Abstract
The role of noradrenergic neurotransmission was analyzed in striatal cholinergic interneurons. Conventional intracellular and whole-cell patch-clamp recordings were made of cholinergic interneurons in rat brain slice preparations. Bath-applied noradrenaline (NA) (1–300 μm) dose-dependently induced both an increase in the spontaneous firing activity and a membrane depolarization of the recorded cells. In voltage-clamped neurons, an inward current was induced by NA. This effect was not prevented by α-adrenoceptor antagonists, whereas it was mimicked by the β-adrenoceptor agonist isoproterenol and blocked by the β1 antagonists propranolol and betaxolol. Interestingly, forskolin, activator of adenylate cyclase, mimicked and occluded the membrane depolarization obtained at saturating doses of both dopamine and NA. Accordingly, SQ22,536, a selective adenylate cyclase inhibitor, reduced the response to NA. Analysis of the reversal potential of the NA-induced current did not provide homogeneous results, indicating the involvement of multiple membrane conductances. Because cAMP is known to modulate Ih, the effects of ZD7288, a selective inhibitor of Ih current, were examined on the NA-induced membrane depolarization/inward current. ZD7288 mostly reduced the response to NA. However, both KT-5720 and H-89, selective protein kinase A (PKA) blockers, failed to prevent the excitatory action of NA. Likewise, calphostin C, antagonist of PKC, genistein, inhibitor of tyrosine kinase, and 8-Bromo-cGMP, blocker of PKG, did not affect the response to NA. Finally, double-labeling experiments combining β1-adrenoceptor and choline acetyltransferase immunocytochemistry by means of confocal microscopy revealed a strongβ1-adrenoceptor labeling on cholinergic interneurons. We conclude that NA depolarizes striatal cholinergic interneurons via β1-adrenoceptor activation, through a cAMP-dependent but PKA-independent mechanism.
Keywords: striatum, slices, noradrenaline, cAMP, TANs, electrophysiology
Introduction
The locus coeruleus, the origin of noradrenergic fibers innervating the forebrain, has been found to be affected in Parkinson's disease (PD) (Bernheimer et al., 1973; Mavridis et al., 1991; Gesi et al., 2000). Clinical and experimental evidence suggests that degeneration of the locus coeruleus might be partially responsible for certain symptoms of PD. Indeed, the significant loss of noradrenergic innervation has been shown to cause behavioral manifestations such as loss of attention and interests, somnolence, and depression (Mavridis et al., 1991; Gesi et al., 2000). In vivo recordings from striatal neurons of behaving monkeys have demonstrated that different neuronal subtypes are enrolled in specific aspects not only related to motor planning and execution, but also to cognitive and motivational functions. Tonically active neurons (TANs) represent a peculiar class of striatal interneurons that respond in a temporally related manner to stimuli that are conditioned by association with primary rewards. There is reasonable evidence that among striatal neuronal subtypes, TANs correspond to the large aspiny cholinergic interneurons (Graybiel et al., 1994; Kawaguchi et al., 1995). These interneurons show an irregular tonic firing of 3–9 Hz in the absence of movements (Kimura, 1990; Wilson et al., 1990; Apicella et al., 1991) and a tendency to discharge synchronously in pairs (Raz et al., 1996, 2001). Although TANs account for the remaining 2% or less of the total neuronal striatal population, they are unique in their responsiveness to behavioral signals in classical conditioning tasks (Apicella et al., 1991, 1997, 1998; Graybiel et al., 1994). The dopaminergic innervation of the striatum is critical for these mechanisms because of its influence on a wide range of high order functions of the brain, including sensorimotor integration attention, memory, and motivational processes that operate in behavioral learning (Graybiel et al., 1994; Watanabe and Kimura, 1998). Accordingly, unilateral dopamine (DA) loss in monkeys treated with the toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine hydrochloride (MPTP), which is able to destroy the nigrostriatal dopaminergic pathway, dramatically reduced the responsiveness of in vivo recorded TANs (Aosaki et al., 1994; Raz et al., 1996). More importantly, the reinstatement of TAN responses by the dopaminergic agonist apomorphine in MPTP-treated monkeys suggests that DA is essential in establishing the expression of motor-related behavior in the striatum (Aosaki et al., 1994).
Previous studies on the distribution of β-adrenergic sites in the human brain have shown that β1 receptors are most enriched in the neostriatum and globus pallidus and β2 sites predominating in the cerebellum (Pazos et al., 1985). Given the importance of catecholaminergic transmission in the control of motor and behavioral aspects of striatal function and the prominent distribution of adrenoceptors in this brain area, we investigated the role of NA on cholinergic interneurons and the possible interactions with the DA system. Such functional interplay might have potential implications in the pharmacological approach to PD and motor complications related to long-term treatment with DA precursors.
We characterized the pharmacological aspects of NA-mediated excitation and identified the adrenoceptor subtype involved. By means of a immunohistochemical approach, we demonstrated the presence of β1-adrenoceptors on this neuronal subtype. Then, the transduction mechanism was analyzed using a pharmacological approach. From these results it is concluded that activation of β1-adrenoceptors depolarizes cholinergic interneurons by modulating multiple membrane conductances via a cAMP-dependent but protein kinase A (PKA)-independent mechanism.
Materials and Methods
Tissue preparation. The animal experimental protocols performed in this study were in accordance with the guidelines of the European Union Council (86/609/EU) and the Animal Act (1986). All efforts were made to minimize animal suffering and the number of animals used. Preparation of striatal slices has been described previously in detail (Calabresi et al., 1998; Pisani et al., 1999, 2000). Male Wistar rats, 3–4 weeks old, were anesthetized and killed by cervical dislocation. After rapid removal of the brain from the skull, corticostriatal coronal slices (180–200 μm) were prepared from tissue blocks with the use of a vibratome in oxygenated Krebs' solution (see composition below) maintained at 33°C. Slices were left to recover for 30–60 min. Then a single slice was transferred in a recording chamber (0.5–1 ml volume) mounted on the stage of an upright microscope (Zeiss Axioskop FS) equipped with a 60×, 0.90 numerical aperture water immersion objective (LUMPlan FI, Olympus) and submerged in a continuously flowing (2–3 ml/min) solution at 33–34°C gassed with 95% O2/5% CO2. At this flow rate, chemicals reached the chamber within 30–50 sec. The composition of the solution was (in mm): 126 NaCl, 2.5 KCl, 1.3 MgCl2, 1.2 NaH2PO4, 2.4 CaCl2, 10 glucose, 18 NaHCO3.
Differential interference contrast infrared videomicroscopy. Individual interneurons were visualized in situ using a differential interference contrast (DIC) (Nomarski) optical system combined with an infrared (IR) filter, a monochrome CCD camera (C3077, Hamamatsu, and an 11 inch monitor (Sony). Cholinergic interneurons were impaled under visual guidance, according to their characteristic shape and size, between 50 and 100 μm beneath the surface of the slice.
Electrophysiological recordings. Intracellular sharp microelectrode recordings were performed in the current-clamp mode with electrodes filled with 2 m KCl. An Axoclamp 2B was used for such experiments. Traces, displayed on an oscilloscope (Gould Classic 6000), were acquired and stored on AxoScope 7.0 (Axon Instruments, Foster City, CA) running on a PC, and off-line analysis was performed with pClamp 8 (Clampfit). Whole-cell patch-clamp recordings were made with borosilicate glass pipettes (1.8 mm outer diameter; 3–5 MΩ) containing (in mm): 125 K +-gluconate, 10 NaCl, 1.0 CaCl2, 2.0 MgCl2, 0.5 BAPTA, 19 HEPES, 0.3 GTP, 1.0 Mg-ATP, adjusted to pH 7.3 with KOH. Membrane currents were monitored using an Axopatch 1D patch-clamp amplifier (Axon Instruments). Whole-cell access resistances measured in voltage clamp were in the range of 5–30 MΩ before electronic compensation (60–80% was routinely used). Voltage ramps and digital subtractions of the resulting currents were obtained by using pClamp 8 software.
To compare correctly current–voltage relationships before and after NA perfusion, series resistance (8–15 MΩ) were monitored by the peak amplitude of the capacitive transient induced by a -5 mV step applied before a voltage ramp (from -120 mm to -40 mV, 6 mV/sec). Neurons in which series resistance changed by >10% during NA application were discarded from the statistics. Intrastriatal synaptic stimulation evoked EPSCs in the recorded cells. The amplitude of averaged EPSCs was measured before and after drug application.
Values given in the text and in the figures are mean ± SEM of changes in the respective cell populations. Student's t test (for paired and unpaired observations) was used to compare the means. Action potential frequency was analyzed off-line using the Mini Analysis Program (Synaptosoft). Values given in the text and in the figures are mean ± SEM of changes in the respective cell populations. Student's t test (for paired and unpaired observations) was used to compare the means.
