Abstract
Development of midbrain dopaminergic neurons is known to depend on inductive signals derived from the ventral midline, including Sonic hedgehog (Shh) as one of the identified molecules. Here we show that in addition to Shh, transforming growth factor (TGF)-β is required for both induction and survival of ventrally located midbrain dopaminergic neurons. Like Shh, TGF-β is expressed in early embryonic structures such as notochord and floor plate, as well as in the area where mibrain dopaminergic neurons are developing. Treatment of cells dissociated from the rat embryonic day (E) 12 mibrain floor with TGF-β significantly increases the number of tyrosine hydroxylase (TH)-positive dopaminergic neurons within 24 hr. Neutralization of TGF-β in vitro completely abolishes the induction of dopaminergic neurons. In the absence of TGF-β, Shh cannot induce TH-positive neurons, and vice versa, neutralizing endogenous Shh abolishes the capacity of TGF-β to induce dopaminergic neurons in vitro. Furthermore, neutralization of TGF-β in vivo during chick E2–7 but not E4–7 resulted in a significant reduction in TH-positive neurons in the ventral midbrain floor but not in the locus coeruleus or diencephalon, which suggests that the TGF-β is required for the induction of mesencephalic dopaminergic neurons with a critical time period at E2/E3. Furthermore, neutralization of TGF-β between E6 and 10, a time period during maturation of mesencephalic dopaminergic neurons when no further inductive cues are required, also resulted in a significant loss of dopaminergic neurons, suggesting that TGF-β is required for the promotion of survival of ventral midbrain dopaminergic neurons as well. Together, our results identify TGF-β as an essential mediator for the induction and maintenance of midbrain dopaminergic neurons.
Keywords: TGF-β, BMP, dopaminergic neurons, Sonic hedgehog, midline, floor plate, phenotype induction
Introduction
Dopaminergic (DA) neurons of the ventral midbrain play important roles in the regulation of motor performances, behavior, and cognition. Neuron losses or functional alterations of dopaminergic systems and DA receptors are linked to disorders, e.g., Parkinson's disease, schizophrenia, and drug addiction (Greenberg et al., 1974; Hirsch et al., 1988; Seeman et al., 1993).
Current knowledge suggests that the development of dopaminergic neurons in the mesencephalon floor relies on signals produced mainly by organizing centers, the floor plate, and the isthmus region (for review, see Hynes and Rosenthal, 1999). In the rat embryo, the first midbrain dopaminergic neurons can be detected by staining for tyrosine hydroxylase (TH) on embryonic day (E) 12.5, adjacent to the floor plate. Using E9 explants of neural plate, Hynes and coworkers (1995a) have shown that TH-positive neurons can be induced ectopically in the dorsal midbrain by floor plate-derived signals. Inductive effects of the floor plate can be mimicked by the secreted morphogen Sonic hedgehog (Shh) and prevented by Shh-blocking antibodies. These results suggested that Shh is necessary for the induction of midbrain dopaminergic neurons (Hynes et al., 1995b).
Similar to midbrain dopaminergic neurons, the development of peripheral catecholaminergic cells requires signals from the floor plate and notochord (Groves and Anderson, 1996). In addition, induction of the catecholaminergic phenotype depends on members of the transforming growth factor (TGF)-β superfamily, bone morphogenetic protein (BMP)-4 and–7 (Reissmann et al., 1996; Shah et al., 1996). BMPs are localized in the wall of the dorsal aorta and have been shown to be essential for the induction of TH in neural crest-derived cells, which aggregate in the vicinity of the dorsal aorta. In addition to BMPs, TGF-β2 and–β3 are also present in the wall of the dorsal aorta. Whether TGF-βs proper (isoforms β1–3) are involved in the induction of catecholaminergic markers has not been firmly established (Howard and Gershon, 1993). In the developing neural tube of the chick embryo, TGF-βs are synthesized and localized in both the floor plate and underlying notochord (Flanders et al., 1991; Unsicker et al., 1996), suggesting their involvement in the specification of neuronal phenotypes, whereas BMP is expressed at the dorsal aspect of the neural tube and functions as a dorsalizing factor (Liem et al., 1995, 1997; Arkell and Beddington, 1997).
The three TGF-β isoforms, β1, β2, and β3, have prominent functions related to morphogenetic events, epithelial–mesenchymal interactions, and differentiation (for review, see Dünker and Krieglstein, 2000; Goumans and Mummery 2000). Mice deficient for each single TGF-β isoform or TGF-β receptor available at present have not yet contributed to the definition of roles of TGF-β in nervous system development (Letterio et al., 1994; Proetzel et al., 1995; Oshima et al., 1996; Sanford et al., 1997).
We now can show that TGF-β2, -β3, and TβR-II are expressed in the ventral mesencephalon of E12.5 rat embryos at locations where the first TH-positive neurons are born. We provide evidence, both in vitro and in vivo, that TGF-β is necessary for the induction of TH-positive neurons. Furthermore, our data show that the capacity of Shh to induce dopaminergic neurons depends on TGF-β.
Materials and Methods
Growth factors and neutralizing antibodies. Recombinant human (rh) N-terminal Shh was kindly provided by Biogen (Cambridge, MA), and rh BMP-4 was provided by Genetics Institute (Cambridge, MA). rh FGF-8 was purchased from R&D Systems (Wiesbaden, Germany), and rh glial cell line-derived neurotrophic factor (GDNF) was from Preprotech (Frankfurt, Germany). TGF-β1 and TGF-β3 were kindly provided by Dr. M. B. Sporn (Dartmouth Medical College, Hanover, NH). The neutralizing monoclonal mouse anti-TGF-β antibody (anti-TGF-β, MAB1835) recognizing all three isoforms was obtained from R&D Systems [specificity described in detail in Krieglstein et al. (1998, 2000)]. Furthermore, the activity of this antibody was determined using the Mv1Lu mink lung epithelial cell system (Combs et al., 2000; Krieglstein et al., 2000). The function-blocking anti-Shh antibody was kindly provided by Biogen (Ericson et al., 1996; Ye et al., 1998).
Cultures of E12 rat ventral mesencephalon. Pregnant Hanover-Wistar rats were killed by CO2 asphyxiation, and E12 embryos were collected in Ca2+–Mg2+-free HBSS (Sigma, St. Louis, MO). The day of vaginal plug identification was designated E0. Small pieces of dissected ventral mesencephalon floor (without the underlying mesenchyme and meninges) were incubated in 0.15% trypsin (Bio-Whittaker, Verviers, Belgium) for 15 min and subsequently dissociated by gentle trituration using fire-polished Pasteur pipettes. Cells in suspension were seeded onto polyornithine (0.1 mg/ml in 15 mm borate buffer, pH 8.4; Sigma) and laminin (1 μg/ml; Sigma) coated glass coverslips (NeoLab) in 24-well plates (Costar, Bodenheim, Germany) at a density of 4000 cells per coverslip (12 mm in diameter). Growth factors were applied at the time of plating to a final volume of 750 μl of high glucose DMEM/F12 medium (Invitrogen, Gaithersburg, MD) supplemented with 0.25% BSA, N1 additives (Sigma), and antibiotics [penicillin, streptomycin, neomycin (PSN); Invitrogen]. Dose–response curves for the applied growth factors were established by titration over a wide concentration range (0.05–40 ng/ml) for each factor (data not shown). Cultures were incubated in a 95% air/5% CO2 atmosphere at 37°C. All experiments were performed at least twice in quadruplicate.
