar labeled t). (4) We defined the “movement-aligned peak” as the highest activity in a 41 msec interval centered on the onset of the pursuit or saccadic eye movement. For individual neurons, we assessed the statistical significance of differences between these measurements, made from individual trials using the Wilcoxon rank sum test (Matlab; The Mathworks, Natick, MA). Because we used slightly different stimulus eccentricities on pursuit and saccade trials, and also because the stimulus moved on pursuit trials but not on saccade trials, the stimulus occupied different locations within the response field on pursuit and saccade trials. For this reason, we did not perform extensive statistical comparisons between pursuit and saccades, but primarily performed statistical comparisons between different conditions for each type of eye movement separately. Finally, for each neuron, we also calculated the trial-by-trial correlation (Pearson's r) between eye movement latency and the pretarget and target-peak activities (Matlab).
We detected the occurrence of saccades by applying a set of amplitude criteria to the eye velocity and acceleration signals, as described previously (Krauzlis and Miles, 1996b). Signals encoding horizontal eye velocity and acceleration were obtained by applying a finite impulse response filter (−3 dB at 54 Hz for monkeys) to the calibrated horizontal eye position signals. This algorithm permitted us to detect saccades with amplitudes as small as ∼0.15o.
The onset of pursuit was estimated from traces of eye velocity on individual trials using a variant of an algorithm described previously (Krauzlis and Miles, 1996b). With this previous technique, the variance associated with a baseline interval was used to detect the beginning of a response interval. A linear regression of the response interval as a function of time was used to determine when the response intersected the baseline; this point in time was defined as the latency of pursuit. The extrapolation used in this method makes the latency estimates sensitive to noise in the response interval. To avoid this extrapolation, we constrained the response interval to immediately follow, and be continuous with, the baseline interval, forming a “hinge.” The baseline interval had a duration of 100 msec, and the response interval had a duration of 75 msec; we tested possible hinge placements ranging from ±40 msec from an initial subjective estimate of pursuit latency. For each of these hinge placements, the slope of the response interval was determined by linear regression, and we measured the mean squared error (MSE) between the data and the model (baseline plus response intervals). The hinge placement that provided the best fit was defined as the latency of pursuit. The MSE values associated with fits of this hinge model typically had values of 2–4 deg2/sec2; trials with large MSE values were interpreted as failures of the hinge model and were excluded from analysis. For both pursuit and saccade latencies, statistical significance of differences across conditions was assessed using the Kruskal–Wallis test for multiple comparisons (SigmaStat; SPSS, Chicago, IL).
Results
On average, the presence of a temporal gap between the offset of the fixation spot and the appearance of the eccentric target reduced the latencies of pursuit by ∼10 msec and the latencies of saccades by ∼20 msec (Table 1). These decreases in latency, although modest, were highly significant (Kruskal–Wallis one-way ANOVA; p < 0.001). For both monkeys, post hoc pairwise comparisons showed that the latencies of pursuit and saccades were significantly shorter under the gap conditions than under the no-gap conditions (Dunn's method; p < 0.01). Pursuit and saccade latencies were also significantly shorter under the blocked-gap condition than under the no-gap condition (Dunn's method; p < 0.01). Thus, under the conditions of our experiment, the introduction of a temporal gap decreased eye movement latencies, although it did not produce a distinct population of short-latency movements, such as express saccades (Fischer and Boch, 1983).
Table 1.
Latencies of pursuit and saccades in the different experimental conditions
|
|
Monkey A |
Monkey W |
|---|---|---|
| Pursuit | ||
| No-gap | 82.7 ± 0.3(n= 1279) | 98.8 ± 0.2(n= 4052) |
| Gap | 75 ± 0.2(n= 1253)* | 92.4 ± 0.2(n= 3864)* |
| Blocked-gap | 73 ± 0.2(n= 1585)* | 85.4 ± 0.2(n= 4356)* |
| Saccade | ||
| No-gap | 102.5 ± 0.3(n= 1168) | 154.5 ± 0.4(n= 3015) |
| Gap | 93.3 ± 0.3(n= 1189)* | 122 ± 0.3(n= 3008)* |
| Blocked-gap
|
96.3 ± 0.3(n= 1519)*
|
126.6 ± 0.4(n= 3351)*
|
Average latencies (in milliseconds plus SD and numbers of observations) are listed according to the type of eye movement, type of trial, and experimental subject. Asterisks indicate significantly lower than no-gap condition (Dunn's method; p < 0.01)
Changes in neuronal activity observed with the gap paradigm
The presence of a temporal gap between the offset of the fixation spot and the appearance of the eccentric target changed the activity of neurons in the rostral SC during both pursuit and saccade trials. An example of one neuron recorded in the right SC during pursuit trials is shown in Figure 3. The starting position of the target spot was 1.6o along the horizontal meridian in the contralateral visual field (Fig. 3A,C,E) or the ipsilateral visual field (Fig. 3B,D,F). Because the target moved back toward the center of the display during the pursuit latent period, the monkey was able to generate pursuit with no corrective saccades, as indicated in Figure 3 by the saccade-free traces of eye velocity. Consistent with previous studies of the rostral SC (Munoz and Wurtz, 1993; Krauzlis et al., 1997, 2000), the neuron showed an increase in activity after the appearance of the target spot in the contralateral visual field (peak in spike density function at ∼100 msec) (Fig. 3A,C,E) and a decrease in activity for the stimulus in the ipsilateral visual field (Fig. 3B,D,F). Before this visual response, the neuron exhibited an increase in activity after the disappearance of the fixation spot on gap and blocked-gap trials (Fig. 3C,E, arrows) that was not present on no-gap trials (Fig. 3A, arrow). This increase in activity was independent of the subsequent target-evoked change in activity, because it occurred before the appearance of the target spot and was evident on trials with either contralateral or ipsilateral targets.
Figure 3.
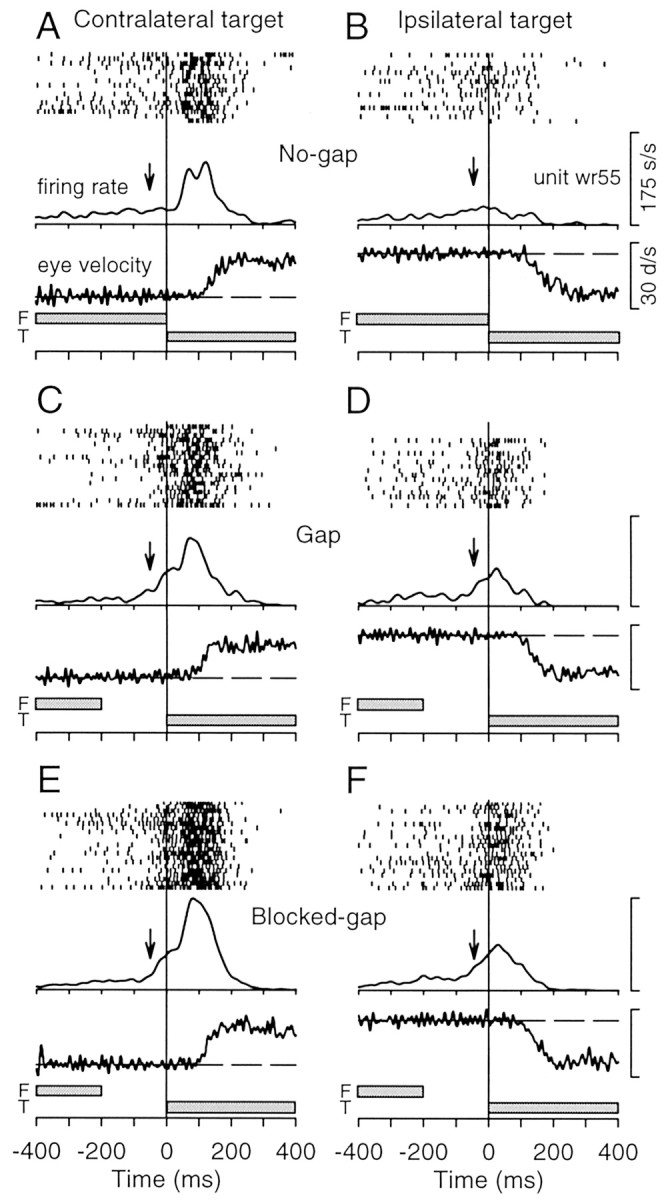
Activity of a sample rostral SC buildup neuron during pursuit trials. A–F, Data obtained on interleaved no-gap trials (A, B), interleaved gap trials (C, D), and blocked-gap trials (E, F) for targets initially appearing within the contralateral (A, C, E) or ipsilateral (B, D, F) visual field. The stack of records within each panel shows, from top to bottom, raster of spike activity, average firing rate, eye velocity from a sample trial, and timing of fixation (F) and target stimuli (T). Vertical arrows are placed at 50 msec before target onset and indicate the increase in tonic activity observed after the disappearance of the fixation spot (C–F) compared with that observed in the presence of the fixation spot (A, B).
