Abstract
Glutamatergic synaptic transmission is mediated primarily through the AMPA-type glutamate receptor (AMPAR); the regulation of this receptor underlies many forms of synaptic plasticity. In particular, phosphorylation of GluR1, an AMPAR subunit, by PKA at serine 845 (S845) increases peak open channel probability and is permissive for both the synaptic expression of the receptor and NMDA-receptor (NMDAR)-dependent long-term potentiation (LTP). Robust NMDAR activation activates PKA as well as other signaling enzymes; however, we find that maximal NMDAR activation dephosphorylates GluR1 at the PKA site S845. Coincident inhibition of phosphatases blocks NMDAR-induced dephosphorylation of S845, but surprisingly does not promote PKA phosphorylation at this site. However, we find that phosphorylation of S845 is increased by the activation of a Gs-coupled receptor, the β1-adrenergic receptor. Interestingly, this divergent signaling occurs despite a more robust coupling of the NMDAR to cAMP generation. In addition, NMDAR activation plays a dominant role in S845 regulation, because activation of β1AR after NMDAR activation has no detectable effect on S845 phosphorylation. These data (1) demonstrate highly specific coupling between these receptors and this substrate, (2) provide an example of a substrate critical in NMDAR-dependent LTP that is incompletely regulated by the NMDAR, and (3) highlight the importance of identifying the physiological signals that regulate these critical synaptic substrates.
Keywords: synaptic plasticity, long-term potentiation, LTP, neuromodulation, neuromodulatory, AMPA receptor, PKA
Introduction
The degree to which heterosynaptic processes contribute to presumptive homosynaptic plasticity has been an important question in neuroscience (Bailey et al., 2000). NMDA-receptor (NMDAR)-dependent synaptic plasticity often requires signals recruited through heterosynaptic neuromodulatory receptors such as the Gs-coupled β1-adrenergic receptor (β1AR) (Thomas et al., 1996; Winder et al., 1999). The β1AR regulates synaptic plasticity through cAMP/PKA and MAPK (mitogen-activated protein kinase)-dependent processes, two cascades that the NMDAR itself activates (Chetkovich et al., 1991; Chetkovich and Sweatt, 1993; English and Sweatt, 1996; Xia and Storm, 1997). Thus, there may exist PKA and/or MAPK substrates that are targeted distinctly by the β1AR.
One potential substrate is glutamate receptor 1 (GluR1), a subunit of the AMPA-type glutamate receptor (AMPAR) that is critically involved in NMDAR-dependent synaptic plasticity. Mice lacking GluR1 exhibit diminished long-term potentiation (LTP) in area CA1 (Zamanillo et al., 1999; Mack et al., 2001) and overexpression of the GluR1 C terminus blocks LTP (Shi et al., 2001). The C terminus contains two well-characterized phosphorylation sites, serine 831 (S831) and serine 845 (S845), the phosphorylation and dephosphorylation of which has been correlated with several forms of NMDAR-dependent synaptic plasticity (Kameyama et al., 1998; Lee et al., 1998, 2000, 2003). Phosphorylation of S845 by PKA regulates peak open probability of the channel (Roche et al., 1996; Banke et al., 2000) is required for subcellular trafficking of GluR1-containing AMPARs into the synaptic membrane and is required for the maintenance of NMDAR-dependent LTP (Ehlers, 2000; Esteban et al., 2003).
To date, the observed forms of LTP that require GluR1 expression or phosphorylation also require NMDAR activation. It is reasonable to predict that the NMDAR mediates the phosphorylation of GluR1 at S845 and/or S831 during LTP, because the NMDAR couples to PKA, Ca2+/calmodulin-dependent protein kinase II (CaMKII), and PKC. However, submaximal NMDAR activation that produces long-term depression (LTD) in area CA1 dephosphorylates S845 (Kameyama et al., 1998; Lee et al., 1998). This may be attributable to a predicted ability of the NMDAR to preferentially couple to phosphatases at lower levels of activation while activating kinases at higher levels of activation. Indeed, higher concentrations of NMDA do elicit marked rises in cAMP through the recruitment of Ca2+-sensitive adenylyl cyclases (AC) (Chetkovich et al., 1991; Chetkovich and Sweatt, 1993; Wong et al., 1999; Suvarna and O'Donnell, 2002), and the NMDAR does regulate cellular substrates, including a voltage-gated Ca2+ channel (Chetkovich et al., 1991) and an after hyperpolarization (AHP) (Blitzer et al., 1995), through PKA activation. However, we report here that a saturating dose of NMDA, which elicits marked increases in cAMP levels and CaMKII/PKC phosphorylation of S831, surprisingly, does not increase PKA phosphorylation of S845 in area CA1, but rather induces dephosphorylation. In contrast, activation of the neuromodulatory, Gs-coupled β1AR does couple to PKA phosphorylation of GluR1 at S845, even with a substantially lower level of cAMP generation compared with the NMDAR. These data support the idea that there is a critical PKA substrate accessible by the β1AR-activated cAMP/PKA signaling cascade but not by the NMDAR-activated cAMP/PKA cascade.
Materials and Methods
Brain slice preparation. Preparation of hippocampal slices was performed as described previously (Winder et al., 1999; Vanhoose et al., 2002). Briefly, hippocampi were dissected from 7- to 13-week-old male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME), and 400 μm thick transverse slices were prepared using a McIlwain chopper. Slices were placed in multiwell submerged chambers or, for Figures 1C,3,and6B, in interface recording chambers with oxygenated artificial CSF (ACSF) containing the following (in mm): 124 NaCl, 4.4 KCl, 2 CaCl2, 1.2 MgSO4, 1 NaH2PO4, 10 glucose, and 26 NaHCO3 at 26–28°C. No differences in NMDA- or isoproterenol-induced changes in GluR1 phosphorylation were observed between the submerged and interface chambers; however, slices in interface chambers were collected for analysis 5 min after drug application because of the difference in the time course of drug delivery between the two chambers. Slices were equilibrated for at least 1 hr before manipulations. After manipulations, slices were transferred directly to a metal surface in dry ice for rapid freezing and microdissection of area CA1. NMDA, isoproterenol, rolipram, betaxolol, (±)-1-[2,3-(dihydro-7-methyl-1H-inden-4-yl)oxy]-3-[(1-methylethyl)amino]-2-butanol hydrochloride (ICI-118,551), and calyculin A were obtained from Tocris Cookson (Ellisville, MO). Cyclosporin A and cypermethrin were obtained from Calbiochem (La Jolla, CA), and tetrodotoxin was obtained from Sigma (St. Louis, MO).