Immunostaining on cultured cells was performed using standard protocols established in our laboratory (Krieglstein et al., 1998). Cultures were fixed in 4% PFA for 10 min at room temperature and incubated overnight at 4°C with the following primary antibodies. Hybridoma supernatants recognizing nestin (Rat 401; 1:50), En-1 (4G11; 1:10), and TGF-β3 (D-β3; 1:20) were obtained from the Developmental Studies Hybridoma Bank (Iowa City, IA). Monoclonal anti-MAP-2 (1:200), anti-A2B5 (1:500), anti-TH (1:200), anti-proliferating cell nuclear antigen (PCNA) (1:200), and anti-vimentin (1:40) antibodies were purchased from Boehringer Mannheim (Mannheim, Germany). Polyclonal antibodies directed against TGF-β2 and -β3 and their receptors (rabbit polyclonal antibodies: TGF-β2, sc-90; TGF-β3, sc-82; TβR-I, sc-398; TβR-II, sc-400; all diluted 1:200) and mouse monoclonal anti-Smad1,2,3 (SC-7960, 1:200) were from Santa Cruz Biotechnology (Santa Cruz, CA) (Krieglstein et al., 1998, 2000). Immunoreactivity (ir) was visualized either by the avidin–biotinylated peroxidase method using a Vectastain ABC Kit and diaminobenzidine fizzing tablets (DAB; Kem-En-Tec, Copenhagen, Denmark) as a chromogen or by immunofluorescence using indocarbocyanine (Cy3)- or carbocyanine (Cy2)-conjugated goat anti-mouse or anti–rabbit, and rabbit anti-goat IgG antibodies (1:500; Dianova, Hamburg, Germany). As control, lack of staining by primary antibody omissions was used. All antibodies obtained from Santa Cruz were additionally tested by preabsorbing the primary antibodies with their corresponding peptides. Nuclear counterstains were performed by 4′,6′-diamidino-2-phenylindole dihydrochloride (DAPI; 1:1000 for 5 min).
For double detection of TH, En-1, and PCNA, cultures were first stained with the monoclonal antibodies recognizing nuclear antigens using the Vectastain ABC kit as described, and subsequently with anti-TH antibody. The first antibody reaction was visualized using DAB chromogen with NiCl2 intensification (black reaction), and second antigens were detected by DAB.
Immunoreactive cells were counted over the whole coverslip. Data are presented as SEM. Statistical comparisons were made with the Student's double t test, ANOVA using MicrocalOrigin software. Differences were considered statistically significant at *p < 0.05, **p < 0.01, and ***p < 0.001.
In ovo treatment of chicken embryos. Fertilized White Leghorn eggs were incubated in a humidified egg chamber at 38°C until E2 [stage according to Hamburger and Hamilton (1951): HH12], E4, or E6. Eggs were windowed 5 × 5 mm in size, and embryos were staged according to Hamburger and Hamilton (1951). Thereafter, eggs were treated daily with the neutralizing pan-anti-TGF-β antibody (10 μg per egg in 50 μl of sterile PBS with 2× PSN) as described previously (Krieglstein et al., 2000; Dünker et al., 2001). Control embryos were treated identically but without addition of the neutralizing antibody or with an antibody of identical isotype (recognizing a myc-tag; kindly provided by Biogen). On the indicated day of incubation, whole embryos were harvested and staged. Whole heads of chicken embryos of different ages were fixed in Bouin's solution for several hours, and the tissue was dehydrated in a graded series of ethanol and embedded in paraffin wax.
Numbers of TH-labeled neurons were counted on the complete series of transverse 10 μm paraffin sections from the diencephalon to hindbrain areas of E7 or E10 chick embryos. A neuron was designated as TH positive if it revealed a darkly labeled cytoplasm and a clearly visible, unstained nucleus. Only cells fulfilling these criteria were included in the cell counts. To avoid double counting the same cell on two sequential sections, only every third section was counted. In all animals, counts were made separately both on the left- and right-hand sides of the brain sections. Statistical analysis did not reveal significant side differences. Neuron counts are given for the right side as mean values ± SEM.
Immunohistochemistry and in situ hybridization. Immunostaining in sections was performed essentially as described by Schober et al. (1999). E12 rats were immersion fixed in 4% PFA overnight at 4°C and either processed for paraffin embedding or cryoprotected in 30% sucrose/PBS overnight at 4°C and further processed for cryosectioning. Paraffin sections (10 μm) were deparaffinized and heated for 30 min in citrate buffer, pH 6, in a microwave oven at 600 W to improve antigen retrieval before immunocytochemistry. Monoclonal anti-TH (Chemicon; 1:200), polyclonal antibodies directed against BMP-4 (goat polyclonal: sc-6896; 1:200), TGF-β2, -β3, and their type II receptor (Krieglstein et al., 1998, 2000), as well as En-1 and 3CB2 [1:10; both obtained from the Developmental Studies Hybridoma Bank (Prada et al., 1995; Ericson et al., 1997)] were visualized by the avidin–biotinylated peroxidase method using the Vectastain ABC Kit and DAB fizzing tablets (Kem-En-Tec, Copenhagen, Denmark) as chromogen. Double immunolabeling was performed as described above.
In situ hybridization using a digoxigenin-labeled chicken Shh RNA probe (kindly provided by Dr. Stefan Heller, Rockefeller University, New York, NY) was performed on a complete series of cryostat sections (20 μm, E7 chick) essentially as described by Ernsberger et al. (1997). Briefly, air-dried sections were hybridized at 68°C overnight and incubated with alkaline phosphatase-conjugated Fab fragments of a monoclonal mouse anti-digoxigenin antibody (overnight at 22°C; 1:1000 in 100 mm maleic acid buffer with 0.1% Tween, pH 7.5). The reaction was visualized using the 5-bromo-4-chloro-3-indolyl phosphate/nitroblue-tetrazolium substrate (Roche, Mannheim, Germany) according to the manufacturer's guidelines.
Bromodeoxyuridine ELISA. Dissociated ventral mesencephalic cells were plated in 96-well plates (Costar) at a density of 1000 cells per well in 100 μl of culture medium containing the indicated factors. Cells were labeled with bromodeoxyuridine (BrdU) for the last 16 hr before being fixed after a total of 24 hr in culture with 70% ethanol in 0.5 m HCl (for 30 min at -20°C) and processed according to the manufacturer's instructions (BrdU ELISA Kit; Roche). After a 30 min nuclease treatment, cells were incubated with an HRP-labeled monoclonal anti-BrdU antibody (1:100) for 30 min at 37°C. After addition of the ABTS substrate, extinction of the samples was measured using a microtiter plate reader (Bio-Rad) at 405 nm (reference wavelength was 490 nm).
Results
Localization of TGF-βs and TGF-β receptor suggests roles in the development of midbrain dopaminergic neurons
Previous studies in chick embryos have revealed prominent TGF-β2 ir and TGF–β3 ir in the notochord and floor plate/midline regions of spinal cord and hindbrain at E3 (Unsicker et al., 1996). Figure 1 extends these findings by showing TGF-β2 and TGF-β3 as well as TβR-II ir in the E12 rat midbrain floor. The localization of both ligands and the receptor coincides with the distribution of TH-ir in the midbrain floor. In contrast, BMP-4, another member of the TGF-β superfamily and a dorsalizing factor, is localized within the mesenchyme adjacent to the neural tube (Fig. 1E). Together, these data indicate that TGF-β2 and TGF–β3 as well as their cognate receptor TβR-II are localized in the early developing midbrain in a manner that implies their putative involvement in the development of mesencephalic dopaminergic neurons.
Figure 1.
