Abstract
The neuropeptides orexins/hypocretins are essential for normal wakefulness and energy balance, and disruption of their function causes narcolepsy and obesity. Although much is known of the role of orexins in sleep/wake behavior, it remains unclear how they stimulate feeding and metabolism. One of the main targets of orexinergic neurons is the arcuate nucleus (ARC) of the hypothalamus, which plays a key role in feeding and energy homeostasis. By combining patch-clamp and RT-multiplex PCR analysis of individual neurons in mouse brain slices, we show that an electrophysiologically distinct subset of ARC neurons coexpress orexin receptors and glutamate decarboxylase-67 and are excited by orexin. Acting on postsynaptic orexin type 2 receptors, orexin activates a sodium—calcium exchange current, thereby depolarizing the cell and increasing its firing frequency. Because GABA is a potent stimulus for feeding, in both the ARC and its main projection site, these results suggest a mechanism for how orexin may control appetite.
Keywords: orexin, hypocretin, arcuate nucleus, sleep, feeding, energy balance, neuropeptides, hypothalamus
Introduction
The arcuate nucleus (ARC) of the hypothalamus is critical for the maintenance of body energy balance (Elmquist et al., 1999, Schwarz et al., 2000). Destruction of the ARC leads to profound metabolic disturbances (Olney, 1969), and genetic defects in ARC function have been linked to obesity in humans (Barsh et al., 2000). ARC neurons express a diverse mixture of neuromodulators that act either within the ARC (Cowley et al., 2001) or at projection targets such as the paraventricular nucleus (PVN) of the hypothalamus (Kalra et al., 1999; Schwarz et al., 2000) and the spinal cord (Elias et al., 1998). Of these, GABA, neuropeptide Y, dynorphin, galanin, β-endorphin, and agouti-related protein stimulate feeding, whereas α-melanocyte-stimulating hormone and cocaine- and amphetamine-regulated transcript suppress feeding (Kalra et al., 1999; Schwarz et al., 2000).
|
Gene |
Accession No. |
Sense |
Antisense |
|---|---|---|---|
| TH | M69200 | CACCTGGAGTACTTTGTGCG (387), | CCTGTGGGTGGTACCCTATG (1525) |
| GAD67 | Z49976 | TGACATCGACTGCCAATACC (731), | GGGTTAGAGATGACCATCCG (1835) |
| OX1 | AF394596 | TGGGTATGGCCTATTTCCAG (306), | ACAAGGACTTGTGTCTGGCA (788) |
| OX2
|
AF394597
|
CTTCCAGCTACCCTCGTTGT (37),
|
CTAATCCGAGCCTTGCTCTG (575)
|
|
Gene |
Sense |
Antisense |
|---|---|---|
| TH | TGCACACAGTACATCCGTCA (936), | TCTGACACGAAGTACACCGG (1312) |
| GAD67 | CATATGAAATTGCACCCGTG (761), | CGGTGTCATAGGAGACGTCA (1462) |
| OX1 | ATTTCCAGATCTTCCGCAAG (318), | GGCACTGTTGGCGTACACTA (670) |
| OX2
|
TTGGTTCTTTGGACAGTCCC (72),
|
CTTTGGGTAAACTTCACCGC (378)
|
To match the release of these modulators to the energy status of the body, ARC neurons integrate a diverse variety of peripheral and central signals (Spanswick et al., 1997; Elias et al., 1998; Elmquist et al., 1999; Kalra et al., 1999; Rauch et al., 2000; Schwarz et al., 2000; Cowley et al., 2001; DeFalco et al., 2001). One of the recently described central inputs to the ARC is from lateral hypothalamic neurons that express orexin-A and orexin-B (Peyron et al., 1998; Horvath et al., 1999; Guan et al., 2001), a recently discovered pair of neuropeptides (de Lecea et al., 1998; Sakurai et al., 1998) also known as hypocretins (de Lecea et al., 1998). Orexins are essential for wakefulness, and disruption of their function leads to narcolepsy (Taheri et al., 2002). They are also important for energy homeostasis, with feeding being stimulated by injection of orexin into the hypothalamus and inhibited by central administration of an orexin antagonist or antibody, or by knockout of the orexin gene (Sakurai et al., 1998; Willie et al., 2001; Cai et al., 2002; Sutcliffe and de Lecea, 2002; Taheri et al., 2002). Both the activity of orexinergic neurons and hypothalamic orexin levels are highest during wakefulness and lowest during sleep (Estabrooke et al., 2001; Kiyashchenko et al., 2002). Thus, orexin appears to stimulate feeding and increase metabolism (Sutcliffe and de Lecea, 2002) during active, awake periods. How orexin mediates its effects on food intake, however, is unclear.
The dense orexinergic innervation of ARC neurons (Peyron et al., 1998; Horvath et al., 1999; Guan et al., 2001), expression of orexin receptors in the ARC (Lu et al., 2000; Guan et al., 2002), and stimulation of feeding after an injection of orexin into the ARC (Willie et al., 2001) suggest that orexins may control energy balance via the ARC. Indeed, extracellular recordings have shown that orexin increases the firing of a subset of ARC neurons (Rauch et al., 2000), although the identity of these neurons and the mechanism of their excitation are unknown. Studies on brain regions involved in wakefulness suggest that orexins can excite neurons by either decreasing or increasing the membrane conductance. For example, the peptide increases the conductance of hypothalamic tuberomammillary neurons by coactivation of the Na+/Ca2+ exchanger and a Ca2+ current (Eriksson et al., 2001a), whereas it decreases the membrane conductance of thalamocortical projection neurons by closing K+ channels (Bayer et al., 2002).
Here we explore the action of orexin on ARC neurons. We use patch-clamp and RT-nested-multiplex-PCR (scRT-PCR) analysis of individual neurons in brain slices (Lambolez et al., 1992; Liss et al., 1999) to determine the location, neurochemical profile, and biophysical properties of orexin-responsive neurons. We then address the mechanism by which orexin modifies the firing rate of ARC neurons.
Materials and Methods
Electrophysiology. Coronal brain slices (250–300 μm) containing the ARC were prepared from 12- to 16-d-old male C57BL/6 mice, as described previously (Burdakov and Ashcroft, 2002).