Drug source and handling. Betaxolol, genistein, propranolol, forskolin, ZD7288, phenylephrine, maprotiline, and desipramine were from Tocris Cookson (Bristol, UK); clonidine, noradrenaline, isoproterenol, TTX, and SQ22,536 were from Sigma (Milan, Italy); H-89, KT-5720, and calphostin C were purchased from Alomone Labs (Jerusalem, Israel). Cocaine was a gift from Dr. N. B. Mercuri (Fondazione Santa Lucia I.R.C.C.S., Rome, Italy).
Drugs were applied by dissolving them to the final concentration in the saline and by switching the perfusion from control saline to drug-containing saline, after a three-way tap had been turned on.
Immunohistochemistry. We evaluated the possible expression of noradrenergic fibers in the rat striatum by means of DA β-hydroxylase (DβH) antibodies and the distribution of β1-adrenoceptors by using selective antibodies for β1 receptors. The cell type-specific expression of β1 receptor subtype was studied in large interneurons of rat striatum by means of double-labeling immunocytochemistry. Animals were anesthetized deeply with chloral hydrate (400 mg/kg, i.p.) and perfused through the ascending aorta with a solution of NaCl for 5 min, followed by 4% paraformaldehyde in 0.1 m PB, pH 7.4, for 30 min. Brains were removed from the skulls, postfixed in 4% paraformaldehyde for 2 hr, and then placed in PBS for 20 min at 4°C. Coronal sections were cut (50 μm thick) with a vibratome and collected in 0.1 m PBS, pH 7.4. For DβH and β1-adrenoceptor immunoreactivity, sections were preblocked by incubating in goat serum (10% in PBS) for 1 hr at room temperature and incubated in a solution containing anti-DβH (mouse anti-DβH, 1:100; Chemicon) or anti-β1-adrenoceptor (rabbit anti-β1, 1:100; Sigma) diluted in 0.1 m PB containing 0.3% Triton X-100 and 2% goat serum for 24 hr at room temperature. Control sections were incubated in preimmune serum instead of primary antibody. After several washes in PBS, the sections were incubated in a solution containing biotinylated anti-mouse or anti-rabbit antibodies (goat anti-rabbit/mouse 1:100; Vectastain Elite) and ABC. Then, the peroxidase reaction was revealed by incubating in PBS plus 3,3′-diaminobenzidine (0.05%) and H2O2 (0.003%). This reaction produced a dark-brown staining of the sections. Then, sections were mounted on gelatin-coated slides and observed at the light microscope. A dual-labeling immunofluorescence study was performed to determine the β1-adrenoceptor immunoreactivity in striatal cholinergic neurons. Thus, sections were incubated in a solution containing anti-β1 receptor (1:100 rabbit, anti-β1-receptor; Sigma) and anti-choline acetyltransferase (ChAT) (1:500, mouse anti-ChAT; Chemicon) diluted in PB 0.1 m–0.3% Triton X-100 for 24 hr at room temperature. After repeated washes in PBS, sections were incubated in a solution containing goat anti-rabbit IgG fluorescein-conjugated secondary antibody (FITC) (Sigma) at a dilution of 1:100 and horse anti-mouse IgG rhodamine-conjugated secondary antibody (TRITC) (Sigma) at a dilution of 1:100 for 2 hr at room temperature and then washed in PBS and mounted in anti-fading medium (Vectashield, Vector). The immunolabeled sections were observed under an epiluminescent microscope (Olympus BX51) and subsequently with a Zeiss LSM 510 confocal laser scanning microscope (CLSM) (Zeiss). From each animal a set of five sections was selected along the rostrocaudal extent of the dorsal striatum. Both hemispheres were examined for each section. Then, striatal labeling for ChAT and β1-adrenoceptor was examined in three separate fields (dorsolateral, medial, and one in the middle, each of 1 mm diameter) using a 20× objective lens (Zeiss). The number of ChAT neurons immunolabeled for or devoid of β1 receptors was counted. The distribution of β1-adrenoceptor immunoreactive neurons among the cholinergic interneurons was evaluated by using a Pearson χ2 test.
Results
Identification of the recorded neurons
The results presented were obtained from 198 striatal interneurons identified as cholinergic large aspiny interneurons. By means of IR-DIC videomicroscopy, the large and polygonal shape of the soma allowed the visual identification of these cells up to ∼80 μm beneath the surface of striatal slices. These neurons displayed electrophysiological characteristics that have been attributed previously to striatal cholinergic interneurons (Kawaguchi, 1993; Kawaguchi et al., 1995; Aosaki et al., 1998; Bennett and Wilson, 1999; Pisani et al., 1999). Approximately half of the recorded cells showed a spontaneous, tonic, irregular action potential discharge. The overall resting membrane potential was close to firing threshold (-60 ± 4.5 mV). A high input resistance (145 ± 56 MΩ) was another distinctive feature of these cells, measured with intracellular sharp electrodes. In the whole-cell configuration, the input resistance was 375 ± 61 MΩ. Injection of small depolarizing current pulses elicited few action potentials (amplitude 68 ± 10 mV; width 1.4 ± 0.5 msec) followed by an afterhyperpolarization of larger amplitude and longer duration (350 ± 130 msec). Hyperpolarizing current pulses (100–400 pA, 1–3 sec) evoked a prominent sag that has been attributed to a hyperpolarization-activated cation current (Ih) (Jiang and North, 1991; Kawaguchi 1993; Aosaki et al., 1998). All of the results shown in the present study were obtained from neurons with these electrophysiological characteristics.
Noradrenaline excites cholinergic interneurons and increases spontaneous firing rate
Brief bath application of NA (1–300 μm) invariably induced a membrane depolarization that was sufficient to trigger a train of action potentials in the recorded neurons (Fig. 1Aa). The depolarizing effect persisted in the presence of 1 μm TTX, excluding an involvement of both TTX-sensitive transmitter release and voltage-gated Na+ channels (Fig. 1Ab). A dose–response curve for the excitatory effect produced by NA revealed an EC50 of 16.8 ± 1.28 μm (Fig. 1B) (n = 49), with a peak effect at 100 μm. Part of the recorded cells exhibited a spontaneous firing activity. In all of these recorded neurons, perfusion with NA (10 μm) induced a rapid increase in the firing activity (Fig. 1C,D)(n = 35; p < 0.001) that slowly returned to control values ∼20 min after drug washout.
Figure 1.
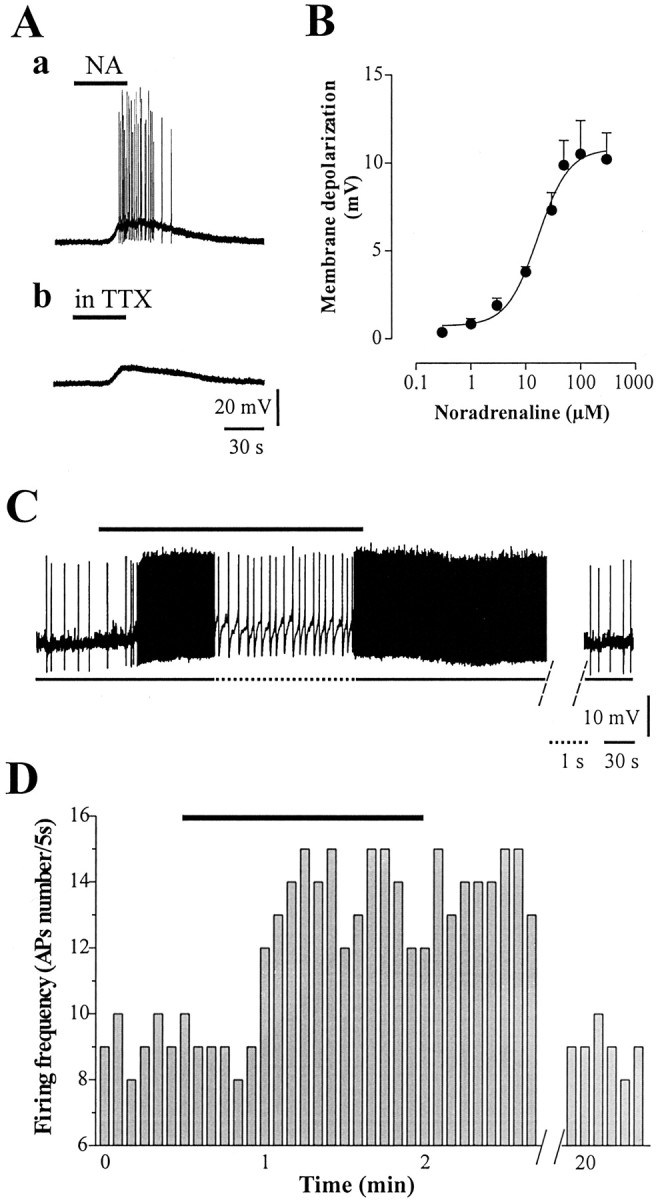
Noradrenaline excites cholinergic interneurons. A, Brief bath perfusion with noradrenaline (NA) (50 μm, 40 sec) induced a membrane depolarization leading the cell to action potential discharge (a). This excitatory action was reversible after drug washout. In the presence of TTX (1 μm), NA produced a similar membrane depolarization, ruling out a TTX-sensitive transmitter release (b). B, Dose—response curve of NA-induced membrane depolarization. EC50 was 16.8 ± 1.28 μm. C, In spontaneously firing interneurons, NA (10 μm, black bar) significantly increased the frequency rate of action potentials (the dotted line shows the trace at higher sweep speed), returning to control 15–20 min after drug washout. Interruption of the trace between two dashed lines indicates >15 min drug wash-out. D, Time-frequency histograms of spontaneous action potentials recorded in current-clamp mode in control condition and in the presence of NA 10 μm (black bar).