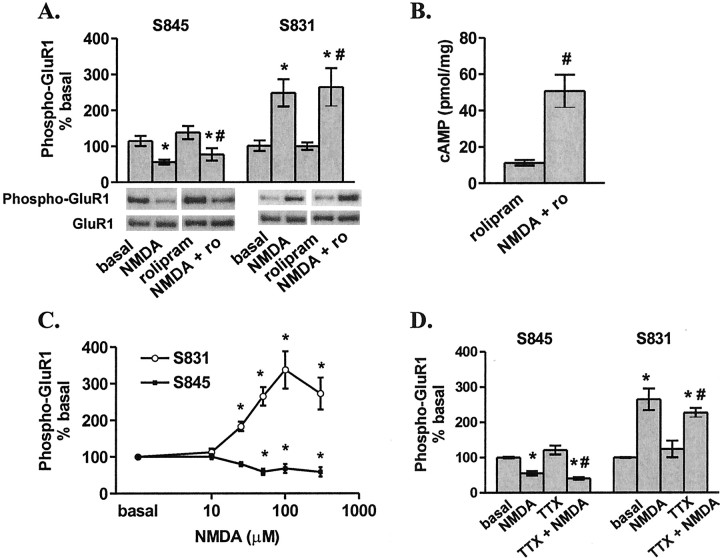
Figure 1.
NMDAR activation decreases the S845 and increases the S831 phosphorylation of GluR1 in area CA1 from mouse hippocampal slices. A, Differences from basal levels in GluR1 phosphorylation at S845 and S831 after pretreatment with rolipram (ro; 0.3μm, 30 min) or vehicle (0.01% DMSO) followed by treatment with NMDA (300μm, 3 min) (n = 4–5). B, NMDA (300μm, 3 min) increases cAMP levels in the presence of rolipram (0.3 μm, 30 min pretreatment) (n = 5). C, Differences from basal levels in GluR1 phosphorylation at S845 and S831 after treatment with varying doses of NMDA (10–300 μm, 3 min) (n = 7–14). D, Differences from basal levels in GluR1 phosphorylation at S845and S831 after pretreatment with tetrodotoxin (TTX;1μm,30 min) followed by treatment with NMDA (300μm, 3 min) (n = 4–7). *p < 0.05 compared with basal levels; #p < 0.05 compared with rolipram or TTX.
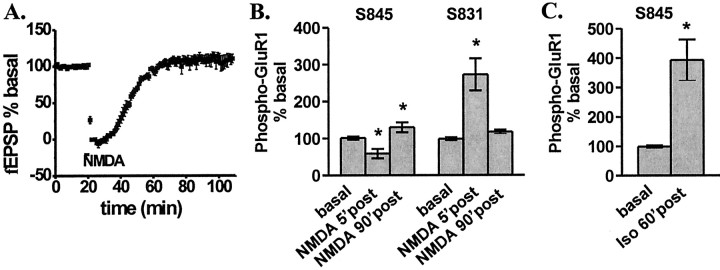
Figure 3.
NMDAR activation induces a transient change in synaptic transmission and GluR1 phosphorylation, whereas βAR activation induces a long-lasting change in GluR1 phosphorylation.A,fEPSP recordings measured in area CA1 after treatment with NMDA (300 μm, 3 min) (n = 5). Differences from basal in GluR1 phosphorylation at S845 and S831 at 5 min and 90 min after treatment with NMDA (300 μm, 3 min) in an interface perfusion chamber (B) (n = 7–19) and at 60 min after treatment with isoproterenol in an interface perfusion chamber (C) (1 μm, 15 min) (n = 11). *p < 0.05 compared with basal levels.
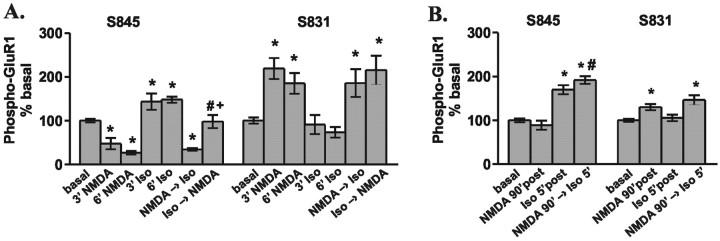
Figure 6.
NMDAR is dominant overβAR in the regulation of GluR1 phosphorylation in area CA1 from mouse hippocampal slices. A, Differences from basal levels in GluR1 phosphorylation at S845 and S831 after treatment with NMDA (300 μm, 3 and 6 min) or isoproterenol (Iso; 1 μm, 3 and 6 min) in submerged chambers. NMDA → isoproterenol indicates that NMDA was added initially (6 min) and isoproterenol was coapplied with NMDA during the final 3 min. Isoproterenol → NMDA indicates the opposite order of drug application (n = 4–6). *p < 0.05 compared with basal levels; #p < 0.05 compared with NMDA 3′; +p < 0.05 compared with isoproterenol 6′. B, Differences from basal levels in GluR1 phosphorylation at S845 and S831 after treatment with NMDA (300μm, 3 min) and isoproterenol (1μm, 3 min) in an interface perfusion chamber at 5 and 90 min after drug application. NMDA 90′ → isoproterenol 5′ indicates that NMDA was added initially, and 90 min later isoproterenol was added (n = 6). *p < 0.05 compared with basal levels; #p < 0.05 compared with NMDA 90′.
Western blotting. Western blotting was performed as described previously (Vanhoose et al., 2002). Briefly, CA1 minislices were homogenized in ice-cold homogenization buffer (TBS, 20 mm; Triton X-100, 0.5%; sodium orthovanadate, 2 mm; NaF, 2 mm). In initial experiments (see Figs. 1 A, 3B) (data not shown), homogenized samples were centrifuged at 20,000 × g for 10 min, and both soluble supernatant and insoluble pellet fractions were analyzed. Similar results were observed in the pellet fractions (data not shown); thus, in subsequent experiments a lighter centrifugation was performed (1500 × g for 10 min) to analyze a more total cellular extract. Protein levels in the soluble fraction were determined by the Bradford method, using a Bio-Rad (Hercules, CA) protein assay kit, diluted to equal concentrations, mixed with an equal volume of sample buffer (Tris-HCl, pH 6.8, 62.5 mm; glycerol, 2% SDS; bromophenol blue, 0.5%; β-mercaptoethanol, 5%), run on a 10% polyacrylamide resolving gel, and transferred to two Immobilon polyvinylidene difluoride membranes in series. The first blot was probed with specific primary antibodies, whereas the second blot was stained for total protein using colloidal gold (Bio-Rad) to verify equal lane loading grossly. In all cases the first blot was stripped and reprobed with additional primary antibodies. The primary antibodies used include anti-phospho-GluR1, S845, 1:2000; anti-phospho-GluR1, S831, 1:1000; and anti-GluR1, 1:2000, (Upstate Biotechnology, Lake Placid, NY).