Procedures involving animals were performed in accordance with the Animals (Scientific Procedures) Act, 1986 (UK). After at least 20 min recovery at 35°C in artificial CSF (ACSF) gassed with 95% O2 and 5% CO2, brain slices were transferred to a recording chamber continuously perfused at 2–4 ml/min with gassed ACSF at room temperature (22–24°C). ACSF contained (in mm): 118 NaCl, 25 NaHCO3, 3 KCl, 1.2 NaH2PO4, 2 CaCl2, 2 MgCl2, 5 glucose, pH 7.25 at 22°C (this corresponds to brain interstitial pH in vivo) (Kintner et al., 2000). “Low Ca2+” extracellular solution contained 0.3 mm Ca2+ and 9 mm Mg2+. “Low Na+” extracellular solution contained 118 mmN-methyl-d-glucamine instead of NaCl. For whole-cell patch-clamp recordings, patch pipettes were filled with internal solution containing (in mm): 120 K-gluconate, 20 KCl, 10 HEPES, 0.1 EGTA (or 10 BAPTA, as indicated), 2 MgCl2, 5 K2-ATP, pH 7.3, adjusted with KOH. For cell-attached recordings, patch pipettes were filled with ACSF. Orexin-A, orexin-B (Sigma, St. Louis, MO), and KB-R7943 (Tocris, Bristol, UK) were made up fresh each day in ACSF from 1000× stock solutions. Several methods of applications were tested: no differences in the response were seen between local or bath application of peptide or drug or between bicarbonate- (ACSF) or HEPES-buffered (Liss et al., 1999) local application solutions. Control experiments showed that the pH of ungassed ACSF in the pipette or the local perfusion system is expected to change <0.5 U during the course of the experiment.
Neurons were visualized using infrared differential interference contrast optics (Stuart et al., 1993), and patch-clamp recordings performed using an EPC-9 amplifier (Heka, Lambrecht, Germany). Patch pipettes were pulled from borosilicate glass (GC150TF/F; Clark, Reading, UK) and had tip resistances of 3–5 MΩ when filled with the internal solution. Data were sampled and filtered with Pulse/Pulsefit software (Heka, Lambrecht, Germany) and analyzed with Pulsefit and Origin (Microcal, Northhampton, MA) software. Data are given as mean ± SEM.
Multiplex and nested single-cell RT-PCR. Single-cell cytoplasm harvesting, reverse transcription, and multiplex and nested PCR of experiments and controls were similar to those described previously (Liss et al., 1999). Patch pipettes had tip resistances 1.3–1.6 MΩ when filled with scPCR solution containing (in mm): 140 KCl, 5 HEPES, 5 EGTA, 3 MgCl2, pH 7.3. Most cells were harvested from the posterior ARC (Bregma coordinates corresponding to between -2.3 and -2.7 mm in adult mice), where the highest numbers of Type-C neurons were found. The 2× Hotstar PCR master mix (Qiagen) was used for all PCR reactions. After reverse transcription, the cDNAs for tyrosine hydroxylase (TH), glutamate decarboxylase-67 (GAD67), orexin receptors 1 and 2 (OX1 and OX2) were simultaneously amplified in a multiplex PCR using the following set of primers (from 5′ to 3′): The nested PCR amplifications were then performed in individual 50 μl reactions, using the following primer pairs: Aliquots (15 μl) of nested PCR products were separated and visualized in ethidium bromide-stained agarose gels (2%) by electrophoresis. The predicted sizes of the PCR amplicons were as follows: TH, 376 bp; GAD, 701 bp; OX1, 352 bp; OX2, 306 bp. All individual PCR products were verified by direct sequencing (n = 2). Highly diluted mouse brain cDNA (<10 fmol) was used as a template for positive controls (Liss et al., 1999).
Superscript RTase was omitted in the RT-minus control to test for possible genomic contaminations (n = 3).
Results
Orexin excites Type-C neurons in the posterior ARC
Three electrophysiologically distinct types of neuron are found in the ARC (Burdakov and Ashcroft, 2002). Type-A cells possess a high-threshold, rapidly inactivating outward current (A-current) and do not have a rebound potential on recovery from hyperpolarizing current injection; Type-B cells possess a low-threshold Ca2+ current and show a depolarization rebound; and Type-C cells have a low-threshold, slowly inactivating A-current and a hyperpolarization rebound (Burdakov and Ashcroft, 2002). These phenotypes resemble those found in the neighboring ventromedial (Miki et al., 2001) and paraventricular (Hoffman et al., 1991) nuclei.
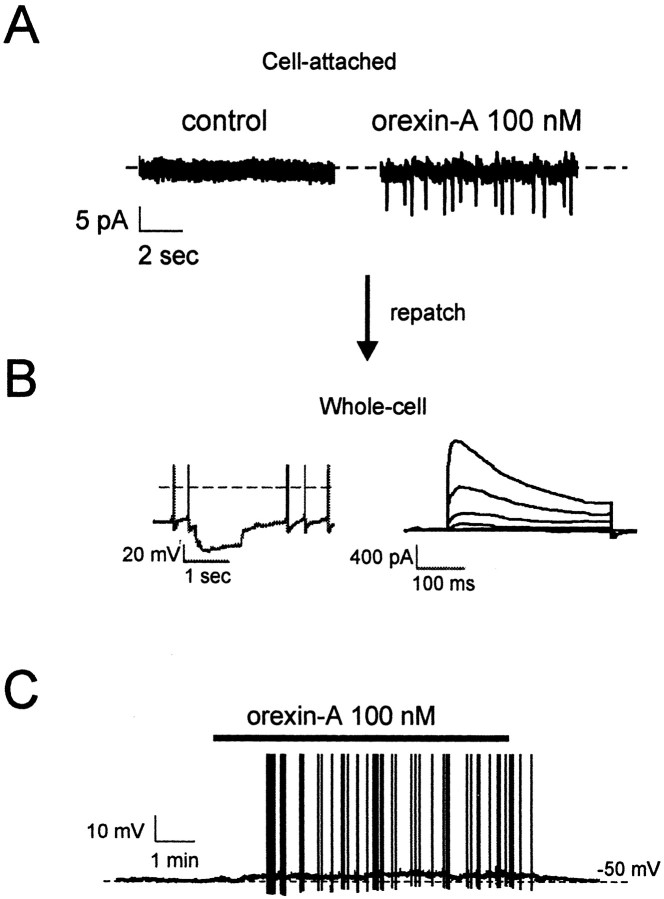
Monitoring the firing rate of ARC neurons in the cell-attached configuration of the patch-clamp method (which leaves the cytosol intact) showed that orexin-A (100 nm) increased the firing of a subset (n = 5/10) of ARC neurons, from 0.6 ± 0.5 to 4 ± 1 Hz (n = 5) (Fig. 1A). These neurons were subsequently repatched using the whole-cell recording configuration to identify their electrical phenotype. This revealed that all orexin-responsive cells corresponded to Type-C neurons (n = 4) (Fig. 1B), whereas the neurons unaffected by orexin belonged to the Type-A class (n = 3). Type-B neurons were encountered too rarely to determine their response to orexin.