The activity of this same neuron recorded during saccade trials is shown in Figure 4. On these trials, the target spot appeared at 3.5o along the horizontal meridian in either the contralateral or ipsilateral visual field but remained stationary. Again, the neuron showed an increase in activity for contralateral targets and a decrease in activity for ipsilateral targets. Consistent with the results obtained during pursuit trials, the neuron also exhibited an increase in activity after the disappearance of the fixation spot on gap and blocked-gap trials that was not present on no-gap trials but was present regardless of the subsequent location of the target spot.
Figure 4.
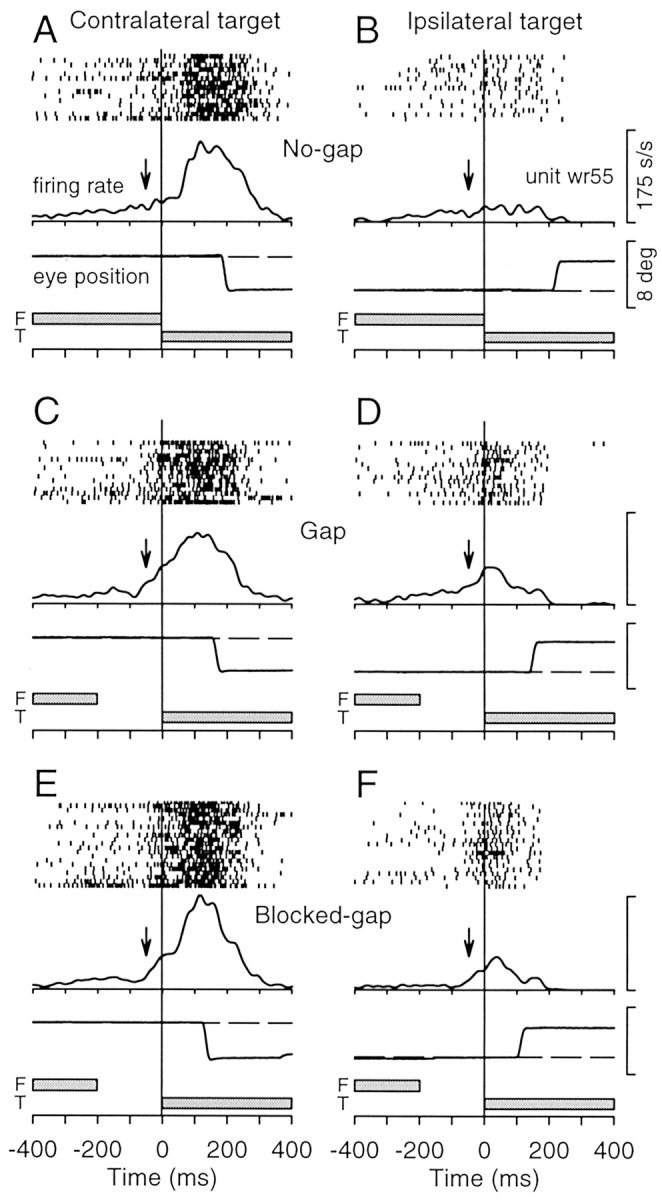
Activity of a sample rostral SC buildup neuron during saccade trials. The panels show the data obtained from the same neuron illustrated in Figure 3. The same conventions described for Figure 3 apply to this figure, except that sample eye position traces are shown in place of eye velocity.
In addition to the increases in tonic activity that occurred after the offset of the fixation spot, we also observed increases in phasic activity evoked by the appearance of the eccentric target spot in the response field of the neuron. For example, in Figure 3, the firing rate reached a peak of 118 spikes per second at 122 msec after the appearance of the target spot on no-gap pursuit trials (Fig. 3A) but reached a peak of 173 spikes per second at 80 msec on blocked-gap pursuit trials (Fig. 3E). Similarly, the firing rate reached a peak of 152 spikes per second at 199 msec no-gap saccade trials (Fig. 4A) but reached a peak of 176 spikes per second at 117 msec on blocked-gap saccade trials (Fig. 4E).
As shown below, we first document the changes in tonic activity observed with the gap paradigm and test whether these changes in activity were correlated with the latencies of pursuit and saccades. We then describe the changes in phasic target-evoked activity and similarly test whether these changes in activity were correlated with latency. Finally, we consider how the changes in phasic activity were related to the antecedent changes in tonic activity.
Changes in tonic neuronal activity
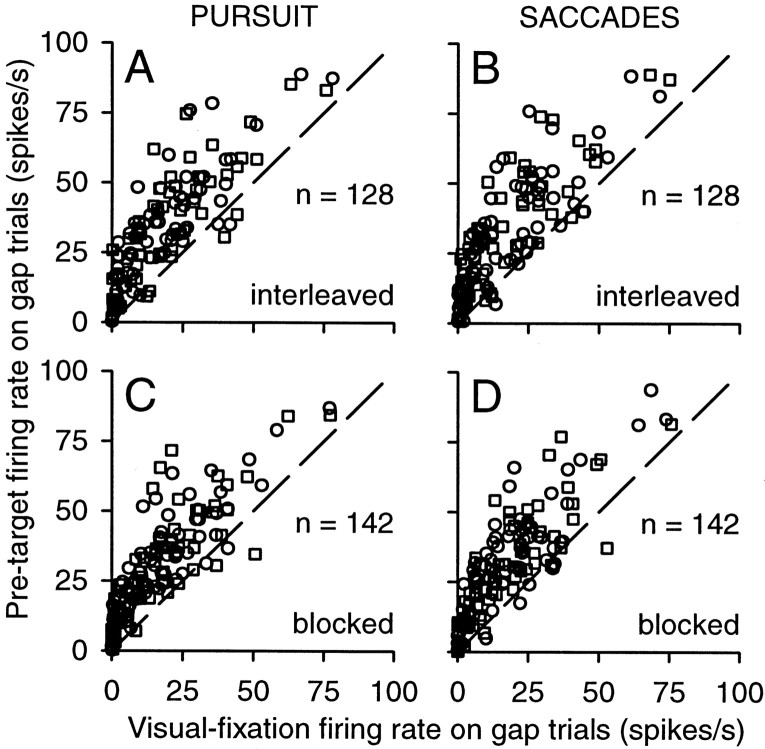
Like the sample neuron illustrated in Figures 3 and 4, most units in our sample exhibited an increase in tonic activity on gap trials shortly after the offset of the fixation spot. We quantified this effect using two different methods. For the first method, following the results of Dorris et al. (1997), we determined how the tonic activity during fixation was altered by the offset of the fixation spot. The average firing rate on gap trials was measured in two intervals: (1) a visual fixation interval, defined as the final 100 msec before the fixation spot was extinguished on gap and blocked-gap trials, and (2) a pretarget interval, defined as a 100 msec interval starting 50 msec before the appearance of the target spot. As shown by the data above the line of unity slope in Figure 5, neuronal activity during the pretarget interval on gap trials was almost always higher than the activity during visual fixation. On pursuit interleaved gap trials (Fig. 5A) randomly interleaved with other no-gap and saccade trials, activity increased significantly (Wilcoxon rank sum test; p < 0.05) for 81% (52 of 64) of the neurons for contralateral targets (circles) and for 83% (53 of 64) of the neurons for ipsilateral targets (squares). On saccade interleaved-gap trials (Fig. 5B), activity increased for 75% (48 of 64) of the neurons for contralateral targets and for 77% (49 for 64) of the neurons for ipsilateral targets.
Figure 5.
Changes in tonic activity caused by the gap: comparison with visual fixation. The average firing rate during the pretarget interval is plotted against the average firing rate during the visual-fixation interval for each neuron and for contralateral (circles) and ipsilateral (squares) target locations. Sixty-four neurons (times 2 locations) were studied on interleaved gap trials during pursuit (A) and saccades (B), resulting in n = 128. Seventy-one neurons (times 2 locations) were studied on blocked-gap trials during pursuit (C) and saccades (D), resulting in n = 142.