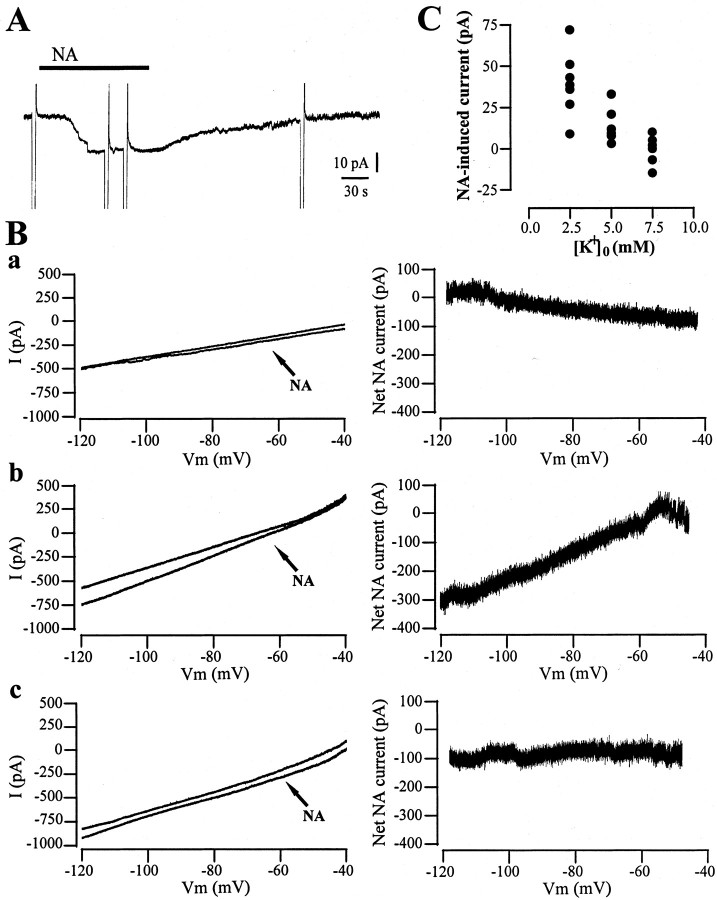
The excitatory effect of NA was also analyzed under whole-cell voltage-clamp at a holding potential of -60 mV. In the presence of TTX (1 μm) in the external solution, NA caused an inward current in all the recorded neurons (n = 26), with an average amplitude of the NA-induced current of 43.2 ± 9.1 pA (Fig. 2A). To identify the conductances underlying the NA-induced inward current, current–voltage relationships were obtained with voltage ramps (from -120 to -40 mV), applied before and during the maximal effect of NA. In 13 of 26 cells, the NA-induced inward current was associated with a reduction in membrane conductance and had an apparent reversal potential close to -120 mV (Fig. 2Ba) (n = 13). Instead, in seven other neurons, the NA-induced inward current was coupled to an increase in membrane conductance and had an estimated reversal potential of -45 to -20 mV (Fig. 2Bb) (n = 7). In the remaining six neurons, no clear changes in membrane conductance were observed, and the current–voltage relation did not appear to cross in the voltage range tested or at more positive or negative values (Fig. 2Bc) (n = 6). These results were confirmed by digital current subtraction in the three distinct groups of neurons to calculate the net NA-induced current (Fig. 2B). To evaluate the relative contribution of potassium currents, in another set of experiments the NA-induced inward current was analyzed by changing the extracellular potassium concentrations ([K+]o) (2.5 mm, n = 7; 5 mm, n = 5; 7.5 mm, n = 5). A dose-dependent reduction in the NA-induced current amplitude was observed, supporting an involvement of potassium ions as major carrier of the NA current (Fig. 2C).
Figure 2.
Multiple conductances underlie the NA-induced response. Voltage ramps (from -120 to -40 mV) were applied to cholinergic interneurons before and during the peak effect produced by NA application (100 μm). A, NA-induced inward current in a voltage-clamped interneuron (holding potential -60 mV). The deflections represent the voltage ramps applied before, during the maximal effect of NA, and after drug washout. B, Representative example of a recording in which the estimated reversal potential was close to -120 mV (a). In another set of recordings the apparent reversal potential was close to -40 mV (b). In the remaining cells, the current—voltage relation did not appear to cross in the voltage range tested or at more positive or negative values (c). The traces shown on the right represent the net NA current recorded at different voltage values obtained by digital subtractions for the corresponding I–V curves shown on the left. C, Changes in extracellular potassium concentration modify the amplitude of the NA-induced current in a dose-dependent manner.
β1-adrenoceptors mediate the noradrenergic response in cholinergic interneurons
To identify the receptor subtype involved in the excitatory effect produced by NA, we used pharmacological agents capable of distinguishing between NA receptor subtypes. In particular, we tested the broad spectrum β-adrenoceptor antagonist propranolol (30 μm, 20 min preincubation) or the selective β1-adrenoceptor antagonist betaxolol (10 μm, 20 min pretreatment). The membrane depolarization induced by NA (30 μm) was nearly abolished by preincubating the slices either in 10 μm betaxolol (Fig. 3Aa,D) or in propranolol (Fig. 3D) (30 μm). Indeed, the membrane depolarization was reduced to 30 ± 11% (p < 0.01) and 6 ± 2.1% (p < 0.001) of control, respectively (Fig. 3D)(n = 22). After betaxolol washout (20 min) the response returned to control levels (data not shown). Similarly, betaxolol (10 μm) fully prevented the increase in firing rate produced by 30 μm NA (Fig. 3B) (n = 11; p < 0.01). In addition, to examine the type of antagonism exerted by betaxolol, dose–response curves were constructed with increasing doses of both NA and betaxolol. This analysis revealed that betaxolol acts as a competitive antagonist, because the dose–response curve to NA was shifted homogeneously to the right by betaxolol (Fig. 3C)(n = 25; p < 0.005). Accordingly, the EC50 calculated in betaxolol was increased dramatically (79.35 ± 1.15 μm). Conversely, as expected for a competitive antagonism, the Hill slope calculated for both curves did not differ significantly (1.47 ± 0.46 and 1.61 ± 0.31, respectively). These results are consistent with the involvement of β1-adrenoceptors in the NA-mediated excitatory effect observed in striatal cholinergic interneurons. Further proof was obtained by bathing the β-adrenoceptor agonist isoproterenol in the perfusing solution (10–30 μm). In the presence of TTX (1 μm), bath application of isoproterenol (30 μm) caused a membrane depolarization of the recorded cells (8 ± 1.4 mV) (Fig. 3Ab) (n = 12). This membrane response was blocked by 10 μm betaxolol (data not shown; p < 0.05). Both the dose–response curve for isoproterenol and the EC50 value resembled those obtained with NA (3 μm = 2.1 ± 0.3 mV; 10 μm = 4 ± 0.2 mV; 30 μm = 8 ± 1.4 mV; 50 μm = 9.4 ± 2 mV; 100 μm = 11.3 ± 2.1 mV; 300 μm = 10.7 ± 1.9 mV; EC50 = 20.4 ± 1.2 μm; n = 12; data not shown). The two curves for NA and isoproterenol were not statistically different (p > 0.05). In another set of experiments we tested selective drugs acting at α-adrenoceptors. The α-1-adrenoceptor antagonist prazosin (0.3 μm) was bathed in the perfusing solution 15–20 min before NA (50 μm) application. However, no detectable effect was obtained on the NA-induced membrane depolarization (Fig. 3D) (control = 10.1 ± 1.2 mV; in prazosin = 9.6 ± 0.9 mV; n = 7; p > 0.05). Similarly, the α-2-adrenoceptor antagonist yohimbine (1 μm, 20 min pretreatment) failed to alter the response to NA (control = 9 ± 2.9 mV; in yohimbine 10.9 ± 1 mV) (Fig. 3D)(n = 5; p > 0.05). Accordingly, bath application of both phenylephrine (10 μm; n = 4) and clonidine (10 μm; n = 4) agonists at the α-1 and α-2-adrenoceptor subtypes, respectively, failed to affect the resting membrane potential as well as the input resistance of the recorded cells (p > 0.05; data not shown). Finally, we analyzed the possible synaptic effects mediated by β1-adrenoceptors on amplitude of synaptic potentials evoked by intrastriatal stimulation. Indeed, because isoproterenol application produced a significant membrane depolarization, these experiments were performed in the whole-cell configuration by analyzing changes in EPSCs amplitude. The synaptic response to isoproterenol, however, was negligible in the recorded cells (30 μm, 5 min; n = 5; p > 0.05; data not shown).