Phospho-GluR1 protein signals were normalized to GluR1 protein signals, and each condition is represented as a percentage of the averaged basal samples within a single blot. Interestingly, in some cases we found that at late (90 min) time points after NMDA application, GluR1 levels were reduced, whereas total protein levels were not (data not shown). However, we observed the same patterns of phosphorylation changes regardless of whether total GluR1 changed. To assess the degree of variability of basal phospho-GluR1 between animals, in initial experiments (Fig. 1 A), all basal samples were loaded together on each gel required to accommodate all numbers of drug-treated samples. On each of the blots from these gels, the basal sample for that experiment was normalized to the average of all basal samples, which were on the blot with that sample, and the degree of variability was observed to be 14%. For all subsequent experiments, as many numbers as possible were loaded together on a gel and the basal for a given number was normalized to the average of all basal samples on the same blot. Results from repeated experiments were averaged together and differences were tested by ANOVA, followed by a Fisher's PLSD or an unpaired Student's t test.
cAMP assay. cAMP RIA assay was performed as described previously (Vanhoose et al., 2002) using a PerkinElmer Life Sciences (Boston, MA) cAMP RIA kit. Sample counts were normalized to an average protein amount determined by the Bradford assay, with CA1 minislices collected in parallel with the CA1 minislices used in the cAMP assay. Results from repeated experiments were then averaged together, and differences were tested by ANOVA followed by a Fisher's PLSD or an unpaired Student's t test.
Electrophysiology. Hippocampal slices were perfused (2 ml/min) in an interface chamber at 28°C. Field EPSP (fEPSP) recordings were obtained with ACSF-filled glass electrodes (1–3 MΩ) positioned in the stratum radiatum of area CA1. A bipolar nichrome stimulating electrode was also placed in the stratum radiatum for stimulation of Schaffer collateral afferents (0.05 msec duration). Test stimuli were applied once every 20 sec at a stimulus intensity that elicits a fEPSP slope that was ∼40% of the maximum. Experiments in which changes in the fiber volley occurred were discarded. NMDA was applied at a 10× concentration at a 1/10 flow rate using a syringe pump (Harvard Apparatus, Holliston, MA).
Results
NMDAR activation does not couple to PKA phosphorylation of GluR1
The GluR1 subunit of the AMPAR contains two phosphorylation sites that alter receptor function and are regulated in correlation with long-lasting alterations in synaptic strength in area CA1 of the hippocampus (Kameyama et al., 1998; Lee et al., 1998, 2000). In particular, phosphorylation at S845, a site targeted by PKA, is required for GluR1-containing AMPAR insertion into the synaptic membrane and for NMDAR-dependent LTP (Esteban et al., 2003). Although submaximal activation of the NMDAR, which induces LTD, signals a decrease in S845 phosphorylation (Kameyama et al., 1998; Lee et al., 1998), we predicted that maximal NMDAR activation that elicits a rise in cAMP would cause an increase in S845 phosphorylation, a signal likely to be associated with LTP. Chetkovich and Sweatt (1993) have shown previously that NMDA (EC50 value, ∼50 μm) induces a rise in cAMP in area CA1 of rat hippocampus; thus, we have explored the effects of a saturating dose of NMDA (300 μm) on GluR1 phosphorylation. Consistent with previous findings, we observe a decrease in S845 phosphorylation in response to low-level activation of the NMDAR. However, surprisingly we find that on application of a saturating dose of NMDA (300 μm) for 3 min, no increase in phosphorylation of S845 was observed, but rather a marked decrease (Fig. 1A). Similar results were observed in the presence of a phosphodiesterase inhibitor (0.3 μm rolipram), a condition in which robust cAMP elevations are observed after NMDA treatment (Fig. 1B). Although NMDAR activation does not increase the phosphorylation of GluR1 at S845, a dramatic rise in CaMKII and/or PKC phosphorylation of GluR1 at S831 does occur (Fig. 1A), suggesting highly specific regulation of both kinases and phosphatases by the NMDAR. A dose–response curve of GluR1 phosphorylation by NMDA illustrates a similar EC50 value of ∼25 μm for both the phosphorylation of S831 and dephosphorylation of S845 (Fig. 1C).
When applying NMDA to an intact hippocampal slice, the possibility that the NMDA-induced alterations in GluR1 phosphorylation could be attributable to indirect, intercellular effects must be considered. Cell firing induced by NMDA could cause an assortment of neurotransmitters to be released into area CA1; subsequent signaling via receptors other than the NMDAR may then account for the observed changes in the phosphorylation of GluR1 after NMDA application. However, this does not appear to be the case, as blockade of action potential firing by pretreatment with TTX (1 μm), a Na+ channel blocker, does not effect the NMDA-induced changes in GluR1 phosphorylation (Fig. 1D). The observed TTX independence suggests that NMDARs intracellularly signal both phosphorylation and dephosphorylation of GluR1.
Taken together, the above data suggest that signaling cascades activated by the NMDAR have access to AMPARs containing GluR1; yet there are mechanisms in place that specifically couple certain signaling cascades (e.g., the PKC or CaMKII cascade) to GluR1 while preventing the coupling of other cascades (e.g., the cAMP/PKA cascade).