We found gap-related increases in activity on pursuit trials even when these trials were performed in blocks consisting of only pursuit and only one starting location (contralateral or ipsilateral). On pursuit blocked-gap trials (Fig. 5C), activity increased significantly for 86% (61 of 71) of the neurons for contralateral targets (circles) and for 89% (63 of 71) of the neurons for ipsilateral targets (squares). Under these conditions, the monkey could predict that a pursuit eye movement would be required and generated saccade-free pursuit at shorter latencies than when the gap trials were interleaved (Table 1); nonetheless, the neuronal gap effect was still present. For comparison, on saccade blocked-gap trials (Fig. 5D), activity increased significantly for 79% (56 of 71) of the neurons for contralateral targets (circles) and for 72% (51 of 71) of the neurons for ipsilateral targets (squares).
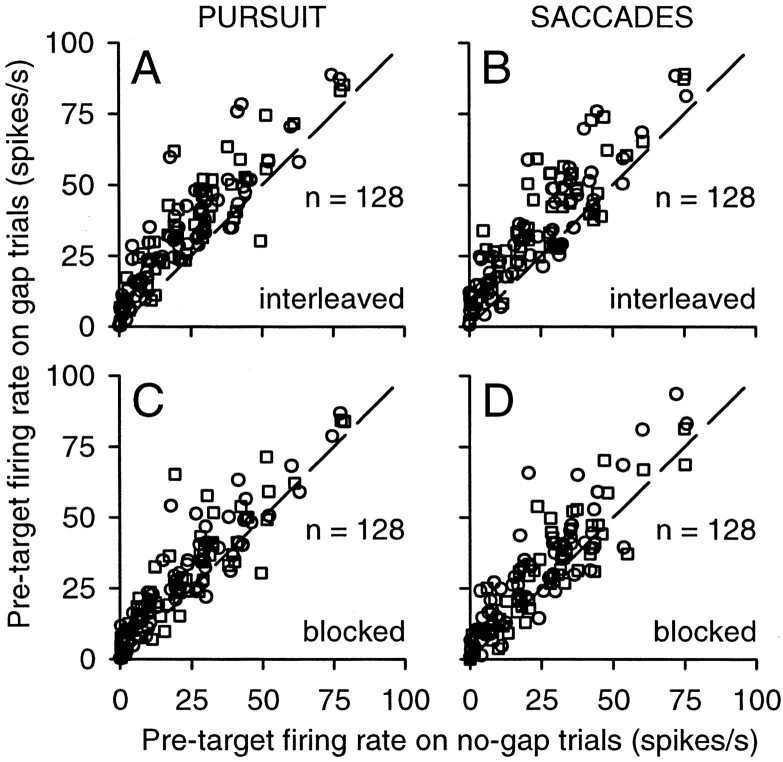
For the second method of quantifying the neuronal gap effect, we compared the firing rate during the pretarget interval on gap trials with the same interval on no-gap trials. These measurements allowed us to compare activity during matched temporal epochs, to isolate gap-related changes in activity from other time-varying influences. As shown in Figure 6, neuronal activity during the pretarget interval was almost always higher on gap trials than on no-gap trials. For interleaved pursuit trials (Fig. 6A), this difference was significant for 66% (42 of 64) and 67% (43 of 64) of the neurons for contralateral targets (circles) and ipsilateral targets (squares), respectively (Wilcoxon rank sum test; p < 0.05). For interleaved saccade trials (Fig. 6B), 56% (36 of 64) and 66% (42 of 64) of the neurons showed significant increases. These effects persisted, albeit at a somewhat lower frequency, when the trials were run in blocks separated according to eye movement type and direction. For blocked pursuit trials (Fig. 6C), activity was significantly higher on gap trials for 56% (36 of 64) and 47% (30 of 64) of the neurons. For blocked saccade trials (Fig. 6D), 48% (31 of 64) and 34% (22 of 64) of the neurons showed significant increases.
Figure 6.
Changes in tonic activity caused by the gap: comparison with no-gap trials. The average firing rate during the pretarget interval on gap trials is plotted against the average firing rate during the same interval on no-gap trials. Other conventions are the same as in Figure 5.
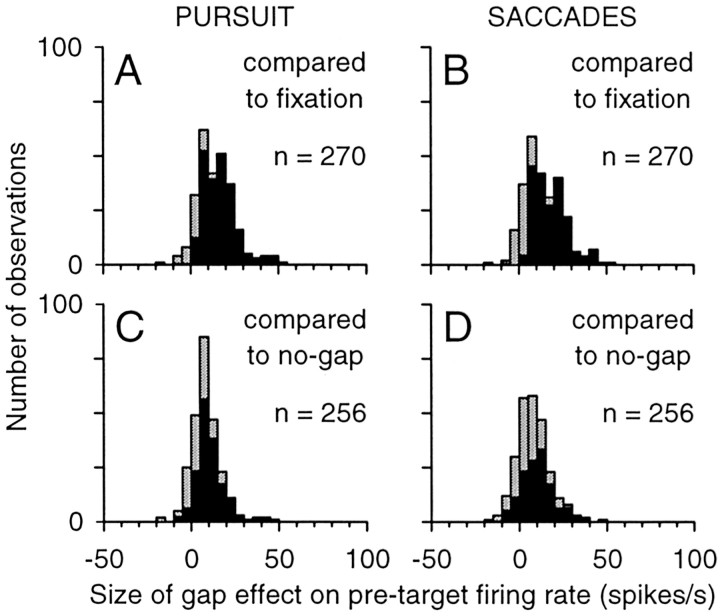
The frequency distributions in Figure 7 summarize the size of the gap effect on the pretarget activity across our sample of neurons. Compared with the firing rate during visual fixation, the firing rate on gap trials increased by an average value of 14.2 spikes per second (median, 13.2) on pursuit trials (Fig. 7A) and 13.9 spikes per second (median, 12.5) on saccade trials (Fig. 7B). The distributions for pursuit and saccades were not significantly different from each other (t test; p = 0.71), but both were significantly greater than zero (t test; p < 0.001). Compared with the pretarget firing rate on no-gap trials, the firing rate on gap trials increased by an average value of 8.5 spikes per second (median, 7.3) on pursuit trials (Fig. 7C) and 7.8 spikes per second (median, 7.2) on saccade trials (Fig. 7D). Again, the distributions for pursuit and saccades were not significantly different from each other (t test; p = 0.45), but both were significantly greater than zero (t test; p < 0.001). In summary, the offset of the fixation spot produced a modest but significant increase in tonic activity regardless of the subsequent target location (ipsilateral or contralateral) or eye movement response (pursuit or saccade).
Figure 7.
Summary of changes in tonic activity caused by the gap. The size of the gap effect was measured as the difference between the firing rate during the pretarget interval on gap trials and either the firing rate during the visual-fixation interval (A, B) or the firing rate during the pretarget interval on no-gap trials (C, D). For the distributions comparing gap effect with fixation for pursuit (A) and saccades (B), we pooled the measurements from interleaved and blocked-gap trials (Fig. 5, A and C for pursuit and B and D for saccades), resulting in n = 270. For the distributions comparing gap effect to no-gap trials for pursuit (C) and saccades (D), we again pooled the measurements from interleaved and blocked-gap trials (Fig. 6, A and C for pursuit and B and D for saccades), resulting in n = 256. Filled bars indicate significant differences (Wilcoxon rank sum test; p < 0.05).
Correlation between eye movement latency and tonic neuronal activity
To test whether the variations in eye movement latency observed under gap and no-gap conditions were correlated with these changes in tonic neuronal activity, we performed linear regression of eye movement latency against the activity during the pre-target interval on a trial-by-trial basis for each neuron. For this analysis, we analyzed only those neurons for which there were no-gap, gap, and blocked-gap trials to pool together (n = 64), to maximize the variance in latency. We analyzed the data for contralateral and ipsilateral targets separately; the minimum number of trials used for this analysis was 28 trials for pursuit (mean, 110) and 41 trials for saccades (mean, 89).