Figure 3.
Pharmacology of the noradrenergic excitation of cholinergic interneurons. A, In the presence of TTX, the membrane depolarization caused by NA (30 μm) was mostly reduced when the slice was pretreated with betaxolol (10 μm) (a). Isoproterenol (30 μm), a β1-adrenoceptor agonist, mimicked the NA-induced membrane depolarization. B, Similarly, time-frequency histograms show the increase in number of action potentials induced by bath-applied NA (30 μm), prevented by pretreatment with the β1-adrenoceptor antagonist betaxolol (10 μm). Pretreatment with betaxolol is indicated by the interruption on the x-axis. C, Dose–response curve of the membrane depolarization, expressed as percentage of maximal response, caused by increasing doses of NA (0.3—1000 μm, filled circles) in control conditions and in the presence of betaxolol (10 μm, open triangles). The rightward shift revealed the competitive nature of its antagonism. The EC50 for the NA response was dramatically increased in betaxolol, whereas the Hill slope did not differ significantly (for details, see Results). D, Summary plot of noradrenergic drugs tested on the NA-induced membrane depolarization: prazosin (0.3 μm), yohimbine (1 μm), propranolol (30 μm), and betaxolol (10 μm). *p < 0.01; **p < 0.001.
Adenylate cyclase activation is required for the excitatory effect of NA
β1-adrenoceptors are known to be positively coupled to Gs class protein and to an adenylate cyclase-cAMP pathway. Hence, it was our aim to test the effects of forskolin, a lipophilic adenylate cyclase activator, on the excitability of these interneurons. In current-clamp recordings, brief bath application of forskolin (3–30 μm) mimicked the membrane depolarization caused by NA in a dose-dependent manner, leading the cell to action potential discharge (Fig. 4A) (n = 20; p < 0.001). In whole-cell voltage-clamp recordings, a saturating dose of forskolin (30 μm) induced a large inward current (88 ± 18.6 pA). Because activation of adenylate cyclase would mimic both DA and NA actions, we performed occlusion experiments, among forskolin, DA, and NA. As shown in Figure 4B, the effects of 100 μm DA were no longer evident after the forskolin-induced inward current reached a steady state. Interestingly, even the addition of NA (100 μm) did not induce any further current (Fig. 4B) (n = 11; p > 0.05). Finally, to verify the involvement of cAMP in the β1-adrenoceptor-mediated effect, the selective adenylate cyclase inhibitor SQ22,536 was tested. Pretreatment with SQ22,536 (300 μm, 15–20 min) significantly reduced both the membrane depolarization (Figs. 4C,6B) (51 ± 11% of control; n = 8; p < 0.001) and the increase in firing frequency induced by 50 μm NA (Fig. 4D) (n = 6; p < 0.0001). These results suggest that adenylate cyclase transduces β1-adrenoceptor-mediated excitation in striatal cholinergic interneurons.
Figure 4.
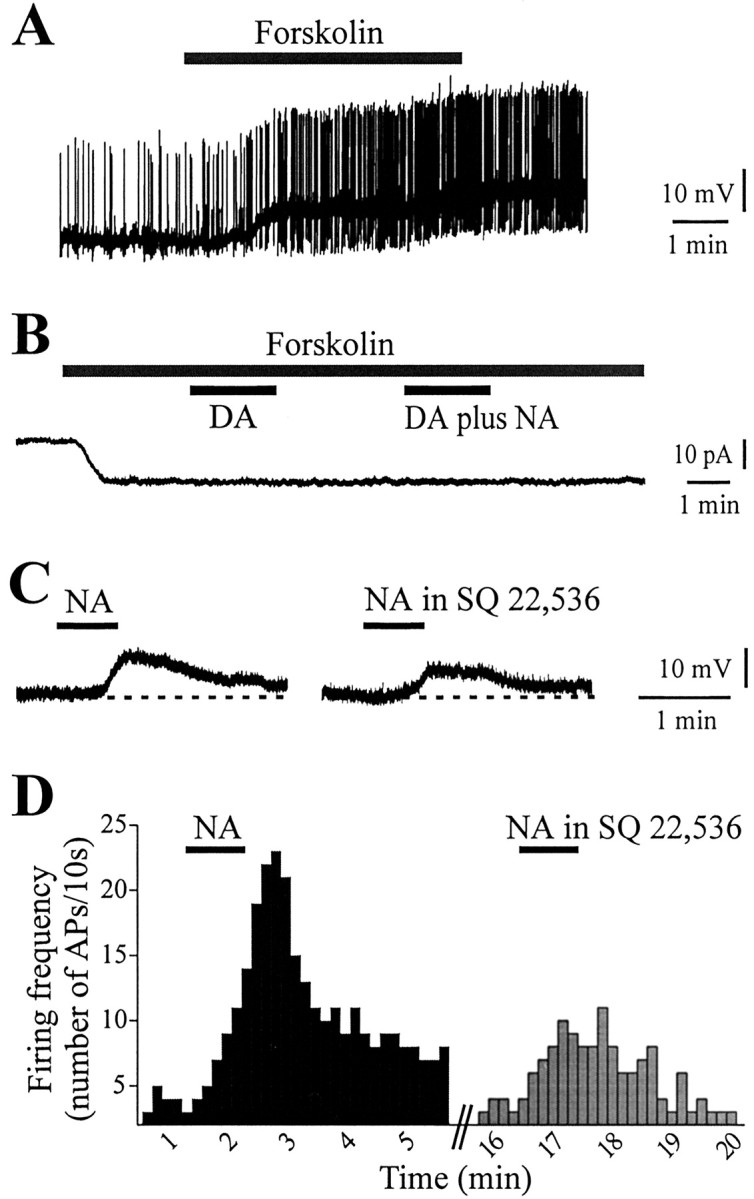
cAMP activation mediates the membrane depolarization induced by noradrenaline. A, The adenylate cyclase activator forskolin (30 μm, 5 min) induced a membrane depolarization and action potential discharge. B, A whole-cell voltage-clamp recording showing the inward current induced by a saturating dose of forskolin (30 μm). When both NA (100 μm) and DA (100 μm) were added to the perfusing solution at the steady-state level of forskolin-induced inward current, no further effect was observed. C, In the presence of the selective adenylate cyclase inhibitor SQ22,536 (300 μm, 20 min preincubation), the response to NA (50 μm, 40 sec) was mostly attenuated. D, Similarly, the increase in firing frequency induced by 50 μm NA was prevented by pretreatment with SQ22,536 (300 μm).
Figure 6.
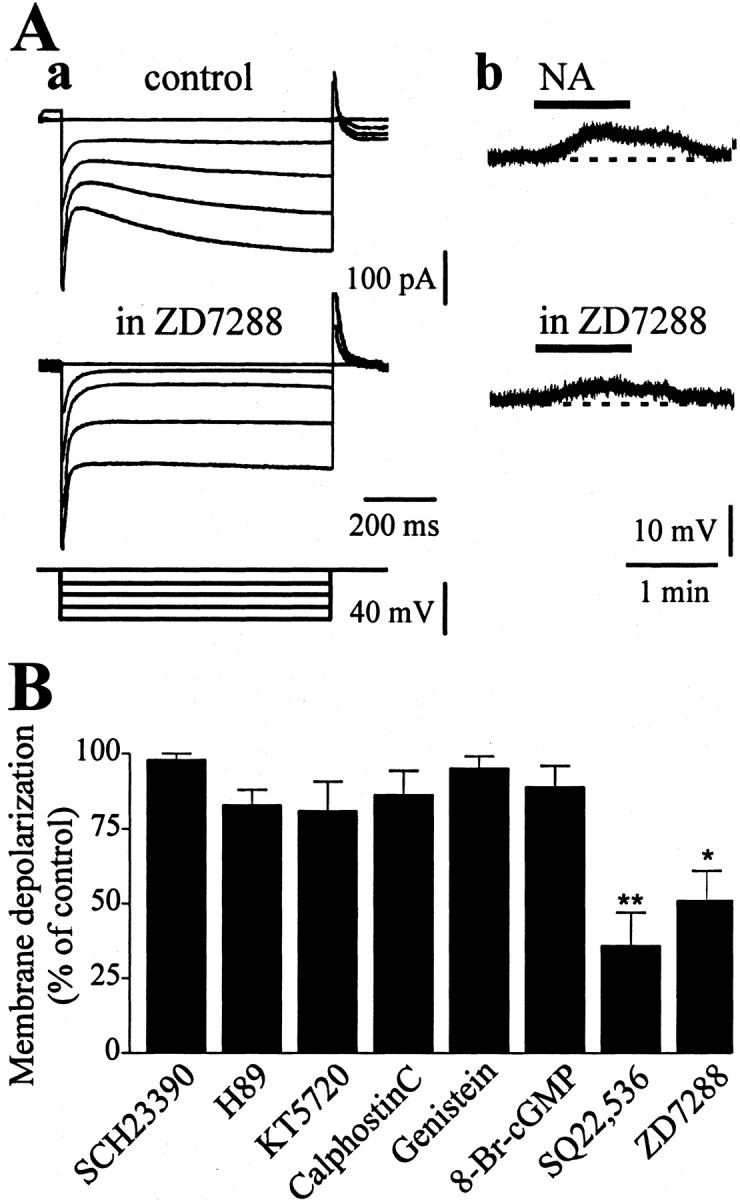
Noradrenaline response involves a modulatory action on Ih. A, Pretreatment of the slice with the selective inhibitor of the Ih current, ZD7288 (20 μm, 20 min), abolished the Ih evoked by hyperpolarizing voltage steps (a). Accordingly, the depolarizing response to 50 μm NA was mostly reduced by 20 μm ZD7288 (b). B, Summary plot of the pharmacological analysis of the post-receptor mechanisms involved in β1-adrenoceptor-mediated response. The adenylate cyclase inhibitor SQ22,536 and the Ih blocker ZD7288 were the only drugs tested that were able to affect the NA-induced excitation. Conversely, a dopamine D1 receptor antagonist SCH23390 (10 μm) and two protein kinase A blockers, H-89 (10 μm) and KT-5720 (2 μm), failed to affect the response to NA. Likewise, the protein kinase C antagonist calphostin C (1 μm) the tyrosine kinase blocker genistein (30 μm), and the intracellularly applied PKG inhibitor 8-bromo-cGMP (10 μm) had no effect on the response to NA (*p < 0.01; **p < 0.001).