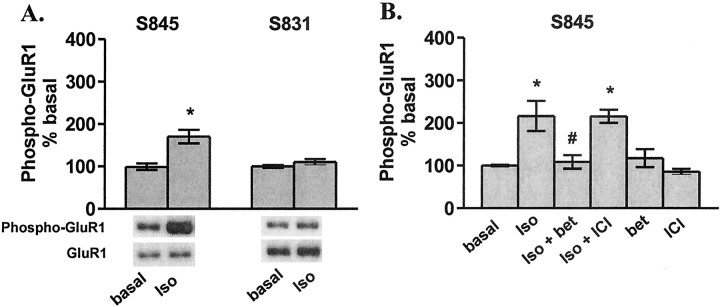
yβ1AR activation couples to PKA phosphorylation of GluR1
The observation of a lack of coupling between NMDAR-induced elevations in cAMP and PKA phosphorylation of GluR1 led us to investigate other potential regulators of PKA phosphorylation of GluR1. It has been observed previously that D1-type dopamine receptor activation signals GluR1 phosphorylation at S845 in striatal tissue (Price et al., 1999; Snyder et al., 2000; Chao et al., 2002a,b); thus, we focused on another Gs-coupled receptor, the βAR, because of its known role in the regulation of NMDAR-dependent synaptic plasticity in area CA1 of hippocampus. Indeed, we report that activation of βARs by the agonist isoproterenol (1 μm) does increase the phosphorylation of GluR1 at S845, and, as expected, βAR activation has no effect on the phosphorylation state of S831 (Fig. 2A), the PKC/CaMKII site. Both β1ARs and β2ARs are expressed in CA1 pyramidal cells (Booze et al., 1993; Davare et al., 2001); however, these two receptor subtypes are differentially targeted within the cell by differing protein–protein interactions (Hu et al., 2000) and facilitation of 5 Hz LTP by isoproterenol is mediated specifically by the β1AR (Winder et al., 1999). Thus, we tested the ability of specific antagonists of these receptors to block the isoproterenol-induced increase in S845 phosphorylation. Pretreatment of slices with the β1AR antagonist betaxolol (10 μm) but not the β2AR antagonist ICI-118,551 (10 μm), completely abolished isoproterenol-induced phosphorylation of GluR1 (Fig. 2B).
Figure 2.
β1AR activation increases S845 phosphorylation of GluR1 in area CA1 from mouse hippocampal slices. A, Differences from basal levels in GluR1 phosphorylation at S845 and S831 after treatment with isoproterenol (iso; 1 μm, 3 min) (n = 3). B, Differences from basal levels in GluR1 phosphorylation at S845 and S831 after pretreatment with betaxolol (bet; 10 μm, 10 min) or ICI-118,551(ICI;10μm,10 min) followed by isoproterenol(100 nm,6 min)(n=4).*p<0.05 compared with basal levels; #p< 0.05 compared with isoproterenol.
A saturating dose of NMDA produces transient regulation of GluR1 phosphorylation, whereasβAR activation produces a long-lasting change in GluR1 phosphorylation
When applying a high dose of NMDA, potential excitotoxicity must be considered; thus, we placed a recording electrode in area CA1 and recorded fEPSPs while applying 300 μm NMDA to the slice for 3 min. The fEPSP was transiently abolished, likely through depolarization block; however, the response returned to baseline within 20 min (Fig. 3A). In addition, the fiber volley, an index of the number of afferents being stimulated, was similarly abolished with a subsequent return to baseline (data not shown). These observations indicate that this application of NMDA does not result in significant deterioration of slice viability during the course of our experiments.
In addition, these data also show that a 3 min application of 300 μm NMDA does not elicit a long-lasting alteration of synaptic strength. Previous studies with either tetanus or lower concentrations of NMDA have shown that NMDAR-dependent LTP is associated with a persistent increase in S831 phosphorylation and that NMDAR-dependent LTD is associated with a persistent decrease in S845 phosphorylation (Kameyama et al., 1998; Lee et al., 1998, 2000). We find that maximal NMDAR activation (300 μm) induces both of these changes in GluR1 phosphorylation; however, these effects are only transient. GluR1 phosphorylation returns to near basal levels by 90 min after NMDAR activation (Fig. 3B), which is consistent with a lack of change in synaptic transmission. However, interestingly, we find that the β1AR regulation of S845 phosphorylation is long-lasting (Fig. 3C).
NMDARs and βARs increase cAMP
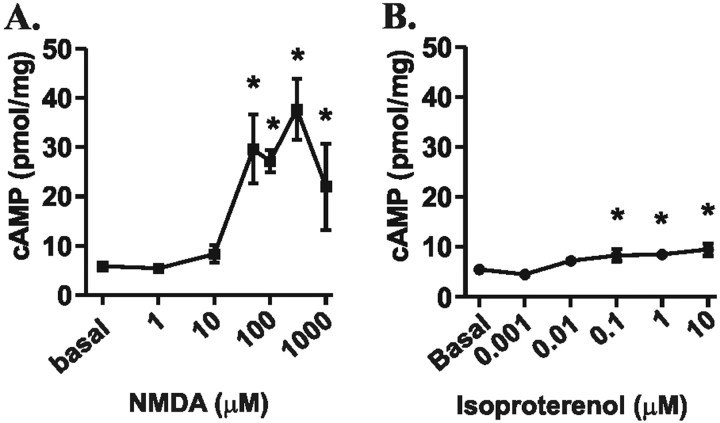
Both the NMDAR and the βAR have been shown to activate ACs and to increase cAMP levels. Thus, the observation that these two receptors signal differentially to a PKA substrate, GluR1, is surprising. One explanation is that the NMDAR-induced rise in cAMP is below the threshold for PKA phosphorylation of GluR1. However, we found that NMDA (300 μm) recruits a rise in cAMP more than three times that of isoproterenol (1 μm) (Fig. 4). This difference persisted in the presence of rolipram (0.3 μm), a phosphodiesterase type IV inhibitor, which shifted potencies of these agonists without significantly altering efficacies (data not shown). Thus, relatively small changes in cAMP levels generated by the β1AR are sufficient to elicit GluR1 phosphorylation, but the higher levels of NMDAR generated cAMP are likely not positioned optimally to signal phosphorylation of GluR1.
Figure 4.
NMDAR and βAR activation dose-dependently increase cAMP levels in area CA1 from mouse hippocampal slices, but to differing magnitudes. A, Dose–response curve of NMDA (1 μm to 1 mm, 3 min) induced changes in cAMP concentration relative to basal levels (n = 3–6). B, Dose–response curve of isoproterenol (1 nm to 10μm, 3 min) induced changes in cAMP concentration relative to basal levels (n = 3–4). *p < 0.05 compared with basal levels.
Blockade of NMDAR-induced dephosphorylation of GluR1 does not promote PKA phosphorylation of GluR1
The NMDAR-induced decrease in GluR1 phosphorylation at S845 in the presence of a dramatic rise in cAMP suggests that the NMDAR also signals robust activation of a phosphatase(s) that may override PKA activity. The NMDAR has been shown to modulate the activity of several phosphatases important for plasticity, including protein phosphatase 2B (PP2B; also known as calcineurin), protein phosphatase 1 (PP1) and protein phosphatase 2A (PP2A) (Winder and Sweatt, 2001). In addition, previous studies have demonstrated that protein phosphatases regulate the S845 phosphorylation state (Kameyama et al., 1998; Snyder et al., 2000). Thus, we tested the possibility that NMDAR activation recruits the activation of a phosphatase(s) that masks NMDAR-induced PKA phosphorylation of GluR1 by pharmacologically inhibiting phosphatases.