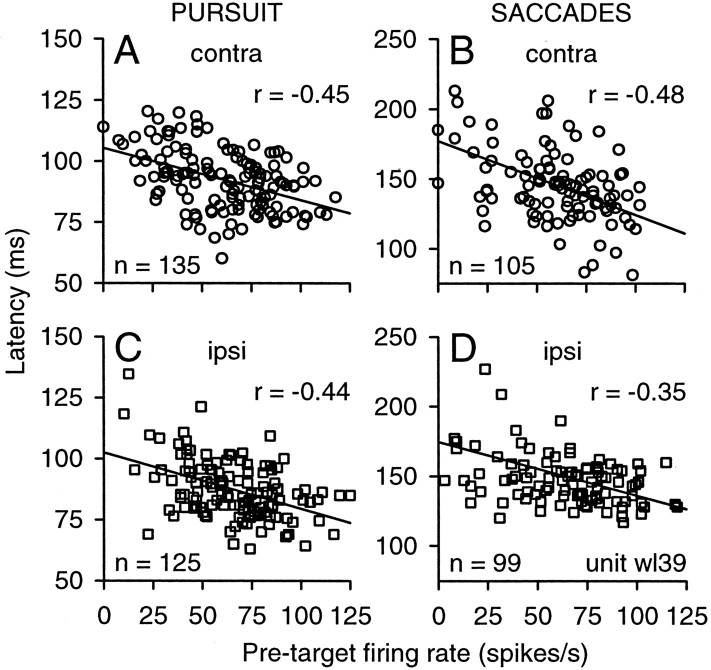
The graphs in Figure 8 show the results for a sample rostral buildup neuron recorded in the left SC. Eye movement latency was inversely related to firing rate, as indicated by the negative regression slopes superimposed on the data. For targets placed in the contralateral hemifield (Fig. 8A,B), the correlation coefficients for pursuit (r =−0.45) and saccades (r =−0.48) were both significant (p < 0.01). This relationship is consistent with the notion that gap-related increases in activity contribute to shorter eye movement latencies by bringing neuronal activity closer to the threshold value necessary for response initiation. However, a similar relationship holds for targets placed in the ipsilateral hemifield (Fig. 8C,D); the correlation coefficients for pursuit (r =−0.44) and saccades (r =−0.35) were again both significant. This result indicates that the gap-related changes in tonic neuronal activity were not specific for the location of the target.
Figure 8.
A–D, Correlation between latency and firing rate during the pretarget interval for pursuit (A, C) and saccades (B, D) for targets located in the contralateral (contra; A, B) or ipsilateral (ipsi; C, D) visual field. Each symbol indicates a pair of measurements taken from one trial from unit wl39. Straight lines indicate the results from the linear regression analysis, and r values indicate the correlation coefficients.
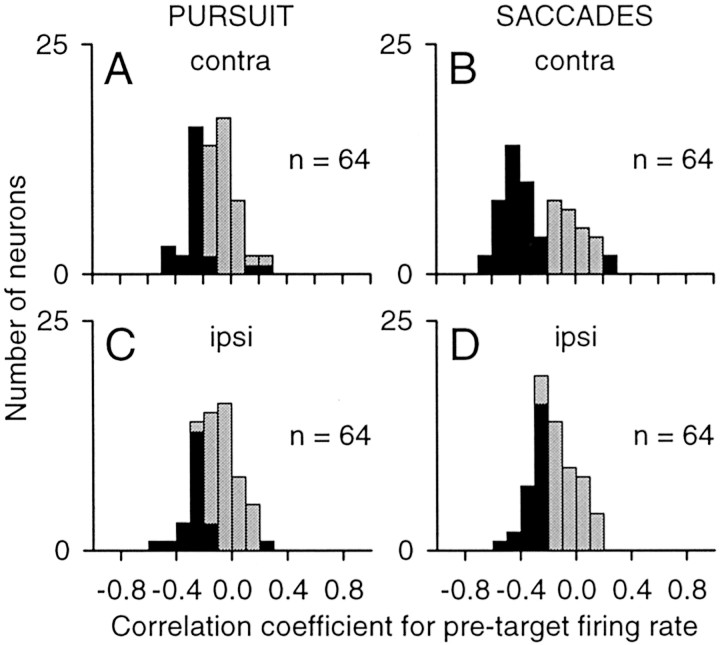
The frequency distributions in Figure 9 summarize the results across our sample of neurons. On pursuit trials, 36% of the neurons (23 of 64) showed significant negative correlations with latency for contralateral targets (Fig. 9A), and 33% (21 of 64) showed significant negative correlations for ipsilateral targets (Fig. 9C). Of the neurons with significant effects, the median correlation coefficients were −0.26 (contralateral) and −0.26 (ipsilateral). Three neurons showed significant positive correlations for pursuit latency; these neurons met the criteria for fixation cells (see Materials and Methods). On saccade trials, 59% of the neurons (38 of 64) showed significant negative correlations with latency for contralateral targets (Fig. 9B), and 41% (26 of 64) showed significant negative correlations for ipsilateral targets (Fig. 9D). The median correlation coefficients were −0.44 (contralateral) and −0.29 (ipsilateral). The two neurons that showed significant positive correlations for saccade latency also satisfied the criteria for fixation cells.
Figure 9.
Summary of the correlation between latency and firing rate during the pretarget interval. A–D, Distributions indicate the range of correlation coefficients obtained for this sample of neurons (n = 64) for pursuit (A, C) and saccades (B, D) for targets located in the contralateral (contra; A, B)or ipsilateral (ipsi; C, D) visual field. Filled bars indicate significant correlations.
Changes in target-evoked neuronal activity
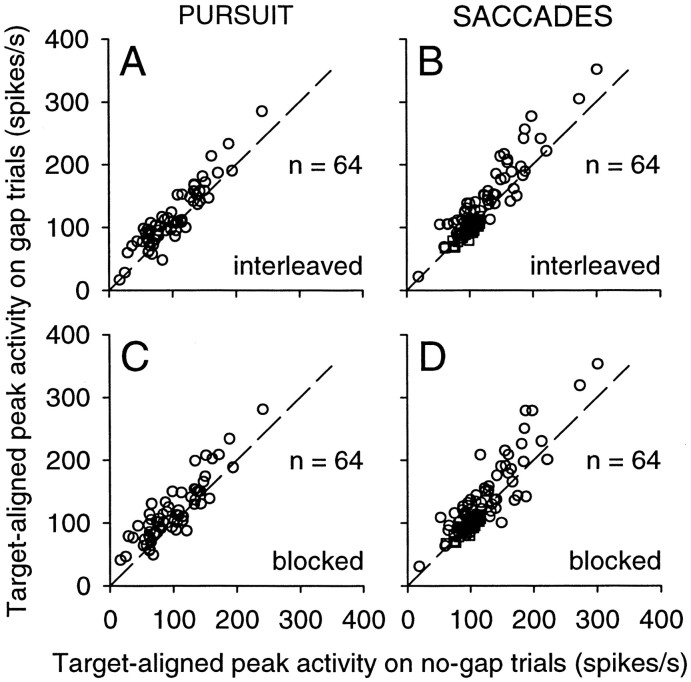
We quantified the phasic activity evoked by the appearance of the target by measuring the peak activity in the interval 60–120 msec after target onset, on a trial-by-trial basis, and also the time of this peak activity. As shown by the data above the line of unity slope in the plot in Figure 10, A and B, the target-aligned peak activity tended to be higher on interleaved gap trials than on no-gap trials. This effect was significant for 50% (32 of 64) of the neurons on pursuit trials and 42% (27 of 64) of the neurons on saccade trials (Wilcoxon rank sum test; p < 0.05). We found slightly larger effects on blocked-gap trials (Fig. 10C,D). The target-aligned peak activity was significantly higher for 53% (34 of 64) and 47% (30 of 64) of the neurons on pursuit and saccade trials, respectively (Wilcoxon rank sum test; p < 0.05). There was also a tendency for this peak activity to occur earlier on gap trials than on no-gap trials. For interleaved gap trials, this difference was significant for 22% (14 of 64) of the neurons during pursuit and 14% (9 of 64) of the neurons during saccades (Wilcoxon rank sum test; p < 0.05). For blocked-gap trials, this difference was significant for 27% (17 of 64 for pursuit) and 19% (12 of 64 for saccades) of the neurons. The average decrease in the time of the peak across all neurons was 3.9 ± 0.7 msec (SD) for pursuit and 2.3 ± 0.6 msec for saccades, and both of these values were significantly greater than zero (t test; p < 0.001).
Figure 10.