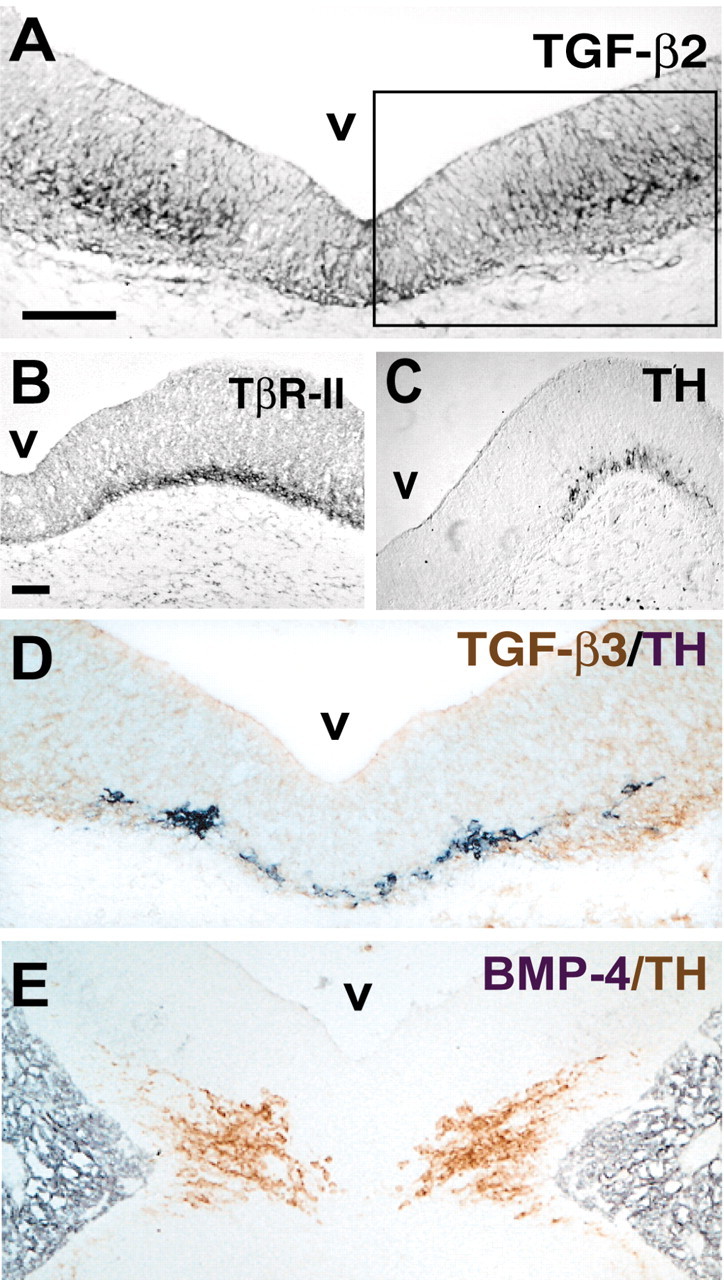
Location of TGF-βs and the ligand-binding receptor, TβR-II, in the E12 rat midbrain analyzed by immunocytochemistry. Coronal paraffin sections were stained for TGF-β2 (A), TβR-II (B), and TH (C) and double labeled with TGF-β3 (brown) and TH (D, black), and BMP-4 (black) and TH (E, brown). A, D, E, Overview of the ventral aspect of the E12 rat mesencephalon stained with different antibodies. The inset in A demarcates the region shown at higher magnification and different stainings in B and C. Note the overlapping expression domain of TH-ir neurons with TβR-II in B and C and with TGF-β3 in D. Here an antibody (D-β3) specifically recognizing the active form of TGF-β3 was used, which indicates that the extracellular matrix is immunoreactive. v, Ventricle of the mesencephalon. Scale bar, 50 μm.
TGF-β induces TH-positive neurons in cultures of E12 rat ventral mesencephalon
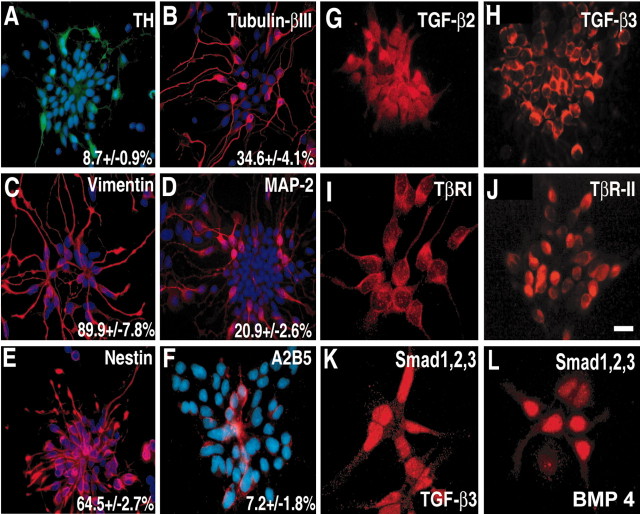
To investigate a putative role of TGF-β in the induction of TH-positive neurons, we established cultures of E12 rat ventral mesencephalic cells. Figure 2 provides information on the characterization of the cultures 24 hr after the cells were seeded. Two-thirds (64.5 ± 2.7%) of the cells were nestin positive, i.e., represent neural progenitor cells. Approximately 90% of the cells stained for vimentin and ∼7% stained for A2B5, both markers of neuron and glial lineages. Approximately one-third of the cells stained for tubulin-βIII, and one-fifth (20.9 ± 2.6%) stained for MAP2, indicating their neuronal character; 8.7% of the cells showed a catecholaminergic phenotype, i.e., were TH positive. In addition, most cells were immunoreactive for TGF-β2, TGF-β3, and TGF-β receptors TβR-I and TβR–II and the intracellular signaling mediator Smad1/2/3, which translocates after TGF-β or BMP stimulation to the nucleus (Fig. 2).
Figure 2.
Cellular composition of low-density cultures of E12 rat ventral mesencephalon after 24 hr in vitro. A–F, Cultures were double stained for the nuclear marker DAPI (blue) and for TH (A, green), tubulin-βIII (B, red), vimentin (C, red), MAP-2 (D, red), nestin (E, red), and A2B5 (F, red). The percentage of positively stained cells is given ± SEM, where the number of DAPI-positive nuclei was set as 100%. Note that most of these cultured cells are immunoreactive for specific markers of the progenitor cells, such as vimentin and nestin. The number of differentiated neuronal cells is between 20 and 30%. TGF-β2 (G), TGF-β3 (H), both receptors, TβR-I (I) and TβR-II (J), and the intracellular mediator Smad1 and Smad2/3 (K, L) are expressed in a dominant cell population of these cultures. Cultures shown in K and L were treated with growth factors for 1 hr to obtain a strong signal in the nucleus [TGF-β3, 1 ng/ml (K); BMP4, 10 ng/ml (L)]. Scale bar: (in J) A–F, 50 μm; G, H, J, 25 μm; I, K, L, 12.5 μm.
The treatment of cultures with a saturating dose of TGF-β3(1 ng/ml; 24 hr; dose–response not shown) increased the number of TH-positive cells twofold (Fig. 3A–C). Having established (Fig. 2G–K) that TGF-β2 and TGF–β3 were present in mesencephalic cells, we wanted to elucidate whether endogenous TGF-β contributed to the acquisition of the TH-positive phenotype. Administration of neutralizing antibodies against all three TGF-β isoforms in an amount sufficient to block 10 ng/ml TGF-β (10 μg/ml) (Krieglstein et al., 2000) almost fully abrogated the TH-positive phenotype (Fig. 3C). This suggests that TGF-β is essentially involved in the induction of the TH-positive phenotype. Several growth factors/cytokines have previously been shown to up-regulate the catecholaminergic phenotype of neural crest-derived or midbrain neuronal cells. As shown in Figure 3C, Shh, FGF-8, and GDNF increased, whereas BMP-4 dramatically decreased the numbers of TH-positive cells. To explore whether enhanced numbers of TH-positive cells were caused by an increase in cell proliferation, we conducted PCNA staining and BrdU ELISAs. As shown in Figure 3, A and B, TH-positive cells did not stain for PCNA. Furthermore, TGF-β did not affect BrdU incorporation (Fig. 3D). To test for a potential role of growth factor-induced cell death or cell survival within the 24 hr time interval, total cell counts were performed. As shown in Figure 3E, there was no significant change in cell numbers in control cultures from seeding up to 24 hr or as a result of any factor treatment, suggesting that neither promotion of cell survival nor induction of cell death is a critical issue during the 24 hr time period chosen. However, during longer cell culture intervals (e.g., 4 d), FGF-8 treatment resulted in a highly significant induction of BrdU incorporation, as well as a doubling of total cell numbers (data not shown). Together, these data indicate that the TGF-β-mediated increase in TH-positive neurons is caused by phenotype induction.
Figure 3.