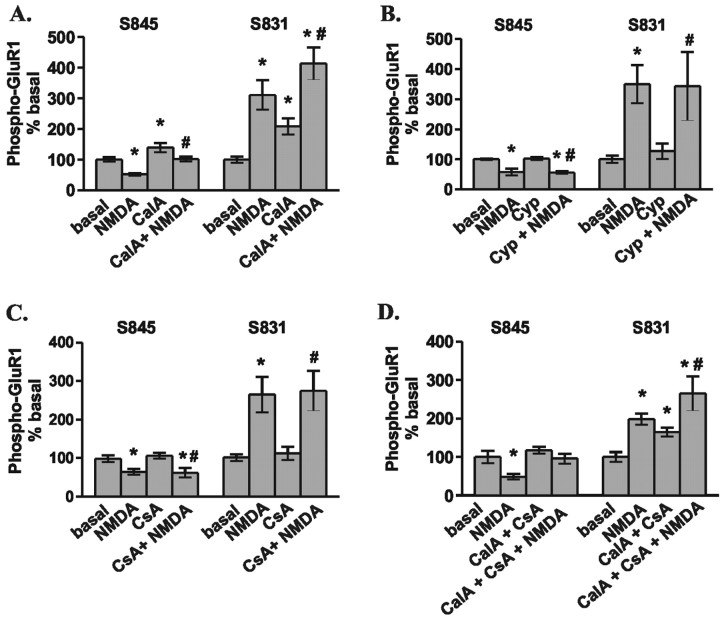
Pretreatment of hippocampal slices for at least 30 min with calyculin A (1 μm), a PP1 and PP2A inhibitor, increased basal GluR1 phosphorylation at both S845 and S831. However, NMDA-induced dephosphorylation at S845 still occurred in the presence of calyculin A, although to a significantly lesser degree (Fig. 5A). These data suggest that PP1 and/or PP2A activity plays a role in maintaining the basal level of GluR1 phosphorylation and at least partially mediates the NMDAR-induced dephosphorylation at S845 of GluR1. Inhibition of PP2B by either of two different inhibitors (cypermethrin, 10 μm; and cyclosporine A, 1 μm) resulted in no change in either basal or NMDAR-induced changes in GluR1 phosphorylation (Fig. 5B,C). Although inhibition of neither phosphatase alone completely blocked NMDAR-induced dephosphorylation of S845, concurrent inhibition of PP1, PP2A, and PP2B does completely block dephosphorylation (Fig. 5D). This suggests that multiple phosphatases have access to and do regulate GluR1 phosphorylation at S845. However, surprisingly, even in the presence of these phosphatase inhibitors, no NMDAR-induced increase in PKA phosphorylation of GluR1 was observed.
Figure 5.
Multiple phosphatases regulate NMDAR-stimulated changes in GluR1 phosphorylation in area CA1 from mouse hippocampal slices. A, Differences from basal levels in GluR1 phosphorylation at S845 and S831 after pretreatment with calyculin A (CalA; 1 μm, 30 min) or vehicle (0.1% DMSO) followed by treatment with NMDA (300 μm, 3 min) (n = 10–11). B, Differences from basal levels in GluR1 phosphorylation at S845 and S831 after pretreatment with cypermethrin (Cyp; 10μm, 30 min) or vehicle (0.1% DMSO) followed by treatment with NMDA (300μm, 3 min) (n = 4–5). C, Differences from basal levels in GluR1 phosphorylation at S845 and S831 after pretreatment with cyclosporinA (CsA;1μm,30 min) or vehicle (0.1%DMSO) followed by treatment with NMDA (300 μm, 3 min) (n = 7). D, Differences from basal levels in GluR1 phosphorylation at S845 and S831 after simultaneous pretreatment with calyculin A plus cyclosporin A (1 μm each, 30 min) or vehicle (0.2% DMSO) followed by treatment with NMDA (300μm, 3 min) (n = 4–5). *p < 0.05 compared with basal levels; #p < 0.05 compared with calyculin A, cypermethrin, cyclosporin A, and calyculin A plus cyclosporin A.
The NMDAR signal to GluR1 is dominant over the βAR signal
We have shown above that in area CA1, one cellular response to NMDAR activation is a decrease in GluR1 phosphorylation at S845. Conversely, β1AR activation elicits an increase in GluR1 phosphorylation at S845. In some forms of synaptic plasticity both of these receptors are activated; thus, we tested the effect of coactivation of these two receptors on GluR1 phosphorylation. We found that the net result of S845 phosphorylation is dependent on the order of NMDAR and βAR activation, whereas S831 phosphorylation remains independent of βAR activation. If the βAR is activated immediately before the NMDAR, there appears to be an additive effect on GluR1 phosphorylation at S845; however, if the NMDAR is activated immediately before the βAR, the βAR signal to GluR1 is completely abolished (Fig. 6A). We have shown above that the NMDAR-induced changes in GluR1 phosphorylation are transient (Fig. 3B); coinciding with this, we find that the NMDAR-induced blockade of βAR coupling to GluR1 phosphorylation is also transient, as activation of the βAR 90 min after NMDA application robustly signals GluR1 phosphorylation at S845 (Fig. 6B). These results suggest first that NMDARs and βARs likely act on a common pool of GluR1, because previous NMDAR signaling to GluR1 blocks βAR signaling to GluR1. Secondly, substrate regulation is refined by multiple incoming signals; previous βAR activation shifts the NMDAR signal to S845 of GluR1, diminishing the NMDAR effect, whereas NMDAR activation completely abolishes subsequent βAR signals to this PKA site on GluR1. Finally, the data also indicate that NMDAR regulation of GluR1 phosphorylation both directly and through the βAR is only a transient signal.
Discussion
We report that in intact hippocampal slices activation of two receptors, both of which are located on CA1 pyramidal cells and elicit rises in cAMP production, nonetheless elicit differential phosphorylation of a specific PKA substrate, S845 of the GluR1 subunit of the AMPAR. NMDAR activation does not signal GluR1 phosphorylation at S845, but rather a decrease in phosphorylation. Conversely, activation of the neuromodulatory β1AR robustly increases PKA phosphorylation of GluR1 at S845. Because phosphorylation of this site is required for NMDAR-dependent LTP, these findings identify this site as a key substrate through which heterosynaptic input may have a key role in regulating homosynaptic plasticity.