Pharmacological analysis of the possible NA/DA interaction
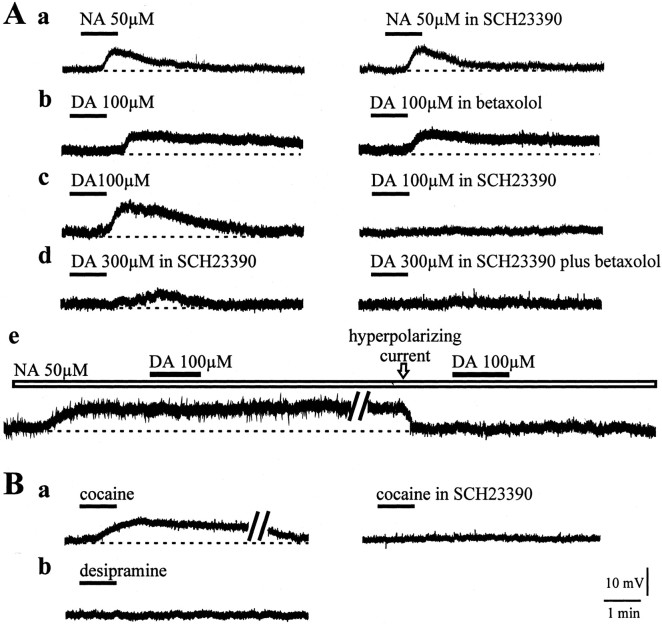
The observation that both DA and NA converge on cAMP activation and, on the other hand, the mismatch between the paucity of noradrenergic innervation and the clear β1-adrenoceptor-dependent pharmacological effect led us to address a crucial issue: do DA and NA interact within the striatum? To answer this question, distinct groups of experiments were performed. First, we tested whether the NA response was affected by blocking D1-like DA receptors. In the presence of 10 μmSCH23390, the membrane depolarization induced by 50 μm NA was unaffected (control = 9.21 ± 1.4 mV; SCH23390 = 9.98 ± 0.7 mV) (Fig. 5Aa) (n = 12; p > 0.05). Then, the response to DA was tested in the presence of betaxolol. DA (100 μm, 1 min) produced a membrane depolarization that was not significantly altered by 10 μm betaxolol (control = 9.5 ± 4.2 mV; betaxolol = 10.1 ± 4.4 mV) (Fig. 5Ab) (n = 9; p > 0.05). As expected, the DA response was fully blocked by the selective D1-like receptor antagonist SCH23390 (Fig. 5Ac) (n = 6; p < 0.001). By increasing DA concentration up to 300 μm in the presence of SCH23390 in the bathing solution, a further, residual depolarizing response could be detected (Fig. 5Ad) (3.6 ± 0.3 mV; n = 11). In this condition, with 10 μm betaxolol in the perfusing medium, the depolarizing effect of 300 μm DA was blocked (Fig. 5Ad)(n = 11; p < 0.05). To further verify a possible DA/NA interaction, we performed occlusion experiments (Fig. 5Ae)(n = 6; p > 0.05). Bath application of a saturating dose of NA (50 μm) depolarized the cell membrane. At the steady-state level of NA-induced depolarization, DA (100 μm) was added to the perfusing solution, but no further depolarizing effect was recorded. With NA in the bathing solution, the recorded cell was repolarized to the control membrane potential by injecting hyperpolarizing current through the recording electrode. In this condition, application of 100 μm DA failed to cause any detectable change in the membrane potential of the cell (Fig. 5Ae).
Figure 5.
Pharmacological analysis of DA/NA interaction. A, NA application (50 μm, 1 min) induced a membrane depolarization that was unaffected by pretreatment with 10 μmSCH23390 (a). Similarly, the excitatory effect caused by 100 μm DA was not prevented by betaxolol (10 μm) (b). Conversely, bath-applied DA (100 μm, 1 min) was able to depolarize the cell via D1 DA receptor activation. In fact, SCH23390 (10 μm) prevented the DA response (c). In the presence of SCH23390, high DA concentrations (300 μm) were still able to induce a residual membrane depolarization. The addition of betaxolol (10 μm) to the perfusing solution blocked the DA-induced depolarizing response (d). The membrane depolarization induced by NA (50 μm) occluded the excitatory response to DA. Indeed, at the steady-state level of the NA response, the cell was repolarized by injecting negative current into the cell (150 pA); in this condition DA application was ineffective (e). B, Bath application of cocaine (30 μm) caused a slow membrane depolarization, fully blocked by pretreatment with the D1-like DA receptor antagonist SCH23390 (10 μm) (a). Instead, the selective NA uptake blocker desipramine (100 μm) caused no change in the resting membrane potential of the recorded interneuron (b).
To investigate the role of endogenous striatal DA and NA, a second group of data were collected by using drugs able to interfere with monoamine reuptake. Bath application of cocaine (30 μm, 1 min) induced a slow and long-lasting membrane depolarization in the recorded interneurons (Fig. 5Ba) (8.6 ± 6.1 mV; n = 10; p < 0.05). Interestingly, this effect was fully prevented by pretreatment with SCH23390, suggesting a DA-mediated response (Fig. 5Ba) (n = 9; p < 0.05). Finally, after perfusion with the selective NA reuptake blocker, desipramine (10–100 μm), no significant change in the membrane potential was detected (Fig. 5Bb) (n = 9; p > 0.05). Similarly, another selective inhibitor of NA reuptake, maprotiline (10–100 μm), was unable to cause any significant change in the membrane properties of the recorded interneurons (data not shown; n = 6; p > 0.05).
The cAMP-dependent effect on Ih partially accounts for the response to NA
In the striatum, the cholinergic interneuron is the only neuronal subtype expressing Ih current (Kawaguchi, 1993; Kawaguchi et al., 1995). Ih is a nonselective cation current activated on hyperpolarization and is widely distributed in neuronal subtypes. This current has been shown to be blocked by cesium ions (Jiang and North, 1991) and by ZD7288 with reasonable selectivity (Chapin and Andrade, 2001). A key feature of Ih is represented by its regulation by cAMP. Indeed, elevation of intracellular cAMP has been shown to shift the voltage dependence of activation of Ih to more depolarized potential, thus resulting in a cell excitation (DiFrancesco and Tortora, 1991). Thus, in another set of experiments, we tested ZD7288 (10–50 μm) on the NA-induced response.
In both current-clamp and voltage-clamp experiments, ZD7288 (20 μm) mostly reduced the hyperpolarization-activated Ih (Fig. 6Aa) (n = 8; p < 0.01). Moreover, pretreatment of the slices (20–30 min) with ZD7288 mostly reduced the membrane depolarization induced by 50 μm NA (Fig. 6Ab,B) (36 ± 8.6% of control; n = 9; p < 0.01). These findings support the hypothesis that at least partially, a cAMP-dependent regulation of Ih current is involved in the β1-adrenoceptor response.
Because elevation of intracellular cAMP has been shown to be involved also in the response of cholinergic interneurons to DA and D1-like agonists (Aosaki et al., 1998), we tested whether the response to DA was affected by blocking Ih current. In experiments performed in the current-clamp mode, the membrane depolarization induced by bath-applied DA (100 μm, 1 min) was significantly reduced by pretreatment with ZD7288 (data not shown; 20 μm;44 ± 11% of control; n = 9; p < 0.005). Similarly, the D1-like DA receptor agonist depolarized the recorded cell SKF38393 (10 μm, 1 min; 9 ± 5.1 mV; n = 5); preincubation with ZD7288 mostly decreased the membrane response to SKF38393 (data not shown; 20 μm;41 ± 8.7% of control; n = 5; p < 0.005). Together, these results support the idea that NA and DA share common transduction mechanisms that are linked, at least in part, to common effects on ion conductances.