Changes in phasic activity caused by the gap: target-aligned activity. The peak firing rate during the target-aligned interval (a 60 msec interval starting 60 msec after target onset) on gap trials is plotted against the peak firing rate during the same interval on no-gap trials. Sixty-four neurons were studied with targets located initially in the contralateral visual field on interleaved gap trials during pursuit (A) and saccades (B) and on blocked-gap trials during pursuit (C) and saccades (D).
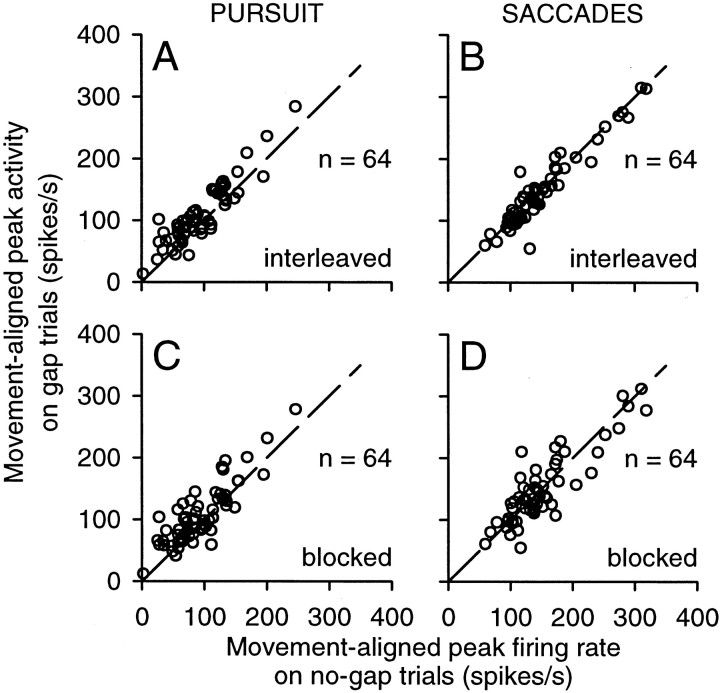
We also tested whether there were changes in the movement-aligned activity, by measuring the peak activity in a 41 msec interval centered on pursuit and saccade onset, on a trial-by-trial basis. For pursuit, the movement-aligned peak activity tended to be higher on gap than on no-gap trials, as shown by the data above the line of unity slope in Figure 11A and C. This effect was significant for 53% (34 of 64) of the neurons on interleaved pursuit trials and 45% (29 of 64) of the neurons on blocked pursuit trials (Wilcoxon rank sum test; p < 0.05). In contrast, for saccades, the movement-aligned peak activity tended to be the same on gap and no-gap trials (Fig. 11B,D). Only 6% (4 of 64) of the neurons showed significant increases with the gap on interleaved saccades trials and 14% (9 of 64) on blocked saccade trials (Wilcoxon rank sum test; p < 0.05). Similar numbers of neurons showed significant decreases: 8% (5 of 64) on interleaved gap trials and 17% (11 of 64) on blocked-gap trials (Wilcoxon rank sum test; p < 0.05).
Figure 11.
Changes in phasic activity caused by the gap: movement-aligned activity. The peak firing rate during the movement-aligned interval (a 41 msec interval starting at movement onset) on gap trials is plotted against the peak firing rate during the same interval on no-gap trials. Other conventions are the same as in Figure 10.
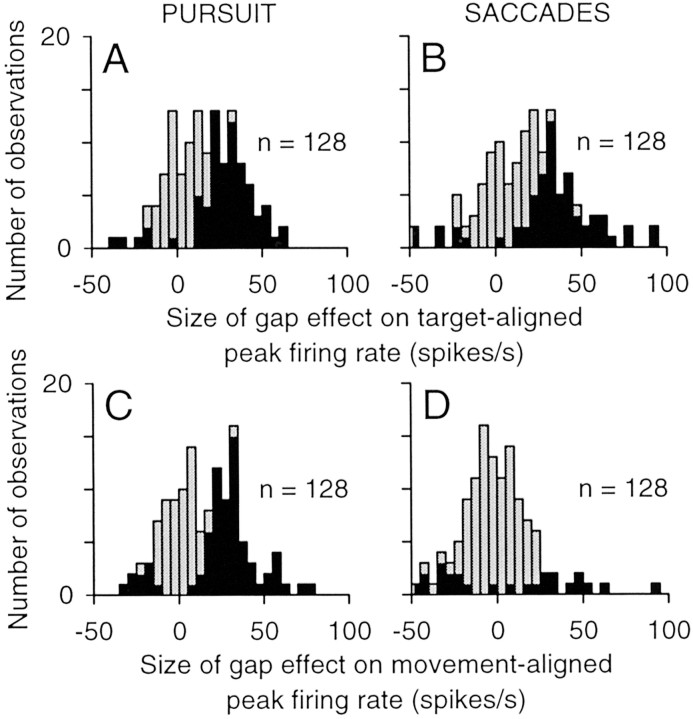
The size of the gap effect on target-aligned and movement-aligned activity across the sample of neurons is summarized by the distributions in Figure 12. The target-aligned peak activity was larger on gap trials than on no-gap trials by an average value of 16.8 spikes per second (median, 16.6) for pursuit (Fig. 12A) and 19.2 spikes per second (median, 19.9) for saccades (Fig. 12B). On average, the increase in target-evoked activity on gap trials was therefore approximately twice as large as the increase in pre-target activity (compare with Fig. 7). This difference in the size of the gap effect between the target-evoked activity and the pretarget activity was highly significant for both pursuit and saccades (Mann–Whitney rank sum test; p < 0.001). This increase in target-evoked activity might have been caused by superimposing a relatively fixed target-evoked response on baseline activity that was elevated beyond that observed in the pretarget interval. Alternatively, the target-evoked activity might have been amplified by the elevated baseline, as if the gap effect involved an increase in the rate of rise of activity as well as a change in the initial level of activity.
Figure 12.
Summary of changes in phasic activity caused by the gap. A, B, Size of gap effect was measured as the difference between the peak firing during the target-aligned interval on gap trials and the peak firing rate during the same interval on no-gap trials for pursuit (A) and saccades (B). C, D, Size of gap effect was measured as the difference between the peak firing during the movement-aligned interval on gap trials and the peak firing during the same interval on no-gap trials for pursuit (C) and saccades (D). For each distribution, we pooled the measurements from interleaved and blocked trials (each with n = 64), resulting in n = 128. Filled bars indicate significant differences (Wilcoxon rank sum test; p < 0.05).
Across the sample of neurons, the movement-aligned peak activity was larger on gap trials than on no-gap trials for pursuit (Fig. 12C) but not for saccades (Fig. 12D). For pursuit, the size of the gap effect on movement-aligned activity (average, 1.4. spikes per second; median, 12.5 spikes per second) was not significantly different from the size of the gap effect on target-aligned activity (t test; p = 0.37), primarily because the timing of pursuit onset was similar to the timing of the visual response. However, for saccades, the gap effect on movement-aligned activity (average, −2.8 spikes per second; median, −2.7 spikes per second was significantly lower than the effect on target-aligned activity (t test; p < 0.001) and was not significantly different from zero (t test; p = 0.20). This difference in the gap effect between pursuit and saccades was attributable to the occurrence of saccade-related bursts that exhibited the same peak firing rate on both gap and no-gap trials (Fig. 11B,D). These bursts were absent on our pursuit trials, because pursuit trials containing saccades were excluded from analysis.