Figure 1.
Orexin excites ARC Type-C neurons. A, Cell-attached patch-clamp recordings in the absence and presence of orexin-A (100 nm). B, Whole-cell recording from the same cell revealed a delayed excitation after hyperpolarizing current injection in current-clamp (left; the dashed line indicates the zero potential) and a low-threshold transient outward current in voltage-clamp (right; holding potential -90 mV, depolarizing steps from -80 to -30 mV in 10 mV increments), the defining features of Type-C neurons (see Results). C, Orexin-A (100 nm) excited ARC Type-C neurons; whole-cell current-clamp recording. Action potentials are truncated at 0 mV.
We next analyzed the effects of orexin on neuronal firing rate in whole-cell current-clamp recordings. Consistent with the cell-attached data, orexin-A (100 nm) depolarized Type-C neurons and either induced or increased sustained firing (n = 25/30) (Fig. 1C) but had no effect on Type-A cells (n = 12). Our subsequent experiments therefore focused on Type-C neurons. Most of the orexin-sensitive Type-C cells were found in the ventral posterior ARC (bregma coordinates corresponding to between -2.3 and -2.7 mm in adult mice) and only occasionally in the other regions of the ARC.
ARC Type-C cells coexpress orexin receptors and GAD67
To determine the molecular fingerprint of Type-C neurons, we used a combined whole-cell patch-clamp and scRT-PCR protocol (Lambolez et al., 1992; Liss et al., 1999) to simultaneously detect mRNAs for the genes GAD67 and TH and the OX1 and OX2 receptors, both of which have been detected in the ARC by immunocytochemistry (Lu et al., 2000; Guan et al., 2002). All four transcripts were detected in ventral mouse brain, with PCR fragments of the sizes predicted by their cDNA sequences (Fig. 2A). ARC Type-C neurons used for scRT-PCR were electrophysiologically identified by their response to hyperpolarizing current pulse injection and the amplitude, time course, and voltage-dependence of currents evoked on depolarization from a holding potential of -90 mV in voltage-clamp (Burdakov and Ashcroft, 2002). All Type-C neurons examined expressed GAD67 (n = 14) (Fig. 2D) but not TH. The OX2 receptor was coexpressed with GAD67 in most Type-C neurons (n = 11/14), whereas the remaining Type-C cells (n = 3) coexpressed GAD67 and OX1 (Fig. 2D). Thus, our results support the idea that in the ARC of the mouse brain, the Type-C electrical signature (Burdakov and Ashcroft, 2002) belongs to a subset of GABAergic neurons, most of which express OX2 receptors and are found in the posterior ARC.
Figure 2.
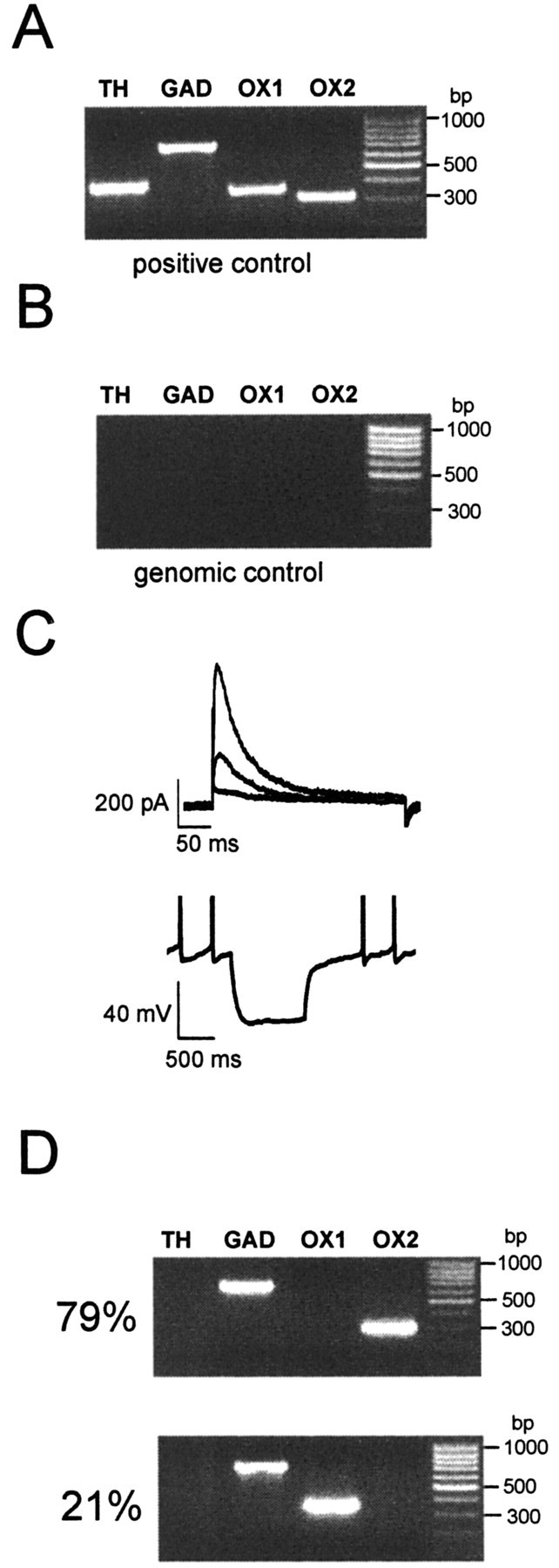
ARC Type-C neurons coexpress GAD67 and orexin receptors. A, Positive control for RT-PCR, performed in parallel with every single-cell PCR round, using highly diluted (<10 fmol) mouse brain cDNA as the template. The amplified fragments were of the predicted sizes (see Materials and Methods). B, Genomic control. Without reverse transcriptase in the reaction mixture, no PCR signals were detected from single-cell mRNA (n = 3). C, Type-C neurons, characterized by low-threshold A-currents (top; currents elicited by voltage steps from -90 mV to -60, -50, and -40 mV) and hyperpolarization rebound (bottom), were found to express either (D) GAD67 and OX2 (79%; n = 11/14) or GAD67 and OX1 (21%; n = 3/14).