TGF-β is necessary for the appearance of the TH-positive phenotype in cultures of E12 rat ventral mesencephalon. A, B, The number of TH-positive neurons is increased after a 24 hr treatment with 1 ng/ml TGF-β3. Control (A) and TGF-β3-treated (B) cultures were double stained for PCNA (A, B, arrowhead; nuclear staining) and TH (A, B, arrow; brown cytoplasmic staining). Note that there is no significant difference between the number of nuclei stained for the proliferation marker in control and TGF-β3-treated cultures. Scale bar, 50 μm. C, Quantitation of the number of TH-positive neurons in E12 mesencephalic cultures after 24 hr treatment with the indicated factors. The appropriate concentrations were defined by dose–response curves over a wide concentration range (data not shown). Results are mean ± SEM (n = 4) from at least three independent experiments. D, TGF-β3 does not change cell proliferation rates in these cultures. Cultures were treated with the same factors at the same concentrations as above and pulsed with BrdU for the last 16 hr of culture, and BrdU ELISA was performed as described. Results are expressed as percentage of control values (set as 100%). Note that Shh at the 1 nm concentration did not induce any cell proliferation of cultured mesencephalic cells that it induced at concentrations >4 nm (data not shown). Results are mean ± SEM (n = 8) from five independent experiments; *p < 0.05, **p < 0.01, ***p < 0.001. E, Quantification of the total number of neurons in E12 mesencephalic control cultures (3, 4, and 24 hr) and cultures after 24 hr treatment with the indicated factors. Cell counts were taken from fixed, DAPI-stained cells plated at a density of 4000 cells per coverslip. No significant differences in total cell numbers between control and treated cultures could be observed. Results are means ± SEM (n = 3) from three independent experiments.
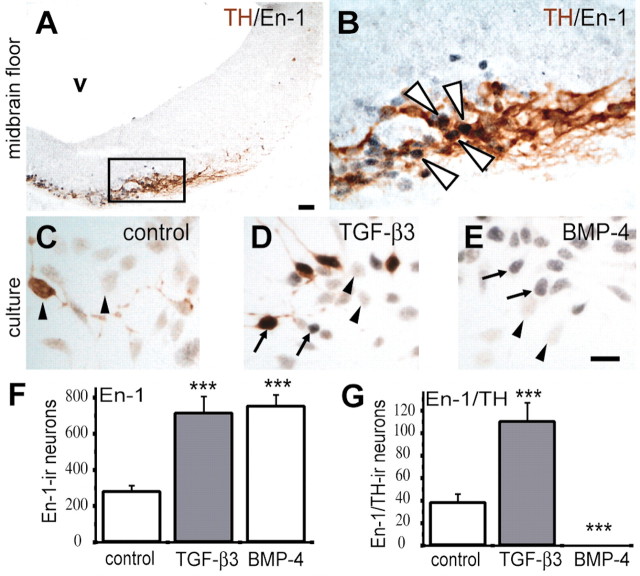
The engrailed genes, En-1 and En-2, code for transcription factors that are important in the patterning of the midbrain and hindbrain territories (Joyner et al., 1985). En-1 and En-2 are expressed in midbrain dopaminergic neurons and are essential for their survival (Simon and O'Leary, 1998). Figure 4, A and B, demonstrates the colocalization of En-1 and TH in the E12 rat midbrain floor. To further support the notion that TGF-β is important for the induction of dopaminergic neurons, we monitored the appearance of En-1 after treatment of mesencephalic cultures. Figure 4C–F shows that TGF-β as well as BMP-4 treatment resulted in increased numbers of En-1-positive cells, whereas only TGF-β resulted in a significant increase of En-1/TH-double-stained cells (Fig. 4G). Although BMP-4 clearly augmented numbers of En-1-positive cells, these cells did not coexpress TH. Together, these data suggest that TGF-β, but not BMP-4, induces ventral midbrain dopaminergic neurons.
Figure 4.
TGF-β specifically induces the expression of En-1 in ventral mesencephalic cultures of the E12 rat. A, B, Colocalization of TH and En-1 in the ventral midbrain floor. Transverse cryostat sections of the E12 rat midbrain were double stained for En-1 (black) and TH (brown). The inset in A demarcates the region shown at higher magnification in B. B, The same section is shown at higher magnification. Nuclei of virtually all TH-positive neurons are stained for En-1, whereas there are a few En-1-positive nuclei without TH expression. Examples of doubled-labeled cells are marked by white arrowheads in B. C–E, Colocalization of TH and En-1 in cultured mesencephalic neurons. Control cultures (C) and cultures treated with 1 ng/ml TGF-β3 (D) or 10 ng/ml BMP-4 (E) for 24 hr were double labeled as in A. Examples for En-1-positive neurons are labeled with black arrows; examples for En-1-negative neurons are marked with black arrowheads. Both TGF-β3 and BMP-4 can induce expression of En-1, but after BMP-4 treatment there are almost no TH-positive neurons. v, Ventricle of the mesencephalon. Scale bars, 50 μm. F, G, Quantitative analysis of the effects of TGF-β3 (1 ng/ml) and BMP-4 (10 ng/ml) on the expression of En-1. Results are presented as total numbers of nuclei immunoreactive for En-1. Both TGF-β3 and BMP-4 (F) increased the number of En-1-positive nuclei, whereas only TGF-β3 treatment (G) enhanced the number of TH-ir neurons expressing En-1. Data are given as mean ± SEM (n = 4). **p < 0.01, ***p < 0.001.
Reduction of endogenous TGF-β in vivo suppresses differentiation of midbrain dopaminergic neurons in the chick embryo
Having shown that TGF-β augmented and neutralization of TGF-β reduced numbers of TH-positive neurons in vitro (Fig. 3), we next investigated whether TGF-β was also required in vivo to induce dopaminergic neurons. We applied an antibody to all TGF-β isoforms to chick embryos from E2, i.e., before the induction of TH-ir neurons, to E7. We had shown previously that these antibodies applied systemically to the chorionic–allantoic membrane of chick embryos completely neutralized endogenous TGF-βs (Combs et al., 2000; Krieglstein et al., 2000). We investigated midbrain TH-positive dopaminergic neurons, noradrenergic neurons in the locus coeruleus, and diencephalic catecholaminergic neurons. Figure 5 reveals that numbers of TH-positive neurons in the midbrain floor were reduced by ∼60%, whereas TH-ir neurons in the locus coeruleus and in the diencephalon were not significantly affected. Because the induction of the dopaminergic phenotype by Shh has been shown to occur within the first 24 hr after the induction of somitogenesis (Ye et al., 1998), we applied the TGF-β neutralizing antibody additionally during the developmental time period from E4 to E7. As shown in Figure 5K, neutralizing TGF-β as late as E4, i.e., after the critical period of dopaminergic phenotype induction (E2–3) had no effect on the numbers of TH+ neurons detectable at E7. These data suggest that TGF-β is essentially required for the induction of midbrain dopaminergic neurons in the chick embryo. Furthermore, they also suggest that TGF-β does not act to stabilize the TH phenotype.
Figure 5.
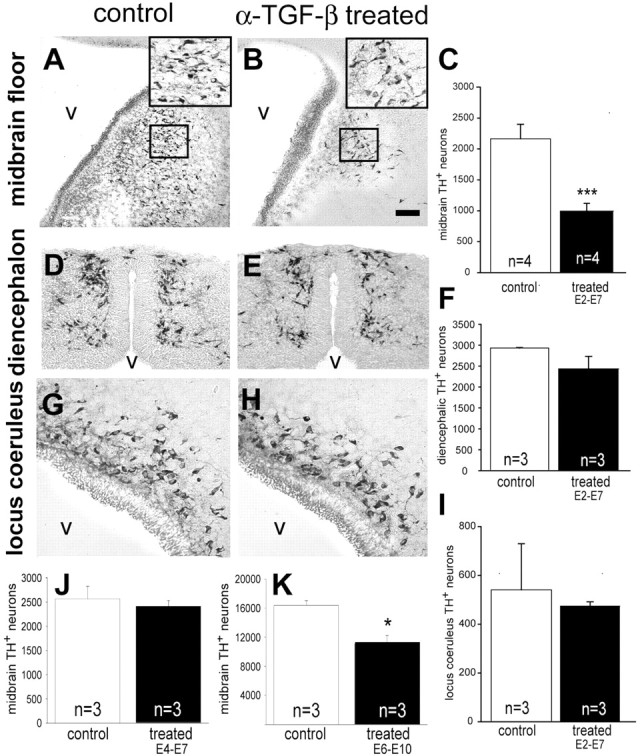
TGF-β signaling is essential for the development of midbrain DA neurons in vivo. Chick embryos were treated with a function-blocking specific anti-TGF-β antibody from E2–7, E4–7, or E6–10, thereafter processed for TH immunohistochemistry, and TH-ir neurons were counted in the midbrain (n = 4), diencephalon (n = 3), and hindbrain (locus coeruleus;n = 3) as described in Materials and Methods. Representative frontal sections are shown, taken at the same level from control embryos, treated with vehicle alone (A, D, G), or embryos treated with neutralizing antibody from E2 to E7 (B, E, H). The small insets in A and B demarcate regions shown at higher magnification in the second, larger inset in the top right corner of the representative pictures. In the absence of TGF-β, there are only a few TH-positive neurons left in the midbrain, whereas in the diencephalon and locus coeruleus there is no significant change. Note that the nucleus tegmenti-pedunculo-pontinus of the chicken is the homolog of the mammalian substantia nigra. v, Ventricle. Scale bar: A, B, D, E, G, H,50 μm. C, F, I, K, L, Quantitation of these experiments is shown. Results are expressed in absolute numbers and given as mean ± SEM. *p < 0.05, ***p < 0.001.