Correlation analyses of the target-evoked changes in neuronal activity
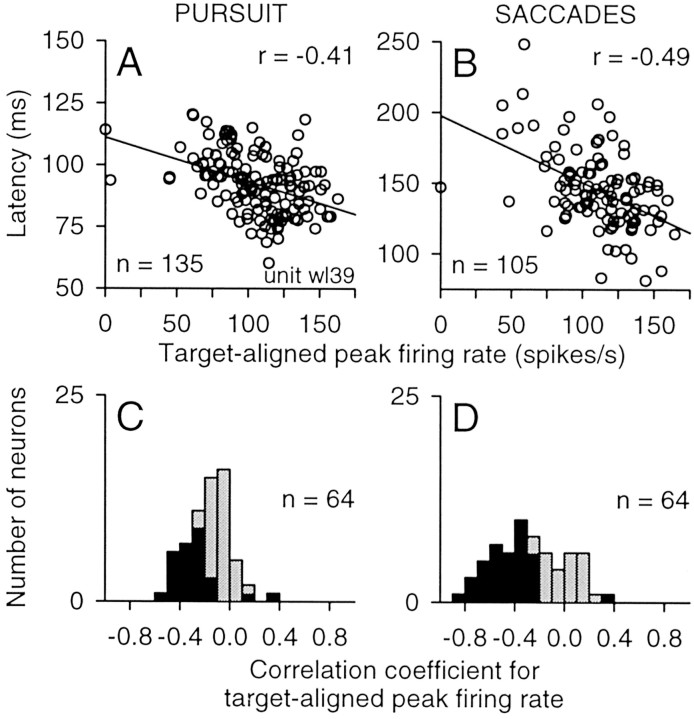
Similar to the analysis of the pretarget activity, we tested whether the variations in eye movement latency were correlated with target-evoked changes in activity by performing a linear regression of eye movement latency against the target-aligned peak activity on a trial-by-trial basis for each neuron. The graphs in Figure 13, A and B, show the results for the same neuron illustrated in Figure 8. Eye movement latency was again inversely related to firing rate, and the regression slopes for these target-aligned changes in activity were somewhat steeper than those found for the pretarget interval. For targets placed in the contralateral hemifield, the correlation coefficients for pursuit (r = −0.41) and saccades (r =−0.49) were both significant (p < 0.001). Not surprisingly, but in contrast to the results found for the pretarget activity (Fig. 9), the changes in target-aligned activity were specific for stimuli placed in the contralateral hemifield (Fig. 13A,B).
Figure 13.
Correlation between latency and firing rate during the target-aligned interval. A, B, Sample correlations for pursuit (A) and saccades (B) for the same neuron shown in Figure 9. Each symbol indicates a pair of measurements taken from one trial from unit wl39. Straight lines indicate the results from the linear regression analysis, and r values indicate the correlation coefficients. C, D, Summary of the correlation between latency and firing rate during the target-aligned interval. Distributions indicate the range of correlation coefficients obtained across this sample of neurons (n = 64) for pursuit (C) and saccades (D) for targets located in the contralateral visual field. Filled bars indicate significant correlations.
The frequency distributions in Figure 13, C and D, summarize the results we obtained for the target-aligned activity across our sample of neurons. On pursuit trials, 41% of the neurons (26 of 64) showed significant negative correlations with latency for contralateral targets, and the median correlation coefficient for these neurons was −0.31. On saccade trials, 59% of the neurons (38 of 64) showed significant negative correlations with latency for contralateral targets, and the median correlation coefficient for these neurons was −0.46. Compared with the results obtained for the activity during the pretarget interval (Fig. 9), the correlations for the target-aligned activity were somewhat higher and reached significance for more neurons, although the two sets of correlation coefficients were not significantly different from each other for either pursuit or saccades (Mann–Whitney rank sum test; p > 0.05).
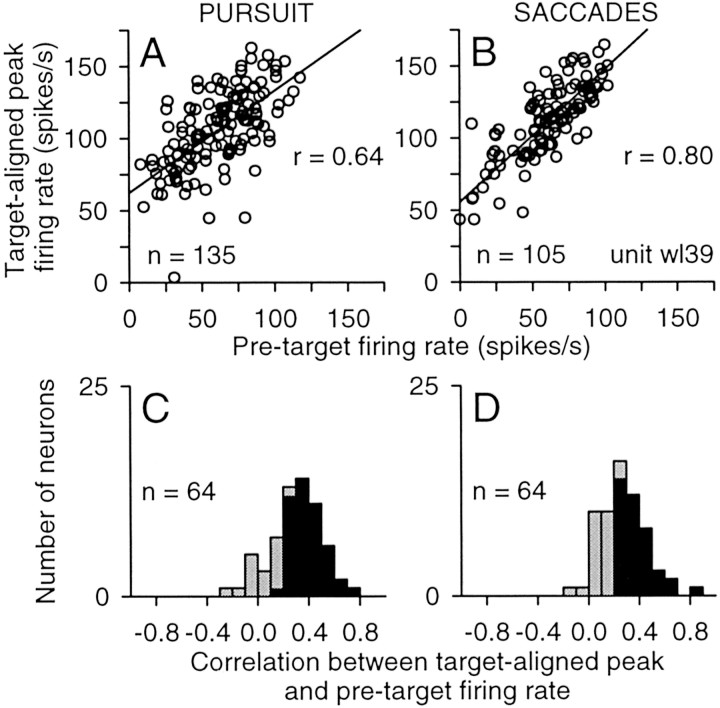
Finally, we tested whether the changes in target-evoked activity were correlated with the preceding tonic activity by performing a linear regression of target-aligned peak activity against the pretarget activity on a trial-by-trial basis for each neuron. The graphs in Figure 14, A and B, show the results for the same neuron illustrated in Figures 8 and 13. The target-aligned peak activity was positively correlated with the pretarget activity; the correlation coefficients for pursuit (r = 0.64) and saccades (r = 0.80) were both significant. The frequency distributions in Figure 14, C and D, summarize the correlation coefficients we obtained across the sample of neurons. On pursuit trials, 73% of the neurons (47 of 64) showed significant positive correlations, and the median correlation coefficient for these neurons was 0.37. On saccade trials, 63% of the neurons (40 of 64) showed significant positive correlations, and the median correlation coefficient for these neurons was 0.36. The strength of these correlations demonstrates that the changes in target-aligned peak activity were primarily dependent on the antecedent changes in tonic activity. In addition, the coefficients obtained with the linear regression analysis also provide information about the likely mechanism underlying the increases in target-evoked activity. Overall, for the neurons with significant correlations, the average slopes of the regressions were 0.92 ± 0.55 (SD) and 0.95 ± 0.52 for pursuit and saccades, respectively, and the average y-intercepts were 89 ± 39 and 120 ± 55. The near-unity slopes indicate that the target-evoked activity was not amplified by the elevated baseline. Instead, the large positive y-intercepts indicate that the target-evoked activity was superimposed on a baseline activity that was elevated by an additional 50–150 spikes per second compared with that observed during the pretarget interval.
Figure 14.
Correlation between the phasic activity during the target-aligned and the tonic activity during the pretarget interval. A, B, Sample correlations for pursuit (A) and saccades (B) for the same neuron shown previously, unit wl39. C, D, Summary of the correlation between phasic and tonic activity. Distributions indicate the range of correlation coefficients obtained across this sample of neurons (n = 64) for pursuit (C) and saccades (D) for targets located in the contralateral visual field. Filled bars indicate significant correlations.
Discussion
We have shown that the firing rate of buildup neurons in the rostral SC is modulated by the removal of a fixated stimulus, and that these changes are related to the time it takes to initiate pursuit and saccades to an eccentric target. Most neurons showed a significant increase in tonic activity starting ∼100 msec after the offset of the fixation spot and, for many neurons, the latencies of pursuit and saccades were correlated with these changes in tonic activity on a trial-by-trial basis. Both the increases in tonic activity and the correlations with eye movement latency were observed regardless of whether the target subsequently appeared in the response field of the neuron or in the opposite visual hemifield. This lack of selectivity suggests that these changes in tonic activity were part of an early stage of response preparation that could be dissociated from the final motor plan. Most neurons also exhibited increases in the phasic activity evoked by the appearance of the target stimulus; these changes were approximately twice as large as the effect on tonic activity. The latencies of pursuit and saccades were correlated with the amplitude of this target-evoked activity, which in turn, was correlated with the level of tonic activity after removal of the fixation stimulus. Finally, the phasic activity around the time of pursuit onset was also increased by the early removal of the fixation spot, but the amplitude of the saccade-related burst was mostly unchanged. These observations provide insight into the role played by buildup activity in the SC in the initiation of pursuit and saccades, and provide a neuronal correlate for the shared behavioral effects observed previously with the gap paradigm during both types of eye movements (Saslow, 1967; Fischer and Boch, 1983; Fischer and Ramsperger, 1984; Fischer et al., 1984; Merrison and Carpenter, 1995; Knox, 1996; Krauzlis and Miles, 1996a,b; Kimmig et al., 2002). We first relate these new results to our previous data on pursuit-related activity in the rostral SC, and then consider how this activity might act to coordinate pursuit and saccades.