The mechanism of orexin excitation of ARC Type-C neurons
In agreement with the expression of orexin receptors by ARC Type-C neurons (Fig. 2D), the orexin-induced depolarization was attributable to a direct postsynaptic action of the peptide on these cells, because it persisted when neurons were synaptically isolated either by using a low Ca2+, high Mg2+ solution (Kelso and Boulant, 1982; Rauch et al., 2000) (n = 5; data not shown) or by inclusion of 1 μm tetrodotoxin in the ACSF (n = 5) (Fig. 3A).
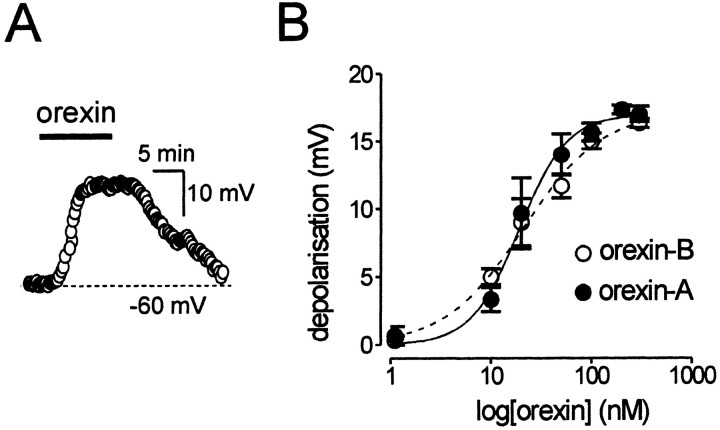
Figure 3.
The excitatory effect of orexin is caused by activation of postsynaptic OX2 receptors. A, Orexin-A (200 nm) reversibly depolarized Type-C neurons; whole-cell current-clamp recording in the presence of tetrodotoxin (1 μm). The membrane potential was set to -60 mV before orexin application by current injection to ensure the same initial conditions for all neurons. B, Concentration—response relations for membrane potential responses (recorded as in A) to orexin-A (n = 3–5) and orexin-B (n = 3–5). The lines were fit to the Hill equation with EC50 of 19 and 21 nm, Hill coefficients of 1.8 and 1.1, and maximal amplitudes of 17.2 and 17 mV, respectively.
The OX1 receptor is selective for orexin-A, whereas the OX2 receptor has a similar affinity for orexin-A and orexin-B (Sakurai et al., 1998). Figure 3B shows that orexin-A and orexin-B were equally effective at depolarizing Type-C neurons, as would be expected if the OX2 receptor is involved (Sakurai et al., 1998). Half-maximal activation (EC50) was produced by 19 ± 2 nm orexin-A and by 21 ± 3nm orexin-B (n = 4). It is noteworthy that the percentage of Type-C neurons expressing OX1 mRNA (Fig. 2D, 21%) closely matched the number of Type-C cells that did not respond to orexin (17%; n = 5/30), suggesting either that the activation of OX1 receptors does not depolarize these neurons or that OX1 mRNA expression does not correspond to the functional protein.
Comparison of the whole-cell steady-state current—voltage relations of Type-C cells before and after orexin-A application (Fig. 4A) revealed that orexin activated a current with an approximately linear current—voltage relation and a reversal potential of -35 ± 2 mV(n = 11) (Fig. 4 B). Because this reversal potential is more positive than the resting potential of ARC Type-C cells (-45 to -50 mV) (Burdakov and Ashcroft, 2002), the orexin current is expected to depolarize the cell and induce excitability. Consistent with this idea, the excitatory actions of orexin were fully reproduced by direct injection of a depolarizing current of similar magnitude into Type-C neurons (performed routinely in most of the cells in which orexin responses were studied; n = 25) (Fig. 4C).
Figure 4.