When endogenous TGF-β is neutralized between E6 and E10, i.e., after the critical period of phenotype induction (E2–3) and acquisition of the TH+ phenotype (E4–7), however, a significant loss of TH+ neurons is detectable (Fig. 5K). This suggests that TGF-β may act as a survival-promoting molecule at later stages of development, as shown by several in vitro studies (Poulsen et al., 1994; Krieglstein et al., 1995, 1998).
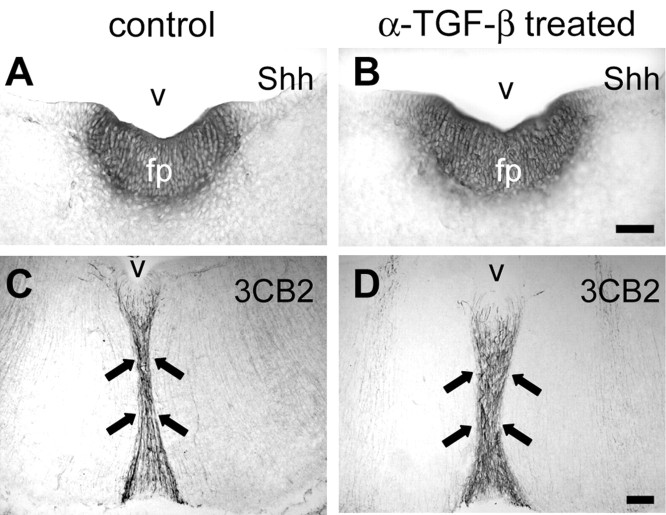
Anti-TGF-β treatment does not interfere with floor plate development and Shh expression
Shh is an important diffusible factor produced by notochord and floor plate cells (Echelard et al., 1993). It is required essentially for the induction of midbrain dopaminergic neurons (Hynes et al., 1995b; Ye et al., 1998). We therefore investigated whether TGF-β antibodies administered in vivo interfered with the development of the floor plate or Shh expression, respectively. Figure 6 shows that anti-TGF-β-treated E7 chick embryos displayed an unaltered midbrain floor plate, as indicated by Shh mRNA expression and radial glia development. This suggests that neutralization of TGF-β did not interfere with the correct development of the floor plate and Shh expression.
Figure 6.
Neutralization of TGF-β in the developing chicken embryo from E2 to E7 does not interfere with the organization of floor plate and the expression of Shh. A, B, Nonradioactive in situ hybridization was performed using a digoxigenin-labeled Shh probe on complete serial cryostat sections of E7 chicken brain. Shh expression in the floor plate does not show significant differences between control (A) and treated (B) embryos, in which TGF-β function was blocked by a neutralizing antibody. C, D, Radial glial structure of the ventral midline is not altered in the absence of functional TGF-β signaling. A specific antibody (3CB2) was used to detect an intermediate filament associated protein selectively labeling radial glial cells at this age of development. v, Ventricle of the mesencephalon; fp, floor plate. Black arrows point to the ventral midline radial glia. Scale bar, 50 μm.
TGF-β and Shh cooperate in the induction of midbrain dopaminergic neurons
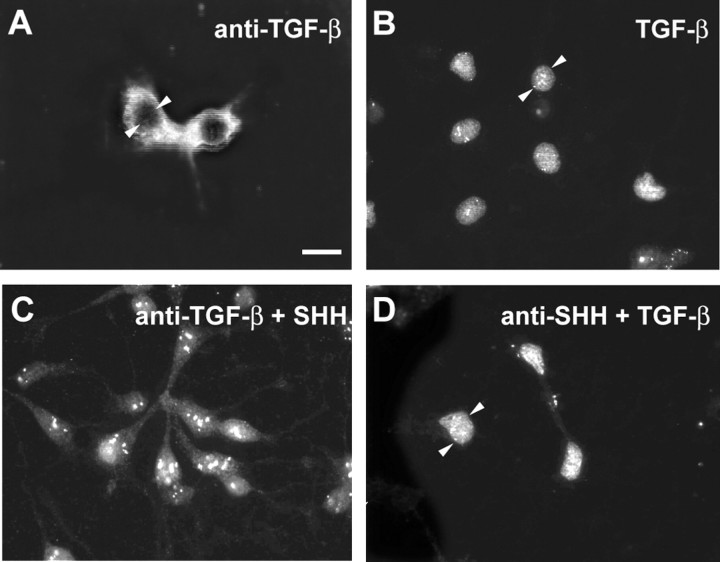
Using dissociated cells from the E12 rat mesencephalon floor, we next investigated whether TGF-β acts downstream of Shh to induce dopaminergic neuron development. If so, neutralization of TGF-β in the presence of Shh should abrogate the inductive effect of Shh, whereas neutralization of Shh in the presence of TGF-β should not interfere with the induction of dopaminergic neurons. Figure 7 reveals that both neutralization of TGF-β in the presence of Shh and neutralization of Shh in the presence of TGF-β abolished dopaminergic neuron development. To exclude the possibility that anti-Shh affected TGF-β binding to the receptor, Smad translocation was used to test whether TGF-β3is still capable of initiating TGF-β signal transduction in the presence of anti-Shh. Figure 8 shows Smad translocation to the nucleus after TGF-β3 application in the absence or presence of anti-Shh antibodies. This suggests that both Shh and TGF-β are essentially required for the induction of dopaminergic neurons. The combination of TGF-β plus Shh further increased numbers of dopaminergic neurons. Together, these data indicate that TGF-β is essentially required and cooperates with Shh to induce ventral mesencephalic dopaminergic neurons.
Figure 7.