The role of SC buildup activity in the initiation of pursuit eye movements
We have shown previously that changes in buildup activity during pursuit, saccades, and fixation are primarily determined by the retinotopic location of the visual target, rather than by the resulting motor outcome (Krauzlis et al., 1997, 2000). Based on these findings, we suggested that these neurons provide a common signal that could aid in the coordination of different types of eye movements, by providing either a motor error signal that modifies the metrics of pursuit and saccades or a retinotopic position signal that contributes to target selection for both eye movements. The observation that buildup activity can occur without a saccade or pursuit eye movement (Munoz and Wurtz, 1995; Krauzlis et al., 2000) indicates that this buildup activity does not represent a motor error signal in the strictest sense; otherwise, it would always cause a corrective motor output. Might buildup activity represent candidate motor error signals for pursuit? Our results argue against this possibility. In the current study, we used a step-ramp paradigm (Rashbass, 1961), in which the target appeared ∼2o in one visual hemifield before it moved smoothly toward and into the opposite visual hemifield. Buildup activity was increased when the initial step was contralateral (i.e., into the response field of the neuron), although the ensuing pursuit moved the eyes toward the ipsilateral side (i.e., away from the response field of the neuron). If this activity represented a motor error signal driving pursuit, it was therefore in the wrong direction.
One might argue that the neurons initially provided a motor error signal promoting contraversive pursuit, but this activity was truncated once tracking started in the ipsiversive direction. However, pursuit-related activity does not typically end at pursuit onset and can persist well into ipsiversive pursuit (Krauzlis and Dill, 2002), contrary to what would be expected if this activity specified the direction of pursuit. Instead, the changes in pursuit-related activity appear to be related to the retinotopic location of the stimulus vis-à-vis the location and extent of the response field of the neuron, consistent with our previous results (Krauzlis et al., 1997, 2000). It therefore seems unlikely that buildup activity provides a motor error signal that modifies the metrics of pursuit but instead provides a retinotopic signal indicating the location of the target. Supporting this idea, we found that pursuit latencies were correlated with the changes in buildup activity. Although modest, the degree of correlation for pursuit was generally similar to the correlation we found for saccades, which were, in turn, similar to the values reported previously for buildup neurons in a saccade gap paradigm (Dorris et al., 1997). Thus, we found similar results for pursuit and saccades, suggesting a similar interpretation: the introduction of a gap reduces the latencies of pursuit and saccades by facilitating selection of the next eye movement goal.
SC activity and the coordination of pursuit and saccades
We have shown recently that during a two-alternative forced-choice task, buildup neurons in the SC exhibit a preference for target over distractor stimuli that emerges before the initiation of pursuit and saccades (Krauzlis and Dill, 2002). By applying a simple decision rule to this change in buildup activity, we demonstrated that this preference could account for the target choices made by the pursuit and saccadic systems and inferred that pursuit uses a less stringent decision criterion than saccades. Based on our current results, we now suggest that this difference in decision criterion could be a consequence of the functional relationship between buildup activity and saccade-related bursts. We found that the offset of a fixated stimulus caused an increase in neuronal excitability that facilitated the effect of subsequent visual inputs for both pursuit and saccades. However, for saccades, this more rapid increase in buildup activity does not appear to directly trigger the saccade, but leads to the faster recruitment of a fixed-amplitude saccade-related burst, which likely provides the effective trigger. Thus, buildup activity might help to define the goal for both pursuit and saccades, but appears to play a permissive role. Pursuit may be triggered if buildup activity exceeds some threshold value, but only if there are motion signals available to drive the pursuit eye movement. Similarly, saccades may occur after an increase in buildup activity, but only if this buildup succeeds in recruiting a high-frequency burst. The relatively higher criterion for saccades and the associated lower error rates and longer latencies may result from the additional excitation required to obtain this saccade-specific burst.
Based on behavioral results with the gap paradigm, we proposed previously that the processes underlying the initiation of pursuit and saccades involve two types of inputs: “selection signals” that are necessary but not sufficient to initiate the movement, and “drive signals” that potentially determine the metrics of the movement (Krauzlis and Miles, 1996b). We also argued that the selection signals were shared between pursuit and saccades but acted through different neural substrates. Our current results provide neuronal correlates for these distinctions. The shared selection inputs may be mediated by the buildup activity that we have shown is related to the initiation of both pursuit and saccades, whereas the initiation of saccades requires a distinct neuronal event, namely the saccade-related burst. This viewpoint also provides an explanation for the increase in the gain and selectivity of pursuit observed in the wake of a targeting saccade (Lisberger, 1998; Gardner and Lisberger, 2001). After the saccade, buildup activity would be elevated at the rostral SC site representing the tracked target, producing a pursuit response selective for the same stimulus targeted by the saccade. If the increase in buildup activity precedes the onset of the saccade by a sufficient interval, this interpretation predicts that pursuit should be enhanced before the saccade as well, and data exist suggesting that this does in fact occur (Gardner and Lisberger, 2001, their Fig. 5; Liston et al., 2002). Indeed, the classic step-ramp paradigm by Rashbass (1961) might be the extreme example of this scenario. This paradigm can evoke a dramatic increase in buildup activity, but with no high-frequency burst, and can produce a pursuit response that is highly selective for the target stimulus but with no saccades (Krauzlis and Dill, 2002).
These selection signals are probably related to the mechanisms of visual attention. During the gap paradigm, the offset of the fixation spot precipitates the disengagement of attention from the location of fixation and facilitates a shift of attention to other spatial locations (Posner, 1980; Mayfrank et al., 1986; Fischer and Breitmeyer, 1987), consistent with the increases in tonic and phasic activity we observed. For saccades, previous studies have shown that buildup activity in the SC can be modulated by symbolic cues or stimulus probability, with corresponding effects on saccade latencies (Kustov and Robinson, 1996; Basso and Wurtz, 1998). Our results indicate that attentional modulation of buildup activity has consequences for pursuit as well, and suggest that attentional modulation of SC activity could play a central role in coordinating these two eye movement systems. This interpretation provides an explanation for the findings that microstimulation of the rostral SC impedes pursuit of stimuli moving toward the ipsilateral side but has only small effects on pursuit of stimuli moving toward the contralateral side (Basso et al., 2000). If such activation mimics the effects of attention, ipsilateral microstimulation would draw attention away from the target and thereby reduce the gain of pursuit, whereas contralateral microstimulation would reinforce the naturally occurring shift of attention toward the target. Our current results also highlight the dynamic nature of these presumptive attentional shifts. The offset of the fixation spot increased the activity of rostral buildup neurons in both SCs, consistent with the potential locations of the upcoming target, but after the target appeared, this small enhancement over a large area narrowed to a large enhancement within a small area around the target. These changes in activity parallel a “spotlight” of attention whose location and size changes as new information becomes available (James, 1890; Posner, 1980; Eriksen and Yeh, 1985; Eriksen and St. James, 1986; Posner and Petersen, 1990). Although attention is often depicted as an auxiliary factor superimposed on the motor control of pursuit and saccades, there is evidence that the orienting of attention involves the same circuits that select targets for eye movements (Rizzolatti et al., 1987; Sheliga et al., 1995). We speculate that the attentional filtering of inputs may be a fundamental component of eye movement preparation rather than an optional feature. If so, the time course of selectivity in eye movements might derive from the temporal dynamics of attention, dynamics that are reflected in the buildup activity of neurons in the SC, among other places.
Footnotes
This research was supported by National Eye Institute Grant EY12212 and The McKnight Foundation. I am grateful for administrative assistance from Cathy Cramer.
Correspondence should be addressed to Richard J. Krauzlis, Salk Institute for Biological Studies, 10010 North Torrey Pines Road, La Jolla, CA 92037. E-mail: rich@salk.edu.