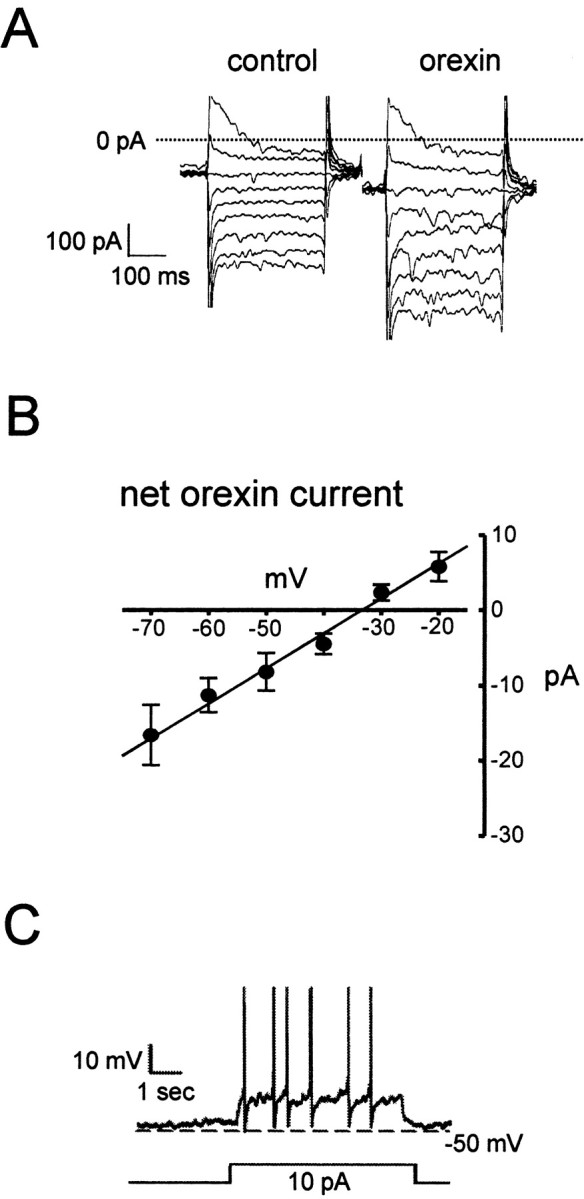
The excitatory effect of orexin is mediated by an inward current. A, Whole-cell currents elicited by voltage steps from -130 to -50 mV (in 10 mV increments) from a holding potential of -60 mV, before and after addition of 200 nm orexin-A. The cells were synaptically isolated with tetrodotoxin (1 μm). B, Mean current—voltage relation of the steady-state difference (control—orexin) current (n = 12). C, Effect of a 10 pA depolarizing current (equivalent to that induced by orexin at -50 mV; see B) on the firing of a Type-C neuron (which responded to orexin). Action potentials are truncated at 0 mV.
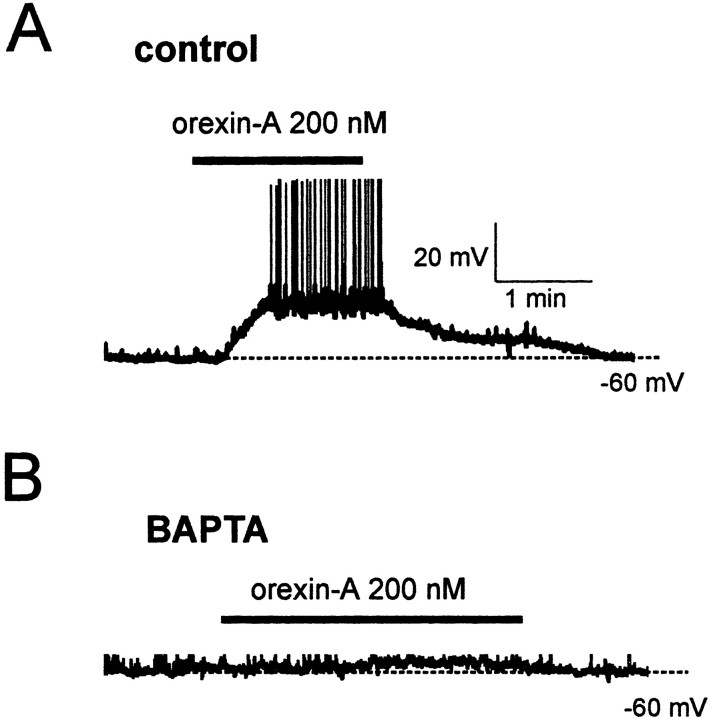
We next investigated the mechanisms underlying the effects of orexin on ARC Type-C cells. Buffering cytosolic Ca2+ increases by inclusion of 10 mm BAPTA in the pipette solution significantly reduced the orexin-induced depolarization (control: 17.5 ± 1.0 mV; BAPTA: 4.5 ± 2.0 mV; p < 0.001; n = 5) (Fig. 5), suggesting that an increase in intracellular Ca2+ is required for orexin action. Suppressing Ca2+ influx using a low Ca2+ extracellular solution did not reduce the magnitude of either the depolarization (n = 5) or current (n = 5) (Fig. 6C) induced by orexin, implying that Ca2+ mobilization from intracellular stores, rather than Ca2+ entry, mediates the effects of orexin.
Figure 5.
Orexin action requires an increase in cytosolic Ca2+. Current-clamp recordings of the response to 200 nm orexin in the absence (A) or presence (B; the scale bars are the same as in A) of 10 mm BAPTA in the pipette solution. The membrane potential was set to -60 mV (by current injection) before orexin application to ensure the same initial conditions for all neurons. Action potentials are truncated at 0 mV.
Figure 6.
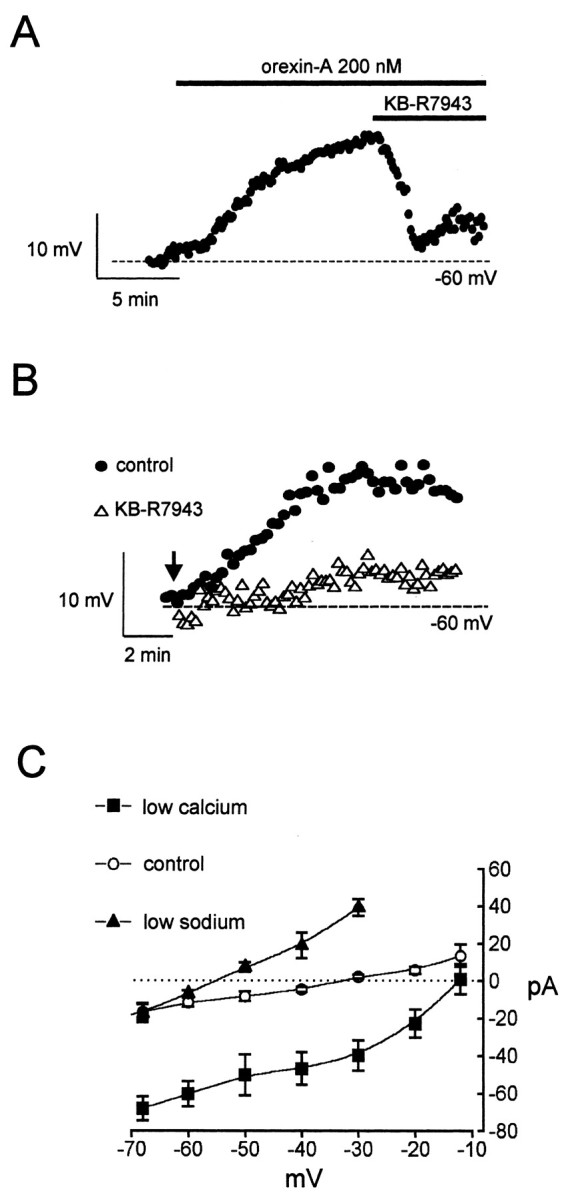
Orexin action is mediated by a Na+/Ca2+ exchange current. Effects of the Na+/Ca2+ exchange inhibitor KB-R7943 (70 μm) on the depolarization induced by 200 nm orexin-A. A, B, The membrane potential was set to -60 mV before orexin application to create the same initial conditions for all neurons. A, KB-R7943 was added after orexin application. B, KB-R7943 was added 5 min before orexin application (arrowed). C, Mean current—voltage relations for the orexin-induced current in control (n = 12), low external Ca2+ (n = 5), and low external Na+ (n = 4) solutions.