Protein kinase activation is not required for the excitatory action of NA
The observation that forskolin mimicked the NA-induced response led us to suppose that a cAMP/PKA cascade would underlie the β1-adrenoceptor-mediated effect. Thus, slices were pretreated with the PKA-selective inhibitors H-89 (1–10 μm) or KT-5720 (1–5 μm). Surprisingly, in the presence of different concentrations of either H-89 or KT-5720, the response to a test NA application (50 μm) was unaffected (control = 11 ± 2.2 mV; H-89 10 μm = 10 ± 1.7 mV; control = 9.87 ± 1.2 mV; KT-5720 5 μm = 10 ± 1.1 mV) (Fig. 6B) (n = 13; p > 0.05), ruling out an involvement of PKA.
To verify whether protein kinases, other than PKA, were involved in the NA-mediated response, we screened other kinase inhibitors. A summary of all the compounds tested is shown in Figure 6B. In detail, the PKC inhibitor calphostin C (1–2 μm) was bathed 20 min before the NA application. However, NA (50 μm) was still able to depolarize the cell membrane (control = 9.5 ± 2.3 mV; calphostin C = 10.1 ± 1 mV) (Fig. 6B) (n = 6; p > 0.05). Similarly, the tyrosine kinase-selective inhibitor genistein (30 μm, 20 min preincubation) failed to alter the response to NA (control = 9.8 ± 1.1 mV; genistein = 9.9 ± 2 mV) (Fig. 6B) (n = 5; p > 0.05). Finally, both the soluble guanylyl cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) (10 μm; n = 3; p > 0.05; data not shown) and the protein kinase G inhibitor 8-Bromo-cGMP (1 μm), applied intracellularly in the recording electrode, had no detectable effect on the membrane depolarization produced by NA (control = 11.1 ± 0.5 mV; ODQ = 9.8 ± 1.4 mV; 8-Bromo-cGMP = 9.7 ± 2 mV) (Fig. 6B) (n = 3; p > 0.05). The lack of effect of ODQ and 8-Bromo-cGMP was reliable because both drugs were able to block the excitatory effect produced by the nitric oxide donor hydroxylamine, which has been shown to act via a cGMP-protein kinase G (PKG)-dependent pathway (Centonze et al., 2001) (data not shown).
Coexpression of ChAT and β1-adrenoceptor immunoreactivity
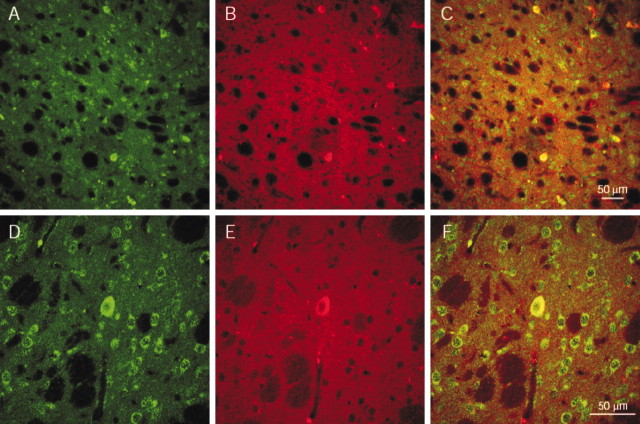
In addition to the pharmacological identification of the receptor subtype involved in the NA-mediated excitatory effect, we analyzed the pattern of β1-adrenoceptor immunoreactivity in striatal cholinergic interneurons identified by ChAT immunoreactivity. The immunohistochemistry for β1-adrenoceptors showed a dense and diffused labeling of the striatum along the rostrocaudal extent of the nucleus. The immunolabeling was localized essentially in the cell body. Most of the β1-receptor immunoreactive cells were medium sized, whereas few of them appeared larger and round in shape, resembling cholinergic interneurons (Fig. 7A,D). These neurons were more numerous in dorsolateral regions of the striatum than in medial and caudal ones. Our immunofluorescence study showed strongly immunopositive neurons for ChAT, distributed without a particular pattern. The fluorescent marker was localized in the cell body of these neurons, although neuropil appeared to be lightly labeled (Fig. 7B,E). When the two fluorescent markers for ChAT and β1-adrenoceptor were merged, a significant superimposition was observed (Fig. 7C,F) (62 ± 1.1%; p < 0.001; n = 124).
Figure 7.
β1-adrenoceptors are expressed, at protein level, on striatal cholinergic interneurons. A–C, CLSM image of dual-label immunofluorescence for ChAT and β1-adrenoceptors in the striatum at low magnification. A, β-1-adrenoreceptor immunoreactivity revealed by FITC (green) fluorescence; medium-sized neurons and a large neuron are immunopositive. B, ChAT immunoreactivity revealed by TRITC (red) fluorescence. C, Merged image. D–F, Same as A–C at high magnification. β1-adrenoceptors colocalize with ChAT in the cholinergic interneuron.
DβH immunoreactivity in the striatum
In a final set of experiments we tested for the DβH immunoreactivity in striatal sections. DβH immunoreactivity showed a diffuse network of varicose nerve fibers in the cerebral cortex and medial septal nuclei, whereas the DβH immunoreactivity was virtually absent in the underlying striatum (data not shown).
Discussion
Pharmacological analysis of the noradrenergic response
Our pharmacological analysis allows the identification of the receptor signaling the NA-induced depolarization in cholinergic interneurons. The first line of evidence derives from the data obtained with isoproterenol, a nonselective β-adrenoceptor agonist that mimicked the effects produced by NA: both the membrane depolarization and the increase in firing rate of the recorded neurons were reproduced by isoproterenol, in a dose-dependent manner. Second, the sensitivity to both propranolol and to the more selective β1-adrenoceptor antagonist betaxolol is consistent with the involvement of β1-adrenoceptors. The competitive nature of betaxolol was confirmed (Cavero et al., 1983; Sharif et al., 2001), showing the rightward shift of the dose–response curve of the membrane depolarization caused by increasing doses of NA in the presence of betaxolol. A third observation suggesting the involvement of β1-adrenoceptors is represented by the finding that forskolin, which increases cAMP levels, mimicked and occluded the effects of NA. Requirement of adenylate cyclase activation for the expression of the effects of NA was also confirmed by the evidence that SQ,22536, an inhibitor of adenylate cyclase activity, mostly reduced the response to NA. Finally, the lack of any significant response on the intrinsic membrane properties of the recorded neurons by selective drugs for α1- and α2-adrenoceptor subtypes ruled out the involvement of α-adrenoceptor subtypes in the noradrenergic effect described.
Ionic and signal transduction mechanisms for the β1-adrenoceptor response
Although the experiments performed by changing the [K+]o are in support of a critical involvement of potassium conductances in the NA response, the data obtained with the voltage ramps indicate that NA-mediated excitation of striatal cholinergic interneurons might involve multiple ionic conductances. The suppression of potassium channels can account for the decrease in membrane conductance observed in the majority of recorded cells, whereas in those cases in which an increase in membrane conductance was obtained, the inward current was most likely caused by an opening of a nonselective cation channel. Notably, NA has already been reported to cause neuronal excitation in other neuronal subtypes by suppressing potassium conductances (Pan et al., 1994; Ansanay et al., 1995) or by opening a nonspecific cationic conductance (Sun and Guyenet, 1990). In striatal cholinergic interneurons, a similar modulation of different membrane conductances has been described for the excitatory effect of DA, via D1-like receptor activation (Aosaki et al., 1998).
In several brain regions, both NA- and serotonin-induced depolarizations are elicited through modulation of the hyperpolarization-activated nonselective cation conductance, termed Ih (McCormick et al., 1991; Shiekhattar and Aston-Jones, 1994; Cardenas et al., 1999; Saitow and Konishi, 2000; Chapin and Andrade, 2001). Therefore, we verified whether the modulation of Ih might contribute, at least partially, to the depolarization evoked by β1-adrenoceptor activation. The evidence that pharmacological blockade of Ih partially reduced the NA-induced excitation supports the involvement of a hyperpolarization-activated current in such an effect. Indeed, a crucial characteristic of Ih is its regulation by cAMP. Elevation of intracellular cAMP, in fact, shifts the voltage dependence of activation of Ih to more depolarized potentials, thereby activating this current even at resting membrane potential values (DiFrancesco and Tortora, 1991). Interestingly, we found that similar to what was observed for the response to NA, both cAMP and activation of Ih are implicated in the excitatory effect of DA and D1-like receptor agonists on this striatal cell subtype.