Copyright © 2003 Society for Neuroscience 0270-6474/03/234333-12$15.00/0
References
- Basso MA, Wurtz RH ( 1998) Modulation of neuronal activity in superior colliculus by changes in target probability. J Neurosci 18: 7519–7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso MA, Krauzlis RJ, Wurtz RH ( 2000) Activation and inactivation of rostral superior colliculus neurons during smooth-pursuit eye movements in monkeys. J Neurophysiol 84: 892–908. [DOI] [PubMed] [Google Scholar]
- Becker W ( 1989) Metrics. In: The neurobiology of saccadic eye movements: reviews of oculomotor research, Vol III (Wurtz RH, Goldberg ME, eds). Amsterdam: Elsevier. [PubMed] [Google Scholar]
- Crist CF, Yamasaki DSG, Komatsu H, Wurtz RH ( 1988) A grid system and a microsyringe for single cell recording. J Neurosci Methods 26: 117–122. [DOI] [PubMed] [Google Scholar]
- Dorris MC, Munoz DP ( 1995) A neural correlate for the gap effect on saccadic reaction times in monkey. J Neurophysiol 73: 2558–2562. [DOI] [PubMed] [Google Scholar]
- Dorris MC, Pare M, Munoz DP ( 1997) Neuronal activity in monkey superior colliculus related to the initiation of saccadic eye movements. J Neurosci 17: 8566–8579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman JA, Keller EL ( 1996) Activity of visuomotor burst neurons in the superior colliculus accompanying express saccades. J Neurophysiol 76: 908–926. [DOI] [PubMed] [Google Scholar]
- Eriksen CW, St. James JD ( 1986) Visual attention within and around the field of focal attention: a zoom lens model. Percept Psychophys 40: 225–240. [DOI] [PubMed] [Google Scholar]
- Eriksen CW, Yeh YY ( 1985) Allocation of attention in the visual field. J Exp Psychol Hum Percept Perform 11: 583–597. [DOI] [PubMed] [Google Scholar]
- Fischer B, Boch R ( 1983) Saccadic eye movements after extremely short reaction times in the monkey. Brain Res 260: 21–26. [DOI] [PubMed] [Google Scholar]
- Fischer B, Breitmeyer B ( 1987) Mechanisms of visual attention revealed by saccadic eye movements. Neuropsychologia 25: 73–83. [DOI] [PubMed] [Google Scholar]
- Fischer B, Ramsperger E ( 1984) Human express-saccades: extremely short reaction times of goal directed eye movements. Exp Brain Res 57: 191–195. [DOI] [PubMed] [Google Scholar]
- Fischer B, Boch R, Ramsperger E ( 1984) Express-saccades of the monkey: effects of daily training on probability of occurrence and reaction time. Exp Brain Res 55: 232–242. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Robinson DA ( 1966) A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol 21: 1068–1070. [DOI] [PubMed] [Google Scholar]
- Gardner JL, Lisberger SG ( 2001) Linked target selection for saccadic and smooth pursuit eye movements. J Neurosci 21: 2075–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH ( 1983) Visual and oculomotor functions of monkey substantia nigra pars reticulata. III. Memory-contingent visual and saccade responses. J Neurophysiol 49: 1268–1284. [DOI] [PubMed] [Google Scholar]
- James W ( 1890) The principles of psychology. New York: Henry Holt.
- Judge SJ, Richmond BJ, Chu FC ( 1980) Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res 20: 535–538. [DOI] [PubMed] [Google Scholar]
- Kimmig H, Biscaldi M, Mutter J, Doerr JP, Fischer B ( 2002) The initiation of smooth pursuit eye movements and saccades in normal subjects and in “express-saccade makers.” Exp Brain Res 144: 373–384. [DOI] [PubMed] [Google Scholar]
- Kingstone A, Klein RM ( 1993) Visual offsets facilitate saccadic latency: does predisengagement of visuospatial attention mediate this gap effect? J Exp Psychol Hum Percept Perform 19: 1251–1265. [DOI] [PubMed] [Google Scholar]
- Knox PC ( 1996) The effect of the gap paradigm on the latency of human smooth pursuit of eye movement. NeuroReport 7: 3027–3030. [DOI] [PubMed] [Google Scholar]
- Kowler E ( 1990) The role of visual and cognitive processes in the control of eye movement. Rev Oculomot Res 4: 1–70. [PubMed] [Google Scholar]
- Krauzlis RJ ( 2001) Extraretinal inputs to neurons in the rostral superior colliculus of the monkey during smooth-pursuit eye movements. J Neurophysiol 86: 2629–2633. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Dill N ( 2002) Neural correlates of target choice for pursuit and saccades in the primate superior colliculus. Neuron 35: 355–363. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Miles FA ( 1996a) Decreases in the latency of smooth pursuit and saccadic eye movements produced by the “gap paradigm” in the monkey. Vision Res 36: 1973–1985. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Miles FA ( 1996b) Release of fixation for pursuit and saccades in humans: evidence for shared inputs acting on different neural substrates. J Neurophysiol 76: 2822–2833. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Basso MA, Wurtz RH ( 1997) Shared motor error for multiple eye movements. Science 276: 1693–1695. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Basso MA, Wurtz RH ( 2000) Discharge properties of neurons in the rostral superior colliculus of the monkey during smooth-pursuit eye movements. J Neurophysiol 84: 876–891. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Dill N, Kornylo K ( 2002) Activity in the primate rostral superior colliculus during the “gap effect” for pursuit and saccades. Ann NY Acad Sci 956: 409–413. [DOI] [PubMed] [Google Scholar]
- Kustov AA, Robinson DL ( 1996) Shared neural control of attentional shifts and eye movements. Nature 384: 74–77. [DOI] [PubMed] [Google Scholar]
- Lisberger SG ( 1998) Postsaccadic enhancement of initiation of smooth pursuit eye movements in monkeys. J Neurophysiol 79: 1918–1930. [DOI] [PubMed] [Google Scholar]
- Liston D, Carello CD, Krauzlis RJ ( 2002) Speed-accuracy tradeoffs for pursuit and saccades in a luminance discrimination task. J Vis 2: 171a. [Google Scholar]
- Mayfrank L, Mobashery M, Kimmig H, Fischer B ( 1986) The role of fixation and visual attention on the occurrence of express saccades in man. Eur Arch Psychiatry Neurol Sci 235: 269–275. [DOI] [PubMed] [Google Scholar]
- Merrison AFA, Carpenter RHS ( 1995) Express smooth pursuit. Vision Res 35: 1459–1462. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH ( 1993) Fixation cells in monkey superior colliculus I: characteristics of cell discharge. J Neurophysiol 70: 559–575. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH ( 1995) Saccade-related activity in monkey superior colliculus I: characteristics of burst and buildup cells. J Neurophysiol 73: 2313–2333. [DOI] [PubMed] [Google Scholar]
- Pare M, Munoz DP ( 1996) Saccadic reaction time in the monkey: advanced preparation of oculomotor programs is primarily responsible for express saccade occurrence. J Neurophysiol 76: 3666–3681. [DOI] [PubMed] [Google Scholar]
- Posner MI ( 1980) Orienting of attention. Q J Exp Psychol 32: 3–25. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE ( 1990) The attention system of the human brain. Annu Rev Neurosci 13: 25–42. [DOI] [PubMed] [Google Scholar]
- Rashbass C ( 1961) The relationship between saccadic and smooth tracking eye movements. J Physiol (Lond) 159: 326–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Hughes HC, Fendrich R ( 1991) The reduction of saccadic latency by prior offset of the fixation point: an analysis of the gap effect. Percept Psychophys 49: 167–175. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Riggio L, Dascola I, Umilta C ( 1987) Reorienting attention across the horizontal and vertical meridians: evidence in favor of a premotor theory of attention. Neuropsychologia 25: 31–40. [DOI] [PubMed] [Google Scholar]
- Saslow MG ( 1967) Effects of components of displacement-step stimuli upon latency for saccadic eye movement. J Opt Soc Am A 57: 1024–1029. [DOI] [PubMed] [Google Scholar]
- Schiller PH, True SD, Conway JL ( 1980) Deficits in eye movements following frontal eye field and superior colliculus ablations. J Neurophysiol 44: 1175–1189. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Sandell JH, Maunsell JHR ( 1987) The effect of frontal eye field and superior colliculus lesions on saccadic latencies in the rhesus monkey. J Neurophysiol 57: 1033–1049. [DOI] [PubMed] [Google Scholar]
- Sheliga BM, Riggio L, Rizzolatti G ( 1995) Spatial attention and eye movements. Exp Brain Res 105: 261–275. [DOI] [PubMed] [Google Scholar]
- Sommer MA ( 1994) Express saccades elicited during visual scan in the monkey. Vision Res 34: 2023–2038. [DOI] [PubMed] [Google Scholar]
- Sparks D, Rohrer WH, Zhang Y ( 2000) The role of the superior colliculus in saccade initiation: a study of express saccades and the gap effect. Vision Res 40: 2763–2777. [DOI] [PubMed] [Google Scholar]
- Swift D, Panish S, Hippensteel B ( 1997) The use of VisionWorks in visual psychophysics research. Spat Vis 10: 471–477. [DOI] [PubMed] [Google Scholar]
- Weber H, Aiple F, Fischer B, Latanov A ( 1992) Dead zone for express saccades. Exp Brain Res 89: 214–222. [DOI] [PubMed] [Google Scholar]