NMDAR couples to cAMP generation but is not coupled to PKA phosphorylation of GluR1 at S845
The observation that maximal NMDAR activation signals dephosphorylation of GluR1 at S845 is surprising for at least a couple of reasons. First, robust NMDAR activation produces a dramatic rises in cAMP levels that activate PKA within area CA1 of the hippocampus (Roberson and Sweatt, 1996). Secondly, NMDAR activation induces a dramatic rise in GluR1 phosphorylation at a neighboring site, S831, that is targeted by CaMKII and PKC.
Consistent with previous studies, we show here that the NMDAR robustly couples to cAMP generation in area CA1 of the hippocampus. In addition, coupling of the NMDAR and cAMP/PKA to cellular substrates has been demonstrated within this brain region. For example, NMDAR activation of the cAMP/PKA cascade induces an increase in voltage-gated Ca2+ channel activity (Chetkovich et al., 1991). Likewise, NMDAR activation suppresses the Ca2+-dependent AHP via PKA activation (Blitzer et al., 1995). Despite findings that the NMDAR signals an increase in cAMP levels, elicits a rise in PKA activation, and exerts a PKA-dependent effect on certain cellular substrates, we show here that the NMDAR does not increase PKA phosphorylation of at least one known PKA substrate, S845 of GluR1. On the contrary, NMDAR activation decreases GluR1 phosphorylation at this PKA site. Multiple phosphatases appear to mediate the NMDAR-induced dephosphorylation of GluR1, because inhibition of both PP1/PP2A and PP2B are required to block the dephosphorylation at S845. However, surprisingly, even in the presence of phosphatase inhibitors NMDAR activation does not signal PKA phosphorylation at S845. The possibility that the NMDAR recruits subthreshold levels of cAMP that are not sufficient to signal PKA phosphorylation of GluR1 is not likely, considering that the βAR signals PKA phosphorylation of GluR1 and produces only a fraction of cAMP compared with the NMDAR. In addition, enhancement of the NMDAR-induced cAMP signal with a phosphodiesterase inhibitor does not alter the inability of the NMDAR to signal PKA phosphorylation of GluR1 at S845. Considering that NMDAR expressed on hippocampal pyramidal neurons has been shown to regulate cellular substrates through PKA activation, but that this PKA activity does not couple to S845 of GluR1 challenges normal tendencies to assume that if a receptor activates a particular signaling cascade, then it must signal all downstream targets.
The observation that a closely neighboring phosphorylation site on GluR1, S831, is heavily phosphorylated by NMDAR activation whereas a simultaneous decrease in phosphorylation at S845 occurs is also very interesting. This is particularly curious given that the EC50 values for NMDAR activation to elicit cAMP generation, S845 dephosphorylation, and S831 phosphorylation are all approximately equal. Thus, these data contrast with the notion that differential recruitment of phosphatases and kinases is accomplished by low- and high-level activation of the NMDAR, respectively. The NMDAR-induced phosphorylation of S831 and dephosphorylation of S845 could be accomplished via signaling CaMKII/PKC and phosphatases to separate pools of GluR1 within the cell. Alternatively, there may be a mechanism in place to target phosphatases specifically to a single GluR1 site.
Receptor–substrate specificity
The specificity of receptor signaling to substrate that we observe here may be attributable to differential localization of signaling complexes associated with these two receptors. Although these two receptors are both expressed on CA1 pyramidal neurons and can be compartmentalized together through PDZ[postsynaptic density-95 (PSD-95)/Discs large (Dlg)/zona occludens-1 (ZO-1)]-binding domain interactions with PSD-95 (Hu et al., 2000), they may potentially activate signaling cascades that are distinctly localized within the cell. The NMDAR activates only a small subset of ACs that are Ca2+-sensitive, whereas the βAR, via Gαs, can elicit activation of a larger host of ACs. Thus, it is conceivable that distinct localization of Ca2+-sensitive ACs, ACI/VIII, allows for the NMDAR to signal to certain PKA substrates, such as voltagegated Ca2+ channels (Chetkovich et al., 1991) and K+ channels (Blitzer et al., 1995), but not others, such as GluR1. However, ACs stimulated by Gαs are presumably spatially located to target GluR1-containing AMPARs. Consistent with the idea of differential AC distribution, immunocytochemical evidence suggests that the Gαs-activated ACs types II and IV are excluded from spines in area CA1, but heavily expressed in dendritic shafts (Baker et al., 1999), whereas Ca2+-sensitive ACs are presumed to be spine-localized (Mons et al., 1995). In addition, overexpressed GluR1 localizes to dendritic shafts before activity-dependent redistribution to the spine, a process that requires PKA phosphorylation of GluR1 at S845 (Esteban et al., 2003).
Another possible explanation for the differential signaling of GluR1 phosphorylation arises from the differential activation of signaling cascades other than the cAMP/PKA cascade by the NMDAR and the βAR. The NMDAR couples to many intracellular enzymes that the βAR does not; it may be the activation of one or more of these addition signaling cascades that blocks PKA coupling to GluR1. Indeed, there is evidence in cultured neurons that NMDAR activation disrupts A-kinase anchoring protein binding to PSD-95 (Gomez et al., 2002), a complex that associates PKA with GluR1 (Colledge et al., 2000) and regulates GluR1 function (Tavalin et al., 2002). Thus, NMDAR activation may induce the disruption of a PKA and GluR1 interaction, removing PKA regulation of GluR1. Our observation that NMDAR activation blocks subsequent βAR-induced GluR1 phosphorylation is consistent with such a mechanism.
Although much work is still required to identify the mechanism(s) these cells use to allow two receptors to initiate a common signaling cascade and have differing effects on a target substrate, the fact that such a mechanism exists reflects the complexity with which cells integrate signals.
Differential phosphorylation of GluR1 by NMDARs and βARs may underlie some forms of synaptic plasticity
NMDAR activation is critical for many long-lasting changes in synaptic transmission that occur in the CNS; however, it is likely that in vivo, additional heterosynaptic signals, such as neuromodulatory influence, are required in concert with NMDAR activation to attain long-lasting alterations in synaptic efficacy. For example, although previous studies using lower concentrations of NMDA demonstrate persistent changes in GluR1 regulation and persistent enhancement or depression of synaptic transmission, we find that with higher doses of NMDA only transient effects on GluR1 phosphorylation and no persistent alteration in synaptic transmission occurs.