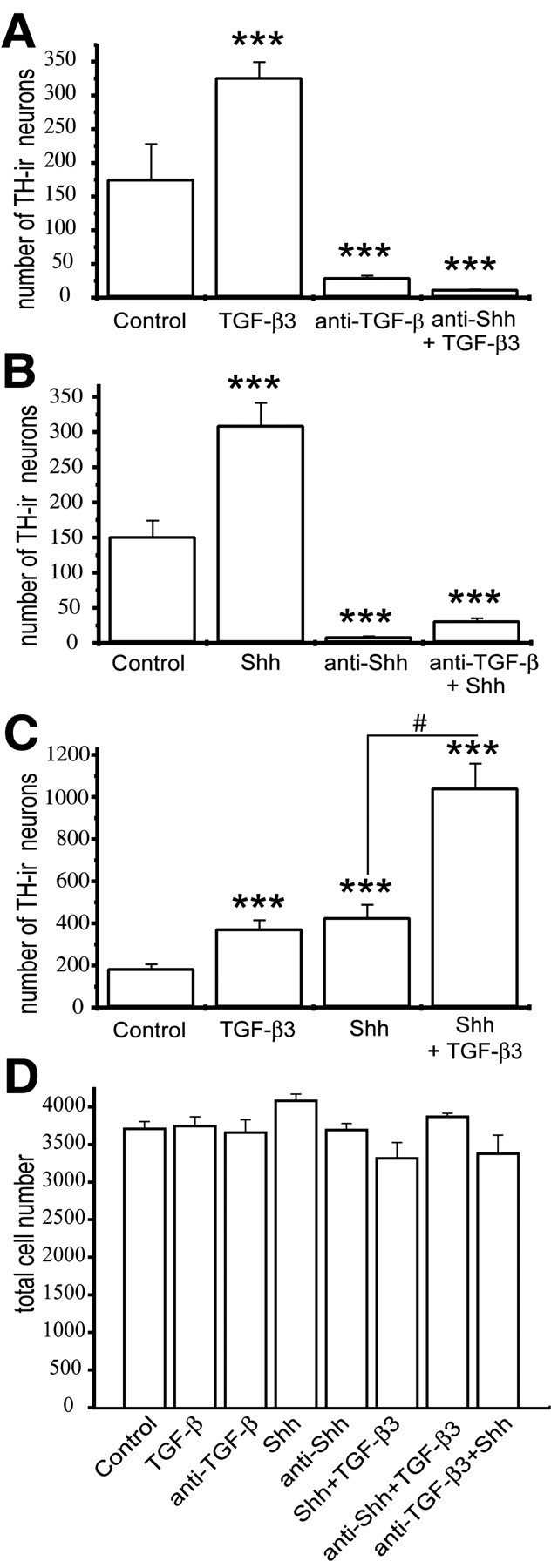
Shh and TGF-β are both essentially required for the induction of DA neurons in cultures of E12 rat mesencephalon. A–C, Quantitation of the cooperative action of TGF-β and Shh in inducing the number of TH-positive neurons. Cultured cells were treated with Shh (20 ng/ml) or TGF-β3 (1 ng/ml) or the function-blocking antibodies against Shh (2.5 μg/ml) or TGF-β (10 μg/ml). DA neurons were almost completely abolished in the absence of either TGF-β (A) or Shh (B) signaling. Furthermore, against the background of absence of Shh signaling (A), TGF-β3 was ineffective in restoring the expression of TH. C, At saturating concentrations of Shh (20 ng/ml) and TGF-β3 (1 ng/ml), these two factors added together induced a significantly higher number of TH-positive neurons than the single-factor treatments. Results are presented as total number of TH-ir neurons per coverslip, and data are given as mean±SEM (n = 4). **p < 0.01, ***p < 0.001. D, Quantification of the total number of neurons in E12 mesencephalic control cultures and cultures after 24 hr treatment with the indicated factors. Cell counts were taken from fixed, DAPI-stained cells plated at a density of 4000 cells per coverslip. No significant differences in total cell numbers were observed between control and treated cultures. Results are means ± SEM (n = 3) from three independent experiments.
Figure 8.
Immunocytochemical detection of Smad localization in dopaminergic midbrain neurons after differential treatments. Cultured cells were treated with anti-TGF-β (10 μg/ml) (A) or TGF-β3 (1 ng/ml) (B) or the combinations of anti-TGF-β plus Shh (20 ng/ml) (C) and anti-Shh (2.5 μg/ml) plus TGF-β3 (1 ng/ml) (D). In cells treated with an anti-TGF-β, a neutralizing antibody to TGF-β (A), the immunocytochemical signal for Smad appears spread throughout the cytoplasm; the nucleus, however, remains free of any Smad signal. By contrast, treatment with recombinant TGF-β3 (B) results in Smad translocation into the nucleus. As confirmed in D, pretreatment of the cells with a neutralizing antibody to Shh followed by recombinant TGF-β3 application does not abolish Smad translocation into the nucleus. Small arrowheads demarcate examples of the cell nucleus. Scale bar: (in A) A–D, 15 μm.
Discussion
By combining in vitro and in vivo experimental models, we have shown that TGF-β is essentially required to induce ventral mesencephalic dopaminergic neurons. To achieve this effect, TGF-β acts in cooperation with Shh.
Shh is a member of a multigene family in vertebrates (Echelard et al., 1993; Krauss et al., 1993; Litingtung and Chiang, 2000a,b) that is related to the Drosophila hedgehog (Nusslein-Volhard and Wieschaus, 1980; Lee et al., 1992). Hedgehog is a secreted protein influencing patterning of cell types within adjacent embryonic tissues. Shh is localized in notochord, floor plate of the neural tube, and posterior margin of limb buds and has proven its organizing properties in transplantation and grafting experiments. Targeted gene disruption of Shh in the mouse has revealed defects in the establishment or maintenance of midline structures, including notochord and floor plate, leading to the absence of ventral cell types in the neural tube (Chiang et al., 1996). Hynes and coworkers (1995a) were the first to describe the influence of the midbrain floor plate as an organizing center for the induction of dopaminergic neurons. They identified Shh as the molecule mediating the inductive effect of the floor plate on dopaminergic neurons (Hynes et al., 1995b).
Our results clearly demonstrate that Shh requires the cooperation of TGF-β to induce ventral midbrain dopaminergic neurons. The important contribution of TGF-β in this process has probably escaped notice, because studies leading to the identification of Shh as the inducer of dopaminergic neurons were conducted in vitro using explants. TGF-β is a widely expressed molecule. As shown by us previously and in the present paper, TGF-β is synthesized and localized in the notochord and floor plate (Unsicker et al., 1996), as well as in the region where dopaminergic neurons develop. This suggests that TGF-β is endogenously present in explants used in the above studies and therefore available as a cofactor for Shh.
Dopaminergic neurons in the midbrain floor are not the only population of catecholaminergic neurons in the midbrain, hindbrain, and diencephalon. We have analyzed three populations of catecholaminergic, TH-positive neurons, including dopaminergic neurons of the ventral midbrain floor, the noradrenergic neurons in the locus coeruleus, and TH-positive neurons in the diencephalon. As shown in this paper, neutralization of TGF-β in vivo resulted in a significant, selective reduction of the ventrally located dopaminergic neurons, without affecting locus coeruleus and diencephalic TH neurons. These results further support the notion of a cooperativity of TGF-β with Shh in the induction of ventrally located dopaminergic neurons. Noradrenergic neurons of the locus coeruleus reside in the ventro-lateral region of the first hindbrain rhombomere. Their identity was established in response to extrinsic signals from the immediate neighborhood (Edlund and Jessell, 1999) identified as FGF8 from the midbrain/hindbrain boundary and as BMP from the epidermal ectoderm (Guo et al., 1999; Vogel-Höpker and Rohrer, 2002). Thus, locus coeruleus neurons apparently do not acquire their identity through immediate contact with Shh/floor plate. How diencephalic TH-positive neurons are instructed to a catecholaminergic fate has not been revealed; however, their localization in the chick does not suggest that inductive signals from the ventral midline are crucial in their development.
The cooperation between Shh and TGF-β raises questions as to putative hierarchies and sequences of their actions. Our cell culture experiments using neutralizing antibodies to Shh and TGF-β, respectively, suggest that neither factor in the absence of the other has the capacity to induce TH. This argues against a sequential mode of action and favors a cooperative model for actions of Shh and TGF-β. Several distinct mechanisms may be conceived to explain the relationship between Shh and TGF-β at molecular levels. For example, TGF-β might be involved in the regulation of Gli. Gli genes belong to the only identified transcription factor family that is directly implicated in Shh signal transduction (Litingtung and Chiang, 2000a,b). Gli genes can function as activators or repressors of Shh function (Aza-Blanc and Kornberg, 1999) and have been implicated in the induction of dopaminergic neurons (Hynes et al., 1997; Litintung and Chiang, 2000a). Smads, which constitute the TGF-β-mediating signaling pathways and form complexes with other transcription factors (Massague and Wotton, 2000; ten Dijke et al., 2000), either may interact with Gli (Liu et al., 1998) or be implied in its transcriptional regulation. An alternative mechanism to explain the requirement of TGF-β might involve a TGF-β-dependent induction of the Shh receptors Patched and Smoothened (Hynes et al., 2000; Ingham et al., 2000). There is one human disease, holoprosencephaly, a common structural defect in the developing forebrain, which can be caused by mutations in the Shh (Roessler et al., 1996) as well as in the TGF-β inducible early gene (TIEG) gene (Gripp et al., 2000). TIEG is a home domain protein and has been shown to act in vivo as a Smad2 transcriptional corepressor (Wotton et al., 1999a,b).