Increases in cytosolic Ca2+ are known to activate the plasmalemmal 3Na+/Ca2+ exchanger, which can significantly alter the electrical activity of excitable cells because of its electrogenicity (Smith and Armstrong, 1996; Keele et al., 1997; Lee and Boden, 1997; Blaustein and Lederer, 1999; Eriksson et al., 2001b). Furthermore, activation of the sodium—calcium exchanger by orexin was demonstrated recently in hippocampal (Wu et al., 2002) and tuberomammillary (Eriksson et al., 2001a) neurons. Therefore we explored whether the orexin current represents enhanced activity of this transporter. The selective Na+/Ca2+ exchange inhibitor KB-R7943 (Iwamoto and Shigekawa, 1998; Eriksson et al., 2001a) reversed the effects of orexin on membrane potential (Fig. 6A), and preincubation with the inhibitor (Fig. 6B) significantly reduced the effect of orexin (control: 18 ± 1.2 mV; KB-R7943: 2.3 ± 1.8 mV; p < 0.001; n = 6). Furthermore, the reversal potential of the mean orexin current was shifted in the negative direction (from -35 mV to approximately -57 mV) in low Na+ solution (n = 4) (Fig. 6C) and in the positive direction (from -35 mV to approximately -12 mV) in low Ca2+ solution (n = 5) (Fig. 6C). This is expected if the current is mediated by the 3Na+/Ca2+ exchanger operating in Ca2+ efflux mode. Finally, thermodynamic equilibrium calculations (Eisner and Lederer, 1989) showed that the 3Na+/Ca2+ exchanger would reverse at the observed value of -35 mV (Fig. 4B) if cytosolic Ca2+ in the presence of orexin is ∼600 nm, a value within the range of orexin-induced Ca2+ rises recorded in hypothalamic neurons (van den Pol et al., 1998).
Discussion
Cellular and molecular basis of orexin action
Orexin, acting on OX2 receptors, has been reported to depolarize neurons and increase their excitability either by activating an inward current (Eriksson et al., 2001a; Wu et al., 2002) or by inhibiting an outward current (Bayer et al., 2002). The former occurs in tuberomammillary (Eriksson et al., 2001a) and hippocampal (Wu et al., 2002) neurons and involves the activation of a Na+/Ca2+ exchange current. The results of our analysis indicate that activation of postsynaptic OX2 receptors also stimulates a Na+/Ca2+ exchange current in ARC Type-C GABAergic neurons, thereby producing membrane depolarization and an increased firing rate. This effect is dependent on an increase in cytosolic Ca2+ (Fig. 5), which is probably derived from intracellular stores (Fig. 6C). Additional support for our conclusions is provided by reports showing that OX2 receptors are coupled to the phospholipase-C/IP3 intracellular Ca2+-mobilizing pathway in a number of cell types (Smart et al., 1999; Mazzocchi et al., 2001; Randeva et al., 2001), including neurons (Holmqvist et al., 2002). In addition, the EC50 values that we obtained for the effects of orexin-A and orexin-B on membrane potential (Fig. 3B) are in close agreement with those found for elevation of cytosolic Ca2+ in response to activation of OX2 receptors heterologously expressed in Chinese hamster ovary cells (Sakurai et al., 1998). Finally, immunocytochemical data show a high density of OX2 receptors in the ARC (Lu et al., 2000), and orexin increases the release of GABA within the ARC (van den Pol et al., 1998).
Implications for energy balance
Our results indicate that ARC Type-C GABAergic cells of the ventral posterior ARC are a major target for orexins. The involvement of the ARC in orexin-induced feeding is suggested by the dense orexinergic innervation of ARC neurons (Peyron et al., 1998; Horvath et al., 1999; Guan et al., 2001). Moreover, ARC OX2 receptors, which our results indicate mediate orexin excitation of ARC GABAergic cells, are selectively upregulated by fasting (Lu et al., 2000), as expected if they mediate feeding (Schwarz et al., 2000). Microinjection of orexin into the ARC has been variously reported to induce feeding (Willie et al., 2001) or to be without effect (Dube et al., 1999). However, in the latter study, orexin was injected into the middle region of the ARC, where we found few Type-C cells, and the extremely small volumes injected (Dube et al., 1999) are unlikely to diffuse to the region where we find the majority of orexin-responsive neurons (which is >800 μm posterior to the site of injection in the rat).
There is accumulating experimental evidence to support a role for activation of ARC GABAergic neurons in orexin-induced feeding. First, GAD67 mRNA levels in the ARC are upregulated by fasting (Leonhardt et al., 1999), as expected if the release of ARC-derived GABA induces food intake. Second, the major projection of the ARC is the PVN (DeFalco et al., 2001), and microinjection of GABA agonists and antagonists into this nucleus stimulates and suppresses feeding, respectively (Grandison and Guidotti, 1977; Kelly and Grossman, 1979). Third, orexin has been shown to increase the local release of GABA within the ARC (van den Pol et al., 1998), where it may act to inhibit appetite-suppressing pro-opiomelanocortin neurons, which are densely innervated by GABAergic synapses (Horvath et al., 1992; Cowley et al., 2001) and are inhibited by GABA (Jegou et al., 1993; Cowley et al., 2001). It is also noteworthy that in the ARC, some GABAergic neurons can coexpress other appetite-stimulating messengers, such as neuropeptide Y (Ovesjo et al., 2001).