One example of converging receptor signals being required for synaptic plasticity is illustrated by prolonged 5 Hz stimulation that leads to a transient depression of synaptic transmission alone, but is converted to dramatic NMDAR-dependent LTP with previous β1AR activation (Thomas et al., 1996; Winder et al., 1999). Previous studies have suggested that the β1AR produces this change in synaptic efficacy through the suppression of phosphatase activity (Thomas et al., 1996; Brown et al., 2000), and the activation of ERK (extracellular signal-regulated kinase) (Winder et al., 1999; Giovannini et al., 2001; Watabe and O'Dell, 2003). However, in both cases this is viewed as an amplification of signal, because NMDAR activation alone is capable of both suppressing phosphatase activity (Blitzer et al., 1998) and activating ERK (English and Sweatt, 1996). Although these mechanisms appear to be important, specific receptor-to-substrate signaling may also play a role in this plasticity. The presently described regulation of GluR1 at S845 is unique in that phosphorylation is not produced by NMDAR activation but is robustly recruited by β1AR activation. Recently, it has been recognized that GluR1 phosphorylation at S845 by PKA is required in addition to CaMKII activation for recruitment of AMPARs to the synapse and for NMDAR-dependent LTP in area CA1 (Esteban et al., 2003). Although the NMDA receptor does activate both the CaMKII and PKA cascades, we show here that the NMDA receptor alone actually decreases PKA phosphorylation of GluR1 at S845. Alternatively, an increase in phosphorylation can be attained by activation of the β1AR, and thus in a physiological setting, a neuromodulatory signal may be required to attain redistribution of GluR1-containing AMPARs into synapses. In this context, it is interesting to note that the β1AR-induced increase in S845 phosphorylation is persistent for at least 1 hr after βAR stimulation. In future studies, it will be critical to determine to what degree these physiologically relevant stimulus patterns recruit phosphorylation of key substrates such as GluR1.
Footnotes
This work was supported by National Institutes of Health Cellular, Biochemical, and Molecular Sciences Training Grant T32 GM08554 (A.M.V.) and National Institute on Drug Abuse Grant DA13699 (D.G.W.). We thank Roger Colbran, Regula Egli, Eric Norman, Nicole Schramm, and Carl Weitlauf for critical comments on this manuscript.
Correspondence should be addressed to Dr. Danny G. Winder, Department of Molecular Physiology and Biophysics, 23rd and Pierce Avenue South, Room 724B, Robinson Research Building, Vanderbilt University School of Medicine, Nashville, TN 37232-0615. E-mail: danny.winder@vanderbilt.edu.
Copyright © 2003 Society for Neuroscience 0270-6474/03/235827-08$15.00/0
References
- Bailey CH, Giustetto M, Huang YY, Hawkins RD, Kandel ER ( 2000) Is heterosynaptic modulation essential for stabilizing Hebbian plasticity and memory? Nat Rev Neurosci 1: 11-20. [DOI] [PubMed] [Google Scholar]
- Baker LP, Nielsen MD, Impey S, Hacker BM, Poser SW, Chan MY, Storm DR ( 1999) Regulation and immunohistochemical localization ofβγ-stimulated adenylyl cyclases in mouse hippocampus. J Neurosci 19: 180-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banke TG, Bowie D, Lee H, Huganir RL, Schousboe A, Traynelis SF ( 2000) Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J Neurosci 20: 89-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitzer RD, Connor JH, Brown GP, Wong T, Shenolikar S, Iyengar R, Landau EM ( 1998) Gating of CaMKII by cAMP-regulated protein phosphatase activity during LTP. Science 280: 1940-1942. [DOI] [PubMed] [Google Scholar]
- Blitzer RD, Wong T, Nouranifar R, Iyengar R, Landau EM ( 1995) Postsynaptic cAMP pathway gates early LTP in hippocampal CA1 region. Neuron 15: 1403-1414. [DOI] [PubMed] [Google Scholar]
- Booze RM, Crisostomo EA, Davis JN ( 1993) β-adrenergic receptors in the hippocampal and retrohippocampal regions of rats and guinea pigs: autoradiographic and immunohistochemical studies. Synapse 13: 206-214. [DOI] [PubMed] [Google Scholar]
- Brown GP, Blitzer RD, Connor JH, Wong T, Shenolikar S, Iyengar R, Landau EM ( 2000) Long-term potentiation induced by theta frequency stimulation is regulated by a protein phosphatase-1-operated gate. J Neurosci 20: 7880-7887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao SZ, Ariano MA, Peterson DA, Wolf ME ( 2002a) D1 dopamine receptor stimulation increases GluR1 surface expression in nucleus accumbens neurons. J Neurochem 83: 704-712. [DOI] [PubMed] [Google Scholar]
- Chao SZ, Lu W, Lee HK, Huganir RL, Wolf ME ( 2002b) D1 dopamine receptor stimulation increases GluR1 phosphorylation in postnatal nucleus accumbens cultures. J Neurochem 81: 984-992. [DOI] [PubMed] [Google Scholar]
- Chetkovich DM, Gray R, Johnston D, Sweatt JD ( 1991) N-methyl-d-aspartate receptor activation increases cAMP levels and voltage-gated Ca 2+ channel activity in area CA1 of hippocampus. Proc Natl Acad Sci USA 88: 6467-6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetkovich DM, Sweatt JD ( 1993) nMDA receptor activation increases cyclic AMP in area CA1 of the hippocampus via calcium/calmodulin stimulation of adenylyl cyclase. J Neurochem 61: 1933-1942. [DOI] [PubMed] [Google Scholar]
- Colledge M, Dean RA, Scott GK, Langeberg LK, Huganir RL, Scott JD ( 2000) Targeting of PKA to glutamate receptors through a MAGUK-AKAP complex. Neuron 27: 107-119. [DOI] [PubMed] [Google Scholar]
- Davare MA, Avdonin V, Hall DD, Peden EM, Burette A, Weinberg RJ, Horne MC, Hoshi T, Hell JW ( 2001) A β2 adrenergic receptor signaling complex assembled with the Ca 2+ channel Cav1.2. Science 293: 98-101. [DOI] [PubMed] [Google Scholar]
- Ehlers MD ( 2000) Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron 28: 511-525. [DOI] [PubMed] [Google Scholar]