Both Shh and TGF-β, which are expressed early in development in the notochord and the floor plate, continue to be expressed beyond periods of phenotype induction. Both proteins have been implicated in the regulation of neuron survival of several neuron populations, including mesencephalic dopaminergic neurons (Poulsen et al., 1994; Krieglstein et al., 1995, 1998; Miao et al., 1997). Here, we provide evidence for the first time that TGF-β indeed promotes dopaminergic neuron survival in vivo, using the developing chick as a model. Furthermore, neutralization of endogenous TGF-β during specific time windows during development provides us with a unique opportunity to distinguish between the various roles of TGF-β in each and every frame of development.
The developmental period E2–7 spans the critical period for induction of dopaminergic neurons until the appearance of the first TH+ neurons. E4–7 lacks the critical period of phenotype induction but includes a long period of development toward a dopaminergic phenotype. Finally, E6–10 represents a time frame in which phenotype stabilization and promotion of survival are regulated. Our experiments clearly demonstrate that endogenous TGF-β is required for both induction of the dopaminergic phenotype (E2–3) and promotion of the survival of these dopaminergic neurons at a later time point (E6–10).
In conclusion, we have provided evidence that TGF-β is required for the induction of ventral midbrain dopaminergic neurons and acts in cooperation with Shh.
Footnotes
This work was supported by grants from the Deutsche Forschungsgemeinschaft. We thank Dr. Alphonse Galdes (Biogen, Cambridge MA) for providing Shh and neutralizing antibodies to SHH, Uwe Ernsberger for help with the in situ hybridizations, and Jutta Fey, Ulla Hinz, and Stephanie Heidrich for excellent technical assistance.
Correspondence should be addressed to Dr. Kerstin Krieglstein, Center of Anatomy, Department of Neuroanatomy, University of Göttingen, Kreuzbergring 36, 37035 Göttingen, Germany. E-mail: kkriegl@gwdg.de.
Copyright © 2003 Society for Neuroscience 0270-6474/03/235178-09$15.00/0
L.M.F. and N.D. have contributed equally to this work.
References
- Arkell R, Beddington RSP ( 1997) BMP-7 influences pattern and growth of the developing hindbrain of mouse embryos. Development 124: 1–12. [DOI] [PubMed] [Google Scholar]
- Aza-Blanc P, Kornberg TB ( 1999) Ci: a complex transducer of the hedgehog signal. Trends Genet 15: 458–462. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA ( 1996) Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 383: 407–413. [DOI] [PubMed] [Google Scholar]
- Combs SE, Krieglstein K, Unsicker K ( 2000) Reduction of endogenous TGF-beta increases proliferation of developing adrenal chromaffin cells in vivo. J Neurosci Res 59: 379–383. [DOI] [PubMed] [Google Scholar]
- Dünker N, Krieglstein K ( 2000) Targeted mutations of transforming growth factor-β genes reveal important roles in mouse development and adult maintenance. Eur J Biochem 267: 6982–6988. [DOI] [PubMed] [Google Scholar]
- Dünker N, Schuster N, Krieglstein K ( 2001) Transforming growth factor beta modulates programmed cell death in the retina of the developing chick embryo. Development 128: 1933–1942. [DOI] [PubMed] [Google Scholar]
- Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP ( 1993) Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 75: 1417–1430. [DOI] [PubMed] [Google Scholar]
- Edlund T, Jessell TM ( 1999) Progression from extrinsic to intrinsic signaling in cell fate specification: a view from the nervous system. Cell 96: 211–224. [DOI] [PubMed] [Google Scholar]
- Ericson J, Morton S, Kawakami A, Roelink H, Jessell TM ( 1996) Two critical periods of Sonic hedgehog signaling required for the specification of motor neuron identity. Cell 87: 661–673. [DOI] [PubMed] [Google Scholar]
- Ericson J, Rashbass P, Schedl A, Brenner-Morton S, Kawakami A, van Heyningen V, Jessell TM, Briscoe J ( 1997) Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell 90: 169–180. [DOI] [PubMed] [Google Scholar]
- Ernsberger U, Patzke H, Rohrer H ( 1997) The developmental expression of choline acetyltransferase (ChAT) and the neuropeptide VIP in chick sympathetic neurons: evidence for different regulatory events in cholinergic differentiation. Mech Dev 68: 115–126. [DOI] [PubMed] [Google Scholar]
- Flanders KC, Ludecke G, Engels S, Cissel DS, Roberts AB, Kondaiah P, Lafyatis R, Sporn MB, Unsicker K ( 1991) Localization and actions of transforming growth factor-betas in the embryonic nervous system. Development 113: 183–191. [DOI] [PubMed] [Google Scholar]
- Goumans M-J, Mummery C ( 2000) Functional analysis of the TGFβ receptor/Smad pathway through gene ablation in mice. Int J Dev Biol 44: 253–265. [PubMed] [Google Scholar]
- Greenberg D, Banerjee SP, Yamamura HI, Snyder SH ( 1974) Drugs, neurotransmitters, and schizophrenia. Science 184: 1243–1253. [DOI] [PubMed] [Google Scholar]
- Gripp KW, Wotton D, Edwards MC, Roessler E, Ades L, Meinecke P, Richieri-Costa A, Zackai EH, Massague J, Muenke M, Elledge SJ ( 2000) Mutations in TGIF cause holoprosencephaly and link NODAL signaling to human neural axis determination. Nat Genet 25: 205–208. [DOI] [PubMed] [Google Scholar]
- Groves AK, Anderson DJ ( 1996) Role of environmental signals and transcriptional regulators in neural crest development. Dev Genet 18: 64–72. [DOI] [PubMed] [Google Scholar]
- Guo S, Brush J, Teraoka H, Goddard A, Wilson SW, Mullins MC, Rosenthal A ( 1999) Development of noradrenergic neurons in the zebrafish hindbrain requires BMP, FGF8, and the homeodomain protein soulless/Phox2a. Neuron 24: 555–566. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton H ( 1951) A series of normal stages in the development of chick embryo. J Morphol 88: 49–92. [PubMed] [Google Scholar]
- Hirsch EC, Graybiel AM, Agid YA ( 1988) Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson's disease. Nature 334: 345–348. [DOI] [PubMed] [Google Scholar]
- Howard MJ, Gershon MD ( 1993) Role of growth factors in catecholaminergic expression by neural crest cells: in vitro effects of transforming growth factor beta 1. Dev Dyn 196: 1–10. [DOI] [PubMed] [Google Scholar]
- Hynes M, Rosenthal A ( 1999) Specification of dopaminergic and serotonergic neurons in the vertebrate CNS. Curr Opin Neurobiol 9: 26–36. [DOI] [PubMed] [Google Scholar]
- Hynes M, Poulsen K, Tessier-Lavigne M, Rosenthal A ( 1995a) Control of neuronal diversity by the floor plate: contact-mediated induction of midbrain dopaminergic neurons. Cell 80: 95–101. [DOI] [PubMed] [Google Scholar]
- Hynes M, Porter JA, Chiang C, Chang D, Tessier-Lavigne M, Beachy PA, Rosenthal A ( 1995b) Induction of midbrain dopaminergic neurons by Sonic hedgehog. Neuron 15: 35–44. [DOI] [PubMed] [Google Scholar]
- Hynes M, Stone DM, Dowd M, Pitts-Meek S, Goddard A, Gurney A, Rosenthal A ( 1997) Control of cell pattern in the neural tube by the zinc finger transcription factor and oncogene Gli-1. Neuron 19: 15–26. [DOI] [PubMed] [Google Scholar]