In summary, our data offer a cellular and molecular explanation for how orexins act to synchronize feeding and wakefulness. This may also be relevant to understanding metabolic imbalances associated with narcolepsy.
Footnotes
D.B. is supported by a Wellcome Prize Studentship and a Gustav Born Scholarship at Saint Peter's College, Oxford. B.L. was supported by a Todd-Bird Fellowship at New College, Oxford, and a Royal Society Dorothy Hodgkin Research Fellowship. F.M.A. holds the Royal Society GlaxoSmithKline Research Professorship.
Correspondence should be addressed to Denis Burdakov, University Laboratory of Physiology, Parks Road, Oxford, OX1 3PT, UK. E-mail: denis.burdakov@physiol.ox.ac.uk.
B. Liss's current address: Philipps University Marburg, Institute for Physiology, Deutschhausstrasse 2, 35037 Marburg, Germany.
Copyright © 2003 Society for Neuroscience 0270-6474/03/234951-07$15.00/0
D.B. and B.L. contributed equally to this work.
References
- Barsh GS, Farooqi IS, O'Rahilly SO ( 2000) Genetics of body weight regulation. Nature 404: 644–651. [DOI] [PubMed] [Google Scholar]
- Bayer L, Eggermann E, Saint-Mleux B, Machard D, Jones BE, Muhlethaler M, Serafin M ( 2002) Selective action of orexin (hypocretin) on nonspecific thalamocortical projection neurons. J Neurosci 22: 7835–7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein MP, Lederer WJ ( 1999) Sodium/calcium exchange: its physiological implications. Physiol Rev 79: 763–854. [DOI] [PubMed] [Google Scholar]
- Burdakov D, Ashcroft FM ( 2002) Cholecystokinin tunes firing of an electrically distinct subset of arcuate nucleus neurons by activating A-type potassium channels. J Neurosci 22: 6380–6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai XJ, Liu XH, Evans M, Clapham JC, Wilson S, Arch JR, Morris R, Williams G ( 2002) Orexins and feeding: special occasions or everyday occurrence? Regul Pept 104: 1–9. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ ( 2001) Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411: 480–484. [DOI] [PubMed] [Google Scholar]
- DeFalco J, Tomishima M, Liu H, Zhao C, Cai X, Marth JD, Enquist L, Friedman JM ( 2001) Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science 291: 2608–2613. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett II FS, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG ( 1998) The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA 95: 322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube MG, Kalra SP, Kalra PS ( 1999) Food intake elicited by central administration of orexins/hypocretins: identification of hypothalamic sites of action. Brain Res 842: 473–477. [DOI] [PubMed] [Google Scholar]
- Eisner DA, Lederer WJ ( 1989) The electrogenic sodium-calcium exchange. In: Sodium-calcium exchange, pp 178–207. New York: Oxford UP.
- Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, Couceyro PR, Kuhar MJ, Saper CB, Elmquist JK ( 1998) Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron 21: 1375–1385. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Elias CF, Saper CB ( 1999) From lesions to leptin: hypothalamic control of food intake and body weight. Neuron 22: 221–232. [DOI] [PubMed] [Google Scholar]
- Eriksson KS, Sergeeva O, Brown RE, Haas HL ( 2001a) Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J Neurosci 21: 9273–9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson KS, Stevens DR, Haas HL ( 2001b) Serotonin excites tuberomammillary neurons by activation of Na(+)/Ca(2+)-exchange. Neuropharmacology 40: 345–351. [DOI] [PubMed] [Google Scholar]
- Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE ( 2001) Fos expression in orexin neurons varies with behavioral state. J Neurosci 21: 1656–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandison L, Guidotti A ( 1977) Stimulation of food intake by muscimol and beta endorphin. Neuropharmacology 16: 533–536. [DOI] [PubMed] [Google Scholar]
- Guan J, Suzuki R, Funahashi H, Wang Q, Kageyama H, Uehara K, Yamada S, Tsurugano S, Shioda S ( 2002) Ultrastructural localization of orexin-1 receptor in pre- and post-synaptic neurons in the rat arcuate nucleus. Neurosci Lett 329: 209–212. [DOI] [PubMed] [Google Scholar]
- Guan JL, Saotome T, Wang QP, Funahashi H, Hori T, Tanaka S, Shioda S ( 2001) Orexinergic innervation of POMC-containing neurons in the rat arcuate nucleus. NeuroReport 12: 547–551. [DOI] [PubMed] [Google Scholar]
- Hoffman NW, Tasker JG, Dudek FE ( 1991) Immunohistochemical differentiation of electrophysiologically defined neuronal populations in the region of the rat hypothalamic paraventricular nucleus. J Comp Neurol 307: 405–416. [DOI] [PubMed] [Google Scholar]
- Holmqvist T, Akerman KE, Kukkonen JP ( 2002) Orexin signaling in recombinant neuron-like cells. FEBS Lett 526: 11–14. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Naftolin F, Leranth C ( 1992) GABAergic and catecholaminergic innervation of mediobasal hypothalamic beta-endorphin cells projecting to the medial preoptic area. Neuroscience 51: 391–399. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Diano S, van den Pol AN ( 1999) Synaptic interaction between hypocretin (orexin) and neuropeptide Y cells in the rodent and primate hypothalamus: a novel circuit implicated in metabolic and endocrine regulations. J Neurosci 19: 1072–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto T, Shigekawa M ( 1998) Differential inhibition of Na+/Ca2+ exchanger isoforms by divalent cations and isothiourea derivative. Am J Physiol 275: C423–C430. [DOI] [PubMed] [Google Scholar]
- Jegou S, Blasquez C, Delbende C, Bunel DT, Vaudry H ( 1993) Regulation of alpha-melanocyte-stimulating hormone release from hypothalamic neurons. Ann NY Acad Sci 680: 260–278. [DOI] [PubMed] [Google Scholar]
- Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS ( 1999) Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev 20: 68–100. [DOI] [PubMed] [Google Scholar]