Recent molecular cloning of hyperpolarization-activated cation channels revealed that the cAMP-binding site located in an intracellular domain of the channel protein plays a key role in the modulation of the channel activity (Ludwig et al., 1998). Indeed, the cAMP-binding site of this channel appeared to be a target of β-adrenoceptors coupled to cAMP formation via adenylate cyclase activation (Madison and Nicoll, 1986; Sun and Guyenet, 1990; Ansanay et al., 1995; Saitow and Konishi, 2000). A recent study examining the gene expression of hyperpolarization-activated cation channels in the mouse brain found that the mRNA for type 4 channel is selectively expressed in large striatal interneurons, most likely cholinergic (Santoro et al., 2000). Notably, one of the distinctive features of type 4 hyperpolarization-activated cation channels is represented by its capability to strongly respond to cAMP elevation (Moosmang et al., 1999), which appears in line with the present results.
The finding that both H-89 and KT-5720, known as selective PKA blockers, failed to affect the response to NA suggests that β1-adrenoceptors transduce their excitatory effect without involvement of PKA-dependent pathways. This notion is in agreement with previous studies showing a β-adrenergic response to be mediated by cAMP but independent of PKA (Saitow and Konishi, 2000). Moreover, our results obtained with several selective kinase inhibitors seem to exclude an involvement of other protein kinase-dependent mechanisms in the β1-adrenoceptor-mediated action.
Possible interpretations of the NA function in the striatum
The catecholaminergic innervation to the striatum is dopaminergic rather than noradrenergic (Lindvall and Bjorklund, 1974; Swanson and Hartman, 1975). Accordingly, our results indicate that the DβH immunoreactivity was sparse throughout the striatum. Despite this observation, in the present work we show that cholinergic interneurons exhibit significant levels of β1-adrenoceptors. These findings corroborate our pharmacological investigation and appear in agreement with previous observations showing an intense labeling of β-adrenoceptors in the striatum (Aoki et al., 1987; Waeber et al., 1991). Moreover, the co-localization of ChAT and β1-adrenoceptors observed previously in other brain areas (Nicholas et al., 1993) strengthens the significance of the pharmacological response to NA. The key issue that remains unsolved is the source of NA in the striatum. Our morphological data obtained with DβH and pharmacological analysis with desipramine and maprotiline seem to suggest low levels of noradrenergic innervation.
The dopamine-mediated excitation in the striatum occurs via activation of D1-like DA receptors, in a cAMP-PKA-dependent manner (Aosaki et al., 1998); conversely, the NA-dependent effect requires adenylate cyclase activation but is independent of PKA involvement. Thus, DA and NA share a common post-receptor pathway by elevating the levels of intracellular cAMP. It seems plausible that cAMP and cAMP-related mechanisms might be the final target of both catecholamine actions. Accordingly, we have shown that both NA and DA exert a modulatory action on Ih current. Moreover, we demonstrated that either DA occludes NA or NA occludes DA and that pretreatment with forskolin occludes the response to both DA and NA.
It is now evident that the phenomenon of “heterologous uptake,” a promiscuous monoamine uptake, takes place in different brain regions, including the dorsal and ventral striatum (Carboni et al., 1990; Di Chiara et al., 1992; Mundorf et al., 2001). In addition, it has been shown that the affinity of DA for NA receptor subtypes is equal if not higher than that of NA for its receptors (Zhang et al., 1999).
Alternative explanations for mismatch between receptor and ligand distribution have been considered. First, NA released from a relatively small group of afferent fibers might act in a paracrine manner (Kuhar, 1985; Herkenham, 1987). In support of this view is our finding that the residual membrane depolarization caused by high doses of DA in the presence of SCH23390 is blocked by β1-adrenoceptor antagonists. We are aware that such concentrations might be considered too high. However, interestingly, it has been demonstrated recently that in response to salient stimuli of behavioral and motivational significance, DA can be phasically released in the striatum, reaching elevated concentrations and recruiting promiscuously β1-adrenoceptors (Robinson et al., 2002).
Pharmacological implications
The demonstration of functional β1-adrenoceptors and the experimental findings supporting a close interaction between DA and NA within the striatum disclose a potential relevance of these receptors from pharmacological and therapeutic points of view. Unilateral dopamine loss in monkeys treated with the toxin MPTP, which destroys the nigrostriatal dopaminergic pathway, dramatically reduces the responsiveness of in vivo recorded cholinergic interneurons (Aosaki et al., 1994). Moreover, after MPTP treatment, both striatal cholinergic interneurons and pallidal neurons exhibit an oscillatory firing pattern in a frequency range that resembles the range of parkinsonian tremor frequencies (Raz et al., 2001). Thus, in a parkinsonian state, these interneurons might serve as a source of oscillations, representing a key element in the generation of parkinsonian tremor (Raz et al., 2001). Propranolol is commonly used in the treatment of tremor (Marijama-Lyons and Koller, 2000; Silverdale et al., 2003); however, the mechanism underlying its therapeutic effect is not known. It might be hypothesized that β1-adrenoceptor blockers would reduce the excitability of striatal cholinergic interneurons, thereby limiting the oscillatory behavior generating tremor.
Another putative therapeutic target of β1-adrenoceptor blockers might be represented by the side-effects caused by long-term treatment with DA precursors. Indeed, on a long-term basis, treatment of PD symptoms with both DA precursors and DA receptor agonists is not devoid of serious complications such as dyskinesias and motor fluctuations. The cellular mechanisms underlying such disabling effects are unknown. One possibility could be that during levodopa treatment DA would exceed physiological levels, recruiting “silent” β1-adrenoceptors, leading to the motor complications. Thus, in PD patients with chronic levodopa treatment who develop such motor fluctuations, β1-adrenoceptor blockers might prove useful.
Footnotes
This work was supported by grants from Ministero Istruzione Università e Ricerca (Cofin 2002, Fondo degli Investimenti della Ricerca di Base), Ministero Salute, Regione Lazio (Progetto Alzheimer) to G.B. and A.P. and a Telethon grant to A.P. (E.0930). We thank Prof. E. Scarnati for critical reading of this manuscript and M. Tolu for his excellent technical support.
Correspondence should be addressed to Antonio Pisani, Clinica Neurologica, Dipartimento di Neuroscienze, Università di Roma “Tor Vergata”, Via di Tor Vergata 135, 00133, Rome, Italy. E-mail: pisani@uniroma2.it.
Copyright © 2003 Society for Neuroscience 0270-6474/03/235272-11$15.00/0
References
- Ansanay H, Dumuis A, Sebben M, Bockaert J, Fagni L ( 1995) cAMP-dependent, long-lasting inhibition of a K+ current in mammalian neurons. Proc Natl Acad Sci USA 92: 6635–6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella P, Scarnati E, Schultz W ( 1991) Tonically discharging neurons of monkey striatum respond to preparatory and rewarding stimuli. Exp Brain Res 84: 672–675. [DOI] [PubMed] [Google Scholar]
- Apicella P, Legallet E, Trouche E ( 1997) Responses of tonically discharging neurons in the monkey striatum to primary rewards delivered during different behavioral states. Exp Brain Res 116: 456–466. [DOI] [PubMed] [Google Scholar]
- Apicella P, Ravel S, Sardo P, Legallet E ( 1998) Influence of predictive information on responses of tonically active neurons in the monkey striatum. J Neurophysiol 80: 3341–3344. [DOI] [PubMed] [Google Scholar]
- Aoki C, Joh TH, Pickel VM ( 1987) Ultrastructural localization of β-adrenergic receptor-like immunoreactivity in the cortex and neostriatum of rat brain. Brain Res 437: 264–282. [DOI] [PubMed] [Google Scholar]
- Aosaki T, Graybiel AM, Kimura M ( 1994) Effect of the nigrostriatal dopamine system on acquired neural responses in the striatum of behaving monkeys. Science 265: 412–415. [DOI] [PubMed] [Google Scholar]
- Aosaki T, Kiuchi K, Kawaguchi Y ( 1998) Dopamine D1-like receptor activation excites striatal large aspiny neurons in vitro. J Neurosci 18: 5180–5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BD, Wilson CJ ( 1999) Spontaneous activity of neostriatal cholinergic interneurons in vitro. J Neurosci 19: 5586–5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F ( 1973) Brain dopamine and the syndromes of Parkinson and Huntington. J Neurol Sci 20: 415–455. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Pisani A, Sancesario G, North RA, Bernardi G ( 1998) Muscarinic IPSPs in rat striatal cholinergic interneurones. J Physiol (Lond) 510: 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni E, Tanda GL, Frau R, Di Chiara G ( 1990) Blockade of the noradrenaline carrier increases extracellular dopamine concentrations in the prefrontal cortex: evidence that dopamine is taken up in vivo by noradrenergic terminals. J Neurochem 55: 1067–1070. [DOI] [PubMed] [Google Scholar]