- Hynes M, Ye W, Wang K, Stone D, Murone M, Sauvage F, Rosenthal A ( 2000) The seven-transmembrane receptor smoothened cell-autonomously induces multiple ventral cell types. Nat Neurosci 3: 41–46. [DOI] [PubMed] [Google Scholar]
- Ingham PW, Nystedt S, Nakano Y, Brown W, Stark D, van den Heuvel M, Taylor AM ( 2000) Patched represses the hedgehog signaling pathway by promoting modification of the smoothened protein. Curr Biol 10: 1315–1318. [DOI] [PubMed] [Google Scholar]
- Joyner AL, Kornberg T, Coleman KG, Cox DR, Martin GR ( 1985) Expression during embryogenesis of a mouse gene with sequence homology to the Drosophila engrailed gene. Cell 43: 29–37. [DOI] [PubMed] [Google Scholar]
- Krauss S, Concordet JP, Ingham PW ( 1993) A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell 75: 1431–1444. [DOI] [PubMed] [Google Scholar]
- Krieglstein K, Suter-Crazzolara C, Fischer WH, Unsicker K ( 1995) TGF-beta superfamily members promote survival of midbrain dopaminergic neurons and protect them against MPP+ toxicity. EMBO J 14: 736–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieglstein K, Henheik P, Farkas L, Jaszai J, Galter D, Krohn K, Unsicker K ( 1998) Glial cell line-derived neurotrophic factor requires transforming growth factor-beta for exerting its full neurotrophic potential on peripheral and CNS neurons. J Neurosci 18: 9822–9834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieglstein K, Richter S, Farkas L, Schuster N, Dünker N, Oppenheim RW, Unsicker K ( 2000) Reduction of endogenous transforming growth factors beta prevents ontogenetic neuron death. Nat Neurosci 3: 1085–1090. [DOI] [PubMed] [Google Scholar]
- Lee JJ, von Kessler DP, Parks S, Beachy PA ( 1992) Secretion and localized transcription suggest a role in positional signaling for products of the segmentation gene hedgehog. Cell 71: 33–50. [DOI] [PubMed] [Google Scholar]
- Letterio JJ, Geiser AG, Kulkarni AB, Roche NS, Sporn MB, Roberts AB ( 1994) Maternal rescue of transforming growth factor-beta 1 null mice. Science 264: 1936–1938. [DOI] [PubMed] [Google Scholar]
- Liem KF, Tremmel G, Roelink H, Jessell TM ( 1995) Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell 82: 969–979. [DOI] [PubMed] [Google Scholar]
- Liem KF, Tremmel G, Jessell TM ( 1997) A role for the roof plate and its resident TGFβ-related proteins in neuronal patterning in the dorsal spinal cord. Cell 91: 127–138. [DOI] [PubMed] [Google Scholar]
- Litingtung Y, Chiang C ( 2000a) Control of Shh activity and signaling in the developing neural tube. Dev Dyn 219: 143–154. [DOI] [PubMed] [Google Scholar]
- Litingtung Y, Chiang C ( 2000b) Specification of ventral neuron types is mediated by an antagonistic interaction between shh and gli3. Nat Neurosci 3: 979–985. [DOI] [PubMed] [Google Scholar]
- Liu F, Massague J, Ruiz i Altaba A ( 1998) Carboxy-terminally truncated Gli3 proteins associate with Smads. Nat Genet 20: 325–326. [DOI] [PubMed] [Google Scholar]
- Massague J, Wotton D ( 2000) Transcriptional control by the TGF-beta/Smad signaling system. EMBO J 19: 1745–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao N, Wang M, Ott JA, D'Allesandro JS, Woolf TM, Buncrot DA, Mahanthappa NK, Pang K ( 1997) Sonic hedgehog promotes the survival of specific CNS neuron populations and protects these cells from toxic insult in vitro J Neurosci 17: 5891–5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslein-Volhard C, Wieschaus E ( 1980) Mutations affecting segment number and polarity in Drosophila Nature 287: 795–801. [DOI] [PubMed] [Google Scholar]
- Oshima M, Oshima H, Taketo MM ( 1996) TGF-beta receptor type II deficiency results in defects of yolk sac hematopoesis and vasculogenesis. Dev Biol 179: 297–302. [DOI] [PubMed] [Google Scholar]
- Poulsen KT, Armanini MP, Klein RD, Hynes MA, Phillips HS, Rosenthal A ( 1994) TGF-beta 2 and TGF beta 3 are potent survival factors for midbrain dopaminergic neurons. Neuron 13: 1245–1452. [DOI] [PubMed] [Google Scholar]
- Prada FA, Dorado ME, Quesada A, Prada C, Schwarz U, de la Rosa EJ ( 1995) Early expression of a novel radial glia antigen in the chick embryo. Glia 15: 389–400. [DOI] [PubMed] [Google Scholar]
- Proetzel G, Pawlowski SA, Wiles MV, Yin M, Boivin GP, Howles PN, Ding J, Ferguson MW, Doetschman T ( 1995) Transforming growth factor-beta 3 is required for secondary palate fusion. Nat Genet 11: 409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissmann E, Ernsberger U, Francis-West PH, Rueger D, Brickell PM, Rohrer H ( 1996) Involvement of bone morphogenetic protein-4 and bone morphogenetic protein-7 in the differentiation of the adrenergic phenotype in developing sympathetic neurons. Development 122: 2079–2088. [DOI] [PubMed] [Google Scholar]
- Roessler E, Belloni E, Gaudenz K, Jay P, Berta P, Scherer SW, Tsui LC, Muenke M ( 1996) Mutations in the human Sonic hedgehog gene cause holoprosencephaly. Nat Genet 14: 357–360. [DOI] [PubMed] [Google Scholar]
- Sanford LP, Ormsby I, Gittenberg-de Groot AC, Sariola H, Friedman R, Boivin GP, Gardell EL, Doetschman T ( 1997) TGFb2 knockout mice have multiple developmental defects that are nonoverlapping with other TGF-β knockout phenotypes. Development 124: 2659–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober A, Hertel R, Arumae U, Farkas L, Jaszai J, Krieglstein K, Saarma M, Unsicker K ( 1999) Glial cell line-derived neurotrophic factor rescues target-deprived sympathetic spinal cord neurons but requires transforming growth factor-β as cofactor in vivo J Neurosci 19: 2008–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P, Guan HC, Van Tol HHM ( 1993) Dopamine D4 receptors elevated in schizophrenia. Nature 365: 441–445. [DOI] [PubMed] [Google Scholar]
- Shah NM, Groves AK, Anderson DJ ( 1996) Alternative neural crest fates are instructively promoted by TGFbeta superfamily members. Cell 85: 331–343. [DOI] [PubMed] [Google Scholar]
- Simon HH, O'Leary DDM ( 1998). Synuclein expression is regulated by engrailed-1 and–2. Soc Neurosci Abstr 24: 606.9. [Google Scholar]
- ten Dijke P, Miyazono K, Heldin CH ( 2000) Signaling inputs converge on nuclear effectors in TGF-beta signaling. Trends Biochem Sci 25: 64–70. [DOI] [PubMed] [Google Scholar]
- Unsicker K, Meier C, Krieglstein K, Sartor BM, Flanders KC ( 1996) Expression, localization, and function of transforming growth factor-betas in embryonic chick spinal cord, hindbrain, and dorsal root ganglia. J Neurobiol 29: 262–276. [DOI] [PubMed] [Google Scholar]
- Vogel-Hökper A, Rohrer H ( 2002) The specification of noradrenergic locus coeruleus (LC) neurones depends on bone morphogenetic proteins (BMPs). Development 129: 983–991. [DOI] [PubMed] [Google Scholar]
- Wotton D, Lo RS, Lee S, Massague JA ( 1999a) A Smad transcriptional corepressor. Cell 97: 29–39. [DOI] [PubMed] [Google Scholar]
- Wotton D, Lo RS, Swaby LAC, Massague JA ( 1999b) Multiple modes of repression by the Smad transcriptional corepressor TGIF. J Biol Chem 274: 37105–37110. [DOI] [PubMed] [Google Scholar]
- Ye W, Shimamura K, Rubenstein JLR, Hynes MA, Rosenthal A ( 1998) FGF8 and Shh signals create inductive centers for dopaminergic and serotonergic neurons in the anterior neural plate. Cell 93: 755–766. [DOI] [PubMed] [Google Scholar]