- Keele NB, Arvanov VL, Shinnick-Gallagher P ( 1997) Quisqualate-preferring metabotropic glutamate receptor activates Na(+)-Ca2+ exchange in rat basolateral amygdala neurones. J Physiol (Lond) 499: 87–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J, Grossman SP ( 1979) GABA and hypothalamic feeding systems. II. A comparison of GABA, glycine and acetylcholine agonists and their antagonists. Pharmacol Biochem Behav 11: 647–652. [DOI] [PubMed] [Google Scholar]
- Kelso SR, Boulant JA ( 1982) Effect of synaptic blockade on thermosensitive neurons in hypothalamic tissue slices. Am J Physiol 243: R480–R490. [DOI] [PubMed] [Google Scholar]
- Kintner DB, Anderson MK, Fitzpatrick Jr JH, Sailor KA, Gilboe DD ( 2000) 31P-MRS-based determination of brain intracellular and interstitial pH: its application to in vivo H + compartmentation and cellular regulation during hypoxic/ischemic conditions. Neurochem Res 25: 1385–1396. [DOI] [PubMed] [Google Scholar]
- Kiyashchenko LI, Mileykovskiy BY, Maidment N, Lam HA, Wu MF, John J, Peever J, Siegel JM ( 2002) Release of hypocretin (orexin) during waking and sleep states. J Neurosci 22: 5282–5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambolez B, Audinat E, Bochet P, Crepel F, Rossier J ( 1992) AMPA receptor subunits expressed by single Purkinje cells. Neuron 9: 247–258. [DOI] [PubMed] [Google Scholar]
- Lee K, Boden PR ( 1997) Characterization of the inward current induced by metabotropic glutamate receptor stimulation in rat ventromedial hypothalamic neurones. J Physiol (Lond) 504: 649–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhardt S, Shahab M, Luft H, Wuttke W, Jarry H ( 1999) Reduction of luteinizing hormone secretion induced by long-term feed restriction in male rats is associated with increased expression of GABA-synthesizing enzymes without alterations of GnRH gene expression. J Neuroendocrinol 11: 613–619. [DOI] [PubMed] [Google Scholar]
- Liss B, Bruns R, Roeper J ( 1999) Alternative sulfonylurea receptor expression defines metabolic sensitivity of K-ATP channels in dopaminergic midbrain neurons. EMBO J 18: 833–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XY, Bagnol D, Burke S, Akil H, Watson SJ ( 2000) Differential distribution and regulation of OX1 and OX2 orexin/hypocretin receptor messenger RNA in the brain upon fasting. Horm Behav 37: 335–344. [DOI] [PubMed] [Google Scholar]
- Mazzocchi G, Malendowicz LK, Aragona F, Rebuffat P, Gottardo L, Nussdorfer GG ( 2001) Human pheochromocytomas express orexin receptor type 2 gene and display an in vitro secretory response to orexins A and B. J Clin Endocrinol Metab 86: 4818–4821. [DOI] [PubMed] [Google Scholar]
- Miki T, Liss B, Minami K, Shiuchi T, Saraya A, Kashima Y, Horiuchi M, Ashcroft F, Minokoshi Y, Roeper J, Seino S ( 2001) ATP-sensitive K channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat Neurosci 4: 507–512. [DOI] [PubMed] [Google Scholar]
- Olney JW ( 1969) Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science 164: 719–721. [DOI] [PubMed] [Google Scholar]
- Ovesjo ML, Gamstedt M, Collin M, Meister B ( 2001) GABAergic nature of hypothalamic leptin target neurones in the ventromedial arcuate nucleus. J Neuroendocrinol 13: 505–516. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS ( 1998) Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18: 9996–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randeva HS, Karteris E, Grammatopoulos D, Hillhouse EW ( 2001) Expression of orexin-A and functional orexin type 2 receptors in the human adult adrenals: implications for adrenal function and energy homeostasis. J Clin Endocrinol Metab 86: 4808–4813. [DOI] [PubMed] [Google Scholar]
- Rauch M, Riediger T, Schmid HA, Simon E ( 2000) Orexin A activates leptin-responsive neurons in the arcuate nucleus. Pflügers Arch 440: 699–703. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M ( 1998) Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92: 573–585. [DOI] [PubMed] [Google Scholar]
- Schwarz MW, Woods SC, Porte Jr D, Seeley RJ, Baskin DG ( 2000) Central nervous system control of food intake. Nature 404: 661–671. [DOI] [PubMed] [Google Scholar]
- Smart D, Jerman JC, Brough SJ, Rushton SL, Murdock PR, Jewitt F, Elshourbagy NA, Ellis CE, Middlemiss DN, Brown F ( 1999) Characterization of recombinant human orexin receptor pharmacology in a Chinese hamster ovary cell-line using FLIPR. Br J Pharmacol 128: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BN, Armstrong WE ( 1996) The ionic dependence of the histamine-induced depolarization of vasopressin neurones in the rat supraoptic nucleus. J Physiol (Lond) 495: 465–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanswick D, Smith MA, Groppi VE, Logan SD, Ashford MLJ ( 1997) Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature 390: 521–525. [DOI] [PubMed] [Google Scholar]
- Stuart G, Dodt H-U, Sakmann B ( 1993) Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflügers Arch 423: 511–518. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JG, de Lecea L ( 2002) The hypocretins: setting the arousal threshold. Nat Rev Neurosci 3: 339–349. [DOI] [PubMed] [Google Scholar]
- Taheri S, Zeitzer JM, Mignot E ( 2002) The role of hypocretins (orexins) in sleep regulation and narcolepsy. Annu Rev Neurosci 25: 283–313. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Gao XB, Obrietan K, Kilduff TS, Belousov AB ( 1998) Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J Neurosci 18: 7962–7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Yanagisawa M ( 2001) To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci 24: 429–458. [DOI] [PubMed] [Google Scholar]
- Wu M, Zhang Z, Leranth C, Xu C, van den Pol AN, Alreja M ( 2002) Hypocretin increases impulse flow in the septohippocampal GABAergic pathway: implications for arousal via a mechanism of hippocampal disinhibition. J Neurosci 22: 7754–7765. [DOI] [PMC free article] [PubMed] [Google Scholar]