- Cardenas CG, Mar LP, Vysokanow AV, Arnold PB, Cardenas LM, Surmeier DJ, Scroggs RS ( 1999) Serotoninergic modulation of hyperpolarization-activated current in acutely isolated rat dorsal root ganglion neurons. J Physiol (Lond) 518: 507–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavero I, Lefevre-Borg F, Manoury P, Roach AG ( 1983) In vitro and in vivo pharmacological evaluation of betaxolol, a new, potent and selective beta-1 adrenoceptor antagonist. In: L.E.R.S. Betaxolol and other beta-1 adrenoceptor antagonists, Vol I (Morselli PL, Kilborn JR, Cavero I, Harrison DC, Langer SZ), pp 31–42. New York: Raven. [Google Scholar]
- Centonze D, Pisani A, Bonsi P, Giacomini P, Bernardi G, Calabresi P ( 2001) Stimulation of nitric oxide-cGMP pathway excites striatal cholinergic interneurons via protein kinase G activation. J Neurosci 21: 1393–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin EM, Andrade R ( 2001) A 5-HT7 receptor-mediated depolarization in the anterodorsal thalamus. II. Involvement of the hyperpolarization-activated current Ih. J Pharmacol Exp Ther 297: 403–409. [PubMed] [Google Scholar]
- Di Chiara G, Tanda GL, Frau R, Carboni E ( 1992) Heterologous monoamine reuptake: lack of transmitter specificity of neuron-specific carriers. Neurochem Int 20: 231S–235S. [PubMed] [Google Scholar]
- DiFrancesco D, Tortora P ( 1991) Direct activation of cardiac pacemaker channels by intracellular cAMP. Nature 351: 145–147. [DOI] [PubMed] [Google Scholar]
- Gesi M, Soldani P, Giorgi FS, Santinami A, Bonaccorsi I, Fornai F ( 2000) The locus coeruleus in the development of Parkinson's disease. Neurosci Biobehav Rev 24: 655–668. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Aosaki T, Flaherty AW, Kimura M ( 1994) The basal ganglia and adaptive motor control. Science 265: 1826–1831. [DOI] [PubMed] [Google Scholar]
- Herkenham M ( 1987) Mismatches between neurotransmitter and receptor localizations in brain: observations and implications. Neuroscience 23: 1–38. [DOI] [PubMed] [Google Scholar]
- Jiang ZG, North RA ( 1991) Membrane properties and synaptic responses of rat striatal neurones in vitro. J Physiol (Lond) 443: 533–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y ( 1993) Physiological, morphological and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci 13: 4908–4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC ( 1995) Striatal interneurons: chemical, physiological and morphological characterization. Trends Neurosci 18: 527–535. [DOI] [PubMed] [Google Scholar]
- Kimura M ( 1990) Behaviorally contingent property of movement-related activity of the primate putamen. J Neurophysiol 63: 1277–1296. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ ( 1985) The mismatch problem in receptor mapping studies. Trends Neurosci 8: 190–191. [Google Scholar]
- Lindvall O, Bjorklund A ( 1974) The organization of the ascending catecholamine neuron systems in the rat brain as revealed by the glyoxylic acid fluorescence method. Acta Physiol Scand [Suppl] 412: 1–48. [PubMed] [Google Scholar]
- Ludwig A, Zong X, Jeglitsch M, Hofmann F, Biel M ( 1998) A family of hyperpolarization-activated mammalian cation channels. Nature 393: 587–591. [DOI] [PubMed] [Google Scholar]
- Madison DV, Nicoll RA ( 1986). Cyclic-adenosine 3,5-monophosphate mediates beta-receptor actions of noradrenaline in rat hippocampal pyramidal cells. J Physiol (Lond) 372: 245–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marijama-Lyons J, Koller W ( 2000) Tremor-predominant Parkinson's disease. Approaches to treatment. Drugs Aging 16: 273–278. [DOI] [PubMed] [Google Scholar]
- Mavridis M, Degryse AD, Lategan AJ, Marien MR, Colpaert FC ( 1991) Effects of locus coeruleus lesions on parkinsonian signs, striatal dopamine and substantia nigra cell loss after 1-methyl-phenyl-1,2,3,6-tetrahydropyridine in monkeys: a possible role for the locus coeruleus in the progression of Parkinson's disease. Neuroscience 41: 507–523. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Pape HC, Williamson A ( 1991) Actions of norepinephrine in the cerebral cortex and thalamus: implications for function of the central noradrenergic system. Prog Brain Res 88: 293–305. [DOI] [PubMed] [Google Scholar]
- Moosmang S, Biel M, Hofmann F, Ludwig A ( 1999) Differential distribution of four hyperpolarization-activated cation channels in mouse brain. J Biol Chem 380: 975–980. [DOI] [PubMed] [Google Scholar]
- Mundorf ML, Joseph JD, Austin CM, Caron MG, Wightman RM ( 2001) Catecholamine release and uptake in the mouse prefrontal cortex. J Neurochem 79: 130–142. [DOI] [PubMed] [Google Scholar]
- Nicholas AP, Pieribone VA, Hokfelt T ( 1993) Cellular localization of messenger RNA for beta-1 and beta-2 adrenergic receptors in rat brain: an in situ hybridization study. Neurosci 56: 1023–1039. [DOI] [PubMed] [Google Scholar]
- Pan ZZ, Grudt TJ, Williams JT ( 1994) Alpha 1 adrenoceptors in rat dorsal raphe neurons: regulation of two potassium conductances. J Physiol (Lond) 478: 437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazos A, Probst A, Palacios JM ( 1985) β-adrenoceptor subtypes in the human brain: autoradiographic localization. Brain Res 358: 324–328. [DOI] [PubMed] [Google Scholar]
- Pisani A, Calabresi P, Centonze D, Marfia GA, Bernardi G ( 1999) Electrophysiological recordings and calcium measurements in striatal large aspiny interneurons in response to combined O2/glucose deprivation. J Neurophysiol 81: 2508–2516. [DOI] [PubMed] [Google Scholar]
- Pisani A, Bonsi P, Centonze D, Calabresi P, Bernardi G ( 2000) Activation of D2-like dopamine receptors reduces synaptic inputs to striatal cholinergic interneurons. J Neurosci 20:RC69( 1–6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A, Feingold A, Zelanskaya V, Vaadia E, Bergman H ( 1996) Neuronal synchronization of tonically active neurons in the striatum of normal and parkinsonian primates. J Neurophysiol 76: 2083–2088. [DOI] [PubMed] [Google Scholar]
- Raz A, Frechter-Mazar V, Feingold A, Abeles M, Vaadia E, Bergman H ( 2001) Activity of pallidal and striatal tonically active neurons is correlated in MPTP-treated monkeys but not in normal monkeys. J Neurosci 21:RC128( 1–5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Heien MLA, Wightman RM ( 2002) Frequency of dopamine concentration transients increases in dorsal and ventral striatum of male rats during introduction of conspecifics. J Neurosci 22: 1047–10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitow F, Konishi S ( 2000) Excitability increase induced by β-adrenergic receptor-mediated activation of hyperpolarization-activated cation channels in rat cerebellar basket cells. J Neurophysiol 84: 2026–2034. [DOI] [PubMed] [Google Scholar]
- Santoro B, Chen S, Luthi A, Pavlidis P, Shumyatsky GB, Tibbs GR, Siegelbaum SA ( 2000) Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J Neurosci 20: 5264–5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif NA, Xu SX, Crider JY, McLaughlin M, David TL ( 2001) Levobetaxolol (Betaxon) and other beta adrenergic antagonists: preclinical pharmacology, IOP-lowering and sites of action in human eyes. J Ocular Pharmacol Ther 17: 305–317. [DOI] [PubMed] [Google Scholar]
- Shiekhattar R, Aston-Jones G ( 1994) Activation of adenylate cyclase attenuates the hyperpolarization following single action potentials in brain noradrenergic neurons independently of protein kinase A. Neuroscience 62: 523–529. [DOI] [PubMed] [Google Scholar]
- Silverdale MA, Fox SH, Crossman AR, Brotchie JM ( 2003) Potential nondopaminergic drugs for Parkinson's disease. Adv Neurol 91: 273–291. [PubMed] [Google Scholar]
- Sun MK, Guyenet PG ( 1990) Excitation of rostral medullary pacemaker neurons with putative sympathoexcitatory function by cyclic AMP and beta-adrenoceptor agonists “in vitro”. Brain Res 511: 30–40. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Hartman BK ( 1975) The central adrenergic system. An immunofluorescence study of the location of cell bodies and their efferent connections in the rat utilizing dopamine-B-hydroxylase as a marker. J Comp Neurol 163: 467–505. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Chang HT, Kitai ST ( 1990) Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. J Neurosci 10: 508–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waeber C, Rigo M, Chinaglia G, Probst A, Palacios JM ( 1991) Beta-adrenergic receptor subtypes in the basal ganglia of patients with Huntington's chorea and Parkinson's disease. Synapse 8: 270–280. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Kimura M ( 1998) Dopamine receptor-mediated mechanisms involved in the expression of learned activity of primate striatal neurons. J Neurophysiol 79: 2568–2580. [DOI] [PubMed] [Google Scholar]
- Zhang W, Klimek V, Farley JT, Zhu M-Y, Ordway GA ( 1999) α2C adrenoceptors inhibit adenylyl cyclase in mouse striatum: potential activation by dopamine. J Pharmacol Exp Ther 289: 1286–1292. [PubMed] [Google Scholar]