- English JD, Sweatt JD ( 1996) Activation of p42 mitogen-activated protein kinase in hippocampal long term potentiation. J Biol Chem 271: 24329-24332. [DOI] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R ( 2003) PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci 21: 21. [DOI] [PubMed] [Google Scholar]
- Giovannini MG, Blitzer RD, Wong T, Asoma K, Tsokas P, Morrison JH, Iyengar R, Landau EM ( 2001) Mitogen-activated protein kinase regulates early phosphorylation and delayed expression of Ca2+/calmodulin-dependent protein kinase II in long-term potentiation. J Neurosci 21: 7053-7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez LL, Alam S, Smith KE, Horne E, Dell'Acqua ML ( 2002) Regulation of A-kinase anchoring protein 79/150-cAMP-dependent protein kinase postsynaptic targeting by NMDA receptor activation of calcineurin and remodeling of dendritic actin. J Neurosci 22: 7027-7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu LA, Tang Y, Miller WE, Cong M, Lau AG, Lefkowitz RJ, Hall RA ( 2000) beta 1-adrenergic receptor association with PSD-95: inhibition of receptor internalization and facilitation of beta 1-adrenergic receptor interaction with N-methyl-d-aspartate receptors. J Biol Chem 275: 38659-38666. [DOI] [PubMed] [Google Scholar]
- Kameyama K, Lee HK, Bear MF, Huganir RL ( 1998) Involvement of a postsynaptic protein kinase A substrate in the expression of homosynaptic long-term depression. Neuron 21: 1163-1175. [DOI] [PubMed] [Google Scholar]
- Lee HK, Kameyama K, Huganir RL, Bear MF ( 1998) NMDA induces long-term synaptic depression and dephosphorylation of the GluR1 subunit of AMPA receptors in hippocampus. Neuron 21: 1151-1162. [DOI] [PubMed] [Google Scholar]
- Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL ( 2000) Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature 405: 955-959. [DOI] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, Yu S, Ding L, He C, Petralia RS, Wenthold RJ, Gallagher M, Huganir RL ( 2003) Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell 112: 631-643. [DOI] [PubMed] [Google Scholar]
- Mack V, Burnashev N, Kaiser KM, Rozov A, Jensen V, Hvalby O, Seeburg PH, Sakmann B, Sprengel R ( 2001) Conditional restoration of hippocampal synaptic potentiation in GluR-A-deficient mice. Science 292: 2501-2504. [DOI] [PubMed] [Google Scholar]
- Mons N, Harry A, Dubourg P, Premont RT, Iyengar R, Cooper DM ( 1995) Immunohistochemical localization of adenylyl cyclase in rat brain indicates a highly selective concentration at synapses. Proc Natl Acad Sci USA 92: 8473-8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Kim P, Raymond LA ( 1999) D1 dopamine receptor-induced cyclic AMP-dependent protein kinase phosphorylation and potentiation of striatal glutamate receptors. J Neurochem 73: 2441-2446. [DOI] [PubMed] [Google Scholar]
- Roberson ED, Sweatt JD ( 1996) Transient activation of cyclic AMP-dependent protein kinase during hippocampal long-term potentiation. J Biol Chem 271: 30436-30441. [DOI] [PubMed] [Google Scholar]
- Roche KW, O'Brien RJ, Mammen AL, Bernhardt J, Huganir RL ( 1996) Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron 16: 1179-1188. [DOI] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R ( 2001) Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell 105: 331-343. [DOI] [PubMed] [Google Scholar]
- Snyder GL, Allen PB, Fienberg AA, Valle CG, Huganir RL, Nairn AC, Greengard P ( 2000) Regulation of phosphorylation of the GluR1 AMPA receptor in the neostriatum by dopamine and psychostimulants in vivo J Neurosci 20: 4480-4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvarna NU, O'Donnell JM ( 2002) Hydrolysis of N-methyl-d-aspartate receptor-stimulated cAMP and cGMP by PDE4 and PDE2 phosphodiesterases in primary neuronal cultures of rat cerebral cortex and hippocampus. J Pharmacol Exp Ther 302: 249-256. [DOI] [PubMed] [Google Scholar]
- Tavalin SJ, Colledge M, Hell JW, Langeberg LK, Huganir RL, Scott JD ( 2002) Regulation of GluR1 by the A-kinase anchoring protein 79 (AKAP79) signaling complex shares properties with long-term depression. J Neurosci 22: 3044-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Moody TD, Makhinson M, O'Dell TJ ( 1996) Activity-dependent β-adrenergic modulation of low frequency stimulation induced LTP in the hippocampal CA1 region. Neuron 17: 475-482. [DOI] [PubMed] [Google Scholar]
- Vanhoose AM, Emery M, Jimenez L, Winder DG ( 2002) ERK activation by G-protein-coupled receptors in mouse brain is receptor identity-specific. J Biol Chem 277: 9049-9053. [DOI] [PubMed] [Google Scholar]
- Watabe AM, O'Dell TJ ( 2003) Age-related changes in theta frequency stimulation-induced long-term potentiation. Neurobiol Aging 24: 267-272. [DOI] [PubMed] [Google Scholar]
- Winder DG, Sweatt JD ( 2001) Roles of serine/threonine phosphatases in hippocampal synaptic plasticity. Nat Rev Neurosci 2: 461-474. [DOI] [PubMed] [Google Scholar]
- Winder DG, Martin KC, Muzzio IA, Rohrer D, Chruscinski A, Kobilka B, Kandel ER ( 1999) ERK plays a regulatory role in induction of LTP by theta frequency stimulation and its modulation by β-adrenergic receptors. Neuron 24: 715-726. [DOI] [PubMed] [Google Scholar]
- Wong ST, Athos J, Figueroa XA, Pineda VV, Schaefer ML, Chavkin CC, Muglia LJ, Storm DR ( 1999) Calcium-stimulated adenylyl cyclase activity is critical for hippocampus-dependent long-term memory and late phase LTP. Neuron 23: 787-798. [DOI] [PubMed] [Google Scholar]
- Xia Z, Storm DR ( 1997) Calmodulin-regulated adenylyl cyclases and neuromodulation. Curr Opin Neurobiol 7: 391-396. [DOI] [PubMed] [Google Scholar]
- Zamanillo D, Sprengel R, Hvalby O, Jensen V, Burnashev N, Rozov A, Kaiser KM, Koster HJ, Borchardt T, Worley P, Lubke J, Frotscher M, Kelly PH, Sommer B, Andersen P, Seeburg PH, Sakmann B ( 1999) Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science 284: 1805-1811. [DOI] [PubMed] [Google Scholar]